
OPERATING INSTRUCTION MANUAL
MODEL 8760CLP
TOTAL FREE CHLORINE & pH ANALYZER
REV. 0
AquaMetrix Inc.
1245 Maple Hill Ct, Unit 7
Newmarket, ON
Canada, L3Y 9E8
Tel: (800) 742-1413
(905) 954-0841
Fax: (905) 954-0415
www.aquametrix.com
N116-58 R.0

Contents
AQUAMETRIX INC.
Contents ……………………………………………….
8760CLP Menus………………………………………
INTRODUCTION…………………………………….
General …………………………………………
Features………………………………………….
Specifications…………………………………….
INSTALLATION…………………………………….
Analyzer Mounting……………………………….
8760CLP Component Identification……………...
Analyzer Wiring…………………………………..
Sensor Mounting………………………………….
Sensor Wiring……………………………………..
Instrument Shop Test Startup……………………..
STARTUP……………………………………………..
Analyzer Startup Tests………………………….
Easy to use menu………………………………...
Remembers Where You Were…………………...
Home Base: Press Sample……………………….
Display Features…………………………………
Arrow Keys……………………………………...
AUTO and MANUAL Keys………………...……
Standby Mode…………………………………….
Output Hold……………………………………….
Edit Mode…………………………………….…...
Temperature °C or °F………………………….…
Real-Time Clock……………………………..…..
Input Damping…………………………………….
APPLICATION INFORMATION…………………..
Chlorine Chemistry……………………………..
Chlorine and the Effect of pH…………………..
Disinfectant Properties of Chlorine……….…….
8760CLP CHLORINE MEASUREMENT………….
Introduction……………………………………...
Galvanic Measuring Cell…………………….….
CHLORINE SENSOR INSTRUCTIONS………..…
Chlorine Sensor, P/N A2104034, Component
Identification…………………………………...…
Assembly of the Chlorine Sensor………………...
Inserting Chlorine Sensor in the Flow Fitting.……
Removing Chlorine Sensor from Flow Fitting……
Zero Test Technique………………………………
Monthly Maintenance……………………………..
Semi-Annual Maintenance………………………..
Chemical Cleaning………………………………..
Sensor Storage…………………………………….
CHLORINE CALIBRATION……………………….
Standardizing Chlorine……………………………
PH and Temperature impact on Chlorine…………
Manual Temperature Compensation……………...
Manual pH Compensation………………………...
pH SENSOR INSTRUCTIONS……………………...
2
3
6
7
7
8
12
12
12
13
13
13
14
15
15
16
16
16
16
17
17
17
17
18
19
19
19
20
20
20
22
23
23
23
24
24
25
27
27
27
28
28
28
29
30
30
32
32
32
33
Inserting pH Sensor into Flow Fitting…………….
Removing pH Sensor from Flow Fitting…………...
Electrode Maintenance……………………………..
Sensor Storage……………………………………...
Monthly Maintenance………………………………
Yearly Maintenance………………………………..
When to Clean Sensor……………………………...
pH CALIBRATION…………………………………...
Selecting a pH Buffer………………………………
PH Buffer Use and Maintenance…………………...
Standardizing – Single-Buffer Calibration…………
Calibrating – Two-Buffer Calibration……………...
Manual Adjustment of Offset and Slope…………...
ERROR MESSAGES………………………………….
Acknowledging an Error Message…………………
Messages for Chlorine Input……………………….
Messages for Temperature Input…………………...
Messages for pH Input……………………………..
Cautions Messages for Alarms…………………….
DISPLAY PROMPTS…………………………………
GLOSSARY……………………………………………
CONFIGURATION OF PROGRAM………………..
OUTPUT SIGNALS…………………………………..
Reversing the 4 mA to 20 mA Output……………...
Simulated 4 mA to 20 mA Output………………….
Units for Outputs…………………………………...
ALARM FUNTIONS………………………………….
Use of Relay Contacts……………………………...
Alarm Indication……………………………………
Manual Alarm Override……………………………
Delayed Relay Activation………………………….
Unit Selection………………………………………
Wiring and NO/NC Contacts……………………....
High or Low Alarm………………………………...
Deviation Alarm……………………………………
Fault Alarm…………………………………………
Using Alarms for On/Off Control………………….
TROUBLESHOOTING………………………………
Analyzer: Electronic Hardware Alignment………..
Chlorine Sensor…………………………………….
pH Sensor…………………………………………..
APPENDIX A – Enabling Security…………………...
APPENDIX B – Default Settings……………………..
APPENDIX C – Installation…………………………..
DRAWINGS……………………………………………
D4040081: Outline and Mounting Dimensions……
D5030269: Main Board Component Location…….
D5980176: Display Board Component Location…..
D5040276: Wiring Diagram………………………..
INDUSTRIAL PRODUCTS WARRANTY………….
INDEX………………………………………………….
33
33
33
33
34
34
34
36
36
37
38
39
39
40
40
41
41
42
43
44
46
47
49
49
49
49
50
50
50
51
51
51
51
52
52
53
53
54
54
56
57
59
62
63
64
64
65
66
67
68
69
1-800-742-1413 www.aquametrix.com 2
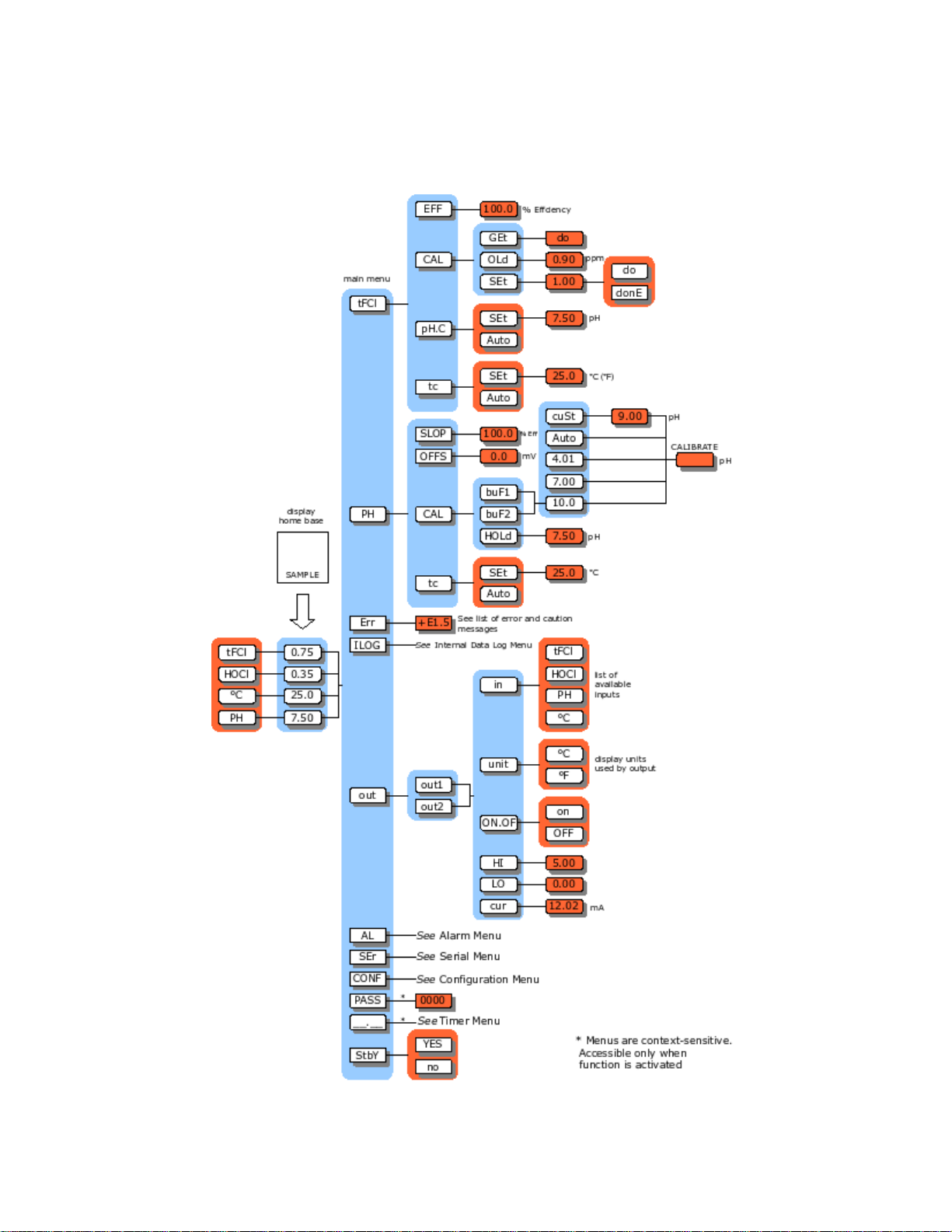
AQUAMETRIX INC.
I
8760CLP Menus
llustration 1: Menu overview
1-800-742-1413 www.aquametrix.com 3
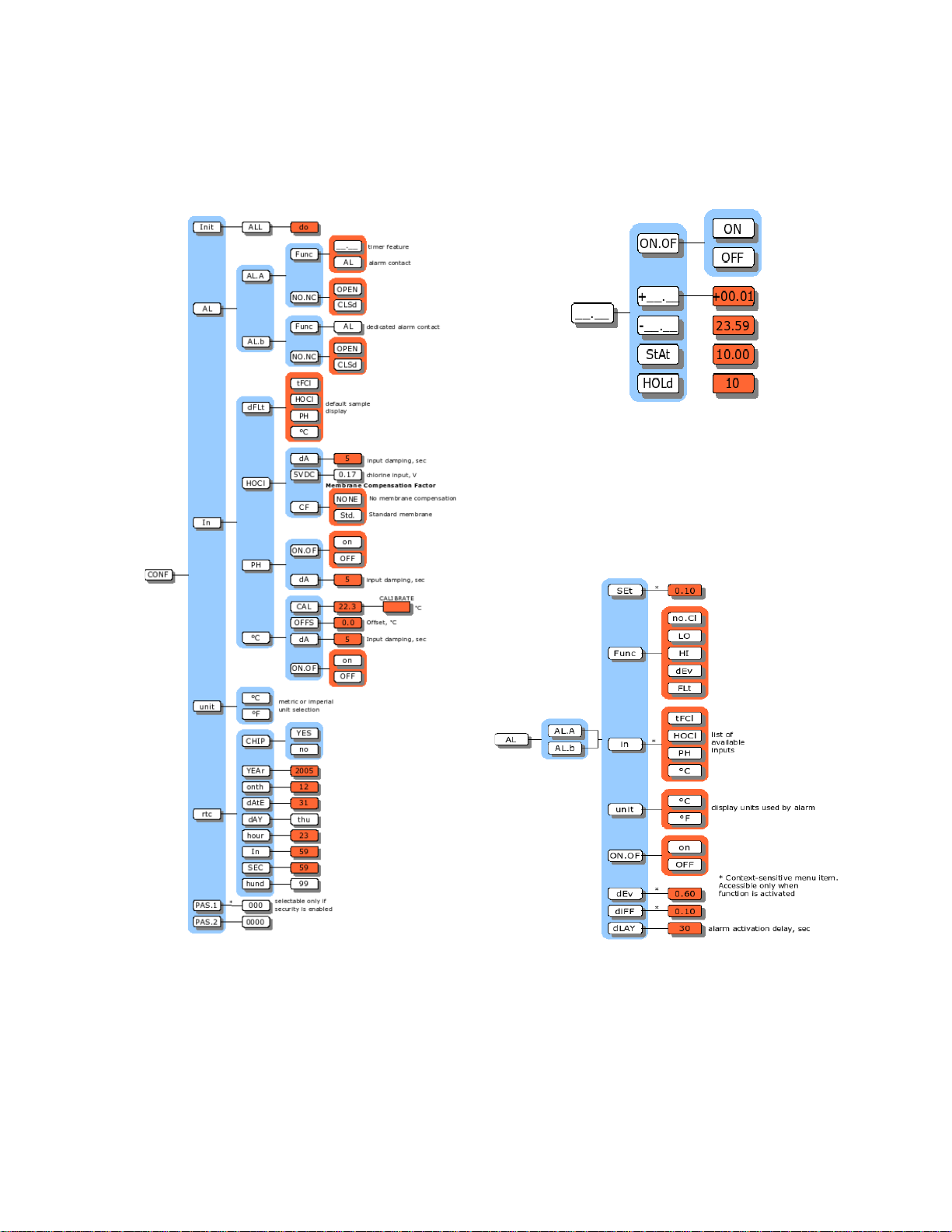
8760CLP Menus
I
I
I
AQUAMETRIX INC.
llustration 3: Timer menu
llustration 2: Configuration menu
1-800-742-1413 www.aquametrix.com
llustration 4 Alarm menu
4
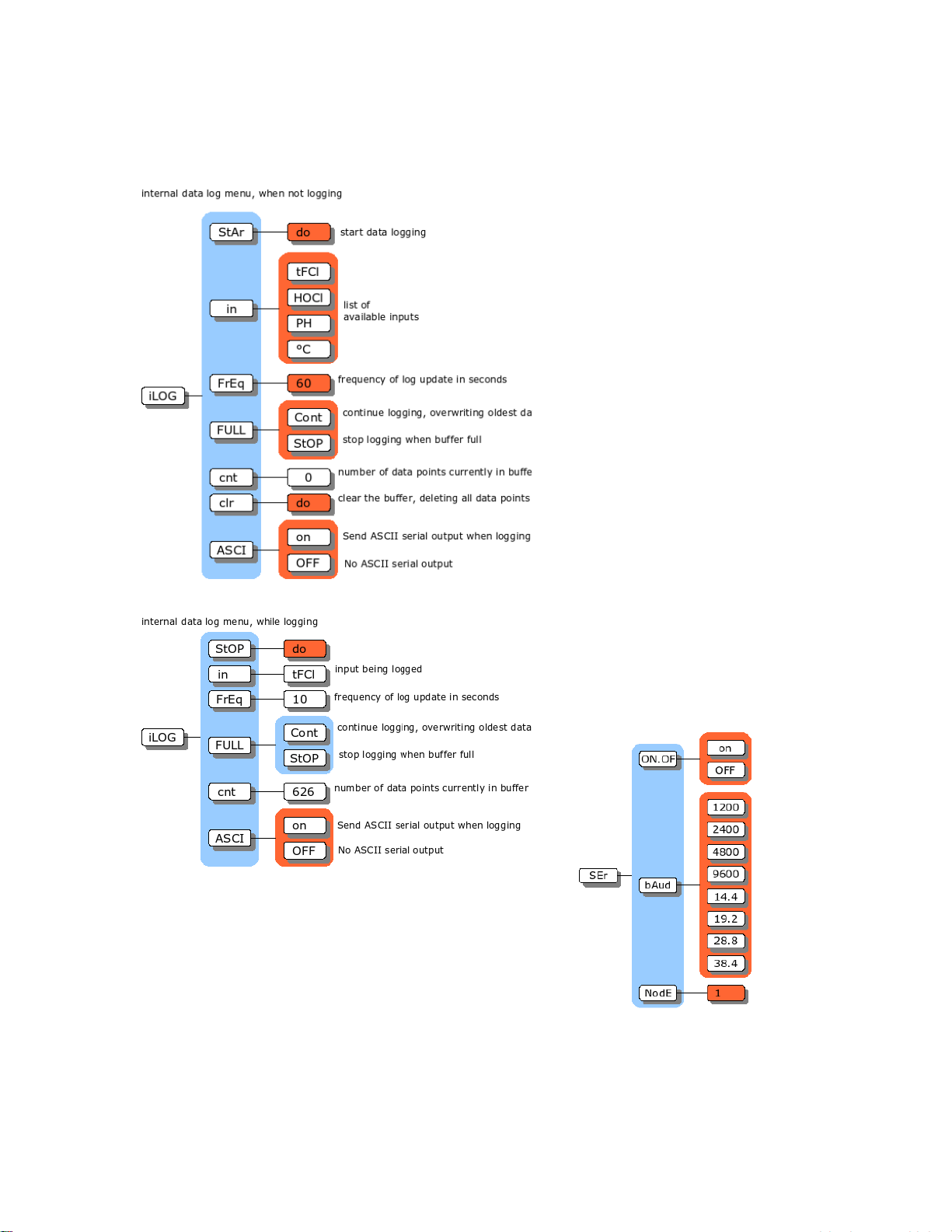
AQUAMETRIX INC.
I
I
llustration 5: Internal data log menu
llustration 6: Serial menu
1-800-742-1413 www.aquametrix.com
5
AQUAMETRIX INC.
INTRODUCTION
The model 8760CLP is AquaMetrix’s industrial quality remote operational total free chlorine and pH
analyzer, designed to provide maximum flexibility, reliability, and ease-of-use. The model 8760CLP
analyzer has been designed to include a pH input to measure sample pH for continual pH
compensation ― ideal for samples with fluctuating pH values. Temperature compensation is obtained
via a temperature sensor in the chlorine sensor.
The chlorine sensor used with the 8760CLP is a galvanic cell that is separated from the process by a
chlorine permeable membrane. As the hypochlorous acid (HOCl) in the process diffuses through the
membrane, a galvanic reaction occurs which produces a current that is proportional to the free available
chlorine concentration. An advantage of the galvanic cell is that an absolute zero measurement can be
obtained; no chlorine present equals no chlorine produced. Many manufacturers use amperometric
technology as opposed to galvanic. Amperometric cells rely on an induced voltage to produce a current.
Since this residual current is always present, an absolute measurement cannot be obtained and the HOCl
concentration measured may be artificially high. Another disadvantage of the amperometric method,
that does not affect galvanic measurement, pertains to iron coating. Polarization attracts iron ions that
may be in the process water which can cause coating of the membrane; iron deposits on the membrane
can skew the chlorine readings.
NOTICE OF COMPLIANCE
US
This meter may generate radio frequency energy and if not installed and used properly, that is, in strict accordance with
the manufacturer’s instructions, may cause interference to radio and television reception. It has been type-tested and
found to comply with the limits for a Class A computing device in accordance with specifications in Part 15 of FCC
Rules, which are designed to provide reasonable protection against such interference in an industrial installation.
However, there is no guarantee that interference will not occur in a particular installation. If the meter does cause
interference to radio or television reception, which can be determined by turning the unit off and on, the user is
encouraged to try to correct the interference by one or more of the following measures:
* Reorient the receiving antenna
* Relocate the meter with respect to the receiver
* Move the meter away from the receiver
* Plug the meter into a different outlet so that the meter and receiver are on different branch
circuits
If necessary, the user should consult the dealer or an experienced radio/television technician for additional suggestions.
The user may find the following booklet prepared by the Federal Communications Commission helpful: How to Identify
and Resolve Radio-TV Interference Problems. This booklet is available from the U.S. Government Printing Office,
Washington, D.C., 20402. Stock No. 004-000-00345-4.
CANADA
This digital apparatus does not exceed the Class A limits for radio noise emissions from digital apparatus set out in the
Radio Interference Regulations of the Canadian Department of Communications.
Le present appareil numérique n’ émet pas de bruits radioélectriques depassant les limites applicables aux appareils
numériques (de la class A) prescrites dans le Règlement sur le brouillage radioélectrique édicté par le ministère des
Communications du Canada.
1-800-742-1413 www.aquametrix.com
6

AQUAMETRIX INC.
General
The 8760CLP is supplied in a corrosion resistant IP65 (NEMA 4X) watertight, dust-tight case. The
analyzer measures the sensor signal corresponding to the actual chlorine with respect to the sample pH
and temperature. The analyzer digitizes the signal for maximum accuracy, conditions it and then sends
it out as a digital output and/or on 4 mA to 20 mA outputs.
The model 8760CLP comes as a complete sample conditioning system. The analyzer is mounted on a
CPVC panel with a dual flow cell containing the pH and chlorine sensors. The sample conditioning
system includes a pressure regulator valve, head tank, sample point and atmospheric drain. The only
installation requirement of the user is to mount the panel and supply plumbing to the inlet and from the
outlet. A chlorine and pH calibration kits are supplied with the unit.
Features
The model 8760CLP total free chlorine and pH analyzer has the following features:
• No reagents required: reagent based analysis typically require a separate waste outlet. Added
reagents also require time for reaction, therefore, there is usually a lag time in response. No reagents
allow for reduced stock and maintenance costs.
• No mechanical parts: since direct measurement does not require reagents to be added and sample
mixing, there is no need for additional pumps, tubing etc., which also reduces the maintenance
required.
• Immediate response without lag time: the direct measurement method used with the model 8760CLP
gives instantaneous results. By comparison, systems that use reagents require time for the sample to
react with the reagent in the sample chamber, thus introducing a lag time.
• Galvanic technology: for better calibration with absolute zero. Galvanic technology does not attract
iron to the sensor tip, therefore, the sensor requires less cleaning and maintenance.
• Easy to replace membrane.
• pH measurement and compensation for better accuracy.
• Intuitive user-friendly program that is easy to use.
• Grab sample calibration for chlorine.
• Self and sensor diagnostics.
• Two programmable 4 mA to 20 mA outputs.
• Two programmable alarms.
• Serial digital output and remote operation.
• Three level security to protect settings.
1-800-742-1413 www.aquametrix.com
7

AQUAMETRIX INC.
Specifications
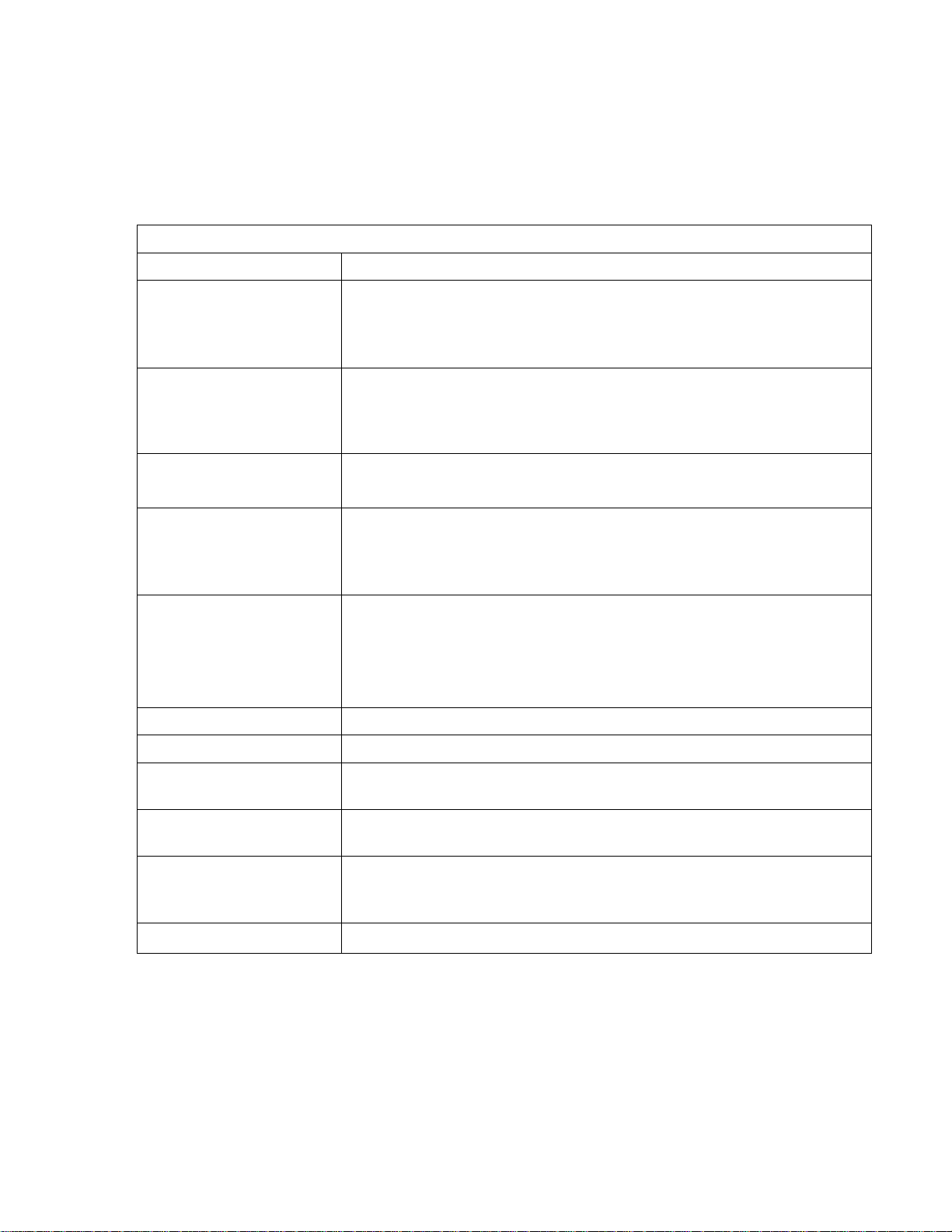
Specifications
Analyzer; 8760CL
Physical Data
PROPERTY CHARACTERISTIC
Display Four LCD digits, 1.5 cm (0.6 in) displays for total free available chlorine
(tFC1) and diagnostic information (back –lit display optional)
Display Ranges Total free available chlorine (tFC1): 0.00 mg/L to 5.00 mg/L
Free available chlorine (HOC): 0.00 mg/L to 2.00mg /L
PH: 0 pH to m14 pH units.
Temperature: -5.0 °C to 105 °C (23.0 °F to 221 °F)
Keypad 8 push button entry keys
LED’S 2 alarms (A and B). 1 auto, 1 error
Analyzer
Dimensions
Panel
Dimensions
Weight 9.1 kg (20.0 lb)
Shipping
Weight
Shipping
Dimensions
PROPERTY CHARACTERISITCS
Temperature
Environment
Ratings
Electrical
Ratings
Electrical
Requirements
12.0cm (H) x 20.0 cm (W) x 7.5 cm (D)
[4.7 in (H) x 7.9 in (W) x 3.0 in (D) ]
36 cm (W) x 66cm (H) [14 in (W) x 26 in (H) ]
11.4 kg (25.0 lb)
71 cm x 41 cm x 20 cm
(28 in x 16 in x 8 in)
Environmental Data
Operational: 5.0 °C to 45 °C (41.0 °F to 113 °F)
Storage: -10 °C to 55 °C (14.0 °F to 55 °F)
Relative Humidity: 95% maximum; non-condensing
Housing: IP (Nema 4X)
Pollution Degree: 2
Installation Category: II
115/230 VAC, 0.25 A, 50/60 Hz
115/230 VAC ± 10%, 50 W
1-800-742-1413 www.aquametrix.com
8
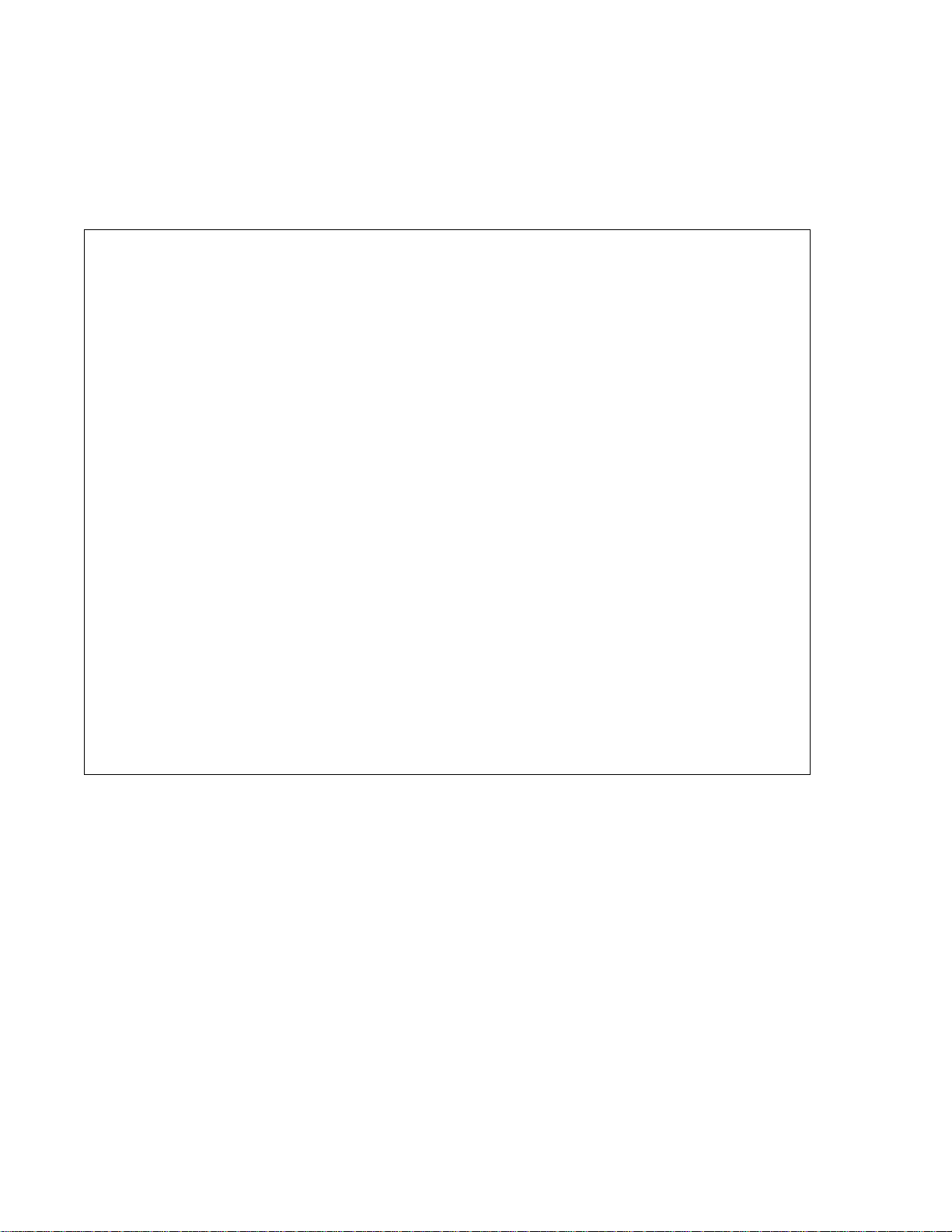
Specifications
Analyzer 8760CL
PROPERTY CHARACTERISTIC
AQUAMETRIX INC.
Operational Data
Accuracy
Precision
Response Time 90% within 5 s (default), function of flow and temperature
Temperature
Compensation
Sample Conditions Flow: 50mL/min to500mL/min
Sample Inlet ½ in barb fitting
Chlorine: ±0.02 mg/L
PH: ±0.04 ph units
Temperature: ±0.1 C
Chlorine: ±0.01 mg/L
PH: ±0.02 pH units
Temperature: ± 0.1 °C
Damping adjust: 3 to 99’s
Automatic temperature compensation via 1000 Ω Pt RTD.
Auto: -5 °C to 105 (23.0 °F to 221 °F)
Manual -5 °C to 105 (23.0 °F to 221 °F)
Temperature: 2 °C to 45 °C (35.0 °F to 113°F)
Pressure: <400 kPa (60 psi, 4 bar)
Drain: Atmospheric
Sample Outlet ½ in barb fitting
Security 3 access-levels security; partial and /or all settings may be protected via 3
and /or 4 digit security code.
Alarms Two continuous, assignable, programmable configurable, fail safe,
NO/NC alarm relays: SPDT, Form C, rated 10 A 115 V/5 A 230 V
Outputs Two continuous, assignable, programmable 4 mA to 20 mA, or 0 mA to
20mA outputs; isolated, max. Load 600 Ω; Convertible from 1 VDC to 5
VDC or 0 VDC to 5 VDC.
Communication
Via RS485 bi-directional serial data port; require IC Net™ 2000 software.
1-800-742-1413 www.aquametrix.com
9
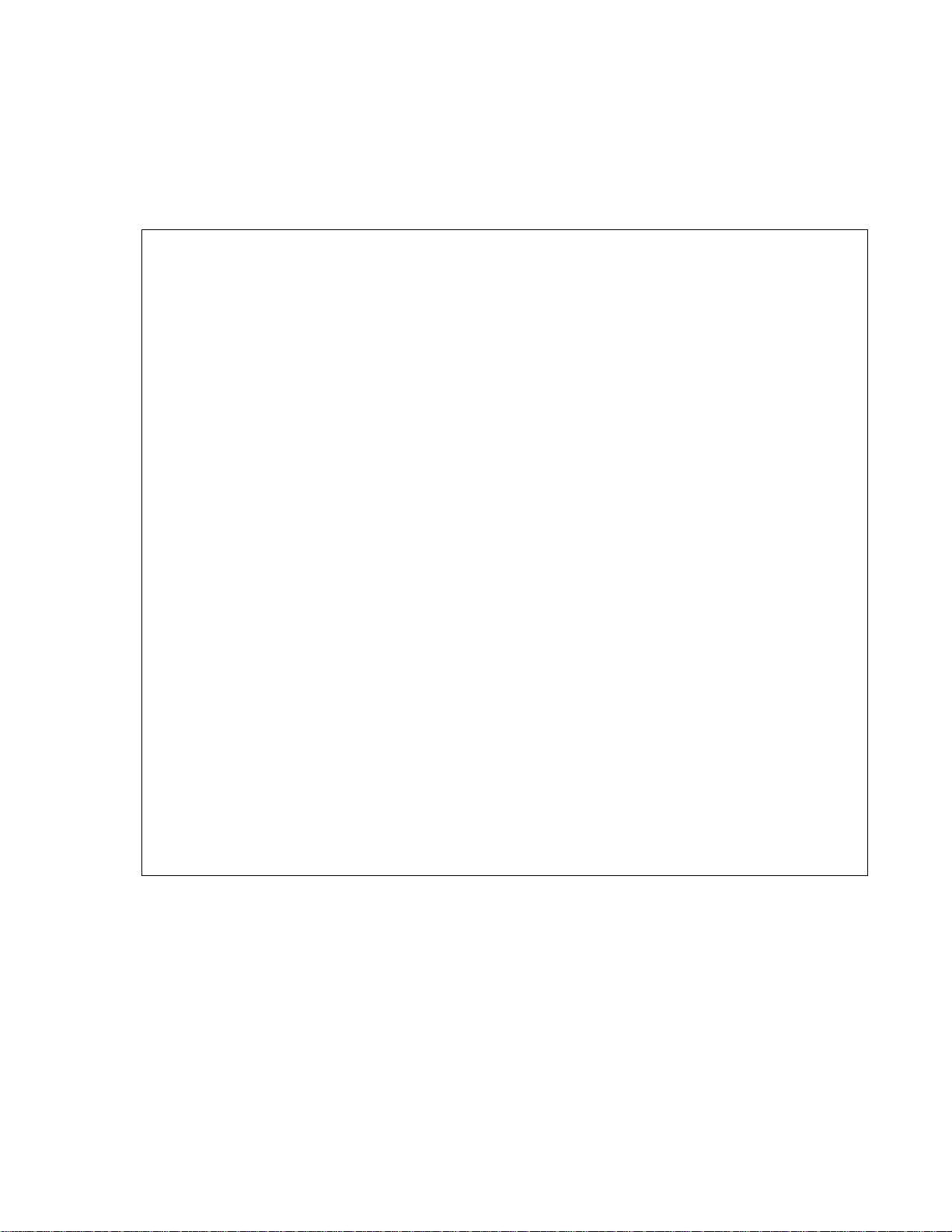
AQUAMETRIX INC.
Specifications
AM-A2104033 pH Sensor
Measurement Range.........................................................................................0 pH to 14 pH units
Minimum Temperature.................................................................................................0 °C (32 °F)
Maximum Temperature..........................................................................................100 °C (212 °F)
Maximum Pressure..............................................................................................689 kPa (100 psi)
Minimum Flow Velocity........................................................................................15 cm/s (0.5 ft/s)
Wetted Materials....................................................................................CPVC, PTFE, Viton, Glass
Electrode Dimensions
Diameter.....................................................................................................................2.3 cm (0.9 in)
Length.......................................................................................................................16.5 cm (6.5 in)
Process Connection..............................................................fixed in the flow cell via a 1 in MNPT
CPVC quick connect insertion fitting
Sensor Cable.........................................................................................2 conductor; 1.5 m (5 ft) in
length with BNC connector
Preamplifier.........................................................................................................................Remote
Weight.........................................................................................................................0.5 kg (1.1 lb)
Shipping Weight........................................................................................................0.9 kg (2.0 lb)
Shipping Dimensions.................................................................................30 cm × 23 cm × 23 cm
(12 in × 9 in × 9 in)
1-800-742-1413 www.aquametrix.com
10
AQUAMETRIX INC.
Specifications
AM-A2104034 Chlorine Sensor
Measurement Range
Free Available Chlorine (HOCl)....................................................................0.00 mg/L to 2.00 mg/L
Total Free Available Chlorine (HOCl + OCl⎯)................................................0.00 mg/L to 5.00 mg/L
Minimum Temperature.................................................................................................0 °C (32 °F)
Maximum Temperature............................................................................................80 °C (176 °F)
Maximum Pressure..............................................................................................621 kPa (90 psi)
Principle of Operation.......................................................................................................Galvanic
Electrode Materials
Cathode......................................................................................................................................Gold
Anode.......................................................................................................................................Silver
Minimum Flow Velocity........................................................................................15 cm/s (0.5 ft/s)
Wetted Materials..............................................................................................CPVC, PTFE, Viton
Electrode Dimensions
Diameter.....................................................................................................................2.3 cm (0.9 in)
Length.......................................................................................................................16.5 cm (6.5 in)
Process Connection..............................................................fixed in the flow cell via a 1 in MNPT
CPVC quick connect insertion fitting
Sensor Cable...............................................................................4 conductor; 1.5 m (5 ft) in length
with 5-pin DIN connector
Weight.........................................................................................................................0.5 kg (1.1 lb)
Shipping Weight........................................................................................................0.9 kg (2.0 lb)
Shipping Dimensions.................................................................................30 cm × 23 cm × 23 cm
(12 in × 9 in × 9 in)
1-800-742-1413 www.aquametrix.com 11

AQUAMETRIX INC.
I
INSTALLATION
Analyzer Mounting
The model 8760CLP comes as a complete sample conditioning system. The analyzer is mounted on a
CPVC panel with a dual flow cell containing the pH and chlorine sensors. The sample conditioning
system includes a pressure regulator valve, sample point and atmospheric drain. The only installation
requirement of the user is to mount the panel and supply plumbing to the inlet and from the outlet.
The panel mounts on a wall via four ⅜ inch bolts at 12¼ inch x 24¼-inch centers; refer to drawing
D4040081 for mounting dimensions. Sample inlet and outlet plumbing hookup is via a ½ in barb fitting.
Analyzer Wiring
8760CLP Component
Identification
A) Identification label; indicates complete model
number and serial number
llustration 7: 8760CLP component identification
B) Analyzer, model 8760CL
C) pH sensor, P/N AM-A2104033
D) Chlorine sensor, P/N AM-A2104034
E) Atmospheric drain
F) Flow cell; a cleaning injection port is located
on the underside of the chlorine sensor flow cell
housing (hidden from view by the pressure
gauge)
G) Pressure gauge
H) Pressure regulator
I) Flow control/shut-off valve
J) Inlet
K) Outlet
1-800-742-1413 www.aquametrix.com
12

AQUAMETRIX INC.
ANALYZER WIRING
Please refer to drawing D5040276 and perform the following:
1. The 8760CLP requires 115 VAC or 230 VAC power to be hooked up to TB400. Power consumed is
less than 1 A so generally 16 gauge wire is OK (consult local electrical codes for verification). For
stable operation, the microprocessor needs a good earth ground.
CAUTION: Confirm that the 115/230 VAC switch is correctly set for your feed.
2. If required, connect the two relay contacts; as supplied, they are not powered. They are typically
used as L1 (HOT) circuit ON-OFF switches, in NO (normally open) configuration to control the
chlorine or acid (pump/valve). Best practice uses a separate circuit to isolate the sensitive sensing
circuits from any pump or solenoid inductive surges however, as a convenience for light loads, a 3 A
circuit fuse can be installed at F402 to feed the 8760CL L1 HOT to COM on relay A.
Alarm A contact TB300, closest to AC lines.
Alarm B contact TB301.
3. If required, connect the two-isolated 4 mA to 20 mA outputs, these are 24 VDC.
Output 1, TB303, closest to the relays.
Output 2, TB304.
4. Connect the inputs.
Chlorine sensor is direct connected to the analyzer via a 5-pin DIN connector.
pH sensor is direct connected to the analyzer via a BNC connector.
Sensor Mounting
Optimum sensor performance with minimum user effort is provided through the use of the factory
integrated sample system; 35.5 cm x 66.0 cm (14 in x 26 in) CPVC sample panel with pressure
regulating valve, flow setting valve, atmospheric break, grab sample point, drain, plus dual flow cell
housing the chlorine and pH sensors. The chlorine sensor and pH sensor are fixed in the flow cell via a
1 in MNPT CPVC quick connect insertion fitting.
The sensors are mounted within the sensor lead length, as near as possible to the chlorine analyzer. The
flow cell is arranged so that the sensors are mounted on a 45-degree rising line, with the sensor's tip
down at an angle anywhere from 15 degrees above horizontal to 15 degrees vertical. 45 degrees above
horizontal is best because air bubbles will rise to the top and grit will sink, both bypassing the sensor.
The pressure-regulating valve installed before the flow cell functions to control and stabilize flow. The
atmospheric drain allows for the collection of representative samples without disturbing sample
conditions and acts as a vent for bubbles. The drain line should be larger than the sample line to allow
for purging of sediments, bubbles, biologicals and other debris.
Sensor Wiring
The basic wiring scheme for AquaMetrix chlorine sensor and pH sensor is shown in drawing D5040276.
This wiring scheme is intended for cable lengths less than 20 meters (65 feet) where electrical
interference is low. The chlorine sensor has a 5-pin DIN connector and the pH sensor has a BNC
connector. This allows the sensors to be connected and disconnected easily at the analyzer.
Take care to route all signal wiring away from AC power lines, to minimize unwanted electrical
interference. Avoid twisting the sensor lead, to minimize possibilities for broken wire. Make sure that
the sensor connections are clean and tight.
1-800-742-1413 www.aquametrix.com
13

AQUAMETRIX INC.
I
k
Instrument Shop Test Startup
1. Apply 115/230 VAC power to the analyzer.
2. Hook up the chlorine sensor to bottom of analyzer via 5-pin DIN connector. Ensure that the shorting strap on
the sensor connector is removed (refer to illustration 8). Keep shorting jack for future use.
3. With the chlorine sensor in air, the 8760CL analyzer should come up reading 0.0 ppm ± 0.05 ppm.
4. Run an “air” zero check; use wires to be field installed and allow 30 minutes warm-up time for the electronics
to stabilize.
5. Run a “span” check. In the [tFCl] menu, change to these settings: [tc] [SEt] [25.0] and [pH.C] [SEt] [7.50].
A fairly accurate 1 ppm chlorine standard can be made from commercially available bleach; use a fresh 5.25%
solution.
a. Pipet 0.1 mL of bleach into a 1.0 L volumetric flask.
b. Fill to mark with deionized water. This will produce a 5 ppm standardizing solution.
c. Pipet 20 mL of the 5 ppm solution into a 100 mL volumetric flask.
d. Fill to mark with deionized water. This solution should be used immediately after prepared
and then discarded after 2 hours.
e. Fill a plastic beaker with the 1-ppm chlorine standard and place the chlorine sensor into the
beaker and stir.
f. Wait 10 minutes; the 8760CL should read 1.0-ppm ± 0.3 ppm.
Return the [tc] and [pH.C] settings back to [Auto].
6. To check for general performance, place the chlorine sensor in running tap water (chlorinated tap water should
be between 0.2 ppm and 1.0 ppm). The display should read in that range.
7. Hook up the pH sensor via the BNC connector on the underside of the 8760CL analyzer and remove orange
protective cap from sensor tip. Keep the cap for future use.
8. With the pH sensor in pH 7 buffer, the pH analyzer should display a reading of 7.0 ± 0.5 pH.
9. Run a “zero” calibration; 7 pH is equivalent to 0.0 mV so use pH 7 buffer.
10. Run a “span” calibration by placing the sensor in pH 4 buffer. The display should read approximately
4.01 ± 0.05 pH.
11. To check for general performance, place the pH sensor in pH 7 buffer again. It should now read approximately
7.0 ± 0.05 pH.
12. The sensor is now ready for field installation.
13. If the application will be in the caustic region, repeat steps 10 & 11 using pH 10 buffers so that the sensor is
tested in the region of use.
14. Before placing the 8760CL analyzer into operation, verify
the settings to ensure that they agree with the intended
setup. Factory defaults are listed in Appendix B. For the 4
mA to 20 mA output, set high limit and low limit.
Set preference for temperature units, ° C or ° F in [CONF]
15.
[unit]; default is ° C.
16. Set desired input signal damping, if known; default is
5 seconds.
17. The analyzer is now ready for field installation.
llustration 8: Pin location for chlorine sensor shorting jac
1-800-742-1413 www.aquametrix.com
14

AQUAMETRIX INC.
STARTUP
If the analyzer is new and has not been installed, follow the procedures described in Installation and
Configuration of Program before mounting. Mounting and wiring procedures for new installations vary
with equipment options — see drawing section for instructions. If the analyzer has been previously
installed, all that is required is to attach the electrode to the analyzer and then to turn on the power.
The analyzer will go through its automatic startup procedure any time power to the analyzer was lost for
more than a few seconds. The startup procedure initializes the analyzer program, performs error
checking, and then proceeds to display the chlorine and operate the analyzer normally.
All program settings, calibration settings, and the analyzer will have remembered defaults, as the
memory is none volatile.
Analyzer Startup Tests
The startup procedure will begin by alternately flashing [tESt] and [——] and blinking the top LED
while performing the memory tests. The analyzer will then display in sequence the analyzer number, in
this case [8760CL], any software option numbers, and the program version number, eg.[2.10]. The
program then proceeds to the display test that will light each of the implemented display segments in
turn. At the same time each of the LEDs will be lighted. If the analyzer passes all the tests, then the
hardware is functioning properly and the analyzer will proceed to display total free chlorine.
If the analyzer displays +Err or -Err, this indicates that the input is off-scale. The error LED will be
lighted as long as any input is off-scale. An off-scale error can indicate that the electrode is not in
solution, is off-scale, or is not connected properly. If the error LED remains lighted, go to the error
display section (select [Err] from main menu) to see what errors the analyzer has detected.
Calibration Settings Retained
If the analyzer was calibrated previously then the analyzer will use the calibration settings from the last
successful calibration, otherwise default settings are used. Error and caution messages generated during
the last calibration will remain in effect. AquaMetrix recommends a full chemical calibration of
chlorine after initial startup. Refer to the Chlorine Calibration section.
Analyzer settings and parameters can be viewed and/or changed at any time. Refer to the menus on
pages 3 to 5; the areas shaded in dark gray indicate program settings.
1-800-742-1413 www.aquametrix.com
15
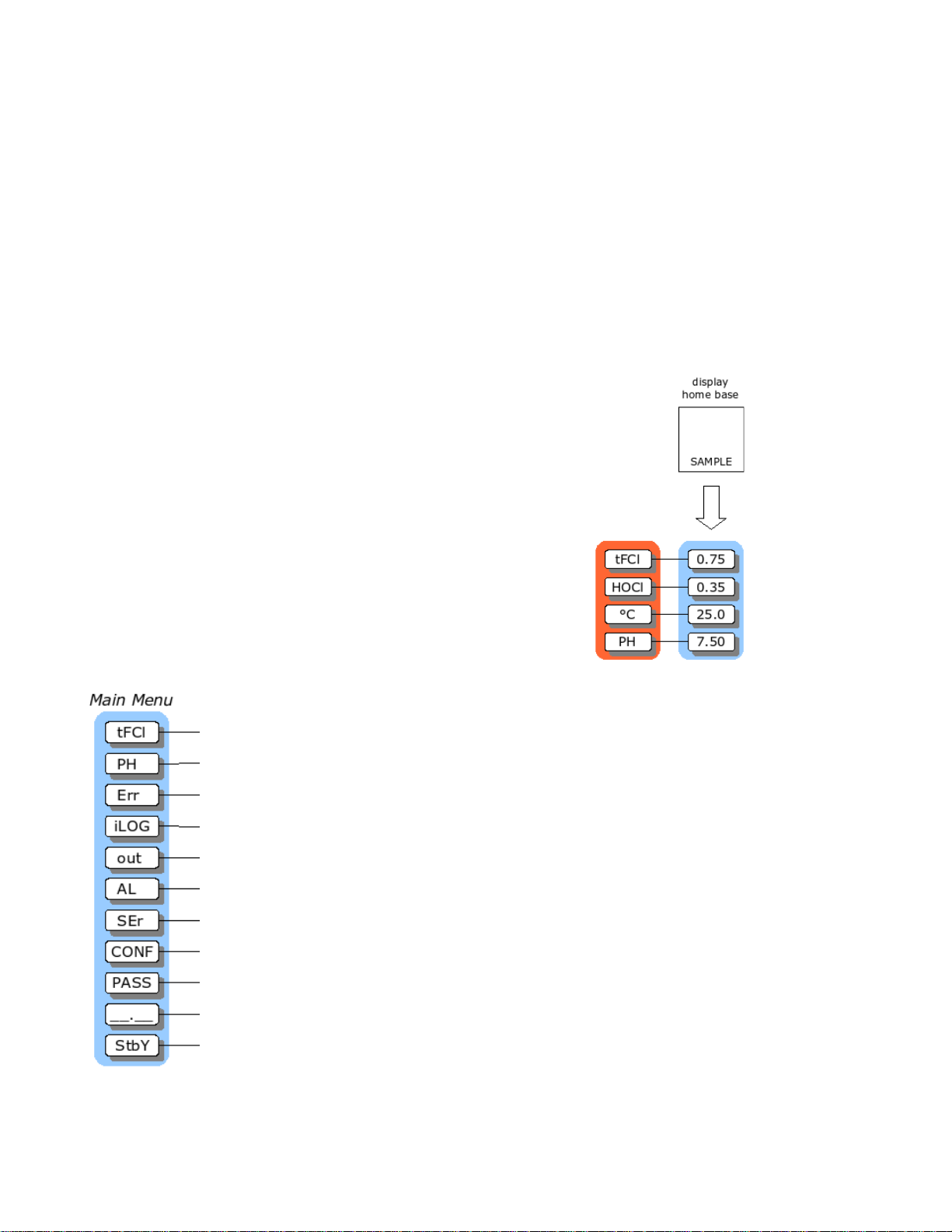
AQUAMETRIX INC.
I
Easy to use Menu
The layout of the program is shown in the menus starting on page 3.
Remembers Where You Were
The analyzer remembers where SAMPLE is. The sample display is
home base for the program. The program also remembers which menu
selections were used last and loops around the columns. The menu can
be accessed using the arrow keys to find any parameter then press
SAMPLE to return to the displayed reading. Then, using the Right
arrow key return to exactly where you were.
Home Base: Press Sample
From anywhere in the menu, the SAMPLE key can be used to return to
displaying tFCl. The program will safely abort whatever it was doing at
the time and return to displaying the tFCl reading.
The tFCl display is the default sample display for the analyzer. The
analyzer's inputs, tFCl, HOCl, pH and temperature, are arranged
underneath each other at the left-hand side of the menu. Use the Up or
Down arrow key to display each of the readings in turn.
Display Features
1. The analyzer has a built-in timer, which returns the program to
displaying tFCl if no key is pressed for 15 minutes. This time-out has
the same effect as pressing the SAMPLE key. If security has been
enabled, then the time-out will change the access level back to 0 or 1
automatically, which gives the user read-only access. The user will
have to enter an appropriate password to go to a higher access level.
2. When the sample value is displayed, pressing the Left arrow key will
show which of tFCL, HOCl, pH or temperature is displayed. Pressing
Right arrow key displays the sample reading again.
3. The main sample, i.e. the input that is displayed first when the SAMPLE
key is pressed, can be changed. By default the main input is [tFCl].
Change the default in [CONF] [in] [dFLt]. Refer to the Configuration
of Program section for further details.
llustration 10: Main menu
Illustration 9: Home base
1-800-742-1413 www.aquametrix.com
16
AQUAMETRIX INC.
I
d
Arrow Keys
The four arrow keys on the keypad are used to move around in the menu.
Example:
Press SAMPLE to make sure that display is at home
base. Press the Right arrow key. One of the prompts
in the column starting with [out] will be displayed.
Use the Up or Down arrow keys to display the prompt
above or below. If the prompt at the top or the bottom
is displayed, the program will loop around. Press the
Up or Down key until [AL] is displayed. Press the
Left key to return to the sample display. Press the
Right key again and [AL] will be displayed.
llustration11: Analyzer keypa
AUTO and MANUAL Keys
The AUTO and MANUAL keys are used to implement the alarm override feature. Refer to the heading
Manual Alarm Override in the Alarm Functions section.
Standby Mode
Standby mode can be selected from the main menu. In standby mode the alarms will not function and
the 4 mA to 20 mA outputs will go to 4.00 mA. When SAMPLE is pressed, all the inputs will show
[StbY] instead of the normal input measurement.
The analyzer will not resume normal operation until the analyzer is
taken out of standby mode. While in standby mode, the entire
menu and all of the settings are accessible to the operator as before.
None of the settings will take effect until the analyzer is returned to
normal operation.
The standby feature is protected by security level 2.
Illustration 12: Standby menu
Output Hold
The 8760CLP features an automatic output hold for the pH input only. Output hold goes into effect as
soon as SELECT is pressed when [CAL] is displayed. The output hold feature avoids false alarms and
erratic signal output that would be caused by a routine calibration. Output hold is not necessary for the
chlorine input as chlorine calibration is performed by grab sample calibration only.
Output hold for the pH input has the following effect:
• 4 mA to 20 mA output signal for pH is frozen at it's current level
• Alarms for pH are temporarily disabled
If the output signal for pH is not acceptable at the value found, it can be changed for the duration of the
calibration. Select [Hold] from the pH menu to display the pH value used by the analyzer to determine
the output signal. Use the normal editing procedure to change the pH value used for output hold.
The output hold remains in effect for the duration of the calibration, that is, the output hold is disabled
when the [CAL] prompt is displayed, the SAMPLE key is pressed, or after no key has been pressed for
15 minutes.
1-800-742-1413 www.aquametrix.com
17

AQUAMETRIX INC.
Edit Mode
Edit mode is used to change a numeric value or to select between different options. Values and settings
that can be edited are identified by the darker shading in the menu. Any frame, which has a white
background, cannot be modified.
Editing by Selecting a Setting
Editing a value is like picking an option from a list; only one item on the list can be seen at a time. To
change the setting, press ENTER to go into edit mode. The display will start blinking. Use the Up or
Down arrow key to switch between the possible options and then press ENTER again to accept the new
setting and leave edit mode.
Example: Turn alarm A off.
From the menu, select [AL] [AL.A] [ON.OF]. The analyzer will now display either [ON] or [OFF],
which are the two choices. To change the setting, press ENTER to go into edit mode. The display will
start blinking. Use the Up or Down arrow key to switch between the possible options. When [ON] is
displayed, press ENTER again to accept the new setting and leave edit mode.
Summary of Key Functions in Edit Mode
Enters edit mode. The entire display or a single digit will blink to indicate that the
analyzer is in edit mode. Press the ENTER key again to leave edit mode and accept the
new value.
Adjusts blinking digit upward or selects the previous item from the list. If a 9 is displayed
then the digit will loop around to show 0.
Adjusts blinking digit downward or selects the next item from the list. If a 0 is displayed
then the digit will loop around to show 9.
Numeric values only: move to the right one digit. If blinking is already at last digit, the
display will loop to the ± sign on the left.
Numeric values: move left one digit. If blinking is at the ± sign then blinking goes to last
character.
Settings: restore the initial value if it was changed. Otherwise leaves edit mode without
doing anything.
Illustration 13: Edit keys
1-800-742-1413 www.aquametrix.com
18

AQUAMETRIX INC.
Temperature °C or °F
By default, the analyzer will use metric units. This means that temperature will be displayed using
degrees Celsius and that the prompt for the temperature input will be [°C]. The analyzer can also use
imperial units. For imperial units, temperature will be displayed using degrees Fahrenheit and the
prompt for the first temperature input will be [°F] instead of [°C].
In this instruction manual, the temperature input is always identified as [°C] throughout the menus.
To select imperial units for the analyzer, select [unit] from the configuration menu, then go into edit
mode and change the [°C] setting to [°F].
Real-Time Clock
The analyzer clock is used for internal date/time stamping of system events and the internal data log.
Both the system events and the internal data log are accessed using the IC Net Intelligent Access
Program, which is available as option -2. Analyzers purchased with option -B have a real-time clock
which will maintain the correct time and date even when the analyzer power is turned off.
Input Damping
The chlorine, pH and temperature measurements can be damped to provide the user with a means to
stabilize rapidly varying or noisy signals. Damping range is 3 s to 99 s. With 0 seconds, there would be
no damping and each reading the analyzer made would be used to directly update the display and 4 mA
to 20 mA output. The factory default of 5 seconds adds the next four seconds of readings to the first and
divides by five ― this gives fast response. Selecting 99 seconds adds the readings for 99 seconds and
divides by 99, providing smooth damping out of turbulent readings. Any selection between 3 s and 99 s
can be made.
Select [CONF] [in] from the menu. Use the Up or down arrow key to select the input to be adjusted,
then select the [dA] frame. Press ENTER, then change the input damping to the new number of seconds.
Press ENTER again to leave edit mode.
1-800-742-1413 www.aquametrix.com
19
AQUAMETRIX INC.
H
q
q
q
q
q
q
q
q
q
I
APPLICATION INFORMATION
Chlorine Chemistry
When chlorine gas is dissolved in water, it hydrolyzes rapidly according to equation 1. This reaction
occurs very rapidly, in only a few tenths of a second at 18 °C.
1)
Cl
2 g
2Oaq
— HOC
l aq
HCl
aq
Since HCl (hydrochloric acid) is a strong acid, the addition of gaseous chlorine to water results in a
lowering of the pH due to the acidic HCl by-product.
The important product of reaction (1) is HOCl or hypochlorous acid. Hypochlorous acid is the
disinfectant form of chlorine in water. Hypochlorous acid is unstable because the chlorine molecule is
weakly bonded and as a result will react quickly.
Hypochlorous acid is also referred to as free available chlorine, or free chlorine. It is taste free and
aggressive against germs and organic compounds.
Chlorine supplied as sodium hypochlorite, calcium hypochlorite, or bleach is in a basic form. When a
base is present, a different reaction sequence occurs:
2)
3)
In any hypochlorite solution, the active ingredient is always hypochlorous acid. Then once HOCl and
OH
4)
NaOCl
Ca OCl
-
are formed an additional reaction occurs:
HOCl
H2O
a
2 a
OH
a
a
2 H2O
1
— OCl
— HOCl
— 2 HOCl
a
1
Na1OH
a
H2O
Ca22 OH
a
a
1
1
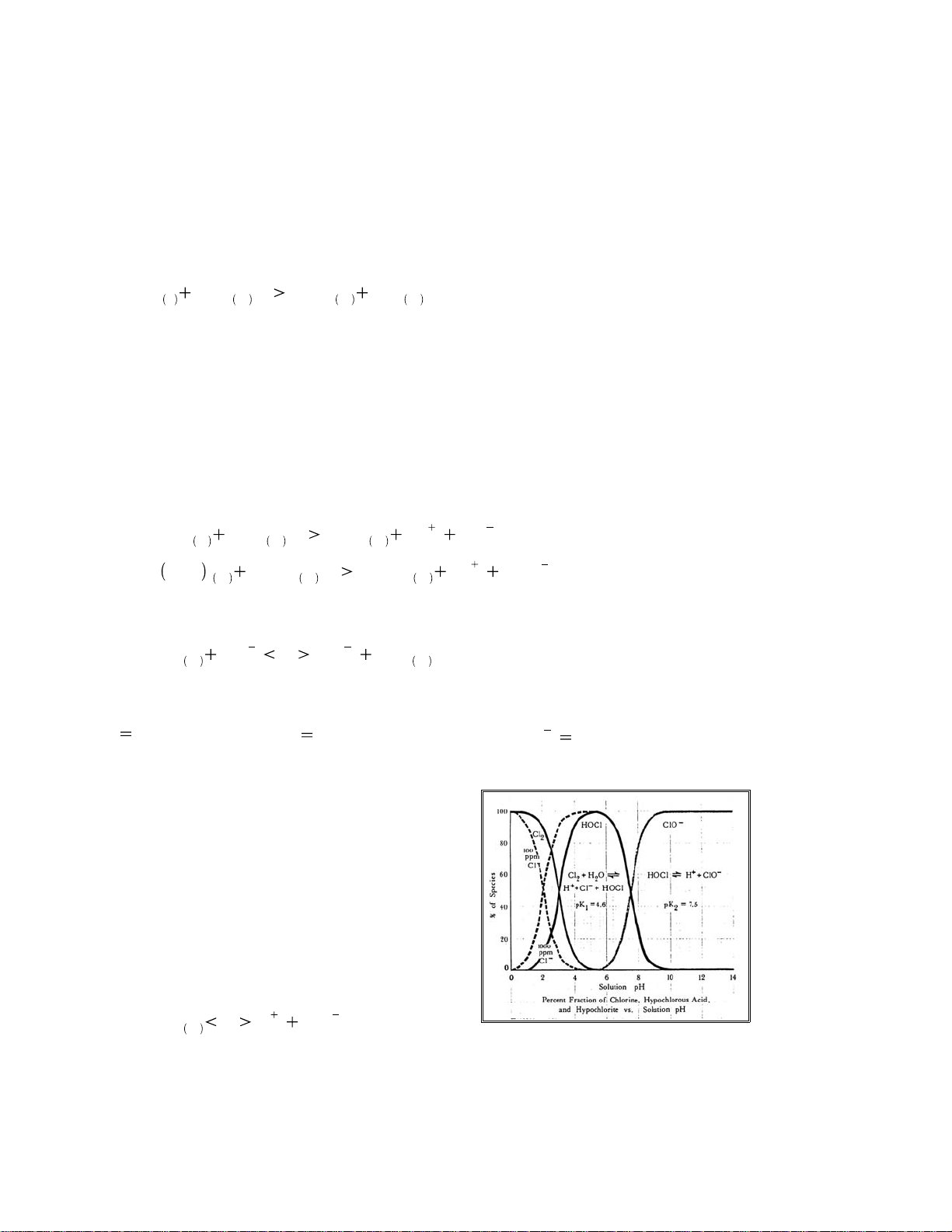
The proportion of chlorine, hypochlorous acid, and hypochlorite ion in solution depends primarily on
pH and somewhat on temperature. The different forms of chlorine are named as follows:
Cl2chlorine
HOCl hypochlorousacid
OCl1hypochlorite ion
At atmospheric pressure and 20 °C, the maximum solubility of chlorine is about 7,395 mg per liter or
7.395 ppm.
Chlorine and the effect of pH
The most important reaction in the chlorination of an
aqueous solution is the formation of hypochlorous acid.
The hypochlorous acid form of chlorine is very
effective for killing germs. Hypochlorous acid is a
‘weak’ acid, meaning that it tends to undergo partial
dissociation to form a hydrogen ion and a hypochlorite
ion. Once in a water environment, HOCl tends to
dissociate into H
5)
HOCl
1-800-742-1413 www.aquametrix.com
+
and OCl- ions.
— H
a
1
OCl
1
llustration 14: Chlorine species change vs. pH
20

AQUAMETRIX INC.
I
In waters between 5 pH and 8.5 pH, the reaction is incomplete and both species are present to some
degree. Since H
+
is one of the ions that is formed and it's concentration is expressed as pH, it follows
that changing pH levels will influence the balance of this reaction and with it the availability of
hypochlorous acid for reaction.
In a water environment, the water pH will affect the chemistry of chlorine due to it's pH sensitivity.
This becomes important as pH rises.
1
6)
H2O —H1OH
(preference is right-to-left)
Three things follow from this form of ionization:
1. Since the tendency of these two ions to react and form
2O is much stronger than the tendency of water to
H
break down into the ions, it follows that as the pH rises
there are fewer H
+
2. The H
released by the breakdown of HOCl (equation
5) react to form water (equation 6) and leave behind
residual OCl
+
ions and more OH- ions.
-
(hypochlorite) ions. Hypochlorite does
not react readily, so the chlorine is weaker.
+
3. If the pH goes down and H
available again, the OCl
-
ions revert to HOCl, which is
ions become readily
the killing form of chlorine. This pH change has been
known to cause surprise downstream fish kills.
Terminology
In the industry, there are a number of terms used to
indicate the various forms of chlorine that are of interest. These terms tend to be used rather loosely and
not necessarily consistently. For that reason, AquaMetrix will define the following terms for purposes
of this instruction manual and the 8760CLP system:
llustration 15: Chlorine concentration vs. pH
FREE AVAILABLE CHLORINE refers to the hypochlorous acid (HOCl) form of chlorine only. It is
said to be free available because it is the free, uncombined form of chlorine that is effective for killing.
TOTAL FREE CHLORINE refers to the sum of hypochlorous acid (HOCl) and hypochlorite ion
-
). The hypochlorite ion is not effective for killing, but it is in a free form. All of the total free
(OCl
chlorine would be in the form of hypochlorous acid if the pH is low enough.
COMBINED CHLORINE refers to chlorine which is not readily available, is not an effective
disinfectant and will not readily convert to hypochlorous acid or hypochlorite ion. For example,
chlorine combined as chloramines or organic nitrogen.
TOTAL RESIDUAL CHLORINE refers to the sum of total free chlorine and combined chlorine. In
environmental studies low total residual chlorine is of particular interest to ensure no downstream
consequences for aquatic life. Total residual chlorine is commonly monitored for final effluent.
1-800-742-1413 www.aquametrix.com
21

AQUAMETRIX INC.
Disinfectant Properties of Chlorine
Chlorine is known to be a good disinfectant; it is able to kill living matter in water such as bacteria,
cysts, and spores. Exactly how chlorine works to kill is not known. Studies do agree, however, that
certain forms of chlorine are more effective disinfectants than others. Whatever the chemical reaction, it
is also generally agreed that the relative efficiency of various disinfecting compounds is a function of
the rate of diffusion of the active agent through the cell wall. Factors which affect the efficiency of
destruction are:
• Nature of disinfectant (type of chlorine residual fraction)
• Concentration of disinfectant
• Length of contact time with disinfectant
• Temperature
• Type and concentration of organisms
• pH
HOCl is the most effective disinfectant of all the chlorine forms and is similar in structure to water. The
germicidal efficiency of HOCl is due to the relative ease with which it can penetrate cell walls. This
penetration is comparable to that of water, and can be attributed to both it's modest size and to it's
electrical neutrality.
The concentration of hypochlorous acid is dependent on the pH, which establishes the amount of
dissociation of HOCl to H
+
and OCl
-
ions. Lowering the temperature of the reacting solution suppresses
the dissociation; conversely raising the temperature increases the amount of dissociation.
-
The rate of dissociation of HOCl is so rapid that equilibrium between HOCl and the OCl
ion is
maintained, even though the HOCl is being continuously used up.
-
The hypochlorite ion (OCl
) form of chlorine is a relatively poor disinfectant because of it's inability to
diffuse through the cell wall of microorganisms. The obstacle is the negative electrical charge.
1-800-742-1413 www.aquametrix.com
22

AQUAMETRIX INC.
g
I
r
8760CLP CHLORINE MEASUREMENT
Introduction
Chlorine in water is a measure of the amount of chlorine, usually thought of as a gas, that is dissolved in
the liquid. Chlorine is widely respected as a leading chemical for the treatment of water to make it
potable or safe to drink. In addition, free available chlorine is often used to control biological agent
growth in water filled industrial systems. The 8760CLP directly measures free available chlorine using
a model (P/N AM-A2104034) galvanic chlorine sensor.
Galvanic Measuring Cell
The chlorine measuring sensor, P/N AM-A2104034, is an electrochemical cell similar to a battery that
produces a current when chlorine is present. By using carefully selected electrodes, in contact with an
appropriate electrolyte, a chemical reaction occurs that uses electrons gained from chlorine molecules to
produce a galvanic current directly proportional to the concentration of chlorine present. Illustration 16
shows how such an electrode system works in a simple laboratory test. Illustration 17 shows how these
scientific principles can be implemented into a working chlorine electrode. Also, unlike an electrolytic
cell in which a flow of current produces the chemical reaction, there is no zero-current as galvanic
current is naturally zero when zero chlorine is present.
The chlorine sensor uses a galvanic cell separated from the sample by a chlorine permeable PTFE
membrane. The cell has a gold cathode in close contact with the PTFE membrane where chlorine gains
electrons (is reduced) to become chloride ions, and a silver anode that produces a fixed potential and
completes the reaction with the chloride to form silver chloride.
The chemical reactions within the cell are;
At the cathode:
At the anode:
Overall:
Cl22 e12 Cl
2 Ag2 A
Cl22 Ag 2 AgCl
1
1
2 e
1
Illustration 2: Basic galvanic cell
llustration 3: Galvanic chlorine senso
1-800-742-1413 www.aquametrix.com
23

AQUAMETRIX INC.
I
CHLORINE SENSOR INSTRUCTIONS
The chlorine sensor is designed for simple maintenance. The sensor is robust and will withstand
difficult applications when properly applied and maintained. Follow the instructions in this section to
promote proper operation.
Chlorine Sensor, P/N A2104034,
Component Identification
A) Membrane
Retainer ring
B)
O-ring
C)
Sensor body
D)
Sensor cable
E)
Silver coils
F)
Gold sensing tip
G)
llustration 18: Chlorine sensor, P/N AM-A2104034, component identificatio n
1-800-742-1413 www.aquametrix.com
24

AQUAMETRIX INC.
I
I
p
I
Assembly of the Chlorine Sensor
This procedure should be done over a sink. Protective eye-wear and plastic or rubber gloves are
recommended when handling the electrolyte, a salt solution. Wash hands with water if the electrolyte
comes in contact with the skin.
1. Galvanic chlorine sensors should have a current drain at all times. Assemble sensor while powered
to analyzer OR with a short; coax center to shield. The chlorine sensor has a 5-pin DIN connector
and the sensor is shipped with a shorting strap across two pins (refer to illustration 8). Remove this
shorting strap prior to connecting to analyzer. Note the location of the pins requiring short for future
sensor storage.
2. Disassemble the chlorine sensor by removing the CPVC membrane retainer (see illustration 20) at
the sensor tip. Pull straight down on the retainer. The retainer holds the membrane in place and
removing the retainer will release the fill solution and expose the silver coils and gold sensing tip.
The fill solution is not hazardous so if any gets on the skin simply rinse with water.
3. Discard the used membrane and rinse the retainer and fill solution cavity thoroughly with deionized
water.
4. Replace the membrane using one of the following procedures:
A. Assembly with Membrane Replacement
Toolkit
Required Materials
i. P/N AM-A2104035 Membrane replacement toolkit
ii. P/N AM-A2104036 Membrane kit
iii. P/N AM-A1100239 Chlorine sensor fill solution
Membrane Replacement Procedure
a) The membrane replacement toolkit, P/N AM-A2104035, consists of two
pieces which fit together into one unit; separate the toolkit into it's
individual components (refer to illustration 19). One piece will be a
cylindrical shape (A) and the other will resemble a "T" (B).
b) Place the membrane retainer, tapered side up, into the larger diameter hole
of the cylindrical component of the membrane
replacement toolkit.
c) Place one membrane from P/N AM-A2104036,
shiny side up, over the membrane retainer.
d) Place the narrow end of the second component
from the membrane toolkit onto the membrane containing component (refer
to illustration 21). Press firmly on top of the second component until the
llustration 21:Step d) of
membrane replacement
rocedure
two components fit together securely.
llustration 19: Membrane toolkit
llustration 20: Membrane
retainer
1-800-742-1413 www.aquametrix.com
25

AQUAMETRIX INC.
Illustration 22: Step f) of membrane replacement
procedure
e) Take the sensor and rinse the fill solution cavity
with fresh fill solution, P/N AM-A1100239. Hold
the sensor in an upright position with the fill solution
cavity facing upwards and fill with P/N
AM-A1100239 so that the gold tip is completely
covered with liquid. Ensure that there are no air
bubbles in the solution.
f) Take the assembled toolkit with the larger
diameter hole and place over top of the fluid filled
sensor tip (refer to illustration 22). Press down
firmly until a stop is felt and a click is heard. Some
fluid will escape; this is normal and to be expected.
g)Remove the toolkit from sensor tip.
h)Dry the chlorine sensor and blot the tip. Examine
the tip — the membrane should be smooth with no
wrinkles or cuts and the surface contours of the gold
electrode should be clear. There should be no lines
from trapped bubbles between the membrane and the
gold electrode. If there are no visible problems as
described here, then the chlorine sensor is ready to be
put into service.
B. Assembly without Membrane Replacement Toolkit
Required Materials
i. P/N AM-A2104036 Membrane kit
ii. P/N AM-A1100239 Chlorine sensor fill solution
Membrane Replacement Procedure
NOTE: Successful membrane replacement without toolkit P/N AM-A2104035 can be difficult. It is
strongly suggested that the toolkit be purchased for ease-of-use.
a) Take the sensor and rinse the fill solution cavity with fresh fill solution, P/N AM-A1100239. Hold
the sensor in an upright position with the fill solution cavity facing upwards and fill with P/N
AM-A1100239 so that the gold tip is completely covered with liquid. Ensure that there are no air
bubbles in the solution.
b) Place one membrane from P/N AM-A2104036, centered and shiny side down, over the filled sensor
tip.
c) Take the membrane retainer (refer to illustration 20) and carefully slide down over the membrane
until a stop is felt. Some fluid will escape; this is normal and to be expected.
d) Dry the chlorine sensor and blot the tip. Examine the tip — the membrane should be smooth with no
wrinkles or cuts and the surface contours of the gold electrode should be clear. There should be no
lines from trapped bubbles between the membrane and the gold electrode. If there are no visible
problems as described here, then the chlorine sensor is ready to be put into service.
1-800-742-1413 www.aquametrix.com
26

AQUAMETRIX INC.
s
I
Inserting Chlorine Sensor in the Flow Fitting
1. Inspect the inside of the quick union fitting for any foreign matter and wipe out any dirt which may
be inside. It should appear clean, shiny and bright.
2. Install the union ring-nut and push sleeve on the assembled chlorine sensor by sliding it down the
lead wire.
3. Check that the sealing O-ring is on the electrode body, on the sensing tip side of the ledge, or in the
O-ring groove of the flow cell.
4. Insert the chlorine sensor into the fitting. Rock the sensor back and forth to pass the O-ring and press
firmly all the way down so that the O-ring firmly seats in its groove.
5. By hand, turn the union-nut until finger tight. For higher pressures it may be necessary to use a
wrench; however, the components are plastic and care is needed to avoid breakage.
CAUTION: Do not use a large wrench to turn the sensor. The plastic components of the chlorine
sensor could be broken or deformed.
Removing Chlorine Sensor from Flow Fitting
1. Stop the sample flow and allow system to drain. Remove the pH sensor as per instructions on
page 33. Removing the pH sensor will reduce the vacuum effect within the flow cell.
CAUTION: Removal of the chlorine sensor from a sealed flow cell will vacuum stretch the thin
ensing membrane. Stretching the membrane will cause slow response and higher readings at low
levels. Parting the membrane will cause chlorine sensor failure.
2. By hand, turn the union-nut until free. For higher pressures it may be necessary to use a wrench to
start turning the nut.
3. Gently rock and pull the chlorine sensor back and forth to ease the O-ring seals back up the
compression throat.
4. When the chlorine sensor has been fully removed, wipe the sensor clean and then proceed to the
calibration procedure or monthly/yearly maintenance, as necessary.
Zero Test Technique
The best way to zero check at the point of use, where all water and even the air contains some chlorine,
is to use a zero chlorine solution available from AquaMetrix as P/N AM-A1100225 in a 500 mL bottle.
CAUTION: If zero standard gets on hands, wash with running water.
Protective eye-wear and gloves are suggested.
1. Pour some of the zero chlorine solution, P/N AM-A1100225, into a clean beaker.
2. Immerse the chlorine sensor into the beaker so that it is about 3 inches below the
surface of the zero check liquid; refer to illustration 23. Provide slow gentle
movement to ensure the chlorine present is consumed. The chlorine sensor
should rapidly fall below 0.1 ppm, thus confirming operation of the sensor.
Make sure that the zero check solution is used within 8 hours because the
scavenger will be used up with exposure to air by also absorbing oxygen. The
remaining zero check solution should be stored tightly capped in it's bottle. The
llustration 23: Zero
check
1-800-742-1413 www.aquametrix.com
zero solution is “single use”, so discard the used zero solution.
27

AQUAMETRIX INC.
I
g
Monthly Maintenance
Certain applications may require occasional sensor cleaning. A monthly maintenance check is
recommended by visual examination of the sensor cell area. If needed, a soft wipe can be used to blot,
plus detergent and water to remove any deposits. Rinse thoroughly after cleaning with water. Run a
calibration and return to service if sensor efficiency is above 50 percent..
White silt inside the sensor cap may not cause problems. However, if after calibration the sensor
response is slow, replace the electrolyte and wipe the coils and surface lightly using a soft wipe, or a
little more vigorous cleaning can be done using a toothbrush. Recharge with fresh electrolyte. Calibrate
and return the sensor to service.
Semi-Annual Maintenance
Replace the membrane, P/N AM-A2104036, and electrolyte solution, P/N AM-A1100239, following the
appropriate membrane replacement procedure in Assembly of the Chlorine Sensor section in Chlorine
Sensor Instructions. Examine the coils for any discoloration or heavy coating. Such coatings should be
removed for best performance (caution the silver coils are soft metal, never use force in cleaning). To
clean the coils, refer to the Chemical Cleaning section for further instructions.
Remove the old membrane from the cell and replace with a new one. Re-assemble the cell, calibrate,
check efficiency and if above 50 percent, place in service.
Chemical Cleaning
Chlorine sensors can be refreshed with AquaMetrix P/N AM-A1100227, chlorine sensor renew solution.
This solution is only available in 30 mL bottles. Due to the acidic nature of this solution, the 30 mL
bottle is packaged in a baking soda packer for non-hazardous shipment.
NOTE: This procedure should be done over a sink. Wear plastic or rubber gloves and protective eye-
wear as the solution is acidic. Wash hands thoroughly with lots of water if the solution comes in contact
with the skin.
1. Disassemble the chlorine sensor to expose the silver coils and gold tip.
2. Immerse in cleaning solution as shown in illustration 24
for about 10 minutes, or until deposits disappear.
3. Remove and rinse in distilled or deionized water; use a
small toothbrush to scrub coils to speed removal, if
necessary.
4. Repeat steps 2 and 3 until coils and tip look clean and have
a shine; re-assemble chlorine sensor with new membrane
and fill solution, calibrate and verify efficiency is above
50%.
5. Repeat steps 2 to 4 as necessary to get at least 50%
efficiency. If not possible, the chlorine sensor should be
replaced.
1-800-742-1413 www.aquametrix.com
llustration 24: Chemical cleanin
28

AQUAMETRIX INC.
Sensor Storage
Short Term: Immerse the sensor tip in tap water. Wet storage can be used up to two weeks. If the
sensor is not connected to the analyzer, the sensor needs to be shorted. Place the shorting strap across
the appropriate pins of the sensor connector (refer to illustration 8).
CAUTION: If a wet sensor dries out in storage, it may become damaged beyond repair.
Long Term: Disassemble the chlorine sensor tip and pour out the fill solution. Rinse the coils, gold
tip, and membrane retainer with deionized water and blot dry with a paper towel. Re-assemble the
chlorine sensor dry, and store dry with the tip covered.
Dry storage can be used for a year or more.
NOTE: The sensor needs to be shorted only when it is charged (filled with electrolyte) and not
connected to a powered analyzer.
1-800-742-1413 www.aquametrix.com
29

AQUAMETRIX INC.
I
I
d
CHLORINE CALIBRATION
The 8760CLP chlorine system is calibrated by grab sample; an easy method of standardizing the
chlorine measurement without taking the electrode out of the sample. Grab sample standardization
method requires the user to determine the actual total free chlorine concentration of the sample using an
alternative method.
When grab sample calibration is used, it
is the responsibility of the user to ensure
that the grab sample taken and the total
free chlorine value recorded for it are
accurate.
A chlorine calibration kit, P/N
AM-A7010001, is supplied with the
model 8760CLP. The calibration kit
uses a reagent which develops a violet
color which is proportional to the
amount of total free chlorine in the
sample. The kit contains 30 ampoules,
sample cup, and low & high range
comparators to measure total free
chlorine in the 0 ppm to 1 ppm and 1
ppm to 5 ppm concentration ranges respectively.
Illustration 25: Chlorine menu
NOTE: Keep the kit closed when not in use. The comparators need
to be stored in the dark.
Standardizing Chlorine
NOTE: The pH input should be calibrated first, prior to chlorine
standardization. Refer to the pH Calibration section.
1. Press SAMPLE to display the [tFCl] reading. Press SELECT to
reach the first menu, then use the Up or Down arrow key to display
[tFCl].
2. Press SELECT then the Up or Down arrow key to display [CAL].
llustration 26 Chlorine calibration
kit, P/N AM-A7010001
4. From the analyzer outlet, obtain a representative grab sample cup full of water, then immediately go
and press ENTER on the analyzer.
5. Take an ampoule from the kit and place the ampoule’s tapered tip into
one of the four depressions in the bottom of the sample cup. Snap the
tip by pressing the ampoule towards the side of the cup. The sample
will fill the ampoule and begin to mix with the reagent. A small bubble
of inert gas will remain in the ampoule to facilitate mixing.
3. Press SELECT then the Up or Down arrow key to [Get]. Then
press SELECT again to display a flashing [do]. LEAVE
ANALYZER FLASHING!
CAUTION: Do not break the tip of the ampoule unless it is completely
immersed in your sample. Accidentally breaking the tip in the atmosphere
1-800-742-1413 www.aquametrix.com
may produce a “jack-hammer” effect, shattering the ampoule. Wear eye
protection when working with these ampoules.
llustration 27: Metho
for breaking the ampoule
30

AQUAMETRIX INC.
I
I
6. Remove the fluid-filled ampoule from the cup. Mix the contents of the ampoule by inverting it several
times, allowing the bubble to travel from end to end each time.
7. Wipe all liquid from the exterior of the ampoule and wait 1 minute.
8. After 1 minute, use the appropriate comparator to determine the level of chlorine in the sample. Write
down the chlorine value.
Low-range Comparator:
0 ppm to 1 ppm
The ampoule is placed in the center tube, flat
end downward. The top of the cylinder is then
directed toward a source of bright light while
viewing from the bottom. Hold the
comparator in a nearly horizontal position and
rotate it until the standard below the ampoule
shows the closest match.
llustration: 28 Using the low-range
comparator
Table 1: How to use the comparators
High-range Comparator:
1 ppm to 5 ppm
The comparator should be illuminated by a
strong white light directly above the
comparator. The filled ampoule should be
placed between the color standards for
viewing. It is very important that the ampoule
be compared by placing it on both sides of the
standard tube before concluding that it is
darker, lighter, or equal to the standard.
llustration 29: Using the high-range
comparator
9. Install the chlorine calibration value, determined in step 8, into the 8760CL as follows:
Press SAMPLE then SELECT to display [tFCl]. Press SELECT to display [CAL], then press SELECT to
display [Get], then press Up arrow to display [SEt]. Press SELECT to display numeric value, then press
ENTER to get the numeric value to flash. Edit the numeric value to the new value determined from step
8. When the flashing value is the chlorine value from step 8, press ENTER to get the analyzer to accept
the value, then press SELECT to display flashing [do]. Press ENTER to get the 8760CL to accept the
chlorine calibration by displaying [Done].
10. Press SAMPLE to display the [tFCl] or total available chlorine reading in mg/L or ppm . Write down this
value.
11. Press Down arrow key to display [HOCl] or free available chlorine in mg/L or ppm. Write down this
value.
12. Press SELECT to display [tFCl], then press SELECT, then the Up arrow key to display [EFF]. Press
SELECT to display the sensor efficiency in percent. Write down this value.
NOTE: Keeping a written calibration record will show how your unit trends over time.
The 8760CL analyzer is now reading chlorine and tracking chlorine changes in the water sample.
1-800-742-1413 www.aquametrix.com
31

AQUAMETRIX INC.
pH and Temperature impact on Chlorine
The measurement of the chlorine concentration is done by the galvanic sensing electrode. However, the
chlorine chemistry of the sample will change with both temperature and pH. Illustration 15 shows how
the relative concentrations of hypochlorous acid and hypochlorite ion shift with a change in the water
pH. This same relationship is also dependent on the temperature of the solution, as the curves will shift
with changes in the temperature. The 8760CLP includes a temperature input and pH input to
compensate for these changes. A pH sensor is provided to measure the pH of the sample and
temperature compensation is provided via a temperature sensor in the chlorine electrode.
A method has been provided in the analyzer program to change the compensation method for
temperature compensation and pH compensation from automatic to manual. Providing a method of
manual temperature compensation and/or manual pH compensation allows the analyzer to continue
measuring free available chlorine and total free chlorine in the event that the temperature sensor and/or
pH sensor are malfunctioning or absent.
Manual Temperature Compensation
From the main menu, select [tFCl] [tc]. At this point either [Auto] (for automatic temperature
compensation), or [SEt] (for manual temperature compensation set-point) will be displayed. To change
the setting from [Auto] to [SEt] press ENTER to edit the current setting. The display will start blinking,
indicating that a selection needs to be made. Use the Up or Down arrow key to display [SEt]. Press
ENTER to select manual temperature compensation.
With [SEt] as the current display, press SELECT to display the temperature setting for manual
temperature compensation. If the current value needs to be changed, press ENTER to edit the current
setting. The display will start blinking. Use the Up or Down arrow keys to display the desired
temperature for manual temperature compensation. Press ENTER to accept the currently displayed
value.
Manual pH Compensation
From the menu select [tFCl] [PH.C]. At this point either [Auto] (for automatic pH compensation), or
[SEt] (for manual pH compensation set-point) will be displayed. To change the setting from [Auto] to
[SEt] press ENTER to edit the current setting. The display will start blinking, indicating that a selection
needs to be made. Use the Up or Down arrow key to display [SEt]. Press ENTER to select manual pH
compensation.
With [SEt] as the current display, press SELECT to display the pH setting for manual pH compensation.
If the current value needs to be changed, press ENTER to edit the current setting. The display will start
blinking. Use the Up or Down arrow keys to display the desired pH value for manual pH compensation.
Press ENTER to accept the currently displayed value.
1-800-742-1413 www.aquametrix.com
32

AQUAMETRIX INC.
pH SENSOR INSTRUCTIONS
Preparation for Use
1. Moisten the pH sensor body with tap water and carefully remove the tape and orange plastic storage
cap. Caution should be used in removing this cap; pull straight down. Do not bend the body of the
pH sensor. This can result in damage to the internal element.
NOTE: Save the lower cap for later use in storage of the pH sensor.
2. Rinse away any deposits on the exposed pH bulb and junction area with tap water.
3. For first time use, or after long term storage, immerse the pH electrode in 4 pH buffer for 30 minutes.
This hydrates the pH bulb and prepares the reference junction for contact with test solutions.
4. If air bubbles are visible inside the pH bulb, shake the electrode downward to fill the bulb with
solution.
5. AquaMetrix electrodes are shipped in a pH electrode storage solution buffered to approximately 7
pH. These electrodes are often ready for use immediately with typical accuracy of ± 0.2 pH without
buffering; however, it is strongly recommended that buffered calibration be performed.
6. The pH sensor is ready to be placed in service.
Inserting pH Sensor into Flow Fitting
Insertion sensors should be examined for good clean sealing surfaces and installed carefully. Clean
seals such as O-rings should be lubricated with silicone grease to ensure liquid tight performance.
Remove the storage cap then carefully push the sensor into the insertion fitting until it is seated against
the stop. Tighten the retainer nut to hold the sensor firmly in place. Let the vessel fill with liquid. The
pH sensor should now read the liquid pH.
Removing pH Sensor from Flow Fitting
Simply turn off the sample flow and allow the pressure to drop to zero, then undo the retaining nut and
carefully remove the pH sensor from the flow cell.
Electrode Maintenance
The pH sensor needs to be calibrated periodically to maintain accurate measurements. AquaMetrix
recommends that the electrode be calibrated every 30 days. Depending on the process, it may need to be
calibrated more frequently, eg. weekly or even daily. Frequent calibration is especially important if
accurate measurements are required.
Over time, electrode performance will degrade. The glass bulb becomes less responsive to pH and the
reference electrode becomes depleted. The electrodes will need to be replaced after several years of use
or, depending on the harshness of the process, after several months.
Sensor Storage
Short Term: Rinse the pH sensor in demineralized water then store in a plastic shipping cap of 4.0 pH
buffer solution.
Long term: Clean the pH sensor in electrode wash solution, AM-A1100091, rinse in demineralized
water, then store in a plastic shipping cap of pH electrode storage solution, AM-A1100090.
1-800-742-1413 www.aquametrix.com
33

AQUAMETRIX INC.
Monthly Maintenance
Remove the sensor from the flow cell, rinse in water, remove any significant deposits, and then check by
calibration in 7 pH for offset and then 4 pH or 10 pH buffer for slope.
If the calibration turns up a caution or error message in the 8760CL analyzer, then follow the
appropriate solution. Also, refer to Troubleshooting section.
If the calibration is good, keep a log of the pH offset and slope at each monthly calibration.
The pH sensor is now ready to return to service.
Yearly Maintenance
Check the pH offset log. If the pH offset has changed more than 30 mV over the past year, it may need
to be chemically cleaned – follow the Chemical Cleaning of Sensor procedure.
Check the pH slope (efficiency) log. If the efficiency has dropped below 85%, it may need to be
chemically cleaned and restored – see Chemical Cleaning of Sensor and/or Restoring Electrode
Response in the Troubleshooting section.
After all the above checks, plus chemical cleaning and/or restoring procedures, follow the monthly
maintenance procedure. Start a new log with the improved values.
When to Clean Sensors
Various factors can affect the pH reading; scale, biological growth, oil, wax, gum, etc., all reduce the
area for hydrogen ion to react with the glass. Biological microbe growths can also produce local pH
environments inside their growth deposit, which can be quite different from the true process pH.
Periodic cleaning of pH sensors will remove these deposits, restore the pH glass surface, reference
junction and thus the pH accuracy.
Mechanical Cleaning of Sensor
The sensor will require cleaning if sludge, slime, or other deposits build up in the internal cavities of the
sensor.
Wherever possible, clean with a soft brush and detergents. General debris, oils, films, biological
growths, and non-tenacious deposits can be removed in this way.
Use a soft flat brush and a beaker or bucket of water with a good liquid detergent. Take care not to
scratch the pH electrode glass surface; it is thin, fragile and easily broken.
All the wetted surfaces of plastic body sensors should be washed with a soft cloth. This will return their
appearance to like-new condition and remove sites for buildups to occur.
When to Chemical Cleaning
After mechanical cleaning, as above, check the sensor against a pH buffer. If the sensor is still not
developing the pH reading properly in the pH buffer, proceed to the Chemical Cleaning procedure;
otherwise return the sensor to the process.
1-800-742-1413 www.aquametrix.com
34

AQUAMETRIX INC.
Chemical Cleaning of Sensor
AquaMetrix offers a pH sensor chemical cleaning kit containing solutions and necessary cleaning items as
P/N AM-A1600054.
NOTE 1: A suitable place to do chemical cleaning is at a counter or bench with a laboratory sink, with a
chemical drain where waste is contained and treated before release.
NOTE 2: AquaMetrix kits are kept small and portable so that they can be taken to installation sites,
together with a plastic bucket of water (for rinsing) and a rag/towel (for drying). Waste materials
(particularly acid leftovers) should be returned to the laboratory for disposal.
CAUTION: Use extra care when handling the cleaning solution as it contains acid. Wear rubber gloves
and adequate facial protection when handling acid. Follow all P/N AM-A1100091 & P/N AM-A1100094
MSDS safety procedures.
a) Set up the cleaning supplies where cleaning is to be performed. Lay out the sensor cleaning brush,
syringe, cleaning solutions and rinse solutions, plus the beakers and sensor.
NOTE: Ensure your cleaning solution beaker is on a firm flat surface since it will contain acid.
b) First remove the pH sensor from the process and examine it for deposits. Use the sensor cleaning brush
and tap water to loosen and flush away any deposits within the measurement area. Detergent can be
added to remove oil films and non-tenacious deposits. Hard scales and other tenacious deposits may
require chemical cleaning.
c) CHEMICAL CLEANING: Fill a beaker ¾ full of pH electrode wash solution, P/N AM-A1100091.
d) Lower the pH sensor into the center of the beaker until the entire tip is submerged.
e) Allow the sensor to sit in this solution for a few minutes and then check to see if the pH electrode and
reference junction appear clean. If not entirely clean, allow sensor to sit in solution until clean. Stubborn
deposits can be removed with the brush and syringe, to squirt wash solution into hard to reach areas.
CAUTION: Use great care when brushing and squirting acid. Wear rubber gloves and facial protection.
f) Rinse the cleaned sensor thoroughly in tap water and then with deionized water for a second rinse prior to
calibration.
g) Check the sensor against a pH buffer close to the application pH. If the sensor is still not reading properly
(± 0.5 pH) in the buffer, clean again using gentle scale remover, P/N AM-A1100094, following steps h) to
l).
h) CHEMICAL DESCALING: Fill a beaker ¾ full with gentle scale remover, P/N AM-A1100094.
i) Lower the pH sensor into the center of the beaker until the entire tip is submerged.
j) Allow the sensor to sit in this solution for a few minutes and then check to see if the pH electrode and
reference junction appear clean. If not entirely clean, allow sensor to sit in solution until clean. Stubborn
deposits can be removed with the brush and syringe to squirt scale remover into hard to reach areas.
CAUTION: Use great care when brushing and squirting acid. Wear rubber gloves and facial protection.
k) Rinse the cleaned sensor thoroughly in tap water and then with deionized water for a second rinse before
calibrating.
l) Check the sensor against a pH buffer solution close to the application pH.
m) A clean and rinsed pH sensor should read near 7 in pH 7 buffer. If it does not, troubleshoot the pH
sensor, wiring and analyzer.
1-800-742-1413 www.aquametrix.com
35

AQUAMETRIX INC.
I
pH CALIBRATION
The pH input is calibrated using one of two methods. A one-point standardization adjusts the electrode
offset while maintaining the previous slope. The two-point calibration combines the results of the
standardization with the results of
the buffer 2 calibration and
calculates the slope as well as the
offset.
A calibration is easily
accomplished by selecting an
appropriate buffer, placing the
electrode in the buffer solution, and
letting the analyzer do the rest.
The analyzer tests for electrode
stability and performs many
diagnostic tests during calibration.
Automatic stability testing takes
llustration: 30 pH menu
acceptable or not. The internal diagnostic tests will activate caution or error messages if faulty operation
is suspected or detected. Errors detected during calibration will not cause the analyzer to lock up.
most of the guesswork out of
deciding whether a reading is
Buffers automatically recognized by the 8760CL are:
Buffer Part Number, 500 mL bottle
4.01 pH, red A35-13
7.00 pH, yellow A35-14
10.0 pH, blue A35-24
Selecting a pH Buffer
pH buffers provide the simplest and most accurate method of calibrating the pH sensor and analyzer.
First Buffer: The first step is to use 7 pH buffer to calculate the mV offset of the electrode from the
theoretically perfect 0 mV. pH 7 buffer is used because it simulates 0 mV thus making it the best standard
since the electronics are also at this 0 mV reference point.
Second Buffer: The next step in the calibration is to use a second buffer (usually 4 pH or 10 pH). When
choosing which buffers to use in calibration, it is best to select buffers that fall on both sides of the normal
operating pH range. By using these two buffers, the slope calculation will encompass the normal pH, thus
giving the most accurate pH measurement. Either of these buffers, pH 4 or pH 10, gives a large enough span
relative to the pH 7 buffer that a good slope can be calculated. When performing the two point calibration, a
percent value will be given in microprocessor based pH analyzers. The closer to 100% the slope is, the better
the efficiency and thus the performance of the electrode.
The model 8760CLP has been programmed to recognize the three buffers most commonly used for
calibration: pH 4, pH 7, and pH 10. To achieve greater accuracy, the temperature compensated values
for these buffers are calculated by the analyzer. Simply place the electrodes in the buffer solution and
the analyzer will select the correct buffer value, allowing for an offset of up to ± 1.3 pH units.
NOTE: [Auto] must be selected in the calibration menu for this feature to work.
1-800-742-1413 www.aquametrix.com
36

AQUAMETRIX INC.
I
p
r
Temperature Dependence of Buffers
The pH of a solution is dependent on temperature.
To achieve greater accuracy, the temperaturecompensated values for the 4 pH, 7 pH and 10 pH
buffers are calculated by AquaMertix analyzers.
The graphs show the temperature-dependence of
the standard buffers. The TC-curves have been
programmed into the AquaMetrix analyzer. The
actual pH value of each of the three standard
buffers will be used.
Example: Calibrate using the pH 4.01 buffer (at
25 °C). The temperature of the buffer is 50 °C.
The analyzer will use the pH value of 4.05.
Incorrect Buffer Selection by the Analyzer
If the offset is known to be greater than ± 77 mV,
or if the analyzer selected the wrong buffer using
automatic buffer recognition, then it is necessary
to specify which buffer is being used. This is
done by selecting [4.01], [7.00], or [10.0] then an
llustration 31: Tem
Illustration 32: Temperature compensated pH 7 buffer
erature compensated pH 4 buffe
offset of ± 4 pH units is allowed and
temperature-compensated values are still used.
Other Buffer Values or Custom Buffers
If a buffer with a pH value other than pH 4, pH 7,
or pH 10 is to be used, select [cuSt] (custom
value), then enter a value between 0 pH and
14 .pH. Buffer values entered this way are not
temperature compensated; the buffer is assumed
to have the specified pH value at the current
temperature. Offsets of up to ± 4 pH units are
allowed.
Illustration 33: Temperature compensated pH 10 buffer
pH Buffer Use and Maintenance
A pH measurement is only as good as the calibration, and the calibration is only as good as the buffers.
The following guidelines for buffer maintenance will ensure accurate pH calibration and thus accurate
pH measurement.
• Buffers have a limited shelf life. AquaMetrix suggests a one (1) year shelf life for unopened pH buffers. Store
buffers at room temperature.
• Discard used buffer - do not return used buffer to the stock bottle.
• Protect buffers from exposure to air as atmospheric carbon dioxide lowers the pH value of alkaline buffers.
Other trace gases found in industrial environments may also affect the buffer pH. Molds resulting from
airborne spores may accumulate in neutral and acidic buffers and change the pH value as well.
• Rinse sensor with demineralized water before placing in buffer to prevent carryover contamination. A few
drops of demineralized water will not visibly alter the pH. Do not wipe the sensor dry as wiping may
induce a static charge which could result in noisy readings.
1-800-742-1413 www.aquametrix.com
37

AQUAMETRIX INC.
I
Standardizing — Single-Buffer Calibration
Standardizing the analyzer causes the analyzer to calculate the offset for the pH electrode; indicated as
[OFFS] in the [PH] menu. The electrode slope value determined during the last buffer 2 calibration will
be maintained; indicated as [SLOP] in [PH] menu.
1. Press SAMPLE to display the [tFCl] reading. Press SELECT to reach the main menu, then use the Up
or Down arrow keys to display [PH]. Press SELECT then use the Up or Down arrow keys to display
[CAL].
2. Press SELECT again, then use the Up or Down arrow keys to display [buF1].
3. Press SELECT again to reach the next menu. A buffer value needs to be
determined with which to calibrate the analyzer. Use either automatic
detection, [Auto], a custom value, [cuSt], or one of the standard buffers,
[4.01], [7.00] or [10.0]. For further details, see Selecting a Buffer for an
explanation of the buffer selection process.
4. Rinse the pH sensor in demineralized water to remove drops of process
liquid.
NOTE: Although pH buffers are formulated to resist pH change, mixing in
strong foreign ions can cause pH shift and resultant calibration to
incorrect pH value. Dirt deposits, biological growths, and any other
contaminants should be removed from the pH sensor body and tip prior to
calibration.
5. Place the electrode in the selected buffer solution, then press SELECT to
start the calibration process. The display will show a flashing pH reading
to indicate that the analyzer is reading pH and is testing for stability.
The calibration procedure is fully automatic from here on. As soon as the
electrode has stabilized, the display will stop flashing, the electrode offset will
be calculated, and the new offset will be entered in memory.
It is, however, possible to override the analyzer. The ENTER key may be
pressed before the electrode has stabilized, forcing the analyzer to calibrate
using the current pH input. Also, the calibration may be redone or started over
at any time. Press CANCEL to display the selected buffer (eg. [Auto]), then
SELECT to restart the calibration.
If the analyzer detects or suspects any problems during calibration, an error
and/or caution message will appear. Refer to Error Messages for a description
of each message.
If an error has occurred, the standardization was not successful. The analyzer
has kept the value from the last successful calibration. Press any key to
acknowledge the error. The analyzer will return to the buffer selection menu
and display the selected buffer, eg. [Auto]. Take corrective action and retry
the calibration.
If a potential problem has been detected; seen by a caution message, then the
analyzer has successfully completed calibration. The caution message simply
informs the user that poor performance is suspected.
Press any key to resume normal operation after a caution
or error message has appeared.
llustration 34: pH standardization
1-800-742-1413 www.aquametrix.com
38

AQUAMETRIX INC.
Calibrating – Two-Buffer Calibration
Calibrating the analyzer involves calculating both the offset and the slope
(electrode efficiency) for a particular electrode pair. The electrode slope will be
calculated as a percentage of Nernstian response.
1. Calibrate the offset with 7 pH buffer as [buF1], buffer 1, by following the
procedure for Standardizing - Single Buffer Calibration. Return to the
calibration menu and display [buF2]. Press SELECT to reach the buffer
selection menu.
2. Use the Up and Down arrow keys to select either automatic detection, [Auto], a
custom value, [cuSt], or one of the standard buffers, [4.01], [7.00] or [10.0].
The second buffer should be at least 2 pH units higher or lower than the buffer
used for the standardize procedure. Refer to the section entitled Selecting a
Buffer for an explanation of the buffer selection process.
3. Rinse the pH sensor in demineralized water to remove drops of pH 7 buffer.
NOTE: Carryover of old buffer into different fresh buffer will decrease the pH
difference between the buffers producing an efficiency calibration error.
4. Place the rinsed sensor into the second buffer and press SELECT to start the
calibration process.
The calibration with the second buffer works similar to a standardization, except
that additional error checking is possible and the electrode efficiency will be
calculated; indicated as [SLOP] in the [PH] menu. If an error occurs at this point,
the settings from the standardization ([buF1] selection) will be kept. Either retry
the calibration with a second buffer ([buF2]), or resume normal operation with the
settings from the standardization.
NOTE: Discard used buffer after calibration. Used buffer usually picked up
carryover buffer and/or contaminants that cause pH error if re-used.
Illustration 35: Buffer 2 calibration
Manual Adjustment of Offset and Slope
It is possible to bypass the regular calibration procedure and edit the slope or offset directly. Offset and
slope are protected by level 1 security, which is the same security as the other calibration procedures.
When the offset or slope are adjusted directly, there is no way for the analyzer to verify the accuracy of
the adjustments made. However, slope and offset warnings are given whenever the adjustments fall
outside the preset ‘safe’ regions. Unlike a normal calibration, the manual adjustments will allow slope
adjustments outside 60% to 110% slope efficiency or offset adjustments greater than ± 4 pH units (about
240 mV). The usual error messages will come up but the specified new values will be installed
nonetheless.
An alternate calibration method, grab sample, may be performed as follows:
1) Take a pH reading using a different method, eg. portable meter.
2) Go to [PH] [CAL] [buF1] [cuSt] and edit the value to the pH value obtained in step (1).
AquaMetrix advises that the operator use one of the regular calibration procedures whenever possible.
1-800-742-1413 www.aquametrix.com
39

AQUAMETRIX INC.
ERROR MESSAGES
Detected errors and/or cautions can be displayed by the analyzer. From the main menu select [Err]. If
there are no error or caution messages, [NONE] will be displayed, otherwise scroll through the error list
using the Up and Down arrow keys. Errors and cautions cannot be removed from this list directly; each
error or caution will be removed automatically when appropriate, eg. errors associated with improper
calibration will be cleared after a
Input/Source Input Number for Error/Caution Messages
Chlorine 1
Temperature 2
pH 3
Alarm A 7
Alarm B 8
Table 2: Input number designation for error messages
not show up in the error menu with the numbered error messages, if any.
successful calibration.
Error messages are numbered. Errors 1
through 5 are identified as [En.e] where
n is the input number and e is the error
number. Messages 6 through 9 are less
serious and are identified as cautions
instead, eg. [CAn.e].
Off-scale errors for chlorine are not
numbered and are identified as [+Err]
and [-Err], depending on whether the
input is at the top or the bottom of the
scale. The off-scale error is displayed
instead of the sample reading and does
Error message indicators can be annoying when one has already been made aware of them. A method
has been provided to turn off the error LED and the fault alarm for a particular error message. Refer to
Acknowledging an Error Message below for the exact procedure.
The error LED will remain on as long as there is an unacknowledged error or caution message or as long
as any input is off-scale. Each source of error must be removed or acknowledged before the error LED
will go off.
Acknowledging an Error Message
Select [Err] from the main menu. Use the Up or Down arrow key until the error message to be
acknowledged is displayed.
Errors are displayed with either a positive (+) sign or a negative sign (-) in front. The + sign is used to
indicate an active or unacknowledged error, the - sign indicates an inactive or acknowledged error.
Acknowledging the error will change the sign from + to -.
Press ENTER to go into edit mode. The + or - sign will be flashing. Use the Up or Down arrow key to
change the sign, then press ENTER again.
An acknowledged error message is cleared for one occurrence of the error only. If the error reappears,
the sign changes from - to + and the error message must be acknowledged again.
1-800-742-1413 www.aquametrix.com
40

AQUAMETRIX INC.
Messages for Chlorine Input
Error Description Causes Solutions
E1.0 Reading is off-
scale. Display
shows [+Err].
E1.2 Electrode
efficiency would
be less than 20%.
Previous setting
retained.
E1.3 Sensor efficiency
would be more
than 300%.
Previous setting
retained.
E1.4 pH compensator
is off-scale.
compensator is
off-scale.
The internal A/D converter is at
the top of the scale. The
analyzer cannot measure higher
chlorine values.
Improper electrode setup or
electrode failure.
No chlorine signal or signal
from sensor very weak.
pH sensor is not connected. Check pH sensor connections and/or select
TC is not connected. Check TC connections or install TC.E1.5 Temperature
Process is outside of TC
operating range of -5 °C to
105 °C.
The analyzer is at the limit of it's
measuring capability. Check the sensor
setup to ensure that the sensor is operating
properly. Service or replace the sensor if
necessary.
The analyzer needs electronic adjustments.
Arrange for servicing.
Set up electrode, then redo calibration.
Also refer to Troubleshooting section.
Check electrode connection, then redo
calibration. Also refer to Troubleshooting
section.
manual pH compensation.
Use manual temperature compensation.
Messages for Temperature Input
Error Description Causes Solutions
E2.1 Temperature reading is off-
scale. Temperature is less
than -5 °C.
E2.2 Temperature reading is off-
scale. Temperature is greater
than 105 °C.
Temperature is less than
-5 °C.
Electronic temperature
calibration necessary.
Temperature compensator is
not attached.
Temperature is greater than
105 °C .
Electronic temperature
calibration necessary.
1-800-742-1413 www.aquametrix.com
Verify process and sensor
location.
Follow procedure in Hardware
Alignment section.
Attach temperature
compensator.
Connect resistor to TC
terminals to simulate a constant
temperature. Refer to
Hardware Alignment section.
Verify process and sensor
location.
Follow procedure in Hardware
Alignment section.
41

AQUAMETRIX INC.
Messages for pH Input
Error Description Causes Solutions
E3.1 Electrode has not stabilized
after 5 minutes of
calibration.
E3.2 Electrode has stabilized, but
offset > ± 1.3 pH units.
This error generated by auto
detection of pH 4, pH 7,
and pH 10 buffers only.
Previous offset is retained.
E3.3 Electrode has stabilized, but
offset > ± 4 pH units.
Previous offset retained.
E3.4 Electrode efficiency less
than 60% or greater than
110% Nernstian response;
slope is too flat or too steep.
Previous calibration is
retained.
Poor electrode performance. Check electrode, redo calibration.
Large offset in electrode.
Wrong buffer used for calibration Only
pH 4, pH 7, and pH 10 buffers can be
detected automatically.
Wrong buffer used for calibration.
Bad electrode. Perform electrode maintenance.
Electrode not connected. Check connections, redo calibration.
[buF2] calibration done before [buF1]
calibration.
Buffers used in [buF1] and [buF2] are too
close together or are the same buffer.
Calibrate specifying custom, pH 4, pH 7, or
pH 10 buffer to allow for offsets of up to ±
4 pH units.
Perform electrode maintenance.
Specify the correct pH value for the
standard and redo the calibration.
Redo calibration specifying correct buffer.
Calibrate using [buF1] for first buffer, then
go to [buF2] to calibrate for slope.
Select buffers which are further apart to
allow for more accurate slope calculation.
Perform [buF1] calibration only and use
default slope.
Wrong buffer specified.
TC is not connected.
off-scale.
CA3.6 Offset > 1.3 pH units.
CA3.7 Slope efficiency less than
85% or greater than 102%
Nernstian response.
Process is outside of TC operating range
of -5 °C to 105 °C.
Large offset in reference electrode or
electrode depleted.
Bad buffer used for calibration. Use fresh buffer.
Poor electrode pair performance.
Bad buffer used for calibration. Use fresh buffer.
Buffers were too close together.
Electrodes did not stabilize.
1-800-742-1413 www.aquametrix.com
Redo calibration with correct buffer.
Check TC connections or use manual TC. E3.5 Temperature compensator is
Redo calibration within TC operating range
or use manual temperature compensation.
Check electrode, service or replace as
required.
Check both the reference and the glass pH
electrode. The glass may need to be etched
or cleaned.
Use buffers which are further apart.
Allow more time for the analyzer to
stabilize, repeat calibration if necessary.
Use buffer closest to pH 7 as first buffer.
42

AQUAMETRIX INC.
Error Description Causes Solutions
+ Err pH reading off-scale;
pH > 14.
- Err pH reading off-scale;
pH < 0.
Process too caustic for accurate
measurement.
Large electrode offset. Service or replace electrode.
Electrode not connected.
Electrode not responding.
Process too acidic to be measured. Verify process.
Caution Messages for Alarms
Caution Number Description
CA7.5 Alarm A, “No Chlorine” alarm
CA7.6 Alarm A, HIGH alarm
CA7.7 Alarm A, LOW alarm
CA7.8 Alarm A, DEVIATION alarm
Verify process.
Connect electrode or check
connections.
Etch glass electrode. Clean
reference electrode.
CA7.9 Alarm A, FAULT alarm
CA8.5 Alarm B, “No Chlorine” alarm
CA8.6 Alarm B, HIGH alarm
CA8.7 Alarm B, LOW alarm
CA8.8 Alarm B, DEVIATION alarm
CA8.9 Alarm B, FAULT alarm
1-800-742-1413 www.aquametrix.com
43

AQUAMETRIX INC.
DISPLAY PROMPTS
[_ . _] Timer menu
[+_ . _] Set time for on-cycle
[-_ . _] Set time for off-cycle
[AL] Alarms
[AL.A] Alarm A
[AL.b] Alarm B
[Auto] Automatic compensation
[bAud] Baud rate
[buF1] Buffer for standardizing or first buffer for calibration
[buF2] Second buffer for calibration
[°C] Temperature in degrees Celsius; temperature input; use metric units
[CAL] Calibrate
[CHIP] Chip. Is this analyzer equipped with a real-time clock chip?
[CF] Membrane compensation factor
[CLSd] Normally closed alarm contact
[CONF] Configuration
[cur] Signal output in mA, or current
[cuSt] Custom buffer value for calibration
[dA] Input damping time in seconds
[dAtE] Date. Real-time clock setting for day of month
[dEv] Deviation alarm
[dFLt] Default
[dLAY] Alarm activation delay
[do] Do — press to do reset/clear action
[donE] Done – reset/clear action has been accepted
[EFF] Efficiency
[Err] Error
[Er.94] RAM checksum failed. Some settings may be lost
[Er.95] EPROM checksum failed
[° F] Temperature in degrees Fahrenheit; temperature input; use imperial units
[FLt] Fault alarm, selectable function for alarm B
[GEt] Get the grab sample calibration reference reading
[HI] High alarm; high limit (20 mA) for 4 mA to 20 mA output window
[HOCl] HOCl, hypochlorous acid, free available chlorine input
[HOLd] Output hold
[hour] Hour. Real-time clock setting
[hund] Hundredth of a second. Real-time clock setting
[iLOG] Internal data log menu; refer to IC Net section
[in] Input – OR – Minute for real-time clock setting
[init] Initialize all program settings to factory defaults
[LO] Low alarm; low limit (4 mA) for 4 mA to 20 mA output window
[no.Cl] No chlorine alarm
[NodE] Node number
[NO.NC] Normally Open/Normally Closed
[NONE] No membrane compensation
[OFF] Off
[OFFS] Offset
[OLd] Old. The grab sample calibration old reading
[ON] On
1-800-742-1413 www.aquametrix.com
44

AQUAMETRIX INC.
[ON.OF] On/off switch
[onth] Month. Real-time clock setting
[OPEN] Normally open alarm contact
[out] 4 mA to 20 mA analog output channel
[out1] Output 1
[out2] Output 2
[PH] pH input
[pH.C] pH compensation
[rtc] Real-time clock
[SEC] Second. Real-time clock setting
[SEr] Serial menu; refer to IC Net section
[SEt] Set-point; select manual compensation value; set grab sample calibration
[SLOP] Calculated slope for pH input, given as % Nernstian response
[StAr] Start
[StAt] Current status of timer
[Stby] Standby mode
[Std.] Standard membrane
[tc] Temperature compensation
[tFCl] Total free available chlorine input; HOCl + OCl
ion
[unit] Display of units used for analog outputs and alarms
[YEAr] Year. Real-time clock setting
-
, hypochlorous acid plus hypochlorite
1-800-742-1413 www.aquametrix.com
45

AQUAMETRIX INC.
GLOSSARY
Electrode
Both a sensing and a reference electrode are needed for the analyzer to measure the process. Commonly these are combined
into one and referred to as a combination electrode. The temperature sensor may be built into the electrode as well.
EPROM
Erasable/Programmable Read Only Memory. The EPROM chip holds the program which determines the functioning of
8760CLP analyzer. Replacing the EPROM chip with a chip containing a new or an updated program changes the way the
analyzer functions. The EPROM chip is programmed by the manufacturer.
Free Available Chlorine
The hypochlorous acid form of chlorine; HOCl.
Hysteresis
The reading at which an alarm is turned on is not the same reading at which the alarm is turned off again. This phenomenon is
referred to as the hysteresis.
LED
Light Emitting Diode. LEDs are used as on/off indicators on the front panel of the 8760CLP.
Menu
The series of prompts which determine the layout of the program used by the analyzer.
Microprocessor
An integrated circuit (chip) which executes the program on the EPROM chip and controls all the input/output functions.
Nernst Equation
Equation which relates the voltage signal produced by the electrodes to the pH of the sample. The equation is temperature
dependent.
NC, Normally Closed
NO, Normally Open.
Normally Closed
Each of the alarm contacts can be wired and configured as normally open or normally closed. A circuit which is wired
normally closed will be closed, ie. the external device wired to it is turned on, when the analyzer is not powered.
Normally Open
A circuit which is wired normally open will be open, ie. the external device wired to it is turned off, when the analyzer is not
powered.
On/off Control
Control response in which the contact is either fully on or fully off.
ppm, Parts per Million.
1 ppm = 1 mg/L. Unit of concentration for chlorine measurement.
RAM
Random Access Memory. Memory in a RAM chip can be both written to and read from. The contents of RAM will disappear
as soon as the RAM chip loses power. The RAM chip has a battery backup device which preserves the contents of the RAM
chip for a considerable time even if the analyzer is turned off. All settings are stored in RAM.
TC, Temperature Compensator.
Temperature Compensation
Correction for the influence of temperature on the sensing electrode. The analyzer reads out concentration as if the process
were at 25 °C, regardless of actual solution temperature.
Total Free Available Chlorine
Sum of the hypochlorous acid (HOCl) and hypochlorite ion (OCl
-
) forms of chlorine.
1-800-742-1413 www.aquametrix.com
46

AQUAMETRIX INC.
CONFIGURATION OF PROGRAM
The 8760CL analyzer has been designed with ease-of-use in mind. In most cases the analyzer has been
configured to ordered specifications at the factory and no configuration of the analyzer is necessary.
However, several hardware options are available and if they are changed the program configuration
settings need to be set accordingly for the program to function properly. Other program adjustments,
which are normally made infrequently or when installing the analyzer, are located in the configuration
menu.
Normally Open or Normally Closed Alarm Contacts
The 8760CL program assumes the alarm contacts are wired normally open. A normally open alarm
contact will be open (inactive) if there is no alarm condition and will be closed (active) when there is an
alarm condition. If the program configuration and the wiring for each alarm do not match then the
incorrectly configured alarm contact will generate an alarm when there is no alarm condition and vice
versa.
Initializing All Program Settings
Occasionally, it may be desirable to
reinitialize all of the program’s settings to
bring them back to default. Executing the
initialization procedure will cause the
analyzer to reset all the program
variables, settings, preferences, and input
calibrations to factory default and then
proceed with the normal startup display.
The initialization procedure is not to be
used unless you are absolutely sure that
you want to restore the analyzer to factory
default configuration.
After the analyzer program has been
initialized, you will need to re-enter the
output signal settings, alarm settings, as
well as the program configuration if it
was different from the factory default
settings.
Select [CONF] [init] [ALL] [do] from the
menu. The display will flash [do].
Nothing will happen if you press
SAMPLE or CANCEL. The analyzer will
re-initialize only if you press ENTER.
The analyzer will then go through it's start
up sequence.
Illustration 36: Configuration menu
1-800-742-1413 www.aquametrix.com
47

AQUAMETRIX INC.
Metric or Imperial Units
By default the analyzer will use metric units. This means that temperature will be displayed using
degrees Celsius and that the prompt for the temperature input will be [°C]. The analyzer can also be
made to use imperial units as the preferred unit. Using imperial units, temperature will be displayed
using degrees Fahrenheit in the sample menu and the prompt for the temperature input will be [°F]
instead of [°C] throughout the program.
For practical reasons, the temperature input is identified as [°C] throughout this instruction manual and
in the menus.
To select imperial units for the analyzer, select [unit] from the configuration menu, then go into edit
mode and change the [°C] prompt to [°F].
Input Damping
The chlorine, pH and temperature measurements can be damped to deal with rapidly-varying or noisy
signals. Damping range is selectable between 3 s to 99 s. With 0 seconds, each reading is used to
directly update the display and 4 mA to 20 mA output. The factory default of 5 seconds adds the next 4
seconds of readings to the first and divides by five; this gives fast response. Selecting 99 seconds
provides a smooth damping out of turbulent readings. Any selection between 3 s and 99 s can be made.
Select [CONF] [in] from the menu. Using Up or Down arrow key find the desired input and press
SELECT. Using Up or Down arrow key find [dA] and press SELECT. Press ENTER to get into edit
mode and change the damping value to the new value. Press ENTER to accept the change and leave edit
mode.
Real-Time Clock
All AquaMetrix analyzers have an internal clock used for date/time stamping of system events and the
internal data log. On power outage, the clock stops, then it continues where it left off when power
returns.
When the 8760CLP is purchased with option -13, a real-time clock will maintain the correct time and
date even with the power turned off. To check if your analyzer has a real-time clock, select [CONF]
[rtc] [CHIP] from the menu. If the display shows [YES], then there is a real-time clock. If the display
shows [no] you can still set the date/time clock, but the time and date will need to be adjusted each time
the analyzer loses power.
To set the real-time clock, select [CONF] [rtc] from the menu. Set the year, month, day (of the month),
hour, minute, and second.
Electronic Calibration of Inputs
Adjustments to the input circuits of chlorine, pH and temperature can be made both electronically and
by making software adjustments.
The program adjustments are made using the configuration section of the menu, ie. by selecting [CONF]
[in] and then the appropriate input from the menu. The procedures are described in detail in the
Electronic Hardware Alignment section.
1-800-742-1413 www.aquametrix.com
48

AQUAMETRIX INC.
I
OUTPUT SIGNALS
Two assignable 4 mA to 20 mA output channels are provided. The user may configure the analyzer to
determine which input signal will be transmitted by each 4 mA to 20 mA output channel. Each output
channel can be independently configured to
transmit a chlorine, pH or temperature signal.
The output channels function independent of
each other. Each output channel has a
separate on/off switch and adjustable low and
high span (or scale) adjustments. This makes
it possible, for example, to transmit both
HOCl and tFCl signals, each using separate
high and low adjustments.
To adjust the output span or output window
for chlorine, pH or temperature signals, set
[LO] to correspond to the low end of the scale
or 4 mA output, and set [HI] to correspond to
the high end of the scale or 20 mA output.
The analyzer will automatically scale the
output according to the new settings.
llustration 37: Output menu
Reversing the 4 mA to 20 mA Output
The low scale setting will normally be lower than the high scale setting. It is possible to reverse the
output or "flip the window" by reversing the settings of the low and high scale.
Simulated 4 mA to 20 mA Output
Select [cur] from the menu to display the output current in mA that is presently being transmitted. The
display will be updated as the output signal changes based on the input signal and the program settings.
From here, one can watch the output respond to the change in the input signal. This is useful for
verifying program settings and for testing the hardware calibration.
In addition, the 8760CLP output can be used to calibrate downstream receivers such as 4 mA to 20 mA
recorders or data acquisition systems. To simulate a different 4 mA to 20 mA output signal press
ENTER to access edit mode. Edit the displayed mA value to display the desired output needed for
testing the output signal. Press ENTER to select the displayed value. The output signal will be adjusted
to put out the desired current. This process can be repeated as often as necessary.
The output signal is held at the displayed level until the program leaves this part of the menu.
Units for Outputs
The output menu will be using different units for its settings, depending on the input selected. Select
[unit] from the output menu to display the units in use for this output.
1-800-742-1413 www.aquametrix.com
49

AQUAMETRIX INC.
I
I
ALARM FUNCTIONS
Two alarms, alarm A and alarm B, are a standard feature. Each alarm has an alarm contact associated
with it which can be used for remote alarm indication or for control functions. The two alarms function
independently of each other. Either alarm can
independently monitor any of the inputs.
Each alarm features an adjustable set-point, userselectable alarm type, and adjustable differential (also
called hysteresis). The alarm types which are
available are “no chlorine”, high, low, deviation, and
fault. Alarms can be set anywhere between 0 ppm
and 2 ppm for HOCl, 0 ppm to 5 ppm for tFCl, 0 pH
to 14 pH for pH and -5 °C to 105 °C for temperature.
Use of Relay Contacts
By default, the relay contacts will be used to indicate
alarm conditions. Alarm conditions are indicated
using both the LED and the relay contact. This usage
of the relay contacts is selected by setting [CONF]
[AL] [AL.A] [FUNC] and [CONF] [AL] [AL.b]
[FUNC] to [AL]. If some other use is selected for the
relay contacts then the alarm cannot simultaneously
use the contact; however, the alarm function
continues using the LED, display messages and serial
llustration 38: Alarm menu
communication.
Alarm Indication
The A and B LEDs on the front panel show the current state of each alarm and alarm contact. In
addition, an alarm condition for an input will cause the sample display for that input to alternate with the
alarm function, [no.Cl], [LO], [HI], [dEv], or [FLt]. An LED that is blinking or on shows that the alarm
has an alarm condition. The status of the alarm contact can also be determined at a glance; the
corresponding alarm contact is activated when the LED is on and is deactivated while the LED is
blinking or off. Note that the alarm LED will blink while the alarm is in MANUAL because this also
deactivates the alarm contacts.
Each alarm will generate a caution number in the error
menu. Refer to Caution Messages for Alarms in the
Error Messages section for the meaning of each alarm
caution. The alarm cautions will not cause the error
LED to come on because the error LED only comes on
if there are any errors. To view alarm caution(s) using
the error menu, select [Err] from the main menu, then
use the Up or Down arrow key to scroll through the list
llustration 39: Alarm status indication, alarm LEDs
written into an internal log which can be accessed using the IC Net Intelligent Access Program. The
IC Net program uses the analyzer's serial communication port to read and display this information.
1-800-742-1413 www.aquametrix.com
of errors and cautions, if any.
Each alarm situation also causes an event tag to be
50

AQUAMETRIX INC.
Manual Alarm Override
For normal alarm operation the alarms are said to operate in auto-mode. If the operator wishes to
intervene and switch off the alarm contacts temporarily while attending to a problem, the alarms can be
switched to manual override using the MANUAL key.
In AUTO mode: the green AUTO LED is on and the analyzer alarms will activate and deactivate the
relay contact as programmed. Press the MANUAL key to temporarily deactivate the alarm contacts.
In MANUAL mode: the green AUTO LED will
blink. The relay contacts are deactivated, but
the alarm LEDs continue to indicate alarm
condition(s). Press the AUTO key to return to
AUTO mode immediately and reactivate the
relays. If no key is pressed for 15 minutes, the
15-minute timeout will return the alarms to
AUTO mode.
Delayed Relay Activation
Alarm relay activation, by default, is immediate upon alarm condition. Alarm relay activation may be
delayed. Activation delay gives the operator a chance to correct alarm situations before the relay
contacts activate, or can eliminate alarms based on temporary or spurious changes in the process.
The delay time is programmable by the operator. To change or view the delay time, select [dLAY] from
the alarm menu. The default value of 0 seconds is for immediate contact activation. The delay time can
be set from 0 s to 9999 s.
Unit Selection
The alarm module will be using different units for it's settings depending on the input selected. Select
[unit] from the alarm menu to display the units in use for this alarm. The [unit] setting affects the setpoint, differential, and deviation settings for the alarm.
The temperature input will use different units depending on whether metric or imperial units are
selected. For temperature, the unit selection can be viewed only. The choice between metric or imperial
units is made in the configuration menu. Refer to the Configuration of Program section for further
details.
Wiring and NO/NC Contacts
The alarm contacts for alarms A and B may be wired as normally open or normally closed. By default,
the analyzer assumes the alarm contacts are wired normally open. A normally open alarm contact will
be inactive if there is no alarm condition and will be active when there is an alarm condition. If the
program configuration and the wiring for each alarm do not match then the incorrectly configured alarm
contact will generate an alarm when there is no alarm condition and vice versa.
1-800-742-1413 www.aquametrix.com
51

AQUAMETRIX INC.
I
I
High or Low Alarm
A high alarm is set when the value of the chlorine, pH or temperature rises above the set-point and is
cleared when that value drops to below the set-point minus the differential (refer to illustration 41). A
low alarm is set when the value of the chlorine, pH or temperature drops below the set-point and is
cleared when that value rises to above the set-point plus the differential (refer to illustration 42). The
differential has the effect of setting the sensitivity of the alarm. The differential provides a digital
equivalent of a hysteresis.
A two-stage alarm can be implemented by choosing the same alarm function, ie. high or low alarm, for
both alarms, but selecting different set-points.
Example:
The HOCl of a critical process may not drop to below 0.5 ppm. Use alarm A as a low alarm set at
0.5 ppm and use alarm B as an advance warning device by configuring it as a low alarm set at 0.75 ppm.
When alarm B is activated there is still time left to take corrective action.
llustration 41: High alarm
llustration 42: Low alarm
Deviation Alarm
A deviation alarm is practical when the process is expected to stay within a certain range. An alarm will
be set if the input deviates too far from a set-point. Note that the [dEv] frame only shows up in the
menu after the alarm function has been changed to deviation alarm; it would have no effect for a high,
low, or fault alarm.
Example:
If the total free chlorine concentration is expected to stay between 0.2 ppm and 1.0 ppm, then we would
set [in] to [tFCl], [Func] to [dEv], [SEt] to 0.6, and [dEv] to 0.4. Effectively, a high alarm at 1.0 ppm
and a low alarm at 0.2 ppm has been set.
The differential setting will continue to function as for high and low alarms.
1-800-742-1413 www.aquametrix.com
52

AQUAMETRIX INC.
Fault Alarm
A fault alarm for an input will be set when anything goes wrong with that input. Something is wrong
with an input if the input is off-scale or an unacknowledged error message exists for that input. Caution
messages do not cause a fault alarm.
To use an alarm as a fault alarm, select [FUNC] from the alarm menu, then select [Flt]. To enable the
alarm, make sure the on/off switch is set to [on]. Also, set the input in the alarm menu to the desired
input, either chlorine, pH or temperature.
The set-point and differential for the alarm have no effect when the alarm is used as a fault alarm.
Using Alarms for On/Off Control
The alarms can also be used for process control; the alarm contacts will then function as on/off signals
for switches controlling a valve, pump, or motor. The set-point determines the control point of the
system and the setting of the differential controls the amount of corrective action before a controlled
shut-off occurs.
1-800-742-1413 www.aquametrix.com
53

AQUAMETRIX INC.
TROUBLESHOOTING
Analyzer: Electronic Hardware Alignment
Devices referred to in the following descriptions are shown on component location drawings D5030269
and D5980176. Proper field wiring for hookup is shown on drawing D5040276. These instructions
assume 115/230 VAC power is hooked up, the calibration of input electronics are operable, and field
wiring is in place.
Alignment of Chlorine Detection Circuit
1. Set up a precision multimeter, Fluke 8051A or equivalent, to read VDC.
2. Use the “CL2” sensor connection, TB201-1, and “COM” sensor, TB200-3, as common. Refer to
wiring diagram.
3. Set the chlorine efficiency constant to 100% by selecting [tFCl] [EFF] from the menu and editing the
value to read 100.0%.
4. Adjust the electronic standardize with blue trimpot VR200, located mid-board above the terminal
block marked D.O., see drawing D5020269. Adjust the trimpot to a reading of 2.50 V at TP200
while inputting 0.250 VDC through a 1 MΩ 1% resistor. 0.250 VDC simulates 1.0 ppm HOCl at
approximately 100% efficiency under above conditions.
Calibration of Temperature Input
The temperature input can be adjusted both by making electronic adjustments and/or by having the
program compensate for differences in offset.
By default the analyzer is shipped with a 1.07 kΩ 1% resistor across the TC terminals. A 1.07 kΩ
resistor across the TC terminals will simulate a temperature of approximately 18 °C or 65 °F.
Software Calibration
To do a software calibration of the temperature input, the correct temperature needs to be known.
1. Select [CONF] [in] [°C] [CAL] from the menu. The actual temperature as measured by the
temperature sensor will be shown. Edit the displayed value to the known, correct temperature. Press
ENTER to leave edit mode, then SELECT to start the calibration.
2. The current temperature will be shown using a flashing display. When it looks like the input is
stable, press ENTER to set the new temperature. The software offset for the temperature input will
be adjusted automatically.
3. The calculated offset in degrees Celsius can be viewed by selecting [CONF] [in] [°C] [OFFS] from
the menu. Whenever the hardware alignment is ‘correct’, the offset will be 0.0. The displayed offset
can be edited.
1-800-742-1413 www.aquametrix.com
54

AQUAMETRIX INC.
Adjusting Electronic Calibration
1. Remove any offset calculated by a previous software calibration of the temperature input. Select
[CONF] [in] [°C] [OFFS] from the menu and edit the offset to read 0.0.
2. Set up a precision multimeter, Fluke 8051A or equivalent, to read VDC.
3. Use TB200, terminal 3, as common. See wiring diagram. Place a 1000 Ω 1% resistor across T+ and
T-. Adjust blue trimpot VR202, located at the top-left side of TB201, for a reading of 0.225 V at
TP202. Refer to wiring diagram and drawing D5030269 for component locations.
4. Place a 1,385 Ω 1% resistor across T+ and T-. Adjust blue trimpot VR203, located at top-right side
of U201, for a reading of 4.80 V at TP202. Refer to drawing D5030269 for component locations.
5. Close case and press SAMPLE key followed by the Down arrow key to display the temperature
reading.
6. Re-insert the 1000 Ω 1% resistor and adjust VR202 until the display reads 0.0 ± 0.1 °C.
7. Re-insert the 1,385 Ω 1% resistor and adjust VR203 until the display reads 100.0 ±0.1 °C.
Calibration of pH Input
Input for measurement circuit zero, 0.00 V, at high impedance BNC connector, normally found on
preamp; refer to drawing D5030269, 876 main board component locations. Measured voltage at TP201
(Pin 1 of U201) should be 2.50 VDC. Adjust voltage using blue trimpot VR204.
NOTE: There is no span adjustment because the chemical calibration always varies it to suit the
electrode.
Calibration of 4 mA to 20 mA Outputs
Use one of the following two approaches to get the analyzer to output the desired current level, and then
make electronic adjustments to calibrate the output.
Approach 1: Simulated 4 mA to 20 mA Output (Self Calibration)
1. Select [cur] from the output 1 menu to display the present output current in mA. The display will be
updated as the output current.
2. To simulate a different 4 mA to 20 mA output signal, press ENTER to enter edit mode. Use the
arrow keys to display the desired output signal. Press ENTER to select the displayed value. The
output signal will be adjusted to put out the desired current. This process can be repeated as often as
necessary to output different signal levels.
3. The output signal is held at the displayed level until the program leaves this menu selection. Make
calibration adjustments while the analyzer shows the output at 20.00 mA.
4. Repeat the above steps for output 2.
Approach 2: Use Voltage Source to Adjust Input
This faster calibration approach requires a voltage source for the input.
1. To calibrate output 1, set [in] = [°C], input a low enough signal to cause analyzer to indicate [- Err];
the analyzer will output 4.00 mA. Reverse the polarity or input a high enough signal to cause the
analyzer to indicate [+ Err]; analyzer will output 20.00 mA.
2. Repeat step 1 for output 2.
Tip: Both outputs can be simultaneously calibrated if you set [in] = [°C] for both inputs.
1-800-742-1413 www.aquametrix.com
55

AQUAMETRIX INC.
Adjusting Electronic Calibration
1. Outputs are isolated from main circuit, therefore, measurements are made with common at the output
2 terminal, TB304.
2. Measure output 1 ‘zero’ at TP301 (pin 8 of U304), while output 1 is outputting 4.00 mA. Reading
should be between -0.870 V and -1.250 V. Adjust #2 voltage with VR300.
3. Change analyzer output to 20.00 mA, switch meter to mA and measure + Terminal (+ terminal of
O/P 1) and adjust VR301 so that the current reads 20.00 mA. Return analyzer output to 4.00 mA and
trim actual output to 4.00 mA using VR300. Check again at 20.00 mA and repeat adjustments until
satisfied.
4. Measure output 2 zero at TP300 (pin 7 of U304), while output 2 is outputting 4.00 mA. The test
point should read between -0.870 V and -1.250 V. Adjust #2 ‘zero’ voltage with VR302.
5. Change output at output 2 to 20.00 mA, switch meter to mA at TB304, + terminal of output 2, and
adjust VR303 (span pot) until the current reads 20.00 mA.
NOTE: Zero and span are very wide range adjustments which show small interactions. Recheck
zero and span to confirm good calibration.
6. If so desired, all software settings can be returned to factory default condition by performing a
reinitialization. Refer to heading Initializing All Program Settings in Configuration of Program
section.
Testing Relay Outputs
1. Relay output operation can be verified by testing for contact closure or continuity at each relay. To
activate a relay, select [CONF] [NO.NC] [AL.A] from the menu. Press ENTER to go into edit mode,
then press the Up or Down arrow key to change the normally open/normally closed configuration
from open to closed. Press ENTER again to accept the new value. A closed contact should open, an
open contact should close.
2. Repeat step 1 for for the Alarm B contact.
3. If so desired, all software settings can be returned to factory default condition by following the
procedure in Re-initializing All Settings in the Configuration of Program section.
Chlorine Sensor
Slow Response — typically due to excessive sample line length and low flow, thus producing long
sample transport lags. Resolve by adding a fast-flow loop with the sensor in a short side stream, or by
shortening the line. Slow response can also be caused by growth of biologicals in the sample line. In
this case, the problem may be alleviated by changing the take-off point.
Readings consistently low or spike low — characteristic of wiring problems between the analyzer and
the chlorine sensor; an open circuit in the field wiring will result in zero cell current and a very low
reading. Review the installation instructions.
Readings gradually falling — the analyzer can no longer be calibrated properly. This problem is
typical of sludge/slime deposits on the face of the chlorine sensor. The sensor will need to be cleaned.
Refer to the Monthly Maintenance and/or Semi-Annual Maintenance procedure in this manual.
Readings trend higher — this problem is typical of pressure gradually stretching the chlorine sensor
membrane which is getting thinner. The membrane will soon fail. Correct by lowering pressure at the
sensor. Change membrane if required.
1-800-742-1413 www.aquametrix.com
56

AQUAMETRIX INC.
pH Sensor
AquaMetrix carries a portable pH analyzer and pH calibrator, model AM-659, for this purpose. The
calibrator can be used to prove the portable pH analyzer before use, or it can be used to prove the
process pH analyzer, in this case the 8760CL, where a problem has been exhibited.
Before testing the pH sensor, be sure the test analyzer is known to be good.
FIRST: Inspect electrodes and if dirty or scaled:
| Clean with a soft cloth.
| Acid clean to remove scale as per Chemical Clean procedure.
SECOND: Run buffer tests in (but do not adjust analyzer):
| pH 7 buffer; write down reading and response time
| pH 4 buffer; write down reading and response time
Slow response? Clean again or acid clean overnight in electrode wash solution, P/N AM-A1100091.
Make sure that after cleaning, response is not longer than 3 minutes.
REFERENCE: If pH 7 reads between 6 pH and 8 pH then the reference is good. If pH reading is
outside pH 6 or pH 8, then the reference is poor or has failed.
pH GLASS: Subtract pH 4 reading from pH 7 reading.
| if result is 2.5 to 3, the glass is good.
| if result is less than 2.5, then pH electrode is failing and should be replaced.
Less responsive pH electrodes can sometimes be regenerated with P/N AM-A1100092, electrode renew
solution. Refer to Restoring Electrode Response.
THIRD: If pH sensor passes the above tests, then it is good.
Place electrode back in the loop and then run a 2 buffer calibration following the instructions in this
manual.
FOURTH: If the sensor fails tests:
| Replace the pH sensor.
Troubleshooting Tips for pH
Reading spike – characteristic of bubbles in the sample line passing through the sensor or sticking to
the pH sensor. Also characteristic of pickup from interference pulses generated from AC lines, when
AC loads go off-line.
Readings gradually drifting away – the pH sensor can no longer be calibrated. This problem is typical
of scale or sludge/slime deposits on the pH glass. The sensor may need to be cleaned.
Readings at maximum – under all conditions.
Either the sensor is in air or there is a problem with the wiring/analyzer setup. Test for shorts by
disconnecting BNC and checking impedance between center pin and outside housing with sensor in air.
Insulation value should exceed 100 MΩ.
If the sensor is OK, use the model AM-659 portable calibrator/analyzer to test the preamp, wiring and
the analyzer. If the problem persists with the AM-659 in place then it is an analyzer problem.
1-800-742-1413 www.aquametrix.com
57

AQUAMETRIX INC.
If the sensor tests as still good, and the analyzer and wiring works with the model AM-659, but the
“+ERR” or over-scale still occur when the analyzer and sensor are hooked up and placed in service, then
the most likely cause is a ground loop short forming, not actually a pH sensor problem. Refer to the
model AM-659 user manual troubleshooting procedures to resolve this pH loop, plant site, interaction
problem.
The above symptoms cover most difficulties associated with pH sensors. The key to isolating problems
in the pH sensor or analyzer is being able to separate the two.
Restoring Electrode Response
Used electrodes which are mechanically intact but low efficiency or slow responding, can often be
restored to full response by one of the following procedures:
1) Scale deposits:
Dissolve the deposit by immersion of the electrode tip, overnight (or over weekend), in electrode
wash solution, P/N AM-A1100091, followed by rinse in tap water. Soak in electrode storage
solution, P/N AM-A1100090 for 1 to 2 hours.
Difficult cases: Repeat substituting gentle scale remover, P/N AM-A1100093, then 15 minute rinse.
2) Oil or grease films:
Wash electrode tip with detergent and water. If film is known to be soluble in a particular organic
solvent, wash with this solvent. Rinse electrode tip with tap water. Let sit in demin water, P/N AMA1100015, for 2 to 4 hours, followed by 2 to 4 hours in electrode storage solution, P/N AMA1100090.
Difficult cases: Repeat using wash in sodium hypochlorite (Javex Bleach) in water solution,
adjusted to pH 6.5 ± 0.5 using vinegar or acid.
3) Plugged or dry reference junction:
Remove the contaminant with one of the above procedures, then soak in electrode storage solution,
P/N AM-A1100090 for 24 hours to one week.
Difficult cases: Repeat but heat almost to boiling for ½ hour first, then soak in electrode storage
solution, P/N AM-A1100090 for 24 hours to one week.
4) Biological growths:
Wash electrode tip with detergent and water.
Difficult cases: Wash with Sodium hypochlorite (Javex Bleach) in water solution, adjusted to pH
6.5 ± 0.5 using vinegar or acid. Use rubber gloves, and wash until deposits fall off or turn white.
Rinse tip with tap water. Let sit in demin water, P/N AM-A1100015 for 2 to 4 hours, then 2 to 4
hours in electrode storage solution, P/N AM-A1100090.
5) Clean, but slow and with less than 85% efficiency:
Wash electrode tip with electrode renew solution, P/N AM-A1100092, for 15 minutes. Rinse
electrode tip using tap water for 15 minutes. Let sit in demin water, P/N AM-A1100015, for 2 to 4
hours, followed by 2 to 4 hours in electrode storage solution, P/N AM-A1100090.
NOTE: If none of the above procedures succeed in restoring electrode response, it is near the end of
the useful life of that sensor and should be replaced.
1-800-742-1413 www.aquamerix.com
58

AQUAMETRIX INC.
APPENDIX A — Enabling Security
The analyzer has a built-in password protection system. This security system is disabled by default and
does not need to be enabled if no password protection is necessary. If you choose not to enable the
password protection system then the user will have unrestricted access to all analyzer settings available
through the menu as described in this manual.
Having security disabled gives the user the same access to the program as being at access-level 2 at all
times.
With security enabled anyone can view settings
anywhere in the program. When you do not have
proper access rights, the program will display
[PASS] for 2 seconds, indicating that a proper
password must be entered before being allowed to
proceed.
This appendix contains instructions for setting
passwords in the configuration section of the menu.
Daily usage of the analyzer by the operator does not
require knowledge of setting passwords in the
configuration section since all passwords are entered
by selecting [PASS] directly from the main menu.
Access-level Description
0 View only access to all settings
1 Access to all settings except for
configuration menu. Usage: operator
access, no changes can be made to
configuration and passwords cannot
be changed.
2 Access to all settings. This gives the
same program access as when
password security is not enabled.
Passwords can be changed. Usage:
installation, management.
ENTERING A PASSWORD
Table1: Security access levels
With security enabled, select [PASS] from the main
menu. The analyzer will display [0000]. Use the arrow keys to display your level 1 or level 2 password,
then press ENTER. The program will display [good], followed by your access level before returning to
the main menu. If an incorrect password was entered, the program displays [bAd] instead. Refer to
illustration 43 to determine how the program validates a password.
You will now have level 1 or level 2 access for as long as you are working with the analyzer. The
access level will automatically be restored to level 0 after no key has been pressed for 15 minutes. This
15-minute timeout will also return to display the main sample.
It is good practice to return the analyzer to level 0 access (or level 1 access if password 1 is set to “000”)
when you have finished using the analyzer. This is accomplished by selecting [PASS] from the main
menu, then pressing ENTER with [0000] displayed.
ENABLING PASSWORD SECURITY
When security is disabled, both password 1 and password 2 are set to “0000.” Security is enabled by
setting password 2 to a non-zero value.
Level 2
Select [CONF] [PAS.2] from the menu. The analyzer will display [0000]. Use the arrow keys to
change the display to the desired password for level 2. You can press SAMPLE at any time to safely
cancel password entry. Press ENTER to enter the password into memory and to enable password
security. The analyzer program automatically returns to the configuration menu.
With only password 2 set to a non-zero value, level 2 access is required to make changes in the
configuration menu but all other settings are unprotected. Effectively the user will always have at least
level 1 access.
1-800-742-1413 www.aquamerix.com
59

AQUAMETRIX INC.
Level 1
At this point, password 1 is still “000.” You may optionally enable operator access control or level 1
security by changing the level 1 password from “000" to a non-zero value. Change the password by
selecting [CONF] [PAS.1] from the menu, then entering an appropriate 3-digit password.
RECORDING YOUR PASSWORDS
You may want to write down the passwords you set and store them in a secure place. Once a password
has been set, there is no way to redisplay it. Since passwords are set in the configuration menu, level 2
access is required to change either password. If you have forgotten the level 2 password, there is no
simple way to regain access to the analyzer. Contact the factory if you find yourself locked out of the
analyzer.
DISABLING PASSWORD SECURITY
Password security can be disabled by setting the level 2 password to “0000.” In order to change the
password you must first have level 2 access to the program.
Select [CONF] [PAS.2] from the menu, then press ENTER when the program displays [0000]. Both
passwords 1 and 2 are set to “0000" and security is now disabled. The main menu will be changed to
exclude the [PASS] frame , and the configuration menu will no longer have the [PAS.1] frame.
Password Validation
1-800-742-1413 www.aquamerix.com
60

AQUAMETRIX INC.
PASSWORD EXAMPLE - A QUICK TOUR
With security disabled, select [CONF] [PAS.2] from the menu. Set the level 2 password to “0002".
Select [CONF] [PAS.1] from the menu. Set the level 1 password to ”001." Security is now enabled.
Select [PASS] from the main menu. Press ENTER with [0000] displayed. The analyzer will display
[ACC.0] to indicate we are now at access level 0.
Try changing the output 1 low setting. Select [out] [out1] [LO] from the menu. The current value will
display. Press ENTER to go into edit mode. The analyzer will display [PASS] for 2 seconds because we
need to enter a password first. Level 1 security is needed to change this setting.
Select [PASS] from the main menu again. Change the displayed value to [0001], which is the level 1
password. Press ENTER. The analyzer will display [good], followed by [ACC.1], indicating that the
password is valid and that we now have level 1 access.
Try changing the output 1 low setting again. You will find that this time we can go into edit mode
unhindered.
Select [PASS] from the main menu again. Enter the level 2 password, which is “0002.” We are going
to set the level 2 password to “0000” again to disable password security. Password 2 is found in the
configuration menu and therefore requires level 2 access before it can be accessed. Select [CONF]
[PAS.2] from the menu. Press ENTER with [0000] displayed. Both passwords are set to “0000” again
and password security is disabled.
1-800-742-1413 www.aquamerix.com
61

AQUAMETRIX INC.
APPENDIX B — Default Settings
The following program settings are the default settings for the analyzer. New analyzers will have these
settings unless the setup has already been customized for your application.
Outputs
Output 1 Output 2
Input to be transmitted tFCl °C
Low setting 0.00 0.0
High setting 2.00 100.0
ON/OFF switch ON ON
Alarms
Alarm A Alarm B
Input for alarm tFCl tFCl
Alarm function HI LO
ON/OFF switch ON ON
Set-point 0.60 ppm 0.20 ppm
Differential 0.10 ppm 0.10 ppm
Unit 1E-6 (ppm) 1E-6 (ppm)
Delay 0 s 0 s
Global Units
Metric units: temperature in degrees Celsius, chlorine concentration in parts per million (ppm)
Alarm Contacts
Configured normally open
Security
Not enabled
pH and Temperature Compensation Method for Chlorine
Automatic TC using temperature input; automatic pH compensation using pH input
Membrane Compensation
Enabled
Timer Feature
Not enabled
1-800-742-1413 www.aquamerix.com
62

AQUAMETRIX INC.
APPENDIX C — Installation
Electrical
The analyzer requires 115/230 VAC power to be hooked up to TB400, found on drawing D5040276.
Connect the two alarm contacts: Alarm A contact: TB300
Alarm B contact: TB301
Connect the two isolated 4 mA to 20 mA outputs: Output 1: TB303
Output 2: TB304
Connect the inputs: Chlorine: direct connect to analyzer via 5-pin DIN connector.
pH: direct connect to analyzer via BNC connector.
Mechanical
Refer to sections entitled Chlorine Sensor Instructions and pH Sensor Instructions of this instruction
manual.
Program
Refer to Appendix B for default settings. Prior to putting the analyzer into operation, verify the settings
to ensure that they agree with the intended setup. For a more detailed description of any program
setting, refer to the appropriate section of this instruction manual.
Change defaults for the alarms. Set alarm function (high, low, deviation, fault), input (chlorine, pH,
temperature), differential, set-point, and on/off switch.
Change defaults for the 4 mA to 20 mA outputs. Set input, high limit, low limit, and on/off switch.
Set preference for metric/imperial units in [CONF] [unit].
Set the normally open/normally closed configuration of the alarm contacts in [CONF] [AL]. The
program setting must reflect the actual NO/NC wiring.
If desired, install password security.
1-800-742-1413 www.aquamerix.com
63

AQUAMETRIX INC.
DRAWINGS
D4040081: Outline and Mounting Dimensions
1-800-742-1413 www.aquametrix.com
64

AQUAMETRIX INC.
D5030269: Main Board Component Location
1-800-742-1413 www.aquametrix.com
65

AQUAMETRIX INC.
D5980176: Display Board Component Location
1-800-742-1413 www.aquametrix.com
66

AQUAMETRIX INC.
D5040276: Wiring Diagram
1-800-742-1413 www.aquametrix.com 67

AQUAMETRIX INC.
INDUSTRIAL PRODUCTS WARRANTY
Industrial instruments are warranted to be free from defects in material and workmanship for a period of
twelve (12) months from the date of installation or eighteen (18) months from the date of shipment from
AquaMetrix whichever is earlier, when used under normal operating conditions and in accordance with
the operating limitations and maintenance procedures in the instruction manual, and when not having
been subjected to accident, alteration, misuse, or abuse. This warranty is also conditioned upon
calibration and consumable items (electrodes and all solutions) being stored at temperatures between 5
°C and 45 °C (40 °F and 110 °F) in a non-corrosive atmosphere. AquaMetrix consumables or approved
reagents must be used or performance warranty is void. Accessories not manufactured by AquaMetrix
are subject to the manufacturer’s warranty terms and conditions.
Limitations and exclusions:
Industrial electrodes, and replacement parts, are warranted to be free from defects in material and
workmanship for a period of three (3) months from the date of installation or eighteen (18) months from
the date of shipment when used under normal operating conditions and in accordance with the operating
limitations and maintenance procedures given in the instruction manual and when not having been
subjected to accident, alteration, misuse, abuse, freezing, scale coating, or poisoning ions.
Chemical solutions, standards or buffers carry an “out-of-box” warranty. Should they be unusable when
first “out-of-box”, contact AquaMetrix immediately for replacement. To be considered for warranty, the
product shall have an RMA (Return Material Authorization) number issued by AquaMetrix service
department for identification and shall be shipped prepaid to AquaMetrix.
In the event of failure within the warranty period, AquaMetrix, or its authorized dealer will, at
AquaMetrix option, repair or replace the product non-conforming to the above warranty, or will refund
the purchase price of the unit.
The warranty described above is exclusive and in lieu of all other warranties whether statutory,
express or implied including, but not limited to, any implied warranty of merchantability or
fitness for a particular purpose and all warranties arising from the course of dealing or usage of
trade. The buyer’s sole and exclusive remedy is for repair, or replacement of the non-conforming
product or part thereof, or refund of the purchase price, but in no event shall AquaMetrix (its
contractors and suppliers of any tier) be liable to the buyer or any person for any special, indirect,
incidental or consequential damages whether the claims are based in contract, in tort (including
negligence) or otherwise with respect to or arising out of the product furnished hereunder.
Representations and warranties made by any person, including its authorized dealers, distributors,
representatives, and employees of AquaMetrix, which are inconsistent or in addition to the terms of this
warranty shall not be binding upon AquaMetrix unless in writing and signed by one of its officers.
1-800-742-1413 www.aquametrix.com
68

INDEX
AQUAMETRIX INC.
Acknowledging error messages 40
Alarms 50
caution messages 43
default settings 62
delay activation 51
deviation 50, 52
differential 50, 53
fault 50, 53
function 50
high 52
indication of 50
low 52
manual override 51
relay contacts 50
sensitivity of 52
set-point 50
two-stage 52
units 51
Ampoules 30
Analyzer
electronic alignment 54pp.
mounting 12
specifications 8p.
startup tests 15
troubleshooting 54
wiring 13
Automatic chemical cleaning 29
Bleach 20
Buffers
automatic recognition of 36
custom 37
selecting 39
temperature dependence of 37
Calcium hypochlorite 20
Calibration
chlorine 30p.
electronic 54pp.
outputs 55p.
pH 36pp.
temperature 54
Celsius 19
Chemical cleaning solution 68
Chlorine
alignment of detection circuit 54
1-800-742-1413 www.aquametrix.com
calibration 30p.
calibration kit 30
chemistry 20p.
combined chlorine 21
disinfectant properties 22
effect of pH 20, 32
free available chlorine 21
grab sample standardization 30
measurement 23
measuring cell 23
pH compensation 32
sensor 24pp.
standardizing 30
temperature compensation 32
temperature effect of 32
total free chlorine 21
total residual chlorine 21
zero test 27
Chlorine calibration kit 30
Chlorine sensor
automatic chemical cleaning 29
chemical cleaning 28
insertion into flow fitting 27
maintenance 28
membrane replacement 25p.
mounting 13
removal from flow fitting 27
specifications 11
storage 29
troubleshooting 56
wiring 13
zero check 27
Combined chlorine 21
Configuration 47, 63
Configuration
clock 48
input damping 48
normally closed 47, 51
normally open 47, 51
program 47, 63
units 48
Current output 49
default settings 62
69

AQUAMETRIX INC.
output hold 17
reversing 49
settings 49
simulating 49
standby mode 17
units 49
Damping, of inputs 19
Default settings 62
Deviation Alarm 52
Display prompts 44
Display prompts 45
Edit Mode
change settings 18
key functions 18
numeric values 18
Electrode 46
Electronic calibration of inputs 48
Er.94 44
Er.95 44
Error messages 40
+/- Err 40, 43
+/- sign 40
acknowledging 40
CA3.6 42
CA3.7 42
clearing 40
E1.0 41
E1.2 41
E1.3 41
E1.4 41
E1.5 41
E2.1 41
E2.2 41
E3.1 42
E3.2 42
E3.3 42
E3.4 42
E3.5 42
Fahrenheit 19
Fault alarm 53
Free available chlorine 21, 46
Galvanic cell 23
Galvanic chlorine sensor 23
Home Base 16
Hypochlorite ion 20
Hypochlorous acid 20
Hysteresis 46
iLOG 44
Input damping 19
Installation 12, 63
Installation
analyzer mounting 12
analyzer wiring 13
sensor mounting 13
sensor wiring 13
shop test startup 14
Keypad
arrow keys 17
AUTO key 17
CANCEL key 18
ENTER key 18
functions 18
MANUAL key 17
SAMPLE key 16
SELECT key 18
LED 46
Manual pH compensation 32
Manual temperature compensation 32
Membrane replacement toolkit 25
Menu 3, 5, 16, 46
Nernst equation 46
Normally closed 46
Normally open 46
Output hold 17
Output signals 49
Password 59pp.
pH
buffers 37
calibration 36pp., 55
compensation 32
Nernst equation 46
Nernstian response 39
offset 36, 38p.
output hold 17
slope 36, 39
standardizing 38
pH compensation 32
pH Sensor
cleaning 34p.
insertion into flow fitting 33
maintenance 33
Mounting 13
1-800-742-1413 www.aquametrix.com
70

AQUAMETRIX INC.
preparation for use 33
removal from flow fitting 33
specifications 10
storage 33
troubleshooting 57p.
wiring 13
ppm 46
Process control 53
Prompts 45
Real-time clock 19
Relays
testing 56
Security
access-level 59
disabling 60
enabling 59
password 59pp.
password 1 59
password 2 59
time-out 16
SEr 45
Sodium hypochlorite 20
Specifications 8pp.
Standby mode 17
Start-up
analyzer tests 15
instrument shop test 14
program initialization 47
Temperature 19
calibration 54
compensation 32
current output 49
default settings 62
units 19, 48
Temperature compensation 32, 46
Terminology
combined chlorine 21
free available chlorine 21
total free chlorine 21
total residual chlorine 21
Timer
15 minute time-out 16
automatic cleaner 64pp.
security time-out 16
Total free available chlorine 46
Total free chlorine 21
Total residual chlorine 21
Troubleshooting 54
analyzer 54
chlorine sensor 56
pH sensor 57
1-800-742-1413 www.aquametrix.com
71
 Loading...
Loading...