Merit Medical VP-210 User Manual
INSTRUCTIONS FOR USE
Y-TEC® VP-210 and VP-211
Implantation System for
Peritoneal Dialysis Catheters
Y-TEC® VP-210 and VP-211
Implantation System for Peritoneal Dialysis Catheter
English
PRODUCT DESCRIPTION
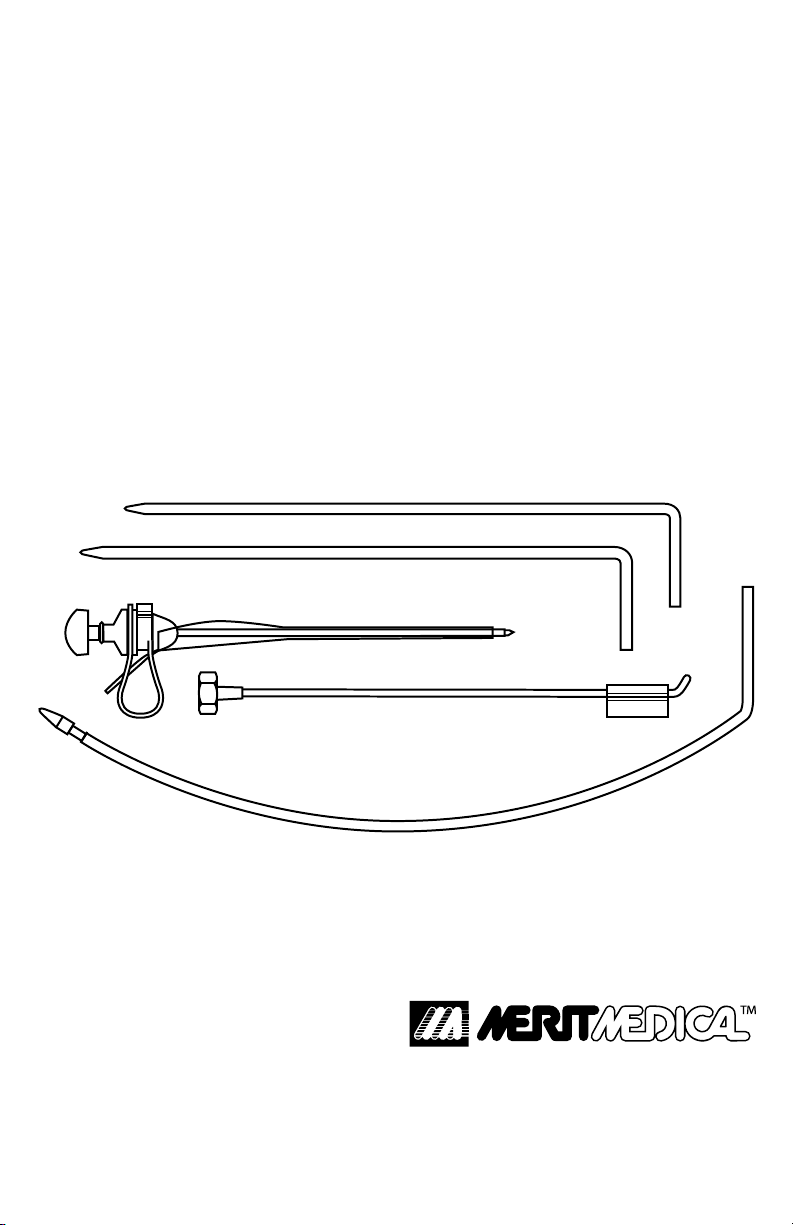
Y-TEC® VP-210 and VP-211
IMPLANTATION SYSTEM CONTAINS:
• Small Dilator
• Large Dilator
• Luke® Guide Assembly:
(Trocar, Cannula and Clip, Luke Guide )
• Cu Implantor™ Tool
• Tunnelor® Tool
INDICATIONS FOR USE
The Y-TEC Implantation System can be used to implant a
peritoneal dialysis catheter in patients who are suitable
candidates for peritoneal dialysis therapy.
CONTRAINDICATIONS
Do NOT use if the patient is not a suitable candidate for
peritoneal dialysis therapy.
Px Only: Caution: Federal (USA) law restricts this device to
sale by or on the order of a physician.
PRECAUTIONS
• Read manufacturer’s instructions prior to use.
• Contents are sterile (via ethylene oxide). Do not use if
packaging is opened, damaged, or broken.
• For single patient use only. Do not reuse, reprocess,
or resterilize. Reuse, reprocessing, or resterilization
may compromise the structural integrity of the device
and/or lead to device failure, which in turn may result
in patient injury, illness, or death. Reuse, reprocessing,
or resterilization may also create a risk of contamination
of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission
of infectious disease(s) from one patient to another.
Contamination of the device may lead to injury, illness,
or death of the patient.
• Do not use after expiration date.
• The medical techniques, procedures, and potential
complications stated herein do NOT give full and/or
complete coverage or descriptions. They are not a
substitute for adequate training and sound medical
judgment by a physician.
• Use an aseptic procedure to open the package and to
remove the contents.
POTENTIAL COMPLICATIONS
Peritoneoscopic and Laparoscopic procedures and general
anesthesia all have inherent risks associated with their use.
All such risks apply to the use of the Y-TEC Implantation
System. Peritoneal dialysis potentially has a number of
complications that may occur, which generally are not
caused by the implantation, but may aect the quality
of therapy. These complications may include, but are not
limited to, the following:
• Infections (exit-site or tunnel)
• Peritonitis
• Sepsis
• Bowel perforation
• Leakage (initial or latent)
• Fluid ow obstruction (inow or outow)
• Bleeding (subcutaneous or peritoneal)
• Ileus
• Proximal exit cu erosion
• Distal (rectus/deep) cu erosion
• Risks normally associated with peritoneoscopic and
laparoscopic procedures.
INSTRUCTIONS FOR USE
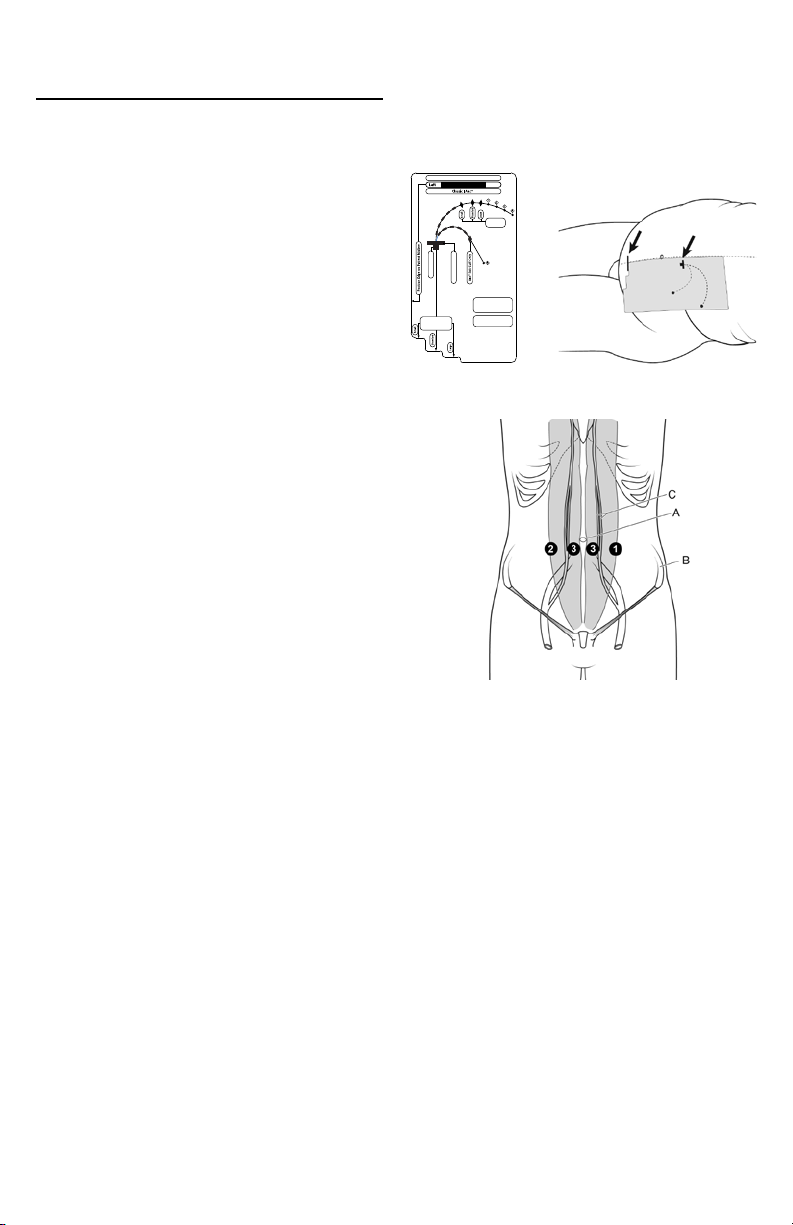
Catheter Implantation Site Options
Locate preferred implantation and exit sites as indicated by
an appropriate Implantation Stencil (Figure 1 and Figure
2) and the anatomical landmarks as indicated in Figure 3.
If using an Implantation Stencil (sold separately), consult
Instructions for Use.
Flex-Neck® Adult PD Catheter
IMPLANTATION STENCIL
Classic Exit
Cu Site
Rectus Cu Site
Primary Incision Site
®
Use with Flex-Neck
Classic & Arc™ Adult
PD Catheter ONLY
For directions, see
Place on Patient’s
Instructions for Use
Cranial Border of
the Pubic Symphysis
Figure 1 Figure 2-Stencil on body
Implantation Stencil
A. Umbilicus
B. Iliac crest
C. Inferior and superior epigastric arteries
Figure 3-Anatomical landmarks
1. Left, lateral border of rectus sheath,
2-3 cm below umbilicus
2. Right, lateral border of rectus sheath,
2-3 cm below umbilicus
3. Medial border of rectus sheath, 2-3 cm below umbilicus
NOTE: Implantation sites should be above superior iliac
crest.
WARNING: Do NOT implant the catheter at the patient’s
beltline, or skin folds.
WARNING: Do NOT place the exit-site in the patient’s skin
folds, or beltline.
PATIENT PREPARATION
1. Sedate patient.
2. Attach appropriate patient monitors.
3. Prepare abdomen and drape patient in standard
sterile manner.
4. Anesthetize primary catheter insertion site.
INSERTING LUkE GUIDE ASSEMbLY
1. Make 3-5 cm long horizontal skin incision.
2. Perform blunt dissection with hemostats to the anterior
surface of the rectus sheath (Figure 4 A & B), use
cauterization device as necessary to control bleeding.
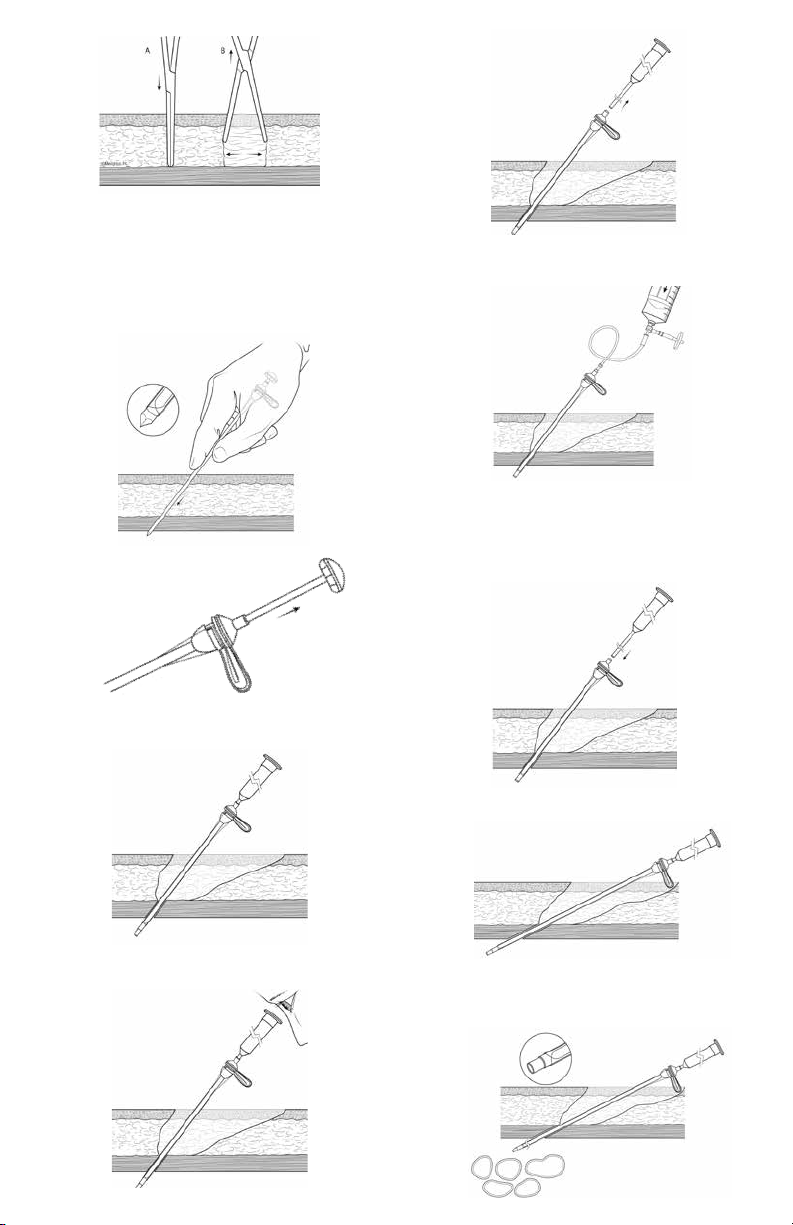
Figure 4
3. Ask patient to tighten abdominal muscles prior to
inserting the Luke Guide Assembly.
4. Insert Luke Guide Assembly at a 45° angle from
horizontal, pointing towards the coccyx (Figure 5).
CAUTION: It is important to maintain a 45° angle to assure
proper anchoring in the rectus muscle and nal catheter
placement.
Figure 5
ChECkING ThE POSITION
1. Remove trocar from assembly (Figure 6).
4. Remove scope (Figure 9).
Figure 9
5. (Optional) Attach Air Insuation Set, (sold separately, not
available in all areas) as needed to cannula (Figure 10).
Figure 10
6. Place patient in typical Trendelenburg position.
7. Insuate ltered room air (approximately 700-1200 cc,
depending on patient size).
8. Detach Air Insuation Set. Place thumb or nger on
cannula to retain air.
POSITIONING ThE CAThETER
1. Re-insert peritoneocope (Figure 11).
Figure 6
2. Insert Y-TEC Peritoneoscope (scope) into cannula and
lock together (Figure 7).
Figure 7
3. Conrm location of distal end of cannula and scope
within the peritoneum (Figure 8).
Figure 8
Figure 11
2. Aim distal tip of scope into the air pocket by
making the scope more parallel with the abdomen
(Figure 12).
Figure 12
3. Examine peritoneum to nd optimal location for
catheter. Note any adhesions or abdominal
characteristics that may impede proper catheter
placement. (Figure 13).
Figure 13
 Loading...
Loading...