Page 1

Medical
BioSphere
®
Instructions for use English 3
Instructions d'utilisation Français (French) 6
Instruções de utilização Português (Portuguese) 9
Instrucciones de utilización Español (Spanish) 12
Z 1759 rev E 06/12
730059002/A
1
Page 2

2
Page 3

ENGLISH
EmboGold®Microspheres in a Prefilled Syringe - For Embolization
CAUTION: Federal (U.S.A.) law restricts this device to use by
or on the order of a licensed physician.
INTENDED USE :
• Embolization of hypervascularized tumors.
• Embolization of arteriovenous malformations (AVM).
CLINICAL APPLICATIONS :
Scientific literature provides extensive documentation of
embolization procedures using a wide variety of agents in both the
neurological and peripheral circulatory systems,including the head,
neck, spine, liver, genitourinary tract, uterus, gastrointestinal
system, limbs, and lungs. A representative bibliography of this
literature is provided following these instructions for use.
DESCRIPTION :
EmboGold Microspheres are part of a family of new embolic
materials based on BioSphere Medical’s proprietary microsphere
technology. These colored spheres are designed to add easy
visualization during handling, while still offering the controlled,
targeted embolization characteristics of the first microsphere
product, Embosphere® Microspheres.
EmboGold Microspheres are biocompatible, hydrophilic, nonresorbable, precisely calibrated microspheres produced from an
acrylic polymer and impregnated with porcine gelatin. EmboGold
Microspheres are available in a range of sizes.
DEVICE PACKAGING :
• Embolic contained in sterile, 20 mL syringe (pre-filled syringe),
packaged in a peel-away tray.
• Each syringe contains approximately 1.0 mL or 2.0 mL of
EmboGold Microspheres in pyrogen-free, sterile, physiological
saline.
CONTRAINDICATIONS :
• Patients intolerant to occlusion procedures
• Vascular anatomy or blood flow that precludes catheter
placement or embolic agent injection.
• Presence or likely onset of vasospasm.
• Presence or likely onset of hemorrhage.
• Presence of severe atheromatous disease.
• Presence of feeding arteries smaller than distal branches from
which they emerge.
• Presence of patent extra-to-intracranial anastomoses or shunts.
• Presence of collateral vessel pathways potentially endangering
normal territories during embolization.
• Presence of end arteries leading directly to cranial nerves.
• Presence of arteries supplying the lesion not large enough to
accept EmboGold Microspheres.
• Vascular resistance peripheral to the feeding arteries precluding
passage of EmboGold Microspheres into the lesion.
• Do not use EmboGold Microspheresin the following applications:
a. Embolization of large diameter arteriovenous shunts (i.e. where
the blood does not pass through an arterial/capillary/venous
transition but directly from an artery to a vein).
b. In the pulmonary arterial vasculature.
c. Use in any vasculature where EmboGold Microspheres could
pass directly into the internal carotid artery, vertebral artery,
intracranial vasculature or the above-listed vessels.
• Patients who have an allergic response to gold.
WARNINGS:
• EmboGold Microspheres contain gelatin of porcine origin, and,
therefore, could cause an immune reaction in patients who are
hypersensitive to collagen or gelatin.Careful consideration should
be given prior to using this product in patients who are suspected
to be allergic to injections containing gelatin stabilizers.
• Studies have shown that EmboGold Microspheres do not form
aggregates, and as a result, penetrate deeper into the vasculature
as compared to similarly sized PVA particles. Care must be taken
to choose larger sized EmboGold Microspheres when embolizing
arteriovenous malformations with large shunts to avoid passage
of the spheres into the pulmonary or coronary circulation.
• Some of the EmboGold Microspheres may be slightly outside of
the range, so the physician should be sure to carefully select the
size of EmboGold Microspheres according to the size of the target
vessels at the desired level of occlusion in the vasculature and after
consideration of the arteriovenous angiographic appearance.
EmboGold Microspheres size should be selected to prevent
passage from artery to vein.
• Because of the significant complications of misembolization,
extreme caution should be used for any procedures involving the
extracranial circulation encompassing the head and neck, and the
physician should carefully weigh the potential benefits of using
embolization against the risks and potential complications of the
procedure. These complications can include blindness, hearing
loss, loss of smell, paralysis and death.
• The color of theEmboGold Microspherescould be visible through
the skin if injected into arteries feeding superficial tissues.
• Serious radiation-induced skin injury may occur to the patient
due to long periods of fluoroscopic exposure, large patient
diameter, angled x-ray projections, and multiple image recording
runs or radiographs. Refer to your facility’s clinical protocol to
ensure the proper radiation dose is applied for each specific type
of procedure performed. Physicians should monitor patients that
may be at risk.
• Onset of radiation-induced injury to the patient may be delayed.
Patients should be counseled on potential radiation side effects
and whom they should contact if they show symptoms.
• EmboGold Microspheres are not cleared for usein uterine fibroid
embolization and are associated with delayed pain and/or rash
when used in these procedures.
• Pay careful attention for signs of mistargeted embolization.
During injection carefully monitor patient vital signs to include
SAO2 (e.g. hypoxia, CNS changes). Consider terminating the
procedure, investigating for possible shunting, or increasing
microsphere size if any signs of mistargeting occur or patient
symptoms develop.
3
Page 4

• Consider upsizing the microspheres if angiographic evidence of
embolization does not quickly appear evident during injection of
the microspheres.
Warnings about use of small microspheres
• Careful consideration should be given whenever use is
contemplated of embolic agents that are smaller in diameter than
the resolution capability of your imaging equipment. The presence
of arteriovenous anastomoses, branch vessels leading away from
the target area or emergent vessels not evident prior to
embolization can lead to mistargeted embolization and severe
complications.
• Microspheres smaller than 100 microns will generally migrate
distal to anastomotic feeders and therefore are more likely to
terminate circulation to distal tissue. Greater potential ischemic
injury results from use of smaller sized microspheres and
consideration must be givento the consequence of thisinjury prior
to embolization. The potential consequences include: swelling,
necrosis, paralysis, abscess and/or stronger post embolization
syndrome.
• Post-embolization swelling may result in ischemia to tissue
adjacent to target area. Care must be given to avoid ischemiaintolerant, nontargeted tissue such as nervous tissue.
PRECAUTION :
• Do not use if the syringe, plunger seal or tray package appears
damaged.
• For single patient use only - Contents supplied sterile - Never
reuse, reprocess, or resterilize the contents of a syringe that has
been opened. Reusing, reprocessing or resterilizing may
compromise the structural integrity of the device and or lead to
device failure, which in turn may result in patient injury, illness or
death. Reusing, reprocessing or resterilizing may also create a risk
of contamination of the device and or cause patient infection or
cross infection including, but not limited to, the transmission of
infectious disease(s) from one patient to another. Contamination of
the device may lead to injury,illness or death of the patient.
All procedures must be performed according to accepted aseptic
technique.
• Do not connect the 20 mL syringe with EmboGold Microspheres
directly to a microcatheter for embolic delivery, as a catheter
occlusion may result.
• The syringe is intended for embolic use only. Do not use for
another application.
• Select the size and quantity of EmboGold Microspheres
appropriate for the pathology to be treated.
• Embolization with EmboGold Microspheres should only be
performed by physicians who have received appropriate
interventional occlusion training in the region intended to be
embolized.
• Patients with known allergy to contrast medium may require
corticosteroids prior to embolization.
• Additional evaluations or precautions may be necessary in
managing periprocedural care for patients with the following
conditions :
– Bleeding diathesis or hypercoagulative state
– Immunocompromise.
POTENTIAL COMPLICATIONS :
Vascular embolization is a high-risk procedure. Complications may
occur at any time during or after the procedure, and may include,
but are not limited to, the following:
• Paralysis resulting from untargeted embolization or ischemic
injury from adjacent tissue edema.
• Undesirable reflux or passage of EmboGold Microspheres into
normal arteriesadjacentto the targeted lesion or through the lesion
into other arteries or arterial beds, such as the internal carotid
artery, pulmonary, or coronary circulations.
• Pulmonary Embolism due to arterial venous shunting.
• Ischemia at an undesirable location, including ischemic stroke,
ischemic infarction (including myocardial infarction), and tissue
necrosis.
• Capillary bed occlusion and tissue damage.
• Vessel or lesion rupture and hemorrhage.
• Neurological deficits, including cranial nerve palsies.
• Vasospasm.
• Death.
• Recanalization.
• Foreign body reactions necessitating medical intervention.
• Infection necessitating medical intervention.
• Clot formation at the tip of the catheter and subsequent
dislodgment.
• Allergic response to gold.
• Complications related to catheterization (e.g., hematoma at the
site of entry, clot formation at the tip of the catheter and
subsequent dislodgment, and nerve and/or circulatory injuries,
which may result in leg injury).
• Allergic reaction to medications (e.g. analgesics).
• Allergic reaction to contrast media or embolic material.
• Pain and/or rash, possibly delayed fromthe time of embolization.
• Blindness,hearing loss, loss of smell.
• Additional information is found in the Warnings section.
STORAGE AND STERILITY :
Inspect packaging prior to use to ensure seal integrity for
maintenance of sterility.
• EmboGold Microspheres must be stored in a cool, dry and dark
place in their original syringe and packaging.
• Use by the date indicated on the syringe label.
• Do not freeze.
• Do not resterilize.
INSTRUCTIONS FOR USE :
• Carefully evaluate the vascular network associated with the
lesion using high resolution imaging prior to beginning the
embolization procedure.
• EmboGold Microspheres are available in a range of sizes.
Because of the potential for misembolization and the inherent
variability in sphere sizes,thephysician should be sure to carefully
select the size of EmboGold Microspheres according to the size of
the target vessels at the desired level of occlusion in the
vasculature.
• When embolizing arteriovenous malformations (AVM),choose an
EmboGold Microspheres size that will occlude the nidus without
passing through the AVM.
4
Page 5
• Choose a delivery catheter based on the size of the target vessel
S
TERILIZE
2
2
STERILE
and the microsphere size being used.EmboGold Microspheres can
tolerate temporary compression of up to 33% in order to facilitate
passage through the delivery catheter.
• EmboGold Microspheres are not radiopaque. It is recommended
that the embolization be monitored using fluoroscopic visualization
by adding the appropriate amount of contrast medium to the
physiologic saline suspension fluid.
• To deliver EmboGold Microspheres:
Match the total volume in the syringe with the same volume of
undiluted contrast, which will result in a 50% microsphere/saline
and 50% contrast solution. Remove all air from the syringe. To
evenly suspend the EmboGold Microsphere/contrast solution,
gently invert the 20mL syringe several times. Attach the 20mL
syringe to one port of the luer-lock 3-way stopcock; attach a 1mL
or 3mL injection syringe to another port on the stopcock, and, if
desired, a delivery catheter may be attached to the remaining port
on the stopcock. Wait several minutes to allow the EmboGold
Microspheres to suspend properly. Draw EmboGold
Microsphere/contrast solution into theinjection syringe slowly and
gently to minimize the potential of introducing air into the system.
Purge all air fromthe system prior to injection. Inject the EmboGold
Microsphere/ contrast solution under fluoroscopicvisualizationwith
the injection syringe, using a slow pulsatile injection, while
observing the contrast flow rate. If there is no effect on the flow
rate, repeat the delivery process with additional injections of
EmboGold Microsphere/contrast solution,or larger sized EmboGold
Microspheres may be considered. If the EmboGold
Microsphere/contrast solution requires resuspension, gently invert
the 20mL syringe several times. Exercise conservative judgment in
determining the embolization endpoint.
• Femoral puncture can result in arterial spasm. This may
predispose to femoral thrombosis (e.g. leg injury). Femoral patency
should be re-assessed prior to final catheter removal.
• Upon completion of the treatment, remove the catheter while
maintaining gentle suction so as not to dislodge EmboGold
Microspheres still within the catheter lumen.
• Apply pressure to the puncture siteuntil hemostatis is complete.
• Discard any open, unused EmboGold Microspheres.
REFERENCES :
1. Bendszus M, Klein R,Burger R, et al.: Efficacy of trisacryl gelatin
microspheresversuspolyvinylalcoholparticles in the preoperative
embolization of meningiomas. AJNR, 21(2):255-61, Feb 2000.
2. Deveikis JP: Endovascular therapy of intracranial arteriovenous
malformations: materials and techniques. Neuroimaging Clin of N
Am, 8(2):401-424, 1998.
3. Frizzel RT, Fisher WS: Cure, morbidity, and mortality associated
with embolization of brain arteriovenous malformations: A review
of 1246 patients in 32 series over a 35-year period. Neurosurg,
37(6):1031-1040, Dec 1995.
4. Gomes, A: Embolization therapy of congenital arteriovenous
malformations;Use of alternative approaches.Radiology,190:1918, Jan 1994.
5.Terada,T; Kinoshita,Y; Yokote, H; Tsuura, M; Itakura, T;Komai,N;
Nakamura,Y;Tanaka,S; Kuriyama, T:Preoperative embolization of
meningiomas fed by ophthalmic branch arteries. Surg Neurol,
45:161-6, 1996.
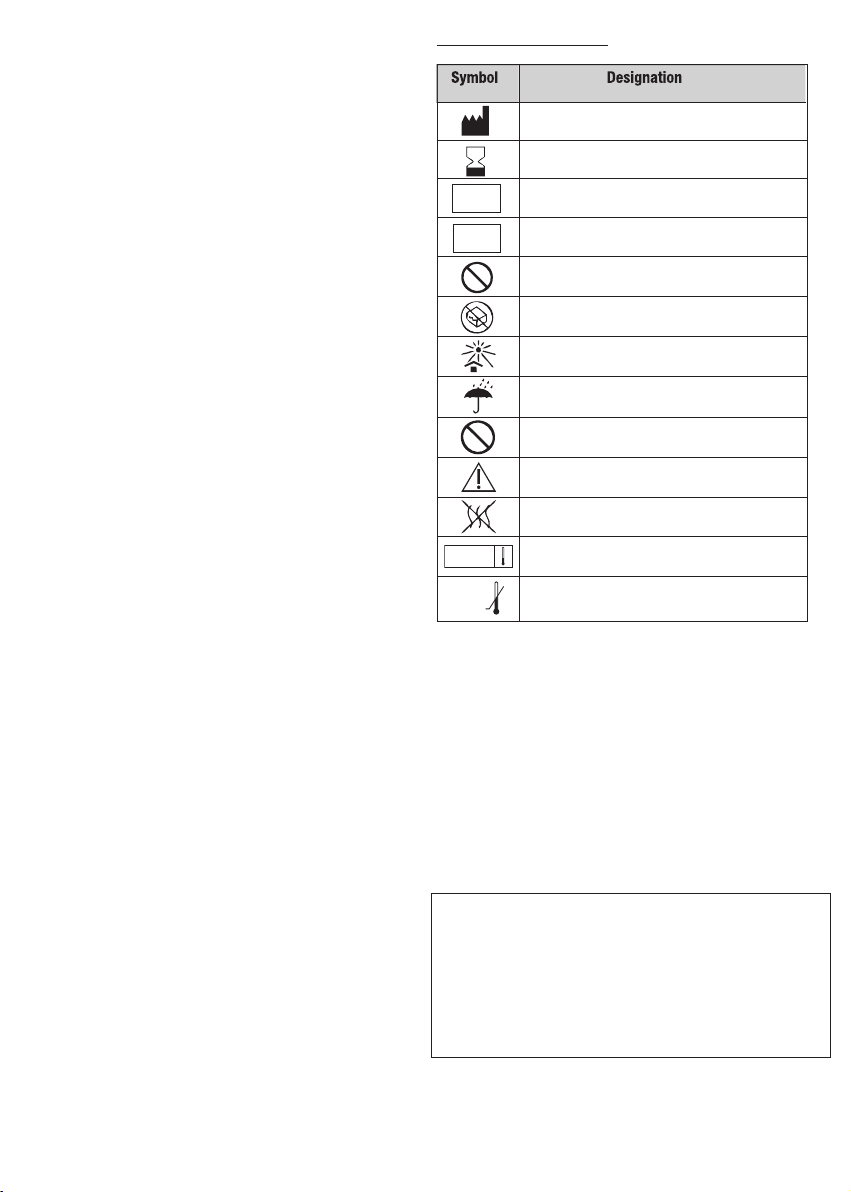
Information on packaging :
Symbol Designation
Manufacturer: Name & Address
Use by date: year-month
LOT
REF
Batch code
Catalogue number
Do not resterilize
Do not use if package is damaged
Keep away from sunlight
Keep dry
Do not re-use
Caution - Refer to Instructions For Use
Non-pyrogenic
Sterilized using steam
0°C
Lower limit of temperature
Distributed in USA by:
Merit Medical Systems, Inc.
Customer Service 1-800-356-3748
781-681-7900
Patent Information
US 5,635,215 and other patent(s) issued and pending.
EmboGold Microspheres is a registered trademark of BioSphere
Medical.
All serious or life-threatening adverse events or deaths associated
with use of EmboGold Microspheres should be reported to the U.S.
Food and Drug Administrationunderthe MedWatchprogramand to
the device manufacturer.Information about the MedWatch program
and forms for reporting adverse events can be obtained at
www.fda.gov/safety/medwatch/howtoreport/ucm053074.htm or
by calling toll free 888-463-6332. Reports to BioSphere Medical,
Inc. can be made by calling toll free 800-394-0295.
5
Page 6

FRANÇAIS
Microsphères EmboGold® en seringue pré-remplie - Pour embolisation
MISES EN GARDE : La loi fédérale américaine limite l’utilisation
de ce dispositif aux médecins autorisés ou sur prescription de
ces médecins.
USAGE RECOMMANDÉ :
• Embolisation des tumeurs hypervascularisées.
• Embolisation de malformations artério-veineuses (MAV).
APPLICATIONS CLINIQUES :
La littérature scientifique compte de nombreux articles sur les
procédures d’embolisation avec différents agents, tant dans le
système nerveux que dans le système circulatoire périphérique,
notamment au niveau de la tête, du cou, du rachis, du foie, de
l’appareil génito-urinaire,del’utérus,dusystème gastro-intestinal,
des membres et des poumons. Vous trouverez une bibliographie
représentative de cette littérature à la fin de ces instructions
d’utilisation.
DESCRIPTION :
Les Microsphères EmboGold font partiede la famille des nouveaux
matériaux emboliques, issus de la technologie brevetée de
microsphères de BioSphere Medical. Ces sphères colorées sont
conçues pour faciliter la visualisation lors de la manipulation, tout
en offrant les mêmes caractéristiques d’embolisation ciblée et
contrôlée que le premier produit,les Microsphères Embosphere®.
Les Microsphères EmboGold sont des microsphères
biocompatibles, hydrophiles, non résorbables, calibrées avec
précision, en polymère acrylique et imprégnées de gélatine
porcine. Les Microsphères EmboGold sont disponibles dans une
gamme de tailles.
CONDITIONNEMENT DU DISPOSITIF :
• Matériau embolique contenu dans une seringue stérile de 20 ml
(seringue pré-remplie), conditionnée dans un blister rigide pelable.
• Chaque seringue contient environ 1,0 ml ou 2,0 ml de
Microsphères EmboGold dans du sérum physiologique stérile
apyrogène.
CONTRE-INDICATIONS :
• Patients intolérants aux procédures d’occlusion.
• Anatomie vasculaire ou flux sanguinempêchant la mise en place
d’un cathéter ou l’injection d’un agent embolique.
• Présence ou risque de vasospasme.
• Présence ou risque d’hémorragie.
• Présence d’une athérosclérose grave.
• Présence d’artères d’irrigation plus petites que les branches
distales dont elles émergent.
• Présence manifeste d’anastomoses extra- ou intracrâniennesou
de shunts.
• Présence de voies vasculaires collatérales susceptibles de
compromettre des zones normales lors de l’embolisation.
• Présence d’artères terminales conduisant directement à des
nerfs crâniens.
• Présence d’artères alimentant la lésion trop étroites pour recevoir
les Microsphères EmboGold.
• Résistance vasculaire à la périphérie des artères d’irrigation
empêchant le passage des Microsphères EmboGold dans la lésion.
• Ne pas utiliser les Microsphères EmboGold dans les applications
suivantes :
a. Embolisation de shunts artério-veineux de grand diamètre (c’està-dire où le sang passe directement de l’artère à la veine sans
transiter par des artères/capillaires/veines).
b. Dans le système artériel pulmonaire.
c. Utilisation dans toute zone vasculaire dans laquel les
Microsphères EmboGold pourraient passer directement dans
l’artère carotide interne, une artère vertébrale, le système
vasculaire intracrânienne ou les vaisseaux indiqués ci-dessus.
• Patients allergiques à l’or.
AVERTISSEMENT :
• Les Microsphères EmboGold contiennent de la gélatine d’origine
porcine et peuvent, par conséquent, entraîner une réaction
immunitaire chez les patients hypersensibles au collagène ou à la
gélatine. Une mûre réflexion est nécessaire avant d’utiliser ce
produit chez les patients pour qui on suspecte une allergie aux
injections contenant des stabilisants en gélatine.
• Des études ont montré que les Microsphères EmboGold ne
forment pas d’agrégats et pénètrent donc plus profondément dans
le système vasculaireque des particules de PVA de taille similaire.
Veiller à choisir des Microsphères EmboGold de plus grande taille
pour l’embolisation de malformations artério-veineuses avec de
grands shunts, pour éviter le passage des microsphères dans la
circulation pulmonaire ou coronaire.
• Il est possible que certaines Microsphères EmboGold soit
légèrementhorsde la gamme de taille, par conséquent,le médecin
devra s'assurer de sélectionner avec soin la taille des microsphères
EmboGold, en fonction de la taille des vaisseaux au niveau désiré
d’occlusion dans le système vasculaire et après considération de
l'aspectangiographique artério-veineux.La taille des microsphères
EmboGold doit être choisie de façon à éviter leur passage de
l'artère à la veine.
• Du fait des complications importantes liées à une mauvaise
embolisation, une prudence extrême doit être appliquée pour de
quelconques interventions impliquant une circulation
extracrânienne englobant la tête et le cou et le médecin doit
sérieusement peser les bienfaits potentiels du recours à
l'embolisation par rapport aux risques et aux complications
potentiels de la procédure. Ces complications peuvent inclure
cécité, perte auditive, perte de l'odorat, paralysie et décès.
• La couleur des Microsphères EmboGoldpeutêtre visibleà travers
la peau si elles sont injectées dans des artères irriguant les tissus
superficiels.
• Le patient peut développer de graves lésions cutanéesinhérentes
à l’irradiation du fait de longues périodes d'exposition à
l'angiographie, de patients de forte corpulence, d’incidences
obliques, de séries répétées d'enregistrement d'images ou de
radiographies multiples. Se reporter au protocole clinique de votre
établissement pour vous assurer que la dosed'irradiation correcte
est utilisée pour chaque type de procédure réalisée. Les médecins
doivent surveiller les patients qui peuvent présenter un risque.
• L’apparition de lésions par irradiation chez le patient peut être
retardée. Les patients doivent être informés des effets potentiels
des rayons, de ce qu'il faut rechercher et de la personne à
contacter en cas d'apparition de symptômes.
• Les Microsphères EmboGold ne sont pas approuvées pour
6
Page 7

l’embolisation de fibrome utérin et leur utilisation dans cette
application est associée à une douleur et/ou une éruption cutanée
différées.
• Apporter une attention particulièreaux signes d'embolisationmal
ciblée. Durant l'injection, suivre attentivement les signes vitaux du
patient, tels que le SaO2 (par ex. l'hypoxie, les changements du
SNC). Envisager d'arrêter la procédure, en cherchant un shunt
éventuel, ou s'orienter vers une taille de microsphères supérieure
si de quelconques signes de mauvais ciblage se produisent ou que
les symptômes du patient s'aggravent.
• Envisager d'utiliser une taille des microsphères supérieure si
l'angiographie ne démontre pas rapidement une embolisation
évidente pendant l'injection des microsphères.
Avertissements relatifsà l’utilisation des petites microsphères
• Une attention toute particulière doit être apportée lorsque les
emboles ont un diamètre inférieur à la capacité de résolution de
votre équipement d'imagerie. La présence d'anastomoses artérioveineuses, de vaisseaux ramifiés conduisant hors de la zone cible
ou de vaisseaux émergents non évidents,peuvent conduire à une
embolisation mal ciblée et à des complications graves.
• Des microsphères de moins de 100 microns effectueront
généralement une migration distale vers les sources
anastomotiques et sont ainsi susceptibles d'emboliser un tissu
distal. L'utilisation de microsphères de taille plus petite peut
conduire à un risque plus élevé de lésion ischémique et les
conséquences de cette lésion doivent être prises en compte avant
l'embolisation. Les conséquences potentielles comprennent le
gonflement, la nécrose, la paralysie, un abcès et/ou un syndrome
post-embolisation plus fort.
• Un gonflement post embolisation peut conduire à une ischémie
du tissu adjacent à la zone cible. Il faut prendre soin d'éviter
l'ischémie d'un tissu intolérant, non ciblé tel que le tissu nerveux.
PRÉCAUTIONS :
• Ne pas utiliser si la seringue, le joint du piston ou le blister est
endommagé. Pour un usage unique. Contenu fourni stérile. Ne
jamais réutiliser, retraiter ou restériliser le contenu d'une seringue
qui a été ouverte. La réutilisation, le retraitement ou la
restérilisation peuvent compromettre l'intégrité structurelle du
dispositif et/ou conduire à une défaillance du dispositif, pouvant
entraîner une lésion, une maladie ou le décès du patient. La
réutilisation, le retraitement ou la restérilisation risquent également
de générer une contamination du dispositif et/ou de causer une
infection ou une infection croisée chez le patient, y compris
notamment, la transmission de maladies infectieuses d'un patient
à l'autre. La contamination du dispositif peut entraîner une lésion,
une maladie ou le décès du patient.Toutes les interventions doivent
être effectuées en respectantune technique aseptique approuvée.
• Ne pas raccorder la seringue de 20 ml contenant les
Microsphères EmboGold directement à un microcathéter pour
l’embolisation car il pourrait en résulterune occlusion du cathéter.
• La seringue est destinée à un usage pour embolisation
uniquement. Ne pas utiliser pour une autre application.
• Choisir la taille et la quantité appropriées deMicrosphères
EmboGold pour la pathologie à traiter.
• Seul un médecin formé aux procédures d’embolisation
interventionnelle dans la région à emboliser peut procéder à une
embolisation avec les Microsphères EmboGold.
• Les patients présentant des allergies connues aux produits de
contraste non ioniques peuvent nécessiter une administration de
corticostéroïdes avant l'embolisation.
• Des examens ou précautions supplémentaires peuvent s'avérer
nécessaires pour les soins péri opératoires pour les patients
atteints des affections suivantes :
– Diathèse hémorragique ou état d’hypercoagulabilité
– Immunodépression.
COMPLICATIONS POTENTIELLES :
L’embolisation vasculaire est une intervention à haut risque. Des
complications peuvent survenir à tout moment, pendant ou après
l’intervention, et peuvent inclure, sans y être limitées, les
complications suivantes :
• Paralysie résultant d’une embolisation non cibléeou d’une lésion
ischémique due à un œdème des tissus adjacents.
• Reflux indésirable ou passage des Microsphères EmboGold dans
des artères saines voisines de la lésionvisée ou après traversée de
la lésion, dans d’autres artères ou lits artériels, tels que la carotide
interne, la circulation pulmonaire, ou coronaire.
• Embolie pulmonaire par shunt artério-veineux.
• Ischémie dans un site non souhaité, y compris accident
ischémique cérébral, infarctus ischémique (dont l’infarctus du
myocarde), et nécrose tissulaire
• Occlusion du lit capillaire et lésions tissulaires.
• Rupture d’un vaisseau ou de la lésion et hémorragie.
• Déficiences neurologiques, notamment paralysie de nerfs
crâniens.
• Vasospasme.
• Décès.
• Reperméabilisation.
• Réactions à un corps étranger nécessitant des soins médicaux.
• Infection nécessitant des soins médicaux.
• Formation de caillots à l’extrémité du cathéter et délogement
consécutif.
• Réaction allergique à l’or.
• Complications associées au cathétérisme (par ex. hématome au
point de ponction, formation d'un caillot à l’extrémité du cathéter
et déplacement secondaire ou lésions nerveuses et/ou vasculaires
pouvant entrainer des troubles au niveau des membres inférieurs)
• Réaction allergique aux médicaments (par ex. analgésiques).
• Réaction allergique au produit de contraste ou aux emboles.
• Douleur et/ou éruption cutanée, pouvant être différée par rapport
au moment de l’embolisation.
• Cécité,perte auditive, perte de l’odorat.
• Informations supplémentaires disponibles dans la section
Avertissements
STOCKAGE ET STÉRILITÉ :
Vérifier l’emballage avant l’utilisation pour vérifier l’intégrité du
scellage afin de s’assurer de la stérilité.
• Les Microsphères EmboGold doivent être conservées dans un
endroit sec, à l’abri de la lumière et de la chaleur, dans leur
seringue et leur emballage d’origine.
• Utiliser avant la date indiquée sur l’étiquette de la seringue.
• Ne pas congeler.
• Ne pas restériliser.
INSTRUCTIONS D’UTILISATION :
• Évaluer soigneusement le réseau vasculaire associé à la lésion
avec une imagerie à haute résolution avant de commencer la
7
Page 8

procédure d’embolisation.
STERILIZE
2
2
STERILE
• Les Microsphères EmboGold sont disponibles en différentes
tailles. Du fait du risque de mauvaise embolisation et de la
variabilité inhérente aux tailles des sphères, le médecin doit
s’assurer qu’il sélectionne avec soin la taille des Microsphères
EmboGold en fonction de la taille des vaisseaux ciblés au niveau
souhaité de l’occlusion du système vasculaire.
• Pour l’embolisation de malformations artério-veineuses (MAV),
choisir une taille de Microsphères EmboGold qui permettra
l’occlusion du nidus sans franchir la MAV.
• Choisir un cathéter d’administration en fonction de la taille du
vaisseau cible et de la taille des microsphères utilisées. Les
Microsphères EmboGold peuvent tolérer une compression
temporaire allant jusqu’à 33 % pour faciliter le passage dans le
cathéter d’administration.
• Les Microsphères EmboGold ne sont pas radio-opaques. Il est
recommandé de surveiller l’embolisation sous visualisation
angiographique en ajoutant la quantité souhaitée de produit de
contraste au sérum physiologique de suspension.
• Injection des Microsphères EmboGold :
Prélever un volume de produit de contraste non dilué égal au
volume total de la seringue, pour obtenir une solution de 50 % de
microsphères/sérum physiologique et 50 % de produit de
contraste. Éliminer tout l’air de la seringue. Pour une suspension
uniforme de solution Microsphère EmboGold/produit de contraste,
basculer délicatement la seringue de 20 ml plusieurs fois. Monter
la seringue de 20 ml sur un port du robinet à 3 voies Luer-lock ;
monter une seringued’injection de 1 ml ou 3 ml à un autreport du
robinet et, le cas échéant, monter un cathéter d’administration sur
le port restant du robinet. Attendre quelques minutes que les
Microsphères EmboGold soient correctement en suspension.
Prélever la solution Microsphère EmboGold/produit de contraste
dans la seringue d’injection lentement et délicatement afin de
minimiser le risque d’introduction d’air dans le système. Purger
tout l’air présent dans le système avant l’injection. Injecter la
solution Microsphère EmboGold/produit de contraste sous
visualisation angiographique avec la seringue d’injection par une
injection lente et intermittente,tout en surveillant le débit du produit
de contraste. S’il n’y a pas d’effet sur ledébit,répéter le processus
d’administration avec des injections supplémentaires de solution
Microsphère EmboGold/produit de contraste, ou envisager
l’utilisation de Microsphères EmboGold de plus grande taille. Si la
solution Microsphère EmboGold/produit de contraste doit être de
nouveau mise en suspension, renverser délicatement la seringue
de 20 ml plusieurs fois. Il convient d’être prudent pour la
détermination du résultat de l’embolisation.
• Une ponction fémorale peut entraîner un spasme artériel. Cela
peut prédisposer à une thrombose fémorale (c.-à-d. une lésion de
la jambe). La perméabilité fémorale doit être réévaluée avant le
retrait final du cathéter.
• Une fois le traitement terminé, retirer le cathéter tout en
maintenant une légère aspiration afin de ne pas déloger les
Microsphères EmboGold qui se trouvent encore dans la lumière du
cathéter.
• Appliquer une pression sur le site de ponction jusqu’à une
hémostase complète.
• Éliminer les Microsphères EmboGold ouvertes non utilisées.
RÉFÉRENCES :
1. Bendszus M, Klein R,Burger R, et al.: Efficacy of trisacryl gelatin
microspheresversuspolyvinylalcoholparticles in the preoperative
embolization of meningiomas. AJNR, 21(2):255-61, Feb 2000.
2. Deveikis JP: Endovascular therapy of intracranial arteriovenous
malformations: materials and techniques. Neuroimaging Clin of N
Am, 8(2):401-424, 1998.
3. Frizzel RT, Fisher WS: Cure, morbidity, and mortality associated
with embolization of brain arteriovenous malformations: A review
of 1246 patients in 32 series over a 35-year period. Neurosurg,
37(6):1031-1040, Dec 1995.
4. Gomes, A: Embolization therapy of congenital arteriovenous
malformations; Use of alternative approaches. Radiology,
190:191-8, Jan 1994.
5.Terada,T; Kinoshita,Y; Yokote, H; Tsuura, M; Itakura, T;Komai,N;
Nakamura,Y;Tanaka,S; Kuriyama, T:Preoperative embolization of
meningiomas fed by ophthalmic branch arteries. Surg Neurol,
45:161-6, 1996.
Informations sur l’emballage :
Symbol Designation
Fabricant : Nom et adresse
Date limite d'utilisation : année-mois
LOT
REF
0°C
Distribué aux États-Unis par :
Merit Medical Systems, Inc.
Service clientèle 1-800-356-3748
Informations sur les brevets
Brevet US 5,635,215 et autre(s) brevet(s) accordé(s) et en cours.
Microsphères EmboGold est une marque déposée de BioSphere
Medical.
Numéro de lot
Référence catalogue
Ne pas restériliser
Ne pas utiliser si l’emballage est endommagé
Conserver à l’abri de la lumière du soleil
Tenir au sec
Ne pas réutiliser
Attention - Consulter la notice d’instructions
Apyrogène
Stérilisé à la vapeur
Limite inférieure de température
781-681-7900
8
Page 9

PORTUGUÊS
Microesferas EmboGold® numa seringa pré-cheia - Para embolização
•
ATENÇÃO : A lei federal dos EUA restringe a venda deste
dispositivo a um médico ou mediante prescrição médica.
UTILIZAÇÃO PREVISTA :
• Embolização de tumores hipervascularizados.
• Embolização de malformações arteriovenosas (MAV).
APLICAÇÕES CLÍNICAS :
A literatura científica fornece documentação exaustiva sobre
procedimentos de embolização queutilizam uma ampla variedade
de agentes nos sistemas circulatórios tanto neurológico como
periférico, incluindo cabeça, pescoço, coluna vertebral, fígado,
aparelho geniturinário, útero, sistema gastrointestinal, membros e
pulmões. Uma bibliografia representativa desta literatura é
fornecida no fim destas instruções de utilização.
DESCRIÇÃO :
As Microesferas EmboGold fazem parte de uma família de novos
materiais embólicos baseados na tecnologia de microesferas
patenteada de BioSphere Medical. Estas esferas coloridas foram
concebidas para facilitara visualização durante o manuseamento,
não deixando de oferecer as características de embolização
controlada e direccionada do primeiro produto de microesferas, as
Microesferas Embosphere®.
As Microesferas EmboGold são microesferas biocompatíveis,
hidrófilas,não-reabsorvíveis e calibradas com precisão, fabricadas
a partir de um polímero acrílico e impregnadas com gelatina de
origem suína.As Microesferas EmboGold estão disponíveis numa
diversidade de tamanhos.
ACONDICIONAMENTO DO DISPOSITIVO :
• Material embólico contido numa seringa estéril de 20 ml (seringa
pré-cheia), acondicionada num tabuleiro de película amovível.
• Cada seringa contém cerca de 1,0 ml ou 2,0 ml de Microesferas
EmboGold em soro fisiológico apirogénico e estéril.
CONTRA-INDICAÇÕES :
• Doentes intolerantes a procedimentos de oclusão.
• Anatomia vascular ou fluxo sanguíneo que impede a colocação
do cateter ou a injecção de um agente embólico.
• Presença ou início provável de vasoespasmo.
• Presença ou início provável de hemorragia.
• Presença de doença ateromatosa grave.
• Presença de artérias aferentes mais pequenas do que as
ramificações distais das quais emergem.
• Presença de anastomoses ou shunts extra a intracranianos
patentes.
• Presença de vias dos vasos colaterais que coloquem
potencialmente em perigo os territórios normais durante a
embolização.
• Presença de artérias terminais que conduzem directamente aos
nervos cranianos.
Presença de artérias que irrigam a lesão, mas que não têm
tamanho suficiente para aceitarem Microesferas EmboGold.
• Resistência vascularperiférica às artérias aferentes que impede
a passagem das Microesferas EmboGold para a lesão.
• Não utilize as Microesferas EmboGold nas seguintes aplicações:
a. Embolização de shunts arteriovenosos de grande diâmetro (ou
seja, quando o sangue não passa através de uma transição
arterial/capilar/venosa mas directamente de uma artériapara uma
veia).
b. Na vasculatura pulmonar arterial.
c. Utilização em qualquer vasculatura na qual as Microesferas
EmboGold podem passar directamente para a artéria carótida
interna, artéria vertebral, vasculatura intracraniana ou os vasos
supra-indicados.
•Doentes com uma resposta alérgica ao ouro.
ADVERTÊNCIAS :
• As Microesferas EmboGold contêm gelatina de origem suína e,
portanto, podem provocar uma reacção imune em doentes que
sejam hipersensíveis ao colagénio ou à gelatina. Deve prestar-se
uma especial atenção antes dese utilizar este produto em doentes
com suspeita de serem alérgicos a injecções que contenham
estabilizadores de gelatina.
• Estudos demonstraram que as Microesferas EmboGold não
formam agregados e, por consequência, penetram mais
profundamente na vasculaturacomparativamente a partículas em
PVA de tamanho semelhante. Deve ter-se cuidado na escolha de
Microesferas EmboGold comtamanho superior quando se efectua
a embolização de malformações arteriovenosas com shunts
grandes,demodoa evitar a passagem de esferas para a circulação
pulmonar ou coronária.
• Algumas das Microesferas EmboGold podem estar ligeiramente
fora da gama, portanto, o médico deve escolher cuidadosamente
o tamanho das Microesferas EmboGold de acordo com o tamanho
dos vasos alvo no nível de oclusão pretendido na vasculatura e
após consideração do aspecto angiográfico arteriovenoso. O
tamanho das Microesferas EmboGold deve ser seleccionado de
modo a impedir a passagem da artéria para a veia.
• Devido às complicações significativas de falha na embolização,
deve ter-se extrema cautela em qualquer procedimento que
envolva a circulação extracraniana incluindoa cabeça e o pescoço,
e o médico deve ponderar cuidadosamente as potenciais
vantagens de utilizar a embolização comparativamente aos riscos
e potenciais complicações do procedimento. Estas complicações
podem incluir cegueira, perda de audição, perda de olfacto,
paralisia e morte.
• A cor das Microesferas EmboGold poderá ser visível através da
pele se forem injectadas em artérias que alimentem os tecidos
superficiais.
• Pode ocorrer lesão grave na pele dodoenteinduzida por radiação
devido a longos períodos de exposição fluoroscópica, diâmetro
grande do doente, projecções angulares de raio-x e diversas séries
de registo de imagens ou radiografias. Consulte o protocolo clínico
da sua instituição para garantir que é aplicada a dose correcta de
radiação para cada tipo específico de procedimento executado. Os
médicos devem monitorizar os doentes que possam estar em
risco.
9
Page 10

• O início da lesão induzida por radiação no doente pode ser
atrasado. Os doentes devem seraconselhados sobre os potenciais
efeitos secundários da radiação e sobre quem devem contactar
caso apresentem sintomas.
• As Microesferas EmboGold não podem ser utilizadas para a
embolização de fibróides uterinos e estão associadas a dor e/ou
exantema cutâneo retardados quando utilizadas nestes
procedimentos.
• Preste especial atenção a sinais de embolização mal
direccionada. Durante a injecção monitorize cuidadosamente os
sinais vitais do doente de modo a incluir SAO2 (por ex., hipoxia,
alterações no SNC). Considere terminar o procedimento,investigar
a possibilidade de existir um shunt ou aumentar o tamanho de
microesferas caso ocorram sinais de mau direccionamento ou se
desenvolvam sintomas no doente.
• Considere aumentar o tamanho das microesferas caso os sinais
angiográficos de embolização não apareçam rapidamente
evidentes durante a injecção das microesferas.
Advertências quanto à utilização de microesferas pequenas
• Deve prestar-se particular atenção sempre que for considerada
a utilização de agentes embólicos cujo diâmetro seja inferior ao
da capacidade de resolução do equipamento de imagiologia. A
presença de anastomoses arteriovenosas, ramificações de vasos
sanguíneos que se afastemda área alvo ou vasos emergentes não
evidentes antes da embolização, podem originar uma embolização
mal direccionada e complicações graves.
• Microesferas com tamanho inferior a 100 mícrones irão
geralmente migrar de forma distal para vasos responsáveis pela
alimentação a anastomóticos e,portanto,têm maior probabilidade
de terminar a circulação ao tecido distal. Um maior potencial para
lesões isquémicas resulta da utilização de microesferas de
tamanho mais pequeno e deve prestar-se atenção à consequência
desta lesão antes da embolização. As possíveis consequências
incluem tumefacção, necrose, paralisia, abcesso e/ou síndrome
pós-embolização mais acentuado.
• A tumefacção pós-embolização pode resultar em isquemia no
tecido adjacente à área alvo.Deve ter-se cuidado de modo a evitar
tecido não tolerante a isquemia e não definido como alvo,tal como
o tecido nervoso.
PRECAUÇÃO :
• Não utilize se a seringa, o vedante do êmbolo ou o blister se
encontrarem danificados. Para utilização numa única doente –
Conteúdo fornecido estéril – Nunca reutilize, reprocesse nem
reesterilize o conteúdo de uma seringa que tenha sido aberta. A
reutilização,reprocessamentooureesterilizaçãopodemcomprometer
a integridade estrutural do dispositivo e/ou resultar na falha do
mesmo,o que, por sua vez,pode resultar em lesão,doença ou morte
do doente.Areutilização,reprocessamentooureesterilização podem
tambémoriginarrisco de contaminaçãododispositivo e/ou provocar
infecção do doente ou infecção cruzada, incluindo, entre outras, a
transmissão de doença(s) infecciosa(s) de um doente para outro. A
contaminação do dispositivo pode provocar lesão,doença ou morte
do doente.Todos os procedimentosdevem ser efectuadosdeacordo
com uma técnica asséptica aceite.
• Não ligue a seringa de 20 ml com Microesferas EmboGold
directamente a um microcateter para a aplicação embólica, dada a
possível ocorrência de oclusão do cateter.
•A seringadestina-seexclusivamenteaumuso embólico.Nãoutilize
para uma outra aplicação.
• Seleccione o tamanho e a quantidade de Microesferas EmboGold
adequados para a patologia a ser tratada.
• A embolização com Microesferas EmboGold só deve ser realizada
por médicos que receberam formação adequada em termos de
oclusão de intervenção na região anatómica que se pretende
embolizar.
• Doentes com alergia conhecida ao meio de contraste podem
necessitar de corticosteróides antes da embolização.
• Podem ser necessárias avaliações ou precauções adicionais no
controlo dos cuidados peri-procedimentos nos doentes com os
seguintes estados:
– Diátese hemorrágica ou estado hipercoagulativo
– Imunocomprometidos.
POSSÍVEIS COMPLICAÇÕES :
A embolização vascular é um procedimento de alto risco. Podem
ocorrer complicações em qualquer altura durante ou após o
procedimento e podem incluiras seguintes,sem limitação:
• Paralisia resultante de embolização não-direccionada ou lesão
isquémica por edema do tecido adjacente.
• Refluxooupassagemindesejados de Microesferas EmboGold para
artérias normais adjacentes à lesão-alvo ou através da lesão para
dentro de outras artérias ou leitos arteriais, como a artéria carótida
interna ou ascirculações pulmonar e coronária.
• Embolia pulmonar causada por shunting venoso arterial.
• Isquemia numalocalização indesejada,incluindo acidente vascular
isquémico, enfarte isquémico (incluindo enfarte do miocárdio) e
necrose tecidular.
• Oclusão do leito capilar e lesão tecidular.
• Ruptura e hemorragia de vaso ou lesão.
• Défices neurológicos, incluindo paralisias do nervo craniano.
• Vasoespasmo.
• Morte.
• Recanalização.
• Reacções a corpo estranho que implicam intervenção médica.
• Infecção que implicaintervenção médica.
• Formação de coágulo na ponta do cateter e subsequente
deslocação.
• Resposta alérgica ao ouro.
• Complicações relacionadas comacateterização(porex.,hematoma
no local de entrada, formação de coágulo na ponta do cateter e
subsequente deslocação e lesões nervosas e/ou circulatórias, que
podem resultar em lesões na perna).
• Reacção alérgica a medicações (por ex., analgésicos).
• Reacção alérgica a meios de contraste ou material embólico.
• Dor e/ou exantema cutâneo, possivelmente retardados desde o
momento da embolização.
• Cegueira,perda de audição e perda de olfacto.
• Pode consultarinformações adicionais na secção Advertências.
CONSERVAÇÃO E ESTERILIDADE :
Inspeccione a embalagem antes da utilização para garantir a
integridade da selagem e, deste modo,garantira
manutenção da esterilidade.
• As Microesferas EmboGold devem ser conservadas num local
fresco, seco e escuro na respectiva seringae embalagemoriginais.
• A utilizar até à dataindicada no rótulo da seringa.
• Não congele.
• Não reesterilizar.
10
Page 11

INSTRUÇÕES DE UTILIZAÇÃO :
STERILIZE
2
2
STERILE
•Avaliecuidadosamentea redevascular associada à lesão,utilizando
exames imagiológicos de alta resolução antes do início do
procedimento de embolização.
• As Microesferas EmboGold estão disponíveisnumadiversidade de
tamanhos.Devido ao potencialde falha da embolização e à inerente
variabilidade dos tamanhos das esferas,o médico deve ter a certeza
de que selecciona cuidadosamente o tamanho das Microesferas
EmboGold de acordo com o tamanho dos vasos visados, no nível
desejado de oclusão na vasculatura.
• Ao efectuar a embolizaçãodemalformações arteriovenosas (MAV),
escolha um tamanho de MicroesferasEmboGoldque ocluda o núcleo
sem passar pelas MAV.
• Escolhaumcateter de administraçãobaseadono tamanhodovasoalvo e no tamanho da microesfera a ser utilizada. As Microesferas
EmboGold podem tolerar uma compressão temporária de até 33%
para facilitar a passagem através do cateter de aplicação.
•AsMicroesferas EmboGoldnãosão radiopacas.Recomenda-seque
a embolização sejamonitorizadautilizandovisualização fluoroscópica
por adição da quantidade adequada do meio de contraste ao soro
fisiológico em suspensão.
• Para aplicar Microesferas EmboGold:
Faça corresponder o volume total na seringa com o mesmo volume
do contraste não-diluído, o que resultará numa solução de
microesfera/soro fisiológico a 50% e numa solução de contraste a
50%. Remova todo o ar da seringa. Para suspender uniformemente
a soluçãodeMicroesfera EmboGold/contraste,invertavárias vezese
com cuidado a seringa de 20 ml.Fixe a seringade20mlaumaporta
da torneira de passagem de 3 vias luer-lock; fixe uma seringa de
injecção de 1 ml ou 3ml a uma outra portanatorneira de passagem
e,seassimodesejar,podeainda fixar umcateterdeaplicaçãoàporta
restante na torneira de passagem. Aguarde vários minutos para
permitir a suspensão adequada dasMicroesferas EmboGold.Lentae
cuidadosamente, extraia a Microesfera EmboGold/solução de
contrastepara a seringa de injecçãodemodo a minimizaropotencial
de introdução de ar no sistema.Elimine todooardosistemaantesda
injecção. Injecte a solução de Microesfera EmboGold/contraste sob
visualização fluoroscópicacom a seringadeinjecção,utilizando uma
injecção pulsátil lenta,enquanto observa o débitodo contraste.Caso
nãoobserve qualquerefeitonodébito,repita o processo de aplicação
com injecções adicionais de solução de Microesfera
EmboGold/contraste ou considere a administração de Microesferas
EmboGold de tamanho superior. No caso de ser necessária uma
ressuspensãoda soluçãodeMicroesfera EmboGold/contraste,inverta
várias vezes a seringa de 20 ml com cuidado. Seja ponderado e
cauteloso ao determinar o objectivoda embolização.
• Um punção femoral poderesultar num espasmoarterial.Isto pode
predispor para a ocorrência de trombose femoral (por ex., lesão na
perna). A permeabilidade femoral deve ser reavaliada antes da
remoção final do cateter.
• No final do tratamento, remova o cateter enquanto mantém uma
aspiração ligeira de modo a não deslocar as MicroesferasEmboGold
ainda presentes no lúmen do cateter.
• Exerça pressão no local da punção até a hemóstase estar
terminada.
• Descarte quaisquer Microesferas EmboGold abertas e não
utilizadas.
BIBLIOGRAFIA:
1. Bendszus M, Klein R, Burger R, et al.: Efficacy of trisacryl gelatin
microspheres versus polyvinyl alcohol particles in the preoperative
embolizationof meningiomas.AJNR, 21(2):255-61,Feb 2000.
2. Deveikis JP: Endovascular therapy of intracranial arteriovenous
malformations:materials andtechniques.NeuroimagingClinofNAm,
8(2):401-424, 1998.
3.Frizzel RT,FisherWS:Cure,morbidity,and mortalityassociatedwith
embolizationofbrainarteriovenousmalformations:A review of 1246
patients in 32 series over a 35-year period. Neurosurg, 37(6):10311040,Dec1995.
4. Gomes, A: Embolization therapy of congenital arteriovenous
malformations; Use of alternative approaches. Radiology,
190:191-8,Jan 1994.
5. Terada, T; Kinoshita, Y; Yokote, H; Tsuura, M; Itakura, T; Komai,
N; Nakamura,Y; Tanaka,S; Kuriyama,T:Preoperative embolization of
meningiomasfed byophthalmicbranch arteries.SurgNeurol,45:1616, 1996.
Informação na embalagem :
Símbolo Designação
Fabricante: nome e morada
Prazo de validade: ano-mês
LOT
REF
0°C
Distribuído nos EUA por:
Merit Medical Systems, Inc.
Serviço de apoio ao cliente 1-800-356-3748
Informações sobre a patente
US 5,635,215 e outra(s) patente(s) emitida(s) e pendente(s).
Microesferas EmboGold é uma marca comercial registada
da BioSphere Medical.
11
Código do lote
Número de catálogo
Não reesterilizar
Não utilizar se a embalagem estiver danificada
Manter afastado da luz solar
Manter seco
Não reutilizar
Atenção – Consultar as Instruções de utilização
Apirogénico
Esterilizado utilizando vapor
Limite de temperatura inferior
781-681-7900
Page 12

ESPAÑOL
Microesferas EmboGold® en una jeringa precargada - Para embolización
AVISO : Las leyes federales estadounidenses restringen el uso
de este dispositivo a médicos o por prescripción facultativa.
USO PREVISTO :
• Embolización de tumores hipervascularizados.
• Embolización de malformaciones arteriovenosas (MAV).
APLICACIONES CLÍNICAS :
La literatura científica proporciona una extensa documentación
sobre los procedimientosde embolizaciónconuna amplia variedad
de agentes, tanto en los sistemas neurológicos como circulatorios
periféricos, incluida la cabeza, cuello, columna, hígado, aparato
genitourinario, útero, aparato digestivo, extremidades y pulmones.
Al final de estas instrucciones de uso se proporciona una
bibliografía representativa de esta literatura.
DESCRIPCIÓN :
Las Microesferas EmboGold forman parte de una gama de nuevos
materiales embólicos que se basan en la tecnología de
microesferas patentada de BioSphere Medical. Estas esferas,
codificadas por color, se han diseñado para visualizarse con más
facilidad durante la manipulación, a la vez que siguen ofreciendo
las características de embolización controladas, selectivas, del
primer producto de microesfera, las Microesferas Embosphere®.
Las Microesferas EmboGold son biocompatibles, hidrofílicas, no
reabsorbibles, calibradas con precisión, producidas a partir de un
polímero acrílico e impregnadas con gelatina porcina. Las
Microesferas EmboGold están disponibles en una amplia gama de
tamaños.
ENVASE DEL DISPOSITIVO :
• Agente embólico incluido en una jeringa estéril de 20 ml (jeringa
precargada), envasada en una bandeja despegable.
• Cada jeringa contiene aproximadamente 1,0 ml o 2,0 ml de
Microesferas EmboGold en una solución salina fisiológica, estéril,
libre de pirógenos.
CONTRAINDICACIONES :
• Pacientes con intolerancia a los procedimientos de oclusión.
• Anatomía vascular o flujo sanguíneo que descarta la colocación
del catéter o la inyección de agente embólico.
• Presencia o probabilidad de aparición de vasoespasmo.
• Presencia o probabilidad de aparición de hemorragia.
• Presencia de enfermedad ateromatosa grave.
• Presencia de arterias nutricias menores que las ramas distales
de las que salen.
• Presencia de anastomosis o fístulas extra a intracraneales
evidentes.
• Presencia de vías de vasos colaterales que potencialmente
ponen en peligro áreas normales durante la embolización.
• Presencia de arterias terminales que se dirigen directamente a
los nervios craneales.
• Presencia de arterias que irrigan la lesión que no son
suficientemente grandes para alojar las Microesferas EmboGold.
• Resistencia vascular periférica a las arterias nutricias que impide
el paso de las Microesferas EmboGold a la lesión.
• No utilizar las Microesferas EmboGold en las siguientes
aplicaciones:
a. Embolización de fístulas arteriovenosas de gran diámetro (esto
es, donde la sangre no pasa por una unión arterio/capilar/venosa
sino directamente desde una arteria a una vena).
b. En la vasculatura arterial pulmonar.
c. Uso en cualquier vasculatura donde las Microesferas EmboGold
podrían pasar directamente a la arteria carótida interna, arteria
vertebral, vasculatura intracraneal o a los vasos enumerados
arriba.
• Pacientes que tengan una reacción alérgica al oro.
ADVERTENCIAS :
• Las Microesferas EmboGold contienen gelatina de origen porcino
y, por lo tanto, podrían provocar una reacción inmunitaria en
pacientes con hipersensibilidad al colágeno o la gelatina. Debe
considerarse previa y detenidamente el uso de este producto en
pacientes que se sospeche que son alérgicos a inyecciones que
contienen estabilizantes de gelatina.
• Los estudios realizados han mostrado que las Microesferas
EmboGold no forman agregadosy, por consiguiente, penetran más
profundamente en la vasculatura en comparación con partículasde
alcoholpolivinílico (PVA)de tamañoparecido. Debe tenersecuidado
al elegir Microesferas EmboGold de mayor tamaño cuando se
embolizan malformaciones arteriovenosas con fístulas grandes
para evitar el paso de las esferas a la circulación pulmonar o
coronaria.
• Algunas de las Microesferas EmboGold podríanestar ligeramente
fuera del rango. Por consiguiente, tras considerar la apariencia
angiográfica arteriovenosa, el médico debe asegurarse de
seleccionar cuidadosamente el tamaño de las Microesferas
EmboGold de acuerdo con el tamaño de los vasos diana y el nivel
de oclusión deseado en la vasculatura. El tamaño de las
Microesferas EmboGold debe seleccionarse de modo que se
impida el paso de la arteria a la vena.
• Debido a las importantes complicaciones de una embolización,
se debe proceder con sumo cuidado en los procedimientos que
implican la circulación extracraneal que abarca la cabeza y el
cuello, y el médico debe sopesar detenidamente los beneficios
potenciales de aplicar embolización frente a los riesgos y las
posibles complicaciones del procedimiento. Estas complicaciones
pueden incluir ceguera, pérdida auditiva, pérdida del olfato,
parálisis y muerte.
• El colorde las Microesferas EmboGoldpodría ser visible a través
de la piel si se inyectan en arterias que llevan sangre a los tejidos
superficiales.
• El paciente puede sufrir una lesión cutánea grave inducida por
la radiación como consecuencia de largos períodos de exposición
radioscópica, por tratarse de un paciente obeso, por proyecciones
radiográficasen ángulo y por varias series de registro de imágenes
o radiografías. Consulte el protocolo clínico de su centro para
asegurar que se aplique la dosis de radiación correcta a cada tipo
específico de procedimiento realizado. Los médicos deben
monitorizar a los pacientes que puedan correr riesgo.
• La lesión inducida por radiación en el paciente podría ser de
aparición diferida.Debe advertirse a los pacientes de los posibles
efectos secundarios de la radiación e indicarles a quién deben
contactar si presentan síntomas.
• Las Microesferas EmboGold no están autorizadas para el uso en
embolización de mioma uterino y están asociadas con un dolor
diferido y/o erupción cutánea cuando se usa en estos
procedimientos.
12
Page 13

• Preste mucha atención a los signos de una embolización de
regiones no diana. Durante la inyección, monitorice
cuidadosamente las constantes vitales del paciente, incluido la
SAO2 (por ejemplo, hipoxia, cambios en el sistema nervioso
central). Si hay signos de embolización de regiones no diana o si
el paciente presenta síntomas, considere terminar el
procedimiento, investigar la presencia de posibles fístulas o
aumentar el tamaño de las microesferas.
• Si durante la inyección de las microesferas no aparecen clara y
rápidamente señales angiográficas de embolización, considere
aumentar el tamaño de las microesferas.
Advertencias sobre el uso de pequeñas microesferas
• Siempre que se contemple el uso de medios embólicos de un
diámetro más pequeño que la capacidad de resolución del equipo
de imágenes, deberá considerarse detenidamente dicha opción.
La presencia de anastomosis arteriovenosas, vasos ramificados
hacia fuera del área diana o vasos emergentes no evidentes antes
de la embolización puedederivar en una embolización deregiones
no diana y complicaciones graves.
• Las microesferasde tamaño inferior a 100 micras generalmente
migran distalmente a las tributarias anastomóticas y, por lo tanto,
tienen más probabilidad de interrumpir la circulación al tejido
distal. Existe una mayor probabilidad de lesión isquémica con el
uso de microesferas de tamaño más pequeño, y deben
considerarse las consecuencias de esta lesión antes de la
embolización. Las posibles consecuencias incluyen: hinchazón,
necrosis, parálisis, absceso y/o síndrome posembolización más
intenso.
• La hinchazón posembolización podría provocar isquemia en el
tejido adyacente al área diana. Deben tomarse precauciones para
evitar el tejido no diana que no tolera la isquemia,como es el caso
del tejido nervioso.
PRECAUCIÓN :
• No utilice si el envase de la jeringa, el protector del émbolo o la
bandeja parecen estar dañados. Para uso en un solo paciente –
Contenido suministrado estéril – No reutilizar nunca,reprocesarni
reesterilizar los contenidos de una jeringa que ya se haya abierta.
Su reutilización,reprocesamiento o reesterilización podrían afectar
a la integridad estructural del dispositivo y/o provocar su fallo, lo
que a su vez podría ocasionar lesión, enfermedad o la muerte del
paciente. Su reutilización, reprocesamiento o reesterilización
podrían constituir también un riesgo de contaminación del
dispositivo o causar infección en el paciente o infección cruzada,
incluida, entre otras cosas, la transmisión de enfermedad/es
infecciosa/s de un paciente a otro. La contaminacióndeldispositivo
puede provocar lesión, enfermedado la muerte del paciente.Todos
los procedimientos deben realizarse de acuerdo con una técnica
aséptica aceptada.
• No conecte la jeringa de 20 ml con las Microesferas EmboGold
directamente al microcatéter para la administración embólica, ya
que se podría producir la oclusión del catéter.
• La jeringa está indicada para uso embólico solamente. No usar
para otra aplicación.
• Seleccione el tamaño y la cantidad de Microesferas EmboGold
apropiadas para la patología que se va a tratar.
• La embolización con las Microesferas EmboGold sólo debe ser
realizada por médicos que han recibido la formación sobre la
oclusión intervencionista apropiada relativa a la región que está
prevista embolizar.
• Las pacientes con alergia conocida al medio de contraste pueden
necesitar corticoides antes de la embolización.
• Pueden ser necesarias evaluaciones u otras precauciones
adicionales en la gestión de la asistencia periprocedimiento para
pacientes con las siguientes afecciones:
– Diatesis hemorrágica o estado hipercoagulativo.
– Inmunodeprimido.
POSIBLES COMPLICACIONES :
La embolización vascular es un procedimiento de alto riesgo. Las
complicaciones pueden ocurrir en cualquier momento, durante o
después del procedimiento y pueden incluir, entre otras, las
siguientes:
• Parálisis que resulta de una embolización no focalizada o lesión
isquémica de edema del tejido adyacente.
• Reflujo no deseado o paso de Microesferas EmboGold a las
arterias normales adyacentes a la lesión diana o a través de la
lesión a otras arterias o lechos arteriales como, por ejemplo, la
arteria carótida interna, pulmonar o circulaciones coronarias.
• Embolismo pulmonar debido a la fístula arterial venosa.
• Isquemia en una ubicación no deseada, incluida ictus isquémico,
infarto isquémico (incluido infarto de miocardio) y necrosis tisular.
• Oclusión del lecho capilar y daño tisular.
• Rotura vascular o lesión y hemorragia.
• Déficits neurológicos, incluidas parálisis del nervio craneal.
• Vasoespasmo.
• Muerte.
• Recanalización.
• Reacciones ante cuerpos extraños que necesitan intervención
médica.
• Infección que necesita la intervención médica.
• Formación de coágulos en la punta del catéter y el consiguiente
desplazamiento.
• Respuesta alérgica al oro.
• Complicaciones relacionadas con el cateterismo (p. ej.hematoma
en el punto de entrada, formación de coágulo en la punta del
catéter y consiguiente desplazamiento y lesiones nerviosas y/o
circulatorias que pueden tener como resultadolesión en la pierna).
• Reacción alérgica a los medicamentos (p. ej. analgésicos).
• Reacción alérgica a los medios de contraste o al material
embólico.
• Dolor y/o erupción cutánea, probablementediferidadel momento
de la embolización.
• Ceguera,pérdida auditiva, pérdida del olfato.
• Información adicional se encuentra en la sección Advertencias.
ALMACENAMIENTO Y ESTERILIDAD :
Inspeccioneel envase antes de su usopara garantizarla integridad
del precinto para el mantenimiento de la esterilidad.
• Las Microesferas EmboGold deben almacenarse en un lugar
fresco, seco y oscuro, en sus jeringas y envases originales.
• Usar antes de la fecha indicada en la etiqueta de la jeringa.
• No congelar.
• No reesterilizar.
INSTRUCCIONES DE USO :
• Evalúe cuidadosamente la red vascular asociada con la lesión
usando imagen de alta resolución antes de iniciar elprocedimiento
de embolización.
• Las Microesferas EmboGold están disponibles en una amplia
gama de tamaños. Debido al potencial de embolización fallida y la
variabilidad de los tamaños de las esferas, el médico debe
asegurarse de seleccionar cuidadosamente el tamaño de las
Microesferas EmboGold según el tamaño de los vasos diana en el
nivel deseado de oclusión en la vasculatura.
• Debe tenerse cuidado para elegir el tamaño apropiado de las
13
Page 14

Microesferas EmboGold que mejor se adapte a la patología (esto
S
TERILIZE
2
2
STERILE
es, diana vascular/tamaño vaso) y proporcione el resultado clínico
deseado.
• Al embolizar las malformaciones arteriovenosas (MAV), elija el
tamaño de las Microesferas EmboGold que ocluyan el nido sin
pasar por la malformación arteriovenosa.
• Elija un catéter portador basándose en el tamaño del vaso diana
y en el tamaño de microesfera que se está usando. Las
Microesferas EmboGold puedentolerar la compresión transitoriade
hasta un 33% para facilitar el paso a través del catéter portador.
• Las Microesferas EmboGold no son radiopacas. Se recomienda
que la embolización sea monitorizada con visualización
radioscópica añadiendo la cantidad apropiada de medio de
contraste al líquidode suspensión de la solución salina fisiológica.
• Para administrar Microesferas EmboGold:
Ajuste el volumen total en la jeringa con el mismo volumen de
contraste sin diluir, lo que originará un 50% de
microesfera/solución salina y un 50% de solución de contraste.
Extraiga todo el aire de la jeringa. Para poner en suspensión
uniforme la solución de MicroesferasEmboGold/contraste,invierta
suavemente la jeringa de 20 ml varias veces.Acople la jeringa de
20 ml a un puerto de la llave de 3 pasos Luer-Lock; acople una
jeringa de inyección de 1 ml o 3 ml a otro puerto de la llave, y, si
se desea, se puede acoplar un catéter portador al puerto restante
de la llave. Espere varios minutos para permitir que las
Microesferas EmboGold se pongan en suspensión correctamente.
Introduzca la solución EmboGold/contraste en la jeringa de
inyección lenta y suavementepara reducir al mínimo laposibilidad
de introducir aire en el sistema. Purgue todo el aire del sistema
antes de inyectar. Inyecte la solución de Microesferas
EmboGold/contraste bajo visualización radioscópica con la jeringa
de inyección, usando una inyección pulsátil lenta, a la vez que se
observa el flujo del contraste. Si no hay efecto sobre el flujo, repita
el proceso de administración con inyecciones adicionales de
soluciónMicroesferas EmboGold/contraste, o se pueden considerar
las Microesferas EmboGold de mayor tamaño. Si la solución de
Microesferas EmboGold/contraste requiere una nueva suspensión,
invierta suavemente la jeringa de 20 ml varias veces. Ejerza un
criterio conservador para determinar el final de la embolización.
• La punción femoral puede tener como resultado un espasmo
arterial.Estopuedepredisponer a la trombosis femoral (p. ej.lesión
en la pierna).Antes de la retirada final del catéter se debe volver a
evaluar la permeabilidad femoral.
• Al completar el tratamiento, retire el catéter mientras mantiene
una suave aspiración de modo que no se desplacen las
Microesferas EmboGold que continúen dentro de la luz del catéter.
• Aplique presión al punto de punción hastaque se ha completado
la hemostasia.
• Deseche cualquier Microesferas EmboGold abierta, no utilizada.
REFERENCIAS :
1. Bendszus M, Klein R,Burger R, et al.: Efficacy of trisacryl gelatin
microspheresversuspolyvinylalcoholparticles in the preoperative
embolization of meningiomas. AJNR, 21(2):255-61, Feb 2000.
2. Deveikis JP: Endovascular therapy of intracranial arteriovenous
malformations: materials and techniques. Neuroimaging Clin of N
Am, 8(2):401-424, 1998.
3. Frizzel RT, Fisher WS: Cure, morbidity, and mortality associated
with embolization of brain arteriovenous malformations: A review
of 1246 patients in 32 series over a 35-year period. Neurosurg,
37(6):1031-1040, Dec 1995.
4. Gomes, A: Embolization therapy of congenital arteriovenous
malformations;Use of alternative approaches.Radiology,190:1918, Jan 1994.
5.Terada,T; Kinoshita,Y; Yokote, H; Tsuura, M; Itakura, T;Komai,N;
Nakamura,Y;Tanaka,S; Kuriyama, T:Preoperative embolization of
meningiomas fed by ophthalmic branch arteries. Surg Neurol,
45:161-6, 1996.
Información en el envase :
Símbolo Designación
Fabricante: Nombre y dirección
Fecha de caducidad: año-mes
LOT
REF
Código de lote
Número de catálogo
No reesterilizar
No utilizar si el envase está dañado
Manténgase protegido de la luz del sol
Manténgase seco
No reutilizar
Precaución - Consultar las Instrucciones de uso
Apirógeno
Esterilizado con vapor
0°C
Límite inferior de temperatura
Distribuido en EE.UU. por:
Merit Medical Systems, Inc.
Servicio de Atención al Cliente 1-800-356-3748
781-681-7900
Información sobre patentes
EE.UU. 5,635,215 y otras patentes emitidas y pendientes.
Microesferas EmboGold es una marca comercial registrada de
BioSphere Medical.
Todos los episodios adversos serios o potencialmente mortales o
muerte asociados ocn el uso de lasMicroesferas EmboGold deben
ser notificados a Food and Drug Administration de EE.UU. bajo el
programa MedWatch y al fabricante del dispositivo. La información
acerca del programa MedWatchy los formularios para informarde
los episodios adversos se pueden obtener en
www.fda.gov/safety/medwatch/howtoreport/ucm053074.htm o
llamando gratuitamente a 888-463-6332. Los informes a
BioSphere Medical,Inc.sepuedenrealizarllamandogratuitamente
a 800-394-0295.
14
Page 15

15
Page 16

Biosphere Medical, S.A.
Parc des Nations - Paris Nord 2
383 rue de la Belle Etoile
95700 Roissy en France
France
16
 Loading...
Loading...