Page 1
24cm PRESSURE ASSISTED DEVICE
INSTRUCTIONS FOR USE
Read instructions prior to use.
Product not made with natural rubber latex.
DEVICE DESCRIPTION
The SAFEGUARD 24cm is a single use disposable device. SAFEGUARD
has a clear medical grade polyurethane window and bladder, a clear
medical grade PVC exible ll tube, and a pressure sensitive, selfadhesive peel backing. A luer valve on the end of the ll tube enables
a syringe to be connected to inate the central bladder with air to
provide pressure to the puncture site. The SAFEGUARD pressure
assisted device has a sterile dressing with a clear window that
facilitates visibility of the access site without removal or manipulation
of the device.
INDICATIONS
The indications for use for the SAFEGUARD 24cm pressure assisted
device are to assist in obtaining and maintaining hemostasis.
The device is also indicated in the reduction of active compression
time in femoral artery cannulation following diagnostic and interven
tional procedures with an ACT of 140 seconds or less, using a 6 Fr. and
smaller sheath size.
CONTRAINDICATIONS
The adhesive portion of the SAFEGUARD device should not be used
over excoriated skin.
CAUTIONS
Px Only. Caution: Federal (U.S.A.) law restricts this device to sale by or
on the order of a physician.
CAUTION: With over-ination i.e., above 40mL’s of air, the bulb
may begin to expand radially and could compromise the adhesive
properties of the device.
CAUTION: Under-ination of device could compromise the ability of
the device to assist in obtaining and maintaining hemostasis.
REUSE PRECAUTION STATEMENT
For single patient use only. Do not reuse, reprocess or resterilize.
Reuse, reprocessing or resterilization may compromise the structural
integrity of the device and/or lead to device failure which, in turn,
may result in patient injury, illness or death. Reuse, reprocessing or
resterilization may also create a risk of contamination of the device
and/or cause patient infection or cross-infection, including, but not
limited to, the transmission of infectious disease(s) from one patient
to another. Contamination of the device may lead to injury, illness or
death of the patient.
PRECAUTIONS
• Use proper aseptic techniques while handling product.
• Do not use if package is damaged
• Inspect device prior to use to verify that no damage has occurred
during shipping.
POTENTIAL ADVERSE EFFECTS
Possible adverse eects that may result from the use of this device:
• Hematoma
• Local bleeding
• Arterio-venous stula or pseudoaneurysm
SAFETY AND EFFECTIVENESS RESULTS
A clinical trial was conducted to evaluate the safety and eectiveness
of the SAFEGUARD Manual Assist Technique (MAT) in reducing active
compression time compared to historical manual compression data.
(Reduced Vascular Complications After Percutaneous Coronary
Interventions with a Non-mechanical Suture Device: Results from the
Randomized RACE Study, Sanborn, TA)
SAFEGUARD MAT Manual Compression
N=100 Historical Control
N=85
Avg. AC/TTH Manual 7.7 ± 3.3 28
Compression (minutes)
Manual Compression
SAFEGUARD Historical
MAT N=101 Control N=85
Major Complications
Access site bleeding
requiring transfusion
Vascular repair or the need
for vascular repair (via
surgery, ultrasound-guided
intervention, transcatheter
embolization, or stent-graft)
Any new ipsilateral lower
extremity ischemia
Surgery for access site
related nerve injury
Access site-related infection
requiring IV antibiotics or
extending hospitalization
Diagnostic
Interventional
0 0 0 0
0 1 0 2
0 0 0 0
0 0 0 0
0 0 0 0
Minor Complications * *
Access site hematoma
>6 cm (after sheath pull)
Bleeding requiring
> 30 minutes to
re-establish hemostasis
Non-treated
pseudoaneurysm
Non-treated arteriovenous
(AV) stula documented
by ultrasound
Skin excoreation at site
of Safeguard after removal
of dressing
Skin erythema
Allergic reaction to adhesive
Ipsilateral lower extremity
arterial emboli, transient loss
of lower extremity pulse, or
deep vein thrombosis
Access site-related vessel
laceration
Transient access-site related
nerve injury
Access site wound dehiscence
Localized access site infection
treated with intramuscular
or oral antibiotics
*None Reported
There is no statistical dierence in the rate of major complications
between the control group and the treated group.
1 1 - -
0 0 - 0 0 - -
0 0 - -
0 0 - -
0 0 - -
0 0 - 0 0 - -
0 0 - -
0 0 - -
0 0 - 0 0 - -
0 0 - -
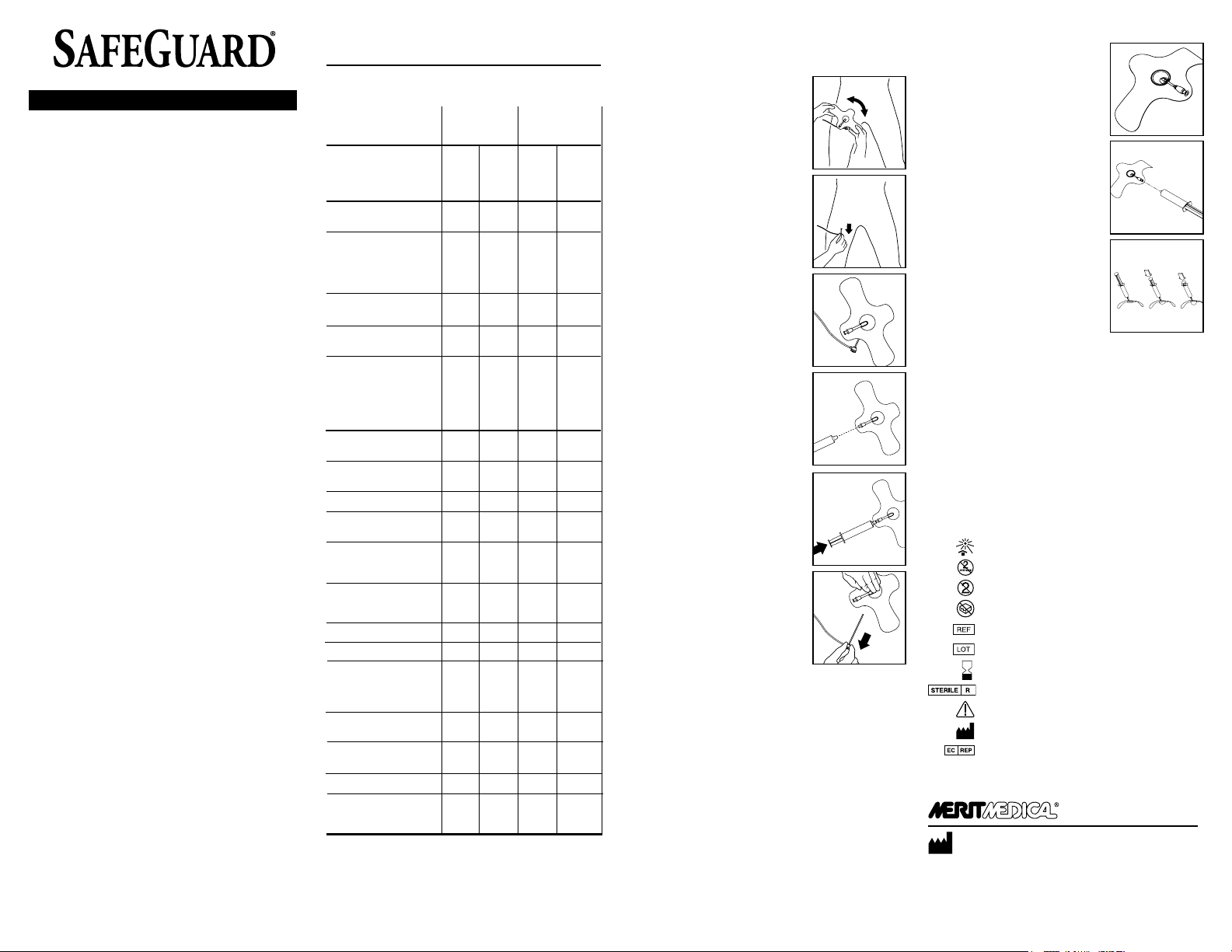
PRE-HEMOSTASIS, or MANUAL ASSIST TECHNIQUE (MAT)
PLACEMENT OF SAFEGUARD
1. Before adhering SAFEGUARD to the
patient, be sure skin is clean and dry.
Determine the appropriate angle for
SAFEGUARD placement to provide easy
access to luer inate/deate port and to
allow for easy sheath removal.
Note: Placement may require adjustment
based on the patient’s anatomy, angle
of the puncture site, and the presence or
absence of a procedural sheath.
Diagnostic
Interventional
2. Consider the point of maximum pulse,
anatomy, angle of puncture and
direction of ow to determine the
appropriate SAFEGUARD position and
verify.
3. Pull the procedural sheath back
approximately 1” (2.5cm) so that when
SAFEGUARD is adhered to the skin the
sheath hub is outside the area of the
SAFEGUARD adhesive.
Note: It is recommended that you aspirate
the sheath prior to removal to prevent distal
embolization from residual clot in sheath.
4. Remove the adhesive backing and place
the bulb where you would position your
ngers to hold manual compression (for
example, in femoral artery procedures,
typically the point of maximum femoral
pulse). Make sure SAFEGUARD is com
pletely adhered to the skin.
5. Attach and completely engage a
standard luer lock syringe to inate the
desired volume a maximum volume
of 40mL’s of air into the bulb to apply
pressure on the arteriotomy site. Syringe
must be completely engaged in the
luer to inate/deate the bulb. Remove
syringe.
Note: Maintain pressure on the plunger
while detaching syringe from the SAFE
GUARD valve. Observe that the desired
pressure is achieved and maintained.
6. Remove sheath, then immediately
apply manual compression directly over
inated bulb.
7. Hold manual compression until hemo
stasis has been achieved.*
• Slowly release manual compression.
• Check distal/proximal pulses to assure
ow is maintained.
• Conrm hemostasis by viewing the site
through the inated bulb window.
* Recommendations (MAT only):
Diagnostic patients - minimum 5 minutes
Interventional patients - minimum 10 minutes
8. Per hospital protocol, periodically check the site through the bulb
window to conrm hemostasis and to manage the bulb volume
and resultant pressure as needed. Continue to check distal/
proximal blood ow to assure patency.
9. Deate bulb every two hours to allow for capillary rell and to
assess the site. Re-inate the bulb if necessary.
10. Deate the bulb by attaching an appropriately sized luer lock sy
ringe to the valve, engage the valve and slowly depress the bulb
allowing the syringe to ll with air. Alternatively, remove plunger
from the syringe, attach syringe and allow air to slowly release
while gently depressing the bulb.
Note: Do not draw negative pressure in the syringe, as this will create
a vacuum on the site.
11. Prior to discharge of the patient, remove SAFEGUARD and apply
sterile dressing per hospital protocol.
-
-
-
POST-HEMOSTASIS TECHNIQUE
1. When hemostasis at the access site has
been achieved, apply the SAFEGUARD
device with the access site visible under
the bulb window of the SAFEGUARD
device. Consider the point of maximum
pulse, anatomy, angle of puncture
and direction of ow to determine the
appropriate SAFEGUARD position and
verify.
Note: Before adhering SAFEGUARD to the
patient, be sure skin is clean and dry. Deter
mine the appropriate angle for SAFEGUARD
placement to provide easy access to luer
inate/deate port.
2. Attach an appropriately sized standard
luer lock syringe to the valve of the
SAFEGUARD device.
Note: Syringe must be completely engaged
in the luer to inate/deate the bulb.
3. Inate the bulb of the SAFEGUARD
device with air to the desired volume of
air (24cm maximum of 40mL’s) to apply
pressure on the arteriotomy site and re
move the syringe. Check distal/proximal
pulses to assure ow is maintained.
Note: Maintain pressure on the plunger
while detaching syringe from the valve of
the SAFEGUARD device. Observe that the
desired volume is achieved and maintained.
4. Per hospital protocol, periodically check the site through the bulb
window to assure hemostasis is maintained and the bulb maintains
pressure.
5. Deate bulb every two hours and assess the site. Re-inate the bulb
if necessary.
6. Deate the bulb by attaching an appropriately sized luer lock
syringe to the valve, engage the valve and slowly depress the bulb
allowing the syringe to ll with air. Alternatively, remove plunger
from the syringe, attach syringe and allow air to slowly release
while gently depressing the bulb.
Note: Do not draw negative pressure in the syringe, as this will create a
vacuum on the site.
7. Prior to discharge of the patient, remove the SAFEGUARD device
and apply sterile dressing per hospital protocol.
Maintain sterile eld during application.
Keep away from sunlight
Do not Resterilize
Do not Reuse
Do not use if package is damaged
Catalog Number
Lot Number
Use By
Sterilized Using Gamma
Caution: Consult accompanying document
Manufacturer
Authorized Representative
-
Manufacturer: Merit Medical Systems, Inc.
1600 West Merit Parkway, South Jordan, Utah 84095 U.S.A. 1-801-253-1600
U.S.A. Customer Service 1-800-356-3748
-
-
www.merit.com ID 010814 400373001/A
 Loading...
Loading...