Page 1
PERCUTANEOUS
PD CATHETER
IMPLANTATION SYSTEM
INSTRUCTIONS FOR USE
VP – 511 and VP-511M
Implantation System for Peritoneal Dialysis Catheters
Product Description:
Implantation System Components:
• 0.038” Guide Wire
• 12 French Dilator
• 14 French Dilator
• 18 Gauge Introducer Needle
• 18 French Peelable Introducer Sheath
• Cu Implantor™
• Faller Trocar
• Scalpel
• 10 mL Syringe
• 4x4 Gauze
• Clip
Indications for Use:
The Percutaneous Implantation Kit can be used to implant
a peritoneal dialysis catheter in patients who are suitable
candidates for peritoneal dialysis therapy.
Contraindications:
• Do NOT use if the patient is not a suitable candidate for
peritoneal dialysis therapy.
Px Only: Caution: Federal (USA) law restricts this device to
sale by or on the order of a physician.
Precautions:
• Read manufacturer’s instructions prior to use.
• Contents are sterile (via ethylene oxide). Do not use if
packaging is opened, damaged, or broken.
• For single patient use only. Do not reuse, reprocess, or
resterilize. Reuse, reprocessing, or resterilization may
compromise the structural integrity of the device and/
or lead to device failure, which in turn may result in
patient injury, illness, or death. Reuse, reprocessing, or
resterilization may also create a risk of contamination
of the device and/or cause patient infection or cross
infection, including, but not limited to, the transmission
of infectious disease(s) from one patient to another.
Contamination of the device may lead to injury, illness,
or death of the patient.
• Do not use after expiration date.
• The medical techniques, procedures, and potential
complications stated herein do NOT give full and/
or complete coverage or descriptions. They are not a
substitute for adequate training and sound medical
judgment by a physician.
• Use an aseptic procedure to open the package and to
remove the contents.
Potential Complications:
Peritoneal Dialysis catheter implantation procedures have
inherent risks associated with their use. All such risks
apply to the use of the Percutaneous Implantation System.
Peritoneal dialysis potentially has a number of complications that may occur, which generally are not caused by
the implantation, but may aect the quality of therapy.
These complications may include, but are not limited to,
the following:
• Infections (exit-site or tunnel)
• Peritonitis
• Sepsis
• Bowel perforation
• Leakage (initial or latent)
• Fluid ow obstruction (inow or outow)
• Bleeding (subcutaneous or peritoneal)
• Ileus
• Proximal exit cu erosion
• Distal (rectus/deep) cu erosion
• Risks normally associated with peritoneoscopic and
laparoscopic procedures
• Allergic reaction
• Abdominal pain
• Infusion pressure/pain
• Organ erosion
• Genital edema
Catheter Implantation Site Options
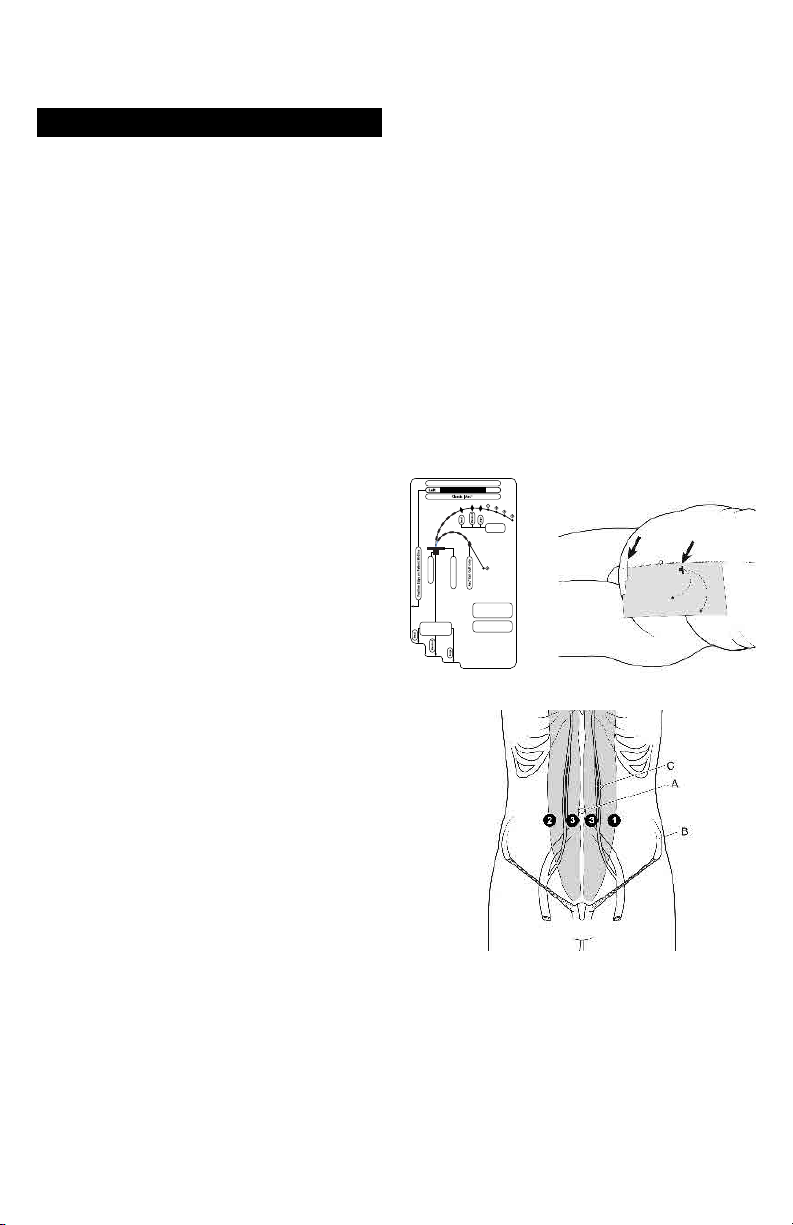
An Implantation Stencil may help to achieve consistent effective catheter placement and assure proper coil location.
Implantation Stencils (Figure 1) are sold separately with the
Flex-Neck® Catheter kits.
PD Catheter Implantation Site Options
Locate preferred implantation, tunnel, and exit sites as
indicated by an appropriate Implantation Stencil (Figure 2).
Please see anatomical landmarks as indicated in Figure 3.
Flex-Neck® Adult PD Catheter
IMPLANTATION STENCIL
Classic Exit
Cu Site
Rectus Cu Site
Primary Incision Site
®
Use with Flex-Neck
Classic & Arc™ Adult
PD Catheter ONLY
For directions, see
Place on Patient’s
Instructions for Use
Cranial Border of
the Pubic Symphysis
Figure 1 Figure 2-Stencil on body
Implantation Stencil
Figure 3 – Potential lower catheter implantation sites
A. Umbilicus
B. Iliac crest
C. Inferior and superior epigastric arteries
1. Left, lateral border of rectus sheath, 2-3 cm below
umbilicus
2. Right, lateral border of rectus sheath, 2-3 cm below
umbilicus
3. Medial border of rectus sheath, 2-3 cm below umbilicus
NOTE: Implantation sites should be above superior iliac
crest.
Page 2
WARNING: Do NOT implant the catheter or place the
exit-site in the patient’s skin folds or beltline.
Patient Preparation:
1. Operating personnel should perform a surgical scrub,
and use sterile hat, mask, gown and gloves according to
hospital protocol.
2. The patient should also wear a mask.
3. Attach appropriate patient monitors and sedate patient.
4. Prepare abdomen and drape patient in standard sterile
manner.
5. Use ultrasound at the intended entrance site to identify
any exclusionary pathology. Duplex ultrasound may
also be useful to identify proper catheter placement site
and avoid injury to the inferior epigastic vessels prior to
needle placement.
6. Anesthetize the proposed tissue tract and primary
catheter insertion site with proper local anesthetic.
Percutaneous Insertion of Introducer Sheath:
7. Make a 2-3cm long horizontal skin incision at selected
catheter implantation site.
8. Use a blunt dissection and cautery device as necessary
to maintain hemostasis. See gure 4.
Figure 4
Warning: If a bowel perforation is identied, the procedure
should be abandoned and the patient should be treated
with antibiotics for an appropriate duration before attempting a repeat catheter placement.
13. Once proper access to the peritoneal space is achieved
and conrmed via contrast, remove syringe from introducer needle and insert the exible end of the guide
wire through the introducer needle. Direct the wire into
the caudal and posterior position. Advance the wire
as appropriate under uoroscopy into the peritoneum.
The guide wire should advance easily into the peritoneal space.
Note: Optional: A hydrophilic, Amplatz or super-sti guide
wire (sold separately) can also be used. Normal saline may
also be infused, when necessary, in order to increase the
space between the abdominal wall and the bowel loops.
Warning: Saline infusion is not recommended in the
presence of ascites.
1
1
Warning: Forcible advancement of the guide wire against
resistance can result in internal organ injury and should be
avoided.
14. Withdraw the introducer needle, leaving the guide wire
positioned in the peritoneum.
15. Further advance the guide wire to the optimal position
in the pelvic gutter.
16. To accommodate catheter passage into the peritoneal
cavity, dilate the rectus muscle with the 12 French and
14 French dilators respectively, under uoroscopic
guidance.
17. Verify that the dilator and introducer sheath are locked
together to prevent separation during insertion.
9. If appropriate, the implantation of the deep cu into
the rectus abdominus muscle can be aided by creating
a small puncture or fasciotomy into the supercial
rectus fascia with a hemostat or scalpel prior to needle
placement.
10. At a 30-45 degree angle from horizontal, using
ultrasound guidance, advance the introducer needle
through the anterior rectus sheath, rectus muscle and
through the posterior rectus sheath.
Note: A non-vascular micropuncture set (sold separately
and available from Merit Medical) may be used to access
the peritoneum. If using a non-vascular micropuncture
set, assure the length is adequate for peritoneal access and
follow manufacturer’s instructions for use.
11. Once access to the peritoneal space is obtained, attach
a 10 mL syringe containing appropriate iodinated contrast material to the needle using exible clear tubing
(sold separately).
12. Under uoroscopy, verify needle placement into the
peritoneal space by identifying the free ow of contrast
outlining regional bowel loops. An amorphous, irregular or striated appearance of injected contrast may
indicate that the needle tip is inappropriately located in
the bowel mesentery, greater omentum, preperitoneal
space or rectus abdominus muscle.
Warning: Do not use barium-based contrast.
Note: Contrast media should outline bowel loops. Contrast
identied within a bowel loop may indicate bowel perforation.
Figure 5
18. Under uoroscopy, advance the 18 French peelable
introducer sheath over the guide wire, gently twisting
it back and forth to assist with passage through the
tissue.
Warning: In order to avoid internal injury, care should be
taken to avoid advancing the introducer or dilators beyond
the tip of the guide wire.
Warning: Care should be taken to avoid creating a kink into
the guide wire with the introducer.
19. Once the sheath is in place, gently remove the dilator
from the peelable introducer sheath. If using the
“Implantation Stylette Technique” as noted below, the
peelable sheath and wire can be removed simultaneously.
Caution: Do not force the introducer into the peritoneum. Take care not to insert further than necessary for the
patient’s size and access site.
Preparing the catheter
20. Prepare the catheter by soaking it in sterile saline,
and squeeze the air out of the cus by rotating the
submerged cus between ngers. See Figure 6.
Page 3

Figure 6 Figure 7
Note: Use the radiopaque stripe as a guide to avoid twisting the catheter. (Figure 7) For optimal catheter placement,
radiopaque stripe should be oriented directly anterior or
directly posterior in the patient.
Implanting the Catheter
Technique #1 – Over-the-Wire using a Peelable
Introducer Sheath
Figure 8
21.
A. Maintaining the position of the distal end of the guide
wire, if placing a coiled catheter, straighten catheter coil
in order to load the catheter over the proximal end of
the wire.
B. Continue to advance the catheter over the guide wire
and through the peelable sheath introducer (Figure 8).
Under uoroscopic guidance, manipulate the guide
wire and catheter in tandem until the distal end of the
wire is in appropriate position.
C. Once optimal catheter positioning is achieved, continue
to “Implanting the Rectus Cu.”
Technique #2 – Implantation Stylette Technique
Note: A Merit catheter straightening stylette (sold separately non-sterile) can be used in place of the guide wire
included in the kit.
Caution: Extreme care should be taken when using the
stylette with or without uoroscopy.
Warning: Organ perforation may occur if uoroscopic
guidance is not utilized.
22.
A. Remove the guide wire and internal sheath dilator
simultaneously, once optimal peritoneal placement is
achieved.
B. Lubricate the facility-sterilized catheter stylette with
sterile gel or saline.
C. Insert the stylette into the catheter.
D. Lubricate the distal part of the catheter with sterile gel
or saline.
E. Insert catheter, with stylette, carefully into the peelable
sheath.
F. Under uoroscopic guidance, advance the catheter
through the sheath, periodically retracting the stylette.
Note: Keep the tip of the stylette within the abdomen to
help the catheter move through the rectus muscle.
Caution: Make sure the catheter is not doubled on itself,
kinked, or twisted (Figure 7).
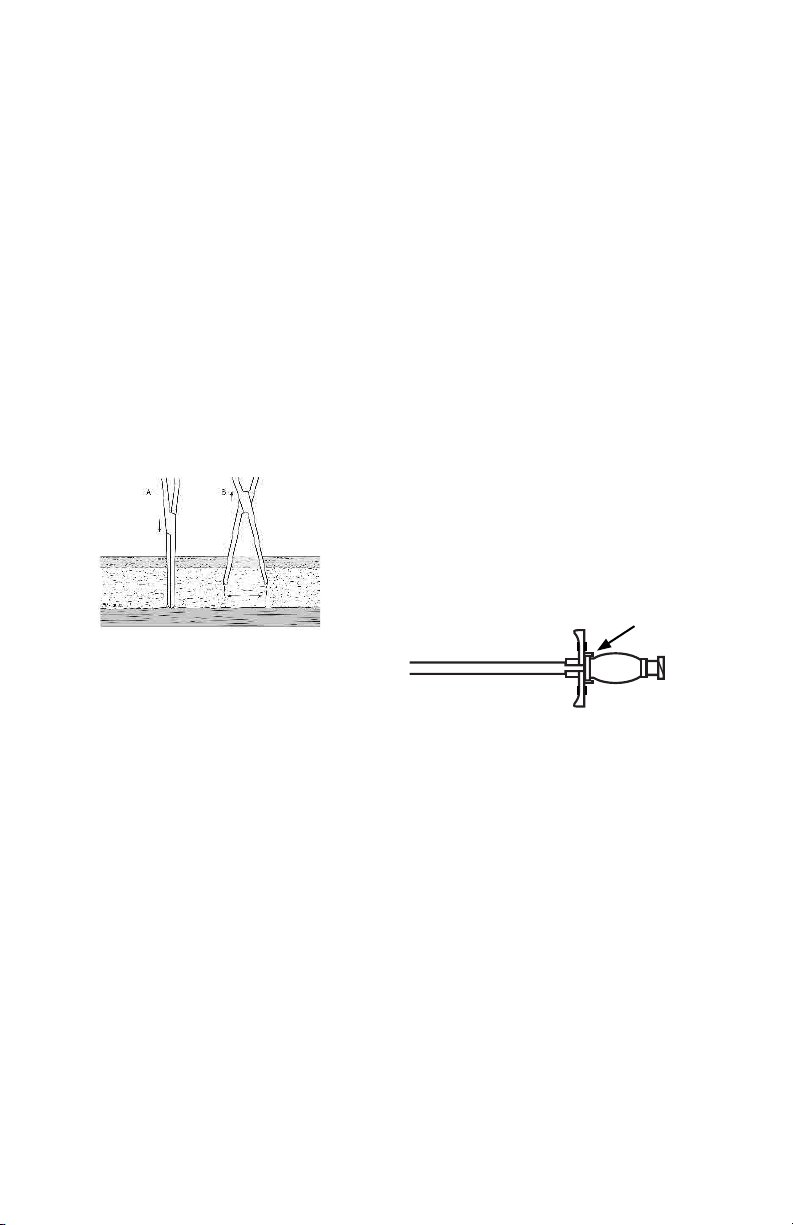
Implanting the Rectus Cu
23. Grasp the tabs of the peelable sheath and crack and
peel the sheath to the level of the anterior rectus
sheath, approximately 5 cm.
24. Place the Cu Implantor between the two cus of the
catheter and advance to the distal (deep) cu.
25. While bracing the cu with the Cu Implantor, advance
the distal cu with the Cu Implantor and peeled part
the sheath into the rectus fascia.
26. Holding the distal cu with the Cu Implantor in the
rectus muscle, peel the remaining sheath with assistance from the procedural assistant.
27. Once the peelable sheath is completely removed, continue to advance the Cu Implantor until the distal cu
is passed through the opening of the anterior rectus
sheath. The bracket on the Cu implantor will keep the
Cu Implantor from passing through the anterior rectus
sheath.
28. Retract the Cu Implantor tool parallel with the catheter, without dislocating or moving the distal cu.
Removing Tools and Tunneling the Catheter
29. Digitally and visually verify that the distal cu is just
below anterior rectus sheath.
Note: To improve visualization of the cu, it is helpful to
retract incision site tissue.
30. Remove the guide wire or stylette.
31. Slide the proximal end of the catheter over the barbed
end of the Faller Trocar, onto the indented section of
the Trocar past the barb.
32. Secure the catheter with a suture by tying the suture
around the catheter to ensure holding strength during
the tunneling process.
33. Insert the sharp end of the Faller Trocar into the initial
implantation site, as indicated by the Implantation
Stencil markings, aiming the sharp tip toward the
planned exit-site as indicated by the Implantation
Stencil markings.
34. Advance the sharp tip of the trocar along the planned
track.
Cautions: The Fallar Trocar is very sharp and can create
sever hematomas or lacerations in the patient or injure the
user if not used correctly by qualied medical personnel.
Warning: Do not twist the catheter.
35. When the trocar tip is close to the exit-site, make a stab
incision not to exceed 5.0 mm with a #11 blade at the
exit-site.
36. Advance the Faller Trocar through the exit-site incision.
Note: Do not twist or kink the catheter.
37. Pull the catheter through the tunnel, and out through
the exit-site and assure the subcutaneous cu is optimally located.
38. Cut the catheter o of the Faller Trocar at the end of the
barbed tip of the trocar.
Page 4

Cautions:
• Verify that the catheter is not twisted or kinked.
• Do not use excessive force as the catheter is pulled.
Excessive force can permanently damage the integrity
of the catheter walls or dislocate the distal part of the
catheter.
• If resistance is noted, carefully use a hemostat inserted
into the primary implantation site to open the tunnel
track for the exit cu.
• Never use a hemostat at the exit-site.
• DO NOT pull the catheter o the tunneler.
• DO NOT cut the suture o. Attempting to cut the suture
o creates a very high risk of damaging the catheter.
39. Inject a small amount of non-ionic contrast to check
that the catheter placement and assure it is not twisted
or kinked.
40. Close the primary incision with sutures.
41. DO NOT suture the exit-site.
42. Conrm placement by infusing 100 to 1000 mL of
warmed sterile saline.
43. If necessary, use clip included in kit to clamp the
catheter to control the ow of saline.
44. Lock the catheter with 10 mL heparin before applying
connector and cap or transfer set (sold separately, not
available from Merit).
45. According to standard hospital protocol, close the
initial catheter insertion site, attach the appropriate
connector, and wound dressing.
References:
1. Abdel-Aal AK, Fluoroscopic and Sonographic Guidance
to Place Peritoneal Catheters: How We Do It, AJR: 2009;
192:1085-1089
Manufacturer:
Merit Medical Systems, Inc.
1600 West Merit Parkway, South Jordan, Utah 84095 U.S.A.
1-801-253-1600 U.S.A. Customer Service 1-800-356-3748
Authorized Representative:
Merit Medical Ireland Ltd,
Parkmore Business Park West, Galway, Ireland
www.merit.com
ID 030414 401059001/A
Page 5

 Loading...
Loading...