Merit Medical Flex-Neck ExxTended Catheter User Manual
FLEXNECK®
EXXTENDED™
CATHETER
IMPLANTATION
PROCEDURE KIT
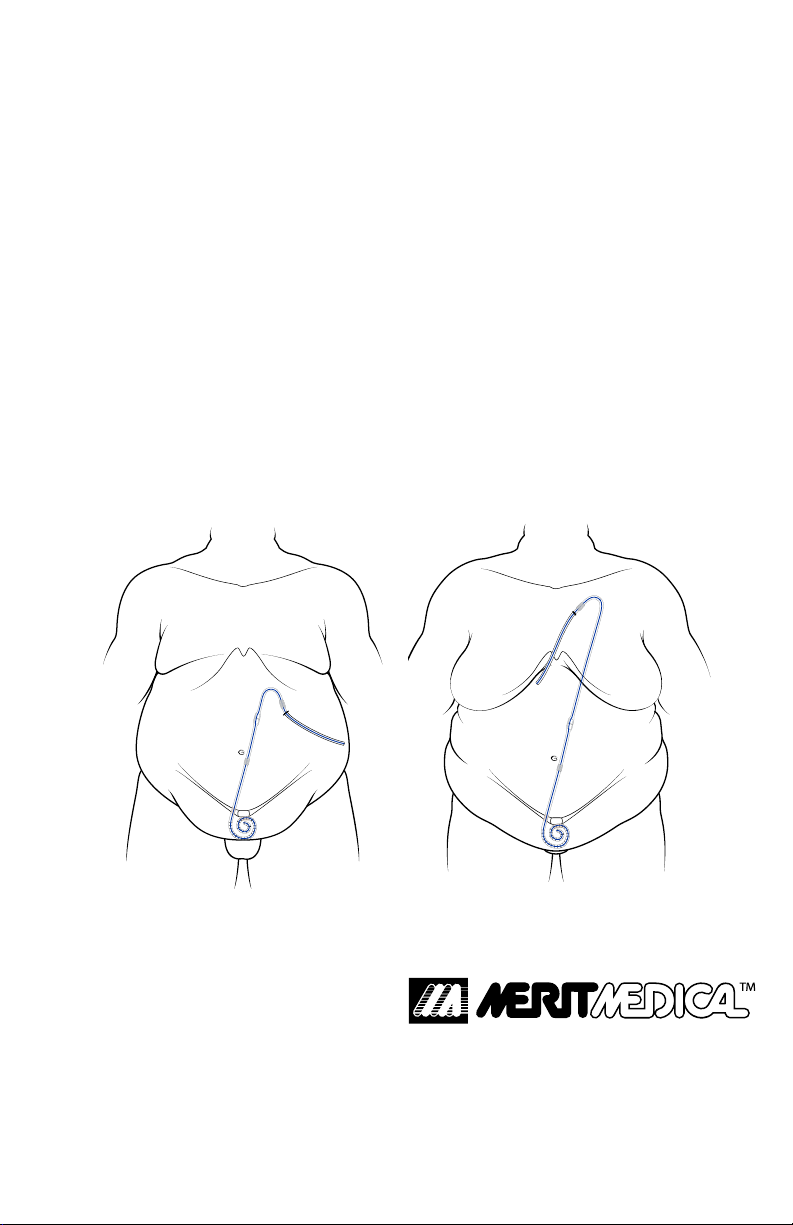
Upper Abdomen Catheter Placement
Upper Chest Catheter Placement
INSTRUCTIONS FOR USE

FLEXNECK EXXTENDED
CATHETER KIT
INSTRUCTIONS FOR USE
Table of Contents
Section A – Instructions for Flex-Neck ExxTended Catheter
Implantation Stencils Kit
Section B – Instructions for Lower Catheter Implantation for
Upper Abdomen and Upper Chest Exit-Site
Section C – Instructions for Upper Abdominal Catheter: Sizing,
Connecting and Placement
Section D – Instructions for Upper Chest Catheter: Sizing,
Connecting and Placement
Section E – Catheter Connector Instructions
Product Description
Each Flex-Neck ExxTended Catheter Kit contains a sealed tray
and a separate sealed pouch.
Tray Contents:
• Lower Catheter
• Upper Catheter
• Titanium tube-to-tube connector, double barbed
(internal use)
• Tape measure
• Flex-Neck® ExxTended™ Catheter Implantation Stencil Set
• Surgical marking pen
• Lubricating gel, water-soluble
• Plastic catheter connector (external use)
• Plastic catheter connector cap (external use)
Pouch Contents:
• Tunneling Tool, curved
• Measuring Rod
Indications for Use
If a patient is a suitable adult candidate for peritoneal dialysis
(PD) therapy, the Flex-Neck ExxTended peritoneal dialysis
catheter can be implanted either surgically, laparoscopically, or
peritoneoscopically for acute or chronic peritoneal dialysis.
Stencils sold with the device, and marketed separately, will be
used to assist the physician to locate the optimum primary
implantation site and the optimum catheter exit site for the
Flex-Neck ExxTended Catheter.
Contraindications for Use
Do not use the ExxTended Catheter if the patient is not a suitable candidate for peritoneal dialysis therapy.
WARNING:
The Upper Chest exit-site location of the ExxTended Catheter
should not be used if the patient has had breast implantation
or breast reconstruction, or has a tracheostomy. However, this
group of patients may be suitable candidates for the Upper
Abdomen exit-site location of the ExxTended Catheter.
Px Only: Caution: Federal (USA) law restricts this device to sale
by or on the order of a physician.
PRECAUTIONS:
• Read manufacturer’s instructions prior to use.
• Contents are sterile (via ethylene oxide). Do not use if packaging is opened, damaged or broken.
• For single patient use only. Do not reuse, reprocess, or resterilize. Reuse, reprocessing, or resterilization may compromise
the structural integrity of the device and/or lead to device
failure, which in turn may result in patient injury, illness, or
death. Reuse, reprocessing or resterilization may also create
a risk of contamination of the device and/or cause patient
infection or cross-infection, including, but not limited to,
the transmission of infectious disease(s) from one patient
to another. Contamination of the device may lead to injury,
illness, or death of the patient.
• Do not use after expiration date.
• The medical techniques, procedures, and potential complications stated herein do NOT give full and/or complete coverage or descriptions. They are not a substitute for adequate
training and sound medical judgment by a physician.
• Use an aseptic procedure to open the package and to
remove the contents.
Potential Complications
Peritoneal dialysis potentially has a number of complications
that may occur, which generally are not caused by the implantation, but may aect the quality of therapy. These complications
may include, but are not limited to, the following:
• Infections (exit-site or tunnel)
• Peritonitis
• Sepsis
• Bowel Perforation
• Leakage (initial or latent)
• Fluid ow obstruction (inow or outow)
• Bleeding (subcutaneous or peritoneal)
• Ileus
• Proximal exit cu erosion
• Distal (rectus/deep) cu erosion
• Risks normally associated with surgical procedures.
Cautions:
• Do no twist or rotate catheter during the implantation procedure.
• ExxTended Stencils cannot be used with other brands of PD
catheters.
• Enclosed Stencils are designed for Flex-Neck, ExxTended,
Coiled Adult Peritoneal Dialysis Catheters ONLY.
• Do NOT use ExxTended stencils with Flex-Neck Adolescent,
Pediatric, or Infant Catheters.
• Do NOT sterilize or reuse Stencils.
• Catheter tubing can tear when subjected to repeated clamping, serrated-jaw forceps, excessive force, or rough tools.
• Do NOT use forceps with a serrated jaw.
• Do NOT use excessive force to lock the forceps closed.
• Use ONLY smooth-jawed forceps or equivalent.
• Do NOT clamp the catheter, or repair tubing repeatedly in
the same area.
• Do NOT clamp near the connector.
Use only catheter connectors and repairs kits which are specifically labeled and approved for use with Flex-Neck Peritoneal
Dialysis Catheters. Approved catheter connectors and repair kits
can be ordered directly from Merit Medical Systems, Inc.
For best results, use the Stencils included with each catheter
kit. If not using the included Stencils, follow generally accepted
standard hospital protocols to make arcuate-shaped tunnels.
Cautions: Use the radiopaque stripe as a guide to keep the catheter straight. The catheter must not be twisted or rotated during
the implantation procedure. Any twist or rotation in the catheter
can lead to kinks, migration, and/or occlusion.
Assumptions to Successful ExxTended™
Catheter Implantation
As with any medical/surgical procedure, there are a number of
assumptions and prerequisites made for the successful implantation of the ExxTended catheter.
1. The implanting physician has had a reasonable number of
successful peritoneal dialysis catheter implantations, preferably
via laparoscopic or peritoneoscopic implantation. The ExxTended catheter can also be implanted via a cut-down method or
guide-wire technique.
2. Preferably, the patient’s lower abdomen and exit-site area
either Upper Chest or Upper Abdomen have been previously
marked by the Flex-Neck ExxTended Catheter Implantation
Stencils, in the clinic or during pre-op evaluation. At a
minimum, the patient should be evaluated preoperatively
to determine a proper, accessible location for the catheter
exit site. These marking should be noted for reference during
implantation.
3. The implanting physician should use the Flex-Neck ExxTended Catheter Stencils (included with each catheter pack) to verify
the primary implantation site, the Upper Catheter tunnel track,
and the exit-site, as previously chosen and marked in the clinic
or pre-op evaluation.
4. For Upper Chest Catheter exit-site procedures, the implanting physician will keep the subcutaneous part of the catheter
and the catheter exit-site o the sternum. Doing so will protect
the integrity of the catheter in the event of future cardiovascular
surgery that may require a midline sternotomy.
Outer Inner Overall Length, Fill
Diameter Diameter Straightened, Volume
Untrimmed
5.1 mm 3.5 mm 62.0 cm (Lower Cath) Maximum: 12.0 mL
62.0 cm (Upper Cath)
To nd ll volume of
Must be trimmed to t trimmed, joined
patient physique and catheter, multiply
exit-site location. total length of joined
catheter (in cm) by
0.096 to get ll
volume in cc.
Section A
INSTRUCTIONS FOR FLEXNECK EXXTENDED CATHETER
IMPLANTATION STENCIL SET
The Implantation Stencils for Flex-Neck ExxTended Peritoneal
Dialysis (PD) Catheters help choose the best implantation and
exit- sites for each patient. Note that these Implantation Stencils
cannot be used with other brands of PD catheters, or with other
sizes or styles of Flex-Neck PD Catheters.
For best results, the Implantation Stencils can also be used
during operative preparations, to mark, coordinate, verify and/or
adopt the markings made during the preoperative examination
as needed after laparoscopic visualization of the peritoneal
space. Please refer to the complete “Implantation Stencils for
Flex-Neck ExxTended Catheter Instructions for Use” distributed
by Merit Medical Systems, for more information.
The Stencil pattern contains essential catheter design information including the distance between the deep cu and the
coil-tip, the shapes of preformed tubing bends, and the distance
between the supercial cu and the exit-site. Additional
features of the Stencil permit its precise orientation on the torso,
according to stable anatomical landmarks: the pubic symphysis,
representing the anterior cranial border of the deep pelvis, and
the anatomical midline of the torso. The Stencils permit accurate
and reproducible association of the catheter design elements
to these vital anatomical landmarks to help determine the
best catheter insertion site and deep cu placement that will
produce the optimal pelvic position of the catheter coil and the
ideal exit-site location, either in the upper abdomen below the
costal margin, or in the upper chest o the sternum.
NOTE:
Each ExxTended Catheter Kit includes three Stencils. Each Stencil
has a reverse side for Right (R) and Left (L) catheter placements.
L-1, L-2 and R-1, R-2 are used for Upper Abdomen Catheter
placement. L-1, L-3 and R-1, R-3 are used for Upper Chest
Catheter placements.
CAUTION
These ExxTended Implantation Stencils are specic for ONLY
Flex-Neck ExxTended Adult Peritoneal Dialysis Catheters.
• Do NOT use these Stencils for other catheter brands.
• Do NOT use these Stencils for Flex-Neck Classic or ARC™
Catheters, in Adult, Adolescent, Pediatric, or Infant sizes.
• Do NOT resterilize these Stencils
• Stencils are available through Merit Medical Systems., Inc.
Patient Marking in the Surgical Suite – Upper Abdomen and
Upper Chest
NOTE:
These instructions are for marking the ExxTended Catheter
Upper Chest and Upper Abdomen congurations.
The following instructions are specic for implanting the
ExxTended Catheter on the patient’s left side. If the ExxTended
Catheter is to be implanted on the patient’s right side, substitute
the R-Series Stencils.
Upper Abdomen Stencil Instructions
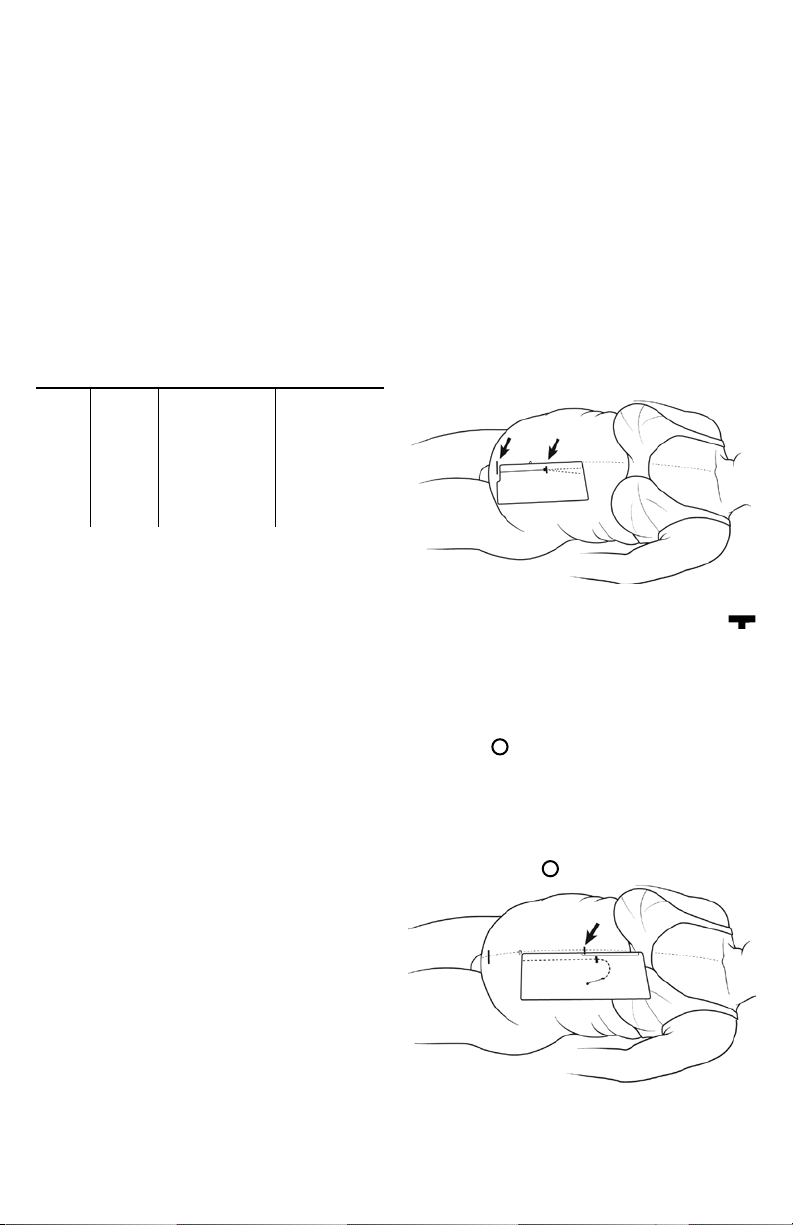
Align the midline edge of the L-1 Stencil on the patient’s abdominal midline. Adjust the Stencil caudally or cranially to position
the notched cutout on the upper border of the pubic symphysis.
This will be the location of the upper extent of the catheter coil
as it lies in the pelvis. See Figure 1.
Figure 1
With the Stencil aligned on the patient’s midline, and the
notched cutout positioned as above, mark the T-bar cutout
which indicates the location of the primary incision site through
which the lower catheter will be inserted during the implantation procedure and species the nal resting position of the
rectus cu.
1. Align the midline edge of the L-2 Stencil with the midline
of the patient’s abdomen. Adjust the Stencil up or down until
exit-site Circle cutout is in desired position according
to pre-procedural patient markings. Conrm that the upper
edge of the catheter tunnel track, or arcuate bend, is below the
costal margin. If the subcutaneous path indicated by the Stencil
overlaps the costal margin, then shift the Stencil caudally until
the rib margin is cleared. The midline edge of the Stencil should
remain parallel to the patient’s midline but may not exactly
coincide with midline due to lateral shift from weight of skin.
Mark the exit-site Circle with the Stencil in this position.
See Figure 2.
Figure 2
Conrm that selected exit-site does not conict with belt line,
skin creases or folds. Exit site should be easily visible to the
patient as indicated by pre-procedural consultation and
markings.
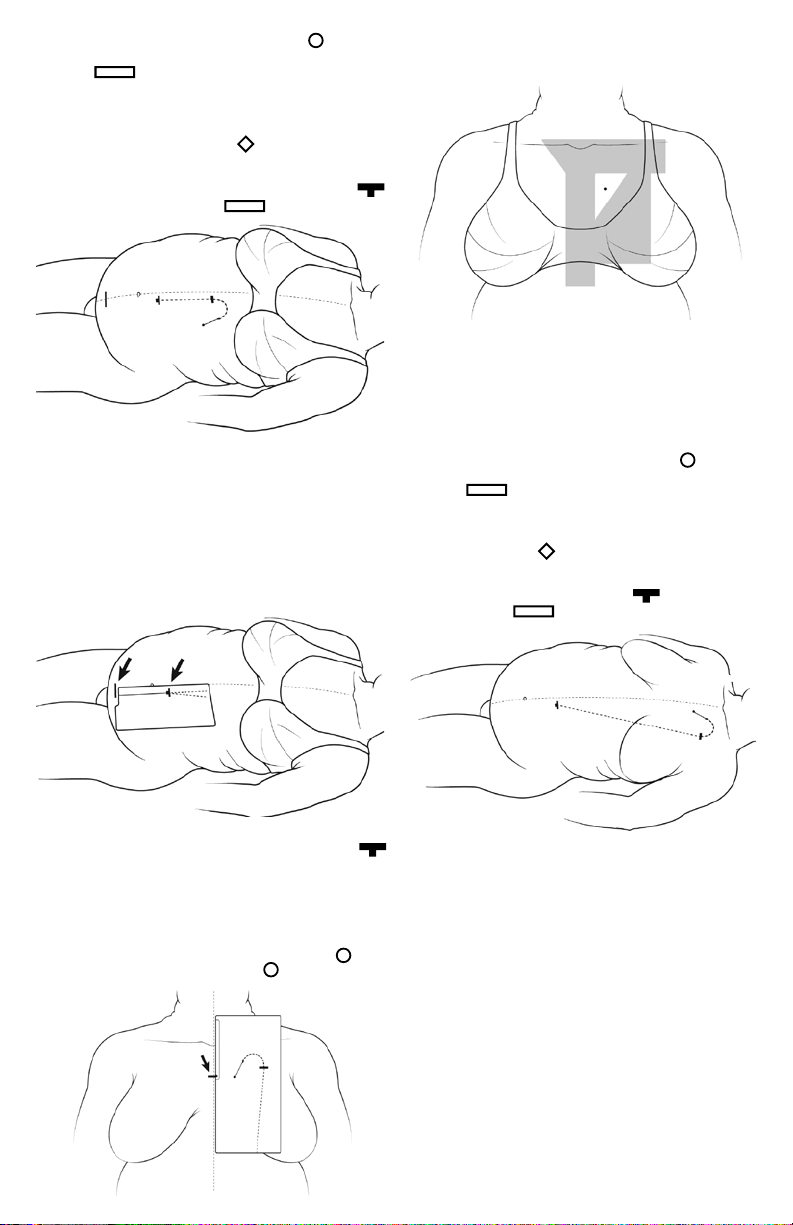
When satisfactory locations for the Exit-Site, Circle , and the
arcuate bend are achieved, mark the secondary incision site,
Rectangle . This is where the Tunneling Tool with the
attached ExxTended Catheter will temporarily emerge. With
proper implantation of the Upper Catheter segment, the black
marker ring will rest at the level of the secondary incision.
Mark the exit cu location, Diamond . Trace the shape of
the arcuate tunnel path on the skin, using the Stencil cutouts
as a guide. The guidelines on Stencils L-1 and L-2 indicate the
planned pathway to connect the primary incision site, T-bar
and secondary incision, Rectangle . See Figure 3.
Figure 3
Upper Chest Stencil Instructions
NOTE: The following Instructions are specic for implanting the
ExxTended Catheter on the patient’s left side. If the ExxTended
Catheter is to be implanted on the patient’s right side, substitute
the R-Series stencils.
Align the midline edge of the L-1 Stencil on the patient’s abdominal midline. Adjust the Stencil caudally or cranially to position
the notched cutout on the upper border of the pubic symphysis.
This will be the location of the upper extent of the catheter coil
as it lies in the pelvis. See Figure 4.
Conrm that selected exit-site is free from open collar area ,
infraclavicular region, median sternotomy zone, and eshy part
of breast . See Figure 6 for overlay of regions.
Figure 6
The midline edge of the Stencil should remain parallel to the
patient’s midline but may not exactly coincide with midline due
to lateral shift from weight of skin. Conrm that the subcutaneous path indicated by the Stencil for the arcuate bend does not
conict with the clavicle. If the subcutaneous path indicated by
the Stencil overlaps the clavicle, then shift the Stencil caudally
until the clavicle is cleared.
When satisfactory locations for the exit-site, Circle , and the
arcuate bend are achieved, mark the secondary incision site,
Rectangle . This is where the Tunneling Tool with the
attached ExxTended Catheter will temporarily emerge. With
proper implantation of the Upper Catheter segment, the marker
ring will rest at the level of the secondary incision. Mark the Exit
Cu location, Diamond . Trace the shape of the arcuate tunnel path on the skin, using the Stencil cutouts as a guide. The
guidelines on Stencils L-1 and L-3 indicate the planned pathway
to connect the primary incision site, T-bar , and secondary
incision, Rectangle . See Figure 7.
Figure 4
With the Stencil aligned on the patient’s midline, and the
notched cutout positioned as above, mark the T-bar cutout
which indicates the location of the primary incision site through
which the lower catheter will be inserted during the implantation procedure and species the nal resting position of the
rectus cu.
Align the midline edge of the L-3 Stencil with midline of chest.
Adjust the Stencil up or down until the Exit-Site, Circle , is in
desired position. Mark the exit-site, Circle . See Figure 5.
Figure 5
Figure 7
Section B
EXXTENDED™ LOWER CATHETER IMPLANTATION FOR
UPPER ABDOMEN AND UPPER CHEST EXITSITE
Implanting the Lower Catheter
There are 3 options for implanting the Lower Catheter:
1. Laparoscopic approach, with or without Y-TEC® catheter
implantation system. This approach is recommended. Y-TEC
peritoneal dialysis catheter implantation systems including Instructions for Use are available through Merit Medical Systems,
Inc.
NOTE: If laparoscopy is used to implant the Lower Catheter,
deate the abdomen before testing catheter patency to avoid
false uid outow rates.
2. Open surgical dissection (cut-down technique).
3. Percutaneous or Modied Seldinger technique, with or
without uoroscopic guidance.
 Loading...
Loading...