Page 1
INSTRUCTIONS FOR USE
EMBEDDING® TOOL
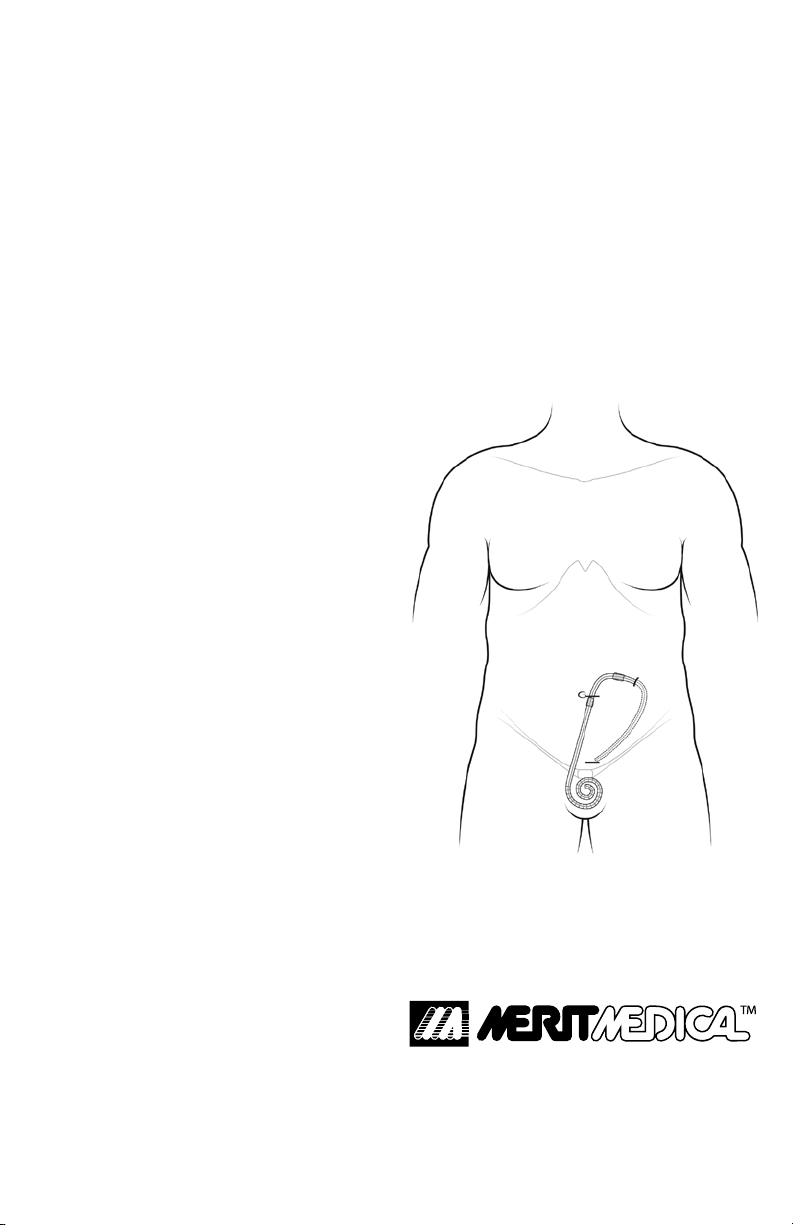
EMBEDDING THE EXTERNAL
PORTION OF A PERITONEAL
DIALYSIS CATHETER
Page 2
EMBEDDING THE EXTERNAL PORTION
262422201816108642
OF A PERITONEAL DIALYSIS CATHETER
English
INTRODUCTION
Merit Medical Systems, Inc.’s Embedding® Tool, TE-1000,
is used to temporarily embed (bury) the external portion
of a peritoneal dialysis (PD) catheter in the patient’s
subcutaneous tissue at the time of implantation. The PD
catheter is implanted and embedded in advance of the need
for peritoneal dialysis. When dialysis treatment is needed, the
catheter is retrieved (exteriorized) in a simple procedure, done
with no additional special tools.
PREFACE
These Instructions make several assumptions that are
critical to know and understand prior to successful use of the
Embedding Tool.
1. The distal catheter coil has been optimally placed in the
peritoneal cavity for good hydraulic function, to avoid
entrapment by omentum, and to minimize catheter
migration.
2. The PD catheter has two polyester felt cus.
3. The distal (deep) cu is correctly positioned within the
rectus muscle.
4. Catheter patency has been clearly established prior to the
embedding procedure.
5. The catheter has been tunneled through an exit-site.
6. The embedding procedure is done during the same
operative procedure as the initial implantation of the
PD catheter itself. There is no adequate way to sterilize
the external limb of a previously implanted catheter,
and then embed it, without signicant risk of infectious
complications.
7. The retrieval procedure is assumed to be done later as a
separate procedure. The retrieval timing is determined
by the patient’s need to start dialysis and by catheter cu
ingrowth, allowing adequate time for tissue ingrowth into
the cus (3 – 5 weeks).
8. The embedding and retrieval procedures are designed to be
performed by a qualied physician.
INSTRUCTIONS FOR USE
Embedding Tool
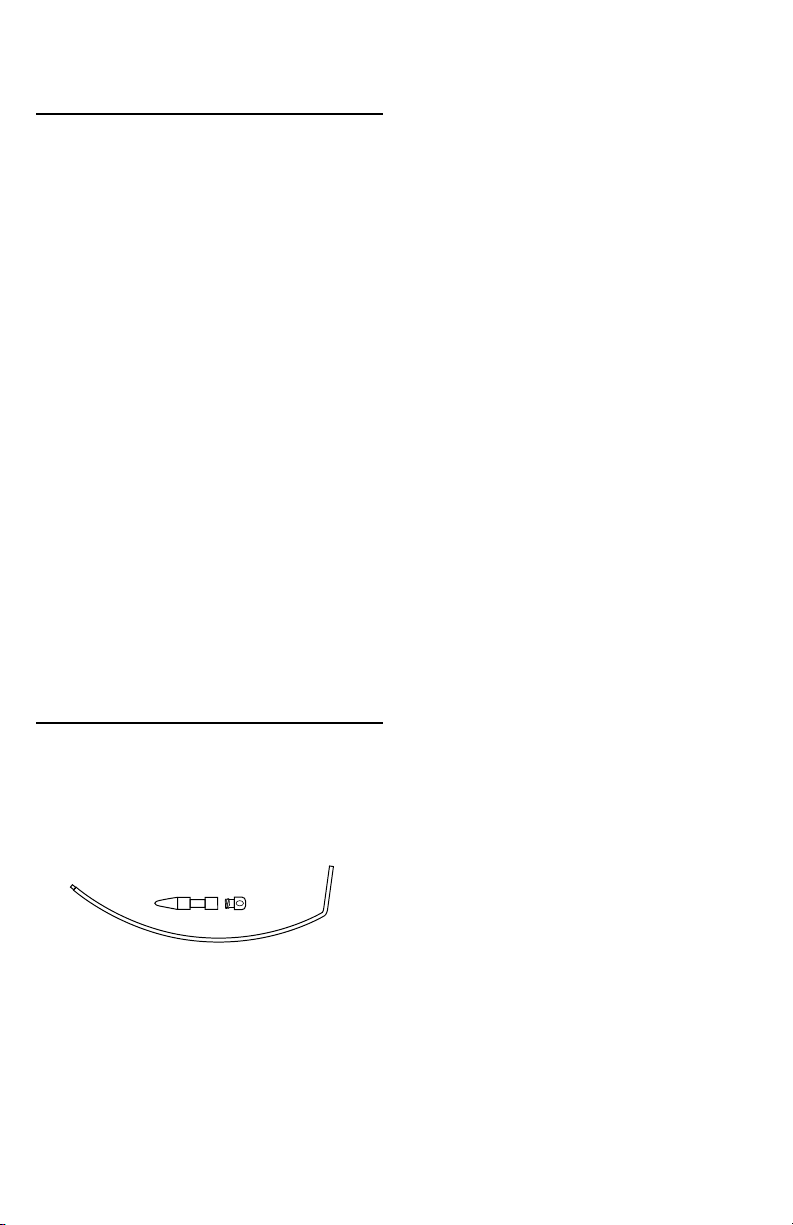
PRODUCT DESCRIPTION
Each box contains one pouch with the following
components;
• Curved plastic handle, (A) with threaded tip (B)
• Titanium Plug (C)
• Titanium Cap (D)
B
A
INDICATIONS FOR USE
This device can be used to embed most known brands
and styles of peritoneal dialysis (PD) catheters when the
nephrologist determines this action is in the best interest
of the patient, however, only immediately after successful
catheter implantation. The Embedding Tool is indicated
for embedding the external portion of most PD catheters
subcutaneously in anticipation of future retrieval of that
part of the catheter, provided that:
• The embedding procedure is done immediately
following PD catheter implantation.
• Catheter patency has been completely established.
• The normally external part of the PD catheter can be
embedded.
C D
• The patient is a candidate for delayed onset of PD
treatment.
• The patient is a candidate for PD.
CONTRAINDICATIONS FOR USE
The use of this device and technique is contraindicated if
one or more of the following conditions exist:
• The PD catheter was implanted at a dierent time or
setting.
• PD catheter patency was not clearly demonstrated prior
to embedding.
• The patient has or will receive a Flex-Neck® Infant
Catheter.
• The patient is not a candidate for delayed onset of PD
treatment.
Px Only: CAUTION: Federal (USA) law restricts this device
to sale by or on the order of a physician.
PRECAUTIONS
• Read manufacturer’s instructions prior to use.
• Contents are sterile (via ethylene oxide). Do not use if
packaging is opened, damaged, or broken.
• For single patient use only. Do not reuse, reprocess, or
resterilize. Reuse, reprocessing, or resterilization may
compromise the structural integrity of the device and/
or lead to device failure, which in turn may result in
patient injury, illness, or death. Reuse, reprocessing, or
resterilization may also create a risk of contamination
of the device and/or cause patient infection or crossinfection, including, but not limited to, the transmission
of infectious disease(s) from one patient to another.
Contamination of the device may lead to injury, illness,
or death of the patient.
• Do not use after expiration date.
• The medical techniques, procedures and potential
complications stated herein do NOT give full and/
or complete coverage or descriptions. They are not a
substitute for adequate training and sound medical
judgment by a physician.
• Use an aseptic procedure to open the package and to
remove the contents.
• This package contains small parts, assure all parts are
placed on sterile area, prior to performing procedure.
POTENTIAL COMPLICATIONS
Peritoneal dialysis potentially has a number of
complications that may occur, which generally are not
caused by the implantation, but may aect the quality
of therapy. These complications may include, but are not
limited to the following:
• Infections (exit-site or tunnel)
• Peritonitis
• Sepsis
• Bowel perforation
• Leakage (initial or latent)
• Fluid ow obstruction (inow or outow)
• Bleeding (subcutaneous or peritoneal)
• Ileus
• Proximal exit cu erosion
• Distal (rectus/deep) cu erosion
• Risks normally associated with peritoneoscopic and
laparoscopic procedures.
CAUTIONS
• Catheter tubing can tear when subjected to repeated
clamping, serrated-jaw forceps, excessive force,
or rough tools.
• Do NOT use forceps with a serrated jaw.
• Do NOT use excessive force to lock the forceps closed.
• Use ONLY smooth-jawed forceps or equivalent.
• Do NOT clamp the catheter repeatedly in the same area.
Page 3

EMBEDDING INSTRUCTIONS
STEP 1: PREPARATION
1. During the PD catheter implantation procedure, implant
and tunnel the catheter according to hospital protocol
or product Instructions for Use, with the supercial cu
3.0 cm from the future exit-site (primary exit-site). The
exit-site incision should be about 1.5 cm long (equal to
three catheter widths).
2. Mark the desired track of the portion of the catheter
to be embedded. The embedding track can be made
wherever there is suitable space. The path of the
subcutaneous embedding track should be straight or
curvilinear in order to facilitate subsequent catheter
retrieval.
NOTE:
A. If using a Flex-Neck Classic or ARC™ catheter, or an
ExxTended catheter with an upper abdomen exit-site,
the shape of the embedding track should form a gentle
curvilinear turn back toward the midline.
B. If using a Flex-Neck ExxTended
Catheter with an upper chest
exit-site, a straight subcutaneous
embedding track down the torso
may be used.
3. Determine that the external length
of the catheter to be embedded
ts the patient’s anatomy. Trim
the external catheter length, if
necessary.
NOTE: If the external part of the
catheter is trimmed, make sure that
the cut is perpendicular to the catheter tube, and that the
face of the cut is smooth.
5. Disassemble the titanium Plug from the Cap. Do NOT
discard the Cap.
6. Thread the Plug onto the plastic Handle. Be careful not
to cross-thread the Plug and Handle threads.
STEP 2: EMBEDDING
1. Lay the external (subcutaneous) part of the catheter
to be embedded on the patient’s abdomen to assure
proper placement.
2. Mark a location on the patient’s abdomen to indicate the
end of the embedding track which also will become the
temporary secondary exit-site.
NOTE: Allow for the extra length of the Plug and Cap. This
is typically 1.0 – 2.0 cm.
3. Make an incision, approximately 0.5 – 0.7 cm, with a #11
blade at the secondary exit-site.
4. Insert the tip of the Handle, with the Plug attached,
through the secondary exit-site and through the middle
of the subcutaneous tissue toward the primary exit-site.
5. Advance the Handle until the entire titanium Plug is
visible.
6. Dry the Plug thoroughly.
7. Infuse heparin or equivalent into the catheter, as per
standard protocol.
8. Insert the Plug into the catheter so that the end of the
catheter is next to (touching) the shoulder of the Plug.
NOTE:
• Do NOT detach or separate the Plug from the Handle.
• A correctly positioned catheter and Plug should form a
relatively smooth and continuous surface from catheter
to Plug.
9. Secure the catheter to the Plug with a permanent, nonabsorbable suture.
10. Retract the Handle and catheter through the
subcutaneous tissue until the Plug is visible at (external
to) the secondary exit-site.
CAUTION: Do NOT twist or kink the catheter.
11. Hold the catheter and Plug stationary.
12. Separate the Handle and the Plug by rotating the
Handle counter-clockwise.
CAUTION: Do NOT twist the catheter or the Plug.
13. Verify that the catheter is not twisted by making sure
that the radiopaque stripe is consistently positioned
from the subcutaneous cu to the end where the plug is
inserted.
14. Thread the titanium Cap onto the Plug and tighten
rmly. There should be no gaps between the Cap and
Plug when completely fastened.
15. Insert the Plug, with the Cap attached, and catheter into
the subcutaneous tissue.
NOTE:
• If necessary, use forceps to push the Plug into the
subcutaneous tissue.
• If necessary, use a second set of forceps at the primary
exit-site to retract the catheter into the subcutaneous
tissue.
• If the catheter (with Plug attached) seems to be too
close to the secondary exit-site, enlarge the secondary
exit-site incision and position the Plug deeper into the
subcutaneous tissue, or trim o additional length of the
catheter and reinsert the Plug.
CAUTION:
• Do NOT retract the catheter more than 2.0 cm back
through the primary exit-site.
• Do NOT dislodge or reposition the supercial
subcutaneous cu.
16. Carefully verify that:
• The catheter is not kinked or twisted.
• The subcutaneous cu is in the proper location relative
to the future primary exit-site.
• The embedding track forms a straight or curvilinear
path that will facilitate pulling the catheter from the
subcutaneous path during the retrieval procedure.
17. Close the original catheter insertion incision, as well
as the primary and secondary exit-site incisions, as per
normal hospital protocol.
Copyright © Merit Medical Systems, Inc. All rights reserved.
Page 4

Manufacturer:
Merit Medical Systems, Inc.
1600 West Merit Parkway, South Jordan, Utah 84095 U.S.A.
1-801-253-1600 U.S.A. Customer Service 1-800-356-3748
Authorized Representative:
Merit Medical Ireland Ltd,
Parkmore Business Park West, Galway, Ireland
www.merit.com
ID 060413 402893001/A
 Loading...
Loading...