Page 1

AtmoCONTROL
FDA Edition Validatable in according to 21CFR
Part 11 U. S. Food and Drug Administration
Version 2.X.X
SOFTWARE MANUAL
100% ATMOSAFE. MADE IN GERMANY.
www.memmert.com | www.atmosafe.net
Page 2

Willi-Memmert-Straße 90–96
We reserve the right to make changes
Page 3

AtmoCONTROL FDA Version
About this manual
target group
This user manual describes the installation and use of the MEMMERT programming software
AtmoCONTROL. It is intended for use by trained personnel of the operator, who have the task
to the appliance. Incorrect use could result in damage to the appliance and/or to the chamber
with AtmoCONTROL and familiarise yourself with it.
This manual should always be kept in a place where those working with the software have
will work with the software are informed as to the whereabouts of this user manual. We rec-
The current version of AtmoCONTROL and this manual are available for download at
Page 4

4 D30396 | Date 09/2014
AtmoCONTROL FDA Version
...........................................................................................................................
.........................................................................................................
..................................................................................................
4. Working with AtmoCONTROL 10
4.1 Starting AtmoCONTROL
....................................................................................................
4.2 Programme interface
.........................................................................................................
4.2.1 Menu bar
.........................................................................................................................
4.2.2 Toolbar
.............................................................................................................................
4.2.3 Status bar
........................................................................................................................
4.3 Installing the device licence
...............................................................................................
4.3.1 Using a USB storage medium (only possible for PLUS appliances)
................................
4.3.2 Via Ethernet with AtmoCONTROL
..................................................................................
4.4 Adding and disconnecting devices
...................................................................................
4.4.1 Adding device connected via Ethernet
...........................................................................
4.4.2 Connecting device using USB storage
............................................................................
4.4.3 Connecting a device using database file
........................................................................
4.4.4 Log file
.............................................................................................................................
4.4.5 Displaying device and licence information
.....................................................................
4.4.6 Disconnecting devices
.....................................................................................................
...................................................................................................
..........................................................................................................
.............................................
...................................................................................................
..........................................................................................
.............................................................................
.....................................................
..................................................
........................................................................................................
............................................................................
.........................................................................
...................................................................................
..............................................................................................
Page 5

AtmoCONTROL FDA Version
...........................................................................................................................
..............................................................................................................................
......................................................................................................................
.........................................................
.............................................................................
..........................................................................................................
..........................................................
....................................................................................................
Page 6

AtmoCONTROL FDA Version
This end user licence agreement forms a legally binding agreement between you, whether a
This licence agreement bindingly regulates the use of software licences of MEMMERT GmbH
your legally binding agreement that you accept the conditions of this licence agreement and
The software is protected by copyright laws, international copyright agreements, as well as by
The software product is licenced, not sold. All property and user rights remain with MEMMERT
The software product is licenced as follows:
As customer you must use the software only for the MEMMERT equipment entered in the
You are not entitled to hire out, lease, loan or otherwise dispose of the software product. You
technological development. The user hereby notes this situation.
All warranty lapses if the user modifies programmes or programme parts or has such changes
from incorrect operation, use of unsuitable operating material, or unusual operating condi-
tions.
Page 7

AtmoCONTROL FDA Version
The user must employ the SOFTWARE PRODUCT only for his own purpose unless expressly
The user must notify MEMMERT GmbH & Co.KG without delay of any faults which occur,
together with a brief description of the fault.
the SOFTWARE PRODUCT is supplied, is free from material faults and manufacturing faults.
The software has been carefully developed and tested by MEMMERT GmbH & Co.KG. There
the use of this software product or any inability to use this software product.
This applies also in case MEMMERT GmbH & Co.KG has been advised of the possibility of such
This exclusion does not apply to damage which has been wilfully caused by MEMMERT GmbH
from such contravention.
The agreement is concluded exclusively on the basis of the General Utilization Conditions of
All legal relations between the parties, including delict law, are subject to the law of the Ger-
Page 8
AtmoCONTROL FDA Version
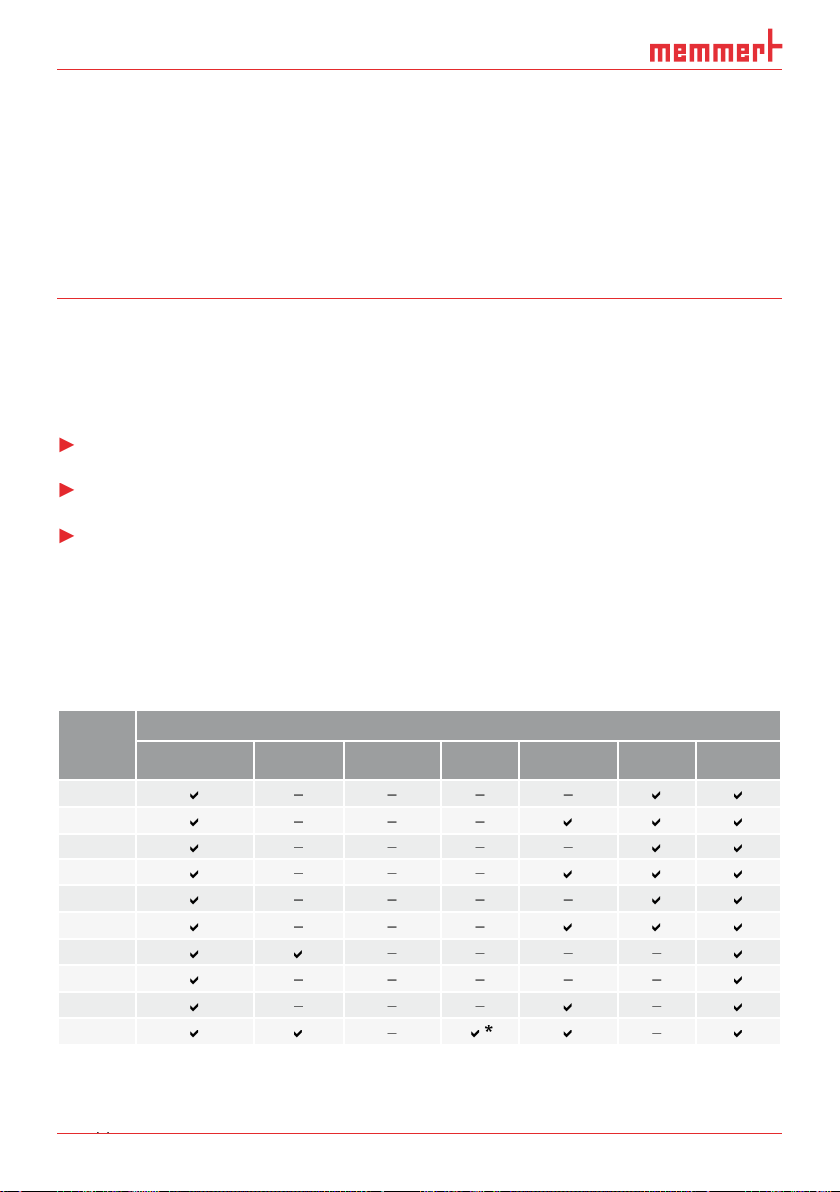
AtmoCONTROL is a software for programming and logging MEMMERT appliances of the
With AtmoCONTROL, you can
Appli-
the appliance itself, and FDA functions are not available.
the appliance itself, and FDA functions are not available.
ance
Temperature Humidity Pressure CO
Programmable main parameter
Fan speed Air fl ap Light*
2
Page 9

AtmoCONTROL FDA Version
Advanced functions of the AtmoCONTROL FDA version
Within a closed system, this specially developed software meets the requirements of the Food
tion processes in the food, chemical and pharmaceutical industry:
►
trail
The regulation 21 CFR (Code of Federal Regulation) part 11 came into force in 1997. It
tion and long-term archiving of the process variables.
The regulation applies to all production and quality assurance documents that had to be pro-
vided signed in paper form before. These documents may as of now be created, stored and
They are stored in:
Audit trails
The FDA compliant software AtmoCONTROL FDA edition fulfils the requirements for the use of
the US Food and Drug Administration (FDA):
Authenticity: The users and administrators of electronic records must be uniquely identifi-
Automatic audit-trailing with timestamp, signature and type of modification to the
Access option to data for the inspectors of regulatory authorities
►
►
►
►
►
►
►
►
►
Page 10
D30396 | Date 09/2014
AtmoCONTROL FDA Version
Category Minimum system requirements
Available free space on
4 GB
VGA graphics and colour monitor
Windows 7, Windows 8
You must have Windows administrator rights to be able to install the AtmoCONTROL FDA
the installation process step by step.
their rights later on (see page 33).
Working with AtmoCONTROL
Which of the following functions are available for you depends on the user rights the
AtmoCONTROL administrator has given you (see page 33).
AtmoCONTROL can be started in two ways:
AtmoCONTROL)
Page 11

AtmoCONTROL FDA Version
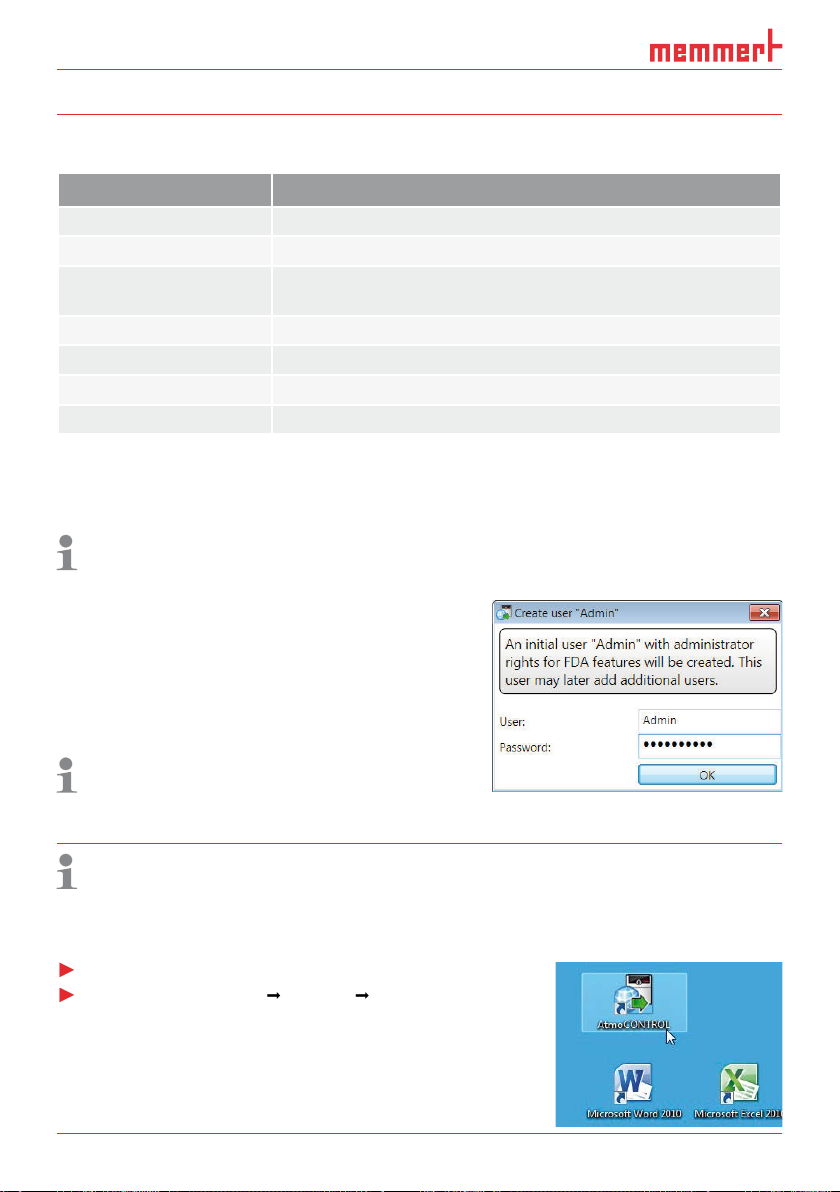
When starting AtmoCONTROL FDA, you have to login as
AtmoCONTROL user with your user name and password. Users
you start AtmoCONTROL FDA for the first time, you are asked
whether these data should be encrypted. This is required for compliance with the FDA require-
The
1
4
Menu bar (see section 4.2.1)
3
Status bar (provides an overview of available appliances, see page 13)
Show/hide status bar
Status bar (provides an overview of available appliances, see page 13)
Status bar (provides an overview of available appliances, see page 13)
5
Editor, simulation and protocol window (only for appliances listed on page 8,
otherwise only protocol window)
Editor, simulation and protocol window (only for appliances listed on page 8,
Editor, simulation and protocol window (only for appliances listed on page 8,
Programming mode switch (for editor/simulation/protocol, see pages 24 and 30)
otherwise only protocol window)
otherwise only protocol window)
You may change the
2
3
6
5
Page 12
D30396 | Date 09/2014
AtmoCONTROL FDA Version
Device Program Protocol Print Options Security Help
Connect to device via
Ethernet (see page 13)
2
protocol data on USB
storage medium (see
protocol data on USB
protocol data on USB
page 14)
3
database file (see page
4
Disconnect selected de-
vice (see page 15)
5
Disconnect all devices
vice (see page 15)
vice (see page 15)
Load a saved programme
8
Save programme
9
Save programme under a
Save programme
Save programme
new name
device via Ethernet (see
page 25)
Export programme to
page 25)
Import protocol from
page 25)
page 25)
page 30)
Export protocol data (see
page 30)
page 30)
page 31)
Print (see page 31)
guage (German/English)
page 31)
Automatic sending of
page 31)
page 31)
emails (see page 32)
Automatic sending of
Automatic sending of
Edit backup folder (see
emails (see page 32)
emails (see page 32)
page 33)
Sign a programme or
protocol electronically
Sign a programme or
Sign a programme or
34)
20
Edit user rights (FDA func-
tion, see page 33)
21
34)
22
tion, see page 34)
23
Display audit trail (FDA
tion, see page 34)
tion, see page 34)
function, see page 35)
Display audit trail (FDA
Display audit trail (FDA
24
Lock AtmoCONTROL
function, see page 35)
function, see page 35)
35)
25
Programme information
26
format
27
device (see page 13)
4.2.2
Toolbar
The toolbar provides rapid access to the most important menu functions:
programme
2
Load programme
programme
programme
from the data
Load programme
Load programme
medium
3
Save new
programme
4
Enlarge view
zoom in)
5
Reduce view
Show full
programme
Connect online via Ethernet
1
Connect offline from USB device
2
Connect offline from database
3
Disconnect device
4
Disconnect all devices
5
New
6
Load
7
Save
8
Save As...
9
Upload to Device
10
Export to USB drive
11
12
13
Import...
Export...
15
Print
14
Language
USER-ID
16
Email options
17
Edit Backup Folder
18
Sign document
19
Edit users
20
Change password
21
Change user
22
Audit trail
23
Lock AtmoCONTROL
24
25
26
27
About...
User Manual
Upload license file to device
1 2 3 4 5 6
Page 13

AtmoCONTROL FDA Version
4.2.3
The status bar gives an overview of the appliances logged on to AtmoCONTROL. Appliances
1 2
Fig. 1
An appliance of type UF 260
(1), named "Laboratory" (2) by the user, is registered via Ether-
net (3) in AtmoCONTROL; current temperature 180.0 °C (4)
Installing the
With AtmoCONTROL FDA, only Memmert appliances with the corresponding electronic licence
There are two methods of installing a licence:
4.3.1
Switch off the respective appliance.
UF 260plus Laboratory
180.0°C
3 4
i
Page 14

D30396 | Date 09/2014
AtmoCONTROL FDA Version
4.3.2
Via Ethernet with AtmoCONTROL
transfer the licence to.
A description of how to set the IP address is
4.
tered) in AtmoCONTROL as described in the following section.
Adding and disconnecting devices
With the AtmoCONTROL FDA edition, only Memmert appliances can be registered which
Adding device connected via Ethernet
vices have at delivery (192.168.100.100). The IP address
A description of how to set the IP address is
When connecting, all data are automatically updated.
Connecting device using USB storage
A description of how to read protocol data on a device is provided in the user
Page 15

AtmoCONTROL FDA Version
4.4.3
Connecting a device using
A window opens, in which you can open the
type.
Log fi le
file Log.txt is also transferred and saved in a sub-
folder of the directory
The log file is structured as shown in the
A
– End of the event
Alarm / event code
Alarm / event description
A detailed list of all event codes is given
from page 34.
4.4.5
When a device has been added, you can
time. To do this, click on the
A window is opened displaying detailed
4.4.6
Disconnecting devices
the status bar, select it and then click on
A B C D
Page 16

D30396 | Date 09/2014
AtmoCONTROL FDA Version
temperature, pressure and humidity), which the appliance then implements from a definable
To be able to create a programme in AtmoCONTROL, the appliance which is to perform the
Two editor threads are always shown for appliances with humidity or pressure control,
want to see the parameter values for a specific point in time, you must change to the simula-
tion mode (see page 24).
Fig. 2
Elements to create a programme
Appliance selected
Elements to create a programme
Elements to create a programme
Available parameters (functions)
Appliance selected
Appliance selected
3
Editor thread
Available parameters (functions)
Available parameters (functions)
Additional editor thread for appliances with humidity or pressure control
2
1
3
4
Page 17

AtmoCONTROL FDA Version
To create a programme, drag the individual parameter icons onto the editor thread one after
the zoom icons in the toolbar list (see section 4.2.2 on page 12) or with the mouse wheel,
you can zoom in or out of the display or have the entire programme displayed.
Ramp 05
Fig. 3
Drag the parameter icon (in this case a tem-
perature change) onto the editor thread while
holding the mouse button down
Fig. 4
Drag other parameters – light and a humidity
change in this case – onto the editor thread.
A red insertion mark helps you to find the
correct position.
The temperature icons (change/hold temperature) may only be placed on the upper editor
thread, humidity and pressure icons only on the lower one.
The meaning of the individual icons and the adjustment options are described from page
Removing a parameter icon from the editor thread
Removing a parameter icon from the editor thread
To remove a parameter
function) from an edi-
tor thread – if you have
with the mouse button
Fig. 5
with the mouse button depressed, to the recycle bin symbol.
10h:00m
11h:30m
Ramp 05
10h:00 m
11h:30 m
LIGHT
21.0 °C 180.0°C
180.0°C
21.0 °C 180.0°C
Increase
40.7%rh 70.6%rh
180.0°C
2h:30 m
0%
Page 18

D30396 | Date 09/2014
AtmoCONTROL FDA Version
To adjust values, click successively on the corresponding fields – in
the example on the right, the setpoint temperature. The value is
The adjustment range depends on the appliance for which the
The main parameters have additional adjustment options, which
fold down icon (Fig. 6, No. 1).
tolerance
Fig. 6
Further adjustment options fold down after clicking on the arrow icon on the right
edge of the window (1)
Fig. 6
Fig. 6
Further adjustment options fold down after clicking on the arrow icon on the right
Further adjustment options fold down after clicking on the arrow icon on the right
Available
Which parameters are available to adjust the programme depends on the appliance for
which a programme is to be created. Only those parameters are available that the appli-
- - h:- - m
- - - .- °C21.0 °C
Ramp 01
21.0 °C
2
Tolerance band
1
0,1 K/min
11h:30m
37.0°C
Tolerance band
0,1 K/min
3
Page 19

AtmoCONTROL FDA Version
Broad
parameter representation
parameter representation
Depiction in
?
temper-
Function
Adjustment options
Adjustment options
)
Temperature to be maintained
Tolerance value above/below
Alarm if limits are exceeded
?
temper-
Function
Adjustment options
Adjustment options
Target (setpoint) temperature
Tolerance value above/below
3
?
15
Function
Adjustment options
Adjustment options
)
tained
Tolerance value above/below
Alarm if limits are exceeded
When run, this is displayed in the
When
3
value is reached, even if the set time has already expired. If this is "off", the programme sequence is
icon bar
Meaning
Depiction on
editor thread
Tolerance band
Tolerance band
Function and
adjustment options
0,1 K/min
70.0%rh
Tolerance band
+
0.0%rh
-
5.0%rh
on off
Alarm
Safe
Hold
12h:00 m
Page 20

D30396 | Date 09/2014
AtmoCONTROL FDA Version
Depiction in
?
15
Function
value.
Adjustment options
Adjustment options
Target (setpoint) humidity
Tolerance value above/below
3
?
15
STAND BY
Function
Adjustment options
Adjustment options
)
Tolerance value above/below
Alarm if limits are exceeded
?
15
STAND BY
Function
Adjustment options
Adjustment options
Target (setpoint) pressure
Tolerance value above/below
3
When run, this is displayed in the
When
3
value is reached, even if the set time has already expired. If this is "off", the programme sequence is
icon bar
Meaning
Depiction on
editor thread
Function and
adjustment options
2h:30 m
40.7%rh 70.6%rh
hold p
decrease
1100mb
2h:30 m
500mb
2h:30 m
500mb
Tolerance bandIncrease
+
5.0%rh
-
5.0%rh
on off
SPWT
Tolerance band
+
20mb
-
10mb
on off
Alarm
Safe
Tolerance band
+
20mb
-
10mb
on off
SPWT
Page 21

AtmoCONTROL FDA Version
Narrow
parameter representation
parameter representation
With the narrow
tive position – and remains active until it is changed by the insertion of a new parameter icon
Depiction
?
15
STAND BY
?
15
STAND BY
15
STAND BY
15
STAND BY
Air flap
15
STAND BY
15
STAND BY
15
STAND BY
Adjustment options: none
Appliance emits an acoustic signal at the
was inserted, for example if a specific setpoint
value is reached or the programme is finished.
in icon bar
Meaning Depiction on
editor thread
CO
2
50%
15
0
O2
CO2
12.0%
FAN
100%
FLAP
100%
LIGHT
Adjustment options/
comments
100%
UV LIGHT
on off
HORN
Page 22

D30396 | Date 09/2014
AtmoCONTROL FDA Version
Depiction
15
STAND BY
Adjustment options: open/close
15
STAND BY
15
STAND BY
Activates the defrosting function of the appli-
15
STAND BY
The programme is repeated each week at the
STAND BY
CALENDAR
Year
30
05
2012
STAND BY
Synchronis-
finished on both editor threads.
finished.
in icon bar
15
Meaning Depiction on
editor thread
DOOR
openclose
SWITCH
A
openclose
DEFROST
CLOCK TIMER
Mo
Tu
We
Th
Fr
Sa
Su
11h:30 m
CALENDAR
Day
18
05
Month
Year
2012
- - - -h: m
22h:30 m
Adjustment options/
comments
SYNC
and or
Page 23

AtmoCONTROL FDA Version
Depiction
STAND BY
The programme jumps back from the insertion
the range that is to be repeated.
... ...
in icon bar
Meaning Depiction on
editor thread
Adjustment options/
comments
STAND BY
JUMP
TARGET
STANDBY
LOOP
JUMP
4 X
TARGET
JUMP
TARGET
LOOP
LOOP
2 X
4 X
Page 24

D30396 | Date 09/2014
AtmoCONTROL FDA Version
While creating the programme, you can display the prospective progression of all parameters
Fig. 7
Programme preview diagram (simulation)
you want to alter the programme.
Saving, loading, transferring and running the programme
The name with which you save the programme is later
Via Program
the saved programmes (*.atpro).
Page 25

AtmoCONTROL FDA Version
Transferring programme via Ethernet
To be able to transfer a programme via Ethernet, the appliance and computer must be
Transferring a programme via USB storage medium
With appliances that have humidity control, make sure that the
water supply tank of the
vals, especially for programmes that run for long periods. The same applies for appliances
with gas supply.
Page 26

D30396 | Date 09/2014
AtmoCONTROL FDA Version
5 841
Fig. 8
maintain this temperature (infinitely
) (4) until it is changed: also Monday to Friday (5) at 6
pm (6) to 50 °C (7) – again continued (infinitely
maintain this temperature (infinitely
maintain this temperature (infinitely
) (4) until it is changed: also Monday to Friday (5) at 6
) (4) until it is changed: also Monday to Friday (5) at 6
) (8) until it is changed again in the morning
at 8 am (2).
120 °C
50 °C
20 °C
2 3
6 7
347
8
Mo. Tu. We. Th. Fr. Sa. Su.
1
Page 27
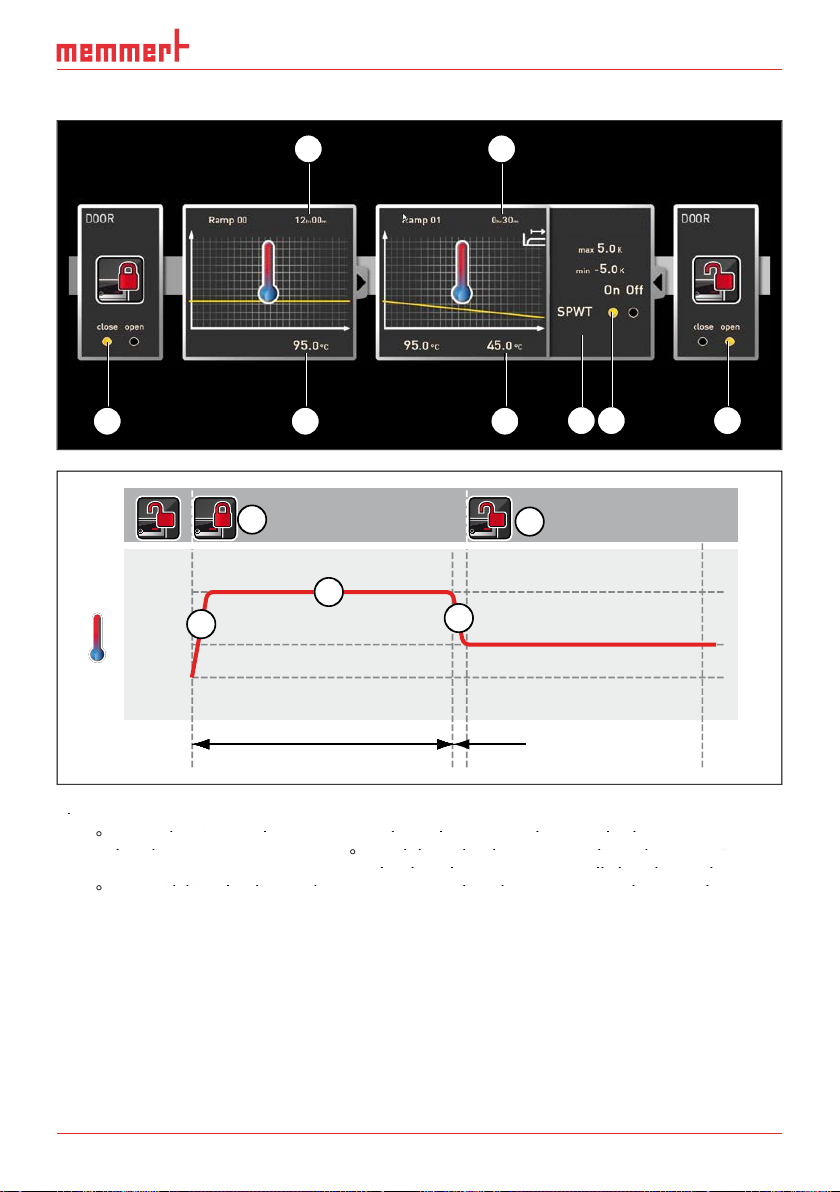
AtmoCONTROL FDA Version
3 4
Fig. 9
95.0
lowered (5) for 30 minutes (4) to 45.0
SPWT
on" (8) ensures that the door is opened only when the temperature really has dropped to
45.0
K/min (7).
1 2 5
Tolerance band
1,7 K/min
8 6
7
95 °C
45 °C
20 °C
1
6
3
2
5
12:00 h ≥ 0:30 h
Page 28

D30396 | Date 09/2014
AtmoCONTROL FDA Version
4
Fig. 10
At the beginning, the fan is switched on to 100 % (1) and the air flap is closed (0 %) (2). Then,
Fig. 10
Fig. 10
the appliance heats up to 180.0
At the beginning, the fan is switched on to 100 % (1) and the air flap is closed (0 %) (2). Then,
At the beginning, the fan is switched on to 100 % (1) and the air flap is closed (0 %) (2). Then,
ting „Safe“ (5) ensures that the sterilisation time does not start (6) before the set tolerance band
the appliance heats up to 180.0
the appliance heats up to 180.0
6
Tolerance band
1 2 3
5
1
0 % 100 % 0 %
2
100 % 0 %
6
180 °C
4
3
20 °C
3:00 h
7
Page 29

AtmoCONTROL FDA Version
9
41
Unload
Fig. 11
First, the appliance heats up to 250.0 °C (2) for one hour (1). Then, the fan begins to run at 80
Fig. 11
Fig. 11
fan is switched off (6). This sequence is repeated from the jump target (7) ten times (8).
250 °C
20 °C
27 3 5
6
0% 80% 0% 80% 0% 80% 0% 80% 0% 80% 0% 80% 0% 80% 0% 80% 0% 80% 0% 80% 0% 80% 0%
235
1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h
1 4
8
6 8
Page 30

D30396 | Date 09/2014
AtmoCONTROL FDA Version
To be able to import a protocol via network, the appliance and computer must be con-
Fig. 12
Protocol display (example)
Importing protocol from USB data medium
At the appliance, protocols can be exported to an USB storage medium and imported in
AtmoCONTROL:
the user manual for the appliance.
TROL.
files on an USB storage medium (see page 14).
Page 31

AtmoCONTROL FDA Version
With Protocol
types *.csv or *.xlsx (Excel), which can be processed in
With the "Print" function, you can print out programmes
You can set the language of the user
With the appliances listed in the table on page 8, it
AtmoCONTROL cannot generate a USER-ID file,
figuration in AtmoCONTROL is also not possible.
There can be only one USER-ID on a USER-ID USB stick.
The settings in this file then apply for all appliances configured.
A USER-ID identifier on a USER-ID USB stick for one (or several) serial numbers can be pur-
Fig. 13
Exporting protocols
Fig. 14
User-ID
Page 32

D30396 | Date 09/2014
AtmoCONTROL FDA Version
with the USER-ID file into the
A window appears with the functions of the logged on appliance
that can be blocked (depending on the appliance type).
4.
Sending
AtmoCONTROL can automatically send an email to a freely definable recipient if an alarm
was triggered, e.g. if the temperature is exceeded. To make the corresponding settings, select
Fig. 15
Settings for the automatic sending of emails in case of alarm
events
Page 33

AtmoCONTROL FDA Version
You can set up a backup folder in which
AtmoCONTROL saves backup copies of
You can either use the default folder or
FDA functions (menu item “Security”)
Adding and deleting users and manage user rights
The FDA version of AtmoCONTROL comprises an own user management, which is independ-
The user management in AtmoCONTROL is accessed via the menu item “Security”
User rights
Fig. 16
Scroll to additional
user rights
password
Add new userChange user
Delete selected
user
Close User
Management
Page 34

D30396 | Date 09/2014
AtmoCONTROL FDA Version
The user management can only be accessed by users the administrator has assigned the
Changing the user password and user
Via the menu item “Security”
Via the menu item “Security”
Signing a document
With the function “Security”
A signature once given cannot be changed or removed
The user rights assigned by the administrator define the
type of signature a user is allowed to provide. The
A user can also sign with several roles, however only with
the corresponding rights. A role can in turn also be signed
Page 35

AtmoCONTROL FDA Version
Traceability by means of a protected audit trail fi le
All actions performed in AtmoCONTROL FDA are
The time span to be displayed can be defined
The audit trail function can be accessed via the
All important functions and activities are saved in
the audit trail. These include:
Adding and removing users
Actions in AtmoCONTROL which may only be performed with the corresponding rights
Actions which are executed via the operating system, as for example deleting files, if the
Event name Description
„Fehler“
„Error“
An error occurred during an action. Examples of this are: Corrupt data; at-
tempt to overwrite a file which has more signatures than the current one.
The action was not performed.
„Info“
„Audit OK“
„Audit fail“
All actions requiring a password or authorisation check.
„Warnung“
„Warning“
The message itself is not critical but may be evidence of an operating er-
To prevent unauthorised use of AtmoCONTROL, for example in case of absence from the
workplace, choose “Security”
Page 36

D30396 | Date 09/2014
AtmoCONTROL FDA Version
Error codes for Generaon 2012 appliances Status: October 09, 2012
Error code
Parameter
Description
101: OS Error
Operang system error
102: File System Error
File system error
103: USB Error
USB error
104: GUI Error
Graphical user interface error
105: IP Stack Error
Ethernet error
106: I2C Bus Error
12C bus error
107: RTC Error
Realme clock error
108: RAM Disk Error
RAM disk error
109: WatchDog Reset
Watchdog error
Power Supply OK
110: Power Supply OK
111: Controller Restart
Restart aer reset
201: Config Error
Configuraon error
202: Calib User Error
Error in calibraon file (user)
203: Calib System Error
Error in calibraon file (calibraon system)
204: PID Config Error
Error in PID parameters
205: User Config Error
Error in user sengs
206: Baery Error
Baery low
207: SD Card Space
Warning at 95% disk space usage
208: SD Card Full
Error, SD card is full
209: SD Card Missing
SD card is missing
210: Failed To Copy Protocol
Error copying the protocol
211: Restauraon Failed
Error restoring LastState
212: Max Count Of Profiles
Maximum number of profiles on SD card reached
301: Fan Error
Fan speed error
302: Heang Error
Heang error
303: Temp Limitor
Temperature limiter has triggered
304: Door open
Door is opened
305: Hzg Err
200000
Triac optocoupler heang 1 power module 1
defecve
020000
Triac optocoupler heang 2 power module 1
defecve
Note:
002000
Triac optocoupler heang 1 power module 2
defecve
000200
Triac optocoupler heang 2 power module 2
defecve
000020
Triac optocoupler heang 1 power module 3
defecve
000002
Triac optocoupler heang 2 power module 3
defecve
100000
Triac heating 1 power module 1 defecve
010000
Triac heang 2 power module 1 defecve
001000
Triac heang 1 power module 2 defecve
000100
Triac heang 2 power module 2 defecve
In case of a hardware error of the oven, the controller displays the following error codes in a status /
error window, as well as logs them in the “Log.txt” file in the “Config” directory on the SD card.
The posion of the error number further specifies the posion of the error.
The posion of the red digit
indicates the defecve stage
of the appliance.
Page 37

AtmoCONTROL FDA Version
000010
Triac heang 1 power module 3 defecve
000001
Triac heang 2 power module 3 defecve
306: Comm Err
1000
Power module 1 is not responding
0100
Power module 2 is not responding
0010
Power module 3 is not responding
0001
Humidity power module is not responding
401: Humidity Sensor
Humidity sensor defecve
402: Humidity Min Al
Humidity below minimum value
403: Humidity Max Al
Humidity maximum value exceeded
405: Temp Sensor Defunct
Temperature sensor defecve
404: Water tank empty
Water tank empty
406: Sensor Alarm
Monitoring sensor defecve
407: Temp Min Alarm
Temperature below minimum value
408: Temp Max Alarm
Temperature maximum value exceeded
409: Temp Auto Alarm
Temperature tolerance band violated
410: Lights Off
Automac lights switch-off
501: Sensor CO2 Error
CO2 sensor defecve
502: CO2 Empty
CO2 supply interrupted / gas cylinder empty
503: CO2 Auto Switch
Noficaon of gas cylinder change
504: CO2 Min Alarm
CO2 below alarm limit
505: CO2 Max Alarm
CO2 alarm limit exceeded
506: Sensor O2 Error
O2 sensor defecve
507: N2 Empty
N2 supply interrupted / gas cylinder empty
508: O2 Min Alarm
O2 below alarm limit
509: O2 Max Alarm
O2 alarm limit exceeded
601: Vacuum Sensor Error
Pressure sensor defecve
801: Program Start
602: No Shelf
No shelf inserted
603: Vacuum Min Alarm
Pressure below alarm limit
604: Vacuum Max Alarm
Pressure alarm limit exceeded
700: Power Min Border
Voltage below minimum limit
701: Device Fail me
me of power failure
702: Device Start Time
me of restart
Programme start
802: Program Cancelled
Programme cancellaon
803: Program End
Programme end
804: Invalid Program
Programme cannot be loaded
Page 38

D30396 | Date 09/2014
AtmoCONTROL FDA Version
Air flap position 21
,
,
,
,
, , ,
,
Target group 3
Tolerance band 18
Toolbar 12
,
W
Water supply tank 25
Zooming 12
Page 39

Page 40

AtmoCONTROL
D30396 | Date 09/2014
englisch
Memmert GmbH + Co. KG
Willi-Memmert-Straße 90-96 | D-91186 Büchenbach
Tel. +49 9122 925-0 | Fax +49 9122 14585
E-Mail: sales@memmert.com
facebook.com/memmert.family
Die Experten-Plattform: www.atmosafe.net
 Loading...
Loading...