Page 1

Battery
testing guide
▪ Why backup batteries are needed
▪ Battery types
▪ Failure modes
▪ Maintenance philosophies
▪ Practical battery testing
▪ Frequently asked questions
▪ Megger products overview
WWW.MEGGER.COM
Page 2

Page 3

Contents
Why backup batteries are needed ................ 4
Why test battery systems .......................................4
Why batteries fail ................................................... 4
Battery types .................................................. 5
Lead-acid overview ................................................5
Nickel-Cadmium Overview .....................................5
Battery construction and nomenclature ..................... 6
Configurations ....................................................... 6
Single post batteries .................................................... 6
Multiple post batteries ................................................ 6
Failure modes ................................................. 7
Lead-acid (flooded) failure modes ..........................7
Lead-acid (VRLA) failure modes ..............................7
Nickel-Cadmium failure modes ..............................8
Maintenance philosophies ............................ 9
How to maintain the battery ..................................... 9
Standards and common practices ........................... 9
IEEE 450 ...................................................................... 9
Inspections .............................................................. 9
Capacity test (discharge test) should be done ........... 9
IEEE 1188 .................................................................. 10
Inspections ............................................................ 10
Capacity test (capacity test) should be done ........... 10
Battery replacement criteria ................................... 10
IEEE 1106 .................................................................. 10
Inspections ............................................................ 10
Capacity test (discharge test) should be done ......... 10
Summary best way to test and evaluate your
battery ................................................................. 10
Test intervals ............................................................. 10
Practical battery testing .............................. 11
Capacity test ...........................................................11
Battery testing matrix – IEEE recommended
practices .............................................................. 11
Procedure for capacity test of vented lead acid
battery ................................................................. 12
Impedance test ....................................................... 13
Impedance theory ................................................13
Intercell connection resistance ................................... 14
Testing and electrical paths ........................................ 15
Voltage ..................................................................... 15
Specific gravity ......................................................... 15
Float current ............................................................. 16
Ripple current ........................................................... 16
Temperature .............................................................. 16
Data analysis ...........................................................17
Locating ground faults on DC systems without
sectionalizing .......................................................... 18
Overview ............................................................. 18
Current test methods ........................................... 18
A better test method ...........................................18
Frequently asked questions ........................ 19
Battery technology summary .................................. 19
Megger products overview ......................... 20
Impedance test equipment ...................................... 20
®
BITE
3 ................................................................20
®
BITE
2 and BITE®2P .............................................21
ProActiv battery database management software ...... 21
®
accessories ....................................................... 21
BITE
Capacity testing ...................................................... 23
TORKEL 820/840/860 ..........................................23
TORKEL accessories ................................................... 23
Ground fault tracing equipment .............................. 24
Battery Ground Fault Tracer (BGFT).......................24
Battery Ground-fault Locator (BGL) ...................... 24
Digital Low Resistance Ohmmeters (DLRO
Microhmmeters (MOM) ..........................................26
DLRO200 and DLRO600 ......................................26
DLRO 247000 series ............................................26
MJÖLNER 200 and MJÖLNER 600 ........................ 27
MOM200A and MOM600A .................................27
MOM690 ............................................................27
Multimeters ............................................................ 28
MMC850 Multi-conductor AC Digital
Clampmeter ........................................................28
Multimeters ......................................................... 28
Insulation Resistance Test Equipment ....................... 29
MIT400 series insulation resistance testers ............ 29
®
) and
BATTERY TESTING GUIDE
3
Page 4

Why backup
batteries are
needed
Batteries are used to ensure that critical electrical equipment
is always on. There are so many places where batteries are
used – it is nearly impossible to list them all. Some of the
applications for batteries include:
▪ Electric generating stations and substations for protection
and control of switches and relays
▪ Telephone systems to support phone service, especially
emergency services
▪ Industrial applications for protection and control
▪ Back up of computers, especially financial data
and information
▪ “Less critical” business information systems
Without battery back-up hospitals would have to close their
doors until power is restored. But even so, there are patients
on life support systems that require absolute 100% electric
power. For those patients, as it was once said, “failure is not
an option.”
Just look around to see how much electricity we use and
then to see how important batteries have become in our everyday lives. The many blackouts of 2003 around the world
show how critical electrical systems have become to sustain
our basic needs. Batteries are used extensively and without
them many of the services that we take for granted would
fail and cause innumerable problems.
Why test battery systems
There are three main reasons to test battery systems:
battery. Nonetheless, a battery, to work the way it is supposed to work must be maintained properly. A good battery
maintenance program may prevent, or at least, reduce the
costs and damage to critical equipment due to an AC mains
outage.
Even thought there are many applications for batteries,
standby batteries are installed for only two reasons:
▪ To protect and support critical equipment during
an AC outage
▪ To protect revenue streams due to the loss of service
The following discussion about failure modes focuses on
the mechanisms and types of failure and how it is possible
to nd weak cells. Below is a section containing a more
detailed discussion about testing methods and their pros
and cons.
Why batteries fail
In order for us to understand why batteries fail, unfortunately a little bit of chemistry is needed. There are two main
battery chemistries used today – lead-acid and nickel-cadmium. Other chemistries are coming, like lithium, which is
prevalent in portable battery systems, but not stationary, yet.
Volta invented the primary (non-rechargeable) battery in
1800. Planté invented the lead-acid battery in 1859 and
in 1881 Faure rst pasted lead-acid plates. With renements over the decades, it has become a critically important
back-up power source. The renements include improved
alloys, grid designs, jar and cover materials and improved
jar-to-cover and post seals. Arguably, the most revolutionary
development was the valve-regulated development. Many
similar improvements in nickel-cadmium chemistry have
been developed over the years.
▪ To insure the supported equipment is adequately backed-
up
▪ To prevent unexpected failures by tracking the battery’s
health
▪ To forewarn/predict death
And, there are three basic questions that battery users ask:
▪ What is the capacity and the condition of the battery
now?
▪ When will it need to be replaced?
▪ What can be done to improve / not reduce its life?
Batteries are complex chemical mechanisms. They have
numerous components from grids, active material, posts,
jar and cover, etc. – any one of which can fail. As with all
manufacturing processes, no matter how well they are made,
there is still some amount of black art to batteries (and all
chemical processes).
A battery is two dissimilar metallic materials in an electrolyte. In fact, you can put a penny and a nickel in half
of a grapefruit and you now have a battery. Obviously, an
industrial battery is more sophisticated than a grapefruit
4 BATTERY TESTING GUIDE
Page 5

Battery types
There are several main types of battery technologies with
subtypes:
▪ Lead-acid
▶ Flooded (wet): lead-calcium, lead-antimony
▶ Valve Regulated Lead-acid, VRLA (sealed): lead-calcium,
lead-antimony-selenium
▶ Absorbed Glass Matte (AGM)
▶ Gel
▶ Flat plate
▶ Tubular plate
▪ Nickel-cadmium
▶ Flooded
▶ Sealed
▶ Pocket plate
▶ Flat plate
Lead-acid overview
The basic lead-acid chemical reaction in a sulphuric acid
electrolyte, where the sulphate of the acid is part of the
reaction, is:
PbO
+ Pb + 2H2SO4 2PbSO4 + 2H2 + 1⁄2 O
2
The acid is depleted upon discharge and regenerated upon
recharge. Hydrogen and oxygen form during discharge and
oat charging (because oat charging is counteracting selfdischarge). In ooded batteries, they escape and water must
be periodically added. In valve-regulated, lead-acid (sealed)
batteries, the hydrogen and oxygen gases recombine to form
water. Additionally, in VRLA batteries, the acid is immobilized by an absorbed glass matte (AGM) or in a gel. The
matte is much like the bre-glass insulation used in houses.
It traps the hydrogen and oxygen formed during discharge
and allows them to migrate so that they react back to form
water. This is why VRLA never need water added compared
to ooded (wet, vented) lead-acid batteries.
A battery has alternating positive and negative plates separated by
micro-porous rubber in ooded lead-acid, absorbed glass matte
in VRLA, gelled acid in VRLA gel batteries or plastic sheeting in
NiCd. All of the like-polarity plates are welded together and to the
appropriate post. In the case of VRLA cells, some compression of
the plate-matte-plate sandwich is exerted to maintain good contact
between them. There is also a self-resealing, pressure relief valve
(PRV) to vent gases when over-pressurization occurs.
2
Nickel-Cadmium Overview
Nickel-Cadmium chemistry is similar in some respects
to lead-acid in that there are two dissimilar metals in an
electrolyte. The basic reaction in a potassium hydroxide
(alkaline) electrolyte is:
2 NiOOH + Cd +2 H
However, in NiCd batteries the potassium hydroxide (KOH) does
not enter the reaction like sulphuric acid does in lead-acid batteries.
The construction is similar to lead-acid in that there are alternating positive and negative plates submerged in an electrolyte. Rarely
seen, but available, are sealed NiCd batteries.
O Ni(OH)2 + Cd(OH)2
2
BATTERY TESTING GUIDE
5
Page 6

Battery construction and
nomenclature
Now that we know everything there is to know about battery
chemistry, except for Tafel curves, ion diffusion, Randles
equivalent cells, etc., let’s move on to battery construction. A
battery must have several components to work properly: a jar
to hold everything and a cover, electrolyte (sulphuric acid or
potassium hydroxide solution), negative and positive plates,
top connections welding all like-polarity plates together and
then posts that are also connected to the top connections of
the like-polarity plates.
All batteries have one more negative plate than positive plate.
That is because the positive plate is the working plate and if
there isn’t a negative plate on the outside of the last positive
plate, the whole outer side of last positive plate will not have
anything with which to react and create electricity. Hence,
there is always an odd number of plates in a battery, e.g., a
100A33 battery is comprised of 33 plates with 16 positive
plates and 17 negative plates. In this example, each positive
plate is rated at 100 Ah. Multiply 16 by 100 and the capacity
at the 8-hour rate is found, namely, 1600 Ah. Europe uses a
little different calculation than the US standards.
In batteries that have higher capacities, there are frequently
four or six posts. This is to avoid overheating of the current-carrying components of the battery during high current
draws or lengthy discharges. A lead-acid battery is a series of
plates connected to top lead connected to posts. If the top
lead, posts and intercell connectors are not sufciently large
enough to safely carry the electrons, then overheating may oc-
2
cur (i
R heating) and damage the battery or in the worst cases,
damage installed electronics due to smoke or re.
To prevent plates from touching each other and shorting
the battery, there is a separator between each of the plates.
Figure 1 is a diagram of a four-post battery from the top
looking through the cover. It does not show the separators.
Configurations
Batteries come in various congurations themselves. Add to
that the many ways that they can be arranged, the number
of possible congurations is endless. Of course, voltage
plays the biggest part in a battery conguration. Batteries
have multiple posts for higher current draws. The more current needed from a battery, the bigger the connections must
be. That includes posts, intercell connectors and buss bars
and cables.
Single post batteries
Smaller battery systems are usually the simplest battery
systems and are the easiest to maintain. They usually have
single post batteries connected with solid intercell connectors. Frequently, they are quite accessible but because they
are small and can be installed in a cubby hole occasionally,
they may be quite inaccessible for testing and maintenance.
Multiple post batteries
Batteries with multiple posts per polarity start to become
interesting quickly. They are usually larger and frequently are
more critical.
Figure 1 Battery construction diagram
6 BATTERY TESTING GUIDE
Page 7

Failure modes
Lead-acid (flooded) failure
modes
▪ Positive grid corrosion
▪ Sediment (shedding) build-up
▪ Top lead corrosion
▪ Plate sulphation
▪ Hard shorts (paste lumps)
Each battery type has many failure modes, some of which
are more prevalent than others. In ooded lead-acid batteries, the predominant failure modes are listed above. Some
of them manifest themselves with use such as sediment
build-up due to excessive cycling. Others occur naturally
such as positive grid growth (oxidation). It is just a matter
of time before the battery fails. Maintenance and environmental conditions can increase or decrease the risks of
premature battery failure.
Positive grid corrosion is the expected failure mode of
ooded lead-acid batteries. The grids are lead alloys (lead-
calcium, lead-antimony, lead-antimony-selenium) that convert to lead oxide over time. Since the lead oxide is a bigger
crystal than lead metal alloy, the plate grows. The growth
rate has been well characterized and is taken into account
when designing batteries. In many battery data sheets, there
is a specication for clearance at the bottom of the jar to
allow for plate growth in accordance with its rated lifetime,
for example, 20 years.
At the designed end-of-life, the plates will have grown suf-
ciently to pop the tops off of the batteries. But excessive
cycling, temperature and over-charging can also increase the
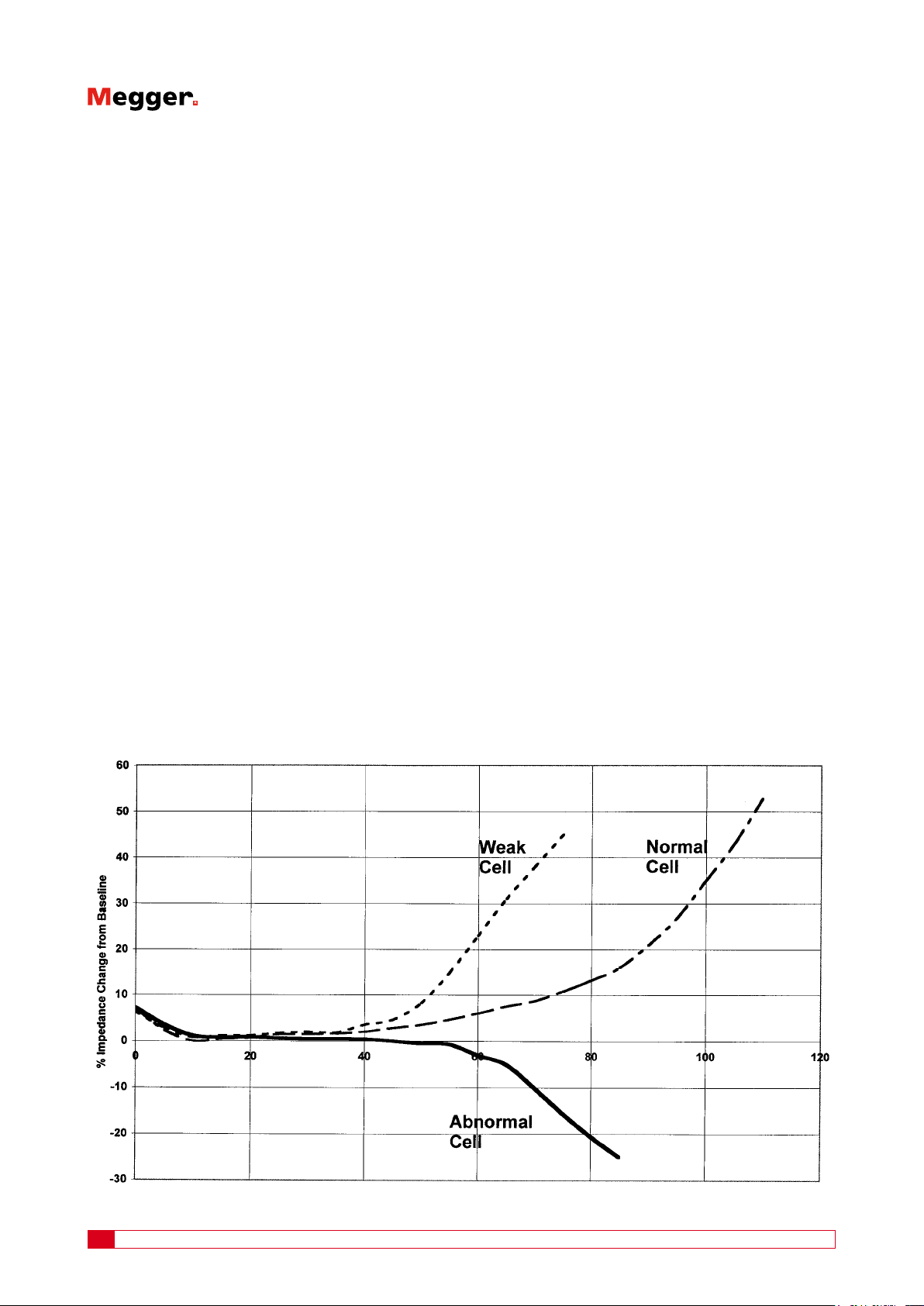
speed of positive grid corrosion. Impedance will increase
over time corresponding to the increase in electrical resistance of the grids to carry the current. Impedance will also
increase as capacity decreases as depicted in the graph in
gure 2.
Sediment build-up (shedding) is a function of the amount
of cycling a battery endures. This is more often seen in UPS
batteries but can be seen elsewhere. Shedding is the sloughing off of active material from the plates, converting to
white lead sulphate. Sediment build-up is the second reason
battery manufacturers have space at the bottom of the jars
to allow for a certain amount of sediment before it buildsup to the point of shorting across the bottom of the plates
rendering the battery useless. The oat voltage will drop and
the amount of the voltage drop depends upon how hard the
short is. Shedding, in reasonable amounts, is normal.
Some battery designs have wrapped plates such that the
sediment is held against the plate and is not allowed to drop
to the bottom. Therefore, sediment does not build-up in
wrapped plate designs. The most common application of
wrapped plates is UPS batteries.
Corrosion of the top lead, which is the connection between
the plates and the posts is hard to detect even with a visual
inspection since it occurs near the top of the battery and is
hidden by the cover. The battery will surely fail due to the
high current draw when the AC mains drop off. The heat
build-up when discharging will most likely melt then crack
open and then the entire string drops off-line, resulting in a
catastrophic failure.
Plate sulphation is an electrical path problem. A thorough
visual inspection can sometimes nd traces of plate sulphation. Sulphation is the process of converting active plate
material to inactive white lead sulphate. Sulphation is due
to low charger voltage settings or incomplete recharge after
an outage. Sulphates form when the voltage is not set high
enough. Sulphation will lead to higher impedance and a
lower capacity.
Lead-acid (VRLA) failure modes
▪ Dry-out (Loss-of-Compression)
▪ Plate Sulphation (see above)
▪ Soft and Hard Shorts
▪ Post leakage
▪ Thermal run-away
▪ Positive grid corrosion (see above)
Dry-out is a phenomenon that occurs due to excessive heat
(lack of proper ventilation), over charging, which can cause
elevated internal temperatures, high ambient (room) temperatures, etc. At elevated internal temperatures, the sealed
cells will vent through the PRV. When sufcient electrolyte
is vented, the glass matte no longer is in contact with the
plates, thus increasing the internal impedance and reducing
battery capacity. In some cases, the PRV can be removed
and distilled water added (but only in worst case scenarios
and by an authorized service company since removing the
PRV may void the warranty). This failure mode is easily
detected by impedance and is one of the more common
failure modes of VRLA batteries.
Soft (a.k.a. dendritic shorts) and Hard shorts occur for a
number of reasons. Hard sorts are typically caused by paste
lumps pushing through the matte and shorting out to the
adjacent (opposite polarity) plate. Soft shorts, on the other
hand, are caused by deep discharges. When the specic
gravity of the acid gets too low, the lead will dissolve into
it. Since the liquid (and the dissolved lead) are immobilized
by the glass matte, when the battery is recharged, the lead
comes out of solution forming threads of thin lead metal,
known as dendrites inside the matte. In some cases, the lead
dendrites short through the matte to the other plate. The
oat voltage may drop slightly but impedance can nd this
failure mode easily but is a decrease in impedance, not the
typical increase as in dry-out. See gure 2, Abnormal Cell.
Thermal run-away occurs when a battery’s internal components melt-down in a self-sustaining reaction. Normally, this
phenomenon can be predicted by as much as four months
or in as little as two weeks. The impedance will increase in
BATTERY TESTING GUIDE
7
Page 8
advance of thermal run-away as does oat current. Thermal
run-away is relatively easy to avoid, simply by using temperature-compensated chargers and properly ventilating the
battery room/cabinet. Temperature-compensated chargers
reduce the charge current as the temperature increases.
Remember that heating is a function of the square of the
current. Even though thermal run-away may be avoided by
temperature-compensation chargers, the underlying cause is
still present.
Nickel-Cadmium failure modes
NiCd batteries seem to be more robust than lead-acid. They are
more expensive to purchase but the cost of ownership is similar
to lead-acid, especially if maintenance costs are used in the cost
equation. Also, the risks of catastrophic failure are considerably
lower than for VRLAs.
The failure modes of NiCd are much more limited than leadacid. Some of the more important modes are:
▪ Gradual loss of capacity
▪ Carbonation
▪ Floating effects
▪ Cycling
▪ Iron poisoning of positive plates
Gradual loss of capacity occurs from the normal aging
process. It is irreversible but is not catastrophic, not unlike
grid growth in lead-acid.
Carbonation is gradual and is reversible. Carbonation is
caused by the absorption of carbon dioxide from the air
into the potassium hydroxide electrolyte which is why it is
a gradual process. Without proper maintenance, carbonation can cause the load to not be supported, which can be
catastrophic to supported equipment. It can be reversed by
exchanging the electrolyte.
Floating effects are the gradual loss of capacity due to long
periods on oat without being cycled. This can also cause
a catastrophic failure of the supported load. However,
through routine maintenance, this can be avoided. Floating
effects are reversible by deep-cycling the battery once or
twice.
NiCd batteries, with their thicker plates, are not well-suited
for cycling applications. Shorter duration batteries generally have thinner plates to discharge faster due to a higher
surface area. Thinner plates means more plates for a given
jar size and capacity, and more surface area. Thicker plates
(in the same jar size) have less surface area.
Iron poisoning is caused by corroding plates and is irreversible.
Figure 2 Changes in impedance as a result of battery capacity
8 BATTERY TESTING GUIDE
Page 9

Maintenance
How to maintain the
philosophies
There are different philosophies and ambition levels for
maintaining and testing batteries. Some examples:
▪ Just replace batteries when they fail or die. Minimum or
no maintenance and testing.
Obviously, not testing batteries at all is the least costly with
considering only maintenance costs but the risks are great.
The consequences must be considered when evaluating
the cost-risk analysis since the risks are associated with
the equipment being supported. Batteries have a limited
lifetime and they can fail earlier than expected. Time
between outages is usually long and if outages are the
only occasions the battery shows its capability risk is high
that reduced or no back-up is available when needed.
Having batteries as back-up of important installations
without any idea of their current health spoils the whole
idea of a reliable system.
▪ Replace after a certain time. Minimum or no maintenance
and testing.
This might also be a risky approach. Batteries can
fail earlier than expected. Also it is waste of capital
if the batteries are replaced earlier than needed.
Properly maintained batteries can live longer than the
predetermined replacement time.
▪ A serious maintenance and testing program in order to
ensure the batteries are in good condition, prolong their
life and to find the optimal time for replacement .
A maintenance program including inspection, impedance
and capacity testing is the way to track the battery’s
state of health. Degradation and faults will be found
before they become serious and surprises can be avoided.
Maintenance costs are higher but this is what you have
to pay for to get the reliability you want for your back-up
system.
The best testing scheme is the balance between maintenance
costs and risks of losing the battery and the supported
equipment. For example, in some transmission substations,
there is upwards of $10 million per hour owing through
them. What is the cost of not maintaining battery systems
in those substations? A $3000 battery is fairly insignicant
compared to the millions of dollars in lost revenues. Each
company is different and must individually weigh the costrisk of battery maintenance.
battery
Standards and common
practices
There are a number of standards and company practices for
battery testing. Usually they comprise inspections (observa-
tions, actions and measurements done under normal oat
condition) and capacity tests. Most well-known are the
IEEE standards:
▪ IEEE 450 for flooded lead-acid
▪ IEEE 1188 for sealed lead-acid
▪ IEEE 1106 for nickel-cadmium
IEEE 450
IEEE 450, “IEEE Recommended Practice for Maintenance, Testing and Replacement of Vented Lead-acid Batteries for Stationary Applications” describes the frequency
and type of measurements that need to be taken to validate
the condition of the battery. The standard covers Inspections, Capacity test, Corrective actions, Battery replacement
criteria etc.
Below is a summarized description for the maintenance, for
the full instructions see the IEEE450 standards.
Inspections
▪ Monthly inspection include appearance and
measurements of string voltage, ripple voltage, ripple
current, charger output current and voltage, ambient
temperature, voltage and electrolyte temperature at pilot
cells, battery float charging current or specific gravity at
pilot cells, unintentional battery grounds etc.
▪ Quarterly inspections include same measurements as
monthly inspection and in addition, voltage of each cell,
specific gravity of 10% of the cells of the battery and float
charging current, temperature of a representative sample
of 10% or more of the battery cells.
▪ Once a year a quarterly inspection should be extended
with, specific gravity of all cells of the battery, temperature
of each cell, cell-to-cell and terminal connection resistance
are performed on the entire string.
Capacity test (discharge test) should be done
▪ At the installation (acceptance test)
▪ Within the first two years of service
▪ Periodically. Intervals should not be greater than 25% of
the expected service life.
BATTERY TESTING GUIDE
9
Page 10

▪ Annually when the battery shows signs of degradation or
has reached 85% of the expected service life. Degradation
is indicated when the battery capacity drops more than
10% from its capacity on the previous capacity test or is
below 90% of manufacturers rating. If the battery has
reached 85% of service life, delivers 100% or greater of
the manufacturer's rated capacity and has no signs of
degradation it can be tested at two-year Intervals until it
shows signs of degradations.
IEEE 1188
IEEE 1188, “IEEE Recommended Practice for Maintenance, Testing and Replacement of Valve-Regulated
Lead-Acid Batteries for Stationary Applications” denes the
recommended tests and frequency.
Below is a summarized description for the maintenance, for
the full instructions see the IEEE1188 standards.
Inspections
▪ Monthly inspection include battery terminal float voltage,
charger output current and voltage, ambient temperature,
visual inspection and DC float current per string.
▪ Quarterly same measurements as for monthly inspection
shall be done and additionally cell/unit impedance value,
temperature of negative terminal of each cell and voltage
of each cell. For applications with a discharge rate of one
hour or less, resistance of 10% of the intercell connections
shall be measured.
▪ Semi-Annual same measurements as for quarterly
inspection shall be done and additionally a check and
record of voltage of each cell/unit, cell/unit internal ohmic
values, temperature of the negative terminal of each cell/
unit of battery.
▪ Yearly and Initial above measurements should be taken
and in addition, cell-to-cell and terminal connection
resistance of entire battery and AC ripple current and/or
voltage imposed on the battery.
Capacity test (capacity test) should be done
▪ At the installation (acceptance test)
▪ Periodically. Intervals should not be greater than 25% of
the expected service life or two years, whichever is less.
▪ Where impedance values has changed significantly
between readings or physically changes has occurred
▪ Annually when the battery shows signs of degradation or
has reached 85% of the expected service life. Degradation
is indicated when the battery capacity drops more than
10% from its capacity on the previous capacity test or is
below 90% of manufacturers rating.
IEEE 1106
IEEE 1106, “IEEE Recommended Practice for Installation,
Maintenance, Testing and Replacement of Vented Nickel-Cadmium Batteries for Stationary Applications”.
Below is a summarized description for the maintenance, for
the full instructions see the IEEE1106 standards.
Inspections
▪ Inspection at least once per quarter include battery
terminal float voltage, appearance, charger output current
and voltage, pilot-cell electrolyte temperature.
▪ Semi-annually general inspection and measurement of
voltage of each cell shall be done.
Capacity test (discharge test) should be done
▪ Within the first two years of service
▪ At 5-year intervals until the battery shows signs of
excessive capacity loss.
▪ Annually at excessive capacity loss
Summary best way to test and
evaluate your battery
Test intervals
1. Make a capacity test when the battery is new as part of
the acceptance test.
2. Make an impedance test at the same time to establish
baseline values for the battery.
3. Repeat the above within 2 years for warranty purpose.
4. Make an impedance test every year on ooded cells and
quarterly on VRLA cells.
5. Make capacity tests at least for every 25% of expected
service life.
6. Make capacity test annually when the battery has
reached 85% of expected service life or if the capacity
has dropped more than 10% since the previous test or is
below 90% of the manufacturers rating.
7. Make a capacity test if the Impedance value has changed
signicantly.
8. Follow a given practice (preferably from the IEEE
standard) for all temperature, voltage, gravity measure-
ments etc. and ll in a report. This will be a great help
for trending and for fault tracing.
Battery replacement criteria
Both IEEE 450 and IEEE 1188 recommend replacing the
battery if its capacity is below 80% of manufacturer’s rating.
Maximum time for replacement is one year. Physical characteristics such as plate condition or abnormally high cell
temperatures are often determinants for complete battery or
individual cell replacements.
10 BATTERY TESTING GUIDE
Page 11

Practical battery
testing
The Battery testing matrix below may help guide even the
most skilled battery testing technician and will help simplify
the recommended practices.
The following is a description of some of the tests or maintenance parameters.
Capacity test
Capacity test is the only way to get an accurate value on the
actual capacity of the battery. While used regularly it can be
used for tracking the battery’s health and actual capacity and
estimating remaining life of the battery. When the battery is
new its capacity might be slightly lower than specied. This
is normal.
There are rated capacity values available from the manufacturer. All batteries have tables telling the discharge current
for a specied time and down to a specic end of discharge
voltage. Table below is an example from a battery manufacturer
End
Volt.
Model
/Cell
DCU/DU-9 100 52 34 26 21 18 15 12 10
1.75
DCU/DU-11 120 66 41 30 25 21 18 15 13
DCU/DU-13 150 78 50 38 31 27 23 19 16
Common test times are 5 or 8 hours and common end of discharge voltage for a lead acid cell is 1.75 or 1.80 V.
During the test it is measured how much capacity (current
x time expressed in Ah) the battery can deliver before the
terminal voltage drops to the end of discharge voltage x
number of cells. The current shall be maintained at a constant value. It is recommended to select a test time that is
approximately the same as the battery’s duty cycle. Common
test times are 5 or 8 hours and common end of discharge
voltage for a lead acid cell is 1.75 or 1.80 V. It is recommended to use the same testing time during the battery’s
Nominal rates at 25º C (77º F)
8 h
Amperes (includes connector voltage drop
Ah
Ratings
1 h 2 h 3 h 4 h 5 h 6 h 8 h 10 h
Battery testing matrix – IEEE recommended practices
Instrument
Parameter
Capacity
Internal ohmic value
Intercell connection
resistance
Voltage of each
cell / pilot cell
Spec. grav. and temp.
of each cell / pilot cell
Corrosion at Terminals
DC Float Current
Unintentional Battery
Grounds
Battery ripple current
Charger AC ripple.
Current and Voltage
Cycling of Ni / Cd
batteries
Structural integrity of
rack / cabinet
Spectrum Analyzer
Bite3 Bite2 DLROs MOM/
Mjölner
▪ ▪
▪ ▪ ▪ ▪
▪ ▪ ▪
▪ ▪ ▪
▪ ▪ ▪
▪ ▪ ▪
▪
DCMs BMM80 M5091 BGFT BGL DMA35 TORKEL Visual
▪ ▪ ▪ ▪
▪ ▪
▪
▪
▪
▪
BATTERY TESTING GUIDE
11
Page 12

lifetime. This will improve accuracy when trending how battery’s capacity changes.
If the battery reaches the end of discharge voltage at the
same time as the specied test time the battery’s actual
capacity is 100% of the rated capacity. If it reaches the end
of discharge at 80% (8 h) or before of the specied 10 h it
is shall be replaced. See gure 3.
Procedure for capacity test of
vented lead acid battery
1. Verify that the battery has had an equalizing charge if
specied by the manufacturer
2. Check all battery connections and ensure all resistance
readings are correct
3. Record specic gravity of every cell
4. Record the oat voltage of every cell
5. Record the temperature of every sixth cell in order to
get an average temperature
6. Record the battery terminal oat voltage
7. Disconnect the charger from the battery
8. Start the discharge. The discharge current should be
corrected for the temperature obtained at point 5 (not if
capacity is corrected afterwards) and maintained during
the entire test.
9. Record the voltage of every cell and the battery terminal
voltage in the beginning of the discharge test
10. Record the voltage of every cell and the battery terminal
voltage one or several times at specied intervals when
the test is running
11. Maintain the discharge until the battery terminal voltage
has decreased to the specied end of discharge voltage
(for instance 1.75 x number of cells)
12. Record the voltage of every cell and the battery terminal
voltage at the end of the test. The cell voltages at the
end of the test have special importance since weak cells
are indicated here.
13. Calculate the actual battery capacity
It is important to measure the individual cell voltages. This
has to be made a couple of times during the test. Most
important is to measure the cells at the end of the discharge
test in order to nd the weak cells.
It is also very important that the time OR the current during
a discharge test is adjusted for the temperature of the battery. A cold battery will give less Ah than a warm. Temperature correction factors and methods are described in the
IEEE standards.
Manufacturers can also specify their batteries at constant
power discharge. This is used where the load has voltage
regulators. Then the current will increase when the voltage drops. Procedure for testing these batteries is the same
but the load equipment must be able to discharge with a
constant power.
Batteries can also be tested at a shorter time than their duty
cycle, for instance at 1 hour. Then the current rate has to be
increased. Advantage is that less capacity is drained from
the battery (valid for lead-acid) and it requires less time to
recharge it. Also less man-hour is needed for the test. Contact your battery manufacturer for more information. At
higher rates it is more important to supervise the battery’s
temperature.
Between load tests, impedance measurement is an excellent
tool for assessing the condition of batteries. Furthermore, it is
recommended that an impedance test be performed just prior to
any load test to improve the correlation between capacity and
impedance.
Figure 3 If the battery reaches the end of discharge at 80%
(8 h) or before of the specified 10 h it is shall be replaced.
12 BATTERY TESTING GUIDE
Figure 4 Replacement of battery is recommended when the
capacity is 80% of rated.
Page 13

Impedance test
Impedance, an internal ohmic test, is resistance in AC terms.
With regard to DC battery systems, impedance indicates
the condition of batteries. Since it tests the condition of
the entire electrical path of a battery from terminal plate to
terminal plate, impedance can nd weaknesses in cells and
intercell connectors easily and reliably.
Basically, impedance test is determined by applying an AC
current signal, measuring the AC voltage drop across the
cell or intercell connector and calculating the impedance
using Ohm’s Law. In practice, not only is the AC voltage
drop measured but so is the AC current. The AC current
is measured because of other AC currents in a battery that
are additive (subtractive). Other AC currents are present
from the charger system. The test is performed by applying
an AC test signal to the terminal plates. Then measure both
the total AC current in the string and the voltage drop of
each unit in the string by measuring each cell and intercell
connector consecutively until the entire string is measured.
The impedance is calculated, displayed and stored. As the
cells age, the internal impedance increases as depicted in
gure 2. By measuring impedance, the condition of each
cell in the string can be measured and trended to determine
when to replace a cell or the string which helps in planning
for budgetary needs.
The impedance test is a true four-wire, Kelvin-type measurement that provides excellent reliability and highly reproducible data on which to base sound decisions with regard
to battery maintenance and replacement. Impedance is able
to nd weak cells so that proactive maintenance can be
performed. After all, the battery is a cost but it is supporting
a critical load or revenue stream. If a single cell goes open
then the entire string goes off line and the load is no longer
supported. Therefore, it is important to nd the weak cells
before they cause a major failure.
The graph in gure 5 shows the effect of decreasing capacity on impedance. There is a strong correlation between
impedance and capacity so that weak cells are ably and
reliably found in sufcient time to take remedial action. The
graph shows the reorganized impedance data in ascending
order with each cell’s corresponding load test end voltage.
(Impedance in milliohms coincidentally is the same scale as
the voltage, 0 to 2.5). This view, that is ascending impedance/descending voltage, groups the weak cells on the right
side of the graph to nd them easily.
Impedance theory
A battery is not simply resistive. There is also a capacitive
term. After all, a battery is a capacitor, a storage device, and
resistors cannot store electricity. gure 6 shows an electrical
circuit, known as the Randles Equivalent Circuit, that depicts a battery in simple terms. There are those who would
have people believe that the capacitive term is not necessary
and that the resistance is the only part that needs measuring.
Impedance measures both the DC resistance (the real
component in impedance) and the reactance (the imaginary components in impedance). Only by measuring both
can the capacitive term start to be understood. The other
argument used against impedance is that frequency is a
variable in the reactance part of the impedance equation.
That is true except that since Megger uses a xed frequency,
namely 50 or 60 Hz depending upon geography, it is always
Figure 5 Ascending impedance with corresponding end voltage
BATTERY TESTING GUIDE
13
Page 14

the same. This variable, 2πω, now becomes a constant and,
therefore, frequency does not affect the nal result in any
way. The only parts that affect the nal result are the parts
that vary within the battery, namely resistance and capacitance, which paint the whole capacity/condition picture.
In the diagram shown in gure 6, Rm is the metallic resistance, Re is the electrolyte resistance, Rct is the charge
transfer resistance, Wi is the Warburg impedance and Cdl is
the capacitance of the double layer. Rm includes all of the
metallic components one post to the other post, i.e., post,
top lead and grids and to a certain degree, the paste. Re
is the resistance of the electrolyte which doesn’t vary that
much on a bulk basis. But at the microscopic level in the
pores of the paste, it can be signicant. Rct is the resistance
of the exchange of ions from the acid to the paste. If the
paste is sulphated, then Rct increases or if that portion of
the paste is not mechanically (electrically) attached to the
grid so that electrons cannot ow out of the cell. Warburg
impedance is essentially insignicant and is a function of
the specic gravity. Cdl is what probably makes the most
important contribution to battery capacity. By only measuring DC resistance, capacitance, an important part of the
cell, is ignored. Impedance measures both DC resistance
and capacitance.
A battery is complex and has more than one electrochemical process occurring at any given time, e.g., ion diffusion,
charge transfer, etc. The capacity decreases during a discharge due to the conversion of active material and depletion of the acid. Also, as the plates sulphate, the resistance
of the charge transfer increases since the sulphate is less
conductive than the active material. (See discussion about
the differences between the thickness of the plates in longduration versus short-duration batteries.)
Intercell connection resistance
Intercell connection resistance is the other half of the battery. A battery is comprised of cells connected in a series
path. If any one component fails the entire series connection fails. Many times batteries fail, not because of weak
cells, but due to weak intercell connections, especially on
lead posts which can cold-ow. Generally, hardware should
be tightened to the low end of the torque scale that is recommended by the battery manufacturer. But torque wrenches are a mechanical means to verify low electrical resistance.
It is far better to actually perform an electrical test using an
appropriate instrument. It is a low electrical resistance that
is desired. This test should be performed before the battery
is commissioned. Proper intercell connections are necessary
to ensure that discharge rates can be met. The instrument
of choice is a DLRO® or a MOM which can easily verify
that all connections have been made properly. It can even
nd minor errors before the battery is commissioned, preventing possible causes of failure or damage to supported
equipment.
Testing intercell connection resistance performs two functions:
▪ Validates intercell connection resistance
▪ Finds possible gross errors with top lead internal to the cell
By following IEEE Recommended Practices, intercell connection resistance can be validated. Those recommended
practices specify that the variation of intercell connection
resistance be less than ten percent. This translates into 7
micro-ohms on a 70-micro-ohm intercell connection resis-
tance. This method can even nd a washer stuck between
the post and the intercell connector whereas torquing will
not. They also specify that ten percent of the intercell connectors be measured quarterly and all intercell connectors
annually.
In multiple post batteries, it is possible to nd those rare
gross errors in a cell’s top lead. (See multiple post bat-
tery diagram in gure 1). On multiple-post cells, measure
straight across both connections, then measure diagonally
to check for balance in the cell and connections. Measuring only straight across does not adequately test for either
intercell connection resistance or for gross top lead defects.
This is due to the parallel circuits for the current.
The graph in gure 7 shows the data obtained from an actual 24-cell telephone (CO) battery The peak at connector
#12 (cell 12 to 13) is an intertier cable connection. Con-
nector #3 was out of specication and it was determined
that one of the two bolts was not properly torqued. It was
retorqued and retested. It came within ten percent of the
string average after retorquing.
The negative plates (odd-numbered plates #1 through 15)
are all connected through negative top lead which is connected to both negative posts. Positive plates (even-numbered) are connected to each other through positive top
lead which is connected to both positive posts. There are
two intercell connectors between neg post 1 and pos post 1
and between neg post 2 and pos post 2.
The higher the current draw the more critical is the proper
sizing of current-carrying components both internal to the
Figure 6 Randles equivalent circuit
14 BATTERY TESTING GUIDE
Figure 7 Intercell connection resistance bar graph
Page 15

cell and external. UPS batteries are usually designed for
a high rate discharge lasting typically only 15-20 minutes.
However, a telecommunications CO battery may have only
a 500 Amp draw but can discharge for up to eight hours. So
either combination can have disastrous effects due to improperly sized and maintained cells and intercell connectors.
Testing and electrical paths
In order to properly test a multiple post cell, one must un-
derstand its construction. Based on the diagram in gure 1,
it can be seen that there are two parallel paths for the test
current to travel. If the test leads are placed on neg post 1
and pos post 1, the two parallel paths are (1) directly from
neg post 1 to pos post 1 through their intercell connectors
and (2) neg post 1 down to the top lead, up to neg post 2
and across the intercell connectors to pos post 2 down to
the pos top lead and back up to pos post 1.The two paths
are parallel circuits and hence indistinguishable. If one bolt
is loose, there isn’t any way to determine that since the test
current will follow the path of least resistance. The better method to measure intercell connection resistance is to
measure diagonally from neg post 1 to pos post 2 and again
from neg post 2 to pos post 1. Compare the two readings
for highest condence. Admittedly, diagonal measurements
are still parallel but the comparison becomes more interest-
ing due to the increased inuence of top lead and loose
hardware. Diagonal measurements do not allow for a direct
connection from post to post. In the case of six-post cells,
measure diagonally across the farthest posts in both directions.
Voltage
Float voltage has traditionally been the mainstay of any
testing procedure. What is voltage? Voltage is the difference,
electrically speaking, between the lead and the lead oxide
on the plates or between the nickel and the cadmium. The
charger is the item that keeps them charged. The sum of all
of the cell voltages must be equal to the charger setting (except for cable losses.) This implies then that voltage merely
indicates the state-of-charge (SOC) of the cells. There is
no indication of a cell’s state-of-health (SOH). A normal
cell voltage doesn’t indicate anything except that the cell is
fully charged. An abnormal cell voltage, however, does tell
you something about the condition of the cell. A low cell
voltage can indicate a shorted cell but only when the volt-
age nally drops to about 2.03. If a cell is low then other
cells must be higher in voltage due to the charger setting.
Remember that the sum of all cell voltages must equal the
charger setting. Those cells that are higher are counteracting the low cell and generally speaking the higher cells are in
better condition because they can tolerate the higher voltage. But those cells are being overcharged which over-heats
them and accelerates grid corrosion and water losses.
Let’s say for the moment that the low voltage cell is not yet
at 2.03, it is at 2.13 V. At 2.13 V it is not low enough to ag
a concern but it is degrading. It may or may not be able to
support the load when an outage occurs. Impedance is able
to nd that weak cell sooner than voltage. In this case, impedance will decrease since it is an impending short circuit.
A similar example can be found in VRLA when it comes to
dry-out or loss-of-compression. Voltage will not nd this
condition until it is far later in the battery’s life, until it is too
late. Impedance nds this condition much earlier so that
remedial action can be performed.
So don’t confuse fully charged with full capacity.
As said above, cell voltage divergence can be caused by a
number of factors and one way to solve this problem could
be to make an equalization charge. In an equalization charge
procedure, the entire battery is charged at a higher (than
normal) voltage for several hours to balance the voltage in
all the cells. The procedure can lead to heating and possibly
water loss. It is recommended to follow the manufacturer’s
procedure to avoid damaging the battery.
Specific gravity
Specic gravity is the measure of the sulphate in the acid of
a lead-acid battery. It is also the measure of the potassium
hydroxide electrolyte in nickel-cadmium battery but since
the potassium hydroxide electrolyte isn’t used in the chemical reaction, it is not necessary to measure it periodically.
Specic gravity traditionally has not provided much value
in determining impending battery failure. In fact, it changes
very little after the initial 3 to 6 months of a battery’s life.
This initial change is due to the completion of the formation process, which converts inactive paste material into
active material by reacting with the sulphuric acid. A low
specic gravity may mean that the charger voltage is set too
low causing plate sulphation to occur.
In a lead-acid battery the sulphate is a closed system in that
the sulphate must be either on the plates or in the acid.
If the battery is fully charged then the sulphate must be
in the acid. If the battery is discharged, the sulphate is on
the plates. The end result is that specic gravity is a mirror
image of voltage and thus state-of-charge. Specic grav-
ity readings should be taken when things are amiss in the
battery to obtain as much information about the battery as
possible.
Different battery applications and geographies have varying
specic gravities to accommodate rates, temperature, etc.
Following is a table that describes some applications and
their specic gravities.
Specific gravities and their applications
Specific gravity Percent acid Application
1.170 25 Tropical stationary
1.215 30 Standard stationary
1.250 35 UPS/high rate
1.280 38 Automotive
1.300 40 VRLA stationary
1.320 42 Motive power
1.400 50 Torpedo
BATTERY TESTING GUIDE
15
Page 16

Float current
Another leg of the Ohm’s Law triangle is current. The
charger voltage is used to keep a battery charged but voltage
is really the vehicle to get current into the battery (or out
of it during discharge). It is current that converts the lead
sulphate back to active material on the grids.
There are two types of DC current on a battery: recharge
current which is the current applied to recharge a battery
after a discharge and oat current which is the current used
to maintain a battery in a fully charged state. If there is a
difference between the charger setting and the battery’s
voltage, that difference will cause a current to ow. When
the battery is fully charged
the oat current which counteracts the self-discharge of
the battery (<1% per week). Since the voltage differential
between the charger and the battery is small, the oat current is small. When there is a large voltage difference such
as after a discharge the current is high and is limited by the
charger until the voltage difference becomes less. When the
current is on the plateau in the graph below, this is called
current limit. When the voltage differential becomes less,
the charge current is reduced as depicted on the downward
sloping charge current line on the graph shown in gure 8.
The charge voltage is the voltage of the battery, not the
charger setting which is why it is increasing.
Float current will vary with battery size. The larger the
battery is, the more oat current it will take to keep it fully
charged. Float current can increase for a couple of reasons:
ground faults on oating battery systems and internal battery faults. Ground faults are discussed later. As a battery’s
internal impedance increases, it takes more current to pass
through that higher impedance. The increase in oat current
can be an indicator of battery faults. In lieu of measuring
oat current, many of the same conditions are found with
impedance.
In VRLA batteries, oat current
of battery problems, namely thermal runaway. Thermal runaway is the result of a battery problem, not the cause. Some
of the causes that can lead to thermal runaway are shorted
cells, ground faults, dry-out, excessive charging and insuf-
Figure 8 Constant-voltage Constant-current charge characteristics
[1]
, the only current owing is
[2,3]
seems to be an indicator
cient heat removal. This process takes anywhere from two
weeks to four months to occur once the oat current starts
its increase. By measuring oat current, it may be possible
to avoid catastrophic failure of the battery and damage to
connected and nearby equipment. Impedance will nd many
of these same errors.
Ripple current
Batteries, as DC devices, prefer to have only DC imposed
on them. The charger’s job is to convert AC into DC but
no charger is 100% efcient. Frequently, lters are added
to chargers to remove the AC current from the DC output.
The AC current on the DC is called ripple current. Battery
manufacturers have stated that more than about 5 A rms
of ripple for every 100 Ah of battery capacity can lead to
premature failure due to internal heating. Ripple voltage is
not a concern since it is the heating effect of the ripple cur-
rent that damages batteries. The 5% ripple current gure is
a rough estimate and depends also on the ambient temperature. Ripple current can increase slowly as the electronic
components in the charger age. Also if a diode goes bad,
the ripple current can increase more dramatically leading to
heating and premature death without anyone knowing it. Although impedance is not a measure of ripple current, ripple
current is measured because of the way Megger designs its
impedance instruments.
There is anecdotal evidence
[4]
that low frequency ripple
(<10Hz) may charge and discharge a battery on a microscale. More research is necessary to prove this hypothesis.
Excessive cycling can lead to premature death of a battery
regardless of the reasons for the cycling, be they outages,
testing or maybe micro-cycling. One thing is true: the lower
the AC is on the battery system, the less the damage is that
can occur. VRLA batteries seem to be more sensitive to
ripple current than their ooded counterparts. It is then
advisable to lter their chargers for ripple current/voltage.
Temperature
It is well known that low temperatures slow up the internal
chemical reactions in any battery; the degrees of reduced
performance vary according to the technology. For example,
at temperatures around freezing, a VRLA may need capacity
compensation of 20%. The lead-calcium cell using 1.215
specic gravity acid will require a doubling of capacity,
while the Ni-Cd will need about 18% increased capacity.
At the other end of the temperature range, high temperature is the killer of all batteries. There will be no surprise
to nd out that this impact varies from one technology to
another. Lead acid at 95˚F will experience a 50% shortened
life, while Ni-Cd will have a 16-18% shortening of life.
By applying what Arrhenius learned about chemical reactions, for every 18º F (10º C) increase in battery tem-
[1] Cole, Bruce, et al., Operational Characteristics of VRLA Batteries Configured in Parallel Strings, GNB Technologies
[2] Brown, AJ, An Innovative Digital Flat Current Measurement Technique - Part Two, Proceedings of BattConn® 2000
[3] Boisvert, Eric, Using Float Charging Current Measurements to Prevent Thermal Runaway on VRLA Batteries, Multitel
[4] Ruhlmann, T., Monitoring of Valve Regulated Lead Acid Batteries, Proceedings of BattConn® 2000
16 BATTERY TESTING GUIDE
Page 17

perature, battery life is halved, battery life can start to be
managed. The increased temperature causes faster positive
grid corrosion as well as other failure modes. By holding a
lead-acid battery at a temperature of 95º F (35º C) instead
of the designed 77º F (25º C), a 20-year battery will last
only ten years, a ten-year battery only ve years and so on.
Increase the temperature by another 18º F to 113º F (45º C),
a 20-year battery will last only ve years!
A battery is rarely held at a certain temperature for its entire
life. A more realistic scenario is for a battery to heat during
the day and cool down at night with higher average temperatures in the summer and lower average temperatures
in winter. It is unfortunate but cooling the battery off to
below 77º F (25º C) will not gain back the life that was lost.
Once the positive grid corrodes, it cannot be converted
back again. Furthermore, positive grid corrosion occurs at
all temperatures, it is merely a matter of speed of the corrosion rate. The end result is to control, as best as possible
(back to cost versus risk), the temperature of the batteries in
the network.
IEEE 450, Annex H offers a method for calculating the
impact of high temperatures on a lead acid battery.
Data analysis
The essence of any testing methodology is how to interpret
the data to make some sense of it all. The same is true of
battery testing. If the data are to be hand-written and led
or if a printout from an instrument is reviewed then led,
then there is no useful analysis except if there is an emergency at that very moment. The real value in battery testing
lies in the trending of data to determine if problems are
imminent or a little farther out. Trending of battery data,
especially impedance and capacity, is an excellent tool for
budgetary planning. By watching the batteries degrade over
time, a decision can be made as to when to replace a battery.
With trending, emergency replacements decrease dramatically.
The rst time a battery’s impedance is tested can cause concern because there is no baseline. In these cases, it is good
to compare each cell against every other cell in the string.
Weak cells stand out. It is these cells which require further
investigation. The table below provides a guideline depending upon the length of time batteries have been tested.
Single Test Trending
% Deviation
from String
Avg
Lead-acid,
Flooded
Lead-acid, VRLA,
AGM
Lead-acid, VRLA,
Gel
NiCd, Flooded 15 10 100
NiCd, Sealed 15 5 80
5 2 20
10 3 50
10 3 50
Cell’s %
Change from
Last Test
Cell’s %
Change
Overall
BATTERY TESTING GUIDE
17
Page 18

Locating ground faults
on DC systems without
sectionalizing
Overview
The main objective of a battery system is to provide standby and emergency power to operate industrial, consumer,
commercial or protective devices. Some of these devices
include emergency lighting units, uninterruptible power
supplies, continuous process systems, operating controls,
switchgear components and protective relays.
In emergency situations, it is essential that these devices be
in proper operating condition. Failure of a DC system or
the battery can result in operational failure of the devices
connected to that system. System failure can lead to loss of
revenue, damage to equipment and/or injured personnel.
It is a common situation for a oating DC system to develop grounds within it. When a battery system is partially
or completely grounded, a short circuit is formed across the
battery and consequently may cause the protective device to
fail to operate when needed.
ground resistance on that feeder. Faults can be traced easily
regardless of the number of distribution panels or circuits
because the “tracer” is merely following the strength of the
AC signal. System integrity is maintained because it is an online AC test and is designed to prevent system trips.
After injection of a low-frequency AC waveform, a resistive
fault on a branch of the battery system will be indicated by
a low-resistance value. For example, if the total resistance
of a battery system showed 10 kΩ, this would indicate a
resistive fault on the battery system. The resistive fault can
be located by clamping on each individual circuit until a
resistive value of 10 kΩ is found.
It is easy to see that this method can be adapted in a straight
forward manner to locate multiple faults by using the theory
of parallel paths. For example, if the total system resistance
indicates 1 kΩ and an individual branch indicates 10 kΩ
resistive fault, the user would know that the system has a
second fault because the total system resistance and the
branch resistance do not match. By using the AC injection
method, ground faults on ungrounded DC systems is easy,
straight-forward and safe.
Current test methods
Traditionally utilities and industrial complexes have gone
to great lengths to nd ground faults within their battery
systems. However, locating these battery grounds proves to
be a very elusive and time-consuming process. The current
ground-fault location method involves sectionalizing, or
interruption, of DC branches to isolate the ground fault.
Sectionalizing disables the system protection and has been
known to cause inadvertent line and generator tripping. For
this reason, many utilities have banned sectionalizing. Until
more recently, though, this had been the only method available to locate ground faults.
A better test method
Developments have led to a better test method; injecting a
low-frequency AC signal and using that AC signal to locate
the ground in the DC system. This method can be performed without sectionalizing the DC system and it reduces
the fault locating time from days to hours. Furthermore, it
allows for system protection to be present at all times.
The AC injection method measures single or multiple
ground faults by rst injecting a low-frequency, 20 Hz AC
signal between the station ground and the battery system.
Second, the resulting current is then measured by using a clamp-on sensing current transformer. From this,
the resistance value can be calculated using the in-phase
component of the circulating current, thus rejecting the
effect of capacitive loads. Therefore, if the signal is injected
at the battery terminal and the clamp-on CT is connected
to the outgoing lead, the instrument will measure the total
ground resistance present on the battery system. If the CT
is clamped on a feeder, then the instrument will measure the
18 BATTERY TESTING GUIDE
Page 19

Frequently asked
questions
What does float voltage of a cell tell me?
Float voltage indicates that the charger is working, that
is, state-of-charge. It does not indicate the state-of-health
(condition) of the cell. It indicates that the cell is fully
charged, but don’t confuse fully charged with full capac-
ity. There have been many times that the oat voltage is
within acceptable limits and the battery fails. A low oat
voltage may indicate that there is a short in the cell. This
is evident by a oat voltage at about 2.06 or below for
lead-acid (if the charger is set for 2.17 V per cell)
In some cases, a cell oats considerably higher than the
average. This may be caused by the high oat voltage cell
compensating for another cell that is weak and is oating low. It is possible that one cell oats much higher to
compensate for several cells oating a little low. The total
of all cells’ voltages must equal the charger setting.
What are the recommended maintenance practices for the
different types of batteries?
IEEE Recommended (Maintenance) Practices cover the
three main types of batteries: Flooded Lead-acid (IEEE
450), Valve-Regulated Lead-acid (IEEE 1188) and NickelCadmium (IEEE 1106). Generally speaking, maintenance
is essential to ensure adequate back-up time. There are
differing levels of maintenance and varying maintenance
intervals depending upon the battery type, site criticality
and site conditions. For example, if a site has an elevated
ambient temperature, then the batteries will age more
quickly implying more frequent maintenance visits and
more frequent battery replacements.
How important is intercell connection resistance?
Our experience has found that many battery failures are
due to loose intercell connections that heat up and melt
open rather than from cell failure. Whether a cell is weak
or an intercell connector is loose, one bad apple does
spoil the whole bushel.
When lead acid batteries are frequently cycled, the negative
terminal may cold ow, thus loosening the connection.
The proper sequence of measuring multiple post batteries is critical. Not all instruments provide valid intercell
connection resistances due to their method of testing.
Megger instruments provide valid data.
How often should impedance readings be taken?
The frequency of impedance readings varies with battery
type, site conditions and previous maintenance practices.
IEEE 1188 Recommended Practices suggests that a baseline shall be taken six months after battery has been in
operation and then semi-annual quarterly. With that said,
Megger recommends that VRLA batteries are measured
quarterly due to their unpredictable nature and semi-annually for NiCd and ooded lead-acid. Impedance reading should also be taken prior to every capacity test.
At what point should I stop changing cells and replace the
entire battery?
In shorter strings (less than 40 cells/jars), the entire
should be replaced when three to ve units have been
replaced. In longer strings, a similar percentage that is
replaced is the criterion.
How can I predict when I need to change a cell?
Even though there is not a perfect mathematical correlation between battery capacity and impedance (or
any other battery test except a load test), the amount of
increase in impedance is a strong indicator of battery
health. Megger has found that a 20 percent increase in im-
pedance for ooded lead-acid generally correlates to 80%
battery capacity. In VRLA, that increase is closer to 50%
from the battery’s initial impedance or from the manufacturer’s baseline values.
Will capacity testing destroy my battery?
The battery system is designed to provide back-up power
during all outages that appear during its lifetime. Performing a capacity test is nothing else than simulating one
outage but in a controlled way. Batteries can normally be
deep discharged (discharged to manufacturer’s end-of
discharge voltage) 100 - 1000 times depending on type
of battery. Using a few of these cycles has no real impact
on the battery’s lifetime. On the other hand there is no
reason to test more frequently than recommended by the
standards.
Can I make a discharge test while my battery is still connected
to the load (on-line)?
Yes it is possible to do. Megger has test equipment that
automatically senses and regulate the discharge current
even when the batteries are connected to the ordinary
load. Most users choose to make a 80% discharge test
when on-line in order to still have some backup time at
the end of the test.
What are some common failure modes?
Failure mode depends upon the type of battery, the site
conditions, application and other parameters. Please refer
the summary on pages 7-8 or to the “Battery Failure
Modes,” which can be found on the Megger website.
Look under the Battery Test Equipment product section.
In the upper right-hand column under “Documents click
for Application Guides, Articles and FAQs.
Battery technology summary
As you can see, there is a lot to a battery. It is a complex
electro-chemical device. There is much more information
available that goes further into the details of Tafel curves
and depolarization but that is beyond this scope. Essentially, batteries need maintenance and care to get the most of
them which is the main reason people spend so much on
batteries – to support far more expensive equipment and
to ensure continuous revenue streams.
BATTERY TESTING GUIDE
19
Page 20

Megger products
Impedance test equip-
overview
Megger offers solutions to ensure system performance with
its comprehensive line of Battery Test Equipment, Low
Resistance Ohmmeters and Micro-ohmmeters, Insulation
Testers, and Multimeters.
An overview of the various products available is described
below. For more information on these and many other Megger products, please contact us at (800) 723-2861,
(214) 333-3201. Visit our web site www.megger.com for the
most up-to-date news, product and service information.
ment
Regardless of whether you are testing ooded lead-acid,
VRLA or Ni-Cd cells, Megger has the right equipment for
your battery maintenance requirements. The products and
associated accessories provides meaningful data on bat-
tery health without signicant expense or any reduction in
remaining battery capacity.
Interruption in service can cause disaster to supported
equipment and facilities. Consequently, a dependable backup
power system is critical so that when AC mains fail, costly
service interruptions can be avoided. The battery impedance
test helps to identify weak cells before they cause problems.
Taking the battery off-line for testing is time consuming and
adds risk to the process. This process is unnecessary with
the on-line testing capabilities of the Megger family of battery test products. The highly repeatable instruments help
reduce downtime.
BITE® 3
20 BATTERY TESTING GUIDE
▪ Determines the condition of lead-acid batteries up to
2000 Ah
▪ On-line testing with Pass/Warning/Fail calculations
▪ Measures impedance, intercell connection resistance, cell
voltage
▪ Windows CE¨ Operating System with more than 16 MB of
memory
▪ Measures float and ripple currents
The BITE 3 is a compact, battery-operated, instrument with
powerful on-board data analysis tools. It is the rst of its
kind instrument in that the ProActiv can download all previous data to provide the best in on-site data analysis like no
other instrument of its kind. The menus are easy to navigate with a bright, backlit LCD. The data display includes
the normal numeric arrangement but adds two graphical
displays to help analyze weak cells.
Page 21

BITE® 2 and BITE®2P
switchgear components, protective relays and continuous
process systems. Failure of a battery system within environments such as utilities, hospitals or manufacturing plants can
result in operational failure of the devices connected to it.
ProActiv assists the user to avoid battery failures, budget for
future battery string and cell replacements, and plan battery
change outs in an orderly manner.
ProActiv utilizes a standard MS Access database format. It
allows the user to organize and manage battery data such as
voltages, impedance, intercell connection resistance, ripple
current, specic gravity, IR thermographs and more.
▪ Determines the condition of lead-acid and nickel-cadmium
batteries up to 7000 Ah
▪ On-board Pass/Warning/Fail indications
▪ On-line testing
▪ Robust, reliable instruments
▪ Built-in printer (BITE 2P)
The BITE 2 and BITE2P Battery Impedance Test Equipment work by applying a test current across the battery
string while on-line, then measuring the impedance, cell
voltage and intercell connection resistance. They also
measure ripple current which indicates the condition of the
charger. The instruments help evaluate the condition of the
entire string from terminal plate to terminal plate and even
the charger.
ProActiv battery database management
software
BITE® accessories
▪ Enhances the capabilities of the BITE line
▪ Full line of accessories
▪ Designed for unique situations
▪ Great for non-standard installations
Megger offers a complete line of accessories to enhance the
capabilities of the BITE product line. There are extension
cables, calibration shunts, etc. Even though we have many
accessories, we are continually evaluating additional products as interest arises.
RopeCT™ is a flexible,
highly accurate current
transmitter for measuring
current flow in larger
battery systems. It comes
in two lengths: 24 in.
(60 cm) and 36 in. (90 cm)
for 8 in. (20 cm) and
12 in. (30 cm) diameters,
respectively. It is designed
specifically for the BITE 2,
BITE2P and EBITE.
▪ Organizes and manages battery data
▪ Performs trending analysis
▪ Assists the user to manage multiple batteries
▪ Prints basic reports
The rst of its kind, ProActiv is a new, powerful, easy to use
battery database management software designed to analyze
each individual battery in a battery system.
Battery testing is crucial to ensure a battery system provides
standby and emergency power to operate devices such
as emergency lighting, UPS systems, operating controls,
Mini-CTs are for measuring
current in smaller gauge
wires and a single cable
tied in a bundle.
The Current Transformer
kit for the BITE 3 is for
measuring the current
in noisy battery systems
and to measure “escape
current” in parallel battery
strings. Competitive
instruments do not
measure current and then,
may provide erroneous
internal ohmic values.
BATTERY TESTING GUIDE
21
Page 22

Lighted Probe Extensions
can be mounted on the
receivers and probes of
the BITE 3, BITE 2 and
BITE 2P. They are ideal
for measuring batteries
in cabinets and hard-toreach places. With these
probe extensions, batteries
needn’t be taken off line
to measure them – a
real time and cost saving
device.
The BITE 3 offers
alternative lead sets for
measuring ELUs and
other smaller battery
applications, spade
terminal batteries and
batteries with harnesses.
They facilitate measuring
the impedance of battery
systems with difficult
access.
Digital Hydrometer
measures specific gravity
and temperature for each
cell and it calculates a
temperature-adjusted
specific gravity to save
time – all in a hand-held
device. It can store up to
256 cells per string in up
to eight strings. No need
to worry about parallax
or hand writing data on
sheets, etc. It is much safer
than bulb hydrometers
and without any spilled
acid to clean up.
22 BATTERY TESTING GUIDE
Page 23

Capacity testing
TORKEL 820/840/860
▪ Batteries can be tested “in service”
▪ Unit adjusts to include load currents in the test parameters
▪ User adjustable alarm and shutdown points to avoid
excessive discharge
Batteries in power plants and transformer substations must
provide the equipment they serve with standby power in the
event of a power failure. Unfortunately, however, the capac-
ity of such batteries can drop signicantly for a number of
reasons before their calculated life expectancy is reached.
This is why it is so important to check batteries at regular
intervals, and the only reliable way of measuring battery
capacity is to conduct a discharge test.
The TORKEL instruments are used for discharge testing.
Tests can be conducted at constant current, constant power,
constant resistance or in accordance with a pre-selected load
prole. For extra discharge capacity there are auxiliary load
units available.
TORKEL 820 can discharge batteries ranging from 12 to
48 volts with currents up to 270 A
TORKEL 840 is used for battery systems ranging from
12 to 250 V.
TORKEL 860 is designed for users who travel from
place to place to maintain battery systems having different voltages. It features excellent discharging capacity plus
a broad voltage range and outstanding portability. The
TORKEL 860 is used for systems ranging from 12 to 480 V.
TXL830/850/870 are extra
load units for providing
higher load currents.
Together, TORKEL and the
TXL extra loads form a
system that can discharge
batteries with currents up
to several kA.
TORKEL accessories
TORKEL Win is a software
for report functions and
remote control of TORKEL.
BATTERY TESTING GUIDE
23
Page 24

Ground fault tracing
equipment
There are two ground fault locating instruments from which
to choose, the Battery Ground Fault Tracer (BGFT) and
the Battery Ground-Fault Locator (BGL). The BGFT has
superior noise elimination while the BGL has an automatic
bridge to differentiate between high capacitance and low
resistance. Here is a brief description of each instrument.
Battery Ground Fault Tracer
(BGFT)
▪ Easily locates ground faults in ungrounded
DC battery systems
▪ Operates in high electrical noise environment
▪ Simplifies fault tracing by identifying fault characteristic
(resistive and capacitive) magnitudes
The Battery Ground-Fault Tracer is an economical, manu-
ally balanced instrument that identies, tracks and locates
ground faults in ungrounded DC battery systems - on-line.
It is particularly effective in high electrical noise environments, as the strength of the test current can be adjusted
up to 80W. The BGFT is particularly useful in any industry
where supply of power for operating measurement, communication and control equipment is critical.
The Battery Ground-Fault Tracer accelerates fault location
by eliminating trial-and-error procedures and because faults
can be located without going off-line. It is line operated and
has a manual bridge. The manual bridge is used to differentiate between true, resistive faults and phantom, capacitive
faults by using a feedback cable to null the capacitance. But
the manual bridge is not required in order to trace faults.
The BGFT works by converting line frequency to 20 Hz. It
then pushes the AC signal through some coupling capacitors to prevent transients on the DC buss and applies the
AC signal into the DC system while on-line. Using the
hand-held tracer, follow the signals with the highest readings
until the fault is found.
BGFT
BGFT
Battery Ground-fault Locator
(BGL)
▪ Ground faults in ungrounded DC battery systems
are easily located
▪ Features an automatic bridge
▪ Battery operated
▪ Simplifies fault tracing by identifying fault characteristic
(resistive and capacitive) magnitudes
The Battery Ground-Fault Locator was developed to detect,
track and locate ground faults on battery systems, without
resorting to sectionalizing! The BGL tracks and locates
ground faults on live or dead battery systems. To save hours
of unnecessary troubleshooting, the BGL readily differentiates between the resistive fault currents and capacitive
charging currents. This feature allows the instrument to
24 BATTERY TESTING GUIDE
BGL
Page 25

detect and track leakage paths, even in the presence of
surge-suppression capacitors.
The BGL works by ltering and applying an AC signal to
the DC buss on-line. The low level output of the BGL
allows it to be battery-operated but is more sensitive to
system noise. It has a built-in automatic bridge to differentiate between real (resistive) and phantom (capacitive) faults
so only the real faults are traced. The BGL is moved from
panel to panel to continue the tracing process until the fault
is found. Since it has an automatic bridge it is very easy to
trace faults and as such is better designed for the novice
user.
BATTERY TESTING GUIDE
25
Page 26

Digital Low Resistance
Ohmmeters (DLRO®) and
Microhmmeters (MOM)
Many times batteries fail not because of weak cells but due
to weak intercell connections. Torquing is a mechanical
method to ensure that the electrical path resistances is very
low. But it does not truly indicate the quality of the electrical path resistance. The only true method is to measure each
intercell connection resistance.
Megger has several DLROs and MOMs that are appropriate
for intercell connection resistance. The portability of the instruments allows effortless mobility around battery strings.
The instruments are built into strong, lightweight cases that
are equally at home in the eld or in the laboratory.
DLRO200 and DLRO600
▪ Small and weighs less than 15kg (33 lbs)
▪ Test currents from 10A to 200 or 600 A DC
▪ 0.1 best resolution
Megger DLRO200 measures resistances between 0.1 µΩ
and 1 Ω, at high currents.This versatile instrument can pro-
vide test currents from 10 amps up to 200 amps subject to
the load resistance and supply voltage. A large liquid crystal
display provides all the information needed to perform a
test.
DLRO 200
DLRO 247000 series
▪ Resolution to 0.1µΩ on 599.9 µΩ range
▪ Standard inaccuracy of ±0.25%
▪ Large, digital LED readout
The 247000 Series of DLROs are a family of highly accurate instruments that provide a simple, practical and reliable
means of making low-resistance tests in the eld. They also
are ideal for production quality control. They operate on the
four-wire measurement principle, thus eliminating lead and
contact resistances. With basic accuracies of ±0.25% and
resolution down to 0.1 µΩ, they are nonetheless designed to
be rugged and portable for use at the job site.
DLRO10 and DLRO10X
▪ Accurate results in under three seconds
▪ Fuse protected to 600 V
▪ Automatically detects continuity in potential and
current connections
▪ Alpha-numeric keypad for entering test notes (DLRO10X)
▪ User configurable high and low limits (DLRO10X)
▪ Printer output and memory (DLRO10X)
The DLRO10 and DLRO10X are fully automatic instruments, selecting the most suitable test current, up to 10 A
DC to measure resistance from 0.1 µΩ to 2000 Ω on one of
seven ranges.
DLRO247001
DLRO10X
26 BATTERY TESTING GUIDE
Page 27

For users who desire more control over the measurement
process, DLRO10X uses a menu system to allow manual
selection of the test current. DLRO10X also adds real-time
download of results and on-board storage for later download to a PC.
MJÖLNER 200 and MJÖLNER 600
▪ True DC ripple free current
▪ Inaccuracy ±0.3
▪ Two displays LED and LCD for visibility in all conditions
▪ Low weight, 8.8 kg (19.4 lbs) and 13.8 kg (30.4 lbs)
▪ Fully automatic testing
MJÖLNER is designed to measure the resistance of circuit
breaker contacts, bus-bar joints, contact elements in busbars and other high-current links. The product has been designed with safety, ease of use and versatility in mind. There
are two models, one 200 A output current and one 600 A.
With MJÖLNER it is possible to make measurements according to the DualGround™ method. This means that the
test object will be grounded on both sides throughout the
test giving a safer, faster and easier workow.
MOM200A and MOM600A
▪ Resolution 1µΩ on 1999 range
▪ Standard inaccuracy of ±1%
MOM200A/600A are ideal for nding poor connections
since they can put out 100 A for extended periods. Its range
extending up to 20 milliohms makes it ideal for measuring
many different types of connections.
MJÖLNER 200
MOM690
▪ Resolution 1µΩ on 200 m range
▪ Standard inaccuracy of ±1%
▪ MOMWin™ software
▪ AC output
In addition to high current capacity, MOM690™ features
microprocessor-based measurement, storage and reporting.
The built-in software enables you to carry out an individual
test or an entire series of tests and store the results.
With the optional MOMWin™ software you can also export the test results to a PC for further analysis and reporting. Ranges are set automatically, resistances are measured
continually and test results can be automatically captured at
a preset test current.
MOM600A
MOM690
BATTERY TESTING GUIDE
27
Page 28

Multimeters
MMC850 Multi-conductor AC
Digital Clampmeter
▪ Single or multiple conductor measurement
▪ Flat or round section cables
▪ Backlit display
▪ Cable centralizing clamp
The MMC850 offers a unique solution to current measurement in multiconductor cables without the need to split
cables. The MMC850 is simply clamped to a multi-conduc-
tor cable and the current owing is then read.
Multimeters
Megger multimeters compliment the solution to measuring
and maintaining battery strings and cells. All instruments
undergo rigorous testing throughout the their design and
manufacture and are suitable for use in eld service applications. All are CE marked and designed to National and International Safety Standard, EN61010-1. They include such
features as large digital displays, automatic power down,
water and dust resistance. There are three series of Megger
multimeters, the M8000, M7000 and AVO300 depending
upon needs and features wanted.
MMC850
M8037
28 BATTERY TESTING GUIDE
Page 29

Insulation Resistance
Test Equipment
Batteries are supposed to be well insulated from adjacent
equipment and metallic objects. The insulation provides
several benets: 1) keeps the charge in the battery rather
than letting it leak, 2) provides for normal oat current, and
3) reduces energy losses. If a battery is leaking electrolyte,
then there may be a path to ground. When a path exists, the
current needed to keep the battery fully charged increases.
It also shortens the length of back-up time of the battery
depending upon the severity of the leak. An insulation resistance test can identify whether there are leaks. The insulation resistance is measured across one of the terminals of
the battery to some ground, presumably the battery rack or
tray. It is a very easy test to perform and provides for a lot
of condence in the overall state of electrical insulation.
This test applies a DC voltage, say 500 Vdc, between the
buss, off-line and the rack. Then measures the DC leakage
current to calculate resistance in MΩ or GΩ. The higher the
resistance is the better. This test is recommended at installation and whenever a leak may be suspected (from tell-tale
signs such as salt build-up.)
Megger offers the MIT400 Series insulation and continuity testers designed for electrical testing by power utilities,
industrials, telecommunication companies, commercial/
domestic electricians and anyone with unique test voltage requirements. The wide range of features also makes
the MIT400 Series ideal for maintenance technicians and
engineers.
These instruments are available from as low as 50 V to as
high as 1 kV. For analytical applications, multiple test voltages are desired.
MIT400 series insulation
resistance testers
▪ Choice of 4 models for commercial and plant applications:
MIT Models 400, 410, 420 and 430
▪ Choice of 3 models for telecom applications: MIT Models
480, 481 and 485
▪ “Special Applications” model for those who need a
unique test voltage, MIT Model 40X offers a variable
insulation test voltage from 10 V to 100 V applications in
1 V stepsÉproviding a solution to practically any unusual
measurement requirement you may have.
The series consists of eight instruments
MIT400 250 V, 500 V and 1000 V
MIT410 50 V, 100 V, 250 V, 500 V and 1000 V plus PI
and DAR
MIT420 Same as Model 410 plus result storage and
download
MIT430 Same as Model 420 plus Bluetooth® download
MIT480 50 V, 100 V
MIT481 50 V, 100 V, 250 V, 500 V, 1000 V plus PI, DAR
and result storage
MIT485 Same as Model 481, plus Bluetooth® download
MIT40X 10 V to 100 V in 1 V steps
MIT410
BATTERY TESTING GUIDE
29
Page 30

30 BATTERY TESTING GUIDE
Page 31

Page 32

Your “One Stop” Source for all your electrical test equipment needs
▪ Battery Test Equipment
▪ Cable Fault Locating Equipment
▪ Circuit Breaker Test Equipment
▪ Data Communications Test Equipment
▪ Fiber Optic Test Equipment
▪ Ground Resistance Test Equipment
▪ Insulation Power Factor (C&DF) Test Equipment
▪ Insulation Resistance Test Equipment
▪ Line Testing Equipment
▪ Low Resistance Ohmmeters
▪ Motor & Phase Rotation Test Equipment
▪ Multimeters
▪ Oil Test Equipment
▪ Portable Appliance & Tool Testers
▪ Power Quality Instruments
▪ Recloser Test Equipment
▪ Relay Test Equipment
▪ T1 Network Test Equipment
▪ Tachometers & Speed Measuring Instruments
▪ TDR Test Equipment
▪ Transformer Test Equipment
▪ Transmission Impairment Test Equipment
▪ Watthour Meter Test Equipment
▪ STATES® Terminal Blocks & Test Switches
▪ Professional Hands-On Technical and
▪ Safety Training Programs
Megger is a world leading manufacturer and supplier
of test and measurement instruments used within the
electric power, building wiring and telecommunication
industries.
With research, engineering and manufacturing
facilities in the USA, UK and Sweden, combined with
sales and technical support in most countries, Megger
is uniquely placed to meet the needs of its customers
worldwide.
UK
Archcliffe Road Dover
CT17 9EN England
T +44 (0) 1304 502101
F +44 (0) 1304 207342
USA
4271 Bronze Way
Dallas, TX 75237-1019 USA
T +1-800-723-2861
F +1-214-331-7399
USA
Valley Forge Corporate Centre
2621 Van Buren Avenue
Norristown, PA 19403 USA
T +1-610 676 8500
F +1-610-676-8610
GERMANY
Megger GmbH
Obere Zeil 2
DE-61440 Oberursel Germany
T +49 6171 929870
F +49 6171 9298719
SWITZERLAND
Megger Schweiz AG
Felsweg 1
Postfach 59
CH-5727 Oberkulm Switzerland
T +41 62 768 20 30
F +41 62 768 20 33
SWEDEN
Megger Sweden AB
Eldarvägen 4, Box 2970
SE-187 29 TÄBY Sweden
T +46 8 510 195 00
F +46 8 510 195 95
E seinfo@megger.com
For more information about Megger and its diversified
line of test and measurement instruments:
www.megger.com
Megger is certified according to ISO 9001 and 14001.
Megger is a registered trademark
Registered to ISO 9001 and 14001
Subject to change without notice.
Art.No. ZP-AD01E • Doc. AD0009CE • 2009
BatteryTestingGuide_AG_en_V03
www.megger.com
Megger is a registered trademark
Price: 5 €, 55 SEK
WWW.MEGGER.COM
 Loading...
Loading...