Medtronic Xomed XPS 3000 User manual

XPS®
Model 3000 System
Service Manual
1
™ Trademarks and ® are Registered marks of Medtronic Xomed Inc.
Patents Pending
Copyright© 2007 Medtronic Xomed, Inc.
Made in U.S.A.
MEDTRONIC XOMED INC.
6743 Southpoint Drive North
Jacksonville, FL 32216 USA
Authoriz
ed Representative (for EC regulatory matters)
Medtronic B.V.
Earl Bakkenstraat 10
6422 PJ Heerlen
The Netherlands
Tel.: +31 (0)45 5668000
Fax: +31 (0)45 566 8668
THIS MANUAL IS PROVIDED PRIMARILY FOR INFORMATION PURPOSES. ALTHOUGH
THERE ARE CERTAIN TROUBLESHOOTING ACTIONS THAT MAY BE ATTEMPTED BY THE
CUSTOMERE AS SPECIFICALLY LISTED IN THIS MANUAL, THE MAJORITY OF REPAIRS
MUST BE UNDERTAKEN BY MEDTRONIC XOMED OR ITS AUTHORIZED REPRESENTATIVE
IT OTHERWISE BEING UNSAFE TO MAINTAIN OR REPAIR THIS DEVICE.
NOTICE
2
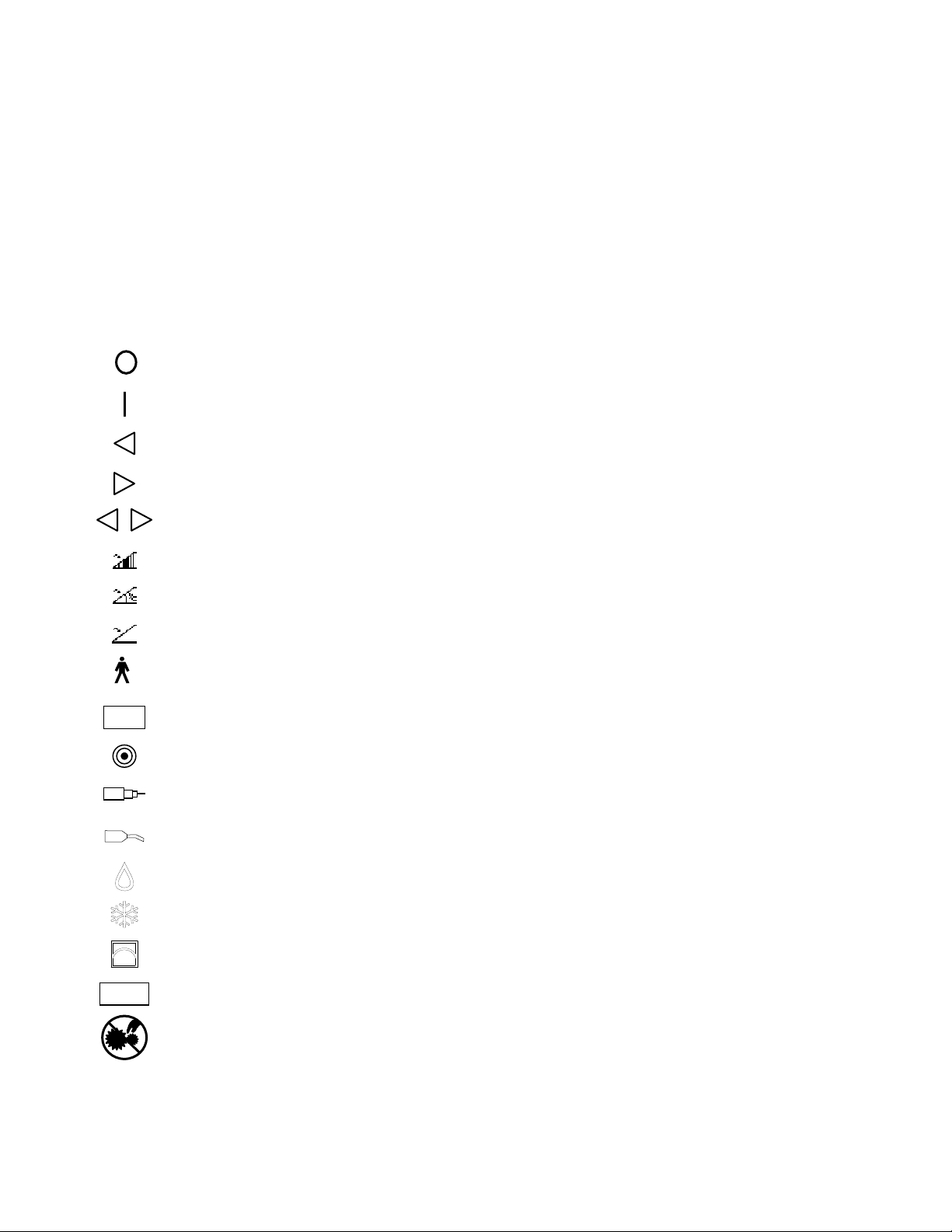
SYMBOLS
A ATTENTION, SEE INSTRUCTIONS FOR USE
C CATALOG NUMBER
N SERIAL NUMBER
D DATE OF MANUFACTURE
B LOT NUMBER
OFF
ON
REVERSE
FORWARD
FOOTSWITCH — VARIABLE MODE
FOOTSWITCH — NORMAL MODE
FOOTSWITCH
TYPE B APPLIED PART
ACCESSORY CONNECTOR
ACC
MANUAL START/STOP
HANDPIECE
SKEETER HANDPIECE
IRRIGANT
COOLANT
PUMP
MENU
MENU
CAUTION: PINCH HAZARD. KEEP FINGERS CLEAR OF ROLLERS.
OSCILLATION
3

WARNINGS AND PRECAUTIONS
IT IS IMPORTANT THAT THE XPS MODEL 3000 OPERATOR BE FAMILIAR WITH THIS MANUAL: ITS PRECAUTIONS,
PROCEDURES AND SAFETY ISSUES
CONDITIONS
, OR PROCEDURES:
“WARNING”
• Identifies conditions or practices that present a risk of injury to the patient and/or user.
" CAUTION / PRECAUTIONS"
y Identifies conditions or practices that could result in damage to the equipment.
"NOTE"
y Identifies special information and/or to clarify/emphasize important instructions.
WARNINGS
DO NOT operate the XPS Model 3000 in the presence of flammable anesthetics.
DO NOT use burs above the speed indicated on the bur label.
Use adequate irrigation. The use of a bur without irrigation may cause an inordinate amount of heat buildup
resulting in thermal injury to tissue.
Always keep the cutting tip away from fingers and loose clothing.
Operate the blade or bur only after the appropriate anatomical landmarks and the intended surgical site have
been confirmed.
Disconnect power to the XPS Model 3000 before cleaning the unit.
Blades, burs and irrigation tubing are disposable and intended for single-use only, UNLESS OTHERWISE
MARKED.
THE FOLLOWING WARNINGS PERTAINING TO SUCTION LIPOPLASTY SHOULD ALSO BE
CONSIDERED:
This device will not, in and of itself, produce significant weight reduction.
This device should be used with extreme caution in patients with chronic medical conditions, such as diabetes;
heart, lung, or circulatory system disease; or obesit y .
The volume of blood loss and endogenous body fluid loss may adversely affect intra and/or po stoperative
hemodynamic stability and patient safety. The capability of providing adequate, timely replacement is essential
for patient safety.
PRECAUTIONS
To achieve electrical grounding reliability, connect the XPS Model 3000 console to ho spital grade receptacles
only.
Only XPS High Speed Burs should be used above 6,000 rpm in forward with the StraightShot Magnum II
Handpiece.
XPS Microresector Blades should be operated in the oscillate mode only. Operating in the forward and reverse
modes may cause damage to the blade. XPS Microresector burs should be operated in the forward mode only.
The Visao/Xcalibur Hi-Speed Water-Cooled Drill is designed to work only with the XPS Model 3000. Use
without water-bag will damage the motor in the handpiece.
All reusable components of the device must be cleaned and sterilized and all disposable components replaced
before using the device system on another patient.
DO NOT allow saline/pump output to drip on console.
Blade and Bur accessories are available for resection of soft tissue and bone for various surgical procedures.
Use of this type of accessory is dependent on the intended application and patient needs. Sharp cutting powered
accessories are capable of inducing bleeding and removal of significant tissue and bone.
Use care in application of the moving cutting end to only appropriate anatomical landmarks and the intended
surgical site when using rotating burs and blades. The use of powered reciprocating instruments may result in
vibration related injury. Use appropriate precautions.
Adequate visualization is to be employed when using rotating burs and blades. Discontinue powered application
in the event of lack of visualization of the surgical site.
. THREE LABELS ARE USED IN THIS MANUAL TO IDENTIFY IMPORTANT CONCERNS,
4

Use methods at the operative site (airway, throat, head, neck or sinus cavity) to control bleeding that could
compromise patient safety during at-risk surgery.
DO NOT IMMERSE OR STERILIZE THE CONSOLE OR FOOTSWITCH.
DO NOT immerse PowerSculpt, StraightShot Magnum, Powerforma, Xcalibur, Skeeter, StraightShot Magnum
II handpieces, motors, or handpiece cables in any solution , except as detailed in the Cleaning section of this
manual.
Always inspect the components before and after use for any damage. If damage is observed, do not use the
system until it is repaired.
DO NOT use any parts other than Medtronic Xomed, Inc. components as damage or substandard operation
could result.
Temperatures higher than those stated may be used for handpiece sterilization when necessary to satisfy
governmental or health care facility requirements so long as the temperature does not exceed 149°C. Heating
above 149°C may damage components and will void the warranty.
Regardless of which type of steam sterilization is used, it is extremely important that the handpiece is rapidly
and completely dried before it is stored. If a vacuum drying cycle is not used following steam sterilization,
moisture may be trapped within the handpiece causing corrosion and residue deposits, resulting in premature
wear and a reduction in the functional life expectancy of the handpiece.
DO NOT use organic solvents such as acetone or isopropyl alcohol to clean the handpiece, motor or cable.
Attempted repair or evidence of attempted repair during the warranty period by anyone other than qualified
Medtronic Xomed, Inc. service personnel will void the equipment warranty.
This medical device complies with EN60601-1-2 safety standard for electromagnetic compatibility,
requirements and test. However, if this equipment is operated in the presence of high levels of EMI or highly
sensitive equipment, interference may be encountered and the user should take whatever steps are necessary to
eliminate or reduce the interference.
THE FOLLOWING PRECAUTIONS PERTAINING TO SUCTION LIPOPLASTY SHOULD ALSO BE
CONSIDERED:
• This device is designed to contour the body by removing localized deposits of excess fat through small
incisions.
• Use of this device is limited to those physicians who, by means of formal professional training or sanctioned
continuing medical education (including supervised operative experience), have attained proficiency in suction
lipoplasty.
• Results of this procedure will vary depending upon patient age, surgical site, and experience of the physician.
• Results of this procedure may or may not be permanent.
• The amount of fat removed should be limited to that necessary to achieve a desired cosmetic effect
5
CUSTOMER SERVICE
U.S. CUSTOMER SERVICE
General customer service and technical support are available toll-free:
800-874-5797 or 904-296-9600
Monday-Friday
8:00 AM - 6:00 PM E.S.T.
www.MedtronicENT.com
INTERNATIONAL SERVICE
International customers should contact their local MEDTRONIC XOMED office:
AUSTRALIA: 61-2-9879-5999
CANADA: 905-826-6020
FRANCE : 33-4-7067-9800
U.K.: 44-44-1923-212213
GERMANY: 49-211-529-3209
NETHERLANDS 31-45-566-8000
BELGIUM 32-2-4560900
THE XPS MODEL 3000 HELP LINE
Should you need immediate help with a technical question or guidance through the appropriate protocol,
just call the XPS Model 3000 Help Line at
1-800-874-5797.
NOTE
HEN CONTACTING OUR CUSTOMER SERVICE AND TECHNICAL SUPPORT, PLEASE HAVE THE
W
APPROPRIATE PRODUCT NUMBER
INQUIRY AVAILABLE
.
Product Number
Serial Number
Date of Purchase
, PRODUCT SERIAL NUMBER, DATE OF PURCHASE, AND NATURE OF
6

WARRANTY AND REPAIR
LIMITED WARRANTY
A. This LIMITED WARRANTY provides assura nce for the customer who purchases a Medtronic Xom ed Prod uct
(hereinafter the “Product”) that should the Product fail to function to Medtronic Xomed’s published specifications
during the term of this LIMITED WARRANTY (one year from the date of shipment for new Product, 90 days from
date of shipment for refurbished or used Product), Medtronic Xomed will either replace, repair, or issue a credit
(adjusted to reflect the age of the Product) for the Product or any portion thereof. This LIMITED WARRANTY is
extended only to the buyer purchasing the Product directly from Medtronic Xomed or from its affiliate or its
authorized distributor or representative.
B. To qualify for this LIMITED WARRANTY, the following conditions must be met:
(1) The Product must be used on or before its “Use By” or “Use Before” date, if applicable.
(2) The Product must be used in accordance with its labeling and may not be altered or subjected to misuse, abuse,
accident or improper handling.
(3) Medtronic Xomed must be notified in writing within thirty (30) days following discovery of a defect.
(4) The Product must be returned to Medtronic Xomed within thirty (30) days of Medtronic Xomed receiving notice
as provided for in (3) above.
(5) Upon examination of the Product by Medtronic Xomed, Medtronic Xomed shall have determined that: (i) the
Product was not repaired or altered by anyone other than Medtronic Xomed or its authorized representative, (ii) the
Product was not operated under conditions other than normal use, and (iii) the prescribed periodic maintenance and
services have been performed on the Product.
C. This LIMITED WARRANTY is limited to its express terms. THIS LIMITED WARRANTY IS IN LIEU OF
ALL OTHER WARRANTIES, EXPRESSED OR IMPLIED WHETHER STAT UTORY OR OTHERWISE,
INCLUDING ANY IMPLIED WARRANTY OF MERC H ANTABILITY OR FITNESS FOR A PARTICULAR
PURPOSE. In no event shall Medtronic Xomed be liable for any consequential, incidental, prospective or other
similar damage resulting from a defect, failure, or malfunction of the Product, whether a claim for such damage is
based upon the warranty, contract, negligence or otherwise.
D. The exclusions and limitations set out above are not intended to, and should not be construed so as to, contravene
mandatory provisions of applicable law. Users may benefit from statutory warranty rights under legislation
governing the sale of consumer goods. If any part or term of this LIMITED WARRANTY is held by any court of
competent jurisdiction to be illegal, unenforceable, or in conflict with applicable law, the validity of the remaining
portion of the LIMITED WARRANTY shall not be affected, and all rights and obligations shall be construed and
enforced as if this LIMITED WARRANTY did not contain the particular part or term held to be invalid.
CAUTION Attempted repair or evidence of attempted repair during the warranty period b y anyone other than
qualified Medtronic Xomed service personnel will void the equipment warranty.
A system that is not functioning properly should not be used until all necessary repairs have been made by
Medtronic Xomed Service Personnel and the unit is tested to ensure that it is functioning in accordance
with Medtronic Xomed specifications.
Domestic Repairs: When returning your system or components of your system for repair, please ensure
that it is carefully packed to avoid any damage during shipping.
Please include the nature of the problem and all information needed to return the repaired system to your
facility.
International Repairs: International customers should contact their local Medtronic Xomed office or local
distributors (See “Customer Service” section).
7

CAUTION
Powered Handpiece Service Note:
Irrigation fluids, especially saline, and residual moisture can accelerate wear and corrosion causing
premature failure of powered handpiece components. Care should be maintained to use a steam sterilizer
with a dry cycle to remove excess moisture.
HE HANDPIECE SHOULD BE EVALUATED PRIOR TO EACH USE FOR SUITABLE OPERATING CONDITION. A
T
HANDPIECE THAT OPERATES AT HIGH HEAT LEVELS
A NOISY CONDITION IS A SIGN THAT SERVICE IS NECESSARY
MEDTRONIC XOMED FOR SERVICE OR REPAIR. IT IS RECOMMENDED THAT A SECONDARY HANDPIECE BE
AVAILABLE TO MINIMIZE ANY DOWNTIME AND INCONVENIENCE TO THE SURGICAL STAFF
, STALLS OR OPERATES INTERMITTENTLY, OR OPERATES IN
. THE HANDPIECE SHOULD BE RETURNED TO
.
8

TABLE OF CONTENTS
Page Number
CHAPTER 1 INTRODUCTION
Introduction................................................................................................................11
CHAPTER 2 SERVICE/REPAIR PROCEDURE
U.S. Customers ..........................................................................................................13
International Customers.............................................................................................13
CHAPTER 3 FUNCTIONAL OVERVIEW
Front Console.............................................................................................................14
Rear Console..............................................................................................................15
XPS 3000 Set-up........................................................................................................15
Cable Connections .....................................................................................................15
Language Selection....................................................................................................15
Console Set-Up ..........................................................................................................16
Footswitches ..............................................................................................................17
CHAPTER 4 TECHNICAL SPECIFICATION
Straightshot Magnum II Handpiece...........................................................................18
Xcalibur Drill.............................................................................................................18
Powersculpt Handpiece..............................................................................................18
Powerforma Drill......................................................................................................19
Skeeter Ultra-Lite Oto-Tool.......................................................................................19
Console ......................................................................................................................19
Multifunction Footswitch...........................................................................................19
Single pedal Multifunction Footswitch......................................................................20
Single Function Footswitch.......................................................................................20
Compliance................................................................................................................20
Recommended Environmental Conditions................................................................20
CHAPTER 5 TROUBLESHOOTING GUIDE...................................................21-22
CHAPTER 6 VERIFICATION TEST
Materials And Equipment..........................................................................................23
Functional Testing .....................................................................................................23
Testing Console With Magnum II Handpiece...........................................................23
Rocker Switch............................................................................................................24
Touchpad Key Functions...........................................................................................24
Dual Footswitch ........................................................................................................25
Testing Console with Xcalibur High Speed Handpiece ............................................25
Xcalibur High Speed Test (60,000 + RPMS)............................................................26
Testing Console with Skeeter Handpiece..................................................................26
Test Endoscrub Pump................................................................................................27
Bio-Tek Safety Test...................................................................................................27
Dielectric Withstand Test ..........................................................................................27
9
 Loading...
Loading...