Page 1

A basic guide to nerve integrity monitoring
NIM™ 2.0 SYSTEMS:
Protocol and Troubleshooting Guide
Page 2
WELCOME
Medtronic ENT has developed this basic guide to nerve integrity monitoring as it
applies to the NIM™ 2.0 System family to assist in simplifying setup procedures. (The
NIM 2.0 System family includes the NIM-Response®2.0 and the NIM-Neuro®2.0.)
Each section gives procedure-specific protocols to follow and includes such information
as: monitoring goals, electrode placement, impedance values, difference values,
threshold and stimulation ranges, as well as samples of responses.
We hope that this booklet will be a helpful guide to follow and we appreciate
your patronage.
Important: This document is not intended to replace the surgeon’s medical judgment or knowledge of
neural anatomy and physiology. Nerve monitoring does not prevent the surgical severance of nerves.
Nerve monitoring with the NIM 2.0 System family or other monitors is only a technical aid and cannot
substitute for the skill, experience and anatomical knowledge of the surgeon.
The user should refer to Medtronic ENT’s Operations Manual for further instruction regarding any
equipment referenced in this document. Requisite training and know-how for performing evoked EMG
monitoring in surgical applications supplements the surgeon’s knowledge of nerve anatomy for
preservation of nerve function during surgical procedures.
Table of Contents
Intracranial (Skull Base)...........................................................................................................2-3
Cerebellopontine Angle Tumor • Vestibular Schwannoma
(acoustic neuroma) • Microvascular Decompression •
Trigeminal Nerve Resection • Vestibular Nerve Section
Intratemporal..............................................................................................................................4-5
Facial Nerve Decompression • Mastoidectomy •
Tympanoplasty • Cochlear Implantation • Translabrynthine
Approach to Posterior Fossa • Labyrinthectomy
Extratemporal.............................................................................................................................6-7
Parotidectomy • Submandibular Gland Dissection •
Head and Neck Dissection • Congenital Aural Atresia
Neck Dissections.......................................................................................................................8-9
Thyroidectomy • Parathyroidectomy • Radical Neck
Dissection • Anterior Cervical Fusion • Substernal Goiter •
Hemithyroidectomy
Monitoring Tips.......................................................................................................................10-13
Tips for Reducing Artifact • Verifying Stimulus Delivery •
Examples of EMG Response: Stimulated EMG Response,
Mechanically Evoked EMG Response, Train Response,
Metal Artifact, Electrode Placement
Troubleshooting Guide .........................................................................................................14-15
1
Page 3

2
Skull base procedures may place at risk
that segment of the facial nerve (Cranial
Nerve VII) from the brainstem through
the internal auditory canal (IAC). Tumors
may be located either adjacent to the
brain stem or within the IAC and are
often intimately involved with the facial
nerve. In order to remove the tumor,
there will be considerable drilling of bone
which may produce heat that may affect
the nerve. Nerve dissections often
involve direct manipulation, stretching or
traction of the facial nerve. The facial
nerve is nonmyelinated at this section,
hence lesser amounts of stimulation will
produce an EMG effect.
If other cranial motor nerves are at risk,
additional electrodes may be placed to
monitor EMG activity in the appropriate
muscle. Additional cranial nerves that
are commonly monitored in skull base
procedures include the trigeminal nerve
(Cranial Nerve V) and the vagus nerve
(Cranial Nerve X).
As a general rule, when shunting current
directly through bone, you will need 1.0mA
of stimulus for every millimeter of bone
above the nerve. Shunting through soft
tissue will usually require a setting of at
least 0.8mA.
Monitoring Goal
Locate, identify, and map the nerve.
Monitor manipulation effect.
It is extremely valuable for the surgeon to verify nerve integrity prior to closing by
stimulating proximal to the tumor site BEFORE and AFTER the surgery.
Cerebellopontine Angle Tumor
Vestibular Schwannoma
(acoustic neuroma)
Microvascular Decompression
Trigeminal Nerve Resection
Vestibular Nerve Section
INTRACRANIAL (SKULL BASE)
Procedures
Page 4
3
INTRACRANIAL (SKULL BASE)
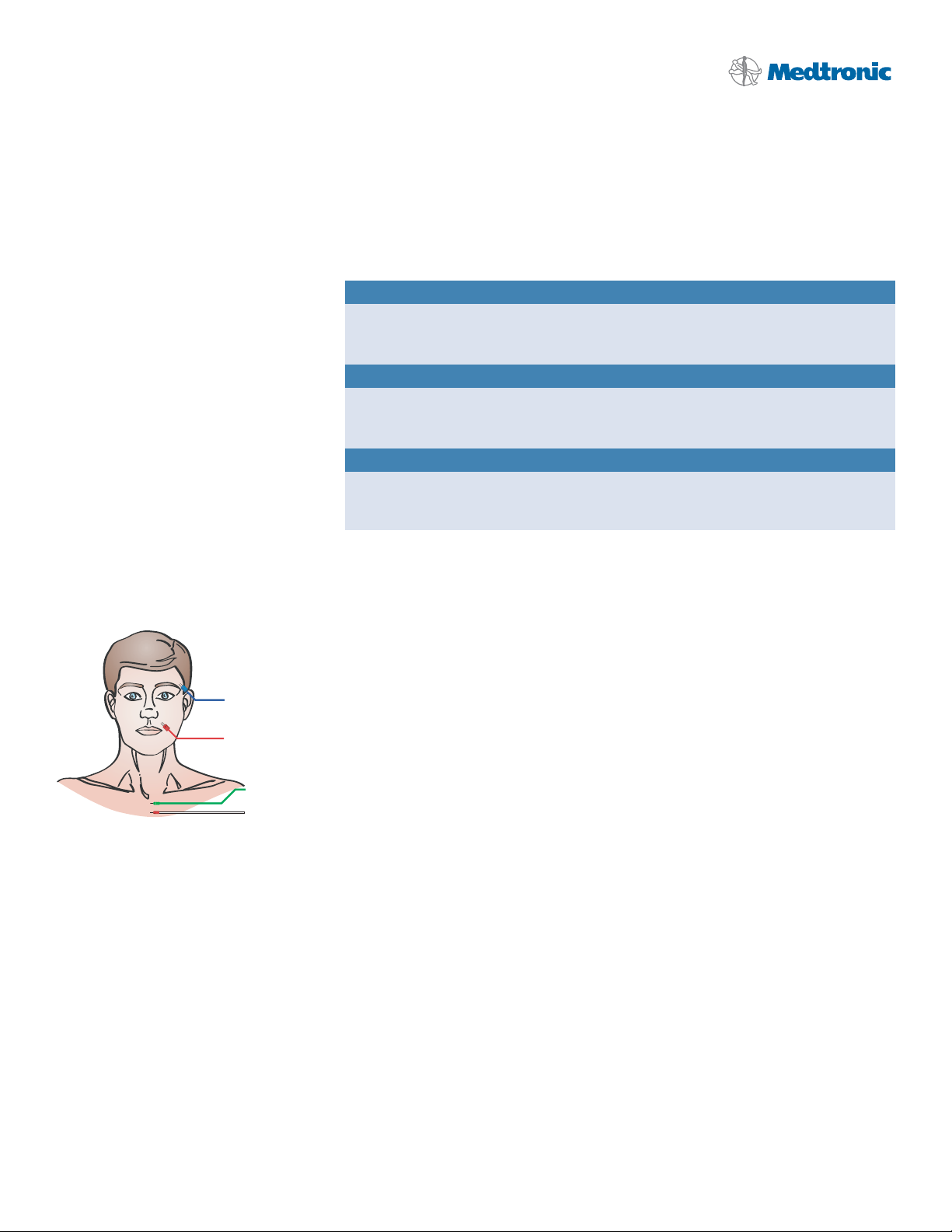
Electrode Placement: Figure 1.
Procedure Specific Protocols
Suggested Electrodes Part No.
• Paired Subdermal Electrodes 8227410
• Prass Paired Electrodes, 18mm 8227304
Suggested Stimulator Probes Part No.
• Prass Standard Monopolar Probe 8225101
• Prass Bipolar Probe 8225451
Suggested Stimulus Instruments Part No.
• Kartush Dissection Set (KSD) 1352400
• Neurotologic Dissection Set (NSD) 1353400
Typical Electrode Readings Channel Values Difference Values
Subdermal needle < 10 KΩ < 1 KΩ
Prass paired needle < 25 KΩ < 5 KΩ
Event Threshold Range
Event Threshold Range is 50uV-100uV. You may wish to increase threshold in case of an
EMG train response.
Stimulation Range
Use 0.8mA to begin, in general, unless otherwise directed by the surgeon at the start
of surgery when the monopolar probe is in use with goal of mapping the nerve.
Increase the stimulus setting until an EMG response has been elicited. At this stage,
there will be tissue and bone through which the current must travel. Once the nerve
has been located, reduce the stimulus level. There is rarely a need to use more than
1.0mA in the cerebellar pontine angle (CPA) because it is possible to place a stimulator
directly on the nerve. The Kartush Bipolar Probe is good to use when the nerve is
exposed because current is not shunted and low stimulus levels can be used.
Operative Side
Orbicularis Oculi m.
Orbicularis Oris m.
Ground
Stim Return (+)
Page 5

4
The facial nerve may be at risk during
surgeries involving the temporal bone
because the exact location of the nerve
varies by patient. This is especially true
for revision cases. These procedures,
generally, are “quiet surgeries” without a
lot of artifact.
Intraoperative facial nerve monitoring
(IFNM) allows the surgeon to avoid the
nerve. In procedures with considerable
bone drilling, heating may affect the
nerve. In nerve decompressions, IFNM
can aid in pinpointing that portion of the
nerve to be decompressed.
Monitoring Goal
Locate, identify, and map the nerve.
Monitor manipulation effect.
It is extremely valuable for the surgeon to verify nerve integrity prior to closing by
stimulating after the surgery.
Facial Nerve Decompression
Mastoidectomy
Tympanoplasty
Cochlear Implantation
Translabrynthine Approach
to Posterior Fossa
Labyrinthectomy
INTRATEMPORAL
Procedures
Page 6
5
INTRATEMPORAL
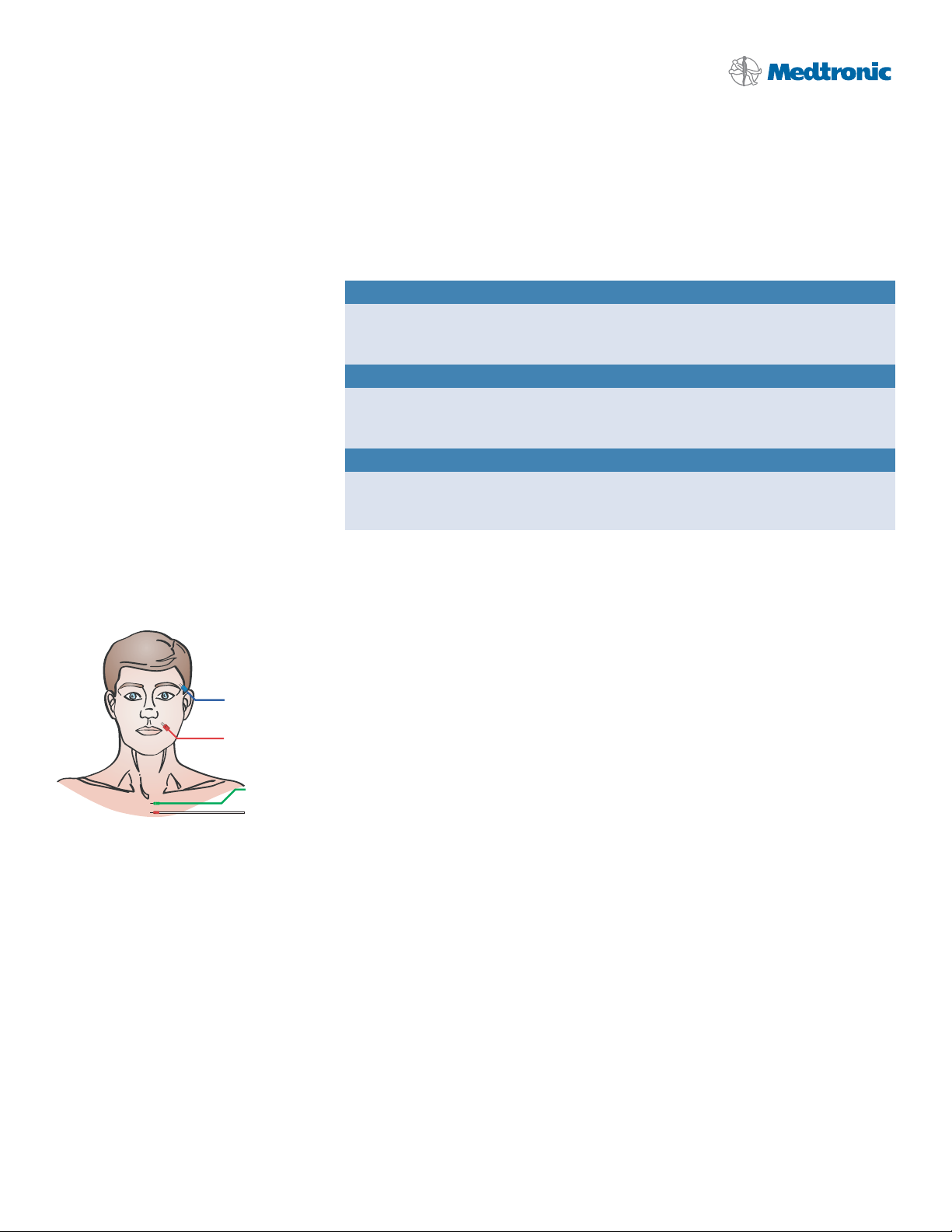
Electrode Placement: Figure 2.
Procedure Specific Protocols
Suggested Electrodes Part No.
• Paired Subdermal Electrodes 8227410
• Prass Paired Electrodes, 18mm 8227304
Suggested Stimulator Probes Part No.
• Prass Standard Monopolar Probe 8225101
• Kartush Side-by-Side Bipolar Probe 8225401
Suggested Stimulus Instruments Part No.
• Kartush Dissection Set (KSD) 1352400
• Neurotologic Dissection Set (NSD) 1353400
Typical Electrode Readings Channel Values Difference Values
Subdermal needle < 10 KΩ < 1 KΩ
Prass paired needle < 25 KΩ < 5 KΩ
Event Threshold Range
Event Threshold Range is 50uV-100uV. May decrease to 50uV to see earliest response.
Stimulation Range
Use 0.8mA at the beginning (mapping) of the procedure or as directed by the surgeon.
Hold the probe tip perpendicular to the tissue for approximately one second to elicit
a response.
There is rarely a need to use more than 1.0mA in the cerebellar pontine angle (CPA)
because it is possible to place a stimulator directly on the nerve. Use lower stimulus
levels as directed by the surgeon and increase slowly until a response is obtained.
Operative Side
Orbicularis Oculi m.
Orbicularis Oris m.
Ground
Stim Return (+)
Page 7

6
Most common in this group for facial
nerve monitoring is the parotidectomy.
The facial nerve enters the parotid gland
as one bundle, then splits into five
different branches within the body of the
parotid gland. To monitor a given nerve
branch, electrodes must be placed in the
muscle that it innervates. The nerve is
myelinated now, hence more stimulus is
usually necessary to evoke a response.
In general, parotidectomies are “noisy
surgeries” due to potential manipulation
of the nerve during blunt dissection.
Also, the electrodes are in close
proximity to the surgical site.
Monitoring Goal
Locate, identify, and map the nerve and branches.
Monitor manipulation effect.
It is extremely valuable for the surgeon to verify nerve integrity prior to closing by
stimulating proximal to the tumor site BEFORE and AFTER the surgery.
Parotidectomy
Submandibular Gland Dissection
Head and Neck Dissection
Congenital Aural Atresia
EXTRATEMPORAL
Procedures
Page 8

7
EXTRATEMPORAL
Electrode Placement: Figure 3.
Procedure Specific Protocols
Suggested Electrodes Part No.
• Paired Subdermal Electrodes 8227410
• Prass Paired Electrodes, 18mm 8227304
Suggested Stimulator Probes Part No.
• Prass Standard Monopolar Probe 8225101
• Kartush Side-by-Side Bipolar Probe 8225401
Typical Electrode Readings Channel Values Difference Values
Subdermal needle < 10 KΩ < 1 KΩ
Prass paired needle < 25 KΩ < 5 KΩ
Event Threshold Range
Event Threshold Range is 100uV-150uV.
Stimulation Range
Use 0.8mA at the beginning of the procedure (mapping) or as directed by the surgeon.
Increase rapidly until a response is evoked. May need to increase to 3.0mA.
The best guideline for setting the stimulus intensity level is to use the lowest amount
of stimulation needed to produce an EMG response large enough for monitoring.
Be prepared to stimulate to as high as 3mA if necessary. Apply probe perpendicular
to tissue for approximately one second to elicit a response.
Operative Side
Frontalis m.
Orbicularis Oculi m.
Orbicularis Oris m.
Mentalis m.
Ground
Stim Return (+)
Page 9

8
Intraoperative monitoring of the
Recurrent Laryngeal Nerve – branch of
the Vagus (Cranial Nerve X). This
surgery may be “noisy” due to potential
manipulation of the nerve during blunt
dissection. Also, the EMG tube is in close
proximity to the surgical site which may
produce some artifact.
Tips for Monitoring during Anterior
Cervical Surgery
The EMG Endotracheal Tube should be
utilized as for thyroidectomy. It may not
be possible to directly stimulate the
Recurrent Laryngeal Nerve as it may
not be exposed. However, the NIM 2.0
System family will passively monitor
traction and pressure status of the nerve
and is especially helpful while the
Thompson Retractor is being positioned
and extended.
Monitoring Goal
Map the nerve, monitor manipulation effect, and verify nerve integrity.
It is extremely valuable for the surgeon to verify nerve integrity prior to moving to the
contralateral lobe dissection and prior to closing. Stimulate proximally and distally to
the tumor site.
Thyroidectomy
Parathyroidectomy
Radical Neck Dissection
Anterior Cervical Fusion
Substernal Goiter
Hemithyriodectomy
NECK DISSECTIONS
Procedures
Page 10

9
NECK DISSECTIONS
NIM™ Standard
Electrode Placement: Figure 4b.
Procedure Specific Protocols
Suggested Electrodes Part No.
NIM™ EMG Reinforced Endotracheal Tubes 8229XXX
See catalog for choice of sizes.
• Usually use one size larger than normal.
• Use only non anaesthetic lubricants like K-Y®Jelly.
• Place EMG tube in the midline & with electrodes in contact with vocal cords.
Secure tube to midline of mouth (not to the side). Temporarily deflate cuff for
adjusting position. Complete instructions are provided in the package insert.
• Visualize electrodes contacting true vocal cords.
Suggested Stimulator Probes Part No.
• Prass Standard Monopolar Probe 8225101
• Kartush Side-by-Side Bipolar Probe 8225401
Typical Electrode Readings Channel Values Difference Values
EMG tube < 10 KΩ < 1 KΩ
Event Threshold Range
Event Threshold Range is 100uV-150uV.
Stimulation Range
The Stimulation Range is approximately 1.0mA. The best guideline for setting the
stimulus intensity level is to use the lowest amount of stimulation needed to produce
an EMG response large enough for monitoring. If stimulation levels are too high, nerve
fibers of adjacent branches may be stimulated. Be prepared to stimulate at a higher
level if necessary. Apply probe perpendicularly to tissue for about a second to elicit
a response.
If there is no response, shunt stimulus in the area just below the Adam’s apple
(cricothyroid), through the muscle. If there is response, the tube is probably too low
through the vocal cords and will need to be repositioned.
NIM Contact™ Tube
Electrode Placement: Figure 4.
MAINTAIN PATIENT’S
EMG TUBE MIDLINE
Operative Side
Right Vocal Fold
Left Vocal Fold
MAINTAIN PATIENT’S
EMG TUBE MIDLINE
Operative Side
Right Vocal Fold
Left Vocal Fold
Ground
Stim Return (+)
Ground
Stim Return (+)
Page 11

10
Verifying Stimulus Delivery
The “Stimulus Tone” or “Stimulus Voice”
can be heard if stimulus is flowing to the
surgical site from the probe tip. Stimulus
delivery can also be confirmed by
comparing the Stimulus setting with the
Stimulus Measure readings (mA). The
stimulus measure reading will be found on
the top right-hand side of the screen.
The value should be approximately the
same as the stimulus setting.
Tips for Reducing Artifact
• Keep the NIM 2.0 Systems away from
the electrosurgical unit and other
electrical equipment.
• Ensure electrode cables are not
intertwined with stim anode return
or with stimulator probe cable.
• Utilize a bipolar stimulating probe
as soon as the nerve is exposed to
minimize artifact. Current is flowing in
a very small area and stim levels can
be reduced accordingly. Also, dry the
surgical area before stimulating, as fluid
can shunt current. Keep bipolar tips dry
to prevent shunting.
• Minimize excessive stimulating current.
Each patient’s tissue conducts
electrical energy differently. To
decrease artifactual interference, it is
better to establish effective levels of
stimulating current as soon as possible
to keep the current to a minimum.
Begin stimulation with the lowest level,
0.05mA, with the Event Threshold at
100uV. Increase stimulus intensity fairly
quickly until raw EMG sound is heard.
This is the lowest level of stimulation
which will evoke a response. Increase
stimulus until an audible event tone
occurs (the four-times-per-second
tone we expect to hear when
stimulating the nerve). Set the
stimulation to at least this value.
• Note the reading next to the amplitude
shown on the screen and adjust the
Event Threshold slightly lower than
that value. This will effectively screen
out unwanted artifactual EMG activity.
• As an option in head and neck
dissections, you may change the
Stimulus Filter on the menu. For
otology and skull base procedures,
verify that the artifact delay marker
is set to 3.1 milliseconds.
• If necessary, owing to frequent artifacts
tones, disable the “Tone Audio”. Yo u
must now rely on raw EMG only.
MONITORING TIPS
Page 12

11
MONITORING TIPS
Procedure Specific Protocols
Example of EMG Responses
Interpretation of events by the surgeon is enhanced by knowing the characteristics of the
waveform, peak-to-peak amplitude, strength of the audio signal, and the surgical context.
Stimulated EMG Response
Cause: Electrical stimulation
Sound: Precisely timed clicks, “machine gun”, four times per second
Mechanically-Evoked EMG Response
Cause: Direct surgical manipulation
Sound: Few clicks synchronous with manipulation
Page 13

12
MONITORING TIPS
Train Response
Cause: Traction, Pressure, Irrigation, Caloric (hot or cold)
Sound: Repetitive asynchronous clicks, “popping corn”
Static Metal-to-Metal Artifact
Cause: Metal-to-metal (“banging of instruments”)
Sound: “Pop”
References
This selection of reference clinical articles is provided for additional background material related to the monitoring procedures herein. The health care professional
should seek and review all other clinical reference materials as dictated by an individual patient’s clinical condition.
1. Neuromonitoring in Otology and Head and Neck Surgery, Jack Kartush, MD and Kenneth R. Bouchard, Ph.D., Raven Press, New York, 1992
2. Handbook of Intraoperative Monitoring, Douglas L. Beck, M.A., Singular Publishing Group, Inc., 1994
3. Intraoperative Neurophysiologic Monitoring. Aage R. Miller, Harwood Academic Publishers, 1995
Page 14

13
MONITORING TIPS
Facial Cranial Nerve VII - 2 Ch.
Facial Cranial Nerve VII - 4 Ch.
Extraocular Cranial Nerve III, IV, VI
Trigeminal Cranial Nerve V
Ground
Stim Return (+)
Operative Side
Glossopharyngeal m.
Glossopharyngeal Cranial Nerve IX
Spinal Accessory Cranial Nerve XI
Hypoglossal Cranial Nerve XII
Procedure Specific Protocols
Electrode Placement
The surgeon will insert electrodes into the appropriate muscle location innervated by the monitored nerve. Additionally, a ground
electrode (green) and a stim return (white) are needed to complete the electrode setup.
Vagus Cranial Nerve X
-(VI N.)
-(IV N.)
-(III N.)
Operative Side
+(VI N.) Sup. oblique m.
+(IV N.) Lat. rectus m.
+(III N.) Inf. rectus m.
Ground
Stim Return (+)
Operative Side
Frontalis m.
Orbicularis Oculi m.
Orbicularis Oris m.
Mentalis m.
Ground
Stim Return (+)
Operative Side
Temporalis m.
Masseter m.
Ground
Stim Return (+)
Operative Side
Orbicularis Oculi m.
Orbicularis Oris m.
Ground
Stim Return (+)
Operative Side
Hypoglossal m.
Ground
Stim Return (+)
MAINTAIN PATIENT’S
EMG TUBE MIDLINE
Operative Side
Right Vocal Fold
Left Vocal Fold
Ground
Stim Return (+)
MAINTAIN PATIENT’S
EMG TUBE MIDLINE
Operative Side
Right Vocal Fold
Left Vocal Fold
Ground
Stim Return (+)
Operative Side
Sternocleidomastoid m.
Trapezius m.
Ground
Stim Return (+)
Page 15

Symptom Cause Solution
TROUBLESHOOTING GUIDE
Please refer to the NIM-Response® 2.0 System User’s Guide (82-50651) or the NIM-Neuro® 2.0
System User’s Guide (82-50650) for complete operating instructions.
No visual display or audio alarms
at power-up.
Touching the screen has
unexpected results.
Electrode impedance is too high.
>10K
Ω for subdermal electrodes
>25K
Ω for Prass Paired electrodes
>10K
Ω for EMG tube
>40K
Ω for hookwire electrodes
Electrode impedance = 0.0K
Ω.
Channel button is flashing.
Electrode reading is “— K
Ω”
or “OFF.”
Electrode difference is greater
than 2KΩ (subdermal electrodes)
or 10K
Ω (Prass Paired electrodes).
Electrosurgical interference.
Interference with anesthesia
equipment.
Excessive muting.
Inadequate muting.
Power cord not connected to outlet or to
the NIM™ 2.0 system.
Power switch not turned on.
Touch screen out of calibration.
Electrode dislodged from patient, but not
completely out.
High resistance in electrode.
Electrode pin not firmly inserted into Patient
Interface.
Positive and negative electrodes touching
below surface of skin.
Extremely low impedance, particularly in EMG
tubes.
Electrode laying on skin surface.
Electrode placement insecure.
Dirty electrode tip.
Electrode cable broken.
Electrode pin disconnected from
Patient Interface.
Dirty electrode.
Mismatched pair.
Unequal placement.
Muting Probe not connected.
Muting Probe input insufficient.
Electrosurgical grounding inadequate.
Source of interference unidentified.
NIM 2.0 system or Patient Interface cable too
close to ESU or its cables.
Lead checking current near anesthesia electrodes.
Unit receiving excessive signal into the Muting
Probe or electrode leads.
Signal from ESU is inadequate to cause muting.
Plug in power cord.
Turn power switch on.
Turn unit off, then press the screen until the screen
calibration test is displayed. Follow the instructions on
the screen to recalibrate.
Insert dislodged electrode; tape down in place.
Remove and replace with new electrode.
Check connection at Patient Interface box.
Remove and relocate electrodes.
Use “tap test” near electrodes to evoke EMG or artifact.
If activity is noted on channel in question, proceed.
Re-insert electrode in question.
Remove and replace electrode in question.
Check connection to Patient Interface box.
Remove and replace electrode for appropriate channel
with highest impedance reading first.
Remove and replace electrode in question.
• Check Muting Probe connections.
• Move input to “MORE MUTE”.
• Check electrosurgical grounding pad on patient.
• Identify source of interference; then eliminate or
separate from the NIM 2.0 system.
• Maintain separation between electrosurgical cable and
the NIM 2.0 system.
• For less coupling, coil up the Muting Probe next to the
NIM 2.0 system.
Have anesthesia try alternate electrode channel.
Turn stimulator to 0.0mA when not stimulating.
Move the Muting Probe connector to a lower number
unit until it stops muting. If it still mutes in position “1,”
disconnect the muting detector completely.
Move the Muting Probe connector to a higher number
until it mutes. If it still does not mute in position “4,”
loop the ESU cable and clip the muting detector over
the doubled cable.
Page 16

TROUBLESHOOTING GUIDE
No response to direct stimulation.
Unexpected responses when
not directly stimulating nerve.
Inadequate stimulus intensity.
Paralyzing anesthetic in use.
White stimulation (+) electrode has fallen out
or is not connected.
Probe not connected.
Patient safety fuse blown.
Not holding probe on nerve long enough.
Nerve not contacted.
Volume control too low.
Event threshold set too high.
Excessive current shunting in surgical field.
No electrodes in innervated muscle.
Nerve not stimulable.
Unexplained continuous “train”
EMG response.
Nerve or monitoring area being stimulated
or manipulated by thermal or mechanical
means.
Metal-to-metal discharge artifact.
Intertwined recording electrode and
stimulator wires.
Inadvertent manipulation of electrode wires,
Patient Interface cable, or recording area on
patient.
Electrical interference from other equipment.
Increase stimulus intensity.
Eliminate paralyzing anesthetic.
• Check that Stimulus Measure is approximately the
same value as the Stimulus setting. Re-insert electrode
in question.
• Secure all recording and stimulating electrode
connections and check the impedance values.
Check stimulator anode(+) and cathode (-) connections.
Check fuse in Patient Interface box (32 mA, x 250V).
Replace if necessary.
Hold probe tip to nerve for at least 1 S.
• Check stimulator tip for obstruction.
Replace if necessary.
• Check location of stimulation.
Check and correct all settings: volume, event threshold,
stimulus intensity.
Remove fluids from surgical stimulating area.
Place channel electrodes in muscle to be monitored.
Identify and eliminate possible source of “train”
stimulation:
• Cold irrigation
• Laser heat
• Retraction on nerve or muscles being recorded
• Patient waking from anesthesia
• Nerve drying
• Ultrasonic aspirator
Identify and eliminate source of inadvertent
manipulation.
Determine response type from waveform pattern on
50 mS screen.
Disentangle recording electrode and stimulator cables.
Check area near recording electrodes for excessive
stretching from tape, drapes, etc.
Check for intermittent stimulation from anesthesiologist
(i.e., hand-held electrical stimulator).
Move NIM 2.0 system away from source of interference.
Make sure Patient Interface cable and electrode wires
do not cross other electrical equipment or cables.
Symptom Cause Solution
Page 17

Medtronic ENT
Medtronic USA, Inc.
6743 Southpoint Drive North
Jacksonville, FL 32216
USA
www.MedtronicENT.com
Toll free: (800) 874-5797
Fax: (800) 678-3995
890403 06.07 2007-341 Rev 1
This literature is intended for the exclusive use of physicians. Rx only.
©2007, Medtronic, Inc.
®
Registered mark of Medtronic, Inc. ™Trademark of Medtronic, Inc.
K-Y
®
is a registered mark of Johnson & Johnson Medical, Inc.
International Telephone Numbers
Australia: 1-800-668-670
Belgium: 32-2456-09-09
Canada: 1-800-217-1617
China: 86-21-50800998
France: 33-470-679-800
Germany: 49-211-5293-209
Hong Kong: 852-2919-1312
India: 91-22-26836733
Italy: 39-02-24137-324
Japan: 81-6-4795-1506
Korea: 82-2-3404-3600
Lebanon: 961-1-370-670
Luxembourg: 32-2456-09-09
Netherlands: 31-45-566-8371
Poland: 48-22-465-6942
Singapore: 65-6776-6255
Spain: 34-91-625-05-40
UK: 44-1923-205-166
USA: 1-904-296-9600
For further information, please call
Medtronic ENT at 800-874-5797 or
904-296-9600. You may also consult
our website at ww.MedtronicENT.com.
 Loading...
Loading...