Page 1

Azure™ S SR MRI SureScan™ W3SR01
MR Conditional single chamber pacemaker with SureScan™ technology and Bluetooth
wireless telemetry (OOE-VVIR)
®
Device Manual
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Page 2

The following list includes trademarks or registered trademarks of Medtronic in the United States and
possibly in other countries. All other trademarks are the property of their respective owners.
Advisa, Advisa DR MRI, Azure, Capture Management, CareAlert, CareLink, EnRhythm, EnRhythm MRI,
Flashback, Integrity, Kappa, Medtronic, Medtronic CareAlert, Medtronic CareLink, Quick Look, Revo MRI,
SureScan
Page 3

Contents
1 System overview 4
1.1 Introduction 4
1.2 System description 4
1.3 Indications and usage 5
1.4 Contraindications 5
1.5 MRI conditions for use 6
1.6 Feature summary 6
1.7 Data security 7
2 Warnings, precautions, and potential adverse events 8
2.1 General warnings and precautions 8
2.2 Explant and disposal 8
2.3 Handling and storage instructions 8
2.4 Lead evaluation and lead connection 9
2.5 Device operation 9
2.6 Potential adverse events 10
3 Clinical data 11
3.1 Adverse events and clinical trial data 11
4 Implant procedure 12
4.1 Preparing for an implant 12
4.2 Selecting and implanting the lead 13
4.3 Testing the lead system 14
4.4 Connecting the lead to the device 15
4.5 Positioning and securing the device 16
4.6 Completing the implant procedure 17
4.7 Replacing a device 17
5 Product specifications 18
5.1 Physical characteristics 18
5.2 Electrical specifications 19
5.3 Replacement indicators 20
5.4 Projected service life 21
6 Device parameters 23
6.1 Emergency settings 23
6.2 Magnet application 23
6.3 Tachyarrhythmia detection parameters 23
6.4 Pacing parameters 24
6.5 Data collection parameters 26
6.6 Medtronic CareAlert parameters 26
6.7 System test parameters 27
6.8 EP Study parameters 27
6.9 Nonprogrammable parameters 29
3
Page 4

1 System overview
1.1 Introduction
This manual describes the Medtronic Model W3SR01 Azure S SR MRI SureScan single chamber, implantable
pulse generator (IPG). It contains model-specific feature information, indications and contraindications, warnings
and precautions, instructions for implanting the device, quick reference specifications, and parameter tables.
The following manuals and documents also contain information about the device:
MRI technical manual – This manual provides MRI-specific procedures and warnings and precautions.
Reference manual – This manual contains information about device features. The reference manual applies to
multiple models of IPG devices.
Programming guide – This manual explains how to use the programmer software to conduct a patient session.
Explanation of symbols – This document defines the symbols that may appear on the device package. Refer to
the package label to see which symbols apply specifically to this device.
Medical Procedure and EMI Warnings and Precautions Manual for Health Care Professionals – This
manual provides warnings, precautions, and guidance for health care professionals who perform medical
therapies and diagnostic procedures on cardiac device patients. The manual also provides patient education
information related to sources of electromagnetic interference (EMI) at home, at work, and in other environments.
Radio regulatory compliance information – This document provides compliance information related to the
radio components of the device.
1.2 System description
The Medtronic Azure S SR MRI SureScan Model W3SR01 single chamber implantable pulse generator (IPG) is
a multiprogrammable cardiac device that monitors and regulates the patient’s heart rate by providing single
chamber rate-responsive bradycardia pacing. This device features Bluetooth wireless technology.
1
The MRI SureScan feature permits a mode of operation that allows a patient with a SureScan system to be safely
scanned by an MRI machine while the device continues to provide appropriate pacing. When programmed to On,
MRI SureScan operation disables arrhythmia detection, magnet mode, and all user-defined diagnostics. Before
performing an MRI scan, refer to the MRI technical manual.
Rate response – Rate response is controlled through an activity-based sensor.
The users of this device include medical professionals (physicians, nurses, technicians, and their supporting staff)
trained in surgery, cardiology, radiology, and magnetic resonance (MR) technology and able to implement the
procedures documented in the instructions for use for this device.
1.2.1 Usage environments
The device is intended to be used in the following environments and conditions:
●
The device will be implanted in a properly equipped, staffed, and sterile surgical environment. Implant will take
place under standard surgical protocols and in the patient population for which the device is indicated.
●
Post-surgical patient and device follow-up care will take place in a properly equipped and staffed cardiology
clinic or office.
●
MRI procedures for patients with this device will take place in a properly equipped and staffed MR facility, and
in consideration of the conditions and requirements described in Section 1.5, “MRI conditions for use”,
page 6.
1
The Bluetooth® word mark is a registered trademark of Bluetooth SIG, Inc. and any use of this mark by
Medtronic is under license.
4
Page 5

●
After having an implant, patients may resume their lives at home, at work, and in other environments with
consideration of the advice and restrictions documented in the Medical Procedure and EMI Warnings and
Precautions Manual for Health Care Professionals and in the patient literature.
1.2.2 System components and accessories
Contents of sterile package – The package contains 1 implantable pulse generator (IPG) and 1 torque wrench.
Implantable device system – The Azure S SR MRI SureScan Model W3SR01 device and the pacing lead
constitute the implantable portions of the device system.
Lead – The lead system used with this device must provide sensing and pacing to the right ventricle (RV). Do not
use any lead with this device without first verifying lead and connector compatibility.
For information about selecting and implanting a SureScan lead for this device, refer to Section 4.2, “Selecting and
implanting the lead”, page 13.
Programmers and software – Medtronic programmers and software are used to program this device.
Programmers from other manufacturers are not compatible with Medtronic devices, but they do not damage
Medtronic devices.
Medtronic pacing system analyzer – A pacing system analyzer is used to measure the electrical characteristics
of the implanted lead to assess its effectiveness for pacing and sensing.
Medtronic patient monitor – Patients use the Medtronic patient monitor, if available, to gather information from
their implanted devices and communicate the information to their physicians through the Medtronic CareLink
Network. For information on using the patient monitor, refer to the patient monitor literature.
1.3 Indications and usage
The Azure S SR MRI SureScan W3SR01 system is indicated for the following conditions:
●
Rate adaptive pacing in patients who may benefit from increased pacing rates concurrent with increases in
activity
●
Accepted patient conditions warranting chronic cardiac pacing, which include:
– Symptomatic paroxysmal or permanent second- or third-degree AV block
– Symptomatic bilateral bundle branch block
– Symptomatic paroxysmal or transient sinus node dysfunctions with or without associated AV conduction
disorders
– Bradycardia-tachycardia syndrome to prevent symptomatic bradycardia or some forms of symptomatic
tachyarrhythmias
1.4 Contraindications
The Azure S SR MRI SureScan Model W3SR01 system is contraindicated for:
●
Concomitant implant with another bradycardia device
●
Concomitant implant with an implantable cardioverter defibrillator
There are no known contraindications for the use of pacing as a therapeutic modality to control heart rate. The
patient’s age and medical condition, however, may dictate the particular pacing system, mode of operation, and
implant procedure used by the physician.
●
Rate-responsive modes may be contraindicated in those patients who cannot tolerate pacing rates above the
programmed Lower Rate.
●
Asynchronous pacing is contraindicated in the presence (or likelihood) of competition between paced and
intrinsic rhythms.
●
Single chamber atrial pacing is contraindicated in patients with an AV conduction disturbance.
5
Page 6

1.5 MRI conditions for use
A complete SureScan pacing system is required for use in the MR environment. A complete SureScan
pacing system includes a SureScan device with a Medtronic SureScan lead. To verify that components are
part of a SureScan system, visit http://www.mrisurescan.com. Any other combination may result in a hazard to the
patient during an MRI scan.
Warning: Do not scan a patient without first programming the MRI SureScan mode to On. Scanning the patient
without programming the MRI SureScan mode to On may result in patient harm or damage to the SureScan pacing
system.
Note: The MRI SureScan mode cannot be programmed to On if the device is recommended for replacement.
Cardiology requirements
Patients and their implanted systems must be screened to meet the following requirements:
●
The patient has no implanted lead extenders, lead adaptors, or abandoned leads.
●
The patient has no broken leads or leads with intermittent electrical contact, as confirmed by lead impedance
history.
●
The SureScan pacing system is implanted in the left or right pectoral region.
●
The pace polarity parameters are set to Bipolar for programming the MRI SureScan mode to On.
●
The SureScan device is operating within the projected service life.
●
For patients whose device will be programmed to an asynchronous pacing mode when the MRI SureScan
mode is programmed to On, no diaphragmatic stimulation is present when the paced lead has a pacing output
of 5.0 V and a pulse width of 1.0 ms.
Caution: It is not recommended to perform an MRI scan if the right ventricular (RV) lead pacing capture threshold
is greater than 2.0 V at 0.4 ms for pacemaker-dependent patients. A higher pacing capture threshold may indicate
an issue with the implanted lead.
Notes:
●
For radiology requirements, refer to the MRI technical manual.
●
Before performing an MRI scan, refer to the MRI technical manual for MRI-specific warnings and
precautions.
Patient monitoring and rescue requirements
●
Continuous patient monitoring is required during the MRI scan.
●
In the event that patient rescue is required, an external defibrillator must be immediately available.
Training requirements
●
A health professional who has completed cardiology SureScan training must be present during the
programming of the MRI SureScan feature.
●
A health professional who has completed radiology SureScan training must be present during the MRI scan.
1.6 Feature summary
The following features are available in this device. For a list of the features that are enabled at shipping, see the
“Shipped” column of the tables in Chapter 6, “Device parameters”, page 23.
1.6.1 Pacing features
Automatic polarity configuration – This device uses lead impedance measurements to automatically configure
pacing and sensing polarities during Implant Detection.
MRI SureScan – This feature allows patients with an implanted MRI SureScan system, including the device and
lead, to have a safe MRI procedure if the requirements provided in the MRI technical manual are followed.
6
Page 7

Rate Profile Optimization – The goal of Rate Profile Optimization is to ensure that the rate response remains
appropriate for the full range of patient activities. This feature monitors the patient’s daily and monthly sensor rate
profiles and adjusts the rate response curves over time to achieve a prescribed target rate profile.
Rate-responsive pacing – This feature varies the pacing rate in response to the patient’s physical motion as
detected by the activity sensor of the device.
RV Capture Management – This feature monitors the right ventricular pacing threshold with daily pacing
threshold searches and, if programmed to do so, adjusts the RV pacing amplitude toward a target amplitude.
Sleep feature – This feature causes the device to pace at a slower rate during a programmed sleep period.
1.6.2 Monitoring and follow-up features
Episode data and EGM storage – The system provides an arrhythmia episode log that enables you to view the
summary and detailed diagnostic data quickly, including stored EGM, for the selected arrhythmia episode.
Flashback memory – This diagnostic feature records intervals that occur immediately prior to tachyarrhythmia
episodes or the most recent interrogation and plots the interval data over time.
Holter telemetry – This function allows the implanted device to transmit an EGM with marker telemetry
continuously for up to 46 hours, regardless of the use of the programming head.
Implant Detection – Implant Detection is a 30 min period, beginning when the device is placed in the surgical
pocket. During this period, the device verifies lead connection by measuring lead impedance. When the Implant
Detection period is completed, various automatic features and diagnostics are activated.
Lead Monitor – This feature measures lead impedances during the life of the implanted device and controls
automatic configuration of lead polarities at implant. If Lead Monitor is programmed to Adaptive, the device
automatically switches bipolar pacing and sensing to unipolar pacing and sensing if the integrity of a bipolar lead
is compromised.
Medtronic CareAlert Monitoring – If the device identifies any programmed or automatic CareAlert conditions,
this feature sends a wireless alert signal to the patient monitor (if available). The patient monitor then transmits the
CareAlert Event data to the Medtronic CareLink Network. If configured to do so, the Medtronic CareLink Network
then sends an alert notification to the clinic.
Rate Histograms report – This report shows heart rate range distributions for the patient.
1.7 Data security
Medtronic has designed safeguards to protect patient information and device data for the
Azure S SR MRI SureScan Model W3SR01 device.
Bluetooth communication system – The device shows its availability through Bluetooth communication.
Critical data accepted or sent through the Bluetooth communication from the device is encrypted by the device
before it is sent over the Bluetooth channel. The device responds only to authorized commands.
Inductive telemetry communication system – The Medtronic inductive telemetry communication system is
used with the clinician programmer to interrogate and program the device. It can also be used to interrogate the
device for remote monitoring, if available. This system uses short-range communication that protects patient
information and device data.
7
Page 8

2 Warnings, precautions, and potential adverse events
2.1 General warnings and precautions
Before performing an MRI scan, refer to the Medtronic MRI technical manual for MRI-specific warnings
and precautions.
Refer to the Medical Procedure and EMI Warnings and Precautions Manual for information about hazards related
to medical therapies and diagnostic procedures on patients with cardiac devices. This manual also includes
information about sources of EMI in the patient’s environment.
Anti-coagulation – Use of the device should not change the application of established anti-coagulation protocols.
Electrical isolation during implant – Do not allow the patient to have contact with grounded electrical equipment
that might produce electrical current leakage during implant. Electrical current leakage may induce
tachyarrhythmias that may result in the patient’s death.
External defibrillation equipment – Keep external defibrillation equipment nearby for immediate use whenever
tachyarrhythmias are possible or intentionally induced during device testing, implant procedures, or post-implant
testing.
Lead compatibility – Although Medtronic device connector modules conform to International Connector
Standards, this device has not been tested for use with non-Medtronic leads. The known potential adverse
consequences of using such a combination may include undersensing of cardiac activity, failure to deliver
necessary therapy, or an intermittent electrical connection.
A complete SureScan pacing system includes a SureScan device connected to a SureScan lead. Before
performing an MRI scan, refer to the Medtronic MRI technical manual for additional information.
2.2 Explant and disposal
Consider the following information related to device explant and disposal:
●
Explant the implantable device postmortem. In some countries, explanting battery-operated implantable
devices is mandatory because of environmental concerns; please check the local regulations. In addition, if
subjected to incineration or cremation temperatures, the device may explode.
●
Medtronic implantable devices are intended for single use only. Do not resterilize and reimplant explanted
devices.
●
Contact Medtronic for Return Mailer Kits to return explanted devices for analysis and disposal. See the back
cover for addresses. Note: Disposal of explanted devices or leads is subject to local, state, and federal
regulations.
2.3 Handling and storage instructions
Carefully observe these guidelines when handling or storing the device.
2.3.1 Device handling
Checking and opening the package – Before opening the sterile package tray, visually check for any signs of
damage that might invalidate the sterility of the package contents.
If the package is damaged – The device packaging consists of an outer tray and an inner tray. Do not use the
device or accessories if the outer or inner packaging tray is wet, punctured, opened, or damaged. Return the
device to Medtronic because the integrity of the sterile packaging or the device functionality may be compromised.
This device is not intended to be resterilized.
If the package information is damaged – If any information on the outer package or the sterile package is
defaced or damaged so that you cannot read it, notify Medtronic so that the device can be replaced.
If the printed manual is illegible – If this manual is supplied in its printed form and any part of it is illegible, contact
Medtronic to request a replacement manual.
8
Page 9

Sterilization – Medtronic has sterilized the package contents with ethylene oxide before shipment. This product
is for single use only and is not intended to be resterilized.
Device temperature – Allow the device to reach room temperature before it is programmed or implanted. Device
temperature above or below room temperature may affect initial device function.
Dropped device – Do not implant the device if it is dropped on a hard surface from a height of 30 cm (12 in) or more
after it is removed from its packaging.
Fluid immersion – Do not immerse the device in fluid or flush the connector ports at the time of implant. Doing so
could adversely affect the performance of the device and lead system.
“Use by” date – Do not implant the device after the “Use by” date because the battery longevity could be reduced.
For single use only – Do not resterilize and reimplant an explanted device.
2.3.2 Device storage
Avoid magnets – To avoid damaging the device, store the device in a clean area away from magnets, kits
containing magnets, and any sources of electromagnetic interference.
Temperature limits – Store and transport the package between –18°C and +55°C (0°F and 131°F). Device reset
may occur at temperatures below –18°C (0°F). Device longevity may decrease and performance may be affected
at temperatures above +55°C (131°F).
2.4 Lead evaluation and lead connection
Refer to the lead technical manuals for specific instructions and precautions about lead handling.
A Medtronic MRI SureScan system includes a Medtronic MRI SureScan device connected to a Medtronic MRI
SureScan lead. Before performing an MRI procedure, refer to the Medtronic MRI technical manual for
additional information.
Torque wrench – Use only the torque wrench supplied with the device. The torque wrench is designed to prevent
damage to the device from overtightening a setscrew. Other torque wrenches (for example, a blue-handled or
right-angled hex wrench) have torque capabilities greater than the lead connector can tolerate.
Lead connection – Consider the following information when connecting the lead and the device:
●
Cap abandoned leads to avoid transmitting electrical signals.
●
Verify the lead connection. A loose lead connection may result in inappropriate sensing.
2.5 Device operation
Lead – A bipolar or unipolar lead may be used with the Azure S SR MRI SureScan Model W3SR01 device, but if
a lead other than bipolar MRI SureScan lead is used, the system is contraindicated for MRI scans.
Accessories – Use this device only with accessories, parts subject to wear, and disposable items that have been
tested to technical standards and found safe by an approved testing agency.
Device status indicators – If any of the device status indicators (for example, Device Reset) are displayed on the
programmer after interrogating the device, inform a Medtronic representative immediately. If these device status
indicators are displayed, therapies may not be available to the patient.
Effects of myopotential sensing in unipolar sensing configurations – In unipolar sensing configurations, the
device may not distinguish myopotentials from cardiac signals. This may result in a loss of pacing due to inhibition.
To address these situations, the device may be programmed to be less sensitive (using higher sensitivity values),
but the sensitivity level must be balanced against the potential to undersense true cardiac signals. Typically, this
balance is easily attained for ventricular sensing using sensitivity values around 2.8 mV, but it may be difficult to
attain for atrial sensing because of the smaller P-wave amplitudes.
Device reset – Temperatures below –18°C (0°F) or strong electromagnetic fields can reset the device. Advise
patients to avoid strong electromagnetic fields. Observe temperature storage limits to avoid exposure of the device
to cold temperatures. If a partial reset occurs, pacing resumes in the programmed mode with many of the
9
Page 10

programmed settings retained. If a full reset occurs, the device operates in VVI mode at 65 bpm. Device reset is
indicated by a programmer warning message that is displayed immediately upon interrogation. To restore the
device to its previous operation, it must be reprogrammed. Inform a Medtronic representative if your patient’s
device has reset.
End of Service (EOS) indicator – Replace the device immediately if the programmer displays an EOS indicator.
The device may soon lose the ability to pace and sense adequately.
False bipolar pathway with unipolar lead – When implanting a unipolar lead, ensure that the tip setscrew is
properly engaged and that all electrical contacts are sealed to prevent electrical leakage. Electrical leakage may
cause the device to inappropriately identify a unipolar lead as bipolar, resulting in loss of output.
Magnets – Placing a magnet over the device suspends tachyarrhythmia detection and initiates asynchronous,
fixed-rate bradycardia pacing. The programming head contains a magnet that can cause magnet operation to
occur. However, magnet operation does not occur if telemetry between the device and the programmer is
established or if the MRI SureScan mode is programmed to On.
Pace polarity – Pace polarity must be bipolar to program the MRI SureScan mode to On.
Pacing and sensing safety margins – Lead maturation (at least one month after implant) may cause sensing
amplitudes to decrease and pacing thresholds to increase, which can cause undersensing or a loss of capture.
Provide an adequate safety margin when selecting values for pacing amplitude, pacing pulse width, and sensitivity
parameters.
Programmers – Use only Medtronic programmers and application software to communicate with the device.
Programmers and software from other manufacturers are not compatible with Medtronic devices.
Rate-responsive modes – Do not program rate-responsive modes for patients who cannot tolerate rates above
the programmed Lower Rate. Rate-responsive modes may cause discomfort for those patients.
Right ventricular apical pacing – Right ventricular apical pacing may be associated with an increased risk of
atrial fibrillation, left ventricular dysfunction, and congestive heart failure.
Maximum output for the RV Capture Management feature – The RV Capture Management feature does not
program right ventricular outputs to values greater than 5.0 V or 1.0 ms. If the patient needs right ventricular pacing
output greater than 5.0 V or 1.0 ms, manually program right ventricular amplitude and pulse width. If a lead
dislodges partially or completely, the RV Capture Management feature may not prevent loss of capture.
Sensitivity setting – Carefully evaluate the possibility of increased susceptibility to EMI and oversensing before
changing the sensitivity from its nominal setting to a more sensitive setting.
Shipping values – Do not use shipping values or nominal values for pacing amplitude and sensitivity without
verifying that the values provide adequate safety margins for the patient.
2.5.1 Pacemaker-dependent patients
OVO pacing mode – Pacing is disabled under the OVO pacing mode. Do not program the OVO mode for
pacemaker-dependent patients. Instead, use the Underlying Rhythm Test to provide a brief period without pacing
support.
Polarity override – Do not override the polarity verification prompt with bipolar polarity when a unipolar lead is
connected. Overriding the polarity verification prompt results in no pacing output.
Underlying Rhythm Test – Use caution when using the Underlying Rhythm Test to inhibit pacing. The patient is
without pacing support when pacing is inhibited.
2.6 Potential adverse events
The following are known potential adverse events associated with the use of pacing systems.
●
Air embolism
●
Allergic reaction
●
Bleeding
10
Page 11

●
Body rejection phenomena including local tissue rejection
●
Cardiac dissection
●
Cardiac perforation
●
Cardiac tamponade
●
Chronic nerve damage
●
Death
●
Embolism
●
Endocarditis
●
Erosion of the device and lead through the skin
●
Excessive fibrosis
●
Extrusion
●
Fibrillation or other arrhythmias
●
Fluid accumulation
●
Formation of cysts
●
Heart block
●
Heart wall rupture
●
Hematoma/seroma
●
Inappropriate acceleration of arrhythmias
●
Infection
●
Keloid formation
●
Lead abrasion and discontinuity
●
Lead migration/dislodgment
●
Muscle and nerve stimulation
●
Myocardial damage
●
Myocardial irritability
●
Myopotential sensing
●
Pericardial effusion
●
Pericardial rub
●
Pneumothorax
●
Threshold elevation
●
Thromboemboli
●
Thrombosis
●
Tissue damage due to heating of device or lead (during an MRI procedure)
●
Transvenous lead-related thrombosis
●
Valve damage
●
Venous occlusion
●
Venous perforation
●
Vein wall rupture
3 Clinical data
3.1 Adverse events and clinical trial data
Information regarding clinical studies and adverse events related to this device is available at
www.medtronic.com/manuals.
The following clinical studies are related to this device:
Advisa DR MRI system study – This clinical study, which evaluated the safety and efficacy of the Advisa DR MRI
SureScan pacing system in the clinical magnetic resonance imaging (MRI) environment, provides support for the
MRI SureScan feature. This study supports removal of the C1-T12 positioning restriction, so that any region of the
body can be scanned when the MR Conditions for Use are followed.
11
Page 12

Kappa 700 clinical study – This study, which evaluated the safety and clinical performance of the Kappa 700
pacemakers, provides support for the Right Ventricular Capture Management feature and other bradycardia
pacing features.
Revo MRI SureScan pacing system clinical study – This clinical study, which evaluated the safety and efficacy
of the EnRhythm MRI SureScan pacing system in the clinical magnetic resonance imaging (MRI) environment,
provides support for the MRI SureScan feature. This study was conducted with the C1 – T12 MRI scan exclusion
zone in place.
SureScan Pacing System Post-Approval Study – This clinical study, which evaluated safety and performance
of approved systems in a magnetic resonance imaging (MRI) environment, provides support for the MRI SureScan
feature.
4 Implant procedure
4.1 Preparing for an implant
The following implant procedures are provided for reference only. Proper surgical procedures and sterile
techniques are the responsibility of the physician. Each physician must apply the information in these procedures
according to professional medical training and experience.
For information about replacing a previously implanted device, see Section 4.7, “Replacing a device”, page 17.
Ensure that you have all of the necessary instruments, system components, and sterile accessories to perform the
implant.
Connect the skin electrodes to the patient if you would like to display surface ECG signals on the programmer. See
the programmer reference manual for more information.
4.1.1 Instruments, components, and accessories required for an implant
The following non-implanted instruments are used to support the implant procedure:
●
Medtronic programmer with a programming head
●
programmer software application for the Azure S SR MRI SureScan Model W3SR01 device
●
Model 2290 Analyzer or equivalent pacing system analyzer
●
external defibrillator
The following sterile system components and accessories are used to perform the implant:
●
implantable device and lead system components
●
programming head sleeve
Note: If a sterilized programming head is used during an implant, a sterile programming head sleeve is not
necessary.
●
pacing system analyzer cables
●
lead introducer appropriate for the lead system
●
extra stylets of appropriate length and shape
4.1.2 Setting up the programmer and starting the application
See the programmer reference manual for instructions about how to set up the programmer. The software
application for the Azure S SR MRI SureScan Model W3SR01 device should be installed on the programmer. Your
Medtronic representative can install this software, if necessary. Establish telemetry with the device and start a
patient session.
12
Page 13

4.1.3 Considerations for preparing for an implant
Review the following information before implanting the lead or device:
Before performing an MRI scan, refer to the Medtronic MRI Technical Manual for additional information.
Warning: A bipolar or unipolar lead may be used with the Azure S SR MRI SureScan Model W3SR01 device, but
if a lead other than a bipolar MRI SureScan lead is used, the system is not approved for MRI scans. Before
performing an MRI scan, refer to the Medtronic MRI technical manual for additional information.
Warning: Do not allow the patient to have contact with grounded electrical equipment that might produce electrical
current leakage during implant. Electrical current leakage may induce tachyarrhythmias that may result in the
patient’s death.
Warning: Keep external defibrillation equipment nearby for immediate use. Potentially harmful spontaneous or
induced tachyarrhythmias may occur during device testing, implant procedures, and post-implant testing.
Caution: The device is intended for implant in the pectoral region with a Medtronic transvenous lead. No claims
of safety and efficacy can be made with regard to other acutely or chronically implanted lead systems that are not
manufactured by Medtronic.
Caution: Do not implant the device after the “Use by” date on the package label. Battery longevity could be
reduced.
To retain the ability to safely scan the SureScan pacing system during MRI scans, the MRI conditions for use in
Section 1.5, “MRI conditions for use”, page 6 must be followed. Refer to the MRI technical manual for additional
information.
4.1.4 How to prepare the device for implant
Before opening the sterile package, perform the following steps to prepare the device for implant:
1. Interrogate the device and print an Initial Interrogation Report.
Caution: If the programmer reports that a device reset occurred, do not implant the device. Contact a
Medtronic representative.
2. Check the Initial Interrogation Report to confirm that the battery voltage is at least 2.85 V at room temperature.
If the device has been exposed to low temperatures, then the battery voltage will be temporarily lower. Allow
the device to warm to room temperature for at least 48 hours and check the battery voltage again. If an
acceptable battery voltage cannot be obtained, contact a Medtronic representative.
Note: The device automatically measures the battery voltage several times a day. The battery voltage
reported on the Battery and Lead Measurements screen is an average of recent automatic measurement
values.
3. Select Params > Data Collection Setup > Device Date/Time… to select the Time Zone for the internal clock
of the device.
4. Program the pacing parameters to values appropriate for the patient.
Note: Do not enable a pacing feature that affects the pacing rate before implanting the device. Doing so may
result in a pacing rate that is faster than expected.
4.2 Selecting and implanting the lead
Use the guidelines in this section to select a lead that is compatible with the device. The appropriate techniques
for implanting the lead may vary according to physician preference and the patient’s anatomy or physical condition.
Consult the technical manuals supplied with the lead for specific implant instructions.
A complete SureScan pacing system is required for use in the MR environment. A complete SureScan
pacing system includes a SureScan device with a Medtronic SureScan lead. To verify that components are
part of a SureScan system, visit http://www.mrisurescan.com. Any other combination may result in a hazard to the
patient during an MRI scan.
13
Page 14

4.2.1 Selecting the lead
The device is typically implanted with 1 bipolar transvenous lead in the right ventricle (RV) for sensing and pacing.
4.2.2 How to verify lead and connector compatibility
Warning: Verify lead and connector compatibility before using a lead with this device. Using an incompatible lead
may damage the connector, resulting in electrical current leakage or resulting in an intermittent electrical
connection.
Note: Medtronic 3.2 mm low-profile leads are not directly compatible with the device IS-1 connector block.
Note: A lead adaptor compromises the ability to safely scan the SureScan pacing system during an MRI scan.
Patients with a lead adaptor are contraindicated for an MRI scan.
Use the information in Table 1 to select a compatible lead.
Table 1. Lead and connector compatibility
Connector port Leads
V IS-1a bipolar or IS-1 unipolar
a
IS-1 refers to the international standard ISO 5841-3.
Warning: If a lead other than a bipolar MRI SureScan lead is used, the system is contraindicated for MRI scans.
4.2.3 Implanting the lead
Implant the lead according to the instructions in the technical manual supplied with the lead, unless a suitable
chronic lead is already in place.
Warning: Pinching the lead can damage the lead conductor or insulation, which may result in the loss of sensing
or pacing therapy.
Transvenous lead – If you use a subclavian approach to implant a transvenous lead, position the lead laterally to
avoid pinching the lead body between the clavicle and the first rib.
4.3 Testing the lead system
After the lead is implanted, test the lead system to verify that the sensing and pacing values are acceptable. Refer
to the literature provided with the pacing system analyzer for instructions.
Note: Do not measure the intracardiac EGM that is telemetered from the device to assess sensing.
Note: The measured pacing lead impedance is a reflection of measuring equipment and lead technology. Refer
to the lead technical manual for acceptable impedance values.
Bipolar lead – When measuring sensing and pacing values, measure between the tip (cathode) and ring (anode)
of the bipolar pacing/sensing lead.
Unipolar lead – When measuring sensing and pacing values, measure between the tip (cathode) of the unipolar
pacing/sensing lead and an indifferent electrode (anode) used in place of the device can.
14
Page 15
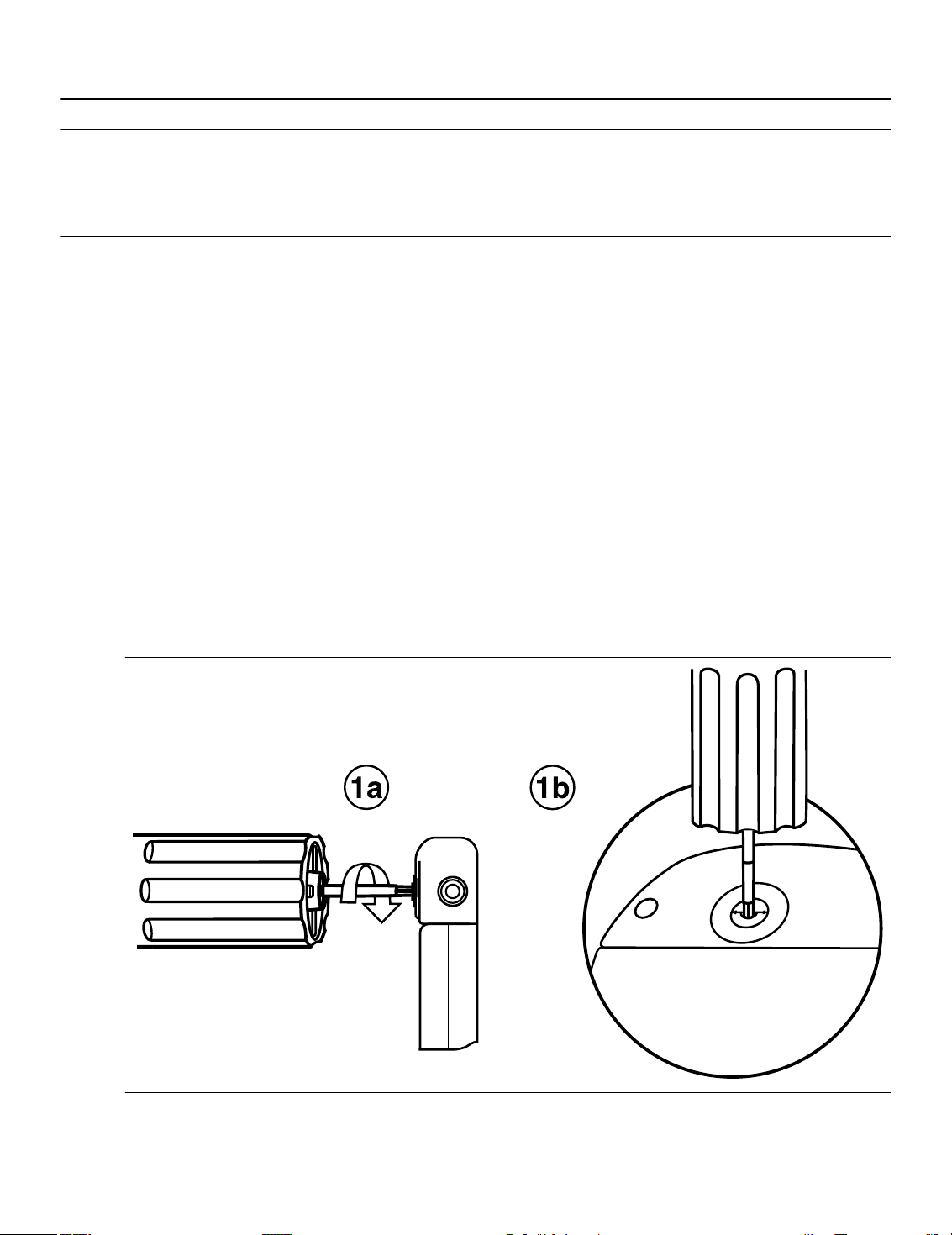
Table 2. Acceptable sensing and pacing values
Measurements required Acute transvenous leads Chronic leads
a
R-wave EGM amplitude ≥ 5 mV ≥ 3 mV
Slew rate ≥ 0.75 V/s ≥ 0.5 V/s
Capture threshold (0.5 ms pulse
≤ 1.0 V ≤ 3.0 V
width)
a
Chronic leads are leads implanted for 30 days or more.
4.4 Connecting the lead to the device
The following procedure describes how to connect the lead to the device, how to confirm that the lead connector
is fully inserted in the connector block, and how to verify that the lead connection is secure.
Warning: After connecting the lead, verify that the lead connection is secure by gently tugging on the lead. A loose
lead connection may result in inappropriate sensing, which can cause false inhibition of pacing.
Caution: Use only the torque wrench supplied with the device. The torque wrench is designed to prevent damage
to the device from overtightening a setscrew.
4.4.1 How to connect a lead to the device
1. Insert the torque wrench into the setscrew.
a. If the setscrew obstructs the port, retract the setscrew by turning it counterclockwise until the port is clear.
Take care not to disengage the setscrew from the connector block (see Figure 1).
b. Leave the torque wrench in the setscrew until the lead connection is secure. This action allows a pathway
for venting trapped air when the lead connector is inserted into the connector port.
Figure 1. Inserting the torque wrench into the setscrew
2. Push the lead connector into the connector port until the lead connector pin is clearly visible in the pin viewing
area. No sealant is required.
15
Page 16
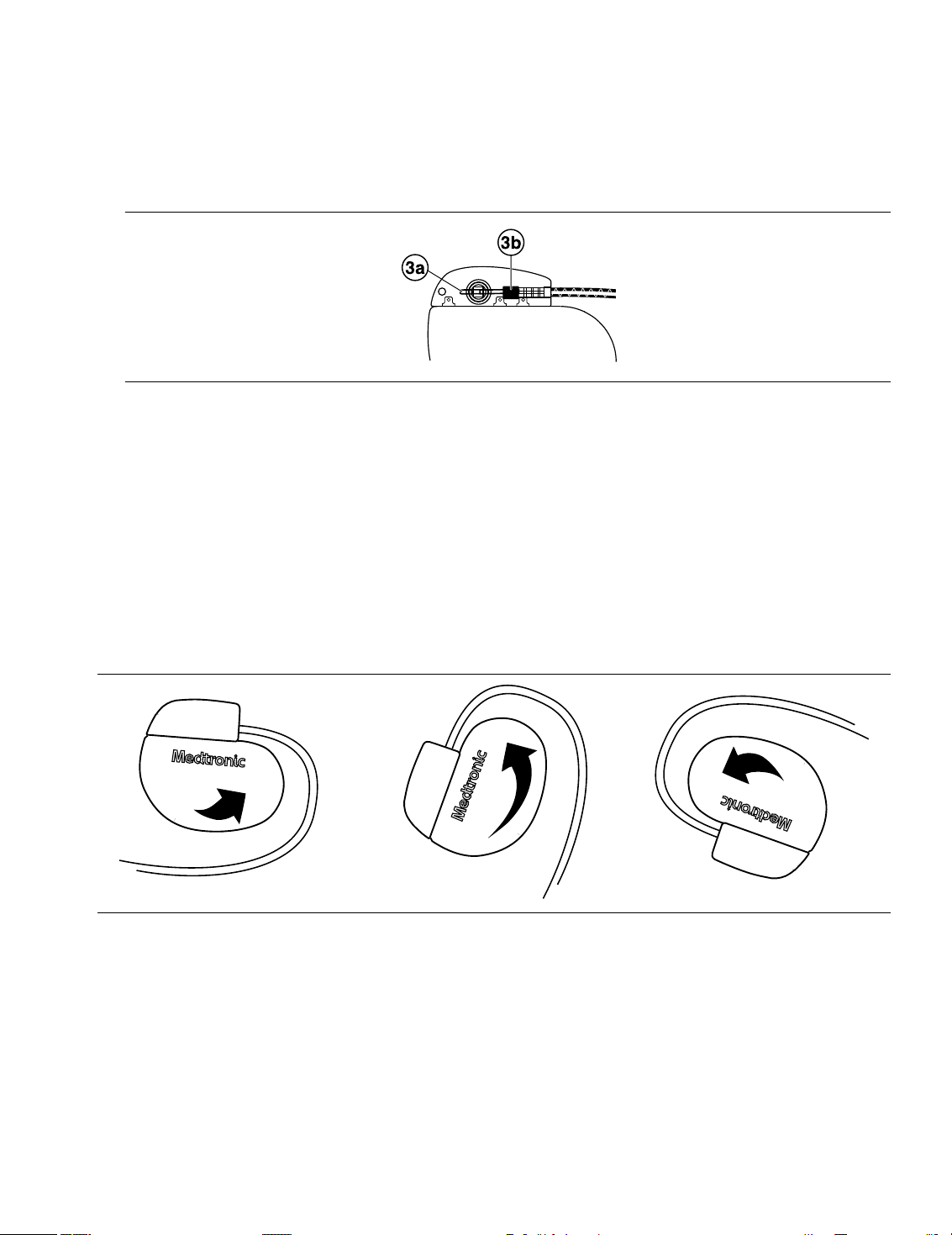
3. Confirm that the lead is fully inserted into the connector pin cavity by viewing the device connector block from
the side or end.
a. The lead connector pin should be clearly visible beyond the setscrew block (see Figure 2).
b. The lead connector ring should be completely inside the spring contact block. There is no setscrew in this
location (see Figure 2).
Figure 2. Confirming the lead connection
4. Tighten the setscrew by turning it clockwise until the torque wrench clicks. Remove the torque wrench.
5. Gently tug on the lead to confirm a secure fit. Do not pull on the lead until the setscrew has been tightened.
4.5 Positioning and securing the device
Note: Implant the device within 4 cm (1.6 in) of the surface of the skin to optimize post-implant ambulatory
monitoring.
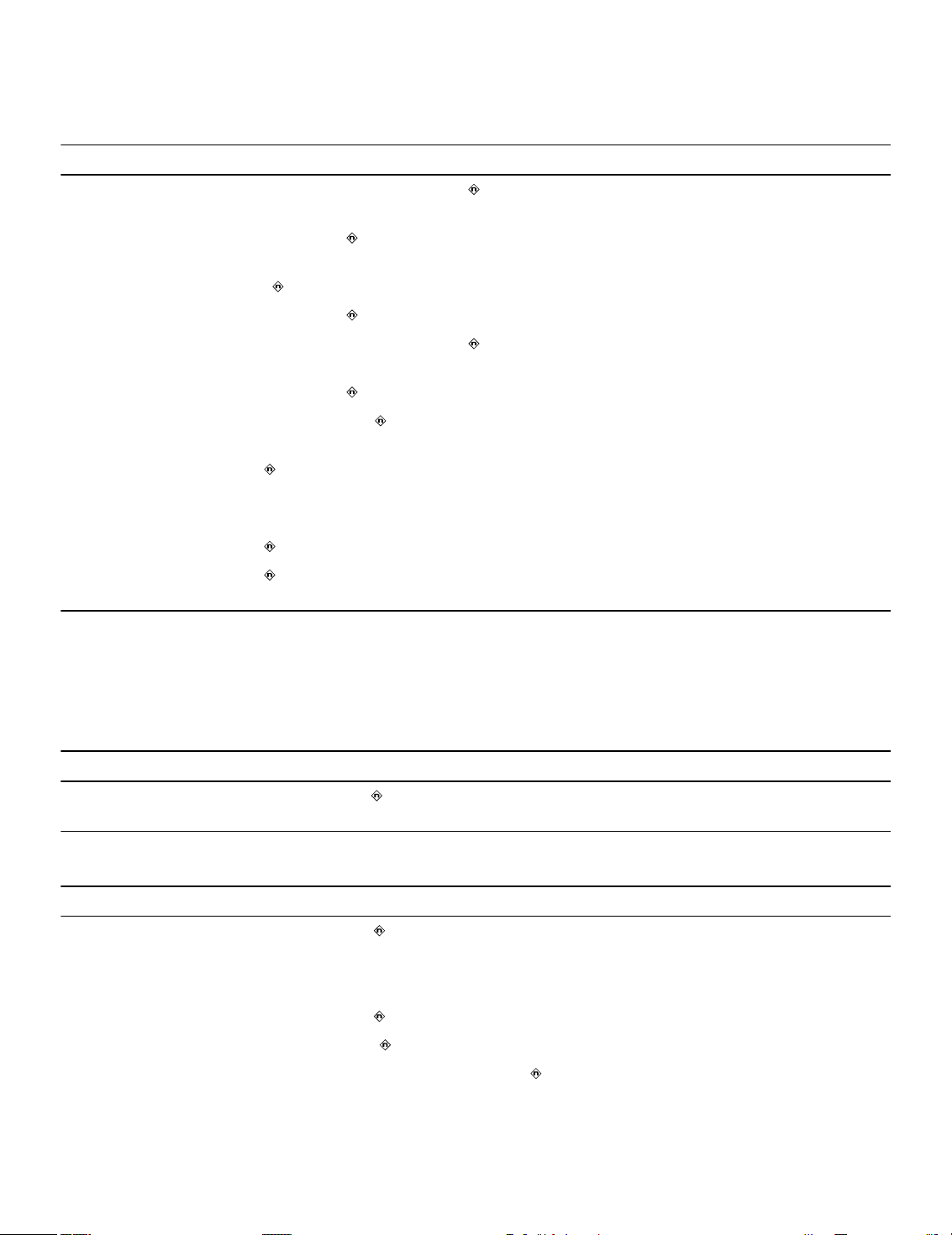
4.5.1 How to position and secure the device
1. Verify that the lead connector pin is fully inserted into the connector port and that the setscrew is tight.
2. To prevent twisting of the lead body, rotate the device to loosely wrap the excess lead length (see Figure 3).
Do not kink the lead body.
Figure 3. Rotating the device to wrap the lead
3. Place the device and the lead into the surgical pocket.
4. Use nonabsorbable sutures to secure the device within the pocket and minimize post-implant rotation and
migration. Use a surgical needle to penetrate the suture hole on the device.
5. Suture the pocket incision closed.
16
Page 17

4.6 Completing the implant procedure
4.6.1 How to complete programming the device
1. If a unipolar lead is implanted, you may want to manually complete the Implant Detection process.
a. Select the Params icon.
b. Program the Pace Polarity and Sense Polarity parameters to Unipolar.
c. Select Additional Features… and program the Implant Detection parameter to Off/Complete.
2. Verify that the pacing and monitor parameters are programmed to values that are appropriate for the patient.
3. Enter the patient’s information.
Note: Be sure to use the Patient Information screen to enter complete information about the implanted lead.
Be sure to use the MRI SureScan System/Other Hardware screen to enter complete information about other
hardware implanted in the patient, including abandoned devices or leads, and lead extenders or adaptors.
This information will be used in the future if the patient needs to be evaluated for an MRI scan. For more
information, see the programming guide.
4. Program the Medtronic CareAlert parameters, if applicable.
5. Program the Data Collection Setup parameters.
4.6.2 How to assess the performance of the device and the lead
After implanting the device, x-ray the patient as soon as possible to verify device and lead placement. Before the
patient is discharged from the hospital, assess the performance of the implanted device and lead.
1. Monitor the patient’s electrocardiogram until the patient is discharged. If the lead dislodges, it usually occurs
during the immediate postoperative period.
2. Check the pacing and sensing values, and adjust the values if necessary. Verify the safety margin for the
pacing threshold.
3. Interrogate the device, and print a Final Report to document the postoperative programmed device status.
4.7 Replacing a device
To retain the ability to safely scan the SureScan pacing system during MRI scans, the MRI conditions for use in
Section 1.5, “MRI conditions for use”, page 6 must be followed. Refer to the Medtronic MRI technical manual for
additional information.
Warning: A bipolar or unipolar lead may be used with the Azure S SR MRI SureScan Model W3SR01 device, but
if a lead other than a bipolar MRI SureScan lead is used, the system is not approved for MRI scans. Before
performing an MRI scan, refer to the Medtronic MRI technical manual for additional information.
Warning: Abandoned leads or previously implanted non-MRI labeled leads compromise the ability to safely scan
the SureScan pacing system during future MRI scans. When implanting a SureScan pacing system, consider the
risks associated with removing previously implanted leads before removing the leads to maintain the ability to
safely scan the SureScan pacing system. Refer to the Medtronic MRI technical manual for additional information.
Warning: Keep external pacing equipment nearby for immediate use. The patient does not receive pacing therapy
from the device when the lead is disconnected, or when the device is removed from the pocket while the device
is operating in unipolar pacing mode.
Note: To meet the implant requirements, you may need to reposition or replace any chronic leads. For more
information, see Section 4.2, “Selecting and implanting the lead”, page 13.
Note: Any unused leads that remain implanted must be capped with a lead pin cap to avoid transmitting electrical
signals. Any capped or unused leads are considered abandoned leads in the MRI conditions for use, and their
presence will contraindicate the system for MRI scanning. Contact your Medtronic representative for information
about lead pin caps.
17
Page 18

4.7.1 How to explant and replace a device
1. Program the device to a mode that is not rate-responsive to avoid potential rate increases while explanting the
device.
2. Dissect the lead and the device free from the surgical pocket. Do not nick or breach the lead insulation.
3. Use a torque wrench to loosen the setscrew in the connector block.
4. Gently pull the lead out of the connector port.
5. Evaluate the condition of the lead (see Section 4.3, “Testing the lead system”, page 14). Replace the lead if
the electrical integrity is not acceptable or if the lead connector pin is pitted or corroded. If you explant the lead,
return the lead to Medtronic for analysis and disposal.
6. Connect the lead to the replacement device (see Section 4.4, “Connecting the lead to the device”, page 15).
Note: A lead adaptor may be needed to connect the lead to the replacement device. Contact a Medtronic
representative for information about compatible lead adaptors.
Note: A lead adaptor compromises the ability to safely perform an MRI scan on the SureScan pacing system
in the future. Patients with lead adaptors are contraindicated for an MRI scan.
7. Position and secure the device in the surgical pocket, and suture the pocket incision closed (see Section 4.5,
“Positioning and securing the device”, page 16).
8. Contact Medtronic for Return Mailer Kits to return explanted devices for analysis and disposal. See the back
cover for addresses. Note: Disposal of explanted devices or leads is subject to local, state, and federal
regulations.
5 Product specifications
5.1 Physical characteristics
Table 3. Physical characteristics
Volume
a
12.25 cm
3
Mass 22.5 g
H x W x D
Radiopaque ID
b
c
42.6 mm x 50.8 mm x 7.4 mm
RNA
Medtronic identifier
Surface area of titanium device can 33.48 cm
Materials in contact with human tissue
d
Titanium, polyurethane, silicone rubber
2
Battery Lithium-hybrid CFx silver vanadium oxide
a
Volume with connector holes unplugged.
b
Grommets may protrude slightly beyond the can surface.
c
The radiopaque ID, which includes a Medtronic-identifier symbol, can be viewed in a fluoroscopic image of the
device.
d
These materials have been successfully tested for the ability to avoid biological incompatibility. The device does
not produce an injurious temperature in the surrounding tissue during normal operation.
18
Page 19

Figure 4. Connector and suture hole
1 IS-1 connector port, V
2 Suture hole
The Model W3SR01 shield graphics are shown in Figure 5.
The IS-1 marking in Figure 5 indicates that the lead connector conforms to ISO 5841-3.
Figure 5. Shield graphics: Model W3SR01
1 V = ventricular
2 IS-1 marking
5.2 Electrical specifications
Table 4. Battery characteristics
Manufacturer Medtronic Energy and Component Center
Model Delta 26H3
Number of battery cells 1
Chemistry Lithium-hybrid CFx silver vanadium oxide
19
Page 20

Table 4. Battery characteristics (continued)
Nominal voltage 3.25 V
Mean usable capacity 1.2 Ah
Mean capacity to RRT 0.97 Ah
Residual usable capacity at RRT 0.23 Ah
Table 5. Current consumption
Current consumption (at 100% pacing)
Current consumption (at 100% inhibition)
a
Current consumption when pacing into 500 Ω ± 1% loads at the Beginning of Service in VVIR mode at 60 bpm,
a
b
7.67 µA
5.81 µA
2.5 V, 0.4 ms.
b
Current consumption when at the Beginning of Service in VVIR mode at 60 bpm, 2.5 V, 0.4 ms, 500 Ω ± 1% .
5.2.1 Output waveforms
Figure 6. Output waveform at nominal conditions (resistive load: 500 Ω)
5.2.2 Variation with temperature
Basic rate, test pulse rate, pulse duration, and pulse amplitude remain within expected tolerances when the device
temperature is between 22°C and 45°C (72°F to 113°F). Sensitivity at nominal conditions as measured at 37°C
(98.6°F) can vary as much as ±1% per °C from 22°C to 45°C (72°F to 113°F).
5.3 Replacement indicators
The battery voltage and messages about replacement status appear on the programmer display and on printed
reports. The Recommended Replacement Time (RRT), Elective Replacement Indicator (ERI), and the End of
Service (EOS) conditions are listed in Table 6.
Table 6. Replacement indicators
Recommended Replacement Time (RRT) ≤ 2.63 V on 3 consecutive daily automatic measure-
ments
Elective Replacement Indicator (ERI) 3 months after RRT
End of Service (EOS) 3 months after ERI
20
Page 21

RRT date – The programmer displays the date when the battery reached RRT on the Quick Look II and Battery and
Lead Measurements screens.
Replace at EOS – If the programmer indicates that the device is at EOS, replace the device immediately.
RRT operation – When the device reaches RRT, it continues to operate with its programmed parameters.
However, placing a magnet over the device initiates asynchronous pacing at 65 bpm rather than at 85 bpm.
ERI operation – When the device reaches ERI, it automatically changes the value of several parameters as shown
in Table 7.
Table 7. Parameter settings after ERI
Pacing Mode VVI
Lower Rate 65 bpm
RV Amplitude as programmed
RV Pulse Width as programmed
Rate Hysteresis Off
Sleep Off
Pre-arrhythmia EGM Off
a
Pre-arrhythmia EGM cannot be reprogrammed after ERI.
a
Note: After ERI, all pacing parameters can be programmed, including mode and rate. Reprogramming the pacing
parameters may reduce the duration of the ERI to EOS period.
Note: When the MRI SureScan mode is programmed to On, battery measurements are taken, but the device does
not report RRT, EOS, or ERI until the MRI SureScan mode has been programmed to Off.
Prolonged Service Period – The Prolonged Service Period (PSP) is the time between the RRT and EOS. The
PSP is defined as 6 months assuming the following conditions: 100% VVI pacing at 60 bpm, 2.5 V RV pacing
amplitude; 0.4 ms pulse width; and 600 Ω pacing load. The EOS may be indicated before the end of 6 months if
the device exceeds these conditions.
5.4 Projected service life
The projected service life in years for the device is shown in Table 8. The data is based on pacing outputs
programmed to the specified amplitude and 0.4 ms pulse width and 60 bpm pacing rate.
The service life of the device is affected by the programmed settings for certain features, such as Pre-arrhythmia
EGM storage.
Projected service life estimates are based on accelerated battery discharge data and device modeling as
specified. These values should not be interpreted as precise numbers.
Table 8. Projected service life in years
500 Ω pacing
impedance
a
2.5 V 3.5 V 2.5 V 3.5 V 2.5 V 3.5 V
Pacing
Pre-arrhythmia
EGM storage
VVI, 0% Off 18.3 18.3 18.3 18.3 18.3 18.3
On 18.2 18.2 18.2 18.2 18.2 18.2
600 Ω pacing
impedance
900 Ω pacing
impedance
21
Page 22

Table 8. Projected service life in years (continued)
Pacing
Pre-arrhythmia
EGM storage
a
500 Ω pacing
impedance
2.5 V 3.5 V 2.5 V 3.5 V 2.5 V 3.5 V
600 Ω pacing
impedance
900 Ω pacing
impedance
VVI, 15% Off 17.5 16.7 17.6 16.9 17.8 17.3
On 17.4 16.6 17.5 16.8 17.7 17.2
VVI, 50%
Off 15.8 13.9 16.1 14.4 16.7 15.4
On 15.7 13.8 16.0 14.3 16.6 15.3
VVI, 100%
Off 13.9 11.2 14.4 11.9 15.4 13.3
On 13.8 11.1 14.3 11.8 15.3 13.2
a
The data provided for programming Pre-arrhythmia EGM storage to On is based on a 6-month period (two
3-month follow-up intervals) over the life of the device. Additional use of Pre-arrhythmia EGM storage reduces
projected service life by approximately 13.6% or 1.6 months per year.
Note: These projections are based on typical shelf storage time (5 months). Assuming worst-case shelf storage
time (18 months), longevity is reduced by approximately 7%.
Medtronic remote monitor transmissions – Additional remote monitoring transmissions reduce the projected
service life of the device. For example, from nominal pacing (at 2.5 V, 0.4 ms, 600 Ω, 60 bpm, 100% ventricular
pacing), a patient can expect a projected service life of 14.3 years. More frequent remote monitoring transmissions
will reduce this projected service life as follows:
●
Monthly transmissions over the life of the device reduce projected service life by 29 days, or <1%.
●
Weekly transmissions over the life of the device reduce projected service life by 153 days, or 2.9%.
●
Daily transmissions over the life of the device reduce projected service life by 956 days, or 18.3%.
Table 9. Projected service life in years per conditions specified in EN 45502-2-1 and ISO 14708-2
EN 45502-2-1 ISO 14708-2
500 Ω ±1% pacing impedance
70 bpm
600 Ω ±1% pacing impedance
60 bpm
Pacing
VVIR, 100%
2.5 V, 0.5 ms 12.4
5.0 V, 0.5 ms 6.4
a
a
2.5 V, 0.4 ms — 14.3
5.0 V, 0.4 ms — 8.7
a
Data storage and diagnostic functions applicable to the pacing mode are On.
22
—
—
a
a
Page 23

6 Device parameters
6.1 Emergency settings
Table 10. Emergency VVI settings
Parameter Selectable values
Pacing Mode VVI
Lower Rate 70 bpm
RV Amplitude
RV Pulse Width
RV Pace Polarity Unipolar
V. Blank Post VP 240 ms
Rate Hysteresis Off
MRI SureScan Off
a
If the programmed RV Amplitude is 8 V, VVI pacing is delivered at 8 V with a pulse width of 1.2 ms.
6.2 Magnet application
When a magnet is placed near the device, the pacing mode changes from the programmed mode to VOO, and the
pacing rate changes to 100 bpm for 5 beats and then changes to 85 bpm or 65 bpm , as described at the end of
this section. Placing a magnet near the device suspends tachyarrhythmia detection. When the magnet is removed,
the device returns to its programmed operation.
a
a
6 V
1.5 ms
The pacing rate will be 85 bpm if the device conditions are normal and it will be 65 bpm if a Recommended
Replacement Time (RRT) indicator or a device reset has occurred.
Note: Magnet operation does not occur if telemetry between the device and programmer is established or if the
MRI SureScan mode is programmed to On.
6.3 Tachyarrhythmia detection parameters
Table 11. Tachyarrhythmia detection parameters
Parameter Programmable values Shipped Reset
VT Monitor Monitor ; Off Monitor Off
VT Monitor Interval (Rate)
RV Sensitivity
a
The measured intervals are truncated to a 10 ms multiple (for example, 457 ms becomes 450 ms). The device
b,c
uses this truncated interval value when applying the programmed criteria and calculating interval averages.
b
This setting applies to all sensing in this chamber for both tachyarrhythmia detection and bradycardia pacing
operations.
c
The device complies with the requirements of ISO 14708-2 when the sensitivity threshold is programmed to
2.0 mV or higher.
a
280; 290 … 360 … 500 ms 360 ms 360 ms
0.45; 0.60; 0.90; 1.20; 2.00; 2.80;
0.90 mV 2.80 mV
4.00; 5.60; 8.00; 11.30 mV
Bipolar: 0.90 mV
Unipolar: 2.80 mV
23
Page 24

6.4 Pacing parameters
Table 12. Modes, rates, and intervals
Parameter Programmable values Shipped Reset
Mode VVIR ; VVI; VOO; OVO VVI VVI
Lower Rate
a
The corresponding Lower Rate interval can be calculated as follows: Lower Rate interval (ms) = 60,000/Lower
Rate.
Table 13. RV parameters
Parameter Programmable values Shipped Reset
RV Amplitude 0.5; 0.75 … 3.5 … 5; 5.5; 6; 8 V
RV Pulse Width 0.03; 0.06; 0.1; 0.2; 0.3; 0.4 … 1.5 ms 0.4 ms 1.5 ms
RV Sensitivity
RV Pace Polarity Bipolar; Unipolar Configure
RV Sense Polarity Bipolar; Unipolar Configure
RV Lead Monitor Monitor Only; Adaptive Monitor Only Monitor Only
Min Limit 200 ; 300; 400; 500 Ω 200 Ω 200 Ω
Max Limit 1000; 1500; 2000; 3000 Ω 3000 Ω 3000 Ω
a
When RV Amplitude is 8 V, RV Pulse Width must be less than 1.3 ms.
b
This setting applies to all sensing in this chamber for both tachyarrhythmia detection and bradycardia pacing
operations.
c
“Configure” is displayed when the device is automatically configuring the lead polarity at implant. It is not a
selectable value.
a
b
30; 35 … 60 ; 70; 75 … 150 bpm 60 bpm
(1000 ms)
a
0.45; 0.60; 0.90; 1.20; 2.00; 2.80; 4.00;
3.5 V 6 V
0.90 mV 2.80 mV
65 bpm
(923 ms)
5.60; 8.00; 11.30 mV
Unipolar: 2.80 mV
Bipolar: 0.90 mV
c
c
Unipolar
Unipolar
Table 14. RV Capture Management parameters
Parameter Programmable values Shipped Reset
RV Capture Management Adaptive ; Monitor; Off Adaptive Off
RV Amplitude Safety Mar-
1.5x; 2.0x ; 2.5x; 3.0x 2.0x 2.0x
gin
RV Minimum Adapted
1.0; 1.5; 2.0 ; 2.5; 3.0; 3.5 V 2 V 2 V
Amplitude
RV Acute Phase Remaining Off; 30; 60; 90; 120 ; 150 days 120 days 120 days
24
Page 25

Table 15. Blanking periods
Parameter Programmable values Shipped Reset
V. Blank Post VP 150; 160 … 200 … 320 ms 200 ms 240 ms
V. Blank Post VS 120 ; 130 … 170; 200; 220; 250; 280; 300;
320 ms
Table 16. Rate Response Pacing parameters
Parameter Programmable values Shipped Reset
Upper Sensor Rate 80; 85 … 130 … 175 bpm 130 bpm 120 bpm
ADL Rate 60; 65 … 95 … 170 bpm 95 bpm 95 bpm
Rate Profile Optimization On ; Off On Off
ADL Response 1; 2; 3 ; 4; 5 3 3
Exertion Response 1; 2; 3 ; 4; 5 3 3
Activity Threshold Low ; Medium Low; Medium High; High Low Medium Low
Activity Acceleration 15; 30 ; 60 s 30 s 30 s
Activity Deceleration Exercise ; 2.5; 5; 10 min Exercise 5 min
ADL Setpoint 5; 6 … 40; 42 … 80 18 18
UR Setpoint 15; 16 … 40; 42 … 80; 85 … 180 40 40
120 ms 120 ms
Table 17. Sleep parameters
Parameter Programmable values Shipped Reset
Sleep On; Off Off Off
Sleep Rate 30; 35 … 50 ; 55; 60; 70; 75 … 100 bpm 50 bpm 50 bpm
Bed Time 00:00; 00:10 … 22:00 … 23:50 22:00 22:00
Wake Time 00:00; 00:10 … 07:00 … 23:50 07:00 07:00
Table 18. MRI SureScan parameters
Parameter Programmable values Shipped Reset
MRI SureScan On; Off Off Off
MRI Pacing Mode VOO; OVO — —
MRI Pacing Rate 60; 70; 75; 80 … 120 bpm — —
Table 19. Additional pacing features
Parameter Programmable values Shipped Reset
Rate Hysteresis Off ; 30; 40 … 80 bpm Off Off
25
Page 26
6.5 Data collection parameters
Table 20. Data collection parameters
Parameter Programmable values Shipped Reset
EGM 1 Source Can to RVring; RVtip to RVring ; RVtip to
RVtip to RVring RVtip to RVring
Can
EGM 1 Range ±1; ±2; ±4; ±8 ; ±12; ±16; ±32 mV ±8 mV ±8 mV
EGM 2 Source Can to RVring; RVtip to RVring; RVtip to
RVtip to Can RVtip to Can
Can
EGM 2 Range ±1; ±2; ±4; ±8 ; ±12; ±16; ±32 mV ±8 mV ±8 mV
EGM 3 Source RVtip to RVring; Can to RVring ; RVTip to
Can to RVring Can to RVring
Can
EGM 3 Range ±1; ±2; ±4; ±8 ; ±12; ±16; ±32 mV ±8 mV ±8 mV
Monitored EGM1 and EGM2 ; EGM1 and EGM3;
EGM2 and EGM3
Pre-arrhythmia
EGM
Off ; On - 1 month; On - 3 months; On Continuous
EGM1 and
EGM1 and EGM2
EGM2
Off Off
Device Date/Timea(select Time Zone) — —
Holter Telemetry Off ; 0.5; 1; 2; 4; 8; 16; 24; 36; 46 hr Off Off
Wireless Teleme-
On ; Off On On
b
try with Monitor
a
The times and dates stored in episode records and other data are determined by the Device Date/Time clock.
b
The reset value may be set to Off if there is an issue with wireless communication that requires it to be disabled.
6.6 Medtronic CareAlert parameters
Table 21. Clinical Management Alerts
Parameter Programmable values Shipped Reset
Monitored VT Episode Detected
Table 22. Lead/Device Integrity Alerts
Parameter Programmable values Shipped Reset
Low Battery Voltage RRT On ; Off On Off
Lead Impedance Out of Range…
Lead Impedance
RV Pacing Enable On ; Off On Off
RV Pacing Less than 200 ; 300; 400; 500 Ω 200 Ω 200 Ω
RV Pacing Greater than 1000; 1500; 2000; 3000 Ω 3000 Ω 3000 Ω
Off ; On Off Off
26
Page 27

Table 22. Lead/Device Integrity Alerts (continued)
Parameter Programmable values Shipped Reset
Capture Management High Threshold…
High Threshold
RV Capture Enable
a
If programmed to On, alert notification is sent if RV capture management has measured high thresholds for 3
a
Off ; On Off Off
consecutive days.
6.7 System test parameters
Table 23. System test parameters
Parameter Selectable values
Pacing Threshold Test parameters
Test Type Amplitude; Pulse Width
Chamber RV
Decrement after 2; 3 … 15 pulses
RV Pace Polarity Unipolar; Bipolar
a
Mode
Lower Rate 30; 35 … 60; 70; 75 … 150 bpm
RV Amplitude 0.25; 0.5 … 5; 5.5; 6; 8 V
RV Pulse Width 0.03; 0.06; 0.1; 0.2 … 1.5 ms
V. Pace Blanking 150; 160 … 320 ms
Sensing Test parameters
a
Mode
Lower Rate 30; 35 … 60; 70; 75 … 120 bpm
a
The selectable values for this parameter depend on the programmed pacing mode.
VVI; VOO
VVI; OVO
6.8 EP Study parameters
Table 24. Fixed Burst induction parameters
Parameter Selectable values
Interval 100; 110 … 600 ms
Amplitude 1; 2; 3; 4 ; 5; 6; 8 V
Pulse Width 0.10; 0.20 … 0.50 … 1.50 ms
Table 25. PES induction parameters
Parameter Selectable values
#S1 1; 2 … 8 … 15
S1S1 100; 110 … 600 … 2000 ms
27
Page 28

Table 25. PES induction parameters (continued)
Parameter Selectable values
S1S2 Off; 100; 110 … 400 … 600 ms
S2S3 Off ; 100; 110 … 600 ms
S3S4 Off ; 100; 110 … 600 ms
Amplitude 1; 2; 3; 4 ; 5; 6; 8 V
Pulse Width 0.10; 0.20 …0.50 … 1.50 ms
Table 26. Shared manual ATP therapy parameters
Parameter Selectable values
Minimum Interval (ventricular ATP) 150; 160 … 200 … 400 ms
Amplitude 1; 2 … 6 ; 8 V
Pulse Width 0.10; 0.20 …1.50 ms
Table 27. Manual Ramp therapy parameters
Parameter Selectable values
Chamber RV
a
RV Ramp therapy parameters
# Pulses 1; 2 … 6 … 15
%RR Interval 50; 53; 56; 59; 63; 66 … 84; 88; 91; 94; 97 %
Dec/Pulse 0; 10 ; 20; 30; 40 ms
a
The chamber value is fixed for this product.
Table 28. Manual Burst therapy parameters
Parameter Selectable values
# Pulses 1; 2 … 8 … 15
%RR Interval 50; 53; 56; 59; 63; 66 … 84; 88 ; 91; 94; 97%
Table 29. Manual Ramp+ therapy parameters
Parameter Selectable values
# Pulses 1; 2; 3 … 15
R-S1 (%RR) 50; 53; 56; 59; 63; 66 … 75 … 84; 88; 91; 94; 97%
S1-S2 (%RR) 50; 53; 56; 59; 63; 66; 69 … 84; 88; 91; 94; 97%
S2-SN (%RR) 50; 53; 56; 59; 63; 66 … 84; 88; 91; 94; 97%
28
Page 29

6.9 Nonprogrammable parameters
Table 30. Nonprogrammable parameters
Parameter Value
Premature event threshold for counting PVCs and Runs of PVCs 69%
Hardware parameters
Pacing rate limit (protective feature) 200 bpm
Input impedance 150 kΩ minimum
Effective pacing capacitance 4 µF
Recommended Replacement Time (RRT)
Battery Voltage Threshold ≤ 2.63 V
Table 31. Nonprogrammable parameters for the MRI SureScan mode
Parameter Value
Pacing amplitude Programmed pacing amplitude value when >5 V;
5 V when programmed pacing amplitude value is ≤5 V
Pulse width Programmed pulse width value when >1 ms;
1 ms when programmed pulse width value is ≤1 ms
Sensitivity Programmed value
Input impedance 150 kΩ
Pacing rate limit 200 bpm
Effective pacing capacitance 4 µF
Blanking period
OVO mode Programmed blanking period value
VOO mode —
29
Page 30

Page 31

Page 32

Medtronic, Inc.
*M994946A001*
710 Medtronic Parkway
Minneapolis, MN 55432
USA
www.medtronic.com
+1 763 514 4000
Medtronic USA, Inc.
Toll-free in the USA (24-hour technical
consultation for physicians and medical
professionals)
Bradycardia: +1 800 505 4636
Tachycardia: +1 800 723 4636
Technical manuals
www.medtronic.com/manuals
© 2019 Medtronic
M994946A001 A
2019-04-15
 Loading...
Loading...