Page 1

System eligibility, battery longevity,
specifications
InterStim™ Systems
Reference manual
! USA
Rx only
Page 2

Medtronic and the Medtronic logo are trademarks of Medtronic. ™* Third party brands
are trademarks of their respective owners. All other brands are trademarks of a
Medtronic company.
2 English System eligibility, battery longevity, specifications 2021-11-01
Page 3

Table of contents
Introduction 5
System eligibility 6
Maximum amplitude 8
Neurostimulator replacement 9
Replacing a Model 3023 Neurostimulator with a Model 3058 Neurostimulator 9
Suggestions for maximizing battery longevity (InterStim Model 3023, InterStim II
Model 3058, and InterStim X Model 97800 Neurostimulators) 11
Cycling (Model 3023 and Model 3058 Neurostimulators only) 11
Example: Impact of cycling intervals on InterStim II Model 3058 battery
longevity 11
Battery longevity (InterStim Micro Model 97810 Neurostimulator) 13
Estimating recharge interval (InterStim Micro Model 97810 Neurostimulator) 14
Battery longevity (InterStim II Model 3058 and InterStim X Model 97800
Neurostimulators) 15
Battery longevity estimation: Model 97800 InterStim X Neurostimulator 15
Battery longevity estimation: Model 3058 InterStim II Neurostimulator 16
Example: Model 3058 Neurostimulator 21
System eligibility, battery longevity, specifications 2021-11-01 English 3
Page 4

Information available for the system:
The information for prescribers manual provides information
about contraindications, warnings, precautions, adverse events,
sterilization, patient selection, individualization of treatment, and
component disposal. For customers in Japan, the appropriate
package insert provides information about safety,
contraindications, warnings, precautions, and adverse events.
The indications sheet provides information about indications and
related information. For customers in Japan, the appropriate
package insert provides information about indications.
The system eligibility, battery longevity, specifications manual
provides information about neurostimulator selection, battery
longevity calculations, and battery characteristics.
The system overview and compatibility insert provides
information about component compatibility.
MRI guidelines provide information about any MRI conditions and
MRI-specific contraindications, warnings, and precautions for MRI
scans with the neurostimulation system.
Product manuals, such as programming guides, recharging
guides, and implant manuals provide device descriptions,
package contents, device specifications, product-specific
warnings and precautions, and instructions for use.
! USA
The clinical summary provides information about the
clinical study results for the neurostimulation system.
4 English System eligibility, battery longevity, specifications 2021-11-01
Page 5

Introduction
This manual provides information about system eligibility, battery longevity and
specifications.
System eligibility describes how to use test stimulation to aid in neurostimulator
▪
selection.
Battery longevity and specifications provides information for use in estimating
▪
recharge interval (the number of days that a rechargeable neurostimulator will
provide stimulation at programmed settings before requiring recharge), and
information for use in estimating battery longevity (the number of months or years
that a neurostimulator is expected to provide stimulation at programmed settings
before requiring replacement). It also provides information about programming
choices that affect recharge interval and battery longevity.
Some product models described in this manual are not available in all geographies.
System eligibility, battery longevity, specifications 2021-11-01 English 5
Page 6
System eligibility
Prior to a new neurostimulator implant or a replacement neurostimulator implant, it is
important that the clinician ensures that a chosen neurostimulation system is appropriate
for the patient.
The InterStim™ Micro Model 97810 Neurostimulator is a rechargeable neurostimulator.
The clinician should assess the ability and willingness of a patient to perform recharging
tasks as part of establishing eligibility for the InterStim Micro system.
The InterStim II Model 3058 Neurostimulator and InterStim X Model 97800
Neurostimulator are not rechargeable. When the battery is depleted, the neurostimulator
must be replaced.
For first implant of a new lead or a new neurostimulation system, the first step in
determining system eligibility is to establish satisfactory patient response during a
therapy evaluation procedure. Refer to the System Overview and Compatibility Insert for
information on compatibility among components.
When using the Verify™ Model 3531 External Neurostimulator, refer to Table 1 for
guidance on determining whether the InterStim Micro Model 97810, InterStim X Model
97800 Neurostimulator, or the InterStim II Model 3058 Neurostimulator is an appropriate
device for the patient.
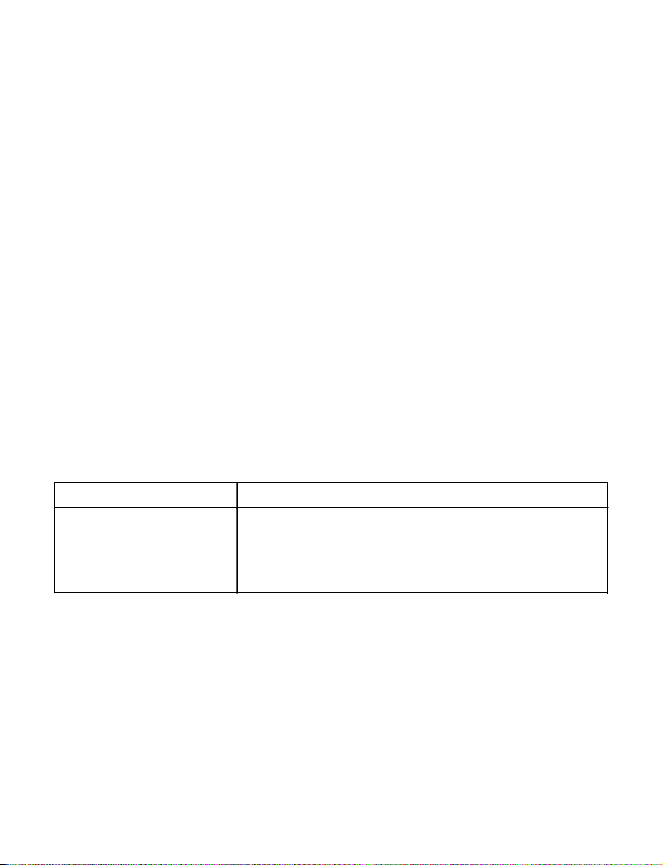
Table 1. Verify Model 3531 External Neurostimulator therapeutic amplitude and
corresponding system eligibility for the InterStim Micro Model 97810, InterStim X Model
97800 or InterStim II Model 3058 Neurostimulators
Therapeutic amplitude System eligibility
Less than 2 mA Settings achievable on the:
InterStim Micro Model 97810 Neurostimulator
•
InterStim X Model 97800 Neurostimulator
•
•
InterStim II Model 3058 Neurostimulator
6 English System eligibility, battery longevity, specifications 2021-11-01
Page 7

Table 1. Verify Model 3531 External Neurostimulator therapeutic amplitude and
corresponding system eligibility for the InterStim Micro Model 97810, InterStim X Model
97800 or InterStim II Model 3058 Neurostimulators (continued)
Therapeutic amplitude System eligibility
2 to 3 mA Settings achievable on the:
InterStim Micro Model 97810 Neurostimulator
•
InterStim X Model 97800 Neurostimulator
•
•
InterStim II Model 3058 Neurostimulator
May require more frequent neurostimulator replacements
(Model 3058 or Model 97800) or recharge (Model 97810)
due to high energy consumption.
Consider adjusting lead placement to obtain therapeutic
•
response at a lower amplitude to achieve lower energy
consumption.
To optimize battery longevity of the InterStim X Model
•
97800, InterStim II Model 3058, or InterStim Model 3023
Neurostimulator, refer to the information provided in
"Suggestions for maximizing battery longevity (InterStim
Model 3023, InterStim II Model 3058, and InterStim X
Model 97800 Neurostimulators)" on page 11.
To optimize recharge interval for the InterStim Micro
•
Model 97810 Neurostimulator, refer to the information
provided in Table 5 on page 14.
Greater than 3 mA Settings achievable on the:
InterStim Micro Model 97810 Neurostimulator
•
•
InterStim X Model 97800 Neurostimulator
Settings may not be achievable on the InterStim II Model
3058 Neurostimulator.
May require more frequent neurostimulator replacements
(Model 3058 or Model 97800) or recharge (Model 97810)
due to high energy consumption.
Consider adjusting lead placement to obtain therapeutic
•
response at a lower amplitude.
Consider adjusting electrode configuration to obtain
•
therapeutic response at a lower amplitude.
When using the Model 3625 Test Stimulator, use Table 2 to determine whether the
patient is eligible to use the InterStim II Model 3058 Neurostimulator. To use Table 2,
locate the intersection of the screening rate and pulse width. The value displayed at this
intersection reflects an amplitude that, if programmed on the Model 3625 Test Stimulator,
will produce an output equivalent to the maximum output of an InterStim II Model 3058
System eligibility, battery longevity, specifications 2021-11-01 English 7
Page 8
Neurostimulator that is programmed to its maximum amplitude, and to that rate and pulse
width combination.
If the screening amplitude exceeds the maximum amplitude value provided in
▪
Table 2 for a rate and pulse width combination, reduce the rate until you are at or
below a rate/pulse width/amplitude combination displayed in the table.
If the screening pulse width value is greater than 450 microseconds, reduce the
▪
pulse width value on the screener to 450 microseconds and gradually increase
amplitude to confirm satisfactory stimulation.
If the screening rate is greater than 130 Hertz, reduce the rate value on the screener
▪
to 130 Hertz and confirm satisfactory stimulation.
If satisfactory stimulation cannot be achieved within the parameters provided in
▪
Table 2, the InterStim II Model 3058 Neurostimulator may not be an appropriate
device for the patient.
If the screening amplitude is close to the maximum amplitude indicated in Table 2,
▪
consider reducing the rate to allow future increases in amplitude.
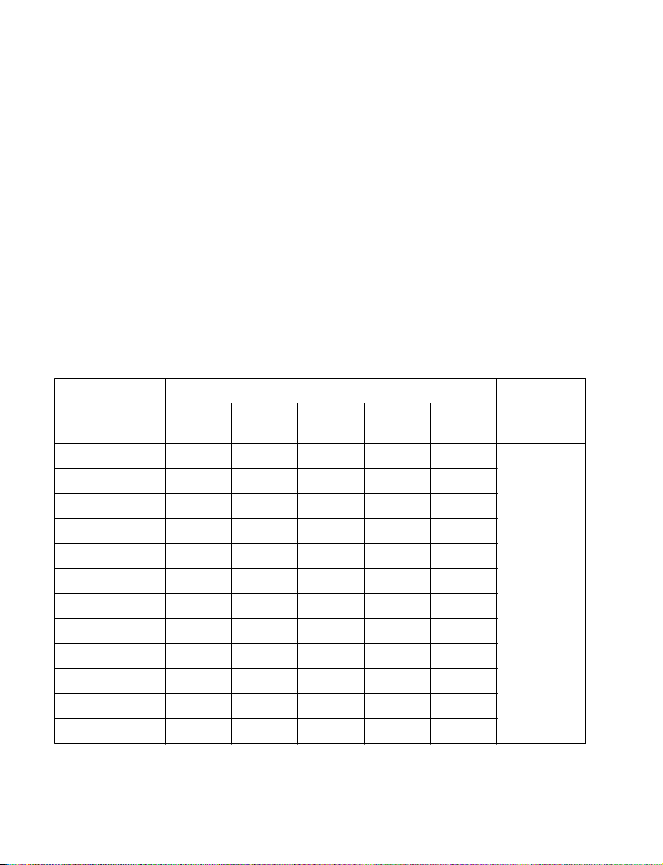
Maximum amplitude
Table 2. Model 3625 Test Stimulator settings for use in therapeutic evaluation of the
maximum amplitude of the InterStim II Model 3058 Neurostimulator
Screening pulse width (microseconds)
Screening rate
(Hz)
130 7.7 7.7 7.7 7.0 6.0 Amplitude
120 7.7 7.7 7.7 7.0 6.0
110 7.7 7.7 7.7 7.0 6.0
100 7.7 7.7 7.7 7.0 6.1
90 7.8 7.8 7.8 7.0 6.2
80 7.8 7.8 7.8 7.1 6.2
70 7.8 7.8 7.8 7.1 6.2
60 7.8 7.8 7.8 7.1 6.3
50 7.8 7.8 7.8 7.2 6.3
40 7.8 7.8 7.8 7.2 6.3
30 7.8 7.8 7.8 7.3 6.4
≤ 20 7.8 7.8 7.8 7.3 6.4
8 English System eligibility, battery longevity, specifications 2021-11-01
60 150 210 330 450
(V)
Page 9

Neurostimulator replacement
Replacing a Model 3023 Neurostimulator with a Model 3058 Neurostimulator
If you are replacing an InterStim Model 3023 Neurostimulator with an InterStim II Model
3058 Neurostimulator, first determine the effective therapeutic amplitude, rate, and pulse
width used by the patient with their Model 3023 Neurostimulator.
Note: Refer to the System overview and compatibility insert for information on
compatibility among components.
Next, use Table 3 to locate the intersection of the therapeutic rate and pulse width. The
value displayed at this intersection reflects an amplitude that, if programmed on the
InterStim Model 3023 Neurostimulator, will produce an output equivalent to the maximum
output of a Model 3058 Neurostimulator that is programmed to its maximum amplitude,
and to that rate and pulse width combination.
Table 3. InterStim Model 3023 Neurostimulator settings for use in therapeutic
evaluation of the maximum output of the InterStim II Model 3058 Neurostimulator
Pulse width (microseconds)
Rate (Hz) 60 150 210 330 450
130 8.5 8.5 8.4 8.0 7.1 Amplitude
120 8.5 8.5 8.4 8.0 7.1
110 8.5 8.5 8.4 8.0 7.1
100 8.5 8.5 8.4 8.0 7.1
90 8.5 8.5 8.5 8.1 7.1
80 8.5 8.5 8.5 8.1 7.1
70 8.5 8.5 8.5 8.1 7.1
60 8.5 8.5 8.5 8.2 7.1
50 8.5 8.5 8.6 8.2 7.2
40 8.5 8.5 8.6 8.2 7.2
30 8.5 8.5 8.6 8.2 7.2
≤ 20 8.5 8.5 8.6 8.2 7.2
(V)
Notes:
System eligibility, battery longevity, specifications 2021-11-01 English 9
Page 10

If the amplitude exceeds the maximum value provided in Table 3 for a rate and pulse
▪
width combination, reduce the rate until you are at or below a rate/pulse width/
amplitude combination displayed in the table.
If satisfactory stimulation cannot be achieved within the parameters provided in
▪
Table 3, the InterStim II Model 3058 Neurostimulator may not be an appropriate
device for the patient.
If the amplitude is close to the maximum value indicated in Table 3, consider
▪
reducing the rate to allow future increases in amplitude.
10 English System eligibility, battery longevity, specifications 2021-11-01
Page 11

Suggestions for maximizing battery longevity
(InterStim Model 3023, InterStim II Model 3058, and
InterStim X Model 97800 Neurostimulators)
To optimize the battery longevity of an implantable neurostimulator, follow these
suggestions:
Place leads in optimal location to achieve effective stimulation.
▪
Use the lowest effective settings for amplitude, rate, and pulse width.
▪
Instruct the patient to use the neurostimulator only when needed and to use the
▪
lowest settings that will give effective symptom relief.
Use the minimum number of electrodes necessary for effective stimulation.
▪
Consider decreasing therapy amplitude if the case electrode is used (Models 3023
▪
and 3058 only).
Cycling (Model 3023 and Model 3058 Neurostimulators only)
Anytime cycling is enabled, the longevity estimate may not be accurate. If cycling is
being used to improve device battery longevity, the information below should be
considered:
For amplitudes less than or equal to 1 Volt, cycling may result in reduced device
▪
battery longevity when compared to continuous mode.
For amplitudes greater than 1 Volt, the following recommended settings may improve
▪
device battery longevity when compared to programming in continuous mode:
Set cycling intervals to greater than or equal to 2 seconds ON and greater than
–
or equal to 2 seconds OFF (without SoftStart/Stop™ enabled).
OR
Set cycling intervals to greater than or equal to 60 seconds ON and greater than
–
or equal to 60 seconds OFF (with SoftStart/Stop enabled and programmed at
greater than or equal to 4 seconds).
Caution: Review information about the impact of cycling intervals on battery
longevity before programming cycling settings to intervals other than the recommended
intervals provided in this manual. Cycling intervals other than the recommended intervals
may result in decreased battery longevity, resulting in more frequent revision surgery.
Example: Impact of cycling intervals on InterStim II Model 3058 battery longevity
Table 4 provides an example of the impact of cycling intervals on battery longevity when
compared to continuous mode. Positive values represent a percentage increase in
battery longevity and negative values represent a percentage decrease in battery
longevity.
System eligibility, battery longevity, specifications 2021-11-01 English 11
Page 12

Table 4. Impact of cycling settings on battery longevity – InterStim II Model 3058
Neurostimulator with SoftStart/Stop enabled
Amplitude
(Volts)
Continuous
mode (no
cycling)
16 s ON
8s OFF
4s ramp
60s ON
60s OFF
4s ramp
a
10min ON
10min OFF
4s ramp
0.5hr ON
23.5hr OFF
0.5 0-10005
1.0 0 -10 0 5 15
2.0 0-15101540
4.0 0 -20 20 30 100
8.0 0 -10 50 60 400
a
All battery longevity calculations are based on a pulse width of 210 microseconds
and a rate of 14 Hz.
Note: Estimates are specific to stated programming parameters. Subsequent changes of
the parameters by the clinician or patient will affect neurostimulator battery longevity.
12 English System eligibility, battery longevity, specifications 2021-11-01
Page 13

Battery longevity (InterStim Micro Model 97810 Neurostimulator)
The InterStim Micro Model 97810 rechargeable Neurostimulator will provide 15 years of
operation before replacement is recommended. When the neurostimulator reaches 15
years of service, the elective replacement indicator (ERI) message will display on the
clinician and patient apps. At this time, the neurostimulator should be replaced.
System eligibility, battery longevity, specifications 2021-11-01 English 13
Page 14

Estimating recharge interval (InterStim Micro Model 97810 Neurostimulator)
The amount of time before the InterStim Micro Model 97810 rechargeable
neurostimulator battery requires charging is affected by the following factors:
programmed parameters (amplitude, rate, pulse width, cycling, and number of active
▪
electrodes)
system impedance
▪
hours per day of stimulation
▪
the degree of patient control over programmable stimulation parameters
▪
Higher stimulation settings will require more frequent recharging sessions. If all
programmable parameters are set to their upper limits, recharge interval could be less
than one day. Patients should define a recharge schedule that meets their individual
needs while maintaining a charge level that is capable of sustaining programmed
stimulation settings.
The table below provides the approximate number of days patients can initially expect
between recharge sessions. Cycling may be used to increase the recharge interval
beyond the values shown. Values shown are for a new device. Over time, the
neurostimulator battery may need more frequent recharges. Like all rechargeable
batteries, use over time will reduce the maximum charge capacity of the neurostimulator
battery.
Table 5. Recharge interval for InterStim Micro Model 97810 Neurostimulator (number of
Amplitude 500 ohms 1200 ohms 1500 ohms
1 mA 19 19 19
3 mA 12 12 11
5 mA 875
7 mA 533
a
Values shown assume bipolar stimulation, a rate of 14 Hz, a pulse width of 210 μs, and
continuous (non-cycling) stimulation.
days between recharge sessions)
a
Impedance
14 English System eligibility, battery longevity, specifications 2021-11-01
Page 15

Battery longevity (InterStim II Model 3058 and InterStim X Model 97800 Neurostimulators)
The purpose of performing the following recommended calculations is to aid the clinician
in selecting a neurostimulation device for each patient.
These calculations apply only to a new device and can only be considered for initial
estimates.
The battery of an implantable neurostimulator can last for months or years, depending on
these factors:
programmed parameters (amplitude, rate, pulse width, cycling, and number of active
▪
electrodes)
system impedance
▪
hours per day of stimulation
▪
the degree of patient control over programmable stimulation parameters
▪
The longevity estimates cannot predict precise battery longevity for a particular patient
because the actual usage and therapy settings (and thus battery life) are affected by the
following:
the patient’s neurostimulator usage: the patient has control of the neurostimulator
▪
usage through the patient control device
the reprogramming of therapy settings
▪
differences in tissue impedance from patient to patient or possibly over time
▪
Note: Changes to programmed parameters after the estimate is calculated will affect
battery longevity.
Battery longevity estimation: Model 97800 InterStim X Neurostimulator
For the estimated longevity for Model 97800, see Table 6.
System eligibility, battery longevity, specifications 2021-11-01 English 15
Page 16

Table 6. Estimated battery longevity for the InterStim X Model 97800 INS, at default rate
and pulse width settings, with two active electrodes
a
System impedance
Amplitude 500 ohms 1200 ohms 1500 ohms
0.1 mA 16.1 16.1 16.1
0.5 mA 12.3 12.3 12.3
1.0 mA 10.2 10.2 10.2
1.5 mA 8.7 8.7 8.7
2.0 mA 7.6 7.6 7.6
b
mA
5.0
a
Values shown within the table represent expected lifetime (in years).
b
See Table 1 for information about use of amplitude settings above 2.0 mA.
4.3 2.6 1.9
Note: Use of more than two active electrodes, a rate that is higher than the default rate
(14 Hz), or a pulse width longer than the default pulse width (210 µs), will result in
reduced battery longevity relative to the values shown in this table. If all programmable
parameters are set to their upper limit, battery longevity could be less than one year.
Values assume the cycling feature is turned off; use of cycling may extend device lifetime
beyond the values shown in this table.
To obtain system impedance value for use in estimating battery longevity, check
impedance following instructions provided in your clinician programming guide.
Battery longevity estimation: Model 3058 InterStim II Neurostimulator
The estimation formula is based on expected programming values and how often the
therapy is used.
Note: Cycling is not included in the battery longevity estimation formula. For more
information on how cycling affects the battery longevity of the Model 3058
Neurostimulator, see "Suggestions for maximizing battery longevity (InterStim Model
3023, InterStim II Model 3058, and InterStim X Model 97800 Neurostimulators)".
1. Record the following expected programming values:
Amplitude: _______ V
Rate: _______ Hz
16 English System eligibility, battery longevity, specifications 2021-11-01
Page 17

Pulse width: _______ μsec
Active electrodes: _______
Stimulation/day: _______
Hours/day
Note: Enter the number of hours/day that the INS is turned
on, if the INS is routinely turned off for part of the day. Time
on or off due to cycling should not be considered in
calculating the hours of stimulation per day.
2. Locate the Energy Use (EU) from Table 7 (using programmed Amplitude, Rate,
Pulse Width):
_______
3. Locate the Electrode Correction Factor (ECF) for the number of active electrodes
from Table 8:
_______
4. Determine the Usage Correction Factor (UCF) using the hours of stimulation per day.
Hours of
stimulation per
day
24 hours Usage
Correction
Factor
_______ ÷24=_______
5. Compute the Adjusted Energy Use value:
EU ECF UCF Adjusted
Energy Use
_______ x _______ x _______ = _______
Step 2
(above)
Step 3
(above)
Step 4
(above)
Formula provides the value to determine longevity estimate
6. Use the Adjusted Energy Use value and determine the estimated battery longevity
from Figure 1:
Longevity estimate _______ years
System eligibility, battery longevity, specifications 2021-11-01 English 17
Page 18

Note: The estimate is applicable to specified programming parameters. Subsequent
changes of the parameters by the clinician or patient will affect neurostimulator
battery longevity.
Table 7. Model 3058 Neurostimulator energy use
a
Pulse width
Amplitude Rate 60 210 450
1.0 14
65
130
2.0 14
65
130
3.0 14
65
130
4.0 14
65
130
5.0 14
65
130
6.0 14
65
130
7.0 14
65
130
8.0 14
a
Use values that are closest to the expected values. For amplitude values not listed in
65
130
12
16
34
27
52
11
47
92
14
74
145
22
109
214
30
153
303
44
206
408
1
6
3
5
4
20
40
12
57
114
21
89
177
34
160
319
59
258
520
98
383
786
127
546
1174
159
773
1484
9
40
80
27
120
240
41
188
377
75
343
699
113
564
1194
164
852
1491
223
993
1513
257
994
1514
the table, round up or down to the nearest whole number as demonstrated in
"Example: Model 3058 Neurostimulator" on page 21.
18 English System eligibility, battery longevity, specifications 2021-11-01
Page 19

Table 8. Electrode correction factor for quadripolar lead when used with the Model 3058
Neurostimulator
Electrode configuration Electrode correction factor
2 active electrodes 1.0
3 active electrodes 1.7
4 active electrodes 2.4
Case +, 1 active electrode 1.9
Case +, 2 active electrodes 3.3
Case +, 3 active electrodes 4.1
Case +, 4 active electrodes 4.8
System eligibility, battery longevity, specifications 2021-11-01 English 19
Page 20

Longevity estimate (years)
Adjusted energy use
Figure 1. Model 3058 Neurostimulator longevity estimates (years) for energy use
Note: If programmed parameters result in less than two years of battery longevity,
consider the risk of increased surgical exposure against the benefit of therapy for the
20 English System eligibility, battery longevity, specifications 2021-11-01
Page 21

patient. If all programmable parameters are set to their upper limit, battery longevity
could be one to two months.
Example: Model 3058 Neurostimulator
The following example shows expected battery longevity based on entering the patient’s
expected programming values into the estimation formula.
Note: Cycling is not included in the battery longevity estimation formula. For more
information on how cycling affects battery longevity, see "Suggestions for maximizing
battery longevity (InterStim Model 3023, InterStim II Model 3058, and InterStim X Model
97800 Neurostimulators)" on page 11.
1. Record the following expected programming values:
Amplitude: 2.6 V
Rate: 14 Hz
Pulse width: 210 μsec
Active electrodes: 2 active electrodes
Stimulation/day: 24
2. Locate the Energy Use (EU) from Table 7 (using programmed Amplitude, Rate,
Pulse Width):
3. Locate the Electrode Correction Factor (ECF) for the number of active electrodes
from Table 8:
_____
_____
________
________________
_______
Hours/day
Note: Enter the number of hours/day that the INS is turned
on, if the INS is routinely turned off for part of the day. Do not
include time off due to cycling.
21
_____
1.0
_____
System eligibility, battery longevity, specifications 2021-11-01 English 21
Page 22

4. Determine the Usage Correction Factor (UCF) using the hours of stimulation per day.
Hours of stimulation per
day
24
_____
24 hours Usage Correction
÷24= 1.0
Factor
_____
5. Compute the Adjusted Energy Use value:
EU ECF UCF Adjusted
21
_____
Step 2
(above)
x1.0
_____
Step 3
(above)
x1.0
_____
Step 4
(above)
Energy Use
=21
_____
Formula provides the value to determine longevity estimate
6. Use the Adjusted Energy Use value and determine the estimated battery longevity
from Figure 1:
Longevity estimate 3.6 years
________
Note: The estimate is applicable to specified programming parameters. Subsequent
changes of the parameters by the clinician or patient will affect neurostimulator
battery longevity.
22 English System eligibility, battery longevity, specifications 2021-11-01
Page 23

System eligibility, battery longevity, specifications 2021-11-01 English 23
Page 24

Manufacturer
Medtronic, Inc.
710 Medtronic Parkway
Minneapolis, MN 55432
USA
www.medtronic.com
Tel. +1-763-505-5000
Authorized Representative
in the European Community
Medtronic B.V.
Earl Bakkenstraat 10
6422 PJ Heerlen
The Netherlands
Tel. +31-45-566-8000
Europe/Africa/Middle East Headquarters
Medtronic International Trading Sàrl
Route du Molliau 31
Case Postale 84
CH - 1131 Tolochenaz
Switzerland
www.medtronic.eu
Tel. +41-21-802-7000
Asia-Pacific
Medtronic International Ltd.
50 Pasir Panjang Road
#04-51 Mapletree Business City
Singapore 117384
Singapore
Tel. +65-6870-5510
*M988757A016*
© Medtronic 2021
All Rights Reserved
2021-11-01
M988757A016 Rev B
 Loading...
Loading...