Page 1

TALENT® THORACIC STENT GRAFT
WITH THE CAPTIVIA DELIVERY SYSTEM
INSTRUCTIONS FOR USE
CAUTION: FEDERAL (USA) LAW LIMITS THIS DEVICE FOR SALE BY OR
ON ORDER OF A PHYSICIAN.
IMPORTANT!
• Do not attempt to use the Talent
before completely reading and understanding the information
contained in this booklet.
• Carefully inspect all product packaging for damage or defects
prior to use. Do not use product if any sign of damage or breach
of the sterile barrier is observed.
• These devices are supplied STERILE for single use only. After
use, dispose of the delivery system in accordance with hospital,
administrative and/or government policies. Do not resterilize.
• Caution: Federal (U.S.) Law restricts this device to sale by or on
the order of a physician.
®
Thoracic Stent Graft System
Page 2

TABLE OF CONTENTS
1.0 INTRODUCTION ................................................................................................................ 5
2.0 DEVICE DESCRIPTION..................................................................................................... 5
2.1 TALENT THORACIC STENT GRAFT SYSTEM ........................................................................ 5
2.2 TALENT THORACIC STENT GRAFT...................................................................................... 5
2.2.1 PROXIMAL MAIN SECTION................................................................................. 6
2.2.2 DISTAL MAIN SECTION ....................................................................................... 7
2.2.3 PROXIMAL EXTENSION ...................................................................................... 7
2.2.4 DISTAL EXTENSION ............................................................................................ 8
2.3 CAPTIVIA DELIVERY SYSTEM............................................................................................. 9
3.0 INDICATIONS FOR USE ................................................................................................. 11
4.0 CONTRAINDICATIONS ................................................................................................... 11
5.0 WARNINGS AND PRECAUTIONS .................................................................................. 11
5.1 GENERAL ....................................................................................................................... 11
5.2 PATIENT SELECTION, TREATMENT AND FOLLOW-UP......................................................... 11
5.3 IMPLANT PROCEDURE ..................................................................................................... 12
5.4 MAGNETIC RESONANCE IMAGING (MRI)........................................................................... 13
6.0 POTENTIAL ADVERSE EVENTS .................................................................................... 14
6.1 ADVERSE EVENT REPORTING.......................................................................................... 14
7.0 SUMMARY OF PIVOTAL US CLINICAL STUDY ............................................................ 15
7.1 SUBJECT ACCOUNTABILITY AND FOLLOW-UP.................................................................... 16
7.2 DEMOGRAPHICS AND BASELINE MEDICAL HISTORY .......................................................... 17
7.3 BASELINE ANEURYSM DATA ............................................................................................ 19
7.4 DEVICES IMPLANTED....................................................................................................... 20
7.5 STUDY RESULTS............................................................................................................. 22
7.5.1 SAFETY............................................................................................................... 22
7.5.2 EFFECTIVENESS ............................................................................................... 32
7.5.3 SUPPLEMENTARY ACUTE PROCEDURAL DATA........................................... 35
7.6 VALOR TEST GROUP RESULTS BY LESION TYPE............................................................. 36
7.6.1 SUBJECT DEMOGRAPHICS AND LESION CHARACTERISTICS ................... 36
7.6.2 PRIMARY AND SECONDARY SAFETY AND EFFECTIVENESS ENDPOINT
ANALYSIS BY LESION TYPE............................................................................ 38
8.0 PATIENT SELECTION ..................................................................................................... 40
8.1 INDIVIDUALIZATION OF TREATMENT.................................................................................. 40
9.0 PATIENT COUNSELING INFORMATION ....................................................................... 40
10.0 HOW SUPPLIED .............................................................................................................. 40
10.1 STERILITY ...................................................................................................................... 40
10.2 CONTENTS ..................................................................................................................... 40
10.3 STORAGE....................................................................................................................... 40
11.0 CLINICAL USE INFORMATION....................................................................................... 41
11.1 RECOMMENDED SKILLS AND TRAINING ............................................................................ 41
11.1.1 PATIENT SELECTION ........................................................................................ 41
11.1.2 PHYSICIAN SKILLS AND EXPERIENCE ........................................................... 41
11.2 MATERIALS RECOMMENDED FOR DEVICE IMPLANTATION .................................................. 41
11.3 PRE-TREATMENT PLANNING AND SELECTION OF STENT GRAFT ........................................ 42
12.0 IMPLANTATION INSTRUCTIONS................................................................................... 44
12.1 PICTORIAL REFERENCES................................................................................................. 44
12.2 VASCULAR ACCESS ........................................................................................................ 44
12.3 INITIAL ANGIOGRAM ........................................................................................................ 44
12.4 DEVICE PREPARATION .................................................................................................... 44
12.5 HYDROPHILIC COATING ACTIVATION................................................................................ 45
12.6 INTRODUCING THE CAPTIVIA DELIVERY SYSTEM............................................................... 45
12.7 POSITIONING THE CAPTIVIA DELIVERY SYSTEM ................................................................ 46
12.8 CONFIRMING STENT GRAFT POSITION AND VERIFY MARKERS........................................... 46
12.9 DEPLOYING THE TALENT THORACIC STENT GRAFT........................................................... 47
12.10 DEPLOYING TIP CAPTURE MECHANISM (FREEFLO STENT GRAFT DELIVERY SYSTEM ONLY)51
2
Page 3

12.11 REMOVING THE DELIVERY SYSTEM.................................................................................. 52
12.12 ANCILLARY BALLOON CATHETER MODELING .................................................................... 54
12.13 IMPLANTING ADDITIONAL COMPONENT SECTIONS............................................................. 55
12.14 TROUBLESHOOTING ........................................................................................................ 56
12.14.1 HANDLE DISASSEMBLY TECHNIQUE FOR PARTIAL STENT GRAFT
DEPLOYMENT ................................................................................................... 56
12.14.2 ALTERNATIVE INSTRUCTION FOR DEPLOYING TIP CAPTURE MECHANISM
............................................................................................................................ 56
13.0 IMAGING GUIDELINES AND POST-OPERATIVE FOLLOW-UP ................................... 57
13.1 GENERAL ....................................................................................................................... 57
13.2 ANGIOGRAPHIC IMAGING ................................................................................................. 57
13.3 CTA/MRA IMAGES ......................................................................................................... 57
13.4 X-RAY ........................................................................................................................... 58
13.5 MRI INFORMATION.......................................................................................................... 58
13.6 ADDITIONAL SURVEILLANCE AND TREATMENT .................................................................. 59
14.0 DEVICE-RELATED ADVERSE EVENTS REPORTING .................................................. 59
15.0 PATIENT MATERIALS AND TRACKING INFORMATION .............................................. 59
16.0 EXPLANATION OF SYMBOLS ........................................................................................ 60
LIST OF FIGURES
Figure 1: Thoracic Stent Graft - Main Section ................................................................................ 6
Figure 2: Thoracic Stent Graft - Additional Distal Main Section ..................................................... 7
Figure 3: Thoracic Stent Graft - Proximal Extension ...................................................................... 7
Figure 4: Thoracic Stent Graft - Distal Extension ........................................................................... 8
Figure 5a: Captivia Delivery System (The FreeFlo Stent Graft Delivery System) ........................ 10
Figure 6: Covered Portion (Top of Fabric) Placement Zones ....................................................... 12
Figure 7- Kaplan-Meier Plot of Freedom from All Cause Mortality at 30 Days and 12 Months:
VALOR Test Group vs. Retrospective Open Surgery Group ........................................ 23
Figure 8 – Kaplan-Meier Plot of Freedom from Major Adverse Events at 30 Days: VALOR Test
Group vs. Retrospective Open Surgery ......................................................................... 27
Figure 9 – Kaplan-Meier Plot of Freedom from Serious Major Adverse Events: VALOR Test
Group Only..................................................................................................................... 29
Figure 10 – Kaplan-Meier Plot of Freedom from Aneurysm-Related Mortality at 12 Months:
VALOR Test Group vs. Retrospective Open Surgery Group ........................................ 31
Figure 11: Regions for Modular Overlaps ..................................................................................... 43
Figure 12: Tip Capture Release Handle in Locked Position ......................................................... 44
Figure 13: Introduce the Captivia Delivery System ....................................................................... 45
Figure 14a: Confirm Stent Graft Position (Proximal Main) ............................................................ 46
Figure 14b: Confirm Stent Graft Position (Distal Main) ................................................................. 46
Figure 15: Covered Portion (Top of Fabric) Placement Zones ..................................................... 48
Figure 16a: Deploying the Proximal End of the Stent Graft (FreeFlo) ......................................... 49
Figure 16b: Deploying the Proximal End of the Stent Graft (Open Web)..................................... 49
Figure 17a: Deploying the Remainder of the Stent Graft (FreeFlo) ............................................. 50
Figure 17b: Deploying the Remainder of the Stent Graft (Open Web) ........................................ 50
Figure 18: Unlocking the Tip Capture Release Handle ................................................................ 51
Figure 19: Deploying the Tip Capture Mechanism ....................................................................... 52
Figure 20: Delivery System Removal ........................................................................................... 53
Figure 21: Balloon Modeling of the Stent Graft ............................................................................ 54
LIST OF TABLES
Table 1 – Stent Graft Materials........................................................................................................ 5
Table 2 - Talent Thoracic Stent Graft Summary.............................................................................. 5
3
Page 4

Table 3 - Subject and Imaging Accountability Table–VALOR Test Group Only ........................... 16
Table 4 - Subject Demographics: VALOR Test Group vs. Retrospective Open Surgery Group .. 17
Table 5 - Subject Anatomic Lesion Type: VALOR Test Group Only............................................. 17
Table 6 - Baseline Medical History: VALOR Test Group vs. Retrospective Open Surgery Group 18
Table 7 - Baseline Modified SVS Classification: VALOR Test Group Only .................................. 18
Table 8 - Baseline Maximum Aneurysm Diameters: VALOR Test Group vs. Retrospective Open
Surgery........................................................................................................................... 19
Table 9 - Baseline Vessel Dimensions (Core Lab Reported): VALOR Test Group Only.............. 19
Table 10- Number of Devices Implanted at Initial Procedure: VALOR Test Group Only.............. 20
Table 11: Number of Main Sections and Number of Extensions Implanted at Initial Procedure:
VALOR Test Group Only ............................................................................................... 20
Table 12 - VALOR Test Group: Talent Thoracic Stent Graft Devices Implanted .......................... 21
Table 13- VALOR Test Group: Distal Main Devices Implanted .................................................... 21
Table 14- All-Cause Mortality at 30 Days and 12 months: VALOR Test Group vs. Retrospective
Open Surgery................................................................................................................. 23
Table 15: Details of Kaplan-Meier Plot of Freedom from All Cause Mortality at 30 Days and 12
Months: VALOR Test Group vs. Retrospective Open Surgery Group........................... 24
Table 16 - Summary of Major Adverse Events for VALOR Test Group vs. Retrospective Open
Surgery Group (30 days) ............................................................................................... 25
Table 17- Freedom from Major Adverse Events (MAE) at 30 days: VALOR Test Group vs.
Retrospective Open Surgery Group .............................................................................. 26
Table 18: Details of Kaplan-Meier Plot of Freedom from Major Adverse Events at 30 Days:
VALOR Test Group vs. Retrospective Open Surgery ................................................... 27
Table 19 -Summary of Serious Major Adverse Events from VALOR Test Group Only ................ 28
Table 20- Freedom from Serious Major Adverse Events (MAE) at 30 days and 12-months:
VALOR Test Group Only ............................................................................................... 28
Table 21: Details of Kaplan-Meier Plot of Freedom from Serious Major Adverse Events: VALOR
Test Group Only............................................................................................................. 29
Table 22- Aneurysm-Related Mortality at 12 Months: VALOR Test Group vs. Retrospective Open
Surgery Group ............................................................................................................... 30
Table 23: Details of Kaplan-Meier Plot of Freedom from Aneurysm-Related Mortality at 12
Months: VALOR Test Group vs. Retrospective Open Surgery Group........................... 31
Table 24- Primary Effectiveness Endpoint: Successful Aneurysm Treatment: VALOR Test Group
....................................................................................................................................... 32
Table 25- Summary of Subjects with Primary Effectiveness Failure: VALOR Test Group ........... 32
Table 26 - Other Effectiveness Data: VALOR Test Group Only ................................................... 33
Table 27 - Supplementary Acute Procedural Data: VALOR Test Group vs. Retrospective Open
Surgery Group ............................................................................................................... 35
Table 28 - Subject Demographics by Lesion Type – VALOR Test Group Only............................ 36
Table 29 - Baseline Vessel Dimensions by Lesion Type: VALOR Test Group Only (Core Lab
Reported
Table 30: Baseline Vessel Shape by Lesion Type (Core Lab Reported1) – VALOR Test Group
Only................................................................................................................................ 38
Table 31: Primary Safety Endpoint: All Cause Mortality by Lesion Type – VALOR Test Group
Only................................................................................................................................ 38
Table 32: Primary Effectiveness Endpoint: Successful Aneurysm Treatment by Lesion Type –
VALOR Test Group Only ............................................................................................... 38
Table 33: Summary of Secondary Endpoints by Lesion Type – VALOR Test Group Only .......... 39
Table 34: Persistent Paraplegia/Paraparesis at 12 Months or last Follow-up by Lesion Type –
VALOR Test Group Only ............................................................................................... 39
Table 3 - Sizing Guidelines............................................................................................................ 42
Table 4 - Order of Deployment When Using Multiple Stent Graft Component Sections............... 43
Table 5 - Imaging Recommendations............................................................................................ 57
Table 6 - CTA Imaging Guidelines ................................................................................................58
1
)...................................................................................................................... 37
4
Page 5

1.0 Introduction
The Talent® Thoracic Stent Graft System is intended for the endovascular repair of fusiform aneurysms and saccular
aneurysms/penetrating ulcers of the descending thoracic aorta. When placed within the target lesion, the stent graft
provides an alternative conduit for blood flow within the patient’s vasculature by excluding the lesion from blood flow and
pressure.
2.0
2.1 Talent Thoracic Stent Graft System
The Talent
• The Talent Thoracic Stent Graft
• The Captivia Delivery System
The Talent Thoracic Stent Graft is pre-loaded into the Captivia Delivery System. The loaded delivery system is inserted
endoluminally via the femoral or iliac artery and tracked through the patient’s vasculature to deliver the stent graft to the
target site.
2.2 Talent Thoracic Stent Graft
The Talent Thoracic Stent Graft is composed of a series of shaped, sinusoidal, self-expanding nitinol wire rings which act
as springs that are stacked in a tubular arrangement to form a self expanding nitinol structure. Proximal and distal springs
of the stent graft are connected by a full-length connecting bar. The self-expanding nitinol structure is covered by a monofilament polyester woven graft. The graft material is sewn to the nitinol structure, which securely incorporates the springs
into the graft. Radiopaque markers, made out of platinum-iridium in shape of a figure eight (known as Figur8), are sewn
to the graft to help visualize and identify: the edge of the graft material, the location of the connecting bar, and the
minimum overlap required when multiple stent grafts are used. A support spring surrounding the proximal edge of the
graft material is also used in some configurations. Table 1 lists the materials comprising the stent graft.
The Talent Thoracic Stent Graft System is a modular device system that accommodates the use of multiple stent graft
sections. Depending on the patient’s anatomy, single or multiple stent grafts may be required to achieve sufficient
coverage and exclude the target lesion. Table 2 summarizes the features of various modular stent graft component
sections. Each component section is described in detail below.
Device Description
®
Thoracic Stent Graft System includes:
Table 1 – Stent Graft Materials
Stent Graft Component Material
Springs Nitinol wire (55% Nickel, balance Titanium with trace elements)
Connecting Bar Nitinol wire (55% Nickel, balance Titanium with trace elements)
Support Spring (FreeFlo™ only) Nitinol wire (55% Nickel, balance Titanium with trace elements)
Stent Fabric High-density woven mono-filament polyester
Sutures Braided polyester suture
Radiopaque Markers Figur8 Platinum Iridium wire
Component
Proximal
Main Section
Distal Main
Section
Proximal
Extension
Distal
Extension
Table 2 - Talent Thoracic Stent Graft Summary
Proximal End
Configuration
FreeFlo (>22mm)
Bare Spring (22mm)
Open Web Closed Web 130mm 110-114mm 26mm – 46mm Tapered Tube
FreeFlo (Bare Spring
with Support Spring)
Open Web Bare Spring 80-90mm 46-54mm 26mm – 46mm Straight Tube
Distal End
Configuration
Closed Web
Open Web 80-90mm 46-54mm 26mm – 46mm Straight Tube
Total
Length
130mm 112-116mm
175mm 157-161mm
215mm 197-201mm
Covered
Length
Available
Diameters
22mm – 46mm Straight Tube
Straight or
Tapered
Tube
5
Page 6

2.2.1 Proximal Main Section
The proximal main section has an uncovered nitinol spring as the proximal end configuration, which allows for transvessel flow. Proximal main stent grafts with a proximal diameter greater than 22mm have a mini-support spring to aid in
sealing. The proximal end configuration in which an uncovered nitinol spring and mini-support spring are present is called
the ‘FreeFlo’ configuration. The proximal end configuration in which an uncovered nitinol spring is present without a minisupport spring is called a ‘Bare Spring’ configuration. The distal end of the stent graft has a Closed Web configuration.
The two proximal markers and two distal markers indicate the ends of the covered portion of the stent graft. The middle
marker indicates the rotational position of the connecting bar. See Figure 1.
Figure 1: Thoracic Stent Graft - Main Section
[GRAPHICAL REPRESENTATION ONLY. MAY APPEAR DIFFERENTLY UNDER FLUOROSCOPY]
6
Page 7

2.2.2 Distal Main Section
Distal main sections are used to increase the length of coverage of the treated vessel when the proximal main section is
inadequate in length to exclude the aneurysm. The proximal end of the distal main section utilizes a configuration in
which the outline of the most proximal spring is covered with fabric leaving a “tulip” effect, called Open Web. The distal
end of the distal main section is a Closed Web configuration. Two alignment markers are used to indicate the 30mm
minimum overlap with the mating graft. The two distal markers indicate the bottom edge of the covered portion of the
stent graft. The middle marker indicates the rotational position of the connecting bar. See Figure 2.
Figure 2: Thoracic Stent Graft - Additional Distal Main Section
MIDDLE MARKER
[GRAPHICAL REPRESENTATION ONLY. MAY APPEAR DIFFERENTLY UNDER FLUOROSCOPY]
2.2.3 Proximal Extension
Proximal extensions are intended to be used when the proximal end of the stent graft requires extension to fully exclude
the target lesion, or to treat proximal Type I endoleaks. The proximal extension is deployed within the proximal end of the
proximal main section. The proximal end of the proximal extension section has a FreeFlo configuration, which allows for
trans-vessel flow. The distal end of the proximal extension section has an Open Web configuration. The two proximal
markers indicate the top edge of the covered stent graft. The single alignment marker is used to indicate the 30mm
minimum overlap with the mating graft, as well as the rotational location of the connecting bar. See Figure 3.
Figure 3: Thoracic Stent Graft - Proximal Extension
FREEFLO
MINI SUPPORT SPRING
[GRAPHICAL REPRESENTATION ONLY. MAY APPEAR DIFFERENTLY UNDER FLUOROSCOPY]
7
Page 8

2.2.4 Distal Extension
Distal extensions are intended to be used when the distal end of the stent graft requires extension to fully exclude the
target lesion, or to treat distal Type I endoleaks. The distal extension is deployed in the distal end of the proximal main or
distal main section and extends distally. The proximal end has an Open Web configuration. The distal end has a bare
spring extending beyond the edge of the fabric, which allows for trans-vessel flow. The single “alignment marker”
indicates the 30mm minimum overlap with the mating graft, as well as the rotational position of the connecting bar. The
two distal markers indicate the bottom edge of the covered portion of the stent graft. See Figure 4.
Figure 4: Thoracic Stent Graft - Distal Extension
[GRAPHICAL REPRESENTATION ONLY. MAY APPEAR DIFFERENTLY UNDER FLUOROSCOPY]
8
Page 9

2.3 Captivia Delivery System
The Captivia Delivery System consists of a single use, disposable catheter with an integrated handle to provide the user
with controlled deployment. The Captivia Delivery System is the generic name for two particular delivery system
configurations:
• The FreeFlo Stent Graft Delivery System – Used with the FreeFlo Straight Proximal Main and Free Flow
Straight Proximal Extension Configuration stent grafts. Features a tip capture mechanism in which the
stent graft is deployed in two stages – (1) deployment of the stent graft with the bare stent of the FreeFlo
configuration still constrained, and (2) release of the bare stent. This particular delivery system allows for
a more controlled deployment of the stent graft in the thoracic aortic environment.
• The Open Web Stent Graft Delivery System – Used with the Open Web Tapered Distal Main and Open
Web Straight Distal Extension Configuration stent grafts. Features a one-stage deployment of the stent
graft.
The Captivia Delivery System is a multi-lumen device, with each lumen serving a different function:
1) The inner member provides a lumen to allow the system to track over a 0.035" (0.89 mm) guidewire.
2) The tip capture tube (for the FreeFlo Stent Graft Delivery System only) provides a lumen to actuate the tip
capture mechanism.
3) The flexible stent stop provides a lumen to aid in tracking the system through tortuous anatomy and maintains
stent graft position during deployment.
4) The graft cover, with stainless steel braid, provides a lumen to contain the stent graft during tracking and from
which the stent graft is released during deployment.
A flexible tapered tip is attached to the end of the inner member and provides a smooth transition from the guidewire to
the outer graft cover. The tapered tip and graft cover are coated with a lubricious hydrophilic coating to facilitate vessel
access and tracking through tortuous anatomy. A distal radiopaque marker indicates the graft cover edge under
fluoroscopy. A hemostasis valve at the proximal end of the delivery system minimizes blood loss and leaking during the
procedure. The stent graft is deployed by rotating or retracting the integrated slider/handle. The tip capture release
handle at the rear of the delivery system is unlocked and retracted to release the bare stent of the FreeFlo configuration,
thereby completing deployment of the stent graft (the tip capture feature is only available on the FreeFlo Stent Graft
Delivery System).
NOTE: THE CAPTIVIA DELIVERY SYSTEM WILL ACCOMMODATE ONLY A 0.035” (0.89 MM) GUIDEWIRE.
9
Page 10

Figure 5a: Captivia Delivery System (The FreeFlo Stent Graft Delivery System)
Figure 5b: Captivia Delivery System
(The Open Web Stent Graft Delivery System)
1. Luer Connector 9. Stent Stop Insert
2. Sideport Extension 10. Tip Capture Mechanism
3. Screw Gear 11. RO Marker Band
4. Slider / Handle 12. Tapered Tip
5. Trigger 13. Back End Lock
6. Front Grip 14. Tip Capture Release Handle
7. Strain Relief 15. Clamping Ring
8. Graft Cover (Introducer
Sheath)
10
Page 11

3.0 Indications for Use
The Talent Thoracic Stent Graft System is intended for the endovascular repair of fusiform aneurysms and saccular
aneurysms/penetrating ulcers of the descending thoracic aorta in patients having appropriate anatomy, including:
• iliac/femoral access vessel morphology that is compatible with vascular access techniques, devices, and/or
accessories;
• non-aneurysmal aortic diameter in the range of 18 – 42mm; and
• non-aneurysmal aortic proximal and distal neck lengths ≥ 20mm
4.0 Contraindications
The Talent Thoracic Stent Graft is contraindicated in:
• Patients who have a condition that threatens to infect the graft.
• Patients with sensitivities or allergies to the device materials (see Table 1).
5.0
5.1 General
5.2 Patient Selection, Treatment and Follow-Up
Warnings and Precautions
• Read all instructions carefully. Failure to properly follow the instructions, warnings and precautions may lead to
serious consequences or injury to the patient.
• The Talent Thoracic Stent Graft System should only be used by physicians and teams trained in vascular
interventional techniques, including training in the use of this device. Specific training expectations are
described in Section 11.1.
• Consider having a vascular surgery team available during implantation or reintervention procedures in the event
that conversion to open surgical repair is necessary.
• Do not attempt to use the Talent Thoracic Stent Graft with the Captivia Delivery System in patients unable to
undergo the necessary preoperative and postoperative imaging and implantation studies as described in
Section 13.0.
• The Talent Thoracic Stent Graft System is not recommended in patients who cannot tolerate contrast agents
necessary for intra-operative and post-operative follow-up imaging.
• The Talent Thoracic Stent Graft System is not recommended in patients exceeding weight and/or size limits
which compromise or prevent the necessary imaging requirements.
• Prior to the procedure, pre-operative planning for access and placement should be performed. See Section
11.3. Key anatomic elements that may affect successful exclusion of the aneurysm include severe neck
angulation, short aortic neck(s) and significant thrombus and/or calcium at the arterial implantation sites. In the
presence of anatomical limitations, a longer neck length may be required to obtain adequate sealing and
fixation.
• The use of this device requires administration of radiographic agents. Patients with preexisting renal
insufficiency may have an increased risk of renal failure postoperatively.
• The safety and effectiveness of this device in the treatment of dissections have not been established. In the
first 10 years of clinical experience (OUS-commercial and US-investigational), there were 39 reported events of
retrograde dissection in patients. Of the 39 reported events, 33 patients had a pre-existing aortic dissection.
• Inappropriate patient selection may contribute to poor device performance.
• The safety and effectiveness of the Talent Thoracic Stent Graft has not been evaluated in the following patient
situations and/or populations in which:
Planned placement of the COVERED (top edge of fabric) portion of the stent graft requires implant to
occur in zones 0 or 1 (See Figure 6).
11
Page 12
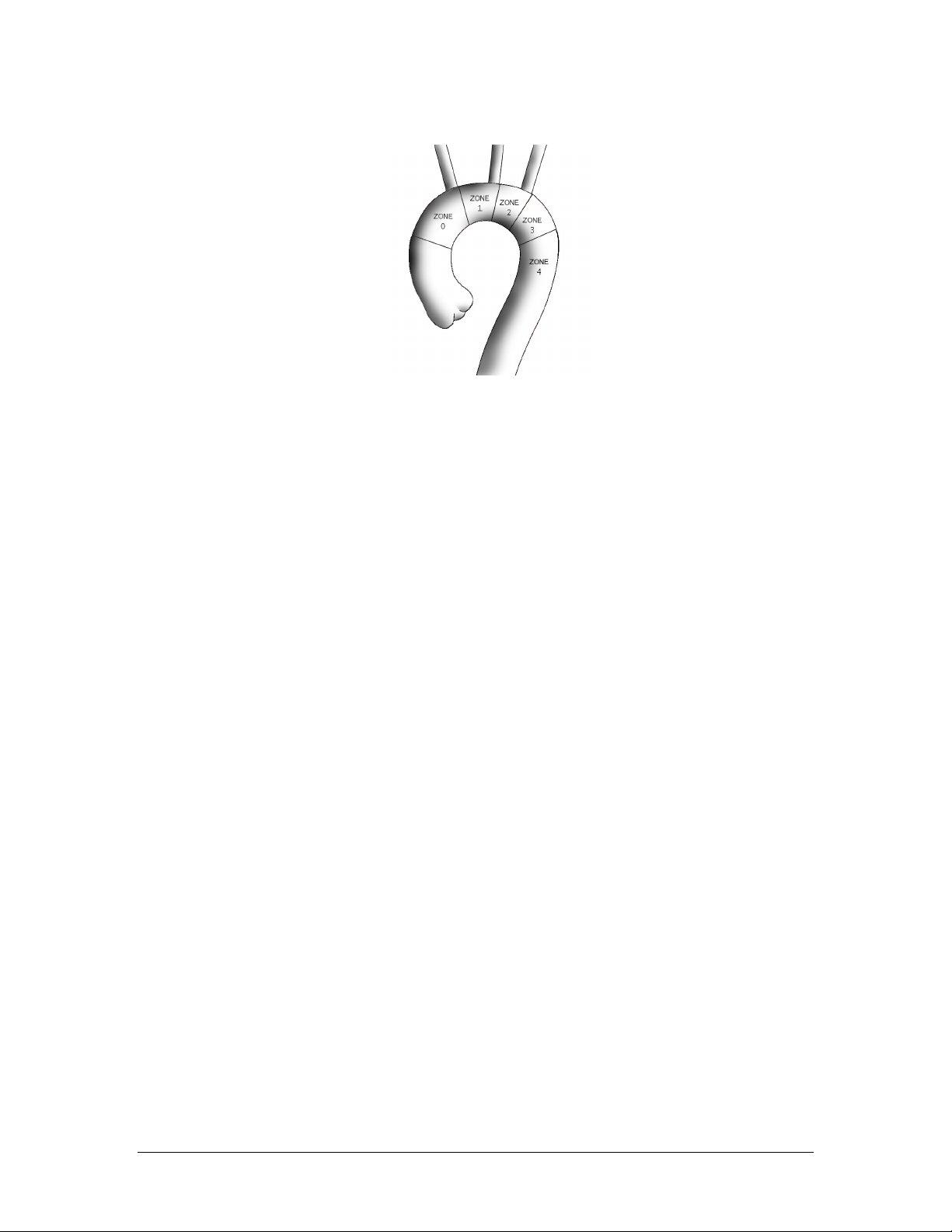
Figure 6: Covered Portion (Top of Fabric) Placement Zones
The patient’s access vessel (as determined by treating physician) precludes safe insertion of the delivery
system.
NOTE: ILIAC CONDUITS MAY BE USED TO ENSURE THE SAFE INSERTION OF THE DELIVERY SYSTEM.
Patient requires a planned aortic conduit.
Patient has a thoracic aneurysm with a contained rupture.
Patient has a connective tissue disease (e.g., Marfan’s syndrome, medial degeneration).
Patient has received a previous stent and/or stent graft or previous surgical repair in the descending
thoracic aortic area.
Patient requires treatment of an infra-renal aneurysm at the time of implant.
Patient has had previous surgical or endovascular treatment of an infra-renal aortic aneurysm.
Patient has a history of bleeding diathesis, coagulopathy, or refuses blood transfusions.
Patient has had a recent (within 3 months) Cerebral Vascular Accident (CVA).
The patient has a known hypersensitivity or contraindication to anticoagulants or contrast media, which is
not amenable to pre-treatment.
The presence of significant and/or circumferential aortic mural thrombus at either the proximal or distal
attachment sites that would compromise fixation and seal of the implanted stent graft.
Pregnant females.
Patients less than 18 years old.
• The long-term safety and effectiveness of this implant have not been established. All patients with
endovascular aneurysm repair must undergo periodic imaging to evaluate the stent graft and aneurysm size.
Significant aneurysm enlargement (>5 mm), the appearance of a new endoleak, or migration resulting in an
inadequate seal zone should prompt further investigation and may indicate the need for additional intervention
or surgical conversion.
• Intervention or conversion to standard open surgical repair following initial endovascular repair should be
considered for patients experiencing enlarging aneurysms and/or endoleak. An increase in aneurysm size
and/or persistent endoleak may lead to aneurysm rupture.
5.3 Implant Procedure
• Oversizing of the stent graft to vessel more than 10% may be unsafe, especially in the presence of dissecting
tissue or intramural hematoma.
• A seal zone less than 20mm could increase the risk of endoleak or migration of the stent graft. Migration may
also be caused by deployment of the proximal spring into a thrombus-filled or severely angled vessel wall.
• Manipulation of wires, balloons, catheters, and endografts in the thoracic aorta may lead to vascular trauma
including aortic dissection and embolization.
• Wrinkling of graft material may promote thrombus formation. Inflate a conformable balloon within the deployed
stent graft lumen to reduce wrinkling of the graft material.
TM
• Use the Reliant
Device. Do not attempt to use the Reliant Stent Graft Balloon Catheter before completely reading and
understanding the information supplied with the Reliant Device.
• Do not use the Reliant Stent Graft Balloon Catheter in patients with history of thoracic dissection disease. Do
not over-inflate the Reliant Stent Graft balloon within or outside of the graft material.
• When expanding a vascular prosthesis using the Reliant Balloon, there is an increased risk of vessel injury
and/or rupture, and possible patient death, if the balloon’s proximal and distal radiopaque markers are not
completely within the covered (graft fabric) portion of the prosthesis.
Stent Graft Balloon Catheter according to the instructions for use supplied with the Reliant
12
Page 13

• Failure to align the connecting bar with the outer bend of the target vessel may increase the likelihood of
endoleaks post implantation.
• During general handling of the Captivia Delivery System, avoid bending or kinking the graft cover because it
may cause the Talent Thoracic Stent Graft to prematurely and improperly deploy.
• It is not recommended to position the device higher in the presence of excessive calcification or thrombus, due
to the increased risk of dislodging material during distal repositioning of the Stent Graft.
• The proximal edge of the covered portion of the Stent Graft should not be placed beyond the origin of the left
common carotid artery (i.e., Zone 0 or Zone 1, See Figure 6).
• Ensure that the proximal and distal springs are placed in an adequate landing zone comprised of healthy tissue.
Healthy tissue is defined as tissue without evidence of circumferential thrombus, intramural hematoma,
dissection, ulceration, and/or aneurysmal involvement. Failure to do so may result in inadequate exclusion or
vessel damage, including perforation.
• The retrieval of the tip must be carefully monitored with fluoroscopic guidance to ensure that the tip does not
cause the Talent Thoracic Stent Graft to be inadvertently pulled down.
• Any endoleak left untreated during the implantation procedure must be carefully followed after implantation.
NOTE: THE RELIANT STENT GRAFT BALLOON CATHETER IS RECOMMENDED FOR USE WITH THE TALENT
THORACIC STENT GRAFT. DATA IS NOT AVAILABLE FOR USE WITH OTHER BALLOONS FOR
REMODELING STENT GRAFTS.
5.4 Magnetic Resonance Imaging (MRI)
MRI may be used on the graft only under specific conditions. See Section 13.5: MRI INFORMATION for details.
13
Page 14

6.0 Potential Adverse Events
Adverse events associated with use of the Talent Thoracic Stent Graft System include, but are not limited to the following:
• Amputation
• Aneurysm Enlargement
• Balloon rupture
• Breakage of the metal portion of the device
• Cardiac Failure/Infarction
• Change in mental status
• Conversion to open surgery
• Death
• Deployment difficulties
• Edema
• Embolization
• Endoleak
• Erectile Dysfunction
• Erosion with fistula or pseudoaneurysm
• Failure to deploy
• Gastrointestinal complications, including: adynamic ileus, bowel (ileus, transient ischemic, infarction, necrosis)
• Graft twisting and/or kinking
• Hemorrhage/Bleeding
• Inaccurate placement
• Infection and fever
• Insertion and removal difficulties
• Intercostal pain
• Neurological complications, including: spinal cord ischemia with paraplegia, paraparesis and/or paresthesia,
Cerebral Vascular Accidents (CVA), Transient Ischemic Attacks (TIA), neuropathy, and blindness
• Prosthetic thrombosis
• Pulmonary complications
• Renal failure
• Rupture of graft material
• Ruptured vessel/aneurysm sac enlargement
• Stent graft migration
• Vascular complications including: thrombosis, thromboembolism, occlusion (arterial and venous), vessel
dissection
• Wound healing complications
1
or perforation, collateral vessel occlusion, vascular ischemia, tissue necrosis, amputation
6.1 Adverse Event Reporting
Any adverse event (clinical incident) involving the Talent Thoracic Stent Graft System should be reported to Medtronic
immediately. To report an incident, call (800) 465-5533 (in the US).
1
Aortic dissection is an infrequent but recognized risk of endovascular repair. In the first 10 years of clinical experience
(OUS-commercial and US-investigational), there were 39 reported events of retrograde dissection in patients. Of the 39
reported events, 33 patients had a pre-existing aortic dissection
14
Page 15

7.0 Summary of Pivotal US Clinical Study
The VALOR Pivotal Study (VALOR Test Group) was a multi-center, non-randomized clinical study conducted within the
United States in order to evaluate the safety and effectiveness of the Talent Thoracic Stent Graft System when used in
the treatment of subjects with descending thoracic aortic aneurysms (fusiform aneurysms and saccular
aneurysms/penetrating ulcers). For the VALOR Test Group, 38 sites enrolled a total of 195 subjects. The primary safety
endpoint was All-Cause Mortality at one year. The All-Cause Mortality rate of TAA repair with the Talent Thoracic Stent
Graft was to be compared to the literature All-Cause Mortality rate for open surgical TAA repair, within one year of the
initial procedure. The primary effectiveness endpoint, Successful Aneurysm Treatment
80%, derived from a control population from the Feasibility studies totaling 21 subjects with 1 year of follow-up, all of
whom met the protocol definition of Successful Aneurysm Treatment.
In the VALOR Test Group, analysis of the primary endpoints used follow-up visits at 1, 6 and 12 months after the implant
procedure and annually for a total of 5 years from the date of the initial implant. Clinical sites sent CT/MR and chest X-ray
(CXR) images to an independent Core Laboratory to provide an assessment of patient data through one year post
implantation. All major adverse events (MAEs) were adjudicated by an independent Clinical Events Committee (CEC) for
device and procedure relatedness.
Original Literature Control
The original literature control compared the All-Cause Mortality rate of TAA repair of the Talent Thoracic Stent Graft with
the literature All-Cause Mortality rate for open surgical TAA repair, within one year of the initial procedure. Based on the
adequacy of information regarding disease etiology, length of follow-up information and definition of events, three articles
were chosen, from which 608 subjects had atherosclerotic lesions that accurately fit the VALOR Test Group’s intended
patient population of descending thoracic aortic aneurysms. Of the 608 patients, the number of patients surviving at 12
months was estimated from the 12 month rates given in the Kaplan-Meier curves included in each article. Using this
method, 181 patients were estimated to have died within one year, establishing an All-Cause Mortality rate of 29.8%. The
result of Primary Safety Endpoint comparison between the VALOR Test Group and the Original Literature Control Group
is included in Section 7.5.1 (page 22) below.
Retrospective Open Surgery Control
After the original VALOR Trial was conducted, additional retrospective open surgical data was gathered from selected
surgical centers to serve as a comparator for Acute Procedural Outcomes and Acute Adverse Events, as well as to further
compare early and 12-Month Mortality and Aneurysm-Related Mortality. This retrospective surgical control group included
189 subjects from 3 centers who matched selected inclusion/exclusion criteria of the VALOR study. The VALOR Test
and Retrospective Open Surgery Groups included surgical candidates diagnosed with a thoracic aortic aneurysm of
degenerative etiology. The VALOR Test Group candidates were of low to moderate risk (SVS 0, 1, and 2). The
Demographics and Baseline Medical History comparison between the VALOR Test Group and Retrospective Open
Surgery Group is included in Section 7.2. Baseline Aneurysm Data comparison is included in section 7.3. Safety
information is compared in section 7.5.1 (page 21 onwards) and effectiveness data and procedural result comparison is
provided in section 7.5.2 and 7.5.3.
2
, was compared to a fixed rate of
2
Successful Aneurysm Treatment was a composite endpoint defined as no aneurysm growth greater than 5 mm at the 12
month follow-up visit when compared to the one (1) month follow-up visit (after the initial Talent Thoracic Stent Graft
implant) AND absence of a Type I endoleak for which a secondary procedure was performed before, at or as a result of
the 12 month follow-up visit.
15
Page 16

7.1 Subject Accountability and Follow-up
For the VALOR Test Group, 38 sites enrolled a total of 195 subjects. One (1) subject had technical failure and did not
receive a stent graft and therefore did not have any imaging follow-up. Four (4) subjects died and one (1) withdrew from
the study before the 1-month visit.
189 subjects were eligible for clinical and imaging follow-up at 1 month follow-up interval. Of these 189 subjects, 80.4%
(152/189) had a clinical follow-up. Please note; three (3) additional patients who were not eligible for clinical follow-up had
imaging follow-up within the expanded time windows (as footnoted within the Table 3 below).
At the 6 month follow-up interval, 173 subjects were eligible for clinical and imaging follow-up. Of these, 74.0% (128/173)
had clinical follow-up and 73.8 % (127/173) had imaging follow-up. CT imaging was performed on 68.2% (118/173)
subjects.
At the 12 month follow-up interval, 157 subjects were eligible for clinical and imaging follow-up. Of these 71.3% (112/157)
had clinical follow-up and 90.4% (142/157) had imaging follow-up. CT imaging was performed on 82.8% (130/157)
patients.
Detailed subject follow-up and accountability for 1, 6, and 12 months is provided in Table 3.
Table 3 - Subject and Imaging Accountability Table–VALOR Test Group Only
Patients with
Patient follow-up
2
imaging
performed at time
interval
(Core Lab)
3
3
3
Patients with adequate
imaging to assess the
3
parameter
3
Patient events occurring before next
4
3
visit
2
Eligible
Clinical
Treatment / Follow-up
Interval
Originally Enrolled 195 1
Events after implant but before
1 Month visit
1 Month 189 152 192 184 189 174 182 161
Events after 1 Month visit but
before 6 Month visit
6 Month 173 128 127 118 114 117 112 117 93
Events after 6 Month visit but
before 12 Month visit
12 Month 157 112 142 130 125 129 123 129 97
0 4 1 0
0 14 2 0
1 13 1 1
Imaging
Follow-up
Follow-up
CT Imaging
Aneurysm
KUB Imaging
Endoleak
size increase
Integrity
Migration
Failure
Technical
Conversion to
Death
Surgery
Withdrawal
1 Data analysis sample size varies for each of the time points above and in following tables. This variability is due to patient availability for follow-
up, as well as, quantity and quality of images available from specific time points for evaluation. For example, the number and quality of images
available for evaluation of endoleak at 6 months is different than the number and quality of images available at 12 months due to variation in the
number of image exams performed, the number of images provided from the clinical site to the Core Lab, and/or the number of images with
acceptable evaluation quality.
2 Protocol-defined time windows were used for clinical follow-up and patient events
1-month : 16days to 44 days
6 month: 153 days to 213 days
12-month: 335 days to 395 days
3 Expanded time windows were used for Imaging follow-up and assessment of imaging-dependant parameters
1-month: 0 days to 122 days
6 month: 153 days to 213 days
12-month: 335 days to 480 days for CT, Endoleak and Aneurysm size increase
335 days to 760days for X-ray and Integrity
4 Number of subjects evaluable for migration assessment were based on CT performed in windows and Slice interval and thickness <5mm for
10mm evaluation
Lost to
Follow-up
16
Page 17

7.2 Demographics and Baseline Medical History
Table 4 to Table 7; provide the demographics of the VALOR Test Group and Retrospective Open Surgery Group subjects.
Table 4 - Subject Demographics: VALOR Test Group vs. Retrospective Open Surgery Group
VALOR Test Group
AGE
Total Population
Mean ± SD (years) 70.2 ± 11.1 69.6 ± 9.1 0.528
Mean ± SD (years) 69.3 ± 11.7 69.9 ± 8.5 0.680
Mean ± SD (years) 71.6 ± 10.1 69.3 ± 9.8 0.130
Gender
Ethnicity
White, non-Hispanic 83.1% (162) 93.7% (177)
Black- non-Hispanic 12.8% (25) 5.8% (11)
Hispanic (White or
Asian/Pacific Islander 1.0% (2) 0% (0)
Native American 0% (0) 0% (0)
1
One subject had Ethnicity specified as “None given”
N 195 189
Median 73.0 71.0
Min-Max 27 - 86 27 - 85
Male
N 115 99
Median 72.0 71.0
Min-Max 27 - 85 40 - 84
Female
N 80 90
Median 74.0 71.0
Min-Max 38 - 86 27 - 85
Males 59.0% (115) 52.4% (99)
Females 41.0% (80) 47.6% (90)
Black)
Other 0.5% (1) 1 0% (0)
2.6% (5) 0.5% (1)
Retrospective Open
Surgery
p-value
0.218
0.007
Table 5 - Subject Anatomic Lesion Type: VALOR Test Group Only
Thoracic Lesion
Fusiform 112 57.4
Saccular/Penetrating Ulcer 70 35.9
Both 13 6.7
The Retrospective Open Surgery Group did not provide patient level data for Anatomic Lesion Type treated.
Note;
N %
17
Page 18

Table 6 - Baseline Medical History: VALOR Test Group vs. Retrospective Open Surgery Group
Body System / Condition
VALOR Test Group
% (m/n)
1
Retrospective Open
Surgery
1
% (m/n)
p-value
Cardiovascular
Angina 14.4% (28/195) 22.8% (26/114) 0.064
Arrhythmias 26.7% (52/195) 20.3% (37/182) 0.182
Carotid artery disease 5.6% (11/195) Not Available N/A
Congestive heart failure (CHF) 8.7% (17/195) 11.2% (21/187) 0.495
Coronary artery bypass grafting (CABG) 10.3% (20/195) 13.3% (25/188) 0.428
Coronary artery disease (CAD) 40.5% (79/195) 49.2% (91/185) 0.099
Hypertension 87.2% (170/195) 88.8% (166/187) 0.641
Myocardial infarction (MI) 13.8% (27/195) 20.9% (39/187) 0.079
Percutaneous coronary intervention (PCI) 5.6% (11/195) Not Available N/A
Peripheral vascular disease (PVD) 16.4% (32/195) 37.4% (70/187) <0.001
Symptomatic thoracic aortic aneurysm 26.2% (51/195) Not Available N/A
Abdominal Aortic Aneurysm (AAA) 19.0% (37/195) 37.0% (70/189) <0.001
Abdominal Aortic Aneurysm Repair 2.1% (4/195) 27.5% (52/189) <0.001
Gastrointestinal conditions 53.8% (105/195) Not Available N/A
Renal insufficiency 17.4% (34/195) 16.0% (30/187) 0.784
Musculoskeletal conditions 53.8% (105/195) Not Available N/A
Neurological
Cerebral vascular accident (CVA) 9.7% (19/195) 13.4% (25/186) 0.267
Paraplegia 1.0% (2/195) 0.5% (1/186) 1.000
Paraparesis 0.5% (1/195) Not Available N/A
Transient ischemic attack (TIA) 7.7% (15/195) Not Available N/A
Pulmonary
Chronic obstructive pulmonary disease (COPD) 36.9% (72/195) 42.6% (80/188) 0.296
Tobacco use 76.9% (150/195) 75.9% (142/187) 0.904
Other abnormal body systems
Hyperlipidemia 43.6% (85/195) Not Available N/A
Diabetes 15.9% (31/195) 8.6% (16/187) 0.030
Bleeding disorders 2.6% (5/195) Not Available N/A
1m = number in category, n = number of known values
Table 7 - Baseline Modified SVS Classification: VALOR Test Group Only
Modified SVS
n
195 4.1% (8) 21.0% (41) 72.8% (142) 2.1% (4)
SVS 0
% (m)
18
SVS 1
% (m)
SVS 2
% (m)
SVS 3
% (m)
Page 19

7.3 Baseline Aneurysm Data
Table 8 lists the initial aneurysm diameter sizes treated.
Table 8 - Baseline Maximum Aneurysm Diameters: VALOR Test Group vs. Retrospective Open Surgery
VALOR Test Group
Aneurysm
Diameter (mm)
Site-Reported
% (m / n)
1
Core Lab
Reported
% (m / n)
2
Retrospective
Open Surgery
%(m/n)
10-17 0% (0/188) 0% (0/187) 0% (0/189)
18-29 0% (0/188) 0.5% (1/187) 0% (0/189)
30-39 4.3% (8/188) 7.5% (14/187) 0% (0/189)
40-49 10.6% (20/188) 20.3% (38/187) 0.5% (1/189)
50-59 34.6% (65/188) 34.8% (65/187) 13.8% (26/189)
60-69 33.5% (63/188) 24.6% (46/187) 40.7% (77/189)
70-79 12.2% (23/188) 10.2% (19/187) 24.3% (46/189)
80-89 3.2% (6/188) 2.1% (4/187) 16.9% (32/189)
90-99 1.1% (2/188) 0% (0/187) 0.5% (1/189)
100-109 0.5% (1/188) 0% (0/187) 1.6% (3/189)
110-119 0% (0/188) 0% (0/187) 0.5% (1/189)
120+ 0% (0/188) 0% (0/187) 1.1% (2/189)
1 Denominator is 188 subjects with site reported data.
2 Denominator is 187 subjects with evaluable scans.
3 This p-value represents a Monte Carlo estimate of the p-value for the exact Mantel-Haenszel Chi-
Square test for trend, based on 100,000 Monte Carlo repetitions.
p-value
Site-Reported
VALOR vs.
Retrospective
Open Surgery
<0.001
3
Table 9 - Baseline Vessel Dimensions (Core Lab Reported): VALOR Test Group Only
Vessel Dimensions (mm) n
1
Mean ± SD Median Min Max
Proximal neck diameter 187 31.2 ± 4.9 31.5 18.5 43.5
Aneurysm diameter 187 55.5 ± 11.6 56.0 26.2 88.8
Distal neck diameter 184 29.7 ± 5.0 29.5 17.0 42.5
Proximal neck length 187 80.0 ± 52.1 77.9 10.0 234.0
Aneurysm length 180 121.4 ± 72.7 107.7 8.0 297.5
Distal neck length 184 90.0 ± 62.9 73.5 9.0 255.0
Right external iliac minimum diameter 122 6.5 ± 1.5 6.5 2.9 11.0
Left external iliac minimum diameter 124 6.6 ± 1.5 6.5 3.3 10.9
1 Denominators are n specified from readable scans.
19
Page 20

7.4 Devices Implanted
Table 10 provides details on the number of devices implanted per subject for the VALOR Test Group.
Table 10- Number of Devices Implanted at Initial Procedure: VALOR Test Group Only
Devices Implanted
Number per subject % (m)1
0
1 19.5% (38)
2 28.7% (56)
3 24.6% (48)
4 17.4% (34)
5 7.2% (14)
6 1.5% (3)
7+ 0.5% (1)
1
m= number of subjects implanted & percentages based on total number of
enrolled subjects (N=195)
Table 11 cross-tabulates the 194 subjects in the VALOR Test Group, who had Talent Stent Grafts implanted by the
number of main sections and the number of extensions. For example, 38 subjects had a single main section implanted
and no extensions, and 5 subjects had one main section and one extension. Similarly, 51 subjects had two main sections
and no extensions and 6 had two main sections and one extension.
0.5% (1)
Table 11: Number of Main Sections and Number of Extensions Implanted at Initial Procedure: VALOR Test Group
1
m (%)
0 1 2 Total
1
2
Number of
Main
Sections
1 m= number of subjects with tabulated number of main sections and extensions. Percentages based on total number of
implanted subjects (N=194)
3
4
5
6
Total
38 (19.59%) 5 (2.58%) 1 (0.52%) 44 (22.68%)
51 (26.29%) 6 (3.09%) 5 (2.58%) 62 (31.96%)
41 (21.13%) 11 (5.67%) 2 (1.03%) 54 (27.84%)
18 (9.28%) 6 (3.09%) 0 (0.00%) 24 (12.37%)
6 (3.09%) 1 (0.52%) 0 (0.00%) 7 (3.61%)
2 (1.03%) 1 (0.52%) 0 (0.00%) 3 (1.55%)
156 (80.41%) 30 (15.46%) 8 (4.12%) 194 (100.00%)
Only
Number of Extensions
20
Page 21

Table 12 and Table 13 provide details on the components (proximal main devices, proximal extension devices, distal main
devices, and distal extension devices) implanted per subject for the VALOR Test Group.
Table 12 - VALOR Test Group: Talent Thoracic Stent Graft Devices Implanted
Diameter (mm)
Proximal Main
% (m)
Stent Graft Modular Component (Number Implanted)
1
Proximal Extension
% (m)
1
Distal Extension
% (m)
22 0.5% (1)
24 1.4% (3)
26 1.9% (4) 0.0% (0) 0.0% (0)
28 2.8% (6) 0.0% (0) 12.0% (3)
30 3.8% (8) 4.8% (1) 4.0% (1)
32 8.1% (17) 14.3% (3) 8.0% (2)
34 11.4% (24) 4.8% (1) 16.0% (4)
36 16.1% (34) 14.3% (3) 8.0% (2)
38 19.4% (41) 19.0% (4) 16.0% (4)
40 11.4% (24) 4.8% (1) 12.0% (3)
42 10.9% (23) 4.8% (1) 8.0% (2)
44 5.2% (11) 9.5% (2) 8.0% (2)
46 7.1% (15) 23.8% (5) 8.0% (2)
Total Catalog Devices
Implanted
211 21 25
1 m=number of subjects implanted with specific type of device within each diameter category & denominator is the
total number of the specific type of device implanted.
1
Table 13- VALOR Test Group: Distal Main Devices Implanted
(mm)
1
Number of Devices
% (m)
2
Diameters
26 – 22 0.4% (1)
28 – 24 0.8% (2)
30 – 26 0.8% (2)
32 – 28 0.4% (1)
34 – 30 2.3% (6)
36 – 32 5.4% (14)
38 – 34 14.0% (36)
40 – 36 16.3% (42)
42 – 38 19.8% (51)
44 – 40 15.1% (39)
46 – 42 14.3% (37)
46 – 44 10.5% (27)
1 Proximal – distal.
2 m=number of subjects implanted and the denominator is 258
implanted distal main devices.
21
Page 22

7.5 Study Results
Results of the safety and effectiveness of the Talent Thoracic Stent Graft are provided in Section 7.5.1 to Section7.5.3.
7.5.1 Safety
Primary Safety Endpoint
All-Cause Mortality at One Year: Talent Thoracic vs. Original Literature Control
The primary safety endpoint was All-Cause Mortality at 12 months. Based on the test of superiority of the All-Cause
Mortality rate in the Test Group to that of the original literature control group with an All-Cause Mortality rate of 181 of 608
subjects, or 29.8% (H
0
: P
TestArm
≥ P
SurgicalGroup
versus HA: P
TestArm
< P
SurgicalGroup
), the VALOR Test Group subjects met the
pre-specified performance goal of 29.8%. The primary safety endpoint of the VALOR Study was met.
Through one year, subjects who received the Talent Thoracic Stent Graft experienced an All-Cause Mortality rate of
16.1% and the subjects who underwent open surgery experienced a rate of 29.8%.
22
Page 23

All-Cause Mortality at 30 days and 12 months: Talent Thoracic vs. Retrospective Open Surgery Group
Table 14 and Figure 7 describe the 30-day mortality rates for the VALOR Test Group as compared to the Retrospective
Open Surgery Group. The VALOR Test Group experienced a lower rate of early mortality (2% vs. 8%).
An analysis of freedom from All-Cause Mortality was performed, and a Kaplan-Meier plot of subject freedom from AllCause Mortality is provided in Table 14 and Figure 7.
Table 14- All-Cause Mortality at 30 Days and 12 months: VALOR Test Group vs. Retrospective Open Surgery
All-cause mortality at 30 days
All-cause mortality at 12 months
VALOR Test Group
% (m/n)
Retrospective Open
Surgery
% (m/n)
95% Exact Confidence
Interval of
Difference
2.1% (4/195) 7.9% (15/189) (-10.9%, -1.3%)
16.1% (31/192) 20.6% (39/189)
3
(-12.4%, -3.4%)
1,2
1 Confidence level was not adjusted for multiplicity. Confidence interval for difference (VALOR Test Group –
Retrospective Open Surgery group) in percentage was calculated by the exact method.
2 Difference represents the (% of patients with mortality from any cause within the period in the population treated
with the test device) - (% of patients with mortality from any cause within the period in the population treated with
open surgery)
3 Of the 39 deaths, this data includes both information from the reporting centers and queries of the National Social
Security Death Index database
Figure 7- Kaplan-Meier Plot of Freedom from All Cause Mortality at 30 Days and 12 Months: VALOR Test Group
vs. Retrospective Open Surgery Group
98.0%
100
90
80
70
±1.0%
83.9%
±2.6%
92.1%
±2.0%
79.4%
±2.9%
60
50
40
30
20
Number at risk
Valor
10
190 176 161
Open Surgery
174 157 149
0
0 30 60 90 120 150 180 210 240 270 300 330 360 390
23
Page 24

Table 15: Details of Kaplan-Meier Plot of Freedom from All Cause Mortality at 30 Days and 12 Months: VALOR
No. at Risk 195 190 176 189 174 157
No. of Events 4 13 14 15 17 7
No. Censored 1 1 1 0 0 1
Kaplan-Meier
Estimate
Test Group vs. Retrospective Open Surgery Group
VALOR Test Group Retrospective Open Surgery
Treatment
to 30 days
0.980 0.912 0.839 0.921 0.831 0.794
31 days to
182 days
183 days to
365 days
Treatment
to 30 days
31 days to
182 days
183 days to
365 days
24
Page 25

Secondary Safety Endpoints
Major Adverse Events (MAE) at 30 days: VALOR Test Group vs. Retrospective Open Surgery Group
Adverse events were categorized by severity in the VALOR Trial and in the Retrospective Open Surgery Group using the
following definitions. A Major Adverse Event (MAE) was defined as the occurrence of any of the following:
• Death:
o due to complications of the procedure, including bleeding, vascular repair, transfusion reaction, or
conversion to open surgical TAA repair
o within 30 days of the baseline implant or surgical procedure
• Respiratory complications (atelectasis / pneumonia, pulmonary embolism, pulmonary edema, respiratory
failure)
• Renal complications (renal failure, renal insufficiency)
• Cardiac: MI, unstable angina, new arrhythmia, exacerbation of congestive heart failure (CHF)
• Neurological: new CVA / embolic events, paraplegia / paraparesis
• Aneurysm rupture
• Gastrointestinal: bowel ischemia
• Major bleeding complication (procedural or post-procedural), coagulopathy
• Vascular complications
Table 16 is a comparison of 30-day MAE for Talent Thoracic subjects versus the Retrospective Open Surgical Group.
Table 16 - Summary of Major Adverse Events for VALOR Test Group vs. Retrospective Open Surgery Group (30
days)
Retrospective Open
Surgery
Major Adverse Events
0-30 days % (m/n)
N=1891
95% Exact Confidence
Interval of Difference
2,3
Category
VALOR Test Group
Major Adverse Events
0-30 days % (m/n)
N=195
Any MAE 41.0% (80/195) 84.4% (151/179) (-51.9%, -34.2%)
Respiratory complications 13.3% (26/195) 46.9% (84/179) (-42.2%, -24.6%)
Renal complications 6.2% (12/195) 29.1% (52/179) (-30.6%, -15.3%)
Cardiac complications 12.3% (24/195) 44.7% (80/179) (-41.0%, -23.5%)
Neurological complications 11.8% (23/195) 20.1% (36/179) (-16.0%, -0.7%)
GI complications 1.0% (2/195) 0.6% (1/179) (-2.1%, 3.2%)
Bleeding complications 15.4% (30/195) 48.0% (86/179) (-41.7%, -23.4%)
Vascular complications 21.0% (41/195) 12.3% (22/179) (1.1%, 16.5%)
Target Lesion Aneurysm Rupture 0.0% (0/195) 0.6% (1/179) (-3.1%, 1.4%)
1 10 patients were followed for less than 16 days without MAE so were eliminated from the analysis
2 Confidence level was not adjusted for multiplicity. Confidence interval for difference (VALOR Test Group –
Retrospective Open Surgery group) in percentage was calculated by the exact method.
3 Difference represents the (% of patients free from MAEs within 30 days in the population treated with the test device)
- (% of patients free from MAEs within 30 days in the population treated with open surgery)
25
Page 26

One or more Major Adverse Events were reported in 80 of the 195 VALOR Test Group subjects available for evaluation,
resulting in a probability of freedom from Major Adverse Events of 59%. In the Retrospective Open Surgery group, 151 of
the 179 subjects had one or more Major Adverse Events, resulting in a freedom from Major Adverse Event rate of 15.6%
in this group.
Table 17- Freedom from Major Adverse Events (MAE) at 30 days: VALOR Test Group vs. Retrospective Open
Parameter VALOR Test Group
Number of subjects at start 195 179
Number of subjects with one or more events 80 151
Probability of freedom from event 59.0% 15.6% (34.2%, 51.9%)
1 Confidence level was not adjusted for multiplicity. Confidence interval for difference (VALOR Test Group –
Retrospective Open Surgery group) in percentage was calculated by the exact method.
2 Difference represents the (% of patients free from MAEs within 30 days in the population treated with the test device)
- (% of patients free from MAEs within 30 days in the population treated with open surgery)
Surgery Group
Retrospective
Open Surgery
95% Exact Confidence
Interval of Difference
Figure 8 provides the Kaplan-Meier analysis of Freedom from Major Adverse Events at 30 Days: VALOR Test Group vs.
Retrospective Open Surgery
1,2
26
Page 27

Figure 8 – Kaplan-Meier Plot of Freedom from Major Adverse Events at 30 Days: VALOR Test Group vs.
100
90
80
70
60
50
40
30
20
10
Number at risk
VALOR 122 118 114
0
Open Surgery 47 29 28
Retrospective Open Surgery
59.0%
± 3.5%
19.6%
± 2.9%
0 5 10 15 20 25 30 35
Table 18: Details of Kaplan-Meier Plot of Freedom from Major Adverse Events at 30 Days: VALOR Test Group vs.
Treatment
No. at Risk 195 122 118 189 47 29
No. of Events 73 4 3 141 9 1
No. Censored 0 0 1 1 9 0
Kaplan-Meier
Estimate
to 5 days
0625 0.605 0.590 0.254 0.203 0.196
Retrospective Open Surgery
VALOR Test Group Retrospective Open Surgery
6 days to 15
days
16 days to
30 days
Treatment
to 5 days
6 days to 15
days
16 days to
30 days
27
Page 28

Serious Major Adverse Events: VALOR Test Group Only
VALOR MAEs were further stratified into more clinically severe events: Serious Major Adverse Events (Serious MAEs).
These Serious MAEs were fatal, life-threatening, required in-patient hospitalization or prolongation of existing
hospitalization, caused persistent or significant disability/incapacity, or resulted in a congenital anomaly/birth defect.
Major Adverse Events (MAE) were reviewed by the CEC and adjudicated as either device- and/or procedure-related as
per the study protocol. A Major Adverse Event (MAE) that was identified as a Serious Adverse Event (SAE) by the clinical
Investigator was defined as a Serious MAE.
The total number of subjects with one or more Serious MAEs in each category is summarized in Table 19.
Table 19 -Summary of Serious Major Adverse Events from VALOR Test Group Only
0-30 days % (m)
Category
N=195
Serious Major
Adverse Events
Any Serious MAE 30.3% (59/195)
Respiratory complications 6.7% (13/195)
Renal complications 3.6% (7/195)
Cardiac complications 5.1% (10/195)
Neurological complications 9.7% (19/195)
GI complications 0.5% (1/195)
Bleeding complications 13.3% (26/195)
Vascular complications 9.2% (18/195)
Target Lesion Aneurysm
Rupture
0.0% (0/195)
0-30 days
95% Exact CI
(23.9%, 37.2%)
(3.6%, 11.1%)
(1.5%, 7.3%)
(2.5%, 9.2%)
(6.0%, 14.8%)
(0.0%, 2.8%)
(8.9%, 18.9%)
(5.6%, 14.2%)
(0.0%, 1.9%)
0-365 days % (m)
1
N=192
Serious Major
Adverse Events
42.7% (82/192)
15.1% (29/192)
6.8% (13/192)
12.0% (23/192)
13.5% (26/192)
1.0% (2/192)
14.6% (28/192)
10.4% (20/192)
0.5% (1/192)
0-365 days
95% Exact CI
(35.6%, 50.0%)
(10.4%, 21.0%)
(3.7%, 11.3%)
(7.7%, 17.4%)
(9.0%, 19.2%)
(0.1%, 3.7%)
(9.9%, 20.4%)
(6.5%, 15.6%)
(0.0%, 2.9%)
1 Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was calculated by the exact
(binomial) method.
During the VALOR Study, 59 of 195 evaluable subjects had one or more Serious Major Adverse Events within 30 days,
giving a rate of Serious MAEs within 30 days of 30.3% (95% CI 23.9-37.2%). Eighty-two (82) of 192 evaluable subjects
had one or more Serious MAEs within 12 months, providing a Serious MAE rate of 42.7% (95% CI 35.6-50.0%).
1
Table 20- Freedom from Serious Major Adverse Events (MAE) at 30 days and 12-months: VALOR Test Group Only
Parameter
Talent Thoracic
Serious MAE at 30 days Serious MAE at 12-months
Number of subjects at start 195 1921
Number of subjects with one or more events 59 82
Probability of freedom from event 69.7% 57.3%
Exact 95% confidence interval for freedom from
2
event
(62.7%, 76.1%) (49.1%, 63.4%)
1 192 subjects followed for the required time frame.
2 Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was calculated by the
exact (binomial) method.
28
Page 29

Figure 9 – Kaplan-Meier Plot of Freedom from Serious Major Adverse Events: VALOR Test Group Only
100
90
80
70
60
50
57.5%
± SE 3.6%
40
30
20
10
0
Table 21: Details of Kaplan-Meier Plot of Freedom from Serious Major Adverse Events: VALOR Test Group Only
No. at Risk 195 135 118
No. of Events 59 13 10
_____ VALOR
Number at risk:
135 118 103
0 30 60 90 120 150 180 210 240 270 300 330 360 390
VALOR Test Group
Treatment to 30 days 31 days to 182 days 183 days to 365 days
No. Censored 1 4 5
Kaplan-Meier Estimate 0.697 0.629 0.575
29
Page 30

Aneurysm-Related Mortality
Table 22 and Figure 10 provide Aneurysm-Related Mortality information for the VALOR Test and Retrospective Open
Surgery Groups. An analysis of freedom from Aneurysm-Related Mortality was performed, and a Kaplan-Meier plot of
subject freedom from Aneurysm-Related Mortality is provided in Figure 10.
Table 22- Aneurysm-Related Mortality at 12 Months: VALOR Test Group vs. Retrospective Open Surgery Group
Retrospective
Open Surgery
% (m/n)
2
95% Exact Confidence
Interval of Difference
Aneurysm-Related Mortality at 12
Months
1
VALOR Test Group
% (m/n)
3.1% (6/192) 11.6% (22/189) (-14.2%, -2.9%)
1 Aneurysm-related mortality was defined as any death within 30 days from initial implantation or occurring as a
consequence of an aneurysm rupture, a conversion to open repair, or any other secondary endovascular procedure
relative to the aneurysm that was treated by the Talent Thoracic Stent Graft System as evidenced by CT,
angiography or direct observation at surgery or autopsy. Excluded are aneurysms in anatomic areas other than the
targeted segment treated by the Talent Thoracic Stent Graft System.
2 The definition for Aneurysm Related Mortality for the Retrospective Open Surgery Group was any death within 30
days from the surgical procedure or any death caused by reintervention of the targeted aortic segment, or by
complications related to the graft or the procedure (i.e., graft infections, rupture, pseudoaneurysm, aorto-eophageal
fistula, aorto-bronchial fistula, etc.)
3 Confidence level was not adjusted for multiplicity. Confidence interval for difference (VALOR Test Group –
Retrospective Open Surgery group) in percentage was calculated by the exact method.
4 Difference represents the (% of patients with aneurysm-related mortality within 12 months in the population treated
with the test device) - (% of patients with aneurysm-related mortality within 12 months in the population treated with
open surgery)
3,4
30
Page 31

Figure 10 – Kaplan-Meier Plot of Freedom from Aneurysm-Related Mortality at 12 Months: VALOR Test Group vs.
100
90
80
70
60
50
40
30
20
Number at risk
10
VALOR
190 176 161
Open Surgery
0
174 157 149
0 30 60 90 120 150 180 210 240 270 300 330 360 390
Retrospective Open Surgery Group
96.9%
±1.3%
88.3%
±2.3%
Table 23: Details of Kaplan-Meier Plot of Freedom from Aneurysm-Related Mortality at 12 Months: VALOR Test
Treatment
No. at Risk 195 190 176 189 174 157
No. of Events 4 1 1 15 7 0
No. Censored 1 13 14 0 10 8
Kaplan-Meier
Estimate
to 30 days
Group vs. Retrospective Open Surgery Group
VALOR Test Group Retrospective Open Surgery
31 days to
182 days
0.980 0.974 0.969 0.921 0.883 0.883
183 days to
365 days
Treatment
to 30 days
31 days to
182 days
183 days to
365 days
31
Page 32

7.5.2 Effectiveness
Primary Effectiveness Endpoint: Successful Aneurysm Treatment
The primary effectiveness endpoint was met. This endpoint, Successful Aneurysm Treatment, was a composite endpoint
consisting of:
• No aneurysm growth greater than 5 mm at the 12 month follow-up visit when compared to the one (1) month
follow-up visit as assessed by the Core Lab (after the initial Talent Thoracic Stent Graft implant); and
• Absence of a Type I endoleak as assessed by the Core Lab for which a secondary procedure was performed
before, at or as a result of the 12 month follow-up visit.
The rate of Successful Aneurysm Treatment in the VALOR Test Group, 89.2%, was higher than the 80% comparator
(which was based on earlier feasibility studies). As shown is Table 24, the Talent Thoracic Stent Graft achieved a
successful aneurysm treatment rate of 89.2%. Table 25 provides details regarding subjects who have failed the
successful aneurysm treatment endpoint.
Table 24- Primary Effectiveness Endpoint: Successful Aneurysm Treatment: VALOR Test Group
Primary Effectiveness Endpoint
Successful Aneurysm Treatment at 12
months
% (m / n)
[95% CI]
1
89.2% (116/130)
[82.6% – 94.0%]
95% Exact
Confidence Interval
2
1 Eligible subjects for Successful Aneurysm Treatment required images depicting a one and
twelve month aneurysm size, or had a Type I endoleak which required endovascular repair to
be included in the analysis. Twenty-nine (29) subjects were missing a 12 month image at the
Core Lab and were excluded from this analysis.
2 Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was
calculated by the exact (binomial) method.
Table 25- Summary of Subjects with Primary Effectiveness Failure: VALOR Test Group
Subjects with Primary Effectiveness Failure
n
Aneurysm growth > 5mm 101
Type I endoleak requiring re-intervention 3
Aneurysm growth > 5mm and
12
Type I endoleak requiring re-intervention
1 Of the 10 subjects, four (4) had secondary procedures. Of the remaining six (6)
subjects, one (1) patient died of cardiac arrest at approximately 24 months, and one died
of cirrhosis at 14 months.
2 This subject is alive at 24 months
32
Page 33

Other Effectiveness Data
Table 26 summarizes the other secondary endpoints from the VALOR Study.
Table 26 - Other Effectiveness Data: VALOR Test Group Only
Secondary Endpoint
Successful deployment and delivery of the stent graft at implantation 99.5% (194/195)2 (97.2%, 100.0%)
Secondary procedures due to endoleak at 30 days 0.0% (0/194) (0.0%, 1.9%)
Conversion to open surgical repair within 12 months post-implantation 0.5% (1/192)3 (0.0%, 2.9%)
Aneurysm rupture within 12 months post-implantation 0.5% (1/192)4 (0.0%, 2.9%)
Stent graft migration between 1 and 12 months 3.9% (4/103)5 (1.1%, 9.6%)
• Proximal stent graft migration > 10 mm proximally
• Proximal stent graft migration > 10 mm distally
• Distal stent graft migration > 10 mm proximally
• Distal stent graft migration > 10 mm distally
1 Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was calculated by the exact
(binomial) method.
2 One (1) subject did not receive a stent graft due to extensive disease and heavy calcification of the iliac arteries.
3 One (1) subject was converted to surgery. The stent graft was explanted 9 months post initial procedure due to an apparent
infection in the stented segment of the aorta.
4 One (1) subject experienced aneurysm rupture at the distal thoracic aorta, at the stent graft seal zone. Review of CT scans
by the Core Lab revealed patient had a thoraco-abdominal aneurysm rather than an isolated descending thoracic aneurysm
as well as an inadequate distal landing zone.
5 Migration is defined as proximal or distal movement of the stent graft (>10mm) relative to fixed anatomic landmarks. The 1-
month CTA/MRA was used as the baseline for this determination.
• Two (2) subjects had no MAEs due to their device migration
• One (1) subject underwent a secondary procedure at Day 273. Two additional proximal main devices were implanted to
resolve migration and cover a pseudoaneurysm. Repair was successful
• One (1) subject had no MAEs due to their device migration. Subject underwent a planned AAA open repair at
approximately 2 months and expired at approximately 14 months from cirrhosis
Incidences
% (m / n)
0.0% (0/103) (0.0%, 3.5%)
1.9% (2/103) (0.2%, 6.8%)
1.9% (2/103) (0.2%, 6.8%)
0.0% (0/103) (0.0%, 3.5%)
95% Exact CI1
33
Page 34

Table 26 - Other Effectiveness Data: VALOR Test Group Only (Continued)
Incidences
Secondary Endpoint
% (m / n)
All endoleaks at 12 months (Core Lab reported) 12.2% (15/123)
• Type I
• Type II
• Type III
• Type IV
• Unknown
4.9% (6/123)
4.9% (6/123)
0.0% (0/123)
0.0% (0/123)
2.4% (3/123)
Secondary procedures due to endoleak between 31 days and 365 days 6.5% (12/186)8
Loss of patency of the stent graft at 12 months 0% (0/107)
Loss of stent graft integrity at 12 months9 2.1% (2/97)10
6
7
95% Exact CI
(7.0%, 19.3%)
(1.8%, 10.3%)
(1.8%, 10.3%)
(0.0%, 3.0%)
(0.0%, 3.0%)
(0.5%, 7.0%)
(3.4%, 11.0%)
(0.0%, 3.4%)
(0.3%, 7.3%)
Change in maximum aneurysm diameter from 1 month image
• Increase > 5 mm
• Stable
• Decrease > 5 mm
8.5% (11/129) (4.3%, 14.7%)
67.4% (87/129) (58.6%, 75.4%)
24.0% (31/129) (16.9%, 32.3%)
1 Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was calculated by the exact
(binomial) method.
6 None of the six (6) subjects with Type I endoleak underwent secondary procedure within 12 months
7 One (1) of the six (6) subjects with Type II endoleak underwent a secondary procedure at 140 days
8 The 12 subjects who received a secondary endovascular procedure are characterized as follows, all secondary repairs
were successful:
• Two (2) patients had endoleaks detected at day 6 and 35, with secondary procedures at Day 84 and 186, respectively.
Proximal mains were placed to correct Type I endoleaks (proximal).
• One (1) patient had endoleak detected at day 55, with secondary procedure at Day 334. Proximal extension was placed
to correct Type I endoleak (proximal).
• Four (4) patients had endoleak detected at days 22, 29, 33 and 38, with secondary procedures at Day 140, 203, 116 and
253, respectively. Distal extensions were placed to correct three Type I endoleaks (distal) and one Type II endoleak.
• One (1) patient had endoleak detected at day 8, with secondary procedure at Day 113. Distal mains were placed to
correct a Type III endoleak.
• Three (3) patients had endoleaks detected at day 19, 27 and 32, with secondary procedures at Day 56, 49 and 42,
respectively. Proximal and distal mains were placed to correct one Type I (distal) endoleak and two Type I (proximal)
endoleaks.
• One (1) patient had endoleak detected at day 155, with secondary procedure at Day 246. Proximal and Distal
extensions were placed to correct a Type I (proximal) endoleak.
9 Loss of stent graft integrity is defined as the absence of stent fractures and/or graft fabric defects.
10 Of the two (2) subjects with loss of stent graft integrity, one was due to a nitinol spring fracture and the second was a
connecting bar fracture. Neither subject had any adverse event related to these fractures. Both subjects are alive at 24
months
1
34
Page 35

7.5.3 Supplementary Acute Procedural Data
Table 27 provides the Acute Procedural Data for VALOR Test Group and Retrospective Open Surgery Group. The
VALOR Test Group showed reduced blood loss, reduced need for transfusions, as well as shorter ICU and hospital stays
when compared to Open Surgery.
Table 27 - Supplementary Acute Procedural Data: VALOR Test Group vs. Retrospective Open Surgery Group
Parameter VALOR Test
Subjects requiring blood transfusion (%) 22.7% (44/194) 93.7% (164/175) (-77.5%, -63.5%)
Blood loss during procedure (ml) (mean ± SD)1 371.2 ± 514.4 3054.9 ± 1702.4 (-2961.1, -2406.2)
Duration of implant procedure (min) (mean ± SD)2 154.2 ± 76.0 303.3 ± 97.6 (-166.9, -131.3)
Time in Intensive Care Unit (hours) for all assessable
subjects (mean ± SD)
Overall hospital stay (days) (mean ± SD)4 6.4 ± 11.5 16.7 ± 15.0 (-12.9, -7.5)
1 189 VALOR Test Group subjects and 57 Retrospective Open Surgery subjects had known data for this parameter
2 194 VALOR Test Group subjects and 178 Retrospective Open Surgery subjects had known data for this parameter
3 193 VALOR Test Group subjects and 168 Retrospective Open Surgery subjects had known data for this parameter
4 195 VALOR Test Group subjects and 186 Retrospective Open Surgery subjects had known data for this parameter
5 Confidence level was not adjusted for multiplicity. Confidence intervals for difference (VALOR Test Group-
Retrospective Open Surgery group) in means were calculated using a t-distribution. Confidence intervals for difference
(VALOR Test Group-Retrospective Open Surgery group) in percentages were calculated by the exact method.
Confidence interval for Time in ICU is not calculated due to a large number of ties in the data (i.e. large number of “0
hours” reported in the Test Group).
6 For Duration of Procedure and Overall Hospital Stay, difference represents the (mean of specific acute procedural
parameter in the population treated with the test device) - (mean of specific acute procedural parameter in the
population undergoing open surgical repair). For Patients Requiring Blood Transfusion, difference represents the (% of
patients with the specific acute procedural parameter for the population treated with the test device) - (% of patients
with the specific acute procedural parameter for the population undergoing open surgical repair).
3
Group
46.8 ± 114.3 185.3 ± 204.7
Retrospective
Open Surgery
95% Confidence
Interval of
Difference
5,6
35
Page 36

7.6 VALOR Test Group Results by Lesion Type
The VALOR Test Group consisted of subjects with the following three groups of lesion types
• Subjects with fusiform thoracic aneurysms
• Subjects with saccular aneurysms and/or penetrating ulcers
• Subjects with multiple types of lesions (fusiform thoracic aneurysms and saccular and/or penetrating ulcers)
Section 7.6.1 and 7.6.2, provide demographic and lesion characteristics as well as safety and effectiveness endpoint
analysis by lesion type.
7.6.1 Subject Demographics and Lesion Characteristics
Table 28 - Subject Demographics by Lesion Type – VALOR Test Group Only
AGE Total Population
N 112 70 13
Mean ± SD (years) 71.7 ± 9.2 68.0 ± 13.4 69.4 ± 11.9
Median 74.0 72.0 74.0
Min-Max 39 – 86 27 – 85 46 – 85
Male
N 63 44 8
Mean ± SD (years) 70.7 ± 8.9 67.8 ± 14.6 67.1 ± 13.9
Median 73.0 72.0 72.5
Min-Max 50 – 85 27 – 85 46 – 85
Female
N 49 26 5
Mean ± SD (years) 73.1 ± 9.3 68.5 ± 11.5 73.0 ± 7.8
Median 75.0 70.5 75.0
Min-Max 39 – 86 38 – 82 64 – 84
Gender
Males 56.3% (63) 62.9% (44) 61.5% (8)
Females 43.8% (49) 37.1% (26) 38.5% (5)
Ethnicity
White, non-Hispanic 84.8% (95) 81.4% (57) 76.9% (10)
Black- non-Hispanic 12.5% (14) 12.9% (9) 15.4% (2)
Hispanic (White or Black) 1.8% (2) 2.9% (2) 7.7% (1)
Asian/Pacific Islander 0% (0) 2.9% (2) 0% (0)
Native American 0% (0) 0% (0) 0% (0)
Other 0.9% (1) 1 0% (0) 0% (0)
1 One subject declined providing ethnicity
Fusiform
Saccular /
Penetrating Ulcer
Multiple Lesion
36
Page 37

Table 29 - Baseline Vessel Dimensions by Lesion Type: VALOR Test Group Only (Core Lab Reported1)
Vessel Dimension n Mean ± SD Median Min Max
Proximal Neck Diameter (mm)
Fusiform 107 32.1 ± 4.7 32.5 19.0 43.5
Saccular/Penetrating Ulcer 67 29.8 ± 5.2 30.5 18.5 43.5
Multiple Lesion 13 31.0 ± 4.1 30.0 25.0 37.7
Max Aneurysm Diameter (mm)
Fusiform 107 60.3 ± 9.1 59.0 43.5 88.8
Saccular/Penetrating Ulcer 68 48.0 ± 11.9 44.8 26.2 79.8
Multiple Lesion 12 55.7 ± 7.1 55.7 44.4 71.3
Distal Neck Diameter (mm)
Fusiform 104 31.0 ± 4.8 30.8 18.5 42.0
Saccular/Penetrating Ulcer 67 27.9 ± 4.9 27.5 17.0 42.5
Multiple Lesion 13 28.4 ± 4.5 26.4 22.0 38.0
Proximal Neck Length (mm)
Fusiform 107 82.4 ± 50.9 78.0 12.9 234.0
Saccular/Penetrating Ulcer 67 76.2 ± 56.0 70.0 10.0 214.0
Multiple Lesion 13 79.9 ± 42.2 78.9 18.0 149.0
Aneurysm Length (mm)
Fusiform 101 145.7 ± 71.6 157.9 30.0 297.5
Saccular/Penetrating Ulcer 66 86.8 ± 63.6 63.0 8.0 258.9
Multiple Lesion 13 107.7 ± 49.5 99.0 34.0 186.0
Distal Neck Length (mm)
Fusiform 104 74.1 ± 51.9 62.2 10.9 225.0
Saccular/Penetrating Ulcer 67 114.5 ± 71.3 105.0 9.0 255.0
Multiple Lesion 13 90.7 ± 60.7 66.7 11.9 180.8
Right External Iliac Min Diameter
(mm)
Fusiform 71 6.5 ± 1.6 6.3 3.5 11.0
Saccular/Penetrating Ulcer 43 6.7 ± 1.5 6.5 2.9 9.7
Multiple Lesion 8 5.5 ± 1.5 5.4 4.0 7.9
Left External Iliac Min Diameter
(mm)
Fusiform 71 6.7 ± 1.5 6.5 4.0 10.9
Saccular/Penetrating Ulcer 45 6.5 ± 1.5 6.5 3.3 9.6
Multiple Lesion 8 5.8 ± 1.5 6.1 3.4 8.0
1 Denominators are n specified from readable scans.
37
Page 38

Table 30: Baseline Vessel Shape by Lesion Type (Core Lab Reported1) – VALOR Test Group Only
Vessel Shape Fusiform
% (m/n)
Proximal Neck Shape
Parallel 14.0% (15/107) 41.8% (28/67) 30.8% (4/13)
Funnel 22.4% (24/107) 22.4% (15/67) 23.1% (3/13)
Inverted Funnel 63.6% (68/107) 35.8% (24/67) 46.2% (6/13)
Distal Neck Shape
Parallel 27.9% (29/104) 49.3% (33/67) 46.2% (6/13)
Funnel 54.8% (57/104) 37.3% (25/67) 38.5% (5/13)
Inverted Funnel 17.3% (18/104) 13.4% (9/67) 15.4% (2/13)
1 Denominators are n specified for readable scans
7.6.2 Primary and secondary safety and Effectiveness endpoint Analysis by Lesion Type
Table 31: Primary Safety Endpoint: All Cause Mortality by Lesion Type – VALOR Test Group Only
Lesion Type % (m/n) [95% CI]
Fusiform
Saccular/Penetrating Ulcer
Multiple Lesion
1 Confidence level was not adjusted for multiplicity. Confidence interval for the
percentage was calculated by the exact (binomial) method.
Saccular/Penetrating Ulcer
% (m/n)
1
15.6% (17/109)
[9.4%-23.8%]
15.7% (11/70)
[8.1%-26.4%]
23.1% (3/13)
[5.0%-53.8%]
Multiple Lesion
% (m/n)
Table 32: Primary Effectiveness Endpoint: Successful Aneurysm Treatment by Lesion Type – VALOR Test Group
Lesion Type % (m/n) [95% CI]
Fusiform
Saccular/Penetrating Ulcer
Multiple Lesion
1 Confidence level was not adjusted for multiplicity. Confidence interval for the
percentage was calculated by the exact (binomial) method.
Only
89.0% (65/73)
[79.5%-95.1%]
88.2% (45/51)
[76.1%-95.6%]
100.0% (6/6)
[54.1%-100.0%]
1
38
Page 39

Table 33: Summary of Secondary Endpoints by Lesion Type – VALOR Test Group Only
Secondary Endpoints Fusiform
Successful deployment and delivery
% (m/n) [95% CI]
1
Saccular /
Penetrating Ulcer
% (m/n) [95% CI]
Multiple Lesion
% (m/n) [95% CI]
1
1
of the stent graft
@ Implant 99.1% (111/112)
[95.1%-100.0%]
“All-cause” Mortality
Within 30 Days 0.0% (0/112)
[0.0%-3.2%]
Aneurysm-related Death
Within 12 months 0.9% (1/109)
[0.0%-5.0%]
Paraplegia/paraparesis
Paraplegia @ 30 Days 0.0% (0/112)
[0.0%-3.2%]
Paraparesis @ 30 Days 9.8% (11/112)
[5.0%-16.9%]
Secondary endovascular procedure
100.0% (70/70)
[94.9%-100.0%]
5.7% (4/70)
[1.6%-14.0%]
7.1% (5/70)
[2.4%-15.9%]
2.9% (2/70)
[0.3%-9.9%]
2.9% (2/70)
[0.3%-9.9%]
100.0% (13/13)
[75.3%-100.0%]
0.0% (0/13)
[0.0%-24.7%]
0.0% (0/13)
[0.0%-24.7%]
7.7% (1/13)
[0.2%-36.0%]
7.7% (1/13)
[0.2%-36.0%]
due to endoleak
Within 30 Days post implantation 0% (0/111)
Between 31 days and 12 months
post implantation
[3.9%-15.4%]
One or more Major Adverse Events
[0.0%-3.3%]
8.4% (9/107)
0% (0/70)
[0.0%-5.1%]
4.5% (3/66)
[0.9%-12.7%]
0% (0/13)
[0.0%-24.7%]
0% (0/13)
[0.0%-24.7%]
(MAE)
Within 30 Days post implantation 46.4% (52/112)
[37.0%-56.1%]
Within 12 Months post implantation 57.8% (63/109)
[48.0%-67.2%]
One or more Serious Major Adverse
35.7% (25/70)
[24.6%-48.1%]
48.6% (34/70)
[36.4%-60.8%]
23.1% (3/13)
[5.0%-53.8%]
46.2% (6/13)
[19.2%-74.9%]
Events (MAE)
Within 30 Days post implantation 34.8% (39/112)
[26.1%-44.4%]
Within 12 Months post implantation 46.8% (51/109)
[37.2%-56.6%]
Conversion to open surgical repair
Within 12 Months post implantation 0.0% (0/109)
[0.0%-3.3%]
Migration of the stent graft
Migration >10 mm between 1 and
12 months
Loss of patency of the stent graft
5.6% (3/54)
[1.2%-15.4%]
At 12 Month visit 0% (0/60)
[0.0%-6.0%]
Aneurysm rupture
Within 12 Months post implantation 0.9% (1/109)
[0.0%-5.0%]
Endoleaks
At 12 Month visit 13.2% (9/68)
[6.2%-23.6%]
24.3% (17/70)
[14.8%-36.0%]
37.1% (26/70)
[25.9%-49.5%]
0.0% (0/70)
[0.0%-5.1%]
2.3% (1/44)
[0.1%-12.0%]
0% (0/42)
[0.0%-8.4%]
0.0% (0/70)
[0.0%-5.1%]
12.2% (6/49)
[4.6%-24.8%]
23.1% (3/13)
[5.0%-53.8%]
38.5% (5/13)
[13.9%-68.4%]
7.7% (1/13)
[0.2%-36.0%]
0.0% (0/5)
[0.0%-52.2%]
0% (0/5)
[0.0%-52.2%]
0.0% (0/13)
[0.0%-24.7%]
0.0% (0/6)
[0.0%-45.9%]
1 Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was calculated
by the exact (binomial) method.
Table 34: Persistent Paraplegia/Paraparesis at 12 Months or last Follow-up by Lesion Type – VALOR Test Group
Only
Safety Endpoint Fusiform
% (m/n) [95% CI]
Paraplegia
(at 12 Months or last Follow-up)
Paraparesis
(at 12 Months or last Follow-up)
1 Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was calculated by the exact (binomial) method.
0.9% (1/109)
[0.0%-5.0%]
5.5% (6/109)
[2.0%-11.6%]
1
Saccular / Penetrating
% (m/n) [95% CI]
Ulcer
2.9% (2/70)
[0.3%-9.9%]
0.0% (0/70)
[0.0%-5.1%]
1
Multiple Lesion
% (m/n) [95% CI]
7.7% (1/13)
[0.2%-36.0%]
0.0% (0/13)
[0.0%-24.7%]
1
39
Page 40

8.0 Patient Selection
8.1 Individualization of Treatment
Medtronic recommends that Talent Thoracic Stent Graft be used according to the Sizing Guidelines (see Table 35). All
lengths and diameters of the devices necessary to complete the procedure should be available to the physician,
especially when pre-operative case planning measurements (treatment diameters/lengths) are not certain. This approach
allows for greater intraoperative flexibility to achieve optimal procedural outcomes. The warnings and precautions
previously described in Section 5.0 should be carefully considered relative to each patient before use of the Talent
Thoracic Stent Graft System. The risks and benefits should be carefully considered for each patient before use of the
Talent Thoracic Stent Graft System.
Patient selection factors to be assessed should include but are not limited to:
• Patient age and life expectancy.
• Co-morbidities (e.g., cardiac, pulmonary or renal insufficiency prior to surgery, morbid obesity, etc.).
• Patient's suitability for open surgical repair.
• Patient's anatomical suitability for endovascular repair.
• The risk of aneurysm rupture compared to the risks of endovascular repair.
• Ability to tolerate general, regional or local anesthesia.
• Iliac/femoral access vessel morphology (minimal thrombus, calcium and/or tortuosity) that is compatible with vascular
access techniques, devices, and/or accessories;
• Non-aneurysmal aortic diameter in the range of 18 – 42mm;
• Non-aneurysmal aortic proximal and distal neck lengths ≥ 20mm.
• The final treatment decision is at the discretion of the physician and patient.
9.0
The physician should consider the following points when counseling the patient about this endovascular device and
procedure:
Medtronic recommends that physicians use the Medtronic Patient Information Booklet to aid in describing risks associated
with use of the Talent Thoracic Stent Graft System with the patient. Additionally Medtronic recommends that detailed
patient specific risks also be discussed.
10.0
10.1 Sterility
Each Talent Thoracic Stent Graft is individually contained within a Captivia Delivery System. The Captivia Delivery
Systems are sterilized using e-beam and are supplied sterile for single use only.
• Do not reuse or attempt to resterilize.
• Do not use if package is opened or damaged.
Patient Counseling Information
• Differences between endovascular repair and open surgical repair.
• Risks related to open surgical repair.
• Risks related to endovascular repair.
• Pros and cons of open surgical repair and endovascular repair.
• Endovascular repair is an option with potential advantages related to its minimally invasive approach.
• It is possible that subsequent endovascular or open surgical repair of the aneurysm may be required.
• The long term effectiveness of endovascular repair has not been established.
• Regular follow-up, including imaging of the device, should be performed at least every 6 to 12 months, or more
frequently in subjects with enhanced surveillance needs (see Section 13.0 for additional imaging
recommendations).
• Details contained in the patient information booklet regarding risks occurring after implantation of the device,
e.g., cardiac complications, neurological complications, etc.
• Symptoms of aneurysm rupture.
How Supplied
10.2 Contents
The following items are supplied in an envelope with the Talent Thoracic Stent Graft System:
• One (1) set Device Registration Packet
10.3 Storage
Store at room temperature in a dark, dry place.
40
Page 41

11.0 Clinical Use Information
WARNING: CONSIDER HAVING A SURGICAL TEAM AVAILABLE DURING IMPLANTATION OR REINTERVENTION
PROCEDURES IN THE EVENT THAT CONVERSION TO OPEN SURGICAL REPAIR IS NECESSARY.
11.1 Recommended Skills and Training
Physicians performing the Talent Thoracic Stent Graft System procedure must be trained in vascular interventional
procedures and in the use of this device.
The recommended skill/knowledge requirements for physicians using the Talent Thoracic Stent Graft System are outlined
below:
11.1.1 Patient Selection
• Knowledge of the natural history of thoracic aortic aneurysms and comorbidities associated with thoracic repair.
• Knowledge of image interpretation, stent graft selection and sizing.
11.1.2 Physician Skills and Experience
Either the individual physician operator or a combined, multidisciplinary team should possess extensive procedural
skills and experience with:
• Angioplasty.
• Appropriate use of contrast material.
• Embolization.
• Endovascular stent graft placement.
• Femoral cutdown, arteriotomy, and repair.
• Live fluoroscopic and angiographic image interpretation.
• Non-selective and selective catheterization.
• Snare techniques.
• Techniques to minimize radiation exposure.
11.2 Materials Recommended for Device Implantation
At the time of surgery, Medtronic recommends that the physicians have available:
• Additional Talent Thoracic Stent Grafts of various lengths and diameters which might be needed to customize the
implant to fit the anatomy of the individual patient.
• Assorted angiographic catheters, angioplasty catheters, graduated pigtail catheters.
• Assorted guidewires of at least 260cm in length.
• At least one additional Talent Thoracic Stent Graft (of the size intended for implantation) in the event that the device is
damaged during attempted placement.
• At least two additional Talent Thoracic Stent Grafts (one size larger and one size smaller) in the event that the original
measurement underestimated or overestimated the vessel size.
• Contrast media.
• Fluoroscope with digital angiography capabilities and the ability to record and recall imaging.
• Heparin and heparinized saline solution.
• Intravascular Ultrasound catheter (IVUS).
• Introducer sheaths for vascular access to access arteries and to perform diagnostic imaging.
• Reliant Stent Graft Balloon Catheter and other materials recommended by the Reliant Instructions for Use.
NOTE: THE RELIANT STENT GRAFT BALLOON CATHETER IS RECOMMENDED FOR USE WITH THE TALENT
• Sterile lubricant.
• Stiff 0.035” diameter guidewires to support the Captivia Delivery System in the aortic vasculature.
THORACIC STENT GRAFT. DATA IS NOT AVAILABLE FOR USE WITH OTHER BALLOONS FOR
REMODELING STENT GRAFTS.
41
Page 42

11.3 Pre-Treatment Planning and Selection of Stent Graft
The specific stent graft diameter used for treatment should be oversized to the non-aneurysmal vessel using the sizing
guidelines to ensure appropriate radial fixation. Table 35, Column 2 describes the stent graft to vessel over-sizing
guidelines.
When multiple components are needed to exclude the target lesion, and the component junctions (overlapping
connections) will not be supported by the vessel (i.e. in the aneurysm sac), a 4mm oversizing between overlapping stent
grafts should be used, as shown in Column 3 of Table 35. If the component junctions will be supported by the vessel,
sizing to the supporting native vessel should be used, as described in Table 35, Column 2. See Figure 11 for a depiction
of supported and unsupported regions.
Oversizing stent grafts greater than the stated guidelines could lead to increased stress on the springs or vessel damage.
Undersizing of the stent graft may lead to device migration.
CAUTION: OVERSIZING THE DEVICE BEYOND THE RECOMMENDED GUIDELINES MAY LEAD TO VESSEL
TRAUMA OR RUPTURE. WHEN OVERLAPPING TWO OR MORE STENT GRAFTS TO EXCLUDE THE
TARGET LESION, OVERSIZING OF THE STENT GRAFT TO THE VESSEL SHOULD BE USED AS PER
THE SIZING TABLE IN THE IFU.
STRICT ADHERENCE TO THE IFU SIZING GUIDE IS STRONGLY RECOMMENDED WHEN SELECTING
THE APPROPRIATE DEVICE SIZE, AS PER TABLE 35 IN THE IFU. APPROPRIATE DEVICE
OVERSIZING HAS BEEN INCORPORATED INTO THE IFU SIZING GUIDE. SIZING OUTSIDE THIS
RANGE CAN POTENTIALLY RESULT IN ENDOLEAK, FRACTURE, MIGRATION, INFOLDING OR
FABRIC WEAR.
MEDTRONIC IS AWARE OF AN INSTANCE FROM TALENT THORACIC STENT GRAFT EXPLANT
OBSERVATIONS, WHERE OVERSIZING THE OVERLAP COMPONENTS BEYOND THE
RECOMMENDED GUIDELINES HAS RESULTED IN GRAFT MATERIAL HOLE AND BROKEN SUTURES.
Table 35 - Sizing Guidelines
Column 1
Native Vessel
Diameter
(mm)
18 22 26
19 22 26
20 24 28
21 24 28
22 26 30
23 26 30
24 28 32
25 28, 30 32, 34
26 30 34
27 30, 32 34, 36
28 32 36
29 32, 34 36, 38
30 34 38
31 34, 36 38, 40
32 36 40
33 38 42
34 38 42
35 40 44
36 40 44
37 42 46
38 42 46
39 44
40 44
41 46
42 46
Column 2
Suggested Talent
Thoracic Oversizing
(mm)
Unsupported Junction with
Column 3
Suggested 4mm
Oversizing for
Graft Sizes from
Column 2
(mm)
42
Page 43

Figure 11: Regions for Modular Overlaps
The order of deployment when using multiple stent graft component sections may vary, depending on the diameter of the
aorta proximal to and distal to the lesion. Table 36 should be followed to determine the order of deployment when using
multiple stent graft component section.
Table 36 - Order of Deployment When Using Multiple Stent Graft Component Sections
First Section
Implanted
(Primary Section)
Second Section
Implanted
(Additional
Section)
Third Section
Implanted
(Additional
Section)
Proximal Aortic Diameter =
Distal Aortic Diameter
Proximal Main Section
implanted at proximal end of
lesion.
Distal Main Section implanted
with correct junction oversizing.
Due to tapered configuration of
distal main section, this fits a
straight aorta correctly.
[Optional] Additional Distal Main
Sections or extensions
implanted with correct
oversizing at junction.
Proximal Aortic Diameter >
Distal Aortic Diameter*
Distal Main Section (or other
configuration if more
appropriate) implanted at
distal end of lesion.
Proximal Main Section
implanted with correct
oversizing at junction with
Distal Main Section.
Proximal telescoping of
devices fits this shape of
aorta.
[Optional] Additional Proximal
Main Sections or extensions
to telescope to fit greater
proximal diameter better.
Proximal Aortic Diameter <
Distal Aortic Diameter
Proximal Main Section
implanted at proximal end of
lesion.
Distal Main Section implanted
with correct oversizing at
junction.
Distal Extension (which is not
tapered) to telescope to
properly fit diameter of distal
landing zone.
* Use this option when implanting the proximal section first to avoid oversizing beyond the recommendations in Table 35.
NOTE: THE END CONFIGURATION FOR DEPLOYMENT WITHIN AN ADJACENT COMPONENT MUST BE A
Correct sizing of the aorta and iliac/femoral vessels must be determined before implantation of the Talent Thoracic Stent
Graft System. Medtronic recommends a Computed Tomography Angiogram (CTA) be performed within 3 months prior to
implantation. These images should be available for review during the procedure.
CLOSED WEB OR OPEN WEB.
43
Page 44

12.0
12.1 Pictorial References
For pictorial references of the Talent Thoracic Stent Graft components and Captivia Delivery System refer to Figure 1 to
Figure 5.
12.2 Vascular Access
Step 1:
primary access artery. A secondary access site can be used for diagnostic and imaging purposes. The secondary
access site is determined by physician discretion (percutaneous, brachial, etc.).
Step 2:
antiocoagulated for the duration of the procedure to achieve an ACT of 250-300 seconds at the discretion of the physician.
Antiplatelet therapy may also be administered at the discretion of the physician.
12.3 Initial Angiogram
Using continuous fluoroscopy, traverse a 0.035" (0.89 mm) guidewire and graduated pigtail angiographic catheter (via the
secondary access site) to confirm the target landing zones. Pre-operative CT measurements (length/diameter) should be
confirmed with angiographic images at this time. Confirm diameter and length of the selected Talent Thoracic Stent Graft
for suitability. The angiographic catheter is left in place during the procedure to aid in confirming the position of the graft.
During the Talent Thoracic Stent Graft implantation, these images may be used for road-mapping.
In order to obtain the most accurate angiogram of the Stent Graft landing zone, the fluoroscope should be positioned such
that the arc of the wire appears as “open” as possible. That is, the fluoroscope will be most perpendicular to the target
landing zone when the image of the wire angle within the anatomy is as wide as possible.
12.4 Device Preparation
Step 1:
Carefully inspect the sterile package for damage or defects before opening. Do not use product after the “Use By” date
on the package. If the integrity of the sterile package has been compromised prior to the product “Use By” date or the
packaging or product is defective, do not use the product and contact your Medtronic representative for return information.
Step 2:
While holding the Talent Thoracic Stent Graft with the Captivia Delivery System upright, flush the graft cover using a
syringe with heparinized saline solution via the sideport (tapping the sheath to aid in releasing air bubbles). If difficult to
flush, continue to apply pressure to the syringe, allowing time for the saline to infuse the stent graft.
Step 3:
Flush the guidewire lumen with heparinized saline via the luer connector.
CAUTION: DO NOT GRIP THE TIP CAPTURE RELEASE HANDLE DURING FLUSHING OF THE DELIVERY
Step 4:
In its locked position, as indicated in Figure 12, the handle should not be able to rotate clockwise.
Implantation Instructions
Establish vascular access for the Captivia Delivery System introduction via a small oblique groin incision over the
Provide systemic anticoagulation. To reduce the risk of thromboembolism, it is recommended that patients be
Inspection Prior to Use
Flush Graft Cover
Flush Guidewire Lumen
SYSTEM.
For the FreeFlo Stent Graft Delivery System - Verify that the tip capture release handle is in its locked position.
Figure 12: Tip Capture Release Handle in Locked Position
CAUTION: INITIATING DEPLOYMENT OF THE STENT GRAFT WITH THE TIP CAPTURE RELEASE HANDLE IN
Step 5:
Inspect the radiopaque markers on the stent graft to identify positioning of the graft within the sheath. Identify the location
of the connecting bar by visualization of the markers described in Section 2.2. Before introducing the system into the
patient’s body, turn the delivery system to align the connecting bar with the outer bend of the target vessel for implantation.
CAUTION: FAILURE TO ALIGN THE CONNECTING BAR WITH THE OUTER BEND OF THE TARGET VESSEL
ITS UNLOCKED POSITION (ROTATED COUNTER-CLOCKWISE) MAY RESULT IN PREMATURE
RELEASE OF THE PROXIMAL BARE STENT OF THE FREEFLO CONFIGURATION.
Identify and Align Connecting Bar
MAY INCREASE THE LIKELIHOOD OF ENDOLEAKS POST IMPLANTATION.
44
Page 45

12.5 Hydrophilic Coating Activation
Step 1:
Saturate a sterile gauze with sterile saline.
Gently wipe the surface of the graft cover with saturated gauze until the graft cover is wet/slippery to touch.
Step 2:
12.6 Introducing the Captivia Delivery System
CAUTION: MANIPULATION OF WIRES, BALLOONS, CATHETERS, AND ENDOGRAFTS IN THE THORACIC
NOTE: CAREFULLY MONITOR THE PATIENT’S VITAL SIGNS THROUGHOUT THE IMPLANTATION
NOTE: IF NECESSARY, OPEN NARROW ILIAC VESSELS WITH PERCUTANEOUS TRANSLUMINAL
Step 1:
and advancement, it is important to align the connecting bar with the outside of the most severe bend of the target
deployment area. Proper orientation of the connecting bar should be monitored during advancement. Proper alignment is
key in order to avoid excessive twisting and manipulation of the Captivia Delivery System.
Step 2:
Stent Graft implantation according to standard endovascular procedures. If necessary, dilate vessel with tapered vessel
dilator. A step-up approach is recommended for vessel dilation (at the discretion of the physician).
AORTA M AY LEAD TO VASCULAR TRAUMA INCLUDING AOR TIC DI SSECTION AND EMBOLIZATION.
PROCEDURE.
ANGIOPLASTY (PTA) CATHETERS PRIOR TO TALENT THORACIC STENT GRAFT SYSTEM
PLACEMENT, OR DILATE VESSELS WITH TAPERED VESSEL DILATORS WITH A STEP-UP
APPROACH. ALTERNATIVELY, AN ILIAC CONDUIT MAY BE SEWN TO THE ILIAC ARTERY TO
FACILITATE PLACEMENT OF THE DELIVERY SYSTEM.
Slowly insert the Captivia Delivery System over a 0.035” stiff or super-stiff guidewire (Figure 13). During insertion
If necessary, open narrow entry vessels with standard PTA catheters or vessel dilators prior to Talent Thoracic
Figure 13: Introduce the Captivia Delivery System
CAUTION: IF AN OBSTRUCTION IN THE VESSEL (E.G., A TORTUOUS BEND, STENOSIS, CALCIFICATION, ETC.)
CAUTION: DO NOT GRIP THE TIP CAPTURE RELEASE HANDLE DURING INTRODUCTION OF THE DELIVERY
CAUTION: DURING GENERAL HANDLING OF THE CAPTIVIA DELIVERY SYSTEM, AVOID BENDING OR KINKING
NOTE: THE CAPTIVIA DELIVERY SYSTEM DOES NOT REQUIRE A SEPARATE INTRODUCER SHEATH FOR
PREVENTS ADVANCEMENT OF THE CAPTIVIA DELIVERY SYSTEM, DO NOT USE EXCESSIVE
FORCE TO ADVANCE THE DELIVERY SYSTEM.
SYSTEM.
THE GRAFT COVER BECAUSE IT MAY CAUSE THE TALENT THORACIC STENT GRAFT TO
PREMATURELY AND IMPROPERLY DEPLOY.
THE PRIMARY ACCESS SITE.
45
Page 46

12.7 Positioning the Captivia Delivery System
Slowly advance the Captivia Delivery System to the targeted landing zone. For patients who do not have excessive
calcification or thrombus it is suggested to position the device more proximal (a few millimeters higher in the vessel) to the
targeted landing zone. In patients with highly tortuous anatomy, it is suggested to position the device even more proximal
to the targeted landing zone, as the stent graft may move distally when the graft cover is initially pulled back, then
proximally when the first covered stent of the stent graft is released.
CAUTION: IT IS NOT RECOMMENDED TO POSITION THE DEVICE HIGHER IN THE PRESENCE OF EXCESSIVE
CAUTION: BE SURE TO AVOID OR COMPENSATE FOR PARALLAX OR OTHER SOURCES OF VISUALIZATION
CAUTION: DO NOT ADVANCE THE CAPTIVIA DELIVERY SYSTEM TIP OR GUIDEWIRE ACROSS THE AORTIC
CAUTION: DO NOT GRIP THE TIP CAPTURE RELEASE HANDLE DURING POSITIONING OF THE DELIVERY
12.8 Confirming Stent Graft Position and Verify Markers
Step 1:
angiogram.
Step 2:
desired location as shown in Figure 14and 14b. While positioning, also verify that the connecting bar is oriented on the
outside of the most severe bend of the vessel. The middle marker indicates the rotational position of the connecting bar.
In the event that exact placement of the distal end of the graft is critical, it is also important to verify that the distal markers
indicating the bottom edge of the fabric are at the desired location.
CALCIFICATION OR THROMBUS, DUE TO THE INCREASED RISK OF DISLODGING MATERIAL
DURING DISTAL REPOSITIONING OF THE STENT GRAFT.
ERROR.
VALVE.
SYSTEM.
Before beginning deployment of the Talent Thoracic Stent Graft, confirm proper position of the device with an
When placing a main section, verify that the proximal markers indicate that the top edge of the fabric is at the
Figure 14a: Confirm Stent Graft Position (Proximal Main)
Confirming position with Proximal and Distal
Figur8 Markers
46
Page 47

Figure 14b: Confirm Stent Graft Position (Distal Main)
Confirming position of overlapping components
with Figur8 Mid-Markers
(An already-implanted FreeFlo
stent graft is shown in this image)
CAUTION: IN THE PRESENCE OF EXCESSIVE CALCIFICATION OR THROMBUS, IT IS NOT RECOMMENDED TO
CAUTION: BE SURE TO AVOID OR COMPENSATE FOR PARALLAX OR OTHER SOURCES OF VISUALIZATION
CAUTION: DO NOT GRIP THE TIP CAPTURE RELEASE HANDLE WHILE CONFIRMING THE POSITION OF THE
NOTE: FOR CONFIRMING STENT GRAFT POSITION OF ADDITIONAL COMPONENTS, THE MINIMUM
12.9 Deploying the Talent Thoracic Stent Graft
WARNING: THE PROXIMAL EDGE OF THE COVERED PORTION OF THE STENT GRAFT SHOULD NOT BE
POSITION THE DEVICE HIGHER AND THEN REPOSITION DISTALLY AFTER PARTIAL STENT GRAFT
DEPLOYMENT, DUE TO THE INCREASED RISK OF DISLODGING MATERIAL.
ERROR.
DELIVERY SYSTEM.
OVERLAP IS ACHIEVED BY ALIGNING THE DISTAL FIGUR8 MARKERS OF THE PROXIMAL GRAFT
WITH THE SINGLE FIGUR8 MID-MARKER OF THE DISTAL GRAFT (SEE SECTION 12.13).
PLACED BEYOND THE ORIGIN OF THE LEFT COMMON CAROTID ARTERY (I.E., ZONE 0 OR ZONE 1).
47
Page 48

Figure 15: Covered Portion (Top of Fabric) Placement Zones
WARNING: ENSURE TH AT THE P ROX IM AL AND DISTAL SPRINGS ARE PLACED I N AN ADEQUATE LANDING
ZONE COMPRISED OF HEALTHY TISSUE. HEALTHY TISSUE IS DEFINED AS TISSUE WITHOUT
EVIDENCE OF CIRCUMFERENTIAL THROMBUS, INTRAMURAL HEMATOMA, DISSECTION,
ULCERATION, AND/OR ANEURYSMAL INVOLVEMENT. FAILURE TO DO SO MAY RESULT IN
INADEQUATE EXCLUSION OR VESSEL DAMAGE, INCLUDING PERFORATION.
Decreasing Mean Arterial Blood Pressure (MAP)
Step 1:
Upon confirmation that the Captivia Delivery System is positioned properly, it may be appropriate to momentarily
decrease the patient’s MAP to approximately 80 mmHg (at the discretion of the physician) to avoid inadvertent
displacement of the Talent Thoracic Stent Graft upon withdrawal of the graft cover.
Deploying Proximal End
Step 2:
First hold the delivery system stationary with one hand on the front grip. Then, slowly withdraw the graft cover with the
other hand by rotating the slider counter-clockwise. It may take multiple rotations before the graft cover separates from
the tip, visualized by movement of the radiopaque marker band.
For the FreeFlo Stent Graft Delivery System (Proximal Mains): The proximal bare stent of the FreeFlo configuration
will be constrained by the tip capture mechanism. Withdraw the graft cover until up to two covered stents are
exposed.
3
For the FreeFlo Stent Graft Delivery System (Proximal Extensions): The proximal bare stent of the FreeFlo
configuration will be constrained by the tip capture mechanism. Withdraw the graft cover until up to one covered
stent is exposed.
For the Open Web Stent Graft Delivery System: Withdraw the graft cover until up to two covered (body) stents are
exposed.
2
2
Stabilize the delivery system during stent graft deployment.
3
In the unlikely event of delivery system failure and concomitant partial stent graft deployment due to graft cover
severance, a “handle disassembly” technique will permit successful deployment of the stent graft. See instruction in
Section 12.14.1 for details of the technique.
48
Page 49

Figure 16a: Deploying the Proximal End of the Stent Graft (FreeFlo)
For the FreeFlo Stent Graft Delivery System,
the proximal bare stent is constrained
by the tip capture mechanism.
Figure 16b: Deploying the Proximal End of the Stent Graft (Open Web)
For the Open Web Stent Graft System, the
proximal end is not constrained.
(An already-implanted FreeFlo)
stent graft is shown in this image)
CAUTION: DO NOT PLACE THE PROXIMAL EDGE OF THE FABRIC BEYOND THE TOP OF THE AORTIC ARCH
CAUTION: IF THE STENT GRAFT IS DEPLOYED HIGHER THAN THE TARGETED LANDING ZONE IT IS
CAUTION: DO NOT RELEASE THE PROXIMAL BARE STENT OF THE FREEFLO CONFIGURATION BEFORE THE
Step 3:
Use angiography to verify the position of the stent graft in relation to the desired location. Use the proximal Figur8
markers to aid in visualizing the top edge of the graft fabric. If the stent graft was deployed higher than the targeted
landing zone, maintain the position of the slider and pull down on the entire delivery system until the proximal Figur8
markers indicating the top edge of the fabric are at the desired position.
OR THE DISTAL EDGE OF THE LEFT COMMON CAROTID ARTERY.
IMPORTANT NOT TO DEPLOY MORE THAN TWO COVERED STENTS PRIOR TO RE-POSITIONING
OF THE STENT GRAFT. FURTHER DEPLOYMENT OF THE GRAFT CAN IMPAIR THE ABILITY TO
MOVE THE GRAFT TO THE DESIRED LANDING ZONE.
ENTIRE STENT GRAFT HAS BEEN DEPLOYED, AS THIS MAY RESULT IN INACCURATE
DEPLOYMENT.
Verifying Stent Graft Position
49
Page 50

Step 4: Deploying Remainder of Stent Graft
Continue withdrawing the graft cover.
For the FreeFlo Stent Graft Delivery System (Proximal Mains) and Open Web Stent Graft Delivery System Only:
To more rapidly deploy the stent graft, place one hand firmly on the grey front grip and hold the system stationary. While
maintaining support on the grey front grip, pull back the grey trigger to engage the quick-release function of the blue slider
handle. Pull the blue slider handle away from the grey front grip until the RO Marker Band on the graft cover is beyond the
distal spring. If excessive force is felt, release the grey trigger and rotate the blue slider handle to complete deployment of
the stent graft
NOTE: At this point on the FreeFlo Stent Graft Delivery System, the proximal bare stent is still constrained by the tip
capture mechanism.
For the FreeFlo Stent Graft Delivery System (Proximal Extensions): Continue to slowly withdraw the graft cover by
rotating the slider counter-clockwise.
Figure 17a: Deploying the Remainder of the Stent Graft (FreeFlo)
For the FreeFlo Stent Graft Delivery
System, the proximal bare stent is
constrained by the tip capture
Figure 17b: Deploying the Remainder of the Stent Graft (Open Web)
mechanism.
For the Open Web Stent Graft System,
the proximal end is not constrained.
(An already-implanted FreeFlo
stent graft is shown in this image)
50
Page 51

CAUTION: WHEN USING THE TRIGGER TO DEPLOY THE STENT GRAFT, BE SURE TO KEEP THE FRONT GRIP
STATIONARY. FAILURE TO DO SO WILL CAUSE MOVEMENT OF THE STENT GRAFT POSITION,
RESULTING IN INACCURATE DEPLOYMENT.
CAUTION: DO NOT ROTATE THE GRAFT COVER DURING DEPLOYMENT, AS THIS MAY TORQUE THE
DELIVERY SYSTEM AND CAUSE THE STENT GRAFT TO TWIST ON DEPLOYMENT.
CAUTION: ONCE THE ENTIRE COVERED PORTION OF THE STENT GRAFT HAS BEEN DEPLOYED, DO NOT
ATTEMPT TO ADJUST THE POSITION OF THE STENT GRAFT PROXIMALLY OR DISTALLY.
CAUTION: IF THE STENT GRAFT IS DEPLOYED HIGHER THAN THE TARGETED LANDING ZONE, IT IS
EXTREMELY IMPORTANT NOT TO DEPLOY MORE THAN THE FIRST TWO STENT SPRINGS (SEE
FIGURE 16A). FURTHER DEPLOYMENT OF THE GRAFT CAN IMPAIR THE ABILITY TO MOVE THE
GRAFT TO THE DESIRED LANDING ZONE.
CAUTION: IF THE GRAFT COVER IS INADVERTENTLY WITHDRAWN, THE TALENT THORACIC STENT GRAFT
WILL PREMATURELY DEPLOY AND WILL BE PLACED INCORRECTLY.
NOTE: IF NECESSARY, THE STENT GRAFT CAN BE RE-POSITIONED DISTALLY TO ITS DESIRED
LOCATION BY RETRACTING IT, AS LONG AS NO MORE THAN TWO OF THE PROXIMAL SPRINGS OF
THE STENT GRAFT HAVE BEEN DEPLOYED.
NOTE: DEPLOYMENT OF THE TALENT THORACIC STENT GRAFT IN THE AORTIC ARCH CAN INCREASE
THE DEPLOYMENT FORCE. DEPLOYMENT FORCES CAN BE FURTHER INCREASED BY EXCESSIVE
TORTUOSITY AND A SMALL RADIUS AORTIC ARCH.
NOTE: IN THE UNLIKELY EVENT OF DELIVERY SYSTEM FAILURE AND CONCOMITANT PARTIAL STENT
GRAFT DEPLOYMENT DUE TO GRAFT COVER SEVERANCE, A “TROUBLE SHOOTING” TECHNIQUE
WILL PERMIT SUCCESSFUL DEPLOYMENT OF THE STENT GRAFT. SEE INSTRUCTIONS IN
SECTION 12.14 FOR DETAILS OF THE TECHNIQUE.
12.10 Deploying Tip Capture Mechanism (FreeFlo Stent Graft Delivery System Only)
Continue to hold the delivery system stationary with one hand on the front grip.
Step 1:
With the other hand, rotate the tip capture release handle counter-clockwise to unlock the handle.
Step 2:
Figure 18: Unlocking the Tip Capture Release Handle
(FreeFlo Stent Graft Delivery System Only)
Step 3:
Pull the tip capture release handle back in a smooth motion until the tip capture mechanism is released, and the
proximal bare stent of the FreeFlo configuration is completely open (see Figure 19). Observe the opening of the bare
stent under fluoroscopy and confirm that the proximal bare stent has been completely deployed.
51
Page 52

Figure 19: Deploying the Tip Capture Mechanism
(FreeFlo Stent Graft Delivery System Only)
CAUTION: KEEP THE DELIVERY SYSTEM STATIONARY WHILE DEPLOYING THE TIP CAPTURE MECHANISM.
DO NOT PULL BACK ON OR PUSH FORWARD ON THE DELIVERY SYSTEM WHILE DEPLOYING THE
TIP CAPTURE MECHANISM, AS IT MAY CAUSE THE ENTIRE GRAFT TO MOVE.
CAUTION: DO NOT PUSH FORWARD ON THE TIP CAPTURE RELEASE HANDLE OR ON THE ENTIRE
DELIVERY SYSTEM UNTIL THE FRONT GRIP HAS BEEN PULLED TOWARDS THE SLIDER (SECTION
12.11, STEP 2). PUSHING FORWARD ON THESE MAY CAUSE THE TIP CAPTURE COMPONENT TO
GET CAUGHT ON THE PROXIMAL BARE STENT.
NOTE: IN THE UNLIKELY EVENT THAT THE PROXIMAL BARE STENT OF THE FREEFLO CONFIGURATION
CANNOT BE DEPLOYED, REFER TO SECTION 12.14.2.
12.11 Removing the Delivery System
Continue to hold the Captivia Delivery System with one hand on the front grip and the other hand on the slider.
Step 1:
Pull back the trigger and hold the slider stationary while bringing the front grip towards the slider as depicted in
Step 2:
Figure 20. Use continual fluoroscopy and watch the proximal end of the Talent Thoracic Stent Graft while slowly pulling
back the tapered tip into the graft cover of the delivery system. It may be necessary to pull the entire delivery system
back into a straight section of the aorta to aid in retraction of the tip.
For the Open Web Stent Graft Delivery System: Proceed to Step 4.
For the FreeFlo Stent Graft Delivery System: After the front grip has been pulled back to rejoin the slider,
Step 3:
push the tip capture release handle forward so that the tip capture component moves toward the radiopaque marker band
of the graft cover. Monitor the movement of the tip capture component using fluoroscopy.
Gently remove the delivery system, using fluoroscopy to ensure that the stent graft does not move during the
Step 4:
withdrawal.
52
Page 53

Figure 20: Delivery System Removal
CAUTION: CAREFULLY MONITOR THE RETRIEVAL OF THE TAPERED TIP WITH FLUOROSCOPY TO ENSURE
THAT THE TIP DOES NOT CAUSE THE TALENT THORACIC STENT GRAFT TO BE INADVERTENTLY
PULLED DOWN.
53
Page 54

12.12 Ancillary Balloon Catheter Modeling
The Reliant Stent Graft Balloon Catheter, packaged separately, may be used to assist in stent graft implantation by
modeling the covered springs and removing wrinkles and folds from the graft material as needed. Sub-optimal apposition
of the self-expanding stent graft may be improved by use of the Reliant Stent Graft Balloon (see Figure 21). Refer to the
Instructions for Use supplied with the Reliant Stent Graft Balloon Catheter for more information.
Figure 21: Balloon Modeling of the Stent Graft
CAUTION: USE THE RELIANT STENT GRAFT BALLOON CATHETER ACCORDING TO THE INSTRUCTIONS FOR
NOTE: THE RELIANT STENT GRAFT BALLOON CATHETER IS RECOMMENDED FOR USE WITH THE
If focal area narrowing of the stent graft is observed, re-balloon. If the area remains narrow following ballooning, place
another Talent Thoracic Stent Graft inside that segment. Do not leave untreated any focal area with significant stent graft
narrowing or abrupt kinks of the connecting bar. This can lead to thrombosis, damage of the stent graft or incomplete
distal seal.
WARNING: WHEN EXPANDING A VASCULAR PROSTHESIS USING THE RELIANT BALLOON, THERE IS AN
WARNING: DO NOT USE THE RELIANT STENT GRAFT BALLOON CATHETER IN PATIENTS WITH HISTORY OF
USE SUPPLIED WITH THE RELIANT DEVICE. DO NOT ATTEMPT TO USE THE RELIANT STENT
GRAFT BALLOON CATHETER BEFORE COMPLETELY READING AND UNDERSTANDING THE
INFORMATION SUPPLIED WITH THE RELIANT DEVICE.
TALENT THORACIC STENT GRAFT. DATA IS NOT AVAILABLE FOR USE WITH OTHER BALLOONS
FOR REMODELING STENT GRAFTS.
INCREASED RISK OF VESSEL INJURY AND/OR RUPTURE, AND POSSIBLE PATIENT DEATH, IF THE
BALLOON’S PROXIMAL AND DISTAL RADIOPAQUE MARKERS ARE NOT COMPLETELY WITHIN
THE COVERED (GRAFT FABRIC) PORTION OF THE PROSTHESIS.
THORACIC DISSECTION DISEASE. DO NOT OVER-INFLATE THE RELIANT STENT GRAFT BALLOON
WITHIN OR OUTSIDE OF THE GRAFT MATERIAL.
54
Page 55

12.13 Implanting Additional Component Sections
If two or more Talent Thoracic Stent Graft component sections are required to exclude the target lesion, use the following
steps.
Step 1 - Device Preparation and Insertion
Complete all steps described above in Sections 12.3, 12.5, 12.6, and 12.7.
Slowly advance the Talent Thoracic Stent Graft System to the targeted landing zone. Advancement of the device within
the previously implanted stent graft must be carefully monitored under fluoroscopy to ensure that the implanted stent graft
does not move. Before deploying the stent graft, confirm proper position of the system.
Step 2 - Confirm Device Position and Verify Markers
When placing a distal main section, radiographically verify that the proximal alignment markers are aligned with or above
the distal markers of the mating graft (see Appendix A). Also, verify that the distal markers that indicate the bottom edge
of the graft material are at the desired location. Verify that the connecting bar is rotationally oriented on the outside of the
most severe bend of the vessel.
When placing a proximal main or proximal extension, radiographically verify that the proximal markers that indicate the top
edge of the graft material are at the desired location (see Appendix B). It may be necessary to perform an angiogram to
ensure this. Also, verify that the alignment-marker of the proximal extension is aligned with, or below, the proximal
markers of the main graft. Radiographically verify that the connecting bar is oriented on the outside of the most severe
bend of the vessel.
When placing a distal extension, radiographically verify that the proximal alignment-marker is aligned with, or above, the
distal markers of the mating graft (see Appendix C). Also, verify that the distal markers that indicate the bottom edge of
the graft material are at the desired location. Verify that the connecting bar is rotationally oriented on the outside of the
most severe bend of the vessel.
CAUTION: AT LEAST A MINIMUM OF 30MM OF OVERLAP IS REQUIRED. HOWEVER, IN AREAS OF
CAUTION: A FREEFLO/BARE SPRING SHOULD NEVER BE PLACED INSIDE THE GRAFT COVERED SECTION
Step 3 - Remaining Steps
Follow procedures previously described in Sections12.8, 12.9, 12.10(if applicable), 12.11, and12.12.
Step 4 – Final Angiogram
Upon completion of the final ballooning procedure, perform angiography to verify stent graft apposition and seals, and
absence of endoleaks (Type I and Type III). The most reliable course of endoleak management (Type I or Type III) is by
remodeling the stent graft with a balloon and, if needed, placing a stent graft extension. A minor leak that does not seal
after re-ballooning may seal spontaneously within several days. If any adjunctive maneuvers were conducted, perform a
final angiogram to confirm successful exclusion of the target lesion. Do not use high-pressure injections at the edges or
within the Talent Thoracic Stent Graft immediately after implantation.
CAUTION: ANY ENDOLEAK LEFT UNTREATED DURING THE IMPLANTATION PROCEDURE MUST BE
Step 5 – Entry Site Closure
Remove all remaining accessories (e.g., guidewire, introducer sheath, angiogram catheter). Close the arteriotomy site by
standard surgical closure techniques.
ANGULATION OR CURVATURE AND/OR IF MORE THAN TWO (2) STENT GRAFTS ARE REQUIRED,
ADDI TIONAL OVERLAP IS RECOMMENDED (AT LEAST AN AD DITIONAL SPRING LENGTH-15MM).
FAILURE TO PROVIDE SUFFICIENT OVERLAP MAY RESULT IN SEPARATION OF THE STENT
GRAFTS AT THEIR JUNCTION. REFER TO Appendix A, Appendix B, AND Appendix C.
OF ANOTHER GRAFT AS DOING SO MAY RESULT IN ABRASION OF THE FABRIC BY THE BARE
SPRING, RESULTING IN A GRAFT MATERIAL HOLE OR BROKEN SUTURES.
MEDTRONIC IS AWARE OF AN INSTANCE FROM TALENT THORACIC STENT GRAFT EXPLANT
OBSERVATIONS, WHERE PUTTING A BARE SPRING INSIDE THE GRAFT COVERED SECTION OF
ANOTHER DEVICE HAS RESULTED IN GRAFT MATERIAL HOLE BROKEN SUTURES AND STENT
FRACTURE GRAFT MATERIAL HOLE AND BROKEN SUTURES.
CAREFULLY FOLLOWED AFTER IMPLANTATION.
55
Page 56

12.14 Troubleshooting
12.14.1 Handle Disassembly Technique for Partial Stent Graft Deployment
In the unlikely event of delivery system failure and concomitant partial stent graft deployment due to graft cover severance,
a “handle disassembly” technique may permit the successful deployment of the stent graft.
Step 1: Pull back the trigger and fully retract the slider. Note: Since the graft cover is severed, the slider can be
retracted without further deploying the stent graft.
Stabilize the delivery system.
Step 2:
Step 3:
Insert the tips of a pair of hemostats into each of the handle disassembly ports on the front grip.
Disengage the front grip from the screw gear by pressing the tips of the hemostats into the handle disassembly
Step 4:
ports and simultaneously advancing the front grip away from the screw gear.
Advance the front grip until it fully clears the screw gear.
Step 5:
Separate the screw gear halves in order to identify the location of graft cover severance.
Step 6:
Grip the graft cover manually or with hemostats and retract until the stent graft is fully deployed.
Step 7:
For the FreeFlo Stent Graft Delivery System: Deploy the tip capture mechanism per Section 12.10.
Step 8:
Remove the delivery system by gripping the screw gear and withdrawing from the patient.
Step 9:
12.14.2 Alternative Instruction for Deploying Tip Capture Mechanism
In the unlikely event of delivery system failure and non-release of the tip capture mechanism, an alternative technique
may permit the successful release of the proximal bare stent.
Step 1:
Ensure the delivery system remains stationary and continue to monitor stent graft position.
Remove the back end lock by turning counter-clockwise and pulling off of the delivery system. It may be
Step 2:
necessary to push the tip capture release handle forward to gain access to the back end lock.
Pull the tip capture release handle back as far as it can go.
Step 3:
Separate the halves of the tip capture release handle and discard.
Step 4:
Remove the clamping ring by turning clockwise and pulling off of the delivery system.
Step 5:
Separate the screw gear halves at the back end in order to identify the location of tip capture tube. The tip
Step 6:
capture tube is the brown tube from which the guidewire lumen emerges.
Grip the tip capture tube with a hemostat and apply tension while rotating the luer connector of the guidewire
Step 7:
lumen clockwise. Continue rotating the luer connector of the guidewire lumen until the proximal bare stent is fully
released from the tip capture mechanism.
Hold the delivery system with one hand on the front grip and the other hand on the slider. Pull back the trigger
Step 8:
and hold the slider stationary while bringing the front grip towards the slider as depicted in Figure 20
Gently remove the delivery system while maintaining backwards tension on the guidewire lumen to keep the
Step 9:
tapered tip seated within the graft cover. Use fluoroscopy to ensure that the stent graft does not move during the
withdrawal.
.
56
Page 57

13.0 Imaging Guidelines and Post-Operative Follow-up
13.1 General
All patients should be advised that endovascular treatment requires life-long, regular follow-up to assess their health and
the performance of their endovascular graft. Patients with specific clinical findings (e.g., endoleaks, enlarging aneurysms,
or changes in the structure or position of the endovascular graft) should receive additional follow-up. Patients should be
counseled on the importance of adhering to the follow-up schedule, both during the first year and at yearly intervals
thereafter. Patients should be informed that regular and consistent follow-up is a critical part of ensuring the ongoing
safety and effectiveness of endovascular treatment of TAAs.
Physicians should evaluate patients on an individual basis and prescribe follow-up relative to the needs and
circumstances of each individual patient. The recommended imaging schedule is presented in Table 37. This schedule
outlines the minimum requirement for patient follow-up and should be maintained even in the absence of clinical
symptoms (e.g., pain, numbness, weakness). Patients with specific clinical findings (e.g., endoleaks, enlarging
aneurysms, or changes in the structure or position of the stent graft) should receive follow-up at more frequent intervals.
Annual imaging follow-up may include chest X-ray and computed tomography angiogram (CTA). Magnetic resonance
angiogram (MRA) may be used in patients with impaired renal function or intolerance to contrast media.
• The combination of contrast and non-contrast CT imaging provides information on aneurysm diameter
change, endoleak, patency, tortuosity, progressive disease, fixation length and other morphological changes
• The chest X-rays provide information on device integrity (separation between components and stent fracture)
Table 37 lists the minimum requirements for imaging follow-up for patients with the Talent Thoracic Stent Graft.
Table 37 - Imaging Recommendations
Visit
Angiogram CTA/MRA
Pre-Treatment X (optional) X1
Treatment X
1 Month X4 X
12 Month (Annually thereafter) X4 X
1 Pre-treatment assessment should be done within 3 months prior to treatment.
2 A six month follow-up with CT Scan and Chest X-ray is recommended if an endoleak is reported at 1 month after the
procedure.
3 Magnetic resonance angiogram (MRA) may be used in patients with impaired renal function or intolerance to contrast
media
4 If a Type I or III endoleak is present, prompt intervention and additional follow-up post-intervention is recommended. See
Section 13.6
Ultimately, it is the physician’s responsibility, based on previous clinical results and the overall clinical picture, to
determine the appropriate imaging schedule for a particular patient.
Imaging Modality
2,3
Chest X-ray2
13.2 Angiographic Imaging
Angiographic images are recommended at pre-treatment (within 3 months of implant) for centers without CTA 3-D
reconstruction capabilities to assist in determining anatomic suitability. Angiographic images are also recommended
during the treatment to evaluate anatomy and device placement.
13.3 CTA/MRA Images
CTA images are recommended pre-treatment (within 3 months prior to implant) to determine anatomic suitability for the
Talent Thoracic Stent Graft. CTA with 3-D reconstruction is recommended in order to accurately assess the patient’s
anatomy.
CTA images are also recommended post-treatment for lesion and device assessment. The triphasic imaging protocol for
follow-up CT should consist of an unenhanced, contrast enhanced and 5 minute delay scan. Please refer to Table 38 for
optimal CTA results. MRA may be indicated for patients with impaired renal function.
• Film sets should include all sequential images at the lowest possible slice thickness (<3mm). Do not perform large
slice thickness (>3mm) and/or omit consecutive CT images/films sets, as this prevents precise anatomical and
device comparisons over time.
• All images should include a scale for each film/image. Images should be arranged no smaller than 20:1 images on
14 inch X 17 inch sheets if film is used.
• Both non-contrast and contrast runs are required, with matching or corresponding table positions.
57
Page 58

• Pre-contrast and contrast run slice thicknesses and intervals must match.
• DO NOT change patient orientation or re-landmark the patient between non-contrast and contrast runs.
Non-contrast and contrast enhanced baseline and follow-up imaging are important for optimal patient surveillance. It is
important to follow accepted imaging protocols during the CT exam. Table 38 lists examples of accepted imaging
protocols.
Table 38 - CTA Imaging Guidelines
Injection Volume (cc or mL) 100-150
Injection Rate (cc/sec or mL/sec)
Bolus Timing SmartPrep, Carebolus, or equivalent
Scan Range Thoracic inlet to aortic bifurcation
Scan Diameter (FOV) Large
DFOV (cm) 24 - 30
Scan Type Helical
Rotation Speed (sec) 0.8
Slice Thickness <2.5
Scan Mode HS
Table Speed (mm/rot) 15
Interval (mm) 1
kVp 120
mA 120 for non-contrast / 200 for contrast portion of study
Reconstruction (mm) 1 (normal body habitus) to 2 (> 220 lbs (99.8 kg))
13.4 X-Ray
Chest X-rays should be used to assess the presence of stent graft fracture. Posterior/anterior (PA) and lateral images are
recommended for visualization of the stent graft. Ensure the entire device is captured on images for device assessment.
3-4 via 20G IV or larger (4-5 for obese pts > 220 lbs
(99.8 kg))
13.5 MRI Information
Non-clinical testing has demonstrated that the Talent Thoracic Stent Graft is MR Conditional. It can be scanned safely in
both 1.5T & 3.0T MR systems under the following conditions:
1.5 Tesla Systems:
• Static magnetic field of 1.5 Tesla
• Spatial gradient field of 1000 Gauss/cm
• Maximum whole-body-averaged specific absorption rate (SAR) of 4 W/kg for 15 minutes of scanning.
Based on non-clinical testing, the device was determined to produce a temperature rise of less than 1
whole body averaged specific absorption rate (SAR) of 4 W/kg for 15 minutes of MR scanning in a 64MHz whole body
transmit coil, which corresponds to a static field of 1.5T. The maximum whole body averaged specific absorption rate
(SAR) was derived by calculation and verified by calorimetry.
3.0 Tesla Systems:
• Static magnetic field of 3.0 Tesla.
• Spatial gradient field of 1000 Gauss/cm.
• Maximum whole-body-averaged specific absorption rate (SAR) of 4 W/kg for 15 minutes of scanning (or the maximum
SAR allowed by the MR System, whatever is less).
Based on non-clinical testing, the device was determined to produce a temperature rise of less than 2
whole body averaged specific absorption rate (SAR) of 4 W/kg for 15 minutes of MR scanning in a 3 Tesla Siemens
TrioTIM (VB 13 Software) MR scanner. The maximum whole body averaged specific absorption rate (SAR) was derived
by calculation and verified by calorimetry.
Image Artifact (1.5 Tesla & 3 Tesla Systems):
MR image quality may be compromised if the area of interest is in the same area or relatively close to the position of the
device. Therefore, it may be necessary to optimize MR imaging parameters for the presence of this implant. The image
artifact extends approximately 5 mm and 8 mm from the device, both inside and outside the device lumen when scanned
in non-clinical testing using the sequence: spin echo and gradient echo, respectively in a 3.0T Siemens TrioTIM (VB 13
Software) MR system with a whole body coil.
Patients with Talent Thoracic Stent Grafts implanted in the thoracic aorta may safely undergo MRI for Normal Mode and
First Level Controlled Operating Mode of the MR System, as defined in IEC Standard 60601-2-33.
o
C at a maximum
o
C at a maximum
58
Page 59

13.6 Additional Surveillance and Treatment
Additional surveillance and possible treatment is recommended for:
• Aneurysms with endoleak.
• Aneurysm enlargement, > 5mm of maximum diameter (regardless of endoleak status).
• Migration.
• Inadequate seal length.
• Fracture.
Consideration for reintervention or conversion to open repair should include the attending physician's assessment of an
individual patient's co-morbidities, life expectancy, and the patient's personal choices. Patients should be counseled that
subsequent re-intervention, including the fact that catheter-based and open surgical conversion may become necessary
following an endograft procedure.
14.0
Any adverse event (clinical incident) involving the Talent Thoracic Stent Graft System should be reported to Medtronic
immediately. To report an incident, call (800) 465-5533 (in the US).
15.0
The Talent Thoracic Stent Graft System is packaged with additional specific information which includes:
Upon receipt of the device tracking form, Medtronic will mail the patient a permanent device implant card. This card
includes important information regarding the implanted stent graft. Patients should refer to this card anytime they visit
health practitioners, particularly for any diagnostic procedures (e.g. MRI). Patients should carry this card with them at all
times. If a patient does not receive their permanent device implant card, or requires changes to the card, call 1-800-551-
5544. In addition a patient information booklet (PIB) will be provided to the physicians during training and additional
copies will be available upon request. The PIB will also be available online on the Medtronic website
(www.medtronic.com). This booklet provides patients with basic information on thoracic aortic aneurysms and
endovascular repair therapy.
Device-Related Adverse Events Reporting
Patient Materials and Tracking Information
• Temporary Device Identification Card that includes both patient and stent graft information. Physicians
should complete this card and instruct the patient to keep this card in their possession at all times. The
patients should refer to this card anytime they visit additional health practitioners, particularly for any additional
diagnostic procedures (e.g. MRI). This temporary device implant card should only be discarded when the
permanent identification card is received.
• Device Tracking Form to be completed by the hospital staff and forwarded to Medtronic for the purposes of
registering the devices to identify all patients who received a Talent Thoracic Stent Graft (as required by
Federal Regulation). The hospital’s submission of the device tracking form to Medtronic is also required for a
patient to receive the permanent device implant card.
59
Page 60

16.0 Explanation of symbols that may occur on product labeling
Use by
Manufacturer
Catalogue number
Serial number
Do not reuse
MR Conditional
Contents: One Talent Thoracic Stent Graft with the Captivia Delivery System
Non-pyrogenic
Sterilized using irradiation
Do not use if package is damaged
Store at room temperature in a dark, dry place
Peel here
Do not use if indicator turns black
Caution: Federal (USA) law restricts this device for sale by or on order of a physician.
Consult instructions for use at www.medtronic.com/manuals
60
Page 61

APPENDIX A
[GRAPHICAL REPRESENTATION ONLY. MAY APPEAR DIFFERENTLY UNDER FLUOROSCOPY]
61
Page 62

APPENDIX B
[GRAPHICAL REPRESENTATION ONLY. MAY APPEAR DIFFERENTLY UNDER FLUOROSCOPY]
62
Page 63

APPENDIX C
[GRAPHICAL REPRESENTATION ONLY. MAY APPEAR DIFFERENTLY UNDER FLUOROSCOPY]
63
Page 64

MANUFACTURER:
MEDTRONIC, INC.
710 Medtronic Parkway NE
Minneapolis, MN 55432U.S.A.
Tel: (763) 514-4000
Fax: (763) 514-4879
www.medtronic.com
© 2012 Medtronic
All Rights Reserved
Protected by one or more of the following United States Patents: 5,591,195; 5,713,917; 6,306,141; 6,344,052; 6,911,039
and 7,105,016.
Rev 1C
64
 Loading...
Loading...