Page 1
9. The TA™ staplers are provided STERILE and are intended for multiple use during a single surgical procedure. Discard
A
H
2
C
5
3
H1
H2
6
4
H4
1
H3
I
2
TA™
Stapler with DST Series™ Technology
PT00167877
D
C
B
E
A
1
F
G
J
H
2
C
BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY.
IMPORTANT!
This booklet is designed to assist in using this product. It is not a reference to surgical techniques.
3
H1
4
H2
5
H3
6
H4
I
2
This device was designed, tested and manufactured for single patient use only. Reuse or reprocessing of this device may lead to its
failure and subsequent patient injury, or death. Reprocessing and/or resterilization of this device may create the risk of contamination
and patient infection. Do not reuse, reprocess or resterilize this device.
DESCRIPTION
The TA™ staplers with DST Series™ technology place a double staggered row of titanium staples and are available in 30 mm, 45 mm,
60 mm and 90 mm staple line lengths for use in various applications. The TA™ 30-V3 stapler with DST Series™ technology places a
triple staggered row of titanium staples. Three staple sizes (2.5 mm, 3.5 mm and 4.8 mm) are available to accommodate various tissue
thicknesses. Please refer to “SPECIFICATION CHART” for availability of staple sizes and instrument / cartridge lengths. Each TA™ stapler
with DST Series™ technology should only be reloaded up to 7 times for a total of 8 firings per instrument.
INDICATIONS
The TA™ staplers with DST Series™ technology have applications in abdominal, gynecological, pediatric and thoracic surgical
procedures for resection or transection of tissue.
CONTRAINDICATIONS
1. This device is not designed, sold, or intended for use except as indicated.
2. The TA™ staplers and loading units should not be used on tissues which, in the opinion of the surgeon, would not be able to
tolerate conventional suture materials or conventional closure techniques.
3. The TA™ staplers must not be used in instances where the retaining pin cannot be positioned securely in the retaining pin hole in
the anvil jaw. Failure to secure the retaining pin may result in improperly formed staples and a compromised staple line, and may
cause bleeding, leakage, or disruption.
4. Tissue thickness should be carefully evaluated before firing any stapler. Refer to “SPECIFICATION CHART” and the “tissue
compression requirement” section. “Tissue compression requirement” refers to the compression requirement for each staple size.
The TA™ 30, 45, 60 and 90 loading units should not be used on tissue that will not comfortably compress, or that compresses to
less than, the specified compression requirements. If these instructions are not followed, closure failure, tissue trauma, dehiscence,
tissue tearing, and displacement may occur, and/or hemostasis may not be obtained.
5. The TA™ staplers should not be used on friable or delicate tissue where the closure of the device might be destructive to the tissues.
WARNINGS AND PRECAUTIONS
1. Ensure that the staple reloads are compatible with the staplers.
2. As with all surgical staplers, surgeons should consider specific patient factors before deciding if the device is suitable for use. For
example, preoperative radiotherapy may result in changes to tissue. These changes may, for example, cause the tissue thickness
to exceed the indicated range for the selected staple size. Careful consideration should be given to any pre-surgical treatment the
patient may have undergone and in corresponding selection of staple size.
3. When positioning the stapler on the application site, ensure that no unintentional obstructions, such as clips, are incorporated into
the instrument jaws. Firing over an obstruction may result in improperly formed staples.
4. There is an increased risk of leak when staple lines are crossed, even if there may be clinical circumstances when a surgeon may
deem it necessary or appropriate to do so.
5. Clamping and unclamping of delicate structures may result in damage to tissue irrespective of stapler firing.
6. Do not use stapler on major vessels without making provisions for proximal and distal control.
7. Visually inspect prior to firing for inclusion of unintended anatomic structures within the staple line.
8. Avoid use of the device on the aorta.Each component in the package must be used in the manner indicated.
after use. Reprocessing and/or resterilization of this device may create the risk of contamination, patient infection,
permanent impairment or life threatening injury. Do not reuse, reprocess or resterilize this device.
10. The loading unit is provided STERILE and is intended for use in a SINGLE procedure only. DISCARD AFTER USE.
Reprocessing and/or resterilization of this device may create the risk of contamination, patient infection, permanent
impairment or life threatening injury. Do not reuse, reprocess or resterilize this device.
11. Do not modify this instrument or loading unit. Use of a modified device may result in improper instrument function.
12. Each loading unit will fit and operate properly in only the instrument(s) designed for use with the loading unit.
Attempting to use a loading unit in any instrument other than that for which it has been designed will result in stapler
malfunction.
13. Compatibility of staple line reinforcement material for use with the device has not been determined.
14. Compatibility of other manufacturer’s staples and staplers has not been determined.
15. Dispose of used instruments and used reloads in accordance with the end-user’s medical and biological waste disposal
requirements.
MRI SAFETY INFORMATION
Non-clinical testing demonstrated that a representative titanium staple is MR Conditional. A patient with these titanium
staples can be scanned safely immediately after placement of the titanium staple under the following conditions:
• Static magnetic field of 1.5T and 3.0T
• Maximum spatial gradient magnetic field of 3000-Gauss/cm (30 T/m)
• Normal Operating Mode of operation for the MRI system (whole body averaged SAR, 2-W/kg) for 15 minutes of
scanning, per pulse sequence.
Under the scan conditions defined above, the titanium staple is expected to produce a maximum temperature rise of less
than 3.2°C after 15 minutes of continuous scanning.
In non-clinical testing, the image artifact caused by the device extends approximately 2 mm from the titanium staple when
imaged with a gradient echo pulse sequence and a 3.0T MRI system.
ADVERSE REACTIONS
Possible adverse reactions and potential complications are: bleeding/hematoma/hemorrhage that may lead to death,
anastomotic leak, sepsis that may lead to death, fistula and infection which includes intraabdominal abscesses, ischemia,
chronic pain, inflammatory reaction, visceral adhesions, small bowel obstruction, stenosis, potential for staple migration
need for future re-operation, peritonitis, allergic reactions and perforation.
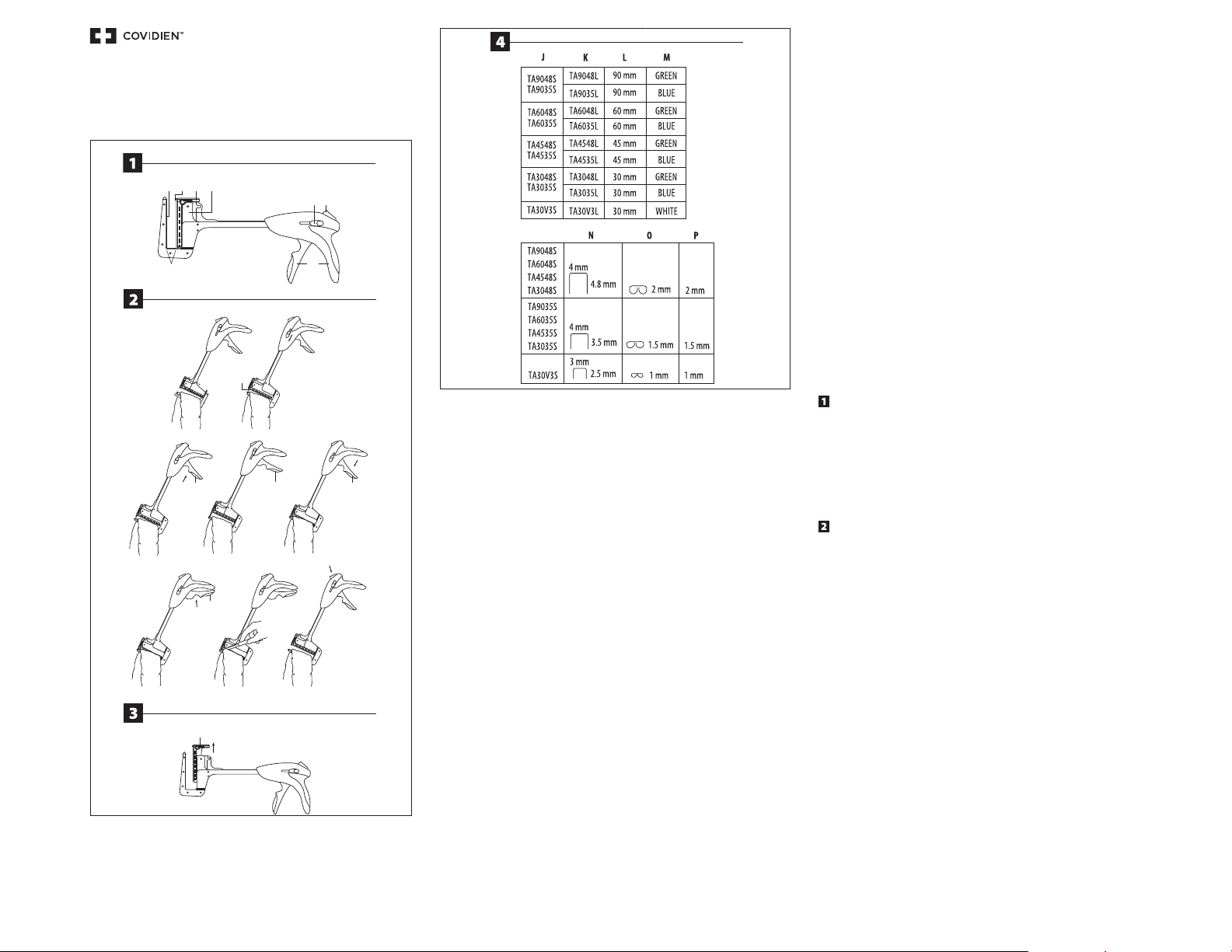
SCHEMATIC VIEW
A) JAWS
B) ANVIL
C) RETAINING PIN
D) LOADING UNIT
E) CARTRIDGE HOUSING
F) RETAINING PIN THUMB BUTTONS
G) RELEASE BUTTON
H) HANDLE
INSTRUCTIONS FOR USE
The TA™ staplers are supplied sterile with the jaws in the open position and with one loading unit in place.
1. Slip the instrument jaws around the tissue to be transected or resected.
2. The retaining pin (C) must be advanced and properly seated prior to closing, or firing, the instrument. The pin can be
manually advanced by pushing the white thumb button forward. If the retaining pin is not manually seated prior to
closing the instrument, the pin will automatically seat as the instrument jaws are closed.
CAUTION: Proper seating of the retaining pin in the anvil hole should be confirmed visually and
by feeling each side of the anvil to ensure that the retaining pin is not misplaced. Pressing the
retaining pin firmly into place will help ensure proper placement. Firing an instrument with a
misplaced retaining pin may result in improperly formed staples, which could result in leakage or
disruption of the staple line.
NOTE: If the retaining pin is manually seated and the release button is activated prior to a complete
handle squeeze, the pin will need to be manually retracted. In all other situations the pin will
retract automatically.
3. Squeeze the handle to begin approximating the tissue. There is a tactile “pre-clamp” position built into the instrument
where the handle can be released without returning to its original position to allow for final positioning of the tissue
within the jaws. After the tissue is properly positioned, continue the handle stroke until the instrument is clamped. The
handle will return to the original position.
H1) INITIAL CLOSING
H2) PRE-CLAMPED POSITION
H3) CLAMPED POSITION-READY TO FIRE
CAUTION: Do not squeeze the handle a second time unless the tissue is properly positioned - a
second squeeze will fire the staples. The black release button may be used to open the instrument
at any point during approximation.
4. To fire the instrument, squeeze the handle a second time until it reaches a solid stop and it locks in the back position.
When fully fired, the handle will remain in the locked position until the black release button is pushed and the
instrument is opened.
H4) FIRED POSITION-HANDLE LOCKED
CAUTION: If the instrument handle is partially squeezed during firing then released, bleeding may
occur as the instrument was not fully fired - the black release button may be used to open the
instrument at any point during approximation
Page 2
5. Prior to opening the fully fired instrument, the cartridge edge may be used as a cutting guide during tissue transection.
6. Press the black release button in the rear of the instrument to open the jaws and unlock the handle. The alignment pin
will automatically retract whether it was manually or automatically advanced.
NOTE: After removing the instrument, always inspect the staple line for hemostasis. Minor bleeding
may be controlled by manual sutures or electrocautery.
RELOADING
1. Ensure that the instrument jaws are in the fully open position, and the alignment pin is fully retracted before removing
the single use loading unit.
2. To remove the loading unit, grasp the finger pads (I) at the top of the loading unit and pull straight up from the jaws.
3. Prior to reloading the instrument, any tissue and/or formed but unused staples must be cleared from the anvil surface
to ensure proper staple formation in subsequent firings.
4. To reload the instrument, grasp the new, unused loading unit by the finger pads, with the staple holes facing the
instrument anvil. Insert the loading unit into the metal cartridge housing and push firmly downward until the single
use loading unit clicks into position.
CAUTION: The instrument jaws will not close if a loading unit has not been loaded into the jaws, if
the loading unit is not fully loaded into the jaws, or if a fired, or partially fired, loading unit is in the
jaw. Always ensure the proper loading unit is being used for the tissue thickness. An empty loading
unit can be identified by the presence of yellow pushers in the front of the staple slots.
5. Repeat the loading procedure as needed.
NOTE: Each TA™ stapler should only be reloaded up to 7 times for a total of 8 firings per instrument.
SPECIFICATION CHART
J) INSTRUMENT REORDER CODES
K) SULU REORDER CODES
L) STAPLE LINE LENGTH
M) COLORS
N) OPEN STAPLE SIZE
O) CLOSED STAPLE SIZE
P) TISSUE COMPRESSION REQUIREMENT
NOTE: “Tissue compression requirement” refers to the compression requirement for each staple
size. If tissue cannot comfortably compress to this requirement, or compresses to less than this
requirement, the tissue may be too thick/thin for the staple size.
Tissue
7
en: Maximum number of reloads
en: Staggered staple pattern
en: Open
en: Intended compressed tissue thickness
en: Sterilized using ethylene oxide
en: Single use
en: Caution: U.S. Federal law restricts this device to sale by or on the order of
a physician.
en: Do not resterilize
en: Do not use if package is damaged.
en: Caution, consult accompanying documents
en: MR Conditional
en: Made from 100% recycled bres. Minimum 35% post-consumer content.
en: High density polyethylene high density polyethylene
en: Package quantity
© 2011 Covidien.
Covidien llc, 15 Hampshire Street, Manseld, MA 02048 USA.
www.covidien.com
+1 800 633 8766
+1 763 514 4000
COVIDIEN, COVIDIEN with logo, and Covidien logo and Positive Results for Life are U.S. and internationally registered
trademarks of Covidien AG. Other brands are trademarks of a Covidien company, ™* brands are trademarks of their
respective owner.
2022 - 06 / 02
 Loading...
Loading...