Page 1

Resolute Integrity™ Zotarolimus-Eluting Coronary Stent System
Rapid Exchange Delivery System
INSTRUCTIONS FOR USE
Th e fo llowing l ist i ncludes trademar ks o r reg ister ed trademar ks o f Medtronic in the United States an d p ossi bly i n o ther
co untries . All o ther trademarks are the p ro perty of th eir r espec tive o wners.
End eavor, Medtronic, Resolute Integrity
CAUTION – Federal (USA) law restricts this device for sale by or on the order of a physician.
Page 2

Table of Contents
1 RESOLUTE INTEGRITY™ ZOTAROLIMUS-ELUTING CORONARY STENT SYSTEM ..................... 1
1.1 DEV ICE COMPONENT DESCRIPTION .......................................................................................... 2
1.2 DRUG COMPONENT DESCRIPTION ............................................................................................ 3
1.2.1 Zotarolimus ................................................................................................................... 3
1.2.2 Polymer System Description ............................................................................................ 3
1.2.3 Product Matrix and Zotarolimus Content ............................................................................ 4
2 INDICATIONS ......................................................................................................................... 5
3 CONTRAINDICATIONS............................................................................................................ 5
4 WARNINGS ............................................................................................................................ 5
5 PRECAUTIONS....................................................................................................................... 5
5.1 PRE- AND POST-P ROCEDURE ANTIPLATELET REGIMEN.................................................................. 6
5.1.1 Oral Antiplatelet Therapy ................................................................................................. 6
5.2 USE OF MULTIPLE STENTS ..................................................................................................... 7
5.3 USE IN CONJUNCTION WITH OTHER PROCEDURES........................................................................ 7
5.4 BRACHYTHERAPY ................................................................................................................. 7
5.5 USE IN SPECIAL POPULATIONS ................................................................................................ 7
5.5.1 Pregnancy..................................................................................................................... 7
5.5.2 Lactatio n ....................................................................................................................... 8
5.5.3 Gender ......................................................................................................................... 8
5.5.4 Ethnicity ........................................................................................................................ 8
5.5.5 Pediatric Use ................................................................................................................. 8
5.5.6 Geriatric Use ................................................................................................................. 8
5.5.7 Lesion/Vessel Characteristics........................................................................................... 8
5.6 DRUG INTERACTIONS ............................................................................................................ 9
5.7 MAGNETIC RESONA NCE IMAGING (MRI)..................................................................................... 9
5.8 STENT HANDLING PRECAUTIONS .............................................................................................. 9
5.9 STENT PLACEMENT PRECAUTIONS ......................................................................................... 10
5.10 STENT/SYSTEM REMOVAL PRECAUTIONS ................................................................................. 10
5.11 POST-P ROCEDURE ............................................................................................................. 11
6 DRUG INFORMATION ........................................................................................................... 11
6.1 MECHA NISMS OF ACTION ..................................................................................................... 11
6.2 METABOLISM..................................................................................................................... 11
6.3 PHARMACOKINETICS OF THE RESOLUTE STE N T ......................................................................... 11
6.4 PHARMACOKINETICS FOLLOWING MUL T I -DOSE INTRAVENOUS ADMINISTRATION OF ZOTAROLIMUS .......... 12
6.5 MUTAGENESIS, CARCINOGENICITY AND REPRODUCTIVE TOXICOLOGY ............................................. 13
6.5.1 Mutagenesis ................................................................................................................ 13
6.5.2 Carcinogenicity ............................................................................................................ 13
6.5.3 Reproductive Toxicology ............................................................................................... 13
6.6 PREGNANCY ..................................................................................................................... 13
6.7 LACTATION ....................................................................................................................... 14
7 OVERVIEW OF CLINICAL TRIALS.......................................................................................... 14
8 ADVERSE EVENTS ............................................................................................................... 19
8.1 OBSERVED ADVERSE EVE NTS ............................................................................................... 19
8.2 POTENTIAL ADVERSE EVENTS ............................................................................................... 21
8.2.1 Potential Adverse Events Related to Zotarolimus .............................................................. 21
8.2.2 Potential Adverse Events Related to BioLinx polymer ........................................................ 21
8.2.3 Potential Risks Associated with Percutaneous Coronary Diagnostic and Treatment Procedures21
i
Page 3

9
CLINICAL STUDIES .............................................................................................................. 22
9.1 RESULTS OF THE RESOLUTE US TRIAL ................................................................................. 22
9.2 RESULTS OF THE RESOLUTE ALL COMERS (AC) CLINICAL TRIAL ................................................. 49
9.3 RESULTS OF THE RESOLUTE INTERNATI ONAL STUDY ................................................................ 55
9.4 RESULTS OF THE RESOLUTE FIM CLINICAL TRIAL.................................................................... 57
9.5 RESULTS OF THE RESOLUTE JAPAN CLINICAL TRIAL................................................................. 62
9.6 RESULTS OF THE RESOLUTE INTEGRITY US POST MARKET STUDY........................................... 67
9.7 SUBJECTS WITH DIABETES MELLITUS IN THE RESOLUTE POOLED ANALYSIS...................................... 71
9.7.1 Subjects with Diabetes Mellitus in the RESOLUTE 38 mm Length Stent Sub-study ................ 74
9.8 SUBJECTS WITH CHRONIC TOTAL OCCLUSION ........................................................................... 75
9.9 POOLED RESULTS OF THE GLOBAL RESOLUTE CLINICAL TRIAL PROGRAM (RESOLUTE FIM,
RESOLUTE US, RES OL UTE AC, RES OL UTE INT, RES OLUTE JAPAN) .................................... 79
9.9.1 Gender Analysis from the RESOLUTE Pooled On-label Dataset.......................................... 83
9.9.2 Subset Analyses from the Resolute Pooled Dataset........................................................... 87
10 PATIENT SELECTION AND TREATMENT ............................................................................... 90
11 PATIENT COUNSELING INFORMATION ................................................................................. 90
12 HOW SUPPLIED ................................................................................................................... 90
13 DIRECTIONS FOR USE ......................................................................................................... 90
13.1 ACCESS TO PACKAGE HOL DING STE RI L E STE N T DELIVERY SYSTE M ............................................... 90
13.2 INSPECTION PRIOR TO USE................................................................................................... 91
13.3 MATERIALS REQUIRED......................................................................................................... 91
13.4 PREPARATION PRECAUTION .................................................................................................. 91
13.4.1 Guidewire Lumen Flush................................................................................................. 91
13.4.2 Delivery System Preparation .......................................................................................... 91
13.5 DELIVERY PROCEDURE........................................................................................................ 92
13.6 DEPLOYMENT PRO CEDURE ................................................................................................... 92
13.7 REMOVAL PROCEDURES ...................................................................................................... 92
13.8 IN-VITRO INFORMATION: ....................................................................................................... 93
13.9 FURTHER DILATATION OF STE NT E D SEGMENT ........................................................................... 93
14 REUSE PRECAUTION S TATEMENT ....................................................................................... 93
DISCLAIMER OF WARRANTY ........................................................................................................... 94
ii
Page 4

THE COMPONENTS OF THE RESOLUTE INTEGRITY ZOTAROLIMUS-ELUTING CORONARY STENT
SYSTEM ARE STERILE.
1 RESOLUTE INTEGRITY™ ZOTAROLIMUS-ELUTING CORONARY STENT SYSTEM
The Med tronic Resolute Integrity™ Zotarolimus-Eluting Coronary Stent System (Resolute Integrity
System) is a device/drug combination product comprised of the following device components: the
Integrity coronary stent and MicroTrac™ delivery systems and a drug component (a formulation of
zotarolimus in a polymer coating). The characteristics of the Resolute Integrity System are described in
Table 1-1.
Table 1-1: Device Component Description and Nominal Dimensions
Resolute Integrity Zotarolimus-Eluting Coronary Stent System
Component
Small Vessel Medium/Large Vessel
Available Stent Diameters (mm): 2.25, 2.5, 2.75 3.0, 3.5, 4.0
Available Stent Lengths
Unexpanded (mm):
Stent Material & Geometry:
Drug Component: A coating of polymers loaded with z otarolimus in a formulat ion applied to t he entire s urface of t he
Delivery System Working Length: 140 c m
Delivery System Luer Adapter Port s: Single access port to the inflation lumen. A guidewire exit port is loc ated approximat ely 25 cm from
Stent Delivery Balloon:
8, 12, 14, 18, 22, 26, 30 9, 12, 15, 18, 22, 26, 30, 34, 38
A cobalt-based alloy conforming to ASTM F562
and ISO 5832-6:1997 with 1.0 mm length
elements, 7.5 alternating crowns and 0.0035”
strut thickness; the s tent utiliz es a single helix
fusion pattern. The coronary stent is formed f rom
a single wire bent into a continuous sinusoid
pattern and then laser fused back ont o itself. The
stents are provided in multiple lengths and
diameters.
stent at a dose of approximat ely 1.6 μg/ mm
380 μg on the largest stent (4.0 x 38 mm).
the tip. Designed for guidewire less than or equal to 0.36 mm (0. 014 inch).
Single-layer Pebax balloon, wrapped over an inner member tubing with 2 radiopaque marker bands
to locate the stent edges.
Rapid Exchange Delivery System
A cobalt-based alloy conforming to ASTM
F562 and ISO 5832-6:1997 with 0.9 mm
length elements, 9.5 alternating crowns and
0.0035” strut thickness; utilizes a helical ujoint fusion pattern. The coronary stent is
formed from a single wire bent into a
continuous sinusoid pattern and then laser
fused back onto itself. The stents are provided
in multiple lengths and diam eters.
2
which results in a maximum nominal drug content of
Balloon Inflation Pressure: Nominal Inflation Pressure: 9 ATM (912 kPa)
Rated Burst Pressure: 16 ATM (1621 kPa) f or 2.25-3.5mm diameters
15 ATM (1520 kPa) for 4.0 mm diameter
Minimum Guide Catheter Inner
Diameter:
Catheter Shaft Outer Diamet er: Proximal OD: 2.1 F (0.69 m m, 0.027 inc h)
≥ 5 F (1.42 mm, 0.056 inch)
Distal Section OD: 2.7 F (0.91 mm, 0.036 inch)
1
Page 5

1.1 Device Component Description
The Med tronic Resolute Integrity Zotarolimus-Eluting Coronary Stent System (Resolute Integ rity
System) consists of a balloon-expandable intracoronary drug-eluting stent pre-mounted on the
MicroTrac Rapid Exchange (RX) stent delivery system. The Resolute Integrity stent is manufactured
f rom a cobalt alloy and is formed from a single wire bent into a continuous sinusoid pattern and then
laser fused back onto itself. The stents are available in multiple lengths and diameters. The d elivery
system has two radiopaque markers to aid in the placement of the stent during fluoroscopy and is
co mpatible with 0.014-inch (0.36mm) guidewires. The MicroTrac RX delivery system (Figure 1-1) has an
ef f ective length of 140 cm.
Figure 1-1: MicroTrac RX Delivery System (with Stent)
The stent is crimped on various size delivery catheter balloons, which are sized from 2.25 to 4.0 mm.
The Resolute Integrity available stent sizes are listed in Table 1-2.
Table 1-2: Resolute Integrity Stent Sizes
Diameter
(mm)
2.25 9 --- 9 9 --- 9 9 9 9 --- ---
2.5 9 --- 9 9 --- 9 9 9 9 --- ---
2.75 9 --- 9 9 --- 9 9 9 9 --- ---
3.0 ---
3.5 ---
4.0 ---
Note: “---“ indicat es sizes not offered; “9” indicates sizes offered.
8 9 12 14 15 18 22 26 30 34 38
9 9
9 9
9 9
Stent Length (mm)
9 9 9 9 9 9 9
---
9 9 9 9 9 9 9
---
9 9 9 9 9 9 9
---
2
Page 6

1.2 Drug Component Description
The drug coating of the Resolute Integrity System consists of the drug zotarolimus (the active ingredient)
and BioLinx
®
polymer system (the inactive ingredient).
1.2.1 Zotarolimus
The active pharmaceutical ingredient utilized in the Resolute Integrity System is zotarolimus. It is a
tetrazole-containing macrocyclic immunosuppressant.
The Chemical name of zotarolimus is:
[3S-[3R*[S*(1R*,3S*,4R*)],6S*,7E,9S*,10S*,12S*,14R*,15E,17E,19E,21R*, 23R*, 26S*,27S*,34aR*]]9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-9,27-dihydroxy-3-[2-[3-methoxy-4(1H-tetrazol-1-yl)cyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy3H-pyrid o[2,1-c] [1,4] oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone.
The chemical structure of zotarolimus is shown in Figure 1-2:
NN
N
N
MeO
N
O
Figure 1-2: Zotarolimus Chemical Structure
Zotarolimus has extremely low water solubility and is a lipophilic compound that is freely soluble in
Propylene glycol, Acetone, Toluene, Acetonitrile, Ethanol, Benzyl alcohol and DMSO. The molecular
formula of zotarolimus is C
52H79N5O12
and its molecular weight is 966.2.
Zotarolimus does not have any ionizable group(s) in the physiological pH range; therefore, its solubility
is exp ected to b e unaltered in this range.
1.2.2 Polymer System Description
The Resolute Integrity stent is comprised of a bare metal stent with a Parylene C primer coat and a
co ating that consists of a blend of the drug zotarolimus and the BioLinx polymer system. BioLinx is a
blend of the Medtronic proprietary components C10 and C19, and PVP (polyvinyl pyrrolidone). The
structural formula of the BioLinx polymer subunits are shown in Figure 1-3:
OO OH
O
O
MeO
OHO
OMe
O
C10 Polymer C19 Polymer PVP Polymer
Figure 1-3: Chemical Structure of the BioLinx Polymer Subunits
3
Page 7

(μg)
1.2.3 Product Matrix and Zotarolimus Content
Table 1-3: Resolute Integrity Zotarolimus-Eluting Coronary Stent System
Product Matrix and Nominal Zotarolimus Doses
Product Number
RX
RSINT22508UX 2.25 8 59
RSINT25008UX 2.5 8 59
RSINT27508UX 2.75 8 59
RSINT30009UX 3.0 9 90
RSINT35009UX 3.5 9 90
RSINT40009UX 4.0 9 90
RSINT22512UX 2.25 12 85
RSINT25012UX 2.5 12 85
RSINT27512UX 2.75 12 85
RSINT30012UX 3.0 12 120
RSINT35012UX 3.5 12 120
RSINT40012UX 4.0 12 120
RSINT22514UX 2.25 14 102
RSINT25014UX 2.5 14 102
RSINT27514UX 2.75 14 102
RSINT30015UX 3.0 15 150
RSINT35015UX 3.5 15 150
RSINT40015UX 4.0 15 150
RSINT22518UX 2.25 18 128
RSINT25018UX 2.5 18 128
RSINT27518UX 2.75 18 128
RSINT30018UX 3.0 18 180
RSINT35018UX 3.5 18 180
RSINT40018UX 4.0 18 180
RSINT22522UX 2.25 22 153
RSINT25022UX 2.5 22 153
RSINT27522UX 2.75 22 153
RSINT30022UX 3.0 22 220
RSINT35022UX 3.5 22 220
RSINT40022UX 4.0 22 220
RSINT22526UX 2.25 26 188
RSINT25026UX 2.5 26 188
RSINT27526UX 2.75 26 188
RSINT30026UX 3.0 26 260
RSINT35026UX 3.5 26 260
RSINT40026UX 4.0 26 260
RSINT22530UX 2.25 30 213
RSINT25030UX 2.5 30 213
RSINT27530UX 2.75 30 213
Nominal Expanded
Stent ID (mm)
Nominal Unexpanded
Stent Length (mm)
Nominal Zotarolimus
Content
4
Page 8

(μg)
Table 1-3: Resolute Integrity Zotarolimus-Eluting Coronary Stent System
Product Matrix and Nominal Zotarolimus Doses
Product Number
RX
RSINT30030UX 3.0 30 300
RSINT35030UX 3.5 30 300
RSINT40030UX 4.0 30 300
RSINT30034UX 3.0 34 340
RSINT35034UX 3.5 34 340
RSINT40034UX 4.0 34 340
RSINT30038UX 3.0 38 380
RSINT35038UX 3.5 38 380
RSINT40038UX 4.0 38 380
2 INDICATIONS
The Resolute Integrity Zotarolimus-Eluting Coronary Stent System is indicated for improving coronary
luminal diameters in p atients, including those with diabetes mellitus, with symptomatic ischemic heart
disease d ue to de novo lesions of length 35 mm in native coronary arteries with reference vessel
diameters of 2.25 mm to 4.2 mm. In addition, the Resolute Integrity Zotarolimus-Eluting Coronary Stent
System is indicated for treatment of de novo chronic total occlusions.
3 CONTRAINDICATI ONS
The Resolute Integrity System is contraindicated for use in:
• Patients with known hypersensitivity or allergies to aspirin, heparin, bivalirudin, clopidogrel,
prasugrel, ticagrelor, ticlopidine, drugs such as zotarolimus, tacrolimus, sirolimus, everolimus, or
similar drugs or any other analogue or derivative.
• Patients with a known hypersensitivity to the cobalt-based alloy (cobalt, nickel, chromium, and
molybdenum).
• Patients with a known hypersensitivity to the BioLinx polymer or its individual components (see
details in Section 1.2.2 – Polymer System Description).
Coronary artery stenting is contraindicated for use in:
• Patients in who m anti-platelet and/or anticoagulation therapy is contraindicated.
• Patients who are judged to have a lesion that prevents complete inflation of an angioplasty balloon
or prop er placement of the stent or stent delivery system.
Nominal Expanded
Stent ID (mm)
Nominal Unexpanded
Stent Length (mm)
Nominal Zotarolimus
Content
4 WARNINGS
• Please ensure that the inner package has not been opened or d amaged as this would indicate the
sterile barrier has b een breached.
• The use o f this product carries the same risks associated with coronary artery stent implantation
procedures which include subacute and late vessel thrombosis, vascular complications, and/or
bleeding events.
• This product should not be used in p atients who are no t likely to comply with the recommended
antiplatelet therapy.
5 PRECAUTIONS
• Only physicians who have received adequate training should perform implantation of the stent.
• Subsequent stent restenosis or occlusion may require repeat catheter-based treatments (including
ballo on d ilatation) of the arterial segment containing the stent. The long-term outcome following
repeat catheter-based treatments of previously implanted stents is not well characterized.
• The risks and benefits of stent implantation should be assessed for patients with a history of severe
reaction to contrast agents.
• Do not expose or wipe the product with organic solvents such as alcohol.
• When d rug eluting stents (DES) are used outside the specified Indications for Use, patient
outcomes may differ from the results observed in the RESOLUTE pivotal clinical trials.
5
Page 9

• Compared to use within the specified Indications for Use, the use of DES in patients and lesions
outside of the labeled indications, including more tortuous anatomy, may have an increased risk of
adverse events, including stent thrombosis, stent embolization, MI, or death.
• Care should be taken to control the position of the guide catheter tip during stent delivery,
deployment, and balloon withdrawal. Before withdrawing the stent delivery system, visually confirm
co mplete balloon deflation by fluoroscopy to avoid guiding catheter movement into the vessel and
sub sequent arterial damage.
• Stent thrombosis is a low-frequency event that is frequently associated with myocardial infarction
(MI) o r d eath. Data from the RESOLUTE clinical trials have been p rospectively evaluated and
adjudicated using the d efinition developed by the Academic Research Consortium (ARC) (see
Section 9.9 Pooled Results of the Global RESOLUTE Clinical Trial Program (RESOLUTE FIM,
RESOLUTE US, RESOLUTE AC, RESOLUTE International, RESOLUTE Japan) for more
inf ormation).
5.1 Pre- and Post-Procedure Antiplatelet Regimen
In the Medtronic RESOLUTE US Clinical Trial, RESOLUTE AC Clinical Trial, RESOLUTE International
Study, RESOLUTE First-In-Man (FIM) Clinical Trial and RESOLUTE Japan Clinical Trial, the protocol
sp ecified administration of clopidogrel or ticlopidine prior to the procedure and for a period of at least 6
months post-procedure and up to 12 months in patients who were not at high risk of bleeding. Aspirin
was administered prior to the procedure concomitantly with clopidogrel or ticlopidine and then continued
indef initely to reduce the risk of thrombosis. In the Medtronic RESOLUTE US Primary Enrollment
Group, 95.9%, 93.8% and 46.6% of the patients remained on dual antiplatelet therapy at 6 months, 12
months and 60 months, respectively. In the RESOLUTE AC Clinical Trial, 93.1%, 84.2% and 11.0% of
the p atients remained on dual antiplatelet therapy at 6 months, 12 months and 60 months, respectively.
In the RESOLUTE International Study, 95.9%, 91.1% and 34.6% of the patients remained on dual
antiplatelet therapy at 6 months, 12 months and 36 months, respectively. In the RESOLUTE FIM
Clinical Trial, 79.1%, 58.1% and 39.4% of the patients remained on dual antiplatelet therapy at 6
months, 12 mo nths and 60 months,
respectively. In the RESOLUTE Japan Clinical Trial, 99.0%, 94.9%
and 62.5% of the patients remained on dual antiplatelet therapy at 6 months, 12 months and 60 months,
resp ectively. In the RESOLUTE 38 mm Length Group, 92.8%, 91.4% and 61.5% of the patients
remained o n d ual antiplatelet therapy at 6 months, 12 months and 60 months, respectively. See
Section
9 - CLINICAL STUDIES for more information.
5.1.1 Oral Antiplatelet Therapy
Dual antiplatelet therapy (DAPT) using a combination treatment of aspirin with a P2YP12 platelet inhibitor
af ter percutaneous coronary intervention (PCI), reduces the risk of stent thrombosis and ischemic cardiac
events, b ut may increase the risk of bleeding complications. The o ptimal duration of DAPT, specifically a
P2Y12 platelet inhibitor in addition to aspirin, following DES implantation is unknown, and DES
thrombosis may still occur despite continued therapy. It is very important that the patient is compliant with
the p ost-procedural antiplatelet recommendations.
1
Per 2016 ACC/AHA guidelines,
a daily aspirin dose of 81 mg is recommended indefinitely after PCI. A
P2Y12 platelet inhibitor should be given daily for at least 6 months in stable ischemic heart disease
patients and for at least 12 months in patients with acute coronary syndrome (ACS).
In p ivo tal trials of current generation DES, subjects were prescribed DAPT f or at least 6 months postprocedure, and most patients who were not at high risk of bleeding used DAPT for at least 12 months.
Consistent with the 2016 ACC/AHA guidelines,
1
and the DAPT Study,2 longer duration of DAPT may be
co nsidered in patients who have tolerated DAPT without a bleeding complication and who are not at
high bleed ing risk. In patients who are at a hig h risk of bleeding or who develop significant bleeding
during DAPT treatment, these guidelines suggest that a shorter DAPT duration may be reasonable.
1
Levine GN, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary
Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice
Guidelines. J Am Coll Cardiol. 2016; doi:10.1016/j.jacc.2016.03.513. F or full text, please refer to the following website:
http://content.onlinejacc.org/article.aspx?doi=10.1016/j.jacc.2016.03.513
2
Mauri L, et al. Twelve or 30 Months of Dual Antiplatelet Therapy After Drug-Eluting Stents. N Engl J Med. 2014;371:2155–66.
6
Page 10

However, definitive evidence supporting the safety of short DAPT duration has not been established in
prospective clinical studies.
Decisions about duration of DAPT are best made on an individual basis and should integrate clinical
judgment, assessment of ischemic and bleeding risks, and patient preference.
Premature discontinuation or interruption of prescribed antiplatelet medication could result in an
increased risk of stent thrombosis, MI or death.
Prio r to PCI, if premature discontinuation of antiplatelet therapy is anticipated, physicians should
caref ully evaluate with the patient whether a DES and its associated recommended DAPT regimen is
the ap propriate PCI choice.
Following PCI, if elective non-cardiac surgery requiring suspension of antiplatelet therapy is considered,
the risks and benefits of the procedure should be weighed against the possible risk associated with
interruptio n of antiplatelet therapy.
Patients who require premature DAPT discontinuation should be carefully monitored for cardiac events.
At the d iscretion of the patient’s treating physician(s), the antiplatelet therapy should be restarted as
so o n as possible.
5.2 Use of Multiple Stents
The lo ng-term effects of zotarolimus are currently unknown. The extent of the patient’s exposure to
zotarolimus drug and the stent and polymer coating is directly related to the number of stents and total
stent length implanted.
When multip le stents are required, stent materials should be of similar composition. Placing multiple
stents of different materials in contact with each other may increase potential for corrosion. To avoid the
possibility of d issimilar metal corrosion, do not implant stents of different materials in tandem where
overlap or contact is possible.
Potential interactions of the Resolute Integrity stent with other drug-eluting or coated stents have not
been evaluated and should be avoided whenever possible.
When using two wires, care should be taken when introducing, torquing and removing one o r both
guidewires to avoid entanglement. In this situation, it is recommended that one guidewire be completely
withd rawn f rom the patient before removing any additional equipment.
5.3 Use in Conjunction with Other Procedures
The safety and effectiveness of using mechanical atherectomy devices (directional atherectomy
catheters, rotational atherectomy catheters) or laser angioplasty catheters in conjunction with Resolute
Integrity stent implantation have not been established.
5.4 Brachytherapy
The safety and effectiveness of the Resolute Integrity stent in target lesions treated with prior
brachytherapy, or the use of brachytherapy to treat in-stent restenosis of a Resolute Integrity stent, have
not been established.
5.5 Use in Special Populations
Information on use of the Resolute Integrity stent in certain special patient populations is derived from
clinical studies of the Resolute stent system, which uses the same drug (zotarolimus) – see Section 7 -
OVERVIEW OF CLINICAL TRIALS for a description of the other features of the Resolute stent system
co mpared to the Resolute Integrity stent system.
5.5.1 Pregnancy
Pregnancy Category C. See Section 6.6 Pregnancy und er Drug Information. There are no well-
co ntrolled studies in p regnant women or men intending to father children. The Resolute Integrity stent
sho uld be used during pregnancy only if the potential benefit outweighs the potential risk to the embryo
or fetus. Effective contraception should be initiated before implanting a Resolute Integrity stent and for 1
year af ter implantation.
7
Page 11

5.5.2 Lactation
It is not known whether zotarolimus is excreted in human milk. The pharmacokinetic and safety profiles
of zotarolimus in infants are not known. Because many drugs are excreted in human milk and because
of the potential for adverse reactions in nursing infants from zotarolimus, a decision should be made
whether to discontinue nursing or to implant a Resolute Integrity stent, taking into account the
imp o rtance of the stent to the mother. See Section 6.7 – Lactation under Drug Information.
5.5.3 Gender
Clinical studies of the Resolute stent did not suggest any significant differences in safety and
ef f ectiveness for male and female patients. See Section 9.9.1 – Gender Analysis from the
RESOLUTE Pooled On-label Dataset.
5.5.4 Ethnicity
Clinical studies of the Resolute stent did not include sufficient numbers of patients to assess for
dif ferences in safety and effectiveness due to ethnicity.
5.5.5 Pediatric Use
The safety and effectiveness of the Resolute Integrity stent in patients below the age of 18 years have
not been established.
5.5.6 Geriatric Use
Clinical studies of the Resolute stent did not have an up per age limit. Among the 1242 p atients treated
with the Resolute stent in the Resolute US Main Study that included 2.25-3.5 mm stents, 617 patients
were ag e 65 o r older and 88 patients were age 80 or older. A post hoc analysis of patients treated with
the Resolute stent showed no significant differences between subjects under age 65 vs. age 65 and
older in the rates of cardiac death, target vessel MI, target lesion revascularization, ARC definite or
probable stent thrombosis, or target lesion failure at 12 months. The rate of all-cause death at 12
months was 0.3% in patients under age 65 vs. 1.8% in patients age 65 or older.
5.5.7 Lesion/Vessel Characteristics
The safety and effectiveness of the Resolute Integrity stent have not been established in the cerebral,
caro tid, or peripheral vasculature or in the f ollowing coronary disease patient populations:
• Patients with coronary artery reference vessel diameters <2.25 mm or >4.2 mm.
• Patients with coronary artery lesions longer than 35 mm or requiring more than one Resolute
Integrity stent.
• Patients with evidence of an acute MI within 72 hours of intended stent implantation.
• Patients with vessel thrombus at the lesion site.
• Patients with lesions located in a saphenous vein graft, in the left main coronary artery, ostial
lesions, or bifurcation lesions.
• Patients with diffuse disease or poor flow distal to identified lesions.
• Patients with tortuous vessels in the region of the target vessel or proximal to the lesion.
• Patients with in-stent restenosis.
• Patients with moderate or severe lesion calcification at the target lesion.
• Patients with 3 vessel disease.
• Patients with a left ventricular ejection fraction of <30%.
• Patients with a serum creatinine of >2.5 mg/dl.
• Patients with longer than 24 months of follow-up.
8
Page 12

5.6 Drug Interactions
The ef fect of potential drug interactions on the safety or effectiveness of the Resolute Integrity stent has
not been investigated. While no specific clinical data are available, drugs, like sirolimus, that act through
the same b inding protein (FKBP12) may interfere with the efficacy of zotarolimus. Zotarolimus is
metabolized by CYP3A4, a human cytochrome P450 enzyme. When administered concomitantly with
200 mg keto conazole bid, a strong inhibitor of CYP3A4, zotarolimus produces less than a 2-fold
increase in AUC
with no ef fect on C
0-i nf
drug interactions when deciding to place a Resolute Integ rity stent in a patient who is taking d rugs that
are known substrates or inhibitors of the cytochrome P450 isoenzyme CYP3A4. Systemic exposure of
zotarolimus should also be taken into consideration if the patient is treated concomitantly with systemic
immunosuppressive therapy.
Formal drug interaction studies have not been conducted with the Resolute Integrity stent.
5.7 Magnetic Resonance Imaging (MRI)
Non-clinical testing has demonstrated the Resolute Integrity stent up to a total length of 120 mm is MR
Conditional. It can be scanned safely under the following conditions:
• Static magnetic field of 1.5 and 3 Tesla.
• Spatial gradient field of 1000 G/cm or less
• Maximum whole b ody averaged specific absorption rate (SAR) of 2.0 W/kg or less under normal
operating mode only, for 15 minutes of scanning.
1.5 T
Based on non-clinical testing and modeling, a 38 mm Resolute Integrity stent was calculated to produce
an in-vivo temperature rise of less than 2.35°C, and overlapped stents with a maximum length of 120
mm were calculated to pro duce an in-vivo temperature rise of less than 3.87°C at a maximum whole
body averaged specific absorption rate (SAR) of 2.0 W/kg for 15 minutes of MR scanning per sequence
in a 64 MHz whole body transmit coil, which corresponds to a static field of 1.5 Tesla. These
calculations do not take into consideration the cooling effects of perfusion and b lood flow. The maximum
whole body averaged specific absorption rate (SAR) was derived by calculation.
. Therefore, consideration should be given to the potential for
max
3 T
Based on non-clinical testing and modeling, a 38 mm Resolute Integrity stent was calculated to produce
an in-vivo temperature rise of less than 3.29°C, and overlapped stents with a maximum length of 120
mm were calculated to pro duce an in-vivo temperature rise of less than 3.95°C at a maximum whole
body averaged specific absorption rate (SAR) of 2.0 W/kg for 15 minutes of MR scanning per sequence
in a 3 T GE SIGNA HDx with software version 14\LX\MR release 14.0.M5A.0828.b. These calculations
do not take into consideration the cooling effects of perfusion and blood flow. The maximum whole body
averag ed specific absorption rate (SAR) was derived by calculation.
1.5 T and 3 T
The Resolute Integrity stent should not move or migrate when exposed to MR scanning immediately
post-implantation. MRI at 3 Tesla and 1.5 Tesla may be performed immediately following the
imp lantation of the stent. Non-clinical testing at field strength greater than 3 Tesla has not been
performed to evaluate stent migration and heating. MR image quality may be compromised if the area of
interest is in the same area or relatively close to the position of the device. Theref ore, it may be
necessary to optimize MR imaging parameters for the presence of the stent. The image artifact extends
approximately 1 cm from the device, both inside and outside the device lumen when scanned in nonclinical testing using the spin echo and gradient echo sequences specified in ASTM F2119-01; the
device lumen was always observed during scanning. This testing was completed using a GE SIGNA
HDx with software version 14\LX\MR release 14.0.M5A.0828.b.
5.8 Stent Handling Precautions
• For single use only. The Resolute Integrity System is provided sterile. Do not resterilize or reuse
this p roduct. Note the “Use By” date on the product label. Do not use if package or product has
been opened or damaged.
• Only the contents of the pouch should be considered sterile. The outside surface of the pouch is not
sterile.
• Do not remove the contents of the pouch until the device will be used immediately.
9
Page 13

• Do not remove the stent from the delivery balloon; removal may damage the stent and polymer
co ating and /or lead to stent embolization. The Resolute Integrity System is intended to perform as a
system. The stent is not designed to be crimped onto another delivery device.
• Special care must be taken not to handle or in any way disrupt the stent on the balloon. This is most
imp o rtant while removing the catheter from the packaging, placing it over the g uidewire, and
advancing it through the rotating hemostatic valve and guide catheter hub.
• Do not try to straighten a kinked shaft or hypotube. Straightening a kinked metal shaft may result in
breakage of the shaft.
• Stent manip ulation (e.g., rolling the mounted stent with your fingers) may cause coating damage,
co ntamination or dislodgement of the stent from the delivery system balloon.
• The Resolute Integ rity System must not be exposed to any direct handling or contact with liquids
prior to preparation and delivery as the coating may be susceptible to d amage or premature drug
elution.
• Use only the appropriate balloon inflation media. Do not use air or any gaseous medium to inflate
the b alloon as this may cause uneven expansion and difficulty in deployment of the stent.
• The Resolute Integ rity stent delivery system should not be used in conjunction with any other stents
or for post-dilatation.
5.9 Stent Placement Precautions
• The vessel must be pre-dilated with an appropriately sized balloon. Refer to the pre-dilatation
ballo on sizing described in Section 13.5 – Delivery Procedure. Failure to do so may increase the
risk o f placement difficulty and procedural complications.
• Do not prepare or pre-inflate the balloon prior to stent deployment other than as directed. Use the
ballo on p urging technique described in Section 13 – DIRECTIONS FOR USE.
• Guide catheters used must have lumen sizes that are suitable to accommodate the stent delivery
system (see Device Component Description in Table 1-1).
• Af ter preparation of the stent delivery system, do not induce negative pressure on the delivery
catheter prior to placement of the stent across the lesion. This may cause premature dislodgment of
the stent f rom the balloon or delivery difficulties.
• Balloon pressures should be monitored during inflation. Do not exceed rated b urst pressure as
indicated on the product label. Use of pressures higher than those specified on the product label
may result in a ruptured balloon with possible intimal damage and dissection.
• In small or diffusely diseased vessels, the use of high balloon inflation pressures may over-expand
the vessel distal to the stent and could result in vessel dissection.
• Imp lanting a stent may lead to a dissection of the vessel distal and/or proximal to the stented portion
and may cause acute closure of the vessel requiring additional intervention (e.g., CABG, further
dilatation, placement of additional stents, or other intervention).
• Do not expand the stent if it is not properly positioned in the vessel (see Section 5.10 -
PRECAUTIONS–Stent/System Removal Precautions).
• Placement of the stent has the potential to compromise side branch patency.
• Do not attempt to pull an unexpanded stent back through the guide catheter, as dislodgement of the
stent from the balloon may occur. Remove as a single unit per instructions in Section 5.10 PRECAUTIONS –Stent/System Removal Precautions.
• Under-expansion of the stent may result in stent movement. Care must be taken to properly size
the stent to ensure that the stent is in full contact with the arterial wall upon deflation of the balloon.
• Stent retrieval methods (e.g., use of additional wires, snares and/or forceps) may result in additional
trauma to the coronary vasculature and/or the vascular access site. Complications may include
bleeding, hematoma, or pseudoaneurysm.
• Ensure full coverage of the entire lesion/dissection site so that there are no g aps between stents.
• Administration of appropriate anticoagulant, antiplatelet and coronary vasodilator therapy is critical
to successful stent implantation.
5.10 Stent/System Removal Precautions
If removal of a stent system is required prior to deployment, ensure that the guide catheter is coaxially
positioned relative to the stent delivery system and cautiously withdraw the stent delivery system into
the g uide catheter. Should unusual resistance be felt at any time when withdrawing the stent towards
10
Page 14

the g uide catheter, the stent delivery system and the guide catheter should be removed as a single unit.
This must be done under direct visualization with fluoroscopy.
When removing the stent delivery system and guide catheter as a single unit:
• Do not retract the stent delivery system into the guide catheter. Maintain guidewire placement across
the lesion and caref ully pull back the stent delivery system until the proximal balloon marker of the
stent delivery system is aligned with the distal tip of the guide catheter.
• The system should be pulled back into the descending aorta toward the arterial sheath. As the distal
end of the guide catheter enters into the arterial sheath, the catheter will straighten, allowing safe
withd rawal of the stent delivery system into the guide catheter and the subsequent removal of the
stent delivery system and the guide catheter from the arterial sheath.
Failure to f ollow these steps and/or applying excessive force to the stent delivery system can potentially
result in loss or damage to the stent and/or stent delivery system components such as the balloon.
5.11 Post-Procedure
• Care must be exercised when crossing a newly d eployed stent with an intravascular ultrasound
(IVUS) catheter, an optical coherence tomography (OCT) catheter, a coronary guidewire or a
ballo on catheter to avoid disrupting the stent placement, apposition, geometry, and/or coating.
• Post-dilatation: All efforts should be made to assure that the stent is not under dilated. If the
deployed stent is not fully apposed to the vessel wall, the stent may be expanded further with a
larger diameter balloon that is slightly shorter (about 2 mm) than the stent. The p ost-dilatation can
be done using a low-profile, high pressure, non-compliant balloon catheter. The b alloon should not
extend outside of the stented region. Do not use the stent delivery balloon for post-dilatation.
• If patient requires MR imaging, refer to Section 5.7 – Magnetic Resonance Imaging (MRI) above.
• Antiplatelet therapy should be administered post-procedure (see Precautions – Section 5.1 - Pre-
and Post-Procedure Antiplatelet Regimen). Patients who require early discontinuation of
antiplatelet therapy (e.g., secondary to active bleeding), should be monitored carefully for cardiac
events. At the discretion of the patient's treating physician, antiplatelet therapy should be restarted
as soon as possible.
6 DRUG INFORMATION
6.1 Mechanisms of Action
The suggested mechanism of action of zotarolimus is to bind to FKBP12, leading to the formation of a
trimeric complex with the protein kinase mTOR (mammalian target of rapamycin), inhibiting its activity.
Inhibition of mTOR results in the inhibition of protein phosphorylation events associated with translation
of mRNA and cell cycle control.
6.2 Metabolism
Zotarolimus undergoes oxidative metabolism in the liver to form the demethyl and hydroxylated
metabolites of the parent drug. Further metabolism can lead to the formation of hydroxyl-demethyl and
dihydroxyl-demethyl metabolites. Enzymes of the CYP3A family are the major catalysts of oxidative
metabolism of zotarolimus. Zotarolimus is a competitive inhibitor of CYP3A-dependent activities;
however, the IC
values (3 μM and ab o ve) are many fold higher than the systemic concentrations
50
exp ected following implantation of a drug -eluting stent. The anticipated zotarolimus blood levels in
stented patients are expected to be less than 0.004 μM, suggesting that clinically significant drug-drug
interactions are unlikely.
6.3 Pharmacokinetics of the Resolute Stent
The pharmacokinetics information for the Resolute Integrity stent system is derived from a study
co nd ucted on the Resolute stent system. The Reso lute Integrity stent system is similar to the Resolute
stent system with regards to the stent design, the stent coating technology (dosing and drug to polymer
ratio), and delivery system design and materials. Given these similarities and supportive bench and
animal study information, the pharmacokinetics information from the RESOLUTE FIM PK Sub-study, as
described b elow, is applicable to the Resolute Integrity stent system.
11
Page 15

The pharmacokinetics (PK) of zotarolimus delivered from the Resolute stent has been determined in
patients with coronary artery disease after stent implantation in the Medtronic RESOLUTE FIM Clinical
Trial. The d o se of zotarolimus was calculated per stent unit surface area and the key pharmacokinetic
parameters determined from these patients are provided in Table 6-1.
Table 6-1: Zotarolimus Pharmacokinetics in the Medtronic RESOLUTE FIM Clinical Trial PK
Sub-study Patients after Implantation of Resolute Zotarolimus-Eluting Coronary Stents
PK
Parameter
Units
Group I
(128 μg)
N = 1†
Group IIa
(180 μg)
N = 11
Group IIIa
(240 μg)
N = 7
Group IVa
Cmax (ng/mL) 0. 129 0.210 ± 0.062 0.300 ± 0.075 0.346 ± 0.133
Tmax (h) 1.00 0.9 ± 0.7 0.9 ± 0.5 0.8 ± 0.5
AUC0-last (ng•h/mL) 15.08 16.04 ± 4.74 35. 89 ± 12.79 31. 19 ± 17.69
AUC0-inf$ (ng•h/mL) 41.89 39.09 ± 11.77 52.41 ± 12.57 80.12 ± 51.00
ȕ$ (1/h) 0.003 0.004 ± 0.001 0.004 ± 0.001 0.003 ± 0.002
‡,#
t½
(h) 263.4 195.5 ± 74.4 167.4 ± 29.7 208.3 ± 144.4
CL/F$ (L/h) 3.06 5.23 ± 2.55 4.80 ± 1.11 5.14 ± 3.55
Vdȕ/F$ (L) 1161.2 1449.3 ± 221.6 1181.2 ± 336.4 1658.6 ± 494.8
Notes
C
Maximum observed blood concentration a Primary dose groups
max
T
Time to C
max
AUC
0-last
AUC
0-inf
t½ Harmonic mean half-life
CL/F Mean apparent clearance
Vdȕ/F Apparent volume of distribution $ Not a true sample
Area under the blood concentration-time curve
(AUC) from time 0 to time of last measurable
concentration
AUC from time 0 to infinity (AUC
† No SD was reported when N = 1
max
‡ Harmonic mean ± pseudo-standard deviation
). #
0-inf
Not a true estimate of the elimination half-life as the drug
release from the stent was not complete during the
course of the pharmacokinetic sampling
The results in Table 6-1 show that the p harmacokinetics of zotarolimus were linear in the primary doseproportionality evaluation (including dose groups with N > 1), 180, 240 and 300 μg, following the
implantation of the Resolute stents as illustrated by dose proportional increases in maximum blood
co ncentration (C
measurable concentration (AUC
clearance (CL/F) and harmonic mean half-life (t
), area under the blood concentration-time curve (AUC) from time 0 to time of last
max
) and AUC from time 0 to infinity(AUC
0-l ast
) for the primary dose groups ranged from 4.80 to 5.23
1/2
). The mean apparent
0-i nf
L/h and 167.4 to 208.3 h, respectively. The mean time to reach peak systemic concentration (T
ranged from 0.8 to 0.9 h after stent implantation.
The data demonstrate dose proportionality and linearity similar to that seen with increasing zotarolimus
doses from the Endeavor stent and intravenous administration. Based on available zotarolimus
pharmacokinetic data, systemic safety margins of 78-fold have been established for the Resolute stent
at 380 μg due to the extended elution of zotarolimus from the BioLinx polymer.
(300 μg)
N = 3
max
)
6.4 Pharmacokinetics following Multi-dose Intravenous Administration of Zotarolimus
Zotarolimus pharmacokinetic activity has been determined following intravenous administration in
healthy subjects. Table 6-2 provides a summary of the pharmacokinetic analysis.
12
Page 16

g
Table 6-2: Pharmacokinetic Parameters (Mean ± Standard Deviation) in Patients Following Multi-
dose Intravenous Administration of Zotarolimus
200 μg QD
PK
Parameters
Cmax (ng/mL) 11.41± 1.38¥ 11.93 ± 1.25 21.99 ± 3.79 23.31± 3.15 37.72 ± 7.00 41.79 ± 6.68
Tmax (h) 1.05 ± 0.04¥ 1.03 ± 0.04 1.00 ± 0.14 1.05 ± 0.04 1.03 ± 0.04 1.03 ± 0.05
AUC0-24
t1/2$ (h) 32.9 ± 6.8 37.6 ± 4.5 36.0 ± 4.7
CLb (L/h)
Notes
¥
N = 16;
$ Harmonic mean ± pseudo-standard deviation
b
Clearance data is calculated using compartmental methods.
All other data presented in Table 6-2 is calculated usin
Units
(ng•h/mL)
34.19 ± 4.39¥ 47.70 ± 6.68 68.43 ± 15.41 100.47 ± 18.02 123.48 ± 13.34 174.43 ± 19.88
4.2 ± 0.6 4.2 ± 0.6 4.0 ± 0.9 4.0 ± 0.9 4.6 ± 0.4 4.6 ± 0.4
N = 15
Day 1 Day 14 Day 1 Day 14 Day 1 Day 14
non-compartmental methods.
400 μg QD
N= 16
When administered intravenously for 14 consecutive days, zotarolimus showed dose proportionality.
Renal excretion is not a major route of elimination for zotarolimus as approximately 0.1% of the dose
was excreted as unchanged drug in the urine per day. In multiple doses of 200, 400 and 800 μg,
zotarolimus was generally well tolerated by the subjects. No clinically significant abnormalities in
physical examinations, vital signs or laboratory measurements were observed during the study.
6.5 Mutagenesis, Carcinogenicity and Reproductive Toxicology
6.5.1 Mutagenesis
Zotarolimus was not genotoxic in the in vitro bacterial reverse mutation assay, the human peripheral
lymphocyte chromosomal aberration assay, or the in vivo mouse micronucleus assay.
800 μg QD
N=16
6.5.2 Carcinogenicity
No long-term studies in animals have been performed to evaluate the carcinogenic potential of
zotarolimus. The carcinogenic potential of the Resolute stent is expected to be minimal based on the
types and quantities of materials present.
6.5.3 Reproductive Toxicology
No effect on fertility or early embryonic development in f emale rats was observed following the IV
administration of zotarolimus at dosages up to 100 μg/kg/day (approximately 19 times the cumulative
blood exposure provided by Resolute stents coated with 300 μg zotarolimus).
For male rats, there was no effect on the fertility rate at IV dosages up to 30 μg/kg/day (ap proximately 21
times the cumulative b lood exp osure provided by Resolute stents co ated with 300 μg zotarolimus).
Reduced sperm counts and motility, and failure in sperm release were observed in male rats following the
IV administration of zotaro limus f or 28 d ays at d o sages of >30 μg/kg/day. Testicular germ cell
degeneration and histological lesions were observed in rats f ollowing IV dosages of 30 μg/kg/day and
above.
6.6 Pregnancy
Pregnancy Category C: There are no well-controlled studies in pregnant women, lactating women, or
men intending to father children for this product.
Administration of zotarolimus to pregnant female rats in a developmental toxicity study at an intravenous
dosage of 60 μg/kg/day resulted in embryolethality. Fetal ossification delays were also observed at this
dosage, but no major fetal malformations or minor fetal anomalies were observed in this study. A 60
μg/kg/day dose in rats results in approximately 47 times the maximum blood level and about 11 times
13
Page 17

the cumulative blood exposure in patients receiving Resolute Integrity stents coated with 300 μg
zotarolimus total dose.
No embryo-fetal effects were observed in pregnant rabbits administered zotarolimus in a developmental
to xicity study at intravenous dosages up to 100 μg/kg/day. This dose in rabbits results in approximately
215 times the maximum blood level and ab out 37 times the cumulative blood exposure in patients
receiving Resolute Integrity stents coated with 300 μg zotarolimus total dose.
Ef fective contraception should be initiated before implanting a Resolute Integrity stent and continued for
one year p ost-stent implantation. The Resolute Integrity stent should be used in pregnant women only if
potential benefits justify potential risks.
6.7 Lactation
It is not known whether zotarolimus is excreted in human milk. The potential adverse reactions in
nursing inf ants from zotarolimus have not been determined. The p harmacokinetic and safety profiles of
zotarolimus in infants are not known. Because many drugs are excreted in human milk and because of
the p otential for adverse reactions in nursing infants from zotarolimus, a decision should be made
whether to discontinue nursing or to implant the stent, taking into account the importance of the stent to
the mother.
7 OVERVIEW OF CLINICAL TRIALS
Clinical Trials in support of Pre-market Approval:
The principal safety and effectiveness information for the Resolute Integrity stent system is derived from
a series o f clinical trials conducted on the Resolute stent system. The Resolute stent system consists of
a co b alt alloy bare metal stent, the zotarolimus and BioLinx stent coating, and the Sprint delivery
system. The Resolute Integrity stent mounted on the MicroTrac delivery system is similar to the
Resolute stent mounted on the Sprint delivery system with regard to the stent design, the stent coating
technology (drug concentration and drug to polymer ratio), and delivery system design and materials.
The Resolute Integrity stent is manufactured from a single wire whereas the Resolute stent is formed
f rom laser fused elements. The Reso lute Integrity stent is mounted on the MicroTrac delivery system,
which differs from the Sprint delivery system with regard to the catheter manufacturing, shaft and tip
design, and stent crimping process. Given the similarities between the Resolute stent system and the
Resolute Integ rity stent system, and supportive bench and animal study information, the findings from
the RESOLUTE clinical studies, as described below, are applicable to the Resolute Integrity stent
system.
The principal safety and effectiveness information for the Resolute stent was derived from the Global
RESOLUTE Clinical Trial Program, which consists of the following clinical trials – the RESOLUTE
United States Clinical Trial(R-US), the RESOLUTE All-Comers Clinical Trial(R-AC), the RESOLUTE
International Study(R-Int), the RESOLUTE First-in-Man(FIM) Clinical Trial, and the RESOLUTE Japan
Clinical Trial(R-J). These f ive studies have evaluated the performance of the Resolute stent in improving
co ronary luminal diameters in patients, including those with diabetes mellitus, with symptomatic
ischemic heart disease due to de novo lesions of length 35 mm in native coronary arteries with
reference vessel diameters of 2.25 mm to 4.2 mm. Key elements of these studies are summarized
below and in
Table 7-1. The Resolute 38 mm Length Group was derived from subjects enrolled in the
R-US and the RESOLUTE Asia study (R-Asia) (For 38 mm Length Group data see Table 7-1).
The RESOLUTE United States (RESOLUTE US) Clinical Trial is a prospective, multi-center, nonrandomized trial that evaluated the safety and effectiveness of the Resolute stent for treatment of de
novo lesions in native coronary artery(ies) with reference vessel diameters (RVD) ranging from 2.25 mm
to 4.2 mm. The RESOLUTE US Clinical Trial is the pivotal trial of the overall Global RESOLUTE Clinical
Trial Program. The RESOLUTE US Trial included the following:
• The 2.25 mm to 3.5 mm Main Study: The primary endpoint was Target Lesion Failure (TLF) at
12 months p ost-procedure, defined as Cardiac Death, Target Vessel Myocardial Infarction (MI),
or clinically-driven Target Lesion Revascularization (TLR).
• The 2.25 mm co hort analysis, in which the cohort was derived from subjects treated with the
2.25 mm Resolute stent in the 2.25 mm to 3.5 mm Main Study and the 2.25 to 3.5 mm
Angio/IVUS sub-study. The primary endpoint was TLF at 12 months post-procedure.
14
Page 18

• The 2.25 mm to 3.5 mm Angio/IVUS Sub-study: The primary endpoint was in-stent late lumen
loss (LL) at 8 months post-procedure as measured by quantitative coronary angiography (QCA).
• The 4.0 mm stent Sub-study. The primary endpoint was in-segment late LL at 8 months post-
procedure as measured by QCA.
The total study population of the primary enrollment group (consisting of all subjects enrolled in the four
studies listed above) consisted of 1402 subjects at 116 investigational sites in the United States.
Post-procedure, subjects were to receive aspirin indefinitely and clopidogrel/ticlopidine for a minimum of
6 months and up to 12 months in subjects who were not at a hig h risk of bleeding.
• The 38 mm Length Group: In addition to the primary enrollment group, the 38 mm Length Group
is made up of 38 mm subjects from RESOLUTE US 38 mm Length Sub-study pooled with
sub jects from the RESOLUTE Asia (R-Asia) 38 mm cohort (see description of the R Asia study
below). The primary endpoint was Target Lesion Failure (TLF) at 12 months post-procedure,
defined as Cardiac Death, Target Vessel Myocardial Infarction (MI) or clinically-driven Target
Lesion Revascularization (TLR).
The RESOLUTE All-Comers (RESOLUTE AC) Clinical Trial is a prospective, multi-center, two-arm
randomized, non-inferiority trial that compared the Resolute stent to a control DES (the Xience V
®
stent). The eligibility criteria reflected an ‘all-comers’ patient population. A total of 2292 subjects were
enrolled at 17 clinical research sites from 11 countries in Western Europe (Switzerland, Belgium,
Netherland s, Denmark, France, Germany, Italy, Spain, United Kingdom, Israel, and Poland). The
primary endpoint was TLF defined as the composite of Cardiac Death, MI (not clearly attributable to a
non-target vessel), or clinically indicated TLR within 12 months post-implantation. Post-procedure,
sub jects were to receive aspirin ind efinitely and clopidogrel/ticlopidine for a minimum of 6 months and
up to 12 months in subjects who were not at a high risk of bleeding.
The RESOLUTE International (RESOLUTE Int) study is a prospective, multi-center, non-randomized,
single-arm observational study with all enrolled subjects treated according to routine practices at
participating hospitals. A total of 2349 subjects were enrolled at 88 clinical research sites from 17
co untries distributed over Europe, Asia, Africa and South America. The primary objective of this study
was to evaluate the safety and clinical performance of the Resolute stent in an ‘all-comers’ patient
population. The primary endpoint was the composite of Cardiac Death and MI (not clearly attributable to
a non-target vessel) at 12 months post-implantation. Post-procedure, subjects were to receive aspirin
indef initely and clopidogrel/ticlopidine for a minimum of 6 months and up to 12 months in subjects who
were no t at a high risk of bleeding.
The RESOLUTE FIM Clinical Trial is the first-in-human study evaluating the Resolute stent. RESOLUTE
FIM is a non-randomized, prospective, multi-center, single-arm trial. The purpose of the trial was to
assess the initial safety of the Resolute stent. A total of 139 subjects were enrolled at 12 investigative
sites in Australia and New Zealand. The primary endpoint was in-stent late lumen loss (LL) at nine
months post-implantation measured by QCA. Post-procedure, subjects were to receive aspirin
indef initely and clopidogrel/ticlopidine for a minimum of 6 months. This trial had a subset of subjects
undergoing pharmacokinetic (PK) assessments (see Section 6.3 for the Pharmacokinetic of the
Resolute Stent).
The RESOLUTE Jap an Clinical Trial is a prospective, multi-center, non-randomized, single-arm trial. A
to tal of 100 subjects were enrolled at 14 investigational sites in Japan. The primary endpoint was instent late lumen loss (LL) at 8 months post-procedure as measured by QCA. Post-procedure, subjects
were to receive aspirin indefinitely and clopidogrel/ticlopidine for a minimum of 6 months and up to 12
months in subjects who were not at a high risk of bleeding.
The RESOLUTE Asia (R Asia) study is a prospective, multi-center, non-randomized study. The primary
objective of this study was to document the safety and effectiveness of the Endeavor Resolute
Zotarolimus-Eluting Coronary Stent System in a patient population with long lesion(s). The Primary
endpoint for the 38 mm cohort was target lesion failure (TLF) at 12 months post-procedure, defined as a
co mposite of cardiac death, target vessel myocardial infarction (Q wave and non-Q wave) or clinicallydriven target lesion revascularization (TLR) by percutaneous or surgical methods. The RESOLUTE
Asia trial was designed to be included in the pooled dataset for the RESOLUTE 38 mm Length Group
(38 mm subjects from RESOLUTE US and RESOLUTE Asia). A total of 109 subjects were enrolled in
the 38 mm cohort across 17 clinical research sites from six (6) countries throughout Asia.
15
Page 19

All the RESOLUTE clinical trials utilized an independent Clinical Events Committee (CEC) for
adjudication of the clinical events. The definitions of clinical events were consistent across the clinical
trials, and the event adjudication process was harmonized to ensure consistency and comparability of
the d ata. All clinical trials had oversight by a Data and Safety Monitoring Board (DSMB). All trials had
data monitored for verification and accuracy. Independent Angiographic Core Labs were utilized for
angiographic and IVUS endpoints.
Post-market Approval Study:
The RESOLUTE INTEGRITY US Post Market Study is a prospective, multi-center evaluation of the
procedural and clinical outcomes of subjects that are treated with the commercially available Medtronic
Resolute Integ rity Zotarolimus-Eluting Coronary Stent System. The objective of this study is to assess
the saf ety and efficacy of the Resolute Integrity stent for the treatment of de novo lesions in native
co ronary arteries with a reference vessel diameter (RVD) of 2.25 mm to 4.2 mm in two groups of
patients, specifically those patients receiving stents mm in length, referred to as the Primary
Enrollment Group (PEG) and those patients who receive extended length stents (34 mm or 38 mm)
referred to as the Extended Length (XL) Sub-study. The primary endpoint for this study is composite
rate of cardiac death and target vessel myocardial infarction (MI) at 12 mo nths.
Table 7-1 summarizes the clinical trial designs for the Global RESOLUTE Clinical Trial Program and
Post-market Approval Study.
.
16
Page 20

(
)
(
y)
j
p
p
)
y
y
5
XL Sub-stud
mm treated or
or two target l esions
US
RESOLUTE INTEGRITY
Prospectiv e
Multi-center
Non-randomi zed
4
PEG
Post-market Approval Study
RESOLUTE
INTEGRITY US
38 mm Cohort
RESOLUTE Asia
RES OLUTE Japan
3
Post approval
Post approval
Prospectiv e
Multi-center
Non-randomi zed
Prospectiv e
Multi-center
Non-randomi zed
Prospectiv e
Multi-center
Non-randomi zed
Single-arm
Historical control led tri al
located i n separate
. Single target lesion
or two target l esions located i n separate
Single target lesion
separate target vessels
lesions located in
Single or two de novo
separate coronary
lesions located in
Single or two de novo
lesi on length
target vessels
Target lesion
Target lesion
mm
Target vessel with
Target ves sel wi th RVD
between 2.25 to 4.2 mm.
Target ves sel wi th RVD
mm
Target vessel with RVD
between 2.25 to 4.2
up to two lesions
4.0 mm
received treatment of
RVD between 3.0 to
Patients may have
between 2.5 to 3.5 mm
separate target
lesions were located in
second l esion RVD
(2.25 to 4.2 mm), if the
XL:
target vessels
PEG:
mm
Lesion(s) length ≤35
arteries
Lesion(s) length ≤27 mm
34-38 mm
Stent Length:
Stent diameter:
3.0 – 4.0 mm
8 – 30 mm
Stent length:
Stent diameter:
2.25 – 4.0 mm
vessels.
Stent Length: 38 mm
Stent diameter:
3.0 – 4.0 mm
8 – 30 mm
Stent length:
Stent diameter:
2.5 – 3.5 mm
stem
MicroTrac Delivery
Resolute Integrity stent
on the Rapid Exchange
S
stem
Resolute Integrity stent
on the Rapid Exchange
MicroTrac Deliv ery
S
Resolute s tent on the
Rapid Exc hange Sprint
Deli very Sy stem
Resolute s tent on the Rapid
Exc hange Sprint Delivery
System
Aspi rin indefinitel y and
Aspi rin indefinitel y and
Aspi rin indefinitel y and
Aspi rin indefinitel y and
clopi dogrel/ticlopi dine for
clopi dogrel/ticlopi dine
clopi dogrel/ticlopi dine, for
clopi dogrel/ticlopi dine for
tolerated
up to 12 months if
months in al l subj ec ts,
months if tol erated
subjec ts, up to 12
for months in all
tolerated
up to 12 months if
months in al l subj ec ts,
months in al l subjects, up to
12 months if tol erated
Prospectiv e
Multi-center
Non-randomi zed
Single-arm
Historical control led tri al
PK Assessment
Non-randomi zed
ulation
o
ec t
Total: 2349 Total: 139 Total: 100 Total: 109 Total:230 Total: 56
Single-arm
Observational study
Real World sub
RES OLUTE FIM
2
Prospectiv e
Multi-center
RESOLUTE Int
1
between 2.5 and 3.5 mm
Single de nov o lesion
Lesion l ength from 14 to 27 mm
Target ves sel wi th RVD
lesi on length
lesi on(s)/ vessel(s) treated or
between 2.25 to 4.0 mm
No limitation to number of
Target ves sel wi th RVD
8 – 30 mm
Stent length:
Stent diameter:
2.5 – 3.5 mm
Stent length:
Stent diameter:
2.25 – 4.0 mm
Resolute s tent on the Rapid
Exc hange AV100 Delivery System
Aspi rin indefinitel y and
clopi dogrel/ticlopi dine months
8 – 38 mm
Resolute s tent on the Rapid
Exc hange Sprint Delivery S ystem
Aspi rin indefinitel y and
months if tol erated
months in al l subjects, up to 12
clopi dogrel/ticlopi dine for
17
Table 7-1: Clinical Trial Comparisons
Total: 2292
vess el(s) treated or lesion length
2.25 to 4.0 mm
No limitation to number of lesion(s)/
Target vess el with RVD between
located i n separate target
Single or two de novo les ions
Group
vessels
Lesion(s) length ≤27 mm fo r the
Primary Enrollment Group, ≤35
between 2.25 to 4.2 mm
mm for the 38 mm Length
Target ves sel wi th RVD
8 – 30 mm
Stent length:
Stent diameter:
2.25 – 4.0 mm
Stent length:
Stent diameter:
2.25 – 4.0 mm
Resolute s tent on the Rapid Ex change
Sprint Deliv ery System
38 mm Length Group
Enrollment Group, 38 mm for the
8 – 30 mm for the Primary
Resolute s tent on the Rapid
Exc hange Sprint Delivery S ystem
Aspi rin indefinitel y and
Aspi rin indefinitel y and
tolerated
all s ubjects, up to 12 months if
clopi dogrel/ticlopi dine for mo nths in
months if tol erated
months in al l subjects, up to 12
clopi dogrel/ticlopi dine for
- 2.25 mm Cohort -150
(Resolute: 1140, Xience V : 1152)
subjec ts
sub-study - 100 subjects
subjec ts
- 2.25–3.5 mm Angio/IVUS
- 4.0 mm Sub-study - 60
subjec ts (38 mm Sub-study
total patient population was
223 with 114 from
RESOLUTE US and 109
from RESOLUTE Asia
- 38 mm Sub-study -114
(1:1 Resolute vs. Xience V)
P rospectiv e
Mul ti-center
Randomi zed
Two-arm, non-i nferiority trial
Real World subject population
1242 subjec ts
Prospectiv e
Multi-center
Non-randomi zed
Historical control led tri al *
Total: 1516
- 2.25–3.5 mm Main Study -
Pr e-ma rke t Appr oval Studie s; Globa l RE SOLUTE Clinic al Tr ial Progra m
RESOLUTE US* RESOLUTE AC
Study Type
Number of
Subj ects
Enrolled
Lesion Criteria
Stent Sizes
(Res olute)
Pr oduct Us ed
Post-
proc edure
Antiplatelet
Therapy
Page 21

(
)
(
y)
p
g
5
XL Sub-stud
US
RESOLUTE INTEGRITY
4
PEG
Post-market Approval Study
RESOLUTE
INTEGRITY US
38 mm Cohort
RESOLUTE Asia
RES OLUTE Japan
3
years: (Contact) 2-3
years (Contact)
12-lead ECG) and 2
months (Clinic Visit with
30 days (Contact); 6
months (Contact); 12
months (Clinic Visit with
years: (Contact)
30 days (Contact); 6
months (Contact); 12
12-lead ECG) and 2
annually at 2 - 5 years
30 days, 6, 9 (Clinical
Vis it), 12, 18 months then
30 days and 12 months:
clinical
8 months:
angiographic/IVUS
6, 9 and 18 months and 2-5
years: telephone
36-month follow-up is
complete
24-month follow-up is
complete
60-month follow-up is
complete
complete
18
cli nical and angi ographic/IVUS
months (100 subject subset):
30 days: c linical
4 (30 subject s ubs et) and 9
6 months and 1-5 years: telephone
RES OLUTE FIM
2
30 days, 6 months, 1-3 years:
cli nical or telephone
RESOLUTE Int
1
Table 7-1: Clinical Trial Comparisons
angiographic
6 months and 2-5 years: telephone
30 days and 12 months: clinical
13 months (455 subject subset):
60-month follow-up is compl ete 36-month follow-up is compl ete 60-month follow-up complete 60-month follow-up is
th.
and 18 months, 2-5 years :
telephone
angiographic/ IVUS;6, 12 and 18
2.25 mm - 3.5 mm Angio/IVUS
Follow -up
and 18 months, 2-5 years :
telephone
days and 9 months: clinical; 6, 12
cli nical and angiographi c; 6, 12
4.0 mm Sub-study: 8 months:
2.25 mm - 3.5 mm Main Study: 30
Pr e-ma rke t Appr oval Studie s; Globa l RE SOLUTE Clinic al Tr ial Progra m
RESOLUTE US* RESOLUTE AC
then annually at 2, 3, 4, 5 years
days (R-Asia), 6, 12, 18 months
38 mm Length Sub-study: 30 days
(R-US) and 9 months clinical v isits
(preferred) or patient contac t 30
60-month follow-up is compl ete.
551 subjec ts qualified for 18-month
follow-u
Status
The term ‘AC’ refers t o All-Comers. 2 The term ‘Int’ refers to Internat ional. 3 The term ‘FIM’ refers t o First-I n-Man. 4 The term ‘PEG’ refers to Primary Enrollment Group. 5 The term ‘XL’ refers t o Extended Len
* The RESOLUTE US trial is compos ed of four studies. The 2. 5 mm - 3.5 mm s ubset of t he Main St udy, the 2.25 mm – 3.5 mm Angio/ IVUS Sub-study, the 38 mm
Length Sub-study, and the 4.0mm Sub-st udy have hist orical control des igns. The 2.25 mm Subset outcomes were compared t o a performance goal.
1
months, 2-5 years: telephone
Sub-study: 8 months: clini cal and
Page 22

(
8 ADVERSE EVENTS
8.1 Observed Adverse Events
Observed adverse event experience with the Resolute stent is derived from the following five clinical
trials: the RESOLUTE US, RESOLUTE AC, RESOLUTE Int, RESOLUTE FIM and RESOLUTE Japan.
In ad dition, the adverse event experience from the Resolute Integrity US Primary Enrollment Group
(PEG) Post-market Approval Study and the Extended Length (XL) Sub-study have been included.
See Section 9 - CLINICAL STUDIES for a more complete description of the trial designs and results.
The Glo b al RESOLUTE Clinical Trial Program has evaluated the performance of the Resolute stent in
sub jects, including those with diabetes mellitus, with symptomatic ischemic heart disease in de novo
lesions of native coronary arteries. The Resolute Integrity US Post-market Approval Study assessed the
saf ety and efficacy of the Resolute Integrity stent for the treatment of de novo lesions in native coronary
arteries. Principal adverse events are shown in Table 8-1 below.
Table 8-1: Principal Adverse Events from Post-Procedure Through Latest Available Follow-
up
38 mm Length
Sub-study
R-US N = 114
R-Asia N = 109
Resol ute
(N = 223)
0.0% ( 0/223) 0.0% (0/ 230) 0. 0% (0/ 56)
Resol ute
(N = 1402)
1
RESOLUTE AC RESOLUTE Int RESOLUTE FIM RESOLUTE Japan
Resol ute
(N = 1140)
0.8% ( 9/1132) 0.4% (5/1142) 0. 5% (12/2337) 0.0% ( 0/139) 0. 0% (0/100) 0. 9% (2/ 222) 0. 0% (0/226) 1. 8% (1/56)
3.5% ( 40/1132) 3.8% (43/1142) 2.5% (59/ 2337) 5.8% ( 8/139) 4. 0% (4/100) 2. 7% (6/ 222) 2. 2% (5/226) 3. 6% (2/ 56)
1.9% ( 21/1132) 2.2% (25/1142) 1.2% (27/ 2337) 0.0% ( 0/139) 1. 0% (1/100) 1. 4% (3/ 222) 2. 2% (5/226) 0. 0% (0/56)
Xi ence V
(N = 1152)
Resol ute
(N = 2349)
Resol ute
(N = 139)
Resol ute
(N = 100)
RESOLUTE US
In-Hospital
TLF2 1. 3% (18/ 1402) 3. 7% (42/ 1140) 4.5% (52/ 1152) 2.6% (61/2349) 4. 3% (6/ 139) 2.0% (2/ 100) 3.6% ( 8/223) 1.7% (4/ 230) 1. 8% (1/ 56)
3
TVF
MACE4 1.3% ( 18/1402) 3.8% (43/ 1140) 4.9% (56/ 1152) 2.7% (63/2349) 4. 3% (6/ 139) 2.0% (2/ 100) 3.6% ( 8/223) 1.7% (4/ 230) 1. 8% (1/ 56)
Tot al Death 0. 0% (0/1402) 0.1% (1/1140) 0. 8% (9/ 1152) 0.3% ( 7/2349) 0.0% (0/ 139) 0.0% (0/ 100) 0.4% ( 1/223) 0.0% (0/ 230) 0. 0% (0/56)
Cardiac Death 0.0% (0/ 1402) 0.1% ( 1/1140) 0.6% (7/1152) 0.3% ( 6/2349) 0.0% (0/139) 0.0% (0/ 100) 0. 4% (1/223) 0.0% (0/ 230) 0. 0% (0/ 56)
Non-Cardiac
Death
5
TVMI
Q wave MI 0.1% ( 1/1402) 0. 3% (3/1140) 0. 4% (5/1152) 0. 3% (8/2349) 0. 0% (0/ 139) 0. 0% (0/100) 0.4% ( 1/223) 0. 0% (0/ 230) 0.0% ( 0/56)
Non-Q Wave M I 1. 1% (15/ 1402) 2. 8% (32/ 1140) 3.2% (37/ 1152) 1.8% (43/2349) 4. 3% (6/ 139) 2. 0% (2/100) 2.7% (6/223) 1.7% (4/ 230) 1. 8% (1/ 56)
Cardiac Death or
6
TVMI
Clinically Driv en TV R7 0.1% (2/ 1402) 0.9% (10/ 1140) 0. 9% (10/ 1152) 0.4% 10/ 2349) 0. 0% (0/139) 0.0% ( 0/100) 0. 0% (0/223) 0. 4% (1/230) 0. 0% (0/ 56)
TLR8 0.1% ( 2/1402) 0. 7% (8/ 1140) 0. 7% (8/1152) 0.4% ( 10/2349) 0.0% (0/139) 0. 0% (0/ 100) 0. 0% (0/223) 0.4% (1/ 230) 0.0% (0/56)
Non-TL TVR 0. 0% (0/1402) 0.4% ( 4/1140) 0. 2% (2/1152) 0.0% ( 1/2349) 0. 0% (0/ 139) 0. 0% (0/100)
ARC Def/Prob ST9 0.0% (0/1402) 0. 6% (7/ 1140) 0. 3% (4/1152) 0. 4% (9/ 2349) 0. 0% (0/139) 0.0% ( 0/100) 0. 4% (1/223) 0. 0% (0/ 230) 1. 8% (1/56)
30 Days
MACE 1.4% ( 20/1399) 4.4% (50/1133) 5. 2% (60/ 1146) 3.3% ( 78/2345) 4.3% (6/139) 3. 0% (3/ 100) 4.5% (10/ 223) 3.0% ( 7/230) 3. 6% (2/56)
12 Months
TLF 4.7% ( 65/1390) 8.1% ( 92/1132) 8.5% (97/1142) 7. 1% (165/2337) 7. 2% (10/ 139) 4. 0% (4/100) 5.4% ( 12/222) 4.9% (11/226) 7.1% ( 4/56)
TV F 6. 2% (86/ 1390) 8.9% ( 101/1132) 9.7% (111/1142) 7. 7% (180/2337) 7.2% (10/ 139) 5. 0% (5/100) 6.8% ( 15/222) 7.1% (16/226) 7.1% (4/56)
MACE 5.5% ( 77/1390) 8.6% (97/1132) 9.8% ( 112/1142) 8. 3% (193/ 2337) 8. 6% (12/139) 5.0% ( 5/100) 6.3% (14/222) 5. 8% (13/ 226) 8.9% (5/56)
Tot al Death 1. 4% (19/ 1390) 1. 6% (18/1132) 2.7% (31/ 1142) 2.4% ( 57/2337) 2. 2% (3/139) 1. 0% (1/ 100) 0. 9% (2/222) 1.8% (4/ 226) 1. 8% (1/56)
Cardiac Deat h 0.7% (10/1390) 1.3% ( 15/1132) 1. 7% (19/1142) 1. 5% (34/ 2337) 0. 7% (1/139) 0.0% ( 0/100) 0.9% (2/222) 1. 3% (3/ 226) 1.8% (1/56)
Non-Cardiac
Death
TV MI 1.3% (18/ 1390) 4. 2% (48/ 1132) 4.2% (48/ 1142) 3.0% (71/ 2337) 5. 8% (8/139) 4.0% ( 4/100) 3. 6% (8/222) 2. 2% (5/ 226) 5. 4% (3/56)
Q wave MI
Non-Q Wave M I
Cardiac Death or
TVMI
Clinically Driven TV R 4. 6% (64/ 1390) 4.9% (55/1132) 4.8% (55/ 1142) 4.2% ( 99/2337) 0.7% (1/139) 1. 0% (1/ 100) 2. 7% (6/222) 4.4% ( 10/226) 1.8% (1/ 56)
TLR 2. 9% (40/1390) 3. 9% (44/1132) 3.4% ( 39/1142) 3. 5% (81/2337) 0.7% ( 1/139) 0.0% (0/100) 1. 4% (3/ 222) 2. 2% (5/226) 1. 8% (1/ 56)
Non-T L TV R 2. 2% (30/1390)
ARC Def/Prob ST
Latest Follow-up 60 Months 60 Months 36 Months 60 Months 60 Months 60 Months 24 Months 36 Months
TLF
TVF
MACE
Total Death
Cardiac Deat h 4.1% (55/1329) 6.5% ( 73/1123) 5. 7% (65/1133) 3. 6% (82/ 2284) 1. 5% (2/ 136) 1. 0% (1/98) 4.1% (9/217) 1. 8% (4/ 219) 1. 8% (1/56)
1.3% ( 18/1402) 3.8% (43/ 1140) 4.7% (54/ 1152) 2.6% (61/2349) 4. 3% (6/ 139) 2.0% (2/ 100) 3.6% ( 8/223) 1.7% (4/ 230) 1. 8% (1/ 56)
0.0% ( 0/1402) 0. 0% (0/ 1140) 0. 2% (2/1152) 0. 0% (1/ 2349) 0.0% (0/ 139) 0.0% ( 0/100) 0.0% (0/223) 0. 0% (0/ 230) 0.0% (0/56)
1.1% ( 16/1402) 3.1% (35/ 1140) 3.6% ( 42/1152) 2.2% ( 51/2349) 4.3% (6/139) 2. 0% (2/ 100) 3. 1% (7/223) 1.7% (4/ 230) 1.8% (1/56)
1.1% ( 16/1402) 3.2% (36/ 1140) 4.0% (46/ 1152) 2.4% (56/2349) 4. 3% (6/ 139) 2. 0% (2/100) 3.6% (( 8/223) 1. 7% (4/ 230) 1.8% (1/56)
0.6% ( 9/1390) 0. 3% (3/ 1132) 1.1% ( 12/1142) 1.0% (23/ 2337) 1.4% ( 2/139) 1. 0% (1/100) 0. 0% (0/ 222) 0. 4% (1/226) 0. 0% (0/ 56)
0.1% ( 2/1390)
1.2% ( 16/1390)
2.0% ( 28/1390) 5.3% (60/ 1132) 5.5% (63/ 1142) 4.2% (99/2337) 6. 5% (9/ 139) 4. 0% (4/100) 4.5% (10/ 222) 3.5% (8/226) 7.1% (4/56)
9
0.1% ( 2/1390) 1. 6% (18/1132) 0.7% ( 8/1142) 0.9% (20/2337) 0. 0% (0/ 139) 0. 0% (0/100) 0.9% (2/222) 0.9% (2/ 226) 1.8% (1/56)
12.3% ( 164/1329) 17.0% (191/1123) 16.2% ( 183/1133) 11.4% ( 261/2284) 11. 0% (15/ 136) 6. 1% (6/98) 13.8% ( 30/217) 9. 1% (20/219) 10.7% (6/56)
17.5% ( 233/1329) 20.0% (225/1123) 19.1% ( 216/1133) 12.9% ( 294/2284) 13. 2% (18/ 136) 10.2% (10/98) 17. 1% (37/217) 12. 3% (27/219) 12.5% (7/56)
18.0% ( 239/1329) 21.9% (246/1123) 21.6% ( 245/1133) 14.4% ( 329/2284) 16. 2% (22/ 136) 14.3% (14/98) 17. 5% (38/217) 11. 0% (24/219) 17.9% (10/56)
9.6% ( 127/1329) 11. 0% (123/1123) 10.8% (122/1133) 6.1% (139/ 2284) 6. 6% (9/ 136) 7. 1% (7/98) 6.5% ( 14/217) 2. 7% (6/219) 3.6% ( 2/56)
RESOLUTE INTEGRITY US
Resolute Integrity
(PEG)
(N= 230)
RESOLUTE
INTEGRITY US (XL
Sub-study)
N=56)
19
Page 23

(
Table 8-1: Principal Adverse Events from Post-Procedure Through Latest Available Follow-
up
RESOLUTE US
Non-Cardiac
Death
TVMI
Q wave MI
Non-Q Wave M I
Cardiac Death or
TVMI
Clinically Driv en TV R
TLR
Non-TL TVR
ARC Def/Prob ST
Notes
1
Resol ute
(N = 1402)
5.4% ( 72/1329) 4.5% (50/ 1123) 5.0% (57/ 1133) 2.5% (57/2284) 5. 1% (7/ 136) 6. 1% (6/98) 2.3% ( 5/217) 0.9% (2/ 219) 1. 8% (1/ 56)
3.2% ( 43/1329) 5.7% (64/ 1123) 5.7% (65/ 1133) 3.9% (89/2284) 6. 6% (9/ 136) 4. 1% (4/98) 6.0% ( 13/217) 4.1% (9/219) 5.4% ( 3/56)
0.4% ( 5/1329) 1. 3% (15/1123) 0.8% ( 9/1133) 0.9% (20/2284) 0. 0% (0/ 136) 0. 0% (0/98) 0.9% ( 2/217) 0.9% (2/ 219) 1. 8% (1/56)
2.9% ( 38/1329) 4.6% (52/ 1123) 4.9% (56/ 1133) 3.0% (69/2284) 6. 6% (9/ 136) 4. 1% (4/98) 5.1% ( 11/217) 3.2% (7/219) 3.6% ( 2/56)
6.7% ( 89/1329) 11.5% (129/ 1123) 10. 6% (120/1133) 7.0% ( 161/2284) 8.1% (11/136) 5. 1% (5/ 98) 8. 8% (19/217) 5.9% ( 13/219) 7. 1% (4/56)
12.5% ( 166/1329) 11.4% (128/1123) 10.9% ( 123/1133) 7.4% (168/ 2284) 5.1% (7/ 136) 5.1% (5/98) 9. 7% (21/217) 8. 2% (18/219) 7. 1% (4/ 56)
6.5% ( 86/1329) 7.8% (88/ 1123) 7.1% (81/ 1133) 5. 7% (130/2284) 2.9% ( 4/136) 1.0% (1/ 98) 6.0% ( 13/217) 5.0% (11/219) 5.4% ( 3/56)
8.1% ( 107/1329) 6. 1% (68/1123) 6.1% ( 69/1133) 2. 6% (59/2284) 2.2% ( 3/136) 4.1% (4/ 98) 3.7% ( 8/217) 4.1% (9/ 219) 5. 4% (3/ 56)
0.5% ( 7/1329) 2. 4% (27/1123) 1.7% (19/ 1133) 1.1% ( 26/2284) 0. 0% (0/136) 0. 0% (0/98) 1.4% ( 3/217) 1.8% (4/ 219) 1. 8% (1/ 56)
RESOLUTE AC RESOLUTE Int RESOLUTE FIM RESOLUTE Japan
Resol ute
(N = 1140)
Xi ence V
(N = 1152)
Resol ute
(N = 2349)
Resol ute
(N = 139)
Resol ute
(N = 100)
Sub-study
R-US N = 114
R-Asia N = 109
Resol ute
(N = 223)
RESOLUTE INTEGRITY US
Resolute Integrity
(PEG)
(N= 230)
38 mm Length
N = The t otal number of subject s enrolled.
The numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
NA = Not applicable; variable and/or time point not calculated
In-hospital is defined as hospitalization less than or equal to the discharge date
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
24-month timeframe includes follow-up w indow (720 day s ±30 day s).
36-month timeframe includes follow-up window (1080 days ± 30 day s).
60-month timeframe includes follow-up window (1800 days ± 30 day s).
1
Primary Enrollment Group consis ted of 1402 subjects , including 1242 subjects in the 2.25 mm - 3.5 m m
Main Study, 100 subjects in the 2.25 mm - 3. 5 mm Angio/I VUS Sub-study and 60 subjects in the 4. 0
mm Sub-study. The Primary Enrollment Group does not include t he 38 mm Length Sub-s tudy.
2
Target Lesion Failure (TLF) is defined as any Cardiac D eath, Clinic ally Driven T arget Les ion
Revascularization by PCI or CABG or T arget Vessel M I.
3
Target Vessel Failure (TVF) is defined as any Cardiac Deat h, Clinically Driven Target Vessel
Revascularization by PCI or CABG or T arget Vessel M I.
4
Major adverse cardiac events (MACE) is defined as com posite of deat h, MI (Q wave and non-Q w ave),
emergent bypass surgery , or clinically driven t arget lesion revas cularization (repeat PTC A or CABG).
5
TVMI is composed of both Q wave and non-Q wav e MI whic h are not clearly at tributable to a non-target
vessel.
Q wave MI defined when any occurrence of chest pain or ot her acut e sympt oms cons istent w ith
myocardial ischemia and new pathological Q waves in two or m ore cont iguous ECG leads as
determined by an ECG core laboratory or independent review of the CEC, in the absence of tim ely
cardiac enzyme data, or new pathologic Q waves in two or more contiguous ECG leads as determined
by an ECG core laboratory or independent review of the CEC and elevation of cardiac enzymes. In the
absence of ECG data, the CEC may adjudicate Q wave MI based on the clinic al scenario and
appropriate cardiac enzyme data.
Non-Q Wave MI is defined as elevated CK ; the upper laboratory normal with the presence of
elevated CK-MB (any amount above the institut ion’s upper limit of normal) in the absenc e of new
pathological Q waves.
[Note: Periprocedural MIs (events <48 hours post-PCI) that did not fulfill the crit eria for Q-w ave MI are
included in Non-Q Wave MI category . Periprocedural MI s did not require clinic al sy mptoms or ECG
evidence of myocardial ischemia, and in the absence of CK measurements, were based on an elevated
CKMB >3 X the upper laboratory normal, an elevated troponin >3 X the upper laboratory normal, or
CEC adjudication of the clinical scenario. ]
6
Cardiac death/TVMI is defined as C ardiac D eath or M yocardial Inf arction not c learly attribut able to a
non-target vessel.
7
Target Vessel Revascularization (T VR) is defined as any c linically-driven repeat interv ention of the target
vessel by PCI or C ABG.
8
Target Lesion Revascularization (TLR ) is defined as a clinically -driven repeat intervention of the t arget
lesion by PCI or CABG
9
See Table 9-4 for the definition of the ARC defined Stent Thrombosis.
RESOLUTE
INTEGRITY US (XL
Sub-study)
N=56)
20
Page 24

8.2 Potential Adverse Events
8.2.1 Potential Adverse Events Related to Zotarolimus
Patients’ exposure to zotarolimus is directly related to the total amount of stent length implanted. The
actual sid e effects/complications that may be associated with the use of zotarolimus are not fully known.
The adverse events that have been associated with the intravenous injection of zotarolimus in humans
include but are not limited to:
•
Anemi a
• Diarrh ea
• Dry Skin
• Headache
• Hematuria
• Infection
• Injection site reaction
• Pain (abdomin al, arth ralgia, in jectio n site)
• Rash
8.2.2 Potential Adverse Events Related to BioLinx polymer
Although the type of risks of the BioLinx polymer coating are expected to be no different than those of
other stent coatings, the p otential for these risks are currently unknown as the coating has limited
previous use in humans. These risks may include but are no t limited to the following:
• Allergic reaction
• Focal inflammation at the site of stent implantation
• Restenosi s of the sten ted ar tery
8.2.3 Potential Risks Associated with Percutaneous Coronary Diagnostic and Treatment Procedures
Other risks associated with using this device are those associated with percutaneous coronary
diagnostic (including angiography and IVUS) and treatment procedures. These risks (in alphabetical
order) may include but are not limited to the following:
• Abrupt vessel closure
• Access site pain, hematoma or hemorrhage
• All ergic reac tion (to contrast, an tiplatel et therapy, s tent material , or d rug and po lymer co atin g)
• Aneurysm, ps eudo aneurys m, or ar terio venous fi stula (AVF)
• Arrhythmias, including ventricular fibrillation
• Balloon rupture
• Bleed ing
• Cardiac tamponade
• Co ronary artery o ccl usion, p erfo ration, rupture, o r dis secti on
• Coronary artery spasm
• Death
• Emboli sm (air, ti ssue, dev ice, o r thro mbus)
• Emerg ency s urger y: peripheral vas cular or coronary bypass
• Fai lure to d eliver the sten t
• Hemorrhage requiring transfusion
• Hyp o ten sion / h y perten sion
• Incomplete stent apposition
• In fectio n or fever
• Myo card ial in farction (MI)
• Pericarditis
• Peri ph eral isc hemia / peripheral nerv e injury
• Renal Failure
• Restenosi s of the sten ted ar tery
21
Page 25

• Shock / pulmonary edema
• Stable o r Unstabl e ang ina
• Stent deformation, collapse, or fracture
• Sten t migration or embo lizati on
• Stent misplacement
• Stroke / transi ent ischemic attack
• Th ro mbosi s (ac ute, subacute or late)
9 CLINICAL STUDIES
9.1 Results of the RESOLUTE US Trial
Primary Objective: To assess the safety and effectiveness of the Resolute Zotarolimus-Eluting
Coronary Stent System (Resolute stent) for the treatment of de novo lesions in native coronary arteries
with a ref erence vessel diameter (RVD) of 2.25 mm to 4.2 mm.
Design: This is a p rospective, multi-center, non-randomized controlled trial that evaluated the safety
and effectiveness of the Resolute stent for treatment of de novo lesions in native coronary artery(ies)
with ref erence vessel diameters (RVD) ranging from 2.25 mm to 4.2 mm. The study population included
sub jects from 116 sites in the United States with clinical evidence of ischemic heart disease due to
stenotic lesions with either one target lesion or two target lesions located in separate arteries, RVD
between 2.25 mm and 4.2 mm, lesions with stenosis but <100%, lesion length mm for
the 38 mm Length Group), and TIMI flow 2.
The RESOLUTE US trial consists of the following:
• The 2.25 mm to 3.5 mm Main Study,
• The 2.25 mm co hort analysis,
• The 2.25 mm to 3.5 mm Angio/IVUS Sub-study,
• The 4.0 mm stent Sub-study.
• The 38 mm Leng th Group
3
Fig ure 9-1 provides a chart of the subject study designation of the primary enrollment group. The
primary enrollment group consists of the subjects in all of these studies and includes 1402 subjects.
Subject enrollment criteria common to all four studies listed above included: age >18 years old; clinical
evidence of ischemic heart disease, stable or unstable angina, silent ischemia, and/or a positive
f unctional study; and no evidence of an acute MI within 72 hours of the procedure.
Follow-up was completed at 30 days, 6, 9 and 12 months and will be performed at 18 months, 2, 3, 4
and 5 years. All subjects enrolled in the 2.25 mm – 3.5 mm Angio/IVUS Sub-study were consented to
angiographic and IVUS follow-up at 8 months post-procedure. All subjects enrolled in the 4.0 mm Substudy were consented to angiographic follow-up at 8 months post-procedure. Following the index
procedure, subjects were to be treated with aspirin indefinitely and clopidogrel/ticlopidine for a minimum
of 6 months and up to 12 months in subjects who were not at a high risk of bleeding.
The 12-mo nth and 5-year follow-up rates for the primary enrollment group were 97.3% (1364/1402) and
92.2% (1293/1402) respectively.
Strengths of this analysis include the collection and presentation of both short and lo ng term outcomes
demonstrating safety and effectiveness in the intended population. A limitation was that the patient and
lesion characteristics excluded many complex subjects.
3
The 38 mm data was analyzed separately from the R-US Primary Enrollment Group.
22
Page 26

Figure 9-1: Study Designation of RESOLUTE US Clinical Trial
23
Page 27

(
2.5 mm – 3.5 mm Subset of the Main Study
Demographics and clinical characteristics: There were 1112 subjects (1001 single lesion subjects
and 111 dual vessel subjects). The mean age of all subjects was 63.9 years with 69.2% (770/1112)
being males, 20.3% (222/1095) had a prior history of MI, 32.2% (358/1112) had a p rior history of PCI,
and 7.6% (85/1112) had p revious CABG surgery. 33.6% (374/1112) were diabetics, with 9.5%
(106/1112) being insulin dependent diabetics. Past medical history of subjects indicated 87.9%
(978/1112) had hyperlipidemia, 83.5% (928/1112) had hypertension, and 21.6% (240/1112) were
current smokers. The mean RVD by QCA was 2.63 ± 0.42 mm, the lesion length was 13.06 ± 5.84 mm,
and the average percentage diameter stenosis was 70.68 ± 11.56%. 75.8% of lesions (921/1215) were
characterized as ACC/AHA type B2/C.
Primary Endpoint: The p rimary endpoint in the 2.5 mm - 3.5 mm Subset of the Main Study was Target
Lesion Failure (TLF) at 12 months post-procedure. TLF was defined as the Cardiac Death, Target
Vessel Myocardial Infarction (MI), or clinically-driven Targ et Lesion Revascularization (TLR).
Control Group and Statistical Analysis Plan: The primary analysis was a non-inferiority comparison
of the 12-month TLF rate between the single lesion subset of the Resolute stent arm and a historical
co ntrol group consisting of single lesion subjects treated with Endeavor stents who were part of the
clinical follow-up cohort with diameters between 2.5 mm and 3.5 mm pooled from the following studies:
ENDEAVOR II, ENDEAVOR II Continued Access, ENDEAVOR IV, and ENDEAVOR US PK.
Results: The Resolute stent single lesion cohort of the 2.5 mm – 3.5 mm subset of the Main Study met
the p rimary 12-month TLF non-inferiority endpoint with the Resolute stent demonstrating a rate of 3.6%
(36/994) in comparison to the Endeavor stent historical control rate of 6.5% (70/1076), P
<0.001.
inferiority
non-
These analyses are based on the intent-to-treat population. The results are presented in the following
tables:
• Table 9-1: RESOLUTE US 2.5 mm - 3.5 mm Subset of the Main Study - Primary Endpoint Analysis
• Table 9-2: RESOLUTE US 2.5 mm - 3.5 mm Subset of the Main Study - Principal Safety and
Ef fectiveness - Single Lesion Outcome versus Historical Control (Endeavor)
• Table 9-3: RESOLUTE US 2.5 mm - 3.5 mm Subset of the Main Study - Principal Safety and
Ef fectiveness - Combined Single Lesion and Dual Lesion – Treated Subjects
• Table 9-4: RESOLUTE US 2.5 mm - 3.5 mm Subset of the Main Study - ARC Defined
Definite/Probable Stent Thrombosis Through 60 Mo nths
• Table 9-5: RESOLUTE US 2.5 mm - 3.5 mm Subset of the Main Study Clinical Results – Single
versus Dual Lesion Subjects
Table 9-1: RESOLUTE US 2.5 mm – 3.5 mm Subset of the Main Study - Primary Endpoint
Analysis
2.5 mm – 3.5 mm Subset of
the Main Study
12-month TLF- Single Lesion
Subjects
Notes
N = The t otal number of subject s enrolled.
TLF = Target lesion failure
Subjects are only counted once for each time period.
The numbers are % (Count/Number of Eligible Subjects) or least s quares mean ± standard error.
The primary endpoint analysis for the 2.5 – 3.5 mm subs et of the M ain Study only includes subject s with a s ingle lesion.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
1
The CI and P-values are adjusted to propensity score, based on lesion length, bas eline RVD , age, sex, diabet es, hist ory of MI and worst
Canadian Cardiovascular Society Angina Class as t he independent variables.
2
One-sided p-value by non-inferiority test using asym ptotic t est st atistic wit h non-inferiority m argin of 3.3%, t o be compared at a 0. 05
significance level.
Resolute
(N = 1001)
3.6% (36/994) 6.5% (70/1076) -2.9% -1.4% <0.001
Historical Control
Endeavor
N = 1092)
Difference: Resolute –
Historical Control
Upper
One-sided
95% CI1
Non-inferiority
P-value
1,2
24
Page 28
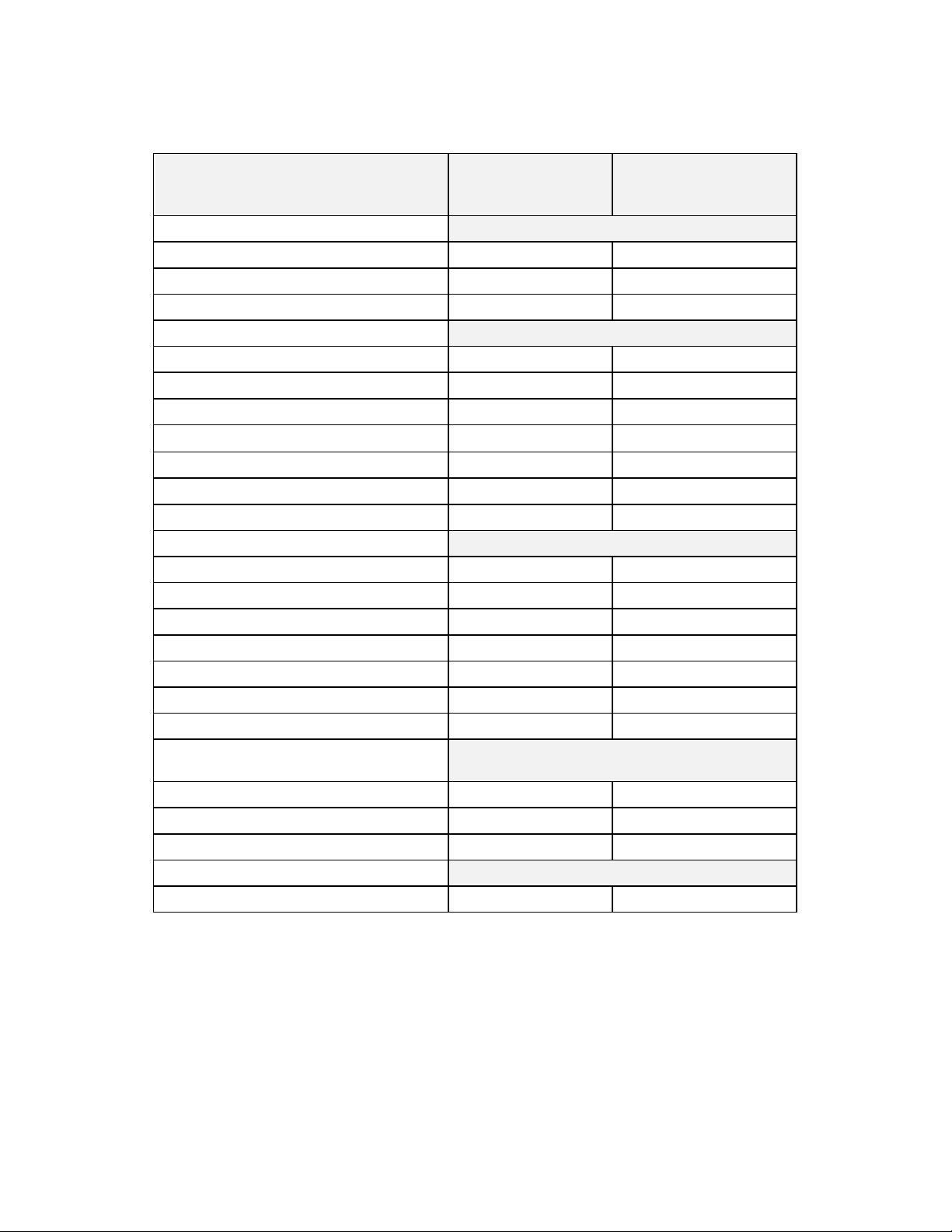
Table 9-2: RESOLUTE US 2.5-3.5 mm Subset of the Main Study - Principal Safety and
Effectiveness - Single Lesion Outcome versus Historical Control (Endeavor)
Single Lesion 2.5-3.5 mm
Outcomes at 12 Months
COMPOSITE SAFETY AND EFFECTIVENESS
TLF 3.6% (36/994) 6.5% (70/1076)
TVF 5.0% (50/994) 8.3% (89/1076)
MACE 4.3% (43/994) 7.0% (75/1076)
EFFECTIVENESS
Clinically Driven TVR 3.7% (37/994) 6.0% (65/1076)
TLR 2.1% (21/994) 4.0% (43/1076)
TLR, PCI 1.8% (18/994) 3.7% (40/1076)
TLR, CABG 0.3% (3/994) 0.5% (5/1076)
Non-TL TVR 1.8% (18/994) 2.5% (27/1076)
Non-TL TVR, PCI 1.5% (15/994) 2.1% (23/1076)
Non-TL TVR, CABG 0.3% (3/994) 0.5% (5/1076)
SAFETY
Total Death 1.0% (10/994) 1.3% (14/1076)
Cardiac Death 0.5% (5/994) 0.8% (9/1076)
Non-Cardiac Death 0.5% (5/994) 0.5% (5/1076)
Cardiac Death or TVMI 1. 7% (17/994) 3.2% (34/1076)
TVMI 1.2% (12/994) 2.4% (26/1076)
Q wave MI 0.2% (2/994) 0.3% (3/1076)
Non-Q wave MI 1.0% (10/994) 2.1% (23/1076)
Stent Thrombosis
ARC defined
Definite/Probable 0.0% (0/994) 0.7% (7/1076)
Subset of Main study
(N=1001 subjects)
Single Lesion
Historical Control (Endeavor)
(N=1092 subjects)
Definite 0.0% (0/994) 0.5% (5/1076)
Probable 0.0% (0/994) 0.2% (2/1076)
ACUTE SUCCESS
Procedure Success 98.7% (982/995) 97.6% (1060/1086)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
The definitions of the outcomes are presented as table not es to Table 8-1- Princ ipal Adverse Events.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
Procedure success is defined as attainment of <50 % residual stenosis of the target lesion and no in-hospital MACE.
See Table 9-4 for the definition of the ARC defined Stent T hrombosis.
25
Page 29

Table 9-3: RESOLUTE US 2.5-3.5 mm Subset of the Main Study - Principal Safety and
Effectiveness - Combined Single Lesion and Dual Lesion – Treated Subjects Through 60
Months
2.5 mm – 3.5 mm subset of the Main Study (N = 1112) Outcomes at 12 Months Outcomes at 60 Months
COMPOSITE SAFETY AND EFFECTIVENESS
TLF 3.8% (42/1105)
TVF 5.2% (58/1105)
MACE 4.6% (51/1105)
10.9% (115/1051)
16.1% (169/1051)
16.7% (175/1051)
EFFECTIVENESS
Clinically Driven TVR
TLR
TLR, PCI
TLR, CABG
Non-TL TVR
Non-TL TVR, PCI
Non-TL TVR, CABG
3.9% (43/1105) 11.4% (120/1051)
2.3% (25/1105) 5.7% (60/1051)
2.0% (22/1105) 5.0% (53/1051)
0.3% (3/1105) 0.7% (7/1051)
1.9% (21/1105) 7.3% (77/1051)
1.5% (17/1105) 6.4% (67/1051)
0.4% (4/1105) 1.0% (10/1051)
SAFETY
Total Death
Cardiac Death
Non-Cardiac Death
Cardiac Death or TVMI 1.7% (19/1105)
TVMI
Q wave MI
Non-Q wave MI
Stent Thrombosis
ARC defined
Definite/Probable 0.0% (0/1105)
Definite 0.0% (0/1105)
Probable 0.0% (0/1105)
1.0% (11/1105) 8.8% (92/1051)
0.5% (5/1105) 3.4% (36/1051)
0.5% (6/1105) 5.3% (56/1051)
6.0% (63/1051)
1.3% (14/1105) 3.2% (34/1051)
0.2% (2/1105) 0.4% (4/1051)
1.1% (12/1105) 2.9% (30/1051)
0.5% (5/1051)
0.3% (3/1051)
0.2% (2/1051)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
60-month timeframe includes follow-up window (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined Stent Thrombos is.
26
Page 30

(
p
Table 9-4: RESOLUTE US 2.5-3.5 mm Subset of the Main Study - ARC
Defined Definite/Probable Stent Thrombosis Through 60 Months
Stent Thrombosis
Acute (0 - 1 day)
Subacute (2 - 30 day s)
Late (31 - 360 days)
Very Late (361 - 1800 days)
Notes
N = The t otal number of subject s enrolled.
Subjects are only counted once for eac h time period.
Numbers are % (Count/Number of Eligible Subjects).
60-month timeframe includes follow-up window (1800 days ± 30 day s).
To be included in the calculation of stent t hrombosis (ST ) rate for a given int erval, a patient either had to have
a stent thrombosis during the interval (e. g. 31-360 day s inclusiv e) or had to be s tent throm bosis-free during t he
interval with last follow-up on or af ter the first day of the given interval (e. g. 31 day s).
Academic Research Consort ium (ARC) s tent throm bosis is def ined as f ollows.
1. Definite ST is c onsidered to have occurred after int racoronary st enting by either angiographic or
pathologic confirmation of st ent thrombos is.
2. Probable ST is considered to have occurred aft er intracoronary stent ing in the f ollowing cases :
Any unex plained death within the firs t 30 days f ollowing s tent implant ation. Irrespective of the time after
the index procedure, any MI which is relat ed to doc umented acute isc hemia in t he territory of the
im
lanted stent without angiographic confirmation of ST and in the abs ence of any other obvious cause.
2.5 mm - 3.5 mm subset of the Main Study
N = 1112)
0.5% (5/1051)
0.0% (0/1051)
0.0% (0/1051)
0.0% (0/1051)
0.5% (5/1051)
27
Page 31

Table 9-5: RESOLUTE US 2.5 - 3.5 mm Subset of the Main Study Clinical Results – Single
versus Dual Lesion Subjects
Single Lesion 2.5 - 3.5
mm Subset of Main
study
(N = 1001 subjects)
Outcomes at 12 Months Outcomes at 60 Months
Dual Lesion* 2.5 - 3.5
mm Subset of Main
study
(N = 111 sub jects)
Single Lesion 2.5 -3.5
mm Subset of Main
study
(N = 1001 subjects)
Dual Lesion* 2.5 -3.5
mm Subset of Main
study
(N = 111 sub jects)
COMPOSITE SAFETY AND
EFFECTIVENESS
TLF
TVF
MACE
3.6% (36/994)
5.0% (50/994)
4.3% (43/994)
EFFECTIVENESS
Clinically Driven TVR
TLR
TLR, PCI
TLR, CABG
Non-TL TVR
Non-TL TVR, PCI
Non-TL TVR, CABG
3.7% (37/994)
2.1% (21/994)
1.8% (18/994)
0.3% (3/994)
1.8% (18/994)
1.5% (15/994)
0.3% (3/994)
5.4% (6/111)
7.2% (8/111)
7.2% (8/111)
5.4% (6/111)
3.6% (4/111)
3.6% (4/111)
0.0% (0/111)
2.7% (3/111)
1.8% (2/111)
0.9% (1/111)
15.7% (149/947) 19.2% (20/104)
16.3% (154/947) 20.2% (21/104)
11.2% (106/947) 13.5% (14/104)
10.3% (98/947) 16.3% (17/104)
5.3% (50/947) 9.6% (10/104)
4.5% (43/947) 9.6% (10/104)
0.7% (7/947) 0.0% (0/104)
7.4% (70/947) 6.7% (7/104)
6.7% (63/947) 3.8% (4/104)
0.7% (7/947) 2.9% (3/104)
SAFETY
Total Death
Cardiac Death
Non-Cardiac Death
Cardiac Death or TVMI
TVMI
Q wave MI
Non-Q wave MI
1.0% (10/994)
0.5% (5/994)
0.5% (5/994)
1.7% (17/994)
1.2% (12/994)
0.2% (2/994)
1.0% (10/994)
0.9% (1/111)
0.0% (0/111)
0.9% (1/111)
1.8% (2/111)
1.8% (2/111)
0.0% (0/111)
1.8% (2/111)
8.8% (83/947) 7.7% (8/104)
3.3% (31/947) 4.8% (5/104)
5.5% (52/947) 2.9% (3/104)
5.9% (56/947) 6.7% (7/104)
3.3% (31/947) 2.9% (3/104)
0.4% (4/947) 0.0% (0/104)
2.9% (27/947) 2.9% (3/104)
Sten t Thro mb osi s
ARC defined
Definite/Probable
0.0% (0/994) 0.0% (0/111)
Definite 0.0% (0/994) 0.0% (0/111)
Probable 0.0% (0/994) 0.0% (0/111)
0.5% (5/947) 0.0% (0/104)
0.3% (3/947) 0.0% (0/104)
0.2% (2/947) 0.0% (0/104))
ACUTE SUCCESS
Procedure Success 98.7% (982/995) 98.2% (108/110)
28
Page 32

Notes
* Included in the 111 subject dual lesion s ubset are 95 subjec ts wit h 1 treated lesion within 2 different vessels , 13 subject s with 2 treated
lesions within a single vessel, 1 subject w ith 3 treated lesions w ithin a s ingle vessel, and 2 subjects with 1 treat ed lesion within a single
vessel plus 2 treated lesions w ithin a dif ferent single vess el.
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
Procedure success is defined as attainment of <50 % residual stenosis of the target lesion and no in-hospital MACE.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
60-month timeframe includes follow-up window (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined Stent Thrombos is.
2.25 mm Cohort
Demographics and clinical characteristics: There were 150 subjects. The mean age of all subjects
was 66.3 years with 64.7% (97/150) being males. 34.0% (49/144) had a prior history of MI, 42.0%
(63/150) had a prio r history of PCI, and 15.3% (23/150) had previous CABG surgery. 41.3% (62/150)
were d iabetics, with 10.7% (16/150) being insulin dependent diabetics. Past medical history of subjects
indicated 90.0% (135/150) had hyperlipidemia, 90.7% (136/150) had hypertension, and 12.7% (19/150)
were current smokers. The mean RVD by QCA was 2.15 ± 0.40 mm, the lesion length was 12.40 ± 6.03
mm and the average percentage diameter stenosis was 72.21 ± 10.45%. 67.9% of lesions (133/196)
were characterized as ACC/AHA type B2/C.
Primary Endpoint: The p rimary endpoint in the 2.25 mm Cohort was Target Lesion Failure (TLF) at 12
months post-procedure, defined as the Cardiac Death, Target Vessel Myocardial Infarction (MI), or
clinically-driven Targ et Lesion Revascularization (TLR).
Control Group and Statistical Analysis Plan: The primary endpoint of 12-month TLF was compared
to a perf ormance goal that was derived from a logistic regression of TLF rates in subjects treated with
Endeavor or Driver stents pooled from the following studies: ENDEAVOR II, ENDEAVOR III, and
ENDEAVOR IV. The performance goal was set at 20%, which was 55% above the expected TLF rate
f or a drug-eluting stent and preserved 50% of the benefit of a drug -eluting stent vs. a bare metal stent.
Results: The Resolute stent 2.25 mm Cohort met the 12-month TLF rate primary endpoint performance
goal of 20%, with a rate of 4.8% (7/147) and an upper one-sided 95% CI of 8.8%. (P-value <0.001).
These analyses are based on the intent-to-treat population. The results are presented in the following
tables:
• Table 9-6: RESOLUTE US 2.25mm Cohort - Primary Endpoint Analysis,
• Table 9-7: RESOLUTE US 2.25mm Cohort - Principal Safety and Effectiveness
• Table 9-8: RESOLUTE US 2.25mm Cohort - ARC Defined Definite/Probable Stent Thrombosis
Through 60 Months
29
Page 33

(
Table 9-6: RESOLUTE US 2.25 mm Cohort - Primary Endpoint Analysis
2.25 mm Cohort
12-month TLF 4.8% (7/147) 20% 8.8%
Notes
N = The t otal number of subject s enrolled.
Subjects are only counted once for eac h time period.
The numbers are % (Count/Number of Eligible Subjects) or least squares mean ± standard error.
The primary endpoint analysis utilized a randomly selec ted lesion from subjec ts who had treatm ent of dual 2.25m m lesions.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
1
One-sided confidence interval using normal approximation.
2
One sided p-value test using asymptot ic test statistic, to be com pared at a 0.05 significanc e level.
Resolute
N = 150)
Performance Goal
Upper One-sided
95% CI1
P-value2
<0.001
30
Page 34

(
Table 9-7: RESOLUTE US 2.25mm Cohort – Principal Safety and Effectiveness
2.25 mm Cohort
N = 150)
COMPOSITE SAFETY AND EFFECTIVENESS 12 Month 60 Months
TLF
TVF
MACE
5.4% (8/147) 18.5% (27/146)
8.2% (12/147) 27.4% (40/146)
6.8% (10/147) 23.3% (34/146)
EFFECTIVENESS
Clinically Driven TVR
TLR
TLR, PCI
TLR, CABG
Non-TL TVR
Non-TL TVR, PCI
6.8% (10/147)
4.1% (6/147)
4.1% (6/147) 8.2% (12/146)
0.0% (0/147)
2.7% (4/147)
2.7% (4/147)
Non-TL TVR, CABG 0.0% (0/147)
SAFETY
Total Death
Cardiac Death
Non-Cardiac Death
Cardiac Death or TVMI
2.7% (4/147) 15.1% (22/146)
1.4% (2/147) 10.3% (15/146)
1.4% (2/147) 4.8% (7/146)
2.0% (3/147)
TVMI 0.7% (1/147)
Q wave MI 0.0% (0/147)
Non-Q wave MI 0.7% (1/147)
Stent Thrombosis
ARC defined
Definite/Probable 1.4% (2/147)
Definite 0.7% (1/147)
Probable 0.7% (1/147)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
60-month timeframe includes follow-up window (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined Stent Thrombos is.
2.25 mm Cohort (N = 150)
19.9% (29/146)
8.2% (12/146)
0.0% (0/146)
13.7% (20/146)
11.6% (17/146)
2.1% (3/146)
12.3% (18/146)
3.4% (5/146)
0.7% (1/146)
2.7% (4/146)
1.4% (2/146)
0.7% (1/146)
0.7% (1/146)
31
Page 35

p
Table 9-8: RESOLUTE US 2.25mm Cohort - ARC Defined Definite/Probable Stent
Thrombosis Through 60 Months
Stent Thrombosis
Acute (0 - 1 day)
Subacute (2 - 30 day s)
Late (31 days - 360 days )
Very Late (361 - 1800 days)
Notes
N = The t otal number of subject s enrolled.
Subjects are only counted once for eac h time period.
Numbers are % (Count/Number of Eligible Subjects).
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
60-month timeframe includes follow-up window (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined St ent Thrombosis.
To be included in the calculation of stent t hrombosis (ST ) rate for a given int erval, a patient either had to have a stent
thrombosis during the interval (e.g. 31-360 days inclus ive) or had to be stent t hrombosis-f ree during the int erval with las t
follow-u
on or after the first day of the given interval (e.g. 31 days).
2.25 mm Cohort
(N = 150)
1.4% (2/146)
0.0% (0/146)
0.7% (1/146)
0.7% (1/146)
0.0% (0/146)
2.25 mm – 3.5 mm Angio/IVUS Sub-study
Demographics and clinical characteristics: There were 100 subjects. The mean age of all subjects
was 64.9 years with 62.0% (62/100) being males. 22.0% (22/100) had a prior history of MI, 29.0%
(29/100) had a prio r history of PCI, and 35.0% (11/100) had previous CABG surgery. 35.0% (35/100)
were d iabetics, with 9.0% (9/100) being insulin dependent diabetics. Past medical history of subjects
indicated 86.0% (86/100) had hyperlipidemia, 84.0% (84/100) had hypertension, and 20.0% (20/100)
were current smokers. The mean RVD by QCA was 2.48 ± 0.38 mm, the lesion length was 14.04 ± 5.87
mm and the average percentage diameter stenosis was 70.75 ± 11.57%. 76.0% of lesions (79/104)
were characterized as ACC/AHA type B2/C.
Primary Endpoint: The p rimary endpoint in the 2.25 mm to 3.5 mm Angio/IVUS Sub-study was in-stent
late lumen loss (LL) at 8 months post-procedure as measured by quantitative coronary angiography
(QCA).
Control group and Statistical Analysis Plan: The primary analysis was a non-inferiority comparison
of the 8-month in-stent late LL in the Resolute stent compared to a historical control population of
sub jects treated with an Endeavor stent in the ENDEAVOR II trial. The non-inferiority margin was set at
0.16 mm.
Results: The 2.25 mm – 3.5 mm Ang io/IVUS Sub-study met the primary non-inferiority endpoint with an
8-month in-stent late LL of 0.39 ± 0.06 mm for the Resolute stent compared to the 8-month in-stent late
LL histo rical control of 0.61 ± 0.03 mm for the Endeavor stent P
These analyses are based on the intent-to-treat population. The results are presented in the following
tables:
• Table 9-9: RESOLUTE US 2.25-3.5 mm Angio/IVUS Sub-study - Primary Endpoint Analysis
• Table 9-10: RESOLUTE US 2.25-3.5 mm Angio/IVUS Sub-study - Principal Safety and
Ef fectiveness
• Table 9-11: RESOLUTE US 2.25-3.5 mm Angio/IVUS Sub-study - ARC Defined Definite/Probable
Stent Thrombosis through 60 Months
• Table 9-12: RESOLUTE US 2.25-3.5 mm Angio/IVUS Sub-study - Ang iographic and IVUS Results
non-i nferi ori ty
<0.001.
32
Page 36

(
g
Table 9-9: RESOLUTE US 2.25 mm – 3.5 mm Angio/IVUS Sub-study - Primary Endpoint Analysis
2.25 mm – 3.5 mm
Angio/IVUS Sub-study
8-month In-Stent Late Lumen
Loss (mm)
Notes
N = The t otal number of subject s enrolled.
M = The t otal number of lesions at baseline.
Subjects are only counted once for each time period.
The numbers are least squares mean ± standard error (number of evaluable lesions).
The primary endpoint analysis utilized a randomly selec ted lesion from subjec ts who had treatm ent of dual lesions .
1
The CI and P-values are adjusted to propensity sc ore, based on lesion length, baseline RVD, age, sex, diabet es, hist ory of MI and worst
Canadian Cardiovascular Society Angina Class as t he independent variables.
2
One-sided p-value by non-inferiority test using asym ptotic t est st atistic wit h non-inferiority m argin of 0.16 mm , to be com pared at a 0.05
si
nificance level.
Resolute
(N = 100, M =104)
0.39 ± 0.06 (90) 0.61 ± 0.03 (264) -0.22 -0.11 <0.001
Historical Control
Endeavor
N = 264, M = 264)
Difference: Resolute -
Historical Control
Upper One-sided
95% CI1
Non-inferiority
P value
1,2
33
Page 37

(
(
Table 9-10: RESOLUTE US 2.25-3.5mm Angio/IVUS Sub-study - Principal Safety and
Effectiveness
2.25 mm - 3.5 mm
Angio/IVUS Sub-study
N = 100)
2.25 mm - 3.5 mm
Angio/IVUS Sub-study
N = 100)
COMPOSITE SAFETY AND EFFECTIVENESS 12 Months 60 Months
TLF
TVF
MACE
12.1% (12/99) 18.6% (18/97)
13.1% (13/99) 20.6% (20/97)
13.1% (13/99) 21.6% (21/97)
EFFECTIVENESS
Clinically Driven TVR
TLR
TLR, PCI
TLR, CABG
Non-TL TVR
Non-TL TVR, PCI
Non-TL TVR, CABG 0.0% (0/99)
10.1% (10/99) 16.5% (16/97)
8.1% (8/99)
7.1% (7/99)
1.0% (1/99) 11.3% (11/97)
4.0% (4/99) 8.2% (8/97)
4.0% (4/99) 7.2% (7/97)
12.4% (12/97)
3.1% (3/97)
2.1% (2/97)
SAFETY
Total Death
Cardiac Death
Non-Cardiac Death
Cardiac Death or TVMI
TVMI
Q wave MI 0.0% (0/99)
Non-Q wave MI 1.0% (1/99)
Stent Thrombosis
ARC defined
Definite/Probable 0.0% (0/99)
Definite 0.0% (0/99)
Probable 0.0% (0/99)
4.0% (4/99) 10.3% (10/97)
3.0% (3/99) 6.2% (6/97)
1.0% (1/99) 4.1% (4/97)
4.0% (4/99)
1.0% (1/99)
7.2% (7/97)
2.1% (2/97)
0.0% (0/97)
2.1% (2/97)
0.0% (0/97)
0.0% (0/97)
0.0% (0/97)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
60-month timeframe includes follow-up w indow (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined Stent Thrombos is.
34
Page 38

(
p
Table 9-11: RESOLUTE US 2.25-3.5 mm Angio/IVUS Sub-study - ARC Defined
Definite/Probable Stent Thrombosis Through 60 Months.
Stent Thrombosis
Acute (0 - 1 day)
Subacute (2 - 30 days)
Late (31 – 360 days)
Very Late (361 - 1440 days)
Notes
N = The t otal number of subject s enrolled.
Subjects are only counted once for eac h time period.
Numbers are % (Count/Number of Eligible Subjects).
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
60-month timeframe includes follow-up window (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined St ent Thrombosis.
To be included in the calculation of stent t hrombosis (ST ) rate for a given int erval, a patient either had to have a stent
thrombosis during the interval (e.g. 31 - 360 days inclusive) or had t o be st ent thrombos is-free during the interval w ith last
follow-u
on or after the first day of the given interval (e.g. 31 days).
2.25 mm - 3.5 mm Angio/IVUS Sub-study
N = 100)
0.0% (0/97)
0.0% (0/97)
0.0% (0/97)
0.0% (0/97)
0.0% (0/97)
35
Page 39

(
Table 9-12: RESOLUTE US 2.25-3.5 mm Angio/IVUS Sub-study -
Angiographic and IVUS Results
2.25 mm - 3.5 mm Angio/IVUS Sub-study
Outcomes at 8 Months
ANGIOGRAPHIC RESULTS
MLD (mm), In-stent
Post-Procedure 2.44 ± 0.39 (104)
8-Month 2.06 ± 0.66 (93)
MLD (mm), In-segment
Post-Procedure 2.06 ± 0.39 (104)
8-Month 1.80 ± 0.58 (93)
% DS, In-stent
Post-Procedure 4.07 ± 10.12 (104)
8-Month 16. 40 ± 23.55 (93)
% DS, In-segment
Post-Procedure 19.41 ± 8.22 (104)
8-Month 26. 86 ± 19.65 (93)
Late Loss (mm)
In-stent 0.36 ± 0.52 (93)
In-segment 0.24 ± 0.43 (93)
Binary Restenosis
In-stent 10.8% (10/93)
In-segment 11.8% (11/93)
IVUS RESULTS
Neointimal Volume (m m3) 7.29 ± 9.30 (63)
% Volume Obstruction 5.34 ± 5.97 (63)
Incomplete Apposition
Persist ent 16.7% (10/60)
Late Acquired 1.7% (1/60)
Notes
N = The t otal number of subject s enrolled.
M = The t otal number of lesions at baseline.
Numbers are % (Count/Number of Evaluable Les ions) or Mean ± SD (N umber of Evaluable
Lesions).
Subjects are only counted once for each time period.
N = 100, M = 104)
36
Page 40

4.0 mm Sub-study
Demographics and clinical characteristics: There were 60 subjects. The mean ag e of all subjects
was 63.7 years with 66.7% (40/60) being males. 20.0% (12/60) had a prior history of MI, 25.0% (15/60)
had a prior history of PCI and 10.0% (6/60) had previous CABG surgery. 36.7% (22/60) were diabetics,
with 10.0% (6/60) being insulin dependent diabetics. Past medical history of subjects indicated 80.0%
(48/60) had hyperlipidemia, 85.0% (51/60) had hypertension, and 23.3% (14/60) were current smokers.
The mean RVD by QCA was 3.25 ± 0.48 mm, the lesion length was 12.83 ± 5.97 mm and the average
percentage diameter stenosis was 67.70 ± 13.09%. 79.1% of lesions (57/72) were characterized as
ACC/AHA type B2/C.
Primary Endpoint: The p rimary endpoint in the 4.0 mm Sub-study was in-segment late LL at 8 months
post-procedure as measured by QCA.
Control Group and Statistical Analysis Plan: The primary analysis was a superiority comparison of
the 8-month in-segment late LL in the Resolute stent compared to a historical control population of
sub jects treated with a Driver bare metal stent of diameters 3.5 mm or 4.0 mm in the Medtronic S8
Driver stent registry (6-month late LL) and the ENDEAVOR II trial (8-mo nth late LL).
Results: The 4.0 mm Resolute stent met the primary superiority endpoint with an 8-month in-segment
late LL of 0.11 ± 0.09 mm, compared with the historical Driver stent control in-segment late LL of 0.66 ±
0.05 mm, P
super iority
<0.001.
These analyses are based on the intent-to-treat population. The results are presented in the following
tables:
• Table 9-13: RESOLUTE US 4.0 mm Sub-study - Primary Endpoint Analyses
• Table 9-14: RESOLUTE US 4.0 mm Sub-study – Principal Safety and Effectiveness
• Table 9-15: RESOLUTE US 4.0 mm Sub-study - ARC Defined Definite/Probable Stent Thrombosis
through 60 Months
• Table 9-16: RESOLUTE US 4.0 mm Sub-study - Angiographic Results
37
Page 41

(
Table 9-13: RESOLUTE US 4.0 mm Sub-study - Primary Endpoint Analyses
4.0 mm Sub-study
8-month In-Segment Late
Lumen Loss (mm)
Notes
N = The t otal number of subject s enrolled.
M = The t otal number of lesions at baseline.
Subjects are only counted once for eac h time period.
The numbers are least squares mean ± standard error (number of evaluable lesions).
The primary endpoint analysis utilized a randomly selec ted lesion from subjec ts who had treatment of dual 4.0 m m lesions
1
The CI and P-values are adjusted to propensity sc ore, based on lesion length, baseline RVD, age, sex, diabet es, hist ory of MI and worst
Canadian Cardiovascular Society Angina Class as t he independent variables.
2
One sided p-value by superiority test using asymptotic test statistic, to be compared at a 0.05 significance level.
Resolute
(N = 60, M = 72)
0.11 ± 0.09 (50) 0.66 ± 0.05 (150) -0.56 -0.38 <0.001
Historical Control
Driver
N = 150, M = 150)
Difference: Resolute -
Historical Control
Upper
One-sided
95% CI1
Superiority
1,2
P-value
38
Page 42

(
(
Table 9-14: RESOLUTE US 4.0 mm Sub-study – Principal Safety and Effectiveness
4.0 mm Sub-Study
N = 60)
COMPOSITE SAFETY AND EFFECTIVENESS 12 Months 60 Months
TLF 6.8% (4/59)
TVF 6.8% (4/59)
MACE 8.5% (5/59)
EFFECTIVENESS
Clinically Driven TVR 3.4% (2/59)
TLR 3.4% (2/59)
TLR, PCI 3.4% (2/59)
TLR, CABG 0.0% (0/59)
Non-TL TVR 1.7% (1/59)
Non-TL TVR, PCI 1.7% (1/59)
Non-TL TVR, CABG 0.0% (0/59)
SAFETY
Total Death 1.7% (1/59)
Cardiac Death 0.0% (0/59)
Non-Cardiac Deat h 1.7% (1/59)
Cardiac Death or TVMI 3.4% (2/59)
TVMI 3.4% (2/59)
Q wave MI 0.0% (0/59)
Non-Q wave MI 3.4% (2/59)
Sten t Thro mb osi s ARC
defined
Definite/Probable 0.0% (0/59)
Definite 0.0% (0/59)
Probable 0.0% (0/59)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
60-month timeframe includes follow-up w indow (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined Stent Thrombos is.
4.0 mm Sub-Study
N = 60)
12.7% (7/55)
14.5% (8/55)
23.6% (13/55)
9.1% (5/55)
7.3% (4/55)
7.3% (4/55)
0.0% (0/55)
7.3% (4/55)
5.5% (3/55)
1.8% (1/55)
10.9% (6/55)
0.0% (0/55)
10.9% (6/55)
5.5% (3/55)
5.5% (3/55)
0.0% (0/55)
5.5% (3/55)
0.0% (0/55)
0.0% (0/55)
0.0% (0/55)
39
Page 43

(e.g
Table 9-15: RESOLUTE US 4.0mm Sub-study - ARC Defined
Definite/Probable Stent Thrombosis Through 60 Months
Stent Thrombosis
Acute (0 - 1 day)
Subacute (2 - 30 day s)
Late (31 - 360 days)
Very Late (361 - 1440 days)
Notes
N = The t otal number of subject s enrolled.
Subjects are only counted once for eac h time period.
Numbers are % (Count/Number of Eligible Subjects).
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
60-month timeframe includes follow-up window (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined Stent T hrombosis.
To be included in the calculation of stent thrombos is (ST) rate f or a given interval, a patient either
had to have a stent throm bosis during the interval (e.g. 31 - 360 day s inclusiv e) or had to be s tent
thrombosis-free during the interval with las t follow-up on or af ter the firs t day of t he given int erval
. 31 days).
4.0 mm Sub-study
(N = 60)
0.0% (0/55)
0.0% (0/55)
0.0% (0/55)
0.0% (0/55)
0.0% (0/55)
40
Page 44

(
j
Table 9-16: RESOLUTE US 4.0 mm Sub-study Angiographic
Results
Outcomes at 8 Months
ANGIOGRAPHIC RESULTS
MLD (mm), In-stent
Post-Procedure 3.12 ± 0.38 (72)
8-Month 2.94 ± 0.65 (60)
MLD (mm), In-segment
Post-Procedure 2.75 ± 0.45 (72)
8-Month 2.60 ± 0.60 (60)
% DS, In-stent
Post-Procedure 4.54 ± 9.36 (72)
8-Month 9.37 ± 19.48 (60)
% DS, In-segment
Post-Procedure 16.62 ± 8.27 (72)
8-Month 20.22 ± 14.79 (60)
Late Loss (mm)
In-stent 0.19 ± 0.56 (60)
In-segment 0.14 ± 0.43 (60)
Binary Restenosis
In-stent 6.7% (4/60)
In-segment 6.7% (4/60)
Notes
N = The t otal number of subject s enrolled.
M = The t otal number of lesions at baseline.
Numbers are % (Count/Number of Evaluable Les ions) or Mean ± SD (N umber of Evaluable
Lesions).
ects are only counted once for each time period.
Sub
4.0 mm Sub-study
N = 60, M = 72)
41
Page 45

RESOLUTE US – Primary Enrollment Group - Gender Analysis
Table 9-17 shows the baseline demographic and clinical characteristics stratified by gender for subjects
in the pooled RESOLUTE US analysis, 445/1402 (31.7%) subjects were female and 957/1402 (68.3%)
were male. Consistent with other DES clinical studies, female patients were older, had a hig her rate of
diabetes and hypertension and had smaller reference vessel diameters (RVD).
Table 9-17: RESOLUTE US Baseline Demographic and Lesion Characteristics –
Male vs. Female
Patient Characteristics
Age (Years)
History of smoking/tobac co use
Prior PCI
Hyperlipidemia
Diabetes Mellitus
Insulin Dependent
Hypertension
Prior MI
Prior CABG
Ejection fraction - Qualitative
<30%
30 - 40%
>40%
Lesion Class
A
B1
B2
C
Moderate/Severe Calcification
Pre procedure RVD
Pre procedure MLD
Pre procedure Diameter Stenosis
Lesion Length
The 12-mo nth rate o f TLF was 4.5% in males and 5.0% in f emales (Table 9-18). This post hoc analysis
sho ws a generally similar treatment effect between genders for the primary endpoint of 12-month TLF.
These data suggest that the safety and effectiveness of the Resolute stent can be generalized to males
and females.
Male
(N=957)
63.14±10.48 (957) 66.23±10.76 (445) <.001
67.0% (641/957) 52.8% (235/445) <.001
34.2% (327/957) 29.4% (131/445) 0.087
88.5% (847/957) 86.1% (383/445) 0.221
31.3% (300/957) 40.9% (182/445) <.001
7.2% (69/957) 14.8% (66/445) <.001
82.2% (787/957) 88.3% (393/445) 0.004
24.6% (232/943) 15.1% (66/436) <.001
10.6% (101/957) 5.2% (23/445) <.001
0.12% (1/823) 0.26% (1/386)
6.68% (55/823) 3.37% (13/386)
93.20% (767/823) 96.37% (372/386)
5.55% (60/1082) 7.64% (37/484)
17.10% (185/1082) 22.11% (107/484)
30.87% (334/1082) 27.48% (133/484)
46.49% (503/1082) 42.77% (207/484)
26.0% (281/1082) 28.9% (140/484) 0.241
2.62±0.48 (1082) 2.53±0.44 (484) <.001
0.75±0.35 (1082) 0.79±0.34 (484) 0.058
71.43±11.57 (1082) 68.98±11.24 (484) <.001
13.27±5.89 (1082) 12.60±5.83 (484) 0.036
Female
(N=445)
p-value
0.028
0.022
42
Page 46
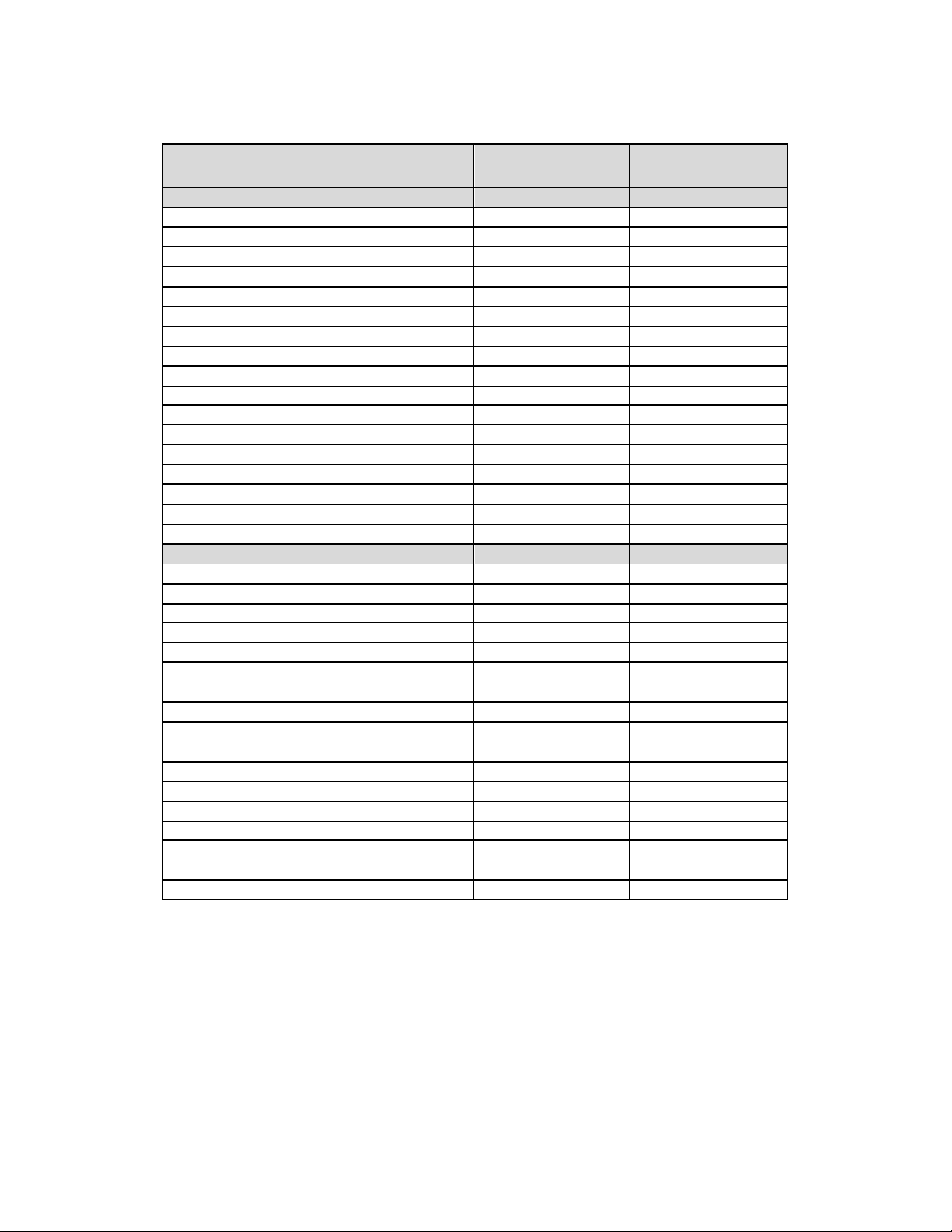
Table 9-18: RESOLUTE US Primary Enrollment Group - Clinical
Endpoints by Gender – Principal Safety and Effectiveness to 60
Months
Male
Safety Measures to 12 Months
COMPOSITE SAFETY AND EFFECTIVENESS
TLF 4.5% (43/952) 5.0% (22/438)
TVF 5.7% (54/952) 7.3% (32/438)
MACE 5.6% (53/952) 5.5% (24/438)
EFFECTIVENESS
Clinically Driven TVR 4.4% (42/952) 5.0% (22/438)
Clinically Driven TLR 3.0% (29/952) 2.5% (11/438)
SAFETY
Death 1.4% (13/952) 1.4% (6/438)
Cardiac Death 0.6% (6/952) 0.9% (4/438)
Non-Cardiac Death 0.7% (7/952) 0.5% (2/438)
TVMI (Extended Historical Definition) 1.1% (10/952) 1.8% (8/438)
Cardiac Death or Target Ves sel MI (T VMI) 1.7% (16/952) 2.7% (12/ 438)
Stent Thrombosis ARC defined
Definite/Probable 0.2% (2/952) 0.0% (0/438)
Definite 0.1% (1/952) 0.0% (0/438)
Probable 0.1% (1/952) 0.0% (0/438)
Safety Measures to 60 Months
COMPOSITE SAFETY AND EFFECTIVENESS
TLF
TVF
MACE
EFFECTIVENESS
Clinically Driven TVR
Clinically Driven TLR
SAFETY
Death
Cardiac Death
Non-Cardiac Death
TVMI (Extended Historical Definition)
Cardiac Death or Target Ves sel MI (T VMI)
Stent Thrombosis ARC defined
Definite/Probable
Definite
Probable
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
60-month timeframe includes follow-up window (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined Stent Thrombos is
(N = 957)
12.4% (113/909) 12.1% (51/420)
17.5% (159/909) 17.6% (74/420)
18.0% (164/909) 17.9% (75/420)
12.7% (115/909) 12.1% (51/420)
6.9% (63/909) 5.5% (23/420)
9.4% (85/909) 10.0% (42/420)
4.0% (36/909) 4.5% (19/420)
5.4% (49/909) 5.5% (23/420)
2.8% (25/909) 4.3% (18/420)
6.2% (56/909) 7.9% (33/420)
0.6% (5/909) 0.5% (2/420)
0.2% (2/909) 0.5% (2/420)
0.3% (3/909) 0.0% (0/420)
Female
(N = 445)
See Section 9.9.1 - Gender Analysis from the RESOLUTE Pooled On-label Dataset for the
co mprehensive gender analysis.
43
Page 47

g
(
RESOLUTE 38 mm Length Group
The 38 mm Length Gro up was designed to demonstrate the safety and effectiveness of the Resolute 38
mm stent and consisted of subjects with ischemic heart disease due to a stenotic lesion in a de novo
native coronary artery with a reference vessel diameter between 3.0 and 4.2 mm and a lesion length ≤
35 mm amenable to percutaneous treatment with a 38 mm Resolute stent. The 38 mm Length Group
was made up of 38 mm subjects pooled from the RESOLUTE US and RESOLUTE ASIA studies. 223
sub jects were enrolled at 47 sites with 114 subjects at 29 sites in the US and 109 subjects at 17 sites in
Asia: Bangladesh, India, Hong Kong, Malaysia, Singapore, and Thailand.
Demographics and clinical characteristics: There were 223 sub jects. The mean age of all subjects
was 60.9 years with 78.9% (176/223) being males. 32.4% (70/216) had a prior history of MI, 27.4%
(61/223) had a prio r history of PCI and 7.2% (16/223) had previous CABG surgery. 37.7% (84/223)
were d iabetics, with 10.3% (23/223) being insulin dependent diabetics. Past medical history of subjects
indicated 58.7% (131/223) had hyperlipidemia, 74.9% (167/223) had hypertension, and 18.8% (42/223)
were current smokers. The mean RVD by QCA was 2.78±0.42 mm, the lesion length was 25.22 ± 8.83
mm and the average percentage diameter stenosis was 71.33 ± 11.61%. 91.2% of lesions (240/263)
were characterized as ACC/AHA type B2/C.
Primary Endpoint: The p rimary endpoint of the 38 mm Length Group was Target Lesion Failure (TLF)
at 12 months post-procedure, defined as Cardiac Death, Target Vessel Myocardial Infarction (MI), or
clinically driven Target Lesion Revascularization (TLR).
Control Group and Statistical Analysis Plan: The primary endpoint of 12-month TLF was compared
to a perf ormance goal that was derived from a logistic regression of TLF rates in subjects treated with
Endeavor or Driver stents pooled from the Endeavor stent clinical program: ENDEAVOR I, ENDEAVOR
II, ENDEAVOR II CA, ENDEAVOR III, ENDEAVOR IV and E NDEAVOR PK . The performance goal was
set at 19%, which was 48% above the expected TLF rate for a drug-eluting stent and preserved 51% of
the b enef it of a drug-eluting stent vs. a bare metal stent.
Results: The 38 mm Leng th Group 12-month TLF rate was 4.5% (10/223) with an upper one-sided 95%
CI of 7.5%, which met the performance goal of 19%, (P-value <0.001). The 12-month and 60-month
f ollow-up rates were 99.1% (221/223) and 93.7% (209/223), respectively.
These analyses are based on the intent-to-treat population. The results are presented in the following
tables:
• Table 9-19: 38 mm Length Group – Primary Endpoint Analyses
• Table 9-20: 38 mm Leng th Group - Principal Safety and Effectiveness Through 60 Months
• Table 9-21: 38 mm Leng th Group – ARC Defined/Probable Stent Thrombosis Through 60 Months
• Table 9-22: Pooled Resolute Analysis including the 38 mm Length Group
Table 9-19: 38 mm Len
38 mm Length Group
12-month TLF
Notes
N = The t otal number of subject s enrolled.
Subjects are only counted once for eac h time period.
The numbers are % (Count/Number of Eligible Subjects).
The primary endpoint analysis utilized a randomly selec ted lesion from subjec ts who had treatment of dual 38 mm lesions.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
1
One-sided confidence interval using normal approximation.
2
One-sided p-value test using asymptotic test statistic, to be compared at a 0.05 significance level
th Group - Primary Endpoint Analysis
Resolute
N=223)
4.5% (10/223) 19.00% 7.5% <0.001
Performance
Goal
Upper One-sided
95% CI1
P-value2
44
Page 48

(
(
(
(
Table 9-20: 38 mm Length Group – Principal Safety and Effectiveness
COMPOSITE SAFETY AND
EFFECTIVENESSS
TLF
TVF
MACE
EFFECTIVENESS
Clinically-driven TVR
TLR
TLR, PCI
TLR, CABG
Non-TL TVR
Non-TL TVR, PCI
Non-TL TVR, CABG
SAFETY
Total Death
Cardiac Death
Non-Cardiac Death
Cardiac Death or TVMI
TVMI
Q wave MI
Non-Q wave MI
38 mm Length Group
N = 223)
Outcomes at 12
Months
5.4% (12/222) 7.1% (8/113) 3.7% (4/109) 13.8% (30/217)
6.8% (15/222) 9.7% (11/113) 3.7% (4/109) 17.1% (37/217)
6.3% (14/222) 8.8% (10/113) 3.7% (4/109) 17.5% (38/217)
2.7% (6/222) 4.4% (5/113) 0.9% (1/109)
1.4% (3/222) 1.8% (2/113) 0.9% (1/109)
1.4% (3/222) 1.8% (2/113) 0.9% (1/109)
0.0% (0/222) 0.0% (0/113) 0.0% (0/109)
1.4% (3/222) 2.7% (3/113) 0.0% (0/109)
1.4% (3/222) 2.7% (3/113) 0.0% (0/109)
0.0% (0/222) 0.0% (0/113) 0.0% (0/109)
0.9% (2/222) 1.8% (2/113) 0.0% (0/109)
0.9% (2/222) 1.8% (2/113) 0.0% (0/109)
0.0% (0/222) 0.0% (0/113) 0.0% (0/109)
4.5% (10/222) 5.3% (6/113) 3.7% (4/109)
3.6% (8/222) 3.5% (4/113) 3.7% (4/109)
0.9% (2/222) 0.9% (1/113) 0.9% (1/109)
2.7% (6/222) 2.7% (3/113) 2.8% (3/109)
Side Branch Occlusion† 5.4% (12/222) 7.1% (8/113) 3.7% (4/109) 5.5% (12/217)
Stent Thrombosis
ARC Defined
Definite/Probable
Definite
0.9% (2/222) 0.9% (1/113) 0.9% (1/109)
0.5% (1/222) 0.0% (0/113) 0.9% (1/109)
Probable 0.5% (1/222)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
The definitions of the outcomes are presented as table notes to Table 8-1: Principal Adverse Events from PostProcedure Through Latest Available Follow-up- Princ ipal Advers e Event s.
12-month timeframe includes follow-up window (360 days ± 30 day s).
60-month timeframe includes follow-up window (1800 days ± 30 day s).
† 5 subjects with side branch oc clusion had a TVM I
See Table 9-4: RESOLUTE US 2.5-3.5 m m Subset of the M ain Study - ARC Def ined Def inite/Probable Stent
Thrombosis Through 60 Months f or the definition of the ARC def ined Stent Throm bosis.
R-US Sub-study
N = 114)
Outcomes at 12
Months
0.9% (1/113)
0.0% (0/109)
R-Asia Cohort
N = 109)
Outcomes at 12
Months
38 mm Length Group
Outcomes at 60 Months
N = 223)
9.7% (21/217)
6.0% (13/217)
5.5% (12/217)
0.5% (1/217)
3.7% (8/217)
3.2% (7/217)
0.5% (1/217)
6.5% (14/217)
4.1% (9/217)
2.3% (5/217)
8.8% (19/217)
6.0% (13/217)
0.9% (2/217)
5.1% (11/217)
1.4% (3/217)
0.5% (1/217)
0.9% (2/217)
45
Page 49

y
Table 9-21: 38 mm Length Group – ARC Defined
Definite/Probable Stent Thrombosis Through 60 Months
Stent Thrombosis 1.4% (3/217)
Acute (0-1 day) 0.0% (0/217)
Subacute (2-30 days) 0.9% (2/217)
Late (31-360 days) 0.0% (0/217)
Very Late (361-1800 days) 0.5% (1/217)
Notes
N = The t otal number of subject s enrolled.
Subjects are only counted once for eac h time period.
Numbers are % (Count/Number of Eligible Subjects).
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
60-month timeframe includes follow-up window (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined St ent Thrombosis.
To be included in the calculation of stent t hrombosis (ST ) rate for a given int erval, a
patient either had to have a stent thrombosis during the interv al (e.g. 31-360 days
inclusive) or had to be stent throm bosis-free during t he interv al with last follow-up on
or after the first da
of the given interval (e.g. 31 days).
38 mm Length Group
(N = 223)
Table 9-22 shows the Pooled Resolute Analysis including the 38 mm Length Groups. The safety and
ef f icacy data from the 38 mm Length Group and five (5) Resolute studies; Resolute FIM, Resolute US
(PEG), Resolute International, Resolute All-Comers and Resolute Japan are presented to 60 Months.
Table 9-22: Pooled Resolute Analysis including the 38 mm Length Groups -
Through 60 Months
Poole d Resolute analysis with the 38mm Length Group
Safety Measures to 12 Months
COMPOSI TE SAFETY AND EFFECTIVENE SS
TLF 6.5% (348/5321)
TVF 7.5% (397/5321)
MACE 7.5% (398/5321)
EFFECTIVENESS
Clinically Drive n TVR 4.2% (226/5321)
Clinically Drive n TLR 3.2% (169/5321)
SAFETY
Death 1.9% (100/5321)
Cardiac Death 1.2% (62/5321)
Non-Cardiac Death 0.7% (38/5321)
TVMI (Extended Historical Definition) 3.0% (157/5321)
Cardiac Death or Target Vessel MI (TVMI) 3.9% (210/5321)
Stent Thrombosis ARC de fined
Definite/Probable 0.8% (42/5321)
Definite 0.6% (30/5321)
Probable 0.3% (14/5321)
Safety Measures to 36 Months
COMPOSI TE SAFETY AND EFFECTIVENE SS
TLF 10.7% (562/5234)
TVF 13.0% (681/5234)
MACE 13.5% (708/5234)
EFFECTIVENESS
Clinically Drive n TVR 7.9% (413/5234)
Clinically Driven TLR 5.3% (276/5234)
(N=5353)
46
Page 50

Table 9-22: Pooled Resolute Analysis including the 38 mm Length Groups -
Through 60 Months
Poole d Resolute analysis with the 38mm Length Group
SAFETY
Death 5.4% (284/5234)
Cardiac Death 3.1% (161/5234)
Non-Cardiac Death 2.4% (123/5234)
TVMI (Extended Historical Definition) 3.8% (200/5234)
Cardiac Death or Target Vessel MI (TVMI) 6.5% (339/5234)
Stent Thrombosis ARC de fined
Definite/Probable 1.1% (56/5234)
Definite 0.7% (38/5234)
Probable 0.4% (20/5234)
Safety Measures to 60 Months*
COMPOSI TE SAFETY AND EFFECTIVENE SS
TLF 14.0% (406/2905)
TVF 18.0% (523/2905)
MACE 19.2% (559/2905)
EFFECTIVENESS
Clinically Drive n TVR 11.3% (327/2905)
Clinically Drive n TLR 6.6% (192/2905)
SAFETY
Death 9.6% (280/2905)
Cardiac Death 4.8% (140/2905)
Non-Cardiac Death 4.8% (140/2905)
TVMI (Extended Historical Definition) 4.6% (133/2905)
Cardiac Death or Target Vessel MI (TVMI) 8.7% (253/2905)
Stent Thrombosis ARC de fined
Definite/Probable 1.3% (37/2905)
Definite 0.8% (23/2905)
Probable 0.5% (15/2905)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
36-month timeframe includes follow-up window (1080 days ± 30 day s).
60-month timeframe includes follow-up w indow (1800 day s ± 30 days).
* Note: R-Int. follow-up ends at three years and is not included in this analysis.
See Table 9-4 for the definition of the ARC defined Stent Thrombos is
(N=5353)
47
Page 51

Table 9-23 shows the baseline demographic and clinical characteristics stratified by gender for subjects
in the 38 mm Leng th Group analysis, 47/223 (21.1%) subjects were female and 176/223 (78.9%) were
male. Consistent with other DES clinical studies, female patients were older, and there was no o ther
significant difference in baseline demographic and clinical characteristics observed between gender
groups.
Table 9-23: RESOLUTE 38 mm Length Group - Baseline Demographic and Lesion
Characteristics Male vs. Female
Male
Patient Characteristics
Age (Years) 60.1±10.7 64.0±10.2 0.028
History of smoking/tobac co use 20.5% (36/176) 12.8% (6/47) 0.469
Prior PCI 25.0% (44/176) 36.2% (17/47) 0.142
Hyperlipidemia 56.3% (99/176) 68.1% (32/47) 0.182
Diabetes Mellitus 36.4% (64/176) 42.6% (20/47) 0.499
Insulin Dependent 9.1% (16/176) 14.9% (7/47) 0.280
Hypertension 72.2% (127/176) 85.1% (40/47) 0.088
Prior MI 32.4% (55/170) 32.6% (15/46) 1.000
Prior CABG 7.4% (13/176) 6.4% (3/47) 1.000
Ejection fraction - Qualitative 0.389
<30% 0.0% (0/144) 0.0% (0/40)
30-40% 4.2% (6/144) 7.5% (3/40)
>40% 95.8% (138/144) 92.5% (37/40)
Lesion Class 0.634
A 1.9% (4/209) 0.0% (0/54)
B1 8.1% (17/209) 3.7% (2/54)
B2 9.6% (20/209) 9.3% (5/54)
C 80.4% (168/209) 87.0% (47/54)
Moderate/Severe Calcification 32.5% (68/209) 44.4% (24/54) 0.111
Pre procedure RVD (mm) 2.79±0.44 2.75±0.37 0.504
Pre procedure MLD (mm) 0.80±0.36 0.80±0.36 0.912
Pre procedure Diameter Stenosis (%) 71.42±11.49 70.98±12.15 0.805
Lesion Length (mm) 25.16±8.45 25.48±10.27 0.808
(N = 176)
The 12-mo nth rate o f TLF was 4.0% in males and 10.9% in f emales (Table 9-24). Although event rates
were numerically higher in women, the number of women in the study was small. Further, the
RESOLUTE 38 mm Length study was not designed or powered to study the safety or effectiveness of
the 38 mm Resolute stent in gender-specific subgroups, so these post hoc analyses are considered
hyp o thesis-generating.
Female
(N = 47) p -value
48
Page 52

Table 9-24: RESOLUTE 38 mm Length Group - 12-Month Clinical Endpoints by
Gender – Principal Safety and Effectiveness
Male (N = 176) Female (N = 47)
COMPOSITE SAFETY AND
EFFECTIVENESS
TLF
TVF
MACE
EFFECTIVENESS
Clinically Driven TVR
TLR
SAFETY
Total Death
Cardiac Death
Non-Cardiac Death
TVMI
Cardiac Death or TVMI
Sten t Thro mb osi s
ARC defined
Definite/Probable
Definite
Probable
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
See Table 9-4 for the definition of the ARC defined St ent Thrombosis
4.0% (7/176) 10.9% (5/46)
5.1% (9/176) 13.0% (6/46)
5.1% (9/176) 10.9% (5/46)
1.7% (3/176) 6.5% (3/46)
0.6% (1/176) 4.3% (2/46)
0.6% (1/176) 2.2% (1/46)
0.6% (1/176) 2.2% (1/46)
0.0% (0/176) 0.0% (0/46)
2.8% (5/176) 6.5% (3/46)
3.4% (6/176) 8.7% (4/46)
0.6% (1/176) 2.2% (1/46)
0.0% (0/176) 2.2% (1/46)
0.6% (1/176) 0.0% (0/46)
9.2 Results of the RESOLUTE All Comers (AC) Clinical Trial
Primary Objective: To compare the Resolute Zotarolimus-Eluting Coronary Stent System (Resolute
stent) with the Ab bott XIENCE V Everolimus-Eluting Coronary Stent System (Xience V stent) in a “real
world” patient population with respect to Target Lesion Failure, composite of Cardiac Death, MI no t
clearly attributable to a non-target vessel, clinically indicated TLR at 12 months.
The data from the RESOLUTE AC trial were used to support the PMA approval of the Resolute Integrity
stent. In particular, the on-label data from the RESOLUTE AC population were pooled with other on-
label RESOLUTE program data to demonstrate the long-term safety of the Resolute stent
Section 8.1 – Observed Adverse Events.
Design: This is a p rospective, multi-center, randomized, two-arm non-inferiority trial that compared the
Resolute stent to the Abbott Xience V stent. A total of 2292 subjects were enrolled at 17 clinical
research sites from 11 countries in Western Europe. Patients were eligible if they had at least one
co ronary lesion with a diameter stenosis >50%, in a vessel with a reference diameter between 2.25 mm
and 4.0 mm. No restriction was placed on the total number of treated lesions, treated vessels, lesion
length or number of stents implanted. The study was designed to enroll patients with symptomatic
co ronary disease including chronic stable angina, silent ischemia, and acute coronary ischemic
49
. See
Page 53

syndromes. Subjects were stratified as being non-complex or complex (based on clinical features and
co ronary anatomy), with complex subjects having one o r more of the following patient or lesion
characteristics: Bifurcation, bypass graft, in stent restenosis, AMI <72 hours, LVEF <30%, unprotected
lef t main, >2 vessels stented, renal insufficiency or failure (serum creatinine >2.5 mg/dl), lesion length
>27 mm, >1 lesion per vessel, lesion with thrombus or total occlusion (pre procedure TIMI = 0)
Follow-up was performed at 30 d ays, 6, 9, 12 and 24 months and will be performed annually out to 5
years. Following the index procedure, subjects were to be treated with aspirin indefinitely and
clopidogrel/ticlopidine for a minimum of 6 months and up to 12 months in those who were not at a high
risk o f bleeding.
Demographics and clinical characteristics: Refer to Table 9-25: R-AC – Baseline Characteristics
Table 9-25: R-AC - Baseline Characteristics
Baseline Characteristics
Mean Age (years) 64.4 64.2
Male Enrollment 76.67% (873/1140) 77.2% (889/1152)
Hx of prior PCI 31.8% (363/1140) 32.1% (370/1152)
Hx of prior MI 28.8% (323/1122) 30.4% (341/1120)
Hx of Diabetes 23.5% (268/1140) 23.4% (270/1152)
Multi-vessel disease
Type B2/C lesions
Syntax Score
Complex*
* Complex was defined as having one or more of the following patient or les ion characteris tics: Bifurcation,
bypass graft, in stent rest enosis, AMI <72 hr, LVEF <30%, unprot ected left main, >2 vessels stented, renal
insufficiency or failure (serum creatinine >2.5 mg/dl), lesion length >27 mm, >1 les ion per ves sel, lesion w ith
thrombus or total occlusion (pre procedure TIMI = 0).
The remaining baseline clinical features were well-matc hed between both arms.
(N = 1140 sub jects; M =
Resolute
(N = 1152 subjects; M =
1661 lesions)
58.4% (666/1140) 59.2% (682/1152)
77.5% (1268/1636) 74.7% (1251/1673)
14.8 ± 9.3 14.6 ± 9.2
67% (764/1140) 65.6% (756/1152)
Xience V
1705 lesions)
Clinical Results: A summary of the results is presented in the following tables:
• Table 9-26: R-AC Princip al Safety and Effectiveness (All subjects)
• Table 9-27: R-AC Princip al Safety and Effectiveness (Complex cohort)
• Table 9-28: R-AC Princip al Safety and Effectiveness (Non-Complex cohort)
• Table 9-29: R-AC ARC Def ined Definite/Probable Stent Thrombosis through 60 Months (Complex
and Non-Co mplex)
The 12-mo nth and 5-year f ollow-up rates for the RESOLUTE All Comers study were 99.5% (2280/2292)
and 98.8% (2265/2292) respectively.
Strengths of this analysis include the collection and presentation of both short and long term outcomes
demonstrating safety and effectiveness in a randomized study in an all comer population. A limitation was
that this trial was not sized to determine the rate of lo w frequency events with a pre-specified precision.
The published RESOLUTE All-Comers trial results are available in Serruys PW, Silber S, Garg S, et al.
Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010; 363: 136-46.
50
Page 54

(
(
(
(
Table 9-26: R-AC Principal Safety and Effectiveness (All subjects)
All subjects
COMPOSITE SAFETY AND
EFFECTIVENESS
TLF
TVF
MACE
EFFECTIVENESS
Clinically Driven TVR
TLR
TLR, PCI
TLR, CABG
Non-TL TVR
Non-TL TVR, PCI
Non-TL TVR, CABG
SAFETY
Total Death
Cardiac Death
Resolute
N = 1140)
8.1% (92/1132) 8.5% (97/1142) 17.0% (191/1123) 16.2% (183/1133)
8.9% (101/1132) 9.7% (111/1142) 20.0% (225/1123) 19.1% (216/1133)
8.6% (97/1132) 9.8% (112/1142) 21.9% (246/1123) 21.6% ( 245/1133)
4.9% (55/1132) 4.8% (55/1142) 11.4% (128/1123) 10.9% (123/1133)
3.9% (44/1132) 3.4% (39/1142) 7.8% (88/1123) 7.1% (81/1133)
3.4% (38/1132) 2.7% (31/1142) 6.9% (77/1123) 6.0% (68/1133)
0.5% (6/1132) 0.8% (9/1142) 1.4% (16/1123) 1.4% (16/1133)
1.9% (21/1132) 2.2% (25/1142) 6.1% (68/1123) 6.1% (69/1133)
1.5% (17/1132) 1.9% (22/1142) 5.2% (58/1123) 5.2% (59/1133)
0.4% (4/1132) 0.4% (4/1142) 0.9% (10/1123) 1.0% (11/1133)
1.6% (18/1132) 2.7% (31/1142) 11.0% (123/1123) 10.8% (122/1133)
1.3% (15/1132) 1.7% (19/1142) 6.5% (73/1123) 5.7% (65/1133)
12 Months
Xience V
N = 1152)
Resolute
N = 1140)
60 Months
Xience V
N = 1152)
Non-Cardiac Death
Cardiac Death or TVMI
TVMI
Q wave MI
Non-Q wave MI
Sten t Thro mb osi s
ARC defined
Definite/Probable 1.6% (18/1132) 0.7% (8/1142) 2.4% (27/1123) 1.7% (19/1133)
Definite 1.1% (13/1132) 0.3% (3/1142)
Probable 0.5% (6/1132) 0.4% (5/1142) 0.9% (10/1123) 0.9% (10/1133)
Notes
N = The t otal number of subject s enrolled.
Subjects are only counted once for eac h time period.
Numbers are % (Count/Number of Eligible Subjects).
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
12-month timeframe includes follow-up window (360± 30 day s)
60-month timeframe includes follow-up window (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined Stent Thrombos is.
0.3% (3/1132) 1.1% (12/1142) 4.5% (50/1123) 5.0% (57/1133)
5.3% (60/1132) 5.5% (63/1142) 11.5% (129/1123) 10.6% (120/1133)
4.2% (48/1132) 4.2% (48/1142) 5.7% (64/1123) 5.7% (65/1133)
0.8% (9/1132) 0.4% (5/1142) 1.3% (15/1123) 0.8% ( 9/1133)
3.5% (40/1132) 3.8% (43/1142) 4.6% (52/1123) 4.9% (56/1133)
1.6% (18/1123) 0.8% (9/1133)
51
Page 55

Table 9-27: R-AC Principal Safety and Effectiveness (Complex cohort)
Complex cohort
12 Months
60 Months
COMPOSITE SAFETY AND
EFFECTIVENESS
TLF 8.8% (67/760) 10.0% (75/750) 18.2% (137/751) 18.4% (137/745)
TVF 9.7% (74/760) 11.3% (85/750) 22.1% (166/751) 21.3% (159/745)
MACE 9.1% (69/760) 11.7% (88/750) 22.5% (169/751) 24.6% (183/745)
EFFECTIVENESS
Clinically Driven TVR 5.5% (42/760) 5.6% (42/750) 13.4% (101/751) 11.7% (87/745)
TLR 4.3% (33/760) 4.1% (31/750) 8.9% (67/751) 8.1% (60/745)
TLR, PCI 3.9% (30/760) 3.2% (24/750) 8.1% (61/751) 6.7% (50/745)
TLR, CABG 0.4% (3/760) 1.1% (8/750) 1.2% (9/751) 1.7% (13/745)
SAFETY
Total Death 1.4% (11/760) 3.3% (25/750) 10.4% (78/751) 13.2% (98/745)
Cardiac Death 1.3% (10/760) 2.1% (16/750) 6.4% (48/751) 7.4% (55/745)
Non-Cardiac Death 0.1% (1/760) 1.2% (9/750) 4.0% (30/751) 5.8% (43/745)
Cardiac Death or TVMI 5.4% (41/760) 6.4% (48/750) 11.9% (89/751) 12.2% (91/745)
TVMI
Q wave MI 0.7% (5/760) 0.5% (4/750) 1.3% (10/751) 0.9% (7/745)
Non-Q wave MI 3.7% (28/760) 4.1% (31/750) 4.8% (36/751) 5.1% (38/745)
Sten t Thro mb osi s
ARC defined
Definite/Probable 1.7%(13/759) 0.9%(7/749) 2.5% (19/751) 2.0% (15/745)
Definite 1.2%(9/759) 0.4%(3/749) 1.7% (13/751) 0.9% (7/745)
Probable 0.7%(5/759) 0.5%(4/749) 0.9% (7/751) 1.1% (8/745)
Notes
N = The t otal number of subject s enrolled.
Subjects are only counted once for eac h time period.
Numbers are % (Count/Number of Eligible Subjects).
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
12-month timeframe includes follow-up window (360 ± 30 days)
60-month timeframe includes follow-up window (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined St ent Thrombos is.
Complex was defined as having one or m ore of the following patient or lesion charact eristics : Bifurcat ion, bypass graft, in s tent restenosis , AMI <72
hr., LVEF <30%, unprot ected left main, >2 vessels stent ed, renal insuff iciency or f ailure (serum creat inine >2.5 mg/dl), lesion lengt h >27 mm, >1
lesion per vessel, lesion with thrombus or tot al occlusion (pre procedure TIMI = 0).
Resolute
(N = 764)
4.2% (32/760) 4.7% (35/750) 5.9% (44/751) 6.0% (45/745)
Xience V
(N = 756)
Resolute
(N = 764)
Xience V
(N = 756)
52
Page 56

(
(
(
(
p
Table 9-28: R-AC Principal Safety and Effectiveness (Non-Complex Cohort)
Non-Complex cohort
COMPOSITE SAFETY AND
EFFECTIVENESS
TLF 6.7% (25/372) 5.6% (22/392) 14.5% (54/372) 11.9% (46/388)
TVF 7.3% (27/372) 6.6% (26/392) 15.9% (59/372) 14.7% (57/388)
MACE 7.5% (28/372) 6.1% (24/392) 20.7% (77/372) 16.0% (62/388)
EFFECTIVENESS
Clinically Driven TVR 3.5% (13/372) 3.3% (13/392) 7.3% (27/372) 9.3% (36/388)
TLR 3.0% (11/372) 2.0% (8/392) 5.6% (21/372) 5.4% (21/388)
TLR, PCI 2.2% (8/372) 1.8% (7/392) 4.3% (16/372) 4.6% (18/388)
TLR, CABG 0.8% (3/372) 0.3% (1/392) 1.9% (7/372) 0.8% (3/388)
SAFETY
Total Death 1.9% (7/372) 1.5% (6/392) 12.1% (45/372) 6.2% (24/388)
Cardiac Death 1.3% (5/372) 0.8% (3/392) 6.7% (25/372) 2.6% (10/388)
Non-Cardiac Death 0.5% (2/372) 0.8% (3/392) 5.4% (20/372) 3.6% (14/388)
Cardiac Death or TVMI 5.1% (19/372) 3.8% (15/392) 10.8% (40/372) 7.5% (29/388)
TVMI
Q wave MI 1.1% (4/372) 0.3% (1/392) 1.3% (5/372) 0.5% (2/388)
Non-Q wave MI 3.2% (12/372) 3.1% (12/392) 4.3% (16/372) 4.6% (18/388)
Sten t Thro mb osi s
ARC defined
Definite/Probable 1.3%(5/372) 0.3%(1/392) 2.2% (8/372) 1.0% (4/388)
Definite 1.1%(4/372) 0.0%(0/392) 1.3% (5/372) 0.5% (2/388)
Probable 0.3%(1/372) 0.3%(1/392) 0.8% (3/372) 0.5% (2/388)
Notes
N = The t otal number of subject s enrolled.
Subjects are only counted once for eac h time period.
Numbers are % (Count/Number of Eligible Subjects).
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
12-month timeframe includes follow-up window (360± 30 day s)
60-month timeframe includes follow-up window (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined St ent Thrombos is.
Complex was defined as having one or m ore of the following patient or lesion charact eristics : Bifurcat ion, bypass graft, in s tent restenosis , AMI <72
hr., LVEF <30%, unprot ected left main, >2 vessels stent ed, renal insuff iciency or f ailure (serum creat inine >2.5 mg/dl), lesion lengt h >27 mm, >1
lesion
er vessel, lesion with thrombus or t otal occlus ion (pre procedure TIMI = 0).
Resolute
N = 376)
4.3% (16/372) 3.3% (13/392) 5.4% (20/372) 5.2% (20/388)
12 Months
Xience V
N = 396)
Resolute
N = 376)
60 Months
Xience V
N = 396)
53
Page 57

(
(
(
(
(
(
g
Table 9-29: R-AC ARC Defined Definite/Probable Stent Thrombosis Through 60 Months (All
Subjects, and Complex and Non-Complex Subjects)
All Subjects Non-Complex Complex
Cumulative Stent Thrombosis
Through 1-Year
Cumulative Stent Thrombosis
Through 5 -Years
Acute (0 - 1 day) 0.4% (5/1123) 0.2% (2/1133) 0.3% (1/372) 0.0% (0/388) 0.5% (4/751) 0.3% (2/745)
Subacute (2 - 30 days) 0.7% (8/1123) 0.4% (4/1133) 0.3% (1/372) 0.3% (1/388) 0.9% (7/751) 0.4% (3/745)
Late (31 - 360 days) 0.6% (7/1123) 0.2% (2/1133) 0.8% (3/372) 0.0% (0/388) 0.5% (4/751) 0.3% (2/745)
Very Late (361 - 1800 days) 0.8% (9/1123) 1.0% (11/1133) 0.8% (3/372) 0.8% (3/388) 0.8% (6/751) 1.1% (8/745)
Notes
N = The t otal number of subject s enrolled.
Subjects are only counted once for eac h time period.
Numbers are % (Count/Number of Eligible Subjects).
12-month timeframe includes follow-up window (360 ± 30 days)
60-month timeframe includes follow-up window (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined St ent Thrombosis.
Complex was defined as having one or m ore of the following patient or lesion charact eristics : Bifurcat ion, bypass graft, in s tent
restenosis, AMI <72 hr., LVEF <30%, unprot ected left main, >2 vessels stented, renal ins ufficienc y or failure (serum creat inine >2.5
m
/dl), lesion length >27 mm, >1 lesion per vessel, lesion with thrombus or t otal occlus ion (pre procedure TIMI = 0).
Resolute
N = 1140)
1.6% (18/1132) 0.7% (8/1142) 1.3% (5/372) 0.3% (1/392) 1.7%(13/760) 0.9%(7/750)
2.4% (27/1123) 1.7% (19/1133) 2.2% (8/372) 1.0% (4/388) 2.5% (19/751) 2.0% (15/745)
Xience V
N = 1152)
Resolute
N = 376)
Xience V
N = 396)
Resolute
N = 764)
Xience V
N = 756)
54
Page 58

9.3 Results of the RESOLUTE International Study
Primary Objective: To evaluate the safety and overall clinical performance of the Resolute
Zotarolimus-Eluting Coronary Stent System (the Resolute stent) in an ‘all-comers’ patient population
requiring stent implantation.
Design: This is a p rospective, multi-center, non-randomized observational study. A total of 2349
sub jects were enrolled at 88 clinical research sites from 17 countries in Europe, Asia, Africa and South
America, where the Resolute stent is commercially available. This study was designed to treat all
enrolled subjects according to routine hospital practice. No restriction was placed on the total number
of treated lesions, treated vessels, lesion length or number of stents implanted. The study enrolled
patients with symptomatic coronary disease (including chronic stable angina, silent ischemia, and acute
co ronary ischemic syndromes). Enrolled subjects were permitted to have complex clinical or anatomic
f eatures as described in Section 9.2 - Results of the RESOLUTE All Comers (AC) Clinical Trial.
Follow-up was performed at 30 d ays, 6 and 12 months and will be performed annually out to 3 years.
Following the index procedure, subjects were to be treated with aspirin indefinitely and clopidogrel/
ticlopidine for a minimum of 6 months and up to 12 months in subjects who were not at a high risk of
bleeding.
Demographics and clinical characteristics: The baseline demographics and clinical characteristics
sho w a mean age of 63.5 years with a male enrollment of 77.8% (1828/2349). Of the subjects enrolled
in this study, 29.6% (696/2349) of subjects had a prior percutaneous coronary revascularization and
8.4% (197/2349) had p revious CABG surgery. In to tal, 30.5% (716/2349) of the subjects had a history of
diabetes mellitus with 9.0% (211/2349) being insulin dependent. Past medical history of subjects
indicated 63.9% (1501/2349) had hyperlipidemia, 68.0% (1598/2349) had hypertension, and 24.2%
(569/2349) were current smokers. The mean RVD was 2.94 ± 0.46 mm, the lesion length was 18.75 ±
10.77 mm, and the average percentage diameter stenosis was 84.50 ± 12.12%. The ACC/AHA lesion
classification was reported by sites as type B2/C for 57.1% (1798/3147) of the lesions.
Results: These analyses are b ased on the intent-to-treat population. The 12 month and 3 year followup rates f or the RESOLUTE International study were 97.7% (2295/2349) and 96.7% (2271/2349)
respectively.
Strengths of this analysis include the collection and presentation of both short and long term outcomes
demonstrating safety and effectiveness in an all comer population. A limitation was that this trial was not
sized to determine the rate of low frequency events with a pre-specified precision.
The results are presented in the following tables:
• Table 9-30: RESOLUTE International - Principal Safety and Effectiveness
• Table 9-31: RESOLUTE International - ARC Defined Definite/Probable Stent Thro mbosis Through
36 Months
55
Page 59

(
(
Table 9-30: RESOLUTE International - Principal Safety and Effectiveness
COMPOSITE SAFETY AND EFFECTIVENESS 12 Months 36 Months
TLF
TVF
MACE
EFFECTIVENESS
Clinically Driven TVR
TLR
TLR, PCI
TLR, CABG
Non-TL TVR
Non-TL TVR, PCI
Non-TL TVR, CABG 0.0% (0/2337) 0.2% (4/2284)
SAFETY
Total Death
Cardiac Death
Non-Cardiac Death
Cardiac Death or MI 4.2% (99/2337) 7.0% (161/2284)
TVMI
Q wave MI
Non-Q wave MI
Stent Thrombosis
ARC defined
Definite/Probable 0.9% (20/2337) 1.1% (26/2284)
Definite 0.6% (15/2337) 0.8% (19/2284)
Probable 0.3% (6/2337) 0.4% (8/2284)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
36-month timeframe includes follow-up window (1080 days ± 30 day s)
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
See Table 9-4 for the definition of the ARC defined Stent Thrombos is.
N = 2349)
7.1% (165/2337) 11.4% (261/2284)
7.7% (180/2337) 12.9% (294/2284)
8.3% (193/2337) 14.4% (329/2284)
4.2% (99/2337) 7.4% (168/2284)
3.5% (81/2337) 5.7% (130/2284)
3.1% (72/2337) 5.2% (118/2284)
0.4% (10/2337) 0.6% (14/2284)
1.2% (27/2337) 2.6% (59/2284)
1.2% (27/2337) 2.5% (56/2284)
2.4% (57/2337)
1.5% (34/2337)
1.0% (23/2337)
3.0% (71/2337)
0.5% (12/2337)
2.5% (59/2337)
N = 2349)
6.1% (139/2284)
3.6% (82/2284)
2.5% (57/2284)
3.9% (89/2284)
0.9% (20/2284)
3.0% (69/2284)
56
Page 60

(
Table 9-31: RESOLUTE International - ARC Defined
Definite/Probable Stent Thrombosis Through 36 Months
Stent Thrombosis 1.1% (26/2284)
Acute (0 - 1 day) 0.1% (3/2284)
Subacute (2 - 30 days) 0.6% (14/2284)
Late (31 - 360 days) 0.1% (3/2284)
Very Late (361 - 1080 days) 0.3% (6/2284)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
36-month timeframe includes follow-up window (1080 days ± 30 day s)
See Table 9-4 for the definition of the ARC defined Stent Thrombos is
9.4 Results of the RESOLUTE FIM Clinical Trial
Primary Objective: To evaluate the safety, effectiveness, and pharmacokinetics (PK) of the Resolute
Zotarolimus-Eluting Coronary Stent (Resolute stent) for the treatment of single de novo lesions in native
co ronary arteries with a reference vessel diameter (RVD) between 2.5 mm and 3.5 mm in diameter.
Design: The RESOLUTE FIM Clinical Trial, the f irst-in-human study for the Resolute stent, is a nonrandomized, prospective, multi-center, single-arm trial. A total of 139 subjects were enrolled at 12
investigative sites in Australia and New Zealand who presented with symptomatic ischemic heart
disease d ue to a de novo stenotic lesion contained within a native coronary artery with a reference
vessel diameter between 2.5 mm and 3.5 mm and a lesion length between 14 mm and 27 mm
amenable to percutaneous treatment with a single stent.
Resolute
N = 2349)
Follow-up was performed at 30 d ays, 4, 9, 12 months and annually at 2, 3 and 4 years. Follow-up will be
performed at 5 years. Thirty subjects were consented to have an angiographic and IVUS follow-up at 4
months post-procedure while an additional 100 subjects were consented to have the same type of
f ollow-up at 9 months post-procedure. Following the index procedure, subjects were to be treated with
asp irin indefinitely and clopidogrel/ticlopidine for a minimum of 6 months.
Primary Endpoint: The primary endpoint was in-stent late lumen loss (LL) at 9 months post-procedure
as measured b y QCA.
Control Group and Statistical Analysis Plan: The primary analysis was a non-inferiority comparison
of the 9-month in-stent late LL in the Resolute stent compared to a historical control population of
sub jects treated with an Endeavor stent in the ENDEAVOR II trial. The non-inferiority margin was set at
0.16 mm.
Demographics and clinical characteristics: The mean age was 60.7 years, with 76.3% (106/139)
men, 17.3% (24/139) d iabetics, 18.7% (26/139) with a history of prior percutaneous coronary
revascularization, 46.4% (64/138) with a history of prior MI and 2.9% (4/139) with a history of prior
CABG. Past medical history of subjects indicated 94.2% (131/139) had hyperlipidemia and 66.9%
(93/139) had hypertension. The mean RVD was 2.81 ± 0.40 mm, the lesion length was 15.61 ± 6.13mm
and the average percentage diameter stenosis was 70.30 ± 11.37%. The ACC/AHA lesion classification
was reported by sites as type B2/C for 81.4% (114/140) of the lesions.
Results: The Resolute stent met the primary non-inferiority endpoint with a 9 months in-stent late LL of
0.22 ± 0.27 mm, compared with the historical Endeavor stent control 8-month in-stent late LL of 0.62 ±
0.45 mm, p
non-i nferi ori ty
<0.001. The 12-month and 5-year follow-up rates were 99.3% (138/139) and 97.1%
(135/139) respectively.
Strengths of this analysis include the collection and presentation of both short and lo ng term outcomes
demonstrating safety and effectiveness in the intended population. A limitation was that it was a single
arm feasibility study.
57
Page 61

(
These analyses are based on the intent-to-treat population. PK results are presented in Section 6.3 for
the Pharmacokinetics of the Resolute Stent. The results are presented in the following tables:
• Table 9-32: RESOLUTE FIM - Primary Endpoint Analysis
• Table 9-33: RESOLUTE FIM - Principal Safety and Effectiveness
• Table 9-34: RESOLUTE FIM - ARC Def ined Definite/Probable Stent Thrombosis through 60 Months
• Table 9-35: RESOLUTE FIM - Angiographic and IVUS Results
Table 9-32: RESOLUTE FIM - Primary Endpoint Result
Primary Endpoint1
9-month In-stent Late Lumen Los s
(mm)
Notes
N is the total number of subjects enrolled.
M is the total number of lesions at baseline.
Numbers are Mean ± SD (number of evaluable lesions).
Subjects are only counted once for each time period.
1
Angiographic Follow-Up for RESOLUTE was at 9 Months and for Endeavor stent from the EN DEAVOR II trial w as at 8 Months.
2
Confidence interval calculated using normal approximation.
3
One-sided p-value by non-inferiority test using t test with non-inferiority margin of 0.16 mm, to be compared at a 0.05 significance level.
RESOLUTE
(N = 139, M = 140)
0.22 ± 0.27 (96) 0.62 ± 0.45 (256) -0.39 [-0.49, -0.30] <0.001
ENDEAVOR II
Endeavor
N = 256, M = 256)
Difference
[95% CI]2
Non-Inferiority
P-value3
58
Page 62

(
(
Table 9-33: RESOLUTE FIM - Principal Safety and Effectiveness
Outcomes at
9 Months
N = 139)
COMPOSITE SAFETY AND
EFFECTIVENESS
TVF 6.5% (9/139) 7.2% (10/139) 13.2% (18/136)
MACE 7.2% (10/139) 8.6% (12/139) 16.2% (22/136)
EFFECTIVENESS
Clinically Driven TVR 0.0% (0/139) 0.7% (1/139) 5.1% (7/136)
TLR 0.0% (0/139) 0.7% (1/139) 2.9% (4/136)
TLR, PCI 0.0% (0/139) 0.7% (1/139) 2.2% (3/136)
TLR, CABG 0.0% (0/139) 0.0% (0/139) 0.7% (1/136)
Non-TL TVR 0.0% (0/139) 0.0% (0/139) 2.2% (3/136)
Non-TL TVR, PCI 0.0% (0/139) 0. 0% (0/139) 2.2% (3/136)
Non-TL TVR, CABG 0.0% (0/139) 0.0% (0/139) 0. 0% (0/136)
SAFETY
Total Death 1.4% (2/139) 2.2% (3/139)
Cardiac Death
Non-Cardiac Death
0.7% (1/139)
0.7% (1/139)
Outcomes at
12 Months
(N = 139)
0.7% (1/139)
1.4% (2/139)
Outcomes at
60 Months
N = 139)
6.6% (9/136)
1.5% (2/136)
5.1% (7/136)
Cardiac Death or MI 6.5% (9/139) 6.5% (9/139) 8.1% (11/136)
MI
Q wave MI
Non-Q wave MI
Sten t Thro mb osi s
ARC defined
Definite/Probable 0.0% (0/139) 0.0% (0/139)
Definite 0.0% (0/139) 0.0% (0/139)
Probable 0.0% (0/139) 0.0% (0/139)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
9-month timeframe includes follow-up window (270 days ± 14 day s).
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
60-month timeframe includes follow-up window (1800 days ± 30 day s).
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
See Table 9-4 for the definition of the ARC defined Stent Thrombos is.
5.8% (8/139)
0.0% (0/139)
5.8% (8/139)
5.8% (8/139)
0.0% (0/139)
5.8% (8/139)
6.6% (9/136)
0.0% (0/136)
6.6% (9/136)
0.0% (0/136)
0.0% (0/136)
0.0% (0/136)
59
Page 63
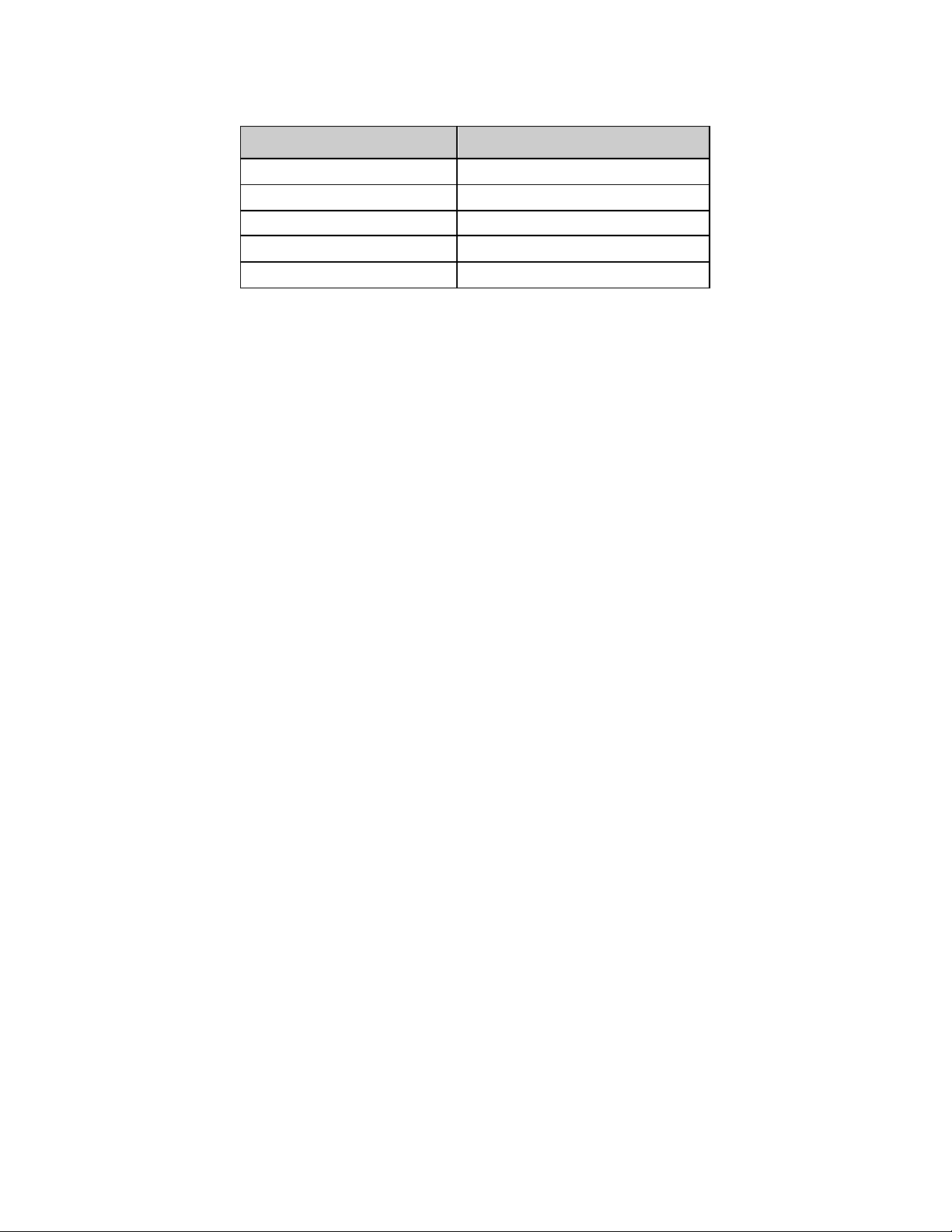
(
Table 9-34: RESOLUTE FIM - ARC Defined Definite/Probable
Stent Thrombosis Through 60 Months
Stent Thrombosis 0.0% (0/136)
Acute (0 - 1 day) 0.0% (0/136)
Subacute (2 - 30 days) 0.0% (0/136)
Late (31 – 360 days) 0.0% (0/136)
Very late (361 - 1800 days) 0.0% (0/136)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
60-month timeframe includes follow-up w indow (1800 day s ± 30 days).
See Table 9-4 for the definition of the ARC defined Stent Thrombos is
Resolute
N = 139)
60
Page 64

Table 9-35: RESOLUTE FIM - Angiographic and IVUS Results
Outcomes at 4 Months
ANGIOGRAPHIC RESULTS
MLD (mm), In-stent
Post-Procedure 2.76 ± 0.39 (140) 2.76±0.39 (140)
Follow Up 2.68±0.39 (30) 2. 51±0.48 (96)
MLD (mm), In-segment
Post-Procedure 2.36 ± 0.43 (140) 2.36±0.43 (140)
Follow Up 2.38±0.40 (30) 2. 21±0.45 (96)
% DS, In-stent
Post-Procedure 3.36±8.54 (140) 3.36±8.54 (140)
Follow Up 7.18±7.86 (30) 10.13±12. 63 (96)
% DS, In-segment
Post-Procedure 17.80±8.24 (140) 17.80±8.24 (140)
Follow Up 17.74±7.57 (30) 21.08±10.62 (96)
Late Loss (mm)
In-stent 0.12±0.26 (30) 0.22±0.27 (96)
In-segment 0.05±0.20 (30) 0.12±0.27 (96)
Binary Restenosis
In-stent 0.0% (0/30) 1.0% (1/ 96)
In-segment 0.0% (0/30) 2.1% (2/96)
IVUS RESULTS
Neointimal Volume (m m3) 3.72±4.21 (24) 6.55±7.83 (88)
% Volume Obstruction 2.23±2.43 (24) 3.73±4.05 (88)
Incomplete Apposition
Persist ent 6.7% (2/30) 17.0% (15/88)
Late Acquired 3.3% (1/30) 6.8% (6/88)
Notes
139 subjects with 140 lesions underwent angiographic follow-up at bas eline.
N = The t otal number of subject s enrolled.
M = The t otal number of lesions at baseline.
Numbers are % (Count/Number of Evaluable Les ions) or Mean ± SD (N umber of Evaluable Lesions).
Subjects are only counted once for each time period.
(N = 30, M = 30)
Outcomes at 9 Months
(N = 100, M = 101)
61
Page 65

9.5 Results of the RESOLUTE Japan Clinical Trial
Primary Objective: To verify the safety and effectiveness of the Resolute Zotarolimus-Eluting Coronary
Stent (Resolute stent) in a Japanese population for the treatment of de novo lesions in native coronary
arteries with a ref erence vessel diameter of 2.5 mm to 3.5 mm and lesion lengths mm.
Design: This is a no n-randomized, prospective, multi-center, single-arm trial. A total of 100 subjects
were enro lled at 14 investigational sites in Japan.
Follow-up was performed at 30 d ays, 6, 9, and 12 months and will be performed annually out to 5 years.
All subjects were scheduled to have angiographic and IVUS follow-up at 8 months post-procedure.
Following the index procedure, subjects were to be treated with aspirin indefinitely and
clopidogrel/ticlopidine for a minimum of 6 months and up to 12 months in subjects who were not at a
high risk of bleeding.
Primary Endpoint: The primary endpoint was in-stent late LL at 8 mo nths post-procedure measured
by QCA.
Control Group and Statistical Analysis Plan: The primary analysis was a non-inferiority comparison
of the 8-month in-stent late LL in the Resolute stent compared to a historical control population of
sub jects treated with a Taxus stent in the ENDEAVOR IV trial. The non-inferiority margin was set at
0.20 mm. If the no n-inferiority endpoint was met, a superiority test would be performed.
Demographics and clinical characteristics: The mean age was 67.7 years with 77.0% (77/100) of
sub jects being males. Of the subjects enrolled, 45.0% (45/100) had diabetes mellitus, 22.0% (22/100)
were current smokers, 25.0% (25/100) had prior MI, 42.0% (42/100) had prior PCI, 81.0% (81/100) had
hyp ertension, and 78.0% (78/100) reported hyperlipidemia. Baseline lesion characteristics include
42.6% (46/108) LAD lesions, a mean lesion length of 15.52 ± 5.37 mm, 52.8% (57/108) ACC/AHA type
B2/C lesions and 18.5% (20/108) lesions involving a bifurcation. The mean RVD was 2.85 ± 0.44 mm
and the percentage diameter stenosis was 69.17 ± 7.80%.
Results: The Resolute stent in-stent late LL at 8 months was 0.13 ± 0.22 mm, which met the primary
non-inf eriority endpoint (and demonstrated superiority) compared with the historical Taxus stent 8month in-stent late LL of 0.42 ± 0.50 mm. The 12 month and 5 year follow-up rates were 100%
(100/100) and 96% (96/100) respectively.
Strengths of this analysis include the collection and presentation of both short and long term outcomes
demonstrating safety and effectiveness in the intended population. A limitation was that the patient and
lesion characteristics excluded many complex subjects.
These analyses are based on the intent-to-treat population. The results are presented in the following
tables:
• Table 9-36: RESOLUTE Japan - Primary Endpoint Analysis
• Table 9-37: RESOLUTE Japan - Principal Safety and Effectiveness
• Table 9-38: RESOLUTE Japan - ARC Defined Definite/Probable Stent Thrombosis through 60
Months
• Table 9-39: RESOLUTE Japan - Angiographic and IVUS Results
62
Page 66

Table 9-36: RESOLUTE Japan - Primary Endpoint Result
Primary Endpoint
8-month In-stent Late
Lumen Loss (mm)
Resolute
(N = 100, M = 108)
0.13 ± 0.22 (99) 0.42 ± 0.5 (135) -0.29 [-0.41, -0.16]
ENDEAVOR IV Taxus
(N = 164, M = 164)
Notes
N = The t otal number of subject s enrolled.
M = The num ber of les ions at baseline.
Numbers are Mean ± SD (Number of Evaluable Lesions).
Subjects are only counted once for eac h time period.
Confidence interval and p values are adjusted using propensity score method.
1
Confidence interval calculated using normal approximation.
2
One-sided p-value by non-inferiority test using asym ptotic t est st atistic wit h non-inferiority m argin of 0.20 mm to be compared at a 0.05
significance level.
3
Two-sided p-value by superiority test using asymptotic test statistic, to be compared at a 0.05 significance level.
Difference
95%CI1
Non- Inferiority
P-value2
<0.001 <0.001
Superiority
P-value3
63
Page 67
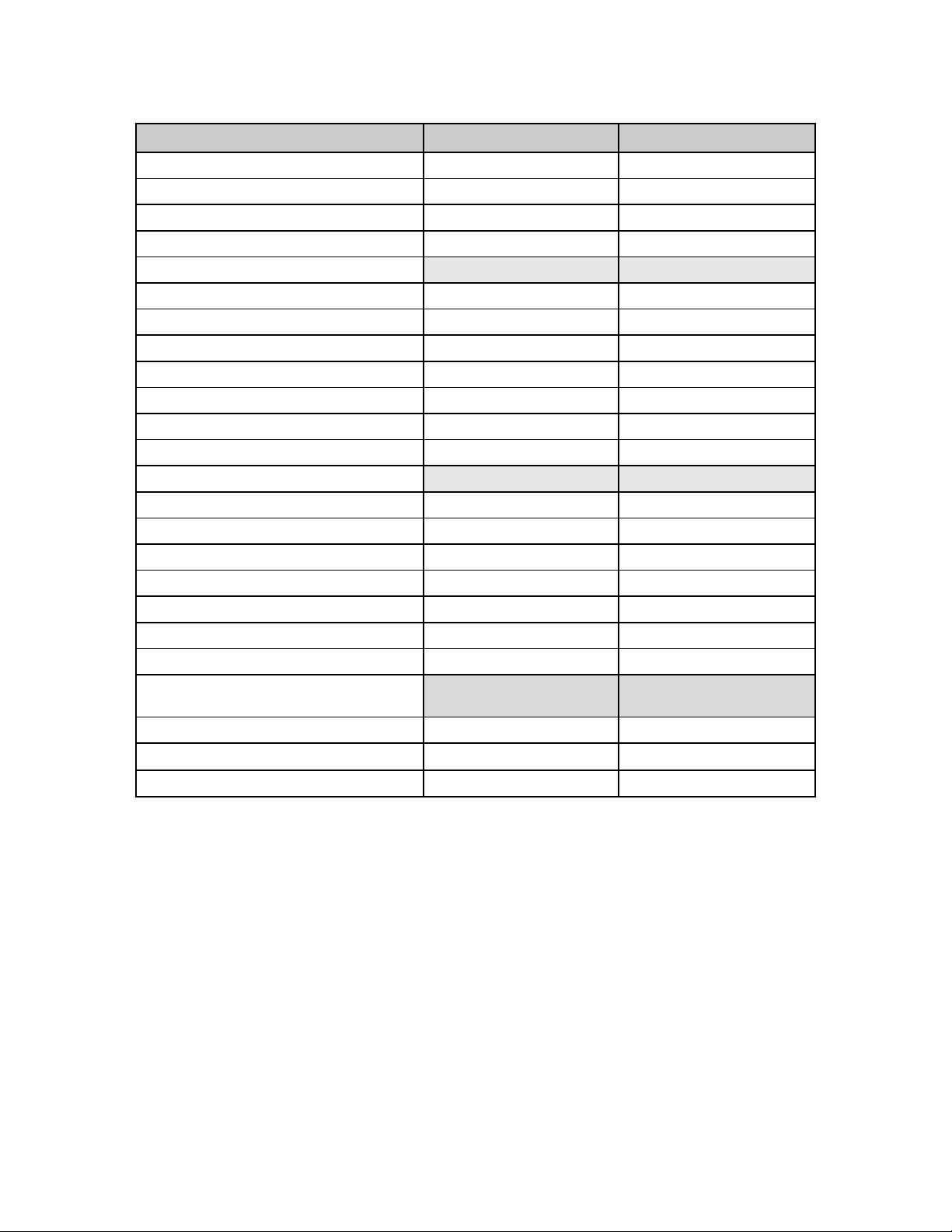
Table 9-37: RESOLUTE Japan - Principal Safety and Effectiveness
(N = 100) (N = 100)
COMPOSITE SAFETY AND EFFECTIVENESS 12 Months 60 Months
TLF
TVF
MACE
EFFECTIVENESS
Clinically Driven TVR
TLR
TLR, PCI
TLR, CABG
Non-TL TVR
Non-TL TVR, PCI
Non-TL TVR, CABG
SAFETY
Total Death
Cardiac Death
Non-Cardiac Death
Cardiac Death or MI 4.0% (4/100) 5.1% (5/98)
TVMI
Q wave MI
Non-Q wave MI
Stent Thrombosis
ARC defined
Definite/Probable
Definite
Probable
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
60-month timeframe includes follow-up window (1800 days ± 30 day s)
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
See Table 9-4 for the definition of the ARC defined Stent Thrombos is.
4.0% (4/100)
5.0% (5/100)
5.0% (5/100)
1.0% (1/100)
0.0% (0/100)
0.0% (0/100)
0.0% (0/100)
1.0% (1/100)
1.0% (1/100)
0.0% (0/100)
1.0% (1/100)
0.0% (0/100)
1.0% (1/100)
4.0% (4/100)
0.0% (0/100)
4.0% (4/100)
0.0% (0/100)
0.0% (0/100)
0.0% (0/100)
6.1% (6/98)
10.2% (10/98)
14.3% (14/98)
5.1% (5/98)
1.0% (1/98)
1.0% (1/98)
0.0% (0/98)
4.1% (4/98)
3.1% (3/98)
1.0% (1/98)
7.1% (7/98)
1.0% (1/98)
6.1% (6/98)
4.1% (4/98)
0.0% (0/98)
4.1% (4/98)
0.0% (0/98)
0.0% (0/98)
0.0% (0/98)
64
Page 68

(
Table 9-38: RESOLUTE Japan - ARC Defined Definite/Probable Stent
Thrombosis Through 60 Months
Stent Thrombosis 0.0% (0/98)
Acute (0 - 1 day)
Subacute (2 - 30 days) 0.0% (0/98)
Late (31 – 360 days) 0.0% (0/98)
Very late (361 - 1800 days) 0.0% (0/98)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
60-month timeframe includes follow-up window (1800 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined Stent Thrombos is.
Resolute
N = 100)
0.0% (0/98)
65
Page 69

(
(
(
(
(
(
(
(
(
(
(
(99)
(99)
Table 9-39: RESOLUTE Japan - Angiographic and IVUS
Results
Outcomes at 8 Months
ANGIOGRAPHIC RESULTS
MLD (mm), In-stent
Post-Procedure
8-Month
MLD (mm), In-segment
Post-Procedure
8-Month
% DS, In-stent
Post-Procedure
8-Month
% DS, In-segment
Post-Procedure
8-Month
Late Loss (mm)
In-stent
In-segment
Binary Restenosis
In-stent
In-segment
IVUS RESULTS
Neointimal Volume (m m3)
% Volume Obstruction
Incomplete Apposition
Persistent
Late Acquired
Notes
N = The t otal number of subject s enrolled.
M = The num ber of les ions at baseline.
Numbers are % (Count/Number of Evaluable Lesions) or Mean ± SD (Number of
Evaluable Lesions).
Subjects are only counted once for each time period.
N = 100, M = 108)
2.79 ± 0.40
2.66 ± 0.46
2.45 ± 0.43
2.35 ± 0.47
3.28 ± 7.19
6.52 ± 9.20
15.23 ± 7.39
17.71 ± 8.72
0.12 ± 0.22
0.10 ± 0.25
0.0% (0/107)
0.0% (0/107)
3.19 ± 4.53
2.33 ± 3.51
7.8% (8/103)
4.9% (5/103)
2.9% (3/103)
108)
107)
108)
107)
108)
107)
108)
107)
107)
107)
66
Page 70

9.6 Results of the RESOLUTE INTEGRITY US Post Market Study
The RESOLUTE INTEGRITY US Post Market Study is a post approval study of the Medtronic Resolute Integrity
Zotarolimus-Eluting Coronary Stent System in the Treatment of De Novo Lesions in Native Coronary Arteries
with a Ref erence Vessel Diameter of 2.25 mm to 4.2 mm.
Primary Objective: The o b jective of this study is to assess the safety and efficacy of the Resolute Integrity
stent for the treatment of de novo lesions in native coronary arteries with a reference vessel diameter (RVD) of
2.25 mm to 4.2 mm in two groups of patients, specifically those patients receiving stents mm in length,
referred to as the Primary Enrollment Group (PEG) and those patients who receive extended length stents (34
mm o r 38 mm) ref erred to as the Extended Length (XL) Sub-study.
Design: The RESOLUTE INTEGRITY US Post Market Study is a p rospective, multi-center evaluation of the
procedural and clinical outcomes of subjects that are treated with the commercially available Medtronic Resolute
Integrity Zotarolimus-Eluting Coronary Stent System.
This stud y enrolled 286 patients with de novo lesions in native coronary arteries who met the eligibility criteria
and signed the informed consent form to participate in this study of which 230 patients were part of the PEG and
56 patients in the XL Sub-study.
For the PEG, centers were allowed to enroll a maximum of 40 subjects per center until study enrollment has
been co mpleted, whichever came first. For the XL Sub-study, centers were allowed to enroll a maximum of 16
sub jects per center until study enrollment was completed, whichever comes first.
The expected time of participation in the studies for each subject is two years for the PEG and three years for
the XL Sub -study.
Patient f ollow up occurs at: 30 days ± 5 days (Contact); 6 months ± 14 days (Contact); 12 months ± 30 days
(Clinic Visit with 12-lead ECG); 24 months ± 30 d ays (Contact). The XL Sub-study has additional visits at 2-3
years ± 30 days (Contact).
Primary Endpoint: The primary endpoint for this study was composite rate of cardiac death and target vessel
myocardial infarction (MI) at 12 months.
Control Group and Statistical Analysis Plan: The primary analysis sample was based on intent-to-treat
(ITT): For this study, all subjects who sign the written informed consent and are enrolled in the study will be
counted in the ITT set, which will be the primary analysis set.
Demographics and Clinical Characteristics for the RESOLUTE Integrity-US PEG: Baseline demographics
and clinical characteristics showed a mean age of 64.4 years with 69.6% (160/230) of subjects being males. Of
the subjects enrolled, 42.2% (97/230) had d iabetes mellitus, 18.3% (42/230) were current smokers, 24.4%
(55/225) had prio r MI, 35.7% (82/230) had prior PCI, 86.5% (199/230) had hypertension, and 82.6% (190/230)
reported hyperlipidemia. Baseline lesion characteristics include 40.9% (94/230) LAD lesions, a mean lesion
length of 13.03 ± 5.76 mm, 82.9% (200/241) ACC/AHA type B2/C lesions and 36.5% (88/241) lesions involving
a bif urcation. The mean RVD was 2.60 ± 0.45 mm and the percentage diameter stenosis was 66.84 ± 10.37%.
Results for the RESOLUTE Integrity-US PEG: These analyses are based on the intent-to-treat population.
The results specifically for the RI-US PEG are presented in the following tables:
• Table 9-40: RESOLUTE Integrity US (PEG) - Primary Endpoint Analysis
• Table 9-41: RESOLUTE Integrity US (PEG) - Principal Safety and Effectiveness
• Table 9-42: RESOLUTE Integrity US (PEG) - ARC Defined Definite/Probable Stent Thrombosis
through 24 Months
Table 9-40: RESOLUTE Integrity US (PEG) - Primary Endpoint Analysis
Primary Endpoint - Cardiac Death/TVMI at 12-month- I TT set 3.5% (8/226) [ 1.5%, 6.9%]
1
The two-sided 95% CI is calculated by binomial (exact ) distribution.
Resolute Integrity US (PEG)
(N=230)
95% Confidence
Interval1
67
Page 71

Table 9-41: RESOLUTE Integrity US (PEG) - Principal Safety and
Effectiveness
RESOLUTE INTEGRITY US (PEG)
Clinical Outcomes
Outcomes at
12 Months
Target Lesion Failure (TLF) 4.9% (11/226) 9.1% (20/219)
Target Vessel Failure (TVF) 7.1% (16/226) 12.3% (27/219)
MACE 5.8% (13/226) 11.0% (24/219)
Cardiac Death or Target Vessel M I (TVMI ) 3.5% (8/226) 5.9% (13/219)
Death 1.8% (4/226) 2.7% (6/219)
Cardiac Death 1.3% (3/226) 1.8% (4/219)
Non-Cardiac Death 0.4% (1/226) 0.9% (2/219)
TVMI (Extended historical definition) 2.2% (5/226) 4.1% (9/219)
Clinically Driven TLR 2.2% (5/226) 5.0% (11/219)
Clinically Driven TVR 4.4% (10/226) 8.2% (18/219)
Stent Thrombosis (ARC) D efinite/Probable 0.9% (2/220) 1.8% (4/219)
Early Thrombosis (30 days) 0.9% (2/226) 0.9% (2/219)
Late Thrombosis (31-360 days) 0.0% (0/226) 0.0% (0/219)
Very Late Thrombosis (361-720 days) N/A 0.9% (2/219)
Effectiveness Measures
Lesion Success (Definition 1)2 100.0% (245/245)
Lesion Success (Definition 2)3 99.2% (243/245)
Device Success (Definition 1)4 100.0% (245/245)
Device Success (Definition 2)5 99.2% (243/245)
Procedure Success (Definition 1)6 98.2% (223/227)
Procedure Success (Definition 2)7 97.4% (221/227)
Device Specific Procedure Success (Definition 1)8 98.2% (223/227)
Device Specific Procedure Success (Definition 2)9 97.4% (221/227)
1
Numera tor (m) is th e numb er of p atie nts (or lesion s) with the spe cific classif ication ,
denominator (n) is the number of patients (or lesions) in the study group with known
values, and percentage (%) was calculated as 100 × (m/n)
2
The attainment of <50% residual stenosis of the target lesion using any percutaneous
method.
3
The attainment of <30% residual stenosis by QCA (or <20% by visual assessment)
AND TIMI flow 3 after the procedure, using any percutaneous method.
4
The attainment of <50% residual stenosis of the target lesion using only the assigned
device.
5
The attainment of <30% residual stenosis by QCA (or <20% by visual assessment)
AND TIMI flow 3 after the procedure, using the assigned device only.
6
The attainment of <50% residual stenosis of the target lesion and no in-hospital MACE.
7
The attainment of <30% residual stenosis by QCA (or <20% by visual assessment)
AND TIMI flow 3 after the procedure, using any percutaneous method without the
occurrence of MACE during the hospital stay.
8
The attainment of <50% residual stenosis of the target lesion using only the assigned
device and no in-hospital MACE.
9
The attainment of <30% residual stenosis by QCA (or <20% by visual assessment)
AND TIMI flow 3 after the procedure, using the assigned device only, and no in-hospital
MACE .
(N=230 Patients)
(N=251 Lesions)
(m/n)1
Outcomes at
24 Months
68
Page 72

(
Table 9-42: RESOLUTE Integrity US (PEG) - ARC Defined
Definite/Probable Stent Thrombosis through 24 Months
Resolute Integrity
Stent Thrombosis 1. 8% (4/219)
Acute (0 - 1 day) 0.0% (0/219)
Subacute (2 - 30 days) 0.9% (2/219)
Late (31 - 360 days) 0.0% (0/219)
Very Late (361 - 720 days) 0.9% (2/219)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
12-month time frame includes follow-up window (360 days ± 30 day s).
24-month time frame includes follow-up window (720 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined Stent Thrombos is
N = 230)
The 12-mo nth and 24-month follow-up rates for the RESOLUTE Integrity US Post Market Study PEG were
94.3% (217/230) and 94.8% (218/230) respectively.
Strengths of this analysis include the collection and presentation of both short and lo ng term outcomes
demonstrating safety and effectiveness in the intended population. A limitation was that the patient and lesion
characteristics excluded many complex subjects.
Results for the RESOLUTE Integrity-US XL Sub-study: These analyses are based on the intent-to-treat
population.
The results specifically for the RESOLUTE Integrity-US XL Sub-study are presented in the following tables:
• Table 9-43: RESOLUTE Integrity US (XL Sub-study) - Primary Endpoint Analysis
• Table 9-44: RESOLUTE Integrity US (XL Sub-study) - Principal Safety and Effectiveness
• Table 9-45: RESOLUTE Integrity US (XL Sub-study) - ARC Defined Definite/Probable Stent
Thrombosis through 36 Months
Table 9-43: RESOLUTE Integrity US (XL Sub-study) - Primary Endpoint Analysis
Resolute Integrity US (XL
Primary Endpoint - Cardiac Death/TVMI at 12-month- ITT set 7.1% (4/56) [2.0%, 17.3%]
1
The two-sided 95% CI is calculated by binomial (exact ) distribution.
Sub-study)
(N=56)
95% Confidence
Interval1
Table 9-44: RESOLUTE Integrity US (XL Sub-study) - Principal Safety and Effectiveness
RESOLUTE INTEGRITY US (XL Sub-study)
Clinical Outcomes
Target Lesion Failure (TLF) 7.1% (4/56) 10.7% (6/56)
Target Vessel Failure (TVF) 7.1% (4/56) 12.5% (7/56)
MACE 8.9% (5/56) 17.9% (10/56)
Cardiac Death or Target Vessel M I (TVMI ) 7.1% (4/56) 7.1% (4/56)
Outcomes at
(N=56 Patients)
(N=69 Lesions) (m/n)1
12 Months
Outcomes of
36 Months
69
Page 73

Table 9-44: RESOLUTE Integrity US (XL Sub-study) - Principal Safety and Effectiveness
RESOLUTE INTEGRITY US (XL Sub-study)
(N=56 Patients)
(N=69 Lesions) (m/n)1
Clinical Outcomes
Outcomes at
12 Months
Outcomes of
36 Months
Death 1.8% (1/56) 3.6% (2/56)
Cardiac Death 1.8% (1/56) 1.8% (1/56)
Non-Cardiac Death 0.0% (0/56) 1.8% (1/56)
TVMI (Extended historical definition) 5.4% (3/56) 5.4% (3/56)
Clinically Driven TLR
1.8% (1/56)
Clinically Driven TVR 1.8% (1/56)
Stent Thrombosis (ARC) D efinite/Probable
1.8% (1/56)
Early Thrombosis (30 days) 1.8% (1/56)
Late Thrombosis (31-360 days)
0.0% (0/56)
Very Late Thrombosis (361-1080 days) N/A
Effectiveness Measures
5.4% (3/56)
7.1% (4/56)
1.8% (1/56)
1.8% (1/56)
0.0% (0/56)
0.0% (0/56)
Lesion Success (Definition 1)2 100.0% (69/69)
Lesion Success (Definition 2)3
98.6% (68/69)
Device Success (Definition 1)4 97.1% (67/69)
Device Success (Definition 2)5
Procedure Success (Definition 1)6
Procedure Success (Definition 2)7
Device Specific Procedure Success (Definition 1)8
95.7% (66/69)
98.2% (55/56)
96.4% (54/56)
94.6% (53/56)
Device Specific Procedure Success (Definition 2)9 92.9% (52/56)
1
Numera tor (m) is th e numb er of p atie nts (or lesion s) with the spe cific classif ication , denominator (n) is the number of patients (or
lesions) in the study group with known values, and percentage (%) was calculated as 100 × (m/n)
2
The attainment of <50% residual stenosis of the target lesion using any percutaneous method.
3
The attainment of <30% residual stenosis by QCA (or <20% by visual assessment) AND TIMI flow 3 after the procedure, using any
percutaneous method.
4
The attainment of <50% residual stenosis of the target lesion using only the assigned device.
5
The attainment of <30% residual stenosis by QCA (or <20% by visual assessment) AND TIMI flow 3 after the procedure, using the
assigned device only.
6
The attainment of <50% residual stenosis of the target lesion and no in-hospital MACE.
7
The attainment of <30% residual stenosis by QCA (or <20% by visual assessment) AND TIMI flow 3 after the procedure, using any
percutaneous method without the occurrence of MACE during the hospital stay.
8
The attainment of <50% residual stenosis of the target lesion using only the assigned device and no in-hospital MACE.
9
The attainment of <30% residual stenosis by QCA (or <20% by visual assessment) AND TIMI flow 3 after the procedure, using the
assigned device only, and no in-hospital MACE.
70
Page 74

(
Table 9-45: RESOLUTE Integrity US (XL Sub-study) - ARC
Defined Definite/Probable Stent Thrombosis through 36
Months
Resolute Integrity
Stent Thrombosis 1.8% (1/56)
Acute (0 - 1 day) 0.0% (0/56)
Subacute (2 - 30 days) 1.8% (1/56)
Late (31 - 360 days) 0.0% (0/56)
Very Late (361 - 1080 days) 0.0% (0/56)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
36-month time frame includes follow-up window (1080 days ± 30 day s).
See Table 9 4 for the definition of the ARC defined Stent Thrombosis
N = 56)
The 12mo nth f ollow-up rate for the RESOLUTE Integrity US Post Market Study XL Sub-study was 96.4%
(54/56).
Strengths of this analysis include the collection and presentation of both short and lo ng term outcomes
demonstrating safety and effectiveness in the intended population. A limitation was that the patient and lesion
characteristics excluded many complex subjects.
9.7 Subjects with Diabetes Mellitus in the Resolute Pooled Analysis
Subjects with diabetes mellitus (DM) comprise an important patient subgroup that is at increased risk for
card iovascular morbidity and mortality
4, 5
. A Global Statistical Analysis Plan (GSAP) was created with a
pre-specified hypothesis to evaluate the safety and effectiveness of the Resolute stent to treat stenotic
lesions in diabetic subjects with coronary artery disease. This section provides an overview of this plan
and the results supporting the indication of the Resolute stent to treat coronary artery disease in
sub jects with diabetes mellitus.
Primary Objective: To assess the safety and effectiveness of the Resolute Zotarolimus-Eluting
Coronary Stent System (Resolute stent) for the treatment of de novo lesions in native coronary arteries
in patients with DM with a ref erence vessel diameter (RVD) of 2.25 mm to 4.2 mm.
Population: The study population for the GSAP was selected by combining subjects with DM from the
Global RESOLUTE Clinical Trial Program. The study population selected for this analysis met predefined general and angiographic inclusion and exclusion criteria. Analysis populations consisted of
co nsecutively enrolled eligible diabetic subjects in the trials noted below.
The f ollowing global RESOLUTE clinical trials contributed subjects to the diabetes mellitus cohort:
• RESOLUTE FIM
• RESOLUTE All-Comers
• RESOLUTE International
• RESOLUTE United States
• RESOLUTE Japan
4
Americ an Heart Associat ion. Heart D isease and Stroke Stat istics - 2008 Update. www.americanheart.org/statistics [Online publication]. Access ed
12 November 2008, 2008.
5
Fang J, Alderm an MH. I mpact of the increasing burden of diabetes on acute my ocardial inf arction in New York City : 1990-2000. Diabetes.
2006;55(3):768-773.
71
Page 75

(
In total, there were 878 subjects included in the RESOLUTE DM cohort. RESOLUTE US provided the
highest p ercentage of subjects at 54.9% (482/878) while RESOLUTE Int contributed 27.6% (242/878),
RESOLUTE AC 9.7% (85/878), RESOLUTE Japan 5.1% (45/878), and RESOLUTE FIM 2.7% (24/878).
Subjects from the 38 mm Length sub-study are not included in this Resolute Pooled Analysis of
Subjects with Diabetes Mellitus. Additional information is provided in Section 9.7.1 for the Resolute US
38 mm Leng th Group for subjects with Diabetes Mellitus.
Design: The Reso lute stent performance for treatment of lesions in patients with DM was compared
with a p erf ormance goal (PG) derived from a meta-analysis of published studies of coronary DES use in
DM subjects and from data from the ENDEAVOR pooled studies.
Inclusio n of study subjects in this analysis were required to have DM defined by either a history of DM or
use o f medications to treat DM (i.e., oral hypoglycemics or insulin) at time of enrollment. The Reso lute
stent DM subjects and those included in the meta-analysis were also required to have clinical
characteristics of an on-label population, consistent with the enrollment criteria of the RESOLUTE US
Clinical Trial. That is, subjects with the following clinical or lesion characteristics were excluded: total
lesion leng th per vessel >27mm, >2 lesions per vessel, unprotected left main lesions, bifurcation
lesions, total occlusions, bypass grafts, acute MI within 72 ho urs of the index procedure, thrombusco ntaining lesions, left ventricular ejection fraction <30%, or renal impairment (serum creatinine >2.5
mg/dl).
The Resolute DM TVF rate at 12-month follow-up was compared to a performance goal to demonstrate
the saf ety and effectiveness of the Resolute stent in diabetic subjects. The objective of the primary
endpoint analysis in the RESOLUTE DM cohort was to assess whether the true p rimary endpoint rate of
12-month Target Vessel Failure (TVF) for the Resolute stent met the PG established as 14.5% (which is
a 31% increase over the expected rate of 11.08% for DES use in DM subjects derived from the metaanalysis). The hypothesis for this analysis accounted for the differences in the protocols of the individual
studies in the published literature, the ENDEAVOR pooled studies, and the Global RESOLUTE Clinical
Trial Program. Specifically, in calculating the meta-analytic PG for DM subjects, adjustments were made
to the 12-month TVF rate based on protocol-req uired follow-up angiography and protocol-required postPCI card iac b iomarker measurements.
Demographics: The mean age of subjects was 65.2 years and 66.4% (583/878) were male. 28.5%
(250/878) of the subjects were insulin dependent diabetics. Of the subjects included in this analysis,
24.9% (216/867) o f the subjects had a prior MI and 28.9% (254/878) were undergoing revascularization
f or unstable angina.
Primary Endpoint: The p rimary endpoint was Target Vessel Failure (TVF) at 12 months following the
intervention. The TVF composite endpoint includes Cardiac Death, MI that cannot be attributed to
vessel(s) other than the target vessel, and clinically driven Target Vessel Revascularization (TVR).
Results: The analysis met the primary endpoint’s performance goal of 14.5%, as the TVF rate of the
DM Cohort was 7.84% at 12 months with an upper bound of the 95% CI of 9.51%.
These analyses are based on the intent-to-treat population. The results are presented in the following
tables:
• Table 9-46: RESOLUTE Diabetes Mellitus Cohort - Primary Endpoint Analysis
• Table 9-47: RESOLUTE Diabetes Mellitus (DM) Cohort: All DM Subjects, Insulin-Dependent DM
Subjects (IDDM), Non-Insulin Dependent DM Subjects (Non-IDDM), and Non-DM Subjects –
Principal Safety and Effectiveness
• Table 9-48: RESOLUTE Diabetes Mellitus Cohort - ARC Defined Definite/Probable Stent
Thrombosis Events through 12 Months
Table 9-46: Resolute Diabetes Mellitus Cohort - Primary Endpoint Analysis
Primary Endpoint
12-month TVF 7.84% (68/867) 9.51% 14.5% <0.001
RESOLUTE DM
N = 878)
Upper Bound of
95%CI
1
Performance
Goal
P-value
2
72
Page 76

Notes
N is the total number of subjects.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
The primary endpoint analysis utilized a randomly selec ted lesion from subjec ts who had treatment of dual lesions.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
1
One-sided confidence interval using exact m ethod.
2
One-sided p-value using exact test stat istic to be compared at a 0.05 significance level.
Table 9-47: RESOLUTE Diabetes Mellitus (DM) Cohort: All DM Subjects, Insulin-
Dependent DM Subjects (IDDM), Non-Insulin Dependent DM Subjects (Non-IDDM), and
Non-DM Subjects – Principal Safety and Effectiveness Through 12 Months
COMPOSITE SAFETY AND
EFFECTIVENESS
TLF
TVF 8.1% (70/867) 11.8% (29/246) 6.6% (41/621) 5.9% (110/1867)
MACE 7.5% (65/867) 11.8% (29/246) 5.8% (36/621) 5.7% (106/1867)
EFFECTIVENESS
Clinically Driven TVR 5.1% (44/867) 6.5% (16/246) 4.5% (28/621) 3.1% (57/1867)
All DM Subjects
(N = 878)
6.6% (57/867) 10.6% (26/246) 5.0% (31/621) 4.9% (92/1867)
IDDM
(N = 250)
Non IDDM
(N = 628)
Non DM
(N = 1903)
TLR 3.3% (29/867) 5.3% (13/246) 2.6% (16/621) 2.0% (38/1867)
TLR, CABG
TLR, PCI 3.1% (27/867)
Non-TL TVR
Non-TL TVR, CABG
Non-TL TVR, PCI 2.1% (18/867) 1.6% (4/246) 2.3% (14/621) 1.1% (20/1867)
SAFETY
Total Death
Cardiac Death 2.0% (17/867) 2.8% (7/246) 1.6% (10/621) 0.4% (8/1867)
Non-Cardiac Death
Cardiac Death or TVMI
TVMI 1.8% (16/867) 4.1% (10/246) 1.0% (6/621) 2.7% (51/1867)
Q wave MI
Non-Q wave MI
Stent Thrombosis
ARC defined
Definite/Probable
Definite 0.2% (2/867) 0.4% (1/246) 0.2% (1/621) 0.2% (4/1867)
0.2% (2/867)
2.2% (19/867)
0.1% (1/867)
2.8% (24/867) 4.1% (10/246) 2.3% (14/621) 1.0% (19/1867)
0.8% (7/867) 1.2% (3/246) 0.6% (4/621) 0.6% (11/1867)
3.6% (31/867) 6.1% (15/246) 2.6% (16/621) 3.2% (59/1867)
0.3% (3/867)
1.5% (13/867)
0.3% (3/867) 0.8% (2/246) 0.2% (1/621) 0.3% (6/1867)
0.8% (2/246) 0.0% (0/621)
4.5% (11/246) 2.6% (16/621)
1.6% (4/246) 2.4% (15/621)
0.0% (0/246) 0.2% (1/621)
0.8% (2/246) 0.2% (1/621)
3.3% (8/246) 0.8% (5/621)
0.3% (6/1867)
1.7% (32/1867)
1.3% (24/1867)
0.2% (4/1867)
0.3% (5/1867)
2.5% (46/1867)
Probable 0.1% (1/867) 0.4% (1/246) 0.0% (0/621) 0.1% (2/1867)
Notes
N = The t otal number of subject s.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
The definitions of the outcomes are presented as table not es to Table 8-1- Princ ipal Adverse Events.
See Table 9-4 for the definition of the ARC defined Stent Thrombos is.
73
Page 77

(
(
(
ys)
Table 9-48: RESOLUTE Diabetes Mellitus Cohort - ARC
Defined Definite/Probable Stent Thrombosis Events
Through 12 Months
Resolute
Stent Thrombosis 0.3% (3/ 867)
Acute (0 – 1 day) 0.1% (1/867)
Subacute (2 - 30 day s) 0.1% (1/867)
Late (31 – 360 days) 0.1% (1/867)
Notes
N is the total number of subjects.
Numbers are % (Count/Number of Eligible Subjects).
12-month time frame includes follow-up window (360 days ± 30 day s).
Subjects are only counted once for eac h time period.
See Table 9-4 for the definition of the ARC defined Stent Thrombos is.
(N = 878)
9.7.1 Subjects with Diabetes Mellitus in the RESOLUTE 38 mm Length Stent Sub-study
Additional information is provided in Table 9-49 for the RESOLUTE US 38 mm Length Group in
sub jects with Diabetes Mellitus.
Table 9-49: RESOLUTE 38 mm Length Group: All 38 mm Subjects, Insulin-Dependent DM
Subjects (IDDM), Non-Insulin Dependent DM Subjects (Non-IDDM), and Non-DM Subjects –
Principal Safety and Effectiveness through 12 Months
COMP OSITE
All Diabetic 38 mm
Length Group Subjects
(N = 8 4 Pa tients)
38 mm Length Group
IDDM
(N = 2 3 Pa tients)
38 mm Length Group –
Non-IDDM
(N = 6 1 Pa tients)
38 mm Length Group –
Non-DM (N = 139
Pa tients)
SAFETY AND
EFFECTIVENESS
TLF 6.0% (5/84) 4.3% (1/23) 6.6% (4/61) 5.1% (7/138)
TVF 7.1% (6/84) 4.3% (1/23) 8.2% (5/61) 6.5% (9/138)
MACE 8.3% (7/84) 4.3% (1/23) 9.8% (6/61) 5.1% (7/138)
EFFECTIVENESS
Clinically-driven TVR 3.6% (3/84) 0.0%
TLR 2.4% (2/84) 0.0% (0/23) 3.3% (2/61) 0.7% (1/138)
SAFETY
Total Death 1.2% (1/84) 0.0% (0/23) 1.6% (1/61) 0.7% (1/138)
Cardiac Death 1.2% (1/84) 0.0% (0/23) 1.6% (1/61) 0.7% (1/138)
Non-Cardiac Death 0.0% (0/84) 0.0% (0/23) 0.0% (0/61) 0.0% (0/138)
Cardiac Death or TVMI 3.6% (3/84) 4.3% (1/23) 3.3% (2/61) 5.1% (7/138)
TVMI 2.4% (2/84) 4.3% (1/23) 1.6% (1/61) 4.3% (6/138)
Stent Thrombosis
ARC defined
Stent Thrombosis (ARC
def/prob)
Early (30 days) 0.0% (0/84) 0.0% (0/23) 0.0% (0/61) 1.4% (2/138)
Late (>30 and 360
da
0/23) 4.9%
0.0% (0/84) 0.0% (0/23) 0.0% (0/61) 1.4% (2/138)
0.0% (0/84) 0.0% (0/23) 0.0% (0/61) 0.0% (0/138)
3/61) 2.2%
3/138)
74
Page 78

9.8 Subjects with Chronic Total Occlusion The PERSPECTIVE Study – RESOLUTE CTO Cohort
The PERSPECTIVE Study included a retrospective and a p rospective study arm. Both arms of this
study enrolled approximately 250 patients at a single center experienced in CTO procedures. The
prospective arm essentially comprised a separate sub-study designed to evaluate procedural and 1year clinical outcomes among consecutive patients undergoing attempted p ercutaneous Chronic Total
Occlusion (CTO) revascularization. The prospective arm of the PERSPECTIVE study included a presp ecified subgroup analysis of patients treated with the Resolute family of drug eluting stents (all were
Resolute Integrity).
Primary Objective: To assess the safety and effectiveness of the Resolute Zotarolimus-Eluting
Coronary Stent System (Resolute ZES) for the treatment of chronic total occlusions
Population: The population consisted of prospectively enrolled subjects undergoing attempted
percutaneous CTO revascularization and treated with the Resolute ZES.
Design: The PERSPECTIVE Study
(Prospective Arm/Prespecified Resolute ZES for CTO Analysis) was
a single-center, investigator-initiated, observational study which prospectively enrolled approximately
250 sub jects undergoing attempted CTO. The assessment of use of Resolute ZES stents in CTO
revascularization was based on prospectively enrolled CTO patients compared to a pre-specified
performance goal.
An estimated MACE rate was derived based on a weighted average of the reported rates for drug-
6
eluting stents from the PRISON II
and EXPERT CTO7 studies. Due to the difference in the definition of
myocardial infarction (MI) used in the PRISON II study, an adjustment for the MACE rate was made to
approximate the MACE rate if the ARC definition of myocardial infarction (MI) had been applied. The
weighted average produced an estimated MACE rate of 16.6% using the ARC definition of MI. The
performance goal (PG) for the pre-specified RESOLUTE CTO Cohort analysis was 25.2% based on the
estimated MACE rate of 16.6% and a one-sided 95% CI.
Demographics: In the RESOLUTE CTO Cohort of the PERSPECTIVE Study, the mean age was 63.4 ±
9.5, 79.8% (146/183) were male, 98.4% (180/183) reported dyslipidemia, 88.5% (162/183) had
hyp ertension, 18.0% (31/172) were current smokers, 35.5% (65/183) were diabetic including 12.6%
(23/182) reported as insulin dependent, 33.3% (61/183) had a prior MI, and 80.9% (140/173) were
classified as having stable angina.
Primary Endpoint: Major adverse cardiac events (MACE) at one year; a composite of death,
myocardial infarction (MI) (ARC defined), and clinically-driven target lesion revascularization (TLR)
Results: The observed MACE rate at one year for the RESOLUTE CTO Cohort was 18.2% (33/181) for
the ITT population. The ITT population met the primary endpoint. The upper limit of the 95% confidence
interval was 23.6% which is lower than the pre-specified performance goal (25.2%). A post hoc gender
sub g roup analysis of the primary endpoint resulted in MACE rates at one year of 18.8% (27/144) in
male subjects and 16.2% (6/37) in female subjects.
The PERSPECTIVE Study results are presented in Table 9-50, Table 9-51, and Table 9-52:
Table 9-50: Primary Endpoint Analysis – MACE at 12 Months (ITT)
One-side upper
RESOLUTE CTO cohort
Primary Endpoint
MACE at 12 months
ITT 18.2% (33/181) 23.6% 25.2%
(N=183 Subjects)
95%
Confidence Interval
Performance
Goal
6
Suttorp MJ, Laarman GJ, R ahel BM, et al. Primary Stenting of Totally Occluded Nat ive Coronary Art eries I I (PRISON II): a random ized comparis on
of bare metal stent implantation with sirolimus-elut ing stent im plantation for the treat ment of t otal coronary oc clusions. Circulation 2006; 114(9); 921
– 928.
7
Kandzari DE, Kini AS, Karmpaliotis D, et al. Safet y and Ef fectiv eness of Ev erolimus-Eluting St ents in Chronic Total Coronary Occlusion
Revascularization: Results From the EXPER T CTO M ulticenter T rial (Evaluation of t he XIEN CE Coronary St ent, Perform ance, and Technique in
Chronic Total Occlusions). J Am Coll C ardiol Int v 2015; 8(6); 761 – 769.
75
Page 79

Table 9-51: Principal Safety and Effectiveness Results
RESOLUTE CTO cohort
(N=183 Subjects)
Safety and Effectiveness Measures
Safety Measures (In-hospital)
TLF 15.3% (28/183)
TVF 15.3% (28/183)
MACE 15.3% (28/183)
Cardiac Death or MI 15.3% (28/183)
Death or MI 15.3% (28/183)
Death 1.1% (2/183)
Cardiac Death 1.1% (2/183)
Non-Cardiac Death 0.0% (0/183)
MI 14.8% (27/183)
TLR 0.0% (0/183)
TVR 0.0% (0/183)
Safety Measures (to 6 Mon ths/183 days)
TLF 17.5% (32/183)
TVF 17.5% (32/183)
MACE 17.5% (32/183)
Cardiac Death or MI 17.5% (32/183)
Death or MI 17.5% (32/183)
Death 2.7% (5/183)
Cardiac Death 2.2% (4/183)
Non-Cardiac Death 0.5% (1/183)
MI 15.8% (29/183)
TLR 0.5% (1/183)
TVR 0.5% (1/183)
All Stent Thrombosis (ARC D ef/Prob/Pos s) 1.6% (3/183)
Stent Thrombosis ARC D efinite/Probable 0.6% (1/183)
Stent Thrombosis ARC Pos sible 1.1% (2/183)
Early Stent Thrombosis (0 to 30 days) 0.6% (1/183)
Definite 0.6% (1/183)
Probable 0.0% (0/183)
Possible 0.0% (0/183)
Late Stent Thrombosis (31 days – 6 months) 1.1% (2/183)
Definite 0.0% (0/183)
Probable 0.0% (0/183)
Possible 1.1% (2/183)
Safety Measures (to 1 year/365 days)
TLF 18.2% (33/181)
TVF 18.2% (33/181)
MACE 18.2% (33/181)
Cardiac Death or MI 17.7% (32/181)
Death or MI 17.7% (32/181)
Death 2.8% (5/181)
Cardiac Death 2.2% (4/181)
Non-Cardiac Death 0.6% (1/181)
MI 16.0% (29/181)
TLR 1.1% (2/181)
TVR 1.1% (2/181)
All Stent Thrombosis (ARC D ef/Prob/Pos s) 1.7% (3/181)
%(m/n)
76
Page 80

Table 9-51: Principal Safety and Effectiveness Results
RESOLUTE CTO cohort
(N=183 Subjects)
Safety and Effectiveness Measures
Stent Thrombosis ARC D efinite/Probable 0.6% (1/181)
Stent Thrombosis ARC Pos sible 1.1% (2/181)
Early Stent Thrombosis (0 to 30 days) 0.6% (1/181)
Definite 0.6% (1/181)
Probable 0.0% (0/181)
Possible 0.0% (0/181)
Late Stent Thrombosis (31 days – 1year) 1.1% (2/181)
Definite 0.0% (0/181)
Probable 0.0% (0/181)
Possible 1.1% (2/181)
Effectiveness Measures
Clinical success
Technical success
1CTO procedural success as defined by achievement of <50% residual stenosis with 7,0, 2 antegrade flow
2
Successful guidewire crossing with placem ent in distal true lumen of CTO target lesion
1
2
%(m/n)
92.3% (169/183)
96.2% (175/182)
Table 9-52: RESOLUTE CTO Cohort – Primary Endpoint Analysis by Gender
Male Subjects
RESOLUTE CTO cohort
Primary Endpoint
MACE at 12 months 18.8% (27/144) 16.2% (6/37)
(N=146 Subjects)
% (m/n)
Female Subjects
RESOLUTE CTO cohort
(N=37 Subjects)
% (m/n)
Global RESOLUTE Clinical Program – RESOLUTE Pooled CTO
Population: In order to provide additional support for the performance of the Resolute family of stents in
the treatment o f CTOs, a retrospective, pooled analysis was performed which was comprised of pooled
CTO patients from the Global RESOLUTE Clinical Program.
The f ollowing Global RESOLUTE Clinical Trials contributed subjects to the CTO cohort:
• RESOLUTE International
The RESOLUTE International Study (R-Int) was a p ro spective, multi-center, non-randomized,
single-arm, observational study of the Resolute stent in a real world subject population. A total
2349 sub jects were enrolled into the study. Subjects were followed for 3 years post-procedure. A
to tal of 186 subjects from the R-Int study were included in the RESOLUTE Pooled CTO analysis.
• RESOLUTE China Randomized Controlled Trial
The RESOLUTE China Randomized Controlled Trial (R-China RCT) was a p rospective, multicenter, rando mized, open-label study designed to assess the non-inferiority of the Resolute stent
co mpared to the Taxus Liberte stent for in-stent late lumen loss. A total of 198 subjects were
treated with the Resolute stent. Subjects were followed for 5 years post-procedure. A total of 15
sub jects from the R-China RCT study were included in the RESOLUTE Pooled CTO analysis.
77
Page 81

• RESOLUTE China Registry
The RESOLUTE China Registry (R-China Registry) was a prospective, multi-center, nonrandomized, single-arm, observational study of the Resolute stent in a real-world patient
population requiring stent implantation. A total of 1800 subjects were treated with the Resolute
stent. Subjects were followed for 5 years post-procedure. A total of 157 subjects from the R-China
Registry were included in the RESOLUTE Pooled CTO Analysis.
Design: The Resolute stent performance for the treatment of CTO lesions was analyzed from data
co llected in the R-Int, R-China RCT, and R-China Registry studies. The results pooled datasets from the
5-year data of R-China RCT, 4-year data of R-China Registry, and 3-year data from R-Int. In total, 358
sub jects were evaluable for this CTO subset.
Demographics: The averag e age in the RESOLUTE Pooled CTO subset (n=358) was 60.4 ± 11.3 years
and 84.4% (302/358) were male. For this population, 37.7% (133/353) experienced a prior MI, 65.1%
(233/358) had hypertension, 50.3% (180/358) had hyperlipidemia and 26.5% (95/358) had diabetes.
Global RESOLUTE Clinical Program results are presented in the following table:
Table 9-53: RESOLUTE Pooled CTO Analysis – Safety and Effectiveness Results
RESOLUTE Pooled CTO
(N=358 Patients)
Safety and Effectiveness Endpoints
Effectiveness Measures
Lesion Success6 100.0% (526/526)
Device Success7 94.1% (496/527)
Procedure Success8 97.5% (348/357)
1 Year
TLF1 4.5% (16/352)
TVF2 4.8% (17/352)
MACE3 5.7% (20/352)
Composite Endpoint4 12.2% (43/352)
Cardiac Death or TVMI 3.1% (11/352)
Death or TVMI 4.0% (14/352)
Death 1.7% (6/352)
Cardiac Death 0.9% (3/352)
Non-Cardiac Death 0.9% (3/352)
TVMI (Extended historical definition) 2.3% (8/352)
Clinically Driven TLR 2.0% (7/352)
Clinically Driven TVR 2.3% (8/352)
Stent Thrombosis (ARC) Definit e/Probable) 0.6% (2/352)
Early Thrombosis(30 days) 0.3% (1/352)
Late Thrombosis(>30 and 360 days) 0.3% (1/352)
Significant Bleeding Complications5 1.1% (4/352)
Stroke 0.9% (3/352)
3 Years
TLF1 8.9% (31/347)
TVF2 10.1% (35/347)
MACE3 10.1% (35/347)
Composite Endpoint4 18.4% (64/347)
Cardiac Death or TVMI 6.6% (23/347)
Death or TVMI 7.8% (27/347)
Death 5.5% (19/347)
Cardiac Death 4.3% (15/347)
Non-Cardiac Death 1.2% (4/347)
TVMI (Extended historical definition) 3.2% (11/347)
(N=527 Lesions) %(m/n)9
78
Page 82

Table 9-53: RESOLUTE Pooled CTO Analysis – Safety and Effectiveness Results
RESOLUTE Pooled CTO
(N=358 Patients)
Safety and Effectiveness Endpoints
Clinically Driven TLR 3.2% (11/347)
Clinically Driven TVR 4.3% (15/347)
Stent Thrombosis (ARC) Definit e/Probable) 1.2% (4/347)
Early Thrombosis (30 days) 0.3% (1/347)
Late Thrombosis (>30 and 360 days) 0.3% (1/347)
Very Late Thrombosis (>360 days) 0.9% (3/347)
Significant Bleeding Complications5 1.2% (4/347)
Stroke 1.7% (6/347)
1.Cardiac death, target ves sel myoc ardial infarction (Q wave and non-Q w ave) or c linically-driven t arget les ion revasculariz ation (TLR) by
percutaneous or surgical methods.
2.Cardiac death, target ves sel myoc ardial infarction or clinically -driven target v essel revas cularization.
3.Death, myocardial infarction, (Q wave and non-Q wav e), emergent coronary by pass surgery , or repeat target lesion revasc ularization (clinic ally
driven/clinically indicated) by percutaneous or surgical met hods.
4.The combined clinical outcom e of (all c ause) mortalit y, my ocardial infarct ion (Q-wave and non-Q wav e), or (any ) revasculariz ation.
5.Bleeding complication is defined as a procedure related hemorrhagic event that requires a transfusion or surgical repair. These may include a
hematoma requiring treatment of retroperitoneal bleed.
Significant Bleeding complication is def ined as t he bleeding com plication that has at least one of the following scenarios:
Bleedings that led to an interruption of anti-platelet medication
Bleedings that require transfusion
I ntracerebral bleedings
Bleedings that resulted in substantial hemodynamic c ompromise requiring treatment
6.The attainment of <50% residual stenosis of the target lesion using any percutaneous method.
7.The attainment of <50% residual stenosis of the target lesion using only the assigned device.
8.The attainment of <50% residual stenosis of the target lesion and no in-hospital M ACE.
9.Numerator (m) is the number of patient s (or les ions) with the spec ific clas sificat ion, denominator (n) is the num ber of patients (or lesions) in the
study group with known values, and percentage () was c alculated as 100 × (m/n)
Extended historical definition of MI is used f or all the c omposite endpoint s.
(N=527 Lesions) %(m/n)9
9.9 Pooled Results of the Global RESOLUTE Clinical Trial Program (RESOLUTE FIM, RESOLUTE US, RESOLUTE AC, RESOLUTE Int, RESOLUTE Japan)
In o rder to better estimate the incidence of low-frequency events or outcomes, a subject-level pooled
analysis was conducted. Table 9-54 below provides the total number of subjects included in the
analyses.
Table 9-54: Subjects Included in the Analyses by Clinical Study
All Subjects On-label
RESOLUTE FIM 139 139
RESOLUTE All-Comers – Resolut e 1140 376
RESOLUTE International 2349 763
RESOLUTE US 1402 1402
RESOLUTE Japan 100 100
Pooled Resolute Dataset 5130 2780
Subjects from the 38 mm L ength sub-stu dy were no t inclu ded in the R ESOLUTE p ooled anal ysis presen ted
here
79
Page 83

The on-label subgroup includes all enrolled subjects except those that had a total occlusion, target
lesions involving a bifurcation lesion, target lesions involving a Saphenous Vein Graft lesion (SVG), an
In-Stent Restenosis (ISR) target lesion, a subject having an Acute Myocardial Infarction (AMI) hrs),
sub jects with a demonstrated Left-Ventricular Ejection Fraction (LVEF) less than 30%, target lesions
located in an unprotected Left Main Artery, subjects with treated vessels, subjects with a serum
creatinine of >2.5 mg/dl, a lesion length >27 mm, 2 or more lesions treated per vessel, and target
lesions with the presence of a thrombus.
It is acknowledged that the results of retrospective pooled analyses have limitations. D efinitive proof of
the presence or ab sence of any dif ferences between sub-groups requires prospectively p owered
assessments in clinical trials. The results are presented in the following tables:
• Table 9-55 : Resolute Pooled Analysis - Principal Safety and Effectiveness
• Table 9-56 Reso lute Pooled Analysis - ARC Defined Definite/Probable Stent Thrombosis* through
60 Months
80
Page 84

(
(
Table 9-55: Resolute Pooled Analysis - Principal Safety and
Effectiveness Through 60 Months
All Subjects
N = 5130)
On-label
N = 2780)
Outcomes at 12 Months
COMPOSITE SAFETY AND
EFFECTIVENESS
TLF
TVF
MACE
6.6% (336/5098) 5.4% (150/2759)
7.5% (382/5098) 6.6% (181/2759)
7.5% (384/5098) 6.3% (174/2759)
EFFECTIVENESS
Clinically Driven TVR
Clinically Drive n TLR 3.3% (166/5098) 2.5% (69/2759)
4.3% (220/5098) 3.7% (103/2759)
SAFETY
Total Death
Cardiac Death
Non-Cardiac Death
TVMI
Cardiac Death or TVMI
Stent Thrombosis
1.9% (98/5098) 1.6% (44/2759)
1.2% (60/5098) 0.9% (26/2759)
0.7% (38/5098) 0.7% (18/2759)
2.9% (149/5098) 2.4% (66/2759)
3.9% (200/5098) 3.3% (90/2759)
ARC defined
Definite/Probable
Definite
Probable
0.8% (40/5098) 0.3% (9/2759)
0.6% (29/5098) 0.2% (6/2759)
0.3% (13/5098) 0.1% (3/2759)
Outcomes at 36 Months
COMPOSITE SAFETY AND
EFFECTIVENESS
TLF
TVF
MACE
10.8% (539/5012) 9.2% (249/2709)
13.0% (652/5012) 12.0% (324/2709)
13.5% (679/5012) 12.0% (325/2709)
EFFECTIVENESS
Clinically Driven TVR
Clinically Drive n TLR 5.3% (267/5012) 4.4% (119/2709)
7.9% (397/5012) 7.5% (204/2709)
SAFETY
Total Death
Cardiac Death
Non-Cardiac Death
TVMI
Cardiac Death or TVMI
5.5% (275/5012) 5.0% (135/2709)
3.1% (156/5012) 2.6% (70/2709)
2.4% (119/5012) 2.4% (65/2709)
3.8% (188/5012) 3.1% (84/2709)
6.5% (324/5012) 5.4% (145/2709)
81
Page 85

(
(
Table 9-55: Resolute Pooled Analysis - Principal Safety and
Effectiveness Through 60 Months
All Subjects
N = 5130)
Stent Thrombosis
On-label
N = 2780)
ARC defined
Definite/Probable
Definite
Probable
Outcomes at 60 Months*
COMPOSITE SAFETY AND
EFFECTIVENESS
TLF
TVF
MACE
EFFECTIVENESS
Clinically Driven TVR
Clinically Drive n TLR 6.7% (179/2688) 5.8% (112/1937)
SAFETY
Total Death
Cardiac Death
Non-Cardiac Death
TVMI
Cardiac Death or TVMI
Sten t Thro mb osi s
1.1% (54/5012) 0.5% (13/2709)
0.7% (37/5012) 0.3% (7/2709)
0.4% (19/5012) 0.2% (6/2709)
14.0% (376/2688) 12.3% (239/1937)
18.1% (486/2688) 16.5% (320/1937)
19.4% (521/2688) 18.2% (352/1937)
11.4% (306/2688) 10.6% (205/1937)
9.9% (266/2688) 9.7% (188/1937)
4.9% (131/2688) 4.3% (83/1937)
5.0% (135/2688) 5.4% (105/1937)
4.5% (120/2688) 3.9% (76/1937)
8.7% (234/2688) 7.5% (145/1937)
ARC defined
Definite/Probable
Definite
Probable
1.3% (34/2688) 0.8% (15/1937)
0.8% (22/2688) 0.5% (9/1937)
0.5% (13/2688) 0.3% (6/1937)
Notes
N = The t otal number of subject s enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
12-month time frame includes follow-up window (360 days ± 30 day s).
36-month timeframe includes follow-up w indow (1080 day s ± 30day s).
60-month timeframe includes follow-up window (1800 days ± 30days)
Note: R-Int. f ollow-up ends at t hree years and is not included in this analy sis.
*
The definitions of the outcomes are presented as table not es to Table 8-1 - Princ ipal Advers e Events.
See Table 9-4 for the definition of the ARC defined Stent Thrombos is.
82
Page 86

(
)
(
)
, which may lead to under-diagnosis and under-referral of
9
N = 2017
On-label*
N = 2781
All Subjects*
1.3% (34/2688) 0.8% (15/1937)
0.3% (8/2688) 0.2% (4/1937)
0.5% (13/2688) 0.2% (3/1937)
0.5% (14/2688) 0.4% (8/1937)
83
Stent Thrombosis Through 60 Months
Table 9-56: Resolute Pooled Analysis - ARC Defined Definite/Probable
Stent Thrombosis
Early (0 - 30 days)
Late (31 - 360 days)
Very Late (361 1800 days)*
Notes
N = The t otal number of subject s enrolled. Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for eac h time period.
* Note: R-Int. follow-up ends at three years and is not included in t his analysis .
60-month timeframe includes follow-up window (1800 days ± 30 day s).
12-month time frame includes follow-up window (360 days ± 30 day s).
See Table 9-4 for the definition of the ARC defined Stent Thrombos is.
9.9.1 Gender Analysis from the RESOLUTE Pooled On-label Dataset
However, it is estimated that only 36% of annual PCIs are performed in women. In PCI clinical trials, women represent only 25 - 35% of
8
and had smaller reference vessel diameters (RVD).
sub jects were female and 1996/2780 (71.8%) were male. Female patients at baseline were older, had higher rates of diabetes and hypertension,
(CAD ).
In the United States, an estimated 17,600,000 adults age 20 and o lder (9.1% of men and 7.0% of women) suffer from coronary artery disease
partly attributable to gender differences in presenting symptoms and pathophysiology
the enrolled populations, and there are relatively little gender-specific data. The disproportionate enrollment distribution in clinical studies may be
f emale patients with CAD. Once diagnosed and treated, poorer revascularization outcomes have been reported in women (compared with men)
peripheral vascular d isease.
due to smaller coronary arteries and increased prevalence of baseline comorbidities including advanced age, diabetes, hypertension, and
Table 9-57 describes the baseline and demographic characteristics by gender for subjects in the pooled on-label analysis. 784/2780 (28.2%)
Subjects from the 38 mm Length sub-study were not included in the RESOLUTE pooled analysis.
symptom evaluation, and gender-opt imized diagnostic s trategies. J Am Coll C ardiol. 2006; 47(3):S4-S20.
Lloyd-Jones D, Adam s RJ, Brown TM, et al. Heart Disease and St roke St atistics—2010 U pdate. A Report From the Am erican H eart Association. Circulation. 2010;121(7):e46-e215. 9 Shaw LJ, Bairey Merz CN, Pepine CJ, et al. Ins ights from the N HLBI-Sponsored W omen’s Is chemia Syndrom e Evaluation (WISE) Study : Part I: gender differences in traditional and novel ris k fact ors,
8
Page 87

Fema le
Mal e
p-value
N=784
84
N=1996
Table 9-57: Baseline Characteristics of Male vs. Female for Pooled On-Label Resolute Patients
Patient Characteristics
and effectiveness profile of the Resolute stent is generalizable to both males and females.
non-cardiac death) at 12 months; although after 60 months are evenly distributed among the two groups. These results suggest that the safety
analysis of the principal safety and effectiveness outcomes through 12 months in subjects treated with Resolute stents for on-label indications
stratif ied by gender. In general, event rates were low for both gender groups. The event rates were numerically higher in women (except for
The pooled Resolute stent on-label use data were evaluated retrospectively for gender-based clinical outcomes. Table 9-58 shows a post-hoc
Age(years) 62.9±10.5 (1996) 67.1±10.6 (784) <.001
History of Smoking/Tobacco use 64.1% (1280/1996) 45.5% (357/784) <.001
Prior PCI 32.4% (646/1996) 28.1% (220/784) 0.029
Hyperlipidemia 78.6% (1568/1996) 80.9% (634/784) 0.194
Diabetes Mellitus 29.2% (582/1996) 37.6% (295/784) <.001
Insulin Dependent 7.0% (140/1996) 13.9% (109/784) <.001
History of Hypertension 75.1% (1499/1996) 84.3% (661/784) <.001
Prior MI 28.1% (556/1977) 18.3% (141/772) <.001
Prior CABG 9.4% (187/1996) 5.7% (45/784) 0.002
Ejection Fraction (%) 0.059
<30% 0.1% (1/1463) 0.2% (1/593)
30-40% 6.9% (101/1463) 4.6% (27/593)
>40% 93.0% (1361/1463) 95.3% (565/593)
Lesion Class ACC/AHA 0.133
A 7.8% (172/2219) 9.5% (81/857)
B1 25.8% (573/2219) 27.5% (236/857)
B2 32.0% (710/2219) 29.8% (255/857)
C 34.4% (764/2219) 33.3% (285/857)
Moderate/Severe Calcification 39.1% (867/2216) 39.2% (335/855) 1.000
Pre procedure RVD (mm) 2.7 ± 0.5 (2201) 2.6±0.5 (855) <.001
Pre procedure MLD (mm) 0.7 ± 0.4 (2212) 0.8±0.4 (856) 0.025
Pre procedure Diameter Stenosis (%) 73.0±13.8 (2212) 70.5±13.5 (856) <.001
Lesion Length (mm) 1 3.8 ±5. 8 (2201) 12.9±5.7 (855) <.001
Page 88

(
)
(
)
N=784
Fema le
Mal e
N=1996
85
and Effectiveness Through 60 Months
Table 9-58: Resolute Pooled On-Label Gender (Male vs. Female) – Principal Safety
Sa fety Outc omes to 12 Months
COMPOSI TE SAFETY AND EFFECTIVENE SS
TLF 4.9% (97/1983) 6.8% (53/776)
TVF 5.8% (115/1983) 8.5% (66/776)
MACE 6.0% (118/1983) 7.2% (56/776)
EFFECTIVENESS
Clinically Drive n TVR 3.3% (66/1983) 4.8% (37/776)
Clinically Drive n TLR 2.3% (46/1983) 3.0% (23/776)
SAFETY
Death 1.5% (30/1983) 1.8% (14/776)
Cardiac Death 0.8% (15/1983) 1.4% (11/776)
Non-Cardiac Death 0.8% (15/1983) 0.4% (3/776)
TVMI (Extended Historical Definition) 2.1% (41/1983) 3.2% (25/776)
Cardiac Death or Target Vessel MI (TVMI) 2.8% (55/1983) 4.5% (35/776)
Stent Thrombosis ARC de fined
Definite/Probable 0.3% (5/1983) 0.5% (4/776)
Definite 0.2% (4/1983) 0.3% (2/776)
Probable 0.1% (1/1983) 0.3% (2/776)
Safety Measures to 36 Months
COMPOSI TE SAFETY AND EFFECTIVENE SS
TLF 8.8% (172/1949) 10.1% (77/760)
TVF 11.4% (222/1949) 13.4% (102/760)
MACE 11.8% (230/1949) 12.5% (95/760)
EFFECTIVENESS
Clinically Drive n TVR 7 .3 % (142/1949) 8.2% (62/760)
Clinically Drive n TLR 4.4% (86/1949) 4.3% (33/760)
SAFETY
Death 5.0% (98/1949) 4.9% (37/760)
Cardiac Death 2.5% (48/1949) 2.9% (22/760)
Non-Cardiac Death 2.6% (50/1949) 2.0% (15/760)
TVMI (Extended Historical Definition) 2.6% (51/1949) 4.3% (33/760)
Cardiac Death or Target Vessel MI (TVMI) 4.8% (94/1949) 6.7% (51/760)
Stent Thrombosis ARC de fined
Definite/Probable 0.4% (7/1949) 0.8% (6/760)
Definite 0.3% (5/1949) 0.3% (2/760)
Probable 0.1% (2/1949) 0.5% (4/760)
Page 89

(
)
(
)
j
g
y
p
y
N=784
Fema le
sis
for the definition of the ARC
ooled anal
Mal e
N=1996
86
were not included in the RESOLUTE
and Effectiveness Through 60 Months
Table 9-58: Resolute Pooled On-Label Gender (Male vs. Female) – Principal Safety
Outcomes to 6 0 Months
COMPOSI TE SAFETY AND EFFECTIVENE SS
TLF 12.4% (169/1363) 12.2% (70/574)
TVF 16.6% (226/1363) 16.4% (94/574)
MACE 18.3% (249/1363) 17.9% (103/574)
EFFECTIVENESS
Clinically Drive n TVR 10.6 % (144/1363) 10.6% (61/574)
Clinically Drive n TLR 5.9% (80/1363) 5.6% (32/574)
SAFETY
Death 9.8% (133/1363) 9.6% (55/574)
Cardiac Death 4.3% (58/1363) 4.4% (25/574)
Non-Cardiac Death 5.5% (75/1363) 5.2% (30/574)
TVMI (Extended Historical Definition) 3.6% (49/1363) 4.7% (27/574)
Cardiac Death or Target Vessel MI (TVMI) 7.3% (99/1363) 8.0% (46/574)
th sub-stud
ects from the 38 mm Len
* Note: R-Int. follow-up ends at three years and is not included in t his analysis . See T able 9-4
36-month timeframe includes follow-up window (1080 days ± 30 day s).
12-month timeframe includes follow-up w indow (360 day s ± 30 days).
The definitions of the outcomes are presented as table notes t o Table 8-1- Principal Adverse Events.
Subjects are only counted once for eac h time period.
Numbers are % (Count/Number of Eligible Subjects).
Notes
Stent Thrombosis ARC de fined
Definite/Probable 0.7% (9/1363) 1.0% (6/574)
Definite 0.4% (6/1363) 0.5% (3/574)
N = The t otal number of subject s enrolled.
Probable 0.2% (3/1363) 0.5% (3/574)
defined Stent Thrombosis.
60-month timeframe includes follow-up w indow (1800 day s ± 30 days).
Sub
sub g roups, so these post hoc analyses are considered hypothesis-generating.
The RESOLUTE clinical trials were not designed or powered to study the safety or effectiveness of the Resolute Integ rity stent in gender-specific
Page 90

(
)
(
)
(
)
(
)
N = 509
mm
Lesion Length
RVD
N = 1956
mm
(N = 3636)
B2/C Lesions
Female
(N = 1287)
87
Male
(N = 3843)
Age
yrs.
N = 2547
1.2% (30/2515) 0.8% (31/3780) 0.6% (7/1264) 0.7% (26/3577) 0.6% (12/1928) 1.4% (7/495)
Lesion
N = 2466
On-label Single
2.3% (57/2428) 2.9% (74/2515) 2.8% (105/3780) 3.6% (45/1264) 3.2% (115/3577) 3.5% (67/1928) 1.8% (9/495)
4.5% (113/2515) 3.6% (137/3780) 4.9% (62/1264) 4.0% (144/3577) 4.4% (84/1928) 3.4% (17/495)
0.3% (7/2428) 0.8% (19/2515) 0.8% (31/3780) 0.7% (9/1264) 0.9% (31/3577) 0.7% (14/1928) 1.0% (5/495)
0.2% (5/2428) 0.5% (12/2515) 0.6% (24/3780) 0.4% (5/1264) 0.7% (25/3577) 0.5% (10/1928) 0.6% (3/495)
0.1% (2/2428) 0.3% (8/2515) 0.2% (9/3780) 0.3% (4/1264) 0.2% (8/3577) 0.3% (6/1928) 0.4% (2/495)
Table 9-59: Resolute Pooled Analysis - Subset Outcomes Through 12 Months
EFFECTIVENESS
COMPOSITE SAFETY AND
TLF 5.3% (128/2428) 7.0% (177/2515) 6.3% (239/3780) 7.4% (94/1264) 6.7% (239/3577) 7.3% (141/1928) 7.9% (39/495)
TVF 6.4% (155/2428) 8.0% (202/2515) 7.1% (270/3780) 8.6% (109/1264) 7.6% (272/3577) 8.5% (164/1928) 8.5% (42/495)
MACE 6.1% (147/2428) 8.4% (211/2515) 7.3% (277/3780) 8.0% (101/1264) 7.6% (271/3577) 8.1% (157/1928) 9.3% (46/495)
EFFECTIVENESS
Clinically Driven TVR 3.6% (88/2428) 4.3% (108/2515) 4.3% (162/3780) 4.4% (55/1264) 4.4% (157/3577) 5.0% (96/1928) 5.7% (28/495)
TLR 2.4% (58/2428) 3.1% (79/2515) 3.3% (124/3780) 3.1% (39/1264) 3.3% (118/3577) 3.7% (71/1928) 5.1% (25/495)
SAFETY
Total Death 1.6% (39/2428) 3.1% (78/2515) 1.9% (70/3780) 2.1% (26/1264) 1.7% (62/3577) 1.7% (32/1928) 3.2% (16/495)
Cardiac Death 0.9% (22/2428) 1.9% (48/2515) 1.0% (39/3780) 1.5% (19/1264) 1.0% (36/3577) 1.0% (20/1928) 1.8% (9/495)
Non-Cardiac Death 0.7% (17/2428)
TVMI
Cardiac Death or TVMI 3.2% (77/2428)
Sten t Thro mb osi s
In o rder to provide the totality of data on the Resolute stent, the clinical outcomes in key patient and lesion subsets are provided. The
9.9.2 Subset Analyses from the Resolute Pooled Dataset
dif ferences between subsets requires prospectively powered assessments in clinical trials.
It is ackno wledged that the results of such retrospective pooled analyses have limitations. Definitive proof of the presence or absence of any
9-59 , Table 9-60 , Table 9-61 ). Subjects from the 38 mm Length sub-study were not included in the RESOLUTE pooled analysis.
characteristics. Clinical outcomes at 12 months in key patient subsets from the pooled Resolute trials are provided in the Tables below (Table
exp and ed use of the Resolute stent beyond those enrolled in the p ivotal RESOLUTE US trial. In the RESOLUTE All-Comers and RESOLUTE
International studies 33% of enrolled subjects who fit the on-label criteria, whereas the remaining 67% had complex subject/lesion
RESOLUTE All-Comers Clinical Trial and the RESOLUTE International Study enrolled an ‘all-comers’ patient population representing an
ARC defined Definite/Probable
Definite
Probable
Page 91

(
)
(
)
(
)
N = 199
Restenosis
BMS In-Stent
N = 770
Stenting
Multi-Vessel
Graft
N = 64
11.1% (22/198)
12.1% (24/198)
9.1% (18/198)
8.1% (16/198)
3.0% (6/198)
1.9% (14/756)
2.0% (4/198)
1.3% (10/756)
1.0% (2/198)
0.5% (4/756)
3.0% (6/198)
3.3% (25/756)
1.5% (3/198)
Saphenous Vein
(N = 644)
Overlapping Stents
Multiple Stents
(N = 1788)
7.8% (137/1758) 7.8% (49/632) 17.2% (11/64) 8.2% (62/756)
2.0% (36/1758) 3.0% (19/632) 3.1% (2/64)
0.8% (14/1758) 1.6% (10/632) 0.0% (0/64)
4.5% (79/1758) 4.4% (28/632) 9.4% (6/64) 4.5% (34/756) 4.0% (8/198)
1.1% (20/1758) 1.1% (7/632) 1.6% (1/64) 1.2% (9/756) 2.5% (5/198)
0.4% (7/1758) 0.6% (4/632) 1.6% (1/64) 0.7% (5/756) 1.0% (2/198)
88
Table 9-60: Resolute Pooled Analysis – Subset Outcomes Through 12 Months
EFFECTIVENESS
COMPOSITE SAFETY AND
TLF
TVF 8.6% (152/1758) 8.7% (55/632) 17.2% (11/64) 8.9% (67/756)
MACE 8.8% (155/1758) 9.3% (59/632) 17.2% (11/64) 9.0% (68/756) 12.1% (24/198)
EFFECTIVENESS
Clinically Driven TVR 5.1% (89/1758) 5.4% (34/632) 10.9% (7/64) 5.0% (38/756)
TLR 4.1% (72/1758) 4.4% (28/632) 7.8% (5/64) 4.4% (33/756)
SAFETY
Total Death
Cardiac Death 1.3% (22/1758) 1.4% (9/632) 3.1% (2/64)
Non-Cardiac Death
TVMI 3.5% (62/1758) 3.3% (21/632) 7.8% (5/64)
Cardiac Death or TVMI
Sten t Thro mb osi s
ARC defined
Definite/Probable
Definite 0.9% (15/1758) 0.6% (4/632) 0.0% (0/64) 0.7% (5/756)
Probable
Page 92
(
)
(
)
(N = 799)
AMI <72 hours
2
Renal
N = 135
Insufficiency
N = 57
Left Main
Unprotected
3.6% (2/56) 2.3% (3/133) 2.2% (17/788)
1.8% (1/56) 0.8% (1/133) 1.5% (12/788)
1.8% (1/56) 1.5% (2/133) 0.8% (6/788)
1
89
(N = 505)
1.0% (5/497)
Total Occlusion
(N = 702)
2.0% (10/497)
10.3% (71/690) 6.2% (31/497) 16.1% (9/56) 12.0% (16/133) 7.5% (59/788)
0.7% (5/690) 0.2% (1/497) 1.8% (1/56) 3.8% (5/133) 0.6% (5/788)
1.0% (5/497)
1.6% (11/690)
Table 9-61: Resolute Pooled Analysis – Subset Outcomes Through 12 Months
Bifurcation
EFFECTIVENESS
COMPOSITE SAFETY AND
TLF
TVF 11.4% (79/690) 6.6% (33/497) 16.1% (9/56) 12.8% (17/133) 8.1% (64/788)
MACE 11.3% (78/690) 6.6% (33/497) 17.9% (10/56) 16.5% (22/133) 8.2% (65/788)
EFFECTIVENESS
Clinically Driven TVR 6.1% (42/690) 4.2% (21/497) 7.1% (4/56) 4.5% (6/133) 5.6% (44/788)
TLR 4.8% (33/690) 3.6% (18/497) 7.1% (4/56) 3.0% (4/133) 4.7% (37/788)
SAFETY
Total Death 2.3% (16/690) 1.2% (6/497) 7.1% (4/56) 10.5% (14/133) 2.2% (17/788)
Cardiac Death 1.6% (11/690) 1.0% (5/497) 5.4% (3/56) 6.8% (9/133) 1.5% (12/788)
Non-Cardiac Death
TVMI 5.9% (41/690) 2.4% (12/497) 7.1% (4/56) 5.3% (7/133) 2.4% (19/788)
Cardiac Death or TVMI 7.1% (49/690) 3.4% (17/497) 10.7% (6/56) 9.8% (13/133) 3.8% (30/788)
Sten t Thro mb osi s
ARC defined
Definite/Probable 2.0% (14/690)
Definite
Probable 0.6% (4/690)
Notes
N = The total number of subjects enrolled.
Numbers are % (Count/Number of Eligible Subjects).
Subjects are only counted once for each time period.
Total Occlusion is defined as pre procedure TIMI = 0. 2Renal Insufficiency is defined as serum creatinine >2.5 mg/dl.
12-month time frame includes follow-up window (360 days ± 30 days).
The definitions of the outcomes are presented as table notes to Table 8-1 - Principal Adverse Events.
See Table 9-4 for the definition of the ARC defined Stent Thrombosis
1
Subjects from the 38 mm Length sub-study were not included in the RESOLUTE pooled analysis
Page 93

10 PATIENT SELECTION AND TREATMENT
See also Section 5.5 Use in Special Populations. The risks and benefits described above should be
caref ully considered for each patient before use of the Resolute Integrity stent system. Factors to be
utilized f or patient selection should include an assessment of the risk of prolonged anti-thrombotic
therapy. In the clinical studies reviewed to support the approval of the Resolute Integrity stent system,
sub jects were prescribed DAPT f or at least 6 months post-procedure, and most patients who were not
at a high risk of bleeding used DAPT f or at least 12 months.
Post-Resolute Integrity stent implantation, aspirin should be continued indefinitely, and a P2Y12 platelet
inhib itor should be given for at least 6 months in stable ischemic heart disease patients and for at least
12 months in patients with acute coronary syndrome (ACS). A longer d uration of DAPT may be
co nsidered in patients who have tolerated DAPT without a bleeding complication and who are not at a
high bleed ing risk. In patients who are at a high risk of bleeding, or who develop significant bleeding
during DAPT treatment, a shorter DAPT duration may be reasonable (see Section
Procedure Antiplatelet Regimen). However, definitive evidence supporting the safety of short DAPT
duration has not been established in prospective clinical studies. The safety and effectiveness of the
Resolute Integ rity stent have not been evaluated in p atients at high bleeding risk.
11 PATIENT COUNSELING INFORMATION
Physicians should consider the following in counseling the patient about this product:
• Discuss the risks associated with stent placement
• Discuss the risks associated with a zotarolimus-eluting stent implant
• Discuss the risks/benefits issues for the particular patient
• Discuss alteration to current lifestyle immediately following the procedure and over the long term
• Discuss the risks of early discontinuation of the antiplatelet therapy
5.1 - Pre- and Post-
The following patient materials will be provided to physicians to educate their patients about the options
available for treating coronary artery disease and pro vide contact information to the patient after their
stent imp lant procedure:
• A Patient Guid e which includes information on the Resolute Integrity Zotarolimus-Eluting Coronary
Stent System, coronary artery disease, and the stent implantation procedure.
• A Stent Patient Implant Card that includes patient information, stent implant information and MRI
guidelines. All patients should be instructed to keep this card in their possession at all times for
procedure/stent identification.
12 HOW SUPPLIED
STERILE: This product is sterilized with ethylene oxide (EO) and is nonpyrogenic. Do not use if the
package is opened or damaged. Do not resterilize. If the product or package is opened or d amaged,
return to Medtronic Returned Goods. Contact your local Medtronic representative for return information.
CONTENTS: Package contains one (1) Resolute Integrity Zotarolimus-Eluting Coronary Stent mounted
on a Rapid Exchange (RX) stent delivery system.
STORAGE: Store in the original container. Store at 25ºC (77ºF); excursions permitted to 15 - 30ºC (59 86ºF). Use by the “Use By” date noted on the package.
DISPOSAL INSTRUCTIONS: After use, dispose of the product and packaging in accordance with
hosp ital, administrative and/ or local government policy.
13 DIRECTIONS FOR USE
13.1 Access to Package Holding Sterile Stent Delivery System
Remove the stent delivery system from the package. Special care must be taken not to handle the stent
or in any way disrupt its placement on the balloon. This is most important during catheter removal from
packaging, placement over guidewire, and advancement through the rotating hemostatic valve and
guiding catheter hub. Excessive manipulation, e.g., rolling the mounted stent, may cause dislodgement
of the stent from the d elivery balloon.
90
Page 94

y
[
p
q
pprop
p
y sy
y
y sy
y
g
13.2 Inspection Prior to Use
Bef ore opening the product, carefully inspect the stent delivery system package, and check for damage
to the sterile barrier. Do not use after the “Use By” date. If the sterile package is intact, carefully remove
the system from the package and inspect it for bends, kinks, and other damage. Do not use the product
if any d amage to the packaging or system is noted.
A protective sheath covers the stent mounted on the balloon. After removal of the sheath, visually
inspect the stent to ensure that it has not been damaged or displaced from its original position (between
proximal and distal marker bands) on the balloon.
13.3 Materials Required
Quantity Material
N/A Guid e catheter [≥5 F (1.42 mm, 0.056 inch) inner diameter]
2-3 20 cc s
1,000 u /500 cc Heparinized normal saline
1 Guidewire
1 Rotating hemostatic valve
N/A Contrast medium diluted 1:1 with he
1 Inflation device
1 Sto pcock (3-way minimum)
1 Tor
N/A A
ringe
inch (0.36 mm) outer diameter]
arinized normal saline
ue device
riate anticoagulation and antiplatelet drugs
13.4 Preparation Precaution
• DO NOT use the product if the protective sheath is not present or the stent is damaged/displaced.
• AVOID manipulation of the stent during flushing of the guidewire lumen, as this may disrupt the
placement of the stent on the b alloon.
• DO NOT apply positive pressure to the balloon during the delivery system preparation.
13.4.1 Guidewire Lumen Flush
Flush the stent system guidewire lumen with heparinized normal saline until the fluid exits the distal tip.
13.4.2 Delivery System Preparation
Step Action
1. Pre
2. Remove the stent deliver
are the guide catheter and guidewire according to the manufacturer’s instructions.
stem from the package.
3. Remove the protective sheath covering from the stent/balloon. Removing the protective sheath
will als o remove the st
lette.
4. Inspect the stent to assure it has not been damaged or displaced from its original position on
the b alloon. Verify that the stent is positioned between the proximal and distal balloon markers.
Verif y that there is no visible damage to the stent or the balloon.
Note: Should there b e movement of or damage to the stent, do not use.
5. Flush the Stent Delivery System guidewire lumen with heparinized normal saline in the routine
manner.
6. Fill a 20 cc syringe with 5 cc of contrast/heparinized normal saline mixture (1:1).
7. Attach to the deliver
8. Slowl
release the pressure to allow negative pressure to draw mixture into the balloon lumen.
stem and apply negative pressure for 20 - 30 seconds.
9. Detach the syringe and leave a meniscus of mixture on the hub of the balloon lumen.
10. Prepare the inf lation device in the standard manner and purge to remove all air from the
syring e and tubing.
11 Attach the inflation device to the catheter, directly ensuring no bubbles remain at the
co nnection.
12 Leave on amb ient pressure (neutral position).
Note: Do not apply negative pressure on the inflation device after balloon preparation and prior to
deliverin
the s tent.
91
Page 95

p
y
y
y
g
p
g
p
g
p
13.5 Delivery Procedure
Step Action
1. Pre
are the vascular ac cess site according to standard practice.
2. Pre-dilate the lesion with a PTCA catheter. Pre-dilatation must be performed using a balloon
with the f ollowing three characteristics:
• A diameter at least 0.5 mm smaller than the treatment stent.
• A length equal to or shorter than the lesion length to be dilated.
• A length shorter than the stent to be implanted.
3. Maintain neutral pressure on the inflation device. Open the rotating hemostatic valve as widely
as p o ssible.
Note: If resistance is encountered, do not force passage. Resistance may indicate a problem
and ma
res ult in d amage to t he stent if it is forced. Remove the system and examine.
4. Ensure guide catheter stability before advancing the Resolute Integrity System into the
co ronary artery. Carefully advance the Resolute Integrity System into the hub of the guide
catheter.
5. Advance the stent delivery system over the guidewire to the target lesion under direct
f luoroscopic visualization. Use the radiopaque balloon markers to position the stent across the
lesion; perform angiography to confirm the position of the stent. If the position of the stent is not
optimal, it should be carefully repositioned or removed (see Precautions – Section 5.10
Stent/System Removal Precautions). Expansion of the stent should not be undertaken if the
stent is not properly positioned in the target lesion segment of the vessel.
6. Suf ficientl
Note: Sho uld unusual res istan ce be felt at any ti me d urin g ei ther l esio n access or r emoval o f the s tent deliv ery
system before stent implantation, do not force passage. Maintain guidewire placement across the lesion and
remove the stent d elivery system as a single unit. See Precautions – Section 5.10 Stent/System Removal
Precautions for specific stent delivery system removal instructions. In the event the stent is not deployed,
contact
our local Medtronic. representative fo r return information and avoid handling sten t with bare hands.
tighten the rotating hemostatic valve. The stent is now ready to be deployed.
13.6 Deployment Procedure
Step Action
1. Prior to stent expansion, utilize high-resolution fluoroscopy to verify the stent has not been
dama
ed or shifted during positioning.
2. Maintain inflation
3. Do not exceed Rated Burst Pressure (RBP). The RBP is 16atm for the 2.25mm – 3.5mm
stent diameters and 15atm for the 4.0mm stent diameter. The Resolute Integrity stents
should not be expanded to a diameter beyond the maximum labeled diameter listed on
the label. Do not dilate the 2.25 mm - 2.75 mm stents to greater than 3.50 mm. Do not
dilate the 3.0 mm - 4.0 mm stents to
4. Fluoroscopic visualization during stent expansion should be used in o rder to properly judge
the o ptimum stent diameter as compared to the proximal and distal native coronary artery
diameters (reference vessel diameters). Optimal stent expansion and proper apposition
requires that the stent be in full contact with the arterial wall.
13.7 Removal Procedures
Step Action
1. Deflate the balloon by pulling negative pressure on the inflation device. Allow adequate time,
at least 30 seco nds, for full balloon deflation. Longer stents may require more time for
deflation. Deflation of the balloon should be confirmed by absence of contrast within the
balloon.
2. O
en the hemostatic valve to allow removal of the delivery system.
3. Maintain the position of the guide catheter and guidewire. Very slowly, withdraw the balloon
f rom the stent, maintaining negative pressure, allowing movement of the myocardium to
ently dislodge the balloon from the stent.
4. Af ter removal of the d elivery system, tighten the hemostatic valve.
5. Re
eat angiography and visually assess the vessel and the stent for proper expansion.
ressure for 15-30 seconds for full expansion of the stent.
reater than 4.75mm.
92
Page 96

13.8
In-vitro
Information:
Table 13-1: Inflation Pressure Recommendations
Pressure
ATM kPa 2.25 2.5 2.75 3.0 3.5 4.0
6 608 2.20 2.45 2.70 2.90 3.30 3.75
7 709 2.20 2.45 2.70 2.95 3.35 3.80
8 811 2.25 2.50 2.75 3.00 3.40 3.90
9 912 Nominal 2.30 2.55 2.80 3.05 3.50 3.95
10 1013 2.30 2.60 2.85 3.10 3.55 4.05
11 1115 2.35 2.60 2.90 3.15 3.60 4.10
12 1216 2.40 2.65 2.95 3.20 3.65 4.15
13 1317 2.40 2.70 3.00 3.20 3.70 4.20
14 1419 2.45 2.70 3.05 3.25 3.75 4.25
15 1520 RBP for 4.0 mm 2.50 2.75 3.10 3.30 3.80 4.30
16 1621 RBP* 2.55 2.80 3.15 3.35 3.85 4.35
17 1723 2.60 2.80 3.20 3.40 3.90 4.40
18 1824 2.60 2.85 3.25 3.45 3.95 4.45
19 1925 2.90 3.30 3.50 4.00 4.50
20 2027 2.95 3.40 3.55 4.05
*Do not exceed the rated burst pressure (RBP). The R BP for 4.0 mm diameter is 15 AT M.
** The shaded cells at pressures 19 ATM and 20 AT M signify that 99% of the balloons did not pass at t he listed press ure beyond
RBP with 95% confidence.
Nominal and Rated
Burst Pressure*
13.9 Further Dilatation of Stented Segment
Stent Nominal Inner Diameter (mm)**
The stent delivery balloon may not be used for post-dilatation. Post-dilatation may be performed at
the p hysician’s discretion with appropriately sized (length and diameter) balloons to ensure that the
stent is in f ull contact with the vessel wall. To achieve this, a balloon to artery ratio of 1.0 to 1.1:1.0
sho uld be used to leave a residual diameter stenosis of near 0% (with a recommended maximum of no
greater than 10%). Whenever possible, avoid the use of grossly oversized balloons (balloon:artery ratio
>1.2).
Precaution: Do not dilate the stent beyond the following limits:
Nominal Stent Diameter Dil atation L imits
2.25 mm 3.50 mm
2.50 mm 3.50 mm
2.75 mm 3.50 mm
3.00 mm 4.75 mm
3.50 mm 4.75 mm
4.00 mm 4.75 mm
All ef forts should be taken to assure that the stent is not under dilated. If the deployed stent size is still
inadequate with respect to vessel diameter, or if full contact with the vessel wall is not achieved, a larger
ballo on may be used to expand the stent further. This further expansion should be performed using a
low profile, high pressure, and non-compliant balloon catheter. If this is required, the stented segment
sho uld be recrossed carefully with a prolapsed guidewire to avoid dislodging or displacing the stent. The
ballo on should be centered within the stent and should not extend outside of the stented region. The
Resolute Integrity stents should not be expanded to a diameter beyond the maximum labeled
diameter listed on the label. Do not dilate the 2.25 mm - 2.75 mm stents to greater than 3.50 mm.
Do not dilate the 3.0 mm - 4.0 mm stents to greater than 4.75 mm.
14 REUSE PRECAUTION STATEMENT
For single use only.
Do no t Resterilize or Reuse.
93
Page 97

DISCLAIMER OF WARRANTY
THE WARNINGS CONTAINED IN THE PRODUCT LABELING PROVIDE MORE DETAILED
INFORMATION AND ARE CONSIDERED AN INTEGRAL PART OF THIS DISCLAIMER OF
WARRANTY. ALTHOUGH THE MEDTRONIC RESOLUTE INTEGRITY ZOTAROLIMUSELUTING CORONARY STENT SYSTEM, HEREAFTER REFERRED TO AS “PRODUCT,” HAS
BEEN MANUFACTURED UNDER CAREFULLY CONTROLLED CONDITIONS, MEDTRONIC
HAS NO CONTROL OVER THE CONDITIONS UND ER WHICH THIS PRODUCT IS USED.
MEDTRONIC, THEREFORE, DISCLAIMS ALL WARRANTIES, BOTH EXPRESS AND
IMPLIED, WITH RESPECT TO THE PRODUCT, INCLUDING, BUT NOT LIMITED TO, ANY
IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR
PURPOSE. MEDTRONIC SHALL NOT BE LIABLE TO ANY PERSON OR ENTITY FOR ANY
MEDICAL EXPENSES OR ANY DIRECT, INCIDENTAL, OR CONSEQUENTIAL DAMAGES
CAUSED BY ANY USE, DEFECT, FAILURE, OR MALFUNCTION OF THE PRODUCT,
WHETHER A CLAIM FOR SUCH DAMAGES IS BASED UPON WARRANTY, CONTRACT,
TORT, OR OTHERWISE. NO PERSON HAS ANY AUTHORITY TO BIND MEDTRONIC TO
ANY REPRESENTATION OR WARRANTY WITH RESPECT TO THE PRODUCT.
The exclusions and limitations set out above are not intended to, and should not be construed so as to,
co ntravene mandatory provisions of applicable law. If any part or term of this disclaimer of warranty is
held to be illegal, unenforceable, or in conflict with applicable law by a court of competent jurisdiction,
the validity of the remaining portions of this disclaimer of warranty shall not be affected, and all rights
and obligations shall be construed and enforced as if this disclaimer of warranty did not contain the
particular part or term held to be invalid.
94
Page 98

MANUFACTURER:
MEDTRONIC, INC.
710 Medtronic Parkway
Minneap o lis, MN 55432
U.S.A.
Tel: (+1-763) 514-4000
Fax: (+1-763) 514-4879
www.medtronic.com
U.S. CUSTOMER SERVICE / PRODUCT INQUIRIES
Tel: (+1-888) 283-7868
Fax: (+1-800) 838-3103
*M724130B001* Rev AA
© 2013, 2014, 2017, 2018, 2019, 2020 Medtronic, Inc.
All Rights Reserved
 Loading...
Loading...