Page 1

Catheter
fr
Cathéter
es
Catéter
pt-br
Cateter
M994065ADOC2 Rev. A (04/2019)
Page 2

English ................................................................... 3
Français .................................................................. 4
Español ................................................................... 5
Português (Brasil) .......................................................... 6
Symbol Glossary ........................................................... 8
2
Page 3

English
en
Instructions for Use
React™ Catheter
DEVICE DESCRIPTION
The catheter is a single lumen, flexible, variable stiffness composite catheter. The catheter shaft has a
hydrophilic coating that spans the distal 40 cm to reduce friction during use. The catheter shaft is visible
under fluoroscopy. The catheter dimensions are included on the individual device label. The catheter is
introduced through a guide catheter or sheath and into the intracranial vasculature and guided over a
neurovascular guidewire and/or microcatheter. The proximal end of the catheter has a luer fitting to allow
attachment of accessories and infusion of liquids through the system. The catheter is provided sterile, nonpyrogenic, and is intended for single use only.
INDICATIONS FOR USE
The React™ 68 Catheter and React™ 71 Catheter are indicated for the introduction of interventional devices
into the peripheral and neuro vasculature.
CONTRAINDICATIONS
There are no known contraindications.
COMPATIBILITY
Refer to product label for device dimensions. Refer to labeling provided with other medical technologies to
determine compatibility.
WARNINGS
- The catheter should only be used by physicians who have received appropriate training in
interventional techniques.
- Do not reuse. The device is intended for single use only. Discard the catheter after one procedure.
Structural integrity and/or function may be impaired through reuse or cleaning. Catheters are
extremely difficult to clean after exposure to biological materials and may cause adverse patient
reactions if reused.
- The catheter has not been tested for use with automated high-pressure contrast injection equipment,
do not use this equipment with the device because it may damage the device.
RECOMMENDED PREPARATION AND USE
1. Flush package hoop with saline before removing catheter.
2. Gently remove the catheter and packaging hoop from the pouch by grasping the packaging hoop and
slowly pulling the catheter and hoop out of the pouch.
3. Remove the catheter from the packaging hoop.
4. Inspect the catheter for kinks or other damage. If any damage is observed, replace with new device.
5. Connect a rotating hemostasis valve (RHV) to the hub of the catheter and flush the inner lumen with
heparinized saline.
6. Introduce the catheter into the vasculature through the sheath, and over a guide wire using a
percutaneous entry technique of choice. A split sheath is provided in the packaging to provide support
and facilitate the introduction of the tip of the catheter into the sheath.
NOTE: The maximum diameter recommended for a guide wire is 0.038” (0.97 mm). When using the
React™ 71 Catheter, the minimum inner diameter recommended for a sheath is 0.087” (2.21 mm).
7. Under fluoroscopic guidance, advance the system over the guide wire until the desired position is
attained.
8. Remove the guide wire prior to the introduction of other intravascular devices.
STORAGE
Store in a cool, dry and dark place. See the product label for the device “Use-by date.” Do not use the device
beyond its labeled “Use-by date”.
LIMITED WARRANTY
Although this product has been manufactured under carefully controlled conditions, the manufacturer
has no control over the conditions under which this product is used. The manufacturer therefore disclaims
all warranties, both expressed and implied, with respect to the product including, but not limited to, any
implied warranty of merchantability or fitness for a particular purpose. The manufacturer shall not be
liable to any person or entity for any medical expenses or any direct, incidental or consequential damages
caused by any use, defect, failure or malfunction of the product, whether a claim for such damages is based
upon warranty, contract, tort or otherwise. No person has any authority to bind the manufacturer to any
representation or warranty with respect to the product. The exclusions and limitation set out above are not
intended to, and should not be construed so as to contravene mandatory provisions of applicable law. If any
part or term of this Disclaimer of Warranty is held illegal, unenforceable or in conflict with applicable law
by a court of competent jurisdiction, the validity of the remaining portions of this Disclaimer of Warranty
shall not be affected, and all rights and obligations shall be construed and enforced as if this Disclaimer of
Warranty did not contain the particular part or term held to be invalid.
PRECAUTIONS
• Do not use open, kinked, or damaged devices.
• Do not autoclave.
• Use the catheter in conjunction with fluoroscopic visualization.
• Inspect the catheter before use to verify that its size and condition are suitable for the specific
procedure.
• Do not advance or withdraw the catheter against resistance without careful assessment of the cause
using fluoroscopy. If the cause cannot be determined, withdraw the device. Moving or torqueing the
device against resistance may result in damage to the vessel or device.
• Maintain a constant infusion of appropriate flush solution.
• If flow through the catheter becomes restricted, do not attempt to clear the lumen by infusion.
Remove and replace the device.
• Extreme care must be taken to avoid damage to the vasculature through which the catheter passes.
The catheter may occlude smaller vessels. Care must be taken to avoid complete blood flow blockage.
• Torqueing the catheter may cause damage which could result in kinking and possible separation along
the catheter shaft. Should the catheter become severely kinked, withdraw the catheter.
• An appropriate anticoagulation therapy should be applied per institutional guidelines.
• Operators should take all necessary precautions to limit X-radiation doses to patients and themselves
by using sufficient shielding, reducing fluoroscopy times, and modifying X-ray technical factors where
possible.
POTENTIAL ADVERSE EVENTS
Possible complications include, but are not limited to, the following:
• acute occlusion
• allergic reaction and anaphylaxis from contrast
media
• complication at puncture site
• complication of radiation exposure (e.g.,
alopecia, burns ranging in severity from skin
reddening to ulcers, cataracts, and delayed
neoplasia)
• embolism
• false aneurysm formation
• infection
• inflammation
• intracranial hemorrhage
• ischemia
• neurological deficits including stroke
• vessel spasm, thrombosis, dissection or
perforation
3
Page 4

Français
fr
Mode d'emploi
Cathéter React™
DESCRIPTION DU DISPOSITIF
Le cathéter est un cathéter composite à rigidité variable, flexible et à lumière unique. Le corps du cathéter
est doté d'un revêtement hydrophile qui couvre 40cm à partir de l'extrémité distale afin de réduire la
friction durant l'utilisation. Le corps du cathéter est visible sous radioscopie. Les dimensions du cathéter
sont incluses sur l'étiquette de chaque dispositif. Le cathéter est introduit à travers un cathéter-guide
ou une gaine dans le système vasculaire intracrânien et guidé sur un fil-guide et/ou un microcathéter
neurovasculaire. L'extrémité proximale du cathéter est dotée d'un raccord luer permettant la fixation
d'accessoires et la perfusion de liquides à travers le système. Le cathéter est fourni stérile, apyrogène et est
exclusivement destiné à un usage unique.
INDICATIONS D'UTILISATION
Le cathéter React™68 et le cathéter React™71 sont indiqués pour l'introduction de dispositifs
interventionnels dans le système vasculaire périphérique et neurovasculaire.
CONTRE-INDICATIONS
Il n'existe aucune contre-indication connue.
COMPATIBILITÉ
Se reporter à l'étiquette du produit pour les dimensions du dispositif. Se reporter à l'étiquetage fourni avec
les autres technologies médicales afin de déterminer la compatibilité.
AVERTISSEMENTS
- Le cathéter doit uniquement être utilisé par des médecins ayant reçu une formation appropriée aux
techniques interventionnelles.
- Ne pas réutiliser. Le dispositif est conçu pour un usage unique exclusivement. Mettre le cathéter au
rebut après une procédure unique. La réutilisation ou le nettoyage risque de compromettre l'intégrité
structurelle et/ou le fonctionnement. Les cathéters sont extrêmement difficiles à nettoyer après une
exposition aux matières biologiques et ils peuvent entraîner des réactions indésirables chez le patient
s'ils sont réutilisés.
- L'utilisation du cathéter avec des équipements automatiques d'injection de produit de contraste
à haute pression n'a pas été testée; ne pas utiliser ces équipements avec le dispositif sous peine
d'endommager ce dernier.
PRÉCAUTIONS
• Ne pas utiliser les dispositifs ouverts, plicaturés ou endommagés.
• Ne pas passer à l'autoclave.
• Utiliser le cathéter conjointement avec la visualisation radioscopique.
• Inspecter le cathéter avant l'utilisation pour vérifier que sa taille et son état conviennent à la procédure
spécifique.
• Ne pas avancer ou ne pas retirer le cathéter en présence d'une résistance sans en évaluer
minutieusement la cause sous radioscopie. Si la cause ne peut pas être déterminée, retirer le dispositif.
Le déplacement ou la torsion du dispositif en présence d'une résistance risque de léser le vaisseau ou
d'endommager le dispositif.
• Maintenir une perfusion constante de la solution de rinçage appropriée.
• Si le débit se restreint à travers le cathéter, ne pas tenter de dégager la lumière par perfusion. Retirer
et remplacer le dispositif.
• Il convient de faire preuve d'une vigilance extrême afin d'éviter toute lésion du système vasculaire
traversé par le cathéter. Le cathéter est susceptible d'obstruer les vaisseaux plus petits. Veiller à éviter
un blocage total du débit sanguin.
• La torsion du cathéter risque de provoquer des dommages qui pourraient résulter en une plicature et
une séparation possible le long du corps du cathéter. En cas de plicature importante du cathéter, le
retirer.
• Une anticoagulothérapie appropriée doit être mise en place conformément aux directives de
l'établissement.
• Les opérateurs doivent prendre toutes les précautions nécessaires pour limiter les doses de rayonsX
reçues par les patients et par eux-mêmes en utilisant une protection suffisante, en réduisant la durée
de radioscopie et en modifiant les facteurs techniques des rayonsX lorsque cela est possible.
ÉVÉNEMENTS INDÉSIRABLES POTENTIELS
Les complications possibles incluent, sans toutefois s'y limiter, ce qui suit :
• Occlusion aiguë
• Réaction allergique et anaphylaxie dues au
produit de contraste
• Complication au niveau du site de ponction
• Complication de l'exposition aux rayonnements
(p.ex., alopécie, brûlures dont la gravité peut
aller d'un rougissement de la peau à des
ulcères, cataracte et néoplasie tardive)
• Embolie
• Formation d'un faux anévrysme
• Infection
• Inflammation
• Hémorragie intracrânienne
• Ischémie
• Déficits neurologiques, y compris un accident
vasculaire cérébral
• Spasme vasculaire, thrombose, dissection ou
perforation
PRÉPARATION ET UTILISATION RECOMMANDÉES
1. Rincer le manchon d'emballage avec une solution saline avant de retirer le cathéter.
2. Retirer délicatement le cathéter et le manchon d'emballage du sachet en saisissant le manchon
d'emballage et en tirant lentement le cathéter et le manchon hors du sachet.
3. Retirer le cathéter du manchon d'emballage.
4. Inspecter le cathéter pour s'assurer qu'il n'est pas plicaturé ou endommagé de quelque manière que ce
soit. Si d'éventuels dommages sont observés, remplacer par un dispositif neuf.
5. Connecter une valve hémostatique rotative (VHR) à l'embase du cathéter et rincer la lumière interne
avec une solution saline héparinée.
6. Introduire le cathéter dans le système vasculaire à travers la gaine et sur un fil-guide selon la technique
d'accès percutané privilégiée. Une gaine fendue est fournie dans l'emballage pour servir de support et
faciliter l'introduction de l'extrémité du cathéter dans la gaine.
REMARQUE: Le diamètre maximum recommandé pour un fil-guide est de 0,038” (0,97mm). Lorsque le
cathéter React™71 est utilisé, le diamètre interne minimum recommandé pour une gaine est de 0,087"
(2,21mm).
7. Sous guidage radioscopique, faire progresser le système sur le fil-guide jusqu'à ce que la position
souhaitée soit atteinte.
8. Retirer le fil-guide avant l'introduction des autres dispositifs intravasculaires.
STOCKAGE
Stocker dans un endroit frais, sec et sombre. Consulter l'étiquette du produit pour la date de péremption du
dispositif. Ne pas utiliser le dispositif au-delà de sa date de péremption mentionnée sur l'étiquette.
GARANTIE LIMITÉE
Bien que ce produit ait été fabriqué dans des conditions soigneusement contrôlées, le fabricant n'a aucun
contrôle sur les conditions dans lesquelles il est utilisé. En conséquence, le fabricant décline toute garantie,
expresse et implicite, relative au produit, dont, entre autres, toute garantie implicite de qualité marchande
ou d'adéquation à un but particulier. Le fabricant ne pourra en aucun cas être tenu pour responsable,
envers aucune personne ou entité, des frais médicaux ou des dommages directs, fortuits ou indirects causés
par tous usages, défectuosités, défaillances ou dysfonctionnements du produit, qu'une plainte pour de
tels dommages soit fondée sur une garantie, une responsabilité contractuelle, délictueuse ou autre. Nul
n'est habilité à lier le fabricant à une représentation ou une garantie quelconque concernant le produit.
Les exclusions et la limitation exposées ci-dessus ne sont pas, et ne doivent pas être, interprétées comme
contraires aux dispositions obligatoires des lois applicables. Si une partie ou une disposition du présent Déni
de garantie devait être considérée comme illégale, non applicable ou contraire à la loi en vigueur par un
tribunal compétent, la validité des autres dispositions du présent Déni de garantie n'en sera pas affectée,
et tous les droits et obligations seront interprétés et appliqués comme si le présent Déni de garantie ne
contenait pas la partie ou la disposition considérée comme non valide.
4
Page 5

Español
es
Instrucciones de uso
Catéter React™
DESCRIPCIÓN DEL DISPOSITIVO
El catéter es un catéter compuesto de rigidez variable, flexible y de una sola luz. El cuerpo del catéter tiene
un revestimiento hidrófilo que abarca la porción distal de 40cm para reducir el rozamiento durante su uso.
El cuerpo del catéter es visible bajo fluoroscopia. Las dimensiones del catéter se indican en la etiqueta de
cada dispositivo. El catéter se introduce a través de un catéter guía o de un introductor hasta la vasculatura
intracraneal y se guía sobre una guía o un microcatéter neurovascular. El extremo proximal del catéter tiene
un adaptador luer para permitir el acoplamiento de accesorios y la infusión de líquidos a través del sistema.
El catéter se suministra estéril y apirógeno y es válido para un solo uso.
INDICACIONES DE USO
El catéter React™68 y el catéter React™71 están indicados para la introducción de dispositivos
intervencionistas en la vasculatura periférica y en la neurovasculatura.
CONTRAINDICACIONES
No se conoce ninguna contraindicación.
COMPATIBILIDAD
Consulte las dimensiones del dispositivo en la etiqueta del producto. Consulte la documentación
proporcionada con otros dispositivos médicos para determinar su compatibilidad.
ADVERTENCIAS
- El catéter solo debe ser utilizado por médicos que hayan recibido una formación apropiada en técnicas
intervencionistas.
- No lo reutilice. El dispositivo es válido para un solo uso. Deseche el catéter después de un
procedimiento. Se pueden deteriorar su integridad estructural y su función al reutilizarlo o limpiarlo.
Los catéteres son sumamente difíciles de limpiar tras la exposición a materiales biológicos, y su
reutilización puede causar reacciones adversas en el paciente.
- No se ha evaluado el uso del catéter con equipos automáticos de inyección de contraste a alta presión,
por lo que no deben usarse estos equipos con el dispositivo, ya que pueden dañarlo.
MEDIDAS PREVENTIVAS
• No use dispositivos abiertos, retorcidos o dañados.
• No esterilice el producto en autoclave.
• Use el catéter bajo visualización fluoroscópica.
• Examine el catéter antes de usarlo para comprobar que su tamaño y su estado son adecuados para el
procedimiento específico que va a realizar.
• Si encuentra resistencia, no haga avanzar ni retire el catéter sin realizar antes una valoración
meticulosa de la causa de la resistencia bajo fluoroscopia. Si no se puede determinar la causa, retire el
dispositivo. Si se mueve o se gira el dispositivo con resistencia, se puede dañar el dispositivo o se puede
producir una lesión del vaso.
• Mantenga una infusión constante de la solución de irrigación adecuada.
• Si se produce una restricción del flujo a través del catéter, no intente eliminar la obstrucción de la luz
mediante infusión. Extraiga y sustituya el dispositivo.
• Debe tener extremo cuidado para evitar producir lesiones en los vasos a través de los cuales pase
el catéter. El catéter puede ocluir los vasos más pequeños. Debe tener cuidado para evitar una
obstrucción completa del flujo sanguíneo.
• Si se gira el catéter este podría dañarse, lo cual puede causar retorcimiento y una posible separación a
lo largo del cuerpo del catéter. Si el catéter está muy retorcido, retírelo.
• Debe administrarse el tratamiento anticoagulante adecuado conforme a la práctica clínica habitual
del centro.
• Los usuarios deben tomar todas las medidas preventivas necesarias para limitar la radiación por
rayosX a los pacientes y a sí mismos mediante el uso de una protección suficiente, la reducción del
tiempo de fluoroscopia y la modificación de los factores técnicos asociados a los rayosX cuando sea
posible.
POSIBLES EFECTOS ADVERSOS
Las posibles complicaciones son, entre otras, las siguientes:
• oclusión aguda
• reacción alérgica y anafilaxia por los medios
de contraste
• complicación en el lugar de punción
• complicaciones por exposición a radiación
(p.ej., alopecia, quemaduras cuya intensidad
varía desde enrojecimiento de la piel a úlceras,
cataratas y neoplasia tardía)
• embolia
• formación de seudoaneurisma
• infección
• inflamación
• hemorragia intracraneal
• isquemia
• déficits neurológicos incluido ictus
• espasmo, trombosis, disección o perforación
vasculares
PREPARACIÓN Y USO RECOMENDADOS
1. Irrigue el aro del envase con solución salina antes de extraer el catéter.
2. Extraiga con cuidado el catéter y el aro del envase de la bolsa sujetando el aro del envase y tirando
lentamente del catéter y del aro para extraerlos de la bolsa.
3. Extraiga el catéter del aro del envase.
4. Examine el catéter por si está retorcido o presenta otros daños. Si observa algún daño, sustituya el
dispositivo por uno nuevo.
5. Conecte una válvula hemostática giratoria (VHG) al conector del catéter e irrigue la luz interna con
solución salina heparinizada.
6. Introduzca el catéter en la vasculatura a través del introductor y sobre una guía mediante la técnica de
entrada percutánea de su elección. En el envase se incluye un introductor dividido para proporcionar
soporte y facilitar la introducción de la punta del catéter en el introductor.
NOTA : El diámetro máximo recomendado para una guía es de 0,97mm (0,038pulgadas). Cuando
se utiliza el catéter React™71, el diámetro interior mínimo recomendado para un introductor es de
2,21mm (0,087pulgadas).
7. Bajo guía fluoroscópica, haga avanzar el sistema sobre la guía hasta alcanzar la posición deseada.
8. Retire la guía antes de introducir otros dispositivos intravasculares.
CONSERVACIÓN
Conserve el producto en un lugar fresco, seco y oscuro. Consulte la fecha de caducidad del producto en su
etiqueta. No utilice el dispositivo después de la fecha de caducidad indicada.
GARANTÍA LIMITADA
Aunque este producto seha fabricado en condiciones cuidadosamente controladas, el fabricante notiene
control sobre las condiciones en las que se utilice. Por tanto, el fabricante no ofrece garantía alguna, ya sea
expresa o implícita, con respecto al producto, incluida entre otras toda garantía implícita de comerciabilidad
o idoneidad para un fin particular. El fabricante no será responsable ante ninguna persona o entidad
por los gastos médicos o daños directos, indirectos o fortuitos causados por el uso, defecto, fallo o mal
funcionamiento del producto, aun cuando la reclamación por dichos daños se base en una garantía,
contrato, responsabilidad extracontractual u otro fundamento legal. Ninguna persona tiene autoridad para
obligar al fabricante a ofrecer alegación o garantía alguna con respecto al producto. Las exclusiones y la
limitación arriba expresadas no pretenden contravenir las disposiciones obligatorias establecidas por la
legislación vigente, ni deben interpretarse de dicha forma. En el supuesto de que cualquier parte o condición
fuera declarada ilegal, inaplicable o contraria a la ley por cualquier tribunal competente, ello no afectará
a la validez del resto de la renuncia de responsabilidad, interpretándose y aplicándose cuantos derechos y
obligaciones se contienen en la misma como si la presente renuncia de responsabilidad no contuviera la
parte o condición considerada no válida.
5
Page 6

Português (Brasil)
pt-br
Instruções de utilização
Cateter React™
DESCRIÇÃO DO DISPOSITIVO
Cateter compósito de lúmen único, flexível, de rigidez variável. A haste do cateter possui um revestimento
hidrofílico que abrange os 40 cm distais para redução da fricção durante o uso. A haste do cateter é visível
sob fluoroscopia. As dimensões do cateter estão incluídas na etiqueta do dispositivo individual. O cateter é
introduzido na vasculatura intracraniana através de um cateter guia ou de uma bainha e guiado sobre um
fio guia e/ou microcateter neurovascular. A extremidade proximal do cateter possui uma conexão Luer para
permitir a conexão de acessórios e a infusão de líquidos através do sistema. O cateter é fornecido estéril,
apirogênico e se destina apenas a uso único.
INDICAÇÕES DE USO
O cateter React™ 68 e o cateter React™ 71 são indicados para a introdução de dispositivos intervencionistas
na vasculatura periférica e na neurovasculatura.
CONTRAINDICAÇÕES
Não existem contraindicações conhecidas.
COMPATIBILIDADE
Consulte a etiqueta do produto para ver as dimensões do dispositivo. Consulte as etiquetas fornecidas com
outras tecnologias médicas para determinar a compatibilidade.
AVISOS
- O cateter deve ser usado apenas por médicos com treinamento adequado em técnicas
intervencionistas.
- Não reutilizar. O dispositivo se destina apenas a uso único. Descarte o cateter após um procedimento.
A integridade estrutural e/ou a função podem ser comprometidas devido à reutilização ou limpeza. Os
cateteres são extremamente difíceis de limpar após a exposição a materiais biológicos e podem causar
reações adversas no paciente caso reutilizados.
- O cateter não foi testado para ser usado com equipamento de injeção de contraste de alta pressão
automatizado, não use este equipamento com o dispositivo, pois o mesmo pode ser danificado.
PRECAUÇÕES
• Não use dispositivos abertos, dobrados nem danificados.
• Não esterilize em autoclave.
• Use o cateter em conjunto com visualização fluoroscópica.
• Antes do uso, inspecione o cateter para verificar se seu tamanho e estado são adequados para o
procedimento específico.
• Se sentir resistência, não avance nem recue o cateter sem uma avaliação cuidadosa da causa usando
fluoroscopia. Se não for possível determinar a causa, retire o dispositivo. O movimento ou a torção do
dispositivo contra resistência pode resultar em danos ao vaso ou ao dispositivo.
• Mantenha uma infusão constante de solução de irrigação apropriada.
• Se o fluxo através do cateter ficar restrito, não tente limpar o lúmen por infusão. Remova e substitua
o dispositivo.
• Deve-se tomar cuidado extremo para evitar danos à vasculatura por onde passa o cateter. O cateter
pode ocluir vasos menores. Deve-se tomar cuidado para evitar o bloqueio total do fluxo sanguíneo.
• A torção do cateter pode provocar danos que podem resultar na dobra e na possível separação ao
longo da haste do cateter. Se o cateter ficar acentuadamente dobrado, retire-o.
• Deverá ser administrada uma terapia anticoagulante adequada segundo as diretrizes da instituição.
• Os operadores devem tomar todas as precauções necessárias para limitar as doses de radiação X sobre
si mesmos e sobre os pacientes usando proteção suficiente, reduzindo o tempo de fluoroscopia e,
sempre que possível, modificando os fatores técnicos do raio X.
EVENTOS ADVERSOS POTENCIAIS
As seguintes possíveis complicações podem ocorrer, entre outras:
• oclusão aguda
• reação alérgica e anafilaxia por causa dos meios
de contraste
• complicação no local da punção
• complicações devido à exposição à radiação
(por exemplo, alopecia, queimaduras de
gravidade variável, desde vermelhidão na pele
até úlceras, cataratas e neoplasia tardia)
• embolia
• formação de falso aneurisma
• infecção
• inflamação
• hemorragia intracraniana
• isquemia
• deficiências neurológicas, incluindo AVC
• espasmo vascular, trombose, dissecção ou
perfuração
PREPARAÇÃO E USO RECOMENDADOS
1. Irrigue o aro protetor com soro fisiológico antes de remover o cateter.
2. Remova suavemente o cateter e o aro protetor da bolsa, segurando o aro e puxando lentamente o
cateter e o aro para fora da bolsa.
3. Remova o cateter do aro protetor.
4. Inspecione o cateter quanto a dobras ou outros danos. Caso observe danos, substitua por um dispositivo
novo.
5. Conecte uma válvula hemostática rotativa (VHR) ao conector do cateter e irrigue o lúmen interno com
soro fisiológico heparinizado.
6. Introduza o cateter na vasculatura através da bainha e sobre um fio guia usando a técnica de entrada
percutânea preferida. É fornecida uma bainha do tipo "peel away" na embalagem para proporcionar
suporte e facilitar a introdução da ponta do cateter na bainha.
OBSERVAÇÃO: o diâmetro máximo recomendado do fio guia é de 0,97 mm (0,038 pol.). Ao usar o
cateter React™ 71, o diâmetro interno mínimo recomendado da bainha é de 2,21 mm (0,087 pol.).
7. Sob orientação fluoroscópica, avance o sistema sobre o fio guia até atingir a posição desejada.
8. Remova o fio guia antes da introdução de outros dispositivos intravasculares.
ARMAZENAMENTO
Armazene em local fresco, seco e ao abrigo da luz. Consulte a etiqueta do produto quanto à "Use-by date"
(Data de validade). Não use o dispositivo depois da data “Use-by date” (Data de validade) indicada na
etiqueta.
GARANTIA LIMITADA
Embora este produto tenha sido fabricado sob condições cuidadosamente controladas, o fabricante não tem
qualquer controle sobre as condições em que o mesmo é usado. Portanto, o fabricante renuncia quaisquer
garantias, expressas ou implícitas, em relação ao produto, incluindo, sem limitação, quaisquer garantias
implícitas de comercialização ou de adequação a um fim específico. O fabricante não será responsável por
qualquer pessoa ou entidade, por quaisquer despesas médicas ou por quaisquer danos diretos, incidentais
ou consequenciais causados por qualquer uso, defeito, falha ou mau funcionamento do produto, quer a
reclamação relativa a tais danos tenha por base a garantia, o contrato, delito ou outros. Nenhum indivíduo
tem qualquer autoridade para vincular o fabricante a qualquer tipo de representação ou garantia relativa
ao produto. As exclusões e limitação acima não se destinam a infringir disposições obrigatórias da lei
aplicável, não devendo ser interpretadas como tal. Se alguma parte ou termo desta Renúncia de Garantia for
considerado ilegal, não executável ou em conflito com a lei aplicável por parte de um tribunal da jurisdição
competente, a validade das partes restantes desta Renúncia de Garantia não deverá ser afetada e todos
os direitos e obrigações devem ser interpretados e executados como se esta RENÚNCIA DE GARANTIA não
contivesse a parte ou o termo particular considerado inválido.
6
Page 7

This page is intentionally left blank.
Cette page est laissée vierge intentionnellement.
Esta página se ha dejado intencionadamente en blanco.
Esta página foi deixada intencionalmente em branco.
7
Page 8
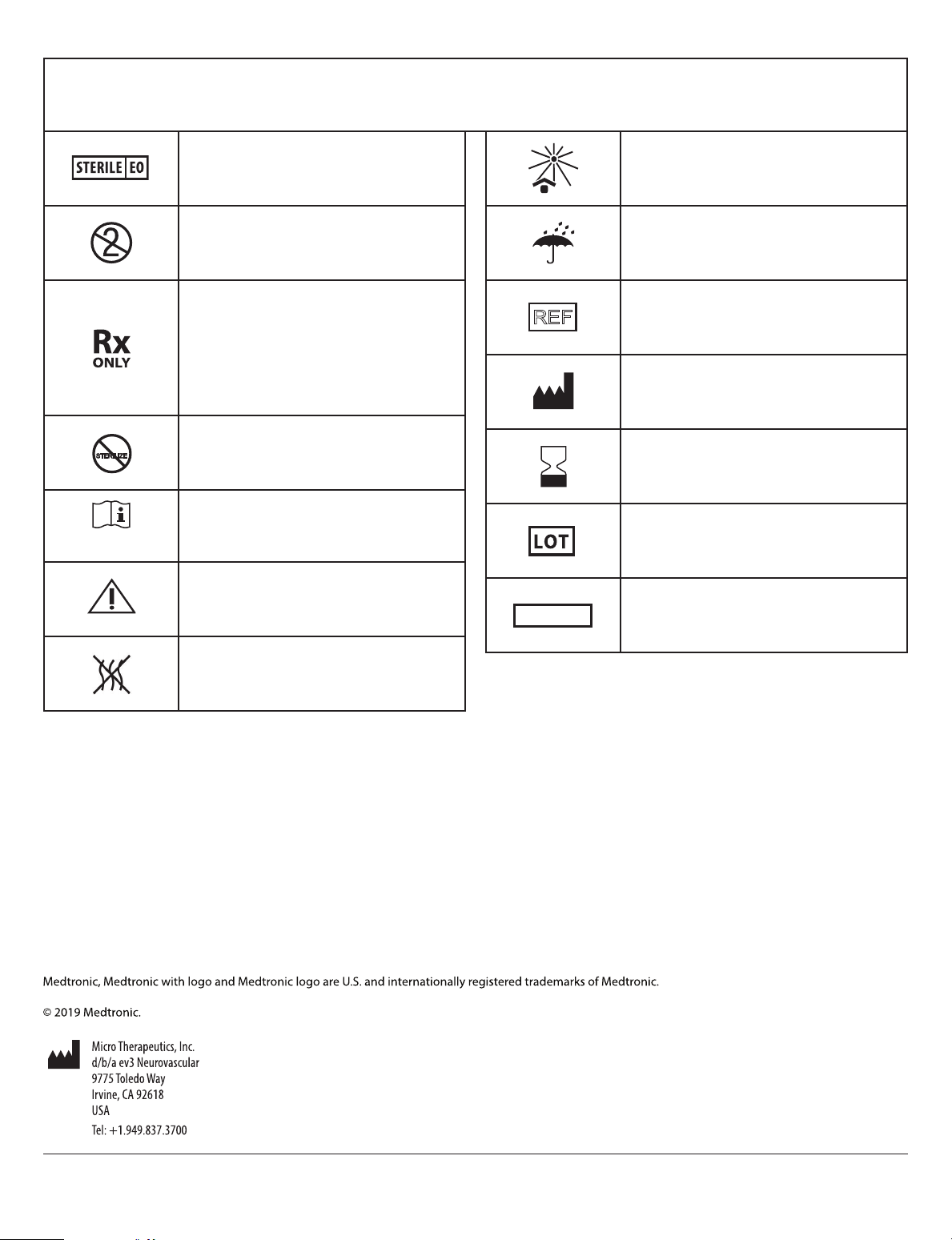
React™ Catheter Symbol Glossary / Glossaire des symboles du cathéter React™ /
www.medtronic.com/manuals
REF
Glosario de símbolos del catéter React™ / Glossário de símbolos do cateter React™
2
STERILIZE
Sterilized using ethylene oxide/ Stérilisé à l’oxyde d’éthylène/
Esterilizado mediante óxido de etileno/ Esterilizado com
óxido de etileno
Do not re-use/ Ne pas réutiliser/ No reutilizar/ Não reutilizar
Caution: Federal (USA) law restricts this device to sale by
or on the order of a physician/ Attention: La loi fédérale
(États-Unis) limite la vente de ce dispositif par un médecin
ou sur ordonnance médicale/ Precaución: Las leyes federales
de los Estados Unidos únicamente permiten la venta de
este producto a un médico o bajo prescripción facultativa./
Advertência: a lei federal americana (EUA) limita a venda
deste dispositivo a médicos ou mediante prescrição médica
Do not resterilize/ Ne pas restériliser/ No reesterilizar/ Não
reesterilizar
Consult instructions for use at this website/ Consulter le mode
d’emploi sur ce site Web/ Consultar las instrucciones de
uso en este sitio web/ Consulte as instruções de utilização
neste sítio da Internet
Caution/ Attention/ Precaución/ Advertência
CONTENTS
Keep away from sunlight/ Conserver à l’abri de la lumière du
soleil/ Mantener alejado de la luz del sol/ Manter longe da
luz do sol
Keep dry/ Conserver au sec/ Mantener seco/ Manter seco
Catalogue number/ Numéro de catalogue/ Número de
referencia/ Número de catálogo
Manufacturer/ Fabricant/ Fabricante/ Fabricante
Use-by date/ Date de péremption/ Fecha de caducidad/ Data
de validade
Batch code/ Code du lot/ Código de lote/ Código do lote
Contents of Package/ Contenu de l’emballage/ Contenido del
envase/ Conteúdo da embalagem
Non-pyrogenic/ Apyrogène/ Apirógeno/ Apirogênico
M994065ADOC2 Rev. A (04/2019)
 Loading...
Loading...