PlasmaBlade™ X 4.0S
Single-Use Device Designed for Use
with Qualied Generators
Instructions For Use

© 2019 Medtronic. All rights reserved. Medtronic, Medtronic logo and
Further, Together are trademarks of Medtronic. All other brands are
trademarks of a Medtronic company.

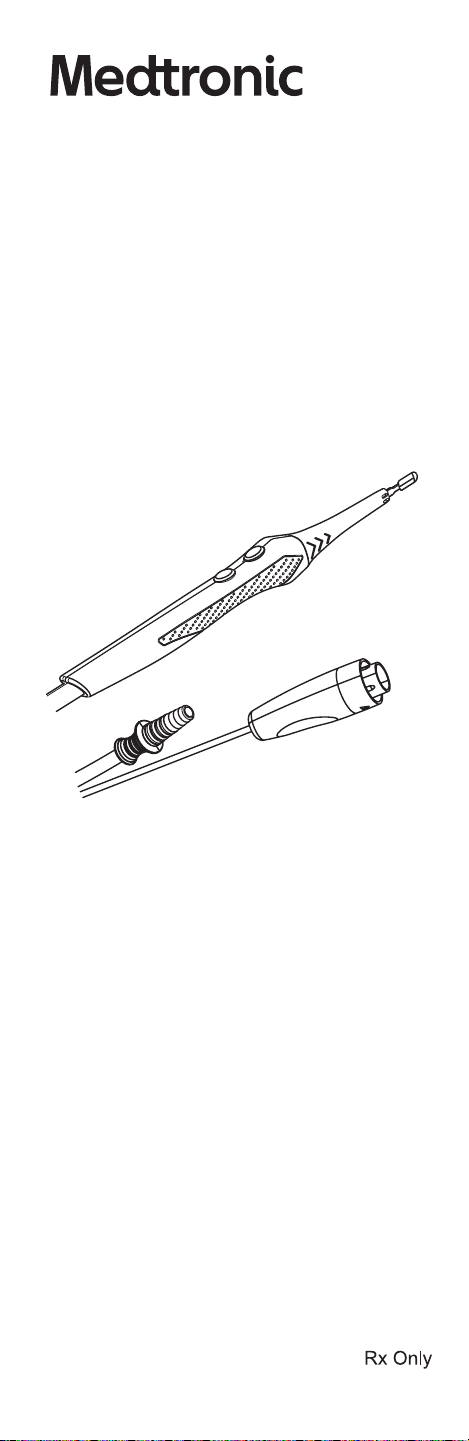
Device Description
The PlasmaBlade™ X 4.0S is a single-use- only device designed for use with
qualified Generators as a System (Surgery System). The PlasmaBlade™ X 4.0S
has integrated handswitch control, or alternatively, may be controlled with a
qualified Footswitch, supplied as an optional accessory to the Generator. The
PlasmaBlade™ X 4.0S consists of a single bendable blade and rotatable nose
piece that can be adjusted by hand. The finger grip also incorporates a suction
lumen for the evacuation of smoke and fluids. The system components are
designed to be used together and operated as a single unit. For a full list of
qualified generators, see IFU insert card.
Blue Button (COAG)
Yellow Button (CUT)
Bendable Blade
Cable
Handle
Rotatable Nose
Indications
The Surgery System is indicated for cutting and coagulation of soft tissue
during General, Plastic and Reconstructive (including but not limited to skin
incisions and development of skin flaps), ENT, Gynecologic, Orthopaedic,
Arthroscopic, Spinal and Neurological procedures.
Contraindications
The PlasmaBlade™ X should not be used on small appendages or body parts,
as in circumcision.
Adverse Events
As a consequence of electrosurgery, damage to surrounding tissue through
iatrogenic injury could occur.
Warnings
• Observe re precautions at all times. An electrosurgical device may
provide an ignition source due to sparking and heating.
• Do not use in the presence of ammable anesthetics or oxidizing gases such
as nitrous oxide and oxygen. Do not activate the device until vapors from
alcohol-based skin prepping agents have dissipated. Naturally occurring
gases that accumulate in body cavities can also be an ignition source.
• Do not touch the electrode tip of the PlasmaBlade™ X device while power is
being applied as this may result in user injury.
• Do not contact metal objects and instruments with the PlasmaBlade™ X
device while power is being applied as unintended tissue damage and
electrode tip damage could occur.
• Activation of the PlasmaBlade™ X device outside the eld of view could
cause patient injury.
• Activation of the handpiece when not in contact with target tissue may
cause capacitive coupling.
• Do not allow patient contact with grounded metal objects, as such
contact may result in patient or user injury.
• Activation of the handpiece simultaneously while aspirating uid may
alter the path of electrical energy away from target tissue.
• Inadvertent patient contact may result in burns. When not in use, place
the device in a dry and nonconductive area away from the patient.
• Ensure that only the active tip of the device is in contact with the patient
during use.
• Monopolar devices require a Patient Return Electrode. Compatible generator
contact quality monitoring (CQM) systems will only function properly with
split-style patient return electrodes. Other patient return electrode products
may not identify loss of safe contact between the return electrode and the
patient, thereby failing to provide auditory or visual alarms and causing
patient injury or product damage. Refer to the manufacturer’s instructions for
application site and placement procedures when applying the Patient Return
Electrode. Do not rely entirely on the impedance sensing feature as it can be
aected by a damaged (shorted) Patient Return Electrode. It is recommended
that the operator verify appropriate placement and contact of the Patient
Return Electrode. Inadequate contact of the Patient Return Electrode may
result in patient alternate site burns or injury. Refer to the selected generator's
Operator's Manual for compatible Patient Return Electrodes.
• Do not reuse, resterilize or reprocess the PlasmaBlade™ X device as it is
supplied sterile and intended for Single Use Only. A device that has been
resterilized or reprocessed may not perform properly and may result in
patient or user injury.
• When bending the blade, do not exceed a 45° angle. Do not bend more
than three times. Excessive bending of the blade may compromise
performance of the device or cause device failure, which could result
in patient or user injury. Use nger force to bend the blade; do not use
forceps as this could damage the device.
• Avoid uid contact with the handle and its interfaces and connections as
this may result in patient or user injury, or device failure.
1

• Position the cable to avoid creating a tripping hazard.
• Position the cable to avoid patient contact to protect against high frequency
current paths to the patient as this may result in patient or user injury.
• The use of electrosurgery in the presence of internal or external electrically
conductive implants is potentially hazardous due to concentration or redirection of HF currents.
• Interference produced by electrosurgical equipment may adversely
inuence the operation of other electronic equipment. Interference with
the function of or damage to cardiac pacemakers or other active implants
may occur. Consult the active implant manufacturer for further information
before proceeding with the surgery.
• Direct contact with implanted leads can cause physical damage to the
leads. Exercise caution around leads associated with any active implant;
particularly those with thin insulation.
• Do not use monopolar electrosurgery on small appendages, such as in
nger surgery, as it can cause thrombosis or other unintended injury to
tissue proximal to the surgical site.
• The device should not be used near electrocardiograph electrodes as it
can cause interference.
• The PlasmaBlade™ device should only be used with a qualied Generator
on output settings with peak voltages equal to or less than the rated
accessory voltage (see Table 1).
• Adequate ventilation to reduce electrosurgical smoke by use of a smokeplume evacuator or other means is recommended.
Precautions
• The PlasmaBlade™ X device should only be used by qualied medical
personnel possessing training in the surgical procedures to be performed.
• Take care when handling the electrode tip to prevent possible damage to
the tip and to prevent user injury.
• Use the lowest power setting and the shortest activation time possible to
achieve the desired end eect.
• Unless the product is being used to spot coagulate a vessel, it is
recommended to keep the electrode tip in motion while activated to
avoid excessive eschar buildup. Excessive eschar buildup can compromise
device performance.
• Do not use sharp or abrasive instruments or materials to clean eschar
buildup on the electrode tip as this may damage the tip.
• When not in use, the device should be placed in the holster securely
fastened to the surgical drape.
• Prior to initial use, ensure that all package inserts, including warnings,
cautions, instructions for use, as well as the selected Generator Operator’s
Manual, are read and understood.
• Prior to use, carefully inspect the package before opening. Do not use
the device if the package appears damaged in any way. Return damaged
packages to Medtronic Navigation, Inc.
• Prior to use, inspect the PlasmaBlade™ X device for any defects. Do not use
if insulation or connectors are damaged.
• The PlasmaBlade™ X device is intended for use only with qualied
Generators.
Equipment List
• PlasmaBlade™ X Compatible Generator
• Optional Generator Compatible Footswitch
• Patient Return Electrode, compatible with selected Generator
Instructions For Use
Read all instructions carefully. Failure to comply may lead to electrical or
thermal injury, or cause device malfunction.
Please refer to the selected Generator Operator’s Manual for step-by-step
instructions regarding initial setup and preparation for surgery. The
PlasmaBlade™ X device may only be used with a qualified Generator.
Before Surgery
1. Inspect the PlasmaBlade™ X package to ensure that it is unopened and
undamaged. If it is damaged or the sterile seal is broken, do not use the
device.
2. Remove the PlasmaBlade™ X device and holster from packaging using
aseptic technique.
3. Inspect the device for any signs of damage.
If the device is damaged it must not be used.
4. Attach the holster to the sterile drape using
a clamp or tape. The PlasmaBlade™ X cable
can be placed into the cutouts of the holster
for convenience.
5. For detailed instructions on the selected
Generator use, please refer to the operator’s
manual. Do not use the PlasmaBlade™ X
device until reading and understanding the directions for the selected
Generator.
6. Switch on the Generator to perform the initial setup and self-test.
7. Plug the PlasmaBlade™ X device into the Monopolar Connector of the
Generator. Upon connection, the unit will alarm if the device is not
recognized as a PlasmaBlade™ X device or if it fails a unit test. In event of an
alarm, replace the device.
8. If using suction, connect the suction tubing from either a central or
portable suction line to the connector on the handpiece.
Cable
cutouts
Electrode
cleaning slot
2

The Generator will display default power level settings for the PlasmaBlade™
X device upon connection. These settings must be verified and adjusted by
the user to ensure the appropriate settings are selected. The lowest power
setting possible should be used to achieve the desired end effect.
Table 1: PlasmaBlade™ X 4.0S Settings
Tissue
Effect
CUT
low
hemostasis,
low collateral
damage
Level
(LCD
display)
1
Mode
Power
[W-RMS]
0.5W 1000
2 2W 1100
3 6W 950
Low Cut
4 10W 1400
****
Maximum
Output
Voltage (Vpk)
5 20W* 1400
high
hemostasis,
higher
collateral
damage
COAG
low
hemostasis,
low collateral
damage
6
Medium Cut
7 35W 550
8 50W** 550
High Cut
9
(Blend 1)
High Cut
10
(Blend 2)
1
2 20W 1400
3 25W 1400
Low Coag
20W 500
25W 1500
50W 1400
15W 1400
4 30W 1400
5 35W*** 1600
high
hemostasis,
higher
collateral
damage
6
30W 2400
7 35W 2400
High Coag
8 40W 2550
9 45W 2600
10 50W 2600
* When Cut level is changed from 5 to 6, there is a change in Cut mode from Low Cut to
Medium Cut.
** When Cut level is changed from 8 to 9, Cut mode changes from Medium Cut to High Cut
(Blend 1). Hence, output power will increase when the Cut level is changed from 9 to 8.
*** When Coag level is changed from 5 to 6, Coag mode changes from Low Coag to High
Coag. Hence, output power will increase when the Coag level is changed from 6 to 5.
**** The listed Maximum Output Voltage values pertain to device utilization with the AEX
generator. The overall Maximum Output Voltage is 2750 Vpk in accordance with device
utilization on the Pulsar II generator.
9. If using the optional footswitch, plug the wireless receiver into the
Footswitch Connector of the selected Generator. The footswitch can only
be used to activate output on a monopolar device if a bipolar device is
not connected.
10. Position the Patient Return Electrode on the patient and then connect
the Patient Return Electrode to the Patient Connector receptacle of the
qualied Generator.
11. The qualied Generators have an Impedance indicator display . Ensure the
impedance indicator is illuminated green before starting surgery. Refer to
the Generator Operator’s Manual for more information on the impedance
sensing feature and approved split-style return electrodes.
12. The qualie d Generators have Impedance indicator displays that
indicate the type and impedance level of the Patient Return Electrode
in monopolar mode. The indicator will remain green with proper return
electrode impedance. The indicator will turn red when the unit does not
detect proper return electrode impedance. Ensure proper return electrode
impedance before starting surgery.
13. Remove the electrode tip protector before activation of device.
Bend
Manipulating the Device
1. Ensure power is not applied while
handling the nose as this may result in
user injury.
NOTE: The electrode tip and shaft may
be rotated for user preference of cutting angle and to allow use of all sides
of the cutting edge.
2. Grasp the device nose, rotate, and release to reseat. The nose can be
rotated eight separate steps at 45° each, for a total of 360°.
3. If needed, use nger force to bend the blade to a maximum of 45°. Inspect
the blade for damage after bending and do not use if damaged. Do not
bend more than three times. Excessive bending of the shaft may cause
device failure and could result in patient or user injury.
Rotate
Grasp nose
3

During Surgery
Place the device in the holster when not in use.
1. To cut, press the yellow button/pedal on the handswitch or footswitch.
2. To coagulate, press the blue button/pedal on the handswitch or footswitch.
NOTE: Eschar buildup on the tip can be removed
manually with gauze pads, or by inserting the tip into
the slot at the front of the holster and drawing the
device backwards through the slot. Inspect the device
for any signs of damage after cleaning.
After Surgery
1. Turn o the Generator.
2. Disconnect the PlasmaBlade™ X device, Footswitch,
and Patient Return Electrode from the Generator.
3. Discard the PlasmaBlade™ X device after use. It is
intended for Single Use Only. Do not reuse, resterilize, or reprocess. A device
that has been resterilized or reprocessed may not perform properly and
may result in patient or user injury.
Cleaning
slot
How Supplied
The PlasmaBlade™ X device is supplied sterile and is intended for Single Use
Only. Contents are sterile unless the package is opened or damaged. Do not
reuse, resterilize, or reprocess the PlasmaBlade™ X device. The product is
sterilized using Ethylene Oxide (EO).
Customer Service
Please call Medtronic Customer Service at +18667779400 if you have any
device returns or questions about Medtronic’s device.
Limited Express Warranty
IF, PRIOR TO THE PRODUCT EXPIRATION DATE, THE STERILIZED DEVICE
IS FOUND TO BE INOPERABLE DURING NORMAL AND PROPER USE
IN ACCORDANCE WITH APPLICABLE INSTRUCTIONS, MEDTRONIC
NAVIGATION, INC WILL REPLACE THE PRODUCT AT NO CHARGE.
This Limited Express Warranty does not extend to a product where the
user has compromised the sterile integrity of the package or the product
is used after the product expiration date. A sterile package that has been
compromised should not be used.
Disclaimer of Implied Warranties
and Consequential Damages
MEDTRONIC NAVIGATION, INC MAKES NO OTHER
WARRANTIES WITH RESPECT TO THE PRODUCT AND
EXPRESSLY DISCLAIMS ALL OTHER WARRANTIES, EXPRESS
OR IMPLIED, AS TO MERCHANTABILITY, FITNESS FOR A
PARTICULAR PURPOSE OR ANY OTHER MATTER. IN NO
EVENT SHALL MEDTRONIC NAVIGATION, INC BE LIABLE FOR
ANY INDIRECT, CONSEQUENTIAL OR SPECIAL DAMAGES
OF ANY KIND. THE REMEDIES SET FORTH IN THE LIMITED
EXPRESS WARRANTY ARE THE EXCLUSIVE REMEDIES
AVAILABLE TO CUSTOMER.
4

Symbols
Follow Instructions for Use
Use by Date
Do Not Reuse
Do Not Resterilize
Humidity Limitation: Non-condensing Humidity
Temperature Limitation
Sterilized by Ethylene Oxide
Non-Pyrogenic
Catalog Number
Lot Number
Manufacturer
Do Not use if the package is damaged.
Caution: Federal law (USA) restricts this device to
sale by or on the order of a physician.
5

Medtronic Navigation, Inc
826 Coal Creek Circle
Louisville, CO 80027 USA
medtronic.com
manuals.medtronic.com
© Copyright 2019
Medtronic Navigation, Inc
70-10-1506 Rev B
2019/09
 Loading...
Loading...