Medtronic PMC10UB305N Operator's Manual

Nellcor™
OxiCable, USB
305 cm
Operator's Manual

Medtronic, Medtronic with rising man logo, and Medtronic logo are trademarks of Medtronic. Third-party trademarks (“TM*”) belong to
their respective owners. The following list includes trademarks or registered trademarks of a Medtronic entity in the United States
and/or in other countries: Nellcor
TM

Symbols
kPa
kPa
°F
°C
°F
°C
47
Prescription only.
Consult instructions for use
Must consult instructions for use
Atmospheric pressure limits (see Environmental Conditions, page 25)
Humidity limits (see Environmental Conditions, page 25)
Temperature limits (see Environmental Conditions, page 25)
Keep dry
Proper waste disposal for electrical and electronic equipment
Defibrillation-proof type BF applied part
Protection against fluid ingress: Protected against solid objects greater than 1mm. Protected against the effects of
submersion in water up to 1 meter deep for up to 30 minutes.
USB (Universal Serial Bus)
CSA – Canadian Standards Association certification mark
Catalog number
Manufacturer
Serial number
Date of manufacture
i

ii

Table of Contents
1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Technical Assistance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
2 Product Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Product Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Monitoring Cable Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
3 Connection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Connection to a Host Monitoring System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Connection to a Nellcor™ Sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
4 Performance Considerations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Nellcor™ Sensor Performance Considerations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Electromagnetic Interference . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
5 Product Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Service and Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
6 Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Nellcor™ Sensor Selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
7 Theory of Operations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Theoretical Principles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Automatic Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Functional Testers and Patient Simulators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Functional versus Fractional Saturation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Measured Versus Calculated Saturation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
System Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
8 Product Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Physical Characteristics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Electrical Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Environmental Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
System Accuracy and Ranges . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Nellcor™ Sensor Optical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Product Compliance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Biocompatibility Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Manufacturer’s Declaration and Guidance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Host Monitoring System Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Essential Performance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Appendix A. Clinical Study . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Methods . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Study Population . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Study Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
Operator's Manual English 1

Conclusion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
Figures
Figure 1. Monitoring Cable Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Figure 2. USB Connector on Monitoring Cable . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Figure 3. Inserting Sensor Connector . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Figure 4. Latch Closed over Sensor Connector . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Figure 5. Check These Areas for Soil . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Figure 6. Check These Areas for Soil When Latch is Removed . . . . . . . . . . . . . . . . . . . . . . . . 14
Figure 7. Ensure All of These Areas are Clean When Latch is Removed . . . . . . . . . . . . . . . 15
Figure 8. Oxyhemoglobin Dissociation Curve . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Figure 9. Modified Bland-Altman for SpO2 - MAXA Sensor: SaO2 vs. (SpO2 - SaO2) . . . . . 33
Figure 10. Modified Bland-Altman for SpO2 - MAXN Sensor: SaO2 vs. (SpO2 - SaO2) . . . 33
Figure 11. Modified Bland-Altman for SpO2 - MAXFAST Sensor: SaO2 vs. (SpO2 - SaO2) 34
Figure 12. Modified Bland-Altman for SpO2 - FLEXMAX Sensor: SaO2 vs (SpO2 - SaO2) . 35
Figure 13. Modified Bland-Altman for SpO2 - SoftCare-A Sensor: SaO2 vs. (SpO2 - SaO2)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
Figure 14. Modified Bland-Altman for SpO2 - DS-100A Sensor: SaO2 vs. (SpO2 - SaO2) . 36
Figure 15. Modified Bland-Altman for SpO2 - DYS-E Sensor: SaO2 vs. (SpO2 - SaO2) . . . . 37
Tables
Table 1. Nellcor™ Sensor Models . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Table 2. System Measurement Ranges . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Table 3. System Accuracy Ranges . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Table 4. Electromagnetic Emissions Guidelines and Compliance . . . . . . . . . . . . . . . . . . . . 27
Table 5. Electromagnetic Immunity Guidelines and Compliance . . . . . . . . . . . . . . . . . . . . . 27
Table 6. Electromagnetic Immunity Compliance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
Table 7. Proximity Field Immunity Compliance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
Table 8. Demographic Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Table 9. SpO2 Accuracy for NellcorTM Sensors vs. CO-Oximeters . . . . . . . . . . . . . . . . . . . . . . 32
Table 10. SpO2 Accuracy for FLEXMAX Sensor vs. CO-Oximetry . . . . . . . . . . . . . . . . . . . . . . 32
Table 11. SpO2 Accuracy per Decade for NellcorTM Sensors vs. CO-Oximeters . . . . . . . . . 32
2 Operator's Manual English

1. Introduction
1.1. Overview
This manual provides information for using the Nellcor™ OxiCable, USB (the “monitoring
cable”). This manual applies to the following product:
PMC10UB305N
1.2. Safety Information
This section contains important safety information for use of the monitoring system. Use
this information in conjunction with the safety information specified in the host monitoring
system documentation.
1.2.1. Safety Symbols
Warning: Warnings alert users to potential serious outcomes (death, injury, or adverse
events) to the patient, user, or environment.
Caution: Cautions alert users to exercise appropriate care for safe and effective use of the
product.
Note: Notes provide additional guidelines or information.
1.2.2. Patient and Operator Safety - General Use
Warning: Shock hazard — Do not immerse or wet the monitoring cable or sensor.
1
Warning: Choking hazard — The monitoring cable contains small detachable parts.
Warning: Disconnect the monitoring cable, sensor, and monitoring system from the patient
during magnetic resonance imaging (MRI) scanning. Objects containing metal can become
dangerous projectiles when subjected to the strong magnetic fields created by MRI
equipment. Also, induced currents could potentially cause burns.
Warning: Do not use the monitoring cable in the presence of flammable anesthetics. This
may cause an explosion or fire.
Warning: Do not use a pulse oximetry sensor on the same extremity as a blood pressure
cuff or other constricting instrument. Such usage can cause inaccurate pulse oximetry
measurements or a loss of signal.
Warning: Do not use any monitoring cable, monitoring system, sensor, cable, or connector
that has a damaged enclosure or any damaged component. Remove any damaged
equipment from service for inspection by a qualified service technician.
Warning: As with all medical equipment, carefully route patient cabling to reduce the
possibility of patient entanglement or strangulation.
Warning: Ensure that the monitoring cable is carefully positioned to prevent tripping and
entanglement.
Caution: Federal law (U.S.A.) restricts this device to sale by or on the order of a physician.
Operator's Manual English 3

1.2.3. System Connection, Compliance, and Interference
Warning: The monitoring cable may cause radio interference or may disrupt the operation
of nearby equipment. Mitigation for such disruption may require re-orienting or relocating
the monitoring cable or shielding the location.
Warning: The use of accessories, sensors, and cables other than those specified may result
in inaccurate readings and increased EMI emissions of the monitoring cable.
Warning: EMI disruption can cause erratic readings, cessation of operation, or other
incorrect functioning.
Caution: This device has been tested and found to comply with the limits for medical
devices related to IEC 60601-1-2: 2014 for Class B Emissions. These limits are designed to
provide reasonable protection against harmful interference in a typical medical installation.
Caution: Anyone who connects the monitoring cable to a host monitoring system is
configuring a medical system and, therefore, is responsible for ensuring the system
complies with the Requirements for Medical Electrical Systems IEC 60601-1:2005+A1:2012
and electromagnetic compatibility IEC 60601-1-2:2014.
Caution: Do not connect the monitoring cable’s USB connector to anything other than a
compatible USB 2.0 host device.
1.2.4. Sensor Use and Performance Considerations
Warning: Certain physical conditions may affect calculation of SpO2 and pulse rate. These
conditions include, but are not limited to: dysfunctional hemoglobin, intravascular dyes,
low perfusion, and darkly pigmented skin, refer to Nellcor™ Sensor Performance
Considerations, page 11.
Caution: Use only Medtronic-approved sensors when connecting to the sensor port.
Connecting any other sensor influences the accuracy of sensor data, which may lead to
adverse results.
1.2.5. Disposal
Caution: Dispose of the monitoring cable in accordance with local requirements and
regulations.
1.3. Technical Assistance
1.3.1. Technical Services
For technical information and assistance, if unable to correct a problem while using the
monitoring cable, or to order parts, contact Medtronic or a local Medtronic representative.
Medtronic Technical Services
15 Hampshire Street
Mansfield, MA 02048 USA
+1.800.635.5267, +1.925.463.4635 (toll)
or contact a local Medtronic representative
www.medtronic.com
4 Operator's Manual English

When calling Medtronic or a local Medtronic representative, have the monitoring cable
serial number available.
1.3.2. Warranty Information
To obtain information, contact Medtronic or a local Medtronic representative.
See Technical Services, page 4.
Purchase of this instrument confers no express or implied license under any Medtronic
patent to use that instrument with any sensor not manufactured or licensed by Medtronic.
1
Operator's Manual English 5

6 Operator's Manual English
2. Product Overview
2.1. Product Description
When used with a host monitoring system, the Nellcor™ OxiCable, USB (the “monitoring
cable”) provides continuous non-invasive monitoring of functional oxygen saturation of
arterial hemoglobin (SpO2) and pulse rate, as measured by Nellcor™ pulse oximetry sensors.
The monitoring cable relies on unique oximetry technology and design to provide
hospitals, clinicians, and caregivers with accurate, timely data.
The monitoring cable provides the following patient data to the host monitoring system:
•
Arterial blood oxygen saturation (SpO2)— Functional measure of oxygenated
hemoglobin relative to the sum of oxyhemoglobin and deoxyhemoglobin.
•
Pulse rate (PR)— Detected pulsations per minute.
•
Plethysmographic waveform (Pleth)— Visual waveform representing detected
pulsations. (Nonnormalized)
•
Operating status— Alarm conditions and operational status.
2
2.2. Indications for Use
The Nellcor™ OxiCable, USB is indicated for prescription use only for spot check or
continuous non-invasive monitoring of functional oxygen saturation of arterial
hemoglobin (SpO2) and pulse rate. It is intended for use with neonatal, pediatric, and adult
patients during both no motion and motion conditions and for patients who are either well
or poorly perfused, in hospitals and hospital-type facilities.
•
Note: Hospital use typically includes such areas as the intensive care unit (ICU),
neonatal intensive care unit (NICU), operating room (OR), post-anesthesia care unit
(PACU), emergency department, and medical/surgical general care floor (GCF).
•
Note: Hospital-type facilities include step-down units and long-term care facilities.
Use with any particular patient requires the selection of an appropriate Nellcor™ sensor.
See Nellcor™ Sensor Selection, page 17.
Operator's Manual English 7

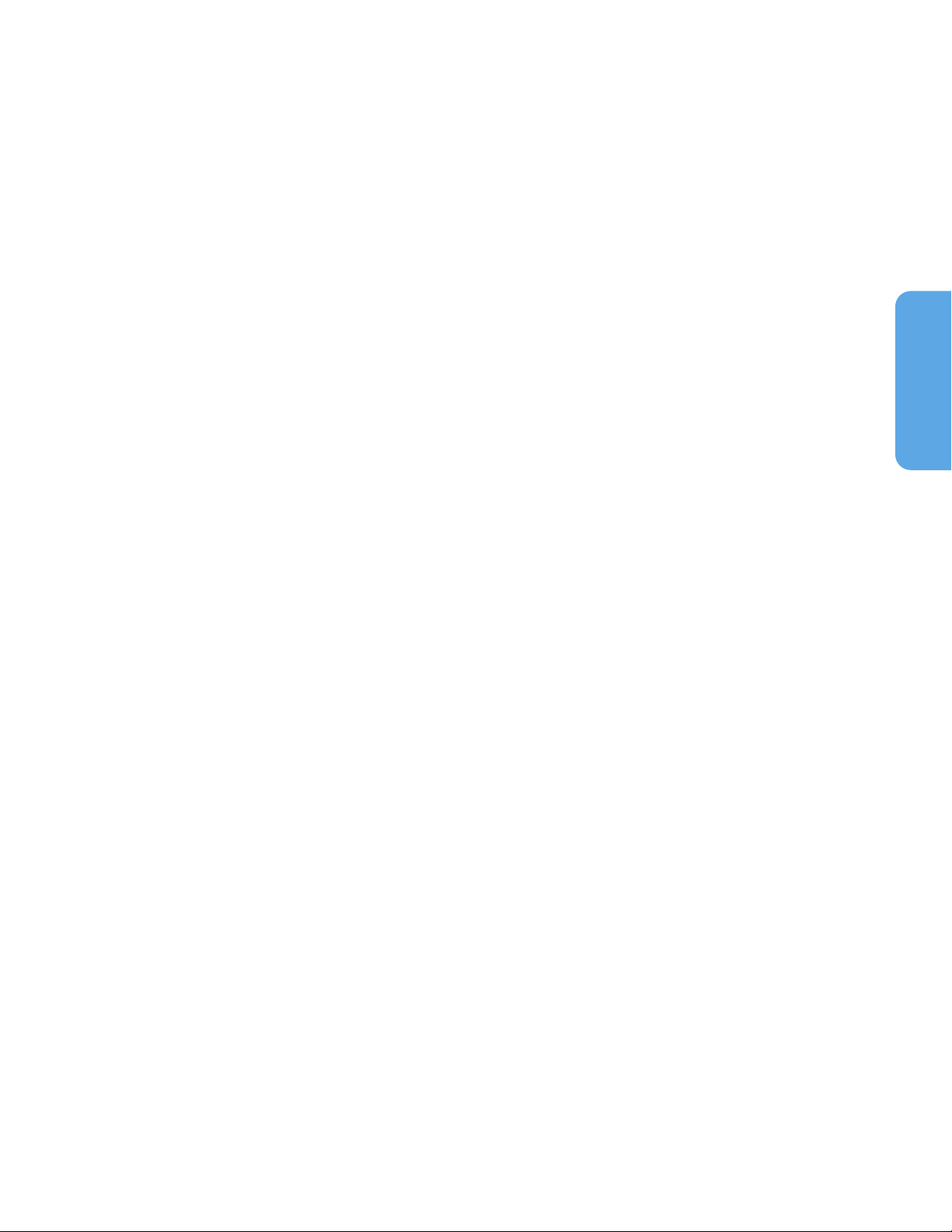
2.3. Monitoring Cable Components
Figure 1. Monitoring Cable Components
1. Sensor Port (to NellcorTM Sensor)
2. Sensor Latch
3. Isolation Module
4. USB Connector (to Host Monitoring
System)
8 Operator's Manual English
3. Connection
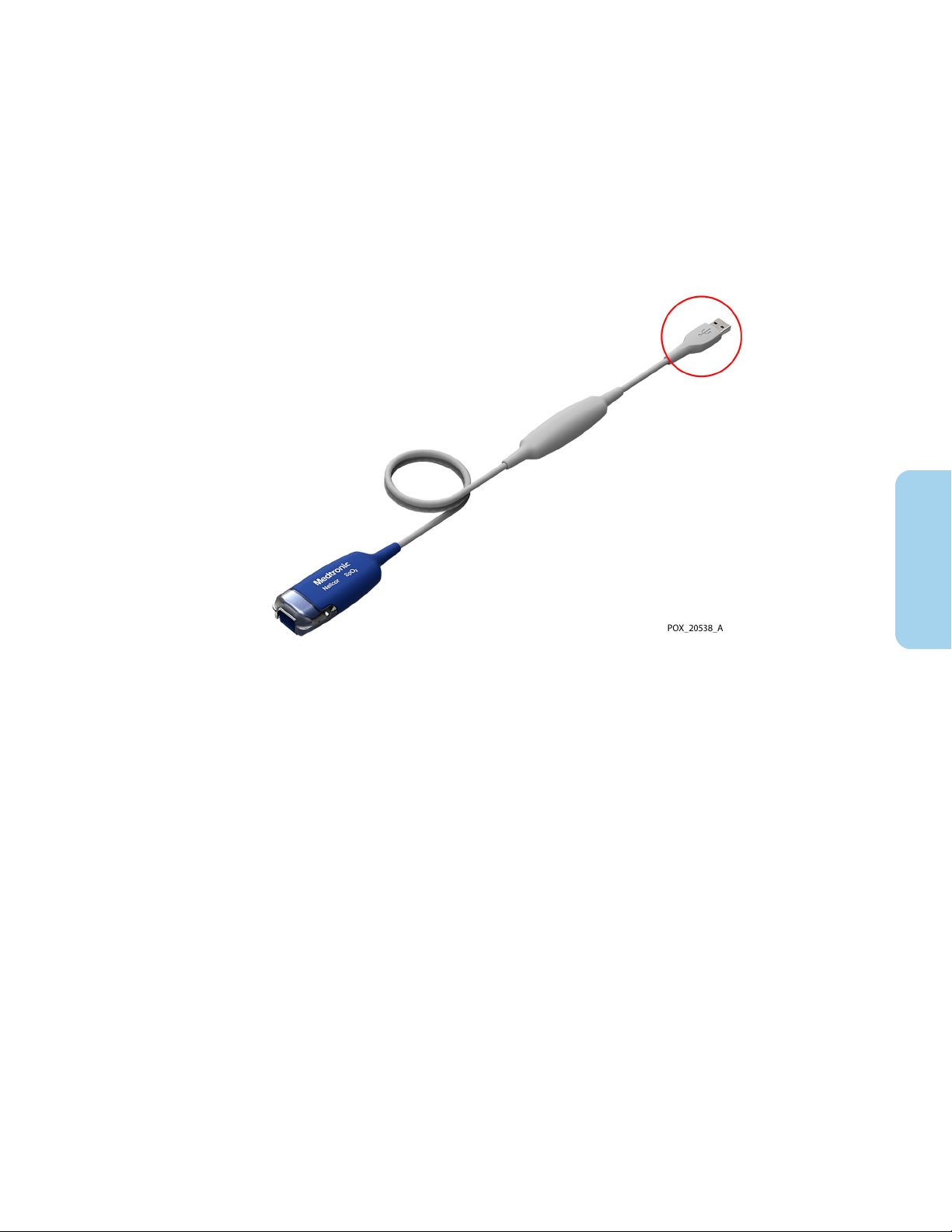
3.1. Connection to a Host Monitoring System
To connect the Nellcor™ OxiCable, USB (the “monitoring cable”) to a host monitoring
system, insert the monitoring cable’s USB connector into a compatible USB port on the
host system.
Figure 2. USB Connector on Monitoring Cable
Note: The monitoring cable derives power from the host monitoring system. The
monitoring cable has no power switch. To ensure that power is removed from the
monitoring cable, disconnect it from the host monitoring system.
3.2. Connection to a Nellcor™ Sensor
Prior to using a Nellcor™ sensor with the monitoring cable:
•
See Nellcor™ Sensor Selection, page 17 for information about selecting the
appropriate sensor for the patient.
•
Read the Instructions for Use accompanying the sensor.
•
See Nellcor™ Sensor Performance Considerations, page 11 for information about
optimizing the performance of the sensor and monitoring cable during patient use.
To connect a Nellcor™ sensor to the monitoring cable:
1. Open the latch at the end of the monitoring cable’s sensor port and firmly insert
the sensor connector. The connector is keyed so that it fits correctly in one
orientation only.
3
Operator's Manual English 9
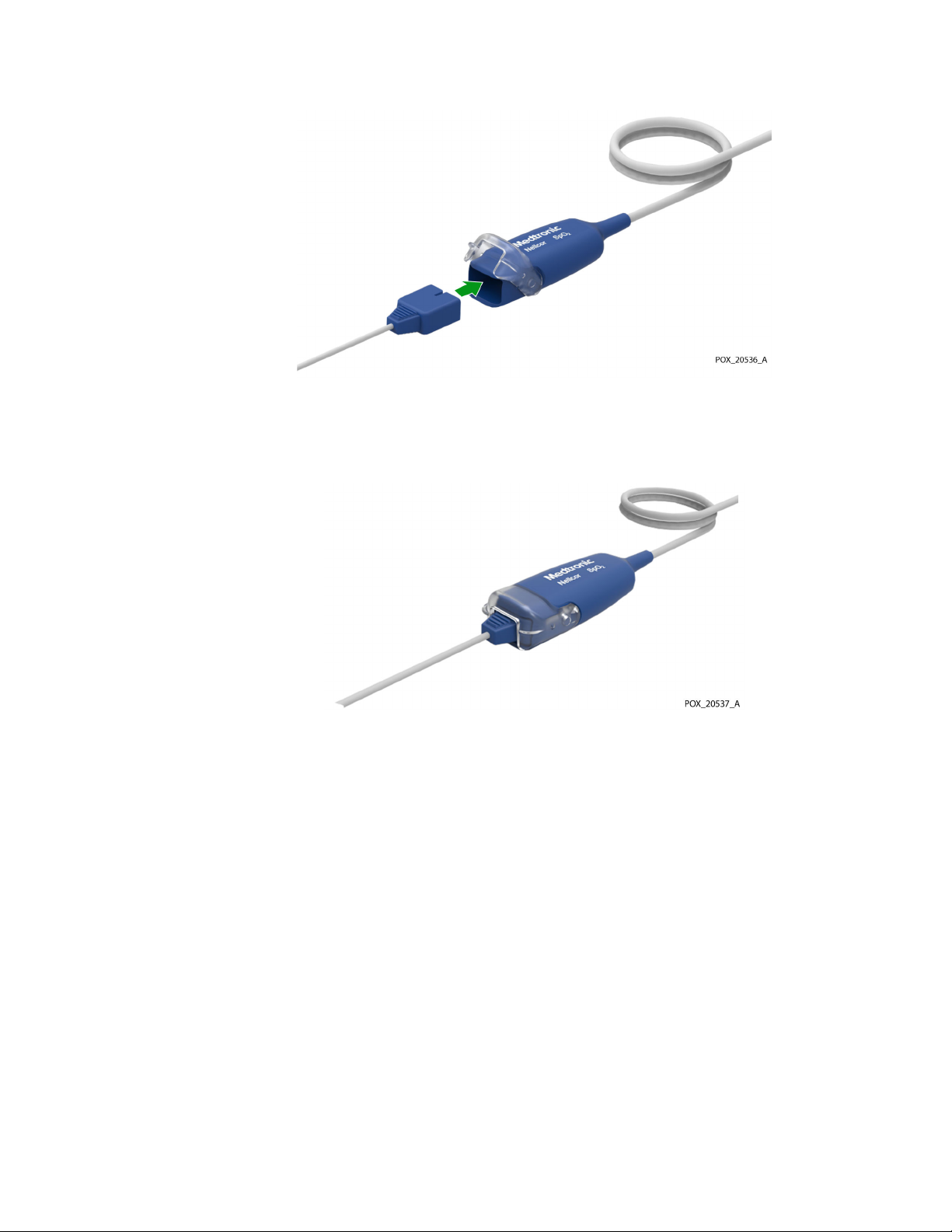
Figure 3. Inserting Sensor Connector
2. Snap the latch over the sensor connector. When the sensor connector is seated
properly, the latch should close completely over the connector.
Figure 4. Latch Closed over Sensor Connector
10 Operator's Manual English
 Loading...
Loading...