Medtronic N’VISION 8840 Technical Manual

™
N’VISION
Clinician Programmer
8840
Technical Manual
Rx Only

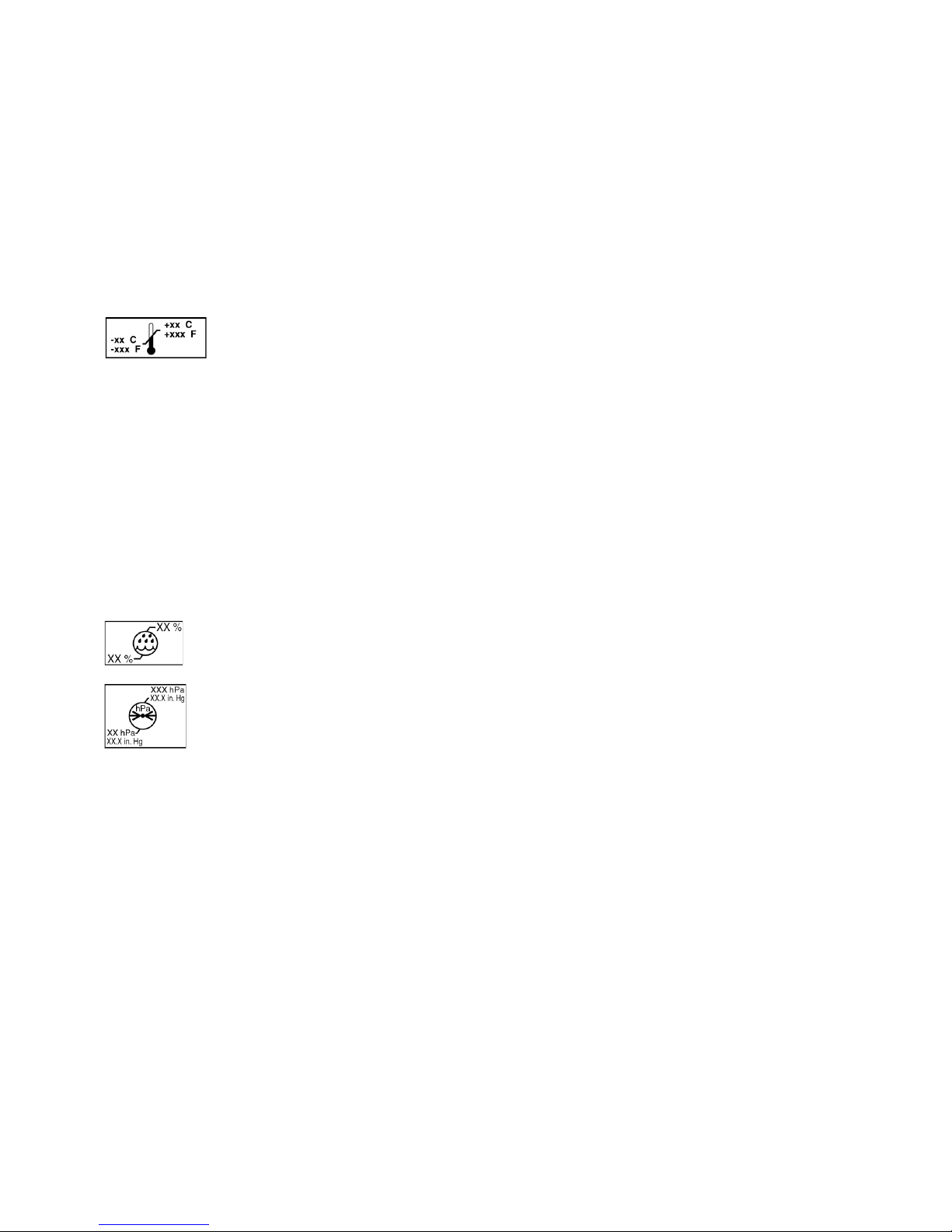
Explanation of Symbols
Serial number
n
Storage temperature
Attention. See accompanying documents
w
IEC 60601-1, Type BF Equipment
y
Caution
#
Relative Humidity
Atmospheric Pressure

Medtronic is a registered trademark of Medtronic, Inc. N’Vision is a
trademark of Medtronic, Inc.

How to Use This Guide
About the Model 8840 N’Vision Clinician Programmer Guide
This guide presents information for users of the N’Vision Clinician
Programmer:
■
Programmer overview
■
Programmer description
■
Programmer use
■
Programmer maintenance
■
Programmer troubleshooting
■
Programmer specifications
■
Glossary
How to Use This Guide
Medtronic N’Vision Clinician Technical Manual iii

How to Use This Guide
iv Medtronic N’Vision Clinician Technical Manual

Table of contents
Table of contents
Explanation of Symbols i
How to Use This Guide iii
1. Programmer Overview
Device Description 2
Supply and Accessories 2
Indications for Use 3
Warnings and Precautions 3
Warnings 3
Precautions 4
2. Programmer Description
Component Identification 2
Interactive Features 5
Graphical Display 5
Display Feature 10
Hard Keys 10
Scroll Wheel 10
Connection Ports and Other Features 11
Application Card Port 11
Telemetry Module 12
IR Port 12
Audio Speaker 12
3. How to Use the N’Vision Programmer
Powering Up the Programmer 2
Inserting the Application Card 5
Stand-by Mode 7
Navigating Menu Options 8
The Programmer Display 8
Using the Display 9
Display Calibration 9
Using the Scroll Wheel and Program Key 9
Programmer Operation 11
Start-up and Self-test Sequence 11
Programmer Status Bar 11
Status Bar Icons 12
Software Application Selection 13
Using the Slider Bar 14
Activating the Slider Bar 14
Slider Bar Icons 14
Medtronic N’Vision Clinician Technical Manual v

Table of contents
Platform Session Data Manager 19
Navigation Tab Bar 20
THERAPY-STOP Key 20
Programming Key 20
Programmer Connections 21
Communicating with a Printer 21
Printing 21
Telemetry Module Use 22
Using the Telemetry Module Attached to the Programmer 22
Using the Telemetry Module Detached from
the Programmer 22
Positioning the Telemetry Head 23
Reeling in the Telemetry Head 25
Connecting the Magnet to the Telemetry Module 27
4. Programmer Maintenance
Changing the Programmer Batteries 2
Battery Replacement 3
Back-up Battery 3
Cleaning the Display 3
Display Calibration 4
Cleaning the Telemetry Module/IR Lens 5
Safety and Technical Checks 5
5. Troubleshooting
Troubleshooting Reference Guide 2
6. Specifications and Warranty
N’Vision Programmer Specifications 2
Electrical and Operating Characteristics 2
Programmer Storage and Operating Conditions 3
Warranty 4
Medtronic
®
Neurological Equipment Limited Warranty (U.S.
Customers Only) 4
A. Glossary
vi Medtronic N’Vision Clinician Technical Manual

Programmer Overview 1
Device Description 1-2
Supply and Accessories 1-2
Indications for Use 1-3
Warnings and Precautions 1-3
1
Medtronic N’Vision Clinician Technical Manual 1-1

Programmer Overview
Device Description
Device Description
The Model 8840 N’Vision Clinician Programmer is a small, portable
device that offers a single programming platform for Medtronic
Neurological implantable therapy devices. The programmer is equipped
with a touchscreen display for data entry, telemetry head for device
programming, and an infrared port through which communications can be
established with compatible printers.
A therapy application card that contains application-specific software is
supplied separately.
Supply and Accessories
The following accessories are used in conjunction with the N’Vision
Programmer:
■
therapy software on an application card (supplied separately)
■
a compatible printer (optional)
■
a magnet (required for programming SynchroMed and
SynchroMed EL Pumps)
1-2 Medtronic N’Vision Clinician Technical Manual

Indications for Use
The N’Vision Programmer is indicated for use with Medtronic Neurological
therapies and devices as provided on Medtronic application cards. Refer
to specific programming guides to determine card compatibility.
Warnings and Precautions
Warnings
■
Refer to the appropriate implant/device manual for instructions
on specific therapy applications and a complete listing of
warnings, precautions, contraindications and instructions for
use for applications.
■
The N’Vision Programmer can only be used to program
Medtronic Neurological devices that correspond with therapy
application software on the application card within the
programmer.
■
Use only fresh batteries (four “AA” alkaline batteries).
■
Do not immerse the N’Vision Programmer in water or other
fluids. Do not expose the programmer to excessive amounts of
water or other fluids. This may damage the programmer.
■
Do not connect the N’Vision Programmer to any equipment not
specifically listed in this technical manual. Connection to non-
specified equipment may result in damage to the programmer
or patient injury.
■
Do not use the N’Vision Programmer if it is damaged.
■
Peripheral equipment connected to the N’Vision Programmer
must be certified according to the respective IEC standards
(e.g., IEC 950 for data processing equipment and IEC 60601 for
medical equipment). Furthermore, the system formed by the
N’Vision Programmer and any connected peripherals must
comply with the requirements of IEC 60601-1-1, safety
requirements for medical electrical systems. Such compliance
is the responsibility of the person who connects the peripheral.
If in doubt, consult the technical services department or your
local representative.
Programmer Overview
Indications for Use
For all peripherals certified to IEC 950, it is the responsibility of
the user to keep the peripheral at least 2 meters from the patient.
This will satisfy the requirements of IEC 60601-1-1.
Medtronic N’Vision Clinician Technical Manual 1-3

Programmer Overview
Warnings and Precautions
■
When using the programmer in a sterile field, place the
programmer and programming head in a sterile bag. The
programmer is not sterile and cannot be sterilized.
■
The magnet is for use with Medtronic SynchroMed and
SynchroMed EL pumps only. Remove the magnet before using
the N’Vision Programmer with Medtronic neurostimulators.
■
Return devices for disposal to Medtronic when the devices are
no longer functional.
■
If the display is inactive, inadvertent programming may occur.
■
If the display is not working, do not use the N’Vision
Programmer. Return the device to Medtronic for repair.
Precautions
■
Power failures during programmer use will reinitialize the
programmer, and application state data will be lost.
■
Do not remove the application card while a therapy is active,
because these circumstances may cause programming
operations to cease.
■
Do not drop the device, because the display may break, causing
injury to the user.
■
Telemetry failures will result in loss of communication between
the programmer and the device. To ensure that telemetry is
established and maintained, keep the telemetry head as close
as practical to the implantable device. Do not move the
telemetry head once telemetry has been established.
■
Do not insert nonMedtronic, generic compact flash cards into
the N’Vision Programmer.
■
The N’Vision Programmer is not certified for use in the
presence of a flammable anesthetic mixture with air or with
oxygen or nitrous oxide. The consequences of using the
N’Vision Programmer near flammable atmospheres are
unknown.
1-4 Medtronic N’Vision Clinician Technical Manual

Programmer Overview
Warnings and Precautions
■
Medtronic neurostimulators controlled by the N’Vision
Programmer may affect the operation of other implanted
devices, such as cardiac pacemakers and ICDs. Physical
proximity of implanted neurostimulators to other implanted
devices may cause sensing problems and inappropriate
responses by these other implanted devices. If the patient
requires concurrent implantable pacemaker and/or defibrillation
therapy, evaluation of any potential interference problems and
careful programming of each system may be necessary to
optimize the patient’s benefit from each device.
■
The THERAPY-STOP function ( ) does not operate unless
an application card is in place in the programmer and a therapy
has been selected.
■
Do not use the N’Vision Programmer or Application Card if they
were transported or stored at temperatures above or below the
specified temperature range of -40° – 149 °F (-40° – 65 °C).
Allow the equipment to stabilize to a temperature within the
operating range of -48° – 105 °F (10° – 40 °C) before using it to
program.
Medtronic N’Vision Clinician Technical Manual 1-5

Programmer Overview
Warnings and Precautions
1-6 Medtronic N’Vision Clinician Technical Manual

Programmer Description 2
Component Identification 2-2
Interactive Features 2-5
Connection Ports and Other
Features 2-11
2
Medtronic N’Vision Clinician Technical Manual 2-1

Programmer Description
Component Identification
Component Identification
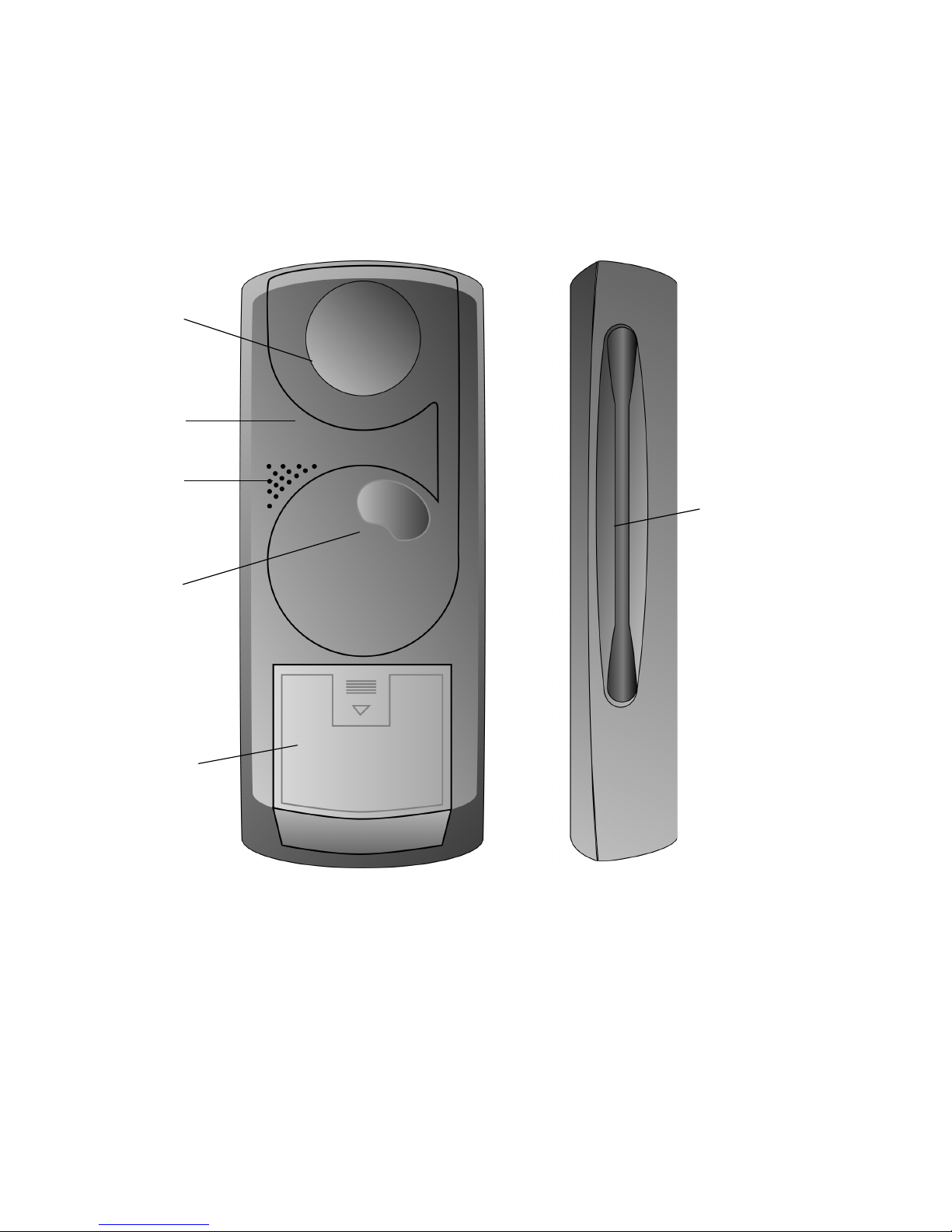
The front of the Model 8840 N’Vision Clinician Programmer is equipped
with a touchscreen display and a THERAPY-STOP “hard key”
(Figure 2-1). The left side of the programmer includes an application card
port, two “hard” keys (to initiate power or programming functions), a scroll
wheel used during programming, and a button used to eject application
cards from the programmer. An infrared transceiver (IR port) is located at
the top of the programmer.
Note: Serial number information for the N’Vision Programmer is
displayed:
■
inside the programmer battery compartment
■
on the programmer Information Screen (refer to Figure 3-11)
2-2 Medtronic N’Vision Clinician Technical Manual

Programmer Description
Component Identification
THERAPY-STOP
Hard Key
Display
IR Port
Scroll Wheel
Programming
Key
Application
Card Port
Application
Card Eject
Button
Front Left Side
Figure 2-1. N’Vision Programmer Front and Left Side
w
Warning: The Expansion Port is planned for use with future applications.
DO NOT use the port to connect the programmer with any other
equipment.
Power Key
Expansion Port
Medtronic N’Vision Clinician Technical Manual 2-3
Programmer Description
Component Identification
Magnet
Te l em e t r y
Module
Audio Speaker
On the back of the N’Vision Programmer are the telemetry module and
magnet, cable reel for the telemetry module, audio speaker, and battery
compartment (Figure 2-2). The stylus is stored in a recessed area on the
right side of the device.
Stylus Storage
Te l em e t r y
Cable Wheel
Battery
Compartment
Right SideRear
Figure 2-2. N’Vision Programmer Back and Right Side Views
2-4 Medtronic N’Vision Clinician Technical Manual

Interactive Features
Graphical Display
The touchscreen of the programmer displays text and graphical
messages that guide the user through all programmer functions. The
display is divided into five sectors:
■
Programmer status bar
■
Title bar
■
Application tabs
■
Primary application area
■
Secondary application area
The significance of each sector is unique.
Programmer Description
Interactive Features
Icons have been placed on these sectors, and these icons represent
either access to status information (“inactive” icons) or links to another
function within the program (“active” icons).
Inactive icons may appear as faded or outlined graphics. Active icons
appear as selectable “buttons” on the screen.
Medtronic N’Vision Clinician Technical Manual 2-5

Programmer Description
Interactive Features
■
Programmer Status Bar:
The programmer status bar provides information on the status of
various programmer functions (Figure 2-3). The following “inactive”
icons present information on the status of the:
connection status (telemetry);
printer;
telemetry function;
and battery.
Figure 2-3. Programmer Status Bar
2-6 Medtronic N’Vision Clinician Technical Manual
Programmer Description
Interactive Features
■
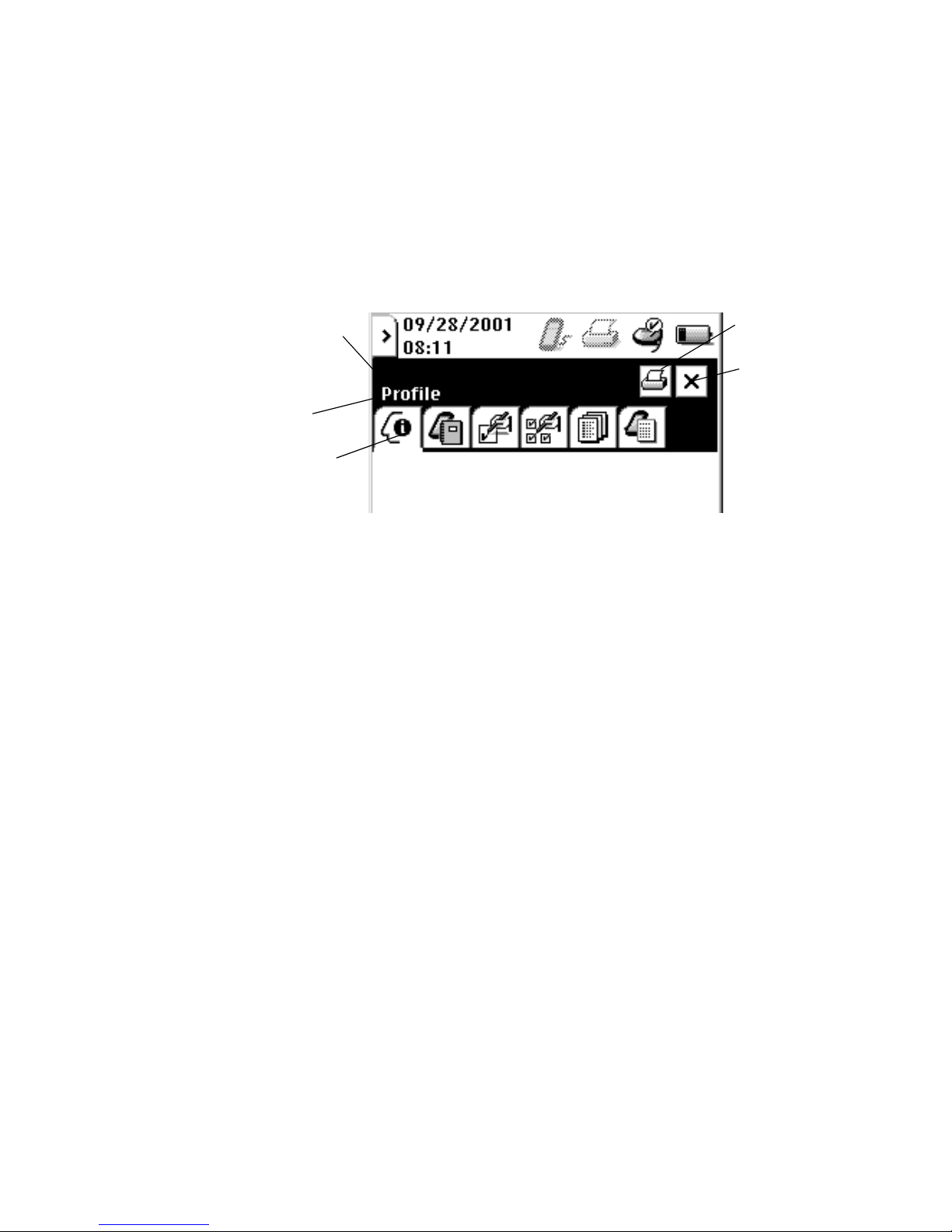
Title Bar and Application Tabs:
When an application is active on the N’Vision Programmer, the Title
Bar identifies the application tab in use, along with the print and close
program icons (Figure 2-4). Application tabs are application-specific
“active” icons, each representing a separate function of the
application in use.
Title Bar
Application Tab in Use
Application Tabs
Print Icon
Close Icon
Figure 2-4. Title Bar and Application Tabs
Print and close icons on the title bar are “active” icons. Proper contact of
the stylus with these “active” icons results in the action associated with the
icon. For example, on the Title Bar, contact of the stylus with the “close”
icon results in termination of the current application.
Medtronic N’Vision Clinician Technical Manual 2-7
Programmer Description
Interactive Features
■
Primary Application Area:
Active application information comprises the largest area of the
display screen (Figure 2-5).
Note: The parameters displayed in Figure 2-5 are generic and do not
represent data from any specific pump or neurostimulator.
Primary Application
Area
Figure 2-5. Primary Application Area of the Display
2-8 Medtronic N’Vision Clinician Technical Manual
 Loading...
Loading...