Medtronic MSW001 Technical Manual

24965
Patient Connector
Technical Manual
Caution: Federal law (USA) restricts this device to sale by or on the order
of a physician.

The following list includes trademarks or registered trademarks of Medtronic in the United States and possibly in other
countries. All other trademarks are the property of their respective owners.
CareLink, Medtronic

Contents
1 Introduction to the 24965 Patient Connector .............. 4
1.1 Explanation of packaging and product symbols ........... 4
1.2 Description ................................ 6
1.3 Intended use ............................... 6
1.4 Contraindications ............................ 6
1.5 Warnings ................................. 6
1.6 Precautions ................................ 6
1.7 Regulatory compliance ......................... 8
1.8 Patient Connector functions ...................... 8
1.9 Security .................................. 9
2 Setup and configuration ........................... 9
2.1 Contents of package ........................... 9
2.2 System components ...........................10
2.3 Compatible components ........................12
2.4 Supported mobile devices .......................12
2.5 Setup .................................... 13
3 Conducting a patient session ....................... 18
3.1 Position the patient connector ..................... 18
3.2 Communicating with an implantable device .............18
3.3 Troubleshooting .............................19
4 Maintaining the patient connector .................... 20
4.1 Cleaning and disinfecting the 24965 patient connector ...... 20
4.2 Software updates ............................21
4.3 Specifications ...............................21
3

1 Introduction to the 24965 Patient Connector
1.1 Explanation of packaging and product symbols
Refer to the package label and product to see which symbols apply to this product.
Consult instructions for use
Conformité Européenne (European Conformity). This symbol means that the device fully complies with applicable
European Union Acts.
Ingress protection
Use only with specified power supply
Type BF applied part
Humidity limitation
Non-ionizing electromagnetic radiation
Do not dispose of this product in the unsorted municipal
waste stream. Dispose of this product according to local regulations. See http://recycling.Medtronic.com for instructions
on proper disposal of this product.
Direct current
Manufacturer
Date of manufacture
Authorized representative in the European community
For US audiences only
Re-order number
Serial number
Package contents
4
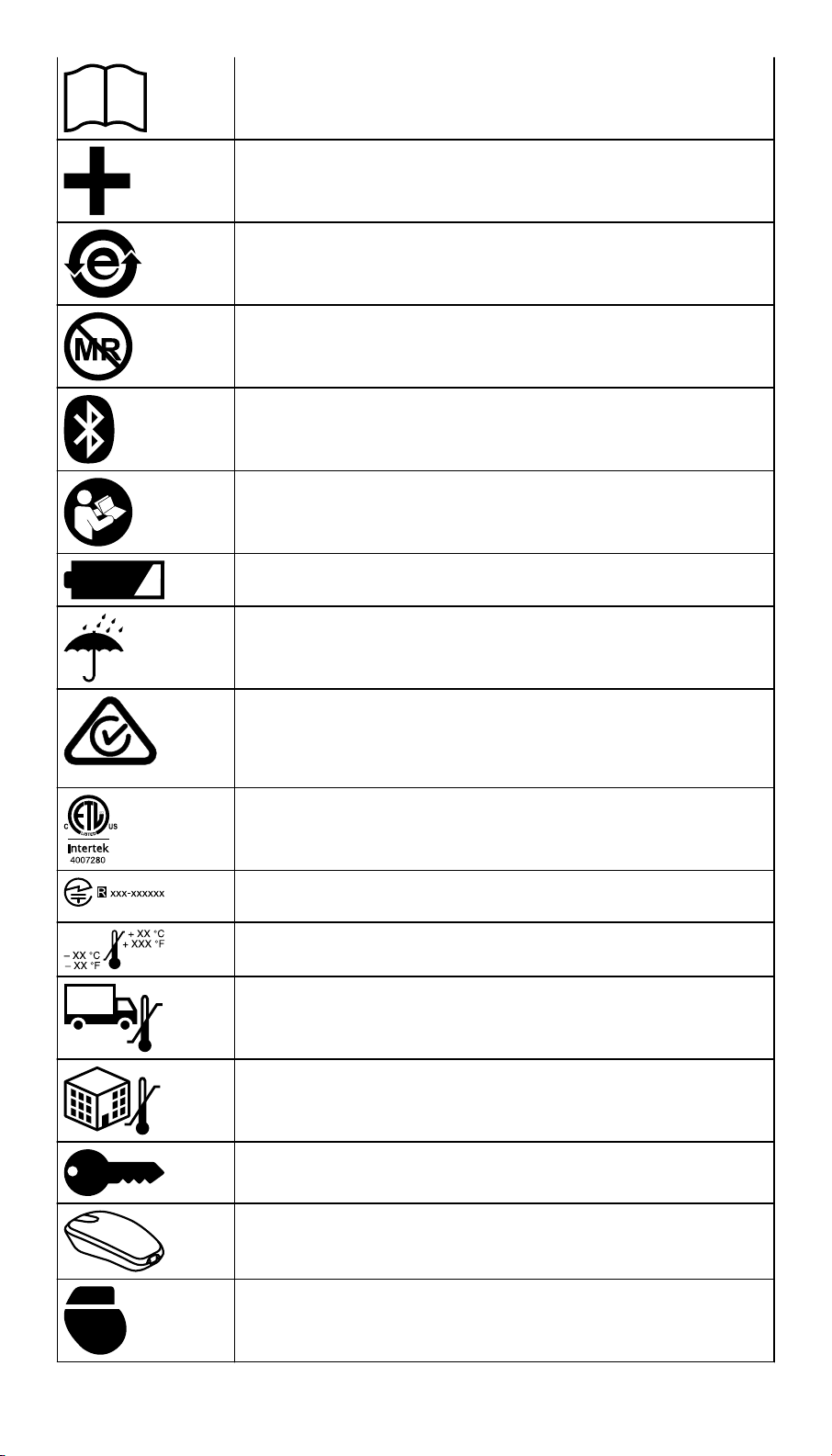
Product documentation
Accessories
China RoHS
Magnetic Resonance (MR) Unsafe
Bluetooth connection
Follow instructions for use (blue)
Low battery
Keep dry
ACMA (Australian Communications and Media Authority)
and the New Zealand Ministry of Economic Development
Radio Spectrum Management compliance mark for Australia
and New Zealand
ETL Listed Mark
Technical Conformity (Ministry of Internal Affairs and Communications) mark for Japan
Operating temperature
Transit temperature
Storage temperature
Security key
Patient Connector
Telemetry
5

Tether kit
Power supply
1.2 Description
The 24965 Patient Connector (referred to from now on as the patient connector),
when paired with Medtronic applications (referred to from now on as apps) on your
mobile device, is used to interrogate and program implantable Medtronic devices.
Interrogated information can be viewed or sent to the CareLink Network.
1.3 Intended use
The patient connector is a portable electronic device using low frequency inductive
telemetry to communicate with Medtronic implantable heart devices. The patient
connector uses Bluetooth technology to transmit implantable heart device data to a
Medtronic Mobile app for further processing.
The patient connector is intended to be used by healthcare personnel only in a
clinical or hospital environment.
1.4 Contraindications
There are no known contraindications for the use of this device.
1.5 Warnings
These warnings apply in general to using the patient connector settings. For more
information related to specific implantable device models, see the reference guides
for the implantable device and the software.
Battery exposure – Exposing the patient connector to cold temperatures may
result in a loss of performance and shortened patient connector service life.
Damage due to impact – Do not use the patient connector if it has sustained
impact damage. Internal components may be damaged or exposed. Use of
damaged equipment may impact user or patient safety.
Magnetic Resonance (MR) Unsafe – The patient connector is MR Unsafe. Do not
bring the patient connector into Zone 4 (magnet room), as defined by the American
College of Radiology.
Modification of equipment – Do not modify this equipment. Modifications may
reduce system effectiveness and impact user or patient safety. Modifying the patient
connector without the approval of Medtronic could void the user’s authority to
operate the equipment.
Unauthorized use – The patient connector can be used with any compatible
mobile device onto which the app is installed. Inappropriate programming could
result if untrained persons obtain the patient connector and a REVEAL LINQ patient
allows them to use it with the patient’s device.
Use of unapproved power supply – Use only the Medtronic-supplied power
supply with the patient connector. Use of an unapproved power supply may damage
equipment or impact user or patient safety.
1.6 Precautions
Attaching the tether kit – Do not overtighten the screw when attaching the tether
kit.
6

Autoclaving – Do not autoclave the patient connector.
Damaged equipment – If the case of the patient connector is cracked, or if the
power supply connector is damaged, contact your Medtronic representative.
Replace the power supply if there is damage to it. Dispose of the damaged power
supply according to local regulations or return the part to Medtronic.
Do not immerse – Take care to prevent liquid from entering the patient connector.
Do not immerse the patient connector or any accessories in any liquid or clean them
with aromatic or chlorinated hydrocarbons.
Maintenance and service – Do not modify or do any maintenance or service on
the patient connector while you are using it. Doing any of these tasks on the patient
connector while it is in use can lower its effectiveness. Contact Medtronic at the
number on the back cover of this manual if your patient connector is not working
properly.
Product and packaging labels and information – If labels or information appear
to be missing from the product or packaging, contact your local Medtronic
representative at the address and telephone number located on the back cover of
this document.
Radio-frequency (RF) interference – Portable and mobile RF communications
equipment can interfere with the operation of the patient connector. There is no
guarantee that the patient connector will not receive interference or that any
particular transmission from this system will be free from interference. To avoid
interference, do not use the patient connector and mobile device within 2 m (6 feet)
of a television, computer monitor or screen, or other wireless communications
equipment such as wireless network devices, mobile phones, cordless telephones
and their base stations, “walkie-talkies”, or radio frequency identification (RFID) and
security systems. Using the patient connector near these devices could interfere
with communication between the implantable heart device and the patient
connector.
Security – Maintain adequate physical security of the patient connector to prevent
unauthorized use that could lead to harm to patients. Bluetooth communication in
the patient connector is encrypted for security. Medtronic inductive telemetry uses
short-range communication to protect patient information. If the patient connector
should fail, there is no risk of patient harm.
Use of wireless devices – The patient connector incorporates radio-frequency
(RF) communications components which may affect other devices and equipment
in the medical environment. The use of wireless devices in the medical environment
must be evaluated and authorized by the responsible organization. RF interference
may affect device performance.
Electromagnetic Compliance (EMC) testing shows that the patient connector
provides reasonable protection against harmful interference and provides EMC
immunity in a typical medical installation. The use of wireless devices in the medical
environment must be evaluated and authorized by the responsible organization.
However, there is no guarantee that interference will not occur in a particular
installation.
If the patient connector does cause harmful interference to other devices or is
negatively impacted by other devices, correct the interference by one or more of the
following measures:
• Reorient or relocate the patient connector and other devices.
• Increase the separation between the patient connector and other devices by at
least two meters (approximately 6 feet). Other devices include, but are not
7

limited to, cellular phones, computer screens, wireless network devices, and
‘walkie-talkies’.
• Turn off any interfering equipment.
1.6.1 Environmental precautions
To ensure safe and effective operation, use the patient connector with care to avoid
damage to the patient connector from environmental factors that may impair its
function. Care is exercised in design and manufacturing to minimize damage to the
patient connector under normal use. However, the patient connector is susceptible
to many environmental stresses including, but not limited to, the following examples.
• The patient connector is designed to be used indoors in a clinical or hospital
environment.
• Do not drop or mishandle the patient connector or damage may occur. Damage
can impair the functionality of the patient connector. Even if the patient
connector works immediately after being dropped, operational damage may
have occurred that may not be observed immediately.
• Do not spill fluid on the patient connector. Fluid incursion can occur and cause
damage to the patient connector.
• The patient connector may be affected by electrostatic discharge (ESD). In an
environment likely to cause ESD, such as a carpeted floor, discharge any
charge collected on your body before touching the patient connector.
• Do not open the case of the patient connector. The patient connector is
constructed to minimize risk from environmental factors. Opening the case of
the patient connector can make it susceptible to environmental factors and can
expose the patient or user to hazardous voltage or current.
• Do not expose the patient connector to rapid temperature changes. Rapid
temperature changes may affect proper operation of the patient connector. If
the patient connector is exposed to rapid temperature changes, allow the
temperature to stabilize before using it.
• Do not store or operate the patient connector for prolonged periods of time in
high humidity. Prolonged storage or operation of the patient connector in high
humidity can affect proper operation.
If the patient connector is damaged, contact Medtronic at the telephone number on
the back cover of this manual.
Other environmental factors can impair the performance of the patient connector.
Always use good health management practices to prevent environmental damage
to the patient connector.
1.7 Regulatory compliance
1.7.1 US Federal Communications Commission (FCC)
FCC ID: LF524965 (for patient connector). Contains FCC ID: T7V1316.
This device complies with part 15 of the FCC Rules. Operation is subject to the
following two conditions: (1) This device may not cause harmful interference, and
(2) this device must accept any interference received, including interference that
may cause undesired operation.
1.8 Patient Connector functions
The patient connector communicates with an implantable device. The patient
connector also communicates with the Medtronic app running on a mobile device.
8
 Loading...
Loading...