Page 1

KYPHON ELEMENT™ Inflatable Bone
M708348B477E Rev. A
Tamp (Size 15/3)
2015-08-05
IMPORTANT INFORMATION ON THE KYPHON ELEMENT™ Inflatable Bone Tamp
(Size 15/3)
INDICATIONS FOR USE
The KYPHON ELEMENT™ Inflatable Bone Tamp (IBT) is intended to be used as a conventional bone tamp for the reduction of
fractures and/or creation of a void in cancellous bone in the spine (including use during a balloon kyphoplasty procedure with a
PMMA-based bone cement that is cleared for use in kyphoplasty procedures), hand, tibia, radius, and calcaneus.
DEVICE DESCRIPTION
See Figure 1.
1. Y-Adapter with EZ Prep
2. Stylet
3. Distal Sleeve
4. IBT Balloon
5. Radiopaque Marker Band
6. Plug
7. Insertion Sleeve
8. Outer Sheath
9. Exit Marker
▪ The working surface of the IBT is designed to compress cancellous bone and/or move cortical bone as it inflates. The
Inflatable Component of the IBT is near the distal tip of the device as shown in Figure 1.
▪ The shaft contains one lumen.
▪ The proximal end of the shaft has a side-arm adapter. The Inflation Port of the adapter is contiguous with the balloon.
▪ The Exit Marker located on the outer lumen is used during IBT insertion.
▪ A Radiopaque Marker Band is located directly proximal to the balloon. The tip of the Stylet is located at the distal end of the
balloon. Fluoroscopic visualization of the deflated balloon will be determined with the Marker Band and the Stylet.
For US Audiences Only
CAUTION: FEDERAL LAW (USA) RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN.
WARNINGS
Breakage of the device may require intervention or retrieval.
PRECAUTIONS
▪ The KYPHON ELEMENT™ Inflatable Bone Tamp is a single use device intended to contact body tissues. Do not reuse,
reprocess, or resterilize. Reusing this device carries the risk of contamination and may cause patient infection or crossinfection, regardless of the reprocessing methods. There is also an increased risk of the deterioration of the device
performance due to the reprocessing steps, which may lead to patient injury or death.
▪ It is important to read the Instructions For Use and these precautions carefully prior to device operation.
▪ Use the IBT prior to the use by date noted on the package.
▪ Do not use if package is opened or damaged because product integrity including sterility may be compromised.
▪ Do not use damaged products. Before use, inspect the IBT and packaging to verify that no damage has occurred.
▪ Prior to use, the IBT should be examined to verify functionality.
▪ Do not use this product if you have not been properly trained. The IBT should only be used by physicians who are trained in
the techniques of bone tamp use. Physicians using the devices should be familiar with the physiology and pathology of the
selected anatomy.
▪ The IBT should be manipulated only while under fluoroscopic observation with radiographic equipment that provides high
quality images.
Page 2
▪ The IBT should only be inflated using an Inflation Syringe having a 20 ml volume capacity.
▪ Only inflate the IBT with liquid contrast medium: a 60% solution is recommended. Follow manufacturer’s instructions for
contrast medium indications, usage and cautions.
▪ Do not use air or other gas to inflate the IBT.
▪ The Inflatable Component of the IBT may fail due to contact with bone splinters, bone cement and/or surgical tools.
▪ The inflation characteristics of the IBT are altered by inflation inside bone.
▪ Reconditioning, refurbishing, repair, modification, or reprocessing of the device to enable further use is expressly prohibited.
NOTE: The Medtronic QL® 1430 Inflation Device provides superior volume control compared to other inflating systems, such
as a standard syringe, and is required for inflation of the IBT. Follow the manufacturer’s Instructions for Use of the Medtronic
QL® 1430 Inflation Device. The VacLok™ Syringe is packaged with the Medtronic QL® 1430 Inflation Device.
ADVERSE EVENTS
Adverse events potentially associated with use of the IBT include:
▪ Embolism of fat, thrombus or other materials resulting in symptomatic pulmonary embolism or other clinical sequelae.
▪ Rupture with fragmentation of the inflatable portion of the IBT resulting in retention of a fragment within the vertebral body.
▪ Rupture of the IBT causing contrast medium exposure, possibly resulting in an allergic reaction or anaphylaxis.
▪ Deep or superficial wound infection.
▪ Retropulsed vertebral body bone fragments which may cause injury to the spinal cord or nerve roots resulting in
radiculopathy, paresis or paralysis.
▪ Bleeding or hematoma.
DIRECTIONS FOR USE
CAUTION: Follow the manufacturer’s Instructions for Use of the Medtronic QL® 1430 Inflation Device.
CAUTION: Contrast media may have different viscosity and precipitation levels that may cause slower inflation and deflation
times. For this reason, the use of 60% contrast medium is recommended.
1. Push plunger all the way into the VacLok™ Syringe. Attach the VacLok™ Syringe to Inflation Port (1) on IBT.
2. Pull the VacLok™ Syringe plunger back. Turn plunger to lock it in position on the last slot in syringe.
3. Detach the VacLok™ Syringe from IBT.
4.
Attach connecting port on Medtronic QL® 1430 Inflation Device tubing to Inflation Port on IBT. The system is now prepared
and ready to use.
Use of the IBT
1. Table 1 defines the inflated diameter (D) and inflated length (L) of the IBT in 37°C water at inflation volume increments to
the maximum inflation volume.
2. These dimensions may vary during product use due to local variation in bone structure.
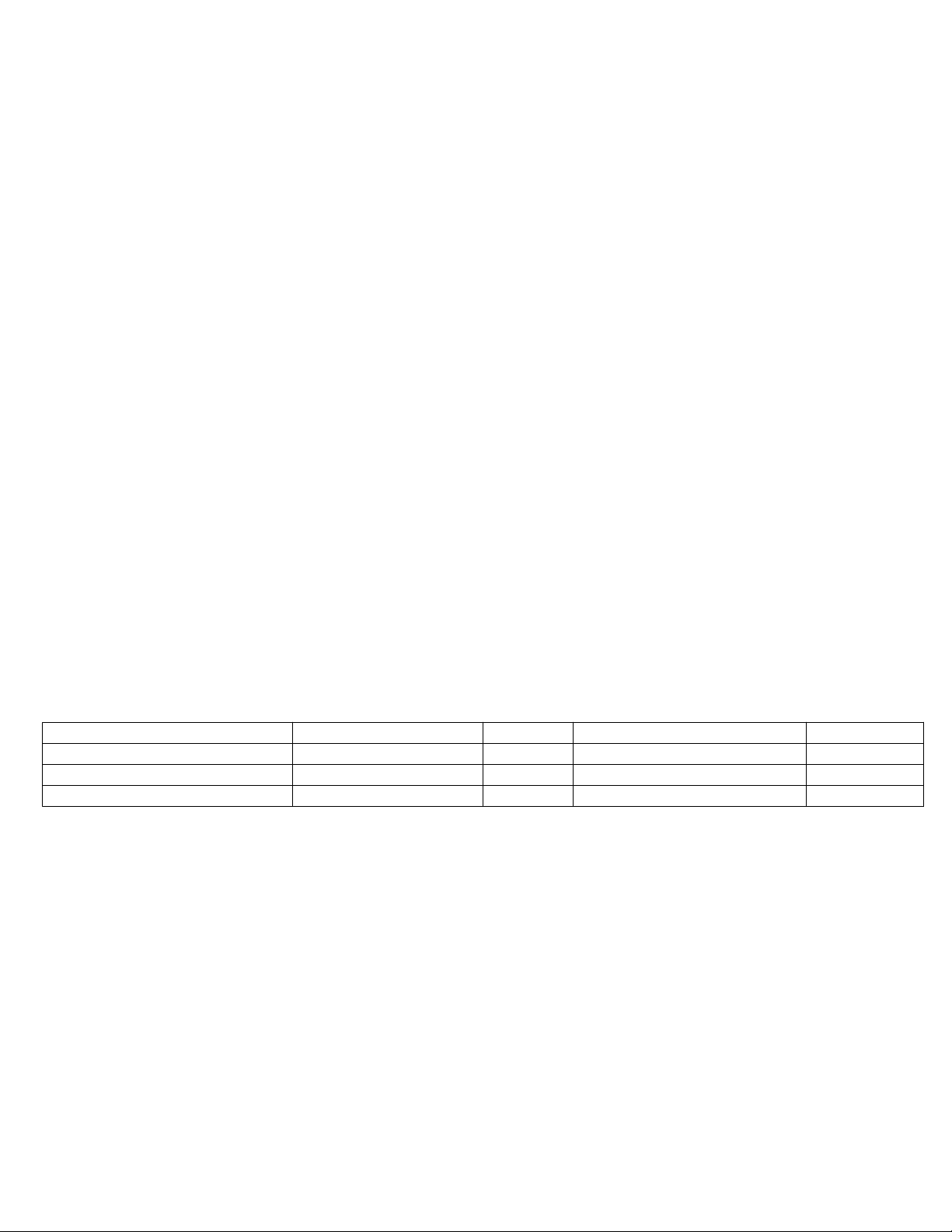
Table 1: KYPHON ELEMENT™ IBT Inflated Dimensions (in 37°C water)
Model Number KR153 Inflated Dimensions
Size 15/3 Volume Diameter (D1, D2) Length (L)
Max. Inflation Volume 4 ml 2 ml 11.7 mm 17.3 mm
Max. Inflation Pressure 400 psi (27 ATM) 4 ml 15.6 mm 23.6 mm
IBT Insertion
1. An access channel is required for IBT placement.
2. Follow the Instructions for Use for the KYPHON ELEMENT™ bone access tools to create an access channel into the bone.
3. Remove and discard Insertion Sleeve prior to use.
NOTE: The distal tip of the deflated IBT has reached the distal end of the cannula when the Exit Marker on the outer lumen
of the IBT enters the proximal end of the cannula.
4. Place the deflated IBT into the access channel and position it under image guidance using the Radiopaque Marker Band
and the distal tip of the Stylet. A gentle twisting motion with the forward push can aid insertion.
5. While holding the IBT in place, inflate to 44 psi (3 ATM) to secure IBT in position.
IBT Inflation
1. Inflate the IBT under continuous image guidance. Use the lateral view to monitor distance from the anterior and posterior
cortex. Use the AP view to monitor the lateral cortices.
2. If continuous imaging is not used, increase the volume in small increments (0.25 – 0.5 ml). Assess tamp position in lateral
and AP views before proceeding to further volume increase.
3. Stop when treatment goal is achieved: any part of the IBT inflated length contacts cortical bone or maximum inflation
volume and/or maximum inflation pressure is attained (see Table 1).
Page 3

IBT Removal
CAUTION: Never withdraw the IBT unless the Inflatable Component is fully deflated. Never withdraw the IBT against resistance.
Determine the cause of resistance under fluoroscopy and take the necessary remedial actions.
Unlock the Medtronic QL® 1430 Inflation Device and evacuate the contrast medium from the IBT until the balloon is completely
deflated. Remove the IBT from the bone through the cannula with a gentle twisting motion.
▪ If there is resistance, connect a 30 ml syringe to the Inflation Port. Pull the syringe plunger back to the “30 ml” mark to
create a vacuum. Resume the IBT removal.
▪ Confirm entry of the inflatable portion of the IBT into the cannula. If the Inflatable Component does not move into the
cannula, advance the cannula over the proximal end of the balloon. Following cannula advancement, withdraw the IBT
through the cannula. If resistance is noted, remove IBT and cannula simultaneously.
Completion of the Balloon Kyphoplasty Procedure
PMMA-based bone cement may be introduced using the KYPHON ELEMENT™ Bone Filler Device following void creation in a
pathological fracture of the vertebral body. Refer to the PMMA-based bone cement and KYPHON ELEMENT™ Bone Filler
Device Instructions for Use.
STERILIZATION
Sterilized with irradiation.
HOW SUPPLIED
The KYPHON ELEMENT™ IBT is supplied sterile in a peel-open package. In the event of damage to the sterile packaging, do
not use and notify the manufacturer.
MATERIALS NOT SUPPLIED
▪ Medtronic QL
®
1430 Inflation Device and VacLok™ Syringe
▪ Contrast Medium (60%)
▪ Small Bowl
NOTE: Medtronic QL® 1430 Inflation Device and VacLok™ Syringe are available from Medtronic.
STORAGE
The IBTs should be stored in their original shipping materials. Proper care should be taken to ensure that the IBTs will not be
damaged. Store in a cool dry place.
LIMITATION OF LIABILITY
Medtronic will not be responsible for any direct, indirect, incidental, consequential, or exemplary damages resulting from reuse
of the KYPHON ELEMENT™ Inflatable Bone Tamp.
In no event shall Medtronic be liable for any direct, indirect, incidental, consequential, or exemplary damages arising out of or in
connection with the KYPHON ELEMENT™ Inflatable Bone Tamp, based upon breach of contract (including breach of warranty).
FURTHER INFORMATION
In case of complaint, or for supplementary information, contact Medtronic.
©2015 Medtronic Sofamor Danek USA, Inc. All rights reserved.
Figure 1: KYPHON ELEMENT™ Inflatable Bone Tamp (IBT)
Page 4

AUSTRALIAN SPONSOR:
Medtronic Australasia Pty Ltd
97 Waterloo Rd
North Ryde, NSW 2113
Australia
EXPLANATION OF SYMBOLS
Authorized representative in the European Community
The device complies with European Directive MDD
93/42/EEC
CAUTION: Federal law (USA) restricts these devices to
sale by or on the order of a physician.
Do not re-use
Batch code
Manufacturer
Catalogue number
Medtronic Sofamor Danek USA, Inc.
1800 Pyramid Place
Memphis, TN 38132
Telephone: 800 933 2635 (USA)
901 396 3133 (Outside USA)
Fax: 901 396 0356
Medtronic B.V.
Earl Bakkenstraat 10
6422 PJ Heerlen
The Netherlands
Tel: + 31 45 566 80 00
For US audiences only
Sterilized using irradiation
Do not resterilize
Do not use if package is damaged
Use-by date
Consult instructions for use
 Loading...
Loading...