Page 1

IN.PACT™ 018
Paclitaxel-coated PTA Balloon Catheter
Instructions for Use
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Page 2

© 2022 Medtronic. All rights reserved. Medtronic and Medtronic logo are trademarks of Medtronic. ™* Third party brands are trademarks of their respective
owners. All other brands are trademarks of a Medtronic company.
Page 3

Symbols
Sterilized using ethylene oxide
Catalogue number
Lot number
Manufacturer
Manufactured in
Use-by date
Quantity
Consult instructions for use at this website: www.medtronic.com/manuals
Consult instructions for use
Do not reuse
Do not resterilize
Keep away from sunlight
Keep dry
Do not use if package is damaged
Outer diameter
Temperature limit
Nonpyrogenic
Do not exceed rated burst pressure
Over the wire
Nominal pressure
Rated burst pressure
Inflation pressure
Balloon diameter
Minimum sheath inner diameter
Maximum guidewire diameter
Balloon length
Usable catheter length
1
Page 4

Table of contents
1. Product Name .................................................................................................................................................................. 2
2. Product Description ........................................................................................................................................................ 3
2.1. Device Component Description .............................................................................................................................. 3
2.2. Drug Component Description ................................................................................................................................. 4
3. Indications for Use .......................................................................................................................................................... 5
4. Contraindications ............................................................................................................................................................ 5
5. Warnings .......................................................................................................................................................................... 5
6. Precautions ...................................................................................................................................................................... 5
6.1. General Precautions ............................................................................................................................................... 5
6.2. Pre-procedure and Post-procedure Medication Regimen ...................................................................................... 6
6.3. Use of Multiple Balloons ......................................................................................................................................... 6
6.4. Use in Conjunction with Other Procedures ............................................................................................................. 6
6.5. Drug Interaction ...................................................................................................................................................... 7
6.6. Balloon Handling and Preparation Precautions ...................................................................................................... 7
6.7. Balloon Placement Precautions .............................................................................................................................. 7
6.8. Balloon Catheter Removal Precautions .................................................................................................................. 7
6.9. Post-procedure Precautions ................................................................................................................................... 7
7. Use in Special Populations ............................................................................................................................................. 7
7.1. Pregnancy and Lactation ........................................................................................................................................ 8
7.2. Gender .................................................................................................................................................................... 8
7.3. Ethnicity .................................................................................................................................................................. 8
7.4. Pediatric Use ........................................................................................................................................................... 8
7.5. Geriatric Use ........................................................................................................................................................... 8
8. Drug Information .............................................................................................................................................................. 8
8.1. Mechanism of Action .............................................................................................................................................. 8
8.2. Pharmacokinetics ................................................................................................................................................... 8
8.3. Metabolism .............................................................................................................................................................. 8
8.4. Carcinogenicity, Genotoxicity, and Reproductive Toxicity ....................................................................................... 8
9. Potential Adverse Effects ............................................................................................................................................... 8
10. Patient Counseling Information ..................................................................................................................................... 9
11. Summary of Clinical Studies ........................................................................................................................................ 10
11.1. Late Mortality Signal for Paclitaxel-Coated Devices ............................................................................................. 10
11.2. IN.PACT SFA Trial ................................................................................................................................................ 10
11.3. IN.PACT SFA Trial Post-Approval Study .............................................................................................................. 21
11.4. Pharmacokinetic Sub-study .................................................................................................................................. 37
11.5. IN.PACT Global Study .......................................................................................................................................... 38
11.6. Summary of Rare Adverse Events ....................................................................................................................... 38
11.7. IN.PACT Admiral DCB ISR Clinical Evaluation ..................................................................................................... 38
11.8. IN.PACT Global DCB Long Lesion Sub-Cohort .................................................................................................... 46
12. How Supplied ................................................................................................................................................................. 63
13. Instructions for Use ....................................................................................................................................................... 63
13.1. Equipment ............................................................................................................................................................. 63
13.2. Balloon Catheter Size Selection ........................................................................................................................... 64
13.3. Recommendations for Optimal Treatment ............................................................................................................ 64
13.4. PTA Preparation .................................................................................................................................................... 64
13.5. IN.PACT 018 DCB Preparation ............................................................................................................................. 64
13.6. Inflation Device Connection to the IN.PACT 018 DCB .......................................................................................... 65
13.7. Delivery and Dilatation Procedure ........................................................................................................................ 65
13.8. Removal Procedure .............................................................................................................................................. 66
13.9. Using Multiple IN.PACT 018 DCBs ....................................................................................................................... 66
14. DISCLAIMER OF WARRANTY ....................................................................................................................................... 68
1. Product Name
IN.PACT™ 018 paclitaxel-coated percutaneous transluminal angioplasty (PTA) balloon catheter
2 Instructions for Use English
Page 5

2. Product Description
The IN.PACT 018 paclitaxel-coated PTA balloon catheter is an over-the-wire (OTW) balloon catheter with a drug-coated balloon
at the distal tip. The drug component, referred to as the FreePac™ drug coating, consists of the drug paclitaxel and the
excipient urea. The device component physically dilates the vessel lumen by PTA, and the drug is intended to reduce the
proliferative response that is associated with restenosis. Product Component Description (Table 1) summarizes the
characteristics of the device, hereafter referred to as IN.PACT 018 DCB.
Table 1. Product Component Description
Balloon Diam-
eter (mm)
Available Balloon
Diameters (mm)
and Lengths
(mm)
Balloon Coating
(Drug Component)
Catheter Design Over-the-Wire (OTW)
Usable Catheter
Lengths
Balloon Inflation
Pressure
Minimum Introducer Sheath
Compatibility
Guidewire Compatibility
4.0 x x x x x x 3 folds
5.0 x x x x x x
7.0 x x x --- --- ---
Note: “---” indicates size not offered; “x” indicates sizes offered
Paclitaxel (Active Pharmaceutical Ingredient) and Urea (excipient)
80 cm, 130 cm, and 200 cm
Nominal Pressure: 8 atm (811 kPa)
Rated Burst Pressure : 10 atm (1013 kPa)
Balloon Diam-
eter (mm)
4.0 mm
6.0 mm
7.0 mm 2.00 6
The catheter is compatible with a guidewire diameter of 0.018 in (0.46 mm).
Max Crossing Profile
Balloon Length (mm)
40 60 80 100 120 150 Balloon Wrap
Configuration
6 folds6.0 x x x x x x
Introducer Sheath (Fr)
(mm)
1.86 55.0 mm
2.1. Device Component Description
The OTW balloon catheter consists of a proximal hub, a coaxial dual-lumen shaft, and a distal dilatation balloon. The central
lumen extends to the distal tip and is used to pass the catheter over a guidewire with a diameter of 0.018 in (0.46 mm). The
balloon-inflation lumen is used to inflate and deflate the balloon with a mixture of contrast medium and saline solution. Two
radiopaque platinum-iridium markers indicate the working length of the balloon to position the balloon across the target lesion
during fluoroscopy. See IN.PACT 018 Paclitaxel-coated PTA Balloon Catheter (Figure 1).
Figure 1. IN.PACT 018 Paclitaxel-coated PTA Balloon Catheter
1. Guidewire Port
2. Hub
3. Inflation Port
4. Strain Relief
5. Shaft
6. Usable Catheter Length
Instructions for Use English 3
Page 6

7. Radiopaque Marker
1
2
12
11
10
9
8
7
6
5
4
3
13
14
20
19
18
17
16
15
3’
2’
1’
8. Balloon
2.2. Drug Component Description
The FreePac™ drug coating on the balloon of the IN.PACT 018 DCB consists of the drug paclitaxel and the excipient urea. The
balloon surface has a nominal paclitaxel dose density of 3.5 μg/mm2.
2.2.1. Paclitaxel
The active pharmaceutical ingredient in the IN.PACT 018 DCB is paclitaxel. The principal mechanism by which paclitaxel
inhibits neointimal growth is through the stabilization of microtubules by preventing their depolymerization during the final G2/M
phase of cell division.
The CAS Registry number of paclitaxel is 33069-62-4. The chemical name of paclitaxel is:
Benzenepropanoic acid, ß-(benzoylamino)-α-hydroxy-,6,12b-bis(acetyloxy)-12-(benzoyloxy) -2a,3,4,4a,5,6,9,10,11,12,12a,12b-
dodecahydro-4,11-dhydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]-oxet-9-ylester,[2aR[2aα,4β,4aβ,6β,9α(αR, βS),11α,12α,12aα,12bα]]
See Chemical Structure of Paclitaxel (Figure 2) below.
Figure 2. Chemical Structure of Paclitaxel
Paclitaxel is a diterpenoid with a characteristic taxane skeleton of 20 carbon atoms, a molecular weight of 853.91 g/mol, and a
molecular formula of C47H51NO14. It is a white powder, has extremely low water solubility, is highly lipophilic, and is freely soluble
in methanol, ethanol, chloroform, ethyl acetate, and dimethyl sulfoxide.
2.2.2. Urea
The coating utilizes the inactive ingredient urea as an excipient to facilitate the release and transfer of paclitaxel into the arterial
wall. See Chemical Structure of Urea (Figure 3) below.
Figure 3. Chemical Structure of Urea
2.2.3. Product Matrix and Paclitaxel Content
Table 2. Product Matrix and Paclitaxel Content
Model number
(80 cm usable
catheter length)
IPU04004008P IPU04004013P IPU04004020P 4.0 40 1995
IPU04006008P IPU04006013P IPU04006020P 4.0 60 2875
IPU04008008P IPU04008013P IPU04008020P 4.0 80 3754
IPU04010008P IPU04010013P IPU04010020P 4.0 100 4634
IPU04012008P IPU04012013P IPU04012020P 4.0 120 5513
IPU04015008P IPU04015013P IPU04015020P 4.0 150 6833
Model number
(130 cm usable
catheter length)
Model number
(200 cm usable
Nominal balloon
diameter (mm)
Nominal balloon
Length (mm)
Nominal paclitaxel
content (μg)
catheter length)
4 Instructions for Use English
Page 7

Model number
(80 cm usable
catheter length)
IPU05004008P IPU05004013P IPU05004020P 5.0 40 2575
IPU05006008P IPU05006013P IPU05006020P 5.0 60 3674
IPU05008008P IPU05008013P IPU05008020P 5.0 80 4774
IPU05010008P IPU05010013P IPU05010020P 5.0 100 5873
IPU05012008P IPU05012013P IPU05012020P 5.0 120 6973
IPU05015008P IPU05015013P IPU05015020P 5.0 150 8622
IPU06004008P IPU06004013P IPU06004020P 6.0 40 3188
IPU06006008P IPU06006013P IPU06006020P 6.0 60 4508
IPU06008008P IPU06008013P IPU06008020P 6.0 80 5827
IPU06010008P IPU06010013P IPU06010020P 6.0 100 7147
IPU06012008P IPU06012013P IPU06012020P 6.0 120 8466
IPU06015008P IPU06015013P IPU06015020P 6.0 150 10445
IPU07004008P IPU07004013P IPU07004020P 7.0 40 3837
IPU07006008P IPU07006013P IPU07006020P 7.0 60 5376
IPU07008008P IPU07008013P IPU07008020P 7.0 80 6916
Model number
(130 cm usable
catheter length)
Model number
(200 cm usable
catheter length)
Nominal balloon
diameter (mm)
Nominal balloon
Length (mm)
Nominal paclitaxel
content (μg)
3. Indications for Use
The IN.PACT 018 paclitaxel-coated PTA balloon catheter is indicated for percutaneous transluminal angioplasty, after
appropriate vessel preparation, of de novo, restenotic, or in-stent restenotic lesions with lengths up to 360 mm in superficial
femoral or popliteal arteries with reference vessel diameters of 4-7 mm.
4. Contraindications
The IN.PACT 018 DCB is contraindicated for use in:
■
coronary arteries, renal arteries, and supra-aortic/cerebrovascular arteries
■
patients who cannot receive recommended antiplatelet and/or anticoagulant therapy
■
patients judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the
delivery system
■
patients with known allergies or sensitivities to paclitaxel
■
women who are breastfeeding, pregnant, or are intending to become pregnant, or men intending to father children. It is
unknown whether paclitaxel will be excreted in human milk and whether there is a potential for adverse reaction in nursing
infants from paclitaxel exposure.
5. Warnings
■
A signal for increased risk of late mortality has been identified following the use of paclitaxel-coated balloons and
paclitaxel-eluting stents for femoropopliteal arterial disease beginning approximately 2-3 years post-treatment
compared with the use of non-drug coated devices. There is uncertainty regarding the magnitude and mechanism
for the increased late mortality risk, including the impact of repeat paclitaxel-coated device exposure. Physicians
should discuss this late mortality signal and the benefits and risks of available treatment options with their
patients. See Section 11 for further information.
■
Use the product prior to the Use-by Date specified on the package.
■
Contents are supplied sterile. Do not use the product if the inner packaging is damaged or opened.
■
Do not use air or any gaseous medium to inflate the balloon. Use only the recommended inflation medium (equal parts
contrast medium and saline solution).
■
Do not move the guidewire during inflation of the IN.PACT 018 DCB.
■
Do not exceed the rated burst pressure (RBP). The RBP is 10 atm (1013 kPa). The RBP is based on the results of in vitro
testing. Use of pressures higher than RBP may result in a ruptured balloon with possible intimal damage and dissection.
■
The safety and effectiveness of using multiple IN.PACT 018 DCBs with a total drug dosage exceeding 34,854 μg of
paclitaxel in a patient has not been clinically evaluated.
6. Precautions
6.1. General Precautions
■
This product should only be used by physicians trained in percutaneous transluminal angioplasty (PTA).
■
Assess risks and benefits before treating patients with a history of severe reaction to contrast agents.
Instructions for Use English 5
Page 8
■
Administer appropriate drug therapy to the patient according to standard protocols for PTA before insertion of the dilatation
catheter.
■
Take precautions to prevent or reduce clotting when any catheter is used. Flush and rinse all products entering the vascular
system with heparinized normal saline or a similar solution. For the IN.PACT 018 DCB catheter, flush the guidewire lumen
through the guidewire port with heparinized normal saline until the fluid exits the distal tip. Do not rinse or wipe the
IN.PACT 018 DCB catheter.
■
Identify allergic reactions to contrast media and antiplatelet therapy before treatment and consider alternatives for
appropriate management prior to the procedure.
■
Prior to the procedure, inspect the product to verify that the product is intact.
■
Handle the product with caution to avoid any damage to the balloon coating or folded balloon.
■
This product is not intended for the expansion or delivery of a stent.
■
Do not use the IN.PACT 018 DCB for pre-dilatation or for post-dilatation.
■
This product is designed for single patient use only. Do not reuse, reprocess, or resterilize this product. Reuse,
reprocessing, or resterilization may compromise the structural integrity of the device and/or create a risk of contamination of
the device, which could result in patient injury, illness, or death.
■
Do not expose the product to organic solvents such as alcohol.
■
To reduce the potential for vessel damage, the inflated diameter of the balloon should approximately match the diameter of
the vessel just distal to the lesion.
■
The use of this product carries the risks associated with percutaneous transluminal angioplasty, including thrombosis,
vascular complications, and/or bleeding events.
6.2. Pre-procedure and Post-procedure Medication Regimen
It is recommended that dual antiplatelet therapy (aspirin with clopidogrel; use ticlopidine as an alternate to clopidogrel in case of
allergy) is administered before the procedure and for a minimum of one month after the intervention, and that aspirin is
continued for a minimum of six months after the procedure. Prolonged antiplatelet therapy can be given at the discretion of the
physician. See Recommended Pre-procedure and Post-procedure Medication Regimen (Table 3).
Table 3. Recommended Pre-procedure and Post-procedure Medication Regimen
Medication Pre-procedure During Procedure Post-procedure
Antiplatelet Aspirin (ASA) 300-325 mg loading
dose within 24 hours
prior to procedure
Antiplateletc,
d
Clopidogrel 75-300 mg within
24 hours prior to proce-
dure or 2 hours post-
e
Ticlopidine
procedure
f
500 mg/day for at least
3 consecutive days prior
NA
b
81-325 mg/day
(6 months minimum)
NA
75 mg/day
(1 month minimum)
NA 500 mg/day
(1 month minimum)
a
to procedure (last dose
within 24 hours of pro-
cedure)
Anticoagulation IV Heparin (or other
Dosing as per institutional standard
g
thrombin inhibitor)
a
For cases of provisional stenting, refer to the published patient management guidelines for dosing instruction.
b
ASA loading dose not required for subjects already on a chronic regimen, defined as at least 81 mg daily for at least 5 consecutive days pre-procedure, with the last dose given/taken within 24 hours prior to
procedure.
c
The safety and efficacy of this dose has not been prospectively studied. Please refer to current package inserts.
d
Subjects on a prasugrel or ticagrelor regimen for acute coronary syndrome (ACS) may continue that regimen as antiplatelet therapy. Please refer to the current package insert for information about risks and
benefits of these medications, as well as for information on concomitant use of ASA and other medications.
e
Clopidogrel loading dose not required for subjects who have been taking 75 mg/day for at least 3 consecutive days before the intervention, with the last dose being taken within 24 hours prior to procedure.
f
Recommended in subjects with allergies to clopidogrel.
g
It is recommended that a bolus of 3000 to 5000 units of heparin be given prior to the angioplasty procedure, and that anticoagulation is given as needed to maintain an activated clotting time (ACT) of
≥250 seconds, or ≥200 seconds where GP IIb/IIIa inhibitors are concomitantly administered.
6.3. Use of Multiple Balloons
The extent of the patient’s exposure to the drug coating is directly related to the number of balloons used. Refer to Using
Multiple IN.PACT 018 DCBs (Section 13.9) and Product Matrix and Paclitaxel Content (Table 2) for details regarding the use of
multiple balloons and a product matrix containing the nominal paclitaxel content for each device size, respectively.
6.4. Use in Conjunction with Other Procedures
The safety and effectiveness of the IN.PACT 018 DCB used in conjunction with other drug-eluting stents or drug-coated
balloons in the same procedure or following treatment failure has not been evaluated.
6 Instructions for Use English
Page 9

6.5. Drug Interaction
Formal drug interaction studies have not been conducted with the IN.PACT 018 DCB. In the clinical pharmacokinetic (PK) substudy, systemic levels of paclitaxel following treatment with DCB(s) were low and cleared rapidly, reducing possible impact of
drug-drug interactions due to concomitant medications (see Section 11.4). Consideration for both systemic and local drug
interactions should be given when deciding to use IN.PACT 018 DCB(s) in a patient who is taking a drug with known
interactions to paclitaxel or when deciding to initiate therapy with such a drug in a patient who has recently been treated with
DCB(s). Please refer to Drug Information (Section 8).
6.6. Balloon Handling and Preparation Precautions
■
Do not remove the device from the pouch until it is needed for immediate use.
■
Handle the device with caution to avoid any damage to the balloon coating or folded balloon.
■
Keep the peel-away balloon protector in place when purging the balloon catheter of air bubbles.
■
Do not use the peel-away balloon protector as an introduction aid or a rewrapping tool.
■
Do not apply positive pressure to the balloon during preparation.
6.7. Balloon Placement Precautions
■
Manipulate the catheter under fluoroscopic observation when it is exposed to the vascular system. Do not advance or
retract the catheter unless the balloon is fully deflated under vacuum.
■
Do not move the guidewire during inflation of the balloon.
■
Do not manipulate the IN.PACT 018 DCB while inflated.
■
Catheter applications vary. Select the technique on the basis of the patient’s condition and the experience of the
interventionalist.
■
Introducer sheaths used must have lumen sizes that are suitable to accommodate the IN.PACT 018 DCB. See Product
Component Description (Table 1) for the introducer sheath compatibility and crossing profile of each device size.
■
If resistance occurs during manipulation, ascertain the cause via fluoroscopy, road mapping, or digital subtraction
angiography (DSA) before moving the IN.PACT 018 DCB backward or forward.
■
Do not manipulate the IN.PACT 018 DCB without sufficient fluoroscopy.
■
Use a pressure-monitoring device to prevent overpressurization. Refer to Product Component Description (Table 1).
■
To ensure full coverage of the entire lesion, the balloon diameter must match the reference vessel diameter distal to the
lesion and the balloon length must exceed the lesion length by approximately 1 cm on both ends. When using multiple
balloons, do so only as described in Using Multiple IN.PACT 018 DCBs (Section 13.9).
■
Never advance the IN.PACT 018 DCB without the guidewire extending from the tip.
■
Maintaining balloon inflation is strongly recommended for 180 seconds. Adequate drug transfer occurs in the first
60 seconds of inflation.
■
Appropriate vessel preparation is required prior to use of the IN.PACT 018 DCB.
Note: Vessel preparation using only pre-dilatation was studied in the clinical study (see Section 11). Other methods of vessel
preparation, such as atherectomy, have not been studied clinically with IN.PACT 018 DCB.
6.8. Balloon Catheter Removal Precautions
■
Prior to withdrawing the balloon catheter from the lesion, completely deflate the balloon under vacuum.
■
Center the IN.PACT 018 DCB relative to the introducer sheath when withdrawing, and use caution when removing the
IN.PACT 018 DCB.
■
Should unusual resistance be felt at any time when withdrawing the balloon catheter back into the introducer sheath,
remove the balloon catheter and the introducer sheath as a single unit to reduce the risk of vascular damage. This must be
done under direct visualization with fluoroscopy.
■
If removal of the IN.PACT 018 DCB is required prior to deployment and a repeat attempt is desired, use a new IN.PACT
018 DCB.
6.9. Post-procedure Precautions
■
Administer post-procedure antiplatelet therapy as described in Pre-procedure and Post-procedure Medication Regimen
(Section 6.2).
7. Use in Special Populations
IN.PACT 018 DCB has not been evaluated for use in special populations. The safety and effectiveness of drug-coated balloon
treatment using paclitaxel has been established based on clinical studies discussed in Section 11.
Instructions for Use English 7
Page 10

7.1. Pregnancy and Lactation
The IN.PACT 018 DCB is contraindicated in women who are pregnant or breast-feeding. It is unknown whether paclitaxel will
be excreted in human milk or whether there is a potential for adverse reaction from paclitaxel exposure in nursing infants.
Pregnancy Category C: See Carcinogenicity, Genotoxicity, and Reproductive Toxicity (Section 8.4).
7.2. Gender
Gender was a predefined subgroup that was analyzed in the pivotal clinical study. The outcomes are shown in Primary Safety
Composite and Primary Effectiveness by Gender (Section 11.2.7, Table 9). The results of an interaction analysis indicate that
the treatment differences between DCB and PTA groups in the pivotal clinical study are consistent between male and female
subjects.
7.3. Ethnicity
Clinical studies (refer to Section 11) did not include a sufficient number of patients to assess for differences in safety or
effectiveness due to ethnicity, regardless of assessment by individual ethnicity categories or assessment by Caucasian or nonCaucasian categories.
7.4. Pediatric Use
The safety and effectiveness of the IN.PACT 018 DCB in pediatric patients has not been established.
7.5. Geriatric Use
The pivotal clinical study (refer to Section 11.2) had an upper age limit of 85 years, and had a predefined study subgroup of
subjects 75 years or older (85 subjects). Within this subgroup, the DCB group showed improvement on the primary safety and
effectiveness endpoints.
8. Drug Information
8.1. Mechanism of Action
The mechanism(s) by which the IN.PACT 018 DCB affects neointimal production has not been fully established. The principal
mechanism by which paclitaxel inhibits neointimal growth is through the stabilization of microtubules by preventing their
depolymerization during the final G2/M phase of cell division. Consequently, the microtubule network may not maintain the
dynamic rearrangement required for a normal mitotic process.
8.2. Pharmacokinetics
The pharmacokinetic profile of paclitaxel following treatment with the paclitaxel DCB was evaluated in 25 patients receiving
2,850 μg to 16,900 μg of paclitaxel. This evaluation was conducted as a sub-study of the randomized clinical trial and is
described in Summary of Clinical Studies (Section 11). Paclitaxel systemic exposure in the treated subjects was low and
cleared rapidly with a bi-phasic decline. The C
11.4 to 128.8 hr*ng/mL. These data indicate that treatment with the IN.PACT 018 DCB provides low systemic exposure of
paclitaxel.
8.3. Metabolism
Metabolic transformation of paclitaxel occurs predominantly in the liver through cytochromes P450 2C8 (CYP2C8) and 3A4
(CYP3A4). Agents which could compete with or inhibit the activity of the CYP2C8 and CYP3A4 isoenzymes may increase
paclitaxel plasma levels. For more information on potential drug interactions, see Drug Interaction (Section 6.5).
ranged from 1.0 to 35.9 ng/mL and the AUC
max
ranged from
0-∞
8.4. Carcinogenicity, Genotoxicity, and Reproductive Toxicity
No long-term studies in animals have been published in peer-reviewed literature to evaluate the carcinogenic potential of
paclitaxel. Paclitaxel was not mutagenic in the Ames test or the CHO/HGPRT gene mutation assay. However, the mechanism
by which paclitaxel interferes with cellular proliferation may give rise to loss of chromosomes during cell division as a result of
microtubule stabilization during cell division. Paclitaxel is an established aneugenic drug in vitro on human normal cells and will
also produce a positive response in the mouse bone marrow micronucleus assay. It has not been established that paclitaxel
exerts any direct action on DNA to induce strand fragmentation.
Reproductive toxicity has been previously evaluated in vivo in both rabbits and rats. When administered during rabbit fetal
organogenesis, paclitaxel doses of 3.0 mg/kg/day caused embryo- and fetotoxicity; maternal toxicity was also observed. No
teratogenic effects were observed at 1.0 mg/kg/day; effects at higher doses could not be assessed due to fetal mortality. In rats,
fertility impairment was observed at doses ≥ 1 mg/kg/day. For comparison, the average dose of paclitaxel in the IN.PACT SFA
PK Sub-study (Section 11.4) was 7454 μg, with an average subject weight of 91 kg, for a theoretical normalized dose of
0.082 mg/kg (assuming all the paclitaxel from the coating enters the systemic circulation).
9. Potential Adverse Effects
Below is a list of the potential adverse effects (eg., complications) associated with the use of the device:
■
Abrupt vessel closure
8 Instructions for Use English
Page 11

■
Access site pain
■
Allergic reaction to contrast medium, antiplatelet therapy, or catheter system components (materials, drugs, and excipients)
■
Amputation/loss of limb
■
Arrhythmias
■
Arterial aneurysm
■
Arterial thrombosis
■
Arteriovenous (AV) fistula
■
Death
■
Dissection
■
Embolization
■
Fever
■
Hematoma
■
Hemorrhage
■
Hypotension/hypertension
■
Inflammation
■
Ischemia or infarction of tissue/organ
■
Local infection at access site
■
Local or distal embolic events
■
Perforation or rupture of the artery
■
Pseudoaneurysm
■
Renal insufficiency or failure
■
Restenosis of the dilated artery
■
Sepsis or systemic infection
■
Shock
■
Stroke
■
Systemic embolization
■
Vessel spasms or recoil
■
Vessel trauma which requires surgical repair
Potential complications of peripheral balloon catheterization include, but are not limited to:
■
Balloon rupture
■
Detachment of a component of the balloon and/or catheter system
■
Failure of the balloon to perform as intended
■
Failure to cross the lesion
These complications may result in adverse effects.
Although systemic effects are not anticipated, potential adverse effects not captured above that may be unique to the paclitaxel
drug coating include, but are not limited to:
■
Allergic/immunologic reaction
■
Alopecia
■
Anemia
■
Gastrointestinal symptoms
■
Hematologic dyscrasia (including leucopenia, neutropenia, thrombocytopenia)
■
Hepatic enzyme changes
■
Histologic changes in vessel wall, including inflammation, cellular damage, or necrosis
■
Myalgia/arthralgia
■
Myelosuppression
■
Peripheral neuropathy
Refer to the Physician’s Desk Reference for more information on the potential adverse effects observed with paclitaxel. There
may be other potential adverse effects that are unforeseen at this time.
10. Patient Counseling Information
Physicians should consider the following when counseling patients about this product:
■
Discuss the risks associated with percutaneous transluminal angioplasty procedures.
■
Discuss the risks associated with the IN.PACT 018 DCB.
Instructions for Use English 9
Page 12

■
Discuss the risks and benefits of the treatment specific to the patient.
■
Discuss short- and long-term post-procedure changes to the patient's lifestyle.
■
Discuss the risks of early discontinuation of the antiplatelet therapy.
11. Summary of Clinical Studies
The safety and effectiveness of the IN.PACT Admiral DCB (.035 in guidewire compatible), as established in the clinical studies
described below that were performed primarily via femoral access, can be considered supportive for the IN.PACT 018 DCB.
The IN.PACT 018 DCB has not been evaluated in a clinical study.
11.1. Late Mortality Signal for Paclitaxel-Coated Devices
A meta-analysis of randomized controlled trials published in December 2018 by Katsanos et. al. identified an increased risk of
late mortality at 2 years and beyond for paclitaxel-coated balloons and paclitaxel-eluting stents used to treat femoropopliteal
arterial disease. In response to these data, FDA performed a patient-level meta-analysis of long-term follow-up data from the
pivotal premarket randomized trials of paclitaxel-coated devices used to treat femoropopliteal disease using available clinical
data through May 2019. The meta-analysis also showed a late mortality signal in study subjects treated with paclitaxel-coated
devices compared to patients treated with uncoated devices. Specifically, in the 3 randomized trials with a total of 1090 patients
and available 5-year data, the crude mortality rate was 19.8% (range 15.9% - 23.4%) in patients treated with paclitaxel-coated
devices compared to 12.7% (range 11.2% - 14.0%) in subjects treated with uncoated devices. The relative risk for increased
mortality at 5 years was 1.57 (95% confidence interval 1.16 - 2.13), which corresponds to a 57% relative increase in mortality in
patients treated with paclitaxel-coated devices. As presented at the June 2019 FDA Advisory Committee Meeting, an
independent meta-analysis of similar patient-level data provided by VIVA Physicians, a vascular medicine organization,
reported similar findings with a hazard ratio of 1.38 (95% confidence interval 1.06 - 1.80). Additional analyses have been
conducted and are underway that are specifically designed to assess the relationship of mortality to paclitaxel-coated devices.
The presence and magnitude of the late mortality risk should be interpreted with caution because of multiple limitations in the
available data, including wide confidence intervals due to a small sample size, pooling of studies of different paclitaxel-coated
devices that were not intended to be combined, substantial amounts of missing study data, no clear evidence of a paclitaxel
dose effect on mortality, and no identified pathophysiologic mechanism for the late deaths.
Paclitaxel-coated balloons and stents improve blood flow to the legs and decrease the likelihood of repeat procedures to
reopen blocked blood vessels compared to uncoated devices. The benefits of paclitaxel-coated devices (e.g., reduced
reinterventions) should be considered in individual patients along with potential risks (e.g., late mortality).
In the IN.PACT SFA IDE Trial, based on the analysis completed for the June 2019 FDA Advisory Committee Meeting using AsTreated cohort and vital status update, the Kaplan Meier cumulative mortality estimates at 2, 3 and 5 years are 7.3% [3.8%,
10.8%], 10.5% [6.4%, 14.6%], 15.7% [10.8%, 20.6%], respectively, for the IN.PACT Admiral DCB treatment device and 0.9%
[0%, 2.7%], 2.8% [0%, 5.9%], 11.2% [5.3%, 17.1%], respectively, for the PTA control device. Additional information regarding
long-term outcomes can be found in Section 11.
11.2. IN.PACT SFA Trial
11.2.1. Primary Objective
The objective of the IN.PACT SFA Trial was to evaluate the safety and effectiveness of the IN.PACT Admiral DCB as compared
with PTA when used to treat atherosclerotic lesions of the superficial femoral artery (SFA) and/or proximal popliteal artery
(PPA).
11.2.2. Study Design
The IN.PACT SFA Trial was designed as a two-phase, multicenter, single-blind, randomized trial. Subjects in the IN.PACT SFA I
phase were enrolled in Austria, Belgium, Germany, Italy, and Switzerland under ISO 14155:2003, Declaration of Helsinki, and
ICH GCP. The second phase, IN.PACT SFA II, was conducted in the United States under an investigational device exemption
(IDE). Subjects were randomized 2:1 to treatment with the IN.PACT Admiral DCB as compared to PTA. Provisional stenting
was used in cases of PTA failure. Follow-up was completed at 30 days, 6 months, 12 months, 24 months, 36 months,
48 months, and 60 months post-index procedure.
The data from the IN.PACT SFA Trial, with greater than 50% subjects coming from the U.S. population (150 subjects Europe
and 181 subjects U.S.), have been pooled and comprise the pivotal trial data. This aggregate data provides statistical power for
the 12-month primary safety and effectiveness endpoints.
The primary endpoints for the IN.PACT SFA Trial are listed below.
■
Primary Safety Composite Endpoint:
■
Freedom from device- and procedure-related death through 30 days post-index procedure and freedom from target limb
major amputation and clinically-driven target vessel revascularization (TVR)1 within 12 months post-index procedure
1
Clinically-driven TVR is defined as any re-intervention within the target vessel due to symptoms or drop of ABI/TBI of ≥ 20% or > 0.15 when compared to post-procedure baseline ABI/TBI
10 Instructions for Use English
Page 13

For the primary safety endpoint, the treatment (πT) and control (πC) groups were compared in a non-inferiority format under the
following hypothesis.
H0: πT ≤ πC - 0.1
HA: πT > πC - 0.1
■
Primary Effectiveness Endpoint:
■
Primary patency within 12 months post-index procedure, defined as freedom from clinically-driven target lesion
revascularization (TLR)2 and freedom from restenosis as determined by duplex ultrasound (DUS)3 peak systolic velocity
ratio (PSVR) ≤ 2.4
4
For the primary effectiveness endpoint, the treatment (pT) and control (pC) groups were compared in a superiority format under
the following hypothesis.
H0: pT = p
HA: pT > p
C
C
The sample size was estimated using the two-group chi-square test for the primary effectiveness endpoint, and it was driven by
the assumptions of a one-sided 0.024995 alpha and at least 80% desired power to show superiority of IN.PACT Admiral DCB
to PTA.
The secondary endpoints for the IN.PACT SFA Trial are listed below.
■
Major Adverse Events (MAE) through 60 months. MAE are defined as all-cause death, clinically-driven TVR, major target
limb amputation, and thrombosis at the target lesion site
■
Death of any cause within 30 days, 6, 12, 24, 36, 48 and 60 months
■
TVR within 6, 12, 24, 36, 48 and 60 months
■
TLR within 6, 12, 24, 36, 48 and 60 months
■
Time to first clinically-driven target lesion revascularization (TLR) through 60 months post-index procedure
■
Major target limb amputation within 6, 12, 24, 36, 48 and 60 months
■
Thrombosis at the target lesion site within 6, 12, 24, 36, 48 and 60 months
■
Primary sustained clinical improvement at 6, 12, 24, 36 months post-procedure
■
Secondary sustained clinical improvement at 6, 12, 24, 36 months post-procedure
■
Duplex-defined binary restenosis (PSVR > 2.4) of the target lesion at 6, 12, 24 and 36 months or at the time of the re-
intervention prior to any pre-specified timepoint
■
Duplex-defined binary restenosis (PSVR > 3.4) of the target lesion at 6, 12, 24 and 36 months or at the time of the re-
intervention prior to any pre-specified timepoint
■
Quality of life assessment by EQ-5D questionnaire at 6, 12, 24, and 36 months as change from baseline
■
Walking distance as assessed by 6 Minute Walk Test at 30 days and at 6, 12, 24, and 36 months as change from baseline
(IN.PACT SFA II phase only)
■
Walking capacity assessment by walking impairment questionnaire (WIQ) at 30 days and at 6, 12, 24, and 36 months
■
Device success defined as successful delivery, balloon inflation and deflation and retrieval of the intact study device without
burst below the rated burst pressure (RBP)
■
Procedural success defined as residual stenosis of ≤ 50% (non-stented subjects) or ≤ 30% (stented subjects) by core
laboratory (if core laboratory was not available then the site-reported estimate was used)
■
Clinical success defined as procedural success without procedural complications (death, major target limb amputation,
thrombosis of the target lesion, or TVR) prior to discharge
■
Days of hospitalization due to the index lesion from procedure through 6, 12, 24, and 36 months
As the four primary endpoint tests passed (and in a superiority manner), each at a critical level of 0.024995, several pre-defined
secondary endpoints were compared on all ITT non-stented subjects between treatment groups sequentially. These secondary
endpoints were analyzed in the following order: (1) CD-TLR at 12 months, (2) primary sustained clinical improvement at
12 months, (3) walking distance at 12 months as assessed by the 6-minute walk test, and (4) duplex-defined binary restenosis
(PSVR >2.4) at 24 months or at the time of reintervention. This sequential approach keeps the family-wise error rate at the
0.024995 level across the set of four secondary endpoints.
The statistical analysis plan included planned primary analysis of all non-stented patients, as well as a secondary analysis of
the intent to treat (ITT) population. The demographics and results provided are for the ITT population, which demonstrated
similar results as the all non-stented patient population.
2
Clinically-driven TLR is defined as any re-intervention at the target lesion due to symptoms or drop of ABI/TBI of ≥ 20% or > 0.15 when compared to post-procedure baseline ABI/TBI
3
Post-index procedure DUS (intended to establish a post-treatment baseline) does not contribute to the primary endpoint determination
4
Restenosis determined by either PSVR > 2.4 as assessed by an independent DUS core laboratory or > 50% stenosis as assessed by an independent angiographic core laboratory
Instructions for Use English 11
Page 14

11.2.3. Patient Population
Subject demographics, medical history, and risk factors of the 331 subjects are summarized in Baseline Demographics and
Medical History (Table 4), which shows similarity between subjects enrolled in both the IN.PACT Admiral DCB and PTA groups.
Table 4. Baseline Demographics and Medical History
IN.PACT DCB PTA p-value
(N=220 Subjects) (N=111 Subjects)
Age (yr) 67.5 ± 9.5 68.0 ± 9.2 0.612
Male 65.0% (143/220) 67.6% (75/111) 0.713
a
Race
White 78.3% (94/120) 83.3% (50/60)
Black 14.2%(17/120) 11.7% (7/60)
Asian 5.8% (7/120) 3.3% (2/60)
Native Hawaiian or Other Pacific Islander 1.7% (2/120) 0.0% (0/60)
0.435
American Indian or Alaska Native 0.0% (0/120) 0.0% (0/60)
Other 0.0% (0/120) 1.7% (1/60)
Obesity (BMI ≥ 30 kg/m2)
27.7% (61/220) 25.2% (28/111) 0.694
Diabetes Mellitus 40.5% (89/220) 48.6% (54/111) 0.161
Hypertension 91.4% (201/220) 88.3% (98/111) 0.431
Hyperlipidemia 84.5% (186/220) 82.0% (91/111) 0.637
Current Smoker 38.6% (85/220) 36.0% (40/111) 0.719
Coronary Heart Disease 57.0% (122/214) 55.0% (60/109) 0.813
Carotid Artery Disease 34.9% (73/209) 31.7% (32/101) 0.610
Renal Insufficiency (baseline serum creatinine
8.3% (18/217) 6.4% (7/109) 0.662
≥ 1.5 mg/dL)
Below-the-knee Vascular Disease of Target Leg (Stenotic/
40.9% (90/220) 53.2% (59/111) 0.036
Occluded)
ABI / TBIb (mmHg ratio) 0.769 ± 0.228 (209) 0.744 ± 0.189 (106) 0.308
Rutherford Category
2 37.7% (83/220) 37.8% (42/111)
3 57.3% (126/220) 55.9% (62/111)
4 5.0% (11/220) 5.4% (6/111)
0.898
5 0.0% (0/220) 0.9% (1/111)
Numbers are % (counts/sample size) unless otherwise stated.
Site reported data.
a
Race and ethnicity data was not collected in IN.PACT SFA I phase (Europe).
b
TBI was not measured in IN.PACT SFA I phase.
The baseline lesion characteristics, as reported by the sites and angiographic core laboratories, have been provided in Lesion
Characteristics (Table 5). The total target lesion length treated was similar between treatment groups (IN.PACT Admiral DCB
8.94 cm, PTA 8.81 cm; p=0.815). Occluded lesions comprised 25.8% of IN.PACT Admiral DCB subject lesions and 19.5% of
PTA subject lesions (p=0.222). Pre-dilatation using a PTA catheter was performed as part of the clinical study to prepare the
vessel and occurred in 96.4% (212/220) of IN.PACT Admiral DCB subjects.
Table 5. Lesion Characteristics
IN.PACT DCB PTA p-value
Baseline Lesion Characteristics
a
(N=220 Subjects) (N=111 Subjects)
Lesion Type
De novo 95.0% (209/220) 94.6% (105/111)
Restenotic (non-stented) 5.0% (11/220) 5.4% (6/111)
Lesion Location b,
c
(N=221 Lesions) (N=113 Lesions)
0.875
Superficial Femoral Artery 97.7% (216/221) 94.7% (107/113) 0.193
Proximal Popliteal Artery 6.8% (15/221) 7.1% (8/113) 1.000
Angiographic Lesion Characteristics
b
(N=221 Lesions) (N=113 Lesions)
Lesion Length (cm) 8.94 ± 4.89 8.81 ± 5.12 0.815
Reference Vessel Diameter (RVD) (mm) 4.647 ± 0.841 4.681 ± 0.828 0.728
12 Instructions for Use English
Page 15

IN.PACT DCB PTA p-value
Minimum Lumen Diameter (MLD) (Pre-procedure) (mm) 0.900 ± 0.776 0.933 ± 0.771 0.711
Diameter Stenosis (Pre-procedure) 81.1% ± 15.5% 81.3% ± 13.7% 0.946
Occluded Lesions (100% Stenosis) 25.8% (57/221) 19.5% (22/113) 0.222
TASC Lesion Type
A 56.6% (125/221) 62.8% (71/113)
B 30.8% (68/221) 26.5% (30/113)
C 12.2% (27/221) 10.6% (12/113)
0.275
D 0.5% (1/221) 0.0% (0/113)
Calcification 59.3% (131/221) 58.4% (66/113) 0.907
Severe Calcification 8.1% (18/221) 6.2% (7/113) 0.662
# Run-off Vessels Occluded
0 41.5% (88/212) 35.7% (40/112)
1 41.5% (88/212) 33.0% (37/112)
2 13.7% (29/212) 26.8% (30/112)
0.042
3 3.3% (7/212) 4.5% (5/112)
Dissections (Post-procedure)
0 (No Dissection) 36.2% (80/221) 38.9% (44/113)
0.360A–C 63.8% (141/221) 60.2% (68/113)
D–F 0.0% (0/221) 0.9% (1/113)
Minimum Lumen Diameter (Post-procedure) (mm) 3.903 ± 0.750 3.862 ± 0.732 0.632
Diameter Stenosis (Post-procedure) 19.9% ± 10.4% 19.1% ± 10.3% 0.535
Procedural Characteristics
d
(N=220 Subjects) (N=111 Subjects)
Pre-dilatation 96.4% (212/220) 85.6% (95/111) <0.001
Post-dilatation 26.8% (59/220) 18.9% (21/111) 0.135
Provisional Stenting 7.3% (16/220) 12.6% (14/111) 0.110
Numbers are % (counts/sample size) or mean ± standard deviation.
Note that four subjects in the trial were assessed by site as having tandem lesions treated during the index procedure and
were assessed by the angiographic core laboratory as having two target lesions treated during the index procedure.
a
Site reported data.
b
Core laboratory reported data. All lesions within artery segment are counted.
c
All lesions within artery segment are counted
d
Required for IN.PACT SFA II phase; not required for IN.PACT SFA I phase.
11.2.4. Primary Safety and Effectiveness Endpoints
The primary safety endpoint of the study, a composite of freedom from device- and procedure-related death through 30 days,
freedom from target limb major amputation within 12 months and freedom from clinically-driven target vessel revascularization
within 12 months, was 95.7% in the IN.PACT Admiral DCB group and 76.6% in the PTA group (p<0.001). The IN.PACT Admiral
DCB group met the predefined 10% non-inferiority margin and showed superiority in safety against the PTA group using a
sequential analysis approach. The primary effectiveness endpoint, primary patency at 12 months, was 82.2% in the IN.PACT
Admiral DCB group and 52.4% for the PTA group (p<0.001). The IN.PACT Admiral DCB group showed statistical superiority
against the PTA group.
See Primary Safety and Effectiveness Endpoints (Table 6). Also see Kaplan-Meier Plot - Event-Free from Primary Safety
Endpoint through 360 Days (Figure 4) and Kaplan-Meier Plot - Primary Patency through 390 Days (Figure 5).
Instructions for Use English 13
Page 16

Table 6. Primary Safety and Effectiveness Endpoints
Outcome IN.PACT DCB
PTA (N=111) Difference [95% CI] p-value
(N=220)
Primary Safety Endpoint 95.7% (198/207) 76.6% (82/107) 19.0% [10.5%,
<0.001
27.5%]
Primary Effectiveness Endpoint – Primary
Patency at 12 Months
■
Primary safety endpoint is defined as freedom from device- and procedure-related death through 30 days, target limb
82.2% (157/191) 52.4% (54/103) 26.2% [15.1%,
37.3%]
<0.001
major amputation within 360 days, and clinically-driven TVR within 360 days.
■
Primary patency is defined as freedom from clinically-driven TLR1 and freedom from restenosis as determined by duplex
ultrasound2 (DUS) peak systolic velocity ratio (PSVR) ≤2.43 within 12 months. Key primary patency endpoint definition
components:
1. Clinically-driven TLR is defined as any reintervention at the target lesion due to symptoms or drop of ABI/TBI of ≥20%
or >0.15 when compared to postprocedure baseline ABI/TBI
2. Post-index procedure DUS is intended to establish a post-treatment baseline and does not contribute to the primary
endpoint determination
3. Restenosis determined by either PSVR >2.4 as assessed by an independent DUS core laboratory or >50% stenosis
as assessed by an independent angiographic core laboratory.
■
Post-index procedure DUS did not contribute to the primary effectiveness endpoint determination. Therefore, effectiveness
results do not reflect four DCB patients who had post-procedure binary restenosis which was later not observed at
12 months.
Statistical references:
■
Numbers are % (counts/sample size). CI - Confidence Interval
■
Analysis sets: Effectiveness - all randomized subjects with multiple imputation performed on missing data for primary
patency are provided in the Difference [95% CI] and p-value columns; Safety - all randomized subjects experiencing at
least one component for the safety endpoint or with follow-up of at least 330 days post-procedure (i.e. the denominator
was adjusted for missing data).
■
Non-inferiority on the primary safety endpoint was tested using the Farrington-Manning approach. The non-inferiority
margin of 10% was met, however, the results shown above are for superiority testing.
Data sources:
All events were adjudicated by the independent Clinical Events Committee and all duplex ultrasound and angiographic
measures were made by the independent core laboratories.
a
all alpha are one-sided with significance of 0.024995 required.
a
14 Instructions for Use English
Page 17

100%
90%
80%
70%
60%
0%
50%
40%
30%
20%
10%
IN.PACT DCB
PTA
Log-rank p < 0.001
0 30 60 90 120 150 180 210 240 270 300 330
Days after Index Procedure
360
Freedom from Primary Safety Endpoint
76.6%
95.6%
From day X
To day Y
IN.PACT DCB (N=220 Subjects)
# Entered
# Censored
# Events
Event-free [%]
Greenwood SE [%]
PTA (N=111 Subjects)
0
0
1
30
31
60
61
90
91
120
121
150
151
180
181
210
211
240
241
270
271
300
301
330
331
360
220 220 215 214 214 212 210 208 206 205 203 197 195
0
0 0 0
0
0 0 0 0
3
3
3
2
2 2 21 1
1
1
1
1 1
1 1
50
100.0%
0.0%
99.1% 99.1% 99.1% 98.6% 98.6% 98.6% 98.1% 98.1% 98.1% 96.7% 96.2% 95.6%
0.6% 0.6% 0.6% 0.8% 0.8% 0.8% 0.9% 0.9% 0.9% 1.2% 1.3% 1.4%
# Entered
# Censored
# Events
Event-free [%]
Greenwood SE [%]
111
111
109 108 108 105 104 101 91 89 89 88 84
0 1 0 1 3 2 0 1
0
1 3 8
1
3
100.0%
0.0%
99.1% 98.2% 98.2% 95.5% 94.6% 91.8% 84.4% 82.6% 82.6% 81.7% 78.9% 76.6%
0.9% 1.3% 1.3% 2.0% 2.2% 2.6% 3.5% 3.6% 3.6% 3.7% 3.9% 4.1%
0 01 0 0 00 0 2 0
2
24
Survival Curves Comparison
Analysis Method Test Chi Square Degr. Freedom p-value
Kaplan-Meier Analysis Log-Rank 27.3314 1 <0.001
All events were adjudicated by the independent Clinical Events Committee.
Figure 4. Kaplan-Meier Plot - Event-Free from Primary Safety Endpoint through 360 Days
Instructions for Use English 15
Page 18

100%
90%
80%
70%
60%
0%
50%
40%
30%
20%
10%
IN.PACT DCB
PTA
Log-rank p < 0.001
0 30 60 90 120 150 180 210 240 270 300 330
Days after Index Procedure
360 390
Primary Patency through 390 Days
78.4%
89.8%
66.8%
49.5%
From day X
To day Y
IN.PACT DCB (N=220 Subjects)
# Entered
# Censored
# Events
Event-free [%]
Greenwood SE [%]
PTA (N=111 Subjects)
0
0
1
30
31
60
61
9091120
121
150
151
180
181
210
211
240
241
270
271
300
301
330
331
360
220 220 215 214 214 212 210 208 207 206 204 200 198
0
0 0 0
0
0 0 0 0
3
1
3
2
2 2 21 1
1
1
0
1 1
1 14
43
100.0%
0.0%
99.1% 99.1% 99.1% 98.6% 98.6% 98.6% 98.6% 98.6% 98.6% 98.1% 97.6% 89.8%
0.6% 0.6% 0.6% 0.8% 0.8% 0.8% 0.8% 0.8% 0.8% 0.9% 1.0% 2.2%
# Entered
# Censored
# Events
Event-free [%]
Greenwood SE [%]
111
111
109 108 108 106 106 103 93 92 92 91 86
0 1 0 0 3 1 0 1
0
1 2 8
1
4
100.0%
0.0%
99.1% 98.2% 98.2% 96.4% 96.4% 93.6% 86.3% 85.3% 85.3% 84.4% 80.7% 66.8%
0.9% 1.3% 1.3% 1.8% 1.8% 2.3% 3.3% 3.4% 3.4% 3.5% 3.8% 4.7%
0 01 0 0 00 0 2 0
13
18
Survival Curves Comparison
Analysis Method Test Chi Square Degr. Freedom p-value
Kaplan-Meier Analysis Log-Rank 33.2068 1 <0.001
All TLR events were adjudicated by the independent Clinical Events Committee.
All DUSs were analyzed by an independent core laboratory.
361
390
141
15
39
78.4%
3.4%
55
49.5%
5.4%
13
9
The primary safety and effectiveness outcomes of all non-stented patients and intent to treat (ITT) population are shown in
Outcomes of All ITT and All Non-stented Populations (Table 7).
Figure 5. Kaplan-Meier Plot - Primary Patency through 390 Days
16 Instructions for Use English
Page 19

Table 7. Outcomes of All ITT and All Non-stented Populations
All ITT All Non-Stented
IN.PACT DCB PTA IN.PACT DCB PTA
Primary Safety Endpoint 95.7% (198/207) 76.6% (82/107) 95.8% (183/191) 77.7% (73/94)
Primary Effectiveness Endpoint –
82.2% (157/191) 52.4% (54/103) 82.9% (145/175) 52.2% (47/90)
Primary Patency at 12 Months
■
Primary safety endpoint is defined as freedom from device- and procedure-related death through 30 days, target limb
major amputation within 360 days, and clinically-driven TVR within 360 days.
■
Primary patency is defined as freedom from clinically-driven TLR1 and freedom from restenosis as determined by duplex
ultrasound2 (DUS) peak systolic velocity ratio (PSVR) ≤2.43 within 12 months. Key primary patency endpoint definition
components:
1. Clinically-driven TLR is defined as any reintervention at the target lesion due to symptoms or drop of ABI/TBI of ≥20%
or >0.15 when compared to postprocedure baseline ABI/TBI
2. Post-index procedure DUS is intended to establish a post-treatment baseline and does not contribute to the primary
endpoint determination
3. Restenosis determined by either PSVR >2.4 as assessed by an independent DUS core laboratory or >50% stenosis
as assessed by an independent angiographic core laboratory.
■
Post-index procedure DUS did not contribute to the primary effectiveness endpoint determination. Therefore, effectiveness
results do not reflect four DCB patients who had post-procedure binary restenosis which was later not observed at
12 months.
Statistical references:
■
Numbers are % (counts/sample size).
■
Analysis sets: Effectiveness - all randomized subjects with as-observed results for primary patency; Safety - all
randomized subjects experiencing at least one component for the safety endpoint or with follow-up of at least 330 days
post-procedure (ie, the denominator was adjusted for missing data).
Data sources:
All events were adjudicated by the independent Clinical Events Committee and all duplex ultrasound and angiographic
measures were made by the independent core laboratories.
11.2.5. Principal Safety and Effectiveness Results
A summary of the principal safety and effectiveness results through 12 months, including major secondary endpoints, have
been shown below in Principal Safety and Effectiveness Results (Table 8). Secondary safety endpoints were more favorable in
the IN.PACT Admiral DCB group. The 12-month major adverse event rate was 6.3% in the IN.PACT Admiral DCB group versus
24.3% in the PTA group (p<0.001). This statistical significance was primarily driven by a dramatic reduction in clinically-driven
target vessel revascularization (CD-TVR) rate. The IN.PACT Admiral DCB group also showed highly statistically significant
results of secondary effectiveness, such as clinically-driven TLR (CD-TLR) and primary sustained clinical improvement both of
which passed hierarchical testing.
Table 8. Principal Safety and Effectiveness Results
IN.PACT DCB PTA Difference p-value
(N=220 Sub-
jects)
(N=111 Sub-
jects)
[95% CI]
a
Safety Parameters
Primary Safety Composite Endpoint – Freedom
from:
Device- and Procedure-related Death
95.7% (198/207) 76.6% (82/107) 19.0% [10.5%,
<0.001
27.5%]
0.0% (0/218) 0.0% (0/111) NA >0.999
through 30 Days
Target Limb Major Amputation within
0.0% (0/207) 0.0% (0/107) NA >0.999
360 Days
Clinically-driven TVR within 360 Days 4.3% (9/207) 23.4% (25/107) -19.0% [-27.5%,
<0.001
-10.5%]
Death (all-cause) within 30 days 0.0% (0/218) 0.0% (0/111) NA >0.999
Effectiveness Parameters
Primary Effectiveness Endpoint – Primary Patency
at 12 Months
82.2% (157/191) 52.4% (54/103) 26.2% [15.1%,
37.3%]
<0.001
Instructions for Use English 17
Page 20
IN.PACT DCB PTA Difference p-value
(N=220 Sub-
jects)
Primary Sustained Clinical Improvement at
85.2% (167/196) 68.9% (73/106) 16.3% [6.2%,
12 Months
Device Success 99.0% (308/311) 98.5% (128/130) 0.6% [-1.8%,
(N=111 Sub-
jects)
[95% CI]
<0.001
26.5%]
0.302
3.0%]
Procedural Success 99.5% (219/220) 98.2% (109/111) 1.3% [-1.3%,
0.111
4.0%]
Clinical Success 99.1% (218/220) 97.3% (108/111) 1.8% [-1.5%,
0.103
5.1%]
Binary Restenosis (PSVR >2.4) at 12 Months 16.5% (31/188) 33.7% (29/86) -17.2% [-28.5%,
0.001
-5.9%]
Binary Restenosis (PSVR >3.4) at 12 Months 7.3% (13/178) 21.4% (18/84) -14.1% [-23.7%,
<0.001
-4.6%]
Cumulative complications within 360 days
MAE Composite (Death, Major Target Limb Amputation, Clinically-driven TVR, Thrombosis)
Death (all-cause) 1.9% (4/207) 0.0% (0/107) 1.9% [0.1%,
6.3% (13/207) 24.3% (26/107) -18.0% [-26.8%,
-9.2%]
<0.001
0.926
3.8%]
Clinically-driven TVR 4.3% (9/207) 23.4% (25/107) -19.0% [-27.5%,
<0.001
-10.5%]
Major Target Limb Amputation 0.0% (0/207) 0.0% (0/107) NA >0.999
Thrombosis 1.4% (3/207) 3.7% (4/107) -2.3% [-6.2%,
0.096
1.7%]
Clinically-driven TLR 2.4% (5/207) 20.6% (22/107) -18.1% [-26.1%,
<0.001
-10.2%]
Any TVR 4.8% (10/207) 23.4% (25/107) -18.5% [-27.1%,
<0.001
-10.0%]
Any TLR 2.9% (6/207) 20.6% (22/107) -17.7% [-25.7%,
<0.001
-9.7%]
■
Primary sustained clinical improvement was defined as freedom from target limb amputation, TVR, and increase in Rutherford class at 12 months post-procedure.
■
Device success defined as successful delivery, inflation, deflation and retrieval of the intact study balloon device without
burst below the RBP.
■
Procedure success defined as residual stenosis of ≤50% (non-stented subjects) or ≤30% (stented subjects) by visual estimate.
■
Clinical success defined as procedural success without procedural complications (death, major target limb amputation,
thrombosis of the target lesion, or TVR) prior to discharge.
■
Clinically-driven TLR/TVR is defined as any reintervention within the target vessel due to symptoms or drop of ABI/TBI of
≥20% or >0.15 when compared to post-procedure baseline ABI/TBI.
■
Major Adverse Events (MAE) defined as all-cause death, clinically-driven TLR/TVR, major target limb amputation, thrombosis at the target lesion site at 360 days.
■
Binary restenosis is defined as duplex restenosis (PSVR >2.4/3.4) or angiographic restenosis of the target lesion at
12 months postprocedure, or at the time of reintervention prior to any prespecified timepoint.
Statistical references:
■
Numbers are % (counts/sample size). CI - Confidence Interval
■
Analysis sets: Effectiveness - all randomized subjects with multiple imputation performed on missing data for primary
patency are provided in the Difference [95% CI] and p-value columns; Safety - all randomized subjects experiencing at
least one component for the safety endpoint or with follow-up of at least 330 days post-procedure (i.e. the denominator
was adjusted for missing data).
Data sources:
All events were adjudicated by the independent Clinical Events Committee, all duplex ultrasound and angiographic measures were made by the independent core laboratories.
a
all alpha are one-sided with significance of 0.024995 required. All tests were for superiority using the chi-square test for binary variables and t-test for continuous variables.
a
18 Instructions for Use English
Page 21
11.2.6. Subgroup Analysis
Relative Risk [95% CI]
Subgroup
IN.PACT
DCB %
Control
PTA %
Overall ITT 95.7% 76.6%
Rutherford Category 2 96.2% 82.9%
Rutherford Category 3 95.8% 74.6%
Rutherford Category 4 90.9% 50.0%
Diabetes Mellitus 92.7% 73.1%
Age ≥75 98.0% 82.1%
Lesion Length <5 cm 97.9% 91.7%
Lesion Length ≥5 cm and <10 cm 98.6% 79.5%
Lesion Length ≥10 cm and <18 cm 92.1% 61.8%
Total Occlusion 96.3% 61.9%
Female Gender 94.6% 68.6%
Male Gender 96.2% 80.6%
Favors Control PTA
Favors IN.PACT DCB
0 1 2 3 4 5
Relative Risk [95% CI]
Subgroup
IN.PACT
DCB %
Control
PTA %
Overall ITT 82.2% 52.4%
Rutherford Category 2 82.2% 43.6%
Rutherford Category 3 82.4% 59.6%
Rutherford Category 4 80.0% 33.3%
Diabetes Mellitus 77.3% 49.0%
Age ≥75 84.4% 42.3%
Lesion Length <5 cm 93.3% 73.9%
Lesion Length ≥5 cm and <10 cm 83.3% 57.1%
Lesion Length ≥10 cm and <18 cm 74.6% 39.4%
Total Occlusion 83.3% 40.9%
Female Gender 75.7% 43.8%
Male Gender 86.0% 56.3%
Favors Control PTA
Favors IN.PACT DCB
0 5 10
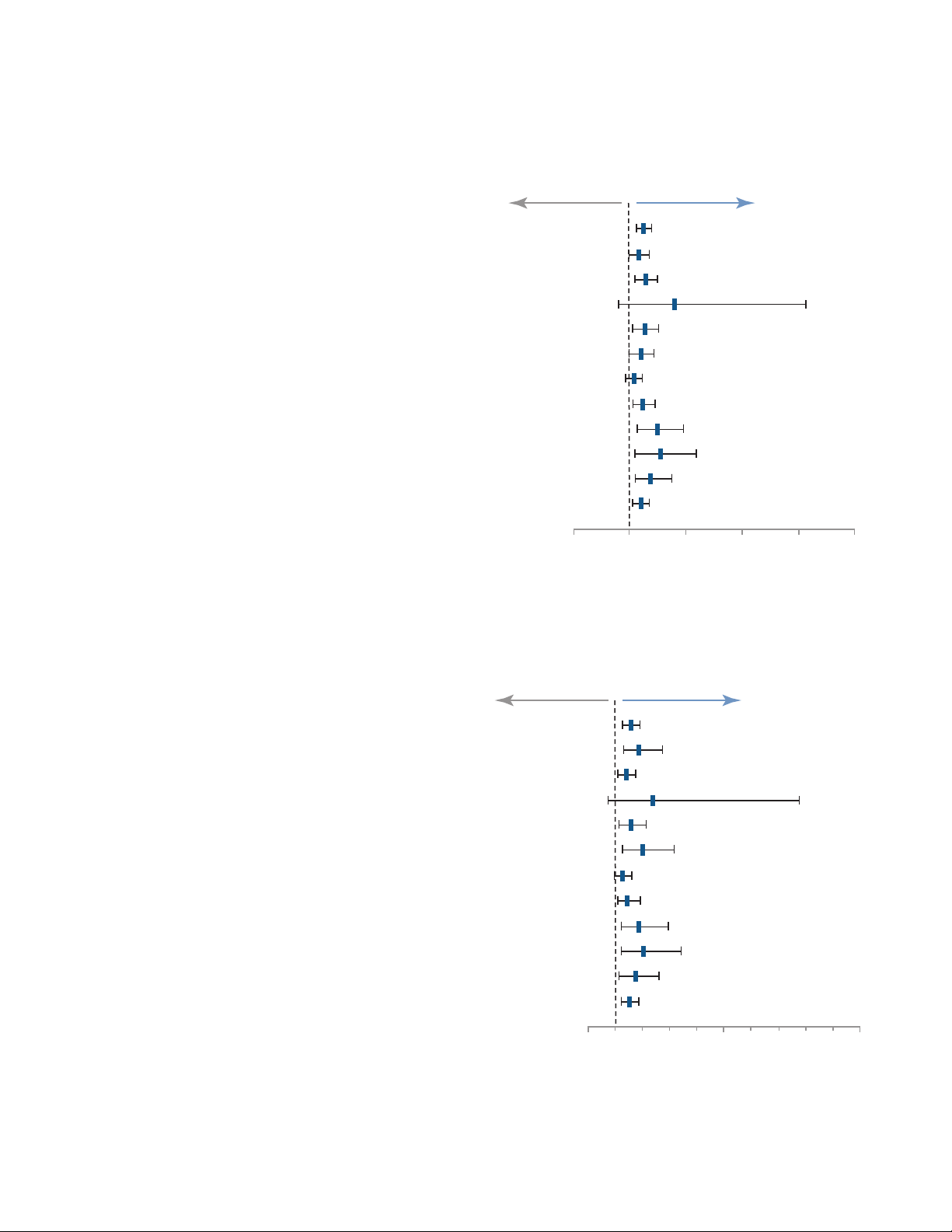
Medtronic has analyzed trial results by different pre-defined subgroups to investigate the consistency of results through
12 months. Primary Safety Endpoint Event at 12 Months (Figure 6), Primary Patency at 12 Months (Figure 7), and Clinicallydriven Target Lesion Revascularization at 12 Months (Figure 8) have been illustrated for each subgroup in the forest plots
below. All data for the subgroup analyses trended in favor of IN.PACT Admiral DCB over PTA.
Figure 6. Primary Safety Endpoint Event at 12 Months
Note: There were no significant treatment-by-subgroup interactions (p>0.15). The 95% confidence intervals were unadjusted
for multiplicity.
Note: There were no significant treatment-by-subgroup interactions (p>0.15). The 95% confidence intervals were unadjusted
for multiplicity.
Figure 7. Primary Patency at 12 Months
Instructions for Use English 19
Page 22

Relative Risk [95% CI]
Subgroup
IN.PACT
DCB %
Control
PTA %
Overall ITT 2.4% 20.6%
Rutherford Category 2 2.6% 17.1%
Rutherford Category 3 1.7% 20.3%
Rutherford Category 4 9.1% 50.0%
Diabetes Mellitus 3.7% 23.1%
Age ≥75 0.0% 17.9%
Lesion Length <5 cm 0.0% 4.2%
Lesion Length ≥5 cm and <10 cm 1.4% 20.5%
Lesion Length ≥10 cm and <18 cm 5.3% 32.4%
Total Occlusion 1.9% 38.1%
Female Gender 4.1% 25.7%
Male Gender 1.5% 18.1%
Favors Control PTA
Favors IN.PACT DCB
0 1 2 3 4 5
Figure 8. Clinically-driven Target Lesion Revascularization at 12 Months
Note: There were no significant treatment by subgroup interactions (p>0.15) except in diabetes mellitus (p=0.027). The 95%
confidence intervals were unadjusted for multiplicity.
11.2.7. Gender Analysis
There were 218 males and 113 females enrolled in the pivotal study. Based on gender subgroup analyses, both female and
male subgroups showed improvement on the primary safety and effectiveness endpoints through 12 months. The results of an
interaction analysis indicate that the treatment differences between IN.PACT Admiral DCB and PTA groups are consistent
between male and female subjects.
Table 9. Primary Safety Composite and Primary Effectiveness by Gender
Females
Outcome IN.PACT DCB Standard PTA Difference
(N=77 Subjects) (N=36 Subjects)
Primary Safety Endpoint 94.6% (70/74) 68.6% (24/35) 26.0%
Primary Effectiveness Endpoint –
75.7% (53/70) 43.8% (14/32) 29.3%
Primary Patency at 12 Months
Males
Outcome IN.PACT DCB Standard PTA Difference
Primary Safety Endpoint
(N=143 Subjects) (N=75 Subjects)
96.2% (128/133) 80.6% (58/72)
15.7%
20 Instructions for Use English
Page 23

Primary Effectiveness Endpoint –
86.0% (104/121) 56.3% (40/71) 25.0%
Primary Patency at 12 Months
■
Primary safety endpoint is defined as freedom from device- and procedure-related death through 30 days, target limb
major amputation within 360 days, and clinically-driven TVR within 360 days.
■
Primary patency is defined as freedom from clinically-driven TLR1 and freedom from restenosis as determined by duplex
ultrasound2 (DUS) peak systolic velocity ratio (PSVR) ≤2.43 within 12 months. Key primary patency endpoint definition
components:
1. Clinically-driven TLR is defined as any reintervention at the target lesion due to symptoms or drop of ABI/TBI of ≥20%
or >0.15 when compared to postprocedure baseline ABI/TBI
2. Post-index procedure DUS is intended to establish a post-treatment baseline and does not contribute to the primary
endpoint determination
3. Restenosis determined by either PSVR >2.4 as assessed by an independent DUS core laboratory or >50% stenosis
as assessed by an independent angiographic core laboratory.
■
Post-index procedure DUS did not contribute to the primary effectiveness endpoint determination. Therefore, effectiveness
results do not reflect four DCB patients who had post-procedure binary restenosis which was later not observed at
12 months.
Statistical references:
■
Numbers are % (counts/sample size).
■
Analysis sets: Effectiveness - all randomized subjects with multiple imputation performed on missing data for primary
patency are provided in the Difference column; Safety - all randomized subjects experiencing at least one component
for the safety endpoint or with follow-up of at least 330 days post-procedure (ie, the denominator was adjusted for
missing data).
Data sources:
All events were adjudicated by the independent Clinical Events Committee and all duplex ultrasound and angiographic
measures were made by the independent core laboratories.
11.2.8. Serious Adverse Events
For additional details on serious adverse events, see Summary of Adverse Events (Section 11.3.7).
11.3. IN.PACT SFA Trial Post-Approval Study
11.3.1. Primary Objective
The IN.PACT SFA Trial Post-Approval Study was designed to evaluate the long-term safety and effectiveness of the IN.PACT
Admiral DCB via a two-year primary patency endpoint and a composite two-year safety endpoint for the treatment of lesions in
the SFA and/or PPA.
11.3.2. Study Design
As a condition of premarket approval, the IN.PACT SFA Trial subjects were followed through 60 months post-index procedure
and assessed for the primary and secondary endpoints listed below. Additional de novo subjects were not enrolled. For
additional details regarding study design, refer to Section 11.2.2, Study Design.
The primary endpoints for the IN.PACT SFA Trial Post-Approval Study are listed below:
■
Primary Safety Endpoint:
■
Freedom from device- and procedure-related death at 30 days and freedom from target limb major amputation and
clinically-driven target vessel revascularization (CD-TVR) at 24 months.
■
Primary Effectiveness Endpoint
■
Primary patency at 24 months, defined as freedom from clinically-driven TLR (CD-TLR) and freedom from restenosis as
determined by duplex ultrasound (DUS) peak systolic velocity ratio (PSVR) ≤ 2.4.
The secondary endpoints for the IN.PACT SFA Trial Post-Approval Study are listed below:
Assessed through 60 months:
■
Major adverse event (MAE) composite and its individual components (all-cause mortality, CD-TVR, major target limb
amputation, and thrombosis at the target lesion site)
■
CD-TLR
■
All TVR
■
All TLR
■
Serious adverse events (SAEs)
Assessed at 24 and 36 months:
Instructions for Use English 21
Page 24

■
Primary sustained clinical improvement
■
Secondary sustained clinical improvement
■
Duplex-defined binary restenosis (PSVR > 2.4) of the target lesion
■
Duplex-defined binary restenosis (PSVR > 3.4) of the target lesion
■
Quality of Life (QoL) assessment by EQ-5D Questionnaire
■
Walking capacity assessment by Walking Impairment Questionnaire (WIQ)
11.3.3. Patient Population
The 331 subjects assessed for the IN.PACT SFA Trial Post-Approval Study were the same subjects as originally enrolled in the
IN.PACT SFA Trial. For additional details on the study population, refer to Section 11.2.3, Patient Population.
Follow-up compliance through the 60-month follow-up visit is presented in Subject Follow-up Compliance through 60 Months
(Table 10). The overall follow-up compliance rates in the IN.PACT Admiral DCB group and the PTA group were greater than
90% from 12 months through 60 months.
Table 10. Subject Follow-up Compliance through 60 Months
Subject Compliance Characteristics IN.PACT DCB (N=220 Subjects) Standard PTA (N=111 Subjects)
12-Month Follow-up
Eligible Subjects
b
Death
Withdrawal
a
202 108
5 0
b
13 3
Follow-up Not Done 5 4
Follow-up Visit Within Window
Follow-up Visit Out of Window
Within Window Follow-up Compliance (%)
Overall Follow-up Compliance (%)
c
c
d
e
188 98
9 6
93.1% 90.7%
97.5% 96.3%
24-Month Follow-up
Eligible Subjects
b
Death
Withdrawal
b
a
187 104
16 1
17 6
Follow-up Not Done 17 10
Follow-up Visit Within Window
Follow-up Visit Out of Window
c
c
Within Window Follow-up Compliance (%)
Overall Follow-up Compliance (%)
e
d
154 83
16 11
82.4% 79.8%
90.9% 90.4%
36-Month Follow-up
Eligible Subjects
b
Death
Withdrawal
b
a
173 99
22 2
25 10
Follow-up Not Done 12 7
Follow-up Visit Within Window
Follow-up Visit Out of Window
c
c
Within Window Follow-up Compliance (%)
Overall Follow-up Compliance (%)
e
d
133 82
28 10
76.9% 82.8%
93.1% 92.9%
48-Month Follow-up
Eligible Subjects
b
Death
Withdrawal
b
a
163 91
24 7
33 13
Follow-up Not Done 14 5
Follow-up Visit Within Window
Follow-up Visit Out of Window
c
c
Within Window Follow-up Compliance (%)
Overall Follow-up Compliance (%)
e
d
146 85
3 1
89.6% 93.4%
91.4% 94.5%
60-Month Follow-up
Eligible Subjects
a
155 88
22 Instructions for Use English
Page 25

Subject Compliance Characteristics IN.PACT DCB (N=220 Subjects) Standard PTA (N=111 Subjects)
b
Death
Withdrawal
b
29 10
36 13
Follow-up Not Done 8 3
Follow-up Visit Within Window
Follow-up Visit Out of Window
c
c
Within Window Follow-up Compliance (%)
Overall Follow-up Compliance (%)
e
d
142 83
5 2
91.6% 94.3%
94.8% 96.6%
Site reported data
a
Eligible subjects are all subjects who either have a follow-up visit form or are past due for their follow-up (beyond upper limit of window on study and did not exit the study before the upper limit of the window)
b
Death and withdrawal are cumulative
c
Within window visits are defined as: 30-days ± 7 days, 6-months ± 30 days, 12-months ± 30 days, 2-years ± 30 days, 3-years ± 30 days, 4-years ± 60 days, 5-years ± 60 days
d
Percentage based on number of subjects who had follow-up visit within window divided by total number of eligible subjects
e
Percentage based on number of subjects who had a follow-up visit within or out of window divided by total number of eligible subjects.
11.3.4. Primary Safety and Effectiveness Endpoints
The primary safety composite endpoint is defined as freedom from device- and procedure-related death through 30 days postprocedure and freedom from target limb major amputation and CD-TVR within 24 months post-index procedure. The primary
safety composite endpoint at 24 months in the IN.PACT Admiral DCB group was 87.4% (173/198) versus 69.8% (74/106) in the
PTA group (p<0.001). The primary effectiveness endpoint is defined as primary patency within 24 months post-index
procedure. Primary patency is defined as freedom from CD-TLR and freedom from restenosis as determined by DUS PSVR ≤
2.4. Primary patency at 24 months in the IN.PACT Admiral DCB group was 69.2% (108/156) versus 50.5% (48/95) in the PTA
group (p=0.005).
See Primary Safety and Effectiveness Endpoints at 24 Months (Table 11). Also see Kaplan-Meier Plot - Event-free from Primary
Safety Endpoint through 720 Days (Figure 9) and Kaplan-Meier Plot - Cumulative Primary Patency through 750 Days
(Figure 10).
Table 11. Primary Safety and Primary Effectiveness Endpoints at 24 Months
Primary Endpoints IN.PACT DCB
Primary Effectiveness Endpoint – Primary
Patency at 24 Months
(N=220 Subjects)
69.2% (108/156) 50.5% (48/95) 18.7% [6.3%,
Standard PTA
(N=111 Subjects)
Difference
[95% CI]
31.1%]
p-value
0.005
Primary Safety Endpoint at 24 Months 87.4% (173/198) 69.8% (74/106) 17.6% [8.5%, ∞] <0.001*
Endpoint definitions:
■
Primary patency is defined as freedom from clinically-driven TLRa and freedom from restenosis as determined by duplex
ultrasoundb (DUS) Peak Systolic Velocity Ratio (PSVR) ≤ 2.4
■
Primary safety endpoint consists of freedom from device- and procedure-related death through 30 days; freedom from
c
target limb amputation within 24 months; and freedom from clinically-driven TVRd within 24 months.
Statistical references:
■
Numbers are % (counts/sample size) unless otherwise stated.
■
CI – Confidence Interval
■
Analysis sets: Effectiveness – all randomized subjects with multiple imputation performed on missing data for Primary
Patency; Safety – all randomized subjects experiencing at least one component for the safety endpoint or with follow-up of
at least 690 days post-procedure, i.e. the denominator was adjusted for missing data.
■
For the primary effectiveness endpoint the Z test of two proportions was used to compare treatment groups for all randomized subjects.
■
∞ means Not Applicable for this one-sided test.
■
*non-inferiority p-value.
■
Non-inferiority on the primary safety endpoint was tested using the Farrington-Manning risk difference between treatments
(calculated as treatment minus control) and its one-sided lower 97.5005% confidence interval. The non-inferiority margin
was 10%.
Data sources:
All events were adjudicated by the independent Clinical Events Committee, all duplex ultrasound and angiographic measures
were made by the independent core laboratories
Key endpoint definition components:
a
Clinically-driven TLR is defined as any re-intervention at the target lesion due to symptoms or drop of ABI/TBI of ≥20% or >0.15 when compared to post-procedure baseline ABI/TBI
b
Post-index procedure DUS is intended to establish a post-treatment baseline and does not contribute to the Primary Endpoint determination
c
Restenosis determined by either PSVR >2.4 (determined by Target Lesion Category of ’50-99%’ or ‘Occluded’) as assessed by an independent DUS core lab or >50% stenosis as assessed by an independent
angiographic core lab
d
Clinically-driven TVR is defined as any re-intervention within the target vessel due to symptoms or drop of ABI/TBI of ≥20% or >0.15 when compared to post-procedure baseline ABI/TBI
Instructions for Use English 23
Page 26

100%
90%
80%
70%
60%
0%
50%
40%
30%
20%
10%
0 60
120 180
210 300 360 420 480 540 600 660 720
Freedom from Primary Safety Endpoint
Days After Index Procedure
IN.PACT DCB
Log-Rank p < 0.001
PTA
From day X
To day Y
IN.PACT DCB (N=220 Subjects)
# Entered
# Censored
# Events
Event-free [%]
Greenwood SE [%]
Standard PTA (N=111 Subjects)
0
0
1
60
61
120
121
180
181
240
241
300
301
360
361
420
421
480
481
540
541
600
601
660
661
720
220 220 215 213 209 206 199 193 175 173 171 166 162
0
0 1 0
4
3 2 1 1
3
2
3
2
4 4 11 2
1
6
12
1 4
0 0
18
100.0%
0.0%
99.1% 98.6% 98.6% 98.2% 96.7% 95.7% 89.6% 89.1% 88.6% 87.5% 87.5% 87.5%
0.6% 0.8% 0.8% 0.9% 1.2% 1.4% 2.2% 2.2% 2.2% 2.3% 2.3% 2.3%
# Entered
# Censored
# Events
Event-free [%]
Greenwood SE [%]
111
111
108 105 101 89 88 83 75 75 75 75 74
0 3 4 1 5 0 0 0
0
2 10 6
1
0
100.0%
0.0%
98.2% 95.5% 91.8% 82.6% 81.7% 77.0% 71.4% 71.4% 71.4% 71.4% 71.4% 70.4%
1.3% 2.0% 2.6% 3.6% 3.7% 4.0% 4.3% 4.3% 4.3% 4.3% 4.3% 4.4%
0 01 0 0 00 2 2 0
1
10
70.4%
87.5%
All events were adjudicated by the independent Clinical Events Committee.
Analysis Method Test Chi Square Degr. Freedom P-Value
Kaplan-Meier Analysis Log-Rank 16.7894 1 <0.001
Figure 9. Kaplan-Meier Plot – Event-free from Primary Safety Endpoint through 720 Days
Survival Curves Comparison
24 Instructions for Use English
Page 27

100%
90%
80%
70%
60%
0%
50%
40%
30%
20%
10%
0 60
120 180
210 300 360 420 480 540 600 660 720 750
Primary Patency
Days After Index Procedure
From day X
To day Y
IN.PACT DCB (N=220 Subjects)
# Entered
# Censored
# Events
Event-free [%]
Greenwood SE [%]
Standard PTA (N=111 Subjects)
0
0
1
60
61
120
121
180
181
240
241
300
301
360
361
420
421
480
481
540
541
600
601
660
661
720
721
750
220 220 215 212 204 194 188 174 154 153 152 147 144
0
0 2 4
4
1 11 0 0
3
2
3
2
5 3 11 4
6
9
11
1 3
0 3
14
100.0%
0.0%
99.1% 98.2% 96.3% 93.4% 92.9% 87.5% 81.8% 81.8% 81.8% 80.7% 80.7% 79.0%
0.6% 0.9% 1.3% 1.7% 1.8% 2.3% 2.7% 2.7% 2.7% 2.8% 2.8% 2.9%
# Entered
# Censored
# Events
Event-free [%]
Greenwood SE [%]
111
111
108 105 90 69 68 60 53 51 51 51 50
0 3 14 1 8 1 0 0
0
2 20 5
1
0
100.0%
0.0%
98.2% 95.5% 82.6% 64.2% 63.2% 55.8% 51.1% 50.1% 50.1% 50.1% 50.1% 50.1%
1.3% 2.0% 3.6% 4.6% 4.6% 4.8% 4.8% 4.8% 4.8% 4.8% 4.8% 4.8%
0 11 0 0 00 1 2 1
0
7
127
8
15
73.8%
3.2%
43
47.7%
4.9%
2
5
50.1%
79.0%
IN.PACT DCB
Log-Rank p < 0.001
PTA
All TLR events were adjudicated by the independent Clinical Events Committee.
All DUSs were analyzed by an independent core laboratory.
11.3.5. Secondary Safety and Effectiveness
A summary of the secondary safety and effectiveness endpoints through 60 months is shown in Secondary Safety and
Effectiveness Endpoints through 60 Months (Table 12).
Within 720 days, the composite MAE rate was 18.6% (38/204) in the IN.PACT Admiral DCB group versus 30.6% (33/108) in the
PTA group. CD-TVR rates were lower in the IN.PACT Admiral DCB group at 12.3% (25/204) versus 29.6% (32/108) in the PTA
group. CD-TLR rates were lower in the IN.PACT Admiral DCB group at 8.8% (18/204) versus 27.8% (30/108) in the PTA group.
Analysis Method Test Chi Square Degr. Freedom P-Value
Kaplan-Meier Analysis Log-Rank 32.0231 1 <0.001
Figure 10. Kaplan-Meier Plot – Cumulative Primary Patency through 750 Days
Survival Curves Comparison
Instructions for Use English 25
Page 28

Within 1080 days, the composite MAE rate was 27.5% (55/200) in the IN.PACT Admiral DCB group versus 36.8% (39/106) in
the PTA group. CD-TVR rates were lower in the IN.PACT Admiral DCB group at 18.5% (37/200) versus 34.9% (37/106) in the
PTA group. CD-TLR rates were lower in the IN.PACT Admiral DCB group at 15.0% (30/200) versus 30.2% (32/106) in the PTA
group.
Within 1440 days, the composite MAE rate was 37.4% (71/190) in the IN.PACT Admiral DCB group versus 40.4% (42/104) in
the PTA group. CD-TVR rates were lower in the IN.PACT Admiral DCB group 26.3% (50/190) versus 35.6% (37/104) in the
PTA group. CD-TLR rates were lower in the IN.PACT Admiral DCB group 23.2% (44/190) versus 30.8% (32/104) in the PTA
group.
Within 1800 days, the composite MAE rate was 42.9% (79/184) in the IN.PACT Admiral DCB group versus 48.1% (50/104) in
the PTA group. CD-TVR rates were lower in the IN.PACT Admiral DCB group 29.3% (54/184) versus 40.4% (42/104) in the
PTA group. CD-TLR rates were lower in the IN.PACT Admiral DCB group 25.5% (47/184) versus 35.6% (37/104) in the PTA
group.
All deaths were adjudicated by the blinded, Clinical Events Committee and none were found to be device- or procedure-related.
The causes of death were varied and found to occur relatively late in the study. One major amputation in the IN.PACT DCB
group occurred 1653 days post-index procedure.
Table 12. Secondary Safety and Effectiveness Endpoints through 60 Months
Description of Event IN.PACT DCB (N=220 Subjects) Standard PTA (N=111 Subjects)
Cumulative Complications Within 720 Days
MAE (Death, Major Target Limb Amputation, Clinically-driven TVR, Thrombosis)
Death (all-cause) 7.8% (16/204) 0.9% (1/108)
Clinically-driven TVR 12.3% (25/204) 29.6% (32/108)
Major Target Limb Amputation 0.0% (0/204) 0.0% (0/108)
Thrombosis 1.5% (3/204) 3.7% (4/108)
Clinically-driven TLR 8.8% (18/204) 27.8% (30/108)
Any TVR 12.7% (26/204) 30.6% (33/108)
Any TLR 9.8% (20/204) 28.7% (31/108)
Cumulative Complications Within 1080 Days
MAE (Death, Major Target Limb Amputation, Clinically-driven TVR, Thrombosis)
Death (all-cause) 10.5% (21/200) 1.9% (2/106)
Clinically-driven TVR 18.5% (37/200) 34.9% (37/106)
Major Target Limb Amputation 0.0% (0/200) 0.0% (0/106)
Thrombosis 2.0% (4/200) 4.7% (5/106)
Clinically-driven TLR 15.0% (30/200) 30.2% (32/106)
Any TVR 19.0% (38/200) 35.8% (38/106)
Any TLR 16.0% (32/200) 33.0% (35/106)
Cumulative Complications Within 1440 Days
MAE (Death, Major Target Limb Amputation, Clinically-driven TVR, Thrombosis)
Death (all-cause) 12.6% (24/190) 6.7% (7/104)
Clinically-driven TVR 26.3% (50/190) 35.6% (37/104)
Major Target Limb Amputation 0.0% (0/190) 0.0% (0/104)
Thrombosis 2.1% (4/190) 4.8% (5/104)
Clinically-driven TLR 23.2% (44/190) 30.8% (32/104)
Any TVR 26.8% (51/190) 36.5% (38/104)
Any TLR 24.2% (46/190) 33.7% (35/104)
Cumulative Complications Within 1800 Days
MAE (Death, Major Target Limb Amputation, Clinically-driven TVR, Thrombosis)
Death (all-cause) 15.8% (29/184) 9.6% (10/104)
Clinically-driven TVR 29.3% (54/184) 40.4% (42/104)
Major Target Limb Amputation 0.5% (1/184) 0.0% (0/104)
Thrombosis 2.2% (4/184) 4.8% (5/104)
18.6% (38/204) 30.6% (33/108)
27.5% (55/200) 36.8% (39/106)
37.4% (71/190) 40.4% (42/104)
42.9% (79/184) 48.1% (50/104)
26 Instructions for Use English
Page 29

Description of Event IN.PACT DCB (N=220 Subjects) Standard PTA (N=111 Subjects)
Clinically-driven TLR 25.5% (47/184) 35.6% (37/104)
Any TVR 29.9% (55/184) 40.4% (42/104)
Any TLR 26.6% (49/184) 37.5% (39/104)
Percentages are based on number of evaluable subjects at each time point, and all events are adjudicated by the CEC.
Cumulative complications within 720 days, 1080 days, 1440 days and 1800 days include both IN.PACT SFA I and SFA II
phase subjects.
All randomized subjects experiencing at least one component for the safety endpoint or with follow-up of at least 690 days for
24-month, 1050 days for 36-month, 1380 days for 48-month, and 1740 days for 60-month post-procedure, were considered as
evaluable subjects for the event rate calculation for each corresponding time point, i.e. the denominator was adjusted for missing data.
Major Adverse Events (MAE) defined as all-cause death, clinically-driven TVR, major target limb amputation, thrombosis at the
target lesion site.
Clinically-driven TLR/TVR is defined as any re-intervention within the target vessel due to symptoms or drop of ABI/TBI of ≥
20% or >0.15 when compared to post-procedure baseline ABI/TBI.
Numbers are % (counts/sample size) unless otherwise stated.
11.3.6. Functional Endpoints
A summary of functional outcomes through 24 months and 36 months are presented in Table 13. These functional tests are
only assessed through the 36-month follow-up.
Primary sustained clinical improvement is defined as freedom from target limb amputation, TVR, and an increase in Rutherford
classification. Outcomes for this endpoint at 24 months in the IN.PACT Admiral DCB subjects were 76.9% (133/173) versus
59.2% (61/103) in the PTA group. Secondary sustained clinical improvement is defined as freedom from target limb amputation
and an increase in Rutherford classification. Outcomes for this endpoint at 24 months in the IN.PACT Admiral DCB subjects
were 86.9% (146/168) versus 86.2% (81/94) in the PTA group.
The QoL assessment uses the EQ-5D Index four-digit scoring algorithm to calculate an overall score, that was generated by
the five individual dimensions (mobility, self-care, activity, pain, and anxiety), where 0.109 represents the worst possible
outcome and 1.000 represents the best possible outcome. At 24 months, the mean change from baseline in the IN.PACT
Admiral DCB subjects was 0.0957 ± 0.2159 versus 0.0545 ± 0.2286 in the PTA group. The WIQ scoring (%) is expressed on a
scale of 0% to 100%, with 0% meaning the patient was unable to perform the assessment due to claudication, and 100%
meaning no impairment. The mean walking impairment score in the IN.PACT Admiral DCB group was 72.5% ± 34.1% versus
67.2% ± 33.6% in the PTA group.
At 36 months, primary sustained clinical improvement in the IN.PACT Admiral DCB subjects was 68.7% (114/166) versus
52.6% (51/97) in the PTA group and secondary sustained clinical improvement in the IN.PACT Admiral DCB subjects was
85.3% (133/156) versus 87.8% (79/90) in the PTA group.
At 36 months, the QoL mean change from baseline in the IN.PACT Admiral DCB subjects was 0.0832 ± 0.2293 versus
0.0662 ± 0.1977 in the PTA group. In the WIQ (%), the mean in the IN.PACT Admiral DCB group was 71.8% ± 34.2% versus
74.7% ± 29.2% in the PTA group.
Table 13. Secondary Safety and Effectiveness Endpoints through 36 Months
IN.PACT DCB (N=220 Subjects) Standard PTA (N=111 Subjects)
Outcomes At 24 Months
Primary Sustained Clinical Improvement 76.9% (133/173) 59.2% (61/103)
Secondary Sustained Clinical Improvement 86.9% (146/168) 86.2% (81/94)
Change in Quality of Life from Baseline by EQ-5D
Index
N 167 92
Mean ± SD 0.0957 ± 0.2159 0.0545 ± 0.2286
Median 0.1040 0.0370
Min, Max -0.786, 0.672 -0.696, 0.554
Walking Impairment by WIQ (%)
N 170 93
Mean ± SD 72.5 ± 34.1 67.2 ± 33.6
Median 100.0 75.0
Min, Max 0, 100 0, 100
Instructions for Use English 27
Page 30

IN.PACT DCB (N=220 Subjects) Standard PTA (N=111 Subjects)
Outcomes At 36 Months
Primary Sustained Clinical Improvement 68.7% (114/166) 52.6% (51/97)
Secondary Sustained Clinical Improvement 85.3% (133/156) 87.8% (79/90)
Change in Quality of Life from Baseline by EQ-5D
Index
N 156 90
Mean ± SD 0.0832 ± 0.2293 0.0662 ± 0.1977
Median 0.0905 0.0620
Min, Max -0.759, 0.693 -0.689, 0.554
Walking Impairment by WIQ (%)
N 158 91
Mean ± SD 71.8 ± 34.2 74.7 ± 29.2
Median 100.0 75.0
Min, Max 0, 100 0, 100
Endpoint definitions:
■
Primary Sustained Clinical Improvement is defined as freedom from target limb amputation, TVR, and increase in Rutherford class of at least one class from baseline at 24 (36) months post-procedure.
■
Secondary Sustained Clinical Improvement is defined freedom from target limb amputation and increase in Rutherford
class of at least one class at 24 (36) months post-procedure.
■
Quality of Life assessment by EQ-5D questionnaire index at 24 (36) months
■
Walking impairment assessed by Walking Impairment Questionnaire (WIQ) at 24 (36) months.
Statistical references:
■
Numbers are % (counts/sample size) unless otherwise stated.
Data Source:
All events were adjudicated by the independent Clinical Events Committee, all duplex ultrasound and angiographic measures
were made by the independent core laboratories, and all other data were site reported.
11.3.7. Summary of Adverse Events
Serious Adverse Event Rates by SOC and Preferred Term through 1-year and through 5-year snapshot (Table 14) shows
serious adverse event rates by subject and stratified by system-organ class (SOC) and preferred term. Serious adverse events
were site-reported, and SOC was assigned via MedDRA version 13.0 coding.
A serious adverse event (SAE) was defined as an adverse event that led to a death or to a serious deterioration in the health of
the subject. A serious deterioration in the health of the subject is defined as one or more of the following:
■
a life-threatening illness or injury
■
a permanent impairment of a body structure or a body function
■
in-patient hospitalization or prolongation of an existing hospitalization
■
a medical or surgical intervention to prevent life-threatening illness or injury or permanent impairment to a body structure or
body function
■
fetal distress, fetal death, or a congenital abnormality or birth defect.
Table 14. Serious Adverse Event Rates by SOC and Preferred Term through 1-year and through 5-year snapshot
Through 1-Year Through 5-Year
Serious Adverse Events IN.PACT DCB
(N=220 Subjects)
SUBJECTS WITH ONE OR MORE
46.4% (102/220) 55.9% (62/111) 79.1% (174/220) 84.7% (94/111)
Standard PTA
(N=111 Subjects)
IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
SERIOUS ADVERSE EVENTS
BLOOD AND LYMPHATIC SYS-
TEM DISORDERS
a
2.3% (5/220) 1.8% (2/111) 8.2% (18/220) 2.7% (3/111)
ANAEMIA 1.8% (4/220) 0.9% (1/111) 5.9% (13/220) 2.7% (3/111)
ANAEMIA OF MALIGNANT DIS-
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
EASE
HAEMORRHAGIC ANAEMIA 0.5% (1/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
LEUKOCYTOSIS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PANCYTOPENIA 0.0% (0/220) 0.9% (1/111) 0.5% (1/220) 0.9% (1/111)
28 Instructions for Use English
Page 31

Through 1-Year Through 5-Year
Serious Adverse Events IN.PACT DCB
(N=220 Subjects)
CARDIAC DISORDERS
ACUTE CORONARY SYN-
a
9.5% (21/220) 6.3% (7/111) 30.5% (67/220) 21.6% (24/111)
0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
Standard PTA
(N=111 Subjects)
IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
DROME
ACUTE MYOCARDIAL INFARC-
1.4% (3/220) 0.0% (0/111) 5.9% (13/220) 1.8% (2/111)
TION
ANGINA PECTORIS 0.9% (2/220) 0.9% (1/111) 2.7% (6/220)) 0.9% (1/111)
ANGINA UNSTABLE 0.0% (0/220) 0.9% (1/111) 1.8% (4/220) 1.8% (2/111)
ARRHYTHMIA 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ATRIAL FIBRILLATION 0.9% (2/220) 1.8% (2/111) 4.1% (9/220) 4.5% (5/111)
ATRIAL FLUTTER 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
ATRIOVENTRICULAR BLOCK
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
COMPLETE
ATRIOVENTRICULAR BLOCK
0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
SECOND DEGREE
BRADYCARDIA 0.0% (0/220) 0.0% (0/111) 1.8% (4/220) 0.0% (0/111)
CARDIAC ARREST 0.5% (1/220) 0.0% (0/111) 2.3% (5/220) 0.9% (1/111)
CARDIAC FAILURE 0.0% (0/220) 0.0% (0/111) 3.2% (7/220) 0.9% (1/111)
CARDIAC FAILURE ACUTE 0.0% (0/220) 0.0% (0/111) 1.4% (3/220) 0.0% (0/111)
CARDIAC FAILURE CONGES-
2.7% (6/220) 0.9% (1/111) 8.2% (18/220) 2.7% (3/111)
TIVE
CARDIOMYOPATHY 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
CORONARY ARTERY DIS-
3.2% (7/220) 0.9% (1/111) 9.1% (20/220) 7.2% (8/111)
EASE
CORONARY ARTERY DISSEC-
0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
TION
CORONARY ARTERY OCCLU-
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.9% (1/111)
SION
CORONARY ARTERY STENO-
0.0% (0/220) 0.0% (0/111) 2.3% (5/220) 0.0% (0/111)
SIS
CORONARY ARTERY THROM-
0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
BOSIS
HEART VALVE STENOSIS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ISCHAEMIC CARDIOMYOP-
0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.9% (1/111)
ATHY
MITRAL VALVE DISEASE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
MITRAL VALVE INCOMPE-
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
TENCE
MYOCARDIAL INFARCTION 0.9% (2/220) 0.9% (1/111) 3.2% (7/220) 2.7% (3/111)
MYOCARDIAL ISCHAEMIA 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PERICARDITIS 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
SINUS BRADYCARDIA 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
SINUS TACHYCARDIA 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
SUPRAVENTRICULAR TACHY-
0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
CARDIA
TRICUSPID VALVE DISEASE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
VENTRICULAR TACHYCARDIA 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
CONGENITAL, FAMILIAL AND
GENETIC DISORDERS
a
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.9% (1/111)
ATRIAL SEPTAL DEFECT 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
HYDROCELE 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
EAR AND LABYRINTH DISOR-
a
DERS
0.5% (1/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
VERTIGO 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
Instructions for Use English 29
Page 32

Through 1-Year Through 5-Year
Serious Adverse Events IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
VERTIGO POSITIONAL 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
EYE DISORDERS
a
0.5% (1/220) 0.0% (0/111) 2.3% (5/220) 1.8% (2/111)
CATARACT 0.0% (0/220) 0.0% (0/111) 1.8% (4/220) 0.0% (0/111)
DIABETIC RETINOPATHY 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
RETINAL DETACHMENT 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
VISUAL IMPAIRMENT 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
GASTROINTESTINAL DISOR-
a
DERS
4.1% (9/220) 0.9% (1/111) 10.9% (24/220) 5.4% (6/111)
ABDOMINAL PAIN 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.9% (1/111)
ANAL FISTULA 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ASCITES 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
COLONIC POLYP 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
DIABETIC GASTROPARESIS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
DIARRHOEA 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
DYSPHAGIA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
GASTRIC PERFORATION 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
GASTRIC ULCER 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
GASTRITIS EROSIVE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
GASTROINTESTINAL HAE-
0.9% (2/220) 0.9% (1/111) 3.2% (7/220) 1.8% (2/111)
MORRHAGE
GASTROINTESTINAL ULCER
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
HAEMORRHAGE
GASTROOESOPHAGEAL
0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
REFLUX DISEASE
HAEMATOCHEZIA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ILEUS 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
IMPAIRED GASTRIC EMPTY-
0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ING
INTESTINAL INFARCTION 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
INTESTINAL ISCHAEMIA 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
INTESTINAL OBSTRUCTION 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
LARGE INTESTINE PERFORA-
0.5% (1/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
TION
MELAENA 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
PANCREATITIS 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PERITONITIS 0.5% (1/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
PROCTITIS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.9% (1/111)
RECTAL HAEMORRHAGE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
SALIVARY GLAND FISTULA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
SALIVARY GLAND MASS 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
SMALL INTESTINAL
0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.9% (1/111)
OBSTRUCTION
UMBILICAL HERNIA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
UPPER GASTROINTESTINAL
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
HAEMORRHAGE
VARICES OESOPHAGEAL 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
GENERAL DISORDERS AND
ADMINISTRATION SITE CONDITIONS
a
5.5% (12/220) 4.5% (5/111) 11.4% (25/220) 9.9% (11/111)
ADVERSE DRUG REACTION 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
CHEST PAIN 0.9% (2/220) 0.9% (1/111) 5.0% (11/220) 4.5% (5/111)
DEATH 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.9% (1/111)
30 Instructions for Use English
Page 33

Through 1-Year Through 5-Year
Serious Adverse Events IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
DEVICE DISLOCATION 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
DEVICE OCCLUSION 0.5% (1/220) 0.9% (1/111) 0.5% (1/220) 0.9% (1/111)
GENERALISED OEDEMA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
IMPAIRED HEALING 0.5% (1/220) 0.9% (1/111) 1.4% (3/220) 0.9% (1/111)
IMPLANT SITE THROMBOSIS 0.5% (1/220) 0.0% (0/111) 0.0% (0/220) 0.0% (0/111)
MASS 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.0% (0/111)
MULTI-ORGAN FAILURE 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
NECROSIS 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
EDEMA PERIPHERAL 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.9% (1/111)
POLYP 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PYREXIA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
SUDDEN DEATH 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
THROMBOSIS IN DEVICE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.9% (1/111)
VESSEL PUNCTURE SITE
0.9% (2/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
HAEMATOMA
HEPATOBILIARY DISORDERS
a
0.9% (2/220) 0.9% (1/111) 1.8% (4/220) 0.9% (1/111)
BILE DUCT OBSTRUCTION 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
CHOLECYSTITIS 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
CHOLECYSTITIS ACUTE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
HEPATIC CIRRHOSIS 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
HEPATIC MASS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
LIVER DISORDER 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PERFORATION BILE DUCT 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PORCELAIN GALLBLADDER 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
IMMUNE SYSTEM DISORDERS
KIDNEY TRANSPLANT REJEC-
a
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
TION
INFECTIONS AND INFESTATIONS
a
3.6% (8/220) 1.8% (2/111) 16.4% (36/220) 13.5% (15/111)
ABSCESS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
APPENDICEAL ABSCESS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
APPENDICITIS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ARTHRITIS BACTERIAL 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ARTHRITIS INFECTIVE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
BILIARY SEPSIS 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
BRONCHIECTASIS 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
BRONCHITIS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
BRONCHOPNEUMONIA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.9% (1/111)
CELLULITIS 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 2.7% (3/111)
CLOSTRIDIAL INFECTION 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
CLOSTRIDIUM DIFFICILE COL-
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ITIS
CYSTITIS 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
DEVICE RELATED INFECTION 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
DIVERTICULITIS 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
EAR INFECTION 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
GANGRENE 0.9% (2/220) 0.0% (0/111) 0.9% (2/220) 0.9% (1/111)
GASTROENTERITIS 0.5% (1/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
HERPES ZOSTER 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
INFECTED LYMPHOCELE 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
INFECTION 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
LOBAR PNEUMONIA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
Instructions for Use English 31
Page 34

Through 1-Year Through 5-Year
Serious Adverse Events IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
LOCALISED INFECTION 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
MEDIASTINITIS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ORCHITIS 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
OSTEOMYELITIS 0.0% (0/220) 0.9% (1/111) 0.9% (2/220) 3.6% (4/111)
PNEUMONIA 0.5% (1/220) 0.0% (0/111) 5.5% (12/220) 2.7% (3/111)
PNEUMONIA PRIMARY ATYPI-
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
CAL
POST PROCEDURAL INFEC-
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
TION
SEPSIS 0.5% (1/220) 0.0% (0/111) 2.3% (5/220) 3.6% (4/111)
SEPTIC SHOCK 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
STAPHYLOCOCCAL OSTEO-
0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
MYELITIS
SUBCUTANEOUS ABSCESS 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
UPPER RESPIRATORY TRACT
0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
INFECTION
URINARY TRACT INFECTION 0.5% (1/220) 0.0% (0/111) 0.9% (2/220) 1.8% (2/111)
UROSEPSIS 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 1.8% (2/111)
WEST NILE VIRAL INFECTION 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
INJURY, POISONING AND PROCEDURAL COMPLICATIONS
a
5.9% (13/220) 12.6% (14/111) 21.8% (48/220) 24.3% (27/111)
ANAEMIA POSTOPERATIVE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ARTERIAL RESTENOSIS 0.5% (1/220) 3.6% (4/111) 5.5% (12/220) 13.5% (15/111)
BRAIN CONTUSION 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
BURNS SECOND DEGREE 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
CLAVICLE FRACTURE 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
CONTUSION 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
DELAYED RECOVERY FROM
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ANAESTHESIA
FACIAL BONES FRACTURE 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
FALL 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
FEMORAL NECK FRACTURE 0.5% (1/220) 0.9% (1/111) 1.4% (3/220) 0.9% (1/111)
FIBULA FRACTURE 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
FRACTURED COCCYX 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
HIP FRACTURE 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.9% (1/111)
IN-STENT ARTERIAL RESTE-
1.4% (3/220) 0.9% (1/111) 7.3% (16/220) 0.9% (1/111)
NOSIS
IN-STENT CORONARY
0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ARTERY RESTENOSIS
INCISIONAL HERNIA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
LUMBAR VERTEBRAL FRAC-
0.5% (1/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
TURE
OVERDOSE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PATELLA FRACTURE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PERIPHERAL ARTERIAL
0.0% (0/220) 3.6% (4/111) 0.9% (2/220) 1.8% (2/111)
REOCCLUSION
POST PROCEDURAL HAE-
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
MORRHAGE
RENAL INJURY 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
RIB FRACTURE 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 2.7% (3/111)
SHUNT MALFUNCTION 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
SHUNT STENOSIS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
32 Instructions for Use English
Page 35

Through 1-Year Through 5-Year
Serious Adverse Events IN.PACT DCB
(N=220 Subjects)
SPINAL COMPRESSION
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.9% (1/111)
Standard PTA
(N=111 Subjects)
IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
FRACTURE
SUBDURAL HAEMATOMA 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
TRACHEAL INJURY 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
UPPER LIMB FRACTURE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
URETHRAL INJURY 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
VASCULAR GRAFT OCCLU-
0.5% (1/220) 0.0% (0/111) 0.9% (2/220) 0.9% (1/111)
SION
VASCULAR PSEUDOANEUR-
1.4% (3/220) 2.7% (3/111) 2.7% (6/220) 2.7% (3/111)
YSM
INVESTIGATIONS
a
GENERAL PHYSICAL CONDI-
0.0% (0/220) 0.9% (1/111) 0.9% (2/220) 1.8% (2/111)
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
TION ABNORMAL
INTERNATIONAL NORMAL-
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ISED RATIO INCREASED
OXYGEN SATURATION
0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
DECREASED
PROSTATIC SPECIFIC ANTI-
0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
GEN INCREASED
METABOLISM AND NUTRITION
DISORDERS
a
1.4% (3/220) 0.0% (0/111) 1.4% (3/220) 0.0% (0/111)
DEHYDRATION 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
DIABETIC FOOT 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
FLUID RETENTION 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
HYPERCALCAEMIA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
HYPERGLYCAEMIA 0.5% (1/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
HYPERKALAEMIA 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
HYPONATRAEMIA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
OBESITY 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
URAEMIC ACIDOSIS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
MUSCULOSKELETAL AND CONNECTIVE TISSUE DISORDERS
a
4.5% (10/220) 4.5% (5/111) 8.2% (18/220) 9.9% (11/111)
ARTHRALGIA 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
BACK PAIN 0.5% (1/220) 0.9% (1/111) 0.5% (1/220) 2.7% (3/111)
BURSITIS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
EXOSTOSIS 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
FISTULA 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
INTERVERTEBRAL DISC PRO-
0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
TRUSION
LUMBAR SPINAL STENOSIS 0.9% (2/220) 0.9% (1/111) 0.9% (2/220) 1.8% (2/111)
MUSCULOSKELETAL PAIN 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.9% (1/111)
OSTEOARTHRITIS 0.9% (2/220) 0.0% (0/111) 1.4% (3/220) 0.9% (1/111)
PAIN IN EXTREMITY 0.9% (2/220) 0.9% (1/111) 3.2% (7/220) 0.9% (1/111)
PATELLOFEMORAL PAIN SYN-
0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
DROME
RHABDOMYOLYSIS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
SPINAL COLUMN STENOSIS 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 1.8% (2/111)
SPINAL OSTEOARTHRITIS 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
SPONDYLOLISTHESIS 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
SYNOVIAL CYST 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
TENOSYNOVITIS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
Instructions for Use English 33
Page 36

Through 1-Year Through 5-Year
Serious Adverse Events IN.PACT DCB
(N=220 Subjects)
NEOPLASMS BENIGN, MALIGNANT AND UNSPECIFIED (INCL
CYSTS AND POLYPS)
a
0.9% (2/220) 4.5% (5/111) 8.6% (19/220) 13.5% (15/111)
Standard PTA
(N=111 Subjects)
IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
ADENOCARCINOMA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ADENOCARCINOMA PAN-
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
CREAS
B-CELL LYMPHOMA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
BASAL CELL CARCINOMA 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
BLADDER CANCER 0.0% (0/220) 0.9% (1/111) 0.5% (1/220) 0.9% (1/111)
BLADDER NEOPLASM 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
BLADDER TRANSITIONAL
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
CELL CARCINOMA
BREAST CANCER 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
BRONCHIAL CARCINOMA 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.9% (1/111)
COLON CANCER 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.9% (1/111)
COLON CANCER METASTATIC 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
GASTROINTESTINAL CARCI-
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
NOMA
KERATOACANTHOMA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
LEUKAEMIA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
LIPOMA 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
LUNG NEOPLASM 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
LUNG NEOPLASM MALIG-
0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 1.8% (2/111)
NANT
LUNG SQUAMOUS CELL CAR-
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 1.8% (2/111)
CINOMA STAGE UNSPECIFIED
MYELOID LEUKAEMIA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
NEOPLASM MALIGNANT 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
NON-SMALL CELL LUNG CAN-
0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
CER
PROSTATE CANCER 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
RECTAL ADENOMA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
RENAL CANCER 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
RENAL CELL CARCINOMA 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
SALIVARY GLAND NEOPLASM 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
SMALL CELL LUNG CANCER
0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
STAGE UNSPECIFIED
TONSIL CANCER 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
a
NERVOUS SYSTEM DISORDERS
5.0% (11/220) 6.3% (7/111) 19.1% (42/220) 14.4% (16/111)
AMNESIA 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
BALANCE DISORDER 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
BRAIN STEM STROKE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
CAROTID ARTERY DISEASE 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 1.8% (2/111)
CAROTID ARTERY STENOSIS 0.5% (1/220) 1.8% (2/111) 3.6% (8/220) 4.5% (5/111)
CARPAL TUNNEL SYNDROME 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
CEREBRAL INFARCTION 0.5% (1/220) 0.9% (1/111) 0.5% (1/220) 0.9% (1/111)
CEREBROVASCULAR ACCI-
0.5% (1/220) 0.0% (0/111) 1.8% (4/220) 1.8% (2/111)
DENT
DEMENTIA 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
DIZZINESS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
EMBOLIC CEREBRAL INFARC-
0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
TION
34 Instructions for Use English
Page 37

Through 1-Year Through 5-Year
Serious Adverse Events IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
ENCEPHALOPATHY 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.9% (1/111)
HAEMORRHAGE INTRACRA-
0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
NIAL
HAEMORRHAGIC STROKE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
HEPATIC ENCEPHALOPATHY 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.9% (1/111)
HYPOAESTHESIA 0.5% (1/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
INTRACRANIAL HAEMATOMA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
LOSS OF CONSCIOUSNESS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
LUMBAR RADICULOPATHY 0.5% (1/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
ORTHOSTATIC INTOLERANCE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PARAESTHESIA 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
PARKINSON'S DISEASE 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
PARTIAL SEIZURES 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PERIODIC LIMB MOVEMENT
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
DISORDER
PRESYNCOPE 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
REVERSIBLE POSTERIOR
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
LEUKOENCEPHALOPATHY
SYNDROME
SYNCOPE 0.5% (1/220) 1.8% (2/111) 2.7% (6/220) 2.7% (3/111)
TRANSIENT ISCHAEMIC
1.4% (3/220) 0.0% (0/111) 1.8% (4/220) 0.0% (0/111)
ATTACK
TREMOR 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
VISUAL PATHWAY DISORDER 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
PSYCHIATRIC DISORDERS
a
0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 2.7% (3/111)
COMPLETED SUICIDE 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
DELIRIUM TREMENS 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
MENTAL STATUS CHANGES 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
RENAL AND URINARY DISOR-
a
DERS
0.5% (1/220) 2.7% (3/111) 8.2% (18/220) 6.3% (7/111)
CALCULUS URETERIC 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
CALCULUS URINARY 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
CYSTITIS HAEMORRHAGIC 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
HAEMATURIA 0.0% (0/220) 0.9% (1/111) 0.9% (2/220) 1.8% (2/111)
HAEMORRHAGE URINARY
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
TRACT
HYDRONEPHROSIS 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
NEPHROLITHIASIS 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
RENAL ARTERY STENOSIS 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.9% (1/111)
RENAL COLIC 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.0% (0/111)
RENAL CYST 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
RENAL FAILURE 0.0% (0/220) 0.9% (1/111) 1.8% (4/220) 2.7% (3/111)
RENAL FAILURE ACUTE 0.5% (1/220) 0.0% (0/111) 3.6% (8/220) 0.9% (1/111)
RENAL FAILURE CHRONIC 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
REPRODUCTIVE SYSTEM AND
BREAST DISORDERS
a
BENIGN PROSTATIC HYPER-
0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.9% (1/111)
0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
PLASIA
POSTMENOPAUSAL HAE-
0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
MORRHAGE
RESPIRATORY, THORACIC AND
MEDIASTINAL DISORDERS
a
1.4% (3/220) 0.9% (1/111) 12.7% (28/220) 9.0% (10/111)
Instructions for Use English 35
Page 38

Through 1-Year Through 5-Year
Serious Adverse Events IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
ACUTE PULMONARY EDEMA 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ACUTE RESPIRATORY FAIL-
0.0% (0/220) 0.0% (0/111) 3.2% (7/220) 0.9% (1/111)
URE
CHRONIC OBSTRUCTIVE
0.5% (1/220) 0.0% (0/111) 2.3% (5/220) 2.7% (3/111)
PULMONARY DISEASE
DYSPNOEA 0.0% (0/220) 0.0% (0/111) 1.4% (3/220) 2.7% (3/111)
DYSPNOEA EXERTIONAL 0.5% (1/220) 0.0% (0/111) 1.4% (3/220) 0.0% (0/111)
HAEMOPTYSIS 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
HAEMOTHORAX 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
PLEURAL EFFUSION 0.0% (0/220) 0.0% (0/111) 1.4% (3/220) 0.0% (0/111)
PNEUMONIA ASPIRATION 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
PNEUMONITIS 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
PNEUMOTHORAX 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PULMONARY CONGESTION 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PULMONARY EMBOLISM 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PULMONARY FIBROSIS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PULMONARY HYPERTENSION 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PULMONARY OEDEMA 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
RESPIRATORY FAILURE 0.5% (1/220) 0.9% (1/111) 1.8% (4/220) 0.9% (1/111)
SLEEP APNOEA SYNDROME 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
SKIN AND SUBCUTANEOUS TISSUE DISORDERS
a
0.0% (0/220) 1.8% (2/111) 1.4% (3/220) 3.6% (4/111)
DECUBITUS ULCER 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
DRY GANGRENE 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
INGROWING NAIL 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
LICHENIFICATION 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
NEUROPATHIC ULCER 0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
SKIN NECROSIS 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
SKIN ULCER 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.9% (1/111)
SUBCUTANEOUS EMPHY-
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
SEMA
SURGICAL AND MEDICAL PROCEDURES
a
0.9% (2/220) 0.9% (1/111) 1.4% (3/220 5.4% (6/111)
ANGIOPLASTY 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
CAROTID ENDARTERECTOMY 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
CORONARY REVASCULARI-
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
SATION
DEBRIDEMENT 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
IMPLANTABLE DEFIBRILLA-
0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
TOR REPLACEMENT
JOINT SURGERY 0.5% (1/220) 0.0% (0/111) 0.0% (0/220) 0.0% (0/111)
PERIPHERAL ARTERY ANGIO-
0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
PLASTY
PERIPHERAL REVASCULARI-
0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
SATION
REMOVAL OF FOREIGN BODY
0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
FROM EYE
THERAPEUTIC EMBOLISA-
0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
TION
VASCULAR DISORDERS
a
22.7% (50/220) 35.1% (39/111) 46.8% (103/220) 57.7% (64/111)
AORTIC ANEURYSM 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.9% (1/111)
AORTIC DILATATION 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
AORTIC STENOSIS 0.0% (0/220) 0.0% (0/111) 0.9% (2/220) 2.7% (3/111)
36 Instructions for Use English
Page 39

Through 1-Year Through 5-Year
Serious Adverse Events IN.PACT DCB
(N=220 Subjects)
ARTERIAL OCCLUSIVE DIS-
0.5% (1/220) 0.0% (0/111) 0.9% (2/220) 0.0% (0/111)
Standard PTA
(N=111 Subjects)
IN.PACT DCB
(N=220 Subjects)
Standard PTA
(N=111 Subjects)
EASE
ARTERIAL STENOSIS LIMB 0.5% (1/220) 2.7% (3/111) 3.2% (7/220) 5.4% (6/111)
ARTERIAL THROMBOSIS LIMB 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 1.8% (2/111)
ARTERIOVENOUS FISTULA 0.0% (0/220) 1.8% (2/111) 0.0% (0/220) 2.7% (3/111)
ARTERY DISSECTION 3.2% (7/220) 1.8% (2/111) 5.5% (12/220) 6.3% (7/111)
DEEP VEIN THROMBOSIS 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
EMBOLISM 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
FEMORAL ARTERIAL STENO-
6.8% (15/220) 9.0% (10/111) 18.6% (41/220) 20.7% (23/111)
SIS
FEMORAL ARTERY DISSEC-
1.8% (4/220) 4.5% (5/111) 1.4% (3/220) 1.8% (2/111)
TION
FEMORAL ARTERY OCCLU-
1.4% (3/220) 4.5% (5/111) 5.9% (13/220) 9.0% (10/111)
SION
HAEMATOMA 0.0% (0/220) 0.9% (1/111) 0.9% (2/220) 0.9% (1/111)
HAEMORRHAGE 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
HYPERTENSION 0.0% (0/220) 0.0% (0/111) 1.4% (3/220) 0.0% (0/111)
HYPERTENSIVE CRISIS 0.0% (0/220) 0.0% (0/111) 2.3% (5/220) 2.7% (3/111)
HYPOTENSION 0.5% (1/220) 0.0% (0/111) 0.9% (2/220) 0.9% (1/111)
ILIAC ARTERY OCCLUSION 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
ILIAC ARTERY STENOSIS 0.9% (2/220) 0.9% (1/111) 5.0% (11/220) 3.6% (4/111)
INTERMITTENT CLAUDICA-
3.2% (7/220) 9.9% (11/111) 9.1% (20/220) 13.5% (15/111)
TION
LABILE HYPERTENSION 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
LYMPHOCELE 0.0% (0/220) 0.0% (0/111) 0.0% (0/220) 0.9% (1/111)
ORTHOSTATIC HYPOTENSION 0.5% (1/220) 0.9% (1/111) 0.5% (1/220) 0.9% (1/111)
PERIPHERAL ARTERIAL
3.6% (8/220) 4.5% (5/111) 9.5% (21/220) 8.1% (9/111)
OCCLUSIVE DISEASE
PERIPHERAL ARTERY DIS-
1.4% (3/220) 3.6% (4/111) 1.8% (4/220) 1.8% (2/111)
SECTION
PERIPHERAL EMBOLISM 0.5% (1/220) 0.9% (1/111) 0.5% (1/220) 0.9% (1/111)
PERIPHERAL ISCHAEMIA 0.9% (2/220) 1.8% (2/111) 1.8% (4/220) 3.6% (4/111)
PERIPHERAL VASCULAR DIS-
0.9% (2/220) 0.0% (0/111) 3.6% (8/220) 3.6% (4/111)
ORDER
SHOCK HAEMORRHAGIC 0.5% (1/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
SUBCLAVIAN ARTERY
0.0% (0/220) 0.9% (1/111) 0.0% (0/220) 0.9% (1/111)
STENOSIS
VENOUS INSUFFICIENCY 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
VESSEL PERFORATION 0.0% (0/220) 0.0% (0/111) 0.5% (1/220) 0.0% (0/111)
TOTAL SERIOUS ADVERSE
195 118 770 363
EVENTS
Numbers are % (counts/sample size).
a
Event verbatim terms are reported by sites. The events listed in this table are then coded using MedDRA version 13.0 and then stratified by System-Organ Class (SOC) and Preferred Term. Patients may be
counted in this table more than once by Preferred Term, but are only counted once in the SOC summary line.
11.4. Pharmacokinetic Sub-study
Human pharmacokinetics was investigated as a sub-study of the IN.PACT SFA Trial. This sub-study was a prospective, multicenter, non-randomized study arm (IN.PACT Admiral DCB) conducted at multiple prespecified investigational sites, designed to
evaluate the levels of paclitaxel in the systemic circulation of subjects at multiple time points. Pharmacokinetic parameters were
determined for a total of 24 subjects (16 male and 8 female). A summary of the pharmacokinetic parameters is presented in
Summary of Pharmacokinetic Parameters (Table 15). The pharmacokinetic sub-study demonstrated low systemic exposure
with rapid clearance of paclitaxel.
Instructions for Use English 37
Page 40

Table 15. Summary of Pharmacokinetic Parameters
Parameter Mean (N=24) Standard Deviation %CV Range
T
(hr) 0.17 0.067 38.8 0.07 – 0.32
max
C
(ng/mL) 7.9 7.70 97.9 1.0 - 35.9
max
AUC
AUC
T
(hr*ng/mL) 29.4 22.06 75.0 3.2 – 91.6
0-last
(hr*ng/mL) 47.8 28.98 60.6 11.4 – 128.8
0-inf
(hr) 72.5 39.70 54.7 8.2 – 153.5
1/2
CL/F (L/hr) 192.2 103.44 53.8 54.7 – 472.7
T
(hr) The timepoint where Cmax is reached
max
C
(ng/mL) Maximum plasma concentration
max
AUC
AUC
T
(hr*ng/mL) Area under plasma concentration-time curve from time zero to time of last measurable concentration
0-last
(hr*ng/mL) Area under the plasma concentration-time curve from time zero extrapolated to infinity
0-inf
(hr) Terminal half-life
1/2
CL/F (L/hr) Apparent clearance
11.5. IN.PACT Global Study
11.5.1. Study Overview
The IN.PACT Global Study is a prospective, multi-center, single-arm study designed to collect and assess global safety and
effectiveness data on the IN.PACT Admiral DCB in treatment of atherosclerotic disease of the superficial femoral and/or
popliteal arteries in a “real-world” population. The study is estimated to continue follow-up through 2019. The study will enroll
approximately 1500 subjects at more than 60 sites in Europe, Australia, Asia, Northern Africa, Canada, the Middle East, and
South America. Follow-up will be completed at 30 days, 6 months, and 12 months and 2, 3, 4, and 5 years. The interim data
from the IN.PACT Global Study that were available at the time were provided to FDA for consideration as part of the PMA
submission.
11.6. Summary of Rare Adverse Events
Medtronic has provided an evaluation of rare adverse events (RAE) in more than 800 subjects from the IN.PACT SFA Trial, the
IN.PACT SFA PK Sub-study, and the IN.PACT Global Study.
The following RAEs were adjudicated by the independent Clinical Events Committees (CEC): paclitaxel-related vessel
thrombosis within 30 days, paclitaxel-related distal embolic events within 360 days, paclitaxel-related neutropenia within
360 days, and paclitaxel-related drug hypersensitivity/reaction within 360 days. The rate of paclitaxel-related thrombosis within
30 days was 0.2% (2/890). There were no paclitaxel-related distal embolic events within 360 days (0/806), paclitaxel-related
neutropenia within 360 days (0/806), or paclitaxel-related drug hypersensitivity/reaction within 360 days (0/806).
The current RAE outcomes demonstrate no increased risk of adverse events due to the paclitaxel coating.
11.7. IN.PACT Admiral DCB ISR Clinical Evaluation
11.7.1. Primary Objective
The objective of this clinical evaluation was to assess the safety and effectiveness of the IN.PACT Admiral DCB as compared
with PTA when used to treat in-stent restenotic (ISR) lesions of the superficial femoral artery (SFA) or popliteal artery.
11.7.2. Design
This clinical evaluation was designed as an observational, propensity score-adjusted, comparative analysis of IN.PACT Admiral
DCB subjects selected from the real-world IN.PACT Global Study (“DCB ISR Cohort”) and PTA subjects provided from the
Society of Vascular Surgery (SVS) Vascular Quality Initiative (VQI) Registry database (“PTA ISR Comparator”).
A total of 164 DCB subjects from the IN.PACT Global Study comprised the DCB ISR Cohort, and a total of 153 PTA subjects
from the SVS VQI Registry comprised the PTA ISR Comparator. Patients in the DCB ISR Cohort were treated at 31 sites in
Austria, Belgium, Canada, Egypt, Germany, Hungary, Italy, The Netherlands, Poland, Singapore, Slovakia, South Korea, and
Switzerland between June 6, 2012 and December 16, 2013, and patients in the PTA ISR Comparator were treated at 23 sites
in the United States between 2011 and 2014.
The objective of this analysis was to demonstrate that the primary endpoint of 12-month target lesion revascularization (TLR)
was significantly lower in the DCB ISR Cohort when compared to the PTA ISR Comparator group for the treatment of ISR.
For the primary effectiveness endpoint of 12-month TLR, the treatment (DCB ISR Cohort) and control (PTA ISR Comparator)
groups were compared in a superiority format. Formally, the hypothesis tested was:
38 Instructions for Use English
Page 41

H0: 12-month TLR rate in subjects in DCB ISR Cohort (pT) is equal to or higher than that for subjects in PTA ISR Comparator
(pC).
H0: pT ≥ p
C
HA: 12-month TLR rate in subjects in DCB ISR Cohort (pT) is lower than that for subjects in PTA ISR Comparator (pC).
HA: pT < p
C
Due to the nature of this non-randomized retrospective comparison, it was necessary to adjust for expected baseline
differences between the subjects in the two groups to ensure objectivity of the clinical evaluation design and the validity of the
results. As pre-specified in the statistical analysis plan, propensity score analysis was performed using clinically relevant
baseline characteristics. The propensity score calculation was carried out by an independent statistician without access to the
outcomes of either group. The calculation results were submitted to the FDA for review and approval prior to performing the
primary endpoint analyses.
The primary endpoint of the powered statistical analysis comparing ISR outcomes in the DCB ISR Cohort and the PTA ISR
Comparator was the incidence of TLR through 12 months.
The clinically relevant secondary endpoints assessed included:
1. All-cause mortality at 30 days, 6 months, and 12 months.
2. Any TVR at 30 days, 6 months, and 12 months.
3. Major target limb amputation at 30 days, 6 months, and 12 months.
4. Time to first TLR through 12 months post-index procedure.
5. Time to all-cause mortality through 12 months post-index procedure.
With regard to success criteria, the study was deemed successful if it demonstrated superiority of the DCB ISR Cohort on the
12-month primary endpoint of target lesion revascularization compared to the PTA ISR Comparator.
11.7.3. Patient Population
Table 16 below presents the baseline demographics and clinical characteristics for the 164 DCB ISR Cohort subjects and the
153 PTA ISR Comparator subjects. These 20 baseline variables were pre-specified as the covariates in the propensity score
analysis, and all of the variables were included in the propensity score calculation except for the TASC lesion type due to a
missing data rate in the DCB ISR Cohort that exceeded the pre-specified cutoff of 20%.
Table 16. Baseline Demographics and Clinical Characteristics
Baseline Characteristics DCB ISR Cohort
(N=164)
PTA ISR Comparator
(N=153)
Propensity Score
Adjusted p-value
Baseline Demographics
Age (yrs)
Mean±SD (N) 66.95±9.84 (163) 66.79±11.23 (153) 0.795
Median (Q1, Q3) 67.00 (60.00,74.00) 66.00 (58.00,75.00)
Range (Min, Max) (39.00,86.00) (44.00,89.00)
Male 72.6% (119/164) 51.6% (79/153) 0.536
BMI (kg/m2)
Mean±SD (N) 26.34±4.39 (164) 28.21±6.06 (153) 0.835
Median (Q1, Q3) 25.94 (23.63,28.57) 28.00 (24.00,30.00)
Range (Min, Max) (16.46,43.26) (13.00,50.00)
Ankle-Brachial Index (ABI) (mmHg ratio)
Mean±SD (N) 0.64±0.22 (147) 0.70±0.41 (140) 0.786
Median (Q1, Q3) 0.65 (0.51,0.77) 0.64 (0.48,0.81)
Range (Min, Max) (0.00,1.43) (0.00,2.00)
Baseline Clinical Characteristics
Hypertension 83.4% (136/163) 87.6% (134/153) 0.972
Diabetes 36.6% (60/164) 49.0% (75/153) 0.920
Insulin-dependent Diabetes 16.5% (27/164) 22.9% (35/153) 0.946
On Dialysis 3.0% (5/164) 1.3% (2/153) 0.579
Coronary artery disease 20.5% (30/146) 24.2% (37/153) 0.964
Current smoker 36.0% (59/164) 32.7% (50/153) 0.915
Previous limb amputation (major or
5.5% (9/164) 4.6% (7/153) 0.980
minor)
a
Instructions for Use English 39
Page 42

Baseline Characteristics DCB ISR Cohort
(N=164)
Renal insufficiency (per serum creatinine
9.5% (14/147) 9.9% (15/151) 0.931
PTA ISR Comparator
(N=153)
Propensity Score
Adjusted p-value
a
≥1.5 mg/dl)
Baseline Lesion and Procedural Characteristics
TASC lesion type 0.906
A 20.2% (21/104) 30.7% (47/153)
B 30.8% (32/104) 27.5% (42/153)
C 36.5% (38/104) 30.1% (46/153)
D 12.5% (13/104) 11.8% (18/153)
Lesion length (cm)
Mean±SD (N) 17.59±10.49 (164) 13.16±9.61 (151) 0.735
Median (Q1, Q3) 16.00 (10.00,27.00) 10.00 (6.00,20.00)
Range (Min, Max) (1.00,47.00) (2.00,47.00)
Total occlusion 40.9% (67/164) 68.7% (103/150) 0.990
Occluded lesion length (cm)
Mean±SD (N) 7.47±11.65 (164) 7.87±9.40 (150) 0.709
Median (Q1, Q3) 0.00 (0.00,10.50) 4.00 (0.00,14.00)
Range (Min, Max) (0.00,42.00) (0.00,45.00)
Pre-op aspirin 95.7% (157/164) 83.0% (127/153) 0.422
Indication of Claudication - Rutherford Clas-
87.8% (144/164) 74.5% (114/153) 0.633
sification or equivalent
Lesion Location: SFA 71.3% (117/164) 86.9% (133/153) 0.706
Provisional stent 15.9% (26/164) 33.3% (51/153) 0.814
Numbers are % (counts/sample size) unless otherwise stated.
Categorical variables between groups were compared using the chi-squared test or Fisher's exact test as appropriate, and
continuous variables were compared using Student's t-test.
Site reported data.
All of the variables in this table were included in the propensity score calculation except TASC lesion type due to a missing
data rate that exceeded the pre-specified cutoff of 20%.
p-values are not adjusted for multiplicity
a
The propensity score adjusted p-value was based on all subjects for each baseline variable. For each variable with missing values (<20%), a gender-specific imputation was performed by replacing the missing
values of the variable with the gender-specific median observed value within each group.
Follow-up compliance for the 12-month follow-up visits is presented in Table 17 for the DCB ISR Cohort subjects. The rate of inwindow follow-up visit completion at 12 months was 92.3%.
Table 17. Subject Follow-up Compliance – IN.PACT Global DCB ISR Cohort Subjects
Subject Compliance Characteristics
a
DCB ISR Cohort
(N=164 Subjects)
12-Month Follow-up
Eligible Subjects
c
Death
Withdrawal
b
155
1
c
8
Follow-up Not Done 7
Follow-up Visit Within Window
Follow-up Visit Out of Window
Follow-up Compliance (%)
a
Site reported data
b
Eligible subjects are all subjects who either have a follow-up visit form or are past due for their follow-up (beyond upper limit of window on study and did not exit the study before the upper limit of the window)
c
Death and withdrawal are cumulative
d
Within window visits are defined as: 12-month ± 60 days.
e
Percentage based on number of subjects who had follow-up visit within window divided by total number of eligible subjects
d
d
e
143
5
92.3%
40 Instructions for Use English
Page 43

11.7.4. Safety and Effectiveness Results
Primary Endpoint Analysis
The results of the powered statistical analysis comparing the 12-month primary endpoint between the DCB ISR Cohort and the
PTA ISR Comparator are shown in Table 18.
The primary endpoint of the clinical evaluation was met, demonstrating superiority of the DCB ISR Cohort over the PTA ISR
Comparator on the primary effectiveness endpoint of target lesion revascularization (TLR) at 12 months (10.13% vs. 35.92%,
p<0.001).
Table 18. Primary Effectiveness Endpoint Results
DCB ISR Cohort
(N=164)
PTA ISR Comparator
(N=153)
Hazard Ratio [95% CI]
p-value
a
Effectiveness Parameters
Target Lesion Revascularization at
10.13% (16) 35.92% (51) 0.258 [0.128, 0.517] <0.001
12 Months
Statistical references:
■
Numbers are cumulative incidence % (number of failures) based on Kaplan-Meier method. CI – Confidence Interval
■
Analysis sets: The primary analysis set was based on the intent-to-treat (ITT) principle. All subjects enrolled through the
selection process specified in SAP Section 3.1 were included as ITT subjects.
a
■
To analyze the treatment differences between the DCB ISR Cohort and PTA ISR Comparator groups in the clinical/safety endpoints such as TLR, a propensity-quintile-stratified Cox proportional hazards model was employed, with
time to event as the dependent variable and treatment group as the independent variable.
The Kaplan-Meier analysis of this primary effectiveness endpoint, presented as freedom from target lesion revascularization, is
shown in Figure 11.
Instructions for Use English 41
Page 44

Target Lesion
Revascularization
DCB
# Entered
# Censored
# Events
Survived [%]
PTA
0 1-60 61-120 121-180 181-240 241-300 301-360 361-425
164 163 154 152 148 143 141 126
0
1
1 3
1
1 3
4
5
0 121 3
2
15
7
99.39%
96.27% 95.65% 93.75% 92.47% 91.82% 89.87% 84.53%
# Entered
# Censored
# Events
Survived [%]
153
151
144 138 125 113 90 56
2 6 13 10 64 10 3
98.69% 96.07% 92.06% 83.39% 76.66% 69.40% 64.08% 59.94%
0 03 13 280 2 17
0 60 120 180 240 300 360 425
100%
90%
80%
70%
60%
50%
40%
30%
20%
10%
0%
Time after Initial Procedure (Days)
Freedom from Target
Lesion Revascularization
PTADCB
64.1%
59.9%
89.9%
84.5%
Figure 11. Kaplan-Meier Plot - Event-free from Target Lesion Revascularization through 360 and 425 Days
Secondary Safety and Effectiveness Endpoints
The results of the secondary endpoints for the IN.PACT Admiral DCB ISR Clinical Evaluation are shown in Table 19.
In both the DCB ISR Cohort and the PTA ISR Comparator, there were high rates of acute success. Since the acute success
definitions differed, these were not able to be directly compared, but the DCB ISR Cohort had high rates of device success
(99.5%), procedural success (99.4%), and clinical success (98.8%), while the PTA ISR Comparator had a high rate of technical
success (97.4%). The definitions of each of these endpoints are provided in the footnotes of Table 19.
Clinical safety and effectiveness outcomes were reported at 30 days, 6 months, and 12 months. All event rates were low in
both groups at 30 days, but lower event rates were observed at 6 months and at 12 months for both the TLR and the target
vessel revascularization (TVR) endpoints in the DCB ISR Cohort. The 12-month TVR rate was 11.41% in the DCB ISR Cohort
compared to 38.07% in the PTA ISR Comparator.
There were no major target limb amputations in the DCB ISR Cohort and three major target limb amputations in the PTA ISR
Comparator within 12 months. Lastly, there was one death in the DCB ISR Cohort and no deaths in the PTA ISR Comparator
within 12 months. One subject in the DCB ISR Cohort experienced a non-cardiac death at day 276 post-index procedure. The
independent clinical events committee determined that the event was not device-related and not procedure-related.
42 Instructions for Use English
Page 45

Table 19. Secondary Safety and Effectiveness Endpoint Results
DCB ISR Cohort
PTA ISR Comparator
(N=164)
Effectiveness Parameters
Device Success
a
Procedural Success
Clinical Success
a
Technical Success
a
a
99.5% (364/366) NA
99.4% (163/164) NA
98.8% (162/164) NA
NA 97.4% (149/153)
Safety Parameters
Cumulative complications within 30 days
Death (all-cause) 0.00% (0) 0.00% (0)
Target Vessel Revascularization 0.61% (1) 2.61% (4)
Major Target Limb Amputation 0.00% (0) 0.00% (0)
Target Lesion Revascularization 0.61% (1) 2.61% (4)
Cumulative complications within 180 days
Death (all-cause) 0.00% (0) 0.00% (0)
Target Vessel Revascularization 6.88% (11) 17.28% (26)
Major Target Limb Amputation 0.00% (0) 1.33% (2)
Target Lesion Revascularization 6.25% (10) 16.61% (25)
Cumulative complications within 360 days
Death (all-cause) 0.65% (1) 0.00% (0)
Target Vessel Revascularization 11.41% (18) 38.07% (54)
Major Target Limb Amputation 0.00% (0) 2.08% (3)
(N=153)
Other Major Secondary Endpoints at 12 Months DCB ISR Cohort
(N=164)
PTA ISR Comparator
(N=153)
Time to all-cause mortality (days)
Mean±SD (N) 276.00 (1) -Median 276.00 -(Min, Max) (276.00,276.00) --
Time to first TLR (days)
Mean±SD (N) 148.44±115.25 (16) 182.33±93.53 (51)
Median 146.50 187.00
(Min, Max) (0.00,328.00) (0.00,335.00)
Endpoint definitions:
■
Device success (assessed for DCB ISR Cohort only) defined as successful delivery, balloon inflation and deflation and
retrieval of the intact study device without burst below the rated burst pressure (RBP).
■
Procedure success (assessed for DCB ISR Cohort only) defined as residual stenosis of ≤ 50% (non-stented subjects) or
≤ 30% (stented subjects) by visual estimate.
■
Clinical success (assessed for DCB ISR Cohort only) defined as procedural success without procedural complications
(mortality, major target limb amputation, thrombosis of the target lesion, or TVR) prior to discharge.
■
Technical success (assessed for PTA ISR Comparator only) defined as the ability to cross the lesion without resulting in
occlusion and having residual stenosis ≤ 30% and resting systolic pressure gradient < 10 mmHg (if measured).
Statistical references:
■
Numbers are cumulative incidence % (number of failures) based on Kaplan-Meier method unless otherwise stated. CI –
Confidence Interval
a
■
Numbers are % (counts/sample size)
■
Analysis sets: The primary analysis set was based on the intent-to-treat (ITT) principle. All subjects enrolled through the
selection process specified in SAP Section 3.1 were included as ITT subjects.
Subgroup Analyses
Medtronic has analyzed the clinical evaluation results by the male and female gender subgroups. Both the male and female
gender subgroups showed favorable trends on the primary effectiveness endpoint of 12-month TLR (male subgroup: 8.72%
DCB vs. 32.94% PTA, and female subgroup: 14.08% DCB vs. 39.11% PTA). Favorable clinical trends were also noted for the
Instructions for Use English 43
Page 46

secondary endpoint of 12-month TVR (male subgroup: 10.48% DCB vs. 34.67% PTA, and female subgroup: 14.08% DCB vs.
41.76% PTA).
Summary of Adverse Events
A serious adverse event was defined in the IN.PACT Global Study protocol as an adverse event that led to death; led to serious
deterioration in the health of the subject that resulted in a life-threatening illness or injury, a permanent impairment of a body
structure or a body function, in-patient or prolonged hospitalization, or medical or surgical intervention to prevent lifethreatening illness or injury or permanent impairment to a body structure of a body function; or led to fetal distress, fetal death
or a congenital abnormality or birth defect.
Table 20 provides a summary of serious adverse event rates by system-organ class (SOC) through 360 days occurring in the
DCB ISR Cohort.
Table 20. Serious Adverse Event Rates by SOC and Preferred Term through 360 Days – IN.PACT Global DCB ISR Cohort
Subjects
Serious Adverse Event DCB ISR Cohort
(N=164 Subjects)
Subjects with One or More Serious Adverse Events 41.5% (68/164)
CARDIAC DISORDERS
a
2.4% (4/164)
ACUTE MYOCARDIAL INFARCTION 0.6% (1/164)
ATRIAL FIBRILLATION 0.6% (1/164)
CONGESTIVE CARDIOMYOPATHY 0.6% (1/164)
CORONARY ARTERY DISEASE 0.6% (1/164)
MYOCARDIAL INFARCTION 0.6% (1/164)
CONGENITAL, FAMILIAL AND GENETIC DISORDERS
a
0.6% (1/164)
CONGENITAL CYSTIC KIDNEY DISEASE 0.6% (1/164)
EYE DISORDERS
a
1.2% (2/164)
CATARACT 0.6% (1/164)
RETINAL ARTERY OCCLUSION 0.6% (1/164)
GASTROINTESTINAL DISORDERS
a
1.2% (2/164)
GASTRITIS 0.6% (1/164)
OESOPHAGEAL HAEMORRHAGE 0.6% (1/164)
GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS
a
1.2% (2/164)
DEATH 0.6% (1/164)
DEVICE BREAKAGE 0.6% (1/164)
HEPATOBILIARY DISORDERS
a
1.2% (2/164)
ACUTE HEPATIC FAILURE 0.6% (1/164)
CHOLECYSTITIS 0.6% (1/164)
CHOLELITHIASIS 0.6% (1/164)
INFECTIONS AND INFESTATIONS
a
3.0% (5/164)
CLOSTRIDIUM DIFFICILE COLITIS 0.6% (1/164)
GANGRENE 0.6% (1/164)
GROIN INFECTION 0.6% (1/164)
PILONIDAL CYST 0.6% (1/164)
PNEUMONIA 0.6% (1/164)
PSEUDOMEMBRANOUS COLITIS 0.6% (1/164)
URINARY TRACT INFECTION 0.6% (1/164)
INJURY, POISONING AND PROCEDURAL COMPLICATIONS
a
7.3% (12/164)
ARTERIAL RESTENOSIS 2.4% (4/164)
IN-STENT ARTERIAL RESTENOSIS 4.3% (7/164)
IN-STENT CORONARY ARTERY RESTENOSIS 0.6% (1/164)
PERIPHERAL ARTERIAL REOCCLUSION 0.6% (1/164)
VASCULAR PSEUDOANEURYSM 1.2% (2/164)
INVESTIGATIONS
a
0.6% (1/164)
INTERNATIONAL NORMALISED RATIO INCREASED 0.6% (1/164)
METABOLISM AND NUTRITION DISORDERS
a
1.2% (2/164)
DIABETES MELLITUS 0.6% (1/164)
44 Instructions for Use English
Page 47

Serious Adverse Event DCB ISR Cohort
(N=164 Subjects)
DIABETIC FOOT 0.6% (1/164)
MUSCULOSKELETAL AND CONNECTIVE TISSUE DISORDERS
a
BACK PAIN 0.6% (1/164)
INTERVERTEBRAL DISC PROTRUSION 0.6% (1/164)
MUSCULOSKELETAL DISORDER 0.6% (1/164)
MYOFASCIAL PAIN SYNDROME 0.6% (1/164)
PAIN IN EXTREMITY 0.6% (1/164)
SPINAL COLUMN STENOSIS 0.6% (1/164)
NEOPLASMS BENIGN, MALIGNANT AND UNSPECIFIED (INCL CYSTS AND POLYPS)
a
LUNG NEOPLASM 0.6% (1/164)
LUNG NEOPLASM MALIGNANT 0.6% (1/164)
MALIGNANT NEOPLASM PROGRESSION 0.6% (1/164)
NEOPLASM SKIN 0.6% (1/164)
OESOPHAGEAL CARCINOMA 0.6% (1/164)
PROSTATE CANCER RECURRENT 0.6% (1/164)
NERVOUS SYSTEM DISORDERS
a
CAROTID ARTERY STENOSIS 0.6% (1/164)
CEREBROVASCULAR ACCIDENT 0.6% (1/164)
FACIAL PALSY 0.6% (1/164)
PARAESTHESIA 0.6% (1/164)
RENAL AND URINARY DISORDERS
a
BLADDER TAMPONADE 0.6% (1/164)
NEPHROLITHIASIS 0.6% (1/164)
RENAL COLIC 0.6% (1/164)
REPRODUCTIVE SYSTEM AND BREAST DISORDERS
a
UTERINE POLYP 0.6% (1/164)
RESPIRATORY, THORACIC AND MEDIASTINAL DISORDERS
a
DYSPNOEA 1.2% (2/164)
SURGICAL AND MEDICAL PROCEDURES
a
LEG AMPUTATION 0.6% (1/164)
VASCULAR DISORDERS
a
25.0% (41/164)
ARTERIAL OCCLUSIVE DISEASE 0.6% (1/164)
ARTERIAL STENOSIS LIMB 0.6% (1/164)
ARTERIAL THROMBOSIS 0.6% (1/164)
ARTERIAL THROMBOSIS LIMB 3.0% (5/164)
ARTERIOSCLEROSIS OBLITERANS 0.6% (1/164)
EMBOLISM 0.6% (1/164)
FEMORAL ARTERIAL STENOSIS 6.7% (11/164)
FEMORAL ARTERY OCCLUSION 4.3% (7/164)
HAEMORRHAGE 0.6% (1/164)
HYPERTENSION 0.6% (1/164)
HYPERTENSIVE CRISIS 0.6% (1/164)
INTERMITTENT CLAUDICATION 3.7% (6/164)
PERIPHERAL ARTERIAL OCCLUSIVE DISEASE 4.3% (7/164)
PERIPHERAL EMBOLISM 0.6% (1/164)
PERIPHERAL ISCHAEMIA 3.0% (5/164)
VESSEL PERFORATION 0.6% (1/164)
3.7% (6/164)
3.7% (6/164)
2.4% (4/164)
1.2% (2/164)
0.6% (1/164)
1.2% (2/164)
0.6% (1/164)
Instructions for Use English 45
Page 48

Serious Adverse Event DCB ISR Cohort
(N=164 Subjects)
Total Serious Adverse Events 123
Numbers are % (counts/sample size) unless otherwise stated.
a
Event verbatim terms are reported by sites. The events listed in this table are then coded using MedDRA version 13.0 and
then stratified by System-Organ Class (SOC) and Preferred Term. Patients may be counted in this table more than once by
Preferred Term, but are only counted once in the SOC summary line.
Site reported data.
11.8. IN.PACT Global DCB Long Lesion Sub-Cohort
11.8.1. Primary Objective
The objective of this clinical evaluation was to assess the safety and effectiveness of the IN.PACT Admiral DCB in the
treatment of long restenotic lesions (lesion length >180 mm) in the superficial femoral and popliteal arteries.
11.8.2. Design
This clinical evaluation was designed as an observational, non-randomized, multi-center, single-arm evaluation that is intended
to assess the safety and effectiveness performance of the IN.PACT Admiral DCB for the treatment of de novo, restenotic, or instent restenotic long lesions (lesion length > 180 mm) in the superficial femoral and/or popliteal artery vessels.
A total of 227 DCB subjects from the IN.PACT Global Study Imaging Cohorts (Long Lesion, In-Stent Restenosis and Chronic
Total Occlusions), and meeting specific post-hoc inclusion criteria (including lesion lengths > 180 mm, Rutherford clinical
category 2-4, and single unilateral treated lesions), comprised the DCB Long Lesion Sub-Cohort. Patients in the DCB Long
Lesion Sub-Cohort were treated at 28 sites from 13 countries, including Austria, Belgium, Canada, Colombia, Germany,
Hungary, Italy, The Netherlands, Singapore, Slovakia, South Korea, Switzerland and the United Kingdom. The 227 DCB
subjects were enrolled at these IN.PACT Global Study sites between 6 June 2012 and 16 December 2013.
The primary effectiveness endpoint is Primary Patency within 12 months post-index procedure, which is defined as:
■
Freedom from clinically-driven TLR, and
■
Freedom from restenosis as determined by DUS Peak Systolic Velocity Ratio (PSVR) ≤ 2.4.
The Primary Safety Endpoint is a composite endpoint through 12 months. Composite Safety Endpoint is defined as freedom
from device- and procedure-related death through 30 days post procedure and freedom from target limb major amputation and
clinically-driven TVR within 12 months post index procedure.
The secondary endpoints include:
■
Major Adverse Events (MAE) through 12 months.
MAE is defined as all-cause death, clinically-driven TVR, major target limb amputation, thrombosis at the target lesion site
■
All-cause mortality at 30 days, 6 months, and 12 months.
■
CD-TLR5 at 30 days, 6 months, and 12 months.
■
Any TVR at 30 days, 6 months and 12 months.
■
CD-TVR at 30 days, 6 months and 12 months.
■
Thrombosis at the target lesion site at 30 days, 6 months and 12 months.
■
Major target limb amputation at 30 days, 6 months and 12 months.
■
Time to first TLR through 12 months post-index procedure.
■
Time to all-cause mortality through 12 months post-index procedure.
■
Primary sustained clinical improvement6 at 6 and 12 months.
■
Secondary sustained clinical improvement7 at 6 and 12 months.
■
Walking impairment evaluation by Walking Impairment Questionnaire (WIQ) at 6 and 12 months.
■
Walking distance as measured by 6 Minute Walk Test at 6 and 12 months.
■
Device success
■
Procedural success
■
Clinical success
■
Binary restenosis within 12 months post-index procedure, which is determined by DUS PSVR > 2.4.
8
9
10
5
Clinically-driven TLR is defined as any re-intervention within the target lesion due to symptoms or drop of ABI of ≥ 20% or > 0.15 when compared to post-index procedure baseline ABI.
6
Primary sustained clinical improvement is defined as sustained upward shift of at least 1 category on Rutherford classification as compared to baseline without the need for repeated TLR or surgical
revascularization in amputation-free surviving subjects.
7
Secondary sustained clinical improvement is defined as sustained upward shift of at least 1 category on Rutherford classification as compared to baseline including the need for repeated TLR or surgical
revascularization in amputation-free surviving subjects.
8
Device success is defined as successful delivery, balloon inflation and deflation and retrieval of the intact study device without burst below the rated burst pressure (RBP).
9
Procedural success is defined as residual stenosis of ≤ 50% (non-stented subjects) or ≤ 30% (stented subjects) by visual estimate.
10
Clinical success is defined as procedural success without procedural complications (mortality, major target limb amputation, thrombosis of the target lesion, or TVR) prior to discharge.
46 Instructions for Use English
Page 49

11.8.3. Patient Population
Subject demographics, medical history, and risk factors of the 227 IN.PACT Global DCB Long Lesion Sub-Cohort subjects are
summarized in Baseline Demographics and Clinical Characteristics (Table 21).
Table 21. Baseline Demographics and Clinical Characteristics- IN.PACT Global DCB Long Lesion Sub-Cohort Subjects
Subject Characteristics
a
IN.PACT Admiral DCB
(N=227 Subjects)
Age (yrs)
N 226
Mean ± SD 68.8 ± 9.7
Median 69.0
Min, Max 37, 90
BMI (kg/m²)
N 222
Mean ± SD 26.7 ± 4.6
Median 26.4
Min, Max 16, 47
Obesity (BMI ≥ 30 kg/m²) 20.7% (46/222)
Male 67.4% (153/227)
Hypertension 86.7% (195/225)
Hyperlipidemia 71.7% (157/219)
Diabetes Mellitus 38.7% (87/225)
Insulin Dependent Diabetes Mellitus 18.2% (41/225)
Carotid Artery Disease 24.0% (49/204)
Coronary Heart Disease 40.6% (89/219)
Current Smoker 42.7% (97/227)
Renal Insufficiency (baseline serum creatinine ≥ 1.5 mg/dl) 12.3% (25/203)
On Dialysis 1.8% (4/226)
Below-the-knee Vascular Disease of Target Leg (Stenotic/Occluded) 48.6% (101/208)
Previous Peripheral Revascularization 58.1% (132/227)
Iliac 21.1% (48/227)
Common Femoral 7.0% (16/227)
Femoral Profunda 1.3% (3/227)
Superficial Femoral 48.9% (111/227)
Popliteal 14.1% (32/227)
Below-the-knee 8.4% (19/227)
Other Location 1.3% (3/227)
Previous Limb Amputation 1.3% (3/227)
Toe 0.4% (1/227)
Transmetatarsal 0.0% (0/227)
Below-the-knee 0.0% (0/227)
Above-the-knee 0.9% (2/227)
Rutherford Category
0 0.0% (0/227)
1 0.0% (0/227)
2 22.5% (51/227)
3 65.2% (148/227)
4 12.3% (28/227)
5 0.0% (0/227)
6 0.0% (0/227)
ABIb (mmHg ratio)
N 198
Mean ± SD 0.625 ± 0.214
Median 0.630
Min, Max 0.00, 1.67
Instructions for Use English 47
Page 50

Subject Characteristics
a
IN.PACT Admiral DCB
(N=227 Subjects)
Numbers are % (counts/sample size) unless otherwise stated.
Rutherford clinical grades and categories:
Grade Category Clinical Description
0 0 Asymptomatic, no hemodynamically significant occlusive dis-
ease
I 1 Mild claudication
I 2 Moderate claudication
I 3 Severe claudication
II 4 Ischemic rest pain
III 5 Minor tissue loss; non-healing ulcer; focal gangrene with dif-
fuse pedal ischemia
III 6 Major tissue loss extending above transmetatarsal level; func-
tional foot no longer salvageable
a
Site reported data
b
ABI for all target limbs treated during the 1st index procedure are included (can be bilateral)
The baseline lesion characteristics, as reported by the sites and angiographic core laboratories, have been provided in
Procedural Lesion Characteristics (Table 22). The mean total target lesion length treated was 28.74 ± 7.11 cm.
Table 22. Procedural Lesion Characteristics – IN.PACT Global DCB Long Lesion Sub-Cohort Subjects
Procedural Lesion Characteristics (per lesion) IN.PACT Admiral DCB (N=227 Subjects) (N=227 Lesions)
Pre-procedure
a
RVD (mm)
N 133
Mean ± SD 4.611 ± 0.896
Median 4.500
Min, Max 2.25, 6.85
MLD (mm)
N 134
Mean ± SD 0.332 ± 0.568
Median 0.000
Min, Max 0.00, 2.65
Occluded Lesion (100% stenosis) 70.1% (157/224)
Diameter Stenosis (%)
N 224
Mean ± SD 94.1 ± 10.7
Median 100.0
Min, Max 54, 100
Lesion Length (cm)
N 227
Mean ± SD 28.74 ± 7.11
Median 27.50
Min, Max 18.5, 53.0
Occluded Lesion Length (cm)
N 207
Mean ± SD 11.67 ± 11.32
Median 10.00
Min, Max 0.0, 45.0
Post-Procedure
b
Diameter Stenosis (%)
N 226
Mean ± SD 11.2 ± 12.3
Median 10.0
Min, Max 0, 60
Total Target Lesion Length Treated with Study Device (cm)
48 Instructions for Use English
Page 51

Procedural Lesion Characteristics (per lesion) IN.PACT Admiral DCB (N=227 Subjects) (N=227 Lesions)
N 226
Mean ± SD 27.46 ± 6.68
Median 26.00
Min, Max 14.5, 47.0
Numbers are % (counts/sample size) unless otherwise stated.
Key Core Laboratory definitions:
Reference Vessel Diameter (RVD) – angiographic measurement
of the normal artery proximal and/or distal to the lesion intended
for treatment.
Minimum Lumen Diameter (MLD) – angiographic measurement
of the tightest area of obstruction or stenosis located within the
segment of interest or the intended area of treatment.
Lesion length – angiographic measurement from the proximal
healthy vessel segment to the distal healthy vessel segment
(e.g. length of obstruction).
a
Angio core lab reported data.
b
Site reported data.
As shown in Table 23 below, a total of 96 IN.PACT Global DCB Long Lesion Sub-Cohort subjects (42.5%) received provisional
stenting. The mean total stent length per subject was 173.7 ± 104.7 mm.
Table 23. Provisional Stenting – IN.PACT Global DCB Long Lesion Sub-Cohort
Subject Characteristics IN.PACT Admiral DCB (N=227 Subjects)
Provisional Stent Rate per Subject 42.5% (96/226)
Total Provisional Stent Length per Subject (mm)
N 96
Mean ± SD 173.7 ± 104.7
Median 150.0
Min, Max 10, 450
Numbers are % (counts/sample size).
Site reported data.
Follow-up compliance through the 12-month follow-up visits is presented in Table 24 for the IN.PACT Global DCB Long Lesion
Sub-Cohort subjects. The rate of in-window follow-up visit completion at 12 months was 84.5%.
Table 24. Subject Follow-up Compliance – IN.PACT Global DCB Long Lesion Sub-Cohort
Subject Compliance Characteristics
a
IN.PACT Admiral DCB (N=227)
30-Day Follow-up
Eligible Subjects
c
Death
Withdrawal
Follow-up Not Done
Follow-up Visit Within Window
Follow-up Visit Out of Window
Within Window Follow-up Compliance (%)
Overall Follow-up Compliance (%)
b
225
0
c
c
d
d
e
f
2
13
197
15
87.6%
94.2%
6-Month Follow-up
Eligible Subjects
c
Death
Withdrawal
Follow-up Not Done
Follow-up Visit Within Window
Follow-up Visit Out of Window
b
c
c
d
d
Within Window Follow-up Compliance (%)
Overall Follow-up Compliance (%)
f
e
218
1
8
27
171
20
78.4%
87.6%
12-Month Follow-up
Instructions for Use English 49
Page 52

Subject Compliance Characteristics
Eligible Subjects
c
Death
Withdrawal
Follow-up Not Done
Follow-up Visit Within Window
Follow-up Visit Out of Window
b
c
c
d
d
Within Window Follow-up Compliance (%)
Overall Follow-up Compliance (%)
a
Site reported data
b
Eligible subjects are all subjects who either have a follow-up visit form or are past due for their follow-up (beyond upper limit of window on study and did not exit the study before the upper limit of the window)
c
Death, withdrawal and follow-up not done are cumulative
d
Within window visits are defined as: 30-day ± 7 days, 6-month ± 30 days, 12-month ± 30 days.
e
Percentage based on number of subjects who had follow-up visit within window divided by total number of eligible subjects
f
Percentage based on number of subjects who had a follow-up visit within or out of window divided by total number of eligible subjects
11.8.4.
Primary Effectiveness and Safety Results
a
IN.PACT Admiral DCB (N=227)
207
5
15
20
175
12
e
f
84.5%
90.3%
The primary effectiveness and safety results are shown in Table 25 and Figure 12 below.
The primary effectiveness endpoint is defined as primary patency within 12 months post-index procedure. Primary patency is
defined as freedom from CD-TLR and freedom from restenosis as determined by DUS PSVR ≤ 2.4. Primary patency at
12 months was 64.9% for all subjects.
The primary safety composite endpoint is defined as freedom from device- and procedure-related death through 30 days postprocedure and freedom from target limb major amputation and CD-TVR within 12 months post-index procedure. The primary
safety composite endpoint at 12 months was 92.9%.
Table 25. Primary Effectiveness and Safety Results through 12 Months – IN.PACT Global DCB Long Lesion Sub-Cohort
Subjects
Parameters IN.PACT Admiral DCB
(N=227 Subjects)
Effectiveness Parameters
Primary Effectiveness Endpoint – Primary Patency at 12 Months 64.9% (98/151)
Safety Parameters
Primary Safety Composite Endpoint – Freedom from: 92.9% (195/210)
Device- and Procedure-related Death through 30 Days 0.0% (0/225)
Target Limb Major Amputation within 360 Days 0.0% (0/210)
Clinically-driven TVR within 360 Days 7.1% (15/210)
Death (all-cause) within 360 days 2.4% (5/210)
The Kaplan-Meier analysis of this primary effectiveness endpoint, presented as primary patency through 420 days, is shown
in Figure 12.
50 Instructions for Use English
Page 53

From day X To day Y
IN.PACT DCB
(N=227 Subjects)
# Censored
# Events
Event-free [%]
# Entered
0
0
1
303160619091120
121
150
151
180
181
210
211
240
241
270
271
300
301
330
331
360
361
390
391
420
227 227 224 215 214 214 213 210 205 203 200 195 190 181 157
0
0
100.0%
2
1
99.6%
5
4
97.7%
1
0
97.7%
0
0
97.7%
0
1
97.3%
2
1
96.8%
4
1
96.4%
1
1
95.9%
0
3
94.5%
4
1
94.0%
3
2
93.0%
1
8
89.1%
4
20
79.1%
1
4
77.1%
Primary Patency
Time after Index Procedure (days)
100%
90%
80%
70%
60%
50%
40%
30%
20%
10%
0%
0 30 60 90 120 150 180 210 240 270 300 330 360 390 420
89.1%
77.1%
Figure 12. Kaplan-Meier Plot - Primary Patency through 420 Days
11.8.5. Secondary Safety and Effectiveness Endpoints
The results of the secondary endpoints for the IN.PACT Global DCB Long Lesion Sub-Cohort Clinical Evaluation are shown
in Table 26.
The primary sustained clinical improvement was 80.9% (152/188) at 12-months post-procedure. The device, procedural and
clinical success was over 99% for the entire subject population in this sub-cohort (99.2%, 99.1% and 99.1% respectively).
The MAE composite for the long lesion sub-cohort at 360-days was 10.5% (22/210). Reported MAEs within 360-days included
2.4% death (5/210), 3.3% thrombosis (7/210) and no cases of major target limb amputation. Reintervention rates were reported
as 7.1% (15/210) clinically-driven TVR and 7.1% (15/210) clinically-driven TLR. The average time to first clinically-driven TLR
was 177.8 days. Other major secondary endpoints included binary restenosis (PSVR>2.4) in 34.2% (51/149) of subjects.
Table 26. Secondary Endpoints through 12 Months – IN.PACT Global DCB Long Lesion Sub-Cohort
Parameters IN.PACT Admiral DCB
(N=227 Subjects)
Effectiveness Parameters
Primary Sustained Clinical Improvement at 12 Months 80.9% (152/188)
Secondary Sustained Clinical Improvement at 12 Months 86.5% (160/185)
Device Success 99.2% (653/658)
Procedural Success 99.1% (224/226)
Clinical Success 99.1% (224/226)
Cumulative complications within 360 days
MAE Composite (Death, Major Target Limb Amputation, Clinically-driven TVR, Thrombosis)
Death (all-cause) 2.4% (5/210)
Clinically-driven TVR 7.1% (15/210)
10.5% (22/210)
Major Target Limb Amputation 0.0% (0/210)
Instructions for Use English 51
Page 54

Parameters IN.PACT Admiral DCB
(N=227 Subjects)
Thrombosis 3.3% (7/210)
Clinically-driven TLR 7.1% (15/210)
Any TVR 7.1% (15/210)
Any TLR 7.1% (15/210)
Other Major Secondary Endpoints at 12 Months
Binary Restenosis (PSVR >2.4) 34.2% (51/149)
Time to First Clinically-driven TLR (days)
N 15
Mean ± SD 177.8 ± 115.9
Median 204.0
Min, Max 21, 356
Walking Impairment by WIQ (%)
N 178
Mean ± SD 73.7 ± 32.5
Median 100.0
Min, Max 0, 100
Endpoint definitions:
■
Clinically-driven TLR is defined as any reintervention at the target lesion due to symptoms or drop of ABI/TBI of ≥20% or
>0.15 when compared to post-procedure baseline ABI/TBI
■
Device success defined as successful delivery, inflation, deflation and retrieval of the intact study balloon device without
burst below the RBP. Results reported as device based summary.
■
Procedure success defined as residual stenosis of ≤ 50% (non-stented subjects) or ≤ 30% (stented subjects) by corelab (if
corelab was not available then the site-reported estimate was used).
■
Clinical success defined as procedural success without procedural complications (death, major target limb amputation,
thrombosis of the target lesion, or TVR) prior to discharge.
■
Primary sustained clinical improvement is defined as sustained upward shift of at least 1 category on Rutherford classification as compared to baseline without the need for repeated TLR or surgical revascularization in amputation-free surviving
subjects.
■
Secondary sustained clinical improvement is defined as sustained upward shift of at least 1 category on Rutherford classification as compared to baseline including the need for repeated TLR or surgical revascularization in amputation-free surviving subjects.
■
Safety composite endpoint consists of: freedom from device- and procedure-related death within 30 days, freedom from
major target limb amputation, and freedom from clinically-driven TVR within 360 days post-index procedure.
■
Clinically-driven TLR/TVR is defined as any re-intervention within the target vessel due to symptoms or drop of ABI/TBI of
≥20% or >0.15 when compared to post-procedure baseline ABI/TBI.
■
Major Adverse Events (MAE) defined as all-cause death, clinically-driven TLR/TVR, major target limb amputation, thrombosis at the target lesion site at 360 days
■
Walking impairment assessed by Walking Impairment Questionnaire (WIQ) at 12 months.
Data sources:
All events were adjudicated by the independent Clinical Events Committee, all duplex ultrasound and angiographic measures
were made by the independent core laboratories, and all other data were site reported.
11.8.6. Subgroup Analysis
Medtronic has analyzed the clinical evaluation results by lesion length grouping, gender, and assigned imaging cohorts.
11.8.7. Lesion Length Grouping Analysis
The outcomes for subjects by respective lesion length grouping are presented below in Table 27. The primary patency by lesion
length group were 75.5% (40/53) for lesions >180-240 mm, 65.1% (28/43) for lesions >240-300 mm, 63.3% (19/30) for lesions
>300-360 mm, and 44.0% (11/25) for lesions > 360 mm.
The primary safety composite by lesion length group were 95.8% (68/71) for lesions >180-240 mm, 93.7% (59/63) for lesions
>240-300 mm, 92.3% (36/39) for lesions >300-360 mm, and 86.5% (32/37) for lesions > 360 mm. There were no device- or
procedure-related deaths through 30 days or major target limb amputations through 12 months.
52 Instructions for Use English
Page 55

Table 27. Principal Effectiveness and Safety Results through 12-months by Lesion Length Groups – IN.PACT Global DCB
Long Lesion Sub- Cohort
Parameters 18-24cm 24-30cm 30-36cm >36cm
(N=77 Subjects) (N=66 Subjects) (N=43 Subjects) (N=41 Subjects)
Lesion Length (cm)
N 77 66 43 41
Mean ± SD 21.65 ± 1.71 27.17 ± 1.88 32.74 ± 1.73 40.37 ± 3.83
Q1 20.0 25.5 31.0 37.5
Median 22.00 26.75 32.50 39.00
Q3 23.0 29.0 34.0 42.0
Min, Max 18.5, 24.0 24.5, 30.0 30.5, 36.0 36.5, 53.0
Effectiveness Parameters
Primary Patency at
12 Months
Primary Sustained Clinical Improvement at
12 Months
Secondary Sustained
Clinical Improvement at
12 Months
Device Success 100.0% (178/178) 99.5% (186/187) 99.3% (136/137) 98.1% (153/156)
Procedural Success 100.0% (76/76) 98.5% (65/66) 97.7% (42/43) 100.0% (41/41)
Clinical Success 100.0% (76/76) 98.5% (65/66) 97.7% (42/43) 100.0% (41/41)
Safety Parameters
Primary Safety Composite Endpoint – Freedom
from:
Device- and Procedurerelated Death through
30 Days
Target Limb Major
Amputation within
360 Days
Clinically-driven TLR
within 360 Days
Death (all-cause) within
30 days
Cumulative complications within 360 days
MAE Composite (Death,
Major Target Limb Amputation, Clinically-driven
TVR, Thrombosis)
Death (all-cause) 0.0% (0/71) 3.2% (2/63) 2.6% (1/39) 5.4% (2/37)
Clinically-driven TVR 4.2% (3/71) 6.3% (4/63) 7.7% (3/39) 13.5% (5/37)
Major Target Limb
Amputation
Thrombosis at Target
Lesion
Clinically-driven TLR 4.2% (3/71) 6.3% (4/63) 7.7% (3/39) 13.5% (5/37)
Any TVR 4.2% (3/71) 6.3% (4/63) 7.7% (3/39) 13.5% (5/37)
Any TLR 4.2% (3/71) 6.3% (4/63) 7.7% (3/39) 13.5% (5/37)
Other Major Secondary
Endpoints at 12 Months
75.5% (40/53) 65.1% (28/43) 63.3% (19/30) 44.0% (11/25)
83.3% (55/66) 83.6% (46/55) 82.9% (29/35) 68.8% (22/32)
86.4% (57/66) 89.1% (49/55) 91.2% (31/34) 76.7% (23/30)
95.8% (68/71) 93.7% (59/63) 92.3% (36/39) 86.5% (32/37)
0.0% (0/76) 0.0% (0/66) 0.0% (0/42) 0.0% (0/41)
0.0% (0/71) 0.0% (0/63) 0.0% (0/39) 0.0% (0/37)
4.2% (3/71) 6.3% (4/63) 7.7% (3/39) 13.5% (5/37)
0.0% (0/76) 0.0% (0/66) 0.0% (0/42) 0.0% (0/41)
5.6% (4/71) 12.7% (8/63) 7.7% (3/39) 18.9% (7/37)
0.0% (0/71) 0.0% (0/63) 0.0% (0/39) 0.0% (0/37)
1.4% (1/71) 3.2% (2/63) 5.1% (2/39) 5.4% (2/37)
Instructions for Use English 53
Page 56

Parameters 18-24cm 24-30cm 30-36cm >36cm
(N=77 Subjects) (N=66 Subjects) (N=43 Subjects) (N=41 Subjects)
Binary Restenosis
24.5% (13/53) 34.9% (15/43) 34.5% (10/29) 54.2% (13/24)
(PSVR >2.4)
Time to First Clinically-
driven TLR (days)
N 3 4 3 5
Mean ± SD 221.0 ± 71.8 150.3 ± 149.2 91.7 ± 98.4 225.6 ± 110.9
Median 242.0 104.5 50.0 241.0
Min, Max 141, 280 37, 355 21, 204 50, 356
Walking Impairment by
WIQ (%)
N 63 54 32 29
Mean ± SD 73.0 ± 34.9 79.2 ± 28.2 75.0 ± 33.6 63.8 ± 32.4
Median 100.0 100.0 100.0 50.0
Min, Max 0, 100 0, 100 0, 100 0, 100
Endpoint definitions:
■
Primary patency is defined as freedom from clinically-driven TLR1 and freedom from restenosis as determined by duplex
ultrasound2 (DUS) Peak Systolic Velocity Ratio (PSVR) ≤ 2.43 within 12 months. Key Primary Patency endpoint definition
components:
1
Clinically-driven TLR is defined as any reintervention at the target lesion due to symptoms or drop of ABI/TBI of ≥20% or
>0.15 when compared to post-procedure baseline ABI/TBI
2
Post-index procedure DUS is intended to establish a post-treatment baseline and does not contribute to the Primary End-
point determination
3
Restenosis determined by either PSVR >2.4 (determined by Target Lesion Category of ’50-99%’ or ‘Occluded’) as
assessed by an independent DUS core lab or >50% stenosis as assessed by an independent angiographic core lab
■
Device success defined as successful delivery, inflation, deflation and retrieval of the intact study balloon device without
burst below the RBP.
■
Results reported as a device-based summary.
■
Procedure success defined as residual stenosis of ≤ 50% (non-stented subjects) or ≤ 30% (stented subjects) by corelab (if
corelab was not available then the site-reported estimate was used).
■
Clinical success defined as procedural success without procedural complications (death, major target limb amputation,
thrombosis of the target lesion, or TVR) prior to discharge.
■
Primary sustained clinical improvement is defined as sustained upward shift of at least 1 category on Rutherford classification as compared to baseline without the need for repeated TLR or surgical revascularization in amputation-free surviving
subjects.
■
Secondary sustained clinical improvement is defined as sustained upward shift of at least 1 category on Rutherford classification as compared to baseline including the need for repeated TLR or surgical revascularization in amputation-free surviving subjects.
■
Safety composite endpoint consists of: freedom from device- and procedure-related death within 30 days; freedom from
major target limb amputation; and freedom from clinically-driven TVR within 360 days post-index procedure.
■
Clinically-driven TVR/TLR is defined as any re-intervention within the target vessel due to symptoms or drop of ABI/TBI of
≥20% or >0.15 when compared to post-procedure baseline ABI/TBI.
■
Major Adverse Events (MAE) defined as all-cause death, clinically-driven TLR/TVR, major target limb amputation, thrombosis at the target lesion site at 360 days.
■
Binary restenosis is defined as duplex restenosis (PSVR > 2.4) of the target lesion at 12 months post-procedure, or at the
time of reintervention prior to any pre-specified timepoint.
■
Walking impairment assessed by Walking Impairment Questionnaire (WIQ) at 12 months.
Data sources:
All events were adjudicated by the independent Clinical Events Committee, all duplex ultrasound and angiographic measures
were made by the independent core laboratories, and all other data were site reported.
11.8.8. Gender Analysis
The results of sub-group analysis on gender are summarized in Table 28 below. The IN.PACT Global DCB Long Lesion SubCohort included 153 males and 74 females at the time of the procedure. The comparison between genders showed the primary
patency at 12 months to be 66.7% (70/105) in males and 60.9% (28/46) in females. The primary safety composite endpoint at
54 Instructions for Use English
Page 57

12 months was reported as 91.5% (129/141) and 95.7% (66/69) in males compared to females respectively. The MAE
composite at 360 days were 10.6% (15/141) in males and 10.1% (7/69) in females.
Table 28. Principal Effectiveness and Safety Results through 12 Months by Gender – IN.PACT Global DCB Long Lesion Sub-
Cohort
Parameters Male Subjects (N=153 Subjects) Female Subjects (N=74 Subjects)
Effectiveness Parameters
Primary Effectiveness Endpoint – Primary
Patency at 12 Months
Primary Sustained Clinical Improvement at
12 Months
Secondary Sustained Clinical Improvement at 12 Months
Device Success 98.9% (455/460) 100.0% (198/198)
Procedural Success 100.0% (153/153) 97.3% (71/73)
Clinical Success 100.0% (153/153) 97.3% (71/73)
Safety Parameters
Primary Safety Composite Endpoint –
Freedom from:
Device- and Procedure-related Death
through 30 Days
Target Limb Major Amputation within
360 Days
Clinically-driven TVR within 360 Days 8.5% (12/141) 4.3% (3/69)
Death (all-cause) within 30 days 0.0% (0/151) 0.0% (0/74)
Cumulative complications within
360 days
MAE Composite (Death, Major Target
Limb Amputation, Clinically-driven TVR,
Thrombosis)
Death (all-cause) 2.8% (4/141) 1.4% (1/69)
Clinically-driven TVR 8.5% (12/141) 4.3% (3/69)
Major Target Limb Amputation 0.0% (0/141) 0.0% (0/69)
Thrombosis 2.8% (4/141) 4.3% (3/69)
Clinically-driven TLR 8.5% (12/141) 4.3% (3/69)
Any TVR 8.5% (12/141) 4.3% (3/69)
Any TLR 8.5% (12/141) 4.3% (3/69)
Other Major Secondary Endpoints at
12 Months
Binary Restenosis (PSVR >2.4) 32.0% (33/103) 39.1% (18/46)
Time to First Clinically-driven TLR (days)
N 12 3
Mean ± SD 195.5 ± 113.5 107.0 ± 116.9
Median 213.0 42.0
Min, Max 21, 356 37, 242
Walking Impairment by WIQ (%)
N 122 56
Mean ± SD 73.0 ± 32.1 75.4 ± 33.5
Median 100.0 100.0
Min, Max 0, 100 0, 100
66.7% (70/105) 60.9% (28/46)
79.7% (102/128) 83.3% (50/60)
86.4% (108/125) 86.7% (52/60)
91.5% (129/141) 95.7% (66/69)
0.0% (0/151) 0.0% (0/74)
0.0% (0/141) 0.0% (0/69)
10.6% (15/141) 10.1% (7/69)
11.8.9. Assigned Imaging Cohorts Analyses
The IN.PACT Global DCB Long Lesion Sub-Cohort was composed of subjects meeting the inclusion criteria specific to this
analysis from the three imaging cohorts (Long Lesion, CTO, and ISR) in the IN.PACT Global study. As such, a breakdown of
the subject outcomes for each of the imaging cohorts was conducted and the results of that analysis are presented in Table 29
below. Slight variability was seen in for the primary effectiveness outcome as the Long Lesion, CTO and ISR imaging groups
reported patency at 12 months of 66.2% (47/71), 68.8% (33/48), and 56.3% (18/32) respectively. The primary safety composite
Instructions for Use English 55
Page 58

endpoint amongst the three imaging cohorts was 94.2% (98/104) for Long Lesion, 92.2% (59/64) for CTO, and 90.5% (38/42)
for ISR. The cumulative MAE rate at 360 days was 10.6% (11/104), 10.9% (7/64), 9.5% (4/42) for the Long Lesion, CTO and
ISR imaging cohorts respectively. It is noted that the reintervention rates (TVR or TLR) were highest in the ISR group compared
to Long Lesions or CTO. Conversely, the ISR group had no reported cases of death or thrombosis compared to the Long
Lesion (2.9% and 4.8% respectively) and the CTO (3.1% and 3.1% respectively) imaging cohorts. Overall, binary restenosis in
the imaging cohorts was 33.8% (24/71) for Long Lesion, 28.3% (13/46) for CTO, and 43.8% (14/32) for ISR.
Table 29. Principal Effectiveness and Safety Results through 12 Months by Assigned Imaging Sub-Cohort – IN.PACT Global
DCB Long Lesion Sub-Cohort
Parameters Long Lesion CTO De novo ISR
(N=114 Subjects) (N=67 Subjects) (N=46 Subjects)
Effectiveness Parameters
Primary Effectiveness Endpoint
– Primary Patency at
12 Months
Primary Sustained Clinical
Improvement at 12 Months
Secondary Sustained Clinical
Improvement at 12 Months
Device Success 99.4% (324/326) 99.0% (191/193) 99.3% (138/139)
Procedural Success 99.1% (112/113) 100.0% (67/67) 97.8% (45/46)
Clinical Success 99.1% (112/113) 100.0% (67/67) 97.8% (45/46)
Safety Parameters
Primary Safety Composite Endpoint – Freedom from:
Device- and Procedure-related
Death through 30 Days
Target Limb Major Amputation
within 360 Days
Clinically-driven TVR within
360 Days
Death (all-cause) within
30 days
Cumulative complications
within 360 days
MAE Composite (Death, Major
Target Limb Amputation, Clinically-driven TVR, Thrombosis)
Death (all-cause) 2.9% (3/104) 3.1% (2/64) 0.0% (0/42)
Clinically-driven TVR 5.8% (6/104) 7.8% (5/64) 9.5% (4/42)
Major Target Limb Amputation 0.0% (0/104) 0.0% (0/64) 0.0% (0/42)
Thrombosis 4.8% (5/104) 3.1% (2/64) 0.0% (0/42)
Clinically-driven TLR 5.8% (6/104) 7.8% (5/64) 9.5% (4/42)
Any TVR 5.8% (6/104) 7.8% (5/64) 9.5% (4/42)
Any TLR 5.8% (6/104) 7.8% (5/64) 9.5% (4/42)
Other Major Secondary Endpoints at 12 Months
Binary Restenosis (PSVR >2.4) 33.8% (24/71) 28.3% (13/46) 43.8% (14/32)
Time to First Clinically-driven
TLR (days)
N 6 5 4
Mean ± SD 182.8 ± 123.3 224.0 ± 124.6 112.5 ± 85.7
Median 191.5 241.0 104.5
Min, Max 50, 355 21, 356 37, 204
Walking Impairment by WIQ
(%)
N 86 53 39
66.2% (47/71) 68.8% (33/48) 56.3% (18/32)
82.6% (76/92) 83.9% (47/56) 72.5% (29/40)
85.7% (78/91) 90.7% (49/54) 82.5% (33/40)
94.2% (98/104) 92.2% (59/64) 90.5% (38/42)
0.0% (0/114) 0.0% (0/66) 0.0% (0/45)
0.0% (0/104) 0.0% (0/64) 0.0% (0/42)
5.8% (6/104) 7.8% (5/64) 9.5% (4/42)
0.0% (0/114) 0.0% (0/66) 0.0% (0/45)
10.6% (11/104) 10.9% (7/64) 9.5% (4/42)
56 Instructions for Use English
Page 59

Parameters Long Lesion CTO De novo ISR
(N=114 Subjects) (N=67 Subjects) (N=46 Subjects)
Mean ± SD 70.3 ± 34.8 79.7 ± 28.2 73.1 ± 32.1
Median 75.0 100.0 100.0
Min, Max 0, 100 0, 100 0, 100
11.8.10. Summary of Serious Adverse Events
A serious adverse event was defined in the IN.PACT Global Study protocol as an adverse event that led to death; led to serious
deterioration in the health of the subject that resulted in a life-threatening illness or injury, a permanent impairment of a body
structure or a body function, in-patient or prolonged hospitalization, or medical or surgical intervention to prevent lifethreatening illness or injury or permanent impairment to a body structure of a body function; or led to fetal distress, fetal death
or a congenital abnormality or birth defect.
As shown in Table 30, there were a total of 246 serious adverse events reported in the IN.PACT Global DCB Long Lesion SubCohort within 360 days. In total, 52.0% of subjects experienced one or more serious adverse events.
Table 30. Number of Subjects with One or More Serious Adverse Events through 360 days by MedDRA System-Organ Class
and Preferred Term – IN.PACT Global DCB Long Lesion Sub-Cohort
Serious Adverse Event
a
IN.PACT Admiral DCB (N=227 Subjects)
Subjects with One or More Serious Adverse Events 52.0% (118/227)
BLOOD AND LYMPHATIC SYSTEM DISORDERS
b
0.4% (1/227)
LEUKOCYTOSIS 0.4% (1/227)
CARDIAC DISORDERS
b
7.9% (18/227)
ACUTE MYOCARDIAL INFARCTION 0.9% (2/227)
ANGINA PECTORIS 0.4% (1/227)
ANGINA UNSTABLE 0.9% (2/227)
ARTERIOSCLEROSIS CORONARY ARTERY 0.4% (1/227)
ATRIAL FIBRILLATION 0.4% (1/227)
ATRIAL FLUTTER 0.4% (1/227)
ATRIAL THROMBOSIS 0.4% (1/227)
CARDIAC FAILURE 1.3% (3/227)
CARDIAC FAILURE ACUTE 0.4% (1/227)
CARDIAC FAILURE CONGESTIVE 0.4% (1/227)
CONGESTIVE CARDIOMYOPATHY 0.4% (1/227)
CORONARY ARTERY DISEASE 2.6% (6/227)
CORONARY ARTERY OCCLUSION 0.4% (1/227)
CORONARY ARTERY STENOSIS 1.3% (3/227)
HYPERTENSIVE HEART DISEASE 0.4% (1/227)
ISCHAEMIC CARDIOMYOPATHY 0.9% (2/227)
EAR AND LABYRINTH DISORDERS
b
0.4% (1/227)
VERTIGO 0.4% (1/227)
ENDOCRINE DISORDERS
b
0.4% (1/227)
GOITRE 0.4% (1/227)
EYE DISORDERS
b
0.9% (2/227)
CATARACT 0.4% (1/227)
RETINAL ARTERY OCCLUSION 0.4% (1/227)
GASTROINTESTINAL DISORDERS
b
3.1% (7/227)
COLITIS 0.4% (1/227)
GASTRITIS 0.4% (1/227)
GASTROINTESTINAL HAEMORRHAGE 1.3% (3/227)
OESOPHAGEAL HAEMORRHAGE 0.4% (1/227)
REFLUX OESOPHAGITIS 0.4% (1/227)
GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS
b
3.5% (8/227)
CHEST PAIN 0.9% (2/227)
DEATH 0.4% (1/227)
DEVICE OCCLUSION 1.3% (3/227)
Instructions for Use English 57
Page 60

Serious Adverse Event
a
IN.PACT Admiral DCB (N=227 Subjects)
IMPAIRED HEALING 0.4% (1/227)
PUNCTURE SITE HAEMORRHAGE 0.4% (1/227)
HEPATOBILIARY DISORDERS
b
0.4% (1/227)
CHOLECYSTITIS 0.4% (1/227)
CHOLELITHIASIS 0.4% (1/227)
INFECTIONS AND INFESTATIONS
b
5.3% (12/227)
BRONCHITIS 0.4% (1/227)
ERYSIPELAS 0.4% (1/227)
GANGRENE 0.4% (1/227)
GASTROENTERITIS VIRAL 0.4% (1/227)
GRAFT INFECTION 0.4% (1/227)
GROIN INFECTION 0.4% (1/227)
INFECTION 0.4% (1/227)
NECROTISING FASCIITIS 0.4% (1/227)
PNEUMONIA 1.8% (4/227)
INJURY, POISONING AND PROCEDURAL COMPLICATIONS
b
11.9% (27/227)
ARTERIAL RESTENOSIS 5.3% (12/227)
IN-STENT ARTERIAL RESTENOSIS 3.1% (7/227)
IN-STENT CORONARY ARTERY RESTENOSIS 0.4% (1/227)
PERIPHERAL ARTERIAL REOCCLUSION 0.9% (2/227)
POSTOPERATIVE THROMBOSIS 0.4% (1/227)
THERMAL BURN 0.4% (1/227)
VASCULAR GRAFT OCCLUSION 0.4% (1/227)
VASCULAR PSEUDOANEURYSM 2.2% (5/227)
INVESTIGATIONS
b
1.3% (3/227)
BLOOD CREATININE INCREASED 0.4% (1/227)
HAEMOGLOBIN DECREASED 0.4% (1/227)
INTERNATIONAL NORMALISED RATIO INCREASED 0.4% (1/227)
METABOLISM AND NUTRITION DISORDERS
b
2.6% (6/227)
DIABETES MELLITUS 0.9% (2/227)
GOUT 0.9% (2/227)
HYPOKALAEMIA 0.4% (1/227)
HYPONATRAEMIA 0.4% (1/227)
MUSCULOSKELETAL AND CONNECTIVE TISSUE DISOR-
b
DERS
4.4% (10/227)
ARTHRITIS 0.4% (1/227)
BACK PAIN 0.9% (2/227)
BURSITIS 0.4% (1/227)
INTERVERTEBRAL DISC PROTRUSION 0.4% (1/227)
NECK PAIN 0.4% (1/227)
OSTEOARTHRITIS 0.4% (1/227)
PAIN IN EXTREMITY 0.9% (2/227)
ROTATOR CUFF SYNDROME 0.9% (2/227)
NEOPLASMS BENIGN, MALIGNANT AND UNSPECIFIED
(INCL CYSTS AND POLYPS)
b
4.0% (9/227)
BLADDER PAPILLOMA 0.4% (1/227)
COLON ADENOMA 0.4% (1/227)
LARYNGEAL CANCER 0.4% (1/227)
LUNG NEOPLASM MALIGNANT 0.4% (1/227)
OESOPHAGEAL CARCINOMA 0.4% (1/227)
PROSTATE CANCER RECURRENT 0.4% (1/227)
SMALL CELL LUNG CANCER STAGE UNSPECIFIED 0.4% (1/227)
SQUAMOUS CELL CARCINOMA 0.4% (1/227)
UNDIFFERENTIATED SARCOMA 0.4% (1/227)
58 Instructions for Use English
Page 61

Serious Adverse Event
NERVOUS SYSTEM DISORDERS
b
a
IN.PACT Admiral DCB (N=227 Subjects)
3.5% (8/227)
BASAL GANGLIA INFARCTION 0.4% (1/227)
BRAIN EDEMA 0.4% (1/227)
CAROTID ARTERY STENOSIS 1.3% (3/227)
CEREBRAL INFARCTION 0.4% (1/227)
HEMIPARESIS 0.4% (1/227)
METABOLIC ENCEPHALOPATHY 0.4% (1/227)
SCIATICA 0.4% (1/227)
SUBARACHNOID HAEMORRHAGE 0.4% (1/227)
PSYCHIATRIC DISORDERS
b
0.4% (1/227)
ALCOHOL WITHDRAWAL SYNDROME 0.4% (1/227)
RENAL AND URINARY DISORDERS
b
0.9% (2/227)
BLADDER TAMPONADE 0.4% (1/227)
HAEMATURIA 0.4% (1/227)
REPRODUCTIVE SYSTEM AND BREAST DISORDERS
b
0.4% (1/227)
POSTMENOPAUSAL HAEMORRHAGE 0.4% (1/227)
RESPIRATORY, THORACIC AND MEDIASTINAL DISOR-
b
DERS
1.3% (3/227)
DYSPNOEA EXERTIONAL 0.4% (1/227)
HYPERVENTILATION 0.4% (1/227)
PLEURAL EFFUSION 0.4% (1/227)
SURGICAL AND MEDICAL PROCEDURES
b
1.3% (3/227)
ILEOSTOMY CLOSURE 0.4% (1/227)
PERIPHERAL ARTERY ANGIOPLASTY 0.4% (1/227)
PERIPHERAL REVASCULARISATION 0.4% (1/227)
VASCULAR DISORDERS
b
31.3% (71/227)
AORTIC ANEURYSM 0.4% (1/227)
ARTERIAL STENOSIS LIMB 3.5% (8/227)
ARTERIAL THROMBOSIS LIMB 2.6% (6/227)
EMBOLISM 0.4% (1/227)
FEMORAL ARTERIAL STENOSIS 11.9% (27/227)
FEMORAL ARTERY DISSECTION 0.4% (1/227)
FEMORAL ARTERY OCCLUSION 5.3% (12/227)
HAEMATOMA 1.3% (3/227)
HYPERTENSION 0.4% (1/227)
HYPERTENSIVE CRISIS 0.4% (1/227)
ILIAC ARTERY STENOSIS 1.3% (3/227)
INTERMITTENT CLAUDICATION 1.3% (3/227)
PERIPHERAL ARTERIAL OCCLUSIVE DISEASE 6.6% (15/227)
PERIPHERAL EMBOLISM 1.8% (4/227)
PERIPHERAL ISCHAEMIA 1.3% (3/227)
PERIPHERAL VASCULAR DISORDER 0.4% (1/227)
VESSEL PERFORATION 1.3% (3/227)
Total Serious Adverse Events 246
Numbers are % (counts/sample size) unless otherwise stated.
a
Site reported data
b
Event verbatim terms are reported by sites. The events listed in this table are then coded using MedDRA version 16.1 and then stratified by System-Organ Class (SOC) and Preferred Term. Patients may be
counted in this table more than once by Preferred Term, but are only counted once in the SOC summary line.
11.8.11. Rare Adverse Events
The Clinical Events Committee (CEC) was responsible for adjudicating rare adverse events, including potential distal embolic
events in the target limb, thrombosis, paclitaxel-related neutropenia, and paclitaxel-related drug hypersensitivity reaction
through 12 months. As reported in Table 31 below, there were no rare adverse events reported for the IN.PACT Global DCB
Long Lesion Sub-Cohort.
Instructions for Use English 59
Page 62

Table 31. Rare Adverse Events - IN.PACT Global DCB Long Lesion Sub-Cohort
Description of Event IN.PACT Admiral DCB/
(N=227 Subjects)
Rare Adverse Events to 180 Days
Paclitaxel-related Thrombosis within 30 Days
a
Paclitaxel-related Distal Embolic Events within 180 Days
Paclitaxel-related Neutropenia within 180 Days
Paclitaxel-related Drug Hypersensitivity/Reaction within
180 Days
d
c
b
0.0% (0/225)
0.0% (0/219)
0.0% (0/219)
0.0% (0/219)
Rare Adverse Events to 360 Days
Paclitaxel-related Thrombosis within 30 Days
a
Paclitaxel-related Distal Embolic Events within 360 Days
Paclitaxel-related Neutropenia within 360 Days
Paclitaxel-related Drug Hypersensitivity/Reaction within
360 Days
d
c
b
0.0% (0/225)
0.0% (0/210)
0.0% (0/210)
0.0% (0/210)
Numbers are % (counts/sample size).
a
Thrombosis is defined as an occlusion due to thrombus formation which is rapidly evolving as confirmed by sudden onset of
symptoms and documented by DUS and/or angiography at the index vessel within 14 days of symptom onset. Occlusions
occurring within 30 days of the index procedure are assumed to be thrombotic, irrespective of symptoms, providing the sheath
or guiding catheter has been removed and the patient has left the catheterization laboratory or angiography suite, and that
they are documented by imaging with DUS and/or angiography of the index vessel. Thrombosis may be categorized as acute
(occurring <1 day post- index), sub-acute (1-30 days) and late (>30 days). For rare adverse events reporting, CEC adjudication will occur through 30-days. Vessel thrombosis reported >30 days will be reported as MAE and CEC adjudicated through
60-months.
b
Distal Embolic Events are defined as embolism with concomitant suggestive clinical signs and symptoms, located separate
from and distal to the target lesion. Classified by probability:
■
Definite Angiographic evidence of distal embolization with a new intraluminal filling defect and/or abrupt occlusion of a runoff vessel distal to a lesion that is clearly not attributable to wire trauma or dissection, irrespective of the time from the
index procedure.
■
Probable: Suggestive clinical signs and symptoms of distal embolization occurring <= 30 days after the index procedure, in
the absence of Angiographic evidence.
■
Possible: Suggestive clinical signs and symptoms of distal embolization occurring >30 days after the index procedure in
the absence of Angiographic evidence.
c
Neutropenia is defined as ANC <1500/mm
■
If no ANC is available, the white blood cell count will be used.
■
If any WBC count is <3,000 cells/mm^super 3 and is a decrease of >50% of the baseline, the event will be adjudicated as
3
neutropenia.
■
If no baseline WBC count is available and site-reported concurrent fever and infection, then any WBC count
<3,000 cells/mm^super 3 will be adjudicated as neutropenia.
d
Drug Hypersensitivity/Reactions is defined as events reported with signs and symptoms which include, but are not limited to
the following: GI upset, Hair loss, Rash, Urticaria (Hives), Erythyroderma, Itchy skin, Pruritus, Vasculitis, Edema, Anaphylaxis /
shock, Asthma / Asthmatic Attack / Bronchospasm, Dyspnea, Chest tightness, Tachycardia, and Eosinophilia. All events were
adjudicated by the independent Clinical Events Committee.
11.8.12. Long Term data (24 month)
The results of the secondary endpoints of the IN.PACT Global DCB Long Lesion Sub-Cohort Clinical Evaluation through
24 months are shown in Table 32 below. The primary sustained clinical improvement was 71.0% (125/176) at 24-months postprocedure. Freedom from CD-TLR by Kaplan Meier estimate was 80.3% through 24-months and can be found below
in Figure 13.
The MAE composite for the long lesion sub-cohort at 720-days was 24.7% (49/198). Reported MAEs within 720-days included
4.5% death (9/198), 5.1% thrombosis (10/198) and one subject had a major target limb amputation (0.5%, 1/198).
Reintervention rates were reported as 20.2% (40/198) clinically-driven TVR and 20.2% (40/198) clinically-driven TLR. The
average time to first clinically-driven TLR was 377.4 days.
60 Instructions for Use English
Page 63

Table 32. Secondary Endpoints through 24 months – IN.PACT Global DCB Long Lesion Sub-Cohort
Parameters IN.PACT Admiral DCB
(N=227 Subjects)
Effectiveness Parameters
Primary Sustained Clinical Improvement at 24 Months 71.0% (125/176)
Secondary Sustained Clinical Improvement at 24 Months 89.2% (149/167)
Device Success 99.2% (653/658)
Procedural Success 99.1% (224/226)
Clinical Success 99.1% (224/226)
Safety Parameters
Primary Safety Composite Endpoint – Freedom from: 79.3% (157/198)
Device- and Procedure-related Death through 30 Days 0.0% (0/225)
Target Limb Major Amputation within 720 Days 0.5% (1/198)
Clinically-driven TVR within 720 Days 20.2% (40/198)
Death (all-cause) within 30 days 0.0% (0/225)
Cumulative complications within 720 days
MAE Composite (Death, Major Target Limb Amputation, Clinically-driven
TVR, Thrombosis)
Death (all-cause) 4.5% (9/198)
Clinically-driven TVR 20.2% (40/198)
Major Target Limb Amputation 0.5% (1/198)
Thrombosis 5.1% (10/198)
Clinically-driven TLR 20.2% (40/198)
Any TVR 20.2% (40/198)
Any TLR 20.2% (40/198)
Other Major Secondary Endpoints at 24 Months
Time to First Clinically-driven TLR (days)
N 40
Mean ± SD 377.4 ± 190.2
Median 394.5
Min, Max 21, 712
Walking Impairment by WIQ (%)
N 157
Mean ± SD 77.7 ± 29.6
Median 100.0
24.7% (49/198)
Instructions for Use English 61
Page 64

Parameters IN.PACT Admiral DCB
(N=227 Subjects)
Min, Max 0, 100
Endpoint definitions:
Clinically-driven TLR is defined as any reintervention at the target lesion due to symptoms or drop of ABI/TBI of ≥ 20% or
>0.15 when compared to post-procedure baseline ABI/TBI
■
Device success defined as successful delivery, inflation, deflation and retrieval of the intact study balloon device without
burst below the RBP.
■
Procedure success defined as residual stenosis of ≤ 50% (non-stented subjects) or ≤ 30% (stented subjects) by corelab (if
corelab was not available then the site-reported estimate was used).
■
Clinical success defined as procedural success without procedural complications (death, major target limb amputation,
thrombosis of the target lesion, or TVR) prior to discharge.
■
Primary sustained clinical improvement is defined as sustained upward shift of at least 1 category on Rutherford classification as compared to baseline without the need for repeated TLR or surgical revascularization in amputation-free surviving
subjects.
■
Secondary sustained clinical improvement is defined as sustained upward shift of at least 1 category on Rutherford classification as compared to baseline including the need for repeated TLR or surgical revascularization in amputation-free surviving subjects.
■
Safety composite endpoint consists of: freedom from device- and procedure-related death within 30 days, freedom from
major target limb amputation, and freedom from clinically-driven TVR within 720 days post-index procedure.
■
Clinically-driven TLR/TVR is defined as any re-intervention within the target vessel due to symptoms or drop of ABI/TBI of
≥ 20% or >0.15 when compared to post-procedure baseline ABI/TBI.
■
Major Adverse Events (MAE) defined as all-cause death, clinically-driven TLR/TVR, major target limb amputation, thrombosis at the target lesion site at 720 days.
■
Walking impairment assessed by Walking Impairment Questionnaire (WIQ) at 24 months.
Data sources:
All events were adjudicated by the independent Clinical Events Committee, all duplex ultrasound and angiographic measures
were made by the independent core laboratories, and all other data were site reported.
62 Instructions for Use English
Page 65

100%
90%
80%
70%
60%
50%
40%
30%
20%
10%
0%
0 6 12 18 24
Freedom from Clinically driven TLR
Time after Index Procedure (months)
93.0%
80.3%
From day X To day Y
IN.PACT DCB
(N=227 Subjects)
# Entered
1
227 227 215 214 210 203 195 189 176 171 166 160 156
# Censored
2
0 7 1 2 5 4 4 5 1 1 2 1 5
# Events 0 5 0 2 2 4 2 8 4 4 4 3 2
Event-free [%]
3
1
Number of subjects at risk at the beginning of each interval.
2
Subjects are censored because their last follow-up has not reached the end of the time interval or because they are lost to follow-up.
3
Estimate made at the end of the time interval.
All events were adjudicated by the independent Clinical Events Committee.
100.0% 97.7% 97.7% 96.8% 95.9% 94.0% 93.0% 89.0% 87.0% 84.9% 82.9% 81.3% 80.3%
0
0
0M
1
60
2M
61
120
4M
121
180
6M
181
240
8M
241
300
10M
301
360
12M
361
420
14M
421
480
16M
481
540
18M
541
600
20M
601
660
22M
661
720
24M
Figure 13. Kaplan-Meier Plot – Event-free from Clinically-driven TLR through 24 months
12. How Supplied
STERILE: The IN.PACT 018 DCB is sterilized by ethylene oxide (EtO) and is nonpyrogenic. It is intended for single use only.
Do not resterilize. Do not use if package is opened or damaged.
CONTENTS: The package contains 1 IN.PACT 018 DCB.
STORAGE: Store the device in the original container. Store between 15°C and 30°C (59°F and 86°F). Use product by the Use-
by Date noted on the package. Do not store near radiation or ultraviolet light sources.
DISPOSAL INSTRUCTIONS: After use, this product may be a biohazard. Handle and dispose of all such devices in
accordance with accepted medical practice and applicable hospital, administrative, and government regulations.
DEVICE RETURN INSTRUCTIONS: In the case of a product failure or malfunction related to the product, contact a Medtronic
Vascular representative for return or replacement. Any ancillary devices involved in the incident should also be returned to
Medtronic, if possible.
13. Instructions for Use
13.1. Equipment
■
0.018 in guidewire
■
Introducer sheath
■
Vessel preparation device
■
Contrast medium
■
Sterile saline
■
Inflation device with manometer
Instructions for Use English 63
Page 66

■
Luer lock syringe for purging
13.2. Balloon Catheter Size Selection
■
The nominal balloon diameter must match the diameter of the vessel distal to the lesion. The balloon length must exceed
the lesion length by about 1 cm on the proximal and distal ends.
■
If the lesion is longer than the longest available IN.PACT 018 DCB, use multiple IN.PACT 018 DCBs to treat the lesion,
using the recommended overlap, as described in Using Multiple IN.PACT 018 DCBs (Section 13.9), Recommended
Overlap When Using Multiple IN.PACT 018 DCBs (Figure 17), and Treatment of a Tandem Lesion with Multiple IN.PACT
018 DCBs (Figure 19).
13.3. Recommendations for Optimal Treatment
■
Appropriate vessel preparation is required prior to the use of the IN.PACT 018 DCB.
Note: Vessel preparation using only pre-dilatation was studied in the clinical study. Other methods of vessel preparation,
such as atherectomy, have not been studied clinically with IN.PACT 018 DCB.
■
When using a PTA balloon for vessel preparation, use a PTA balloon with a diameter 1 mm smaller than the reference
vessel diameter to facilitate the passage of the appropriately sized IN.PACT 018 DCB.
Note: Following vessel preparation, if the lesion cannot be crossed with the first inserted IN.PACT 018 DCB, the second
attempt must be made with a new IN.PACT 018 DCB in order to ensure effective drug delivery.
■
When using a device other than a PTA balloon for vessel preparation, use the device per its Instructions for Use.
■
As noted in Delivery and Dilatation Procedure (Section 13.7), for optimal mechanical dilatation of the vessel, an inflation
time of 180 seconds is strongly recommended for the IN.PACT 018 DCB. Adequate drug transfer occurs in the first
60 seconds of inflation.
■
Post-dilatation should be completed according to the physician’s discretion. If adequate PTA results are not obtained after
the IN.PACT 018 DCB(s) balloon inflation, post-dilatation using a non–drug-coated PTA balloon of shorter length than the
previously used IN.PACT 018 DCB is recommended.
Note: In the randomized trial, provisional stenting with bare-metal stents was completed in cases where adequate results
could not be obtained after using post-dilatation balloons, such as in the case of remaining residual stenosis [≥ 50%] or
major [≥ Grade D] flow-limiting dissection after post-dilatation.
■
It is important to provide drug delivery to the entire length of the treated artery prior to post-dilatation or provisional stenting.
13.4. PTA Preparation
1. Prepare the inflation device, introducer sheath, and guidewire according to the manufacturer's instructions. See Minimum
Introducer Sheath Compatibility (Table 33) for help selecting the appropriately sized introducer sheath.
Table 33. Minimum Introducer Sheath Compatibility
Balloon Diameter (mm) Max Crossing Profile (mm) Introducer Sheath (Fr)
4.0
1.86 55.0
6.0
7.0 2 6
Note: Use of a long introducer sheath extending beyond the iliac bifurcation is recommended if a contralateral approach is
used.
Note: Use of a long introducer sheath extending beyond the renal arteries ostium is recommended if a radial approach is
used.
2. Administer the appropriate medication to the patient prior to treatment as described in Pre-procedure and Post-procedure
Medication Regimen (Section 6.2).
3. Prepare the vascular access site according to standard practice.
4. Insert a guidewire through the hemostatic valve following the manufacturer's instructions or standard practice. Advance the
guidewire carefully into the introducer sheath.
5. Attach a torque device to the wire, if desired. Under fluoroscopy, advance the guidewire to the desired vessel, then across
the stenosis. Remove the torque device once the guidewire is positioned.
Note: If treating an in-stent restenosis, ensure the guidewire has traversed the lesion intraluminally.
13.5. IN.PACT 018 DCB Preparation
1. The catheter is packaged in a protective blister. Verify that the catheter and sterile packaging have not been damaged in
shipment. After all preparation has been completed, carefully remove the catheter from the package. Do not remove the
IN.PACT 018 DCB from the packaging until it is ready for insertion.
64 Instructions for Use English
Page 67

Note: Avoid exposing the balloon drug coating to excessive handling or contact with liquids prior to preparation and
delivery as the coating may be susceptible to damage or premature drug release.
2. The folded balloon catheter may contain air that should be purged prior to use. Connect a stopcock to the balloon port of
the catheter hub. Connect a luer-lock syringe partially filled with saline solution to the stopcock. Open the stopcock.
Keeping the syringe in a downward vertical position, draw back the plunger of the syringe and create a vacuum for
30 seconds in the balloon inflation line until air is completely evacuated.
Caution: If the air bubbles cannot be completely evacuated, there may be a leak in the catheter. Discard the device and
select a new IN.PACT 018 DCB.
Note: It is important to maintain the vacuum seal in order to keep the balloon profile tight before insertion into the
introducer sheath.
Note: Keep the peel-away balloon protector in place during the purging procedure.
3. After air is completely evacuated, close the stopcock and remove the syringe.
4. Connect the filled syringe to the guidewire port. Flush the guidewire lumen through the guidewire port with heparinized
normal saline until the fluid exits the distal tip.
Note: Drops of saline must emerge from the device tip.
Note: To minimize the introduction of air, aspirate and flush the system and keep a tight catheter connection throughout the
procedure.
13.6. Inflation Device Connection to the IN.PACT 018 DCB
1. Fill the inflation device with 15 mL of saline-contrast mixture. Do not use air or any gaseous medium to inflate the balloon.
Use only the recommended inflation medium (equal parts contrast medium and saline solution). Do not apply positive
pressure to the balloon during preparation.
2. Evacuate all air present in the inflation device.
Note: The inflation device should have no air bubbles present, either in the tube or in the cylinder; to remove any air
lodged, keeping the tip upward, purge approximately 1cc of saline-contrast mixture.
3. With the stopcock connected to the balloon port in the closed position, securely couple the inflation device to the stopcock.
Verify that no air is evident in both the stopcock and the inflation device's connection.
13.7. Delivery and Dilatation Procedure
1. Slide the peel-away balloon protector proximally to expose the tip of the device. Insert the tip of the prepositioned
guidewire, which has been placed through the lesion, into the balloon distal tip and track the device over the guidewire until
it exits the luer. See Figure 14.
2. Separate tabs of peel-away balloon protector away from catheter shaft. Using one hand to support the peel-away balloon
protector, push the balloon through the peel-away balloon protector into the introducer sheath. Do not insert the peel-away
balloon protector into the hemostatic valve. Ensure that the catheter is not kinked during this process. Do not use the peelaway balloon protector as an introduction aid or rewrapping tool. See Figure 14.
Figure 14. Insert the balloon into the introducer sheath
3. Advance the catheter under direct fluoroscopic visualization. To avoid kinking, advance the catheter through the hemostatic
valve slowly and in small increments while the stopcock is closed. Open the hemostatic valve to allow for easy passage of
the balloon and to prevent damage to the balloon coating. Once the balloon has passed through, the hemostatic valve
should be closed as much as is needed to prevent blood return while still permitting easy movements of the catheter.
Note: If significant resistance is encountered, do not advance the catheter through the introducer sheath.
4. If required to reach lesion, remove the peel away balloon protector from the catheter by gripping the tabs and pulling
outward until it has fully separated.
Instructions for Use English 65
Page 68

Figure 15. Remove the peel away balloon protector
5. Under fluoroscopy, use the balloon radiopaque markers to position the balloon within the lesion to be dilated. If the inflation
device has not already been connected, connect the inflation device according to instructions in Inflation Device
Connection to the IN.PACT 018 DCB (Section 13.6).
6. Open the stopcock and inflate the balloon to the appropriate pressure as described in the Compliance Chart included in the
device packaging, then close the stopcock to maintain pressure. For optimal mechanical dilatation of the vessel, balloon
inflation time of 180 seconds is strongly recommended. Adequate drug transfer occurs in the first 60 seconds of inflation. If
the IN.PACT 018 DCB was inflated for at least 60 seconds but the vessel requires additional dilatation due to suboptimal
PTA results, a plain PTA balloon of the operator's choice can be used (PTA balloon should be of shorter length compared to
the IN.PACT 018 DCB).
Warning: Do not exceed rated burst pressure as indicated on the device label. Use of pressures higher than those
specified on the device label may result in a ruptured balloon with possible intimal damage and dissection.
Note: The IN.PACT 018 DCB is intended for single inflation only.
13.8. Removal Procedure
1. Open the stopcock and deflate the balloon by applying negative pressure to the inflation device. For all balloon lengths,
allow approximately 60 seconds for full balloon deflation. Larger balloons may require more time for deflation. Deflation of
the balloon should be confirmed by absence of contrast medium within the balloon.
Note: The balloon must be completely deflated before removal.
2. Upon confirmation of full deflation, disconnect the inflation device, then open the hemostatic valve and withdraw the
deflated balloon catheter from the introducer sheath, through the hemostatic valve. Tighten the knurled knob on the
hemostatic valve.
3. If necessary, the balloon catheter can be exchanged for different balloon types or sizes using the guidewire/ introducer that
remains in the vessel.
Note: If further dilatation is required, post-dilatation should be performed with a non–drug-coated PTA balloon of shorter
length than the IN.PACT 018 DCB.
4. When complete, withdraw the guidewire/introducer, and close the hemostatic valve.
Note: After use, this device may be a biohazard. Handle and dispose of all such devices in accordance with accepted
medical practice and applicable hospital, administrative, and government regulations.
13.9. Using Multiple IN.PACT 018 DCBs
Warning: The safety and effectiveness of using multiple IN.PACT 018 DCBs with a total drug dosage exceeding 34,854 μg
paclitaxel in a patient has not been clinically evaluated.
Additional IN.PACT 018 DCBs should be used to treat a lesion only under either of the following circumstances:
■
The first IN.PACT 018 DCB bursts prior to 60 seconds of inflation time.
■
The lesion length requires more than 1 IN.PACT 018 DCB to fully cover the lesion and extend about 1 cm at both the
proximal and distal edges.
If multiple IN.PACT 018 DCBs are required due to a lesion length greater than the longest available DCB, the balloons must
overlap by at least 1 cm. The size of additional DCBs should not be longer than required to allow for this overlap and complete
the lesion coverage with about 1 cm extended beyond the lesion both proximally and distally. Proper size selection is important
to avoid excessive overlap. Refer to Figure 16 through Figure 19 for further guidance.
Note: In order to reduce procedure-related complications, use only the minimum number of devices needed to cover the
lesion(s).
66 Instructions for Use English
Page 69

1 cm
1 cm
1
1
1. approximately 1 cm
1 cm
2
1
1
1 cm
1 cm
Figure 16. Treatment of a Single Lesion
Figure 17. Recommended Overlap When Using Multiple IN.PACT 018 DCBs
1. approximately 1 cm
2. at least 1 cm balloon overlap
Instructions for Use English 67
Page 70

1 cm
1 cm
≤ 3 cm
1
1
2
Figure 18. Treatment of a Tandem Lesion with a Single IN.PACT 018 DCB
3
1
2
1 cm
1 cm
1 cm
≤ 3 cm
1
1. approximately 1 cm
2. lesion gap ≤ 3 cm
Figure 19. Treatment of a Tandem Lesion with Multiple IN.PACT 018 DCBs
1. approximately 1 cm
2. lesion gap ≤ 3 cm
3. at least 1 cm balloon overlap
14. DISCLAIMER OF WARRANTY
The warnings contained in the product labeling provide more detailed information and are considered an integral part
of this disclaimer of warranty. Although the product has been manufactured under carefully controlled conditions,
Medtronic has no control over the conditions under which this product is used. Medtronic, therefore, disclaims all
warranties, both express and implied, with respect to the product, including, but not limited to, any implied warranty
of merchantability or fitness for a particular purpose. Medtronic shall not be liable to any person or entity for any
medical expenses or any direct, incidental, or consequential damages caused by any use, defect, failure, or
malfunction of the product, whether a claim for such damages is based upon warranty, contract, tort, or otherwise. No
person has any authority to bind Medtronic to any representation or warranty with respect to the product.
68 Instructions for Use English
Page 71

The exclusions and limitations set out above are not intended to, and should not be construed so as to, contravene mandatory
provisions of applicable law. If any part or term of this disclaimer of warranty is held by any court of competent jurisdiction to be
illegal, unenforceable or in conflict with applicable law, the validity of the remaining portion of the disclaimer of warranty shall
not be affected, and all rights and obligations shall be construed and enforced as if this disclaimer of warranty did not contain
the particular part or term held to be invalid.
Instructions for Use English 69
Page 72

Medtronic, Inc.
*M003176C001*
710 Medtronic Parkway
Minneapolis, MN 55432 USA
www.medtronic.com
Medtronic Ireland
Parkmore Business Park West
Galway, Ireland
US CUSTOMER SERVICE/PRODUCT INQUIRIES:
Tel. (+1-888) 283-7868
Fax (+1-800) 838-3103
© 2022 Medtronic
M003176C001 Rev. A
2022-04-25
 Loading...
Loading...