Page 1

User Guide
Page 2

6025901-022_a
REF MMT-7745
© 2016 Medtronic MiniMed, Inc. All rights reserved.
Enlite™, Sen-serter™, Sof-sensor™ are trademarks of Medtronic MiniMed, Inc.
CareLink® and iPro® are registered trademarks of Medtronic MiniMed, Inc.
Page 3

Contacts:
Africa:
Medtronic Africa (Pty) Ltd.
Tel: +27 (0) 11 677 4800
Australia:
Medtronic Australasia Pty. Ltd.
Tel: 1800 668 670 (product orders)
Tel: 1800 777 808 (customer help)
Azerbaijan:
Albatros Health Care
Tel: +994 12 498 9537
Bangladesh
Sonargaon Healthcare Pvt Ltd.
Mobile: (+91)-9903995417
or (+880)-1714217131
Belarus:
Badgin Ltd
Tel: +375 (172) 665128
België/Belgique:
N.V. Medtronic Belgium S.A.
Tel: 0800-90805
Bosnia and Herzegovina
Intermedical
Tel: +387 33 202 183
Fax: +387 33 202 183
Brasil:
Medtronic Comercial Ltda.
Tel: +(11) 3707-3707
Bulgaria
Interagro-90 Ltd
Tel: +359 888 636 033
Canada:
Medtronic of Canada Ltd.
Tel: 1-800-284-4416 (toll free/sans-frais)
China:
Medtronic (Shanghai) Ltd.
24 Hour Help (Cell): +86 400-820-1981
24 Hour Help (Landline): +86 800-820-1981
Croatia
Oktal Pharma
Tel: +385 1 659 57 77
Fax: +385 1 659 57 01
Croatia
Medtronic B.V.
Tel: +385 1 488 11 20
Fax: +385 1 484 40 60
Danmark:
Medtronic Danmark A/S
Tel: +45 32 48 18 00
Deutschland:
Medtronic GmbH
Geschäftsbereich Diabetes
Telefon: +49 2159 8149-370
Telefax: +49 2159 8149-110
24-Stdn-Hotline: 0800 6464633
Eire:
Accu-Science LTD.
Tel: +353 45 433000
España:
Medtronic Ibérica S.A.
Tel: +34 91 625 05 42
Fax: +34 91 625 03 90
24 horas: +34 901 120 335
Europe:
Medtronic Europe S.A. Europe, Middle East and Africa
Headquarters
Tel: +41 (0) 21-802-7000
France:
Medtronic France S.A.S.
Tel: +33 (0) 1 55 38 17 00
Hellas:
Medtronic Hellas S.A.
Tel: +30 210677-9099
Hong Kong:
Medtronic International Ltd.
Tel: +852 2919-1300
To order supplies: +852 2919-1322
24-hour helpline: +852 2919-6441
India:
India Medtronic Pvt. Ltd
Tel: (+91)-80-22112245 / 32972359
Mobile: (+91)-9611633007
Indonesia:
Medtronic International Ltd.
Tel: +65 6436 5090
or +65 6436 5000
Israel:
Agentek
Tel: +972 3649 3111
Italia:
Medtronic Italia S.p.A.
Tel: +39 02 24137 261
Fax: +39 02 24138 210
Servizio assistenza tecnica:
Nº verde 24h: 800 20 90 20
Japan:
Medtronic Japan Co. Ltd.
Tel: +81-3-6430-2019
24 Hr. Support Line: 0120-56-32-56
Kazakhstan:
Medtronic Kazakhstan B.V.
Tel: +77273110580
Latin America:
Medtronic, Inc.
Tel: 1(305) 500-9328
Fax: 1(786) 709-4244
Latvija:
Ravemma Ltd.
Tel: +371 7273780
Macedonia:
Kemofarm
Tel: +389 2 260 36 03
Fax: +389 2 260 36 49
Magyarország:
Medtronic Hungária Kft.
Tel: +36 1 889 0688
Malaysia:
Medtronic International Ltd.
Tel: +603 7946 9000
Middle East and North Africa:
Regional Office
Tel: +961-1-370 670
Montenegro:
Glosarij
Tel: +382 20 642 495
Fax: +382 20 642 540
Page 4

Nederland, Luxembourg:
Medtronic B.V.
Tel: +31 (0) 45-566-8291
Gratis: 0800-3422338
New Zealand:
Medica Pacifica
Phone: 64 9 414 0318
Free Phone: 0800 106 100
Norge:
Medtronic Norge A/S
Tel: +47 67 10 32 00
Fax: +47 67 10 32 10
POCCИЯ:
Medtronic B. V.
Tel: +7 495 580 73 77
24h: 8-800-200-76-36
Philippines:
Medtronic International Ltd.
Tel: +65 6436 5090
or +65 6436 5000
Polska:
Medtronic Poland Sp. Z.o.o.
Tel: +48 22 465 6934
Portugal:
Medtronic Portugal Lda
Tel: +351 21 7245100
Fax: +351 21 7245199
Puerto Rico:
Medtronic Puerto Rico
Tel: 787-753-5270
Republic of Korea:
Medtronic Korea, Co., Ltd.
Tel: +82.2.3404.3600
Romania:
Trustmed SRL
Tel: +40 (0) 21 220 6477
Schweiz:
Medtronic (Schweiz) AG
Tel: +41 (0)31 868 0160
24-Stunden-Hotline: 0800 633333
Fax Allgemein: +41 (0)318680199
Serbia
Epsilon
Tel: +381 11 311 5554
Fax: +381 11 311 5554
Singapore:
Medtronic International Ltd.
Tel: +65 6436 5090
or +65 6436 5000
Slovenija:
Zaloker & Zaloker d.o.o.
Tel: +386 1 542 51 11
24-h pomoč: 386 51 316 560
Slovenská republika:
Medtronic Slovakia o.z.
Tel: +421 26820 6986
Fax: +421 268 206 999
Sri Lanka
Swiss Biogenics Ltd.
Mobile: (+91)-9003077499
or (+94)-777256760
Suomi:
Medtronic Finland Oy
Tel: +358 20 7281 200
Help line: +358 400 100 313
Sverige:
Medtronic AB
Tel: +46 8 568 585 20
Fax: +46 8 568 585 11
Taiwan:
Medtronic-Taiwan Ltd.
Tel: +886.2.2183.6068
Toll Free: +886.0800.005.285
Thailand:
Medtronic (Thailand) Ltd.
Tel: +662 232 7400
Turkiye:
Medtronic Medikal Teknoloji
Ticaret Ltd. Sirketi.
Tel: +90 216 4694330
USA:
Medtronic Diabetes Global Headquarters
Tel: +1-800-646-4633
24 Hour HelpLine: +1-800-826-2099
To order supplies: +1-800-843-6687
Ukraine:
Med EK Service Ltd.
Tel: +380445457705
United Kingdom:
Medtronic Ltd.
Tel: +44 1923-205167
Österreich:
Medtronic Österreich GmbH
Tel: +43 (0) 1 240 44-0
24 – Stunden – Hotline: 0820 820 190
Česká republika:
Medtronic Czechia s.r.o.
Tel: +420 233 059 401
Non-stop help line:
+420 233 059 059
Page 5

Contents
Chapter 1 1 Introduction
2 iPro2 system
3 User safety
4 Indications for use
4Contraindications
4Warnings
4Precautions
5 Meters supported by CareLink iPro for uploading
5 Compliance information
6 Interference from wireless devices
6Assistance
Chapter 2 7 One-time device setup
8 One-time iPro2 activation
10 Key notes about iPro2
Chapter 3 11 Patient setup
12 Preparing for study
13 Wiping the iPro2 with alcohol before a patient study
14 Tips for a successful patient study
15 Preparation for sensor insertion
15 Inserting the sensor
16 Briefing the patient
17 Meter use
17 First day
17 Remaining days
17 Care and wearing instructions
18 Preparing to connect the iPro2 (after briefing the patient)
18 Connecting the iPro2 to the sensor
Chapter 4 21 Uploading data to CareLink iPro
22 Before you begin
22 Disconnecting the iPro2 and removing the sensor
iPro2 CGM User Guide Contents v
Page 6

22 Disconnecting the iPro2 from the sensor
22 Removing the sensor from the patient
23 Cleaning and disinfecting the iPro2
24 One-time CareLink iPro software and computer setup
24 Uploading iPro2 data
Chapter 5 28 System maintenance
29 Cleaning the iPro2
29 Cleaning the Dock
29 Components that cannot be cleaned
30 Charging the iPro2 between studies
31 Storage and organization tips
Appendix A 33 Troubleshooting
33 Troubleshooting reference
36 Checking the iPro2 connector pins
37 Dock lights quick reference
38 Resetting the iPro2
Appendix B 39 Enlite sensor performance
39 In Vivo performance
39 Results
39 Site comparison
40 Mean and Median Absolute Relative Difference
40 Clarke error grid analysis
42 Percent agreement
43 Sensor Life
43 Interference
43 Limitations
Appendix C 44 Specifications and notices
44 iPro2 system specifications
46 Guidance and manufacturer's declaration
50 Warranty
51 Icon table
Glossary 53
Index 55
iPro2 CGM User Guide Contents vi
Page 7
Introduction
1
iPro2 system
user safety
1
Welcome to iPro2 Continuous Glucose Monitoring (CGM)
Thank you for your trust in Medtronic products and services. We hope you will find iPro2 to be
the simplest and most convenient CGM product that you have ever used.
• This User Guide provides the information that you need for setting up and using the iPro2
CGM system.
• You will find a page like this at the beginning of each chapter. This page gives you a basic
overview of that chapter, and the steps you will take to complete each task.
assistance
32
• You will also see a “Key Notes” area on each chapter overview page. These are the important
points for you to remember from that chapter.
iPro2 CGM User Guide Introduction 1
Page 8
iPro2 system
iPro2 DockiPro2 cleaning
Dock USB
Cable
wall-powered
adapter
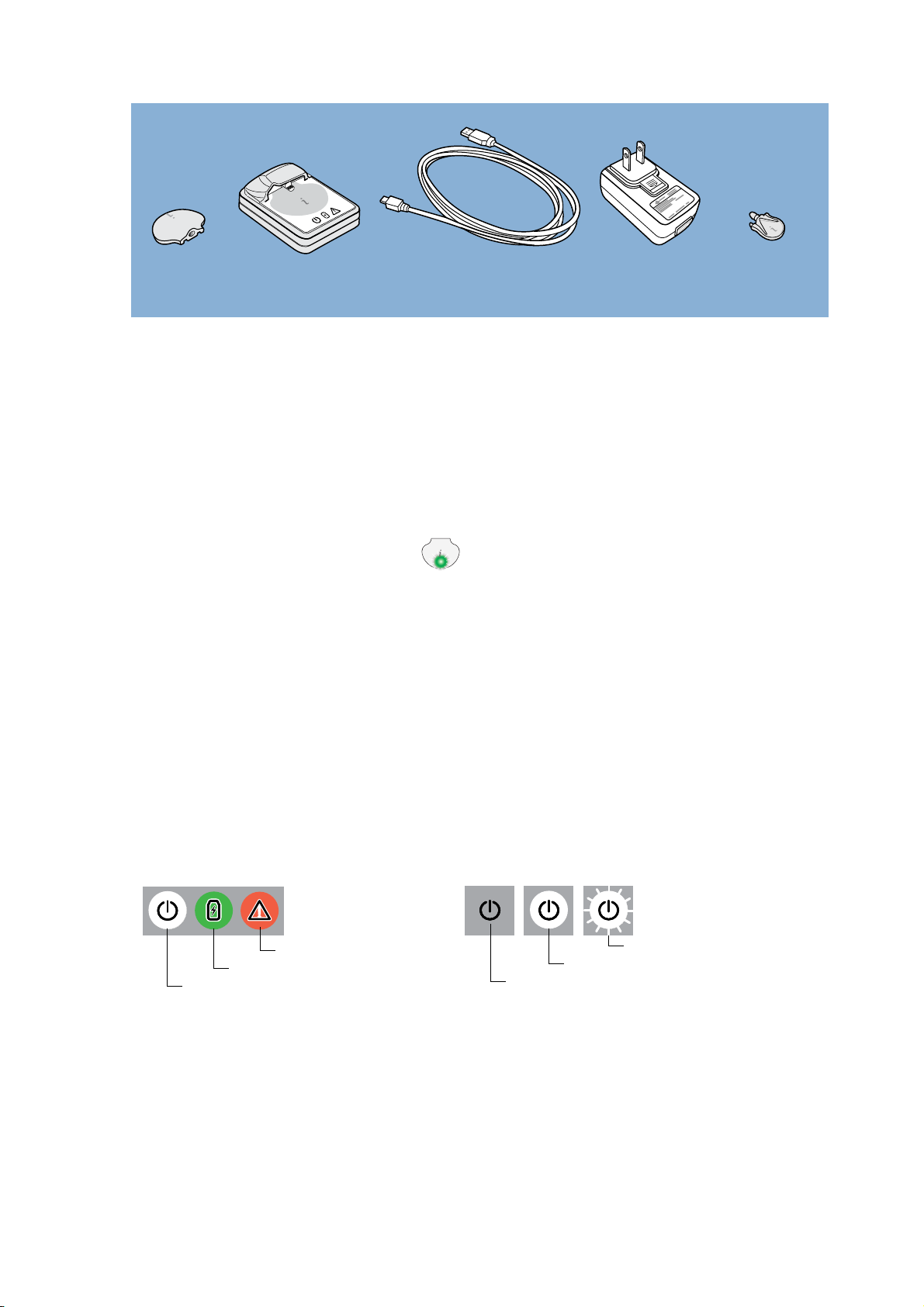
These are the components of the iPro2 CGM system:
• iPro®2 digital recorder, MMT-7741 (iPro2)
The iPro2 collects and stores data from a glucose sensor. The data can be uploaded into
CareLink iPro® Therapy Management Software for Diabetes (CareLink iPro, MMT-7340), to
generate reports and store the data. The iPro2 can collect up to seven 24-hour periods of
data, after which it shuts off automatically.
The iPro2 has an internal green light.
This light flashes when you connect the iPro2
to an inserted glucose sensor. It will only flash if the iPro2 detects an adequately hydrated
sensor, is fully charged, and does not already contain any data.
• iPro®2 Docking Station, MMT-7742 (Dock)
The Dock has two main functions: charging the iPro2 and uploading data from the iPro2 to
CareLink iPro. The Dock has three lights to provide status information. The white Dock power
plug
light indicates whether power is supplied to the Dock. When you connect the iPro2 to the
Dock, the green charging light and the red warning light indicate the status of the iPro2. If
the green charging light is on, the iPro2 is 100% ready to use.
In this User Guide, you will see the three Dock lights described using the following
conventions. Each light is always either off, on, or flashing.
!
!
Red Warning Light
Green Charging Light
White Dock Power Light
On
Off
Flashing
• iPro®2 Dock USB cable (refer to MMT-7747 if re-ordering)
The small end of the Universal Serial Bus (USB) cable connects to the Dock. The other end
of the cable connects to a USB port on a computer, so that you can upload data into CareLink
iPro® and charge the iPro2. You can also connect the USB cable to a wall-powered adapter.
• Wall-powered adapter (refer to MMT-7747 if re-ordering)
iPro2 CGM User Guide Introduction 2
Page 9

The wall-powered adapter lets you charge the iPro2 by connecting the Dock to a regular
electrical socket, instead of a computer.
The wall-powered adapter comes with four (4) interchangeable power plugs. Connect the
appropriate power plug to the wall-powered adapter.
• Three (3) iPro®2 Cleaning Plugs, MMT-7744 (cleaning plug)
The cleaning plugs provide a watertight seal to protect the connector pins on the iPro2.
Always use a cleaning plug when cleaning and disinfecting the iPro2.
Do not clean the o-rings on the cleaning plug, as this can damage the o-rings.
The cleaning plug can be used to clean the iPro2 30 times. Keep track of cleaning plug uses
and discard the cleaning plug after 30 uses. If you continue to use the cleaning plug beyond
30 times, the iPro2 connector pins could be damaged, because the cleaning plug cannot
continue to provide a watertight seal.
Contact your local representative when you need to order more cleaning plugs.
You will also need the following:
• Serter, MMT-7500 or MMT-7510
• Sof-sensor™, MMT-7003A or Enlite™ sensor, MMT-7008A (Glucose sensor)
• A computer with Internet access to CareLink iPro, MMT-7340 (http://ipro.medtronic.com)
• Patient Log Sheet
•Patient Consent Form
• Patient Instructions Sheet
• Clinic Equipment Log Sheet
• Clinic Checklist (for patient setup and for uploading iPro2 data and printing reports)
• Occlusive adhesive dressing
User safety
This section includes important safety information such as indications, contraindications,
warnings, and precautions.
iPro2 CGM User Guide Introduction 3
Page 10

Indications for use
This iPro2 digital recorder is intended to continuously record interstitial glucose levels in persons
with diabetes mellitus. This information is intended to supplement, not replace, blood glucose
information obtained using standard home glucose monitoring devices. The information
collected by the iPro2 digital recorder may be uploaded to a computer (with Internet access)
and reviewed by healthcare professionals. The information may allow identification of patterns
of glucose-level excursions above and below a desired range, facilitating therapy adjustments,
which may minimize these excursions.
This iPro2 system:
• is intended for prescription use only.
• does not allow data to be made available directly to patients in real time.
• provides data that will be available for review by physicians after the recording interval.
• is intended for occasional rather than everyday use.
• is to be used only as a supplement to, and not a replacement for, standard invasive
measurement.
Contraindications
None known.
Warnings
• This product contains small parts and may pose a choking hazard for young children.
• The glucose sensor should be removed if redness, bleeding, pain, tenderness, irritation, or
inflammation develops at the sensor insertion site, or if the patient experiences unexplained
fever.
• An optional occlusive adhesive dressing should be removed if irritation or reaction to the
tape develops.
• The glucose sensor may create special needs regarding your patients' medical conditions or
medications. Healthcare professionals should discuss this with their patients before they use
the glucose sensor.
• Do not modify this product, as modification could result in a safety hazard.
Precautions
• Do not expose the iPro2 to Magnetic Resonance Imaging (MRI) equipment, x-ray
equipment, Computed Tomography (CT) scanners, Intensity-Modulated Radiation Therapy
(IMRT), or other devices that generate strong magnetic fields or ionizing radiation. If the
iPro2 is inadvertently exposed to a strong magnetic field, discontinue use and contact your
local country representative.
• If performing multiple iPro2 studies on the same patient, establish a rotation schedule for
choosing new sensor sites.
iPro2 CGM User Guide Introduction 4
Page 11

• Avoid inserting a sensor in areas on the body that are constrained by clothing, have scar
tissue, or are subject to rigorous movement during exercise.
• If the Enlite sensor (MMT-7008A) was inserted, wait five minutes before connecting the
iPro2. If a Sof-sensor (MMT-7003A) was inserted, wait 15 minutes before connecting the iPro2.
- Make sure that the sensor insertion site is not bleeding before connection. If you find
blood on top of the sensor adhesive, do not connect the iPro2. This is to prevent body
fluids from getting into the connector of the iPro2. If blood gets inside the iPro2's
connector, it may not be properly cleaned out without damaging the connector, so the
iPro2 will have to be discarded.
- If bleeding occurs, apply steady pressure with a sterile gauze or cloth at the insertion
site until bleeding stops. After bleeding stops, attach the iPro2 to the sensor.
- If bleeding persists after three minutes, remove the sensor and discard. Insert a new
sensor in a different location.
• If body fluid comes into contact with the cleaning plug's connector or the Dock's
connector, the contaminated device must be discarded to prevent contamination of the
iPro2.
• Do not allow fluids (including water, cleaning fluids, and disinfectants) on the iPro2's
connector opening or connector pins. Fluids can cause the connector pins to corrode and
may affect the iPro2's performance.
Meters supported by CareLink iPro for uploading
For a list of supported meters, see your CareLink iPro Software User Guide.
Compliance information
The iPro2 and Dock comply with the United States Federal Communications Commission (FCC)
and international standards for Electromagnetic Compatibility. For the specific regulations and
test results for your area, please contact your local representative.
These devices comply with Part 15 of the FCC Rules. Operation is subject to the following two
conditions:
1 These devices may not cause harmful interference.
2 These devices must accept any interference received, including interference that may cause
undesirable operation.
These standards are designed to provide reasonable protection against excessive radio frequency
interference and prevent undesirable operation of the device from unwanted electromagnetic
interference.
iPro2 CGM User Guide Introduction 5
Page 12

Interference from wireless devices
Common wireless consumer devices, such as cellular (mobile) phones or cordless phones, may
disrupt communication during iPro2 uploads to the computer. It is likely that other wireless
devices using similar frequency ranges will have a similar effect. This interference, however, will
not cause any incorrect data to be sent, and will not cause any harm to your iPro2 system.
To reduce the likelihood of data communication errors, you should relocate either the wireless
device or the iPro2 system devices. Testing conducted with several different cellular phones
suggests that interference will not be a problem if the phone is at least 30 centimeters (12 inches)
from the iPro2 system devices.
Assistance
Please contact your Medtronic representative.
iPro2 CGM User Guide Introduction 6
Page 13
One-time device setup
activate iPro2
1
2
!
!
Key Notes:
• The reset button on the Dock is used to wake up (or activate) the iPro2 because it is shipped
in a special sleep mode. This is a one-time task. In the future, doing this will erase all sensor
data that is on the iPro2.
• Never connect an iPro2 to any device other than the Dock, sensor, or cleaning plug.
• For cleaning, use only the cleaning plug.
!!
!!
iPro2 CGM User Guide One-time device setup 7
Page 14
One-time iPro2 activation
!
!
The iPro2 is shipped in a special sleep mode to protect its battery. You need to wake it up by
following this one-time procedure. This should be done a minimum of eight hours before your
first iPro2 patient setup.
CAUTION: Do not perform this procedure if you already have sensor data on the iPro2. If
you press the reset button while the iPro2 is connected to the Dock, all sensor data on the
iPro2 will be erased. This procedure is only for activating the iPro2 for the first time.
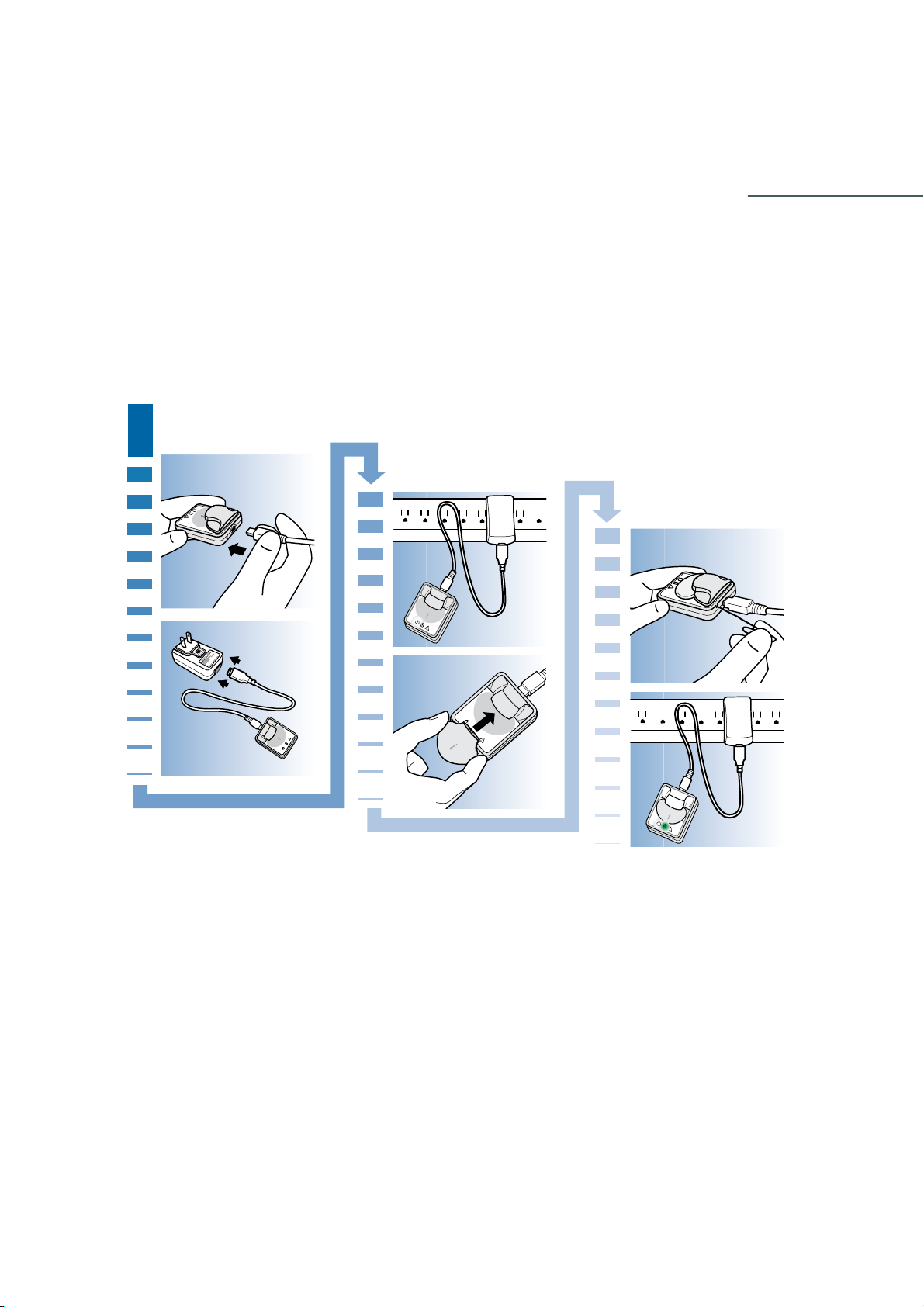
1 Connect the small end of the USB cable to the Dock.
2 Connect the other end of the USB cable to the wall-powered adapter.
!
!
3 Connect the wall-powered adapter into an electrical socket. The three lights on the Dock
will flash once, and then the white Dock power light will remain on, indicating that the Dock
is plugged in.
!
!
!
iPro2 CGM User Guide One-time device setup 8
Page 15

4 Place the iPro2 into the Dock.
!
!
The green charging light will start flashing.
NOTE: The red warning light may turn on if you do not immediately complete the next
steps. This is normal because the iPro2 has not been activated. You can continue to
follow these instructions even if you see the red warning light.
5 Find the small hole on the back of the Dock, next to the USB cable. This is the reset button.
6 Insert the end of a small paper clip into the hole about 0.30 cm (1/8 inch). Push the reset
button once and release. The white Dock power light will flash
. After a few seconds,
the green light on the iPro2 will flash.
The iPro2 is now activated. It will never return to sleep mode.
7 Leave the iPro2 on the Dock to continue charging. During charging, the white Dock power
light will be on, and the green charging light will flash.
!
iPro2 CGM User Guide One-time device setup 9
Page 16

8 Allow up to eight (8) hours for the iPro2 to fully charge. Once the iPro2 is charged, the green
!
!
charging light on the Dock will stop flashing and will remain on. This means that the iPro2
is fully charged.
!
When charged regularly after each sensor use, the iPro2 will become fully charged in only
about 30 minutes.
Key notes about iPro2
• The reset button on the Dock is used to wake up (or activate) the iPro2 because it is shipped
in a special sleep mode. This is a one-time task. In the future, doing this will erase all sensor
data that is on the iPro2.
• Never connect an iPro2 to any device other than the Dock, sensor, or cleaning plug.
!
!
• For cleaning, use only the cleaning plug.
iPro2 CGM User Guide One-time device setup 10
Page 17
Patient setup
3
prepare for patient
1
!
Key Notes:
insert sensor
connect iPro2
32
• Use universal precautions when handling the sensor and iPro2.
• Do not use sticky skin preparation prior to sensor insertion. It can damage the sensor. Sticky
skin preparation solutions may not be available in Japan.
• If the Enlite sensor (MMT-7008A) was inserted, wait five minutes before connecting the
iPro2. If a Sof-sensor (MMT-7003A)was inserted, wait 15 minutes before connecting the
iPro2. Use this time to give instructions to the patient.
• Before setting up any patients on iPro2, make sure that your clinic has completed the onetime CareLink software and computer setup instructions in the previous chapter.
iPro2 CGM User Guide Patient setup 11
Page 18

Preparing for study
Before the patient arrives in your office, make sure that all the necessary equipment and supplies
are available and ready.
!
Materials needed for patient setup:
•Cleaning plug
•Alcohol swabs
•Gloves
•Serter
• Glucose sensor
• Sharps container
• iPro2, charged and disinfected. The green charging light on the Dock must be on
flashing) before you remove the iPro2 from the Dock.
• Patient Log Sheets
•Patient Consent Form
•Patient Instructions
• Clinic Equipment Log
• Occlusive adhesive dressing
• Optional: Clinic Checklist
NOTE: Use universal precautions when handling the sensor and iPro2.
(not
iPro2 CGM User Guide Patient setup 12
Page 19
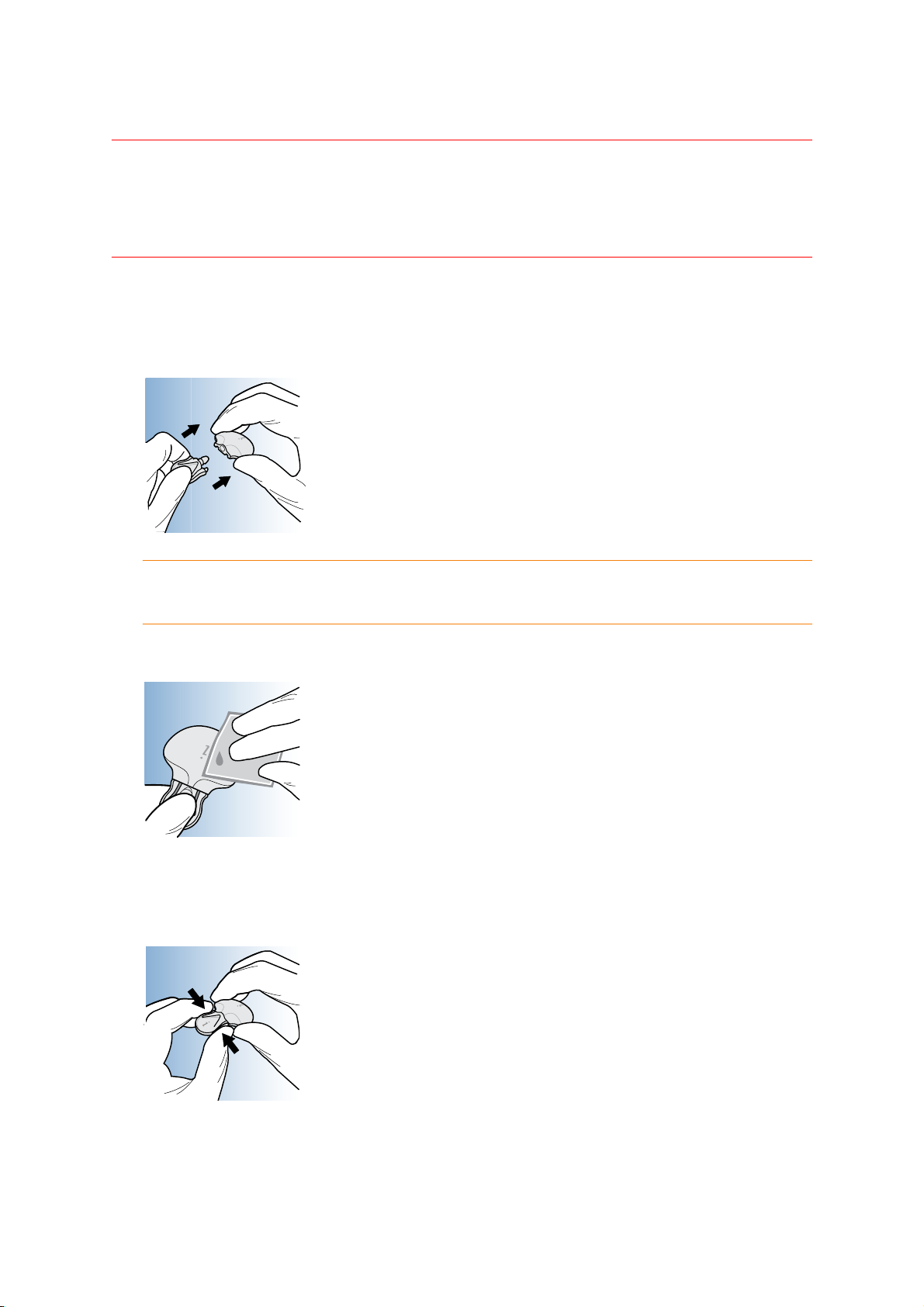
Wiping the iPro2 with alcohol before a patient study
The iPro2 is intended for multiple patient use. Follow this procedure before each patient use.
WARNING: If there is any body fluid inside the connector, the iPro2 must be discarded. Do
not discard the iPro2 in a medical waste container. The iPro2 contains a battery which may
explode upon incineration. Dispose of the iPro2 according to the local regulations for
battery disposal (non-incineration). See Precautions for additional information.
1 While wearing gloves, attach the cleaning plug to the iPro2 to make sure that fluids do not
contact the iPro2's connector opening. Fluids can cause the connector pins to corrode and
affect the iPro2's performance.
CAUTION: Do not twist the cleaning plug while it is attached to the iPro2. This will
damage the iPro2.
2 Wipe the iPro2 with an alcohol swab or rinse with alcohol.
3 Disconnect the cleaning plug from the iPro2 by gently squeezing the arms of the cleaning
plug.
iPro2 CGM User Guide Patient setup 13
Page 20

CAUTION: The o-rings on the cleaning plug have lubricant to help make a watertight
seal with the iPro2. This lubricant may wear off after approximately 30 uses. At that
time, the cleaning plug must be discarded. Keep only one unwrapped cleaning plug at
hand, so that you can keep track of its use and will know when to unwrap a new
cleaning plug.
Tips for a successful patient study
• Keep the sensor hydrated and fully inserted throughout the study:
- Make sure to follow the sensor insertion instructions carefully.
- Choose a good sensor insertion site.
- Use the proper angle for insertion.
- Apply an adhesive dressing over the sensor and iPro2.
• If you see gaps in sensor data, it could be caused by any of the following reasons:
- The sensor was partially removed during the study, which means that no data was being
collected for that period of time.
- The iPro2 lost its connection with the sensor. If the iPro2 is disconnected from the sensor
and then reconnected during the study, it will continue recording. However, there will
be a gap in the sensor data. The length of the gap depends on how long the iPro2 was
disconnected.
- The sensor was not continuously hydrated while connected to the body. It is possible
for the sensor to lose hydration and then regain it, even if it does not pull out.
- CareLink iPro does not have good BG meter readings within 12 hours of each other to
calibrate all of the sensor data.
• Emphasize to the patient, ideally by using a Patient Instructions Sheet, the importance of
following instructions for blood glucose testing throughout the study. Patients should
complete at least four BG meter readings per day to avoid data gaps. If a patient does
not record accurate BG meter readings frequently enough, CareLink iPro will not have
enough BG meter readings to fully calibrate the sensor data. This can cause gaps in data on
the patient's reports. CareLink iPro needs at least one BG meter reading within an expected
range every 12 hours. Erroneous BG meter readings may be ignored by CareLink iPro and
may stop the sensor plot until the next good BG meter reading.
• Make sure that your patient tests blood glucose at least one hour after the iPro2 is connected
to the sensor. The iPro2 takes one hour to start up a sensor. If the patient does the first BG
meter reading too soon, sensor data will not be available for calibration. Therefore, the
sensor trace in the reports will begin at the time of the next BG meter reading. This will be
apparent in CareLink reports because the data will begin later than you expect.
• Make sure that the patient does another BG meter reading two hours after the first one.
This BG meter reading is a backup, in case the first BG meter reading was a few minutes
too early.
iPro2 CGM User Guide Patient setup 14
Page 21

• Mid-study upload: Uploading sensor data from an iPro2 clears the data from the iPro2. The
first upload will be shown as its own study in CareLink iPro. When the iPro2 is reconnected
to the sensor, it will begin the one-hour start up again and start a new study, assuming that
it also has enough charge to start a new study. You cannot combine two separate uploads
into one set of reports in CareLink iPro.
• Do not change the sensor during the study. The iPro2 will keep recording, but the values
on the second sensor will vary widely for many hours because the iPro2 will not properly
start the second sensor. For the best results, upload data after each sensor use.
Preparation for sensor insertion
1 Ask your patient about sleeping position and about his or her normal daily routine. Does
the patient exercise or do a lot of bending or lifting at work? What kind of clothing does
the patient normally wear? Are there other activities that could disturb a sensor site, such
as prolonged sitting in a driving position in a car? Choose a site that will be protected. Refer
to the appropriate sensor/serter user guide for information on where to insert the sensor.
2 Wash your hands thoroughly.
3 Put on gloves.
4 Ask the patient to stand.
5 Clean the insertion site with alcohol and allow to air dry.
NOTE: Do not use sticky skin preparation solutions before inserting the sensor. A sticky
preparation solution may be used after the sensor is inserted, and before applying an
occlusive adhesive dressing, to help the adhesive stick to the patient's skin. Sticky skin
preparation solutions may not be available in Japan.
Always refer to the instructions that came with the glucose sensor and the sensor insertion
device.
Inserting the sensor
CAUTION: Healthcare professionals should wear gloves when handling the sensor.
1 Always refer to the sensor insertion device user guide (Sen-serter™ or Enlite™ Serter) for
instructions on how to insert the sensor.
iPro2 CGM User Guide Patient setup 15
Page 22

CAUTION: If you see body fluid on the metal sensor contacts or black o-rings, do not
connect the iPro2. Remove and dispose of the sensor, and insert a new sensor. This will
prevent contamination of the iPro2.
2 Make an entry on the Clinic Equipment Log and the Patient Log Sheet. Make sure to write
down the serial number (SN) of the iPro2, the patient’s name or ID, and the date that you
placed it on the patient.
3 You now need to wait at least five minutes for Enlite sensor (MMT-7008A) before connecting
the iPro2, to allow the sensor to become hydrated with interstitial fluid. If you are using Sofsensor (MMT-7003A), wait 15 minutes before connecting the iPro2. Take this time to brief
your patient on what to do when he or she goes home.
Briefing the patient
The patient must receive detailed instructions on wearing the sensor and iPro2, study
compliance, meter use and maintaining a log sheet. Ideally, provide the patient with a Patient
Log Sheet and a Patient Instructions Sheet. Go over the items listed on each of the documents
and make sure that your patient understands his or her responsibilities to ensure a successful
study.
Key points:
• Wear the iPro2 continuously while following normal daily activities.
• Record meals, blood glucose, exercise or strenuous activities, and medications on a Patient
Log Sheet.
• Keep the Patient Log Sheet accessible at all times so that information can immediately be
written down after each event. Record the time and date within five minutes of each BG
meter reading.
• Use the same glucose meter and the same lot of strips for the entire study.
• Do not let anyone else use the meter during the study.
• Do not use control solution during the study.
• Do not change the date, time, or any other settings on the meter during the study.
• Take at least four blood glucose (BG) meter readings per day, such as before each meal and
before bed.
iPro2 CGM User Guide Patient setup 16
Page 23

• Take the first BG meter reading at least one hour after leaving the office, and another about
two hours after the first one.
• Only BG values between 40 and 400 mg/dL (2.2 and 22.2 mmol/L) will be used for
calibration. If a meter reading is outside of this range, it does not count, and another BG
meter reading will be needed when the patient's blood glucose is within the range.
CAUTION: The patient must return the iPro2 to the clinic within 10 days of the end of the
study. After 10 days, if the iPro2 is not connected to a powered Dock, the iPro2 battery may
lose its charge, and all data on the iPro2 could be lost. Make sure to schedule the patient's
return of the iPro2 well within this time period.
What to do while briefing the patient
1 Give the patient the materials they need, including at least one Patient Log Sheet and a
Patient Instructions Sheet.
2 On the Patient Log Sheet, write the patient's name, iPro2 serial number, meter brand, meter
ID, and the times for the first two BG meter readings.
3 Make sure that the patient's blood glucose meter has a good battery that will last for the
entire length of the study.
4 Check the date and time on the blood glucose meter.
Meter use
Instruct the patient that BG meter readings are required to calibrate the sensor data, and that
for successful study data, the patient must follow these guidelines for meter use.
First day
The patient must do three blood glucose (BG) meter readings on the first day at these times:
• At least one hour after you connect the iPro2 and the patient leaves the office (but not any
sooner than one hour). Write this time on the front of the Patient Log Sheet.
• Two hours after the first BG meter reading (three hours after the iPro2 is connected)
• Once more before midnight
Remaining days
• For the remaining days of the study, collect at least four BG meter readings per day,
preferably before breakfast, lunch, dinner, and bedtime.
• The patient should do at least three BG meter readings on the last day before the sensor is
removed.
Care and wearing instructions
The patient can shower and swim without removing the iPro2 or sensor. The iPro2 and sensor
are watertight for up to 30 minutes, up to a depth of 2.4 meters (8 feet). There is no time limit
for swimming on the surface of the water or showering.
iPro2 CGM User Guide Patient setup 17
Page 24

The patient should periodically check the sensor site to ensure that the sensor and iPro2 are
tightly connected, that the sensor is fully inserted and that there is no bleeding or irritation at
the sensor site.
• If the sensor is partly pulled out, attempt to gently push it back into place.
• Remove the sensor if there is redness, pain, tenderness, or swelling at the site. The patient
should notify the physician’s office if experiencing any of these symptoms.
Insulin should be injected at least 8 centimeters away from the sensor insertion site, and insulin
pump infusion should be at least 8 centimeters from the sensor insertion site.
The iPro2 must be removed (but the sensor can be left in) prior to an x-ray, CT scan or MRI.
Simply reconnect the iPro2 afterward.
Make sure that the patient can return the iPro2 to the clinic well within 10 days of the end of
the study. After 10 days, if the iPro2 is not connected to a powered Dock, the iPro2 battery may
lose its charge, and all data on the iPro2 could be lost.
Preparing to connect the iPro2 (after briefing the patient)
1 If bleeding has occurred:
a. When bleeding stops, attach the iPro2 to the sensor.
CAUTION: If bleeding does NOT stop, do NOT connect the iPro2 to the sensor.
2 If bleeding does not stop after three minutes, do the following:
a. Remove the sensor and discard.
b. Reapply pressure using a sterile gauze or cloth until the bleeding stops.
c. Insert a new sensor in a different location.
Connecting the iPro2 to the sensor
Important: The iPro2 must be fully charged and cleared of data before connecting to a
sensor. You can verify this by connecting the iPro2 to the Dock. When you connect the iPro2
to the Dock, if the green charging light is on (not flashing), as shown below, the iPro2 is fully
ready to use.
!
1 If the Enlite sensor (MMT-7008A) was inserted, make sure that it has been at least five
minutes since you inserted the sensor. If a Sof-sensor (MMT-7003A) was inserted, make sure
that it has been at least 15 minutes.
2 Touch the end of the inserted sensor to prevent it from moving during connection.
iPro2 CGM User Guide Patient setup 18
Page 25

3 Hold the iPro2 as shown. The flat side of the iPro2 should face the skin.
4 Push the iPro2 onto the sensor until the sensor's flexible side arms snap into the notches
on the iPro2. If the iPro2 is properly connected, and if the sensor has had enough time to
become hydrated, within 10 seconds the iPro2's green light will flash six times. The flashing
takes about 10 seconds.
5 If the iPro2's green light flashes, then the sensor is fully hydrated and the iPro2 has
successfully started the study.
6 If the iPro2's green light does not flash, and the Dock displayed a solid green charging light
before you removed the iPro2 from it, then the sensor is not fully hydrated. You can
do either of the following:
a. If the Enlite sensor (MMT-7008A) was inserted: remove the iPro2 from the sensor, wait
five minutes, and then try connecting the iPro2 again. This can be repeated every five
minutes until the sensor is hydrated.
If a Sof-sensor (MMT-7003A) was inserted, remove the iPro2 from the sensor, wait five
minutes, and then try connecting the iPro2 again. This can be repeated every 15 minutes
until the sensor is hydrated.
b. Remove the sensor from the patient’s body and insert a sensor in a new site on the
body. If the Enlite sensor (MMT-7008A) was inserted, wait five minutes for the new
sensor to become hydrated before connecting the iPro2 again.
If a Sof-sensor (MMT-7003A) was inserted, wait 15 minutes for the new sensor to become
hydrated before connecting the iPro2 again.
7 If you are using Enlite sensor:
iPro2 CGM User Guide Patient setup 19
Page 26

Gently cover the iPro2 with the adhesive tab.
If you are using Sof-sensor:
After you successfully connect the iPro2 to the sensor, it is highly recommended to place
an occlusive adhesive dressing over the iPro2 and the sensor. This may help to keep the
sensor in place under the skin.
Tip: When applying the adhesive dressing, secure the sensor firmly but comfortably, and
secure the iPro2 loosely to allow some movement.
Important: If the sensor is pulled out by more than a millimeter, the iPro2 will stop collecting
data until the sensor is pushed back in place. When the sensor is pushed back in, the iPro2
will start collecting data 30 minutes later.
iPro2 CGM User Guide Patient setup 20
Page 27

Uploading data to CareLink iPro
4
remove iPro2 and
1
sensor
Key Notes:
• Always clean and disinfect the iPro2 as described in Cleaning and disinfecting the iPro2 before
connecting it to the Dock. Always discard used gloves immediately after disinfecting the
iPro2. The Dock connector cannot be disinfected.
clean and disinfect
2
iPro2
upload data
3
!
• If you see any body fluid in the iPro2 connector opening, do not connect the iPro2 to the
Dock. Instead, you must discard the iPro2 after disinfecting it as described in Cleaning and
disinfecting the iPro2 on page 23.
• Always protect the iPro2's connector pins with a water-tight cleaning plug when cleaning
and disinfecting. Replace the cleaning plug after 30 uses to maintain a water-tight seal.
• Do not connect more than one Dock or blood glucose meter to the computer at one
time. Make sure that both ends of the Dock USB cable are completely connected.
iPro2 CGM User Guide Uploading data to CareLink iPro 21
Page 28

Before you begin
When the patient returns after wearing the iPro2, you will need the following:
• Items from patient:
- iPro2 (which has been worn by the patient)
- Patient's blood glucose meter
- Completed Patient Log Sheet(s)
•Gloves
•Cleaning plug
•Optional: adhesive remover
• Mild liquid soap
• Quaternary ammonium compound
• Gauze pad or cloth
• 70% isopropyl alcohol
• Bio-waste container
• Clinic Equipment Log (if used by your office)
• Dock, with the USB cable connected to a computer with Internet access
• Meter manufacturer's cable
Disconnecting the iPro2 and removing the sensor
Disconnecting the iPro2 from the sensor
1 Put on gloves.
2 Carefully remove any adhesive dressing from the iPro2 and sensor assembly.
3 Hold iPro2 as shown, and pinch the flexible side arms of the sensor between your thumb
and forefinger. Do not twist the iPro2 relative to the sensor.
4 Gently pull the iPro2 away from the sensor assembly.
Removing the sensor from the patient
While wearing gloves, gently lift the sensor's adhesive tape away from the patient’s body to
remove the sensor. Place the sensor in a bio-waste container.
iPro2 CGM User Guide Uploading data to CareLink iPro 22
Page 29

Cleaning and disinfecting the iPro2
The iPro2 is intended for multiple patient use. Always clean and disinfect the iPro2 before
connecting it to the Dock. The Dock cannot be disinfected.
WARNING: If there is any body fluid inside the connector, the iPro2 must be discarded. Do
not discard the iPro2 in a medical waste container. The iPro2 contains a battery which may
explode upon incineration. Dispose of the iPro2 according to the local regulations for
battery disposal (non-incineration). See Precautions for additional information.
1 While wearing gloves, attach the cleaning plug to the iPro2 to make sure that fluids do not
contact the iPro2's connector. Fluids can cause the connector to corrode and affect the
iPro2's performance.
CAUTION: Do not twist the cleaning plug while it is attached to the iPro2. This will
damage the iPro2.
CAUTION: The o-rings on the cleaning plug have lubricant to help make a watertight
seal with the iPro2. This lubricant may wear off after approximately 30 uses. At that
time, the cleaning plug must be discarded. Keep only one unwrapped cleaning plug at
hand, so that you can keep track of its use and will know when to unwrap a new
cleaning plug.
2 If there is adhesive residue on the iPro2, you can remove it with adhesive remover between
each patient use.
iPro2 CGM User Guide Uploading data to CareLink iPro 23
Page 30

3 Dampen a clean cloth with a mild liquid soap solution. Wipe the outside of the iPro2.
4 Rinse the iPro2 under warm tap water.
5 Apply three to four drops of a quaternary ammonium compound disinfectant on a clean,
dry cloth and wipe the iPro2.
6 Hold the cleaning plug and wipe the iPro2 with 70% isopropyl alcohol.
7 Disconnect the cleaning plug from the iPro2 by gently squeezing the arms of the cleaning
plug.
8 Place the iPro2 on a clean, dry, non-shedding cloth and air dry completely.
One-time CareLink iPro software and computer setup
Before uploading iPro2 data, make sure that your office has completed the one-time CareLink
software and computer setup. For more information, see your CareLink iPro User Guide.
Uploading iPro2 data
NOTE: Always navigate using the buttons and links in CareLink iPro.
iPro2 CGM User Guide Uploading data to CareLink iPro 24
Page 31

1 Verify that the iPro2 you are about to upload is for the patient whose record you are viewing
in CareLink iPro:
a. Find the serial number on the Clinic Equipment Log and on the Patient Log Sheet. These
should match the serial number on the back of the iPro2.
b. On the Clinic Equipment Log, indicate that the iPro2 has been returned.
CAUTION: Always make sure to verify that you are uploading the correct iPro2.
2 Click the Upload iPro2 button.
3 Follow the on-screen instructions.
If you see a security warning asking if you want to continue, this is asking if you trust that
the content of this system is safe. Your trust is based on the fact that Medtronic MiniMed
has stated that is safe. Select the check box Always trust content from this publisher, and
then click Yes.
4 Make sure that the Dock is connected to the computer by checking both ends of the Dock
USB cable for a complete connection. The white Dock power light
indicates that it is
connected to a power source such as a computer or wall-powered adapter.
!
If you do not see the white Dock power light, the Dock may have insufficient power to
operate. If it is the only device connected, try plugging the Dock into a different USB port
directly on the computer. Not all USB ports may get sufficient power for the Dock to operate.
You can also connect the Dock to the computer using a USB hub. However, if the white
Dock power light does not turn on, then try using a powered USB hub, which has its own
electrical plug that is connected to an electrical socket.
iPro2 CGM User Guide Uploading data to CareLink iPro 25
Page 32

5 When instructed by CareLink iPro, connect the iPro2 to the Dock.
!
!
CAUTION: Do not connect more than one Dock to the computer at one time. Only
connect the iPro2 associated with the opened patient record to the Dock.
The three lights on the Dock will flash once when you connect the iPro2. Then the green
charging light on the Dock will start flashing
. This indicates that the iPro2 contains
data that needs to be uploaded (or that the iPro2 is charging).
!
!
!
!
6 Click Continue. CareLink iPro tells you when the upload is successfully completed.
If you see a message that instructs you to see the User Guide, please look up that message
in Troubleshooting reference on page 33.
7 Check the green charging light on the Dock.
- If the green charging light on the Dock is on and no longer flashing, the iPro2 is charged
and ready for the next patient.
!
- If the green charging light is still flashing after the upload, leave the iPro2 on the Dock
to charge it, so that it is ready for the next patient.
!
iPro2 CGM User Guide Uploading data to CareLink iPro 26
Page 33

- You can also choose to move the Dock to the wall-powered adapter for charging the
iPro2, or move the iPro2 to another Dock that is connected to a wall-powered
adapter, if you have multiple iPro2 systems.
iPro2 CGM User Guide Uploading data to CareLink iPro 27
Page 34

System maintenance
5
cleaning and
1
disinfecting
Key Notes:
• Always connect the cleaning plug to the iPro2 before cleaning.
storing equipment
2
!
!
!
• When not in use, leave the iPro2 connected to the Dock, so it will be ready for use with the
next patient.
• If an iPro2 is unused for several weeks, you must store it on a powered Dock. Otherwise,
the iPro2 battery could become damaged.
• Keep extra Patient Log Sheets and other iPro2 supplies in an organized cabinet.
iPro2 CGM User Guide System maintenance 28
Page 35

Cleaning the iPro2
Always clean and disinfect the iPro2 after removing it from a patient. Make sure to connect the
cleaning plug to the iPro2 before cleaning and disinfecting. For complete instructions, see
Cleaning and disinfecting the iPro2 on page 23.
Cleaning the Dock
WARNING: Always clean and disinfect the iPro2 after removing it from the patient and
before attaching it to the Dock. If the Dock's connector comes in contact with blood, the
Dock must be discarded because the Dock's connector cannot be disinfected. Dispose of the
Dock according to the local regulations for electronic devices.
CAUTION: The Dock is not watertight. Do not immerse in water or any other cleaning agent.
Do not allow liquid to come in contact with the Dock's connector. Repeated exposure to
liquid could damage the connector and affect the performance of the device. If liquid comes
in contact with the connector, allow the Dock to air dry before proceeding with the cleaning
instructions.
1 Disconnect the Dock USB cable from the computer or wall-powered adapter.
2 Disconnect the Dock from the USB cable.
3 Use a damp cloth with liquid dishwashing detergent to clean any dirt or foreign material
from the outside of the Dock. Never use organic solvents such as paint thinner or acetone
to clean the Dock.
4 Place the Dock on a clean, dry cloth and allow it to air dry completely.
5 When the Dock is completely dry, you can reconnect it to the computer or wall-powered
adapter with the USB cable.
Components that cannot be cleaned
You cannot clean the following components of the iPro2 system:
• Cleaning plugs (discard each cleaning plug after 30 uses)
• Wall-powered adapter
iPro2 CGM User Guide System maintenance 29
Page 36

•Dock USB cable
Charging the iPro2 between studies
Charge the iPro2 in the Dock. The Dock can be connected to the computer or to the wallpowered adapter, which lets you use a regular power outlet for charging. While the iPro2 is
charging, the green charging light on the Dock is flashing, as shown:
!
Between patient studies, the iPro2 should take less than 30 minutes to reach a full charge. When
the iPro2 is fully charged, the green charging light on the Dock remains on:
!
CAUTION: If the green charging light continues to flash and never turns solid, this indicates
that the iPro2 contains patient data that you have not uploaded. You cannot use the iPro2
for another study until you upload the data. If you need to clear the data without
uploading it, you can perform a reset. For details, see Resetting the iPro2 on page 38.
Always leave the iPro2 connected to a powered Dock when not in use. This maintains the life
of the iPro2 battery and keeps the iPro2 ready for the next patient study.
If your clinic has only one iPro2, you can leave the Dock connected to the computer and connect
the iPro2 to the Dock when not in use. The computer supplies enough power to charge the
iPro2, as long as the computer is on and the white Dock power light is on
!
.
If you have multiple iPro2s, you can use the wall-powered adapters to keep them charged at
power outlets, and leave one Dock connected to the computer at all times so that it is ready to
upload data.
iPro2 CGM User Guide System maintenance 30
Page 37

Tip: To extend the life of your Docks, mark your calendar to periodically exchange the Dock that
you have connected to the computer with a Dock that is connected to an electrical socket. The
Dock connected to the computer gets the most use, and the connector pins can wear out over
time.
!
!
!
Storage and organization tips
When not in use, store the iPro2 on the Dock and keep the Dock plugged in, so that the iPro2
remains charged. Otherwise, the iPro2 battery could become damaged.
You can organize your other iPro2 supplies in a small drawer organizer, such as the one shown
here. These are some of the items that you will want to keep on hand and ready for the next
patient:
•Serter
• Glucose sensors
• Occlusive adhesive dressings
•Alcohol swabs
• Liquid dishwashing detergent
•Adhesive remover
•Gloves
• Documents and forms, including:
- Patient Log Sheets
- Patient Consent Forms
- Patient Instructions Sheets
-Clinic Equipment Log sheets
-Clinic Checklists
iPro2 CGM User Guide System maintenance 31
Page 38

- A printed copy of this User Guide
• Cleaning plugs
•Gauze pads
• Quaternary ammonium compound
• 70% isopropyl alcohol
iPro2 CGM User Guide System maintenance 32
Page 39

A
Troubleshooting
This appendix contains troubleshooting information for the iPro2 CGM System. Please refer to
these instructions before contacting your local representative.
Troubleshooting reference
Problem Possible causes What to do
Did you take the iPro2 directly from a powered Dock, and did
the Dock display a solid green charging light?
• If yes, then the iPro2 may not be connected properly, or the
sensor may not be fully hydrated. Disconnect and reconnect
the iPro2. If this does not work, wait another five minutes
and then connect the iPro2 to Enlite sensor. If the iPro2 still
does not flash, wait another five minutes and try again. If
you are using Sof-sensor, wait 15 minutes. In some cases, it
can take up to two hours for the sensor to become hydrated.
I connected the iPro2
to the sensor, and
the iPro2 did not
flash after 10
seconds.
Either the sensor is
not adequately
hydrated, the iPro2 is
not connected
properly, or the iPro2
is not ready to begin
a study.
If the iPro2 still does not flash after two hours, you can
remove the sensor and insert a new sensor in a different site
on the body.
• If no, or if you are not sure, the iPro2 may not be fully
charged, or may still contain data from a previous study. In
these cases, the green light will not flash when connected
to the sensor.
Disconnect the iPro2 from the sensor. Clean and disinfect it
(see Cleaning and disinfecting the iPro2 on page 23), and then
connect it to the Dock. If the green charging light on the
Dock turns solid after two minutes, the iPro2 is ready to start
a study on a new patient. If not, the iPro2 needs to be
charged or still contains patient data from the previous
study.
If these steps do not work, use the Dock to reset the iPro2. For
instructions, see Resetting the iPro2 on page 38.
iPro2 CGM User Guide Troubleshooting 33
Page 40

Troubleshooting reference
Problem Possible causes What to do
• Check the Clinic Equipment Log or Patient Log
Sheets to find out which patient's data was last
The iPro2 has been
connected to the Dock
with adequate power for
two to three hours, but the
green charging light keeps
flashing.
The iPro2 most likely
contains data that has not
been uploaded.
collected. Open CareLink iPro and check to see
if a study was uploaded for the dates on the log
sheet. If there is no study, upload the iPro2 into
that patient's record in CareLink iPro. CareLink
iPro clears the data off of the iPro2 as part of
the upload process. You must then wait for the
green charging light on the Dock to turn solid
before the iPro2 is ready to use for the next
patient.
!
• If you are unable to identify which patient's data
is still on the iPro2, or if you are unable to
upload the iPro2 successfully, you may need to
reset the iPro2. For instructions, see Resetting
the iPro2 on page 38.
Try connecting the Dock to a different USB port on
the computer. Wait for all three lights to flash,
followed by a solid white light. If the Dock is
connected to the computer but none of the lights
turn on, there may be other USB devices connected
that are using up power. Disconnect other devices.
Do not connect more than one Dock at a time to a
computer. You can also try connecting the Dock to
another computer.
If the white Dock power light is on, but the three
lights do not flash when you connect the iPro2,
check the iPro2 connector pins for damage or
I connected the iPro2 to
the Dock and no lights
came on.
The Dock may not be
connected to the
computer, or it may not
have sufficient power. The
white Dock power light
must be on before
moisture. For assistance in locating the connector
connecting the iPro2.
pins, see Checking the iPro2 connector pins on
page 36.
If the pins are damaged or corroded, the iPro2
cannot communicate with the Dock or CareLink
iPro. Contact your local representative. It may be
time to replace the iPro2.
iPro2 CGM User Guide Troubleshooting 34
Page 41

Troubleshooting reference
!
!
!
!
Problem Possible causes What to do
I connected the iPro2 to
the Dock and all three
lights are flashing on and
off repeatedly.
!
!
The iPro2 is connected to
the Dock and the red
warning light is on.
!
!
!
This could mean that the
iPro2 is not properly
connected to the Dock.
This could mean that the
iPro2 is not properly
connected to the Dock or
needs to be reset. It also
could mean that there is
damage to the iPro2
battery, circuitry, or
connector pins. The iPro2
may need to be replaced.
Disconnect and reconnect the iPro2 to the Dock.
Disconnect the iPro2 and check the connector pins
for damage, corrosion, or moisture. For assistance in
locating the connector pins, see Checking the iPro2
connector pins on page 36. After you confirm that
the pins are not damaged or corroded, reconnect
the iPro2 to the Dock. If another Dock is available,
try connecting the iPro2 to the other Dock.
If there is sensor data on the iPro2, upload the
sensor data using CareLink iPro.
If the red warning light turns on again, perform a
reset as described in Resetting the iPro2 on
page 38. Allow the iPro2 to charge for 20 minutes.
Please note that by performing a reset, all iPro2
sensor data will be erased.
If the red warning light continues to turn on, or if
the iPro2 pins are damaged or corroded, contact
your local representative. It may be time to replace
the iPro2.
iPro2 CGM User Guide Troubleshooting 35
Page 42

Checking the iPro2 connector pins
If the troubleshooting reference advises you to check the connector pins of the iPro2, use the
following image to assist you. This image is an example of how the connector pins should look.
connector opening
connector pins
housing
Look inside the iPro2’s connector opening to make sure that the connector pins are not damaged
or corroded. If the connector pins are damaged or corroded, the iPro2 cannot communicate with
the Dock or CareLink iPro. Contact your local representative. It may be time to replace the iPro2.
Also look for moisture inside the connector opening. If you see any moisture, allow the iPro2 to
dry for at least one hour. Moisture inside the connector opening could cause the iPro2 to not
work properly, and could cause corrosion and damage over time.
To help prevent damage to the pins:
• Make sure to carefully connect the cleaning plug or sensor to the iPro2.
• Do not twist or bend the cleaning plug or sensor when connecting to the iPro2.
For instructions on how to properly clean the iPro2 using the cleaning plug, see Cleaning and
disinfecting the iPro2 on page 23. For instructions on how to properly connect the iPro2 to a
sensor, see Connecting the iPro2 to the sensor on page 18.
iPro2 CGM User Guide Troubleshooting 36
Page 43

Dock lights quick reference
!
!
!
!
Dock lights Description What it means
!
!
!
!
!
!
!
All of the lights are off.
The white Dock power
light is on.
All three lights flash once.
The white Dock power
light is on and the green
charging light is flashing
continuously.
The white Dock power
light and green charging
light are on.
The white Dock power
light flashed five times and
the green charging light is
flashing continuously.
The Dock is not plugged into an electrical outlet or
computer USB port. If it is plugged in, it may not
be receiving enough power.
The Dock is connected to power. If connected to an
electrical outlet, it is ready to charge an iPro2. If
connected to a computer USB port, it is ready to
charge an iPro2 or upload data from an iPro2. The
iPro2 is not connected to the Dock.
All of the Dock lights flash once when you first
connect the Dock to a sufficient power source, or
when you connect the iPro2 to the Dock.
The iPro2 is charging or the iPro2 contains data
that must be uploaded using CareLink iPro. After
you upload data, if the green charging light
continues to flash, the iPro2 is still charging and is
not ready to begin a new patient study.
All previous data has been cleared from the iPro2.
The iPro2 is fully charged and ready for the next
patient study.
The white Dock power light will flash five times
after you press the reset button. The green
charging light will continue to flash as the iPro2
charges. When the iPro2 is fully charged, the green
charging light will stop flashing and remain on.
The white Dock power
!
!
!
light and the red warning
light are on.
There may be a problem with the iPro2. See
Troubleshooting reference on page 33 for details.
iPro2 CGM User Guide Troubleshooting 37
Page 44

Resetting the iPro2
CAUTION: This procedure erases all patient data from the iPro2. Do not perform these steps
unless you have already uploaded the last patient study, or you are prepared to erase any
data that may be on the iPro2.
1 Connect the Dock to power and make sure that the white Dock power light is on.
2 Place the iPro2 into the Dock.
!
!
3 Find the small hole on the back of the Dock, next to the USB cable.
4 Insert the end of a small paper clip into the hole about 0.30 cm (1/8 inch). Push the reset
button once and release. The white Dock power light will flash
. After a few seconds,
the green light on the iPro2 will flash.
5 Wait for the Dock to show a solid green charging light . This indicates that the data has
been cleared, and the iPro2 is fully charged and ready for the next patient study.
iPro2 CGM User Guide Troubleshooting 38
Page 45

B
Enlite sensor performance
In Vivo performance
The performance of the glucose sensor was assessed using post-hoc analysis of a 2-month
1
study
with a prospective correlational design without controls. The sensor data from a total of
64 subjects contributed to the analysis. Subjects had type 1 diabetes and were 18–75 years of
age. People with tape allergies, skin abnormalities, and some serious concomitant conditions
were excluded from enrollment.
Subjects wore two Enlite sensors attached to the abdomen and buttock areas over two periods
of seven days. Sensors that failed before the end of day 7 were removed and not replaced.
Subjects tested their capillary blood glucose level a minimum of 4 times per day using a
commercially available home blood glucose (BG) meter.
The accuracy of the sensor using the iPro2 retrospective algorithm was the primary efficacy
variable. The raw sensor data and the BG meter readings were post-processed using the iPro2
retrospective algorithm to generate glucose values. To assess sensor accuracy, the retrospectively
calibrated glucose values provided by the sensor (sensor values) were compared to the glucose
values provided by the BG meter reference values (also called self-monitoring of blood glucose
or SMBG).
Results
Site comparison
Sensors were used in both abdominal and buttock sites, and showed similar performance. The
overall mean and median absolute relative difference (MARD) was calculated for sensors located
in both the abdomen and buttock areas compared to a SMBG reference. As shown in the table
below, the results are similar. Therefore, abdominal and buttock data have been combined to
present the overall CGM performance results in the remainder of this CGM Performance section.
iPro2 CGM User Guide Enlite sensor performance 39
Page 46

Absolute relative difference (%) compared to SMBG
Mean Median
Abdominal 11.6 7
Buttocks 10.4 6.7
Combined 11.0 6.9
In addition to the SMBG comparison, a precision analysis compared data from sensors inserted
in the abdomen with data from sensors inserted in the buttocks. This analysis resulted in a MARD
of 15.8%.
Mean and Median Absolute Relative Difference
The overall mean absolute relative difference (ARD) was 11.0% (standard deviation or SD, 13.3),
the median ARD was 6.9%, and the per-day mean ARD ranged from 9.5% to 13.5%. The mean
numerical bias was and the median numerical bias was .
Sensor Mean and Median Absolute Percent Difference Compared to SMBG
Overall Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7
40-80 mg/dL
(2.2-4.4 mmol/L)
(n=958) *
81–120 mg/dL
(4.5-6.7 mmol/L)
(n=1513)
121–240 mg/dL
(6.7-13.3 mmol/L)
(n=3244)
241-400 mg/dL
(13.4-22.2 mmol/L)
(n=1019)
All Ranges
(n=6734)
* For the low range, mean and median are expressed as mean absolute difference in mg/dL. For the other
glucose ranges, mean and median are expressed as absolute relative difference in percentages.
Mean 11.7 15.1 10.4 11.9 10.7 9.1 12.2 11.1
Median81178.58677
Mean 12.3 16.1 11.4 9.6 10.5 13.9 12.1 11.1
Median 8.3 11 8 6.9 7.2 9.7 8.6 8.6
Mean 9.2 11 8.5 8.4 8.2 8.3 10.0 9.7
Median 6.3 8 5.9 5.9 6.2 5.2 6.8 6.2
Mean 7.6 7.2 6.7 5.9 7.9 9.4 7.6 8.9
Median 4.3 4.9 3.9 4.2 4.6 5.2 4.9 3.9
Mean 11 13.5 10.1 9.5 9.7 10.7 11.6 10.9
Median 6.9 8.9 6.1 6.3 6.5 6.6 7.3 6.8
Clarke error grid analysis
The Clarke Error Grid was used to assess the clinical relevance of the differences between the
iPro2 readings and the comparative blood glucose meter measurements. The Clark Error Grid
separates paired observations into five zones (A, B, C, D, and E). The presence and severity of
possible treatment error based on interstitial glucose evaluated by the sensor is defined by the
following zones:
iPro2 CGM User Guide Enlite sensor performance 40
Page 47

Zone Description
A Clinically accurate, would have led to correct treatment decisions.
B Would have led to benign decisions or no treatment.
C Would have led to over-correction of normal glucose levels.
D Failure to detect a glycemic level that might have required action by the patient to correct.
E Erroneous data point. If acted upon, could have been harmful.
In order to evaluate differing levels of accuracy at various blood glucose levels, summary statistics
(n, %) are calculated in each of the five zones. Summary statistics for each of the zones are
reported for each stratum of the referenced values.
Temporally paired glucose measurements from the iPro2 and reference meter BG were plotted
as Clarke Error Grid (CEG) scatter plots. A total of 6582 (97.7%) of paired points were in zones A
and B of the Clarke Error Grid. These results of both algorithms exceed the threshold for clinical
acceptability.
Zone
A+B 6582 (97.7) 846 (88.3) 1511 (99.9) 3236 (99.8) 989 (97.1)
A 5802 (86.2) 740 (77.2) 1242 (82.1) 2886 (89.0) 934 (91.7)
B 780 (11.6) 106 (11.1) 269 (17.8) 350 (10.8) 55 (5.4)
C 7 (0.1) 1 (0.1) 2 (0.1) 4 (0.1) 0 (0.0)
D 141 (2.1) 111 (11.6) 0 (0.0) 0 (0.0) 30 (2.9)
E 4 (0.1) 0 (0.0) 0 (0.0) 4 (0.1) 0 (0.0)
Overall 6734 (100.0) 958 (14.2) 1513 (22.5) 3244 (48.2) 1019 (15.1)
40-400 mg/dL
(2.22-22.20
mmol/L)
n (%) n (%) n (%) n (%) n (%)
40-80 mg/dL
(2.22-4.44
mmol/L)
81-120 mg/dL
(4.50-6.66
mmol/L)
121-240 mg/dL
(6.72-13.32
mmol/L)
241-400 mg/dL
(13.38-22.20
mmol/L)
iPro2 CGM User Guide Enlite sensor performance 41
Page 48

The scatter plot of the 6734 paired sensor and meter BG reference values for adult subjects with
an overlay of the Clarke Error Grid analysis is presented in the following graph.
Percent agreement
The accuracy of the CGMS was evaluated by classifying sensor and reference meter readings into
three categories across five concentration groupings shown below. The three categories are:
1) percentage of CGMS readings within 20% of reference, 2) percentage of CGMS readings within
30% of reference, and 3) percentage of CGMS readings within 40% of reference.
Sensor Ranges 20% Agreement 30% Agreement 40% Agreement
40-400 mg/dL
(2.22-22.20 mmol/L)
40-80 mg/dL
(2.2-4.4 mmol/L)
81-120 mg/dL
(4.5-6.7 mmol/L)
121-240 mg/dL
(6.7-13.3 mmol/L)
241-400 mg/dL
(13.4-22.2 mmol/L)
87.1 94.7 97.4
84.1 93.8 96.8
82.1 92.2 96.2
89.0 95.5 98.0
91.7 96.3 97.6
iPro2 CGM User Guide Enlite sensor performance 42
Page 49

Sensor Life
After calibration, 87.7% of sensors operated until they were removed on or after the seventh day
of wear. Five sensors out of 261 did not calibrate properly. Finally, four sensors were removed
after first calibration event because the companion sensor was removed.
Interference
In vitro and in vivo testing suggests that usual pharmacologic levels of acetaminophen and
ascorbic acid have a minimal effect on the function of the iPro2 system. In vitro testing suggests
that normal physiological levels of uric acid do not affect sensor function. The impact of oral
hypoglycemic agents, lipids, bilirubin and other potential interfering substances have not been
studied.
Limitations
Since the iPro2 system requires calibration using a blood glucose value obtained from a home
glucose meter, any inaccuracy in value obtained from the reference meter will affect the accuracy
of the value calculated by the iPro2 system. Also, since the sensitivity of the sensor may
change, failure to recalibrate the sensor by taking BG meter readings at least three times
daily may result in inaccurate glucose readings.
In vitro testing has indicated that the iPro2 may record inaccurate glucose readings during
exposure to electromagnetic fields of 3 V/m or stronger. Therefore, the use of the iPro2 system
in close proximity to strong electromagnetic sources, such as medical imaging
equipment, television transmitters, high-voltage power lines and high-power radio
transmitters, is not recommended.
1. Medtronic Diabetes, An Inpatient Performance Evaluation of a New Subcutaneous Glucose Sensor, August 2010.
iPro2 CGM User Guide Enlite sensor performance 43
Page 50

Specifications and notices
iPro2 system specifications
iPro2: 57.6kPa - 106kPa (4,880 to -400 meters [16,000 to -1,300
Atmospheric pressure range
feet] elevation)
Dock: 62kPa - 106kPa (3,965 to -400 meters [13,000 to -1,300
feet] elevation)
C
Applied Parts
Biocompatibility iPro2: Complies with ISO 10993-1 for long-term body contact
Operating Conditions
Storage Conditions
iPro2 Battery Life
iPro2 Dimensions and Weight
iPro2 (MMT-7741)
Sensor (MMT-7003)
iPro2 temperature: -5° to +45°C (+23° to +113°F)
iPro2 relative humidity: 5% to 95% with no condensation
Dock temperature: -5° to +45°C (+23° to +113°F)
Dock relative humidity: 5% to 95% with no condensation
iPro2 temperature: -25° to +55°C (-13° to +131°F)
iPro2 relative humidity: 10% to 100% with no condensation
Dock temperature: -25° to +55°C (-13° to +131°F)
Dock relative humidity: 10% to 100% with no condensation
7 days of continuous glucose monitoring (CGM) immediately
following a full charge, plus 10 days of additional battery life
immediately following a CGM study. Any data on the device will
be lost when the battery loses its charge.
Width: 3.5 centimeters (1.4 inches)
Length: 2.8 centimeters (1.1 inches)
Height: 0.9 centimeters (0.4 inches)
Weight: 5.7 grams (0.2 ounces)
Width: 5.1 centimeters (2 inches)
Dock Dimensions and Weight
Length: 6.4 centimeters (2.5 inches)
Height: 2.8 centimeters (1.1 inches)
Weight: 22.7 grams (0.8 ounces)
• The iPro2 is an internally powered device. The mode of operation is continuous. The iPro2
is not suitable for use in the presence of a flammable anaesthetic mixture with air or with
oxygen or nitrous oxide.
iPro2 CGM User Guide Specifications and notices 44
Page 51

• All components of the iPro2 CGM system are suitable for use in a clinical environment. The
iPro2 recorder is suitable for use with a glucose sensor in the patient environment.
iPro2 CGM User Guide Specifications and notices 45
Page 52

Guidance and manufacturer's declaration
Guidance and Manufacturer's Declaration - Electromagnetic Emissions
The iPro2 CGM system is intended for use in the electromagnetic environment specified below. The customer or
the user of the iPro2 CGM system should assure that it is used in such an environment.
Emissions Test Compliance Electromagnetic Environment - Guidance
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/flicker
emissions
IEC 61000-3-3
Group 1
Class B
Complies by
exemption
Complies by
exemption
The iPro2 CGM system does not use RF energy for system
communication functions.
The iPro2 CGM system is suitable for use in all
establishments, including domestic establishments and
those directly connected to the public low-voltage power
supply network that supplies buildings used for domestic
purposes.
iPro2 CGM User Guide Specifications and notices 46
Page 53

Guidance and Manufacturer's Declaration - Electromagnetic Immunity
The iPro2 CGM system is intended for use in the electromagnetic environment specified below. The customer or
the user of the iPro2 CGM system should assure that it is used in such an environment.
Immunity Test IEC 60601 Test Level Compliance Level
Electrostatic discharge
(ESD)
±8 kV indirect
±8 kV, 30%–60%
relative humidity
Electromagnetic
Environment - Guidance
Floor should be wood,
concrete or ceramic tile. If
floors are covered with
IEC 61000-4-2 ±8 kV air
Electrical fast transient/
burst
IEC 61000-4-4
±2 kV for power supply
lines
±1 kV for input/output
lines
±22 kV air
(<5% relative
humidity)
±2 kV
±1 kV
synthetic material, the
relative humidity should be
at least 30%.
Mains power should be
that of a typical
commercial or hospital
environment.
Surge ±1 kV line(s) to line(s) ±1 kV Mains power should be
that of a typical
IEC 61000-4-5 ±2 kV line(s) to earth ±2 kV
<5% U
(>95% dip in UT)
T
for 0.5 cycle
<5% U
T
commercial or hospital
environment.
Mains power should be
that of a typical
commercial or hospital
Voltage dips, short
interruptions and voltage
variations on power supply
lines
IEC 61000-4-11
(60% dip in UT)
T
for 5 cycles
70% UT (30% dip in UT)
for 25 cycles
<5% UT (>95% dip in UT)
for 5 seconds
40% U
70% U
<5% U
T
T
T
environment. If the user of
the iPro2 CGM system
requires continued
operation during power
mains interruptions, it is
recommended that the
iPro2 CGM system be
powered from
uninterruptible power
40% U
supply or battery.
Power frequency
(50/60 Hz) magnetic field
IEC 61000-4-8
NOTE: U
is the a.c. mains voltage prior to application of the test level.
T
3 A/m 3 A/m
Power frequency magnetic
fields should be at levels
characteristic of a typical
location in a typical
commercial or hospital
environment.
iPro2 CGM User Guide Specifications and notices 47
Page 54

Guidance and Manufacturer's Declaration - Electromagnetic Immunity
The iPro2 CGM system is intended for use in the electromagnetic environment specified below. The customer or
user of the iPro2 CGM system should assure that it is used in such an environment.
Immunity Test IEC 60601
Level
Conducted RF
3 Vrms
150 kHz to
IEC 61000-4-6
Radiated RF
80 MHz
3 V/m
80 MHz to
IEC 61000-4-3
6.0 GHz
Compliance Level
3 Vrms
3 V/m
Electromagnetic Environment Guidance
Portable and mobile RF communications
equipment should be used no closer to any
part of the iPro2 CGM system, including
cables, than the recommended separation
distance calculated from the equation
applicable to the frequency of the transmitter.
Recommended separation distance:
d=1.2 P
d=1.2 P
d=2.3 P
80 MHz to 800 MHz
800 MHz to 6.0 GHz
Where P is the maximum output power rating
of the transmitter in watts (W) according to
the transmitter manufacturer and d is the
recommended separation distance in meters
(m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site
a
survey
, should be less than the compliance
level in each frequency range
b
.
Interference may occur in the vicinity of
equipment marked with the following
symbol:
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption, and
reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land
mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically
with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site
survey should be considered. If the measured field strength in the location in which the iPro2 CGM system is
used exceeds the application RF compliance level above, the iPro2 CGM system should be observed to verify
normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the iPro2 CGM system.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
iPro2 CGM User Guide Specifications and notices 48
Page 55

Recommended separation distances between portable and mobile RF communications equipment and the
iPro2 CGM system
This section provides information on the recommended separation distance between portable and mobile RF
communications equipment and the iPro2 CGM system. The iPro2 CGM system is intended for use in an
electromagnetic environment in which radiated RF disturbances are controlled. The customer or users of the
iPro2 digital recorder can help prevent electromagnetic interference by maintaining a minimum distance between
portable and mobile RF communications equipment (transmitters) and the iPro2 digital recorder as recommended
below, according to the maximum output power of the communications equipment.
Separation distance according to the frequency of transmitter (m)
Rated maximum
output power of
transmitter (W)
150 kHz to 80 MHz
d=1.2 P
80 MHz to 800 MHz
d=1.2 P
800 MHz to 6.0 GHz
d=2.3 P
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.74
1 1.2 1.2 2.3
10 3.8 3.8 7.4
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in
meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where p is the
maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption, and
reflection from structures, objects and people.
iPro2 CGM User Guide Specifications and notices 49
Page 56

Warranty
Medtronic Diabetes warrants the iPro2 and Dock to the purchaser of the product against defects
in material and workmanship for a period of one year from the date of purchase.
During the warranty period, Medtronic Diabetes will repair or replace, at its discretion, any
defective iPro2 or Dock, subject to the conditions and exclusions stated herein. This warranty
applies only to new devices. In the event a iPro2 or Dock is repaired or replaced, the warranty
period will not be extended past its original expiration date.
This warranty is valid only if the iPro2 or Dock is used in accordance with the manufacturer's
instructions. Without limitation, this warranty will not apply:
• If damage results from changes or modifications made to the iPro2 or Dock by the user, or
third parties, after the date of sale;
• If service or repairs are performed by any person or entity other than the manufacturer;
•If damage results from a Force Majeure or other event beyond the control of the
manufacturer;
• If damage results from negligence or improper use, including but not limited to: improper
storage, submersion in fluid, physical abuse (such as dropping); or
• If fluid has entered the inside of the iPro2 connector opening or the Dock.
This warranty shall be personal to the original user. Any sale, rental or other transfer or use of
the product covered by this warranty to or by a user other than the original user shall cause this
warranty to immediately terminate. This warranty does not apply to glucose sensors and other
accessories.
The remedies provided for in this warranty are the exclusive remedies available for any defects
in material or workmanship in the product. Neither Medtronic Diabetes nor its suppliers or
distributors shall be liable for any incidental, consequential, punitive or special damages of any
nature or kind caused by or arising out of a defect in the product.
All other warranties, expressed or implied, are excluded and specifically disclaimed, including,
but not limited to, any warranty of merchantability or fitness for a particular purpose.
iPro2 CGM User Guide Specifications and notices 50
Page 57

Icon table
Description Icon
Follow instructions for use
Attention: Read all warnings and precautions in
instructions for use.
Stand-by power
Charging/uploading status
Date of manufacture (year - month)
Manufacturer
Batch code
Catalogue number
Device serial number
Configuration
Storage humidity range
Storage temperature range
Fragile product
Ingress protection safety rating. An object one
millimeter in diameter cannot penetrate the
device and cause harm to the user, property, or
the environment. This device can withstand
immersion under water for 30 minutes at a
depth of 2.4 meters (8 feet).
Type BF equipment (Protection from electrical
shock)
Recycle
One per container/package
Three per container/package
IP48
(3X)
European conformity. This symbol means that
the device fully complies with MDD 93/42/EEC
(NB 0459) and R&TTE Directive 1999/5/EC.
Keep dry
iPro2 CGM User Guide Specifications and notices 51
Page 58

Description Icon
MEDICAL EQUIPMENT WITH RESPECT TO
ELECTRICAL SHOCK, FIRE AND MECHANICAL
HAZARDS ONLY IN ACCORDANCE WITH UL
60601-1, CAN/CSA C22.2 No. 601 and IEC
60601-1-1.
iPro2 CGM User Guide Specifications and notices 52
Page 59

Glossary
Area Under the Curve (AUC) - Indicates the amount in high and low excursions as determined by
preset values. Excursion data indicates the frequency of highs or lows. AUC indicates the
magnitude of events by showing how far out of range and for how long.
BG - Blood Glucose
BG reading - Blood glucose measurement that is taken by a blood glucose meter.
Calibrate - Check, adjust, or set to a standard. Sensor data is calibrated using BG meter readings.
Cleaning plug - Small plastic plug that you connect to the iPro2 before cleaning and disinfecting
it. The cleaning plug protects the iPro2's connector pins from being damaged by water or
cleaning fluids.
Docking Station (Dock) - Device that performs two functions: uploading glucose sensor data
from an iPro2 to CareLink iPro; and charging the iPro2. The Dock can be connected to a
computer or to an electrical socket.
iPro2 Recorder (iPro2) - Device that continuously records sensor glucose data while connected to
a glucose sensor. You can upload the data to CareLink iPro by connecting the iPro2 to a Dock,
and view the sensor data on reports.
Logbook - A screen in CareLink iPro that lets you manually enter events such as BG meter
readings, meals, exercise, and medication taken, so that these events show up on reports. The
Logbook also displays BG meter readings, and possibly other events, that you upload from a
supported blood glucose meter into CareLink iPro.
Mean Absolute Difference % (MAD%) - Represents the level of accuracy in calibration of the
sensor to BG meter readings. The lower this number, the greater the calibration accuracy. MAD
% is calculated by taking the difference between closely occurring pairs of sensor glucose and
BG meter readings, dividing by the BG meter reading, and then averaging across all pairs.
Mean Absolute Difference (MAD) - Represents the level of accuracy in calibration of the sensor to
BG meter readings. The lower this number, the greater the calibration accuracy. MAD is
calculated by taking the difference between closely occurring pairs of sensor glucose and BG
meter readings and then averaging across all pairs.
Meter - A medical device for determining the approximate concentration of glucose in the blood.
A small drop of blood is placed on a disposable test strip, which the meter reads and uses to
calculate the blood glucose level. The meter then displays the level in mg/dL or mmol/L.
Sen-serter - The Sen-serter is indicated as an aid for insertion of the Medtronic Diabetes glucose
sensor.
Study - The period of time that a patient wears a glucose sensor and iPro2. This word also refers to
an upload of glucose sensor data from an iPro2 into CareLink iPro, along with any meter upload
and Logbook entries for that iPro2 upload. Each study has its own set of reports.
iPro2 CGM User Guide Glossary 53
Page 60

Upload - The process of transferring diabetes device data to the CareLink iPro server.
iPro2 CGM User Guide Glossary 54
Page 61

Index
A
accuracy, sensor 39
activating iPro2 7
alcohol wiping 13
assistance 6
attaching iPro2 to sensor 18
B
before connecting iPro2 18
BG meter
patient instructions 17
blood glucose meter use, patient
instructions 17
blood glucose meters
supported 5
briefing patient 16
C
care and wearing instructions 17
CareLink iPro
about uploading data 21
meters supported 5
uploading data 24
charging iPro2 30
cleaning
cleaning plug 29
Dock 29
iPro2 23, 29
USB cable 29
wall-powered adapter 29
cleaning iPro2
about 29
before patient study 13
cleaning plug
about 2
clearing iPro2 data 38
compliance information 5
connecting iPro2
preparing 18
connecting iPro2 to sensor 18
contraindications 4
D
data
uploading iPro2 24
device setup, first time 7
devices that connect to iPro2 10
disconnecting iPro2 from patient 22
disinfecting iPro2 23, 29
Dock
about 2
cleaning 29
lights, quick reference 37
watertightness 29
Dock lights
about 2
E
electromagnetic immunity 47
equipment log
using 16, 25
erasing iPro2 data 38
F
FAQs 33
first day of study 17
first patient visit 11
first time setup
device setup 7
flashing light 37
frequently asked questions 33
G
glucose sensor
connecting iPro2 18
inserting 15
green light 37
iPro2 CGM User Guide Index 55
Page 62

guidance and manufacturer's declaration 46
I
preparing for patient study
about 12
wiping iPro2 with alcohol 13
preparing to connect iPro2 18
icons 51
indications for use 4
inserting sensor 15
insertion
preparation 15
instructing patient 16
interference from wireless devices 6
iPro2
about 2
charging 30
cleaning and disinfecting 13, 23, 29
connecting to sensor 18
disconnecting from patient 22
preparing to connect to patient 18
removing from patient 22
resetting 38
setup, first time 7
system components 2
uploading data 24
iPro2 instructions
care and wearing instructions 17
L
lights, quick reference 37
M
maintenance, system 28
meter use, patient instructions 17
meters
supported 5
N
Q
quick reference, Dock lights 37
R
red light 37
removing
iPro2 from patient 22
sensor from patient 22
reset button, key notes 10
resetting iPro2 38
S
sensor
connecting iPro2 18
inserting 15
removing from patient 22
uploading data 24
sensor performance
about 39
interference 43
setup
iPro2 recorder 7
patient 11
specifications 44
storage and organization tips 31
study
preparing for 12
supported blood glucose meters 5
symbols 51
system maintenance 28
system overview 2
notices
warranty 50
P
patient
briefing 16
instructions 16
setup 11
patient instructions
care and wearing 17
meter use, first day 17
meter use, remaining days 17
patient return visit 21
patient study
tips for success 14
performance, sensor 39
precautions 4
preparation for sensor insertion 15
T
tips for patient studies 14
tips for storage and organization 31
troubleshooting
about 33
connector pins 36
troubleshooting reference 33
U
uploading
iPro2 data 24
supported meters 5
uploading data
about 21
iPro2 CGM User Guide Index 56
Page 63

user safety
about 3
contraindications 4
indications for use 4
precautions 4
warnings 4
W
wall-powered adapter
about 2
warnings 4
warranty 50
watertightness
Dock 29
white light 37
wireless devices, interference 6
iPro2 CGM User Guide Index 57
 Loading...
Loading...