Page 1
1
2
4
5
3
A
E
F
H
I
J
B
1
2
H
G
G
GIA™ Auto Suture™
Stapler with DST Series™ Technology
PT00167843
1
3
4
K
GIA6025S
C
B
A
F
B
1
H
G
D
H
E
I
J
GIA6038S
2
G
GIA6048S
GIA8038S
5
GIA8048S
2
G
GIA10038S
GIA10048S
BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY.
IMPORTANT!
This booklet is designed to assist in using this product. It is not a reference to surgical techniques.
This device was designed, tested and manufactured for single patient use only. Reuse or reprocessing of this device may lead to its
failure and subsequent patient injury. Reprocessing and/or resterilization of this device may create the risk of contamination, patient
infection, permanent impairment or life threatening injury. Do not reuse, reprocess or resterilize this device.
DESCRIPTION
The GIA™ stapler with DST Series™ technology places two double staggered rows of titanium staples and simultaneously cut and
divide tissue between the two double rows. The staplers and single use loading units (SULU) are available in 60 mm, 80 mm, and
100 mm lengths.
The staplers are available in three staple sizes to accommodate various tissue thicknesses: 2.5 mm, 3.8 mm and 4.8 mm. Please refer
to the “SPECIFICATION CHART” for availability of staple sizes and cartridge lengths.
Each stapler may be reloaded up to 7 times for a total of 8 firings per instrument.
INDICATIONS
The reloadable staplers have applications in abdominal and thoracic surgical procedures for resection, transection and creation of
anastomosis.
CONTRAINDICATIONS
1. The instrument should not be used on tissue such as liver or spleen where compressibility is such that closure of the instrument
would be destructive.
2. The instrument should not be used on tissues that are necrotic, friable, or have altered integrity, e.g., ischemic or edematous tissues.
3. Tissue thickness should be carefully evaluated before firing any stapler. Refer to “SPECIFICATION CHART” and the “Tissue
Compression Requirements” section. “Tissue Compression Requirement” refers to the tissue compression requirement for each
staple size. If the tissue cannot comfortably compress to the specified minimum requirement, or compresses to less than this
requirement, the tissue may be too thick or too thin for the selected staple size.
L
GIA6025L
GIA6038L
GIA6048L
GIA6025L
GIA6038L
GIA6048L
GIA6025L
GIA6038L
GIA6048L
GIA8038L
GIA8048L
GIA8038L
GIA8048L
GIA10038L
GIA10048L
GIA10038L
GIA10048L
M
60 mm
60 mm
60 mm
60 mm
60 mm
60 mm
60 mm
60 mm
60 mm
80 mm
80 mm
80 mm
80 mm
100 mm
100 mm
100 mm
100 mm
N
WHITE
BLUE
GREEN
WHITE
BLUE
GREEN
WHITE
BLUE
GREEN
BLUE
GREEN
BLUE
GREEN
BLUE
GREEN
BLUE
GREEN
2.84 mm
2.84 mm
2.84 mm
2.84 mm
2.84 mm
2.84 mm
2.84 mm
2.84 mm
2.84 mm
2.84 mm
2.84 mm
2.84 mm
2.84 mm
2.84 mm
2.84 mm
2.84 mm
2.84 mm
O
2.5 mm
3.8 mm
4.8 mm
2.5 mm
3.8 mm
4.8 mm
2.5 mm
3.8 mm
4.8 mm
3.8 mm
4.8 mm
3.8 mm
4.8 mm
3.8 mm
4.8 mm
3.8 mm
4.8 mm
P Q
1 mm
1.5 mm
2 mm
1 mm
1.5 mm
2 mm
1 mm
1.5 mm
2 mm
1.5 mm
2 mm
1.5 mm
2 mm
1.5 mm
2 mm
1.5 mm
2 mm
1 mm
1.5 mm
2 mm
1 mm
1.5 mm
2 mm
1 mm
1.5 mm
2 mm
1.5 mm
2 mm
1.5 mm
2 mm
1.5 mm
2 mm
1.5 mm
2 mm
WARNINGS AND PRECAUTIONS
1. The instrument should not be used to staple tissue outside of the intended compressed tissue thickness.
2. Avoid use of the instrument on the aorta.
3. Surgeons should consider specific patient factors before deciding if the instrument is suitable for use. For example
preoperative radiotherapy may result in changes to tissue. These changes may, for example, cause the tissue thickness
to exceed the indicated range for the selected staple size. Careful consideration should be given to any pre-surgical
treatment the patient may have undergone and in corresponding selection of staple size.
4. The GIA ™ 60, 80 and 100 staplers are designed to be fully disposable and should not be fired more than 8 times per
instrument. Additional applications may cause improperly formed staples resulting in leakage or disruption of the
staple line.
5. When using the instrument more than once during the same procedure, be sure that the anvil is clear of tissue, blood
and staples following each application.
6. The instrument will cut and staple any structure included in the jaws. Use caution to ensure that only structures to be
cut and stapled are within the instrument jaws.
7. Prior to firing, ensure that no unintentional obstructions, such as clips, are incorporated into the instrument jaws when
positioning the stapler on the application site, and that firing over an obstruction may result in incomplete cutting
action and/or improperly formed staples.
8. There is an increased risk of leak when staple lines are crossed, even if there may be clinical circumstances when a
surgeon may deem it necessary or appropriate to do so.
9. Where practical, proximal control of blood vessels is recommended prior to stapling. Methods of blood vessel control
should be in place in the event of stapler failure. When dividing major vascular structures, be sure to adhere to the basic
surgical principles of proximal and distal control.
10. Clamping and unclamping of delicate structures may result in damage to tissue irrespective of stapler firing.
11. When used in the abdomen, make sure no omental or mesenteric vessels are caught between the cartridge and anvil
assembly prior to locking the instrument.
12. The GIA™ 60, 80 and 100 staplers are provided STERILE and are intended for multiple use during a SINGLE procedure.
DISCARD AFTER USE. Reprocessing and/or resterilization of this device may create the risk of contamination, patient
infection, permanent impairment or life threatening injury. Do not reuse, reprocess or resterilize this device.
13. The SULUs are provided STERILE and are intended for use in a SINGLE procedure only. DISCARD AFTER USE. Reprocessing
and/or resterilization of this device may create the risk of contamination, patient infection, permanent impairment or
life threatening injury. Do not reuse, reprocess or resterilize this device.
14. Bioabsorbable staple line reinforcements such as GORE™* SEAMGUARD™* bioabsorbable staple line reinforcement
and Baxter Peri-Strips Dry™* staple line reinforcement materials have been tested for use with the GIA™ stapler with
DST Series™ technology.
15. If using a reinforcement product, include the thickness of the respective product into the overall tissue thickness to
determine the appropriate staple size that will be applied.
16. When using a staple line buttressing material, follow the instructions provided by the manufacturer of the buttress
material, as performance of the stapler may be affected when using buttress materials.
17. Ensure that the staple reloads are compatible with the staplers. The compatibility of other manufacturers’ staples and
staplers has not been determined.
18. Ensure to select a SULU with the appropriate staple size for the tissue thickness. Overly thick or thin tissue may result in
unacceptable staple formation.
19. After firing, always inspect the staple line for hemostasis. Minor bleeding may be controlled by electrocautery or
manual sutures. Minor leakage may be controlled by manual sutures. At the surgeon’s discretion, a laparotomy or
thoracotomy may be performed.
20. Failure to advance the handle completely may result in incomplete staple formation, which may compromise the
integrity of the staple line.
21. Do not rotate the firing knob during firing. Rotating the knob during firing may cause damage and possible instrument
malfunction.
22. Dispose of used instruments and used reloads in accordance with the end-user’s medical and biological waste disposal
requirements.
MRI SAFETY INFORMATION
Non-clinical testing demonstrated that a representative titanium staple is MR Conditional. A patient with these titanium
staples can be scanned safely immediately after placement of the titanium staple under the following conditions:
• Static magnetic field of 1.5T and 3.0T
• Maximum spatial gradient magnetic field of 3000-Gauss/cm (30 T/m)
• Normal Operating Mode of operation for the MRI system (whole body averaged SAR, 2-W/kg) for 15 minutes of
scanning, per pulse sequence.
Under the scan conditions defined above, the titanium staple is expected to produce a maximum temperature rise of less
than 3.2°C after 15 minutes of continuous scanning.
In non-clinical testing, the image artifact caused by the device extends approximately 2 mm from the titanium staple when
imaged with a gradient echo pulse sequence and a 3.0T MRI system.
ADVERSE REACTIONS
Adverse reaction and potential complications associated with the use of the GIA™ Auto Suture™ stapler with DST Series™
technology are: anastomotic leak, infection, bleeding, fistula, adhesions, stenosis/stricture, tissue damage, organ
perforation, staple migration and allergic reaction.
Page 2

SCHEMATICVIEW
A) CARTRIDGE FORK
B) SHOULDER
C) LOCKING LEVER HANDLE-CARTRIDGE
D) RELEASE BUTTON-CARTRIDGE
E) FIRING KNOB
F) ANVIL FORK
G) SHIPPING WEDGE ( TOP VIEW)
H) SINGLE USE LOADING UNIT (SULU)
I) KNIFE BLADE ASSEMBLY
J) SAFETY LOCKOUT
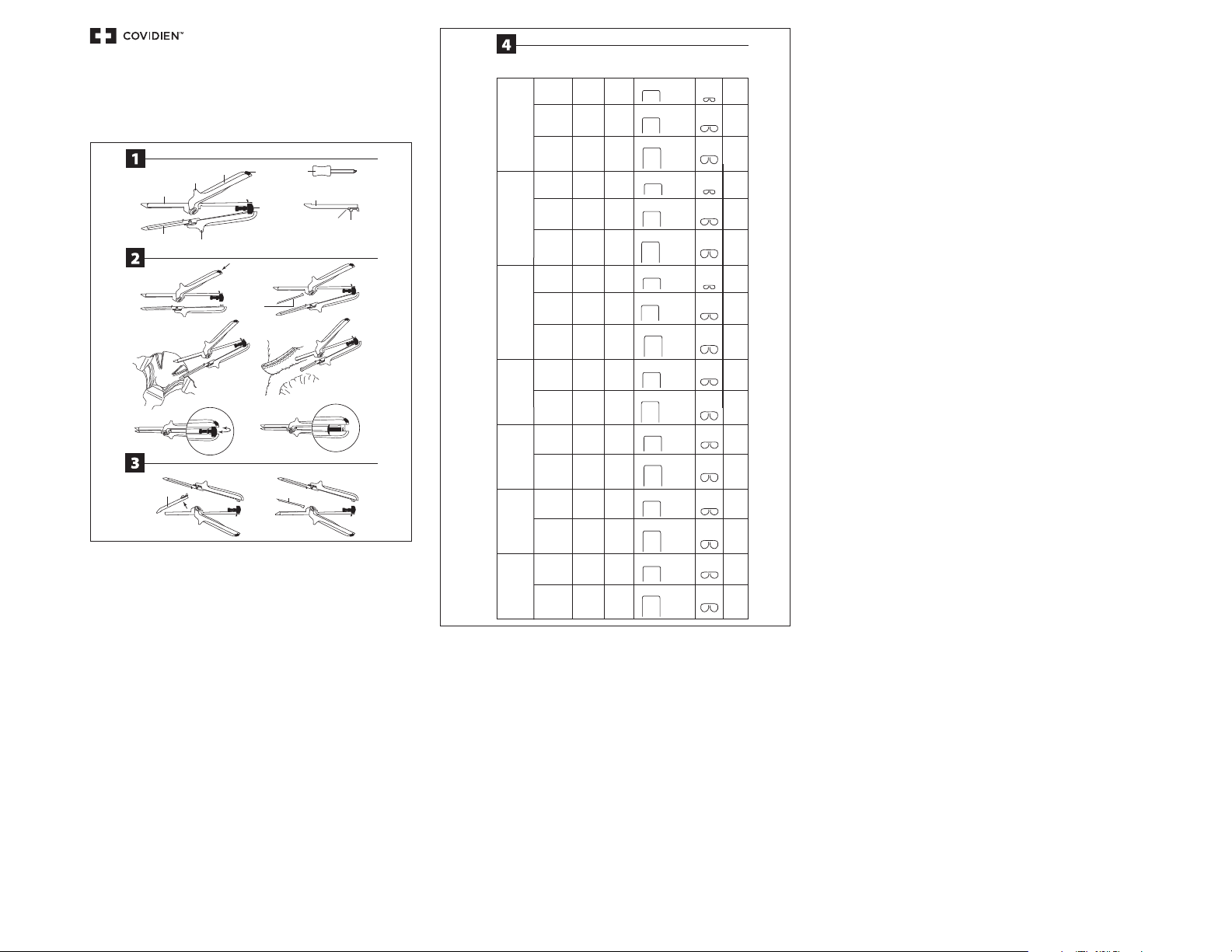
INSTRUCTIONS FOR USE
FIRING INSTRUCTIONS
CAUTION: Ensure to select a SULU with the appropriate staple size for the tissue thickness. Overly
thick or thin tissue may result in unacceptable staple formation.
1. Open the instrument by pushing in on the release button on the cartridge fork lever handle (located at end of
instrument).
2. Remove the shipping wedge (G) from SULU channel by lifting and pulling it out at the finger tabs. (Remove shipping
wedge after SULU is fully loaded. SULU will “float” up and down in final loaded position.)
3. Position the tissue to be stapled between the instrument cartridge and anvil fork; or, if the tissue is to be anastomosed,
place the cartridge fork in one lumen and the anvil fork into the other lumen as appropriate. Align tissue edges equally
on cartridge and anvil. This may be accomplished with the instrument halves separated or hinged at the rear. Close the
instrument by moving the lever handle towards the instrument body until the audible click is heard.
4. With the instrument securely closed, rotate the firing knob to either side of the instrument.
NOTE: In its pre-fire position, the firing knob should rotate to either side of the instrument.
5. Place a thumb behind the firing knob and two fingers on the instrument shoulders. Fire the instrument by sliding the
firing knob forward to a complete stop.
CAUTION: Do not rotate the firing knob during firing. Rotating the knob during firing may cause
damage and possible instrument malfunction.
CAUTION: Failure to advance the handle completely may result in incomplete staple formation,
which may compromise the integrity of the staple line.
6. After firing, return firing knob all the way back to its pre-fire position.
7. Push in the cartridge release button to open the instrument (refer to step 1). Remove the instrument from the surgical
site.
CAUTION: After firing, always inspect the staple line for hemostasis. Minor bleeding can be
controlled by electrocautery or manual sutures. Minor leakage may be controlled by manual
sutures. At the surgeon’s discretion, a laparotomy or thoracotomy may be performed.
NOTE: When a fired instrument is opened, the lockout device will deploy. The safety lockout
prevents clamping of the instrument with a SULU that has been fired.
RELOADING INSTRUCTIONS
1. To remove the fired SULU (H), separate the instrument halves. Holding the cartridge-half of the instrument, grasp the
proximal end of the SULU tabs and pull up and out to remove SULU from instrument.
2. To place a new SULU into the instrument, hold the SULU by the proximal end tabs and insert into the cartridge fork at a
30 to 45 degree angle from the distal end down until unit snaps into place. Remove shipping wedge (G) after SULU is
fully loaded. SULU will “float” up and down in final loaded position.
CAUTION: Ensure to select a SULU with the appropriate staple size for the tissue thickness. Overly
thick or thin tissue may result in unacceptable staple formation.
CAUTION: The reloadable staplers should only be reloaded seven (7) times per instrument for a total
of eight (8) firings.
SPECIFICATION CHART
K) INSTRUMENT REORDER CODES
L) SULU REORDER CODES
M) STAPLE LINE LENGTH
N) SULU COLOR
O) OPEN STAPLE SIZE
P) CLOSED STAPLE SIZE (TYPICAL)
Q) TISSUE COMPRESSION REQUIREMENT
Tissue
en: Maximum Number of Reloads
en: Open
en: Staple rows, staggered staple pattern
en: Intended compressed tissue thickness
en: Sterilized using ethylene oxide
en: Single Use
en: Caution: U.S. Federal law restricts this device to sale by or on the order
of a physician.
en: Do not resterilize
en: Do not use if package is damaged
en: Caution consult accompanying documents
en: MR Conditional
en: High density
en: Made from 100% recycled fibres.Minimum 35% post-consumer content.
© 2011 Covidien.
Covidien llc, 15 Hampshire Street, Mansfield, MA 02048 USA.
www.covidien.com
+1 800 633 8766
+1 763 514 4000
COVIDIEN, COVIDIEN with logo, and Covidien logo and Positive Results for Life are U.S. and internationally registered
trademarks of Covidien AG. Other brands are trademarks of a Covidien company, ™* brands are trademarks of their
respective owner.
2022 - 06 / 03
 Loading...
Loading...