Page 1
Endo GIA™ single use articulating Vascular/Medium reload with Tri-Staple™ technology:
1
2
3
5
Endo GIA™ Ultra
6
Universal, Universal Short, and Universal XL Single
Use Staplers, and Reloads
PT00167982
B
A
C
D
F
E
9
N
10
11
G
H
I
P
12
J
L
N
O
Q
K1
M
P
E
13
R
S
mm
mm
mm
mm
mm
mm
mm
mm
mm
mm
2
2
3
2
2
3
4
2
3
4
Y
.75 - 1 mm
.88 - 1.8 mm
1.5 - 2.25 mm
.75 - 1 mm
.88 - 1.8 mm
1.5 - 2.25 mm
2.25 - 3.0 mm
.88 - 1.8 mm
1.5 - 2.25 mm
2.25 - 3.0 mm
T
U
K1
2
3
K2
F
C
1
G
1
2
3
BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY.
IMPORTANT!
This booklet is designed to assist in using this product. It is not a reference to surgical techniques.
This device was designed, tested and manufactured for single patient use only. Reuse or reprocessing of this device may lead to its
failure and subsequent patient injury. Reuse, reprocessing and/or resterilization of this device may create the risk of contamination,
patient infection, permanent impairment or life threatening injury. Do not reuse, reprocess or resterilize this device.
DESCRIPTION
The Endo GIA™ Ultra universal short, Endo GIA™ Ultra universal and Endo GIA™ Ultra universal XL staplers place staggered rows of
titanium staples and simultaneously divide the tissue so that three staggered rows of staples are placed on either side of the cut line.
The size of the staples is determined by the selection of the single use reload:
Endo GIA™ single use articulating Extra Thin/Vascular reload:
Gray reload and gray curved tip reload - three rows of 2.0 mm titanium staples on either side of the cut line.
V W X
30 mm Gray
30 mm
30 mm
Purple
45 mm Gray
45 mm
45 mm
Purple
45 mm
Black
60 mm
60 mm
Purple
60 mm
Black
2
mm2mm
3
Tan
mm
4
mm
2
mm2mm
3
Tan
mm
4
mm
5
mm
3
Tan
mm
4
mm
5
mm
2.5
mm
3.5
mm
2.5
mm
3.5
mm
4.5
mm
2.5
mm
3.5
mm
4.5
mm
Tan reload and tan curved tip reload - three height progressive rows of 2.0 mm, 2.5 mm, 3.0 mm titanium staples on either
side of the cut line.
Endo GIA™ single use articulating Medium/Thick reload with Tri-Staple™ technology:
Purple reload and purple curved tip reload - three height progressive rows of 3.0 mm, 3.5 mm and 4.0 mm titanium staples
on either side of the cut line.
Endo GIA™ single use articulating Extra Thick reloads with Tri-Staple™ technology:
Black reload - three height progressive rows of 4.0 mm, 4.5 mm and 5.0 mm titanium staples on either side of the cut line.
The Endo GIA™ Ultra universal short stapler is designed for open procedures only. All instructions, warnings, and
precautions referring to trocars or laparoscopic procedures apply to the Endo GIA™ Ultra universal and the Endo GIA™ Ultra
universal XL staplers only, and do not pertain to the Endo GIA™ Ultra universal short stapler.
The Endo GIA™ Ultra universal short, Endo GIA™ Ultra universal and Endo GIA™ Ultra universal XL staplers will
accommodate any of the single use reload sizes that are available in the 30 mm, 45 mm and 60 mm lines.
The Endo GIA™ Ultra universal and Endo GIA™ Ultra universal XL staplers with either the gray, tan, or purple single use
reload, including curved tip reload is designed for introduction and use through a 12 mm trocar sleeve or larger, with the
use of a converter. This does not pertain to the Endo GIA™ Ultra universal short stapler.
The Endo GIA™ Ultra universal and Endo GIA™ Ultra universal XL staplers with the black single use reload is designed for
introduction and use through a 15 mm trocar sleeve or larger, with the use of a converter. This does not pertain to the Endo
GIA™ Ultra universal short stapler.
The instrument may be reloaded and fired up to 25 times in a single procedure.
NOTE: Each instrument can accommodate the 30 mm length, gray, gray curved tip, tan, tan curved
tip, and purple single use reloads; the 45 mm length gray, gray curved tip, tan, tan curved tip,
purple, purple curved tip, and black reload and the 60 mm length tan, tan curved tip, purple, purple
curved tip and black reload.
The curved tip on the distal end of the curved tip reload can be used to dissect and manipulate tissue/vessels when locating
target tissue for subsequent firing and placement of staples.
The flexible introducer provided with the curved tip reload can be mounted on the curved tip and used to guide the anvil
underneath the vessel/tissue that is to be transected.
COMPATIBILITY
Endo GIA™ reloads are compatible with the following Covidien™ staplers: Endo GIA™ Universal, Endo GIA™ Ultra, iDrive,
and Signia™ platforms. Covidien™ systems listed above are compatible with the following Covidien™ reloads: Endo GIA™
universal single use reloads, Endo GIA™ single use reloads with Tri-Staple™ technology, Tri-Staple™ 2.0 reloads, Signia™
intelligent loading units with Tri-Staple™ 2.0 cartridges.
Some features of the Signia™ platform are only compatible when used with Tri-Staple™ 2.0 single use reloads, Signia™ intelligent
loading units.
Refer to the respective IFUs of the stapler or reloads for complete information on the use of the devices.
Bioabsorbable staple line reinforcements such as GORE™* SEAMGUARD™* bioabsorbable staple line reinforcement and
Baxter Peri-Strips Dry™* staple line reinforcement materials have been tested for use with Endo GIA™ single use purple and
black reloads with Tri-Staple™ technology.
Compatibility of other manufacturer’s staples and staplers has not been determined.
INDICATIONS
The Endo GIA™ Ultra universal short, Endo GIA™ Ultra universal and Endo GIA™ Ultra universal XL staplers have applications
in abdominal, gynecologic, pediatric and thoracic surgery for resection, transection and creation of anastomosis. They may
be used for transection and resection of liver substance, hepatic vasculature and biliary structures, and for transection and
resection of pancreas.
The Endo GIA™ Ultra universal short, Endo GIA™ Ultra universal and Endo GIA™ Ultra universal XL staplers when used with
the Endo GIA™ curved tip single use reloads can be used to blunt dissect or separate target tissue from other tissue.
CONTRAINDICATIONS
1. Do not use the Endo GIA™ Ultra universal short stapler for laparoscopic procedures.
2. Do not place the Endo GIA™ Ultra universal short stapler through a trocar, as the shaft of the handle will not be long
enough to safely exit the trocar and allow the jaws of the stapler to open.
3. Do not use the Endo GIA™ gray reload and gray curved tip reload on any tissue that compresses to less than 0.75 mm in
thickness, on any tissue that cannot comfortably compress to 1.0 mm or on the aorta.
4. Do not use the Endo GIA™ tan reload and tan curved tip reload on any tissue that compresses to less than 0.88 mm in
thickness, on any tissue that cannot comfortably compress to 1.8 mm or on the aorta.
5. Do not use the Endo GIA™ purple reload and purple curved tip reload on any tissue that compresses to less than 1.5
mm in thickness, on any tissue that cannot comfortably compress to 2.25 mm or on the aorta.
6. Do not use the Endo GIA™ black reload on any tissue that compresses to less than 2.25 mm in thickness, on any tissue
that cannot comfortably compress to 3.0 mm or on the aorta.
7. The Endo GIA™ reloads/SULUs should not be used on friable or delicate tissue where the closure of the device might be
destructive to the tissues.
8. Do not use the Endo GIA™ Ultra universal short, Endo GIA™ Ultra universal or Endo GIA™ Ultra universal XL stapler
where adequacy of hemostasis cannot be verified visually after applications.
9. Do not use any linear cutter on major vessels without making provisions for proximal and distal control.
10. Do not use the instrument on ischemic or necrotic tissue.
11. The use of curved tip reloads is contraindicated on tissue or structures that cannot fit completely within the jaws
proximal to the transitional angle of the curved tip.
12. These devices are provided STERILE and are intended for use in a SINGLE procedure only. DISCARD AFTER USE. DO NOT
RESTERILIZE.
WARNINGS
1. Ensure that the staple reloads are compatible with the staplers.
2. As with all surgical staplers, surgeons should consider specific patient factors before deciding if the device is suitable for
use. For example, preoperative radiotherapy may result in changes to tissue. These changes may, for example, cause the
tissue thickness to exceed the indicated range for the selected staple size. Careful consideration should be given to any
Page 2
pre-surgical treatment the patient may have undergone and to the corresponding selection of staple size.
3. Always select a reload with the appropriate staple size for the tissue thickness. Overly thick or thin tissue may result in
unacceptable staple formation.
4. Ensure tissue has not extended (extruded) beyond the tissue stop proximally. Tissue forced into the instrument beyond
the tissue stop may be transected without stapling.
5. When positioning the stapler on the application site, ensure that no unintentional obstructions, such as clips, are
incorporated into the instrument jaws. Firing over an obstruction may result in incomplete cutting action and/or
improperly formed staples.
6. There is an increased risk of leak when staple lines are crossed, even if there may be clinical circumstances when a
surgeon may deem it necessary or appropriate to do so.
7. A thorough understanding of the principles involved in laser and electrosurgical procedures is essential to avoid shock
and burn hazards to both patient and operator(s), and damage to the instrument.
8. Clamping and unclamping of delicate structures may result in damage to tissue irrespective of stapler firing.
9. When dividing major vascular structures, be sure to adhere to the basic surgical principles of proximal and distal control.
10. When manipulating tissue with the curved tip reloads, avoid exerting excessive pressure on fragile structures with the
curved tip of the device.
11. The instrument will cut and staple any structure included in the jaws. Use caution to ensure that only structures to be
cut and stapled are within the instrument jaws.
12. Failure to completely fire the reload will result in an incomplete cut and/or incomplete staple formation, which may
result in poor hemostasis and/or leakage.
13. The instrument and single use reload are provided STERILE and are intended for use in a SINGLE procedure only.
DISCARD AFTER USE. DO NOT RESTERILIZE. Reprocessing or resterilization may compromise the device integrity and
could lead to patient injury, illness, or death. Reuse, even after resterilization, may create a risk of contamination and
lead to patient infection or the transmission of infectious disease(s).
PRECAUTIONS
1. When using the Endo GIA™ Ultra universal instrument with a black (4.0 mm, 4.5 mm or 5.0 mm) reload, the
instrument MUST be inserted into a 15 mm trocar. A smaller size trocar will not accept the black (4.0 mm, 4.5 mm or
5.0 mm) reload. This does not pertain to the Endo GIA™ Ultra universal short stapler.
2. Always include the combined thickness of the tissue and of any staple line reinforcement material in use when
choosing the proper staple cartridge.
3. When using a staple line reinforcement material, follow the instructions provided by the manufacturer of the staple line
reinforcement material, as performance of the stapler may be affected when using staple line reinforcement material.
4. When using a staple line reinforcement material, verify compatibility with access ports.
5. When using the Endo GIA™ Ultra universal stapler with a reinforced reload to grasp or manipulate target tissue multiple
times, the reinforcement material may move and/or dislodge.
6. Do not attempt to remove the yellow shipping wedge until the reload is loaded into the instrument. Failure to do so
may result in reload jaws not opening after clamping and firing on tissue. This may cause the inability to fire the device
which can delay treatment and potentially cause tissue damage. The shipping wedge should be retained until the
completion of the case.
7. Do not clamp instrument prior to removing shipping wedge.
8. Always close the jaws of the Endo GIA™ Ultra universal or Endo GIA™ Ultra universal XL stapler prior to introducing and
removing the stapler from the trocar sleeve (or the incision if using the Endo GIA™ Ultra universal short stapler).
9. After firing and removal of the instrument, always inspect the staple line and the surrounding site for hemostasis and/
or leakage. Minor bleeding may be controlled by electrocautery or manual sutures. Minor leakage may be controlled
by manual sutures.
10. Placement of tissue proximal to the tissue stops (on the reload) may result in stapler malfunction. Any tissue extending
beyond the cut line will not be transected.
11. When using the stapler more than once during a SINGLE surgical procedure, be sure to remove the empty Endo GIA™
single use reload and load a new one. A safety interlock is provided that prevents an empty single use reload from
being fired a second time. Do not attempt to override the safety interlock. Overriding the safety interlock will cause
device malfunction.
12. Endoscopic procedures should be performed only by physicians having adequate training and familiarity with
endoscopic techniques. Prior to performance of any endoscopic procedures, consult the medical literature relative to
techniques, complications and hazards.
13. When endoscopic instruments and accessories from different manufacturers are employed together in a procedure,
verify compatibility and ensure that electrical isolation or grounding is not compromised.
14. The anvil must be completely visible, (past the trocar sleeve) prior to opening the reload within the body cavity. This
does not pertain to the Endo GIA™ Ultra universal short stapler.
15. In case of a curved tip reload, ensure that the tissue/vessel to be transected does not extend beyond the black cut line
on the reload. The device will only cut to the black cut line. Tissue contained within the jaws distal to this line will not
be transected.
16. Hold the loop handle while pushing the green firing button. This will enable the loop handle to gradually move forward
to the firing position. If the loop handle is not held while pushing the green button, it will move forward with a snap
and may cause the instrument to shake.
17. Do not squeeze the instrument handle while pulling back the black return knob.
18. Dispose of used instruments and used reloads in accordance with the end-user’s medical and biological waste disposal
requirements.
19. The device may become locked on tissue and may result in tissue trauma and/or unanticipated tissue loss.
20. Compatibility of other manufacturer’s staples and staplers has not been determined.
MRI SAFETY INFORMATION
Non-clinical testing demonstrated that a representative titanium staple is MR Conditional. A patient with these titanium
staples can be scanned safely immediately after placement of the titanium staple under the following conditions:
• Static magnetic field of 1.5T and 3.0T
• Maximum spatial gradient magnetic field of 3000-Gauss/cm (30 T/m)
• Normal Operating Mode of operation for the MRI system (whole body averaged SAR, 2-W/kg) for 15 minutes of scanning,
per pulse sequence.
Under the scan conditions defined above, the titanium staple is expected to produce a maximum temperature rise of less than 3.2°C
after 15 minutes of continuous scanning.
In non-clinical testing, the image artifact caused by the device extends approximately 2 mm from the titanium staple when imaged
with a gradient echo pulse sequence and a 3.0T MRI system.
ADVERSE EVENTS
Possible adverse reactions and potential complications include: seroma/hematoma, bleeding/hemorrhage/leak, ischemia, chronic
pain, infection, allergic reactions, inflammatory reaction, visceral adhesions, fistula, nerve entrapment, tissue erosion, small bowel
perforation, and need for future operation.
SCHEMATIC VIEW
A) PIN
B) SHAFT
C) ARTICULATION LEVER
D) ROTATION COLLAR
E) GREEN FIRING BUTTON
F) BLACK RETURN KNOB
G) LIGHT BLUE UNLOAD BUTTON
H) LOOP HANDLE
I) SINGLE USE RELOAD (30, 45, or 60mm lengths)
J) SHIPPING WEDGE
K1) “LOAD” ALIGNMENT INDICATOR (reload)
K2) “LOAD” ALIGNMENT INDICATOR (shaft)
L) ANVIL
M) TISSUE STOP
N) END OF CUT LINE
O) END OF STAPLE LINE
P) LOWER CLAMP COVER
Q) INCREMENT MARKINGS
R) STAPLE CARTRIDGE
S) SINGLE USE CURVED TIP RELOAD (30, 45, 60)
T) FLEXIBLE INTRODUCER
U) FLEXIBLE INTRODUCER MOUNTING CLIP
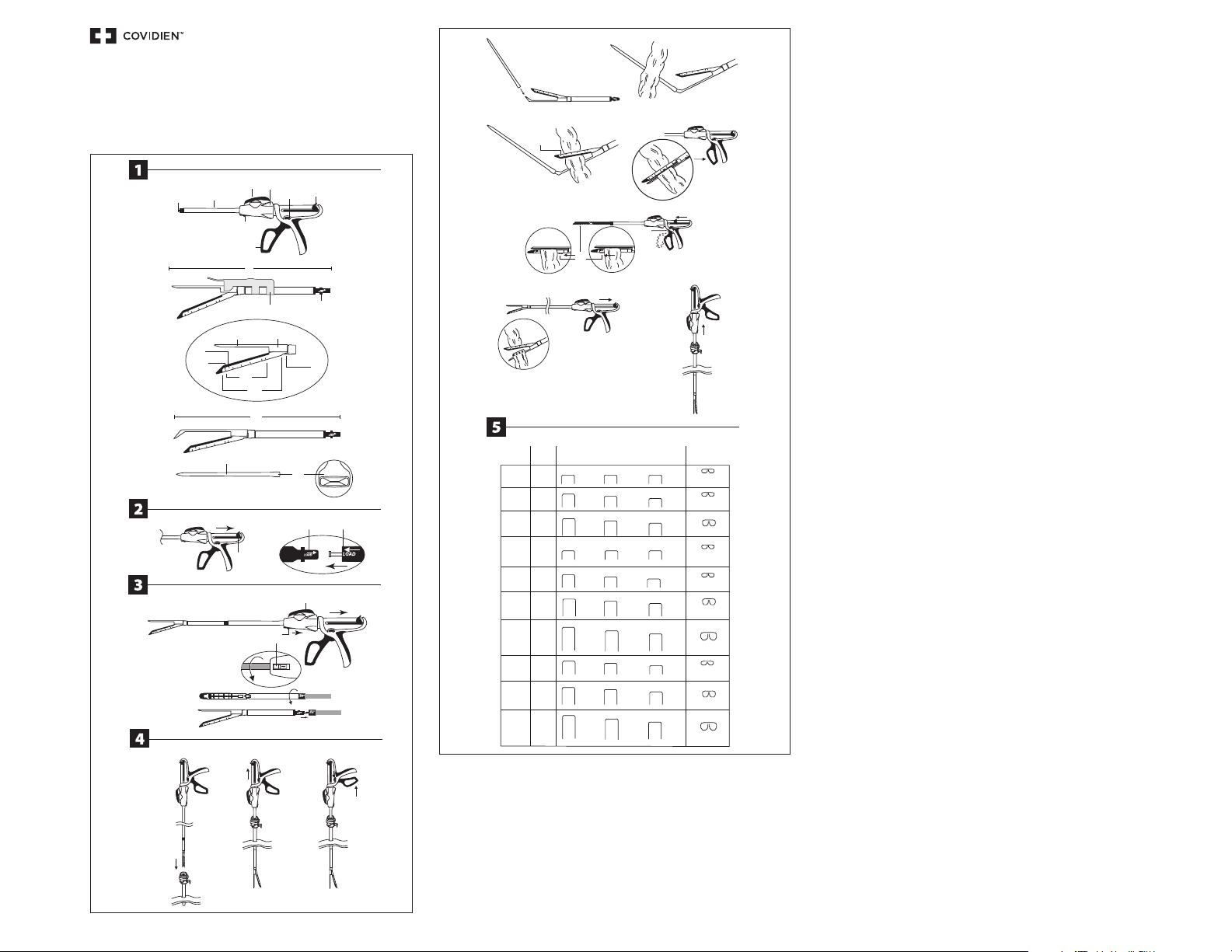
LOADING
1. The reload is packaged in the open position. Do not attempt to close the reload.
WARNING: Always select a reload with the appropriate staple size for the tissue thickness. Overly thick or
thin tissue may result in unacceptable staple formation.
CAUTION: Do not attempt to remove the yellow shipping wedge until the reload is loaded into the
instrument. Failure to do so may result in reload jaws not opening after clamping and firing on tissue. This
may cause the inability to fire the device which can delay treatment and potentially cause tissue damage.
The shipping wedge should be retained until the completion of the case.
2. Ensure that the black return knob (F) on the instrument is pulled back completely and the articulation lever (C) is neutral (0°
relative) to the instrument.
3. To load the Endo GIA™ Ultra universal short, Endo GIA™ Ultra universal or Endo GIA™ Ultra universal XL stapler with the
appropriate reload; insert the pin located at the distal end of the instrument shaft into the reload. Ensure that the “LOAD” alignment
indicator on the reload aligns with the “LOAD” alignment indicator on the shaft. Push the reload in and twist clockwise 45° relative
to the instrument, so that the reload will lock into place and an audible click is heard. When the reload is loaded properly into the
stapler the blue unload button underneath the shaft is seated in place without showing any red coloration underneath.
K1) “LOAD” ALIGNMENT INDICATOR (reload)
K2) “LOAD” ALIGNMENT INDICATOR (shaft)
4. Remove the shipping wedge from the reload. Cycle the instrument to confirm proper loading of the reload. To do so, squeeze the
handle once to close the jaws of the reload. Push the black loop handle all of the way forward or pull back on the black return knob
and confirm that the reload jaws open fully. If the jaws do not open fully the device is not loaded properly. Remove the reload and
repeat steps 1-5 with a new reload. If the new reload does not open fully during cycling, do not continue to use the instrument.
CAUTION: Do not clamp instrument prior to removing shipping wedge.
5. Close the instrument jaws prior to inserting the instrument into the trocar (or the incision if using the Endo GIA™ Ultra universal
short stapler).
UNLOADING
To unload a reload from the stapler, the articulating lever (C) must be in the neutral position. Ensure that the jaws of the reload are
open by pulling the black return knob back completely. Pull the light blue unload button (G) (located on the underside of the shaft
near the loop handle) back towards the instrument, twist the reload counterclockwise 45° and remove the reload from the shaft of
the instrument.
INSTRUCTIONS FOR USE
NOTE: The jaws of the reload must be closed prior to introducing the instrument into the trocar sleeve (or the
incision if using the Endo GIA™ Ultra universal short stapler). To do so, squeeze the handle. Do not attempt
to insert or remove the instrument from the incision, or trocar sleeve if the instrument is in the articulated
position.
1. Insert the Endo GIA™ Ultra universal or Endo GIA™ Ultra universal XL stapler into an appropriately sized trocar sleeve or larger, with
the use of a converter. This does not pertain to the Endo GIA™ Ultra universal short stapler.
CAUTION: The anvil must be completely visible, (past the trocar sleeve) prior to opening the RELOAD
within the body cavity. This does not pertain to the Endo GIA™ Ultra universal short stapler.
The Endo GIA™ Ultra universal stapler enables articulation and rotation of the connected reload.
Use the articulation lever (C) to perform left and right articulation of the connected reload at discrete angles of 0°, 15°, 25°,
30°, 45° in either direction.
Use the rotation collar (D) to perform 360° CW and CCW rotation of the connected reload.
2. Once inside the body cavity, open the jaws of the instrument by pulling the black return knob completely back.
CAUTION: Do not squeeze the instrument handle while pulling back the black knob.
3. Once the jaws are open the stapler will be in grasp mode. In grasp mode, tissue can be atraumatically grasped within
the reload jaws by squeezing the handle to close the jaws. To ungrasp the tissue, push the handle forward to open the
reload jaws. To release the grasp mode squeeze the handle all the way back, hold and push the green firing button
down (the handle will return to the firing position). You are now ready to fire.
4. In case of the curved tip reload, the curved tip of the anvil can be used to manipulate the tissue and will be clearly
visible when the reload is clamped on the tissue/vessel that has to be transected.
WARNING: When manipulating tissue with the curved tip reloads, avoid exerting excessive pressure
on fragile structures with the curved tip of the device.
CAUTION: In case of a curved tip reload, ensure that the tissue/vessel to be transected does not
extend beyond the black cut line on the reload. The device will only cut to the black cut line. Tissue
contained within the jaws distal to this line will not be transected.
5. The curved tip reload can be used with or without the flexible introducer provided with the reload. Surgeons may
mount the introducer on the curved tip and use it to guide the anvil into position and underneath the vessel/tissue
that is to be transected.
The introducer is mounted to the curved tip by pushing the mounting clip end of the introducer onto the curved tip
of the anvil.
CAUTION: The curved tip reload cannot be used for blunt dissection with the Introducer attached.
6. Pass the introducer behind the structure to be stapled; an appropriately sized right angled clamp may be used to assist
passing the introducer.
CAUTION: Do not apply excessive force to the Introducer, excessive force may separate it from the
anvil.
NOTE: The introducer and its mounting clip are radiopaque in the event that either inadvertently
detaches from the curved tip reload.
7. Use the introducer to guide the anvil behind the structure to be stapled.
WARNING: The instrument will cut and staple any structure included in the jaws. Use caution to
ensure that only structures to be cut and stapled are within the instrument jaws.
8. The instrument may be fired with the introducer in place; or the introducer may be removed prior to firing.
9. Apply the Endo GIA™ Ultra universal short, Endo GIA™ Ultra universal or Endo GIA™ Ultra universal XL stapler across
the tissue to be transected.
WARNING: When positioning the stapler on the application site, ensure that no unintentional
obstructions (such as clips) are incorporated in the instrument jaws. Firing over an obstruction may
result in incomplete cutting action and/or improperly formed staples.
CAUTION: The instrument will not cut tissue beyond the black cut line (N) indicated on the single use
reload. More than one application of the stapler may be necessary for tissue exceeding the length
of the reload (30 mm, 45 mm or 60 mm).
CAUTION: Placement of tissue proximal to the tissue stops (on the reload) may result in stapler
malfunction. Any tissue extending beyond the cut line will not be transected.
WARNING: Ensure tissue has not extended (extruded) beyond the tissue stop proximally. Tissue
forced into the instrument beyond the tissue stop may be transected without stapling.
10. Close the jaws of the reload across the tissue to be transected by squeezing the handle completely. The stapler is
equipped with a safety interlock; the instrument will not fire the staples and cut tissue unless the green firing button
is pushed.
NOTE: Pausing between the clamping and firing steps with the device handle squeezed may assist
in providing additional tissue compression.
CAUTION: When using the stapler more than once during a SINGLE surgical procedure, be sure to
remove the empty Endo GIA™ single use reload and load a new one. A safety interlock is provided
that prevents an empty single use reload from being fired a second time. Do not attempt to
override the safety interlock. Overriding the safety interlock will cause device malfunction.
The jaws of the reload may be repositioned on the tissue prior to firing by pulling the black return knob completely back,
allowing the jaws to open. Once the jaws are opened the stapler will be in grasp mode. After grasping and repositioning
the stapler on the tissue, release the grasp mode by squeezing the handle all the way back, hold the handle and push the
green firing button down. The handle will return to the firing position and the instrument is now ready to fire.
CAUTION: Hold the loop handle while pushing the green firing button. This will enable the loop
handle to gradually move forward to the firing position. If the loop handle is not held while
pushing the green button, it will move forward with a snap and may cause the instrument to shake.
11. In order to fire the instrument, once the green firing button (E) has been activated, squeeze the handle sequentially
until the lower clamp cover (P) reaches the distal end of the cartridge slot, and the handle locks. Each sequential full
squeeze equates to approximately 15mm. A partial squeeze will be incrementally less than 15mm. One full squeeze is
when the handle moves through its full range of motion.
Sequential squeezes of the handle are required to fully fire the reload.
The total number of squeezes is relative to the length of the reload (30, 45, or 60mm lengths).
Page 3

WARNING: Failure to completely fire the reload will result in an incomplete cut and/or incomplete
staple formation, which may result in poor hemostasis and/or leakage.
12. Once the instrument has been completely fired, open the jaws by pulling the black return knob completely back. Gently
remove the instrument from the tissue.
CAUTION: After firing and removal of the instrument, always inspect the staple line and
the surrounding site for hemostasis and/or leakage. Minor bleeding may be controlled by
electrocautery or manual sutures. Minor leakage may be controlled by manual sutures.
13. After completely firing the reload, close jaws of the instrument and remove the Endo GIA™ Ultra universal short, Endo
GIA™ Ultra universal or Endo GIA™ Ultra universal XL stapler from the body cavity to unload the single use reload from
the instrument.
NOTE: Do not attempt to insert or remove the instrument from the incision, or trocar sleeve if the
instrument is in the articulated position.
The Endo GIA™ Ultra universal short, Endo GIA™ Ultra universal or Endo GIA™ Ultra universal XL may be reloaded and fired
up to 25 times in a single procedure.
STAPLE SPECIFICATIONS
V) STAPLE LINE LENGTH
W) COLOR
X) OPEN STAPLE SIZE
Y) INTENDED TISSUE THICKNESS R ANGE
STORE AT ROOM TEMPERATURE.
C
B
A
en: Staple rows, staggered staple pattern
A
B
C
en: Open
Tissue
Ready
25
en: Intended compressed tissue thickness
en: Ready
en: Maximum number of reloads
en: Sterilized using ethylene oxide
en: Single Use
en: Caution: U.S. Federal law restricts this device to sale by or
on the order of a physician.
en: Do not resterilize
en: Do not use if package is damaged
en: Caution, consult accompanying documents
en: MR conditional
en: Package quantity
© 2011 Covidien.
Covidien llc, 15 Hampshire Street, Mansfield, MA 02048 USA.
www.covidien.com
+1 800 633 8766
+1 763 514 4000
COVIDIEN, COVIDIEN with logo, and Covidien logo and Positive Results for Life are U.S. and internationally registered
trademarks of Covidien AG. Other brands are trademarks of a Covidien company, ™* brands are trademarks of their
respective owner.
2022 - 07 / 03
 Loading...
Loading...