Page 1

CareLink SmartSync™ Micra™ AV Application Help
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Page 2

Medtronic, Medtronic with rising man logo, and Medtronic logo are trademarks of Medtronic. Third-party trademarks (“TM*”) belong to their respective owners. The
following list includes trademarks or registered trademarks of a Medtronic entity in the United States and/or in other countries.
Capture Management™, CareLink SmartSync™, CareLink™, Micra™, Paceart Optima™, Paceart™, Quick Look™, SessionSync™, SureScan™
Page 3

Contents
1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
1.1 Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
1.2 Intended use of the implantable device app . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
1.3 Warnings and precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
1.4 Potential adverse events . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
1.5 Download or order the instructions for use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
1.6 IT network, tablet, and data information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
1.7 Reporting errors and serious incidents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
2 Overview of the interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
2.1 Areas . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
2.2 Status indicators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
3 Reinterrogating the implanted device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
3.1 Reinterrogate the implanted device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
4 Responding to device status indicator warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
4.1 About device status indicator warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
4.2 Respond to the WARNING – DEVICE ELECTRICAL RESET warning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
4.3 Respond to the SERIOUS DEVICE ERROR warning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
5 Using the Live Rhythm Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
5.1 About the Live Rhythm Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
5.2 About the A. Sensing waveform trace . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
5.3 Markers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
5.4 Adjust the Live Rhythm Monitor display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
5.5 Freeze live waveform traces . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
6 Using the eStrip recorder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
6.1 About the eStrip recorder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
6.2 Modify the display of waveform traces . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
6.3 Configure waveform strip preferences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
6.4 Access waveform strips . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
6.5 Change the length of a waveform strip . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
6.6 Measure time intervals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
6.7 Draw notes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
6.8 Edit the title of a waveform strip . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
6.9 Use the Holter feature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
7 Viewing summary data using the Quick Look screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
7.1 About the Quick Look screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
7.2 View the Quick Look screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
8 Using emergency VVI pacing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
8.1 Enable emergency VVI pacing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
9 Programming patient information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
9.1 Program the patient information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
10 Programming implantable device settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
10.1 Parameter symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
10.2 Program the parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
10.3 Create custom parameter sets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
10.4 Retrieve parameter sets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
10.5 Program data collection preferences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
10.6 View parameter changes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
3
Page 4

11 Viewing and analyzing diagnostic data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
11.1 View clinical diagnostic data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
11.2 View device diagnostic data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
12 Performing system tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
12.1 Configure the test preferences for the Live Rhythm Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
12.2 Perform the Device Measurements Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
12.3 Perform a Sensing Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
12.4 Perform an Impedance Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
12.5 Perform a pacing Threshold Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
12.6 Perform a Temporary Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
12.7 Start the Atrial Sensing Setup process . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
12.8 Perform a Manual Atrial Mechanical Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
12.9 Perform an Exercise Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
13 Using the SessionSync feature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
13.1 View the SessionSync connection status . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
13.2 Send device data and reports to the Paceart Optima system . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
13.3 End the patient session with the automatic SessionSync feature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
14 Using session tools . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
14.1 Connect to the base . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
14.2 Save the implantable device data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
14.3 About Read From File sessions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
14.4 End the patient session . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
15 Working with reports and saved device data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
15.1 Configure the report preferences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
15.2 Generate reports . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
15.3 View or export saved reports and implantable device data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
4
Page 5

1 Introduction
1.1 Description
The CareLink SmartSync Micra AV Application (referred to as the implantable device app) allows you to program the settings of a
Micra AV device and view stored device data.
Use the implantable device app to perform the following tasks:
• Review the presenting rhythm
• Verify the status of the implantable device
• Assess the clinical effectiveness of the implantable device
• View or enter patient information
• Program parameters
• Save or export data
The implantable device app is a component of the CareLink SmartSync device manager.
Note: The CareLink SmartSync Micra AV Application model number is D00U007.
1.2 Intended use of the implantable device app
1.2.1 Intended use
The intended purpose is to interrogate, program, or execute tests on a Medtronic cardiac device.
1.2.2 Intended users
The implantable device app is intended for use by healthcare professionals or Medtronic representatives in a clinical or hospital
environment.
1.2.3 Intended patient population
The implantable device app is intended for use with patients who either have or are receiving a supported implantable device.
1.2.4 Expected clinical benefits
The clinical benefit of the implantable device app is the ability to interrogate and program Medtronic implantable devices.
1.2.5 Indications for use
For information about the indications for the implantable device, refer to the device manual for the implantable device.
1.2.6 Contraindications
There are no known contraindications for the use of the implantable device app.
Note: For information about contraindications for the implantable device, refer to the device manual.
1.3 Warnings and precautions
These warnings and precautions apply when using the implantable device app in combination with the other device manager
components.
Note: For warnings and precautions about the use of the implantable device, refer to the device manual.
Importance of instructions for use – Before using the implantable device app, Medtronic recommends that you do the following:
• Read the implantable device instructions for use.
• Read the device manager instructions for use.
• Carefully assess the patient’s condition and the implantable device system to determine the appropriate settings for tests and
device programming.
Improper use of the implantable device app could result in erroneous programming, inadvertent pacing, improper operation of
telemetry, or incorrect operation of measurement functions.
Tablet and app interaction – Due to the dynamic nature of the tablet environment, operating system events such as notifications,
alarms, and messaging can take priority and, therefore, move the implantable device app to the background. Tapping, pressing
buttons, and using gestures on the tablet can also result in moving the implantable device app to the background or closing the
implantable device app. For example, the implantable device app moves to the background if you lock the tablet.
When the implantable device app moves to the background or closes, telemetry with the implantable device is paused or lost. If a test
is in progress, whether the test continues or stops depends on the type of test. For more information, refer to the section on performing
system tests in this app help.
5
Page 6

When you restore the implantable device app from the background, the implantable device app attempts to re-establish
communication with the implantable device and displays the system status. If the implantable device app was closed, you must
interrogate the implantable device to re-establish communication with the implantable device.
Electromagnetic interference – If electromagnetic interference (EMI) occurs during a telemetry session, EMI can prevent the
proper programming or confirmation of values. For more information about EMI, refer to the device manual.
1.4 Potential adverse events
There are no known potential adverse events related to the use of this implantable device app.
For information about potential adverse events related to the use of the implantable device, refer to the device manual.
1.5 Download or order the instructions for use
To view, download, print, or order a PDF version of this app help, go to www.medtronic.com/manuals, or contact a Medtronic
representative.
The PDF version of this app help can be viewed using a current version of any major internet browser. For best results, use Adobe™*
Acrobat™* Reader software with the browser.
Paper copies of this app help are available to customers free of charge. They should arrive in 3 to 7 days. To order, go to
www.medtronic.com/manuals or contact a Medtronic representative.
1.6 IT network, tablet, and data information
1.6.1 Required IT network characteristics and configuration
To use the implantable device app, your tablet must have Bluetooth® wireless technology1. An Internet connection is optional.
Bluetooth wireless technology
You must enable Bluetooth wireless technology on your tablet. The Bluetooth connection allows the hardware components of the
device manager to communicate with the device manager app that is installed on the tablet.
Failure to provide Bluetooth communication access prevents the device manager components from communicating with each other
and with implantable devices. As a result, the device manager app is unable to establish a Bluetooth connection with the patient
connector. The patient connector is used to interrogate and program the implantable device.
Internet
To configure your network, follow the processes and policies of your organization.
Internet access is not required to export and print reports. However, failure to provide access to an information technology (IT) network
(for example, a Wi-Fi™* or cellular network) results in the inability to export and print reports using a wireless connection.
1.6.2 Supported tablets and technical specifications
The tablet on which the device manager app is installed must meet the requirements in the CareLink SmartSync Tablet Compatibility
Technical Manual. To download or order the CareLink SmartSync Tablet Compatibility Technical Manual, go to
www.medtronic.com/manuals, or contact a Medtronic representative.
Note: The device manager app may not be compatible with the most current version of the tablet operating system.
1.6.3 Intended information flows
Data from the implantable device flows through the device manager components in the following sequential order:
1. Implantable device
2. Patient connector
3. Implantable device app
All information in transit is protected for security.
1.7 Reporting errors and serious incidents
If a serious incident related to the CareLink SmartSync app occurs, immediately report the incident to Medtronic and the applicable
competent authority or regulatory body.
If you find information in this app help that is incorrect, contact a Medtronic representative.
1
The Bluetooth® word mark is a registered trademark of Bluetooth SIG, Inc. Any use of the word mark by Medtronic is under license.
6
Page 7

2 Overview of the interface
2.1 Areas
The implantable device app is divided into 3 areas:
• The 2 status bars at the top of the screen display status information about the device manager components and the patient
session. The top status bar also displays the EMERGENCY button and the
• The Live Rhythm Monitor, which appears below the status bars, displays real-time waveform traces.
• The work area, which is the largest area of the screen, displays the parameters, fields, and controls for the current window.
2.2 Status indicators
The status bar at the top of the screen displays the status of the base, the tablet, the patient connector, and the implantable device.
For more information, tap on the status bar.
Table 1. Base status indicators
Indicator Description
The base is connected to the device manager app.
The base is connected to the device manager app, and an analyzer session is in progress.
There is no base connected to the device manager app.
Note: A green dot indicates that a base was recently connected
to the device manager app, but is not connected now.
(Menu) button.
Table 2. Tablet status indicators
Indicator Description
The tablet is connected to an IT network.
Note: The status indicator shows the remaining percentage of
the tablet battery.
The tablet is not connected to an IT network.
Note: The status indicator shows the remaining percentage of
the tablet battery.
Table 3. Patient connector status indicators
Indicator Description
The patient connector is connected to the device manager app.
The patient connector battery is good.
The patient connector is connected to the device manager app.
The patient connector is charging.
The patient connector is connected to the device manager app.
The patient connector battery is low.
The patient connector is connected to the device manager app.
The patient connector battery is critically low.
There is no patient connector connected to the device manager
app.
Note: A green dot indicates that a patient connector was recently
connected to the device manager app, but is not connected now.
7
Page 8

Table 4. Implantable device status indicators
Indicator Description
The implantable device is connected to the patient connector.
The connection is either strong or moderate. To improve the connection, move the implantable device and the instruments closer
together and away from anything else that causes interference.
The implantable device is connected to the patient connector.
The connection is weak. To improve the connection, move the
implantable device and the instruments closer together and away
from anything else that causes interference.
The connection with the implantable device has been lost. The
device manager attempts to establish the connection and restore
communication. Move the tablet closer to the base or the patient
connector and away from anything else that causes interference.
Table 5. Connection status indicators
Indicator Description
The Bluetooth connection between the device manager app and
the base or the patient connector is strong.
The Bluetooth connection between the device manager app and
the base or the patient connector is moderate.
To improve the connection, move the tablet closer to the base or
the patient connector and away from anything else that causes
interference.
The connection between 2 system components has been lost.
Move the 2 system components closer together and away from
anything else that causes interference. The device manager
attempts to establish the connection and restore communication.
There is a USB connection between the base and the patient
connector.
The connection with the implantable device is weak.
To improve the connection, move the implantable device and the
instruments closer together and away from anything else that
causes interference.
The connection with the implantable device is moderate.
To improve the connection, move the implantable device and the
instruments closer together and away from anything else that
causes interference.
The connection with the implantable device is strong.
3 Reinterrogating the implanted device
3.1 Reinterrogate the implanted device
During a device implant or a patient follow-up appointment, you can reinterrogate the implanted device.
Note: For information on how to perform an initial interrogation, refer to the device manager app help.
To reinterrogate the implanted device, tap > INTERROGATE.
4 Responding to device status indicator warnings
4.1 About device status indicator warnings
The implantable device automatically monitors for internal conditions that affect implantable device operation and require attention.
If any such conditions occur, the implantable device saves the status indicator to its memory. The implantable device app displays the
status indicator warning in a message window when you interrogate the implantable device. The status indicator warning is also
displayed in the OBSERVATIONS area on the Quick Look screen.
8
Page 9

Caution: If the implantable device app displays a status indicator warning for the implantable device, contact a Medtronic
representative.
4.2 Respond to the WARNING – DEVICE ELECTRICAL RESET warning
If the device is not yet implanted, do not implant the device. Contact a Medtronic representative.
A device reset is a safety feature that can automatically change parameter values or clear diagnostic data in response to a problem
with the implantable device memory. If a device status indicator warning for a reset appears, you must clear the device status indicator.
You may need to reprogram the implantable device to the desired parameters.
After a device reset, the device records a status indicator. For a device reset that requires attention, the status indicator warning for
the implantable device describes how the reset affected device data. Read the message accompanying the indicator and follow the
on-screen instructions carefully. If the message indicates that the reset affected implantable device parameters, you must reprogram
the implantable device to restore the previous settings.
1. Respond to the status indicator warning:
a. Remove any sources of electromagnetic interference (EMI).
b. Notify a Medtronic representative.
c. To clear the status indicator, tap CLEAR in the window.
A confirmation window appears, indicating that all previously interrogated data in the implantable device app will be
cleared.
d. Tap CONTINUE.
Note: If a device reset occurred while the MRI SureScan parameter was programmed to On, the MRI SureScan window
appears. Program the MRI SureScan parameter to Off before continuing with the next step.
e. Interrogate the implantable device.
2. Determine the events leading up to the device reset:
a. To determine the time and date of the device reset, note the time and date when counter data was last cleared.
b. If the device is implanted, determine what the patient was doing at the time and date of the device reset.
c. Save the implantable device data.
d. Send the implantable device data to a Medtronic representative.
3. Reprogram the implantable device:
a. Verify the programmed device parameters and reprogram them as necessary.
Note: If the reset affected the parameters, the implanted device is automatically programmed to either Device Off or VVI
mode until the parameters are reprogrammed. In VVI mode, the implanted device paces at 65 bpm.
b. Verify that the implantable device date and time are correct. If necessary, reprogram the date and time.
c. To verify that the battery voltage of the implantable device is acceptable, check the BATTERY AND DEVICE
MEASUREMENTS window.
d. Conduct an electrode impedance test, a sensing test, and a pacing threshold test as desired.
4.3 Respond to the SERIOUS DEVICE ERROR warning
If the implantable device app displays a SERIOUS DEVICE ERROR status indicator warning, contact a Medtronic representative. An
immediate new device implant is recommended.
To respond to the SERIOUS DEVICE ERROR status indicator warning, complete the following actions:
1. To clear the status indicator, tap CLEAR in the window.
A confirmation window appears, indicating that all previously interrogated data in the implantable device app will be cleared.
2. Tap CONTINUE.
3. To verify that the battery voltage of the implantable device is acceptable, check the BATTERY AND DEVICE MEASUREMENTS
window.
4. Verify the programmed device parameters and reprogram them as necessary.
Note: The implantable device is automatically programmed to Device Off until the parameters are reprogrammed.
5 Using the Live Rhythm Monitor
5.1 About the Live Rhythm Monitor
The Live Rhythm Monitor displays markers, the A. Sensing waveform trace, and the telemetered EGM waveform trace from the
implanted device. If the device manager app is connected to the base, the Live Rhythm Monitor displays ECG waveform traces from
the base.
9
Page 10
Note: ECG signals are required to assess AV synchrony.
During patient sessions, you can view live waveform traces, freeze waveform traces, and access waveform strips.
In addition to waveform traces, the Live Rhythm Monitor displays the following information:
• The current heart rate and interval measured by the implanted device
• Annotations above the waveform trace showing when programming occurred (if parameters have been programmed)
The display of waveform traces in the Live Rhythm Monitor varies, depending on the values that you select for the A. Sensing Vector
and Live Waveform Display parameters.
5.2 About the A. Sensing waveform trace
The Live Rhythm Monitor displays the A. Sensing waveform trace, which shows atrial activity in the form of mechanical signals sensed
by the device accelerometer.
The waveform labelled A. Sensing shows sensed atrial activity. In an atrial sensing mode, the selected vector for the Live Waveform
Display is also shown.
5.3 Markers
Markers on the waveform trace indicate events such as pacing and sensing.
Note: Any interruption in telemetry with the implanted device can result in missing markers on the waveform trace display.
Markers that indicate atrial events appear above the waveform trace. Markers that indicate ventricular events appear below the
waveform trace.
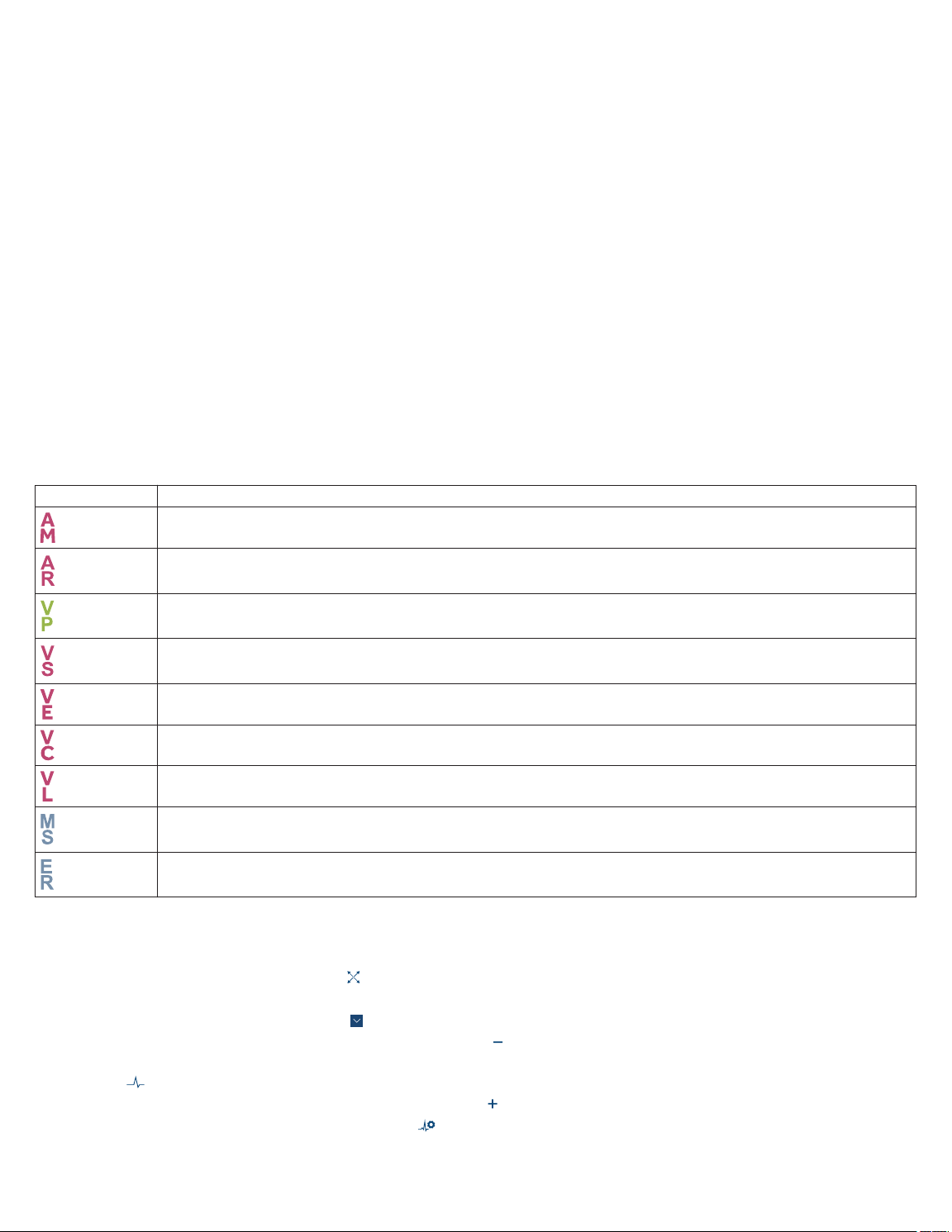
Table 6. Pacing markers
Marker Description
Atrial mechanical sense
Atrial refractory sense
Ventricular pace
Ventricular sense
Ventricular end (A3 Window End)
Ventricular capture
Ventricular loss of capture
Mode switch
Unrecognized marker
5.4 Adjust the Live Rhythm Monitor display
To change the size, order, and presentation of waveforms, complete the following actions:
1. To expand the Live Rhythm Monitor, tap .
2. To change the size, color, and order of the waveform traces, complete the following actions:
• To change the waveform source, tap on the waveform source list and select a source.
• To decrease the size of the displayed waveform trace, tap .
• To adjust the size of the displayed waveform trace to its maximum size without clipping or overlapping other waveform traces,
tap .
• To increase the size of the displayed waveform trace, tap .
• To change the color of the waveform trace, tap and select a color, then close the window.
3. Configure the following additional adjustment options:
10
Page 11

Option Description
Clipping When ON, truncates the tops and bottoms of waveforms that
have high amplitudes.
ECG Filter When ON, can improve the clarity of the ECG in the presence
of interference.
Artifacts When ON, displays line boundaries at the beginning and end
of each wave. This feature is also known as pacing artifact
enhancement.
Sweep Speed Allows you to control how quickly the waveform trace is drawn
across the screen. When you select a fast sweep speed, the
waveform trace appears wide. When you select a slow sweep
speed, the waveform trace appears narrow.
NORMALIZE Adjusts the size of all displayed waveform traces to their max-
imum size without clipping or overlapping.
CALIBRATE Adds a reference signal to the waveform trace of ECG.
OK Closes the adjustment options.
4. To minimize the Live Rhythm Monitor, tap .
5.5 Freeze live waveform traces
To capture a waveform strip and to generate a report, complete the following actions:
1. From the Live Rhythm Monitor, tap .
2. To modify the waveform strip, use the options on the FROZEN STRIP window.
3. To generate a report of the waveform strip, complete the following actions:
a. Tap the PDF button.
b. Select the strips that you want to include in the report.
c. Tap GENERATE REPORT > OK.
6 Using the eStrip recorder
6.1 About the eStrip recorder
You can use the eStrip recorder to view waveform strips, add and modify waveform strips, and generate reports of waveform strips.
To open the eStrip recorder, tap > ESTRIP RECORDER. You can also open the eStrip recorder by freezing live waveform traces
( ) from the Live Rhythm Monitor.
When you open the eStrip recorder, the FROZEN STRIP window appears and displays the last 30 min of all waveform traces from the
Live Rhythm Monitor. You can scroll horizontally along the waveform traces, or you can quickly navigate a waveform trace by using the
Holter feature. Highlights on the waveform traces indicate waveform strips.
Waveform strips are available to view for the duration of the patient session, including strips that are older than 30 min. To view the
waveform strips, use the strips list or the Holter feature.
6.2 Modify the display of waveform traces
Modify the display of waveform traces using the following options from the FROZEN STRIP window:
• To change the sweep speed for the waveform traces, tap
When you select a fast sweep speed, the waveform trace appears wide. When you select a slow sweep speed, the waveform trace
appears narrow.
• To change the waveform source, tap on the waveform source list and select a source.
• To increase the size of the displayed waveform trace, tap . This option decreases the mV/mm value.
• To decrease the size of the displayed waveform trace, tap . This option increases the mV/mm value.
6.3 Configure waveform strip preferences
To set clipping and artifacts options, or to set the default duration for new waveform strip highlights, configure waveform strip
preferences:
1. From the FROZEN STRIP window, tap .
2. Use the following options:
on the sweep speed list and select a value.
11
Page 12
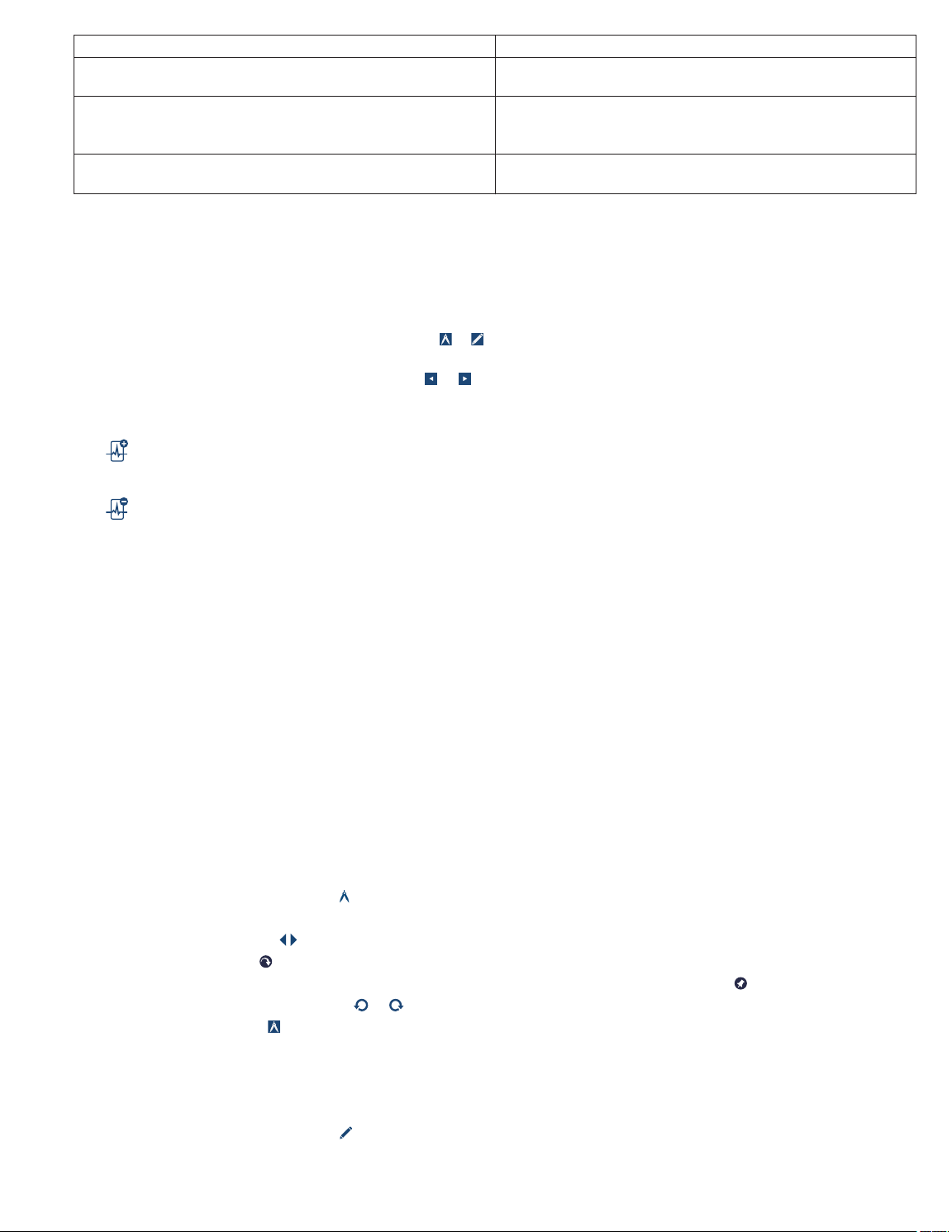
Option Description
Clipping When ON, truncates the tops and bottoms of waveforms that
have high amplitudes.
Show Artifacts When ON, displays line boundaries at the beginning and end
of each wave. This feature is also known as pacing artifact
enhancement.
HIGHLIGHT DURATION Allows you to set the default duration for all new waveform strip
highlights.
3. To save your preferences, tap OK.
6.4 Access waveform strips
To view, modify, and generate reports of waveform strips, complete the following actions in the FROZEN STRIP window:
1. To view waveform strips, perform one of the following actions:
• To select a waveform strip, tap STRIPS, then tap a waveform strip from the list.
Note: In the STRIPS list, the NOTES field displays or when the waveform strip includes a pinned caliper measurement
or annotation.
• To view the previous or next waveform strip, tap or .
2. To add or remove a waveform strip, perform one of the following actions:
• To add a waveform strip, tap the following button:
• To remove a waveform strip, tap its green header, then tap the following button:
Note: You cannot remove test strips or any strips that were automatically generated when you started the session.
3. To modify the waveform strip, use the options on the FROZEN STRIP window.
4. To generate a report of the waveform strip, complete the following actions:
a. Tap the PDF button.
b. Select the strips that you want to include in the report.
c. Tap GENERATE REPORT > OK.
6.5 Change the length of a waveform strip
To change the length of a waveform strip, complete the following actions:
Note: You cannot change the length of test strips or any strips that were automatically generated when you started the session.
1. From the FROZEN STRIP window, choose a strip, then tap its green header.
2. Drag the vertical border of the waveform strip to make it longer or shorter.
If you want to move the waveform strip, drag the horizontal border to the right or left.
6.6 Measure time intervals
To measure time intervals on the waveform strip, use the caliper tool:
1. From the FROZEN STRIP window, tap .
2. Use the following options:
• To adjust the caliper, drag .
• To walk the caliper, tap .
• To pin the caliper and include the caliper measurement in a strip report that you generate, tap .
• To undo or redo a pinned caliper, tap or .
3. To close the caliper tool, tap .
6.7 Draw notes
To annotate the waveform strip, draw notes on the waveform strip. If you generate a report of the strip, the notes that you draw on the
waveform strip are included in the report.
1. From the FROZEN STRIP window, tap
2. Draw on the waveform strip.
.
12
Page 13
3. Optionally, tap or to remove or reinsert drawings.
4. To disable the drawing mode, tap .
6.8 Edit the title of a waveform strip
To edit the title of a waveform strip, complete the following actions:
Note: You cannot edit the titles of test strips or any strips that were automatically generated when you started the session.
1. From the FROZEN STRIP window, choose a strip.
2. From the green header of the strip, tap the strip title.
3. Tap the strip title again to display the keyboard and enter a new title.
6.9 Use the Holter feature
To navigate quickly along a waveform trace, use the Holter feature in the FROZEN STRIP window:
1. To select the waveform trace that you want to view, tap
2. Tap HOLTER.
The blue rectangle indicates the section of the waveform trace that is displayed in the FROZEN STRIP window. A green
rectangle indicates a waveform strip.
3. Use the following options:
• To navigate along the waveform trace, tap and .
• Tap an area of the waveform trace to display that area in the FROZEN STRIP window.
on the top waveform source list and select a source.
7 Viewing summary data using the Quick Look screen
7.1 About the Quick Look screen
The Quick Look screen provides a summary of the most important indicators of the implanted device operation and the patient’s
condition since the last patient session. The screen includes links to more detailed status and diagnostic information stored in the
implanted device.
Use the Quick Look screen to view the following information:
• Device status information that indicates whether the implanted device is operating as expected
• Information about provided therapies, which helps to assess the patient’s clinical status since the last follow-up appointment
• System-defined observations about unexpected conditions, along with suggestions on how to optimize the implanted device
settings
Note: The Quick Look screen displays the information collected and stored in the implanted device memory since the last patient
session. However, the OBSERVATIONS section can also reflect programming changes made during the current patient session.
To update the Quick Look data during a patient session, reinterrogate the implanted device.
7.2 View the Quick Look screen
To view the Quick Look screen, tap
Section Description
REMAINING LONGEVITY Displays the estimated time remaining until Recommended Replacement Time (RRT).
IMPEDANCE (Ω)
THRESHOLD (V @ ms)
AMPLITUDE (mV)
% OF TIME Displays information that helps you to evaluate the effectiveness of programmed implan-
> Quick Look. You can view the following information:
To view more details, tap REMAINING LONGEVITY.
Displays information about the implanted device status, which allows you to assess the
performance and integrity of the device electrode and identify any unusual conditions.
The graphs display electrode impedance, capture threshold, and sensing amplitude
measurements recorded over the last 12 months. The graph legends show the most
recent measurement for each device performance variable.
Use the following options:
• To view detailed device trend data, tap TRENDS.
• To view more details about the most recent measurements, tap LAST MEASURED.
ted device settings.
13
Page 14

Section Description
RATE HISTOGRAMS Opens the RATE HISTOGRAMS window, which displays information about heart rates
recorded between patient sessions. The data can help you to monitor the patient’s condition and assess the effectiveness of therapies.
OBSERVATIONS Displays observations that are based on an analysis of programmed parameters and data
collected since the last patient session. The OBSERVATIONS section can also reflect
programming changes made during the current patient session. Observations alert you to
unexpected conditions related to implanted device status, parameter settings, and clinical
status.
When you select one of the displayed observations, the arrow next to the OBSERVA-
TIONS section title becomes active if more information about the selected observation is
available. To view the relevant details, tap OBSERVATIONS.
8 Using emergency VVI pacing
8.1 Enable emergency VVI pacing
To quickly enable high-output ventricular pacing, program emergency VVI pacing:
Note: When you program emergency VVI pacing, the implantable device app disables MRI SureScan operation.
1. Verify that telemetry is established between the implanted device and the patient connector.
2. Tap EMERGENCY.
3. Tap PROGRAM.
The implanted device delivers emergency VVI pacing and the EMERGENCY PROGRAM - SUCCESSFUL window appears.
4. Close the EMERGENCY PROGRAM - SUCCESSFUL window.
5. When the emergency is resolved, tap EXIT EMERGENCY to close the EMERGENCY - VVI PACING window.
6. Reprogram the implanted device settings to values appropriate for the patient.
9 Programming patient information
9.1 Program the patient information
To store information about the patient and the implantable device for later use, enter and program the patient information into the
implantable device memory. When you program the information into the memory, the implantable device app includes the patient
name, the patient ID, and the serial number of the implantable device on reports.
Typically, you enter the patient information at the time of implant, but you can revise it at any time.
Note: The PATIENT INFORMATION screen should not be used in place of the patient’s medical chart. The PATIENT INFORMATION
screen is provided as an informational tool for the end user. The user is responsible for accurate input of patient information into the
software. Medtronic makes no representation as to the accuracy or completeness of the patient information that end users enter into
the PATIENT INFORMATION screen. MEDTRONIC SHALL NOT BE LIABLE FOR ANY DIRECT, INDIRECT, INCIDENTAL, OR
CONSEQUENTIAL DAMAGES TO ANY THIRD PARTY WHICH RESULT FROM THE USE OF THE PATIENT INFORMATION
SUPPLIED BY END USERS TO THE SOFTWARE.
1. Tap > PATIENT INFORMATION.
2. On the PATIENT INFORMATION screen, tap each field, then enter or select the information.
Field Description
Patient Opens the Patient window, which allows you to enter the patient’s name.
Note: There is a limit of 30 characters.
ID Opens the ID window, which allows you to enter the patient ID.
Note: There is a limit of 15 characters.
Date of Birth Allows you to select the patient’s date of birth.
Serial Number Displays the serial number of the implantable device.
Implant… Opens the IMPLANT window, which allows you to enter or select the test values for R Wave Amplitude
(mV), Electrode Impedance (Ω), and Threshold. Also select the Pulse Width value.
History… Opens the HISTORY window, which allows you to select the patient’s clinical conditions.
Physician Opens the PHYSICIAN NAME/PHONE window, which allows you to select the physician’s name and phone
number from a list.
To add physician information to the list, tap MODIFY LIST and add the information.
14
Page 15

Field Description
Phone Opens the PHYSICIAN NAME/PHONE window, which allows you to select the physician’s name and phone
number from a list.
To add physician information to the list, tap MODIFY LIST and add the information.
Hospital Opens the HOSPITAL window, which allows you to select the hospital.
To add hospital information to the list, tap MODIFY LIST and add the information.
Last Update Displays the last date on which changes to patient information were programmed into the implantable device
memory.
Notes Opens the Notes window, which allows you to enter notes about the patient or other information.
Note: There is a limit of 80 characters.
Note: When the entries are too long to display in the fields, the implantable device app displays truncated versions of the entries.
For example, the Patient field displays a truncated version of the patient name if the name does not fit in the display field. The
Patient Information Report displays the full entry.
3. Tap PROGRAM.
4. To create the Patient Information Report, tap the PDF button.
10 Programming implantable device settings
10.1 Parameter symbols
The implantable device app can display symbols next to parameter values to convey their status or other information.
Table 7. Symbols that appear with parameter values
Symbol Name Description
Interlock The parameter value conflicts with the setting of another present or pending value. Select another
value or resolve the conflicting parameter value before programming the parameter.
Warning A warning message exists regarding that value. To view the message, tap the message symbol next
to the PROGRAM button or reselect that parameter.
Adaptive The programmed value can be changed automatically by the implantable device. The symbol does
not necessarily indicate that the parameter value has been adapted from a previously programmed
value, only that it is able to be adapted.
Nominal The value is the Medtronic nominal value.
Note: If the nominal value is also the programmed value, the Programmed symbol appears instead
of the Nominal symbol.
Programmed Indicates that the value is the programmed value.
The implantable device app displays message symbols next to the PROGRAM button. When you tap the message symbol, additional
parameter information appears.
If there are multiple messages about the pending parameter values, the symbol for the most significant message appears.
Table 8. Message symbols
Symbol Name Description
Interlock A parameter interlock exists. Programming is restricted until the interlock conflict is resolved.
Warning There is a warning associated with programming 1 or more of the pending parameter values.
Informational There is an informational message regarding 1 or more of the parameter values.
10.2 Program the parameters
To control the implantable device functions and data collection capabilities, program the parameters.
The parameters that you can view and program appear as active fields. Some active fields pertain to only 1 parameter, while other
fields provide access to groups of parameters. If a parameter cannot be programmed, no active field appears next to its name.
1. Tap > PARAMETERS.
2. Tap each field and change the value.
The implantable device app displays the new values with a dashed border. The dashed border signifies that the values are
pending.
15
Page 16
3. Tap PROGRAM.
The pending values are programmed to the implantable device memory.
10.3 Create custom parameter sets
Create and save sets of parameter values for retrieval in either the current patient session or in subsequent patient sessions.
You can save and access a custom set of parameter values for a particular clinical situation. For example, you may want to save a set
of parameter values for an initial implant setting, for a specific disease state, or for situations in which you must repeatedly program
a particular set of parameters. The set of parameter values that you save can include both programmed and pending values.
1. Tap > PARAMETERS.
2. On the PARAMETERS screen, make the desired parameter selections.
3. Tap SAVE & GET… > ADD NEW.
4. Enter a name for the parameter set.
5. Tap OK > SAVE.
If a parameter set exists with that name, confirm that you want to replace the existing set with a new set or change the name of
the new parameter set.
10.4 Retrieve parameter sets
Parameter sets are collections of parameter values.
The implantable device app includes 3 types of parameter sets:
• Medtronic Nominals – Parameter values that Medtronic suggests for the implantable device. You cannot customize or delete
Medtronic Nominals.
• Initial Interrogation Values – Permanently programmed parameter values as determined by the first interrogation of the
implantable device during the patient session. You cannot customize or delete Initial Interrogation Values.
• Custom – Sets of parameter values that you create for a particular clinical situation. For example, you may want to save a set of
parameter values for an initial implant setting, for a specific disease state, or for situations in which you must repeatedly program
a particular set of parameters.
1. Tap > PARAMETERS > SAVE & GET….
2. Tap the parameter set you want to retrieve, then tap SET PENDING.
3. On the PARAMETERS screen, tap PROGRAM.
The pending values are programmed to the implantable device memory.
10.5 Program data collection preferences
To adjust the date and time of the implantable device and to enable the Holter telemetry feature, program the data collection
preferences:
1. Tap
2. Configure the following options:
3. To program any pending changes, tap OK > PROGRAM.
> PARAMETERS > Data Collection Setup….
Option Description
Device Date/Time… Allows you to program the date and time of the implantable device to the date and
time of your tablet.
• Device Date/Time – Displays the date and time that the implantable device
currently uses.
• Tablet Date/Time – Displays the date and time that the tablet currently uses.
• SET TO TABLET TIME – Allows you to program the new date and time that the
implantable device will use based on the date and time of your tablet.
Note: The implantable device app expresses time in the 24-hour format or in the
12-hour format, depending on your tablet settings.
Holter Telemetry Duration Allows you to either enter the duration for Holter telemetry or to disable Holter
telemetry. When you enable Holter telemetry, the implanted device continuously
transmits EGM and marker data for the selected duration, regardless of the presence of the patient connector.
Note: Enabling Holter telemetry results in a higher consumption of the implanted
device battery. Use of a customized Holter monitor (provided by Medtronic) is
required for monitoring the device markers and EGM.
16
Page 17
10.6 View parameter changes
Review the list of parameter changes that you made during the patient session:
1. Tap > SESSION > CHANGES THIS SESSION.
2. Review the list of parameter changes.
3. To create a report, tap the PDF button.
11 Viewing and analyzing diagnostic data
11.1 View clinical diagnostic data
11.1.1 About clinical diagnostic data
The implanted device collects and stores diagnostic data, which you can use to assess the patient’s clinical conditions and the
effectiveness of therapies.
11.1.2 View rate histogram data
View the heart rate data that the implanted device records between patient sessions. Use the heart rate data to monitor the patient’s
condition and the effectiveness of therapies.
1. Tap > DATA > RATE HISTOGRAMS.
2. Select Atrial Ventricular or Ventricular from the Rate Histogram list for the type of heart rate data that you want to view.
3. To create a report, tap the PDF button.
11.2 View device diagnostic data
11.2.1 About device diagnostic data
The implanted device automatically measures and records daily device performance data.
11.2.2 View battery and device measurement data
To assess the most recent measurements and trended measurements of implanted device performance, view the battery and device
measurement data.
Warning: The implantable device app displays an End of Service (EOS) indicator when the device battery no longer has adequate
capacity to provide therapy to the patient and the device has reached the EOS condition. When the battery reaches the EOS condition,
the device deactivates pacing permanently.
Note: If the implantable device app displays the Recommended Replacement Time (RRT) indicator, contact a Medtronic
representative and the patient to schedule a procedure for a new device implant.
1. Tap
2. Select the type of data you want to view:
3. To create a report, tap the PDF button.
11.2.3 View electrode impedance trend data
To analyze the automatic daily electrode impedance measurements, view the electrode impedance trend data:
1. Tap > DATA > ELECTRODE IMPEDANCE TREND.
2. To create a report, tap the PDF button.
The graph displays up to 15 daily measurements and up to 80 weekly summary measurements. Gaps in the graph occur when the
implanted device fails to complete automatic electrode impedance measurements.
Note: Significant and sudden changes in electrode impedance can indicate a problem with the implanted device fixation or the
electrode.
> DATA > BATTERY AND DEVICE MEASUREMENTS.
• Remaining Longevity / Battery Voltage
• Sensing Integrity Counter
• Electrode Impedance
• Capture Threshold
• Sensing
11.2.4 View capture threshold trend data
To evaluate Capture Management operations and the effectiveness of the pacing output values, view and analyze the capture
threshold trend data:
17
Page 18

1. Tap > DATA > CAPTURE THRESHOLD TREND.
2. To view the last 15 days of threshold measurement data, tap LAST 15 DAYS DETAIL.
3. To create a report, tap the PDF button.
The graph displays up to 15 daily measurements and up to 80 weekly summary measurements. Gaps in the graph occur when the
implanted device fails to complete daily capture threshold measurement.
The implanted device measures the capture threshold data only when the Capture Management parameter is configured as
Adaptive or Monitor.
Note: Significant and sudden changes in the pacing threshold can indicate a problem with the implanted device fixation or the
electrode.
11.2.5 View R-wave amplitude trend data
View and analyze the daily R-wave sensing amplitude measurements:
1. Tap > DATA > R WAVE AMPLITUDE TREND.
2. To create a report, tap the PDF button.
The graph displays up to 15 daily measurements and up to 80 weekly summary measurements. The daily measurements are the
median values of the amplitudes of 5 normal intrinsic ventricular sensed events. Gaps in the graph occur when the implanted device
is unable to collect 5 amplitude measurements on a given day.
Note: Significant and sudden changes in the R-wave amplitude can indicate a problem with the implanted device fixation or the
electrode.
11.2.6 View A4 amplitude trend data
View and analyze the daily A4 sensing amplitude measurements:
1. Tap > DATA > A4 AMPLITUDE TREND.
2. To create a report, tap the PDF button.
The graph displays up to 15 daily measurements and up to 80 weekly summary measurements. The daily measurements are the most
common measured A4 amplitude of the day. Gaps in the graph occur when the implanted device is unable to collect enough amplitude
measurements on a given day.
Notes:
• The A4 Amplitude Trend measures the maximum acceleration in the A4 window, which may not be the true A4 amplitude if the A4
signal does not occur in the A4 window.
• Significant and sudden changes in the A4 amplitude can indicate a problem with effective VDD pacing therapy.
12 Performing system tests
12.1 Configure the test preferences for the Live Rhythm Monitor
To view the EGM or A. Sensing waveform, configure the test preferences for the Live Rhythm Monitor:
1. Tap > SESSION > PREFERENCES > TESTS.
2. Select one of the following options:
• To display the EGM or A. Sensing waveform, tap Auto-arrange waveforms.
• To leave the waveform display unchanged during a test, tap Do not auto-arrange waveforms.
3. Tap OK.
12.2 Perform the Device Measurements Tests
The implantable device app provides 3 assessment tools to measure device electrical performance for your patient. These tests, the
Sensing Test, the Impedance Test, and the Threshold Test, can be performed individually or in an automated sequence.
Note: If telemetry between the implantable device app and the implanted device is paused or lost during a Sensing Test, Impedance
Test, or Threshold Test, the test stops and the implanted device parameters revert to permanently programmed values.
To select the tests you wish to perform in an automated sequence, complete the following actions:
1. Tap
2. Select each test that you wish to perform.
3. Make your pre-test parameter changes for the Sensing Test or the Threshold Test. The Impedance Test has no parameters.
4. Tap START Tests to perform the selected device measurements tests.
> TESTS > DEVICE MEASUREMENTS.
18
Page 19

5. Optionally, complete 1 or more of the following actions:
• To create a report, tap the PDF button.
• To save the test values to the implanted device memory, tap SAVE… > PROGRAM. The test values appear when you open
the IMPLANT window.
Note: SAVE... only appears within 10 days of implant.
• To clear the test results, tap CLEAR… > CONTINUE.
12.3 Perform a Sensing Test
To assess sensing performance, perform the Sensing Test, which measures R-wave amplitudes.
The Sensing Test allows you to temporarily program pacing parameters to increase the likelihood that sensed events will occur.
Sensing amplitude measurements taken during a Sensing Test may include events that are atypical or a result of oversensing (for
example, noise interference). These events are excluded from the daily automatic sensing amplitude measurements that the
implanted device collects and reports in the R-wave amplitude trend. Because of the difference in measurement operations, Sensing
Test results can differ from the measurements reported in the R-wave amplitude trend data.
Warning: Before you start the Sensing Test, select a temporary pacing rate that allows intrinsic sensed events that can be well
tolerated by the patient. If the patient shows poor tolerance to the selected pacing rate during the test, tap STOP. To complete the
Sensing Test, the device must detect 2 consecutive ventricular sensed events, and the interval between them must be at least 500 ms
(120 bpm). If after 10 s this interval is not detected between 2 consecutive ventricular sensed events, the Sensing Test stops. If you
cannot select a pacing rate that is well tolerated by the patient, consider withholding the Sensing Test.
Caution: Use caution when selecting temporary settings for pacemaker-dependent patients. These patients may not receive
adequate pacing support while sensing amplitude measurements are being obtained.
Notes:
• If you need to conduct consecutive Sensing Tests to collect data, reduce the pacing rate gradually to avoid or minimize patient
symptoms associated with abrupt changes in heart rate.
• If telemetry between the implantable device app and the implanted device is paused or lost during a Sensing Test, the test stops
and the implanted device parameters revert to permanently programmed values.
1. Tap > TESTS > DEVICE MEASUREMENTS.
2. Clear the Impedance Test and Threshold Test checkboxes.
3. Verify or change the TEST VALUE parameter values for Mode and Lower Rate.
4. Tap START Tests.
5. Observe the Live Rhythm Monitor for an intrinsic rhythm.
• If the patient shows poor tolerance to the test pacing rate, tap STOP to stop the test manually before it completes.
• If you see an intrinsic rhythm at a rate that is within the normal rate range for the patient, the test is successful. When the test
successfully completes, it automatically stops. The implantable device app displays the R-wave measurement value and
the pacing settings return to their programmed values.
6. Optionally, complete 1 or more of the following actions:
• To compare the Sensing Test R-wave measurement value with the automatic daily sensing R-wave amplitude
measurements, tap > DATA > R WAVE AMPLITUDE TREND.
• To view the test strip, tap the test strip icon next to the R-Wave measurement value.
• To create a report, tap the PDF button.
• To save the test value to the implanted device memory, tap SAVE… > PROGRAM.
• To clear the test results, tap CLEAR… > CONTINUE.
Note: Do not adjust the RV Sensitivity value based on the results of the Sensing Test. For more information, refer to the reference
manual for the implanted device.
12.4 Perform an Impedance Test
To test the integrity of the implanted device by measuring the impedance of the pacing electrode, use the Impedance Test.
Impedance measurements are made by delivering a pacing pulse. If the intrinsic heart rate is faster than the programmed pacing rate,
the implanted device increases the pacing rate to be slightly faster than the intrinsic rate for 1 interval.
Notes:
• The upper limit for the pacing rate can vary depending on the programmed pacing mode.
• During a sequence of electrode impedance measurements, the implanted device may pace at a rate faster than the programmed
value for the lower rate for 1 or more pacing cycles.
19
Page 20

• If telemetry between the implantable device app and the implanted device is paused or lost during an Impedance Test, the test
stops and the implanted device parameters revert to permanently programmed values.
1. Tap > TESTS > DEVICE MEASUREMENTS.
2. Clear the Sensing Test and Threshold Test checkboxes.
3. Tap START Tests.
When the Impedance Test completes, the implantable device app displays the measured impedance value.
To stop the test manually before it completes, tap STOP.
4. Optionally, complete 1 or more of the following actions:
• To compare the test result to daily automatic impedance measurements, tap > DATA > ELECTRODE IMPEDANCE
TREND.
• To create a report, tap the PDF button.
• To save the test value to the implanted device memory, tap SAVE… > PROGRAM.
• To clear the test results, tap CLEAR… > CONTINUE.
12.5 Perform a pacing Threshold Test
12.5.1 Perform an automatic pacing Threshold Test
To determine the patient’s pacing thresholds, use the automatic (Capture Management) Threshold Test. The Capture Management
test checks the pacing stimulation thresholds at different pacing amplitude settings. Use the test results to help you select amplitude
and pulse width settings that ensure capture while minimizing output to maximize battery longevity.
Note: If telemetry between the implantable device app and the implanted device is paused or lost during a Threshold Test, the test
stops and the implanted device parameters revert to permanently programmed values.
1. Tap > TESTS > DEVICE MEASUREMENTS.
2. Clear the Sensing Test and Impedance Test checkboxes.
3. Select Capture Management from the Threshold Test list.
4. Tap START Tests.
When the test completes, the implantable device app displays the Threshold measurement value.
To stop the test manually before it completes, tap STOP.
5. Ensure that the amplitude and pulse width values provide an adequate safety margin above the pacing threshold.
6. Optionally, complete 1 or more of the following actions:
• To compare the Threshold measurement value to the automatic daily threshold measurement values, tap > DATA >
CAPTURE THRESHOLD TREND.
• To view the test strip, tap the test strip icon next to the Threshold measurement value.
• To create a report, tap the PDF button.
• To save the test values to the implanted device memory, tap SAVE… > PROGRAM.
• To clear the test results, tap CLEAR… > CONTINUE.
12.5.2 Perform a manual pacing Threshold Test
To determine the patient’s pacing thresholds, use the manual pacing Threshold Test. The manual pacing Threshold Test allows you
to program your own mode and pacing settings to identify the pacing stimulation thresholds. Use the test results to help you select
amplitude and pulse width settings that ensure capture while minimizing output to maximize battery longevity.
Note: If telemetry between the implantable device app and the implanted device is paused or lost during a Threshold Test, the test
stops and the implanted device parameters revert to permanently programmed values.
1. Tap > TESTS > DEVICE MEASUREMENTS.
2. Clear the Sensing Test and Impedance Test checkboxes.
3. Select Amplitude - Auto Decrement from the Threshold Test list.
4. Tap START Tests to display the TESTS - PACING THRESHOLD - AMPLITUDE - AUTO DECREMENT window.
5. Verify or change the values:
• Verify or change the value for Decrement After.
• To change the parameters applied during the test, select new values in the TEST VALUE column.
Note: The programmable and default values depend on the programmed values for pacing therapy.
6. Press and hold TEST Press and Hold, then observe the Live Rhythm Monitor for loss of capture.
7. When loss of capture occurs, release TEST Press and Hold.
The implanted device resumes its original pacing values.
20
Page 21

8. In the ENDING VALUE column, verify the detected RV Amplitude at which loss of capture occurred.
9. In the THRESHOLD column, adjust the RV Amplitude threshold value as needed.
10. Ensure that the amplitude and pulse width values in the PERMANENT column provide an adequate safety margin above the
pacing threshold.
11. To change the programmed RV Amplitude or RV Pulse Width values, complete the following actions:
a. In the PERMANENT column, tap the value.
b. On the RV Amplitude or RV Pulse Width window, select the desired value.
c. Tap PROGRAM.
12. View the ending value and permanent value for the V. Pace Blanking parameter.
13. To view a test strip from the Threshold Test, complete the following actions:
a. Tap the Test Strip icon.
b. Close the window to return to the results window.
14. When you have finished viewing or changing data on the RV AMPLITUDE THRESHOLD TEST - RESULTS window, tap
CLOSE to return to the DEVICE MEASUREMENTS screen.
15. Optionally, complete 1 or more of the following actions:
• To compare the Threshold measurement value to the automatic daily threshold measurement values, tap > DATA >
CAPTURE THRESHOLD TREND.
• To view the test strip, tap the test strip icon next to the Threshold measurement value.
• To create a report, tap the PDF button.
• To save the test values to the implanted device memory, tap SAVE… > PROGRAM.
• To clear the test results, tap CLEAR… > CONTINUE.
12.6 Perform a Temporary Test
The Temporary Test evaluates device operation during temporary changes to parameter settings. The changes are in effect only while
the test is in progress.
The Temporary Test allows you to evaluate changes to these parameters:
• Mode
• Lower Rate
• Amplitude
• Pulse Width
• Sensitivity
Note: If telemetry between the implantable device app and the implanted device is paused or lost during a Temporary Test, the test
stops and the implanted device parameters revert to permanently programmed values.
1. Tap
2. To change the parameters applied during the test, select new values in the TEST VALUE column.
3. Press and hold TEST Press and Hold.
4. Observe changes in the waveform.
5. Release TEST Press and Hold.
6. To view a test strip from the most recent Temporary Test, tap the Test Strip icon.
7. To create a report, tap the PDF button.
> TESTS > TEMPORARY.
Note: Be aware of temporary parameter values that can inhibit pacing in pacemaker-dependent patients.
Warning: High-rate stimulation of the ventricle could result in ventricular tachycardia or fibrillation. Apply temporary high-rate
pacing under careful patient monitoring and control.
12.7 Start the Atrial Sensing Setup process
12.7.1 Initiate the Atrial Sensing Setup process
The implanted device can be set up for AV synchrony so the atrial mechanical signal is properly sensed in order to know when to pace
the ventricle. The Atrial Sensing Setup process collects atrial sensing data and then sets the atrial sensing parameters to
patient-specific values based on the collected data. If Atrial Sensing Setup was not used after implant to set up the atrial sensing
parameters, you can initiate the Atrial Sensing Setup process during the follow-up session to reset the atrial sensing parameters.
Note: The implanted device can reprogram some parameters or record relevant observations in the Quick Look screen in response
to its analysis of the cardiac cycle collected during the Atrial Sensing Setup process.
Note: For more information on atrial sensing setup, see the reference manual for the implanted device.
21
Page 22

1. Program the pacing mode to VDD.
2. Program the Atrial Sensing Setup parameter to On/Restart. As shown in the status bar, the pacing mode switches to VDI.
3. Remove the patient connector from the patient for at least 3 min. To stop the Atrial Sensing Setup process at any time, program
the Atrial Sensing Setup parameter to Off/Complete. When the Atrial Sensing Setup process completes, the implanted
device programs the Atrial Sensing Setup parameter to Off/Complete.
4. Following Atrial Sensing Setup, you should interrogate the implanted device to verify AV synchrony performance.
12.7.2 Atrial Sensing Setup status messages
The implantable device app displays Atrial Sensing Setup status messages in the status bar above the Live Rhythm Monitor.
Message title Description
Atrial Sensing Setup Scheduled The Atrial Sensing Setup is scheduled, or is rescheduled if the
Atrial Sensing Setup was suspended, and will start when the
patient connector is removed for at least 3 min.
Atrial Sensing Setup in Progress The Atrial Sensing Setup process is in progress.
The Atrial Sensing Setup process is complete when there is no longer a status message in the status bar above the Live Rhythm
Monitor. If the process failed to complete successfully, an observation that describes the cause will appear on the Quick Look screen.
Note: Programming parameters or running tests suspends the Atrial Sensing Setup process. For other suspension causes, see the
reference manual for the implanted device.
12.8 Perform a Manual Atrial Mechanical Test
The Manual Atrial Mechanical Test allows you to view the A3 signal and A4 signal on the implantable device app and program the atrial
sensing parameters as you choose.
You can observe and program the atrial sensing parameters with the assistance of the Manual Atrial Mechanical Test.
The Manual Atrial Mechanical Test displays the patient’s ECG signal and the device accelerometer signal. Your settings for the A3
Threshold, the A3 Window End, and the A4 Threshold parameters are represented as lines superimposed on the accelerometer
signals. The accelerometer blanking and threshold windows are labeled PVAB, A3, and A4.
The Manual Atrial Mechanical Test can be used to adjust the A3 Threshold, the A4 Threshold, and the A3 Window End parameters
relative to the A3 and A4 signals to optimize AV synchrony. See the reference manual for the implanted device for more details.
Note: Initial test values are set to currently programmed values whenever possible. If the currently programmed values are not valid,
default or previously programmed values will be used as the initial test values.
Note: If telemetry between the implantable device app and the implanted device is paused or lost during a Manual Atrial Mechanical
Test, the test stops and the implanted device parameters revert to permanently programmed values.
1. Attach a surface ECG to the patient to monitor and assess atrial sensing and AV synchrony.
Note: ECG signals are required to assess AV synchrony.
2. Tap Tests > Manual Atrial Mechanical.
3. Verify or change the TEST VALUE parameter values.
4. Press and hold TEST Press and Hold for multiple cardiac cycles shown on the ECG. Collect cycles where the P-wave and
R-wave appear to be synchronous and the A3 and A4 signals can be distinguished from each other.
Note: Up to 10 s of cardiac cycles are collected.
Note: The Manual Atrial Mechanical Test disables all advanced sensing and pacing features during the test operation, including
the auto adjusting features, the mode switch features, and Rate Smoothing.
5. Release TEST Press and Hold. The recorded test cardiac cycles display in the test results window. The cardiac cycles are
displayed in the reverse order that they were recorded. For example, if you record 9 cycles, the first cycle you will see in the test
results window is labeled 9/9.
6. Tap
7. Repeat Step 3 through Step 6 as needed to optimize AV synchrony.
8. To program your updated parameter settings, complete the following actions:
9. To view a test strip from the most recent Manual Atrial Mechanical Test, tap the Test Strip icon.
10. To create a report, tap the PDF button.
and on the test results window to see the collected cardiac cycles.
a. Tap ADJUST PERMANENT.
b. Tap PROGRAM.
22
Page 23

12.9 Perform an Exercise Test
12.9.1 About the Exercise Test
When the pacing mode is programmed to VVIR, the Exercise Test helps you to assess the patient’s settings for rate response. The
rate-response settings are applied when the implanted device is operating in either VVIR or VDIR mode.
As an alternative to automatic Rate Profile Optimization, you can perform an Exercise Test from the implantable device app to set the
rate-response values for the Lower Rate, ADL Rate, and Upper Sensor Rate. If Rate Profile Optimization is programmed to Off,
the rate-response parameters remain at their programmed values. When Rate Profile Optimization is programmed to On, this
feature adjusts the 3 rate-response parameters once each day. For more information about rate-responsive pacing, see the reference
manual for the implanted device.
The Exercise Test allows you to evaluate the rate-response settings for the patient and optimize the rate-response control parameters:
• The LR Setpoint (lower rate setpoint) value determines the activity counts required to pace at a rate higher than the lower rate.
• The ADL Setpoint (activities of daily living setpoint) value determines the minimum sensor response to pace at the ADL Rate,
which falls within the ADL rate range.
• The UR Setpoint (upper rate setpoint) value determines the minimum sensor response to pace at the Upper Sensor Rate, which
is at the upper limit of the exertion rate range.
• The Activity Vector value determines the accelerometer vector used for rate response. For more information about choosing the
Activity Vector, see the reference manual for the implanted device.
Note: The programmed LR Setpoint setting must be lower than the ADL Setpoint setting, and the ADL Setpoint setting must be
lower than the UR Setpoint setting.
12.9.2 Performing an Exercise Test
Perform an Exercise Test to help you assess the patient’s settings for rate response.
Note: If telemetry between the implantable device app and the implanted device is paused or lost during an Exercise Test, the
implanted device continues to record test data. The test completes when the test duration is reached.
1. Program the pacing mode to VVIR.
2. Tap > TESTS > EXERCISE.
3. Tap Duration and select the test duration.
4. Tap Activity Vector, select a value, and tap PROGRAM.
Note: Do not change the currently programmed values for LR Setpoint, ADL Setpoint, and UR Setpoint. These values
provide a baseline to determine the necessity to adjust the patient’s rate-response setpoints in consideration of the results of the
Exercise Test.
5. Tap START Test.
If test data from a previous Exercise Test is in the device memory, the WARNING - DATA WILL BE LOST message appears. The
message warns you that the current test results will be overwritten if you start the Exercise Test.
Note: Create a report or otherwise record the current values if you want to compare them to the test values.
6. To start the test, tap CONTINUE.
When the test completes, the EXERCISE window displays the Test Complete message.
To stop the test manually before it completes, tap Stop and Retrieve. The data recorded up to that point will be saved.
7. Re-establish telemetry between the implanted device and the patient connector.
The test results appear.
12.9.3 Interpreting Exercise Test data and adjusting rate-response setpoints
The implanted device collects the Exercise Test data. You can view the test data on the EXERCISE screen to interpret the test data
and adjust rate-response setpoints.
1. From the EXERCISE screen, view the test data. The test data is displayed as the Activity Counts graph.
2. To view the patient’s heart rate, sensor rate, and the device activity that was recorded during the Exercise Test, tap Activity
Counts and select Rate Graph.
3. Examine the Exercise Test results shown on the Activity Counts graph. Compare the patient activity count data to the
programmed values for LR Setpoint, ADL Setpoint, and UR Setpoint.
To determine if you need to adjust the rate-response setpoint values, consider the activity that the patient was engaged in during
the Exercise Test:
• The LR Setpoint (the lower rate set point) corresponds to when the patient is at rest at the beginning of the test. Following
the test, tap LR Setpoint and select a value slightly higher than the highest activity count for when the patient is at rest.
23
Page 24

• The ADL Setpoint (the activities of daily living set point) corresponds to when the patient is engaged in moderate activity.
Following the test, tap ADL Setpoint and select the average activity count for when the patient is engaged in moderate
activity.
• The UR Setpoint (the upper rate setpoint) corresponds to when the patient is engaged in vigorous activity. Following the
test, tap UR Setpoint and select a value slightly lower than the average activity count for when the patient is engaged in
vigorous activity.
4. Tap PROGRAM to program the updated rate-response values.
Note: The rate-response setpoint values can also be programmed when the implanted device is programmed to VDD mode.
5. To create a report, tap the PDF button.
13 Using the SessionSync feature
13.1 View the SessionSync connection status
If your clinic uses a Paceart Optima system, the SessionSync feature enables you to transfer saved implantable device data and
reports to that system.
To view the SessionSync connection status, use the DATA SYNCHRONIZATION STATUS window.
Notes:
• The SessionSync connection status is only visible if you configure the SessionSync feature. To configure the SessionSync
feature, refer to the device manager app help.
• The Paceart Optima system is only available in supported regions.
1. On the status bar, tap
2. From DATA EXPORT STATUS, tap DETAILS….
3. On the DATA SYNCHRONIZATION STATUS window, view the SessionSync connection status:
• Available – The SessionSync feature is enabled and there is a connection between the device manager app and the
Paceart Optima system.
• Disconnected – The SessionSync feature is enabled, but the tablet is disconnected from the network.
• Not Available – The SessionSync feature is enabled, but the connection to the Paceart Optima system is unavailable.
The DATA SYNCHRONIZATION STATUS window also shows the following information.
Field Description
Clinic Name Name of the clinic that receives SessionSync transfers.
Gateway address IP address or hostname of the SessionSync gateway.
Transfers The implantable device data and reports that the SessionSync feature transfers to
.
the Paceart Optima system. The Transfers table displays the most recent transfer
at the top of the table.
• GENERATED – Date and time of the transfer
• RECEIVING CLINIC – Name of the clinic that receives the transfer
• STATUS – Status of the transfer
13.2 Send device data and reports to the Paceart Optima system
If your clinic uses a Paceart Optima system, the SessionSync feature enables you to transfer implanted device data and reports to that
system without ending the patient session:
1. Tap > SESSION > SessionSync > TRANSFER.
2. To close the message, tap OK.
13.3 End the patient session with the automatic SessionSync feature
If your clinic uses a Paceart Optima system, end the current session and use the automatic SessionSync feature to transfer
implantable device data and reports from the device manager to the Paceart Optima system:
1. Tap
2. Ensure that the Automatic SessionSync checkbox is selected.
3. Tap END NOW > TRANSFER.
4. On the transfer message window, tap END NOW.
> END SESSION.
24
Page 25

14 Using session tools
14.1 Connect to the base
Connect to the base to view ECG waveforms during a patient session:
1. Plug in the base to the AC power outlet (AC mains).
2. Verify that the base is disconnected from any previously connected device manager app.
3. To turn on the Bluetooth wireless technology in the base, press the grey button on the base.
When the base is available for a connection with the device manager app, the Bluetooth light on the base slowly flashes.
4. On the status bar, tap
5. Near the base status indicator, tap SELECT.
6. Tap CONTINUE.
7. From the device manager app, follow the prompts to complete the base connection. For more information, refer to the device
manager app help.
For information on connecting the ECG cable to the base, refer to the base technical manual and the ECG cable IFU.
14.2 Save the implantable device data
Save the interrogated device data from a patient session to the SAVED REPORTS window. The save operation generates a PKG file
that includes the implantable device data (PDD file) and any reports that you generated during the session.
Implantable device data automatically saves when you end the patient session. However, implantable device data does not save when
the implantable device app closes from the tablet operating system. To avoid permanent loss of implantable device data, save the
implantable device data.
Note: During the save operation, the EMERGENCY button is available. However, if an error occurs during a save operation, there may
be a delay in initiating the EMERGENCY - VVI PACING window. Do not save implantable device data while performing a system test
or when it is possible that the Emergency function will be needed immediately. If the Emergency function is used during a save
operation, the implantable device app aborts the save operation.
1. Tap > INTERROGATE.
2. When the interrogation is complete, tap > SESSION > SAVE SESSION.
The implantable device data saves to the SAVED REPORTS window.
3. On the SESSION DATA SAVED window, tap OK.
.
14.3 About Read From File sessions
Use a Read From File session to view saved implantable device data, to save and export reports, and to display all programmed
parameter values.
To start a Read From File session, end the patient session and refer to the device manager app help.
Warning: A Read From File session is designed only for viewing saved implantable device data while no patient session is in progress.
You cannot program an implantable device or deliver emergency therapies from a Read From File session.
A Read From File session presents implantable device data in a slightly different way than what is seen during a patient session.
Because you are not in a live patient session, the Live Rhythm Monitor is replaced with the device model and the words Read From
File.
When you generate reports during a Read From File session, the retention of those reports is the same as the saved implantable
device data.
14.4 End the patient session
When you finish with the patient session, end the session.
Note: After 45 min of inactivity, the implantable device app displays a message that prompts you to extend or end the session. If you
do not respond to the message and there is 60 min of inactivity, the patient session ends automatically.
1. Tap
2. To change the setting for clearing the session data, select one of the following options from the Pacemaker Data list:
3. Tap END NOW.
> END SESSION.
• Select Clear Now to immediately clear the session data at the end of the session.
• Select 1 hour after session end to clear session data 1 hour after the end of the session.
• Select Do not clear if you want data collection to continue as if there were no device interrogation. Data collection ends
when the session data is cleared.
25
Page 26

15 Working with reports and saved device data
15.1 Configure the report preferences
15.1.1 Configure the Initial Interrogation Report preferences
Enable the Initial Interrogation Report, then select the reports that you want to include in the Initial Interrogation Report:
1. Tap
2. Complete the following actions:
To generate an Initial Interrogation Report for a patient session that is in progress, end and restart the patient session.
15.1.2 Configure the Final Report preferences
Select the reports that you want to include in the Final Report:
1. Tap > SESSION > PREFERENCES > FINAL REPORT.
2. Complete the following actions:
15.2 Generate reports
15.2.1 Generate a report using the PDF button
A PDF button appears on many windows and screens throughout the implantable device app. To generate a report from one of these
screens or windows, tap the PDF button.
> SESSION > PREFERENCES > INITIAL REPORT.
a. Select the Automatically generate initial interrogation report after first interrogation checkbox.
b. Select the reports to include in the Initial Interrogation Report.
Note: The Quick Look Report is always included in the Initial Interrogation Report.
c. Tap OK.
a. Select the reports to include in the Final Report. If you are configuring Final Report preferences for the first time, select All
Settings in the PARAMETERS section.
Note: The Session Summary Report is always included in the Final Report.
b. Tap OK.
15.2.2 Generate a final report
To view summaries of selected data at the end of a session, generate the Final Report.
> SESSION > FINAL REPORT.
Tap
15.2.3 Generate a set of reports
To generate available reports, select a set of reports:
1. Tap > SESSION > AVAILABLE REPORTS.
2. Select the reports that you want to generate, then tap GENERATE REPORTS.
15.3 View or export saved reports and implantable device data
When you generate a report or save implantable device data during a session, the report or data is saved to the SAVED REPORTS
window. From the SAVED REPORTS window, you can view or export the reports and data. The export options depend on the email,
network, and printing apps or connections set up on your tablet.
1. Tap
2. On the SAVED REPORTS window, view reports or export reports and device data:
Note: You are responsible for the management of patient and device data that you export from the SAVED REPORTS window.
Examples of patient and device data include printed paper reports, data transferred to a hospital network, and emailed attachments.
> SAVED REPORTS / DATA.
• To view a report, tap VIEW next to the report.
• To export reports, select the reports, tap SEND TO…, then select the export option or location.
• To export device data and the reports associated with the device data, select the PKG file, tap SEND TO…, then select the
export option or location.
Notes:
• To select all files, tap the checkbox at the top of the list.
• When you select multiple reports to export, the reports export as a single PDF file.
26
Page 27

Page 28

Medtronic, Inc.
*M002751C001*
710 Medtronic Parkway
Minneapolis, MN 55432
USA
www.medtronic.com
+1 763 514 4000
Medtronic USA, Inc.
Toll-free in the USA (24-hour technical consultation for physicians and
medical professionals)
Bradycardia: +1 800 505 4636
Tachycardia: +1 800 723 4636
Technical manuals
www.medtronic.com/manuals
© 2021 Medtronic
M002751C001 A
2021-04-19
 Loading...
Loading...