Page 1

CGMS® iProTM Continuous Glucose Recorder
User Guide
Page 2

©2007, Medtronic MiniMed, Inc. All rights reserved.
CGMS®, Paradigm®, Paradigm Link® and Sen-serter® are registered trademarks of Medtronic MiniMed, Inc.
iPro™ is a trademark of Medtronic MiniMed, Inc.
Cavicide® is a registered trademark of Metrex.
Detachol® is a registered trademark of Ferndale Laboratories, Inc.
®
UltraLink™ is a trademark and OneTouch
FreeStyle Flash
®
is a registered trademark of Abbott Laboratories.
and Ultra® are registered trademarks of LifeScan, Inc.
This product is covered by at least the following U.S. Patent Nos: 5,586,533; 5,954,643; 6,248,067; 6,368,141; 6,418,332;
6,809,653. U.S., International and/or foreign patents are pending.
6025286-0U1 061608
REF MMT 7708
Page 3

Contents
Using the system . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
CGMS iPro Continuous Glucose Recorder components . . . . . . . . . . . . . 3
Optional components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Indications for use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
CGMS iPro Magnetic Wand interference . . . . . . . . . . . . . . . . . . . . 6
CGMS iPro Magnetic Wand interference with insulin pumps . . . . . . . 6
CGMS iPro Magnetic Wand interference with PC hard drives . . . . . . . 6
Other electromagnetic interference . . . . . . . . . . . . . . . . . . . . . . 6
Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
FCC notice . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
RF interference from other wireless devices . . . . . . . . . . . . . . . . . 7
Assistance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
United States . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Other countries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Charger . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Installing a new charger battery . . . . . . . . . . . . . . . . . . . . . . . . . 9
Charging the digital recorder . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Hardware setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Supported meters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Serial switch box . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Using the CGMS iPro digital recorder and CGMS iPro Wand . . . . . . . . 13
Communicating with Solutions CGMS iPro . . . . . . . . . . . . . . . . . . . . 14
Setting up the digital recorder . . . . . . . . . . . . . . . . . . . . . . . . . 14
Connecting the glucose sensor . . . . . . . . . . . . . . . . . . . . . . . . . 14
Downloading data from the digital recorder . . . . . . . . . . . . . . . . 16
Calibrating Solutions CGMS iPro . . . . . . . . . . . . . . . . . . . . . . . . 16
Bathing and swimming . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
CGMS® iProTM Continuous Glucose Recorder User Guide 1
Page 4

Disconnecting from the glucose sensor . . . . . . . . . . . . . . . . . . . . 17
Tester . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Connecting the tester . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Disconnecting the tester . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
Cleaning the CGMS iPro Digital Recorder . . . . . . . . . . . . . . . . . . . . 20
Storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Guidance and manufacturer’s declaration . . . . . . . . . . . . . . . . . . . 22
Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Warranty, United States . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Warranty, Other countries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
Icon table . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Index . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
2 CGMS® iProTM Continuous Glucose Recorder User Guide
Page 5

Using the system
The CGMS® iProTM Continuous Glucose Recorder continuously collects and records
®
patient glucose data. This data is then downloaded to the Solutions
®
CGMS
iProTM Continuous Glucose Recorder where it can be used to create clinical
reports. Performance data for this device can be found in the Solutions Software user
guide.
Software for
Digital Recorder Tester Charger CGMS iPro Wand
CGMS iPro Continuous Glucose Recorder components
A complete CGMS iPro Continuous Glucose Recorder kit includes:
•CGMS iPro Digital Recorder (MMT-7709) • Charger (MMT-7705)
• Sen-serter® device (MMT-7500)
(not shown)
• Blue Tester (MMT-7706) • Occlusive dressing (not shown)
• AAA alkaline battery(ies), size E92,
type LR03 (not shown)
You will also need the following components:
•COMLINKTM CGMS iProTM (MMT-7304NPRO/MMT-7304WPRO)
•CGMS iPro Magnetic Wand
(MMT-7712)
CGMS® iProTM Continuous Glucose Recorder User Guide 3
Page 6

• Solutions® Software for CGMS® iProTM Continuous Glucose Recorder (MMT-7319)
Optional components
The following optional components allow you to connect up to four serial devices to
your PC at once. For example, you can connect a meter and a COMLINK iPro to your PC
at the same time.
• Manual serial data switch box (Part number: 1112058-003)
• Serial interface cable for the manual serial data switch box (MMT-7324)
Indications for use
The CGMS iPro Digital Recorder is intended to continuously record interstitial glucose
levels in persons with diabetes mellitus. This information is intended to supplement,
not replace, blood glucose information obtained using standard home glucosemonitoring devices. The information collected by the digital recorder may be
downloaded and displayed on a computer and reviewed by healthcare professionals.
This information may allow identification of patterns of glucose-level excursions above
or below the desired range, facilitating therapy adjustments which may minimize these
excursions.
The CGMS iPro Digital Recorder:
• Is intended for prescription use only.
• Will not allow readings to be made available directly to patients in real time.
• Provides readings that will be available for review by physicians after the recording
interval (72 hours).
• Is currently intended for occasional rather than everyday use.
• Is to be used only as a supplement to, and not a replacement for, standard invasive
measurement.
• Is not intended to change patient management based on the numbers generated, but
to guide future management of the patient based on response to trends noticed.
That is, these trends or patterns may be used to suggest when to take fingerstick
glucose measurements to better manage the patient.
The glucose sensor, tester, charger, and CGMS iPro Wand are intended for use with the
®
CGMS iPro Digital Recorder. The Sen-serter
device is indicated only for insertion of the
Medtronic MiniMed glucose sensor.
4 CGMS® iProTM Continuous Glucose Recorder User Guide
Page 7

Contraindications
Do not use magnetic mattress pads while wearing the CGMS iPro Digital Recorder. The
magnetic mattress pad will cause the digital recorder to frequently enter a
transmission mode that will quickly drain the rechargeable battery and will temporarily
disable data collection. Therefore, there will be sensor data gaps and the digital
recorder is likely to not function for the entire 3-day duration. The magnetic mattress
pad will not, however, affect the accuracy of the sensor readings that are collected,
nor will it lead to any unsafe conditions.
Do not expose your digital recorder to MRI equipment or other devices that generate
strong magnetic fields. If your digital recorder is inadvertently exposed to a strong
magnetic field, discontinue use and contact your physician.
Warnings
Product contains small parts and may pose a choking hazard for young children.
Sensor
The glucose sensor should be removed if redness, bleeding, pain, tenderness, irritation,
or inflammation develops at the insertion site, or if you experience unexplained fever.
An optional occlusive dressing should be removed if irritation or reaction to the tape
develops.
The glucose sensor may create special needs regarding your patients’ medical
conditions or medications. Healthcare professionals should discuss this with their
patients before they use the glucose sensor.
Wait five minutes after glucose sensor insertion before setting up the CGMS iPro Digital
Recorder with the Solutions CGMS iPro.
• Make sure that the site is not bleeding before connection. If any blood gets inside
the digital recorder connector, the digital recorder must be discarded.
• If bleeding occurs, apply steady pressure with a sterile gauze or clean cloth at the
insertion site until bleeding stops. After bleeding stops, attach the digital recorder
to the glucose sensor.
• If bleeding persists after three minutes, remove the glucose sensor and discard.
Insert a new glucose sensor in a different location.
Contact your local country representative or the 24 Hour HelpLine (US) if you
experience any adverse reactions associated with the digital recorder or glucose sensor.
CGMS® iProTM Continuous Glucose Recorder User Guide 5
Page 8

CGMS iPro Magnetic Wand interference
Do not store the CGMS iPro Magnetic Wand or any other magnet within 1.5 inches (3.8
cm) of the CGMS iPro Digital Recorder. Premature battery discharge may occur.
Do not place the CGMS iPro Magnetic Wand or any other magnet within 1.5 inches (3.8
cm) of the CGMS iPro Digital Recorder except while performing patient set-up and data
download procedures. Premature battery discharge may occur.
CGMS iPro Magnetic Wand interference with insulin pumps
Do not place the CGMS iPro Magnetic Wand directly on any insulin pump. Incorrect
pump operation may occur.
Do not insert the glucose sensor with attached CGMS iPro Digital Recorder closer than
two to three inches (5.08 - 7.62 cm) from the insulin pump. The use of the CGMS iPro
Magnetic Wand may inadvertently cause incorrect operation of the pump.
CGMS iPro Magnetic Wand interference with PC hard drives
Do not place the CGMS iPro Magnetic Wand on your PC or external hard drives, as the
magnetic field may impact the integrity of the data files stored there.
Other electromagnetic interference
The CGMS iPro Digital Recorder is designed to withstand common electromagnetic
interference, including airport security systems.
Precautions
If performing multiple CGMS iPro Digital Recorder studies on the same patient, establish
a rotation schedule for choosing new glucose sensor sites. Avoid sites that are
constrained by clothing, have scar tissue, or are subject to rigorous movement during
exercise.
If any blood gets inside the digital recorder connector, the digital recorder must be
discarded.
If you do not plan to use the digital recorder for at least 30 days, connect it to the
charger for storage. The charger will not keep the digital recorder charged, but it will
slow the digital recorder’s battery discharge. Remove the digital recorder from the
charger at least once every 60 days, wait at least one minute, then reconnect the
digital recorder to the charger. To use the digital recorder after it has been stored,
disconnect it from the charger, wait at least one minute, then reconnect it again to
start the charging process. It may take up to eight hours to fully charge a digital
recorder that has been stored for more than 30 days.
6 CGMS® iProTM Continuous Glucose Recorder User Guide
Page 9

FCC notice
This device complies with the United States Federal Communications Commission (FCC)
and international standards for electromagnetic compatibility.
This device complies with Part 15 Rules. Operation is subject to the following two
conditions: (1) this device may not cause harmful interference, and (2) this device must
accept any interference received, including interference that may cause undesirable
operation.
The CGMS iPro Digital Recorder does not interfere with any radio frequency signals
transmitted from outside sources. These FCC standards are designed to provide
reasonable protection against excessive radio frequency interference and prevent
undesirable operation of the device from unwanted electromagnetic interference.
Important: Changes or modifications not expressly approved by the party
responsible for compliance could void the user’s authority to operate the
equipment.
RF interference from other wireless devices
Common consumer wireless devices that transmit in the same 916.5MHz/868.35MHz
frequency band used by the MMT-7709 CGMS iPro Digital Recorder (DR) transmitter may
prevent the DR transmitter from downloading appropriately to Solutions CGMS iPro
through the MMT-7304NPRO/MMT-7304WPRO COMLINK iPro receiver. Most cellular
(mobile) phones and 900 MHz cordless phones, when transmitting or receiving, may
cause significant disruption to communication between the DR transmitter and the
COMLINK iPro receiver. It is likely that other wireless devices co-existing in similar
frequency ranges will have a similar effect. This interference, however, will not cause
any incorrect data to be sent and will not cause any harm to your digital recorder.
Communication problems between the iPro DR transmitter and COMLINK iPro receiver
can usually be resolved or corrected by:
• making sure that the transmitter and receiver are as close to each other as possible,
and no further apart than six feet (1.8 meters),
• turning off, or moving away from, other RF wireless transmitting devices,
• reorienting or relocating the DR transmitter and/or COMLINK iPro receiver.
Testing conducted with several different cellular phones suggests that interference will
not be a problem if the phone is at least 12 inches (31 cm) from the DR transmitter or
CGMS® iProTM Continuous Glucose Recorder User Guide 7
Page 10
COMLINK iPro receiver when used (greater separation distance may be required for
certain devices).
Assistance
United States
Medtronic provides a 24 Hour HelpLine for product assistance for U.S. users. The 24
Hour HelpLine is staffed with technicians who are trained in the setup and use of the
CGMS iPro Continuous Glucose Recorder and are able to answer your questions.
Department Telephone number
24 Hour HelpLine, if calling from within the
United States
24 Hour HelpLine if calling from outside the
United States
MiniMed Web site www.medtronicdiabetes.com
(800) 646-4633
818-576-5555
Other countries
For product assistance outside the U.S., please contact your local country
representative. Refer to the enclosed Medtronic Diabetes International Contact Card
for the Help Line in your area.
8 CGMS® iProTM Continuous Glucose Recorder User Guide
Page 11
Charger
WARNING: Always clean the digital recorder after removing it from the patient
and before attaching it to the charger. See the cleaning instructions on page 20.
The digital recorder contains a non-replaceable, rechargeable battery that you can
recharge as needed with the charger. The charger has a green light that shows the
charging status and a red light that indicates any problems during charging. If you see a
red light, see the Troubleshooting section on page 18. The charger needs one AAA
battery, size E92, type LR03, to operate. A new AAA battery contains enough power to
recharge the digital recorder more than 40 times.
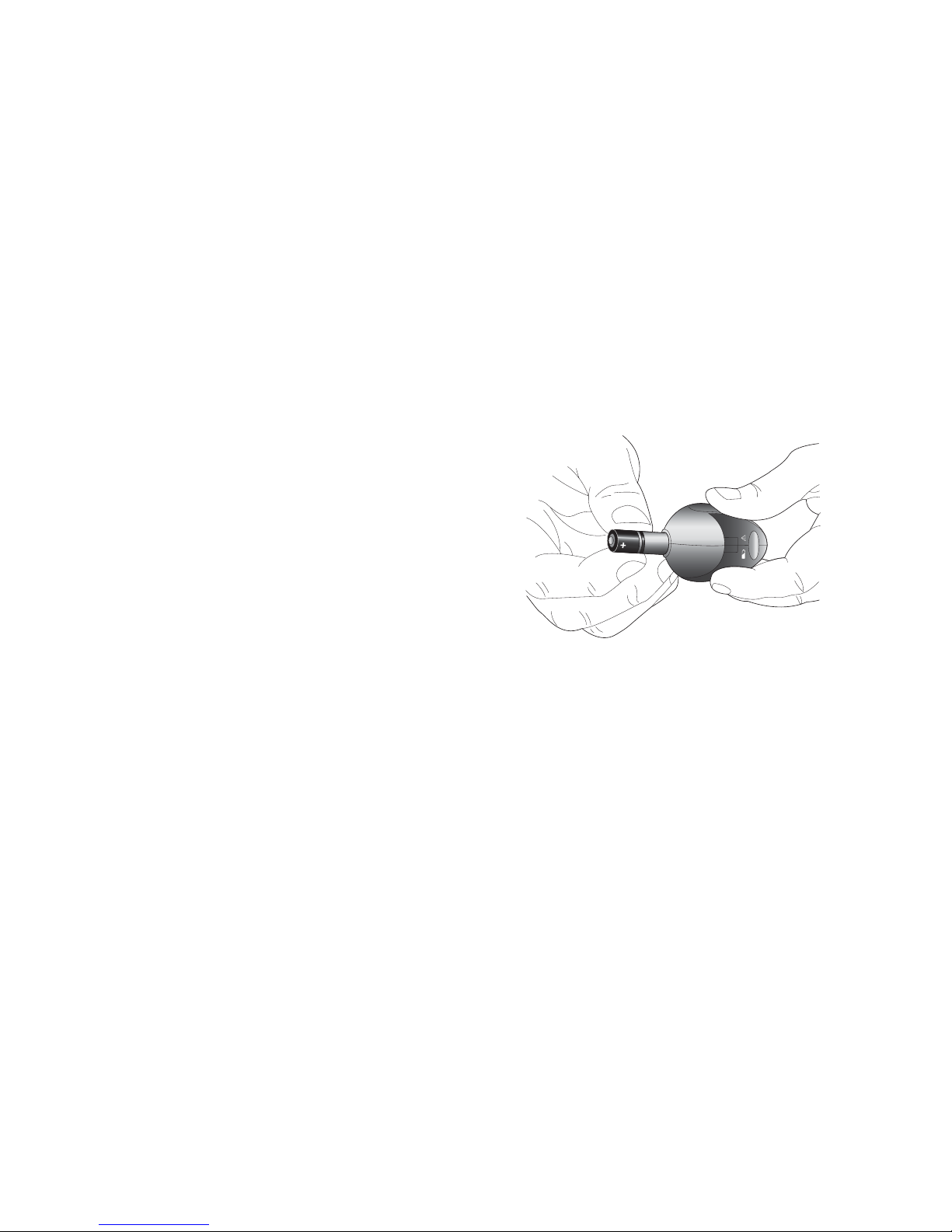
Installing a new charger battery
1. Remove the charger battery cap by turning it
counter-clockwise 1/4 turn using a coin in the
groove of the cap.
2. Insert a new AAA battery with the flat (-) end
first. Make sure that you align the small bumps
on the battery cap with the small notches in the
charger’s battery opening. Push in the cap all
the way using a coin. Turn the cap clockwise 1/4
turn to close.
3. If the battery is installed incorrectly or is low, the charger will not work. Repeat the
steps above using a new battery.
Charging the digital recorder
Before using the digital recorder for the first time, you must fully charge the digital
recorder battery which may take up to eight hours. It is recommended to recharge the
digital recorder after each glucose sensor use. If you choose to recharge the digital
recorder after a 3-day glucose sensor use, the charging time will be less than 20
minutes.
CGMS® iProTM Continuous Glucose Recorder User Guide 9
Page 12

1. If a green light on the digital recorder is lit or flashing, do not connect it to the charger.
The digital recorder will not charge with its green light on. Wait for the green light to
turn off (approximately 30 seconds), then connect the digital recorder to the charger.
2. Connect the digital recorder to the
charger by lining it up, flat side down,
with the charger. Push the two
components together fully. Always
allow at least one minute before
disconnecting the digital recorder
from the charger or the digital
recorder may not work properly. If you
disconnect the digital recorder before
one minute, reconnect it to the
Green light
charger for at least one minute.
3. Within 10 seconds after the digital
recorder is connected, a green light on
the charger will flash for 1-2 seconds as
the charger powers on. For the rest of
the charging time, the charger’s green
light will flash in a continuing pattern of
four flashes, pause, four flashes, pause.
4. When charging is complete, the green
light on the charger will stay on, without
Green light
flashing, for 15-20 seconds and then turn off.
5. After the green charger light turns off,
disconnect the digital recorder from the
charger. The green light on the digital recorder
will flash for about five seconds, then turn off.
6. If the green light on the digital recorder does not
flash, reconnect it to the charger for at least one
minute.
7. After removing the digital recorder from the charger, wait at least one minute before
connecting it to a sensor or tester.
10 CGMS® iProTM Continuous Glucose Recorder User Guide
Page 13

Hardware setup
The following diagram provides an example of how you will connect the components
used with the CGMS iPro Digital Recorder. For information on setting up Solutions CGMS
iPro, see the Solutions Software for CGMS iPro Continuous Glucose Recorder User Guide.
Make sure that your ComLink label says COMLINK CGMS iPro. A standard COMLINK will
not work with the digital recorder.
PC with
Solutions
CGMS iPro
Serial switch
box (optional)
COMLINK
Serial
interface
cable
(optional)
CGMS iPro
Paradigm
Link meter
(with USB
cable)
OneTouch®
®
Ultra
(with serial cable)
The example above shows the Paradigm Link
USB cable. This meter can also be connected with a serial cable.
CGMS® iProTM Continuous Glucose Recorder User Guide 11
Freestyle
Flash
OneTouch®
Ultra® 2
®
meter connecting to the PC with a
OneTouch®
UltraLink™
CGMS iPro
Digital
Recorder
CGMS
iPro Wand
Page 14

Supported meters
The following meters are supported:
•Medtronic Paradigm Link
• LifeScan OneTouch® Ultra
• Abbott Freestyle Flash
®
• LifeScan OneTouch® UltraLink™
®
• LifeScan OneTouch® Ultra® 2
You can use a USB cable with the Paradigm Link meter only. You must use a serial cable
to connect all other meters (even if the meter comes with an optional USB cable).
Serial switch box
The optional four-port serial switch box allows you to connect up to four serial devices
to your PC.
• Connect the device’s serial cable to the A,
B, C or D port on the back of the serial
switch box. The example shows two device
serial cables connected.
• Connect one end of the serial switch box
cable to the I/O port (in the center).
Connect the other end to the serial port on
your PC.
Rear view
• To communicate with the PC, turn the
switch box dial to the letter for the serial
port (A, B, C or D) you want to use. For
example, if a meter is connected to serial
Port A and the COMLINK iPro to Port D, you
can switch between A and D accordingly.
12 CGMS® iProTM Continuous Glucose Recorder User Guide
Front view
Page 15

Using the CGMS iPro digital recorder and CGMS iPro Wand
The CGMS iPro Digital Recorder contains a built-in two-way wireless radio that
communicates with the COMLINK iPro during patient setup and data download. The
radio operates as both a receiver and a transmitter.
The radio receiver is turned on by the iPro Wand that comes with your digital recorder.
The silver end of the iPro Wand is a powerful magnet so it is important to keep it away
from computers, credit cards, insulin pumps, glucose monitors and all other electronic
media that can be affected by magnetic fields.
It is also important to use the iPro Wand correctly in order
to turn on the digital recorder to set up a new patient or
download data from the digital recorder. Only use the
supplied CGMS iPro Wand to swipe the digital recorder. Do
not use any other magnet. When prompted by the
Solutions software, follow the wand swiping technique
described below to properly activate the CGMS iPro radio
receiver:
blue tester
a
1. Place the silver, magnetic tip of the iPro wand flat
b
against the surface of the iPro digital recorder at point
(a) or point (b). You may swipe in either direction.
2. Move the wand slowly along the edge of the digital
recorder from point to point. Take at least five seconds
to complete the swiping process. You may swipe more
than once, and change direction while swiping.
3. Always refer to the Solutions Software for CGMS iPro Continuous Glucose Recorder
User Guide for complete instructions.
CGMS® iProTM Continuous Glucose Recorder User Guide 13
Page 16

Communicating with Solutions CGMS iPro
You u se S ol u ti o ns C GM S iPro to set up a new patient and to download data from the
digital recorder. You should always refer the Solutions Software for CGMS iPro
Continuous Glucose Recorder User Guide for complete instructions. The digital recorder
and Solutions CGMS iPro must communicate during initialization and data transfer. This
section provides an overview of how the components are used for each process.
CAUTION:This procedure describes how to connect the glucose sensor to the digital
recorder. It is very important that you follow the sequence provided by Solutions
CGMS iPro when setting up the device. Solutions CGMS iPro will indicate when to
connect the devices. See your Solutions Software for CGMS iPro Continuous Glucose
Recorder User Guide for more details.
Setting up the digital recorder
1. Enter the digital recorder serial number into the
Solutions software. The serial number is on the flat
side of the digital recorder, as shown on the right.
Enter only the seven numbers, not the letters, when
setting up a new patient.
Connecting the glucose sensor
CAUTION: Do not connect the digital recorder to the
glucose sensor until you are prompted to do so by
Solutions CGMS iPro.
1. It is recommended that the digital recorder be fully charged before connecting it to the
glucose sensor. If it is not fully charged, it may not flash when connected to the sensor
and may not operate the sensor for the entire three days.
2. First insert the glucose sensor (follow the instructions provided with the glucose
sensor). Check for bleeding. Make sure that bleeding has stopped (see Warnings on
page 5 for details). Do not connect the digital recorder at this time.
3. The glucose sensor must be inserted in the patient’s body for at least five minutes
before connecting the digital recorder.
4. When prompted by the Solutions software, connect the digital recorder to a glucose
sensor already inserted into the body. The digital recorder will not work if connected to
a dry sensor (not inserted).
14 CGMS® iProTM Continuous Glucose Recorder User Guide
Page 17

5. When connecting the digital recorder to the glucose sensor, touch the inserted glucose
sensor at the back of the assembly to prevent movement.
6. Hold the digital recorder as shown. Line up the
two notches on both sides with the flexible side
arms of the glucose sensor. The digital
recorder flat side with the label should face
the skin.
7. Slide the digital recorder onto the glucose
sensor. Push it in firmly until the flexible side
Sensor
arms of the glucose sensor snap into the
notches on both sides of the digital recorder. In
the next 20 seconds the digital recorder will
flash green for about 10 seconds. This indicates
that the digital recorder is properly connected to a sensor and that it has enough
battery power to work for at least three days.
8. If the digital recorder light does not flash, disconnect it from the glucose sensor. Wait
for at least one minute and then reconnect. If the digital recorder light still does not
flash, charge the digital recorder.
9. After the digital recorder light flashes green, you
will be prompted by Solutions iPro to swipe the wand
across the digital recorder. Please refer to Using the
CGMS iPro Recorder and iPro Wand, on page 13, for
correct swiping technique.
10. Solutions CGMS iPro will tell you if you have
successfully started communication or if you need to
repeat the start process.
11. After the digital recorder is successfully initialized
by Solutions CGMS iPro, the Solutions software will
prompt you to apply the recommended occlusive dressing over the digital recorder and
the glucose sensor.
CGMS® iProTM Continuous Glucose Recorder User Guide 15
Page 18

Downloading data from the digital recorder
Follow the Solutions software instructions to download the patient’s data from the
digital recorder.
1. Setup the COMLINK iPro. Make sure that the COMLINK iPro is connected to your PC or
the optional serial switch box.
2. If you are using the serial switch box, make sure that you select
the communications port that corresponds to the COMLINK iPro.
If the digital recorder has been disconnected from the sensor,
then connect it to the blue tester. Whichever way you choose to
download, make sure that the digital recorder is as close as
possible to the COMLINK iPro to ensure a good RF connection.
Click NEXT to continue.
3. When the software prompts you, swipe the digital recorder with
the iPro Wand to start communication and begin the download.
4. After a successful data download, clean and dry the digital recorder, remove the tester,
and connect the digital recorder to the charger.
Calibrating Solutions CGMS iPro
The Solutions software must be calibrated with fingerstick measurements to maximize
sensor reading accuracy. The Solutions software requires at least four fingerstick
measurements per 24-hour period for each of the three-day study interval (a minimum
total of 12 fingerstick measurements).
Be sure patients know to take fingerstick glucose measurements when their blood
glucose is stable - such as before meals and at bedtime, and to avoid times such as right
after eating, exercising or insulin delivery.
The Solutions software only accepts BG meter values between 2.2-22 mmol/L (40 and
400 mg/dL).
If patients use one of the Solutions-compatible BG meters listed in this user guide, the
BG measurements will automatically download from the meter into the Solutions
software during the download process.
Patients who use a non-compatible BG meter must log each BG meter value in the BG
Meter and/or in their Patient Log sheet as soon as they take a reading. This allows the
16 CGMS® iProTM Continuous Glucose Recorder User Guide
Page 19

data to be manually entered into the Solutions software when the patient goes back to
the doctor’s office at the end of the three-day study.
Bathing and swimming
After the digital recorder and the glucose sensor are connected, they form a watertight seal to a depth of eight feet (2.4 meters) for up to 30 minutes. You can shower,
bathe or swim without removing them. However, an occlusive dressing is recommended
in order to reduce the risk of the sensor being pulled from the body.
Disconnecting from the glucose sensor
1. Hold the digital recorder as shown, and pinch the
flexible side arms of the glucose sensor between your
thumb and forefinger.
2. Gently pull the digital recorder away from the
glucose sensor assembly.
Tester
The blue tester is used to test the digital recorder to make sure it is working. It is also
used in place of an inserted sensor when the digital recorder is communicating with
Solutions CGMS iPro (primarily during the data download process).
Connecting the tester
1. Make sure the tester is clean before you use it.
2. Hold the digital recorder and the tester as shown. Line
up the flat side of the tester with the flat side of the
digital recorder.
3. Push the tester into the digital recorder. The flexible
side arms of the tester should click into the notches on
both sides of the digital recorder.
4. Within 20 seconds the green light on the digital recorder
will flash for about 10 seconds when properly connected.
5. Check that the digital recorder is communicating with Solutions CGMS iPro. See your
Solutions Software for CGMS iPro Continuous Glucose Recorder User Guide for more
details.
CGMS® iProTM Continuous Glucose Recorder User Guide 17
Page 20

Disconnecting the tester
1. Hold the digital recorder body as shown and pinch
the side arms of the tester.
2. With the tester arms pinched, gently pull the
digital recorder away from the tester.
3. To save the digital recorder’s battery life,
disconnect the tester when you are finished
communicating with Solutions CGMS iPro.
Troubleshooting
Question: Why did the flashing green charger light turn off and a longer flashing red
charger light turn on during charging?
Digital recorder low battery
about 2 secs about 2 secs about 2 secs
Answer: The digital recorder battery is very low. Leave the digital recorder on the
charger for eight hours to completely recharge. If the red light is still flashing after
eight hours, call the 24 Hour HelpLine or your local representative. It may be time to
replace your digital recorder.
Question: Why do I see slow flashing red lights on the charger?
Charger low battery
about 2 secs about 2 secs
Answer: Your charger battery is low. Make sure the digital recorder is not connected to
the charger and replace the charger battery with a new AAA battery.
18 CGMS® iProTM Continuous Glucose Recorder User Guide
Page 21

Question: Why do I see a mix of quick and long flashing red lights on the charger?
Charger and digital recorder low battery
about
1 sec
about
1 sec
about 2 secs
about
1 sec
about
1 sec
Answer: You r ch a rg e r and digital recorder batteries are very low. Replace the charger’s
AAA battery. If you now get the pattern for very low digital recorder battery, leave the
digital recorder on the charger for eight hours to recharge. If the red light is still
flashing after eight hours, call the 24 Hour HelpLine or your local representative. It may
be time to replace your digital recorder.
Question: I had my digital recorder on the charger for a day. Will this damage my
digital recorder?
Answer: It will not damage the digital recorder. You cannot overcharge it.
Question: What should I do if the digital recorder green light did not flash after
removing it from the charger?
Answer: Reconnect the digital recorder to the charger for at least one minute.
Remove it and watch the digital recorder green light flash and then turn off.
Question: What should I do if the digital recorder green light does not flash when
connected to the glucose sensor?
Answer: Is the glucose sensor inserted in the body? If so, has it been inserted for at
least five minutes?
•If it is not inserted, the digital recorder will not flash green or record glucose data.
•If it has been inserted for less than five minutes, the digital recorder may not
flash green or record glucose data. In this case, you need to disconnect the digital
recorder from the glucose sensor. Wait five minutes and then reconnect. If the green
light still does not flash, charge the digital recorder.
•If it has been inserted for more than five minutes, you need to disconnect the
digital recorder from the glucose sensor. Wait for at least one minute and then
reconnect. If the green light still does not flash, charge the digital recorder.
CGMS® iProTM Continuous Glucose Recorder User Guide 19
Page 22

Question: Why didn’t I see the digital recorder green light flash after connecting it to
the blue tester?
Answer: Check the connection. If you still do not see a green light flash, fully recharge
the digital recorder battery. Test the digital recorder with the tester, as described on
page 17 (make sure the tester is clean before using). If you still do not see a green light
flash, clean the tester connector. If you still do not see a green light flash, call the 24
Hour HelpLine or your local representative. It may be time to replace your digital
recorder.
Cleaning the CGMS iPro Digital Recorder
The CGMS iPro Digital Recorder is intended for multiple patient use. Follow this
procedure to clean the digital recorder after each patient use.
CAUTIONS:
• The charger and the tester are NOT watertight. Do NOT immerse in water.
• Do NOT discard the digital recorder in a medical waste container or otherwise
subject it to incineration. The digital recorder contains a battery which may explode
upon incineration.
1. Attach the tester to the digital recorder to make sure that NEITHER water NOR
disinfectant NOR alcohol gets inside the digital recorder’s connector.
2. Dampen a clean cloth with a mild liquid soap solution. Wipe the outside of the digital
recorder.
3. Rinse the digital recorder under warm tap water but do NOT get water inside the
connector. If you get water inside the connector, shake the water out and allow it to air
dry.
4. Apply three to four drops of a quaternary ammonium compound disinfectant (for
example, Cavicide®) on a clean, dry cloth and wipe the digital recorder.
5. Hold the digital recorder and rinse with 70% isopropyl alcohol.
6. If there is adhesive residue on the digital recorder, you can remove it with Detachol
®
adhesive remover between each patient use.
7. Disconnect the tester from the digital recorder.
8. Place the digital recorder on a clean, dry, non-shedding cloth and air dry.
20 CGMS® iProTM Continuous Glucose Recorder User Guide
Page 23

9. After it is completely dry, connect it
Cleaning label
to a charger and place it in a sealed
bag labeled with the cleaning date
(see label example to the right).
CAUTION: If there is any blood inside
the connector, the digital recorder
Device:
Date:
Method of decontamination:
Wash:
Disinfectant:
must be discarded. Dispose of the
digital recorder according to the local
regulations for battery disposal (nonincineration).
Storage
If you do not plan to use the digital recorder for at least 30 days, connect it to the
charger for storage. The charger will not keep the digital recorder charged, but it will
slow the digital recorder’s battery discharge. Remove the digital recorder from the
charger at least once every 60 days, wait at least one minute, then reconnect the
digital recorder to the charger. To use the digital recorder after it has been stored,
disconnect it from the charger, wait at least one minute, then reconnect it again to
start the charging process. It may take up to eight hours to fully charge a digital
recorder that has been stored for more than 30 days.
CGMS® iProTM Continuous Glucose Recorder User Guide 21
Page 24

Guidance and manufacturer’s declaration
Guidance and Manufacturers Declaration – Electromagnetic Emissions
The CGMS iPro Digital Recorder (MMT-7709) is intended for use in the electromagnetic environment
specified below. The customer or the user of the CGMS iPro Digital Recorder should assure that it is
used in such an environment.
Emissions Test Compliance
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/flicker emissions
IEC 61000-3-3
Electromagnetic Environment
Guidance
Group 1 The CGMS iPro Digital Recorder uses RF
energy only for system communication
functions. Therefore, its RF emissions
are very low and are not likely to cause
any interference in nearby electronic
equipment.
Class Bv The CGMS iPro Digital Recorder is
suitable for use in all establishments
other than domestic and those directly
Not applicable
Not applicable
connected to the public low-voltage
power supply network that supplies
buildings used for domestic purposes.
NOTE: The preceding statement is
required by IEC 60601-1-2 for Group
1, Class B devices. However, since
the CGMS iPro Digital Recorder is
battery powered, its emissions will
not be affected by the establishment
power supply and there is no
evidence of any issues associated
with the use of the system in
domestic establishments.
22 CGMS® iProTM Continuous Glucose Recorder User Guide
Page 25

Guidance and Manufacturers Declaration – Electromagnetic Immunity
The CGMS iPro Digital Recorder (MMT-7709) is intended for use in the electromagnetic environment
specified below. The customer or the user of the CGMS iPro Digital Recorder should assure that it is
used in such an environment.
Immunity Test
Electrostatic
discharge (ESD)
IEC 61000-4-2 ±8 kV air ±30 kV air
Electrical fast
transient/burst
IEC 61000-4-4 ±1 kV for input/
Surge ±1 kV line(s) to
IEC 61000-4-5 ±2 kV line(s) to
Voltage dips, short
interruptions and
voltage variations on
power supply lines
IEC 61000-4-11
IEC 60601 Test
Level
±6 kV contact Not applicable The CGMS iPro Digital Recorder should
±2 kV for power
supply lines
output lines
line(s)
earth
<5% U
T
(>95% dip in UT)
for 0.5 cycle
40% U
T
(60% dip in UT)
for 5 cycles
Compliance
Level
(<5% relative
humidity)
Not applicable
Not applicable
Not applicable
Not applicable
Not applicable
Not applicable
Electromagnetic Environment
Guidance
not be affected by electrostatic
discharge that might occur under normal
conditions of use.
Power frequency
(50/60 Hz) magnetic
field
IEC 61000-4-8
NOTE:
U
is the a.c. mains voltage prior to application of the test level.
T
70% U
T
(30% dip in UT)
for 25 cycle
<5% U
T
(>95% dip in UT)
for 5 seconds
3 A/m 3 A/m Power frequency magnetic fields should
Not applicable
Not applicable
be at levels characteristic of a typical
location in a typical commercial or
hospital environment.
CGMS® iProTM Continuous Glucose Recorder User Guide 23
Page 26

Guidance and Manufacturers Declaration – Electromagnetic Immunity
The CGMS iPro Digital Recorder (MMT-7709) is intended for use in the electromagnetic environment
specified below. The customer or user of the CGMS iPro Digital Recorder should assure that it is used in
such an environment.
Immunity
Test
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
IEC 60601
Level
3Vrms
150kHz to
80MHz
3 V/m
80MHz to 6GHz
Compliance
Level
Not
applicable
3 V/m
Electromagnetic Environment Guidance
Portable and mobile RF communications equipment
should be used no closer to any part of the CGMS iPro
Digital Recorder, including cables, than the
recommended separation distance calculated from
the equation applicable to the frequency of the
transmitter.
Recommended separation distance:
d 1.2 P=
d 2.3 P=
Where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in meters (m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey
should be less than the compliance level in each
frequency range.
Interference may occur in the vicinity of equipment
marked with the following symbol:
80 MHz to 800 MHz
800 MHz to 6 GHz
a
,
L
NOTE:
NOTE: These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption, and reflection from structures, objects and people.
a. Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones
and land mobile radios, amateur radio, AM and FM radio broadcasts and TV broadcast cannot be
predicted theoretically with accuracy. To access the electromagnetic environment due to fixed RF
transmitters, an electromagnetic site survey should be considered. If the measured field strength in the
location in which the CGMS iPro Digital Recorder is used exceeds the applicable RF compliance level
above, the CGMS iPro Digital Recorder should be observed to verify normal operation. If abnormal
performance is observed, additional measures may be necessary, such as re-orienting or relocating the
CGMS iPro Digital Recorder.
24 CGMS® iProTM Continuous Glucose Recorder User Guide
At 80 MHz and 800 MHz, the higher frequency range applies.
Page 27

Recommended separation distances between portable and mobile RF communications
equipment and the CGMS iPro Digital Recorder (MMT-7709)
This section provides information on the recommended separation distance between portable and
mobile RF communications equipment and the CGMS iPro Digital Recorder. The CGMS iPro Digital
Recorder is intended for use in an electromagnetic environment in which radiated RF disturbances are
controlled. The customer or users of the CGMS iPro Digital Recorder can help prevent electromagnetic
interference by maintaining a minimum distance between portable and mobile RF communications
equipment (transmitters) and the CGMS iPro Digital Recorder as recommended below, according to
the maximum output power of the communications equipment.
Separation distance according to the frequency of transmitter (m)
Rated maximum output
power of transmitter (W)
0.01 0.12 0.23
0.1 0.38 0.74
11.2 2.3
10 3.8 7.4
100 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation
distance d in meters (m) can be estimated using the equation applicable to the frequency of the
transmitter, where p is the maximum output power rating of the transmitter in watts (W) according to
the transmitter manufacturer.
NOTE: At 80 MHz and 800 MHz, the separation distance for the higher frequency range
applies.
NOTE: These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects and people.
80MHz to 800MHz
d 1.2 P=
800MHz to 6GHz
d 2.3 P=
CGMS® iProTM Continuous Glucose Recorder User Guide 25
Page 28

Specifications
Biocompatibility
Operating
Conditions
Storage
Conditions
Battery Life
Digital Recorder
Frequency
Digital Recorder
Dimensions and
Weight
Digital Recorder: Complies with ISO 10993-1 for body contact
Digital Recorder Temperature: +32° to +122°F (0° to 50°C)
Digital Recorder Relative Humidity: 10% to 95% with no condensation
Charger Temperature: +50° to +104°F (10° to 40°C)
Charger Relative Humidity: 30% to 75% with no condensation
Digital Recorder Temperature: -4° to +131°F (-20° to +55°C)
Digital Recorder Relative Humidity: 10% to 100% with condensation
Charger Temperature: +14° to +122°F (-10° to +50°C)
Charger Relative Humidity: 10% to 95% with no condensation
Digital Recorder: 14 days of continuous glucose monitoring
immediately following a full charge
Charger: Completes 40 typical charging operations using a new AAA
battery
MMT-7709NA - 916.5 MHz
MMT-7709WW - 868.35MHz
Width: 1.40 inches (3.56 centimeters)
Length: 1.12 inches (2.84 centimeters)
Height: 0.37 inches (0.94 centimeters)
Weight: 0.2 ounces (5.67 grams)
ComLink iPro
Frequency
26 CGMS® iProTM Continuous Glucose Recorder User Guide
MMT-7304NPRO - 916.5 MHz
MMT-7304WPRO - 868.35 MHz
Page 29

Warranty, United States
Medtronic MiniMed warrants the Medtronic CGMS iPro Digital Recorder and charger to
the purchaser of the product against defects in material and workmanship for a period
of six months from the date of purchase.
During the warranty period, Medtronic MiniMed will repair or replace, at its discretion,
any defective CGMS iPro Digital Recorder or charger, subject to the conditions and
exclusions stated herein. This warranty applies only to new devices. In the event a
CGMS iPro Digital Recorder or charger is repaired or replaced, the warranty period will
not be extended past its original expiration date.
This warranty is valid only if the Medtronic CGMS iPro Digital Recorder or charger is
used in accordance with the manufacturer's instructions. Without limitation, this
warranty will not apply:
• If damage results from changes or modifications made to the CGMS iPro Digital
Recorder or charger by the user, or third parties, after the date of sale;
• If service or repairs are performed by any person or entity other than the
manufacturer;
•If damage results from a Force Majeure or other event beyond the control of the
manufacturer;
• If damage results from negligence or improper use, including but not limited to:
improper storage, submersion in fluid, physical abuse (such as dropping); or
• If blood or water has entered the inside of the CGMS iPro Digital Recorder connector.
This warranty shall be personal to the original user. Any sale, rental or other transfer or
use of the product covered by this warranty to or by a user other than the original user
shall cause this warranty to immediately terminate. This warranty does not apply to
glucose sensors and other accessories.
The remedies provided for in this warranty are the exclusive remedies available for any
defects in material or workmanship in the product. Neither Medtronic MiniMed nor its
suppliers or distributors shall be liable for any incidental, consequential, punitive or
special damages of any nature or kind caused by or arising out of a defect in the
product.
All other warranties, expressed or implied, are excluded and specifically disclaimed,
including, but not limited to, any warranty of merchantability or fitness for a particular
purpose.
CGMS® iProTM Continuous Glucose Recorder User Guide 27
Page 30

Warranty, Other countries
Medtronic MiniMed warrants the Medtronic CGMS iPro Digital Recorder and charger to
the purchaser of the product against defects in material and workmanship for a period
of six months from the date of purchase.
During the warranty period, Medtronic MiniMed will repair or replace, at its discretion,
any defective CGMS iPro Digital Recorder or charger, subject to the conditions and
exclusions stated herein. This warranty applies only to new devices. In the event a
CGMS iPro Digital Recorder or charger is repaired or replaced, the warranty period will
not be extended past its original expiration date.
This warranty is valid only if the Medtronic CGMS iPro Digital Recorder or charger is
used in accordance with the manufacturer's instructions. Without limitation, this
warranty will not apply:
• If damage results from changes or modifications made to the CGMS iPro Digital
Recorder or charger by the user, or third parties, after the date of sale;
• If service or repairs are performed by any person or entity other than the
manufacturer;
•If damage results from a Force Majeure or other event beyond the control of the
manufacturer;
• If damage results from negligence or improper use, including but not limited to:
improper storage, submersion in fluid, physical abuse (such as dropping); or
• If blood or water has entered the inside of the CGMS iPro Digital Recorder connector.
This warranty shall be personal to the original user. Any sale, rental or other transfer or
use of the product covered by this warranty to or by a user other than the original user
shall cause this warranty to immediately terminate. This warranty does not apply to
glucose sensors and other accessories.
The remedies provided for in this warranty are the exclusive remedies available for any
defects in material or workmanship in the product. Any statutory rights granted to
consumers under any applicable legislation are reserved. Neither Medtronic MiniMed
nor its suppliers or distributors shall be liable for any incidental, consequential,
punitive or special damages of any nature or kind caused by or arising out of a defect in
the product.
All other warranties, except any applicable mandatory statutory warranties, expressed
or implied, are excluded and specifically disclaimed, including, but not limited to, any
warranty of merchantability or fitness for a particular purpose.
28 CGMS® iProTM Continuous Glucose Recorder User Guide
Page 31

Icon table
Serial Number:
Catalogue Number: REF
One per container/package:
Date of Manufacture:
Manufacturer:
Fragile Product:
Attention: See Instructions for use:
Temperature Range:
SN
(1X)
Radio communication:
Configuration:
Type BF Device (Protection from electrical shock):
CGMS iPro Digital Recorder: Protected against the effects of continuous
immersion in water (eight feet or 2.4 meters immersion for 30 minutes):
Contains magnet:
Signifies compliance with Industry Canada EMC and Radio communications
requirements:
CGMS® iProTM Continuous Glucose Recorder User Guide 29
IPX8
Page 32

CE mark by notified body as a medical device:
CE mark as a radio transmitter under the R&TTE1999/5/EC/directives.
Applies to 868.35 MHz (MMT-7709WW)
Signifies compliance to Australian EMC and Radio communications
requirements
0459
0976
30 CGMS® iProTM Continuous Glucose Recorder User Guide
Page 33

Index
Numerics
24 Hour HelpLine 8
A
AAA battery 9
addition components required 3
assistance 8
B
bathing 17
battery life 26
biocompatibility 26
box contents 3
box symbols 29
C
cables 11
Charger 9
battery type 9
green light 10
install battery 9
red lights 18
when to charge 9
charging the recorder 9
charging times 9
cleaning 21
communication setup 16
connecting devices
Connecting the glucose sensor
contact numbers 8
contraindications
11
14
5
initiating communication
specifications 26
storing
swimming with
water 20
digital recorder
charging 10
disconnect from glucose sensor 17
green light 10
magnetic wand 16
disconnect glucose sensor 17
21
17
16
E
electromagnetic emissions 22
electromagnetic environment 22, 25
electromagnetic immunity 23
emissions 22
emissions tests 22
F
FCC notice 7
frequency 26
G
Glucose Sensor
disconnecting 17
warnings 5
H
hardware connection 11
hardware requirements
help line 8
3
D
Digital Recorder
bathing with
cleaning
17
20
CGMS® iProTM Continuous Glucose Recorder User Guide 31
I
icons 29
indications for use 4
Page 34

initiating communication 16
install charger battery 9
interference 7
K
kit contents 3
M
magnetic wand 16
O
operating conditions 26
overview 3
P
precautions 6
problems 18
product overview 3
R
radio frequency
communications 25
emissions 22
interference 7
red lights on charger 18
required components 3
U
using in water 17
W
wand 16
warnings 5
water
17, 20
S
sensor warnings 5
Setting up the digital recorder 14
software requirements 3
specifications 26
storage 21, 26
support calls 8
swimming 17
T
Tester 17
connecting
disconnecting
troubleshooting
32 CGMS® iProTM Continuous Glucose Recorder User Guide
17
18
18
Page 35

Page 36

 Loading...
Loading...