Page 1
Part Number M708348B092 Rev C 2017-01
KYPHON® ActivOs™ 10
Bone Cement with Hydroxyapatite
Instructions for use
Distributed by:
Medtronic Sofamor Danek USA, Inc.
1800 Pyramid Place
Menphis, TN 38132
Tel: 800 933 2635 ( In USA)
Tel: 901 396 3133 ( Outside of USA)
Fax: 901 396 0356
Manufactured in France by :
TEKNIMED S.A.S
8, rue du Corps Franc-Pommiès
65500 VIC EN BIGORRE - FRANCE
Tél. (33)5 62 96 88 38
Fax (33)5 62 96 28 72
www.teknimed.com
CAUTION: U.S. federal law restricts this device to sale by or on the order of a
physician (or properly licensed practitioner) or on the physician's prescription.
Before using this product, the operator must be thoroughly familiarized with the
directions for use, as well as the information relating to the product (description,
operating technique, brochure, etc.). The corresponding information can be
obtained from Medtronic. The operator should also be informed of the residual risk
of this product.
Implantation of KYPHON
carried out by qualified operators with in-depth knowledge and perfect command of the
specific operating techniques of Medtronic products. The operating techniques can be
acquired from the distributors.
The surgeon is responsible for any complications or harmful consequences which may
arise due to an incorrect indication or operating technique, improper use of the material
and failure to observe the safety regulations provided in the directions for use. These
complications cannot be imputed either to the manufacturer or Medtronic.
IN NO EVENT SHALL MEDTRONIC BE LIABLE FOR ANY DIRECT, INDIRECT,
INCIDENTAL, CONSEQUENTIAL, OR EXEMPLARY DAMAGES ARISING OUT OF OR IN
CONNECTION WITH KYPHON® ACTIVOS™ 10 BONE CEMENT WITH
HYDROXYAPATITE, BASED UPON BREACH OF CONTRACT (INCLUDING BREACH OF
WARRANTY).
This product is a fast-setting acrylic cement for kyphoplasty and vertebroplasty procedures.
It is available in the form of an ampoule of sterile liquid and a packet of sterile powder
(liquid monomer and powder polymer).
Powder:
Polymethylmethacrylate 64.4%
Benzoyl peroxide 0.6%
Barium sulfate 25.0%
Hydroxyapatite 10.0%
Liquid:
Methyl Methacrylate 97.6%
N-N dimethyl-p-toluidine 2.4%
Hydroquinone 20 ppm
INSTRUCTIONS FOR THE PREPARATION AND CLINICAL USE OF KYPHON
Timing for the Preparation and Application of KYPHON® ActivOs™ 10 Bone Cement
The handling characteristics of bone cements are affected by operating room conditions,
including the room temperature, temperature of the cement components prior to mixing,
humidity, the geometry of the mixing apparatus, time spent mixing, and the geometry of
the delivery device. Any change in one or more of these conditions can alter the
handling characteristics of the bone cement, including the following:
- handling period - the time it takes for the bone cement to reach the doughy state (the
cement has reached the doughy state when it no longer sticks to surgical gloves)
- working period - the time the bone cement remains in the doughy state and can be
delivered
- hardening period - the time it takes for the bone cement to harden or until it can no
longer be delivered
Under specific conditions in our laboratory, KYPHON
Hydroxyapatite had a mixing and handling period of 8 minutes at a room temperature of
23°C. The bone cement remained in the doughy state for the next 11 minutes at 23°C,
to allow sufficient time for careful, minimally-invasive surgical introduction.
Lower temperatures or other changes in operating room conditions can increase the
handling, doughy (working) and hardening periods. Conversely, higher temperatures or
other changes in operating room conditions can decrease the handling, doughy (working)
and hardening periods.
Handling Characteristics of KYPHON
Hydroxyapatite at 23°C in our Laboratory
Period
Mixing
Handling
Working (doughy state)
Hardening
Note: These cumulative time periods will vary depending on temperature and other
factors. For example, the colder the environment, the longer the time necessary for
the cement to develop the required doughy consistency. Warmer temperatures
require more rapid preparation and handling. (see Figure 1) Ensure the cement's
viscosity is high enough (doughy) before delivery begins. The cement has reached
the doughy state when it no longer sticks to surgical gloves.
* These times are based on cement prepared by Medtronic. Times were obtained
from bone cement mixed in a KYPHON® Mixer and delivered through a KYPHON®
Bone Filler Device (Size 3, inner diameter = 0.114"). Times may vary when other
mixing methods, delivery devices and/or vacuum are used.
ACTIVOS™ 10 BONE CEMENT WITH HYDROXYAPATITE
GENERAL INFORMATION
®
ActivOs™ 10 Bone Cement with Hydroxyapatite should only be
COMPOSITION
®
with Hydroxyapatite
®
ActivOs™ 10 Bone Cement with
®
ActivOs™ 10 Bone Cement with
Approximate Cumulative
Activity
Mix liquid and powder
Transfer into delivery system
Fill cavity in vertebral body
Wait before completing procedure
Time
From Initiation of Mixing
Through a KYPHON
Filler Device*
45 seconds - 8 minutes
®
Bone
0 - 45 seconds
8 - 19 minutes
19 - 21 minutes
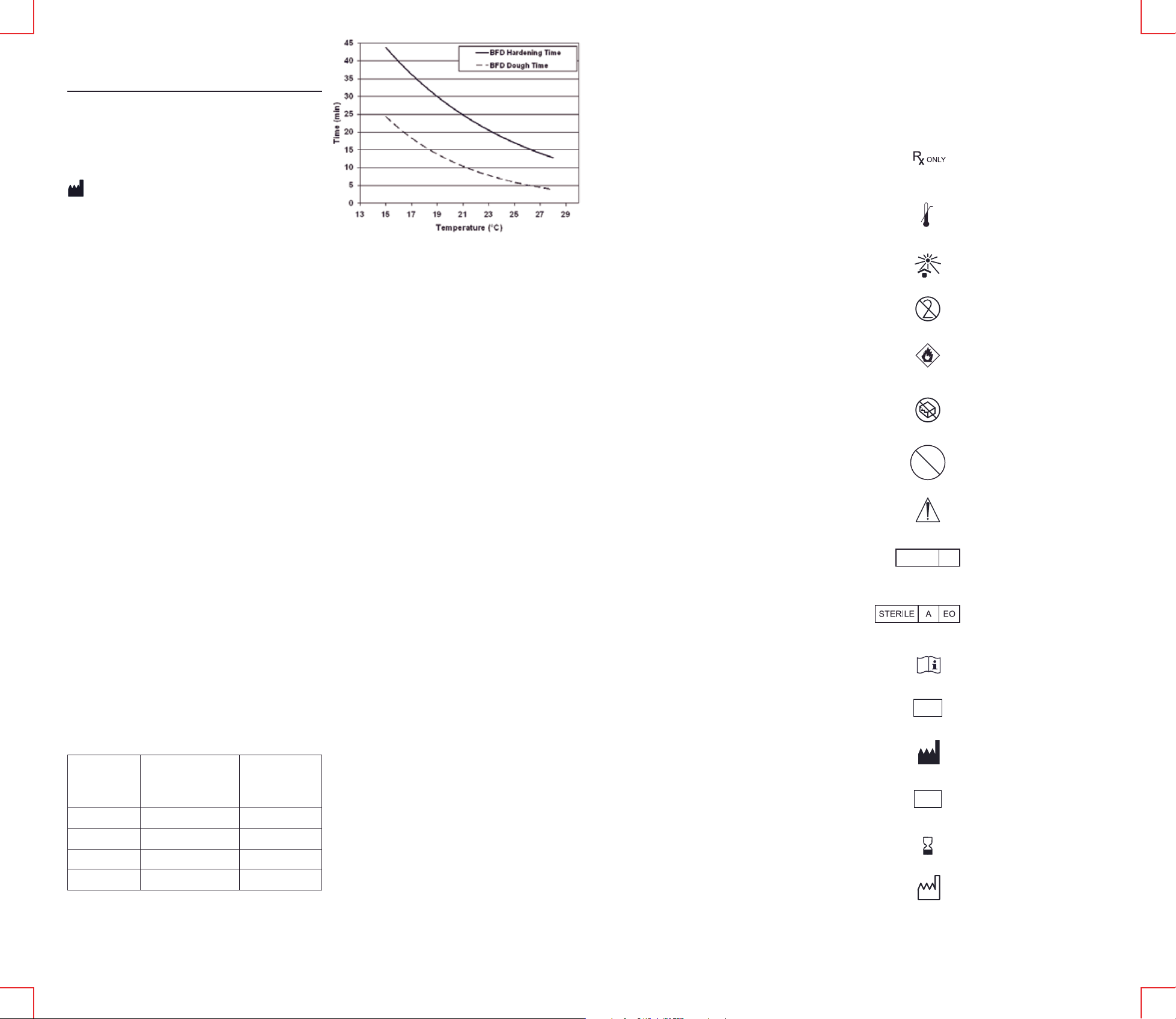
Figure 1. The effect of room temperature on the handling characteristics of KYPHON®
ActivOs™ 10 Bone Cement with Hydroxyapatite is shown in the above time vs.
temperature graph. At room temperatures of 15°C, 19°C, 23°C, and 28°C, units of
®
ActivOs™ 10 Bone Cement with Hydroxyapatite were mixed in a KYPHON®
KYPHON
Mixer and transferred to KYPHON
hardening time out of the BFD were measured, and exponential curves were fitted to
these data. The dashed curve represents BFD dough time (when cement dispensed out
of a BFD no longer sticks to a surgical glove) and the solid curve represents BFD
hardening time (when cement is too hard to dispense out of a BFD).
Preparation Procedures
- Per the note above, temperature can affect the handling of the cement. Prior to use, it
is advised to keep the product at a temperature of 23 ± 1°C for a period of 24 hours.
- Prior to use, KYPHON
should be examined for damage and the presence of all required components.
- Maintain aseptic transfer surgical technique to prevent possible infection.
- Maintain strict adherence to the instructions for mixing the powder and liquid to prevent
possible dermatitis from the liquid monomer during handling.
- Assure that the inner package is undamaged, that the powder is not discolored (yellow
or brown) and the liquid is not syrupy. These conditions indicate that the product has
not been stored correctly.
- Assure that the preparation accessories are specifically compatible with the bone
cement product.
- Do not open the vial of liquid over the mixing bowl to avoid the risk of glass fragments
entering the dough.
- Never add other substances or foreign bodies to the acrylic resin. Never modify the
ratios between the liquid and solid components.
- Care should be taken in the mixing of the liquid and powder components such that the
entire contents of the vial and packet are utilized. The liquid monomer and the powder
component should be thoroughly mixed.
- The length of phases of application depends on room temperature and that of
components, but also on the degree of hygrometry in the operating room. A high
temperature reduces hardening time. A low temperature extends this time.
- Read instructions carefully prior to use.
- The operator should have specific training and experience to be thoroughly familiar with
the properties, handing characteristics, application of the product and percutaneous
cement delivery.
- Follow cement handling, mixing and preparation instructions carefully.
- It is strictly forbidden to re-sterilize products. Single use only. Sterile only if package
is unopened and undamaged.
- Monitor patients carefully for any change in blood pressure during and immediately
following the application of bone cement. Adverse patient reactions affecting the
cardiovascular system, including Bone Cement Implantation Syndrome (BCIS), have been
associated with the use of bone cements. Hypotensive reactions have occurred between
10 and 165 seconds following application of bone cement; they have lasted from 30
seconds to 5 or more minutes. Some have progressed to cardiac arrest. Patients should
be monitored carefully for any change in blood pressure during and immediately
following the application of bone cement, especially those potentially at increased risk for
peri-operative death, including elderly patients, patients with underlying cardiac or
pulmonary compromise, and patients being treated for multiple vertebral body fractures in
one procedure.
- Methyl methacrylate may cause hypersensitivity among high-risk persons, which can
result in an anaphylactic reaction.
- Due to insufficient data, the safety of this cement has not been established with regard
to pregnant women and children.
- The addition of HA has not been shown to provide any improved clinical results.
- We do not recommend using these products on patients that do not suffer from
pathological condition, such as primary or secondary osteoporosis or a tumor. This
could impair the ability of the patient to recover using conservative treatment methods.
- Established surgical principles and techniques must be strictly observed. Deep wound
infection is a serious postoperative complication that may require complete removal of
the bone cement. Deep wound infection may be latent and may not become apparent
until several years after the operation.
- Caution should be exercised to prevent excessive exposure to the concentrated fumes
of the monomer, which may irritate the respiratory tract, eyes and even the liver.
- Always check the condition of the liquid before carrying out the procedure. Do not use
the liquid component if it shows any sign of thickening or premature polymerization.
- Do not allow the liquid component to come into contact with rubber or latex gloves.
The liquid component is a powerful lipid solvent. Should contact occur, the gloves
might dissolve, causing tissue damage. Wearing two pairs of gloves may reduce the
possibility of hypersensitivity reactions.
- Do not allow personnel wearing contact lenses to be near or involved in mixing of the
bone cement.
- Use appropriate imaging techniques to verify that the instruments are correctly
positioned, that no damage has been caused to surrounding structures, and that the
injected bone cement has been correctly located. Use an imaging technique, such as
fluoroscopy, to assess the capacity of the vertebra to contain the injected bone cement.
- Avoid over-pressurizing the bone cement, as this may cause the bone cement to leak
beyond the site of its intended application. Cement leakage may cause tissue damage,
and nerve or circulatory problems, and other serious adverse events.
- Leaks may also occur during the injection if the instruments are in a vein or if there
are undetected micro-fractures.
- If bone cement is detected outside the vertebral body or in the circulatory system
during the procedures, stop the injection immediately.
- Inadequate fixation or unanticipated postoperative events may affect the cement / bone
interface and may lead to micromotion between the cement and bone surface. A layer
of fibrous tissue may then develop between the cement and the bone, and loosening of
the bone cement may occur leading to failure. Long-term regular supervision is
therefore recommended for all patients.
- The final polymerization stage occurs in situ and is an exothermic reaction with
considerable liberation of heat. According to ISO 5833 standard, the temperature can
reach 95°C. The released heat may damage bone or other tissues surrounding the
implant. Maintain patient's position until the polymerization process has been completed,
so as to obtain proper fixation. An additional 1 to 2 hours or more may be necessary,
depending on the patient's medical condition and the operator.
- The long term effects of the bone cement in the spine have not been established.
- Difficulty in swallowing and blistering of the throat, alleged to be an allergic reaction to
the use of bone cement, have also been reported.
- DO NOT mix more than one vial of liquid and one packet of powder together at any
one time. Never modify the ratios between the liquid and solid components. Doing so
could affect bone cement properties, including handling characteristics.
- Modifying the polymerization time by either warming or cooling the bone cement and/or
associated delivery devices has not been tested and could affect bone cement
properties, including handling characteristics.
®
Bone Filler Devices (BFD, size 3). Dough time and
®
ActivOs™ 10 Bone Cement with Hydroxyapatite packaging
WARNINGS
- Only physicians thoroughly trained in the surgical use of bone cement and balloon
kyphoplasty should use KYPHON® ActivOs™ 10 Bone Cement with Hydroxyapatite. The
operator should have specific training and be familiar with the properties, handling
characteristics, and application of the bone cement and adhere to the instructions for use.
- Adverse events may occur whenever you fail to comply with the instruction leaflet.
- The handling characteristics of bone cements are affected by operating room conditions,
including the room temperature, temperature of the cement components prior to mixing,
humidity, the geometry of the mixing apparatus, time spent mixing, and the geometry of
the delivery device. Any change in one or more of these conditions can alter the
handling characteristics of the bone cement, including the following:
- handling period - the time it takes for the bone cement to reach the doughy state
(the cement has reached the doughy state when it no longer sticks to surgical gloves)
- working period - the time the bone cement remains in the doughy state and can be delivered
- hardening period - the time it takes for the bone cement to harden or until it can no
longer be delivered
- During the application of KYPHON® ActivOs™ 10 Bone Cement with Hydroxyapatite,
radiological control is essential so that the operator can follow the progress of the filling
and stop the procedure if the slightest leakage of cement is detected.
- A thorough preoperative check-up of the patient must be carried out prior to the operation.
- Make sure the operating room is properly ventilated to eliminate monomer fumes as
much as possible. The monomer is a volatile and flammable liquid. Ignition of
monomer fumes caused by the use of electrocautery devices in surgical sites near
freshly implanted bone cement has been reported.
- The insertion of a foreign body into the tissues increases the normal risk of infection
associated with surgery during the postoperative period.
KYPHON® ActivOs™ 10 Bone Cement with Hydroxyapatite is indicated for the fixation of
pathological fractures of the vertebral body using vertebroplasty or kyphoplasty procedures.
Painful vertebral compression fractures of the vertebral body may result from osteoporosis,
benign lesions (hemangioma), or malignant lesions (metastatic cancers, myeloma).
- Cases of active or incompletely treated infection
- Coagulation disorders, or severe cardiopulmonary disease
- Spinal stenosis (>20% by retropulsed fragments)
- Compromised of the vertebral fractures due to posterior involvement
- Non-pathological, acute traumatic fractures of the vertebra
- Vertebral plana (collapse>90%)
- Compromise of the vertebral body or the walls of the pedicles
- Unstable vertebral fractures due to posterior involvement
- Hypersensitivity to one of the constituents of the product.
Serious adverse events, some with fatal outcome, associated with the use of acrylic bone
cements include:
- cardiac arrest
- cerebrovascular accident
- myocardial infarction
- pulmonary embolism
- cardiac embolism
Although the majority of these adverse events present early within the post-operative
period, there have been some reports of diagnoses beyond a year or more after the
procedure.
Other reported adverse events relevant to the anatomy being treated with acrylic bone
cements for vertebroplasty and kyphoplasty include:
- Leakage of the bone cement beyond the site of its intended application with introduction
into the vascular system resulting in embolism of the lung and/or heart or other clinical
sequelae.
- deep or superficial wound infection
- fistula
- hematoma
- hemorrhage
- heterotopic new bone formation
- extravasation of bone cement potentially resulting in but not limited to:
- compression or irritation of nerve structures, such as the spinal cord or nerve roots,
causing radiculopathy, parasthesia, paraplegia or paralysis
- introduction into the vascular system resulting in embolism or other clinical sequelae
- intradiscal leakage
- pyrexia due to allergy to bone cement
- short-term conduction irregularities
- thrombophlebitis
- transitory fall in blood pressure
- migration of hardened bone cement bolus
Other reported adverse events include:
- pneumonia
- intercostal neuralgia,
- collapse of the vertebra adjacent to the one injected due to an osteoporotic disease
- pneumothorax
- fracture of a pedicle
- rib fracture in patients with diffuse osteopenia due to the significant downward force
exerted during needle insertion.
The liquid in the ampoule is sterilized by ultra-filtration, and the ampoule's blister pack is sterilized
using ethylene oxide. The powder in a double pouch is sterilized using gamma irradiation.
Before using, check the protective wrapping carefully to ensure that it has not suffered
any damage which could compromise its sterility.
When removing the product from its wrapping be sure to follow asepsis rules.
The cement is supplied sterile, ready for use in the operating room.
It is strictly forbidden to re-sterilize the product. Single use only.
Sterile only if package is unopened and undamaged.
Do not use after the expiry date.
- Percutaneous vertebroplasty or kyphoplasty procedures should only be performed in
medical settings in which emergency decompressive surgery is available.
- Adverse reactions affecting the cardiovascular system have been attributed to acrylic
cement. Recent data indicate that the monomer undergoes rapid hydrolysis into
methacrylic acid, and that a significant fraction of the circulating methacrylate is present
in the form of free acid rather than methyl ester. Correlation between changes in
circulating concentrations of methyl methacrylate/methacrylic acid and changes in blood
pressure has not been established.
- The doctor is responsible for any complication or harmful consequences, which may
result from an erroneous indication or operating technique, from inappropriate use of the
material (including re-use), or non-observation of the safety instructions that figure in the
directions for use.
- Additives (such as antibiotics) are not to be mixed with the bone cement, as this will
alter cement properties.
The patient should be informed by the doctor of the potential consequences of the factors
mentioned in the following paragraphs: contraindications and adverse events, that is,
those liable to hinder the success of the operation, as well as possible complications
which may arise. The patient should also be informed of the measures to be taken to
diminish the possible consequences of these factors.
Designation Powder (g) Liquid (g)
®
KYPHON
ActivOs™ 10 Bone Cement with Hydroxyapatite 21g 9.2g
The cement should be stored unopened in its original packaging, in a dry, clean place
away from the light, at a maximum temperature of 25°C.
-Allow the cement to harden before disposal with other medical waste. Comply with local
regulations in force relating to medical waste for safe handling and disposal of the cement.
-Regarding separate disposal of the liquid or of the powder, comply with local regulations
in force relating to handling and disposal of the cement.
PRECAUTIONS FOR USE
INDICATIONS FOR USE
CONTRAINDICATIONS
ADVERSE EVENTS
STERILIZATION
IMPORTANT PHYSICIAN INFORMATION
PATIENT INFORMATION
PACKAGING AND STORAGE
RECOMMENDATIONS FOR DISPOSAL
For further information, please contact Customer Service, Medtronic Sofamor Danek USA,
Inc., 1800 Pyramid Place, Menphis, Tennessee, 38132, Telephone: 800 933 2635 ( in
U.S.A), 901 396 3133 ( outside of U.S.A), Fax: 901 396 0356
© 2015 Medtronic Sofamor Danek USA, Inc. All rights reserved.
REQUESTS FOR INFORMATION
CAUTION: U.S. Federal Law restricts
this device to sale by or on the order of
a physician (or properly licensed practitioner).
Upper limit of temperature
Keep away from sunlight
Do not reuse
Flammable product
Do not use if package
is damaged
2
STERILIZE
Do not resterilize
Caution
STERILE R
Sterilized using irradiation
Product compound,
using alternative way of sterilization
Consult instructions for use
REF
Catalog number
Manufacturer
LOT
Batch code
Use by date YYYY-MMM-DD
Date of manufacture YYYY-MM-DD
Réf. : TF410407 ind 3 / 01-2017
 Loading...
Loading...