
autoLog
®
Autotransfusion System
Including Associated Disposables
Operator’s Manual
Caution: Federal law (USA) restricts this device to sale by or
on the order of a physician.

PROPRIETARY INFORMATION
The entire contents of this manual are copyrighted and the property of Medtronic, Inc. No part of
this book may be used or reproduced in any form or by any means, or stored in a database or
retrieval system, without the prior written authorization of Medtronic, Inc.
®
Classified by Underwriters Laboratories, Inc.
with respect to electric shock, fire and mechanical
hazards only in accordance with UL 2601-1 and CSA/CAN C22.2 no. 601.1.
®
autoLog
is a registered trademark of Medtronic, Inc.
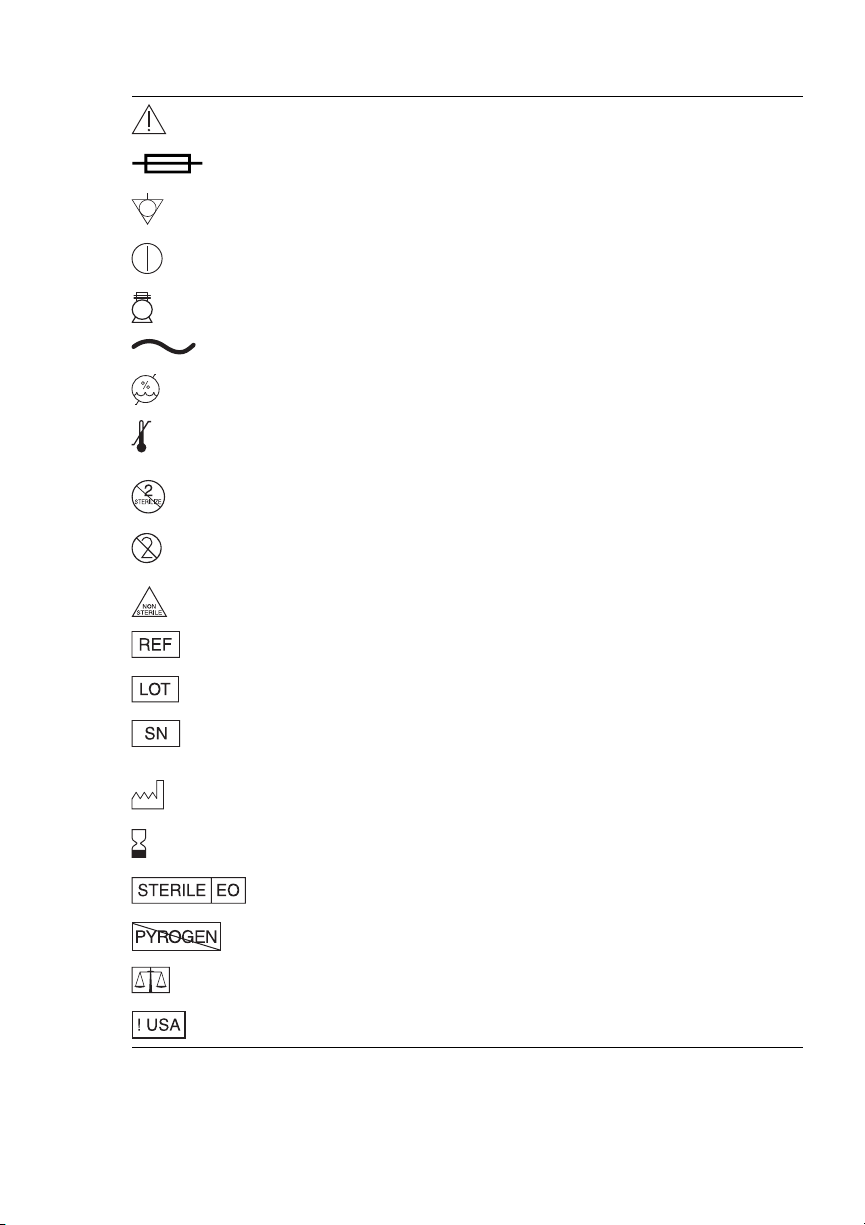
Explanation of symbols on package labeling
Attention, See Instructions for Use
Fuse
Equipotentiality
On/Off
Power - Vacuum Pump
Alternating Current
Humidity Limitation
Temperature Limitation
Do Not Resterilize
Do Not Reuse
Nonsterile
Catalog Number
Lot Number
Serial Number
Date of Manufacture
Use By
Sterilized Using Ethylene Oxide
Nonpyrogenic Fluid Path
Quantity
For US Audiences Only
Operator’s Manual 1

Conformité Européenne (European Conformity). This symbol means
that the device fully complies with European Council Directive 93/42/
EEC.
autoLog Autotransfusion System
Medical equipment with respect to electric shock, fire and mechanical
hazards only in accordance with UL2601-1 and CAN/CSA C22.2
No. 601.1.
Do not dispose of this product in the unsorted municipal waste stream.
Dispose of this product according to local regulations. See
http://recycling.medtronic.com for instructions on proper disposal of
this product.
This Way Up
Keep Dry
Fragile, Handle with Care
Corrugated Recycles
Protective Earth Ground
China RoHS Standard (SJ/T11364-2006) Electric Information Products
Pollution Control Symbol. The number represents the years the device
can be used before it must be recycled (environmental protection use
period).
Contains di(2-ethylhexyl)phthalate (DEHP)
2 Operator’s Manual
Manufacturer
Consult Instructions for Use
Authorized Representative in the European Community

Introduction
Autologous blood is blood that is derived from the same individual. Therefore, an autologous
transfusion is one in which the patient receives only his/her own blood. Autotransfusion is a
procedure in which the blood lost by, or removed from, a patient (autologous blood) is
subsequently returned to the patient’s circulation.
Advantages of Autotransfusion Over Allogeneic Transfusion
Because of concern over blood-related diseases, increasing numbers of physicians and patients
are focusing their attention on the risks of allogeneic transfusion, which has resulted in increased
interest in autotransfusion. There are several benefits:
■
Hepatitis risk is eliminated, as well as other blood-transmitted diseases.
■
Cross-matching errors are eliminated.
■
Use of autologous blood provides additional assurance when performing surgery on patients
with multiple red blood cell antibodies or rare blood phenotypes.
■
Valuable allogeneic blood is conserved.
Intended Use
The autoLog Autotransfusion System is intended for use in the collection, concentration,
washing, and reinfusion of autologous blood. Such areas of application may include, but are not
limited to, the following:
■
General, cardiovascular, orthopedic, vascular, plastic/reconstructive, obstetric/gynecologic
and neurosurgical surgery
■
Postoperative treatment areas
Principles of Operation
The autoLog Autotransfusion System operates by separating whole blood into its individual
components by centrifugation. Blood is an ideal biologic mixture for such a technique, because
it is a suspension of heterogeneous elements of significantly different densities and, thus, is easy
to separate. When subjected to a centrifugal force, the blood components will migrate relative to
their respective densities, with the higher density blood components moving farther from the axis
of rotation. An itemized list of recovered and removed material is shown here:
Recovered:
■
washed, packed red blood cells
Removed examples include (materials less dense than red blood cells):
■
lipids and fats
■
plasma-free hemoglobin
■
pharmacologic agents
■
activated platelets
■
irrigation solutions
■
activated clotting factors
As blood continues to enter the spinning bowl, the amassing red cell pack begins to occupy more
of the bowl volume and the excess plasma is pushed ahead of the red cells. When the total liquid
volume of the bowl has been exceeded, the excess plasma exits the bowl through the effluent
fluid outlet to the waste bag via connecting tubing.
To rid the red cell pack of contaminants, it is washed with isotonic saline (0.9% sodium chloride
solution).
Operator’s Manual 3
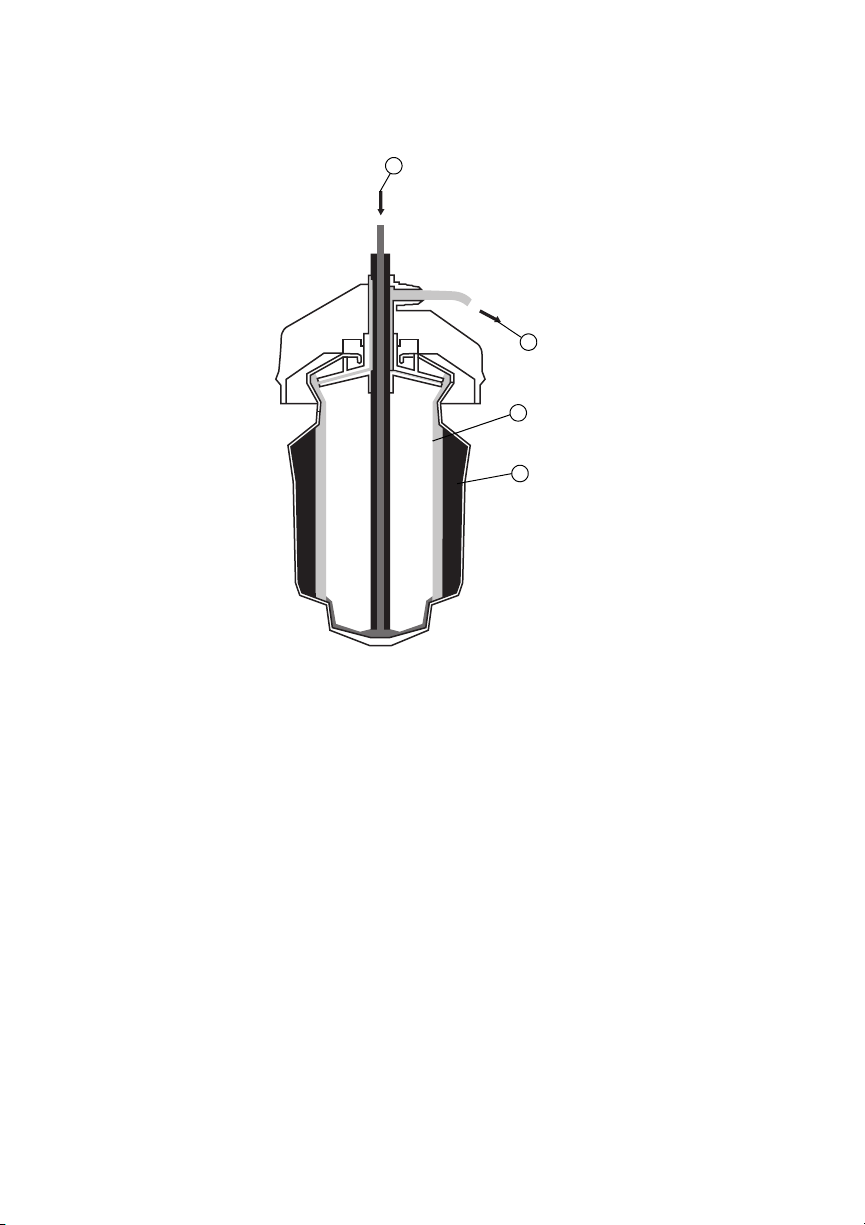
At the termination of washing, the clean, packed red cells are transferred to the holding bag. This
2
3
1
4
is accomplished by reversing the fluid pump rotation, which draws blood from the base of the
bowl and transfers it to the holding bag via the attached tubing. The blood is transferred to a
blood transfer bag and then to the patient.
4 Operator’s Manual
Figure 1.
1. Effluent Fluid Outlet
2. Plasma
3. Red Cells
4. Whole Blood Inlet

Warnings, Precautions, Contraindications, and Possible Complications
Read this Operator’s Manual completely prior to using the autoLog Autotransfusion System.
Warnings and Precautions
1. Direct Patient Reinfusion: Do not use the autoLog Autotransfusion System for direct
patient reinfusion (ie, from the machine directly into the patient) or direct patient draw (ie, from
the patient directly into the machine). Adequate safeguards do not exist to protect the
patient in these situations.
2. Reinfusion of washed red cells can be carried out by gravity or pressure infusion after
transferring processed blood to blood transfer bags. Do not directly reinfuse processed blood
from the holding bag to the patient. Directly reinfusing the blood from the holding bag exposes
the patient to the risk of possible air embolism.
3. Caution: Federal law (USA) restricts this device to sale by or on the order of a
physician. Actual performance results may vary depending on many in-use variables. It is
important to read and understand this Operator’s Manual and understand the principles of cell
washing before undertaking clinical operation of the autoLog Autotransfusion System. The
responsibility for the use of this device in all cases belongs solely to the physician ordering its
use.
4. The safe operation of all cell washing equipment requires the presence of a dedicated
operator. It is the responsibility of the hospital to ensure that the individuals assigned to this
task are well trained in the operation of the autoLog Autotransfusion System and alert to
potential problems. Never leave the machine unattended during operation as
irrecoverable damage to the blood may occur.
5. This device is intended for autotransfusion use in clinical patient care areas such as operating
rooms, intensive care, or recovery rooms. This device is NOT intended for use in blood banks
or apheresis centers, or for use where the blood bank has to handle, label, store, hold, or
otherwise process the blood for later reinfusion into the same patient.
6. The disposable components utilized with this device are for single patient use only. This
disposable was designed for single patient use only. Do not reuse, reprocess, or resterilize
this product. Reuse, reprocessing, or resterilization may compromise the structural integrity
of the device and/or create a risk of contamination of the device, which could result in patient
injury, illness, or death. Only Medtronic
with the autoLog Autotransfusion System. Maintain a sterile field at the collection site. It is
important that aseptic technique be used to minimize the possibility of contamination to the
disposables and/or the patient.
7. Due to the presence of phthalates in the product, the clinician must weigh the medical benefits
of product use against the drawbacks of phthalate exposure for male children and pregnant
or nursing women.
8. Do not attempt to reuse the disposables. Reuse may adversely affect the performance of this
system and compromise patient safety.
9. The disposables must be used immediately after the removal of the protective packaging.
Visually inspect the contents of the disposables. Should any evidence of damage to
components be found during inspection or setup, do not use the disposable and return to
Medtronic for replacement. Do not use silicone oils or greases near the disposables.
®
sterilized disposable kits are approved for patient use
#
Operator’s Manual 5

10.The disposables are sterile (ethylene oxide) and nonpyrogenic as long as package integrity
has not been violated. Do not use if the package is damaged or open. Store all disposables
in a dry place away from extremes of temperature.
11.The autoLog Autotransfusion System must not be used in the presence of flammable agents.
12.The basic concept of cell washing involves the removal of the contaminated plasma and
debris, while leaving red cells suspended in clean saline. Removal of large amounts of
plasma during autotransfusion can cause patient hypovolemia. Since platelets and
coagulation factors are contained in the plasma, this plasma removal may also reduce
coagulation factors or platelet levels below normal levels. It is also possible that inadequate
washing of salvaged blood may result in insufficient removal of anticoagulant and/or the
development of coagulopathies upon return of that blood to the patient. Therefore, careful
monitoring of the patient’s coagulation status is important to prevent complications.
13.Medtronic advises that all autologous collected blood be washed prior to reinfusion.
14.Medtronic does not have sufficient data to support the safety and efficacy of returning washed
cells from partially filled bowls and therefore cannot recommend that practice.
15.Blood may be salvaged from body cavities, joint spaces, and other operative sites or trauma
sites only if there is no clinical evidence of sepsis, malignancy, or wound contamination.
16.Never transfuse blood that is suspected of having high hemolysis.
®1
17.Make sure no water or other irrigation, such as Betadine
, is aspirated into the
autotransfusion system at any time, as this will hemolyze the red cells. Use an adequately
sized suction tip to minimize hemolysis.
18.When using a hard-shell cardiotomy or blood collection reservoir, vacuum levels should not
exceed 150 mm Hg.
19.Do not use any hot solutions over 42°C (108°F), since high heat can destroy red cells.
20.To avoid overheating the centrifuge, which could cause hemolysis, do not use the autoLog
Autotransfusion System at temperatures higher than 30°C (86°F).
21.Plastic materials used in the autoLog Autotransfusion System and its disposable kits may be
sensitive to chemicals (such as solvents and certain detergents). Under certain adverse
conditions, exposure to these chemicals (including vapors) may cause the plastics to fail or
malfunction.
22.Treat all blood and fluids using universal bloodborne pathogen precautions.
23.In the unlikely event of a power loss or other failure during the wash portion of the cycle, a
lower than normal hematocrit will result. The blood should be tested for hematocrit so that the
operator knows what is being given to the patient.
24.Do not restrict the flow in any tubing line. If a tubing line is inadvertently clamped or kinked
during operation, pressure may build up in the centrifuge bowl causing failure or leakage.
Always check the entire disposable kit to confirm that all tubing is free of any kinks, twists or
flat areas. Double check the pump head and wash kit to ensure that all components are in the
proper flow direction.
25.The standard waste bag for the autoLog Autotransfusion System holds approximately 10 L.
Periodically check the waste bag volume and empty as required. The waste bag may be
emptied at any time; however, a small amount of fluid (100–200 mL) should be left in the bag
to provide for proper expansion during filling and emptying. Avoid the introduction of room air
into the waste bag. A full waste bag will cause back pressure and bowl leaks to occur.
1
Betadine® is a registered trademark of Purdue Frederick Company.
6 Operator’s Manual

26.The AABB Standards for Perioperative Blood Collection and Administration (Third Edition,
2007), Reference Standard 5.1.8A, recommends the expiration period for blood recovered
interoperatively with processing be stored at room temperature no longer than 4 hours from
the time of collection. Interoperative blood collected with processing can be stored for
24 hours at 1°C and 6°C, if the storage is begun within 4 hours of completion of processing.
The transfusion of shed blood collected under postoperative or post-traumatic conditions
shall begin within 6 hours of initiating the collection. In the unlikely event of power failure,
these guidelines should be strictly adhered to. If the blood is less than 4 hours old, it is
permissible to continue processing and transfuse the blood to the patient.
27.Medtronic recommends the use of a blood transfusion filter between the reinfusion container
and the patient in compliance with The AABB Standards for Perioperative Blood Collection
and Administration (Third Edition, 2007), Reference Standard 5.4.5.1, which states,
“Perioperative products intended for transfusion shall be transfused through a filter designed
to retain particles that are potentially harmful to the patient.”
28.Inside the autoLog Autotransfusion System cabinet there are various electrical components
and wiring. Physical contact with any of these components while the unit is plugged in could
result in severe electrical shock. Always turn off and unplug the unit prior to working inside
the cabinet or changing any fuses. For continued protection against risk of fire, replace fuses
only with the same type and rating. Internal grounding is provided for safety.
29.Although this system was tested for EMC compliance and passed, the potential exists that, in
some situations, the autoLog Autotransfusion System and other devices might
electromagnetically interfere with each other. Take steps to minimize this possibility.
30.The autoLog Autotransfusion System includes a centrifuge that rotates at 10,000 rpm. Parts
that turn at high speeds may be dangerous. Safety rules related to the use of centrifuges must
be followed. Do not open the centrifuge or remove the bowl before it comes to a complete
stop.
31.Current leakage is a primary indicator of electrical shock hazard to personnel making contact
with any exposed portion of the equipment. Each autoLog Autotransfusion System is checked
during the final quality inspection to verify that current leakage is less than 100 µA. Have
current leakage checked at least yearly, or as required by the operating facility’s biomedical
engineering department, or other qualified service technician. In addition, particular attention
should be given to checking the current leakage and insulation after an event such as a fluid
spill or major voltage surge in the power source has occurred, or after any machine repair.
32.Maintain the autoLog Autotransfusion System in good working order and calibrate it on a
regular basis.
Transportation of Device
1. To avoid potential damage during transit, use the autoLog Autotransfusion System’s original
shipping packaging.
2. Never lay the autoLog Autotransfusion System on its side as this can damage the centrifuge.
3. Be careful not to move the autoLog Autotransfusion System by the IV pole. Over time this can
cause the top panel of the machine to loosen and cause permanent damage.
Contraindications and Complications
1. The use of citrate-based anticoagulant in patients with impaired liver function may require
additional monitoring and may, in certain circumstances, be contraindicated. Improperly
processed red cells may contain residual citrate-based solution which, in excess quantities,
could cause citrate toxicity, depression of serum calcium, or bleeding tendencies.
Operator’s Manual 7

2. Gross contamination and/or septic procedures.
3. Surgery within the malignant area that may allow dissemination of
tumor/malignant cells, if aspirated, into the autotransfusion system.
4. Caesarean sections (presence of amniotic fluid).
5. Presence of high concentrations of prostatic fluid.
6. Contamination of salvaged blood with drugs not intended for intravenous administration.
®2
7. Collagen-based hemostatic agents, such as Gelfoam
, should not be used in combination
with any autotransfusion system. In their presence, temporarily discontinue salvage during
the time the agent is being used. After the agent has been given time to initiate hemostasis
in the wound, irrigate the area copiously with saline and aspirate to non-autotransfusion
collection containers before autologous blood salvage is continued. Failure to flush the area
thoroughly could result in the hemostatic agent being drawn into the collected blood. This
could result in coagulation of the collection blood or possible disseminated intravascular
coagulopathy (DIC) complications in the patient.
8. Coagulopathy.
9. Morbidity and mortality in autotransfusion, as in allogeneic transfusions, are directly related
to the volume of blood infused, if plasma and platelets are not concurrently transfused.
2
Gelfoam® is a registered trademark of Pharmacia & Upjohn Company.
8 Operator’s Manual

Device Description and Specifications
Features and Specifications
Note: Technical data, features, and options referenced in this manual are based on the latest
information available at the time of printing. Medtronic reserves the right to change specifications
without notice.
Electrical Classification:
Class I, Ordinary, Continuous Operation
Power:
Voltage: 110 – 120 / 220 – 240 V~
Frequency: 50 – 60 Hz
Phase: Single
Current: 1.6 / 0.8 A (depending upon voltage selection)
Fuses: 4 A / 250 V~ / T
Power Cord: 2 wires plus ground (earth) connector
3 prong hospital grade (USA only)
Speed and Flow Rate Specifications:
Centrifuge: 0 – 10,000 rpm (± 5%)
Pump: 0 – 600 mL/min (± 5%)
Vacuum: 150 – 200 mm Hg
Dimensions:
Width: 33 cm (13 in)
Height: 75 cm (30 in)
Depth: 22 cm (9 in)
Weight:
32 kg (70 lb)
Temperature Limit:
Operational: 10°C – 30°C (50°F – 86°F)
Storage: 5°C – 50°C (41°F – 122°F)
Humidity Range:
Operational: 10 – 95% noncondensing
Storage: 10 – 95% noncondensing
Operator’s Manual 9
