Page 1

Talent™ Abdominal Stent Graft System
with Xcelerant
®
Hydro Delivery System
Instructions for Use
• Do not attempt to use the Talent Abdominal Stent Graft with Xcelerant Hydro
Delivery System before completely reading and understanding the information
contained in this booklet.
• Carefully inspect all product packaging for damage or defects prior to use. Do not
use this product if any sign of damage or breach of the sterile barrier is observed.
• These devices are supplied STERILE for single use only. After use, dispose of the
delivery catheters in accordance with hospital, administrative, and/or government
policy. Do not resterilize.
• Caution: Federal (U.S.) Law restricts this device to sale by or on the order of a
physician
IMPORTANT!
Page 2

Page 3

Page 4

M708500B001
Talent™ Abdominal Stent Graft with Xcelerant Hydro Delivery System
Instructions for Use: Table of Contents
Section Page
1.0 DEVICE DESCRIPTION ......................................................................................................5
1.1 Device Components .........................................................................................................7
1.2 Xcelerant Hydro Delivery System (HDS) ..........................................................................9
2.0 INDICATIONS ....................................................................................................................10
3.0 CONTRAINDICATIONS.....................................................................................................10
4.0 WARNINGS AND PRECAUTIONS ...................................................................................10
4.1 General...........................................................................................................................10
4.2 Patient Selection, Treatment, and Follow-Up .................................................................10
4.3 Implant Procedure ..........................................................................................................11
4.4 Magnetic Resonance Imaging (MRI) Safety Section......................................................12
5.0 ADVERSE EVENTS...........................................................................................................12
5.1 Observed Adverse Events..............................................................................................12
5.2 Potential Adverse Events ...............................................................................................12
5.3 Device-Related Adverse Events Reporting ....................................................................12
6.0 SUMMARY OF CLINICAL STUDY ....................................................................................12
6.1 Stent Graft Analysis........................................................................................................12
6.2 Delivery System Analysis ...............................................................................................13
6.3 Patient Accountability And Follow-Up.............................................................................13
6.4 Demographic and Baseline Medical History Data ..........................................................16
6.5 Baseline Aneurysm Data ................................................................................................18
6.6 Devices Implanted ..........................................................................................................20
6.7 Study Results .................................................................................................................20
6.8 Safety .............................................................................................................................20
6.9 Effectiveness ..................................................................................................................28
6.10 Acute Procedural Data .................................................................................................31
6.11 CoilTrac Delivery System Performance Data ...............................................................33
7.0 PATIENT SELECTION ......................................................................................................34
7.1 Individualization of Treatment.........................................................................................34
8.0 PATIENT COUNSELING INFORMATION.........................................................................34
9.0 HOW SUPPLIED ...............................................................................................................34
9.1 Contents .........................................................................................................................34
9.2 Sterility and Storage .......................................................................................................35
10.0 CLINICAL USE INFORMATION ......................................................................................35
10.1 Recommended Skills and Training...............................................................................35
10.2 Materials Recommended for Device Implantation ......................................................35
10.3 Pre-Treatment Planning ...............................................................................................36
11.0 DIRECTIONS FOR USE – TALENT ABDOMINAL STENT GRAFT WITH
XCELERANT HDS ...........................................................................................................37
11.1 Pictorial References .....................................................................................................37
1
Page 5

M708500B001
For pictorial references of the Talent Abdominal Stent Graft components with the
Xcelerant HDS refer to Figure 1 and Figure 5 respectively..................................................37
11.2 Vascular Access and Arteriotomy ................................................................................37
11.3 Implantation of the Bifurcated Stent Graft ....................................................................37
11.4 Deploy Distal End .........................................................................................................41
11.5 Delivery System Removal ............................................................................................41
11.6 Preparation of the Contralateral limb with the Xcelerant HDS .....................................42
11.7 Implantation of the Contralateral Limb .........................................................................42
11.8 Confirm Position ...........................................................................................................43
11.9 Deploy Stent Graft ........................................................................................................44
11.10 Remove Delivery System ...........................................................................................44
11.11 Stent Graft Balloon Modeling......................................................................................45
11.12 Procedure Completion................................................................................................46
11.13 Aortic and Iliac Extensions .........................................................................................47
11.14 Handle Dis-assembly Technique................................................................................48
12.0 IMAGING GUIDELINES AND POST-OPERATIVE FOLLOW-UP...................................48
12.1 General.........................................................................................................................48
12.2 Contrast And Non-Contrast CT Recommendations .....................................................49
12.3 Abdominal Radiographs ...............................................................................................49
12.4 Ultrasound ....................................................................................................................50
13.0 MRI SAFETY AND COMPATIBILITY...............................................................................50
14.0 ADDITIONAL SURVEILLANCE AND TREATMENT .......................................................50
15.0 DEVICE-RELATED ADVERSE EVENTS REPORTING..................................................51
16.0 DEVICE REGISTATION PACKET ...................................................................................51
17.0 CONFIGURATIONS AVAILABLE ....................................................................................52
18.0 EXPLANATION OF SYMBOLS........................................................................................55
2
Page 6

M708500B001
List Of Tables
Table 1: Stent Graft Materials ..................................................................................................5
Table 2: Patient and Imaging Accountability – Test Group
1
................................................... 14
Table 3: Patient Accountability – SVS Control .......................................................................15
Table 4: Patient Demographics, Test Group vs. SVS Control ...............................................16
Table 5: Baseline Medical History, Test Group vs. SVS Control ...........................................17
Table 6: Baseline SVS Classification, Test Group Only......................................................... 18
Table 7: Baseline Maximum Aneurysm Diameters, Test Group vs. SVS Control (Site
Reported) ...............................................................................................................................18
Table 8: Distribution of Baseline Maximum Aneurysm Diameters,........................................ 18
Table 9: Baseline Aneurysm Characteristics, Test Group ..................................................... 19
Table 10: Total Number of Talent Abdominal Stent Grafts Implanted at Initial Procedure ....20
Table 11: Primary Safety Endpoint: Freedom from MAEs within 30 Days, Test Group vs. SVS
Control.................................................................................................................................... 20
Table 12: Primary Safety Endpoint: MAE Components within 30 Days, Test Group vs.
SVS Control ...........................................................................................................................21
Table 13: Freedom from MAEs within 365 Days, ..................................................................21
Table 14: MAE Components within 365 Days,....................................................................... 22
Table 15: Details of Kaplan-Meier Estimates of Freedom from MAEs (0 to 365 Days),
Test Group vs. SVS Control................................................................................................... 23
Table 16: Freedom from All-Cause Mortality within 30 Days, Test Group vs. SVS
Control.................................................................................................................................... 24
Table 17: Freedom from Aneurysm-Related Mortality within 365 Days, Test Group vs.
SVS Control ...........................................................................................................................24
Table 18: Details of Kaplan-Meier Estimates of Freedom from Aneurysm-Related
Mortality within 365 Days, Test Group vs. SVS Control......................................................... 25
Table 19: Freedom from All-Cause Mortality within 365 Days, Test Group vs. SVS
Control.................................................................................................................................... 26
Table 20: Details of Kaplan-Meier Estimates of Freedom from All-Cause Mortality
within 365 Days...................................................................................................................... 27
Table 21: Primary Effectiveness Endpoint: Successful Aneurysm Treatment, Test Group... 28
Table 22: Primary Effectiveness Endpoint: Successful Aneurysm Treatment, Test Group... 28
Table 23: Migration-Free at 12 Months, Test Group (Core Lab)............................................ 29
Table 24: Stent Graft Patency at 12 Months, Test Group (Core Lab).................................... 29
Table 25: Freedom from Secondary Endovascular Procedures within 365 Days, Test
Group .....................................................................................................................................29
Table 26: Loss of Stent Graft Integrity at 12 Months, Test Group (Core Lab) ....................... 30
Table 27: Type I/III Endoleak-Free at 12 Months, Test Group (Core Lab) ............................30
Table 28: Summary of All Endoleaks at 1 Month and 12 Months, Test Group (Core Lab).... 31
Table 29: Aneurysm Rupture within 365 Days, Test Group................................................... 31
Table 30: Aneurysm Change from 1 Month to 12 Months, .................................................... 31
Table 31: Acute Procedural Data, Test Group and SVS Control ...........................................32
Table 32: CoilTrac Delivery System: Delivery and Deployment Success ............................. 33
Table 33: CoilTrac Delivery System: Patients with Clinically Relevant Adverse Events [Within
30 Days] ................................................................................................................................. 33
Table 34: Talent Abdominal Stent Graft with Xcelerant HDS Oversizing Guidelines ............ 36
Table 35: Recommended Imaging Schedule for Endovascular Graft Patients...................... 49
Table 36: Accepted Imaging Protocols .................................................................................. 49
Table 37: Bifurcated Stent Grafts with the Xcelerant HDS Delivery System..........................52
Table 38: Contralateral Limbs with the Xcelerant HDS Delivery System............................... 53
Table 39: Iliac Extension Cuffs with the Xcelerant HDS Delivery System ............................. 53
Table 40: Aortic Extension Cuffs with the Xcelerant HDS Delivery System...........................54
3
Page 7

M708500B001
List of Figures
Figure 1: Overview of Talent Abdominal Stent Graft Components ..........................................6
Figure 2: Talent Abdominal Bifurcated Stent Graft .................................................................. 7
Figure 3: Talent Abdominal Contralateral Iliac Limb ................................................................8
Figure 4: Talent Abdominal Iliac (Left) and Aortic (Right) Extension Cuffs.............................. 8
Figure 5: Talent AAA Stent Graft with the Xcelerant HDS ....................................................... 9
Figure 6: Kaplan-Meier Estimates of Freedom from MAEs (0 to 365 Days), Test Group
vs. SVS Control...................................................................................................................... 23
Figure 7: Kaplan-Meier Estimates of Freedom from Aneurysm-Related Mortality within
365 Days, Test Group vs. SVS Control................................................................................. 25
Figure 8: Kaplan-Meier Estimates of Freedom from All-Cause Mortality within 365 Days,
Test Group vs. SVS Control................................................................................................... 27
Figure 9: Introduce the System for the Bifurcated Segment ..................................................38
Figure 10: Position the System .............................................................................................. 39
Figure 11: Deploy the Proximal End ......................................................................................40
Figure 12: Deploy the Distal End............................................................................................41
Figure 13: Use the Quick Disconnect to Retract the Tapered Tip .........................................42
Figure 14: Introduce the Contralateral Limb .......................................................................... 43
Figure 15: Position Contralateral Limb................................................................................... 44
Figure 16: Remove the Delivery System................................................................................ 45
Figure 17: Iliac Extension Cuffs .............................................................................................47
Figure 18: Orienting the Aortic Extension Cuff....................................................................... 47
4
Page 8
M708500B001
1.0 DEVICE DESCRIPTION
The Talent™ Abdominal Stent Graft with the Xcelerant
components: an implantable stent graft and a disposable delivery system. The pre-loaded stent graft is advanced
to the aneurysm location over a guidewire and, upon retraction of the graft cover, expands to the indicated
diameter. During deployment and expansion, the stent graft is intended to form proximal and distal seal zones
surrounding the aneurysm location.
The Talent Abdominal Stent Graft System is modular and consists of four stent graft component configurations:
• Bifurcated (aorto-iliac)
• Contralateral iliac limb
• Iliac extension cuff
• Aortic extension cuff
Each component is introduced separately into the patient’s vascular system. Each stent graft component is
comprised of nitinol metal springs attached to polyester fabric graft material. For all configurations the proximal
and distal springs are attached to connecting bars to provide additional columnar strength to the stent graft. The
springs are sewn to the polyester fabric graft using polyester suture material. Radiopaque markers, made out of
platinum-iridium in the shape of a figure eight (aka, Figur8), are sewn onto the stent graft to aid in visualization of
the stent graft under fluoroscopy and to facilitate accurate placement of the device. See Table 1 for a listing of
stent graft materials and Figure 1 for an overview of stent graft components.
The stent graft is designed to be placed in the native vessel such that the unconstrained stent graft diameter is
larger than the diameter of the native vessel into which it is to be placed. This “oversizing” helps to exclude the
aneurysm from aortic blood flow and ensure that the stent graft is held in place. The amount of oversizing required
is dependent on the diameter of the native vessel. See Table 34 for oversizing guidelines and Section 16.0 for
available device configurations
®
Hydro Delivery System (HDS) comprises of two main
Table 1: Stent Graft Materials
Stent Graft Component Material
Springs Nitinol wire (Nickel-Titanium alloy)
Connecting Bar Nitinol wire (Nickel-Titanium alloy)
Mini-Support Spring (FreeFlo only) Nitinol wire (Nickel-Titanium alloy)
Stent Fabric Woven polyester
Sutures Braided polyester suture
Figur8 Radiopaque Markers Platinum-Iridium wire
5
Page 9

M708500B001
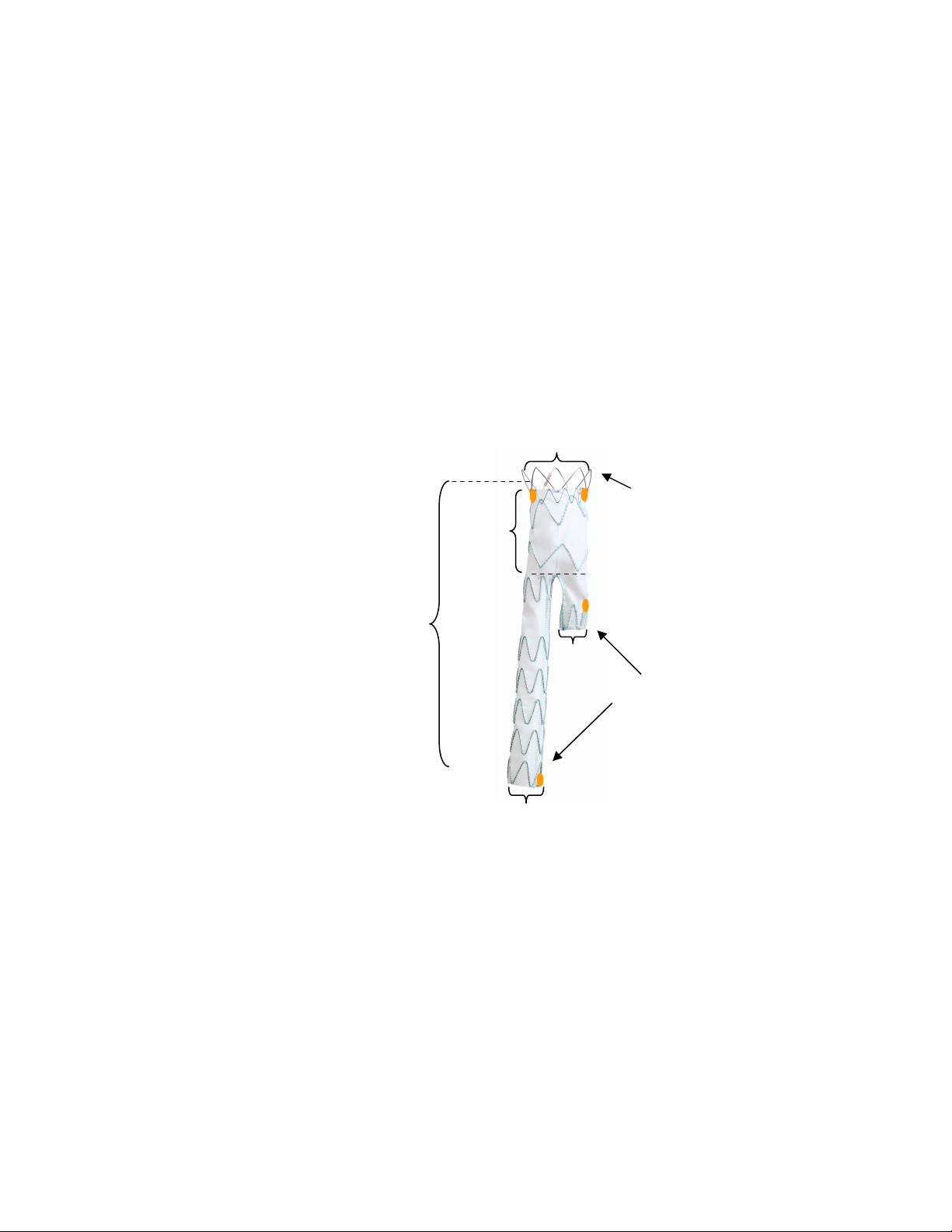
Figure 1: Overview of Talent Abdominal Stent Graft Components
Mini-support Spring
Note: Figure 1 and Figure 2 are representations only. The Talent Abdominal Stent Graft may appear differently
when viewed under fluoroscopy.
.
6
Page 10
M708500B001
1.1 Device Components
Each of the four stent graft configurations is described in the following section.
1.1.1 Bifurcated Stent Graft
The bifurcated component (Figure 2) is the primary component which is inserted into the patient’s
aorta. The proximal end of all bifurcated stent grafts has a bare spring that is not covered with graft
material to allow for supra-renal fixation. Bifurcated stent grafts with a proximal diameter greater
than 22mm have a mini-support spring to aid in sealing. The proximal end configuration in which a
bare spring and mini-support spring are present is called the ‘FreeFlo’ configuration. The proximal
end configuration in which a bare spring is present without a mini-support spring is called a ‘Bare
Spring’ configuration.
The stent graft bifurcates into two smaller iliac diameters; one of which is placed into the ipsilateral
iliac artery, and the other of which is available to receive the contralateral iliac component. The
distal end of the short contralateral leg is 14mm in diameter for all sizes of stent grafts so that it can
receive all available contralateral limb stent graft configurations. In contrast the distal end of the
ipsilateral leg is available in 12, 14, 16, 18 and 20mm diameters. The distal iliac ends of the stent
graft have Closed Web configurations.
1.1.2 Contralateral iliac limb
The contralateral iliac limb component (Figure 3) is implanted after the bifurcated component to
provide a conduit for blood flow into the contralateral iliac artery. The contralateral iliac limb is
introduced though the patient’s contralateral iliac artery and mated to the short contralateral stub leg
on the bifurcated stent graft.
The proximal end of the contralateral iliac limb has an Open Web configuration in which the outline
of the most proximal spring is covered. The proximal diameter is 14mm for all limb sizes, so that all
limbs can dock with all available bifurcated stent graft configurations. The distal end of the limb has
a Closed Web configuration.
140-170mm
Figure 2: Talent Abdominal Bifurcated Stent Graft
22-36mm
50mm
FreeFlo Configuration Shown
[22mm size has Bare Spring configuration
without mini-support spring (not shown in
the figure)]
14mm
Closed Web
Configuration
12-20mm
7
Page 11

M708500B001
1.1.3 Aortic and Iliac Extension Cuffs
The aortic and iliac extension cuff components (Figure 4) are used to extend the lengths of implanted
devices as needed based on the patient’s anatomy.
Open Web
Configuration
Closed Web
Configuration
Open Web Configuration
Closed Web Configuration
Figure 4: Talent Abdominal Iliac (Left) and Aortic (Right) Extension Cuffs
8-24mm
Figure 3: Talent Abdominal Contralateral Iliac Limb
14mm
75-105mm
8-24mm
10-22mm
FreeFlo Configuration Shown
[22mm size has Bare Spring
configuration without mini-support
spring (not shown in the figure)]
74 - 140mm
Open Web Configuration
22-36mm
26-30mm
22-36mm
8
Page 12

M708500B001
1.2 Xcelerant Hydro Delivery System (HDS)
The stent graft is loaded inside the Xcelerant HDS. The Xcelerant HDS is a hydrophilic coated delivery
system as shown in
Figure 5, which facilitate the placement of the stent graft via the arterial vasculature (e.g., femoral
arteries). Using fluoroscopic guidance, the Xcelerant HDS is properly positioned within the patient’s
vasculature and the stent graft is deployed from the Xcelerant HDS.
The Xcelerant HDS consists of:
• A single use, disposable system with an integrated handle to provide the user with controlled
deployment.
• A flexible catheter assembly compatible with a 0.035” guidewire.
• Three concentric single lumen polymer shafts (an outer hydrophilic coated graft cover shaft with an
inner member shaft, and a guidewire lumen).
• A polymeric, atraumatic tip attached at the distal end to facilitate tracking through tortuous and
calcified vessels.
• A radiopaque tip, a marker at the distal end of the stent graft, and a marker on the distal end of the
graft cover to aid in fluoroscopic visualization.
• O-rings contained within the delivery system to maintain hemostasis during the procedure.
Retraction of the graft cover allows deployment of the self-expanding stent grafts. Post deployment, the
physician will recapture the tip of the delivery system by retracting the inner member.
Figu re 5: Tal en t AAA Sten t G raft w ith the X celeran t HDS
5
6
7
8
10
11
2
A
B
1
Detail A
12
13
Detail B
9
3
4 2
1. Stent Stop 8. Trigger
2. Graft Cover 9. Front Grip
3. RO Marker 10. Handle
4. RO Tapered Tip Disassembly Ports
5. Rear Grip 11. Strain Relief
6. Screw Gear 12. Touhy Bourst
7. Slider 13. Quick Disconnect
9
Page 13

M708500B001
INDICATIONS
The Talent Abdominal Stent Graft with Xcelerant HDS is indicated for the endovascular treatment of abdominal
aortic aneurysms with or without iliac involvement having:
• Iliac/femoral access vessel morphology that is compatible with vascular access techniques, devices,
and/or accessories;
• A proximal aortic neck length of ≥ 10mm;
• Proximal aortic neck angulation ≤ 60°;
• Distal iliac artery fixation length of ≥ 15mm;
• An aortic neck diameter of 18–32mm and iliac artery diameters of 8–22mm; and
• Vessel morphology suitable for endovascular repair.
2.0 CONTRAINDICATIONS
The Talent Abdominal Stent Graft is contraindicated in:
• Patients who have a condition that threatens to infect the graft.
• Patients with sensitivities or allergies to the device materials (see Table 1)
3.0 WARNINGS AND PRECAUTIONS
3.1 General
• Read all instructions carefully. Failure to properly follow the instructions, warnings and precautions may
lead to serious consequences or injury to the patient
• The Talent Abdominal Stent Graft System should only be used by physicians and teams trained in
vascular interventional techniques, including training in the use of the device. Specific training
expectations are described in Section 9.1.
• Always have a vascular surgery team available during implantation or reintervention procedures in the
event that conversion to open surgical repair is necessary
3.2 Patient Selection, Treatment, and Follow-Up
• The Talent Abdominal Stent Graft System is not recommended in patients unable to undergo or who will
not be compliant with the necessary preoperative and postoperative imaging and implantation studies as
described in Section 11.0.
• The Talent Abdominal Stent Graft System is not recommended in patients who cannot tolerate contrast
agents necessary for intra-operative and post-operative follow-up imaging.
• The Talent Abdominal Stent Graft System is not recommended in patients exceeding weight and/or size
limits which compromise or prevent the necessary imaging requirements
• Prior to the procedure, pre-operative planning for access and placement should be performed. See
Section 9.3. Key anatomic elements that may affect successful exclusion of the aneurysm include
severe proximal neck angulation (> 60 °); short proximal aortic neck (< 10mm); and thrombus and/or
calcium at the arterial implantation sites, specifically the proximal aortic neck and distal iliac artery
interface. Irregular calcification and/or plaque may compromise the fixation and sealing of the
implantation sites. Necks exhibiting these key anatomic elements may be more conducive to graft
migration.
• Iliac conduits may be used to ensure the safe insertion of the delivery system if the patient’s access
vessels (as determined by treating physician) preclude safe insertion of the delivery system.
• Inappropriate patient selection may contribute to poor device performance.
• The safety and effectiveness of the Talent Abdominal Stent Graft has not been evaluated in patients
who:
Are less than 18 years of age
Are pregnant or lactating
Have a dominant patent inferior mesenteric artery and an occluded or stenotic celiac and/or
superior mesenteric artery
Have aneurysmal involvement or occlusion (surgically performed or naturally occurring) of the
bilateral internal iliac arteries
Have vessels and/or aneurysm dimensions that cannot accommodate the Talent Abdominal
Stent Graft as per the indications in Section 0.
Have no distal vascular bed (one vessel lower extremity run-off required)
Have contraindications for use of contrast medium or anticoagulation drugs
Have an uncorrectable coagulopathy
Have a mycotic aneurysm
Have circumferential mural thrombus in the proximal aortic neck
Have had a recent (within 3 months) myocardial infarction (MI), cerebral vascular accident
(CVA), or major surgical intervention
Have traumatic aortic injury
Have leaking, pending rupture or ruptured aneurysms
Have pseudoaneurysms resulting from previous graft placement
Require a revision to previously placed endovascular stent grafts..
Have genetic connective tissue disease (e.g., Marfan's or Ehlers-Danlos' Syndromes)
Have concomitant thoracic aortic or thoracoabdominal aneurysms
Are patients with active systemic infections
10
Page 14

M708500B001
• The long-term performance of endovascular grafts has not yet been established. All patients should
be advised that endovascular treatment requires lifelong, regular follow-up to assess their health
and the performance of their endovascular graft. Patients with specific clinical findings (e.g.,
endoleaks, enlarging aneurysms or changes in the structure or position of the endovascular graft)
should receive enhanced follow-up. Specific follow-up guidelines are described in Section 11.0.
• After endovascular graft placement, patients should be regularly monitored for perigraft flow,
aneurysm growth or changes in the structure or position of the endovascular graft. At a minimum,
annual imaging is required, including: 1) abdominal radiographs to examine device integrity (stent
fracture, separation between bifurcated device and proximal cuffs or limb extensions, if applicable),
and 2) contrast and non-contrast CT to examine aneurysm changes, perigraft flow, patency,
tortuosity and progressive disease. If renal complications or other factors preclude the use of
image contrast media, abdominal radiographs and duplex ultrasound may provide similar
information.
• Patients experiencing reduced blood flow through the graft limb and/or leaks may be required to
undergo secondary interventions or surgical procedures.
• Intervention or conversion to standard open surgical repair following initial endovascular repair
should be considered for patients experiencing enlarging aneurysms and/or endoleak. An increase
in aneurysm size and/or persistent endoleak may lead to aneurysm rupture.
3.3 Implant Procedure
• Exercise care in handling and delivery technique to aid in the prevention of vessel rupture.
• Studies indicate that the danger of micro-embolization increases with increased duration of the
procedure
• Renal complications may occur:
From an excess use of contrast agents.
As a result of embolic or misplaced stent graft. The radiopaque marker along the edge of the
stent graft should be aligned immediately below the lower-most renal arterial origin.
• Inadequate seal zone may result in increased risk of leakage into the aneurysm or migration of the
stent graft. Other possible causes of migration are deployment of the proximal spring into a
thrombus-filled or severely angled vessel wall.
• Systemic anticoagulation should be used during the implantation procedure based on hospital and
physician preferred protocol. If heparin is contraindicated, an alternative anticoagulant should be
considered.
• Minimize handling of the constrained endoprosthesis during preparation and insertion to decrease
the risk of endoprosthesis contamination and infection.
• Improper placement of the stent graft may also cause an endoleak or occlusion of arteries (other
than the renals), which may prevent blood flow necessary to organs and extremities, necessitating
surgical removal of the device.
• During general handling of the Xcelerant HDS, avoid bending or kinking the graft cover because it
may cause the Talent Abdominal Stent Graft to prematurely and improperly deploy.
• Never advance or retract the Xcelerant HDS from the vasculature without the use of fluoroscopy.
• Do not continue advancing any portion of the delivery system if resistance is felt during
advancement of the guidewire or delivery system. Stop and assess the cause of resistance. Vessel
or catheter damage may occur. Exercise particular care in areas of stenosis, intravascular
thrombosis or in calcified or tortuous vessels.
• When aligning the position of the Xcelerant HDS so that the Talent Abdominal Stent Graft is in
proper position for deployment within the vessel, be sure that the fluoroscope is angled
perpendicularly to the center line of the infrarenal aorta to avoid parallax or other sources of
visualization error. Align the target area/fixation zone (e.g., neck) in the center of the field. Some
cranial-caudal angulation of the i-i tube may be necessary to achieve this, especially if there is
anterior angulation of the aneurysm neck.
• Before initial deployment, it is suggested to position the stent graft slightly higher than the targeted
location.
• Do not retract the graft cover before placing the delivery system in the proper anatomical position,
as this will initiate deployment of the stent graft. The Talent Abdominal Stent Graft cannot be
reconstrained or drawn back into the graft cover, even if the stent graft is only partially deployed. If
the graft cover is accidentally withdrawn, the device will prematurely deploy and could be placed
too high or too low.
• When using the trigger to rapidly deploy the stent graft, be sure to hold the front grip of the delivery
system stationary. Do not rotate the graft cover during this step.
• Do not rotate the graft cover during deployment, as this may torque the device and cause it to spin
on deployment or cause twisting of the iliac limb.
• High pressure injections of contrast media made at the edges of the stent graft immediately after
implantation can cause endoleaks.
• Any endoleak left untreated during the implantation procedure must be carefully monitored after
implantation.
11
Page 15

M708500B001
3.4 Magnetic Resonance Imaging (MRI) Safety Section.
MRI may be used on the graft only under specific conditions. See Section 12.0 for details.
4.0 ADVERSE EVENTS
4.1 Observed Adverse Events
The clinical study for the Test Group was a multicenter, prospective study conducted at 13 sites across the
US, which included 166 test patients. Major adverse events observed in this study are provided in Section 4.0.
4.2 Potential Adverse Events
Adverse events that may occur and/or require intervention include, but are not limited to:
• Amputation
• Anesthetic complications and subsequent attendant problems (e.g., aspiration)
• Aneurysm enlargement
• Aneurysm rupture and death
• Aortic damage, including perforation, dissection, bleeding, rupture and death
• Arterial or venous thrombosis and/or pseudoaneurysm
• Arteriovenous fistula
• Bleeding, hematoma or coagulopathy
• Bowel complications (e.g., ileus, transient ischemia, infarction, necrosis)
• Cardiac complications and subsequent attendant problems (e.g., arrhythmia, myocardial infarction,
congestive heart failure, hypotension, hypertension)
• Claudication (e.g., buttock, lower limb)
• Death
• Edema
• Embolization (micro and macro) with transient or permanent ischemia or infarction
• Endoleak
• Fever and localized inflammation
• Genitourinary complications and subsequent attendant problems (e.g., ischemia, erosion, fistula,
incontinence, hematuria, infection)
• Hepatic failure
• Impotence
• Infection of the aneurysm, device access site, including abscess formation, transient fever and pain
• Lymphatic complications and subsequent attendant problems (e.g., lymph fistula)
• Neurologic local or systemic complications and subsequent attendant problems (e.g., confusion, stroke,
transient ischemic attack, paraplegia, paraparesis, paralysis)
• Occlusion of device or native vessel
• Pulmonary/respiratory complications and subsequent attendant problems (e.g., pneumonia, respiratory
failure, prolonged intubation)
• Renal complications and subsequent attendant problems (e.g., artery occlusion, contrast toxicity,
insufficiency, failure)
• Stent graft: improper component placement; incomplete component deployment; component migration;
suture break; occlusion; infection; stent fracture; graft twisting and/or kinking; insertion and removal
difficulties; graft material wear; dilatation; erosion; puncture and perigraft flow
• Surgical conversion to open repair
• Vascular access site complications, including infection, pain, hematoma, pseudoaneurysm,
arteriovenous fistula, dissection.
• Vascular spasm or vascular trauma (e.g., iliofemoral vessel dissection, bleeding, rupture, death)
• Vessel damage
• Wound complications and subsequent attendant problems (e.g., dehiscence, infection, hematoma,
seroma, cellulitis)
4.3 Device-Related Adverse Events Reporting
See Section 14.0
5.0 SUMMARY OF CLINICAL STUDY
5.1 Stent Graft Analysis
The clinical study for the Test Group was a multicenter, prospective study conducted at 13 sites across the
US. The Test Group included patients diagnosed with abdominal aortic aneurysms, with or without
involvement of the iliac arteries. A total of 166 patients were enrolled in this study. An independent core lab
reviewed CT scans and abdominal x-rays to assess aneurysm changes, device position and integrity, and
endoleaks. A Clinical Events Committee (CEC) adjudicated Major Adverse Events (MAEs) for the Test
Group.
The Control Group (SVS Control) was a compilation of the pivotal open surgical control groups from three
approved abdominal aortic aneurysm (AAA) endograft Premarket Approval (PMA) submissions. The SVS
Control represented a change from the original IDE protocol, and was used because the SVS Control was
more comprehensive than the original IDE Control Group. The data aggregation and analysis were conducted
12
Page 16

M708500B001
under the auspices of the Society for Vascular Surgery (SVS). Outcomes from a total of 243 patients treated
at facilities across the US were included in the SVS Control.
The pivotal analysis included endpoints that were modified from the endpoints listed in the original IDE
protocol to endpoints and other metrics that are consistent with current literature and other EVAR clinical
studies. The primary safety endpoint for this analysis was the proportion of patients free from a MAE within
30 days of the index procedure (based on a composite MAE rate), compared to the open surgical control. The
primary effectiveness endpoint for this analysis was successful aneurysm treatment
and analyses were presented based on follow-up at pre-discharge, 1 month, 6 months, and 12 months.
5.2 Delivery System Analysis
Subsequent to enrollment in the pivotal trial, the delivery system was updated to the CoilTrac Delivery
System. In order to evaluate the clinical performance of the CoilTrac Delivery System, a single-center cohort
of 137 patients from an independent data set was evaluated.
The analysis of this independent data set supports the clinical performance of the CoilTrac Delivery System,
demonstrated by delivery and deployment success rate, as well as, clinically relevant adverse events rates
observed within the 30 day post-procedure period.
5.3 Patient Accountability And Follow-Up
For the Test Group, 13 sites enrolled a total of 166 patients. Four (4) patients had technical failure and did
not receive a stent graft and therefore did not have any imaging follow-up. 162 patients who received the
stent graft were eligible for clinical and imaging follow-up at 1 month follow-up interval. Of these 162 patients,
100% (162/162) had a clinical follow-up and 98.8% (160/162) had imaging follow-up. CT imaging was
performed on 96.3% (156/162) patients.
At the 6 month follow-up interval, 152 patients were eligible for clinical and imaging follow-up. Of these, 90.1%
(137/152) had clinical follow-up and 81.6 % (124/152) had imaging follow-up. CT imaging was performed on
78.9% (120/152) patients.
At the 12 month follow-up interval, 142 patients were eligible for clinical and imaging follow-up. Of these
97.2% (138/142) had clinical follow-up and 93.0% (132/142) had imaging follow-up. CT imaging was
performed on 91.5% (130/142) patients.
Detailed patient accountability and follow-up is provided in Table 2
1
. Other study endpoints
1
Successful aneurysm treatment was a composite endpoint including patients who had technical success
(successful delivery and deployment of the Talent Stent Graft) at the initial procedure and were free from:
• Aneurysm growth > 5mm at 12 months, as evaluated by the core lab; and
• Post-operative interventions to correct Type I/III endoleaks at anytime up to 12 months (Type II
endoleaks are generally considered to be non-device related).
13
Page 17

M708500B001
Patient follow-up
Table 2: Patient and Imaging Accountability – Test Group1
Patients
with
imaging
performed
at time
Patients with adequate
imaging to assess the
parameter
Patient events occurring before
interval
(Core Lab)
next visit
Interval
(Analysis
Window)
Originally
Enrolled
166
Eligible
Clinical
Imaging
Follow-up
Follow-up
KUB
CT Imaging
Imaging
size
increase
Aneurysm
Endoleak
Migration
Integrity
Failure
Technical
Conversion
4
Death
to Surgery
Withdrawal
Lost to
Events after
implant but
before a
0 0 0 0
1Month visit
1 Month
(Day 1-90)
162 162 160 156 141
150 143 136
Events after 1
Month visit but
before a 6
0 5 5 0
Month visit
6 Month
(Day 91-304)
152 137 124 120 103 118 114 120 101
Events after 6
Month visit but
before a 12
0 5 5 0
Month visit
12 Month
(≥ Day 305
1
Data analysis sample size varies for each of the timepoints above and in the following tables. This variability is due to
142 138 132 130 112 128 120 128 110
2
)
patient availability for follow-up, as well as, quantity and quality of images available from specific timepoints for evaluation.
For example, the number and quality of images available for evaluation of endoleak at 6 months is different than the
number and quality of images available at 12 months due to variation in the number of image exams performed, the
number of images provided from the clinical site to the Core Lab, and/or the number of images with acceptable evaluation
quality.
Follow-up
2
In cases where 12 month imaging follow-up data were not available, subsequent imaging follow-up data were used.
The SVS Control included 243 patients. Detailed patient accountability and follow-up is provided in Table 3
below. At the 1 month follow-up interval, 239 patients were eligible and 98.7% (236/239) had clinical followup. At the 6 month follow-up interval, 230 patients were eligible and 90.9% (209/230) had clinical follow-up.
At the 12 month follow-up interval, 219 patients were eligible and 97.7% (214/219) had clinical follow-up.
14
Page 18

M708500B001
Table 3: Patient Accountability – SVS Control
Patient follow-up
Patients with events occurring
before next visit
Interval
(Analysis Window)
Originally enrolled 243
Events after procedure but
before 1 Month visit
1 Month visit
(Day 1-90)
Events after 1 Month visit
but before 6 Month visit
6 Month visit
(Day 91-304)
Events after 6 Month visit
but before 12 Month visit
12 Month visit
(≥ Day 305)
Eligible
4 0
239 236
7 2
230 209
5 6
219 214
Clinical Follow-
up Death
Withdrawal/ Lost
to Follow-up
15
Page 19

M708500B001
5.4 Demographic and Baseline Medical History Data
Table 4 through Table 6 provides the demographics and baseline medical characteristics of the Test Group
and SVS Control patients. Medtronic observed that the Test Group was older and had more co-morbidities
than the patients within the SVS Control.
Parameter Statistics/Category Test Group SVS Control p-value
Age (years)
n 166 243
Table 4: Patient Demographics, Test Group vs. SVS Control
Gender % (m/n)
Male 91.6% (152/166) 81.5% (198/243) 0.004
Ethnicity % (m/n)
Mean ± SD 74.1 ± 7.49 70.1 ± 7.49 < 0.001
Median 76.0 70.0
Min, max 51, 89 46, 86
White, non-Hispanic 92.8% (154/166) 94.9% (168/177)
Non-White 7.2% (12/166) 5.1% (9/177)
0.501
16
Page 20

M708500B001
Table 5: Baseline Medical History, Test Group vs. SVS Control
Body System / Condition
Cardiovascular
Angina 16.9% (28/166) 17.4% (23/132) > 0.999
Arrhythmia 44.0% (73/166) 11.5% (28/243) < 0.001
Test Group
%(m/n) 1
SVS Control
%(m/n) 1 p-value
Cardiac revascularization2 38.6% (64/166) 46.1%
Congestive heart failure 28.3% (47/166) 4.9% (12/243) < 0.001
Coronary artery disease 56.0% (93/166) 61.3%
Hypertension 83.7%
Myocardial infarction 38.6% (64/166) 34.2% (83/243) 0.401
Peripheral vascular disease 46.4% (77/166) 15.6% (38/243) < 0.001
Renal3
Renal insufficiency 54.8% (91/166) N/A N/A
Renal failure N/A 4.1% (10/243) N/A
Neurological3
Cerebral vascular accident 22.9% (38/166) N/A N/A
Cerebrovascular disease N/A 12.8% (31/243) N/A
Other abnormal body systems
Diabetes 15.7% (26/166) 11.9% (29/243) 0.303
Chronic obstructive pulmonary disease 39.2% (65/166) 30.0% (73/243) 0.070
Tobacco use 84.9%
1
Denominator is 166 patients in the Test Group and 243 patients in the SVS Control.
2
Cardiac Revascularization includes Coronary Artery Bypass Grafting (CABG) or PTCA.
3
SVS Control reported "Renal Failure" and "Cerebrovascular Diseases", but Test Group reported "Renal
Insufficiency” and "Cerebral Vascular Accident", respectively. These categories are not comparable.
(139/166)
(141/166)
(112/243)
(149/243)
66.7%
(162/243)
85.6%
(208/243)
0.154
0.306
< 0.001
0.887
17
Page 21

M708500B001
Table 6: Baseline SVS Classification, Test Group Only
SVS Classification
SVS 0 6.0% (10/166)
SVS 1 47.6% (79/166)
SVS 2 41.0% (68/166)
SVS 3 5.4% (9/166)
5.5 Baseline Aneurysm Data
Table 7 through Table 9 provide the baseline aneurysm diameters and morphologies of the Test Group and
SVS Control
Table 7: Baseline Maximum Aneurysm Diameters, Test Group vs. SVS Control (Site Reported)
Aneurysm Characteristics Statistics
n 166 214
Test Group
%(m/n)
Test Group
Site Reported
SVS Control
Site Reported p-value
Maximum aneurysm diameter (mm)
Table 8: Distribution of Baseline Maximum Aneurysm Diameters,
Maximum Aneurysm Diameter
< 30mm 0.0% (0/166) 0.0% (0/214)
30-39mm 0.0% (0/166) 2.3% (5/214)
40-49mm 14.5% (24/166) 21.5% (46/214)
50-59mm 51.8% (86/166) 42.5% (91/214)
60-69mm 22.3% (37/166) 20.1% (43/214)
70-79mm 8.4% (14/166) 8.4% (18/214)
80-89mm 3.0% (5/166) 3.3% (7/214)
≥ 90mm 0.0% (0/166) 1.9% (4/214)
Mean ± SD 57.1±8.49 56.9±11.59 0.826
Median 55.0 54.8
Min, max 43, 87 31, 100
Test Group vs. SVS Control (Site Reported)
Test Group
Site-Reported
%(m/n)
SVS Control
Site-Reported
%(m/n)
18
Page 22

Table 9: Baseline Aneurysm Characteristics, Test Group
Dimension Statistics Site Reported
n 166 156
M708500B001
Core Lab
Reported
Maximum aneurysm diameter (mm)
Proximal neck diameter (mm)
Right iliac diameter (mm)
Left iliac diameter (mm)
Proximal neck length (mm)
Mean ± SD 57.1 ± 8.49 55.0 ± 9.26
Median 55 53
Min, Max 43, 87 38, 88
n 165 156
Mean ± SD 25.6 ± 3.35 25.3 ± 3.58
Median 26 26
Min, Max 16, 32 16, 32
n 164 155
Mean ± SD 9.3 ± 1.55 9.2 ± 1.53
Median 9 9
Min, Max 6, 16 6, 14
n 164 155
Mean ± SD 9.3 ± 1.46 9.3 ± 1.55
Median 9 9
Min, Max 6, 14 6, 15
n 166 154
Mean ± SD 23.9 ± 12.88 22.9 ± 12.48
Median 20 21
Min, Max 3, 85 3, 75
n 157 127
Aortic neck angle (°)
Mean ± SD 18.7 ± 15.40 30.5 ± 15.80
Median 19 30
Min, Max 0, 60 0, 72
19
Page 23

M708500B001
5.6 Devices Implanted
Table 10 provides a breakdown of the number of Talent Abdominal Stent Grafts implanted per patient.
Table 10: Total Number of Talent Abdominal Stent Grafts Implanted at Initial Procedure
Test Group
Number of Devices Implanted
%(m/n)1
1 0.0% (0/162)
2 42.0% (68/162)
3 32.7% (53/162)
4 22.2% (36/162)
5 3.1% (5/162)
≥ 6 0.0% (0/162)
1
Denominator is 162 patients with implanted devices.
5.7 Study Results
Results for the safety and effectiveness of the Talent Abdominal Stent Graft are presented in Section 5.8 and
5.9 below.
5.8 Safety
Primary Safety Endpoint: Freedom from MAEs within 30 Days
Through 30 days, patients who received the Talent Abdominal Stent Graft experienced a lower rate of MAEs
than patients treated with open surgery. Table 11 and Table 12 provide an analysis of freedom from MAEs
within 30 days.
Table 11: Primary Safety Endpoint: Freedom from MAEs within 30 Days, Test Group vs. SVS Control
SVS
Freedom from Major Adverse Event
(MAE) within 30 Days
Test Group
N = 166
% (m/n)
Control
N = 243
% (m/n)
95% Exact
Confidence
Interval of Difference
Freedom from MAEs within 30 Days 89.2% (148/166) 44.0% (107/243) (36.9%, 52.6%)
1
Confidence level was not adjusted for multiplicity. Confidence interval for difference (Test - SVS Control) in
percentage was calculated by the exact method.
2
Difference represents the (% of patients free from MAEs within 30 days in the population treated with the test
device) - (% of patients free from MAEs within 30 days in the population undergoing open surgical repair)
1,2
20
Page 24

M708500B001
Table 12: Primary Safety Endpoint: MAE Components within 30 Days, Test Group vs. SVS Control
SVS
Control
N = 243
%(m/n)
95% Exact
Confidence
Interval of Difference
Major Adverse Event (MAE) within
30 Days1
Test Group
N = 166
%(m/n)
2,3
MAE rate at 30 days 10.8%
(18/166)
All-cause Death 1.8%
(3/166)
Myocardial Infarction 1.8%
(3/166)
Renal Failure 1.8%
(3/166)
Respiratory Failure 3.0%
(5/166)
Paraplegia 0.0%
(0/166)
Stroke 1.2%
(2/166)
Bowel Ischemia 0.6%
(1/166)
Procedural Blood Loss ≥ 1000cc 5.4%
(9/166)
1
A patient may report multiple MAEs; hence, number of patients with any MAE may not be the sum of those in
56.0%
(136/243)
2.9%
(7/243)
5.3%
(13/243)
2.9%
(7/243)
5.8%
(14/243)
0.4%
(1/243)
1.2%
(3/243)
0.0%
(0/243)
51.0%
(124/243)
N/A
(-4.4%, 2.8%)
(-7.6%, 0.4%)
(-4.4%, 2.8%)
(-7.0%, 1.7%)
(-2.3%, 2.0%)
(-2.6%, 3.3%)
(-1.0%, 3.6%)
(-52.6%, -38.1%)
each MAE category.
2
Confidence level was not adjusted for multiplicity. Confidence intervals for difference (Test - SVS Control) in
percentage were calculated by the exact method.
3
Difference represents the (% of patients with MAEs within 30 days in the population treated with the test
device) - (% of patients with MAEs within 30 days in the population undergoing open surgical repair)
Freedom from MAEs within 365 Days
At 365 days, treatment with the Talent Abdominal Stent Graft continued to perform favorably when compared to
open surgery. Table 13 and Table 14 provide an analysis of freedom from MAEs at 365 days, and Figure 6 and
Table 15 depict the corresponding Kaplan-Meier plot.
Table 13: Freedom from MAEs within 365 Days,
Test Group vs. SVS Control
SVS
Freedom from MAEs within 365
Days
Test Group
N = 166
% (m/n)
Control
N = 243
% (m/n)
95% Exact
Confidence Interval
of Difference
1,2
Freedom from MAEs within 365 Days 80.4% (123/153) 41.7% (100/240) (29.4%, 47.2%)
1
Confidence level was not adjusted for multiplicity. Confidence interval for difference (Test - SVS Control) in
percentage was calculated by the exact method.
2
Difference represents the (% of patients free from MAEs within 365 days in the population treated with the test
device) - (% of patients free from MAE within 365 days in the population undergoing open surgical repair)
21
Page 25

M708500B001
Table 14: MAE Components within 365 Days,
Test Group vs. SVS Control
SVS
MAEs within 365 Days1
Test Group
N = 166
% (m/n)
Control
N = 243
% (m/n)
95% Exact
Confidence Interval
of Difference
MAE rate at 365 days 19.6% (30/153) 58.3% (140/240) N/A
All-cause Death 6.5% (10/153) 7.5% (18/240) (-6.1%, 5.0%)
Myocardial Infarction 3.9% (6/153) 7.9% (19/240) (-8.9%, 1.4%)
Renal Failure 3.3% (5/153) 2.9% (7/240) (-3.2%, 5.0%)
Respiratory Failure 3.9% (6/153) 6.3% (15/240) (-6.8%, 3.0%)
Paraplegia 0.0% (0/153) 0.4% (1/240) (-2.4%, 2.2%)
Stroke 2.6% (4/153) 1.7% (4/240) (-2.1%, 5.0%)
Bowel Ischemia 0.7% (1/153) 0.0% (0/240) (-0.9%, 3.9%)
Procedural Blood Loss ≥1000 cc 5.9% (9/153) 51.7% (124/240) (-52.9%, -38.1%)
1
A patient may report multiple MAEs; hence, number of patients with any MAE may not be the sum of those in
each MAE category.
2
Confidence level was not adjusted for multiplicity. Confidence intervals for difference (Test - SVS Control) in
percentage were calculated by the exact method.
3
Difference represents the (% of patients with MAEs within 365 days in the population treated with the test device)
- (% of patients with MAEs within 365 days in the population undergoing open surgical repair)
2,3
22
Page 26

M708500B001
Figure 6: Kaplan-Meier Estimates of Freedom from MAEs (0 to 365 Days),
Test Group vs. SVS Control
Note: eLPS, as described in the figure above, refers to the Test Group.
No. at Risk 166 142 136 243 107 105
No. of Events 18 4 8 136 2 2
No. Censored 6 2 8 0 0 7
Kaplan-Meier
Estimate 0.891 0.866 0.813 0.440 0.432 0.424
Table 15: Details of Kaplan-Meier Estimates of Freedom from MAEs (0 to 365 Days),
Test Group SVS Control
Treatment
to 30 days
31 days to
182 days
Test Group vs. SVS Control
183 days to
365 days
Treatment
to 30 days
31 days to
182 days
183 days to
365 days
23
Page 27

M708500B001
Freedom from All-Cause Mortality within 30 Days
Table 16 provides the summary of patients with freedom from all-cause mortality at 30 days for the Test Group and
SVS Control.
Table 16: Freedom from All-Cause Mortality within 30 Days,
Secondary Endpoint
Test Group vs. SVS Control
Test Group
%(m/n)
SVS
Control
%(m/n)
95% Exact
Confidence
Interval of
Difference
1,2
Freedom from All-Cause Mortality
98.2% (163/166) 97.1% (236/243) (-2.8%, 4.4%)
within 30 Days
1
Confidence level was not adjusted for multiplicity. Confidence interval for difference (Test - SVS Control) in
percentage was calculated by the exact method.
2
Difference represents the (% of patients free from all-cause mortality within 30 days in the population treated
with the test device) - (% of patients free from all-cause mortality within 30 days in the population undergoing
open surgical repair)
Freedom from Aneurysm-Related Mortality within 365 Days
Table 17 and Figure 7 provide the analysis and Kaplan-Meier plot of freedom from aneurysm-related mortality at
365 days. Additional detail is provided in Table 18.
Notably, there were no conversions to surgery or aneurysm ruptures in the Test Group within 365 days. See Table
29 for aneurysm rupture results.
Table 17: Freedom from Aneurysm-Related Mortality within 365 Days,
Test Group vs. SVS Control
Secondary Endpoint
Test Group
N = 166
% (m/n)
SVS
Control
N = 243
% (m/n)
95% Exact
Confidence
Interval of
Difference
1,2
Freedom from Aneurysm-Related
97.9% (143/146) 96.4% (217/225) (-2.8%, 5.4%)
Mortality within 365 Days
1
Confidence level was not adjusted for multiplicity. Confidence interval for difference (Test - SVS Control) in
percentage was calculated by the exact method.
2
Difference represents the (% of patients free from aneurysm-related mortality within 365 days in the population
treated with the test device) - (% of patients free from aneurysm-related mortality within 365 days in the
population undergoing open surgical repair)
24
Page 28

M708500B001
Figure 7: Kaplan-Meier Estimates of Freedom from Aneurysm-Related Mortality within
Test Group vs. SVS Control
365 Days,
Note: eLPS, as described in the figure above, refers to the Test Group.
Table 18: Details of Kaplan-Meier Estimates of Freedom from Aneurysm-Related Mortality within 365
No. at Risk 166 157 151 243 232 227
No. of Events 3 0 0 7 1 0
No. Censored 6 6 12 4 4 21
Kaplan-Meier
Estimate 0.982 0.982 0.982 0.971 0.967 0.967
Treatment
to 30 days
Days, Test Group vs. SVS Control
Test Group SVS Control
31 days to
182 days
183 days to
365 days
Treatment
to 30 days
31 days to
182 days
25
183 days to
365 days
Page 29

M708500B001
Freedom from All-Cause Mortality within 365 Days
Table 19 and Figure 8 provide the analysis and Kaplan-Meier plot of freedom from all-cause mortality at 365 Days.
Additional detail is provided in Table 20.
Table 19: Freedom from All-Cause Mortality within 365 Days, Test Group vs. SVS Control
95% Exact
Confidence
Interval of
Difference
1,2
Related Analysis
Test Group
% (m/n)
SVS
Control
% (m/n)
Freedom from All-Cause Mortality
93.5% (143/153) 92.5% (222/240) (-5.0%, 6.1%)
within 365 Days
1
Confidence level was not adjusted for multiplicity. Confidence interval for difference (Test - SVS Control) in
percentage was calculated by the exact method.
2
Difference represents the (% of patients free from all-cause mortality within 365 days in the population treated
with the test device) - (% of patients free from all-cause mortality within 365 days in the population undergoing
open surgical repair)
26
Page 30

M708500B001
Figure 8: Kaplan-Meier Estimates of Freedom from All-Cause Mortality within 365 Days,
Test Group vs. SVS Control
Note: eLPS, as described in the figure above, refers to the Test Group.
Table 20: Details of Kaplan-Meier Estimates of Freedom from All-Cause Mortality within 365 Days,
No. at Risk 166 157 151 243 232 227
No. of Events 3 3 4 7 4 7
No. Censored 6 3 8 4 1 14
Kaplan-Meier
Estimate 0.982 0.963 0.937 0.971 0.954 0.924
Treatment
to 30 days
Test Group vs. SVS Control
Test Group SVS Control
31 days to
182 days
183 days to
365 days
Treatment
to 30 days
31 days to
182 days
27
183 days to
365 days
Page 31

M708500B001
5.9 Effectiveness
Primary Effectiveness Endpoint: Successful Aneurysm Treatment
The primary effectiveness endpoint, successful aneurysm treatment, was a composite endpoint including
patients who had technical success (successful delivery and deployment of the Talent Stent Graft) at the
initial procedure and
were free from:
• Aneurysm growth > 5mm at 12 months, as evaluated by the core lab; and
• Post-operative interventions to correct Type I/III endoleaks at anytime up to 12 months (Type II
endoleaks are generally considered to be non-device related).
Other clinically relevant measures (see Table 23 through Table 30) of stent graft effectiveness were also
evaluated and are provided separately in the sections below.
As shown in Table 21, the Talent Abdominal Stent Graft achieved a successful aneurysm treatment rate of
90.2%. Table 22 provides details regarding patients who have failed the successful aneurysm treatment
endpoint.
Table 21: Primary Effectiveness Endpoint: Successful Aneurysm Treatment, Test Group
Test Group
Primary Effectiveness Endpoint
%(m/n)
Successful Aneurysm Treatment 90.2% (110/122) (83.4%, 94.8%)
1
Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was
calculated by the exact (binomial) method.
95% Exact
Confidence
Interval
1
Table 22: Primary Effectiveness Endpoint: Successful Aneurysm Treatment, Test Group
Test Group
Patients with Primary Effectiveness Failure
%(m/n)
Unsuccessful (Failure) Aneurysm Treatment 9.8% (12/122)
Technical Failure1 3.3% (4/122)
Aneurysm Growth > 5mm at 12 Months (Core Lab) 2.5% (3/122)2
Post-Operative Interventions To Correct Type I/III Endoleaks 4.1% (5/122)
1
All four technical failures were due to access difficulties. Note: These failures were associated
with a prior iteration delivery system.
2
Of these three patients, two died at day 600 and 692, respectively. One patient death was
attributed to a possible device–related cause (patient refused further treatment). No additional
adverse events were identified with the other patient death.
28
Page 32

Other Effectiveness Data
Other Effectiveness Data
Migration-Free at 12 Months1 99.2% (128/129) 2 (95.8%, 100.0%)
1
Migration is defined as evidence of proximal or distal movement of the stent graft > 10mm
relative to fixed anatomic landmarks.
2
At three-year follow-up, the patient was admitted for endovascular repair of Type I endoleak
(proximal).
3
Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was
calculated by the exact (binomial) method.
Other Effectiveness Data
Stent Graft Patency at 12 Months 100.0% (120/120) (97.0%, 100.0%)
1
Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was
calculated by the exact (binomial) method.
Table 23: Migration-Free at 12 Months, Test Group (Core Lab)
Test Group
%(m/n)
Table 24: Stent Graft Patency at 12 Months, Test Group (Core Lab)
Test Group
%(m/n)
95% Exact
Confidence
Interval3
95% Exact
Confidence
Interval 1
M708500B001
Table 25: Freedom from Secondary Endovascular Procedures within 365 Days, Test Group
Other Effectiveness Data
Secondary Endpoint: Freedom from Secondary
Endovascular Procedures within 365 days
1
The 5 patients who received a secondary endovascular procedure are characterized as follows:
Three (3) patients had endoleaks detected at day 1, 1, and 32, with secondary procedures at Day
69, 74, and 95, respectively. Aortic cuffs were placed to correct Type I endoleaks (proximal).
Repairs were successful.
One (1) patient had endoleak detected at day 103, with a secondary procedure at day 168. Two
(2) iliac limb extensions were placed to correct the Type I endoleak (distal). Repair was
successful.
One (1) patient had graft-blush detected post-procedure, with a secondary procedure at day 183.
An aortic cuff and iliac extension were placed to correct graft blush and stitch hole endoleak.
Repair was successful.
2
Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was
calculated by the exact (binomial) method.
Test Group
%(m/n)
96.5% (138/143) 1 (92.0%, 98.9%)
95% Exact
Confidence
Interval 2
29
Page 33

M708500B001
Loss of Stent Graft Integrity at 12 Months1 2.7% (3/110)2 (0.6%, 7.8%)
1
Loss of stent graft integrity is defined as the occurrence of stent graft wire and/or connecting bar
fracture. Of these 3 patients, 2 had a connecting bar fracture – one at the proximal main body
and the other at the level of the left iliac (source for locations is patient files). The third patient
had a graft wire fracture, located on the second spring row at the proximal aspect of the graft.
2
Of the 3 patients with loss of stent graft integrity, one patient expired at approximately 2 years
due to stroke (CVA). The stent graft did not cause or contribute to the patient death. Another
patient had no endoleak reported at the 1, 6 or 12 month visits. At the 4 year follow-up there were
no endoleaks reported. The remaining patient withdrew from the study 2 years and four months
following the procedure. This patient had no clinical sequelae reported during follow-up.
3
Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was
calculated by the exact (binomial) method.
Endoleak-Free (Type I/III) at 12 Months1 93.4% (113/121)
1
Endoleak-free (Type I/III) at 12 months is defined as patients who did not have Type I/III
endoleak at 12 months time point and did not have a secondary endovascular intervention to
treat a Type I/III endoleak.
2
The 8 patients that were not endoleak-free, include 5 patients that required a secondary
endovascular procedure to treat their endoleaks (previously referenced in Table 22 and Table 25)
and 3 patients that did not require secondary procedures.
3
One (1) patient had a secondary procedure to correct an endoleak at 6 months post implant.
However this patient was not assessable for endoleak at the 12 month follow-up visit. This
represents an increase of 1 in the denominator in the above table as compared to the number of
patients assessable for endoleaks in Table 2
4
Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was
calculated by the exact (binomial) method.
Table 26: Loss of Stent Graft Integrity at 12 Months, Test Group (Core Lab)
95% Exact
Other Effectiveness Data
Test Group
%(m/n)
Confidence
Interval3
Table 27: Type I/III Endoleak-Free at 12 Months, Test Group (Core Lab)
95% Exact
Other Effectiveness Data
Test Group
%(m/n)
2, 3
(87.4%, 97.1%)
Confidence
Interval4
30
Page 34

M708500B001
Table 28: Summary of All Endoleaks at 1 Month and 12 Months, Test Group (Core Lab)
Core Lab
Reported at
Endoleaks
at 12 Months
1 Month1
%(m/n)
Endoleaks of any type 19.3% (29/150) 9.2% (11/120)
Type I 9.3% (14/150) 2.5% (3/120)
Type II 8.7% (13/150) 5.8% (7/120)
Type III 0.0% (0/150) 0.0% (0/120)
Type IV 0.0% (0/150) 0.0% (0/120)
Indeterminate 1.3% (2/150) 0.8% (1/120)
1
Endoleaks reported are not cumulative but represent the number of endoleaks present at each time point.
2
Of these 3 patients, one patient withdrew from the study (post a three year follow-up) prior to a secondary
procedure to treat the endoleak. For the remaining two patients no secondary procedures were reported and
no additional clinical sequelae were reported. All three Type I endoleaks at 12 months were persistent from a
previous follow-up visit, of which one was a secondary endoleak.
3
The 5 patients that required secondary procedures to treat their endoleaks (previously referenced in Table
22 and Table 25) are not captured in this table because their endoleaks had been resolved prior to the 12
month time point.
Core Lab
Reported at
12 Months1
%(m/n)
2,3
Table 29: Aneurysm Rupture within 365 Days, Test Group
95% Exact
Other Effectiveness Data
Test Group
%(m/n)
Confidence
Interval1
Aneurysm rupture within 365 days post implantation 0.0% (0/143) (0.0%, 2.5%)
1
Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was
calculated by the exact (binomial) method.
Table 30: Aneurysm Change from 1 Month to 12 Months,
Test Group (Core Lab and Site-Reported)
Change in Maximum Aneurysm Diameter from 1
Month to 12 Months
Site Reported
%(m/n)
Core Lab Reported
%(m/n)
Increase More than 5mm 4.5% (6/133) 2.3% (3/128)
Stable1 60.9% (81/133) 64.1% (82/128)
Decrease More than mm 34.6% (46/133) 33.6% (43/128)
1
Stable refers to no change (increase or decrease) of more than 5 mm.
5.10 Acute Procedural Data
As shown below, the clinical utility measures of the Talent Abdominal Stent Graft are improved as compared
to surgery with respect to procedure duration, blood loss, length of time in the ICU and hospital, and usage of
general anesthesia. See Table 31 for further information.
31
Page 35

M708500B001
Table 31: Acute Procedural Data, Test Group and SVS Control
Acute Procedural Data Statistics Test Group SVS Control
N 166 241
Duration of procedure (min)
Mean ± SD 167.3 ± 53.17 196.4 ± 82.99 (-43.5, -14.8)
Median 155.0 180.0
Min, max 85, 417 57, 498
N 163
Mean ± SD 152.7 ± 81.50
Contrast Use (cc)
Median 150.0
Patients receiving general
anesthesia
Min, max 15, 370
% (m/n) 40.4% (67/166) 98.7% (222/225) (-65.7%, -50.4%)
N 165 241
Mean ± SD 335.0 ± 282.36 1347.5 ± 1346.91
Estimated blood loss (cc)
Median 250.0 1000.0 (-800.0, -600.0)
Min, max 25, 1750 50, 10763
95% Confidence
Interval of
Difference
1,2
Patients requiring blood
transfusion
% (m/n) 18.2% (30/165) 56.8% (75/132) (-48.6%, -28.0%)
N 166 243
Mean ± SD 19.3 ± 73.88 74.3 ± 178.41
Time in ICU (hours)
Median 0.0 36.0
Min, max 0, 864 0, 1728
Overall
hospita
l stay
(days)
n 166 225
Mean ± SD 3.6 ± 6.38 8.2 ± 7.97 (-6.1, -3.2)
Median 2.0 6.0
Min, max 1, 79 0, 72
1
Confidence level was not adjusted for multiplicity. Confidence intervals for difference (Test-SVS Control) in
means were calculated using a t-distribution. Confidence intervals for difference (Test-SVS Control) in
percentages were calculated by the exact method. Confidence intervals for difference (Test-SVS Control) in
medians were calculated using Hodges-Lehmann estimation of location shift. Confidence interval for Time in
ICU is not calculated due to a large number of ties in the data (i.e. large number of “0 hours” reported in the
Test Group).
2
For Duration of Procedure and Overall Hospital Stay, difference represents the (mean of specific acute
procedural parameter in the population treated with the test device) - (mean of specific acute procedural
parameter in the population undergoing open surgical repair). For Patients Receiving General Anesthesia
and Patients Requiring Blood Transfusion, difference represents the (% of patients with the specific acute
procedural parameter for the population treated with the test device) - (% of patients with the specific acute
procedural parameter for the population undergoing open surgical repair). For Estimated Blood Loss,
difference represents the median shift of estimated blood loss between the two treatment groups (Test-SVS
Control).
32
Page 36

M708500B001
5.11 CoilTrac Delivery System Performance Data
5.11.1 Delivery and Deployment Success
Subsequent to enrollment in the pivotal trial, the delivery system was updated to the CoilTrac Delivery
System. In order to evaluate the clinical performance of the CoilTrac Delivery System, a single-center
cohort of 137 patients from an independent data set was evaluated. The analysis of this independent data
set supports the clinical performance of the CoilTrac Delivery System, demonstrated by delivery and
deployment success rate, as well as, clinically relevant adverse events rates observed within the 30 day
post-procedure period.
Table 32 presents the rate of successful delivery and deployment of the Talent Abdominal Stent Graft using
the CoilTrac Delivery System. A 100% success rate was achieved in 137 patients treated. Successful
delivery and deployment was defined as an initial successful implant procedure that was not aborted and did
not involve delivery system malfunction.
Table 32: CoilTrac Delivery System: Delivery and Deployment Success
Device
Talent Abdominal
Stent Graft with the
CoilTrac Delivery
System
1
Confidence level was not adjusted for multiplicity. Confidence interval for the percentage was
calculated by the exact (binomial) method.
5.11.2 Clinically Relevant Adverse Events Within 30 Days
Table 33 presents the clinically relevant adverse events occurring intra-and peri-operatively for the
patients implanted with the Talent Abdominal Stent Graft using the CoilTrac Delivery System.
The overall rate of patients with at least one clinically relevant adverse event is 15.3% (21/137) with a
two-sided 95% exact confidence interval (9.7%, 22.5%). There were no reports of rupture, surgical
conversion, branch vessel occlusion or migration.
Table 33: CoilTrac Delivery System: Patients with Clinically Relevant Adverse Events [Within 30 Days]
Performance Measure
(Site-Reported)
Successful Stent Graft
Delivery and Deployment
Category
N = 137
% (m/n)
100.0% (137/137) (97.3%, 100.0%)
95% Exact
Confidence
Interval
N = 137
%(m/n)
1
All-cause mortality 1.5% (2/137) 1
AAA rupture 0.0% (0/137)
Conversion to open repair 0.0% (0/137)
Branch vessel occlusion: renal artery/superior mesenteric artery 0.0% (0/137)
Stent graft occlusion 1.5% (2/137)
Stent graft migration 0.0% (0/137)
Device-specific endoleaks 8.8% (12/137) 2
Access site wound infection 2.2% (3/137)
Access site wound hematoma 3.6% (5/137)
1
Both deaths were unrelated to the aneurysm, procedure, or device.
2
Type I endoleak = 7 patients, Type III endoleak = 0 patients, Unknown Type endoleak = 5 patients
33
Page 37

M708500B001
6.0 PATIENT SELECTION
6.1 Individualization of Treatment
Medtronic recommends that the Talent Abdominal Stent Graft component diameters be selected as
described in Table 34. The length of the Talent Abdominal Stent Graft should extend from the distal edge
of the lowest renal artery to just above the origin of the internal iliac (hypogastric) artery. In addition, the
aortic length should be > 1.0cm longer than the main body portion of the chosen bifurcated model. All
lengths and diameters of the devices necessary to complete the procedure should be available to the
physician, especially when pre-operative case planning measurements (treatment diameters/lengths) are
not certain. This approach allows for greater intraoperative flexibility to achieve optimal procedural
outcomes. The warnings and precautions previously described in Section 3.0 should be carefully
considered relative to each patient before use of the Talent Abdominal Stent Graft System. Additional
considerations for patient selection include, but are not limited to:
• Patient's age and life expectancy
• Co-morbidities (e.g., cardiac, pulmonary or renal insufficiency prior to surgery, morbid obesity)
• Patient's suitability for open surgical repair
• Patient's anatomical suitability for endovascular repair
• The risk of aneurysm rupture compared to the risks of endovascular repair
• Ability to tolerate general, regional or local anesthesia
• Iliofemoral access vessel size and morphology (minimal thrombus, calcium and/or tortuosity)
should be compatible with vascular access techniques of the various delivery catheter profiles.
• The Talent Abdominal Stent Graft with Xcelerant HDS is delivered through a vascular graft
cover.
• Adequate iliac/femoral access compatible with the required delivery systems (a diameter of > 7
mm)
• Non-aneurysmal aortic neck between the renal arteries and the aneurysm:
A proximal aortic neck length of >
Proximal aortic neck angulation ≤ 60°
An aortic diameter of 18–32mm
• Common iliac artery distal fixation site:
Distal iliac artery fixation length of >
Iliac artery diameters of 8–22mm
• Freedom from significant femoral/iliac artery occlusive disease that would impede flow through
the vascular graft.
The final treatment decision is at the discretion of the physician and patient.
10mm
15mm
7.0 PATIENT COUNSELING INFORMATION
The physician should consider the following points when counseling the patient about this endovascular
device and procedure:
• Differences between endovascular repair and open surgical repair
• Pros and cons of open surgical repair and endovascular repair, including the fact that endovascular
• The long term effectiveness of endovascular repair has not been established
• Symptoms of aneurysm rupture
• Further counseling information can be found in the Patient Information Booklet
Medtronic recommends that physicians use the Medtronic Patient Information Booklet to aid in describing
risks associated with use of the Talent Abdominal Stent Graft with Xcelerant HDS with the patient.
Additionally Medtronic recommends that detailed patient specific risks also be discussed.
• Risks related to open surgical repair
• Risks related to endovascular repair
• Risks related to non-interventional treatment (medical management)
repair possesses potential advantages related to its minimally invasive approach. It is possible that
subsequent endovascular or open surgical repair of the aneurysm may be required. Regular follow-up,
including imaging of the device, should be performed as recommended in Table 36 (Section11.2), or
more frequently in patients with enhanced surveillance needs.
8.0 HOW SUPPLIED
8.1 Contents
The Talent Abdominal Stent Graft System components are available in the configurations identified in Section
1.0
In addition to the device, each carton contains:
• One (1) set of patient tracking materials
• One (1) instructions for use reference
34
Page 38

8.2 Sterility and Storage
• Never attempt to resterilize a Talent Abdominal Stent Graft or Xcelerant HDS. Resterilization may
adversely affect the proper mechanical function of the stent graft or delivery system and could
result in patient injury and/or conversion to an open surgical procedure.
• For single use only. Delivery systems are disposable; do not reuse.
• Store at room temperature in a dark, dry place
9.0 CLINICAL USE INFORMATION
9.1 Recommended Skills and Training
Physicians using the Talent Abdominal Stent Graft with Xcelerant HDS must be trained in vascular
interventional procedures and in the use of this device.
The recommended skill/knowledge requirements for physicians using the Talent Abdominal Stent Graft
with Xcelerant HDS are outlined below:
9.1.1 Patient selection:
• Knowledge of the natural history of abdominal aortic aneurysms and comorbidities associated with
abdominal repair; and
• Knowledge of image interpretation, stent graft selection and sizing.
9.1.2 Physician skills and experience
Either the individual physician operator or a combined, multidisciplinary team should possess extensive
procedural skills and experience with:
• Femoral cutdown, arteriotomy, and repair;
• Non-selective and selective catheterization;
• Live fluoroscopic and angiographic image interpretation;
• Embolization;
• Angioplasty;
• Endovascular stent graft placement;
• Snare techniques;
• Appropriate use of contrast material; and
• Techniques to minimize radiation exposure.
9.2 Materials Recommended for Device Implantation
At the time of surgery, it is recommended that physicians have available:
• At least one additional set of Talent Abdominal Stent Grafts (of the sizes intended for implantation)
in the event that a device is contaminated or damaged during attempted placement
• Additional Talent Abdominal Stent Grafts (one size larger and one size smaller) in the event that the
original measurement underestimated or overestimated vessel sizes
• Additional aortic and iliac extension cuffs of various lengths and diameters to customize the implant
in order to fit the anatomy of the individual patient
• Fluoroscope - Freely-angled C-arm with digital angiography capabilities and the ability to record
and recall imaging
• Contrast media
• Introducer sheaths for vascular access to access arteries and to perform diagnostic imaging
• Assorted angiographic catheters, angioplasty catheters, graduated pigtail catheters
• Assorted guidewires
• Reliant® Stent Graft Balloon Catheter and other materials recommended by the Reliant Instructions
for Use
• Heparin and heparinized saline solution
• Inflation device with pressure gauge
• Radiopaque ruler with centimeter increments or radiopaque marker board
• Power injector and extension tubing
• Selection of 2-way and 3-way stopcocks
• Embolic coils
• Puncture needles
• Sterile markers (to mark skin, endvascular instruments or monitoring screen)
• Snare devices
• Surgical instruments and supplies
• Sterile normal saline ( for hydrophilic coating activation)
• Sterile gauze
Intravascular ultrasound (IVUS) imaging equipment may also be useful if available, but only if the
physician is experienced with its use. IVUS measurements performed at the time of treatment can
provide supplementary data regarding vessel size
M708500B001
35
Page 39

M708500B001
The Reliant Stent Graft Balloon Catheter is packaged separately. This compliant balloon is used to assist
in stent graft implantation by modeling the covered springs and to remove wrinkles and folds from the
graft material. Sub-optimal expansion of self-expanding stent grafts may be improved by use of the
Reliant Stent Graft Balloon Catheter.
WARNING: DO NOT USE THE RELIANT STENT GRAFT BALLOON IN THE TREATMENT OF
DISSECTIONS.
NOTE: The Reliant Balloon is recommended for use with the Talent AAA Stent Graft. Data is not
available for use with other balloons for remodeling stent grafts.
9.3 Pre-Treatment Planning
Correct sizing of the aorta and iliac vessels must be determined before implantation of the Talent
Abdominal Stent Graft with Xcelerant HDS. Medtronic Vascular recommends using spiral computer
aided tomography (CT) as well as angiograms of both the iliacs and aorta. These images should be
available for review during the procedure.
CAUTION: Do not withdraw the Talent AAA Stent Graft from the graft cover before placing it in the
proper anatomical location. The Talent AAA Stent Graft cannot be reconstrained or drawn back into the
graft cover, even if the stent graft is only partially deployed.
CAUTION: Never advance or retract equipment from the vasculature without the use of fluoroscopy.
Each Talent Abdominal Stent Graft must be sized appropriately to fit the patient's anatomy. Sizing must
be to the vessel wall, not thrombus. Proper sizing of the device is the responsibility of the physician. See
the recommended oversizing guidelines in Table 34.
• Vessel over-distension and damage may be caused by excessive oversizing of the stent graft in
relation to the diameter of the blood vessel.
• Undersizing of the stent graft may lead to device migration and/or endoleaks.
Physicians may consult Medtronic Vascular for guidance in determining proper device dimensions based
on the physician's assessment of the patient's anatomical measurements.
Relevant materials should be readily available as listed in Section 9.2. Cutdown and vessel access are
required and in some cases vessel by-pass may be required. A vascular surgical team should be readily
available (i.e., within the same facility) in case of emergency conversion to an open surgical repair.
To reduce the risk of thromboembolism, it is recommended that patients are anticoagulated during the
procedure, at the discretion of the physician.
If necessary, open narrow iliac vessels with standard Percutaneous Transluminal Angioplasty (PTA)
catheters prior to Talent Abdominal Stent Graft with Xcelerant HDS placement (according to standard
endovascular procedures). If necessary, dilate the vessel with a tapered vessel dilator. A step-up
approach is recommended for vessel dilation.
Table 34: Talent Abdominal Stent Graft with Xcelerant HDS Oversizing Guidelines
(mm)
8 8
9-10 10
11-12 12
13-14 14
15 16
16-17 18
18-19 20 22
20-21 22 24
22 24
23
24-25 28
26-27 30
28-29 32
30-31 34
32
Recommended Talent Diameter (mm) Native Vessel Diameter
Iliac Aorta
26
36
36
Page 40

M708500B001
10.0 DIRECTIONS FOR USE – TALENT ABDOMINAL STENT GRAFT WITH XCELERANT
HDS
10.1 Pictorial References
For pictorial references of the Talent Abdominal Stent Graft components with the Xcelerant HDS refer to
Figure 1 and Figure 5 respectively.
10.2 Vascular Access and Arteriotomy
Following aseptic procedural guidelines perform arteriotomies at the access sites. Place a guidewire in
the ipsilateral femoral artery and advance it above the renal arteries. From the contralateral side femoral
artery, place a second guidewire directed to the abdominal aorta. Over the guidewire, place an
angiography catheter above the renal arteries.
10.3 Implantation of the Bifurcated Stent Graft
10.3.1 Preparation of the Xcelerant HDS
10.3.1.1 Carefully inspect the sterile package for damage or defects before opening.
10.3.1.2 Do not use product after the “Use By” date on the package. If the integrity of the
sterile package has been compromised or the packaging or product is defective, do not
use the product. Contact your Medtronic Vascular representative for return information.
10.3.1.3 Place an angiographic flush catheter above the renal arteries and take an angiogram
10.3.1.4 Flush the guidewire lumen with heparinized saline.
10.3.1.5 Align the stent graft radiopaque markers with the patient’s anatomy
10.3.1.6 Before inserting the device into the vasculature, visualize the radiopaque markers on
the stent graft to identify positioning of the device within the sheath.
10.3.2 Hydrophilic Coating Activation:
10.3.2.1 Saturate sterile gauze in sterile saline.
10.3.2.2 Gently wipe the surface of graft cover with saturated gauze until graft cover is
wet/slippery to touch.
NOTE: It is important to keep the graft cover surface wet/slippery to touch during insertion of the delivery system.
Once the hydrophilic coating on the delivery system is activated the delivery system will be wet/slippery.
NOTE: Prior to insertion, it is advisable to view the delivery system under fluoroscopy to visualize the radiopaque
markers on the stent graft. The radiopaque markers indicate the location of the connecting bar and the edge of the
graft material. Turn the graft cover to align the radiopaque marker on the short stub leg (Figure 2) with the patient’s
contralateral iliac artery (Figure 3). Observe the position of the dot on the front grip of the Xcelerant HDS. This will
serve as a reference for the orientation of the short stub leg.
10.3.3 Introduce System
CAUTION: Never advance or retract the Xcelerant HDS from the vasculature without the use of fluoroscopy.
CAUTION: Do not continue advancing any portion of the delivery system if resistance is felt during advancement of
the guidewire or delivery system. Stop and assess the cause of resistance. Vessel or catheter damage may occur.
Exercise particular care in areas of stenosis, intravascular thrombosis or in calcified or tortuous vessels.
CAUTION: The Talent Abdominal Stent Graft cannot be reconstrained or drawn back into the delivery system,
even if the stent graft is only partially deployed.
CAUTION: Before initial deployment, it is suggested to position the stent graft slightly higher than the targeted
location.
10.3.3.1 Slowly insert the Xcelerant HDS. Advance the delivery system over the guidewire so
that the most proximal spring of the stent graft and the radiopaque markers are visualized
at the target location in the proximal aortic neck (Figure 9)
37
Page 41

M708500B001
Figure 9: Introduce the System for the Bifurcated Segment
10.3.3.2 Inject contrast media into the abdominal aorta and mark the position of the target
location, either on the imaging screen or with radiopaque marker or angiographic ruler on
the patient’s body. Adjust the position of the stent graft such that the top edge of the graft
fabric is just below the lowest renal artery. See Figure 10.
38
Page 42

Figure 10: Position the System
M708500B001
TOP EDGE OF
GRAFT FABRIC
STENT STOP
POSITION
NOTE: In cases of suprarenal fixation, ensure graft material is below both renal arteries. If the top edge
of the graft fabric is to be placed very close to the renal arteries, contrast media may be injected to
identify the location of the lower renal artery and verify the position before fully deploying device. Once
proximal position has been identified, do not move patient or imaging equipment. The angiographic
catheter can be removed prior to deployment. However, if the angiographic catheter is not removed until
after deployment, ensure that the tip is straightened (pigtail catheter) with a guidewire before removal so
that the stent graft is not pulled down.
CAUTION: When aligning the position of the Xcelerant HDS so that the Talent Abdominal Stent Graft is
in proper position for deployment within the vessel, BE SURE THAT THE FLUOROSCOPE IS ANGLED
PERPENDICULARLY TO THE CENTER LINE OF THE INFRARENAL AORTA TO AVOID PARALLAX
OR OTHER SOURCES OF VISUALIZATION ERROR. ALIGN THE TARGET AREA/FIXATION ZONE
(E.G., NECK) IN THE CENTER OF THE FIELD. Some cranial-caudal angulation of the I-I tube may be
necessary to achieve this, especially if there is anterior angulation of the aneurysm neck.
RADIOPAQUE
MARKER FOR
SHORT STUB LEG
10.3.4 Confirm Position
10.3.4.1 Ensure that the distal portion of the contralateral stub leg is above the aortic
10.3.5 Deploy Proximal End
10.3.5.1 In order to deploy the proximal end of the stent graft, first hold the delivery system
bifurcation and within the aneurysmal sac, and not within the iliac vessel. Rotate the graft
cover until the radiopaque marker on the distal-most spring of the short leg is aligned with
the contralateral iliac artery [Note: If the right leg is the entry limb, the radiopaque marker
should be aligned with the left limb]. See Figure 10.
stationary with one hand on the front grip. Then, slowly withdraw the graft cover with the
other hand by rotating the slider counter clockwise, until the proximal most spring has
39
Page 43

M708500B001
FRONT
GRIP
SLIDER
been fully deployed. Be sure to keep the delivery system as straight as possible.2 See
Figure 11.
Figure 11: Deploy the Proximal End
10.3.5.2 It is recommended that the physician rotate the slider until the contralateral stub leg is
deployed. At any point, use thumb to pull the trigger on the slider back in order to more
rapidly deploy the stent graft. See
CAUTION: When using the trigger to rapidly deploy the stent graft, be sure to hold the front grip of the
delivery system stationary. Do not rotate the graft cover during this step.
2
In the unlikely event of delivery system failure and concomitant partial stent graft deployment due to graft cover
severence, a “handle dis-assembly” technique will permit successful deployment of the stent graft. See instructions
at the end of this section for details of the technique.
40
Page 44

TRIGGER
Figure 12: Deploy the Distal End
M708500B001
10.3.6 Use angiography to verify the position of the stent graft in relation to the renal arteries.
10.3.7 If the position is too high, maintain the position of the slider and pull down on the entire delivery
system.
CAUTION: If the graft cover is accidentally withdrawn, the device will prematurely deploy and be incorrectly
positioned.
10.4 Deploy Distal End
10.4.1 While holding the delivery system stationary, withdraw the graft cover until the distal spring of the ipsilateral
iliac segment is completely deployed.
CAUTION: Do not rotate the graft cover during deployment, as this may torque the device and cause it to spin
on deployment or cause twisting of the iliac limb.
10.5 Delivery System Removal
10.5.1 Depress the Quick Disconnect and retract the tapered tip. Use continual fluoroscopy and watch the top of
the Talent Abdominal Stent Graft while slowly pulling back the tapered tip into the graft cover of the Xcelerant
HDS.
NOTE: To prevent the stent graft from being caught between the graft cover and the tapered tip, ensure that
the graft cover is fully retracted. See Figure 13.
41
Page 45

M708500B001
QUICK
DISCONNECT
Figure 13: Use the Quick Disconnect to Retract the Tapered Tip
10.5.2 Gently remove the Xcelerant HDS. Do not use excessive force. Use fluoroscopy to ensure that the
stent graft does not move during the withdrawal.
NOTE: Maintain vessel access until all Talent stent graft segments are placed.
10.6 Preparation of the Contralateral limb with the Xcelerant HDS
10.7 Implantation of the Contralateral Limb
10.7.1 Introduce System
See Section 10.3.1 for device preparation.
10.7.1.1 To insert the limb graft cover into the patient’s contralateral side, insert a guidewire
through the short stub leg and the aortic neck portion of the previously placed Talent
Bifurcated Stent Graft.
10.7.1.2 Place the Xcelerant HDS over the guidewire and into the short stub leg of the
deployed bifurcated stent graft. See Figure 14.
42
Page 46

M708500B001
Figure 14: Introduce the Contralateral Limb
10.8 Confirm Position
10.8.1 Insert the contralateral limb into the short stub leg of the bifurcated stent graft. The proximal spring of the
iliac mating section should be inside and completely above the distal spring of the short stub leg.
10.8.2 To ensure proper docking of the contralateral limb, align the short stub leg radiopaque marker with the
proximal contralateral limb radiopaque marker (or at least 3 cm of overlap). See Figure 15.
CAUTION: Position the connecting bar to align with the medial aspect of the iliac artery.
CAUTION: Ensure by fluoroscopic examination that aortic segment is not pulled down when deploying
contralateral limb in the short stub leg (contralateral side).
43
Page 47

M708500B001
Figure 15: Position Contralateral Limb
RADIOPAQUE
MARKER
ALIGNMENT
10.9.1 While holding the delivery system stationary, slowly withdraw the graft cover as described in Section 3 and
10.10.1 Depress the Quick Disconnect and retract the tapered tip as shown in Figure 10. Use continual fluoroscopy
10.10.2 Gently remove the Xcelerant HDS. Do not use excessive force. Use fluoroscopy to ensure that the stent
10.9 Deploy Stent Graft
depicted Figure 11 and Figure 12. Verify that the proximal spring is deploying in the correct position within the
short stub leg. Do not rotate the graft cover during deployment.
CAUTION: Ensure by fluoroscopic examination that aortic segment is not pulled down when straightening
contralateral short stub leg.
10.10 Remove Delivery System
and watch the top of the Talent AAA Stent Graft while slowly pulling back the tapered tip into the graft cover of
the Xcelerant HDS. This establishes a smooth transition between the tapered tip and the graft cover. To
prevent the stent graft from being caught between the graft cover, ensure that the graft cover is fully retracted.
graft does not move during the withdrawal. See Figure 16.
3
3
In the unlikely event of delivery system failure and concomitant partial stent graft deployment due to graft cover
severence, a “handle dis-assembly” technique will permit successful deployment of the stent graft. See instructions
at the end of this section for details of the technique.
44
Page 48

Figure 16: Remove the Delivery System
M708500B001
NOTE: Care should be taken when inflating the balloon, especially with calcified, tortuous, stenotic, or otherwise
diseased vessels. Inflate slowly. It is recommended that a backup balloon be available.
10.11 Stent Graft Balloon Modeling
The Reliant Stent Graft Balloon Catheter can be used to assist in stent graft implantation by modeling
the covered springs and to remove wrinkles and folds from the graft material. Sub-optimal expansion of
the self-expanding stent graft(s) may also be improved by use of the Reliant Stent Graft Balloon. Refer to
the Instructions for Use supplied with the Reliant Stent Graft Balloon Catheter for more information.
45
Page 49

M708500B001
L
G
DISTAL END
OF
IPSILATERAL
LIMB
PROXIMAL SPRIN
CONTRALATERAL
LIMB JUNCTION
DISTAL END OF
CONTRALATERA
LIMB
10.12 Procedure Completion
10.12.1 Final Angiogram
10.12.1.1 At the completion of the procedure, perform angiography to assess the Talent AAA
Stent Graft for proximal and distal endoleaks and to verify the position of the implanted
stent graft in relation to the aneurysm and renal arteries. Leaks at the attachment or
connection sites should be treated by using the balloon to remodel the stent graft against
the vessel wall. Major leaks that cannot be corrected by re-ballooning may be treated by
adding Talent Stent Graft Extension Cuff(s) to the previously placed stent graft.
CAUTION: Any leak left untreated during the implantation procedure must be carefully
monitored after implantation.
10.12.1.2 In the event that an extension (iliac or aortic cuff) is used, the mating sections are
joined by aligning the radiopaque markers. Radiopaque markers indicate the MINIMUM
recommended overlap. The radiopaque markers used for mating are the radiopaque
markers that are offset 30 mm from the overlapping end of the extension. The edge of
the graft material and the connecting bar are indicated by the proximal and distal
radiopaque markers. See Figure 17 and Figure 18 for orientation of iliac and aortic cuffs.
CAUTION: When deploying the aortic cuff, it is highly recommended to rotate the slider until
the cuff is fully deployed as described in SECTION 10.3, IMPLANTATION OF THE
BIFURCATED SEGMENT OF THE STENT GRAFT and depicted in Figure 11 and Figure 12.
46
Page 50

M708500B001
10.13 Aortic and Iliac Extensions
10.13.1 Usage of Radiopaque Markers to Ensure Minimum Overlap
In the event that an extension (iliac or aortic extension cuff) is used, the mating sections are joined
by aligning specific radiopaque markers. These radiopaque markers indicate the MINIMUM
recommended overlap. The radiopaque markers used for mating are offset 30mm from the end of
the extension. The edges of the graft material and the connecting bar are indicated by the proximal
and distal radiopaque markers.
Figure 17: Iliac Extension Cuffs
Radiopaque Markers
Align Figur8
Figure 18: Orienting the Aortic Extension Cuff
NOTE: Figure 17 and are graphical representations only. May appear differently when viewed under
fluoroscopy.
10.13.2 Close the Entry Site
Remove the introducer and the guidewire. Repair the entry site with standard closure techniques.
If, during placement of the Talent Abdominal Stent Graft, the arteries used for access to the aorta are
injured, additional endovascular and/or surgical procedures to repair the injury will need to be
Align Figur8
Radiopaque Markers
47
Page 51

M708500B001
performed. If vascular repair becomes necessary, follow appropriate institutional guidelines,
including guidelines regarding continuation or termination of the overall stent graft procedure.
10.14 Handle Dis-assembly Technique
In the unlikely event of delivery system failure and concomitant partial stent graft deployment due to
graft cover severance, a “handle dis-assembly” technique will permit the successful deployment of the
stent graft. See instructions below.
10.14.1 Pull back the trigger and fully retract the slider. Note: Since the graft cover is severed, the slider can be
retracted without further deploying the stent graft.
10.14.2 Stabilize the Delivery System.
10.14.3 Insert the tips of a pair of hemostats into each one of the handle disassembly ports on the front grip.
10.14.4 Disengage the front grip from the screw gear by pressing the tips of the hemostats into the handle
disassembly ports and simultaneously advancing the front grip away from the screw gear.
10.14.5 Advance the front grip until it fully clears the screw gear.
10.14.6 Separate the screw gear halves in order to identify the location of graft cover severance.
10.14.7 Manually retract the graft cover with your fingers or with hemostats until the stent graft is fully deployed.
10.14.8 Follow the standard instructions for use for delivery system removal.
11.0 IMAGING GUIDELINES AND POST-OPERATIVE FOLLOW-UP
11.1 General
All patients should be advised that endovascular treatment requires life-long, regular follow-up to assess
their health and the performance of their endovascular graft. Patients with specific clinical findings (e.g.,
endoleaks, enlarging aneurysms, or changes in the structure or position of the endovascular graft)
should receive additional follow-up. Patients should be counseled on the importance of adhering to the
follow-up schedule, both during the first year and at yearly intervals thereafter. Patients should be
informed that regular and consistent follow-up is a critical part of ensuring the ongoing safety and
effectiveness of endovascular treatment of AAAs.
Physicians should evaluate patients on an individual basis and prescribe follow-up relative to the needs
and circumstances of each individual patient. The recommended imaging schedule is presented in Table
35. This schedule outlines the minimum requirement for patient follow-up and should be maintained even
in the absence of clinical symptoms (e.g., pain, numbness, weakness). Patients with specific clinical
findings (e.g., endoleaks, enlarging aneurysms, or changes in the structure or position of the stent graft)
should receive follow-up at more frequent intervals.
Annual imaging follow-up may include abdominal radiographs and both contrast and non-contrast CT
examinations and duplex ultrasounds. If renal complications or other factors preclude the use of image
contrast media, abdominal radiographs, non-contrast CT, and duplex ultrasound should be used.
• The combination of contrast and non-contrast CT imaging provides information on aneurysm
diameter change, endoleak, patency, tortuosity, progressive disease, fixation length and other
morphological changes.
• The abdominal radiographs provide information on device integrity (separation between
components and stent fracture).
• Duplex ultrasound imaging may provide information on aneurysm diameter change, endoleak,
patency, tortuosity and progressive disease. In this circumstance, a non-contrast CT may be
performed to use in conjunction with the ultrasound, since ultrasound may be less reliable.
Ultrasound may be a less reliable and sensitive diagnostic method compared to CT.
Table 35 lists the minimum requirements for imaging follow-up for patients with the Talent Abdominal
Stent Graft.
48
Page 52

M708500B001
Table 35: Recommended Imaging Schedule for Endovascular Graft Patients
Interval Angiogram
[Contrast & Non-Contrast]
CT1
Abdominal
Radiographs
Pre-procedure X2
Procedural X
1 Month X
12 Months (annually thereafter) X
1
A six month follow-up with CT Scan is recommended if an endoleak is reported at 1 month after the procedure
2
Imaging should be performed within 6 months before the procedure.
3
Duplex ultrasound may be used for those patients experiencing renal failure or who are otherwise unable to undergo
contrast enhanced CT scan. W ith ultrasound, non-contrast CT is still recommended.
4
If a Type I or III endoleak is present, prompt intervention and additional follow-up post-intervention is recommended. See
3,4
3,4
X
Section 13.0.
Ultimately, it is the physician’s responsibility, based on previous clinical results and the overall clinical
picture, to determine the appropriate imaging schedule for a particular patient.
11.2 Contrast And Non-Contrast CT Recommendations
• Film sets should include all sequential images at the lowest possible slice thickness (<3mm). Do
not perform large slice thickness (>3mm) and/or omit consecutive CT images/films sets, as this
prevents precise anatomical and device comparisons over time.
• All images should include a scale for each film/image. Images should be arranged no smaller than
20:1 images on 14 inch X 17 inch sheets if film is used.
• Both non-contrast and contrast runs are required, with matching or corresponding table positions.
• Pre-contrast and contrast run slice thicknesses and intervals must match.
• DO NOT change patient orientation or re-landmark the patient between non-contrast and contrast
runs.
Non-contrast and contrast enhanced baseline and follow-up imaging are important for optimal patient
surveillance. It is important to follow accepted imaging protocols during the CT exam. Table 36 lists
examples of accepted imaging protocols.
Table 36: Accepted Imaging Protocols
Non-Contrast Contrast
IV contrast No Yes
Acceptable
machines
Spiral capable of > 40 seconds Spiral capable of > 40 seconds
Injection volume N/A 150cc
Injection rate N/A > 2.5cc/sec
Injection mode N/A Power
Bolus timing N/A
Test bolus: SmartPrep, C.A.R.E. or
equivalent
Coverage - start Diaphragm 1 cm superior to celiac axis
Coverage - finish Proximal femur Profunda femoris origin
Collimation <3mm <3mm
Reconstruction
Axial DFOV
2.5 mm throughout - soft
algorithm
32cm 32cm
2.5mm throughout - soft algorithm
Post-injection runs None None
11.3 Abdominal Radiographs
The following views are suggested:
• Four films: supine-frontal (AP), cross-table lateral, 30 degree LPO and 30 degree RPO views
centered on the umbilicus.
• Record the table-to-film distance and use the same distance at each subsequent examination.
Ensure the entire device is captured on each single image (formatted lengthwise).
If there is any concern about the device integrity (e.g., kinking, stent breaks, migration), it is
recommended to use magnified views. The attending physician should evaluate films for device integrity
(entire device length including components) using 2-4X magnification visual aid.
49
Page 53

M708500B001
11.4 Ultrasound
Ultrasound imaging may be performed in place of contrast CT when patient factors preclude the use of
image contrast media. In order to help support accurate evaluation, ultrasound images should be paired
with non-contrast CT images. A complete aortic duplex should be videotaped and analyzed for maximum
aneurysm diameter, endoleaks, stent patency and stenosis. Included on the videotape should be the
following information as outlined below:
• Transverse and longitudinal imaging should be obtained from the level of the proximal aorta,
including complete imagery from the mesenteric and renal arteries to the iliac bifurcations to
determine if endoleaks are present. Utilize color flow and color power angiography (if available).
• Spectral analysis confirmation should be performed for any suspected endoleaks.
• Transverse and longitudinal imaging of the maximum aneurysm should be obtained.
12.0 MRI SAFETY AND COMPATIBILITY
Non-clinical testing has demonstrated that the Talent Abdominal Stent Graft is MR Conditional. It can be
scanned safely in both 1.5T & 3.0T MR systems under the following conditions:
1.5 Tesla Systems:
• Static magnetic field of 1.5 Tesla
• Spatial gradient field of 1000 Gauss/cm
• Maximum whole-body-averaged specific absorption rate (SAR) of 4W/kg for 15 minutes of
scanning.
Based on non-clinical testing, the device was determined to produce a temperature rise of less than 1°C
at a maximum whole body averaged specific absorption rate (SAR) of 4 W/kg for 15 minutes of MR
scanning in a 64MHz whole body transmit coil, which corresponds to a static field of 1.5T. The
maximum whole body averaged specific absorption rate (SAR) was derived by calculation and verified by
calorimetry.
3.0 Tesla Systems:
• Static magnetic field of 3.0 Tesla
• Spatial gradient field of 1000 Gauss/cm
• Maximum whole-body-averaged specific absorption rate (SAR) of 4W/kg for 15 minutes of scanning
(or the maximum SAR allowed by the MR System, whatever is less).
Based on non-clinical testing, the device was determined to produce a temperature rise of less than 1°C
at a maximum whole body averaged specific absorption rate (SAR) of 4 W/kg for 15 minutes of MR
scanning in a 3 Tesla Siemens TrioTIM (VB 13 Software) MR scanner. The maximum whole body
averaged specific absorption rate (SAR) was derived by calculation and verified by calorimetry.
Image Artifact (1.5 Tesla & 3 Tesla Systems):
MR image quality may be compromised if the area of interest is in the same area or relatively close to
the position of the device. Therefore, it may be necessary to optimize MR imaging parameters for the
presence of this implant. The image artifact extends approximately 5 and 8mm from the device, both
inside and outside the device lumen when scanned in non-clinical testing using the sequence: spin echo
and gradient echo, respectively in a 3.0T Siemens TrioTIM (VB 13 Software) MR system with a whole
body coil.
Patients with Talent Abdominal Stent Grafts implanted in the abdominal aorta may safely undergo MRI
for Normal Mode and First Level Controlled Operating Mode of the MR System, as defined in IEC
Standard 60601-2-33.
13.0 ADDITIONAL SURVEILLANCE AND TREATMENT
Additional surveillance and possible treatment is recommended for:
• Aneurysms with endoleak
• Aneurysm enlargement, > 5mm of maximum diameter (regardless of endoleak status)
• Migration
• Inadequate seal length
• Fracture
Consideration for reintervention or conversion to open repair should include the attending physician's
assessment of an individual patient's co-morbidities, life expectancy, and the patient's personal choices.
Patients should be counseled that subsequent re-intervention, including the fact that catheter-based and
open surgical conversion may become necessary following an endograft procedure.
50
Page 54

M708500B001
14.0 DEVICE-RELATED ADVERSE EVENTS REPORTING
Any adverse event (clinical incident) involving the Talent Abdominal Stent Graft System should be reported to
Medtronic Vascular immediately. To report an incident, call (800) 465-5533 (in the US).
15.0 DEVICE REGISTATION PACKET
The Talent Abdominal Stent Graft System is packaged with additional specific information which includes:
Upon receipt of the device tracking form, Medtronic will mail the patient a permanent device identification card.
This card includes important information regarding the implanted stent graft. Patients should refer to this card
anytime they visit health practitioners, particularly for any diagnostic procedures (e.g. MRI). Patients should carry
this card with them at all times. If a patient does not receive their permanent device identification card, or requires
changes to the card, call 1-800-551-5544. In addition a patient information booklet (PIB) will be provided to the
physicians during training and additional copies will be available upon request. The PIB will also be available
online on the Medtronic website (www.medtronic.com
abdominal aortic aneurysms and endovascular repair therapy.
• Temporary Device Identification Card that includes both patient and stent graft information.
Physicians should complete this card and instruct the patient to keep this card in their possession at
all times. The patients should refer to this card anytime they visit additional health practitioners,
particularly for any additional diagnostic procedures (e.g. MRI). This temporary identification card
should only be discarded when permanent identification card is received.
• Device Tracking Form to be completed by the hospital staff and forwarded to Medtronic for the
purposes of tracking all patients who received a Talent Abdominal Stent Graft (as required by
Federal Regulation). The hospital’s submission of the device tracking form to Medtronic is also
required for a patient to receive the permanent identification card.
). This booklet provides patients with basic information on
51
Page 55

M708500B001
16.0 CONFIGURATIONS AVAILABLE
Table 37: Bifurcated Stent Grafts with the Xcelerant HDS Delivery System
OD
(Fr.)
24
Bifurcated
(mm x mm)
36x20
36x18
34x20
34x18
34x16
32x20
32x18
32x16
32x14
30x20
30x18
30x16
30x14
28x20
28x18
28x16
28x14
Covered
Length
(mm)
155, 170
140, 155,
170
Proximal
Configuration
FreeFlo
Distal
Configuration
Closed Web
26x18
22
The delivery system working length is 52cm. The total length of the stent graft can be determined by adding
approximately 15mm to the covered length shown above.
26x16
26x14
26x12
24x14
24x12
22x14
22x12
140, 155 Bare Spring
52
Page 56

Table 38: Contralateral Limbs with the Xcelerant HDS Delivery System
OD
(Fr.)
20
Contralateral
Limb
(mm x mm)
14x24
14x22
Covered
Length
(mm)
Proximal
Configuration
Distal
Configuration
M708500B001
14x20
14x18
14x16
18
The delivery system working length is 52cm. The total length of the stent graft can be determined by adding
approximately 15mm to the covered length shown above.
Table 39: Iliac Extension Cuffs with the Xcelerant HDS Delivery System
OD
(Fr.)
20
14x14
14x12
14x10
14x8
Iliac
Extension
(mm x mm)
22x22 79
22x18 74
18x24
18x22
18x18
18x16
18x14
18x12
20x16 74
20x20 79
75, 90,
105
105
Covered
Length
(mm)
80
140
Open Web Closed Web
Proximal
Configuration
Distal
Configuration
18x20
18x18
18x16
18x14 75
18
The delivery system working length is 52cm. The total length of the stent graft can be determined by adding
approximately 15mm to the covered length shown above.
18x12
16x16
16x12 75
14x14 80
14x10 75
12x12 81
12x08 75
10x10 81
80
80
Open Web Closed Web
53
Page 57

M708500B001
The catheter working length is 52cm. The total length of the stent graft can be determined by adding
approximately 30mm to the covered length shown above.
Table 40: Aortic Extension Cuffs with the Xcelerant HDS Delivery System
OD
Aortic Extension
(Fr.)
22
20
(mm x mm)
36x36 26
34x34
32x32
30x30
28x28 29
26x26
24x24
22x22
Covered
Length
(mm)
28
30
Proximal
Configuration
FreeFlo
Bare Spring
Distal
Configuration
Open Web
54
Page 58

17.0 EXPLANATION OF SYMBOLS
Explanation of symbols that may appear on product labeling.
M708500B001
Contents: One (1) Talent Abdominal Stent Graft System with Xcelerant HDS and
One (1) Device Registration Packet
Consult instructions for use at. www.medtronic.com/manuals
Do not use if package is damaged
Non-pyrogenic
Peel here
Pull tab to open
Store at room temperature in a dark, dry place
MR Conditional
CAUTION: Federal (USA) law restricts this device for sale by or on order of a physician.
Sterilized using irradiation
Do not use if indicator turns black
55
Page 59

56
Page 60

MANUFACTURER:
MEDTRONIC, INC.
710 Medtronic Parkway NE
Minneapolis, MN 55432-5604
U.S.A.
Tel: (763) 514-4000
Fax: (763) 514-4879
www.medtronic.com
U. S. CUSTOMER SERVICE / PRODUCT INQUIRIES
Tel: (800) 961-9055
Fax: (800) 929-2133
© 2008 Medtronic
All Rights Reserved
Protected by one or more of the following United States patents: 5,591,195; 5,713,917; 6,306,141; 6,344,052;
6,911,039; and 7,105,016. Licensed under U.S. Patent 5,871,536.
Rev B
 Loading...
Loading...