Page 1

System Eligibility
Battery Longevity
Neurostimulation systems for pain
Reference manual
! USA
Rx only
Page 2

Page 3

Explanation of symbols on product and package labeling.
Conformité Européenne (European Conformity). This symbol means that the device fully
complies with applicable European Directives.
Manufacturer
EC
! USA
REP
Authorized Representative in the European Community
For USA audiences only
System Eligibility, Battery Longevity 2021-02-15 English 3
Page 4

Medtronic, Medtronic logo, and Further, Together are trademarks of Medtronic. AdaptiveStim™,
Intellis™, IntelliStim™, InterStim™, Itrel™, Pisces-Quad™, PrimeAdvanced™, Restore™,
RestoreAdvanced™, RestorePrime™, RestoreSensor™, RestoreUltra™, Sequentia™, SureScan™,
Vanta™, and Vectris™ are trademarks of a Medtronic company.
4 English System Eligibility, Battery Longevity 2021-02-15
Page 5

Table of contents
System eligibility 7
Trial stimulation devices 7
Trial stimulation for Vanta with AdaptiveStim™ Technology or Sequentia LT using the Model 97725
Wireless External Neurostimulator 10
Trial stimulation for Intellis or Intellis with AdaptiveStim™ Technology using the Model 97725 Wireless
External Neurostimulator 10
Trial stimulation for RestoreSensor, RestoreSensor SureScan MRI, or RestoreUltra SureScan MRI
using the Model 97725 Wireless External Neurostimulator 11
Trial stimulation for PrimeAdvanced, PrimeAdvanced SureScan MRI, Restore, RestoreAdvanced
SureScan MRI, or RestorePrime using the Model 97725 Wireless External Neurostimulator 11
Trial stimulation for Itrel 4 using the Model 97725 Wireless External Neurostimulator 12
Trial stimulation for Intellis or Intellis with AdaptiveStim™ Technology using the Model 37022 External
Neurostimulator 12
Information available for the system:
The information for prescribers manual provides information about contraindications,
warnings, precautions, adverse events, sterilization, patient selection, individualization of
treatment, and component disposal. For customers in Japan, the appropriate package insert
provides information about safety, contraindications, warnings, precautions, and adverse
events.
The indications sheet provides information about indications and related information. For
customers in Japan, the appropriate package insert provides information about indications.
The system eligibility and battery longevity manual describes programming considerations
and provides battery longevity information to aid in the appropriate neurostimulator selection.
MRI guidelines provide information about any MRI conditions and MRI-specific
contraindications, warnings, and precautions for MRI scans with the neurostimulation system.
Product manuals, such as programming guides, recharging guides, and implant manuals
provide device descriptions, package contents, device specifications, product-specific
warnings and precautions, and instructions for use.
! USA
The clinical summary provides information about the clinical study results for the
neurostimulation system.
System Eligibility, Battery Longevity 2021-02-15 English 5
Page 6

Trial stimulation for RestoreSensor, RestoreSensor SureScan MRI, or RestoreUltra SureScan MRI
using the Model 37022 External Neurostimulator 12
Trial stimulation for PrimeAdvanced, PrimeAdvanced SureScan MRI, Restore, RestoreAdvanced
SureScan MRI, or RestorePrime using the Model 37022 External Neurostimulator 13
Trial stimulation for Itrel 4 using the Model 37022 External Neurostimulator 14
Trial stimulation for PrimeAdvanced, PrimeAdvanced SureScan MRI, Restore, RestoreAdvanced
SureScan MRI, or RestorePrime using the Model 37021 External Neurostimulator 14
Battery longevity and maintenance 16
Intellis and Intellis with AdaptiveStim™ Technology 16
RestoreSensor, RestoreSensor SureScan MRI, and RestoreUltra SureScan MRI 16
Restore and RestoreAdvanced SureScan MRI 17
PrimeAdvanced, PrimeAdvanced SureScan MRI, RestorePrime, Sequentia LT, and Vanta with
AdaptiveStim™ Technology 19
Estimating battery longevity for Vanta with AdaptiveStim™ Technology or Sequentia LT 20
Estimating battery longevity for PrimeAdvanced, PrimeAdvanced SureScan MRI, and
RestorePrime 20
Itrel 4 27
Estimating battery longevity for Itrel 4 27
Estimating battery longevity examples 32
Example 1: PrimeAdvanced, PrimeAdvanced SureScan MRI, or RestorePrime Neurostimulator
and 4 programs with low-impedance leads 32
Example 2: Itrel 4 Neurostimulator with standard-impedance lead 34
6 English System Eligibility, Battery Longevity 2021-02-15
Page 7

System eligibility
A trial or test stimulation period is used to determine if a new or replacement Medtronic neurostimulation
system can deliver adequate paresthesia to a patient. With the trial stimulation devices designed to test
for a number of implantable neurostimulators, make sure that the number of programs and parameter
settings used during trial stimulation are evaluated against the features of the neurostimulators
considered for implant.
This approach ensures:
the number of programs needed for therapy does not exceed the ability of the neurostimulator.
▪
the parameter settings on the trial stimulation device are converted if the trial stimulation device
▪
accepts:
pulse width or rate settings that are higher than the settings accepted by a specific implantable
–
neurostimulator.
amplitude (intensity) settings, at a specific rate and pulse width, which exceed the maximum
–
amplitude setting a specific implantable neurostimulator can use to produce paresthesia. Even if
the amplitude setting is accepted by the implantable neurostimulator, the amplitude output will
not exceed the maximum amplitude listed in the table.
Notes:
When choosing a replacement neurostimulator, remember that all neurostimulators do not have
▪
identical features.
The Itrel 4 Models 37703 and 37704 Neurostimulators are the only Medtronic neurostimulators for
▪
pain with a unipolar mode. When replacing an Itrel 4 Neurostimulator with another model of
Medtronic neurostimulator, ensure that unipolar stimulation is not required.
Use trial stimulation leads (also called trial leads) for trial stimulation that are the same impedance
▪
type (ie, low impedance, standard impedance) as the leads intended to be implanted later with the
fully implanted neurostimulation system.
Using the same impedance-type leads for trial stimulation as the lead intended at the time of full
system implant allows for more accurate longevity estimates for the implanted neurostimulator.
For instance, using a low impedance lead for trial stimulation and later implanting a standard
impedance lead for the fully implanted system may require more energy use. Furthermore, patients
who required high parameter settings during trial stimulation could require parameter values that
exceed the maximum system capability of their fully implanted neurostimulation system.
Trial stimulation devices
The Model 97725 Wireless External Neurostimulator, Model 37022 External Neurostimulator, and Model
37021 External Neurostimulator can each test for more than 1 implantable neurostimulator.
System Eligibility, Battery Longevity 2021-02-15 English 7
Page 8

Use Table 1–Table 3 to determine:
which trial stimulation devices can test for which neurostimulator.
▪
before trial stimulation starts, how to configure program settings for the external neurostimulator if
▪
you know which implantable neurostimulator the patient is likely to receive.
at the end of trial stimulation, which implantable neurostimulators to consider based on the number
▪
of programs that trial stimulation results required and the stimulation settings the patient found most
effective.
If you are using the tables to help configure programs, find the name of the implantable neurostimulator in
the Implantable neurostimulator column, then set up programs for trial stimulation according to the
program setting guidelines in the corresponding Program setting guidelines column.
If you are using the table to select an implantable neurostimulator at the end of the trial, look at the
Program setting guidelines column and find the number of programs and the program settings that are
most similar to the ones the patient used during trial stimulation. Then look at the corresponding
Implantable neurostimulator column to see which implantable neurostimulator can deliver those program
settings.
Table 1. The Model 97725 Wireless External Neurostimulator (ENS) and implantable neurostimulators (INS)
Implantable
neurostimulator
Vanta with AdaptiveStim™
Technology
Program setting guidelines Refer to
•
You must have a pulse width of ≤450 μs and a program rate of
≤130 Hz.
page 10
Sequentia LT •
Intellis
Intellis with AdaptiveStim™
Technology
RestoreSensor
RestoreSensor SureScan
MRI
RestoreUltra SureScan MRI
8 English System Eligibility, Battery Longevity 2021-02-15
You must have a pulse width of ≤450 μs and a program rate of
≤130 Hz.
• Electrode redistribution and IntelliStim are not available.
You may have only 1 program.
•
You may configure 2–8 electrodes.
•
•
No special program configuration considerations required.
Electrode redistribution and IntelliStim are not available for the
•
Intellis Model 97716 Neurostimulator.
•
Electrode redistribution and IntelliStim are not available for these
implantable neurostimulators.
• All active programs must have the same rate.
At least 1 active program may have a pulse width >450 µs and the
•
rate that applies to all the active programs may be >130 Hz.
page 10
page 10
page 11
Page 9

Table 1. The Model 97725 Wireless External Neurostimulator (ENS) and implantable neurostimulators (INS) (continued)
Implantable
Program setting guidelines Refer to
neurostimulator
PrimeAdvanced
PrimeAdvanced SureScan
MRI
RestoreAdvanced SureScan
MRI
Restore
RestorePrime
•
Electrode redistribution and IntelliStim are not available for these
implantable neurostimulators.
• All active programs must have the same rate.
•
If trial stimulation results require 1–2 programs, you must have a
pulse width of ≤450 µs and a program rate of ≤130 Hz.
• If trial stimulation results require 3 programs, you must have a pulse
width of ≤450 µs and a program rate of ≤85 Hz.
page 12
• If trial stimulation results require 4 programs, you must have a pulse
width of ≤450 µs and a program rate of ≤65 Hz.
Itrel 4 •
Table 2. The Model 37022 External Neurostimulator (ENS) and implantable neurostimulators (INS)
Implantable
neurostimulator
Intellis
Intellis with AdaptiveStim™
Technology
RestoreSensor
RestoreSensor SureScan
MRI
RestoreUltra SureScan MRI
PrimeAdvanced
PrimeAdvanced SureScan
MRI
RestoreAdvanced SureScan
MRI
Restore
RestorePrime
Electrode redistribution and IntelliStim are not available for these
page 12
implantable neurostimulators.
• All active programs must have the same rate.
You must have a pulse width of ≤450 µs and a program rate of
•
≤130 Hz.
•
You may have only 1 program.
Program setting guidelines Refer to
•
You may have 1–4 programs. page 12
•
You may have 1–4 programs. page 12
•
If trial stimulation results require 1–2 programs, you must have a
page 13
maximum pulse width of ≤450 µs and a maximum program rate of
≤130 Hz.
• If trial stimulation results require 3 programs, you must have a
maximum pulse width of ≤450 µs and a maximum program rate of
≤85 Hz.
• If trial stimulation results require 4 programs, you must have a
maximum pulse width of ≤450 µs and a maximum program rate of
≤65 Hz.
Itrel 4 •
You may have 1 program. page 14
System Eligibility, Battery Longevity 2021-02-15 English 9
Page 10

Table 3. The Model 37021 External Neurostimulator (ENS) and implantable neurostimulators (INS)
Implantable
neurostimulator
PrimeAdvanced
PrimeAdvanced SureScan
MRI
RestoreAdvanced SureScan
MRI
Restore
RestorePrime
Program setting guidelines Refer to
•
You may have 1–4 programs. page 14
Trial stimulation for Vanta with AdaptiveStim™
Technology or Sequentia LT using the Model 97725
Wireless External Neurostimulator
If you are considering implanting a Vanta with AdaptiveStim™ Technology Model 977006 or Sequentia
LT Model 977005 Neurostimulator, you may use the Model 97725 Wireless External Neurostimulator to
deliver trial stimulation.
For these implantable neurostimulators, the maximum settings available are 450 μs for pulse width and
130 Hz for program rate.
For the Sequentia LT Model 977005 Neurostimulator:
electrode redistribution and IntelliStim are not available.
▪
you may have only one program.
▪
you may configure 2–8 electrodes.
▪
Trial stimulation for Intellis or Intellis with
AdaptiveStim™ Technology using the Model 97725
Wireless External Neurostimulator
If you are considering implanting an Intellis with AdaptiveStim™ Technology Model 97715 or Intellis
Model 97716 Neurostimulator, you may use the Model 97725 Wireless External Neurostimulator to
deliver trial stimulation. There are no special program configuration considerations.
Note: Electrode redistribution and IntelliStim are not available for the Intellis Model 97716
Neurostimulator.
10 English System Eligibility, Battery Longevity 2021-02-15
Page 11

Trial stimulation for RestoreSensor, RestoreSensor
SureScan MRI, or RestoreUltra SureScan MRI using
the Model 97725 Wireless External Neurostimulator
If you are considering implanting any of the following neurostimulators, you may use the Model 97725
External Neurostimulator to deliver trial stimulation:
• RestoreSensor Model 37714
RestoreSensor SureScan MRI Model 97714
•
For these implantable neurostimulators:
the rate must be the same across active programs.
▪
electrode redistribution and IntelliStim are not available.
▪
At least one active program may have a pulse width greater than 450 µs and the rate that applies to all
the active programs may be greater than 130 Hz.
•
RestoreUltra SureScan MRI Model 97712
Trial stimulation for PrimeAdvanced, PrimeAdvanced
SureScan MRI, Restore, RestoreAdvanced SureScan
MRI, or RestorePrime using the Model 97725 Wireless
External Neurostimulator
If you are considering implanting any of the following neurostimulators, you may use the Model 97725
External Neurostimulator to deliver trial stimulation:
• PrimeAdvanced Model 37702
PrimeAdvanced SureScan MRI Model 97702
•
Restore Model 37711
•
For these implantable neurostimulators:
the rate must be the same across active programs.
▪
electrode redistribution and IntelliStim are not available.
▪
For PrimeAdvanced, PrimeAdvanced SureScan MRI, and RestoreAdvanced SureScan MRI, the
maximum settings available are 450 µs for pulse width and 130 Hz for rate for 1 to 2 programs; 450 µs for
pulse width and 85 Hz for rate for 3 programs; and 450 µs for pulse width and 65 Hz for rate for 4
programs.
•
RestoreAdvanced SureScan MRI Model 97713
RestorePrime Model 37701
•
System Eligibility, Battery Longevity 2021-02-15 English 11
Page 12

Trial stimulation for Itrel 4 using the Model 97725 Wireless External Neurostimulator
If you are considering implanting an Itrel 4 Model 37703 or Model 37704 Neurostimulator, you can use
the Model 97725 Wireless External Neurostimulator to deliver trial stimulation.
For these implantable neurostimulators:
the rate must be the same across active programs.
▪
electrode redistribution and IntelliStim are not available.
▪
the maximum settings available are 450 μs for pulse width and 130 Hz for rate for 1 program.
▪
While delivering trial stimulation, make sure that the number of programs used on the trial stimulation
device does not exceed the number of programs available on the neurostimulator being considered for
implant.
Higher pulse width and rates decrease the programmable amplitude in Itrel 4 neurostimulators.
Trial stimulation for Intellis or Intellis with
AdaptiveStim™ Technology using the Model 37022
External Neurostimulator
If you are considering implanting an Intellis with AdaptiveStim™ Technology Model 97715 or Intellis
Model 97716 Neurostimulator, you may use the Model 37022 External Neurostimulator to deliver trial
stimulation.
You may have 1–4 active programs. There are no other special program configuration considerations.
Trial stimulation for RestoreSensor, RestoreSensor
SureScan MRI, or RestoreUltra SureScan MRI using
the Model 37022 External Neurostimulator
If you are considering implanting any of the following neurostimulators, you may use the Model 37022
External Neurostimulator to deliver trial stimulation:
• RestoreSensor Model 37714
•
RestoreSensor SureScan MRI Model 97714
The RestoreSensor, RestoreSensor SureScan MRI, and RestoreUltra SureScan MRI Neurostimulation
Systems may have limited stimulation adjustments available if the therapy parameters are near the high
12 English System Eligibility, Battery Longevity 2021-02-15
RestoreUltra SureScan MRI Model 97712
•
Page 13

output interlocks. If the trial stimulation is near the high output interlocks, approximate the trial stimulation
effect by lowering the amplitude setting and adjusting 1 or more of the following:
Decrease the rate
▪
Increase the pulse width(s)
▪
Change the selected electrodes
▪
Reposition the lead(s)
▪
Trial stimulation for PrimeAdvanced, PrimeAdvanced
SureScan MRI, Restore, RestoreAdvanced SureScan
MRI, or RestorePrime using the Model 37022 External
Neurostimulator
If you are considering implanting any of the following neurostimulators, you may use the Model 37022
External Neurostimulator to deliver trial stimulation:
• PrimeAdvanced Model 37702
•
PrimeAdvanced SureScan MRI Model 97702
Restore Model 37711
•
The clinician programmer will not display the Restore Neurostimulator or RestorePrime Neurostimulator
in the longevity estimate results.
RestoreAdvanced SureScan MRI Model 97713
•
•
RestorePrime Model 37701
However, if the longevity test is successful for the PrimeAdvanced, PrimeAdvanced SureScan MRI, or
RestoreAdvanced SureScan MRI Neurostimulator, the Restore Neurostimulator or RestorePrime
Neurostimulator may be used.
The PrimeAdvanced, PrimeAdvanced SureScan MRI, and RestoreAdvanced SureScan MRI
Neurostimulation Systems may have limited stimulation adjustments available if the amplitude exceeds
9.5 V. If the trial stimulation exceeds 9.5 V, approximate similar stimulation by lowering the amplitude
setting and adjusting 1 or more of the following:
Decrease the rate
▪
Increase the pulse width(s)
▪
Change the selected electrodes
▪
Reposition the lead(s)
▪
For PrimeAdvanced, PrimeAdvanced SureScan MRI, and RestoreAdvanced SureScan MRI, the
maximum settings available are 450 µs for pulse width and 130 Hz for rate for 1 to 2 programs; 450 µs for
pulse width and 85 Hz for rate for 3 programs; and 450 µs for pulse width and 65 Hz for rate for 4
programs. A battery longevity test can be performed with the external neurostimulator to verify that a
System Eligibility, Battery Longevity 2021-02-15 English 13
Page 14

particular setting is available to the neurostimulator. If the setting is not available, the clinician
programmer indicates that the setting is not applicable to the neurostimulator.
Trial stimulation for Itrel 4 using the Model 37022 External Neurostimulator
If you are considering implanting an Itrel 4 Model 37703 or Model 37704 Neurostimulator, you may use
the Model 37022 External Neurostimulator to deliver trial stimulation.
The Itrel 4 Neurostimulation System may have limited stimulation adjustments available if the therapy
parameters exceed 9.5 V or are near the high output interlocks. If the trial stimulation exceeds 9.5 V or is
near the high output interlocks, approximate the trial stimulation effect by lowering the amplitude setting
and adjusting 1 or more of the following:
Decrease the rate
▪
Increase the pulse width(s)
▪
Change the selected electrodes
▪
Reposition the lead(s)
▪
Table 4. Trial stimulation maximum amplitude (V) for Itrel 4 using the Model 37022 External neurostimulator
No. of
programs
intended for
use
1 50 10.5 V 10.5 V 10.5 V 10.5 V 10.5 V 10.5 V
1 70 10.5 V 10.5 V 10.5 V 10.5 V 10.5 V 10.0 V
1 100 10.5 V 10.5 V 10.5 V 10.5 V 10.5 V 9.3 V
1 125 10.5 V 10.5 V 10.5 V 10.5 V 10.15 V 8.75 V
Rate (Hz) Pulse width (µs)
60 120 180 240 330 450
Note: The lead configuration is 1x4 and only one program is defined.
Trial stimulation for PrimeAdvanced, PrimeAdvanced
SureScan MRI, Restore, RestoreAdvanced SureScan
MRI, or RestorePrime using the Model 37021 External
Neurostimulator
If you are considering implanting any of the following neurostimulators, you may use the Model 37021
External Neurostimulator to deliver trial stimulation:
14 English System Eligibility, Battery Longevity 2021-02-15
Page 15

• PrimeAdvanced Model 37702
PrimeAdvanced SureScan MRI Model 97702
•
•
Restore Model 37711
RestoreAdvanced SureScan MRI Model 97713
•
RestorePrime Model 37701
•
The external neurostimulator and these implantable neurostimulators produce comparable paresthesia
when the same number of programs are set to the same parameter settings.
The PrimeAdvanced, PrimeAdvanced SureScan MRI, RestoreAdvanced SureScan MRI, Restore, and
RestorePrime Neurostimulation Systems may have limited stimulation adjustments available if the
amplitude exceeds 9.5 V. If the trial stimulation exceeds 9.5 V, approximate similar stimulation by
lowering the amplitude setting and adjusting 1 or more of the following:
Decrease the rate
▪
Increase the pulse width(s)
▪
Change the selected electrodes
▪
Reposition the lead(s)
▪
System Eligibility, Battery Longevity 2021-02-15 English 15
Page 16

Battery longevity and maintenance
The following neurostimulators have rechargeable batteries:
• Intellis
Intellis with AdaptiveStim™ Technology
•
•
Restore
RestoreAdvanced SureScan MRI
•
The following neurostimulators have nonrechargeable batteries:
• Itrel 4
PrimeAdvanced
•
•
PrimeAdvanced SureScan MRI
Intellis and Intellis with AdaptiveStim™ Technology
The Intellis with AdaptiveStim™ Technology Model 97715 and Intellis Model 97716 Neurostimulator
rechargeable battery requires a recharge session at least once per year for the battery to reach maximum
longevity.
The amount of time before the neurostimulator battery requires charging is affected by the number of
active programs and the stimulation settings in each program. The majority of patients that use rate
settings around 60 Hz can expect to recharge their neurostimulators every 4 or more days. Higher
stimulation settings will require more frequent recharging sessions. Patients should define a recharge
schedule that meets their individual needs while maintaining a charge level that is capable of sustaining
programmed stimulation settings.
RestoreSensor
•
RestoreSensor SureScan MRI
•
•
RestoreUltra SureScan MRI
RestorePrime
•
Sequentia LT
•
•
Vanta with AdaptiveStim™ Technology
The Intellis with AdaptiveStim™ Technology Model 97715 and Intellis Model 97716 Neurostimulators will
provide at least 9 years of operation before replacement is recommended. Over time, the neurostimulator
battery will need more frequent recharges. Like all rechargeable batteries, use over time and repeated
recharge cycles reduce the maximum charge capacity of the neurostimulator battery.
When the neurostimulator reaches 9 years of service, the elective replacement indicator (ERI) message
will display on the Model A710 Intellis clinician programmer application and on the Model 97745
Controller screen. At this time, the neurostimulator should be replaced.
RestoreSensor, RestoreSensor SureScan MRI, and RestoreUltra SureScan MRI
The following neurostimulators have a rechargeable battery and require that a charge be maintained in
order for the battery to reach maximum longevity:
RestoreSensor Model 37714
▪
16 English System Eligibility, Battery Longevity 2021-02-15
Page 17

RestoreSensor SureScan MRI Model 97714
▪
RestoreUltra SureScan MRI Model 97712
▪
The amount of time before the battery requires charging is affected by patient use, the number of
programs, stimulation settings in each program, and the use of cycling. All these factors are variable and
may result in increased frequency of recharge sessions or reduced battery longevity. Monitor your
patient's battery charge level regularly. The majority of patients can expect to recharge their
neurostimulators every 10 days. Higher stimulation settings will require more frequent recharging
sessions. Patients should define a recharge schedule that meets their individual needs while maintaining
a charge level that is capable of sustaining programmed stimulation settings.
The battery for the RestoreSensor, RestoreSensor SureScan MRI, and RestoreUltra SureScan MRI
Neurostimulators will provide 9 years of operation. Over time, the neurostimulator battery will need more
frequent recharges. Like all rechargeable batteries, use over time and repeated recharge cycles reduce
the maximum charge capacity of the neurostimulator battery.
When the neurostimulator battery reaches 8 years of operation, the elective replacement indicator (ERI)
message is displayed in the Observations box on the clinician programmer screen or on the patient
programmer screen. A replacement neurostimulator is needed within 6 months. At 9 years, the end of
service (EOS) message is displayed in the Observations box on the clinician programmer screen or on
the patient programmer screen. At this time, the neurostimulator no longer provides stimulation and a
replacement neurostimulator is needed.
Restore and RestoreAdvanced SureScan MRI
The rechargeable battery for the following neurostimulators requires that a charge be maintained in order
for the battery to reach maximum longevity:
Restore Model 37711
▪
RestoreAdvanced SureScan MRI Model 97713
▪
The amount of time before the battery requires charging is affected by the number of active programs
and the stimulation settings in each program (refer to Table 5).
System Eligibility, Battery Longevity 2021-02-15 English 17
Page 18

Table 5. Time until battery requires recharging for Restore and RestoreAdvanced SureScan MRI
a
1 program
10.5 V
10.0 V
9.0 V
8.0 V
7.0 V
6.0 V
5.0 V
4.0 V
Highest programmed amplitude
3.0 V
2.0 V
1.0 V
a
Time until battery requires recharging is based on 24-hour per day stimulation using the following settings: rate = 60 Hz, pulse
width = 350 μs, impedance = 350 Ω, mode = Cycling off.
3 weeks
1 month
2 program 3 program 4 program
1.5 days2 days3 days6 days
1 week2 weeks
1 week
2 weeks
3 weeks
2 weeks
3 weeks1 month
1 month
1 week
2 weeks
3 weeks
1 month
Battery longevity for the Restore and RestoreAdvanced SureScan MRI Neurostimulators can be
evaluated using the following criteria:
EOS (End of service)—9 years after implant, stimulation is not available and an EOS message
▪
appears if you try to interrogate the neurostimulator.
ERI (Elective replacement indicator)—8.5 years after implant, an ERI message is displayed in the
▪
Observations box on the clinician programmer screen.
Time until daily recharging require—Refer to Table 6 for time until daily recharging is required. If the
▪
battery requires daily recharging, a charge daily message appears in the Observations box on the
clinician programmer screen. Recharging more than once daily may be inconvenient for the patient
to maintain therapy.
If the battery needs daily recharging, you may want to adjust the stimulation settings to increase the time
between battery charges or prepare to replace the neurostimulator.
18 English System Eligibility, Battery Longevity 2021-02-15
Page 19
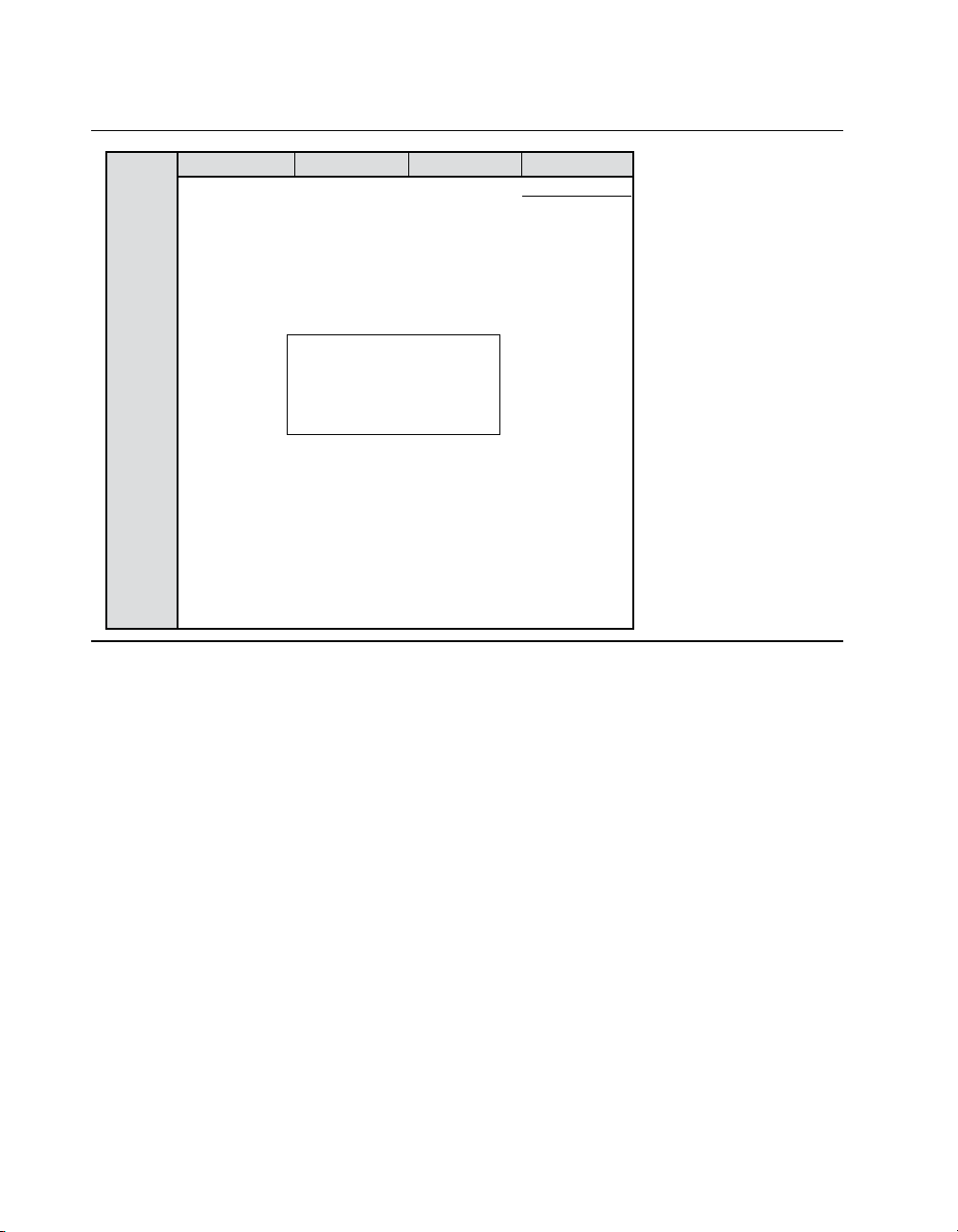
Table 6. Time until battery requires daily recharging for Restore and RestoreAdvanced SureScan MRI
a
2 program1 program
10.5 V
10.0 V
9.0 V
8.0 V
7.0 V
6.0 V
5.0 V
4.0 V
Highest programmed amplitude
3.0 V
2.0 V
1.0 V
a
Time until daily recharging required is based on 24-hour per day stimulation using the following settings: rate = 60 Hz, pulse
width = 350 μs, impedance = 350 Ω, mode = Cycling off.
Device will reach EOS before
needing to be fully charged
more than once per day.
3 program
4 program
8 years
PrimeAdvanced, PrimeAdvanced SureScan MRI,
RestorePrime, Sequentia LT, and Vanta with
AdaptiveStim™ Technology
The battery of an implantable neurostimulator can last for months or years, depending on the following
factors:
Programmed parameters (ie, amplitude, rate, pulse width, number of active electrodes used)
▪
System impedance
▪
Hours per day of stimulation
▪
The degree of patient control over programmable stimulation parameters
▪
To optimize the battery longevity of an implantable neurostimulator, follow these tips:
Place the leads in the optimal location to achieve paresthesia.
▪
System Eligibility, Battery Longevity 2021-02-15 English 19
Page 20

Use fewer programs.
▪
Use the minimum number of electrodes necessary for effective stimulation.
▪
Use the lowest effective settings for amplitude, rate, and pulse width.
▪
Instruct the patient to use the neurostimulator only when needed.
▪
Consider implanting low-impedance leads and extensions.
▪
Estimating battery longevity for Vanta with AdaptiveStim™ Technology or Sequentia LT
The A71200 Vanta/Sequentia LT Clinician Programmer Application and A71300 Stimulation Trialing
Clinician Programmer Application each include a battery longevity feature that estimates battery life
based on program and group settings, along with the number of hours per day the patient might use each
group. Refer to the clinician programmer application manuals for detailed instructions on how to use the
battery longevity estimators.
Estimating battery longevity for PrimeAdvanced, PrimeAdvanced SureScan MRI, and RestorePrime
If you choose to implant a PrimeAdvanced Model 37702 Neurostimulator, PrimeAdvanced SureScan MRI
Model 97702 Neurostimulator, or RestorePrime Model 37701 Neurostimulator after trial stimulation using
the Model 97725 Wireless External Neurostimulator, perform the following steps to estimate battery
longevity:
1. Connect the Model 37022 External Neurostimulator and applicable accessories to the lead(s).
2. Adjust the program settings using the clinician programmer to simulate the trial stimulation achieved
with the Model 97725 Wireless External Neurostimulator.
3. Perform battery longevity estimation. Refer to the appropriate battery longevity section below for
more information.
The estimation formula provided approximates the period that a battery in a new PrimeAdvanced Model
37702 Neurostimulator, PrimeAdvanced SureScan MRI Model 97702 Neurostimulator, or RestorePrime
Model 37701 Neurostimulator will last.
The estimation is based on the settings for one group and on the expected programmed values, the
modes of operation, and how often the therapy is used.
Notes:
The use of cycling may cause a reduction in longevity depending on programmed settings. Refer to
▪
steps on estimating battery longevity and the Adjusted Energy Use graph Figure 1 for additional
information.
20 English System Eligibility, Battery Longevity 2021-02-15
Page 21

The estimation formulas include values from reference tables. Ensure the appropriate tables are
▪
referenced based on the type of lead(s) used:
For low-impedance leads (<2 ohms for 10 cm) (eg, 1x8), use Table 7 and Table 8.
–
For standard-impedance leads (eg, Vectris SureScan MRI, Pisces-Quad), use Table 9 and
–
Table 10.
For a mixed pair of leads, use Table 7 and Table 8.
–
Estimating battery longevity
1. Determine the expected programmed values for each program, the expected modes of operation,
and the expected use.
Note: Rates for all programs will be the same.
Expected programmed values
Program 1 Program 2 Program 3 Program 4
Amplitude (V): _____ Amplitude (V): _____ Amplitude (V): _____ Amplitude (V): _____
Rate (Hz): _____ Rate (Hz): _____ Rate (Hz): _____ Rate (Hz): _____
Pulse width (μs): _____ Pulse width (μs): _____ Pulse width (μs): _____ Pulse width (μs): _____
Active
electrodes:
Hours of stimulation per day (including Scheduled
_____ Active
electrodes:
_____ Active
electrodes:
Modes of operation and use
_____
Therapy):
_____ Active
electrodes:
_____
2. Calculate the Program Factor for each program (P1F, P2F, P3F, P4F).
a. Locate the Energy Use (EU) for Program 1 (P1EU) from Table 7 or Table 9.
Note: Use the amplitude, rate, and pulse width values entered in Step 1.
P1EU = ______
b. Locate the Electrode Correction Factor (ECF) for Program 1 (P1ECF) from Table 8 or Table 10.
Note: Use the number of active electrodes entered in Step 1.
P1ECF = _____
c. Calculate the Program 1 Factor (P1F):
P1EU _____ × P1ECF _____ = P1F _____
d. For a second program, repeat steps a, b, and c for Program 2.
P2EU = ______
P2ECF = ______
System Eligibility, Battery Longevity 2021-02-15 English 21
Page 22

P2EU _____ × P2ECF _____ = P2F _____
e. For a third program, repeat a, b, and c for Program 3.
P3EU = ______
P3ECF = ______
P3EU _____ × P3ECF _____ = P3F _____
f. For a fourth program, repeat steps a, b, and c for Program 4.
P4EU = ______
P4ECF = ______
P4EU _____ × P4ECF _____ = P4F _____
3. Calculate the multi-program factor (MPF).
Note: Enter 0 for any unused programs.
P1F _____ + P2F _____ + P3F _____ + P4F _____ = MPF _____
4. Calculate the Usage Correction Factor (UCF).
Hours of stimulation per day _____ ÷ 12 hours = UCF _____
5. Calculate the Adjusted Energy Use.
MPF _____ × UCF _____ = Adjusted Energy Use _____
6. Determine the battery longevity from Figure 1 using the Adjusted Energy Use value.
22 English System Eligibility, Battery Longevity 2021-02-15
Page 23
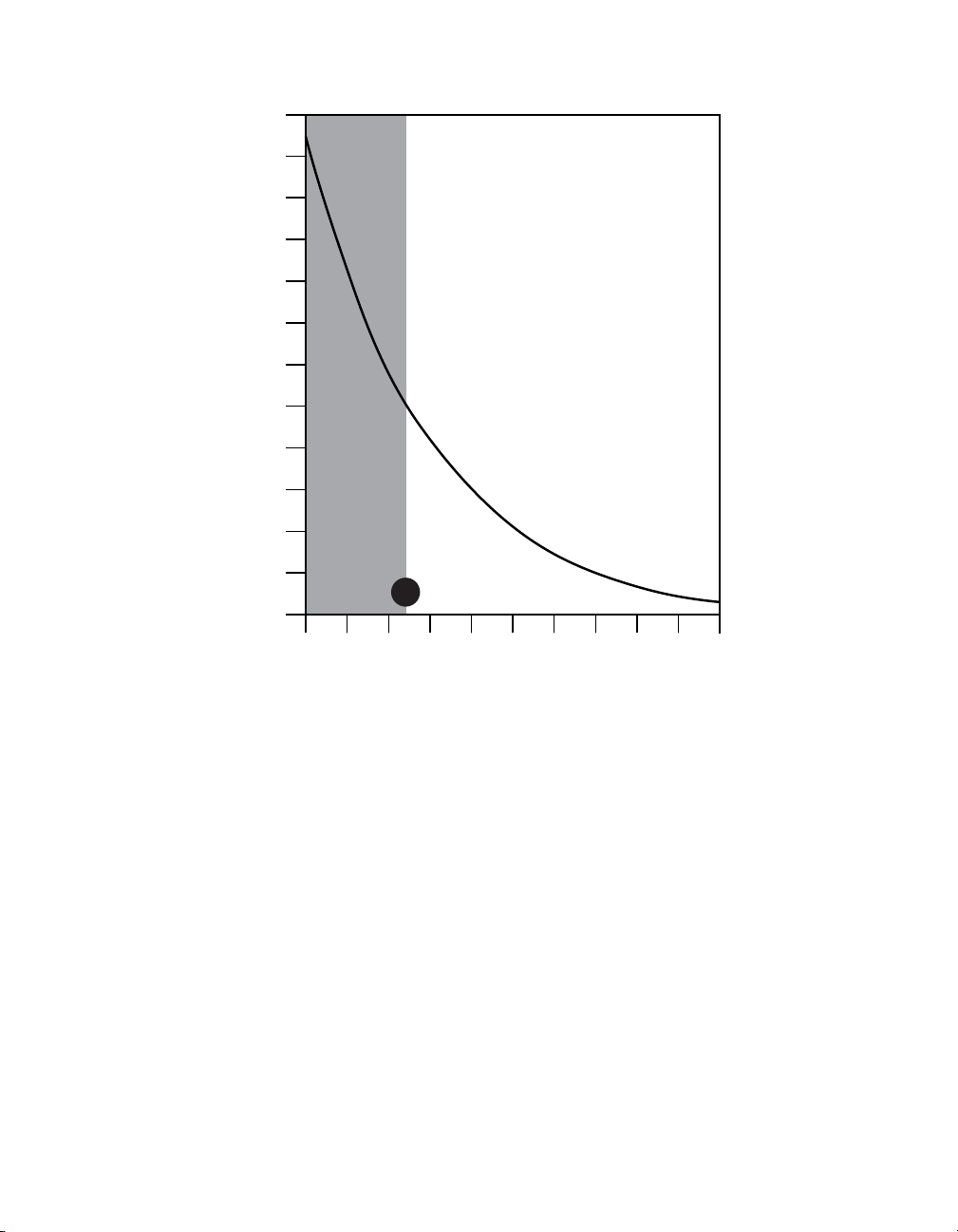
12
11
10
9
8
7
6
When calculated adjusted energy
use values are at or above 122,
cycling at intervals less than
15 seconds on and 15 seconds
off may reduce battery longevity.
5
Longevity Estimate (years)
4
Cycling is not
recommended
3
for use when
calculated
adjusted energy
2
use values
are less than 122.
1
122
0
5025 90 140 300 500 700 1000 1500 2000
200
Adjusted Energy Use
Figure 1. Longevity estimates for PrimeAdvanced, PrimeAdvanced SureScan MRI, and RestorePrime.
System Eligibility, Battery Longevity 2021-02-15 English 23
Page 24

Table 7. For low-impedance leads and PrimeAdvanced, PrimeAdvanced SureScan MRI, or RestorePrime
Neurostimulators:
a
Energy Use
for 2 active electrodes/programsb for 12 hours/day usage
Pulse width (µs)
Amplitude (V) Rate (Hz) 60 210 330 450
30 5 8 11 14
1.0 70 12202633
130 23 37 49 61
30 6 131823
2.0 70 16304356
130 29 57 79 103
30 12 26 38 51
3.0 70 29 62 89 117
130 54 115 163 214
30 18 44 64 82
4.0 70 42 102 148 192
130 78 188 272 353
30 22 54 78 105
5.0 70 51 124 181 243
130 96 231 332 439
30 33 81 117 154
6.0 70 76 186 268 358
130 140 344 493 648
30 41 108 159 219
7.0 70 95 251 384 507
130 174 479 761 979
30 56 143 212 281
8.0 70 127 334 494 649
130 233 616 908 1186
30 66 166 240 355
9.0 70 149 382 625 825
130 275 772 1147 1505
24 English System Eligibility, Battery Longevity 2021-02-15
Page 25

Table 7. For low-impedance leads and PrimeAdvanced, PrimeAdvanced SureScan MRI, or RestorePrime
Neurostimulators:
a
Energy Use
for 2 active electrodes/programsb for 12 hours/day usage (continued)
Pulse width (µs)
Amplitude (V) Rate (Hz) 60 210 330 450
30 82 224 337 397
10.0 70 188 527 695 907
130 347 888 1286 1660
a
Use values that are closest to the expected values (round to the next highest value).
b
If the number of active electrodes or hours of use is different than these values, adjustments are made in the formula.
Table 8. For low-impedance leads and PrimeAdvanced, PrimeAdvanced SureScan MRI, or RestorePrime
Neurostimulators: Electrode Correction Factor
Number of active negative electrodes
1234567
Number of 1 1.0 1.4 1.5 1.6 1.6 1.6 1.7
active 2 1.4 1.9 2.2 2.3 2.4 2.5 --
positive 3 1.5 2.2 2.5 2.8 2.9 -- --
electrodes 4 1.6 2.3 2.8 3.1 -- -- --
5 1.62.42.9--------
6 1.6 2.5 -- -- -- -- --
7 1.7------------
Table 9. For standard-impedance leads and PrimeAdvanced, PrimeAdvanced SureScan MRI, or RestorePrime
a
Neurostimulators: Energy Use
for 2 active electrodes/programsb for 12 hours/day usage
Pulse width (µs)
Amplitude (V) Rate (Hz) 60 210 330 450
30 5 7 9 11
1.0 70 11172227
130 21 32 41 50
30 5 101418
2.0 70 13253443
130 25 47 64 80
30 10 21 30 38
3.0 70 24507090
130 45 92 129 164
30 15 35 50 65
4.0 70 36 82 117 151
System Eligibility, Battery Longevity 2021-02-15 English 25
Page 26
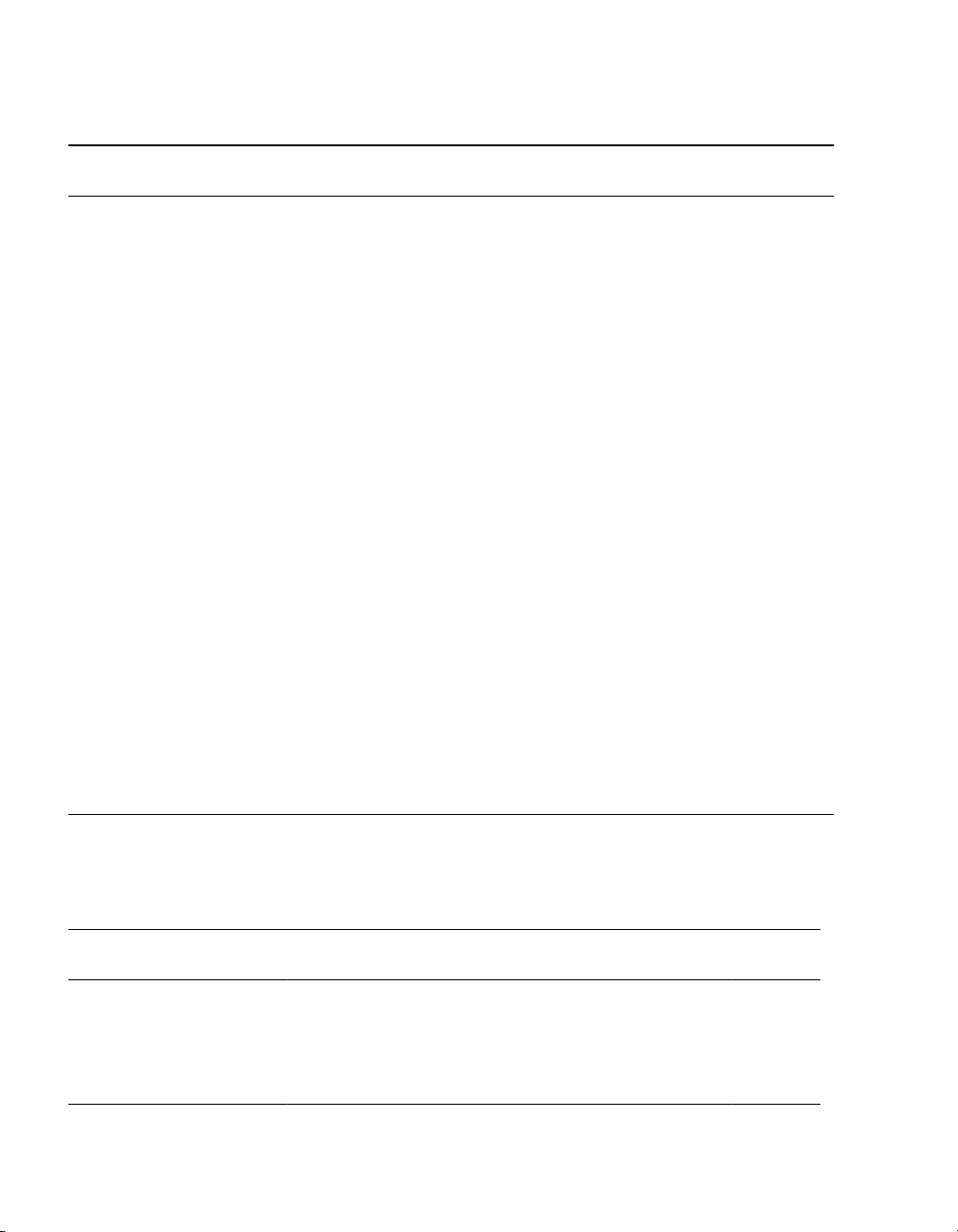
Table 9. For standard-impedance leads and PrimeAdvanced, PrimeAdvanced SureScan MRI, or RestorePrime
a
Neurostimulators: Energy Use
for 2 active electrodes/programsb for 12 hours/day usage (continued)
Pulse width (µs)
Amplitude (V) Rate (Hz) 60 210 330 450
130 67 151 214 276
30 17 42 61 79
5.0 70 41 98 142 185
130 76 180 260 336
30 26 62 91 118
6.0 70 60 144 210 273
130 110 266 384 499
30 34 86 125 165
7.0 70 79 197 289 378
130 145 362 529 688
30 46 115 167 219
8.0 70 105 263 386 503
130 194 483 704 918
30 50 126 184 243
9.0 70 114 292 428 561
130 209 534 885 1158
30 67 157 231 307
10.0 70 135 359 534 703
130 248 660 976 1278
a
Use values that are closest to the expected values (round to the next highest value).
b
If the number of active electrodes or hours of use is different than these values, adjustments are made in the formula.
Table 10. For standard-impedance leads and PrimeAdvanced, PrimeAdvanced SureScan MRI, or
RestorePrime Neurostimulators: Electrode Correction Factor
Number of active negative electrodes
1234567
Number of 1 1.0 1.5 1.6 1.7 1.7 1.8 1.8
active 2 1.5 2.0 2.3 2.5 2.6 2.7 --
positive 3 1.6 2.3 2.8 3.0 3.2 -- --
electrodes 4 1.7 2.5 3.0 3.4 -- -- --
5 1.72.63.2--------
26 English System Eligibility, Battery Longevity 2021-02-15
Page 27

Table 10. For standard-impedance leads and PrimeAdvanced, PrimeAdvanced SureScan MRI, or
RestorePrime Neurostimulators: Electrode Correction Factor (continued)
Number of active negative electrodes
1234567
6 1.8 2.7 -- -- -- -- --
7 1.8------------
Itrel 4
Estimating battery longevity for Itrel 4
If you choose to implant an Itrel 4 Model 37703 or Model 37704 Neurostimulator after trial stimulation
using the Model 97725 Wireless External Neurostimulator, perform the following steps to estimate battery
longevity:
1. Connect the Model 37021 External Neurostimulator and applicable accessories to the lead(s).
2. Adjust the program settings using the clinician programmer to simulate the trial stimulation achieved
with the Model 97725 Wireless External Neurostimulator.
3. Perform battery longevity estimation. Refer to the battery longevity section below for more
information.
The estimation formula provided approximates the period that a new Itrel 4 Model 37703 or Model 37704
neurostimulator battery will last. The estimation is based on the expected programmed values, the modes
of operation, and how often the therapy is used.
Notes:
The use of cycling may cause a reduction in longevity depending on programmed settings. Refer to
▪
Figure 2 for additional information.
The estimation formulas include values from reference tables. Ensure the appropriate tables are
▪
referenced based on the type of lead(s) used:
For low-impedance leads (<2 ohms for 10 cm) (eg, Pisces Z Quad), use Table 11 and Table 12.
–
For standard-impedance leads (eg, Vectris SureScan MRI, Pisces-Quad), use Table 13 and
–
Table 14.
Estimating battery longevity
1. Determine the expected programmed values, the expected modes of operation, and the expected
use.
System Eligibility, Battery Longevity 2021-02-15 English 27
Page 28

Expected programmed values
Amplitude (V): _____
Rate (Hz): _____
Pulse width (μs): _____
Active electrodes: _____
Modes of operation and use
Hours of stimulation per day: _____
If estimating battery longevity when using the Cycling feature,
contact your Medtronic representative for assistance.
2. Locate the Energy Use (EU) from Table 11 or Table 13.
Note: Use the amplitude, rate, and pulse width values entered in Step 1.
EU = ______
3. Locate the Electrode Correction Factor (ECF) from Table 12 or Table 14.
Note: Use the number of active electrodes entered in Step 1.
ECF = _____.
4. Calculate the Usage Correction Factor (UCF).
Hours of stimulation per day _____ ÷ 12 hours = Usage Correction Factor_____
5. Calculate the Adjusted Energy Use:
EU _____ × ECF _____ × UCF _____ + [12.2 × (2 - UCF _____)] = Adjusted Energy Use _____
6. Determine the battery longevity from Figure 2 using the Adjusted Energy Use value.
28 English System Eligibility, Battery Longevity 2021-02-15
Page 29
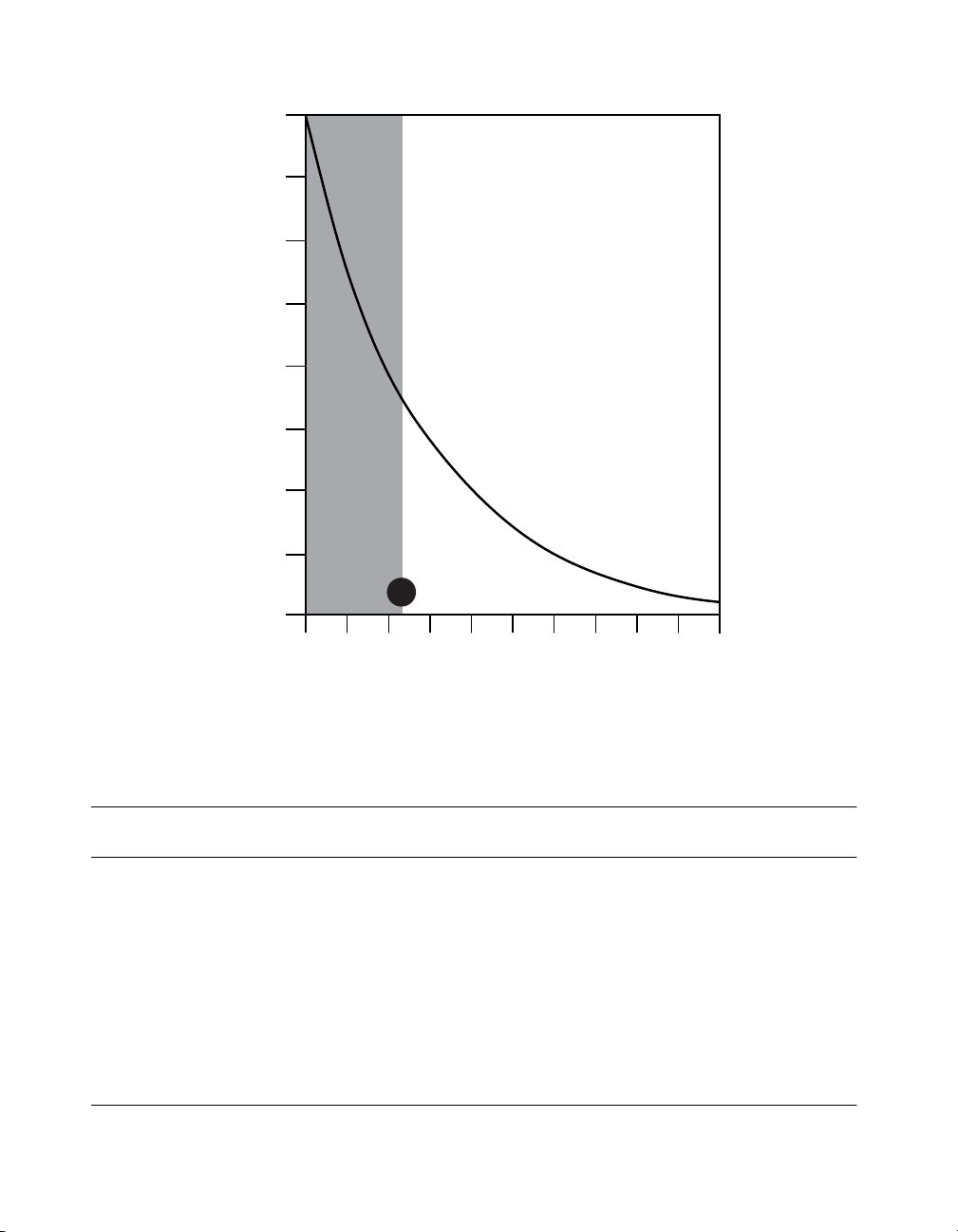
8
7
6
5
4
3
Longevity Estimate (years)
2
1
Cycling is not
recommended
for use when
calculated
adjusted energy
use values
are less than 118.
When calculated adjusted energy
use values are at or above 118,
cycling at intervals less than 15
seconds on and 15 seconds off
may reduce battery longevity.
118
0
5025 90 140 300 500 700 1000 1500 2000
200
Adjusted Energy Use
Figure 2. Itrel 4 Neurostimulator
longevity estimates (years) for energy use.
Table 11. For low-impedance leads and Itrel 4 Neurostimulators: Energy Use
12 hours/day usage
Pulse width (µs)
Amplitude (V) Rate (Hz) 60 210 330 450
30 3 6 9 11
1.0 70 7 15 21 27
130 12 27 38 49
a
for 2 active electrodesb for
30 4 111621
2.0 70 10253749
130 18 47 68 89
30 9 243546
3.0 70 21 55 81 106
System Eligibility, Battery Longevity 2021-02-15 English 29
Page 30
a
Table 11. For low-impedance leads and Itrel 4 Neurostimulators: Energy Use
for 2 active electrodesb for
12 hours/day usage (continued)
Pulse width (µs)
Amplitude (V) Rate (Hz) 60 210 330 450
130 38 101 148 194
30 14 39 60 80
4.0 70 33 92 139 185
130 60 171 256 337
30 20 54 81 107
5.0 70 44 126 188 248
130 82 231 344 453
30 27 75 112 148
6.0 70 61 173 259 343
130 113 318 476 662
30 37 104 156 206
7.0 70 83 239 360 476
130 152 440 659 869
30 50 140 209 276
8.0 70 113 321 482 637
130 207 590 882 1161
30 59 169 254 337
9.0 70 132 388 595 809
130 242 728 1116 1475
30 70 203 318 423
10.0 70 158 487 737 978
130 289 897 1394 1912
a
Use values that are closest to the expected values (round to the next highest value).
b
If the number of active electrodes or hours of use is different than these values, adjustments are made in the formula.
Table 12. For low-impedance leads and Itrel 4 Neurostimulators: Electrode Correction Factor (ECF)
Number of active negative electrodes
Number of active positive
1234
electrodes
Case 1.6 2.7 3.5 4.1
30 English System Eligibility, Battery Longevity 2021-02-15
Page 31

Table 12. For low-impedance leads and Itrel 4 Neurostimulators: Electrode Correction Factor (ECF) (continued)
Number of active negative electrodes
Number of active positive
1234
electrodes
1 1.0 1.3 1.5 --
2 1.3 2.0 -- --
3 1.5------
a
Table 13. For standard-impedance leads and Itrel 4 Neurostimulators: Energy Use
for 2 active electrodesb for
12 hours/day usage
Pulse width (µs)
Amplitude (V) Rate (Hz) 60 210 330 450
30 2579
1.0 70 6 12 17 22
130 11 22 31 40
30 3 9 13 16
2.0 70 8 20 29 38
130 15 37 54 70
30 8 192736
3.0 70 17436383
130 32 79 116 151
30 11 29 43 57
4.0 70 26 67 100 135
130 47 123 185 250
30 15 40 60 81
5.0 70 33 93 143 191
130 62 175 265 349
30 22 59 87 115
6.0 70 50 135 201 265
130 92 247 366 481
30 30 81 121 160
7.0 70 67 186 278 367
130 123 340 506 666
30 38 104 159 213
System Eligibility, Battery Longevity 2021-02-15 English 31
Page 32

Table 13. For standard-impedance leads and Itrel 4 Neurostimulators: Energy Use
Amplitude (V) Rate (Hz) 60 210 330 450
8.0 70 86 246 372 491
130 158 457 678 891
30 47 131 196 260
9.0 70 105 300 451 597
130 193 550 824 1128
30 57 158 236 314
10.0 70 127 361 551 751
130 231 672 1034 1362
a
Use values that are closest to the expected values (round to the next highest value).
b
If the number of active electrodes or hours of use is different than these values, adjustments are made in the formula.
12 hours/day usage (continued)
Pulse width (µs)
a
for 2 active electrodesb for
Table 14. For standard-impedance leads and Itrel 4 Neurostimulators: Electrode Correction Factor (ECF)
Number of active positive
electrodes
Case 1.7 2.9 3.9 4.6
1 1.0 1.3 1.5 --
2 1.3 2.0 -- --
3 1.5------
Number of active negative electrodes
1234
Estimating battery longevity examples
Example 1: PrimeAdvanced, PrimeAdvanced SureScan
MRI, or RestorePrime Neurostimulator and 4 programs
with low-impedance leads
1. Based on the patient's medical condition, the expected programmed values and modes of operation
are as follows:
Expected programmed values
Program 1 Program 2 Program 3 Program 4
Amplitude: 3.0 V Amplitude: 5.0 V Amplitude: 5.0 V Amplitude: 5.0 V
32 English System Eligibility, Battery Longevity 2021-02-15
Page 33

Expected programmed values
Program 1 Program 2 Program 3 Program 4
Rate: 30 Hz Rate: 30 Hz Rate: 30 Hz Rate: 30 Hz
Pulse width: 330 μs Pulse width: 210 μs Pulse width: 210 μs Pulse width: 210 μs
Active
electrodes:
Hours of stimulation per day (including Scheduled
0+, 6−,
remaining
Off
Active
electrodes:
3−, 4−, 5+,
remaining
Off
Modes of operation and use
Therapy):
Active
electrodes:
12
7−, 8−, 9+,
remaining
Off
Active
electrodes:
10−, 11−,
12+,
remaining
Off
2. Program Factors for each program (P1F, P2F, P3F, P4F):
a. Energy Use for Program 1 from Table 7:
P1EU = 38
b. Electrode Correction Factor for Program 1 from Table 8:
P1ECF = 1.0
c. Program 1 Factor:
P1EU × P1ECF = P1F
38 × 1.0 = 38
d. Program 2 Factor:
P2EU × P2ECF = P2F
54 × 1.4 = 75.6
e. Program 3 Factor:
P3EU × P3ECF = P3F
54 × 1.4 = 75.6
f. Program 4 Factor:
P4EU × P4ECF = P4F
54 × 1.4 = 75.6
3. Multi-Program Factor (MPF):
P1F + P2F + P3F + P4F = MPF
38 + 75.6 + 75.6 + 75.6 = 264.8
4. Usage Correction Factor (UCF):
a. UCF = hours of stimulation ÷ 12 hours
12 ÷ 12 = 1.0
5. Adjusted Energy Use:
MPF × UCF = Adjusted Energy Use
264.8 × 1.0 = 264.8
6. The battery longevity from Figure 1 = approximately 2.5 years.
System Eligibility, Battery Longevity 2021-02-15 English 33
Page 34

Example 2: Itrel 4 Neurostimulator with standardimpedance lead
1. Based on the patient's medical condition, the expected programmed values and modes of operation
are as follows:
Expected programmed values
Amplitude (V): 4.2
Rate (Hz): 30
Pulse width (μs): 450
Active electrodes: Case+, 2−, remaining Off
Modes of operation and use
Hours of stimulation per day: 18
2. Energy Use from standard-impedance Table 13:
EU = 57
3. Electrode Correction Factor (ECF) from standard-impedance Table 14:
ECF = 1.7
4. Usage Correction Factor (UCF):
a. UCF = Hours of stimulation ÷ 12 hours
18 ÷ 12 = 1.5
5. Adjusted Energy Use:
Adjusted Energy Use = EU × ECF × UCF + [12.2 × (2-UCF)]
57 × 1.7 × 1.5 + [12.2 × (2-1.5)] =
57 × 1.7 × 1.5 + 6.1 = 151.5
6. The battery longevity from Figure 2 = approximately 2.8 years.
34 English System Eligibility, Battery Longevity 2021-02-15
Page 35

System Eligibility, Battery Longevity 2021-02-15 English 35
Page 36

Manufacturer
Medtronic, Inc.
710 Medtronic Parkway
Minneapolis, MN 55432
USA
www.medtronic.com
Tel. +1-763-505-5000
REP
Authorized Representative
in the European Community
Medtronic B.V.
Earl Bakkenstraat 10
6422 PJ Heerlen
The Netherlands
Tel. +31-45-566-8000
Europe/Africa/Middle East Headquarters
Medtronic International Trading Sàrl
Route du Molliau 31
Case Postale 84
CH - 1131 Tolochenaz
Switzerland
www.medtronic.eu
Tel. +41-21-802-7000
Asia-Pacific
Medtronic International Ltd.
50 Pasir Panjang Road
#04-51 Mapletree Business City
Singapore 117384
Singapore
Tel. +65-6870-5510
EC
*MA00152A189*
© Medtronic 2020
All Rights Reserved
2021-02-15
MA00152A189 Rev A
 Loading...
Loading...