
Freestyle™
995CS, 995MS Bioprosthesis
Instructions for Use
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.

Trademarks may be registered and are the property of their respective owners.

Explanation of symbols on package labeling
Refer to the device labeling to see which symbols apply to this product.
Use-by date
Serial number
Do not reuse
Sterile LC: Device has been sterilized using liquid chemical sterilants according to EN/ISO 14160.
Temperature limitation
Size
For US audiences only
Catalog number
MR Safe
Authorized representative in the European Community
Manufacturer
Do not resterilize
Quantity
Nonpyrogenic
Do not use if indicator turns black
Manufactured in
Model
Date of manufacture
1

Figure 1. Opening the valve container
Figure 2. Removing the retainer from the jar
Figure 3. Removing the cap from the valve retainer body
Figure 4. Removing the bioprosthesis from the retainer
2
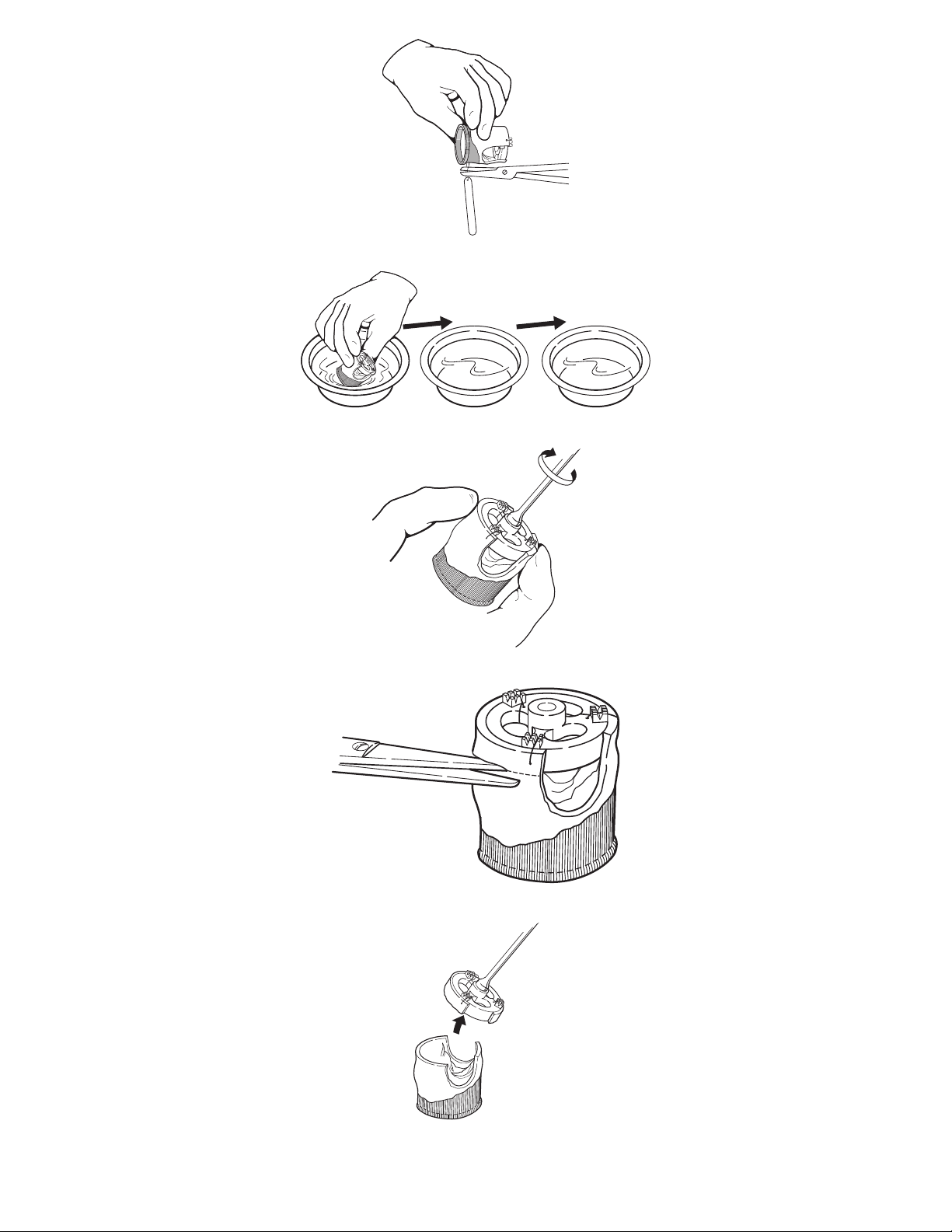
Figure 5. Releasing the identification tag (serial number)
Figure 6. Rinsing the bioprosthesis
Figure 7. Screwing the valve holder onto the handle
Figure 8. Removing the valve holder, method 1
Figure 9. Removing the valve holder, method 1
3
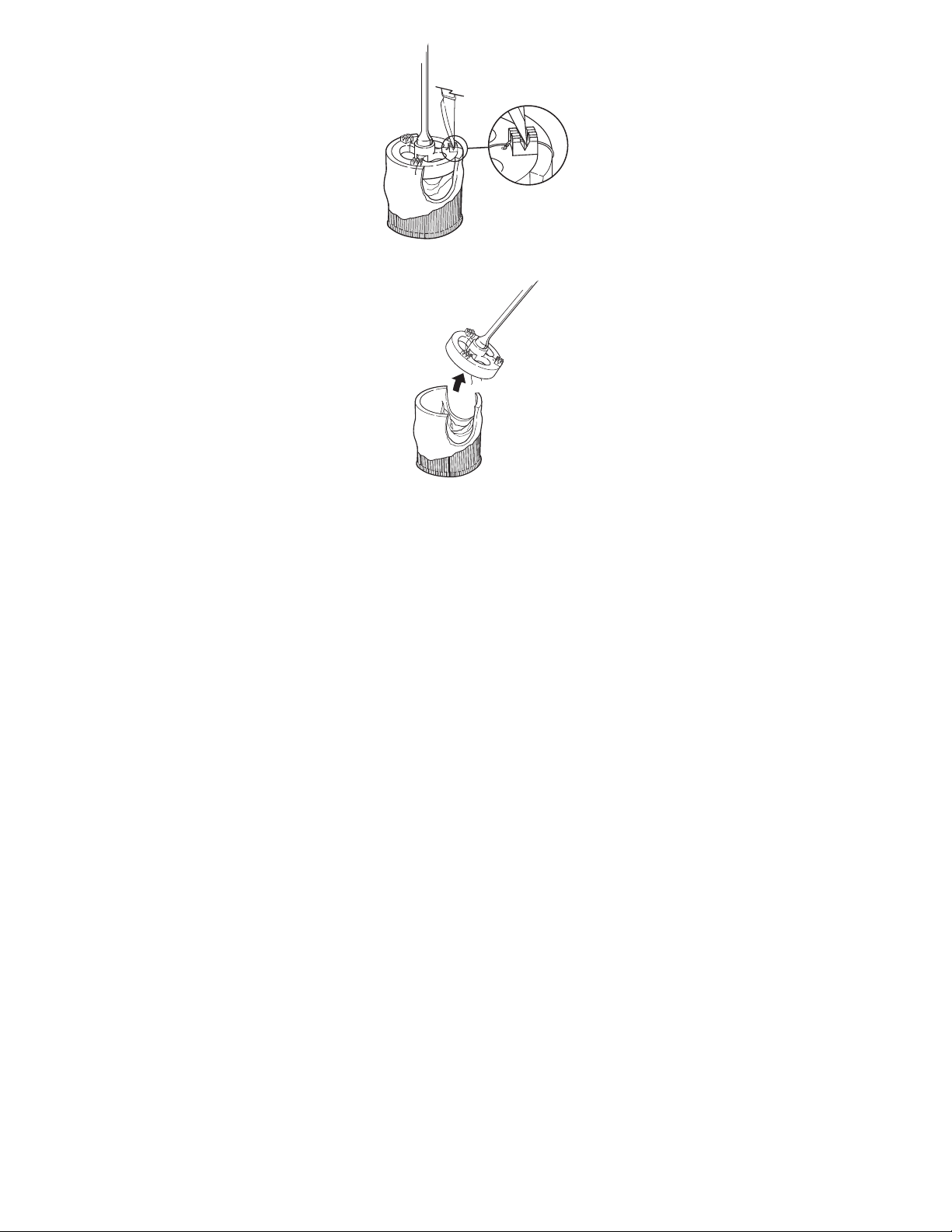
Figure 10. Removing the valve holder, method 2
Figure 11. Removing the valve holder, method 2
4
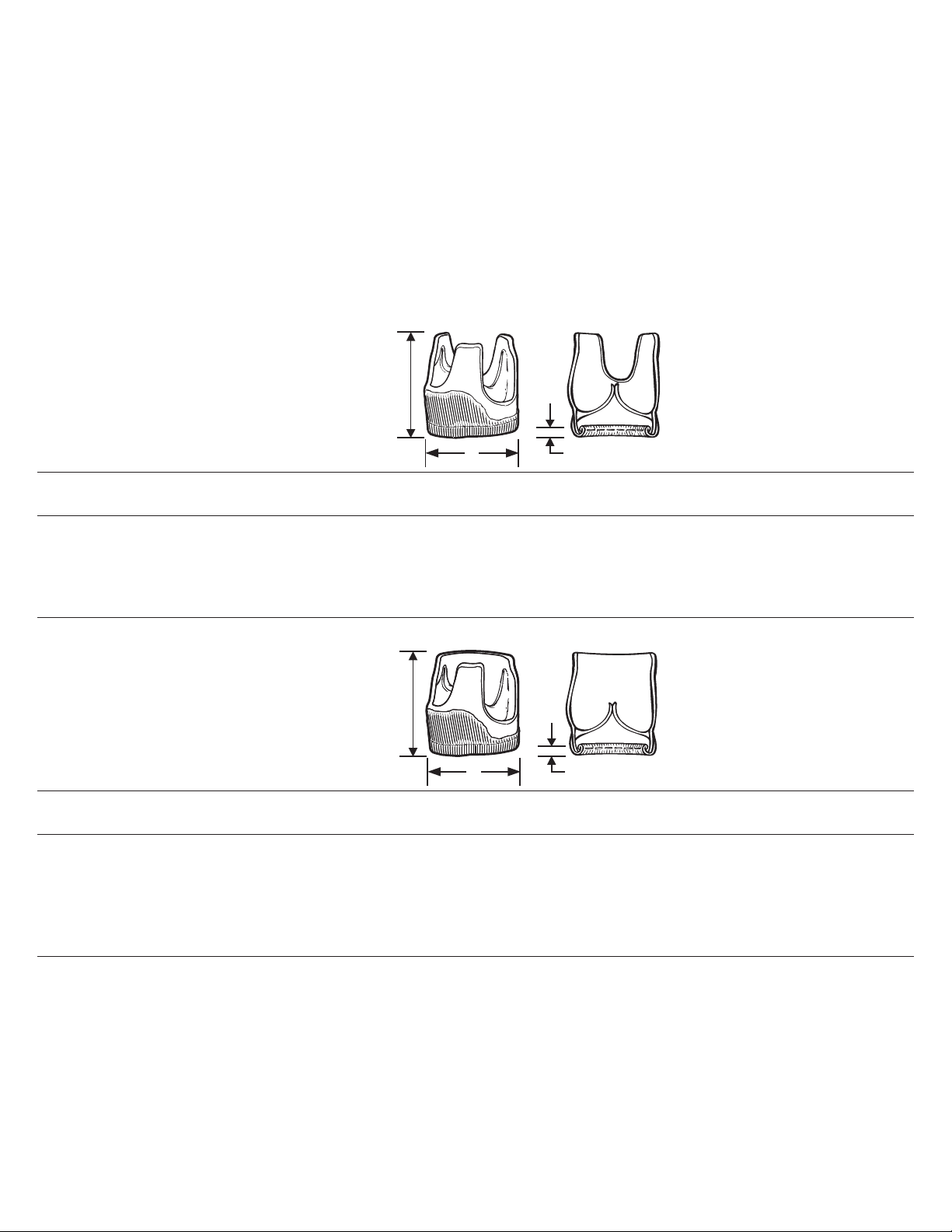
Bioprosthesis
3 mm ± 0.5
A
Valve height
minimum 9 mm
above highest
commissure
3 mm ± 0.5
A
Valve height
minimum 9 mm
above highest
commissure
1. Device description
The Freestyle™ modified subcoronary bioprosthesis, Model 995MS, and the Freestyle™ complete subcoronary bioprosthesis,
Model 995CS, consist of a porcine aortic root scalloped for aortic valve replacement. The modified subcoronary bioprosthesis is
scalloped at the right and left coronary sinuses. The complete subcoronary bioprosthesis is scalloped at each of the 3 sinuses.
The Freestyle subcoronary bioprostheses are preserved in buffered 0.2% glutaraldehyde with a cloth covering added to
strengthen the proximal (inflow) suture line and to cover any exposed porcine myocardium. The fixation and preservation with
buffered glutaraldehyde solutions minimize the immunogenic potential on the porcine tissue.
The Freestyle subcoronary bioprostheses are treated with an alpha amino oleic acid antimineralization process, AOA™, which
has been shown to mitigate porcine leaflet calcification in animal studies.
The Freestyle subcoronary bioprostheses are available in the diameters and sizes shown in Table 1 and Table 2. Each
bioprosthesis is supplied with a valve holder to aid in valve handling before and during the implantation procedure.
Table 1. Freestyle complete subcoronary bioprosthesis, Model 995CS available sizes and dimensions
Size (mm) A
Outside diameter (+0.5 mm-0.0 mm)
19 19.0
21 21.0
23 23.0
25 25.0
27 27.0
Table 2. Freestyle modified subcoronary bioprosthesis, Model 995MS available sizes and dimensions
Size (mm) A
Outside diameter (+0.5 mm-0.0 mm)
19 19.0
21 21.0
23 23.0
25 25.0
27 27.0
29 29.0
2. Indications for use
The Freestyle subcoronary bioprostheses are indicated for the replacement of malfunctioning native or prosthetic aortic valves.
3. Contraindications
No contraindications for use of this device are known.
Instructions for Use English 5

4. Warnings and precautions
4.1. Warnings
This device was designed for single patient use only. Do not reuse, reprocess, or resterilize this product. Reuse, reprocessing,
or resterilization may compromise the structural integrity of the device and/or create a risk of contamination of the device, which
could result in patient injury, illness, or death.
Check the shipping temperature indicator inside the carton. If the shipping temperature indicator window is black, the valve is
not suitable for clinical use.
Do not resterilize the valve by any method. Exposure of the bioprosthesis and container to irradiation, steam, ethylene oxide or
other chemical sterilants will render the bioprosthesis unfit for use.
Do not use the bioprosthesis in any of the following circumstances:
■
The bioprosthesis has been dropped, damaged, or mishandled in any way
■
The Use-by date has elapsed
■
All tamper strips on the glass jar and lid container are damaged
■
The serial number tag does not match the number on the container label
■
The shipping temperature indicator window has turned black
■
The glutaraldehyde storage solution does not completely cover the bioprosthesis
Do not expose the bioprosthesis to solutions other than the storage solution in which it was shipped, the sterile isotonic saline
solution used during the rinsing procedure, or the sterile isotonic saline solution used to irrigate the bioprosthesis.
Do not add antibiotics to either the storage or the rinse solution.
Do not apply antibiotics to the bioprosthesis.
Do not allow the tissue of the bioprosthesis to dry. Maintain tissue moisture with irrigation or immersion in normal saline solution
during surgery.
Do not attempt to repair a damaged bioprosthesis.
Do not use cutting needles, as they may cause structural damage to the fabric of the bioprosthesis.
Do not pass a catheter, surgical instrument, or transvenous pacing lead through the bioprosthesis, as this may damage the
valve.
4.2. Precautions
■
Accelerated deterioration due to calcific degeneration of bioprostheses may occur in the following individuals:
■
Children, adolescents, or young adults
■
Patients with altered calcium metabolism (eg, chronic renal failure, hyperparathyroidism)
■
When selecting a bioprosthesis size, the cardiac anatomy must be considered, and care must be taken to select a
bioprosthesis that adequately provides for the patient's hemodynamic requirements.
■
Implanting physicians must be familiar with the techniques for implanting an unstented bioprosthesis. These techniques are
similar to those required for allograft implantation.
■
In vitro testing of the Freestyle subcoronary bioprosthesis has only been performed in less compliant, simulated aortae
comparable to those of middle aged or older patients. Data from clinical or in vitro testing are not available from more
compliant, simulated aortae comparable to those of younger patients.
■
Do not invert the valve. Inversion will damage valve tissue.
Note: The leaflets of the Freestyle bioprosthesis are in an open position. Use extreme caution to avoid cutting or puncturing
the leaflets when tailoring and suturing the bioprosthesis.
■
Use of pledgets anywhere within the interior aspect of the bioprosthesis is not recommended.
■
Trim suture ends close to the knot to prevent abrasion of leaflet tissue.
5. Adverse events
5.1. Original premarket application (PMA) data (complete subcoronary configuration: sizes 19-27 mm)
A prospective, nonrandomized, multicenter, international study evaluated the Freestyle bioprosthesis with patient follow-up out
to 3 years. A total of 882 patients received the bioprosthesis. Patients were monitored throughout the entire postoperative
period for possible adverse events. The cumulative follow-up was 1246 patient-years with a mean follow-up of 17 months
(standard deviation [SD] = 12 months, range = 0 to 42 months).
Observed adverse events
Subcoronary technique (sizes 19-27 mm)
Note: All clinical results reflect data from the original PMA submission for the Freestyle bioprosthesis, Model 995,
which was custom trimmed by the implanting physician.
6 Instructions for Use English

A total of 640 Freestyle bioprostheses were implanted with the subcoronary technique in 640 patients at 15 centers. Nine of the
640 patients were excluded from the data summary of adverse events for the following reasons: 5 patients had their
bioprosthesis removed and replaced with another prosthesis during the initial surgery due to difficulty sizing a small aortic root,
high mean gradient, or patient prosthesis mismatch; and 4 patients had either a preexisting or concomitant implantation of a
mitral valve prosthesis. The adverse event rates were based on 631 bioprostheses implanted in 631 patients. The cumulative
follow-up was 913 patient-years with a mean follow-up of 17 months (SD = 11 months, range = 0 to 42 months).
Table 3. Observed Adverse Events (AEs) for the Subcoronary Technique. All patients analyzed, N = 631, cumulative follow-up
= 913 patient-years.
Early Events
N % of Patients N % /Patient- Year
a
Late Events
b
All Deaths 31 4.9% 31 3.6%
Bioprosthesis-Related or Unexplained 4 0.6% 9 1.0%
Study Bioprosthesis-Related AEs
Thromboembolism
c
14 2.2% 13 1.5%
Permanent Neurological Event 11 1.7% 6 0.7%
Transient Neurological Event 3 0.5% 6 0.7%
Bioprosthetic Thrombosis 0 0.0% 1 0.1%
Structural Deterioration
Nonstructural Dysfunction
d
d
0 0.0% 0 0.0%
0 0.0% 0 0.0%
Major Antithromboembolic- Related Hemorrhage 10 1.6% 7 0.8%
Primary Paravalvular Leak 3 0.5% 8 0.9%
Endocarditis 2 0.3% 8 0.9%
Primary Hemolysis
d
0 0.0% 0 0.0%
Reoperation 0 0.0% 7 0.8%
Explantation 0 0.0% 6 0.7%
Actuarial Freedom by Kaplan-Meier (%)
1 Year (95% CI) 3 Years (95% CI)
All Deaths 91.6%
[89.0% - 94.2%]
Bioprosthesis-Related or Unexplained 97.9%
[96.5% - 99.3%]
85.9%
[77.5%-94.3%]
97.5%
[93.5% - 100.0%]
Study Bioprosthesis-Related AEs
Thromboembolism
c
Permanent Neurological Event 97.3%
Transient Neurological Event 98.5%
Bioprosthetic Thrombosis 99.8%
Structural Deterioration
Nonstructural Dysfunction
d
d
Major Antithromboembolic- Related Hemorrhage 97.0%
Primary Paravalvular Leak 98.6%
Endocarditis 98.5%
Primary Hemolysis
d
95.6%
[93.6% - 97.6%]
[95.8% - 98.8%]
[97.3% - 99.7%]
[99.4% - 100.0%]
100.0%
[99.1% - 100.0%]
100.0%
[99.1% - 100.0%]
[95.4% - 98.6%]
[97.5% - 99.7%]
[97.3% - 99.7%]
100.0%
[99.1% - 100.0%]
94.6%
[88.8% - 100.0%]
96.6%
[91.9% - 100.0%]
98.2%
[94.7% - 100.0%]
99.8%
[98.6% - 100.0%]
100.0%
[93.7% - 100.0%]
100.0%
[93.7% - 100.0%]
97.0%
[92.6% - 100.0%]
97.6%
[93.6% - 100.0%]
97.9%
[94.2% - 100.0%]
100.0%
[93.7% - 100.0%]
Instructions for Use English 7

Reoperation 98.9%
[97.9% - 99.9%]
Explantation 99.0%
[98.0% - 100.0%]
a
Hospital or 30-day event for death or 30-day event for adverse events.
b
Calculations were based on 864 late patient-years.
c
One late event was a peripheral arterial embolus.
d
The number of patients remaining at risk at 1 year (N = 415) and 3 years (N = 57) was used for N in the calculations of the lower confidence limits for the actuarial estimates. The calculation methods are
described in the following note:
98.6%
[95.6% - 100.0%]
98.7%
[95.8% - 100.0%]
Notes:
■
AEs = Adverse Events
■
Adverse event rates were calculated as the percentage of patients for early events. For late adverse events, the linearized
rates (%/ patient-year) were calculated. For time to first event (early or late), actuarial rates using the Kaplan-Meier method
and confidence intervals were calculated. For adverse events with no occurrences, the lower two-sided 95% confidence
limits for the Kaplan-Meier estimates were calculated as (1-maximum risk), where (1- maximum risk) is equal to (0.025)1/N.
If there was no censoring, N would be the total sample size. Since there was censoring, the number of patients remaining
at risk at 1 and 3 years was used for N. Using the number of patients remaining at risk ignores the experience of all the
patients who were censored before the relevant time points and, therefore, overestimates the maximum risk.
5.2. Supplemental data (modified and complete subcoronary configurations: sizes 19-27 mm) Note: All clinical results reflect data from the Post-Approval Study for the Freestyle bioprosthesis, Model 995, which was
custom trimmed by the implanting physician.
A prospective, nonrandomized, multicenter, international study evaluated the Freestyle bioprosthesis with patient follow-up out
to 6 years. A total of 502 patients received the bioprosthesis with the subcoronary technique. Patients were monitored
throughout the entire postoperative period for possible adverse events.
Two patients were excluded from the data summary of adverse events for the following reasons: 1 had a preexisting mitral
valve prosthesis, and 1 had a concomitant implantation of a mitral valve prosthesis. The cumulative follow-up for the remaining
500 patients was 1518 patient-years with a mean follow-up of 3.0 years (SD = 1.7 years, range = 0 to 6.4 years). Two additional
patients were excluded from the data summary of adverse events because data for the number of sinuses scalloped were not
indicated.
Observed adverse events
Complete configuration
A total of 153 Freestyle bioprostheses were implanted with the complete configuration of the subcoronary technique in
153 patients.
Table 4. Observed Adverse Event (AE) Rates: Complete Configuration. All patients analyzed: N = 153 cumulative follow-up =
495.7 patient-years.
Early Events
n % of
a
Late Events
n %/ Pt.-
Pts.
All Deaths 6 3.9 27 5.6 90.2
Valve-Related or Unexplained 1 0.7 5 1.0 97.3
Yrs.
b
Freedom From Event (%) [95% CI]
1 Year 3 Years 5 Years
84.6
[85.5, 94.9]
[78.1, 91.1]
[57.2, 85.8]
97.3
[94.6, 100.0]
[94.2, 100.0]
[87.0, 100.0]
c
71.5
95.0
Valve-Related AEs
Thromboembolism 2 1.3 14 2.9 95.1
[91.6, 98.6]
Permanent Neurological Event 2 1.3 6 1.2 97.3
[94.6, 100.0]
Transient Neurological Event 0 0.0 8 1.7 97.9
[95.5, 100.0]
Thrombosis 0 0.0 1 0.2 99.3
[97.9, 100.0]
Structural Valve Deterioration 0 0.0 1 0.2 100.0 99.0
91.2
[85.7, 96.7]
95.7
[91.8, 99.6]
95.5
[91.4, 99.6]
99.3
[97.7, 100.0]
[97.0, 100.0]
87.3
[75.5, 99.1]
93.0
[83.8, 100.0]
92.7
[83.3, 100.0]
99.3
[96.2, 100.0]
99.0
[95.3, 100.0]
Nonstructural Valve Dysfunction 0 0.0 0 0.0 100.0 100.0 100.0
8 Instructions for Use English

Early Events
n % of
a
Late Events
n %/ Pt.-
Pts.
Endocarditis 0 0.0 1 0.2 99.3
Primary Paravalvular Leak 1 0.7 5 1.0 97.2
Major Antithromboembolic- Rela-
4 2.6 6 1.2 95.9
ted Hemorrhage
Yrs.
b
Freedom From Event (%) [95% CI]
1 Year 3 Years 5 Years
99.3
[97.9, 100.0]
[97.7, 100.0]
[96.2, 100.0]
95.6
[94.5, 99.9]
[91.7, 99.5]
[88.0, 100.0]
94.3
[92.6, 99.2]
[89.8, 98.8]
[82.2, 100.0]
c
99.3
95.6
92.4
Primary Hemolysis 0 0.0 0 0.0 100.0 100.0 100.0
Reoperation 0 0.0 2 0.4 99.3
[97.9, 100.0]
Explantation 0 0.0 2 0.4 99.3
[97.9, 100.0]
a
Early deaths occurred within 30 days of implantation if the patient was discharged from the hospital, or at any time after implantation if the patient was not discharged from the hospital. Early valve-related
adverse events occurred within the first 30 days of implantation. Early event rates were calculated as the percentage of patients.
b
Late deaths occurred after 30 days postoperation, if the patient was discharged from the hospital. Late valve-related adverse events occurred after 30 days postoperation. Late event rates were calculated as
linearized rates (%/patient-year). Calculations for late death rates were based on 483.2 late patient-years. Calculations for late valve-related adverse event rates were based on 483.6 late patient-years.
c
Freedom from event (early or late) rates were calculated using the Kaplan-Meier method. Peto’s formula was used for the calculation of the standard error of the Kaplan-Meier estimate for the confidence
interval (CI) for adverse events with at least 1 occurrence.
Modified
configuration
98.3
[95.8, 100.0]
98.3
[95.8, 100.0]
98.3
[93.6, 100.0]
98.3
[93.6, 100.0]
A total of 347 Freestyle bioprostheses were implanted with the modified configuration of the subcoronary technique in
347 patients. Two of the 347 patients were excluded from the data summary of adverse events for the following reasons: 1 had
a preexisting mitral valve prosthesis, and 1 had a concomitant implantation of a mitral valve prosthesis.
Table 5. Observed Adverse Event (AE) Rates: Complete Configuration. All patients analyzed: N = 153 cumulative follow-up =
495.7 patient-years.
Early Events Late Events Freedom From Event (%) [95% CI]
N % of
Pts
b
N %/
Patient-
Year
1 Year
c
[95% CI]
All Deaths 19 5.5 32 3.2 92.3
[89.4, 95.2]
Valve-Related or Unexplained 1 0.3 6 0.6 98.7
[97.3, 100.0]
4 Years
[95% CI]
87.4
[82.9, 91.9]
98.3
[96.5, 100.0]
a
10 Years
[95% CI]
79.2
[70.4, 88.0]
97.2
[93.3, 100.0]
Valve-Related AEs
Thromboembolism
Permanent Neurological Event 4 1.2 10 1.0 98.1
Transient Neurological Event
Thrombosis 0 0.0 1 0.1 99.7
d
d
6 1.7 17 1.7 95.3
[92.9, 97.7]
[96.5, 99.7]
2 0.6 5 0.5 97.8
[96.0, 99.6]
[99.1, 100.0]
94.4
[91.1, 97.7]
97.3
[94.9, 99.7]
97.8
[95.6, 100.0]
99.7
[98.9, 100.0]
91.3
[84.6, 98.0]
94.1
[88.4, 99.8]
97.8
[94.3, 100.0]
99.7
[98.3, 100.0]
Structural Valve Deterioration 0 0.0 0 0.0 100.0 100.0 100.0
Nonstructural Valve Dysfunction 0 0.0 0 0.0 100.0 100.0 100.0
Endocarditis 1 0.3 4 0.4 99.0
[97.8, 100.0]
Primary Paravalvular Leak 4 1.2 4 0.4 98.1
[96.5, 99.7]
Major Antithromboembolic- Related Hemorrhage
4 1.2 5 0.5 97.8
[96.0, 99.6]
98.2
[96.2, 100.0]
97.7
[95.5, 99.9]
97.4
[95.0, 99.8]
98.2
[95.1, 100.0]
97.1
[93.0, 100.0]
95.9
[91.0, 100.0]
Primary Hemolysis 0 0.0 0 0.0 100.0 100.0 100.0
Instructions for Use English 9

Early Events Late Events Freedom From Event (%) [95% CI]
N % of
Pts
Reoperation 1 0.3 5 0.5 98.7
Explant 1 0.3 3 0.3 99.0
a
Freedom from event (early or late) rates were calculated using the Kaplan-Meier method. Peto’s formula was used for the calculation of the standard error of the Kaplan-Meier estimate for the confidence
interval (CI) for adverse events with at least 1 occurrence.
b
Early deaths occurred within 30 days of implantation if the patient was discharged from the hospital, or at any time after implantation if the patient was not discharged from the hospital. Early valve-related
adverse events occurred within the first 30 days of implantation. Early event rates were calculated as the percentage of patients.
c
Late deaths occurred after 30 days postoperative, if the patient was discharged from the hospital. Late valve-related adverse events occurred after 30 days postoperative. Late event rates were calculated as
linearized rates (%/patientyear). Calculations for late death rates were based on 990.5 late patient-years. Calculations for late valve-related adverse events were based on 991.1 late patient-years.
d
One late event was a peripheral arterial embolus, and 1 was a myocardial infarction.
b
N %/
Patient-
Year
c
1 Year
[95% CI]
[97.3, 100.0]
[97.8, 100.0]
4 Years
[95% CI]
98.7
[97.1, 100.0]
99.0
[97.6, 100.0]
a
10 Years
[95% CI]
96.3
[91.8, 100.0]
97.9
[94.4, 100.0]
5.3. Supplemental data (modified subcoronary configuration: size 29 mm) Note: All clinical results reflect data from the Post-Approval Study for the Freestyle bioprosthesis, Model 995, which was
custom trimmed by the implanting physician.
A prospective, nonrandomized, multicenter, international study evaluated the Freestyle bioprosthesis with patient follow-up out
to 3 years. A total of 23 patients received the bioprosthesis with the modified subcoronary configuration. Patients were
monitored throughout the entire postoperative period for possible adverse events. Two patients were excluded from the data
summary of adverse events because each had a preexisting valve prosthesis. The cumulative follow-up for the remaining
21 patients was 33.2 patient-years with a mean follow-up of 1.6 years (SD = 0.8 year, range = 0.3 to 3.5 years).
Observed Adverse Events A total of 23 Freestyle bioprostheses were implanted with the modified configuration of the
subcoronary technique in 23 patients. Two patients were excluded from the data summary of adverse events because they
both had a preexisting valve prosthesis.
Table 6. Observed Adverse Event (AE) Rates: Modified Configuration. All Patients Analyzed: N = 21, Cumulative Follow-Up =
33.2 Patient-Years.
Early Events
a
Late Events b,
c
N % of Patients N
All Deaths 0 0.0% 1
Valve-Related or Unexplained 0 0.0% 0
Valve-Related AEs
Thromboembolism 1 4.8% 0
Permanent Neurological Event 0 0.0% 0
Transient Neurological Event 1 4.8% 0
Thrombosis 0 0.0% 0
Structural Valve Deterioration 0 0.0% 0
Nonstructural Valve Dysfunction 0 0.0% 0
Endocarditis 0 0.0% 0
Primary Paravalvular Leak 0 0.0% 1
Major Antithromboembolic-Related
Hemorrhage 0 0.0% 0
Primary Hemolysis 0 0.0% 0
Reoperation 0 0.0% 0
Explantation 0 0.0% 0
a
Early deaths occurred within 30 days of implantation if the patient was discharged from the hospital, or at any time after implantation if the patient was not discharged from the hospital. Early valve-related
adverse events occurred within the first 30 days of implantation. Early event rates were calculated as the percentage of patients.
b
Late deaths occurred after 30 days postoperation if the patient was discharged from the hospital. Late valve-related adverse events occurred after 30 days postoperation.
c
Due to the small amount of follow-up, linearized rates (%/patient-year) and freedom-from-event rates were not provided.
5.4. Potential adverse events
Adverse events potentially associated with the use of bioprosthetic heart valves include:
■
Angina
■
Cardiac dysrhythmias
■
Death
■
Endocarditis
■
Heart failure
■
Infection, other than endocarditis
10 Instructions for Use English

■
1000
800
600
400
200
0
0 1 2 3 4
Total
Subcoronary
Full root
Root inclusion
Follow-up (years)
Number of patients
Hemolysis
■
Hemolytic anemia
■
Hemorrhage, anticoagulant/antiplatelet-related
■
Leak, transvalvular or paravalvular
■
Myocardial infarction
■
Nonstructural dysfunction (pannus, suture, inappropriate sizing, other)
■
Structural deterioration (calcification, leaflet tear, intracuspal hematoma, other)
■
Thromboembolism
■
Valve thrombosis
■
Acute kidney injury
■
Renal failure
It is possible that these complications could lead to the following:
■
Reoperation
■
Explantation
■
Permanent disability
■
Death
6. Clinical studies
6.1. Original premarket application (PMA) data (complete subcoronary configuration: sizes 19-27 mm) Note: All clinical results reflect data from the original PMA submission for the Freestyle bioprosthesis, Model 995,
which was custom trimmed by the implanting physician.
A prospective, nonrandomized, multicenter, international study evaluated the Freestyle bioprosthesis with patient follow-up out
to 3 years. A total of 882 patients received a bioprosthesis. Patients were evaluated preoperatively, within 30 days
postoperatively, at 3 to 6 months, and annually. The cumulative follow-up was 1246 patient-years with a mean follow-up of
17 months (SD = 12 months, range = 0 to 42 months).
The following graph shows the number of patients who underwent bioprosthesis implantation by duration of follow-up, and the
subsequent table shows the breakdown of duration of follow-up by valve size.
Figure 12. The number of patients who underwent bioprosthesis implantation by duration of follow-up
Table 7. The breakdown of duration of follow-up by valve size
Duration of follow-up (years)
0 1 2 3 3.5
Number of patients by year
Total 882 552 348 96 1
Subcoronary 640 416 255 58 1
Size Number of patients by valve size by year
19 mm 29 17 9 2 0
21 mm 120 80 49 10 0
23 mm 193 125 76 17 1
Instructions for Use English 11

Duration of follow-up (years)
0 1 2 3 3.5
25 mm 169 113 68 17 0
27 mm 129 81 53 12 0
Table 8. Patient Characteristics: Subcoronary Technique. All patients analyzed, N=631.
Age at implantation in years (mean ± SD, N [min., max.]) 71±8, 631 [32, 91]
Gender (% male /% female) 53% /47%
Etiology
Stenosis—percent of patients with stenosis alone [% (num-
43% (272/631)
ber in subgroup/N)]
Insufficiency—percent of patients with insufficiency alone [%
6% (39/631)
(number in subgroup/N)]
Mixed—percent of patients with stenosis and insufficiency [%
50% (318/631)
(number in subgroup/N)]
Othera—percent of patients with etiology other than stenosis
0% (2/631)
or insufficiency [% (number in subgroup/N)]
a
One patient had incidental replacement of a previously implanted prosthesis, and 1 had endocarditis without lesion.
Table 9. Effectiveness Outcomes, Functional NYHAa: Subcoronary Technique. All patients analyzed: N=631, mean ± SD
(number), percent (numerator/N).
Endpoint Preoperation 3-6 Months 1 Year
Functional NYHA 2.9 ± 0.6
(631)
I - % of pts. in NYHA class I 2%
(14/631)
II - % of pts. in NYHA class II 20%
(129/631)
III - % of pts. in NYHA class III 63%
(400/631)
IV - % of pts. in NYHA class IV 14%
(88/631)
a
New York Heart Association
1.2 ± 0.5
(525)
80%
(419/525)
18%
(96/525)
2%
(10/525)
0%
(0/525)
1.2 ± 0.5
(454)
84%
(380/454)
14%
(64/454)
2%
(8/454)
0%
(2/454)
Table 10. Effectiveness Outcomes, Hemodynamics, Valvular Regurgitation: Subcoronary Technique. All patients analyzed: N =
631, mean ± SD (number), percent (numerator/N).
Endpoint ≤30 days 3-6 Months 1 Year
Valvular Regurgitation
a
0.3 ± 0.4 (552) 0.3 ± 0.5 (523) 0.3 ± 0.4 (456)
0 % of pts. with no Rg. 65% (358/552) 62% (326/523) 65% (296/456)
<1+ % of pts. with <mild Rg. 15% (82/552) 17% (87/523) 20% (92/456)
1+ % of pts. with mild Rg. 20% (108/552) 19% (101/523) 13% (61/456)
2+ % of pts. with mod Rg. 1% (3/552) 2% (8/523) 1% (5/456)
3+/4+ % of pts. with mod/ severe Rg. 0% (1/552) 0% (1/523) 0% (2/456)
a
The data reflect regurgitation noted at all locations combined. Data coded as “trivial/mild” were included in the category “<1+, < mild regurgitation." Data in the category "<1+" were coded as “0.5” for the
calculation of mean ± SD.
Note: Rg. = Regurgitation
Table 11. Effectiveness Outcomes, Hemodynamics, Mean Pressure Gradient: Subcoronary Technique. All patients analyzed: N
= 631, number in subgroup/N, mean ± SD [min., max.].
Endpoint ≤30 days 3-6 Months 1 Year
Mean Pressure Gradient (mm Hg)
19 mm 19/27, 17.6 ± 7.6
[8.0, 42.0]
21 mm 100/117, 14.6 ± 8.1
[1.0, 48.0]
23 mm 165/191, 12.9 ± 6.9
[2.0, 39.0]
19/27, 12.0 ± 5.7
[2.0, 23.0]
91/117, 9.0 ± 6.2
[1.0, 47.0]
156/191, 8.9 ± 5.9
[1.0, 35.0]
18/27, 11.7 ± 4.7
[5.0, 19.0]
83/117, 9.8 ± 7.4
[0.8, 51.0]
138/191, 8.8 ± 6.8
[0.0, 57.0]
12 Instructions for Use English

Endpoint ≤30 days 3-6 Months 1 Year
500
400
300
200
100
0
0 1 2 3 4
All Subcoronary
Modified
Complete
Follow-up (years)
Number of patients
5 6 7
25 mm 145/167, 9.1 ± 4.6
[1.0, 28.0]
27 mm 112/129, 7.3 ± 4.1
[1.0, 22.0]
146/167, 5.5 ± 3.3
[1.0, 19.0]
110/129, 5.0 ± 3.7
[1.0, 30.0]
119/167, 5.1 ± 3.3
[0.0, 18.0]
92/129, 4.4 ± 2.9
[0.7, 13.0]
Table 12. Effectiveness Outcomes, Hemodynamics, Effective Orifice Area: Subcoronary Technique. All patients analyzed: N =
631, number in subgroup/N, mean ± SD [min., max.].
Endpoint ≤30 days 3-6 Months 1 Year
Effective Orifice Area (cm2)
19 mm 19/27, 0.9 ± 0.2
[0.5, 1.4]
21 mm 97/117, 1.3 ± 0.4
[0.5, 2.4]
23 mm 160/191, 1.4 ± 0.5
[0.5, 3.7]
25 mm 143/167, 1.8 ± 0.6
[0.4, 3.9]
27 mm 110/129, 2.2 ± 0.7
[0.8, 5.1]
19/27, 1.1 ± 0.3
[0.6, 1.7]
91/117, 1.5 ± 0.5
[0.8, 4.3]
154/191, 1.7 ± 0.5
[0.6, 3.6]
146/167, 2.0 ± 0.5
[0.9, 3.5]
109/129, 2.4 ± 0.6
[1.2, 4.1]
18/27, 1.1 ± 0.3
[0.7, 1.7]
82/117, 1.4 ± 0.4
[0.4, 3.1]
137/191, 1.7 ± 0.5
[0.8, 3.9]
119/167, 2.0 ± 0.5
[0.8, 3.5]
92/129, 2.5 ± 0.7
[1.3, 4.4]
6.2. Supplemental data (complete and modified configurations: sizes 19-27 mm) Note: All clinical results reflect data from the Post-Approval Study for the Freestyle bioprosthesis, Model 995, which was
custom trimmed by the implanting physician.
A prospective, nonrandomized, multicenter, international study evaluated the Freestyle bioprosthesis with patient follow-up out
to 6 years. A total of 502 patients received the bioprosthesis with the subcoronary technique. Patients were evaluated
preoperatively, within 30 days postoperatively, at 3 to 6 months, and annually. Two patients were excluded from the data
summaries for the following reasons: 1 had a preexisting mitral valve prosthesis, and 1 had a concomitant implantation of a
mitral valve prosthesis. The cumulative follow-up for the remaining 500 patients was 1518 patient-years with a mean follow-up
of 3.0 years (SD = 1.7 years, range = 0 to 6.4 years). Two patients were excluded from the data summaries because data for
the number of sinuses scalloped were not indicated.
A total of 153 Freestyle bioprostheses were implanted with the complete configuration of the subcoronary technique in
153 patients. A total of 347 Freestyle bioprostheses were implanted with the modified configuration of the subcoronary
technique in 347 patients. Two of these patients were excluded from the data summaries for the following reasons: 1 had a
preexisting mitral valve prosthesis, and 1 had a concomitant implantation of a mitral valve prosthesis.
The following graph shows the number of patients implanted with the subcoronary technique by configuration versus duration of
follow-up, and Table 13 shows the breakdown of duration of follow-up for the subcoronary technique by configuration and valve
size.
Figure 13. Number of patients by duration of follow-up and subcoronary implant technique configuration: all patients implanted,
approved sizes, N = 502.
Instructions for Use English 13

Table 13. The breakdown of duration of follow-up for the subcoronary technique by configuration and valve size.
Subcoronary
Technique
Configuration Duration of follow-up (years)
0 1 2 3 4 5 6
Number of patients by subcoronary technique configuration by year
Subcoronary 502 434 362 294 202 101 17
Modified 347 296 241 190 133 68 16
Complete 153 137 120 103 68 33 1
Size Number of patients by size by year
19 mm 32 25 24 19 7 3 0
21 mm 103 80 65 52 35 16 2
23 mm 133 121 105 78 54 22 3
25 mm 138 119 96 79 61 35 6
27 mm 96 89 72 66 45 25 6
Note: Subcoronary technique and size information includes data for 2 patients from whom the number of sinuses scalloped
was not indicated.
Table 14. Patient Demographics: Complete Configuration. All patients analyzed: N = 153.
Age at implantation in years (mean + SD, N [min., max.]) 72 + 7, 153 [51, 91]
Gender (% male /% female) 52% / 48%
Etiology
Stenosis—percent of patients with stenosis alone [% (num-
57% (87/152)
ber in subgroup/N)]
Insufficiency—percent of patients with insufficiency alone [%
3% (5/152)
(number in subgroup/N)]
Mixed—percent of patients with stenosis and insufficiency [%
40% (60/152)
(number in subgroup/N)]
Table 15. Patient Demographics: Modified Configuration. All patients analyzed: N = 345.
Age at implantation in years (mean + SD, N [min., max.]) 72 + 7, 345 [36, 88]
Gender (% male /% female) 53% / 47%
Etiology
Stenosis—percent of patients with stenosis alone [% (num-
38% (132/345)
ber in subgroup/N)]
Insufficiency—percent of patients with insufficiency alone [%
8% (26/345)
(number in subgroup/N)]
Mixed—percent of patients with stenosis and insufficiency [%
54% (187/345)
(number in subgroup/N)]
Table 16. Effectiveness Outcomes, Functional NYHAa: Complete Configuration. All patients analyzed: N = 153.
NYHA
Preoperation 3-6 Months 1 Year 3 Years 5 Years
Class
n/N % n/N % n/N % n/N % n/N %
I 8/153 5% 115/144 80% 103/134 77% 72/102 71% 26/34 77%
II 23/153 15% 21/144 15% 27/134 20% 24/102 24% 6/34 18%
III 99/153 65% 5/144 4% 3/134 2% 3/102 3% 1/34 3%
IV 23/153 15% 0/144 0% 1/134 1% 0/102 0% 0/34 0%
Unable to
0/153 0% 3/144 2% 0/134 0% 3/102 3% 1/34 3%
Assess
a
New York Heart Association
14 Instructions for Use English

Table 17. Effectiveness Outcomes, Functional NYHAa: Modified Configuration. All patients analyzed: N = 345.
NYHA
Preoperation 3-6 Months 1 Year 3 Years 5 Years
Class
n/N % n/N % n/N % n/N % n/N %
I 7/344 2% 243/301 81% 258/291 89% 144/185 78% 45/61 74%
II 110/344 32% 51/301 17% 26/291 9% 30/185 16% 11/61 18%
III 196/344 57% 2/301 1% 2/291 1% 3/185 2% 3/61 5%
IV 31/344 9% 0/301 0% 1/291 0% 3/185 2% 0/61 0%
Unable to
0/344 0% 5/301 2% 4/291 1% 5/185 3% 2/61 3%
Assess
a
New York Heart Association
Table 18. Effectiveness Outcomes, Hemodynamics, Valvular Regurgitation: Complete Configuration. All patients analyzed: N =
153, percent (numerator/N).
Endpoint ≤ 30 days 3-6 Months 1 Year 3 Years 5 Years
Valvular Regurgitation
% of pts. with no regurgitation
% of pts. with trivial
regurgitation
% of pts. with trivial /
mild regurgitation
% of pts. with mild regurgitation
% of pts. with mod regurgitation
% of pts. with mod
severe regurgitation
% of pts. with severe
regurgitation
58%
(85/146)
14%
(21/146)
0%
(0/146)
27%
(39/146)
1%
(1/146)
0%
(0/146)
0%
(0/146)
53%
(75/141)
17%
(24/141)
0%
(0/141)
26%
(37/141)
4%
(5/141)
0%
(0/141)
0%
(0/141)
55%
(73/132)
23%
(31/132)
0%
(0/132)
18%
(24/132)
3%
(4/132)
0%
(0/132)
0%
(0/132)
51%
(47/93)
33%
(31/93)
0%
(0/93)
12%
(11/93)
4%
(4/93)
0%
(0/93)
0%
(0/93)
50%
(11/22)
27%
(6/22)
0%
(0/22)
18%
(4/22)
5%
(1/22)
0%
(0/22)
0%
(0/22)
Note: Data reflect transvalvular, paravalvular, and indeterminate regurgitation noted at all locations combined.
Table 19. Effectiveness Outcomes, Hemodynamics, Mean Pressure Gradient: Complete Configuration. All patients analyzed: N
= 153, number in subgroup/N, mean ± SD [min., max.].
Endpoint ≤ 30 days 3-6 Months 1 Year 3 Years 5 Years
Mean Pressure
Gradient (mm Hg)
19 mm 7/10
18.9 ± 10.6
[12.0, 42.0]
21 mm 26/27
13.2 ± 7.5
[2.0, 41.0]
23 mm 43/45
13.1 ± 5.8
[5.0, 32.0]
25 mm 37/40
9.7 ± 5.2
[3.0, 25.0]
27 mm 27/31
8.1 ± 4.1
[2.0, 22.0]
8/10
11.6 ± 6.0
[6.0, 23.0]
22/27
6.7 ± 3.0
[3.0, 15.0]
43/45
9.0 ± 4.4
[3.0, 20.0]
39/40
6.1 ± 4.2
[1.5, 19.0]
28/31
4.9 ± 3.1
[1.0, 13.0]
7/10
12.0 ± 6.2
[5.0, 19.0]
19/27
8.9 ± 5.7
[2.0, 23.0]
42/45
10.0 ± 4.7
[4.0, 24.0]
36/40
5.7 ± 4.0
[0.0, 17.6]
26/31
5.4 ± 3.1
[0.7, 12.0]
4/10
14.5 ± 11.0
[4.0, 25.0]
11/27
9.9 ± 5.5
[4.0, 23.0]
31/45
9.0 ± 3.7
[1.0, 17.0]
28/40
5.9 ± 3.3
[0.0, 14.0]
17/31
6.2 ± 3.8
[2.0, 13.0]
1/10
4.0
[4.0, 4.0]
3/27
9.0 ± 5.3
[5.0, 15.0]
6/45
7.8 ± 3.5
[3.0, 13.0]
8/40
7.6 ± 4.9
[2.0, 16.0]
4/31
8.0 ± 4.3
[2.0, 12.0]
Instructions for Use English 15

Table 20. Effectiveness Outcomes, Hemodynamics, Effective Orifice Area: Complete Configuration. All patients analyzed: N =
153, number in subgroup/N, mean ± SD [min., max.].
Endpoint ≤ 30 days 3-6 Months 1 Year 3 Years 5 Years
Effective Orifice
Area (cm2)
19 mm 7/10
0.9 ± 0.3
[0.5, 1.4]
21 mm 25/27
1.3 ± 0.4
[0.8, 2.5]
23 mm 42/45
1.3 ± 0.3
[0.8, 2.5]
25 mm 36/40
1.6 ± 0.3
[1.2, 2.3]
27 mm 27/31
2.1 ± 0.6
[1.4, 4.7]
Table 21. Effectiveness Outcomes, Hemodynamics, Valvular Regurgitation: Modified Configuration. All patients analyzed: N =
8/10
1.1 ± 0.4
[0.6, 1.7]
21/27
1.4 ± 0.3
[1.0, 2.1]
42/45
1.5 ± 0.3
[0.9, 2.3]
39/40
1.9 ± 0.4
[1.0, 2.7]
27/31
2.2 ± 0.4
[1.6, 3.4]
345, percent (numerator/N).
7/10
1.0 ± 0.3
[0.7, 1.7]
19/27
1.4 ± 0.2
[0.9, 1.7]
42/45
1.5 ± 0.4
[0.9, 2.7]
36/40
1.9 ± 0.4
[1.3, 3.0]
25/31
2.3 ± 0.4
[1.7, 3.1]
4/10
1.1 ± 0.5
[0.7, 1.8]
11/27
1.2 ± 0.3
[0.8, 1.7]
31/45
1.5 ± 0.3
[1.0, 2.2]
28/40
1.9 ± 0.4
[1.0, 2.8]
17/31
2.2 ± 0.5
[1.3, 2.9]
1/10
1.0
[1.0, 1.0]
3/27
1.5 ± 0.5
[1.1, 2.1]
6/45
1.6 ± 0.4
[1.2, 2.2]
8/40
1.6 ± 0.4
[1.0, 2.1]
4/31
2.4 ± 0.8
[1.6, 3.3]
Endpoint ≤ 30 days 3-6 Months 1 Year 3 Years 5 Years
Valvular Regurgitation
% of pts. with no regurgitation
% of pts. with trivial
regurgitation
% of pts. with trivial /
mild regurgitation
% of pts. with mild regurgitation
% of pts. with mod regurgitation
% of pts. with mod
severe regurgitation
% of pts. with severe
regurgitation
Note: Data reflect transvalvular, paravalvular, and indeterminate regurgitation noted at all locations combined.
Table 22. Effectiveness Outcomes, Hemodynamics, Mean Pressure Gradient: Modified Configuration. All patients analyzed: N
Endpoint ≤ 30 days 3-6 Months 1 Year 3 Years 5 Years
Mean Pressure
Gradient (mm Hg)
19 mm 17/21
17.1 ± 7.2
[8.0, 37.0]
21 mm 63/76
15.6 ± 8.0
[2.0, 38.0]
64%
(207/323)
16%
(51/323)
0%
(1/323)
19%
(60/323)
1%
(4/323)
0%
(0/323)
0%
(0/323)
= 345, number in subgroup/N, mean ± SD [min., max.].
62%
(186/298)
15%
(46/298)
0%
(1/298)
20%
(61/298)
1%
(4/298)
0%
(0/298)
0%
(0/298)
17/21
12.8 ± 4.8
[6.7, 23.0]
61/76
10.3 ± 5.6
[2.0, 24.0]
62%
(175/283)
18%
(52/283)
0%
(1/283)
18%
(52/283)
1%
(3/283)
0%
(0/283)
0%
(0/283)
16/21
13.1 ± 5.7
[6.0, 30.0]
55/76
11.9 ± 7.1
[2.0, 29.0]
57%
(94/164)
29%
(47/164)
0%
(0/164)
11%
(18/164)
2%
(4/164)
1%
(1/164)
0%
(0/164)
11/21
13.1 ± 5.1
[6.0, 24.0]
31/76
10.5 ± 6.6
[1.0, 25.0]
50%
(24/48)
35%
(17/48)
0%
(0/48)
13%
(6/48)
2%
(1/48)
0%
(0/48)
0%
(0/48)
0/21
8/76
12.3 ± 6.0
[5.0, 23.0]
16 Instructions for Use English

Endpoint ≤ 30 days 3-6 Months 1 Year 3 Years 5 Years
23 mm 80/86
13.4 ± 6.2
[4.0, 39.0]
25 mm 94/97
10.4 ± 4.6
[2.0, 25.0]
27 mm 62/65
9.0 ± 4.3
[3.0, 22.0]
Table 23. Effectiveness Outcomes, Hemodynamics, Effective Orifice Area: Modified Configuration. All patients analyzed: N =
345, number in subgroup/ N, mean ± SD [min., max.].
Endpoint ≤ 30 days 3-6 Months 1 Year 3 Years 5 Years
Effective Orifice
Area (cm2)
19 mm 17/21
1.0 ± 0.2
[0.5, 1.4]
21 mm 64/76
1.2 ± 0.3
[0.5, 2.2]
23 mm 79/86
1.4 ± 0.3
[0.6, 2.4]
25 mm 94/97
1.7 ± 0.4
[0.8, 3.0]
27 mm 61/65
2.0 ± 0.5
[1.1, 3.5]
76/86
9.5 ± 5.5
[0.9, 35.0]
84/97
6.9 ± 3.6
[2.0, 18.0]
58/65
6.0 ± 3.9
[2.0, 23.0]
17/21
1.2 ± 0.2
[0.8, 1.8]
60/76
1.4 ± 0.4
[0.8, 2.5]
76/86
1.6 ± 0.4
[0.9, 2.6]
84/97
1.9 ± 0.4
[1.1, 3.3]
58/65
2.3 ± 0.6
[1.3, 4.1]
74/86
9.2 ± 5.0
[1.0, 26.3]
74/97
6.5 ± 3.8
[1.0, 19.0]
60/65
5.8 ± 4.1
[1.0, 19.0]
16/21
1.2 ± 0.2
[0.9, 1.6]
53/76
1.4 ± 0.3
[0.6, 2.2]
74/86
1.7 ± 0.4
[0.8, 2.4]
73/97
2.1 ± 0.6
[1.0, 3.9]
59/65
2.4 ± 0.6
[1.3, 4.2]
41/86
9.0 ± 5.7
[2.0, 27.0]
39/97
5.4 ± 3.9
[1.0, 20.0]
40/65
4.3 ± 3.0
[0.0, 14.0]
11/21
1.2 ± 0.3
[0.8, 1.8]
30/76
1.4 ± 0.4
[0.6, 3.0]
41/86
1.7 ± 0.5
[0.9, 3.0]
39/97
2.0 ± 0.4
[1.3, 3.1]
40/65
2.5 ± 0.5
[1.2, 3.7]
12/86
9.6 ± 6.2
[2.0, 23.0]
13/97
5.2 ± 4.9
[1.0, 19.0]
12/65
3.6 ± 2.4
[1.0, 10.0]
0/21
8/76
1.2 ± 0.3
[0.7, 1.5]
11/86
1.5 ± 0.4
[1.0, 2.2]
13/97
1.9 ± 0.6
[0.8, 2.5]
12/65
2.5 ± 0.5
[1.8, 3.3]
6.3. Supplemental data (modified configuration: size 29 mm) Note: All clinical results reflect data from the Post-Approval Study for the Freestyle bioprosthesis, Model 995, which was
custom trimmed by the implanting physician.
A prospective, nonrandomized, multicenter, international study evaluated the Freestyle bioprosthesis with patient follow-up out
to 3 years. A total of 23 Freestyle bioprostheses were implanted with the modified configuration of the subcoronary technique in
23 patients. Patients were evaluated preoperatively, within 30 days postoperatively, at 3 to 6 months, and annually. Two
patients were excluded from the data summaries because they both had a preexisting valve prosthesis in another position. The
cumulative follow-up for the remaining 21 patients was 33.2 patient-years with a mean follow-up of 1.6 years (SD = 0.8 year,
range = 0.3 to 3.5 years).
Table 24. Patient Demographics: Modified Configuration. All patients analyzed: N = 21.
Age in years at implantation (mean + SD, N [min., max.]) 55 + 16, 21 [31, 90]
Gender (% male /% female) 95%/5%
Etiology
Stenosis: % of pts. with stenosis alone (% [number in subgroup/N])
Insufficiency
% of pts. with insufficiency alone (% [number in subgroup/N]) 29% (6/21)
Mixed
% of pts. with stenosis and insufficiency (% [number in subgroup/N])
10% (2/21)
62% (13/21)
Instructions for Use English 17

Table 25. Effectiveness Outcomes, Functional NYHAa: Modified Configuration. All Patients Analyzed: N = 21.
NYHA
Preoperative 3-6 Months 1 Year 2 Years 3 Years
Class
n/N % n/N % n/N % n/N % n/N %
I 3/21 14% 18/20 90% 17/18 94% 8/9 89% 2/2 100%
II 12/21 57% 2/20 10% 1/18 6% 1/9 11% 0/2 0%
III 4/21 19% 0/20 0% 0/18 0% 0/9 0% 0/2 0%
IV 2/21 10% 0/20 0% 0/18 0% 0/9 0% 0/2 0%
a
New York Heart Association
Table 26. Effectiveness Outcomes, Hemodynamics, and Valvular Regurgitation: Modified Configuration. All Patients Analyzed:
N = 21, % (n/N).
Endpoint ≤ 30 days 3-6 Months 1 Year 2 Years
Valvular Regurgitation % of Pts
(n/N)
None 72%
(13/18)
Trivial 22%
(4/18)
Trivial/Mild 0%
(0/18)
Mild 0%
(0/18)
Moderate 6%
(1/18)
Moderate/ Severe 0%
(0/18)
Severe 0%
(0/18)
% of Pts
(n/N)
85%
(17/20)
0%
(0/20)
0%
(0/20)
10%
(2/20)
5%
(1/20)
0%
(0/20)
0%
(0/20)
% of Pts
(n/N)
72%
(13/18)
17%
(3/18)
0%
(0/18)
6%
(1/18)
6%
(1/18)
0%
(0/18)
0%
(0/18)
% of Pts
(n/N)
89%
(8/9)
0%
(0/9)
0%
(0/9)
11%
(1/9)
0%
(0/9)
0%
(0/9)
0%
(0/9)
Note: Data reflect transvalvular, paravalvular, and indeterminate regurgitation noted at all locations combined.
Table 27. Effectiveness Outcomes, Hemodynamics, Mean Pressure Gradient: Modified Configuration All Patients Analyzed: N
= 21, number in subgroup/N, mean ± SD [min., max.].
Endpoint ≤ 30 days 3-6 Months 1 Year 2 Years
Mean Pressure Gradient (mm Hg)
29 mm 18/21
8.7 ± 4.7
[2.0, 19.0]
16/21
6.9 ± 3.8
[2.0, 17.0]
14/21
5.8 ± 4.0
[3.0, 19.0]
6/21
6.5 ± 3.2
[3.0, 10.3]
Table 28. Effectiveness Outcomes, Hemodynamics, Effective Orifice Area: Modified Configuration All patients analyzed: N =
21, number in subgroup/ N, mean ± SD [min., max.].
Endpoint ≤ 30 days 3-6 Months 1 Year 2 Years
Effective Orifice Area
(cm2)
29 mm 17/21
1.9 ± 0.5
[0.9, 2.5]
17/21
2.3 ± 0.8
[1.1, 4.2]
14/21
2.5 ± 1.0
[1.1, 4.4]
8/21
2.7 ± 0.9
[1.8, 4.1]
7. Individualization of treatment
Consider long-term anticoagulant or antiplatelet therapy for patients with a dilated left atrium, a history of thromboembolic
events, or a cardiac rhythm of atrial fibrillation or atrial flutter.
7.1. Specific patient populations
The safety and effectiveness of the Freestyle subcoronary bioprosthesis has not been established for the following specific
populations because it has not been studied in these populations:
18 Instructions for Use English

■
Patients in whom the Freestyle subcoronary bioprosthesis has been implanted for longer than 5 years (Sections 5 and 6,
Adverse Events and Clinical Studies)
■
Patients who are pregnant
■
Patients who are breast-feeding
■
Patients with chronic renal failure
■
Patients with aneurysmal aortic degenerative conditions (for example, cystic medial necrosis, Marfan’s Syndrome)
8. Patient counseling information
Patients may require anticoagulation or antiplatelet therapy for an indefinite period based on each patient’s condition. Patients
with bioprostheses are at risk for bacteremia (for example, undergoing dental procedures) and should be advised about
prophylactic antibiotic therapy.
Encourage patients to carry the implanted Device Identification Card, provided by Medtronic, with them at all times.
9. How supplied
9.1. Available sizes
The Freestyle subcoronary bioprosthesis is designed only for the aortic position and is available in the following sizes: 19 mm,
21 mm, 23 mm, 25 mm, 27 mm, and 29 mm.
9.2. Packaging
The Freestyle subcoronary bioprosthesis is chemically sterilized and is supplied sterile in a buffered 0.2% glutaraldehyde
storage solution. Sterility is compromised if the glass jar and lid container is opened or damaged. The outside of the container
is not sterile and should not be placed in the sterile field.
9.3. Storage
Store the Freestyle subcoronary bioprosthesis between 5°C and 25°C (41°F and 77°F). Refrigeration is not required, and
freezing may damage the bioprosthesis. Room temperature storage up to 25°C (77°F) is satisfactory provided the bioprosthesis
is not exposed to sunlight or other ultraviolet light sources or placed where significant temperature fluctuations may occur.
Maintain appropriate inventory control so that bioprostheses with earlier Use-by dates are implanted first to avoid expiration
dates.
10. Instructions for use
10.1. Physician training
The function of a stentless bioprosthetic valve is sensitive to surgical implantation techniques. Implanting physicians must be
familiar with the techniques for implanting an unstented bioprosthesis. These techniques are similar to those required for
allograft implantation.
10.2. Device features
The Freestyle subcoronary bioprosthesis features surgeon’s flags, which are located 120 degrees apart at the inflow aspect of
the bioprosthesis to facilitate uniform placement of stitches for the proximal suture line. Colored stitching, circumferentially
placed around the cloth cover, indicates the upper limit for insertion of the proximal sutures. The size of the bioprosthesis is
determined by the outside diameter of the inflow edge. The Freestyle subcoronary bioprosthesis is designed for the aortic
position only.
The bioprosthesis is packaged with a disposable valve holder that comes attached to the distal end of the bioprosthesis. The
holder is provided to aid in valve handling during implantation. Use the disposable holder with the reusable Medtronic valve
handle, Model 7639. The handle is also used with the Freestyle® aortic obturators.
10.3. Handling and preparation instructions
Proper size selection of the bioprosthesis is critical to heart valve replacement. The internal diameter of the patient’s aortic root
at the annulus and supracommissural areas may be measured preoperatively or during diastole, using angiographic or
echocardiographic techniques. Use Freestyle™ aortic obturators, Model 7990, to select the appropriately sized bioprosthesis.
For further information, refer to the Freestyle aortic obturator Instructions for Use.
Within the sterile operative field, prepare 3 rinse basins, each containing 500 mL of sterile isotonic saline solution. The exterior
of the device container and lid are nonsterile. Examine the tamper strips to verify that the container has not been damaged or
previously opened. Do not use if all the tamper strips are damaged. Turn the lid counterclockwise, and open the container
(Figure 1).
The bioprosthesis and all internal packaging components within the container are sterile and must be handled accordingly. With
the thumb and index finger, grasp the retainer and slowly lift the bioprosthesis out of the container, allowing for drainage of the
glutaraldehyde storage solution (Figure 2).
Instructions for Use English 19

Open the cap from the valve retainer body and place the bioprosthesis directly into the free hand (Figure 3 and Figure 4).
Record the valve identification number in the patient’s record.
Carefully cut the identification tag from the bioprosthesis and discard the tag (Figure 5).
Note: Be careful not to cut the cloth or tissue of the bioprosthesis when removing the identification tag. Remove any remnants
of the identification tag suture from the bioprosthesis.
Submerge the bioprosthesis into the first rinse basin. Do not touch the valve leaflets or squeeze the bioprosthesis during the
rinsing procedure. Gently swirl the bioprosthesis in the solution for a minimum of 2 minutes in each of the 3 rinse basins
(Figure 6). The bioprosthesis should remain in the third rinse basin until required by the surgeon. If preferred, the Medtronic
valve handle may be attached to the valve holder as an aid during the rinsing procedure. Screw the handle into the valve holder
while lightly grasping the distal end of the valve (Figure 7).
The bioprosthesis is supplied with a valve holder as an option for use during suturing. The valve holder may be removed either
before or after suturing the inflow end of the valve in the patient’s annulus. The valve holder may be removed by either of the
following methods: Method 1: Using straight scissors, carefully place the scissor blade adjacent to the bottom edge of the valve
holder (Figure 8). Cut through the aortic wall tissue so that the valve holder is completely detached from the valve (Figure 9).
Be careful to avoid cutting the leaflet attachment tissue at the valve commissures. Discard the valve holder and aortic wall
remnants.
Method 2: Cut the 3 valve holder retaining sutures with scissors or a scalpel (Figure 10). After cutting the 3 sutures, hold the
valve in place use the handle to gently pull the holder away (Figure 11). The holder and retaining sutures will pull free from the
valve. Examine the valve holder and the area of the aortic wall to which the holder was attached. Make sure that no suture
remnants remain with the valve. Unscrew the holder from the handle and discard the holder.
10.4. Device implantation
The patient’s aortic valve is excised for subcoronary implantation of the bioprosthesis. The coronary sinuses of the Freestyle
subcoronary bioprostheses have been scalloped to allow for clearance of the native coronary ostia commencing at the
sinotubular junction. The complete subcoronary bioprosthesis is scalloped at each of the 3 sinuses. The modified subcoronary
bioprosthesis is scalloped at the left and right coronary sinus. The noncoronary sinus is left intact.
Care should be exercised when placing sutures through the sewing rim and aortic wall to prevent stitching through, or
perforation of, the valve cusps. The colored suture line at the inflow identifies the area for placing sutures in the sewing rim.
Sutures should only be placed proximal to this demarcation line.
Use of pledgets anywhere within the interior aspect of the bioprosthesis is not recommended.
During implantation, periodically irrigate the valve with sterile normal saline to prevent drying of the delicate valve tissue.
Do not use cutting needles as they may cause structural damage to the bioprosthesis.
Take care not to evert (roll outward) the inflow end of the bioprosthesis when suturing the valve to the patient’s annulus.
Eversion could damage valve tissue.
Use extreme caution if additional tailoring of the bioprosthesis is needed to fit the anatomical requirements of a particular
patient’s coronary sinuses or ostia. Improper trimming may result in immediate or delayed damage to or dysfunction of the
bioprosthesis.
In some patients, the height of the cloth covering the right muscle bar may exceed the height of the origin of the patient’s right
coronary artery. Forcing the bioprosthesis to fit in this situation could cause buckling of the porcine leaflets or aortic wall.
Rotation of the bioprosthesis in such cases may be necessary. In the event of rotation, the noncoronary sinus on Model 995MS
must be trimmed. Use caution when trimming the noncoronary sinus to avoid damage to the valve leaflets.
The potential for damage to the bioprosthesis should be considered before passing surgical instruments through the valve.
10.5. Catheterization
Passage of a catheter through any bioprosthesis may damage the valve and, therefore, is not recommended.
10.6. Accessories
Use only Freestyle aortic obturators, Model 7990, and the Medtronic valve handle, Model 7639, to determine the appropriate
Freestyle subcoronary bioprosthesis size.
Caution: Do not use the obturators or handles until they have been thoroughly cleaned and sterilized. Refer to the appropriate
instructions for use for further instructions.
Caution: Do not use other manufacturers’ valve obturators or obturators for another Medtronic prosthesis to size Freestyle
subcoronary bioprostheses.
20 Instructions for Use English

11. Postoperative information
11.1. MRI safety information
The Freestyle subcoronary bioprosthesis is magnetic resonance (MR) Safe. The device will not cause any harm to the patient
when exposed to MR scanning immediately after implantation.
11.2. Return of explanted bioprostheses
Medtronic is interested in obtaining recovered Freestyle subcoronary bioprostheses. When determined to be appropriate,
explants will be studied by a consulting pathologist. A written report summarizing the findings will be returned to the physician.
Product return kits, including an explant information form, are available by contacting Medtronic distribution centers or a
Medtronic sales representative. It is important that the explant form be completely filled out. If a kit is not available, place the
explanted bioprosthesis in a container of glutaraldehyde or 10% buffered formalin immediately after excision. For further
instructions on the return of an explanted device, contact a Medtronic sales representative.
12. Patient information
12.1. Registration information Note: Patient registration does not apply in countries where patient privacy laws conflict with providing patient information,
including countries from the European Union.
A patient registration form is included in each device package. After implantation, please complete all requested information.
The serial number may be found on the package and on the identification tag attached to the bioprosthesis. Return the original
form to the Medtronic address indicated on the form and provide the temporary identification card to the patient prior to
discharge.
An Implanted Device Identification Card is provided to the patient. The card contains the name and telephone number of the
patient’s physician as well as information that medical personnel would require in the event of an emergency.
12.2. Patient manual
Medtronic has prepared a Patient Information Pamphlet that the physician should provide to the patient prior to discharge.
Copies of these pamphlets may be obtained from a Medtronic sales representative.
13. Disclaimer of warranty
THE FOLLOWING DISCLAIMER OF WARRANTY APPLIES TO UNITED STATES CUSTOMERS ONLY:
ALTHOUGH THE FREESTYLE SUBCORONARY BIOPROSTHESES, MODELS 995MS AND 995CS, HEREAFTER
REFERRED TO AS “PRODUCT,” HAVE BEEN MANUFACTURED UNDER CAREFULLY CONTROLLED CONDITIONS,
MEDTRONIC HAS NO CONTROL OVER THE CONDITIONS UNDER WHICH THIS PRODUCT IS USED. MEDTRONIC,
THEREFORE, DISCLAIMS ALL WARRANTIES, BOTH EXPRESS AND IMPLIED, WITH RESPECT TO THE PRODUCT,
INCLUDING, BUT NOT LIMITED TO, ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A
PARTICULAR PURPOSE. MEDTRONIC SHALL NOT BE LIABLE TO ANY PERSON OR ENTITY FOR ANY MEDICAL
EXPENSES OR ANY DIRECT, INCIDENTAL, OR CONSEQUENTIAL DAMAGES CAUSED BY ANY USE, DEFECT,
FAILURE, OR MALFUNCTION OF THE PRODUCT, WHETHER A CLAIM FOR SUCH DAMAGES IS BASED UPON
WARRANTY, CONTRACT, TORT, OR OTHERWISE. NO PERSON HAS ANY AUTHORITY TO BIND MEDTRONIC TO ANY
REPRESENTATION OR WARRANTY WITH RESPECT TO THE PRODUCT.
The exclusions and limitations set out above are not intended to, and should not be construed so as to, contravene mandatory
provisions of applicable law. If any part or term of this DISCLAIMER OF WARRANTY is held to be illegal, unenforceable, or in
conflict with applicable law by a court of competent jurisdiction, the validity of the remaining portions of this DISCLAIMER OF
WARRANTY shall not be affected, and all rights and obligations shall be construed and enforced as if this DISCLAIMER OF
WARRANTY did not contain the particular part or term held to be invalid.
Instructions for Use English 21

Medtronic, Inc.
*1220021001*
710 Medtronic Parkway
Minneapolis, MN 55432
USA
www.medtronic.com
+1 763 514 4000
LifeLine Technical Support, 24-hour consultation service:
1 877 526 7890
Medtronic Heart Valves Division
1851 E. Deere Avenue
Santa Ana, CA 92705
USA
Medtronic B.V.
Earl Bakkenstraat 10
6422 PJ Heerlen
The Netherlands
+31 45 566 8000
Canada
Medtronic of Canada Ltd
99 Hereford Street
Brampton, Ontario L6Y 0R3
Canada
1 800 268 5346
© 2004-2018 Medtronic
1220021001 Rev. 1C
2018-09-24
 Loading...
Loading...