
Bio-Probe™
Blood Flow Monitoring System: Transducer, Disposable Insert
Blutflussüberwachungssystem: Messumformer, Einwegeinsatz
Système de contrôle du débit sanguin : capteur, raccord jetable
Sistema di monitoraggio del flusso ematico: trasduttore, inserto monouso
Flowbewakingssysteem: transducer, wegwerp-insert
TX50, TX50P, DP38, DP38P, CB2980POLY, CB4627, DP38T, DP38PT, BBDP38, BBDP38P
DP38T, DP38PT: These devices are not CE-marked. These devices are not available for
distribution in the European Union and associated countries.
TX50, TX50P, DP38, DP38P, CB2980POLY, CB4627, BBDP38, BBDP38P: These devices are
CE-marked.
DP38T, DP38PT: Diese Produkte haben keine CE-Kennzeichnung. Diese Produkte sind nicht für
den Vertrieb in der Europäischen Union und zugehörigen Ländern erhältlich.
TX50, TX50P, DP38, DP38P, CB2980POLY, CB4627, BBDP38, BBDP38P: Diese Produkte
haben eine CE-Kennzeichnung.
DP38T, DP38PT : ces appareils ne comportent pas le marquage CE. Ces appareils ne sont pas
disponibles pour la distribution dans l'Union européenne et les pays associés.
TX50, TX50P, DP38, DP38P, CB2980POLY, CB4627, BBDP38, BBDP38P : ces appareils ne
comportent pas le marquage CE.
DP38T, DP38PT: questi dispositivi non sono dotati di marchio CE. Questi dispositivi non sono
disponibili per la distribuzione nell'Unione Europea e nei relativi Paesi.
TX50, TX50P, DP38, DP38P, CB2980POLY, CB4627, BBDP38, BBDP38P: questi dispositivi
sono dotati di marchio CE.
DP38T, DP38PT: Deze producten hebben geen CE-markering. Deze producten zijn niet
verkijgbaar in de Europese Unie en geassocieerde landen.
TX50, TX50P, DP38, DP38P, CB2980POLY, CB4627, BBDP38, BBDP38P: Deze producten
hebben geen CE-markering.
Operator Manual • Benutzerhandbuch • Manuel de l'utilisateur • Manuale dell’operatore •
Gebruikershandleiding
Caution: Federal law (USA) restricts this device to sale by or on the order of
a physician.

Trademarks may be registered and are the property of their respective owners.
Marken sind eventuell eingetragen und sind das Eigentum ihrer jeweiligen Inhaber.
Les marques commerciales peuvent être déposées et appartiennent à leurs propriétaires respectifs.
I marchi di fabbrica possono essere registrati e sono di proprietà dei rispettivi titolari.
Handelsmerken kunnen zijn geregistreerd en zijn het eigendom van de desbetreffende eigenaren.

Explanation of symbols on product or packaging / Erläuterung der auf dem Produkt oder
der Verpackung angebrachten Symbole / Explication des symboles indiqués sur le produit
ou l'emballage / Spiegazione dei simboli presenti sul prodotto o sulla confezione /
Verklaring van de symbolen op het product of de verpakking
Refer to the appropriate product to see which symbols apply. / Auf den Produkten sind nur die für das jeweilige
Produkt gültigen Symbole angebracht. / Se référer au produit approprié pour savoir quels symboles
s'appliquent. / Fare riferimento al prodotto specifico per individuare i simboli che lo riguardano. / Controleer het
desbetreffende product om te zien welke symbolen van toepassing zijn.
Do not use if package is damaged / Nicht verwenden, wenn die Verpackung beschädigt
ist / Ne pas utiliser si l'emballage est endommagé / Non utilizzare se l'imballaggio non è
integro / Niet gebruiken als de verpakking beschadigd is
Open here / Hier öffnen / Ouvrir ici / Aprire qui / Hier openen
Nonpyrogenic fluid path / Pyrogenfreier Flüssigkeitsweg / Trajet des fluides apyrogène /
Percorso per il liquido apirogeno / Niet-pyrogeen vloeistoftraject
Do not reuse / Nicht wiederverwenden / Ne pas réutiliser / Non riutilizzare / Niet opnieuw
gebruiken
Do not sterilize / Nicht sterilisieren / Ne pas stériliser / Non sterilizzare / Niet steriliseren
Do not use alcohol or alcohol-based fluids on any surface of this device / Weder Alkohol
noch Flüssigkeiten auf Alkoholbasis dürfen mit irgendeiner Oberfläche dieses Geräts in
Berührung kommen / Ne pas utiliser d'alcool ou de liquides à base d'alcool sur les
surfaces de cet appareil / Non utilizzare alcol né detergenti a base di alcol su alcuna
superficie di questo dispositivo / Geen alcohol of vloeistoffen op basis van alcohol op het
oppervlak van dit product gebruiken
Do not immerse / Nicht in Flüssigkeit tauchen / Ne pas immerger / Non immergere / Niet
onderdompelen
This direction for fluid path / Diese Richtung für den Flüssigkeitsweg / Trajet des fluides
dans cette direction / Direzione del percorso del liquido / Richting van de vloeistoftraject
Sterilized using ethylene oxide / Mit Ethylenoxid sterilisiert / Stérilisé à l'oxyde
d’éthylène / Sterilizzato a ossido di etilene / Gesteriliseerd met ethyleenoxide
Consult instructions for use / Gebrauchsanweisung beachten / Consulter le mode
d'emploi / Consultare le istruzioni per l'uso / Zie gebruiksaanwijzing
Authorized representative in the European Community / Autorisierter Repräsentant in der
Europäischen Gemeinschaft / Représentant agréé dans la Communauté européenne /
Rappresentante autorizzato nella Comunità europea / Geautoriseerd vertegenwoordiger
in de Europese Gemeenschap
Date of manufacture / Herstellungsdatum / Date de fabrication / Data di fabbricazione /
Productiedatum
Manufacturer / Hersteller / Fabricant / Fabbricante / Fabrikant
1
Manufactured in / Hergestellt in / Lieu de fabrication / Fabbricato in / Vervaardigd in
Do not resterilize / Nicht resterilisieren / Ne pas restériliser / Non risterilizzare / Niet
opnieuw steriliseren
Upper limit of temperature / Obere Temperaturgrenze / Limite supérieure de
température / Limite superiore di temperatura / Maximale temperatuur
Temperature limit / Temperaturbereich / Limite de température / Limiti di temperatura /
Temperatuurbereik
Use-by date / Verwendbar bis / Date de péremption / Utilizzare entro / Uiterste
gebruiksdatum
Lot number / Chargennummer / Numéro de lot / Numero di lotto / Partijnummer
Serial number / Seriennummer / Numéro de série / Numero di serie / Serienummer
Quantity / Menge / Quantité / Quantità / Aantal
Catalog number / Katalognummer / Numéro de référence / Numero di catalogo /
Catalogusnummer
Type CF applied part / Anwendungsteil vom Typ CF / Pièce appliquée de type CF / Parte
applicata di tipo CF / Toegepast onderdeel type CF
For US audiences only / Gilt nur für Leser in den USA / Ne s'applique qu'aux États-Unis /
Esclusivamente per il mercato statunitense / Alleen van toepassing voor de VS
Conformité Européenne (European Conformity). This symbol means that the device fully
complies with European Council Directive MDD: 93/42/EEC. / Conformité Européenne
(Europäische Konformität). Dieses Symbol besagt, dass das Gerät allen Vorschriften der
europäischen Richtlinie 93/42/EWG für Medizinprodukte entspricht. / Conformité
Européenne. Ce symbole signifie que l’appareil est entièrement conforme à la Directive
européenne MDD : 93/42/CEE. / Conformité Européenne (Conformità europea). Questo
simbolo indica che il dispositivo è conforme alla Direttiva del Consiglio europeo MDD
93/42/CEE. / Conformité Européenne (Europese Conformiteit). Dit symbool betekent dat
het product volledig voldoet aan de Europese Richtlijn MDD 93/42/EEG.
2

Do not dispose of this product in the unsorted municipal waste stream. Dispose of this
product according to local regulations. See http://recycling.medtronic.com for instructions
on proper disposal of this product. / Dieses Produkt darf nicht mit dem
Restmüll/Hausmüll entsorgt werden. Bei der Entsorgung dieses Produkts sind die
einschlägigen Vorschriften zu beachten. Anweisungen zur korrekten Entsorgung dieses
Produkts finden Sie auf unserer Webseite unter http://recycling.medtronic.com. / Ne pas
mettre ce produit au rebut dans une décharge municipale ne pratiquant pas le tri des
déchets. Respecter la réglementation locale en vigueur en la matière. Consulter le site
http://recycling.medtronic.com pour obtenir des instructions sur la mise au rebut
adéquate de ce produit. / Non eliminare questo prodotto insieme ai rifiuti solidi urbani
non differenziati. Attenersi alle normative in vigore per il corretto smaltimento. Per
istruzioni sul corretto smaltimento del prodotto, consultare il sito web
http://recycling.medtronic.com. / Niet met het gewone huisvuil wegwerpen. Werp dit
product weg volgens de lokale afvalverwerkingsregels. Zie
http://recycling.medtronic.com voor instructies voor de juiste afvoer van dit product.
Humidity limitation / Luftfeuchtigkeitsbereich / Limite d'humidité / Limiti di umidità /
Vochtigheidsbereik
3

Table of Contents
1. About the Bio-Probe™ blood flow monitoring system 4
1.1. Indications for use 4
1.2. Contraindications 4
1.3. Description 4
1.4. Package contents 5
1.5. Instrument inspection 5
1.6. Environmental limits 5
1.7. Compatibility with the Bio-Console™ extracorporeal blood pumping console 5
1.8. Compatibility of transducers with disposable inserts 5
1.9. Adverse effects 6
1.10. Warnings and precautions 6
2. Assembling 6
3. Emergency 10
4. Troubleshooting 12
5. Cleaning 13
6. Servicing 14
7. Equipment limited warranty 14
8. Limited warranty 15
9. Equipment limited warranty 16
10. Limited warranty 17
1. About the Bio-Probe™ blood flow monitoring system
Before using this system, read these instructions.
1.1. Indications for use
The Bio-Probe™ blood flow monitoring system is to be used with an appropriate model Bio-Console™
extracorporeal blood pumping console to measure directly the blood flow in the extracorporeal perfusion circuit.
1.2. Contraindications
Use the device only as indicated.
1.3. Description
The Bio-Probe blood flow monitoring system consists of a flow transducer and a sterile, single-use insert. A
transducer mount may be used.
The TX50 and TX50P transducer models are reusable. The arrow on the transducer cover indicates the correct
direction of fluid flow, and the color of the arrow indicates whether the transducer should be used with an adult
or pediatric patient:
■
Model TX50: The adult transducer has a gray arrow on the cover and a black cable.
■
Model TX50P: The pediatric transducer has a blue arrow on the cover and a blue cable.
The disposable inserts are available uncoated or with a biocompatible coating. Contact your Medtronic customer
service representative for more information.
The disposable inserts are sterilized using ethylene oxide.
The transducer may be placed on a transducer mount and attached to a pole during a perfusion procedure or
when storing the transducer. Instructions for using the transducer mount are described in the operator manual
for the appropriate model of the Bio-Console extracorporeal blood pumping console.
4 Instructions for Use English

1.4. Package contents
Included in the package is an operator manual and either an adult transducer (Model TX50) or a pediatric
transducer (Model TX50P).
The disposable insert is not included but is a necessary part of the Bio-Probe blood flow monitoring system.
1.5. Instrument inspection
This section provides a list of inspection checks on the Bio-Probe blood flow monitoring system for technical
personnel trained and skilled in all device functions. Users must read and understand this manual completely
before operating the Bio-Probe blood flow monitoring system and performing these checks. Operating functions
of this device should be checked before use as stated in this manual. Device instrument inspection, as
described in this section, should be checked at least once per 12 months.
In all events, should problems occur, contact your Medtronic service representative.
The following checks should be made:
■
Visual inspection
■
Inscriptions, information and warning signs properly and completely fixed
■
Mechanical damage to the device, accessories and all other cables
1.6. Environmental limits
Operating temperature (TX50, TX50P) 18°C to 33°C (64°F to 92°F)
Operating humidity (TX50, TX50P) 30% to 75% R.H. noncondensing
Storage temperatures
■
TX50, TX50P
■
DP38, DP38P, DP38T, DP38PT, BBDP38,
-34°C to 66°C (-29°F to 150°F)
-34°C to 50°C (-29°F to 122°F)
BBDP38P
■
CB2980POLY, CB4627
-34°C to 40°C (-29°F to 104°F)
Storage Humidity (TX50, TX50P) 15% to 95% R.H. noncondensing
1.7. Compatibility with the Bio-Console™ extracorporeal blood pumping console
Before using the Bio-Probe blood flow monitoring system, ensure that you have the appropriate transducer for
the console being used. Refer to the appropriate console operator manual for additional information.
1.7.1. Models 550 and 560
These console models were designed for the transducer Models TX50 and TX50P.
1.8. Compatibility of transducers with disposable inserts
Refer to the Bio-Probe disposable insert instructions for use for additional information pertaining to coatings.
Table 1.
Use with transducer Model TX50
(adult)
Use with transducer Model
TX50P (pediatric)
Uncoated insert DP38 DP38P
Cortiva™ BioActive Surface insert CB2980POLY CB4627
a
Trillium™ Biosurface coated insert
Balance™ Biosurface coated insert
a
Technology licensed under agreement from BioInteractions, Limited, United Kingdom.
b
Technology licensed under agreement from BioInteractions, Limited, United Kingdom.
DP38T DP38PT
b
BBDP38 BBDP38P
Instructions for Use English 5

1.9. Adverse effects
The following adverse effects are associated with the use of the Bio-Probe blood flow monitoring system: blood
loss, coagulopathy, excessive blood component activation/thrombogenicity, hemolytic anemia, hypotension,
infection, ischemia, neurological dysfunction, and organ dysfunction.
1.10. Warnings and precautions
Read all warnings, precautions, and instructions for use carefully prior to use. Failure to read and follow all
instructions, or failure to observe all stated warnings, could cause serious injury or death to the patient.
1.10.1. Warnings
■
Before using this system, read the operator manual for the appropriate model of the Bio-Console
extracorporeal blood pumping console.
■
A standby transducer should be available during perfusion. If the transducer must be replaced during a
procedure, follow the instructions in the Emergency section.
1.10.2. Precautions
■
The disposable inserts were designed for single patient use only. Do not reuse, reprocess, or resterilize this
product. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device and/or
create a risk of contamination of the device, which could result in patient injury, illness, or death.
■
If it is necessary to lubricate the insert while attaching the tubing, use only sterile water or saline. Do not use
alcohol-based fluids on the disposable insert.
■
Before opening the package of the insert, carefully inspect the packaging. If the package is damaged or
opened, or if the insert appears damaged, do not use the insert. Return the insert to your local Medtronic
representative.
■
Inspect the transducer, cable, and cable connector for mechanical damage. If damage is found, use a
standby transducer and contact a qualified Medtronic service technician.
■
Only use the transducers with their compatible disposable inserts (see Table 1). If the incorrect disposable
insert is used, the transducer may become damaged.
■
Handle the transducers with care. Avoid sharp bends, kinks, or twists, or other stresses on the transducer
cable. Hold and carry the transducer gently; do not carry the transducer by the cable. Do not use scalpels,
hemostats, tubing clamps, or other surgical tools on the transducer or its cable.
■
For optimal flow accuracy, attach a primed Bio-Probe blood flow monitoring system to the console.
■
Verify that the pins of the transducer are straight and clean.
■
Ensure that the disposable insert pins contact the transducer pins (underneath the transducer cover).
■
Dispose of the devices in accordance with local hospital, administrative, and other government policies.
2. Assembling
Before placing a patient on cardiopulmonary bypass, connect an assembled and primed Bio-Probe blood flow
monitoring system to the console. Refer to the appropriate Bio-Console extracorporeal blood pumping console
operator manual for instructions about turning on the console and attaching the Bio-Probe blood flow monitoring
system to the console.
Caution: Before assembling the system, ensure that an adult disposable insert is used only with the adult
transducer Model TX50. If a pediatric disposable insert is used, the adult transducer may be damaged when the
transducer cover is closed. See Table 1.
6 Instructions for Use English

1
2
1. Adult
2. Pediatric
Figure 1
1. Using sterile technique, remove the disposable insert from its sterile package.
Note: The pin spacing between the adult disposable insert differs from that of the pediatric insert.
Figure 2
2. Connect the arterial line to one side of the disposable insert.
Caution: Use only sterile saline or sterile water to lubricate the tubing and the insert. Do not use alcohol or
alcohol-related solutions.
Instructions for Use English 7
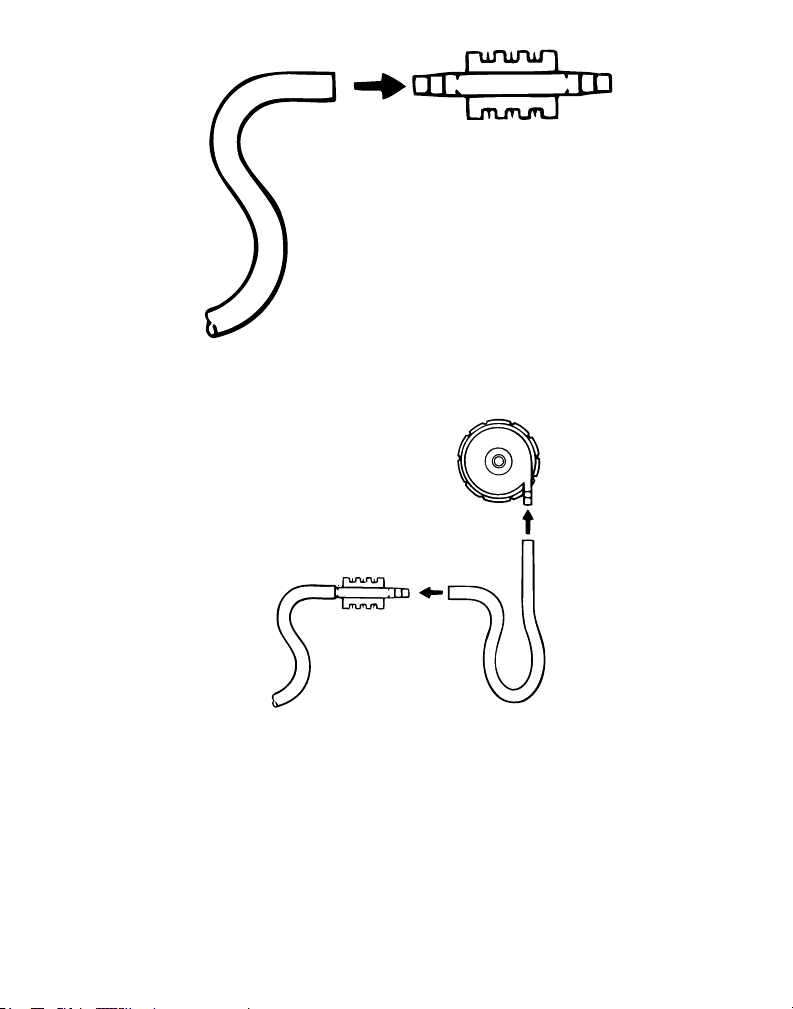
Figure 3
a
b
3. Maintaining sterile technique, connect at least 15 cm (6 in) of tubing to the other side of the disposable
insert (a) and to the outlet of the centrifugal blood pump (b).
Figure 4
4. Push the transducer cover open and align the disposable insert with the fluid flow direction on the
transducer cover.
Note: If the transducer is aligned incorrectly with the fluid path, a negative flow value will be displayed on
the console.
8 Instructions for Use English

Figure 5
5. Firmly push the insert into the transducer.
Figure 6
6. Push the transducer cover closed until the latch engages.
Instructions for Use English 9
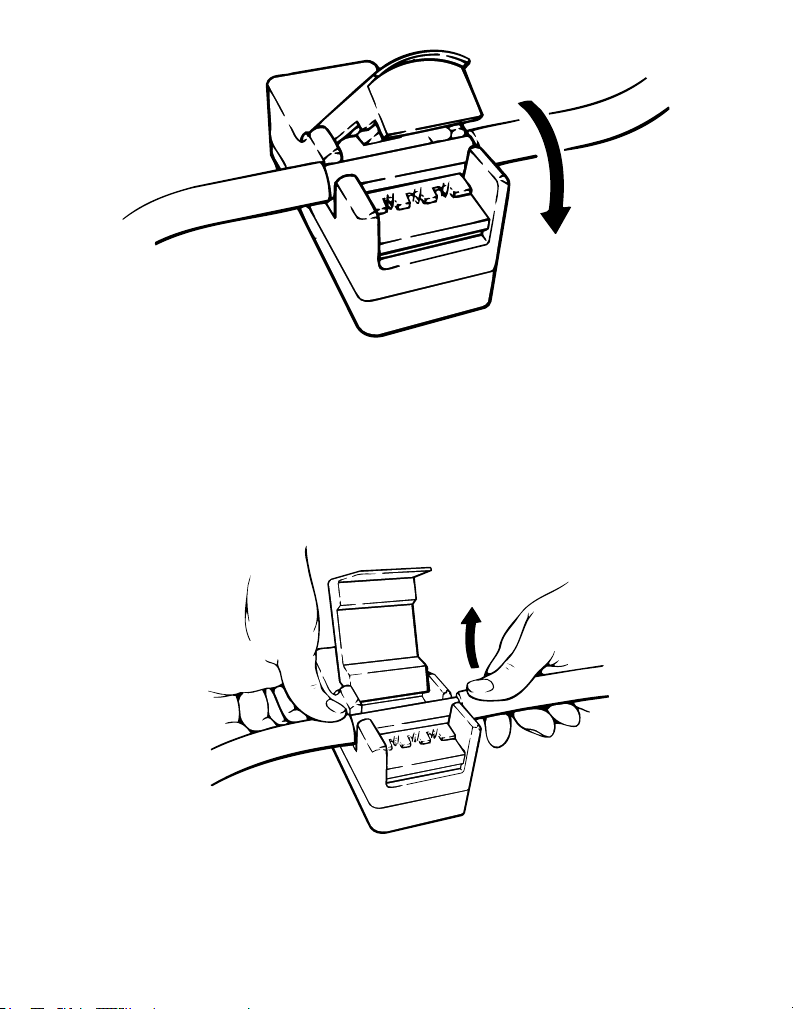
Figure 7
The Bio-Probe blood flow monitoring system is assembled. Refer to the Bio-Console extracorporeal blood
pumping console operator manual for instructions about attaching the transducer, priming the circuit (with a
balanced electrolyte or normal saline solution), and zeroing the transducer.
3. Emergency
Should the transducer stop functioning, replace it with the standby tranducer:
1. Disconnect the nonfunctioning transducer from the console and connect the standby transducer to the
console.
2. Remove the disposable insert with attached tubing from the nonfunctioning transducer.
Figure 8
3. Push open the transducer cover of the standby transducer. Align the disposable insert with the fluid flow
direction on the transducer cover of the functioning transducer.
10 Instructions for Use English
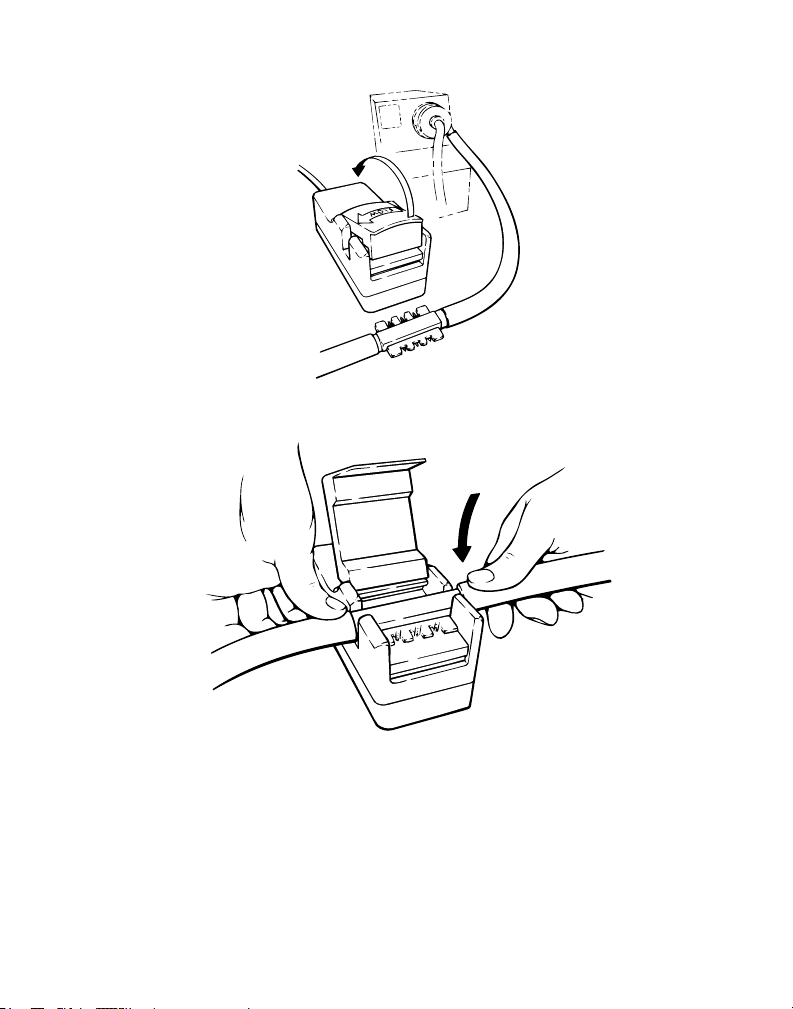
Note: If the transducer is aligned incorrectly with the fluid path, a negative flow value will be displayed on
the console.
Figure 9
4. Firmly push the insert into the standby transducer.
Figure 10
5. Push the transducer cover closed until the latch engages.
Instructions for Use English 11
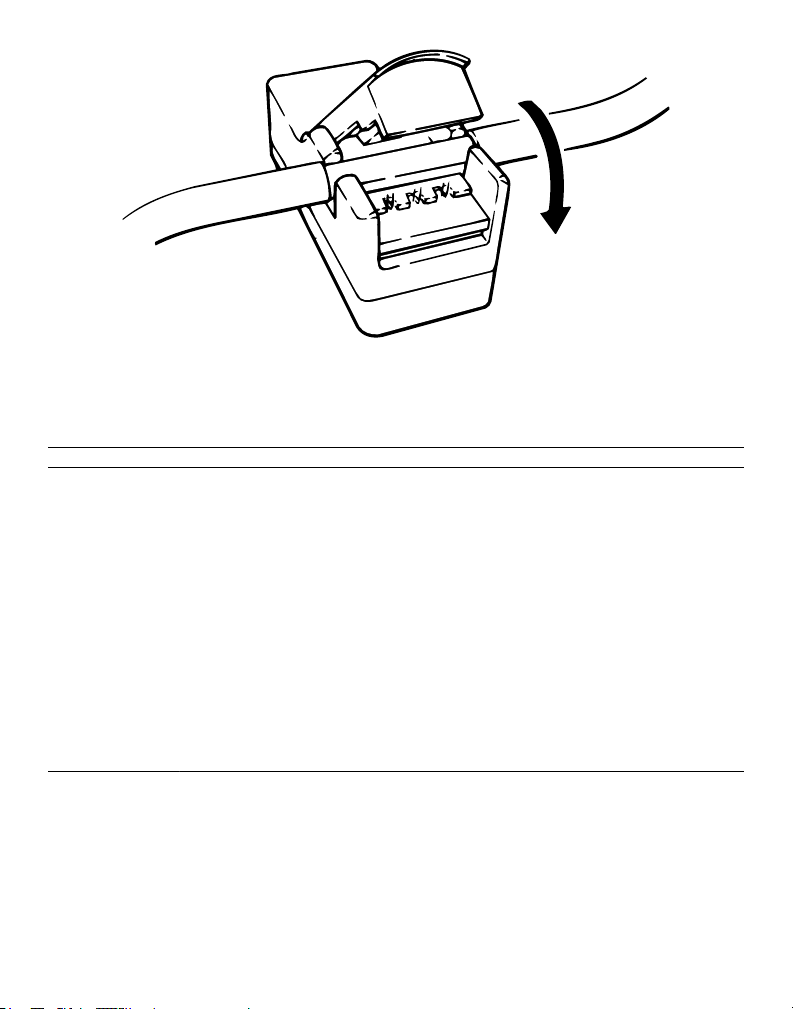
Figure 11
The Bio-Probe blood flow monitoring system is now reassembled with the standby transducer. Refer to the BioConsole extracorporeal blood pumping operator manual for instructions about zeroing the transducer.
4. Troubleshooting
Condition Cause Corrective Action
Flashing digital display
The priming solution is not a balanced
electrolyte or normal saline.
The contact between the pins of the disposable insert and the pins under the
transducer cover is disrupted.
The pins under the transducer cover are
misaligned.
The transducer cable is not connected
properly.
A cautery unit, lamp, or other ambient
electrical current is interfering.
The disposable insert pins or the pins
inside the transducer cover are wet.
Use a balanced electrolyte or normal saline
as the priming solution.
Ensure that the transducer cover (over the
disposable insert) is securely closed.
Verify proper pin contact between the transducer and the disposable insert.
Verify that the transducer pins are free from
dirt or fluid.
Use the correct size of disposable insert for
this transducer model number.
Ensure that the transducer cable is connected into the console.
Check the transducer cable for breakage.
Determine the cause and eliminate it, if pos-
sible.
Dry the pins.
12 Instructions for Use English
Condition Cause Corrective Action
Negative flow
meter reading with
a known positive
pump output or flow
Other problems Unknown Consult with a qualified Medtronic service
The transducer is reversed in the flow
path. (Notice the arrow on the transducer
cover.)
The flow display was not zeroed correctly. Refer to the zeroing or balancing procedure
Open the transducer cover, lift out the disposable insert, turn the transducer (or disposable insert) 180°, place the disposable
insert back under the transducer cover, and
rezero the transducer.
for the flow transducer in the appropriate
operator manual for the Bio-Console extracorporeal blood pumping console.
technician.
5. Cleaning
The transducer will perform more efficiently and last longer if it is cleaned and stored as described:
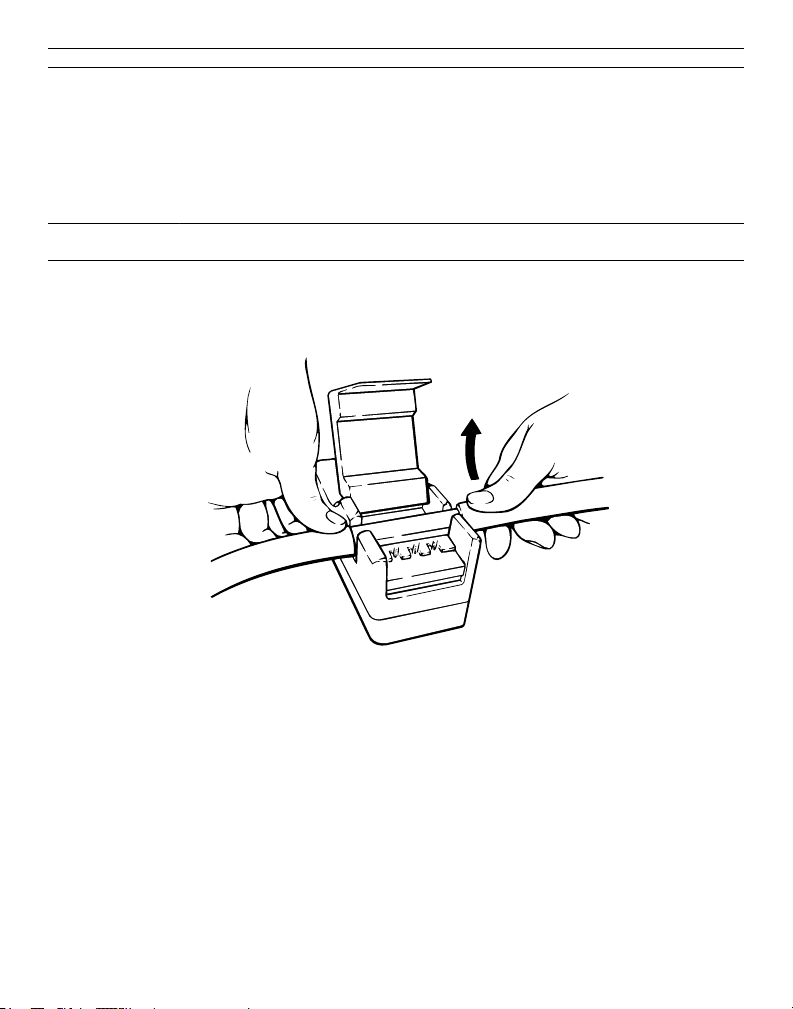
1. After a perfusion procedure, push open the transducer cover and discard the disposable insert.
Caution: The disposable insert should never be resterilized or reused.
Figure 12
2. Clean the transducer, transducer pins (underneath the transducer cover), and cable by wiping them with a
cloth moistened with water and mild soap. Avoid strong or abrasive cleaners. Wipe off the soap using a
cloth or sponge moistened with clear water.
Note: Do not immerse any part of the transducer, cable, or cable connector.
Instructions for Use English 13

Figure 13
Note: The transducer does not require sterilization.
3. Dry the transducer, transducer pins, and cable with a cloth.
4. Ensure that the pins underneath the transducer cover are straight.
If a pin is not straight, notify a qualified Medtronic service technician.
5. To store the transducer, either place it on the transducer mount or unplug the transducer and place it in its
protective plastic case.
Instructions for using the transducer mount are described in the operator manual for the appropriate model of
the Bio-Console extracorporeal blood pumping console.
6. Servicing
If the transducer is damaged, contact the Medtronic Customer Service Department. You must receive
authorization from Medtronic before returning a transducer for repair.
Note: Do not repair the transducer; the warranty will be voided.
Medtronic maintains a professional staff of consultants to provide technical consultation for product users. To
find answers for a question or to schedule product training, contact your local Medtronic service representative.
7. Equipment limited warranty
The following limited warranty 1 applies to United States customers only:
A. This LIMITED WARRANTY provides the following assurance to the purchaser of the Medtronic Models
TX50 and TX50P flow transducers for the Bio-Probe blood flow monitoring system, hereafter referred to as
the “Transducer”:
(1) Should the Transducer fail to function within normal tolerances due to a defect in materials or
workmanship within a period of 1 year, commencing with the delivery of the Transducer to the
purchaser, Medtronic will at its option: (a) repair or replace any part or parts of the Transducer; (b) issue
a credit to the purchaser equal to the purchase price, as defined in Subsection A(2), against the
1
This Limited Warranty is provided by Medtronic, 710 Medtronic Parkway, Minneapolis, MN 55432. It applies only in the United
States. Areas outside the United States should contact their local Medtronic representative for exact terms of the Limited
Warranty.
14 Instructions for Use English

purchase of the replacement Transducer; or (c) provide a functionally comparable replacement
Transducer at no charge.
(2) As used herein, purchase price shall mean the lesser of the net invoiced price of the original, current
functionally comparable, or replacement Transducer.
B. To qualify for the repair, replacement or credit set forth in Section A, the following conditions must be met:
(1) The Transducer must be returned to Medtronic within 30 days after discovery of the defect. (Medtronic
may, at its option, repair the Transducer on site).
(2) The Transducer must not have been repaired or altered outside of Medtronic’s factory in any way which,
in the judgment of Medtronic, affects its stability and reliability. The Transducer must not have been
subjected to misuse, abuse, or accident.
C. This LIMITED WARRANTY is limited to its express terms. In particular:
(1) Except as expressly provided by this LIMITED WARRANTY, MEDTRONIC IS NOT RESPONSIBLE
FOR ANY DIRECT, INCIDENTAL, OR CONSEQUENTIAL DAMAGES BASED ON ANY DEFECT,
FAILURE, OR MALFUNCTION OF THE TRANSDUCER, WHETHER THE CLAIM IS BASED ON
WARRANTY, CONTRACT, TORT, OR OTHERWISE.
(2) This LIMITED WARRANTY is made only to the purchaser of the Transducer. AS TO ALL OTHERS,
MEDTRONIC MAKES NO WARRANTY, EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED
TO, ANY IMPLIED WARRANTY OF MERCHANTABILITY, OR FITNESS FOR A PARTICULAR
PURPOSE WHETHER ARISING FROM STATUTE, COMMON LAW, CUSTOM OR OTHERWISE. NO
EXPRESS OR IMPLIED WARRANTY TO THE PATIENT SHALL EXTEND BEYOND THE PERIOD
SPECIFIED IN A(1) ABOVE. THIS LIMITED WARRANTY SHALL BE THE EXCLUSIVE REMEDY
AVAILABLE TO ANY PERSON.
(3) The exclusions and limitations set out above are not intended to, and should not be construed so as to,
contravene mandatory provisions of applicable law. If any part or term of this LIMITED WARRANTY is
held to be illegal, unenforceable, or in conflict with applicable law by a court of competent jurisdiction,
the validity of the remaining portions of the LIMITED WARRANTY shall not be affected, and all rights
and obligations shall be construed and enforced as if this LIMITED WARRANTY did not contain the
particular part or term held to be invalid. This LIMITED WARRANTY gives the purchaser specific legal
rights. The purchaser may also have other rights which vary from state to state.
(4) No person has any authority to bind Medtronic to any representation, condition, or warranty except this
LIMITED WARRANTY.
8. Limited warranty
(FOR SINGLE-USE PRODUCT)
The following limited warranty 2 applies to United States customers only:
A. This LIMITED WARRANTY provides the following assurance to the patient who receives the Medtronic Bio-
Probe disposable inserts for the Bio-Probe blood flow monitoring system, hereafter referred to as the
“Product”:
(1) Should the Product fail to function within normal tolerances due to a defect in materials or workmanship
prior to its use-by date, Medtronic will at its option: (a) issue a credit to the purchaser equal to the
purchase price, as defined in Subsection A(2), against the purchase of the replacement Product; or (b)
provide a functionally comparable replacement Product at no charge.
(2) As used herein, purchase price shall mean the lesser of the net invoiced price of the original, or current
functionally comparable, or replacement Product.
B. To qualify for the LIMITED WARRANTY, these conditions must be met:
(1) The Product must be used prior to its use-by date.
2
This Limited Warranty is provided by Medtronic, 710 Medtronic Parkway, Minneapolis, MN 55432. Areas outside the United
States should contact their local Medtronic representative for exact terms of the Limited Warranty.
Instructions for Use English 15

(2) The unused portion of the Product must be returned to Medtronic and shall be the property of
Medtronic.
(3) The Product must not have been altered or subjected to misuse, abuse, or accident.
(4) The Product must be used in accordance with the labeling and instructions for use provided with the
Product.
C. This LIMITED WARRANTY is limited to its express terms. In particular:
(1) Except as expressly provided by this LIMITED WARRANTY, MEDTRONIC IS NOT RESPONSIBLE
FOR ANY DIRECT, INCIDENTAL, OR CONSEQUENTIAL DAMAGES BASED ON ANY DEFECT,
FAILURE, OR MALFUNCTION OF THE PRODUCT, WHETHER THE CLAIM IS BASED ON
WARRANTY, CONTRACT, TORT, OR OTHERWISE.
(2) This LIMITED WARRANTY is made only to the patient in whom the Product was used. AS TO ALL
OTHERS, MEDTRONIC MAKES NO WARRANTY, EXPRESS OR IMPLIED, INCLUDING, BUT NOT
LIMITED TO, ANY IMPLIED WARRANTY OF MERCHANTABILITY, OR FITNESS FOR A
PARTICULAR PURPOSE WHETHER ARISING FROM STATUTE, COMMON LAW, CUSTOM, OR
OTHERWISE. NO EXPRESS OR IMPLIED WARRANTY TO THE PATIENT SHALL EXTEND BEYOND
THE PERIOD SPECIFIED IN A(1) ABOVE. THIS LIMITED WARRANTY SHALL BE THE EXCLUSIVE
REMEDY AVAILABLE TO ANY PERSON.
(3) The exclusions and limitations set out above are not intended to, and should not be construed so as to,
contravene mandatory provisions of applicable law. If any part or term of this LIMITED WARRANTY is
held to be illegal, unenforceable, or in conflict with applicable law by a court of competent jurisdiction,
the validity of the remaining portions of the LIMITED WARRANTY shall not be affected, and all rights
and obligations shall be construed and enforced as if this LIMITED WARRANTY did not contain the
particular part or term held to be invalid. This LIMITED WARRANTY gives the patient specific legal
rights. The patient may also have other rights which vary from state to state.
(4) No person has any authority to bind Medtronic to any representation, condition, or warranty except this
LIMITED WARRANTY.
9. Equipment limited warranty
(FOR REPAIRABLE EXTERNAL EQUIPMENT/OUTSIDE THE US)
The following limited warranty 3 applies to customers outside the United States:
A. This LIMITED WARRANTY provides the following assurance to the purchaser of the Medtronic Models
TX50 and TX50P flow transducers for the Bio-Probe blood flow monitoring system, hereafter referred to as
the “Transducer”, that should the Transducer fail to function within normal tolerances due to a defect in
materials or workmanship within a period of one 1 year, commencing with the delivery of the Transducer to
the purchaser, Medtronic will at its option: (a) repair or replace any defective part or parts of the Transducer;
(b) issue a credit equal to the original Transducer purchase price (but not to exceed the value of the
replacement Transducer), against the purchase of replacement Transducer; or (c) provide functionally
comparable replacement Transducer at no charge.
B. To qualify for this repair, replacement, or credit, the following conditions must be met:
(1) The Transducer must be returned to Medtronic within 60 days after discovery of the defect. (Medtronic
may, at its option, repair the Transducer on site).
(2) The Transducer must not have been repaired or altered by someone other than Medtronic in any way
which, in the judgment of Medtronic, affects it stability and reliability.
(3) The Transducer must not have been subjected to misuse, abuse, or accident.
3
This Limited Warranty is provided by Medtronic, 710 Medtronic Parkway, Minneapolis, MN 55432. Areas outside the United
States should contact their local Medtronic representative for exact terms of the Limited Warranty.
16 Instructions for Use English

C. This LIMITED WARRANTY is limited to its express terms. In particular, Medtronic is not responsible for any
incidental or consequential damages based on any use, defect, or failure of the Transducer, whether the
claim is based on warranty, contract, tort, or otherwise.
D. The exclusions and limitations set out above are not intended to, and should not be construed so as to,
contravene mandatory provisions of applicable law. If any part or term of this LIMITED WARRANTY is held
by any court of competent jurisdiction to be illegal, unenforceable, or in conflict with applicable law, the
validity of the remaining portion of the LIMITED WARRANTY shall not be affected, and all rights and
obligations shall be construed and enforced as if this LIMITED WARRANTY did not contain the particular
part or term held to be invalid.
10. Limited warranty
(FOR SINGLE-USE PRODUCT/OUTSIDE THE US)
The following limited warranty 4 applies to customers outside the US:
A. This LIMITED WARRANTY provides assurance for the purchaser who receives the Medtronic Bio-Probe
disposable inserts for the Bio-Probe blood flow monitoring system, hereafter referred to as the “Product”,
that should the Product fail to function to specification, Medtronic will issue a credit, equal to the original
Product purchase price (but not to exceed the value of the replacement Product) against the purchase of
any Medtronic replacement Product used for that patient. THE WARNINGS CONTAINED IN THE
PRODUCT LABELING ARE CONSIDERED AN INTEGRAL PART OF THIS LIMITED WARRANTY.
CONTACT YOUR LOCAL MEDTRONIC REPRESENTATIVE TO OBTAIN INFORMATION ON HOW TO
PROCESS A CLAIM UNDER THIS LIMITED WARRANTY.
B. To qualify for the LIMITED WARRANTY, these conditions must be met:
(1) The Product must be used prior to its use-by date.
(2) The Product must be returned to Medtronic within 60 days after use and shall be the property of
Medtronic.
(3) The Product may not have been used for any other patient.
C. This LIMITED WARRANTY is limited to its express terms. In particular:
(1) In no event shall any replacement credit be granted where there is evidence of improper handling,
improper implantation, or material alteration of the replaced Product.
(2) Medtronic is not responsible for any incidental or consequential damages based on any use, defect, or
failure of the Product, whether the claim is based on warranty, contract, tort, or otherwise.
D. The exclusions and limitations set out above are not intended to, and should not be construed so as to,
contravene mandatory provisions of applicable law. If any part or term of this LIMITED WARRANTY is held
by any court of competent jurisdiction to be illegal, unenforceable, or in conflict with applicable law, the
validity of the remaining portion of the LIMITED WARRANTY shall not be affected, and all rights and
obligations shall be construed and enforced as if this LIMITED WARRANTY did not contain the particular
part or term held to be invalid.
4
This Limited Warranty is provided by Medtronic, 710 Medtronic Parkway, Minneapolis, MN 55432. Areas outside the United
States should contact their local Medtronic representative for exact terms of the Limited Warranty.
Instructions for Use English 17

Inhaltsverzeichnis
1. Das Bio-Probe™ Blutflussüberwachungssystem 18
1.1. Indikationen 18
1.2. Kontraindikationen 18
1.3. Beschreibung 18
1.4. Verpackungsinhalt 19
1.5. Inspektion des Instruments 19
1.6. Umgebungsbedingungen 19
1.7. Kompatibilität mit der extrakorporalen Bio-Console™ Blutpumpenkonsole 19
1.8. Kompatibilität der Messumformer mit Einwegeinsätzen 19
1.9. Unerwünschte Nebenwirkungen 20
1.10. Warnhinweise und Vorsichtsmaßnahmen 20
2. Montage 21
3. Verhalten in Notfällen 24
4. Problemlösung 27
5. Reinigung 27
6. Wartung 29
7. Garantieerklärung für das Produkt 29
8. Garantieerklärung 30
1. Das Bio-Probe™ Blutflussüberwachungssystem
Lesen Sie diese Anleitung vor der Verwendung des Systems sorgfältig durch.
1.1. Indikationen
Das Bio-Probe™ Blutflussüberwachungssystem wird mit einem geeigneten Modell der extrakorporalen BioConsole™ Blutpumpenkonsole für die direkte Messung des Blutflusses im extrakorporalen Perfusionskreislauf
eingesetzt.
1.2. Kontraindikationen
Verwenden Sie das Gerät nur gemäß den Indikationen.
1.3. Beschreibung
Das Bio-Probe Blutflussüberwachungssystem besteht aus einem Flussmessumformer und einem sterilen
Einwegeinsatz. Die Verwendung eines Messumformerhalters ist zulässig.
Die Messumformer Modell TX50 und Modell TX50P sind wiederverwendbar. Der Pfeil auf der
Messumformerabdeckung zeigt die korrekte Richtung des Flüssigkeitsflusses an, und an der Farbe des Pfeils
ist zu erkennen, ob der Messumformer für die Verwendung bei erwachsenen oder bei pädiatrischen Patienten
vorgesehen ist:
■
Modell TX50: Der Messumformer für Erwachsene hat einen grauen Pfeil auf der Abdeckung und ein
schwarzes Kabel.
■
Modell TX50P: Der pädiatrische Messumformer hat einen blauen Pfeil auf der Abdeckung und ein blaues
Kabel.
Die Einwegeinsätze sind unbeschichtet oder mit einer biokompatiblen Beschichtung erhältlich. Weitere
Informationen erhalten Sie von Ihrem Medtronic Kundendienst.
Die Einwegeinsätze sind mit Ethylenoxid sterilisiert.
Der Messumformer kann in einen Messumformerhalter eingesetzt und für den Perfusionseingriff oder zur
Aufbewahrung an einem Ständer angebracht werden. Gebrauchshinweise für den Messumformerhalter sind
18 Gebrauchsanweisung Deutsch

dem Benutzerhandbuch für das entsprechende Modell der extrakorporalen Bio-Console Blutpumpenkonsole zu
entnehmen.
1.4. Verpackungsinhalt
Die Verpackung enthält ein Benutzerhandbuch und einen Messumformer für Erwachsene (Modell TX50) oder
einen pädiatrischen Messumformer (Modell TX50P).
Der Einwegeinsatz liegt nicht bei, wird jedoch für die Nutzung des Bio-Probe Blutflussüberwachungssystems
zwingend benötigt.
1.5. Inspektion des Instruments
Dieser Abschnitt enthält eine Übersicht über die Prüfungen, die im Rahmen der Inspektion des Bio-Probe
Blutflussüberwachungssystems durch geschulte und mit allen Funktionen des Geräts vertraute Techniker
durchzuführen sind. Sie müssen dieses Handbuch vollständig durchgelesen und verstanden haben, bevor Sie
das Bio-Probe Blutflussüberwachungssystem in Betrieb nehmen und diese Prüfungen durchführen. Vor der
Verwendung müssen die Betriebsfunktionen des Geräts wie in diesem Handbuch ausgeführt überprüft werden.
Die in diesem Abschnitt beschriebene Inspektion des Geräts sollte mindestens alle 12 Monate durchgeführt
werden.
Wenden Sie sich beim Auftreten von Problemen jeglicher Art an den für Sie zuständigen Medtronic
Kundendienst-Repräsentanten.
Die nachfolgenden Prüfungen sollten durchgeführt werden:
■
Sichtprüfung
■
korrekt und vollständig angebrachte Aufschriften, Informationen und Warnhinweise
■
mechanische Beschädigungen des Geräts, der Zubehörteile sowie aller weiteren Kabel
1.6. Umgebungsbedingungen
Betriebstemperatur (TX50, TX50P) 18 °C bis 33 °C (64 °F bis 92 °F)
Luftfeuchtigkeit bei Betrieb (TX50, TX50P) 30 % bis 75 % rel. LF., nicht kondensierend
Lagerungstemperatur
■
TX50, TX50P
■
DP38, DP38P, DP38T, DP38PT, BBDP38,
BBDP38P
■
CB2980POLY, CB4627
Luftfeuchtigkeit bei Lagerung (TX50, TX50P) 15 % bis 95 % rel. LF., nicht kondensierend
1.7. Kompatibilität mit der extrakorporalen Bio-Console™ Blutpumpenkonsole
Bevor Sie das Bio-Probe Blutflussüberwachungssystem verwenden, achten Sie darauf, dass Sie den
passenden Messumformer für die verwendete Konsole haben. Nähere Informationen finden Sie in dem
Benutzerhandbuch der verwendeten Konsole.
1.7.1. Modell 550 und 560
Diese Konsolenmodelle wurden für die Messumformer Modell TX50 und Modell TX50P konzipiert.
1.8. Kompatibilität der Messumformer mit Einwegeinsätzen
Weitere Informationen zur Beschichtung sind der Gebrauchsanweisung des jeweiligen Bio-Probe
Einwegeinsatzes zu entnehmen.
-34 °C bis 66 °C (-29 °F bis 150 °F)
-34 °C bis 50 °C (-29 °F bis 122 °F)
-34 °C bis 40 °C (-29 °F bis 104 °F)
Gebrauchsanweisung Deutsch 19

Tabelle 1.
Verwendung mit Messumformer Modell TX50 (erwachsene
Patienten)
Verwendung mit Messumformer
Modell TX50P (pädiatrische
Patienten)
Unbeschichteter Einsatz DP38 DP38P
Einsatz mit bioaktiver Cortiva™ Ober-
CB2980POLY CB4627
fläche
Mit Trillium™ Biosurface beschichteter
a
Einsatz
Mit Balance™ Biosurface beschichteter
b
Einsatz
a
Die Verwendung dieser Technologie unterliegt einer Lizenzvereinbarung mit BioInteractions, Limited, Großbritannien.
b
Die Verwendung dieser Technologie unterliegt einer Lizenzvereinbarung mit BioInteractions, Limited, Großbritannien.
DP38T DP38PT
BBDP38 BBDP38P
1.9. Unerwünschte Nebenwirkungen
Die folgenden unerwünschten Nebenwirkungen können im Zusammenhang mit der Verwendung des Bio-Probe
Blutflussüberwachungssystems auftreten: Blutverlust, Koagulopathie, übermäßige Aktivierung der
Blutkomponenten/Thrombogenität, hämolytische Anämie, Hypotonie, Infektion, Ischämie, neurologische
Fehlfunktionen und Organfunktionsstörungen.
1.10. Warnhinweise und Vorsichtsmaßnahmen
Lesen Sie sich vor Gebrauch alle Warnhinweise, Vorsichtsmaßnahmen und Anweisungen aufmerksam durch.
Werden nicht alle Anweisungen und Warnhinweise gelesen und befolgt, kann dies zu ernsthaften
Verletzungen oder zum Tod des Patienten führen.
1.10.1. Warnhinweise
■
Bevor Sie dieses System verwenden, lesen Sie bitte das Benutzerhandbuch für das entsprechende Modell
der extrakorporalen Bio-Console Blutpumpenkonsole durch.
■
Während der Perfusion muss ein Ersatz-Messumformer bereitgehalten werden. Wenn der Messumformer
während eines Eingriffs ausgetauscht werden muss, befolgen Sie die Anweisungen im Abschnitt „Verhalten
in Notfällen“.
1.10.2. Vorsichtsmaßnahmen
■
Die Einwegeinsätze sind nur auf die Verwendung bei einem einzigen Patienten ausgelegt. Das Produkt darf
nicht wiederverwendet, wiederaufbereitet oder resterilisiert werden. Wiederverwendung, Aufbereitung oder
Resterilisation können die strukturelle Integrität des Geräts beeinträchtigen und/oder unter Umständen eine
Kontamination des Geräts bewirken, die wiederum zu Verletzung, Erkrankung oder zum Tod des Patienten
führen kann.
■
Muss der Einsatz für das Anschließen der Schläuche gleitfähig gemacht werden, darf hierfür nur steriles
Wasser oder sterile Kochsalzlösung verwendet werden. Keine alkoholhaltigen Flüssigkeiten an den
Einwegeinsatz gelangen lassen.
■
Vor dem Öffnen der Verpackung des Einsatzes die Verpackung sorgfältig inspizieren. Verwenden Sie den
Einsatz nicht, wenn die Verpackung beschädigt oder geöffnet ist oder der Einsatz beschädigt zu sein
scheint. Senden Sie den Einsatz an den für Sie zuständigen Medtronic Repräsentanten zurück.
■
Überprüfen Sie den Messumformer, das Kabel und den Kabelstecker auf mechanische Beschädigungen.
Wenn eine Beschädigung festgestellt wird, verwenden Sie einen Ersatz-Messumformer und wenden Sie
sich an einen qualifizierten Medtronic Servicetechniker.
■
Die Messumformer nur mit den für sie vorgesehenen kompatiblen Einwegeinsätzen (siehe Tabelle 1)
verwenden. Wenn der falsche Einwegeinsatz verwendet wird, kann der Messumformer beschädigt werden.
20 Gebrauchsanweisung Deutsch

■
1
2
Vorsichtig mit den Messumformern umgehen. Starkes Biegen, Knicken, Verdrehen und sonstige
Beanspruchungen des Messumformerkabels vermeiden. Messumformer vorsichtig anfassen und
transportieren; den Messumformer nicht am Kabel tragen. Keine Skalpelle, Gefäßklemmen,
Schlauchklemmen oder anderen chirurgischen Instrumente am Messumformer oder an seinen Kabeln
verwenden.
■
Zur Erzielung optimaler Flussgenauigkeit ein vorgefülltes Bio-Probe Blutflussüberwachungssystem an die
Konsole anschließen.
■
Überprüfen Sie, dass die Stifte des Messumformers gerade und sauber sind.
■
Sorgen Sie dafür, dass die Stifte des Einwegeinsatzes mit den Stiften des Messumformers (unter der
Messumformerabdeckung) in Kontakt stehen.
■
Entsorgen Sie die Produkte unter Einhaltung der Vorschriften des Krankenhauses sowie anderer lokaler
und/oder staatlicher Vorgaben.
2. Montage
Bevor ein Patient an die Herz-Lungen-Maschine angeschlossen wird, schließen Sie ein montiertes und
vorgefülltes Bio-Probe Blutflussüberwachungssystem an die Konsole an. Anweisungen zum Einschalten der
Konsole und zum Anschließen des Bio-Probe Blutflussüberwachungssystems an die Konsole sind dem
Benutzerhandbuch der entsprechenden extrakorporalen Bio-Console Blutpumpenkonsole zu entnehmen.
Vorsicht: Vergewissern Sie sich vor der Montage des Systems, dass ein Einwegeinsatz für Erwachsene
ausschließlich mit dem Messumformer für Erwachsene Modell TX50 verwendet wird. Wird ein pädiatrischer
Einwegeinsatz verwendet, kann der Messumformer für Erwachsene beim Schließen der
Messumformerabdeckung beschädigt werden. Siehe Tabelle 1.
1. Erwachsener Patient
2. Pädiatrischer Patient
Abbildung 1
1. Den Einwegeinsatz unter Anwendung steriler Techniken aus seiner Sterilverpackung nehmen.
Hinweis: Der Einwegeinsatz für Erwachsene und der pädiatrische Einwegeinsatz haben einen
unterschiedlichen Stiftabstand.
Gebrauchsanweisung Deutsch 21

Abbildung 2
2. Die arterielle Leitung an eine Seite des Einwegeinsatzes anschließen.
Vorsicht: Zum Gleitfähigmachen der Schläuche und des Einsatzes nur sterile Kochsalzlösung oder steriles
Wasser verwenden. Weder Alkohol noch alkoholhaltige Lösungen verwenden.
Abbildung 3
3. Schließen Sie unter Beibehaltung steriler Techniken mindestens 15 cm (6 Zoll) Schlauchleitung an die
andere Seite des Einwegeinsatzes (a) und an den Auslass der Zentrifugalblutpumpe (b) an.
22 Gebrauchsanweisung Deutsch

a
b
Abbildung 4
4. Die Messumformerabdeckung öffnen und den Einwegeinsatz der auf der Messumformerabdeckung
angegebenen Flüssigkeitsflussrichtung entsprechend ausrichten.
Hinweis: Wird der Messumformer relativ zur Flussrichtung falsch ausgerichtet, wird an der Konsole ein
negativer Flusswert angezeigt.
Abbildung 5
5. Den Einsatz fest in den Messumformer drücken.
Gebrauchsanweisung Deutsch 23

Abbildung 6
6. Die Messumformerabdeckung zudrücken, bis die Verriegelung einrastet.
Abbildung 7
Das Bio-Probe Blutflussüberwachungssystem ist jetzt fertig montiert. Wie der Messumformer angeschlossen,
das Kreislaufsystem (mit einer Vollelektrolyt- oder physiologischen Kochsalzlösung) vorgefüllt und der
Messumformer auf null gesetzt wird, ist dem Benutzerhandbuch für die extrakorporale Bio-Console
Blutpumpenkonsole zu entnehmen.
3. Verhalten in Notfällen
Sollte der Messumformer seine Funktion einstellen, ersetzen Sie ihn durch den Ersatz-Messumformer:
1. Den nicht funktionierenden Messumformer von der Konsole lösen und den Ersatz-Messumformer an die
Konsole anschließen.
24 Gebrauchsanweisung Deutsch

2. Den Einwegeinsatz mit den angebrachten Schlauchleitungen aus dem nicht funktionierenden
Messumformer herausnehmen.
Abbildung 8
3. Die Messumformerabdeckung des Ersatz-Messumformers öffnen. Den Einwegeinsatz der auf der
Messumformerabdeckung des funktionierenden Messumformers angegebenen Flüssigkeitsflussrichtung
entsprechend ausrichten.
Hinweis: Wird der Messumformer relativ zur Flussrichtung falsch ausgerichtet, wird an der Konsole ein
negativer Flusswert angezeigt.
Abbildung 9
4. Den Einsatz fest in den Ersatz-Messumformer drücken.
Gebrauchsanweisung Deutsch 25

Abbildung 10
5. Die Messumformerabdeckung zudrücken, bis die Verriegelung einrastet.
Abbildung 11
Das Bio-Probe Blutflussüberwachungssystem ist jetzt mit dem Ersatz-Messumformer fertig montiert. Wie der
Messumformer auf null gesetzt wird, ist dem Benutzerhandbuch der extrakorporalen Bio-Console
Blutpumpenkonsole zu entnehmen.
26 Gebrauchsanweisung Deutsch

4. Problemlösung
Symptom Ursache Abhilfemaßnahme
Digitalanzeige
blinkt
Negativer Flussmesswert, obwohl
Pumpenleistung
bzw. -fluss mit
Sicherheit positiv
sind
Sonstige Probleme Unbekannt Setzen Sie sich mit einem qualifizierten
Die Vorfülllösung ist keine Vollelektrolytlösung oder physiologische Kochsalzlösung.
Der Kontakt zwischen den Stiften des
Einwegeinsatzes und den Stiften unter
der Messumformerabdeckung ist unterbrochen.
Die Stifte unter der Messumformerabdeckung sind nicht korrekt ausgerichtet.
Das Messumformerkabel ist nicht ordnungsgemäß angeschlossen.
Störung durch ein Kauterinstrument, eine
Lampe oder einen anderen elektrischen
Strom in der Umgebung.
Die Stifte des Einwegeinsatzes oder die
Stifte innerhalb der Messumformerabdeckung sind feucht.
Der Messumformer ist falsch ausgerichtet
in den Flüssigkeitsweg eingesetzt. (Pfeil
auf der Messumformerabdeckung beachten.)
Die Flussmesseranzeige war nicht ordnungsgemäß auf null gesetzt.
Vollelektrolyt- oder physiologische Kochsalzlösung als Vorfülllösung verwenden.
Sicherstellen, dass die Messumformerabdeckung (über dem Einwegeinsatz) fest verschlossen ist.
Korrekten Stiftkontakt zwischen dem Messumformer und dem Einwegeinsatz sicherstellen.
Sicherstellen, dass die Messumformerstifte frei
von Schmutz oder Flüssigkeit sind.
Einwegeinsatz in der für dieses Messumformermodell richtigen Größe verwenden.
Vergewissern Sie sich, dass das Messumformerkabel an die Konsole angeschlossen ist.
Kontrollieren Sie das Messumformerkabel auf
Bruch.
Die Ursache feststellen und nach Möglichkeit
beseitigen.
Die Stifte trocknen.
Die Messumformerabdeckung öffnen, den Einwegeinsatz herausheben, den Messumformer
(oder den Einwegeinsatz) um 180° drehen,
den Einwegeinsatz wieder unter der Messumformerabdeckung einsetzen und den Messumformer erneut auf null setzen.
Siehe Nullsetzungs- bzw. Abgleichverfahren
für den Flussmessumformer in dem entsprechenden Benutzerhandbuch für die extrakorporale Bio-Console Blutpumpenkonsole.
Medtronic Servicetechniker in Verbindung.
5. Reinigung
Der Messumformer funktioniert effizienter und hält länger, wenn er wie nachstehend beschrieben gereinigt und
aufbewahrt wird:
1. Nach einem Perfusionseingriff die Messumformerabdeckung öffnen und den Einwegeinsatz entsorgen.
Vorsicht: Der Einwegeinsatz darf niemals resterilisiert oder wiederverwendet werden.
Gebrauchsanweisung Deutsch 27

Abbildung 12
2. Den Messumformer, die Messumformerstifte (unter der Messumformerabdeckung) und das Kabel zur
Reinigung mit einem mit Wasser und milder Seife befeuchteten Tuch abwischen. Starke oder scheuernde
Reinigungsmittel vermeiden. Die Seife mit einem mit klarem Wasser befeuchteten Tuch oder Schwamm
abwischen.
Hinweis: Kein Teil vom Messumformer, Kabel oder Kabelstecker darf eingetaucht werden.
Abbildung 13
Hinweis: Ein Sterilisieren des Messumformers ist nicht erforderlich.
3. Trocknen Sie Messumformer, Messumformerstifte und Kabel mit einem Tuch ab.
4. Vergewissern Sie sich, dass die Stifte unter der Messumformerabdeckung gerade sind.
Wenn ein Stift nicht gerade ist, wenden Sie sich an einen qualifizierten Medtronic Servicetechniker.
28 Gebrauchsanweisung Deutsch

5. Setzen Sie zur Lagerung den Messumformer in den Messumformerhalter ein oder ziehen Sie den Stecker
vom Messumformer ab und packen Sie den Messumformer in den für die geschützte Aufbewahrung des
Messumformers vorgesehenen Kunststoffbehälter.
Gebrauchshinweise für den Messumformerhalter sind dem Benutzerhandbuch für das entsprechende Modell
der extrakorporalen Bio-Console Blutpumpenkonsole zu entnehmen.
6. Wartung
Wenn der Messumformer beschädigt ist, wenden Sie sich bitte an die Kundendienstabteilung von Medtronic.
Vor dem Einsenden eines Messumformers zur Reparatur müssen Sie eine entsprechende Genehmigung von
Medtronic einholen.
Hinweis: Reparieren Sie den Messumformer nicht; dies führt zum Erlöschen der Garantie.
Medtronic verfügt über ein professionelles Beraterteam, um die Benutzer der Produkte technisch zu
unterstützen. Wenn Sie Antworten auf eine Frage wünschen oder eine Produktschulung planen möchten,
wenden Sie sich bitte an den für Sie zuständigen Medtronic Kundendienst-Repräsentanten.
7. Garantieerklärung für das Produkt
(FÜR REPARATURFÄHIGE EXTERNE PRODUKTE/AUSSERHALB DER USA)
Die folgende Garantieerklärung 3 gilt für Abnehmer außerhalb der USA:
A. Aufgrund dieser GARANTIE wird Medtronic dem Käufer eines Medtronic Messumformers Modell TX50 oder
Modell TX50P für das Bio-Probe Blutflussüberwachungssystem – nachfolgend als
„Messumformer“ bezeichnet – für den Fall, dass der Messumformer aufgrund eines Material- oder
Verarbeitungsfehlers innerhalb einer Frist von einem (1) Jahr ab dem Tag der Auslieferung des
Messumformers an den Käufer nicht innerhalb der normalen Toleranzwerte arbeitet, nach eigener Wahl:
(a) das jeweilige defekte Teil oder die defekten Teile des Messumformers reparieren oder ersetzen,
(b) beim Kauf eines Ersatz-Messumformers eine Gutschrift in Höhe des ursprünglichen Kaufpreises des
Messumformers, höchstens jedoch in Höhe des Kaufpreises des Ersatz-Messumformers gewähren oder
(c) kostenlos einen funktionell vergleichbaren Messumformer liefern.
B. Diese(r) Reparatur, Ersatz oder Gutschrift kann ausschließlich unter folgenden Bedingungen in Anspruch
genommen werden:
(1) Der Messumformer muss innerhalb von 60 Tagen nach Auftreten des Schadens an Medtronic
zurückgeschickt werden. (Medtronic kann den Messumformer nach eigenem Ermessen vor Ort
reparieren.)
(2) Der Messumformer darf nicht in einer Weise, die nach Auffassung von Medtronic die Stabilität und
Verlässlichkeit des Messumformers beeinflusst, von jemand anderem als Medtronic repariert oder
verändert worden sein.
(3) Es darf weder eine vorschriftswidrige Benutzung noch ein Missbrauch des Messumformers noch ein
Unfall vorliegen.
C. Diese GARANTIE ist auf ihren ausdrücklichen Wortlaut beschränkt. Insbesondere haftet Medtronic nicht für
mittelbare oder Folgeschäden, die sich aus dem Gebrauch, Defekt oder Funktionsausfall des
Messumformers ergeben, unabhängig davon, ob der Anspruch auf Haftungsbeschränkung, Vertrag,
unerlaubte Handlung oder eine andere Anspruchsgrundlage gestützt wird.
D. Die hier aufgeführten Haftungsausschlüsse und -beschränkungen sollen nicht gegen geltendes Recht
verstoßen und sind nicht dahingehend auszulegen. Sollte ein zuständiges Gericht feststellen, dass diese
GARANTIE ganz oder teilweise unwirksam, nicht durchsetzbar oder im Widerspruch zu geltendem Recht
steht, berührt dies die Gültigkeit der restlichen Bestimmungen nicht, und alle Rechte und Pflichten aus
3
Diese Garantie wird durch Medtronic, 710 Medtronic Parkway, Minneapolis, MN 55432, USA gewährt. Kunden außerhalb der
USA müssen sich an den zuständigen Medtronic Repräsentanten wenden, um die genauen Bestimmungen der Garantie für das
betreffende Land zu erfragen.
Gebrauchsanweisung Deutsch 29

dieser GARANTIE sind so auszulegen und durchzusetzen, als sei der für ungültig erklärte Teil oder die
ungültige Bestimmung in der GARANTIE nicht enthalten.
8. Garantieerklärung
(FÜR EINWEGPRODUKTE/AUSSERHALB DER USA)
Die folgende Garantieerklärung 4 gilt für Abnehmer außerhalb der USA:
A. Aufgrund dieser GARANTIE wird Medtronic dem Kunden, der einen Medtronic Bio-Probe Einwegeinsatz für
das Bio-Probe Blutflussüberwachungssystem – nachfolgend als „Produkt“ bezeichnet – erhalten hat, für den
Fall, dass das Produkt nicht gemäß den Spezifikationen funktioniert, beim Kauf eines Ersatzprodukts von
Medtronic zur Verwendung bei dem betreffenden Patienten eine Gutschrift in Höhe des ursprünglichen
Kaufpreises des Produkts, höchstens jedoch in Höhe des Kaufpreises des Ersatzprodukts gewähren. DIE
WARNHINWEISE IN DER PRODUKTINFORMATION SIND WESENTLICHER BESTANDTEIL DIESER
GARANTIE. INFORMATIONEN DARÜBER, WIE SIE IHRE FORDERUNGEN AUFGRUND DIESER
GARANTIE GELTEND MACHEN KÖNNEN, SIND BEI IHREM ÖRTLICHEN MEDTRONIC
REPRÄSENTANTEN ERHÄLTLICH.
B. Diese GARANTIE kann ausschließlich unter folgenden Bedingungen in Anspruch genommen werden:
(1) Das Produkt muss vor Ablauf des Verfallsdatums benutzt worden sein.
(2) Das Produkt muss innerhalb von 60 Tagen nach seiner Benutzung an Medtronic zurückgeschickt
werden und in das Eigentum von Medtronic übergehen.
(3) Das Produkt darf nicht für einen weiteren Patienten verwendet worden sein.
C. Diese GARANTIE ist auf ihren ausdrücklichen Wortlaut beschränkt. Insbesondere gilt:
(1) Bei nachweislich falscher Handhabung, nicht sachgerechter Implantation oder wesentlicher
Veränderung des Produkts wird keine Gutschrift für den Kauf eines Ersatzprodukts gewährt.
(2) Medtronic haftet weder für unmittelbare Schäden noch für Folgeschäden, die durch den Gebrauch,
durch einen Defekt oder durch eine Fehlfunktion des Produkts entstehen, unabhängig davon, ob sich
der Anspruch auf Schadensersatz auf eine Garantie, einen Vertrag, eine unerlaubte Handlung oder eine
andere Anspruchsgrundlage stützt.
D. Die hier aufgeführten Haftungsausschlüsse und -beschränkungen sollen nicht gegen geltendes Recht
verstoßen und sind nicht dahingehend auszulegen. Sollte ein zuständiges Gericht feststellen, dass diese
GARANTIE ganz oder teilweise unwirksam, nicht durchsetzbar oder im Widerspruch zu geltendem Recht
steht, berührt dies die Gültigkeit der restlichen Bestimmungen nicht, und alle Rechte und Pflichten aus
dieser GARANTIE sind so auszulegen und durchzusetzen, als sei der für ungültig erklärte Teil oder die
ungültige Bestimmung in der GARANTIE nicht enthalten.
4
Diese Garantie wird durch Medtronic, 710 Medtronic Parkway, Minneapolis, MN 55432, USA gewährt. Kunden außerhalb der
USA müssen sich an den zuständigen Medtronic Repräsentanten wenden, um die genauen Bestimmungen der Garantie für das
betreffende Land zu erfragen.
30 Gebrauchsanweisung Deutsch

Table des matières
1. À propos du système de contrôle du débit sanguin Bio-Probe™ 31
1.1. Indications d'utilisation 31
1.2. Contre-indications 31
1.3. Description 31
1.4. Contenu de l'emballage 32
1.5. Inspection de l'appareil 32
1.6. Limites ambiantes 32
1.7. Compatibilité avec la console de pompe à sang pour circulation extracorporelle Bio-Console™
32
1.8. Compatibilité des capteurs avec des raccords jetables 32
1.9. Effets indésirables 33
1.10. Avertissements et précautions 33
2. Assemblage 34
3. Mesures d'urgence 37
4. Problèmes et solutions 40
5. Nettoyage 40
6. Service 42
7. Garantie limitée pour l'équipement 42
8. Garantie limitée 42
1. À propos du système de contrôle du débit sanguin Bio-Probe™
Lire les instructions suivantes avant d'utiliser ce système.
1.1. Indications d'utilisation
Le système de contrôle du débit sanguin Bio-Probe™ doit être utilisé avec le modèle approprié de console de
pompe à sang pour circulation extracorporelle Bio-Console™ pour mesurer directement le débit sanguin dans le
circuit de perfusion extracorporelle.
1.2. Contre-indications
Utiliser l'appareil uniquement comme indiqué.
1.3. Description
Le système de contrôle du débit sanguin Bio-Probe se compose d'un capteur de débit et d'un raccord stérile à
usage unique. Ce système permet d'utiliser un support pour capteur.
Les modèles de capteur TX50 et TX50P sont réutilisables. La flèche figurant sur le couvercle du capteur indique
la direction correcte du débit de fluide et la couleur de la flèche indique si le capteur doit être utilisé chez un
patient adulte ou pédiatrique :
■
Modèle TX50 : le capteur pour patient adulte présente une flèche grise sur le couvercle et un câble noir.
■
Modèle TX50P : le capteur pour patient pédiatrique présente une flèche bleue sur le couvercle et un câble
bleu.
Les racords jetables sont disponibles sans revêtement ou avec un revêtement biocompatible. Contacter un
représentant du service clientèle de Medtronic pour de plus amples informations.
Les raccords jetables sont stérilisés à l'oxyde d'éthylène.
Le capteur peut être placé sur un support pour capteur et fixé à un pôle au cours d'une procédure de perfusion
ou lors du stockage de l'appareil. Les instructions relatives à l'utilisation du support pour capteur figurent dans le
Mode d’emploi Français 31

manuel de l'utilisateur du modèle approprié de la console de pompe à sang pour circulation extracorporelle BioConsole.
1.4. Contenu de l'emballage
Sont inclus dans l'emballage un manuel de l'utilisateur et un capteur pour patient adulte (Modèle TX50) ou un
capteur pour patient pédiatrique (Modèle TX50P).
Le raccord jetable n'est pas inclus, mais est un élément indispensable du système de contrôle du débit sanguin
Bio-Probe.
1.5. Inspection de l'appareil
Cette section fournit une liste des contrôles à effectuer sur le système de contrôle du débit sanguin Bio-Probe
par des techniciens formés et expérimentés dans l'utilisation de toutes les fonctions de l'appareil. Les
utilisateurs doivent lire et comprendre l'intégralité de ce manuel avant d'utiliser le système de contrôle du débit
sanguin Bio-Probe et d'effectuer ces vérifications. Les caractéristiques fonctionnelles de cet appareil doivent
être vérifiées avant l'utilisation comme décrit dans ce manuel. L'inspection de l'appareil, telle que décrite dans
cette section, doit être réalisée au moins une fois tous les 12 mois.
Si des problèmes devaient se présenter quelles que soient les circonstances, contacter le représentant du
service technique de Medtronic.
Les vérifications suivantes doivent être effectuées :
■
Inspection visuelle
■
Inscriptions, informations et avertissements correctement apposés
■
Dommage mécanique causé à l'appareil, aux accessoires et à l'ensemble des autres câbles
1.6. Limites ambiantes
Température de fonctionnement (TX50, TX50P) 18 °C à 33 °C (64 °F à 92 °F)
Humidité de fonctionnement (TX50, TX50P) 30% à 75% d'humidité relative, sans condensation
Températures de stockage
■
TX50, TX50P
■
DP38, DP38P, DP38T, DP38PT, BBDP38,
BBDP38P
■
CB2980POLY, CB4627
Humidité de stockage (TX50, TX50P) 15% à 95% d'humidité relative, sans condensation
1.7. Compatibilité avec la console de pompe à sang pour circulation extracorporelle Bio-Console™
Avant l'utilisation du système de contrôle du débit sanguin Bio-Probe, s'assurer de disposer du capteur
approprié pour la console utilisée. Pour toute information supplémentaire, se reporter au manuel de l'utilisateur
de la console approprié.
1.7.1. Modèles 550 et 560
Ces modèles de console ont été conçus pour le capteur Modèles TX50 et TX50P.
1.8. Compatibilité des capteurs avec des raccords jetables
Se reporter au mode d'emploi du raccord jetable Bio-Probe pour toute information supplémentaire concernant
les revêtements.
-34 °C à 66 °C (-29 °F à 150 °F)
-34 °C à 50 °C (-29 °F à 122 °F)
-34 °C à 40 °C (-29 °F à 104 °F)
32 Mode d’emploi Français

Tableau 1.
À utiliser avec le capteur
Modèle TX50 (patient adulte)
À utiliser avec le capteur
Modèle TX50P (patient pédiatrique)
Raccord sans revêtement DP38 DP38P
Raccord enduit de surface bioactive
CB2980POLY CB4627
Cortiva™
a
Raccord enduit de biosurface Trillium™
Raccord enduit de biosurface
Balance™
a
b
b
Technologie concédée sous licence en accord avec BioInteractions, Limited, Royaume-Uni.
Technologie concédée sous licence en accord avec BioInteractions, Limited, Royaume-Uni.
DP38T DP38PT
BBDP38 BBDP38P
1.9. Effets indésirables
Les effets indésirables suivants sont associés à l'utilisation du système de contrôle du débit sanguin Bio-Probe :
perte de sang, coagulopathie, activation excessive des composants du sang/thrombogénicité, anémie
hémolytique, hypotension, infection, ischémie, dysfonctionnement neurologique et dysfonctionnement
d'organes.
1.10. Avertissements et précautions
Lire attentivement l'ensemble des avertissements et des précautions ainsi que l'intégralité du mode d'emploi
avant toute utilisation. Ne pas lire ni suivre toutes les instructions ou ne pas respecter tous les
avertissements indiqués pourrait entraîner des blessures graves ou le décès du patient.
1.10.1. Avertissements
■
Avant l'utilisation de ce système, lire le manuel de l'utilisateur du modèle adéquat de console de pompe à
sang pour circulation extracorporelle Bio-Console.
■
Un capteur de réserve doit être disponible pendant la perfusion. Si le capteur doit être remplacé en cours de
procédure, suivre les instructions de la section "Mesures d'urgence".
1.10.2. Précautions
■
Les raccords jetables ont été conçus pour une utilisation sur un seul patient. Ne pas réutiliser, retraiter ni
restériliser ce produit. La réutilisation, le retraitement ou la restérilisation risque de compromettre l'intégrité
de la structure de l'appareil et/ou de contaminer l'appareil, ce qui pourrait entraîner des blessures, une
maladie ou le décès du patient.
■
S'il est nécessaire de lubrifier le raccord lors de la fixation de la tubulure, utiliser exclusivement de l'eau
stérile ou une solution saline. Ne pas utiliser de liquides à base d'alcool sur le raccord jetable.
■
Préalablement à l'ouverture de l'emballage du raccord, examiner attentivement l'emballage. Si l'emballage
est endommagé ou ouvert, ou si le raccord semble endommagé, ne pas utiliser le raccord. Retourner le
raccord au représentant local de Medtronic.
■
Inspecter le capteur, le câble et le connecteur du câble afin de détecter tout dommage mécanique. En cas
de dommage, utiliser un capteur de réserve et contacter un technicien qualifié de Medtronic.
■
Utiliser uniquement les capteurs avec leurs raccords jetables compatibles (voir tableau 1). L'utilisation d'un
raccord jetable inadéquat est susceptible d'endommager le capteur.
■
Manipuler les capteurs avec soin. Éviter l'incurvation excessive du câble du capteur, son entortillement, sa
torsion ou toute autre contrainte. Maintenir et porter le capteur délicatement ; ne pas porter le capteur par le
câble. Ne pas utiliser de scalpels, de pinces hémostatiques, de clamps pour tubulures ou tout autre
instrument chirurgical au niveau du capteur ou de son câble.
Mode d’emploi Français 33

■
1
2
Pour une précision optimale du débit, raccorder un système de contrôle du débit sanguin Bio-Probe amorcé
à la console.
■
Vérifier que les broches du capteur sont droites et propres.
■
S'assurer que les broches du raccord jetable sont en contact avec les broches du capteur (sous le couvercle
du capteur).
■
Mettre les appareils au rebut conformément aux procédures hospitalières, administratives et
gouvernementales.
2. Assemblage
Avant de procéder à la circulation extracorporelle du patient, connecter un système de contrôle du débit sanguin
Bio-Probe assemblé et amorcé à la console. Se référer au manuel de l'utilisateur de la console de pompe à
sang pour circulation extracorporelle Bio-Console approprié pour des instructions concernant la mise sous
tension de la console et la fixation du système de contrôle du débit sanguin Bio-Probe à la console.
Attention : Avant d'assembler le système, veiller à ce qu'un raccord jetable pour patient adulte soit utilisé
exclusivement avec le capteur pour patient adulte Modèle TX50. Si un raccord jetable pour patient pédiatrique
est utilisé, le capteur pour patient adulte risque d'être endommagé lorsque le couvercle du capteur est fermé.
Voir le tableau 1.
1. Patient adulte
2. Patient pédiatrique
Figure 1
1. En appliquant une technique stérile, retirer le raccord jetable de son emballage stérile.
Remarque : L'écartement des broches du raccord jetable pour patient adulte diffère de celui du raccord
pour patient pédiatrique.
34 Mode d’emploi Français

Figure 2
2. Connecter la ligne artérielle à un côté du raccord jetable.
Attention : Utiliser exclusivement de l'eau stérile ou une solution saline stérile pour lubrifier les tubulures et
le raccord. Ne pas utiliser d'alcool ou de solution alcoolisée.
Figure 3
3. En appliquant une technique stérile, connecter la tubulure, sur 15 cm (6 pouces) au moins, à l'autre côté du
raccord jetable (a) et à l'orifice de sortie de la pompe sanguine centrifuge (b).
Mode d’emploi Français 35

a
b
Figure 4
4. Ouvrir le couvercle du capteur en le poussant et aligner le raccord jetable sur la direction du débit de fluide
indiquée sur le couvercle du capteur.
Remarque : Si le capteur est incorrectement aligné sur le sens du trajet des fluides, un débit négatif
s'affichera sur la console.
Figure 5
5. Enfoncer fermement le raccord dans le capteur.
36 Mode d’emploi Français

Figure 6
6. Appuyer sur le couvercle du capteur pour le refermer en engageant le loquet.
Figure 7
Le système de contrôle du débit sanguin Bio-Probe est à présent assemblé. Se référer au manuel de l'utilisateur
de la console de pompe à sang pour circulation extracorporelle Bio-Console pour toute instruction relative à la
fixation du capteur, à l'amorçage du circuit (à l'aide d'une solution électrolytique équilibrée ou d'une solution
saline normale) et à la manière d'effectuer la remise à zéro du capteur.
3. Mesures d'urgence
Si le capteur venait à cesser de fonctionner, le remplacer par le capteur de réserve :
1. Déconnecter le capteur défectueux de la console et connecter à celle-ci le capteur de réserve.
2. Retirer le raccord jetable et la tubulure du capteur défectueux.
Mode d’emploi Français 37

Figure 8
3. Appuyer sur le couvercle du capteur de réserve pour l'ouvrir. Aligner le raccord jetable sur la flèche
indiquant la direction du trajet des fluides et figurant sur le couvercle du capteur opérationnel.
Remarque : Si le capteur est incorrectement aligné sur le sens du trajet des fluides, un débit négatif
s'affichera sur la console.
Figure 9
4. Enfoncer fermement le raccord dans le capteur de réserve.
38 Mode d’emploi Français

Figure 10
5. Appuyer sur le couvercle du capteur pour le refermer en engageant le loquet.
Figure 11
Le système de contrôle du débit sanguin Bio-Probe est à nouveau assemblé et connecté cette fois au capteur
de réserve. Se référer au manuel de l'utilisateur du système de circulation extracorporelle Bio-Console pour
toute instruction relative à la manière d'effectuer la remise à zéro du capteur.
Mode d’emploi Français 39

4. Problèmes et solutions
État Cause Action correctrice
Écran d'affichage
numérique clignotant
Débitmètre indiquant
une valeur négative
alors que le débit à la
pompe ou le débit est
connu pour être positif
Autres problèmes Inconnue Consulter un technicien qualifié de Medtronic.
La solution d'amorçage ne consiste
pas en une solution électrolytique
équilibrée ou en une solution saline
normale.
Le contact entre les broches du raccord jetable et les broches sous le
couvercle du capteur est interrompu.
Les broches situées sous le couvercle
du capteur ne sont pas correctement
alignées.
Le câble du capteur n'est pas correctement connecté.
Une unité de cautérisation, une lampe
ou tout autre courant électrique
ambiant présente des interférences.
Les broches du raccord jetable ou celles situées sous le couvercle du capteur sont mouillées.
La direction du trajet des fluides du
capteur est inversée. (Vérifier la flèche
figurant sur le couvercle du capteur.)
Le débitmètre n'a pas été initialisé correctement.
Utiliser une solution électrolytique équilibrée
ou une solution saline normale comme solution d'amorçage.
S'assurer que le couvercle du capteur (couvrant le raccord jetable) est correctement
fermé.
Vérifier si le contact des broches entre le capteur et le raccord jetable est bien établi.
S'assurer que les broches du capteur sont
exemptes de souillure ou de fluides.
Utiliser un raccord jetable de dimension adéquate pour ce modèle de capteur.
S'assurer que le câble du capteur est connecté à la console.
Vérifier si le câble du capteur ne présente pas
de rupture.
Déterminer la cause et la supprimer, si possible.
Sécher les broches.
Ouvrir le couvercle du capteur, soulever le raccord jetable, faire pivoter le capteur (ou le raccord jetable) de 180°, replacer le raccord jetable sous le couvercle du capteur dans la direction adéquate et procéder à la remise à zéro
du capteur.
Se référer à la procédure de remise à zéro ou
d'équilibrage du capteur de débit figurant dans
le manuel de l'utilisateur approprié relatif à la
console de pompe à sang pour circulation
extracorporelle Bio-Console.
5. Nettoyage
Le capteur présentera de meilleures performances et une longévité accrue s'il est nettoyé et stocké comme
décrit :
1. Après une procédure de perfusion, ouvrir le couvercle du capteur en le poussant et jeter le raccord jetable.
Attention : Le raccord jetable ne doit pas être restérilisé ni réutilisé.
40 Mode d’emploi Français

Figure 12
2. Nettoyer le capteur, ses broches (sous le couvercle du capteur) et son câble en les essuyant au moyen d'un
chiffon humidifié d'eau et de savon doux. Éviter d'utiliser des produits de nettoyage puissants ou abrasifs.
Essuyer le savon au moyen d'un chiffon ou d'une éponge humidifié d'eau claire.
Remarque : Ne pas immerger les pièces du capteur, le câble ou le connecteur du câble.
Figure 13
Remarque : Le capteur ne requiert aucune stérilisation.
3. Sécher le capteur, les broches du capteur et le câble au moyen d'un chiffon.
4. S'assurer que les broches situées sous le couvercle du capteur sont droites.
Si une broche n'est pas droite, contacter un technicien qualifié de Medtronic.
5. Pour stocker le capteur, le placer sur son support ou le débrancher et le ranger dans son étui protecteur en
plastique.
Mode d’emploi Français 41

Les instructions relatives à l'utilisation du support pour capteur figurent dans le manuel de l'utilisateur du modèle
approprié de la console de pompe à sang pour circulation extracorporelle Bio-Console.
6. Service
Si le capteur est endommagé, contacter le service clientèle de Medtronic. Il est indispensable de recevoir
l'autorisation de Medtronic avant de renvoyer un capteur pour réparation.
Remarque : Ne pas réparer le capteur ; la garantie serait annulée.
Medtronic dispose de conseillers professionnels chargés de fournir une assistance technique aux utilisateurs
des produits. Pour toute réponse à des questions ou pour programmer une formation produits, contacter le
représentant local de Medtronic.
7. Garantie limitée pour l'équipement
(POUR L'ÉQUIPEMENT EXTERNE RÉPARABLE/EN DEHORS DES ÉTATS-UNIS)
La garantie limitée suivante 3 s'applique aux clients hors des États-Unis :
A. La présente GARANTIE LIMITÉE certifie à l'acheteur des capteurs de débit Modèles TX50 et TX50P de
Medtronic pour le système de contrôle du débit sanguin Bio-Probe, ci-après le "Capteur", qu'en cas de
défaillance du Capteur dans des tolérances normales due à un défaut de matériaux ou de main-d'œuvre au
cours d'une période d'un (1) an à compter de la date de livraison du Capteur à l'acheteur, Medtronic, à sa
seule décision, optera pour : (a) réparer ou remplacer la ou les pièces défectueuses du Capteur ; (b)
émettre un crédit équivalent au prix d'achat initial du Capteur (sans toutefois excéder la valeur du Capteur
de remplacement) contre l'achat d'un Capteur de remplacement ; ou (c) fournir, sans aucun frais, un
Capteur de remplacement d'une fonctionnalité similaire.
B. Pour pouvoir bénéficier de cette réparation, de ce remplacement ou de ce crédit, les conditions suivantes
doivent être remplies :
(1) Le Capteur doit être retourné à Medtronic dans un délai de 60 jours suivant la découverte du défaut.
(Medtronic, à sa seule décision, pourra opter pour réparer le Capteur sur place.)
(2) Le Capteur ne devra pas avoir été réparé ou modifié par une personne autre que Medtronic d'une façon
telle que, de l'avis de Medtronic, sa stabilité et sa fiabilité en soient affectées.
(3) Le Capteur ne doit pas avoir fait l'objet d'un usage inadéquat ou d'un usage excessif et ne doit pas avoir
été endommagé ou détérioré.
C. La présente GARANTIE LIMITÉE est limitée à ses dispositions expresses. En particulier, Medtronic ne peut
pas être tenue responsable de tout dommage fortuit ou indirect résultant de tout usage, défaut ou
défaillance du Capteur, que la demande se fonde sur une garantie, une responsabilité contractuelle,
délictueuse ou autre.
D. Les exclusions et les limitations mentionnées ci-dessus ne sont pas, et ne doivent pas être, interprétées
comme contraires aux dispositions obligatoires des lois applicables. Si une partie ou une disposition de la
présente GARANTIE LIMITÉE devait être considérée comme illégale, non applicable ou contraire à la loi en
vigueur par un tribunal compétent, la validité des autres dispositions de la GARANTIE LIMITÉE n'en sera
pas affectée et tous les droits et obligations seront interprétés et appliqués comme si la présente
GARANTIE LIMITÉE ne contenait pas la partie ou la disposition considérée comme illégale.
8. Garantie limitée
(POUR LE PRODUIT À USAGE UNIQUE/EN DEHORS DES ÉTATS-UNIS)
La garantie limitée suivante 4 s'applique aux clients hors des États-Unis :
3
La présente Garantie limitée est fournie par Medtronic, 710 Medtronic Parkway, Minneapolis, MN 55432. Pour les pays hors des
États-Unis, les clients doivent contacter leur représentant local de Medtronic pour connaître les dispositions exactes de la
Garantie limitée.
42 Mode d’emploi Français

A. La présente GARANTIE LIMITÉE certifie à l'acheteur qui reçoit les raccords jetables Bio-Probe de
Medtronic pour le système de contrôle du débit sanguin Bio-Probe (ci-après, le "Produit") qu'en cas de
défaillance du Produit dans ses spécifications, Medtronic émettra un crédit équivalent au prix d'achat initial
du Produit (sans toutefois excéder la valeur du Produit de remplacement) contre l'achat d'un Produit de
remplacement de Medtronic qui sera utilisé pour le même patient. LES AVERTISSEMENTS CONTENUS
SUR LES ÉTIQUETTES DU PRODUIT SONT CONSIDÉRÉS COMME FAISANT PARTIE INTÉGRANTE
DE LA PRÉSENTE GARANTIE LIMITÉE. CONTACTER LE REPRÉSENTANT MEDTRONIC LOCAL AFIN
DE SE RENSEIGNER SUR LA FAÇON DE PRÉSENTER UNE RÉCLAMATION SUR LE FONDEMENT DE
CETTE GARANTIE LIMITÉE.
B. Pour pouvoir bénéficier de la GARANTIE LIMITÉE, les conditions suivantes doivent être remplies :
(1) Le Produit doit être utilisé avant sa date de péremption.
(2) Le Produit doit être retourné à Medtronic dans un délai de 60 jours après usage et deviendra la
propriété de Medtronic.
(3) Le Produit ne doit pas avoir été utilisé pour un autre patient.
C. La présente GARANTIE LIMITÉE est limitée à ses dispositions expresses. En particulier :
(1) En aucun cas une note de crédit de remplacement ne sera émise s'il est démontré que le produit a fait
l'objet d'une manipulation inadéquate, d'une implantation inadéquate ou d'une altération matérielle du
Produit remplacé.
(2) Medtronic ne sera pas tenue responsable de tous dommages fortuits ou indirects résultant de tous
usages, défauts ou défaillances du Produit, que la demande se fonde sur une garantie, une
responsabilité contractuelle, délictuelle ou autre.
D. Les exclusions et les limitations mentionnées ci-dessus ne sont pas, et ne doivent pas être, interprétées
comme contraires aux dispositions obligatoires des lois applicables. Si une partie ou une disposition de la
présente GARANTIE LIMITÉE devait être considérée comme illégale, non applicable ou contraire à la loi en
vigueur par un tribunal compétent, la validité des autres dispositions de la GARANTIE LIMITÉE n'en sera
pas affectée et tous les droits et obligations seront interprétés et appliqués comme si la présente
GARANTIE LIMITÉE ne contenait pas la partie ou la disposition considérée comme illégale.
4
La présente Garantie limitée est fournie par Medtronic, 710 Medtronic Parkway, Minneapolis, MN 55432. Pour les pays hors des
États-Unis, les clients doivent contacter leur représentant local de Medtronic pour connaître les dispositions exactes de la
Garantie limitée.
Mode d’emploi Français 43

Sommario
1. Informazioni sul sistema di monitoraggio del flusso ematico Bio-Probe™ 44
1.1. Indicazioni per l'uso 44
1.2. Controindicazioni 44
1.3. Descrizione 44
1.4. Contenuto della confezione 45
1.5. Ispezione dello strumento 45
1.6. Limiti ambientali 45
1.7. Compatibilità con la console per pompaggio ematico extracorporeo Bio-Console™ 45
1.8. Compatibilità dei trasduttori con gli inserti monouso 45
1.9. Effetti indesiderati 46
1.10. Avvertenze e precauzioni 46
2. Assemblaggio 47
3. Emergenza 50
4. Risoluzione dei problemi 53
5. Pulizia 53
6. Assistenza tecnica 55
7. Garanzia limitata dell’apparecchiatura 55
8. Garanzia limitata 55
1. Informazioni sul sistema di monitoraggio del flusso ematico Bio-Probe™
Prima di utilizzare il sistema, leggere attentamente le presenti istruzioni.
1.1. Indicazioni per l'uso
Il sistema di monitoraggio del flusso ematico Bio-Probe™ deve essere utilizzato in associazione all’adeguato
modello di console per pompaggio ematico extracorporeo Bio-Console™, per la misurazione diretta del flusso
ematico nel circuito di perfusione extracorporea.
1.2. Controindicazioni
Utilizzare il dispositivo soltanto nel modo indicato.
1.3. Descrizione
Il sistema di monitoraggio del flusso ematico Bio-Probe comprende un inserto monouso sterile e un trasduttore
di flusso. È possibile utilizzare un supporto per il trasduttore.
I modelli di trasduttore TX50 e TX50P sono riutilizzabili. La freccia sul coperchio del trasduttore indica la
direzione corretta del flusso del liquido. Il colore della freccia indica se il trasduttore dovrà essere utilizzato per
un paziente adulto o pediatrico:
■
Modello TX50: il trasduttore per adulti presenta una freccia di colore grigio sul coperchio e un cavo nero.
■
Modello TX50P: il trasduttore pediatrico presenta una freccia di colore azzurro sul coperchio e un cavo
azzurro.
Gli inserti monouso sono disponibili sia senza rivestimento che con rivestimento biocompatibile. Contattare il
rappresentante del servizio assistenza Medtronic per ulteriori informazioni.
Gli inserti monouso sono sterilizzati con ossido di etilene.
Il trasduttore può essere montato su un apposito supporto e fissato a un’asta durante la procedura di perfusione
o per essere riposto quando non viene utilizzato. Le istruzioni per l’uso del supporto per il trasduttore sono
incluse nel manuale dell’operatore del modello specifico di console per pompaggio ematico extracorporeo
Bio-Console.
44 Istruzioni per l’uso Italiano

1.4. Contenuto della confezione
La confezione contiene un manuale dell’operatore e un trasduttore per adulti (modello TX50) o un trasduttore
pediatrico (modello TX50P).
L’inserto monouso non è compreso nella confezione, ma costituisce un elemento essenziale del sistema di
monitoraggio del flusso ematico Bio-Probe.
1.5. Ispezione dello strumento
In questa sezione viene fornito un elenco delle ispezioni di verifica che devono essere eseguite sul sistema di
monitoraggio del flusso ematico Bio-Probe da parte del personale tecnico addestrato ed esperto in tutte le
funzioni del dispositivo. Prima di utilizzare il sistema di monitoraggio del flusso ematico Bio-Probe e di eseguire
queste verifiche, gli utenti devono leggere e comprendere questo manuale in tutte le sue parti. È necessario
verificare il funzionamento di questo dispositivo prima dell'uso, come indicato nel presente manuale. L'ispezione
del dispositivo, descritta in questa sezione, deve essere effettuata almeno una volta ogni 12 mesi.
In ogni caso, se si dovessero verificare problemi, rivolgersi al rappresentante locale Medtronic per assistenza.
È necessario effettuare i seguenti controlli:
■
Ispezione visiva
■
Corretta e completa applicazione di scritte, informazioni e avvertenze
■
Danni meccanici al dispositivo, agli accessori e a tutti i cavi
1.6. Limiti ambientali
Temperatura di funzionamento (TX50, TX50P) Da 18 °C a 33 °C (da 64 °F a 92 °F)
Umidità di funzionamento (TX50, TX50P) Da 30% a 75% di umidità relativa, senza condensa
Temperature di conservazione
■
TX50, TX50P
■
DP38, DP38P, DP38T, DP38PT, BBDP38,
BBDP38P
■
CB2980POLY, CB4627
Umidità di conservazione (TX50, TX50P) Dal 15% al 95% di umidità relativa, senza condensa
1.7. Compatibilità con la console per pompaggio ematico extracorporeo Bio-Console™
Prima di utilizzare il sistema di monitoraggio del flusso ematico Bio-Probe, assicurarsi di disporre del trasduttore
appropriato alla console in uso. Per ulteriori informazioni, fare riferimento al manuale dell'operatore della
console appropriata.
1.7.1. Modelli 550 e 560
Questi modelli di console sono stati progettati per i trasduttori modello TX50 e TX50P.
1.8. Compatibilità dei trasduttori con gli inserti monouso
Per ulteriori informazioni sui rivestimenti, consultare le istruzioni per l'uso dell'inserto monouso Bio-Probe.
Per l'uso con il trasduttore
modello TX50 (pazienti adulti)
Inserto non rivestito DP38 DP38P
Inserto con superficie bioattiva Cor-
tiva™
CB2980POLY CB4627
Da -34 °C a 66 °C (da -29 °F a 150 °F)
Da -34 °C a 50 °C (da -29 °F a 122 °F)
Da -34 °C a 40 °C (da -29 °F a 104 °F)
Tabella 1.
Per l'uso con il trasduttore
modello TX50P (pazienti pediatrici)
Istruzioni per l’uso Italiano 45

Per l'uso con il trasduttore
modello TX50 (pazienti adulti)
Per l'uso con il trasduttore
modello TX50P (pazienti pediatrici)
Inserto con superficie biopassiva Tril-
a
lium™
Inserto con superficie biopassiva
Balance™
a
b
b
Tecnologia impiegata con licenza d'uso di proprietà di BioInteractions, Limited, Regno Unito.
Tecnologia impiegata con licenza d'uso di proprietà di BioInteractions, Limited, Regno Unito.
DP38T DP38PT
BBDP38 BBDP38P
1.9. Effetti indesiderati
Gli effetti indesiderati associati all'utilizzo del sistema di monitoraggio del flusso ematico Bio-Probe sono: perdita
ematica, coagulopatia, trombogenicità/attivazione dei componenti ematici eccessiva, anemia emolitica,
ipotensione, infezione, ischemia, disfunzione neurologica e degli organi.
1.10. Avvertenze e precauzioni
Leggere attentamente tutte le avvertenze, le precauzioni e le istruzioni per l'uso prima di utilizzare il dispositivo.
La mancata lettura e osservanza di tutte le istruzioni o delle avvertenze indicate potrebbe causare
lesioni gravi o il decesso del paziente.
1.10.1. Avvertenze
■
Prima di utilizzare questo sistema, leggere il manuale dell’operatore relativo al modello appropriato di
console per pompaggio ematico extracorporeo Bio-Console.
■
Durante la perfusione tenere sempre a portata di mano un trasduttore di riserva. Se nel corso della
procedura fosse necessario sostituire il trasduttore, seguire le istruzioni riportate nella sezione Emergenza.
1.10.2. Precauzioni
■
Gli inserti monouso sono progettati per l'utilizzo su un singolo paziente. Non riutilizzare, rigenerare o
risterilizzare il prodotto poiché ciò potrebbe comprometterne l'integrità strutturale e/o comportare un rischio
di contaminazione che potrebbe provocare lesioni, insorgenza di malattie o decesso del paziente.
■
Se è necessario lubrificare l’inserto durante il collegamento dei tubi, utilizzare esclusivamente acqua sterile
o soluzione fisiologica. Non utilizzare liquidi a base alcolica sull’inserto monouso.
■
Esaminare attentamente la confezione dell’inserto prima di aprirla. Se la confezione è danneggiata o aperta
o se l'inserto appare danneggiato, non utilizzare l’inserto. Restituire l'inserto al rappresentante locale
Medtronic.
■
Esaminare il trasduttore, il cavo e il connettore del cavo per individuare eventuali danni meccanici. In caso di
danni, utilizzare un trasduttore di riserva e contattare un tecnico qualificato dell'assistenza Medtronic.
■
Utilizzare i trasduttori esclusivamente con i relativi inserti monouso compatibili (vedere la tabella 1). L’uso di
un inserto monouso non adatto può danneggiare il trasduttore.
■
Maneggiare i trasduttori con cura. Evitare piegature ad angolo acuto, attorcigliamenti, torsioni o altre tensioni
a carico del cavo del trasduttore. Afferrare e trasportare il trasduttore maneggiandolo con cura. Non
trasportare il trasduttore tenendolo per il cavo. Non utilizzare bisturi, pinze emostatiche, clamp per tubi o altri
strumenti chirurgici sul trasduttore o sul relativo cavo.
■
Per ottenere una precisione ottimale del flusso, effettuare il priming del sistema di monitoraggio del flusso
ematico Bio-Probe prima di collegare quest'ultimo alla console.
■
Verificare che i pin del trasduttore siano diritti e puliti.
■
Assicurarsi che i pin dell’inserto monouso siano in contatto con i pin del trasduttore (sotto il coperchio del
trasduttore).
■
Smaltire i dispositivi in conformità alle norme ospedaliere, amministrative e statali locali vigenti.
46 Istruzioni per l’uso Italiano

2. Assemblaggio
1
2
Prima di sottoporre il paziente a un bypass cardiopolmonare, assemblare il sistema di monitoraggio del flusso
ematico Bio-Probe, effettuarne il priming e quindi collegarlo alla console. Per istruzioni sull’attivazione della
console e sul collegamento del sistema di monitoraggio del flusso ematico Bio-Probe alla console, consultare
l'appropriato manuale dell’operatore della console per pompaggio ematico extracorporeo Bio-Console.
Attenzione: prima di assemblare il sistema, assicurarsi di utilizzare un inserto monouso per adulti unicamente
con il trasduttore per adulti modello TX50. L’uso di un inserto di tipo pediatrico può danneggiare il trasduttore
per adulti al momento della chiusura del coperchio del trasduttore. Vedere la tabella 1.
1. Per pazienti adulti
2. Per pazienti pediatrici
Figura 1
1. Utilizzando una tecnica sterile, estrarre l’inserto monouso dalla confezione sterile.
Nota: la distanza tra i pin dell’inserto monouso per adulti è diversa da quella dell’inserto per pazienti
pediatrici.
Istruzioni per l’uso Italiano 47

Figura 2
2. Collegare la linea arteriosa a un lato dell’inserto monouso.
Attenzione: utilizzare esclusivamente una soluzione fisiologica sterile o acqua sterile per lubrificare i tubi e
l’inserto. Non utilizzare alcol o soluzioni contenenti alcol.
Figura 3
3. Sempre utilizzando la tecnica sterile, collegare un tubo lungo almeno 15 cm (6") all'altro lato dell’inserto
monouso (a) e all’uscita della biopompa centrifuga (b).
48 Istruzioni per l’uso Italiano

a
b
Figura 4
4. Aprire il coperchio del trasduttore e allineare l’inserto monouso con la direzione del flusso del liquido
indicata sul coperchio del trasduttore.
Nota: se il trasduttore non viene allineato correttamente con il percorso del liquido, sulla console verrà
visualizzato un valore di flusso negativo.
Figura 5
5. Introdurre saldamente l’inserto nel trasduttore.
Istruzioni per l’uso Italiano 49

Figura 6
6. Chiudere il coperchio del trasduttore fino allo scatto della levetta di blocco.
Figura 7
Il sistema di monitoraggio del flusso ematico Bio-Probe è ora assemblato. Per istruzioni sul collegamento del
trasduttore, il priming del circuito (con soluzione fisiologica normale o bilanciata) e l’azzeramento del trasduttore,
consultare il manuale dell’operatore della console per pompaggio ematico extracorporeo Bio-Console.
3. Emergenza
Qualora il funzionamento del trasduttore dovesse interrompersi, sostituirlo con il trasduttore di riserva:
1. Scollegare il trasduttore non funzionante dalla console e collegarvi il trasduttore di riserva.
2. Rimuovere l’inserto monouso con il relativo tubo dal trasduttore non funzionante.
50 Istruzioni per l’uso Italiano

Figura 8
3. Aprire il coperchio del trasduttore di riserva e allineare l’inserto monouso con la direzione del flusso del
liquido indicata sul coperchio del trasduttore funzionante.
Nota: se il trasduttore non viene allineato correttamente con il percorso del liquido, sulla console verrà
visualizzato un valore di flusso negativo.
Figura 9
4. Introdurre saldamente l’inserto nel trasduttore di riserva.
Istruzioni per l’uso Italiano 51

Figura 10
5. Chiudere il coperchio del trasduttore fino allo scatto della levetta di blocco.
Figura 11
Il sistema di monitoraggio del flusso ematico Bio-Probe è così riassemblato con il trasduttore di riserva. Per
istruzioni sull'azzeramento del trasduttore, consultare il manuale dell’operatore della console per pompaggio
ematico extracorporeo Bio-Console.
52 Istruzioni per l’uso Italiano

4. Risoluzione dei problemi
Problema Causa Azione correttiva
Il display digitale lampeggia
Viene riportato un
valore negativo sul
flussometro in presenza di flusso positivo in uscita dalla
pompa
Altri problemi Ignota Consultare un tecnico qualificato del servizio
La soluzione di priming non è costituita
da soluzione fisiologica normale o bilanciata.
I contatti fra i pin dell’inserto monouso e i
pin sotto il coperchio del trasduttore
sono interrotti.
I pin sotto il coperchio del trasduttore
non sono allineati correttamente.
Il cavo del trasduttore non è collegato
correttamente.
Probabile interferenza da parte di un’unità di cauterizzazione, lampada o altra
corrente elettrica presente nell’ambiente.
I pin dell’inserto monouso o i pin all’interno del coperchio del trasduttore sono
bagnati.
Il trasduttore è invertito rispetto al percorso del flusso (notare la freccia sul
coperchio del trasduttore).
Il display del flusso non è stato azzerato
correttamente.
Utilizzare come soluzione di priming una
soluzione fisiologica normale o bilanciata.
Verificare che il coperchio del trasduttore
(sopra l’inserto monouso) sia ben chiuso.
Verificare il corretto contatto dei pin fra il trasduttore e l’inserto monouso.
Verificare che i pin del trasduttore siano puliti
e che non presentino residui di liquidi.
Utilizzare l’inserto monouso della dimensione
adatta per il modello di trasduttore.
Assicurarsi che il cavo del trasduttore sia collegato alla console.
Verificare che il cavo del trasduttore non presenti rotture.
Identificare la causa ed eliminarla, se possibile.
Asciugare i pin.
Aprire il coperchio del trasduttore, estrarre
l’inserto monouso, ruotare il trasduttore (o
l’inserto monouso) di 180°, rimettere l’inserto
sotto il coperchio del trasduttore e azzerare
nuovamente il trasduttore.
Consultare la procedura di azzeramento o di
bilanciamento del trasduttore di flusso nel
manuale dell’operatore del modello appropriato di console per pompaggio ematico
extracorporeo Bio-Console.
di assistenza Medtronic.
5. Pulizia
Il trasduttore funzionerà in modo più efficiente e durerà più a lungo se pulito e conservato secondo le procedure
descritte di seguito:
1. Dopo una procedura di perfusione, aprire il coperchio del trasduttore ed eliminare l’inserto monouso.
Attenzione: l’inserto monouso non deve mai essere risterilizzato o riutilizzato.
Istruzioni per l’uso Italiano 53

Figura 12
2. Pulire il trasduttore, i pin del trasduttore (sotto il coperchio) e il cavo utilizzando un panno inumidito con
acqua e sapone neutro. Evitare l’uso di detergenti aggressivi o abrasivi. Rimuovere il sapone con un panno
o una spugna imbevuti di acqua pulita.
Nota: non immergere alcun componente del trasduttore, né il cavo o il connettore del cavo.
Figura 13
Nota: il trasduttore non necessita di sterilizzazione.
3. Asciugare il trasduttore, i pin del trasduttore e il cavo con un panno.
4. Assicurarsi che i pin sotto il coperchio del trasduttore siano diritti.
Qualora un pin non fosse diritto, informare un tecnico qualificato dell’assistenza Medtronic.
5. Per conservare il trasduttore, collocarlo sull’apposito supporto o scollegarlo e riporlo nella relativa custodia
protettiva in plastica.
54 Istruzioni per l’uso Italiano

Le istruzioni per l’uso del supporto per il trasduttore sono incluse nel manuale dell’operatore del modello
specifico di console per pompaggio ematico extracorporeo Bio-Console.
6. Assistenza tecnica
Nel caso in cui un trasduttore fosse danneggiato, contattare il servizio clienti Medtronic. Prima di rispedire un
trasduttore per la riparazione, è indispensabile ricevere l'autorizzazione di Medtronic.
Nota: non procedere personalmente alla riparazione del trasduttore; ciò invaliderebbe automaticamente la
garanzia.
Presso Medtronic è costantemente disponibile uno staff di consulenti altamente qualificati, in grado di fornire
consulenza tecnica agli utenti dei prodotti. Contattare il rappresentante locale dell'assistenza Medtronic se si
desidera ottenere risposta a determinati quesiti oppure organizzare delle sessioni di formazione sul prodotto.
7. Garanzia limitata dell’apparecchiatura
(PER LE APPARECCHIATURE ESTERNE RIPARABILI/FUORI DAGLI STATI UNITI)
La seguente garanzia limitata 3 è valida per i clienti al di fuori degli Stati Uniti:
A. La presente GARANZIA LIMITATA assicura all'acquirente dei trasduttori di flusso di Medtronic modello
TX50 e TX50P per l'uso con il sistema di monitoraggio del flusso ematico Bio-Probe, di seguito denominati
"Trasduttore", che, nel caso in cui il Trasduttore non dovesse funzionare entro i normali limiti di tolleranza in
seguito a un difetto dei materiali o di fabbricazione entro il periodo di un (1) anno a partire dalla data di
consegna del Trasduttore all'acquirente stesso, Medtronic provvederà a sua discrezione a: (a) riparare o
sostituire qualsiasi parte difettosa del Trasduttore; (b) accordare un credito pari al prezzo di acquisto del
Trasduttore originale (ma non superiore al valore del Trasduttore sostitutivo) per l'acquisto di un Trasduttore
sostitutivo, oppure, (c) fornire gratuitamente un Trasduttore sostitutivo con funzionalità comparabili.
B. La riparazione, la sostituzione o il credito potranno essere invocati laddove ricorrano le seguenti condizioni:
(1) Il Trasduttore deve essere stato restituito a Medtronic entro 60 giorni dalla scoperta del mancato
funzionamento. (Medtronic potrà, a propria discrezione, effettuare la riparazione del Trasduttore in loco).
(2) Il Trasduttore non deve essere stato riparato od alterato da un soggetto diverso da Medtronic che, a
giudizio di quest’ultima, possa aver inciso sulla sua stabilità ed affidabilità.
(3) Il Trasduttore non deve essere stato soggetto a gestione e uso impropri o a incidente alcuno.
C. La presente GARANZIA LIMITATA è limitata a quanto in essa specificato. In particolare, Medtronic non è
responsabile per eventuali danni incidentali o consequenziali risultanti dall'uso, da difetti o dal mancato
funzionamento del Trasduttore, indipendentemente dal fatto che la richiesta di risarcimento si basi su
garanzia, contratto, fatto illecito o altro.
D. Le esclusioni e le restrizioni di cui sopra non sono intese, né devono essere interpretate in quanto tali, come
contravvenenti alle norme inderogabili della legislazione vigente. Nel caso in cui una parte o un termine
della presente GARANZIA LIMITATA venga dichiarato illegale, inapplicabile o in conflitto con la legislazione
vigente da un organo giudiziario competente, la validità delle rimanenti parti della presente GARANZIA
LIMITATA non verrà compromessa e tutti i diritti e gli obblighi saranno interpretati ed applicati come se la
presente GARANZIA LIMITATA non contenesse la parte o i termini dichiarati non validi.
8. Garanzia limitata
(PER I PRODOTTI MONOUSO/FUORI DAGLI STATI UNITI)
La seguente garanzia limitata 4 è valida per i clienti al di fuori degli Stati Uniti:
3
La presente Garanzia limitata è fornita da Medtronic, 710 Medtronic Parkway, Minneapolis, MN 55432. I clienti al di fuori degli
Stati Uniti sono pregati di rivolgersi al locale rappresentante Medtronic per i termini esatti della presente garanzia limitata.
4
La presente Garanzia limitata è fornita da Medtronic, 710 Medtronic Parkway, Minneapolis, MN 55432. I clienti al di fuori degli
Stati Uniti sono pregati di rivolgersi al locale rappresentante Medtronic per i termini esatti della presente garanzia limitata.
Istruzioni per l’uso Italiano 55

A. La presente GARANZIA LIMITATA garantisce all'acquirente degli inserti monouso Bio-Probe di Medtronic
per l'uso con il sistema di monitoraggio del flusso ematico Bio-Probe, di seguito denominati "Prodotto", che,
in caso di mancato funzionamento del Prodotto secondo le specifiche, Medtronic accorderà un credito pari
al prezzo di acquisto del Prodotto originale (ma non superiore al valore del Prodotto sostitutivo) per
l'acquisto di qualsiasi Prodotto Medtronic sostitutivo utilizzabile per il paziente specifico. LE AVVERTENZE
CONTENUTE NELLE ETICHETTE DEL PRODOTTO SONO DA CONSIDERARSI PARTE INTEGRANTE
DELLA PRESENTE GARANZIA LIMITATA. PER ULTERIORI INFORMAZIONI IN CASO DI RECLAMO NEI
TERMINI DELLA PRESENTE GARANZIA LIMITATA, CONTATTARE IL LOCALE RAPPRESENTANTE
MEDTRONIC.
B. Per poter beneficiare della presente GARANZIA LIMITATA, devono essere soddisfatte le seguenti
condizioni:
(1) Il Prodotto deve essere stato utilizzato prima della data di scadenza indicata.
(2) Il Prodotto deve essere riconsegnato a Medtronic entro 60 giorni dall'uso e diverrà di proprietà di
Medtronic.
(3) Il Prodotto non deve essere stato utilizzato per altri pazienti.
C. La presente GARANZIA LIMITATA è limitata a quanto in essa specificato. In particolare:
(1) Nel caso in cui si riscontrino gestione ed impianto impropri o alterazioni dei materiali del Prodotto
sostituito, non verrà concesso alcun rimborso per le spese di sostituzione.
(2) Medtronic non è responsabile di eventuali danni diretti, incidentali o consequenziali causati da uso,
difetti, guasti o malfunzionamento del Prodotto, indipendentemente dal fatto che la richiesta di
risarcimento di tali danni sia basata su garanzia, contratto, fatto illecito o altro.
D. Le esclusioni e le restrizioni di cui sopra non sono intese, né devono essere interpretate, come
contravvenenti a norme ritenute inderogabili dalla legislazione vigente. Nel caso in cui una parte o un
termine della presente GARANZIA LIMITATA venga dichiarato illegale, inapplicabile o in conflitto con la
legislazione vigente da un organo giudiziario competente, la validità delle rimanenti parti della presente
GARANZIA LIMITATA non verrà compromessa e tutti i diritti e gli obblighi saranno interpretati ed applicati
come se la presente GARANZIA LIMITATA non contenesse la parte o i termini dichiarati non validi.
56 Istruzioni per l’uso Italiano

Inhoudsopgave
1. Over het Bio-Probe™-flowbewakingssysteem 57
1.1. Gebruiksindicaties 57
1.2. Contra-indicaties 57
1.3. Beschrijving 57
1.4. Inhoud van de verpakking 57
1.5. Instrumentinspectie 58
1.6. Omgevingsvereisten 58
1.7. Compatibiliteit met de extracorporele Bio-Console™-bloedpompconsole 58
1.8. Compatibiliteit van transducers met wegwerp-inserts 58
1.9. Bijwerkingen 59
1.10. Waarschuwingen en voorzorgsmaatregelen 59
2. Montage 60
3. Noodsituaties 63
4. Problemen oplossen 66
5. Reiniging 66
6. Onderhoud 68
7. Beperkte garantie bij apparatuur 68
8. Beperkte garantie 68
1. Over het Bio-Probe™-flowbewakingssysteem
Lees deze instructies voordat u dit systeem gebruikt.
1.1. Gebruiksindicaties
Het Bio-Probe™-flowbewakingssysteem dient gebruikt te worden met een geschikt model extracorporele BioConsole™-bloedpompconsole om de bloedflow in het extracorporele perfusiecircuit rechtstreeks te meten.
1.2. Contra-indicaties
Gebruik het apparaat uitsluitend volgens voorschrift.
1.3. Beschrijving
Het Bio-Probe-flowbewakingssysteem bestaat uit een flowtransducer en een steriele wegwerp-insert. Er kan
een transducersteun gebruikt worden.
De transducers Model TX50 en TX50P zijn herbruikbaar. De pijl op het deksel van de transducer geeft de juiste
richting van de vloeistofstroom aan en de kleur van de pijl geeft aan of de transducer bedoeld is voor gebruik bij
een volwassen patiënt dan wel bij een kind:
■
Model TX50: De transducer voor volwassenen heeft een grijze pijl op het deksel en een zwarte kabel.
■
Model TX50P: De transducer voor kinderen heeft een blauwe pijl op het deksel en een blauwe kabel.
De wegwerp-inserts zijn verkrijgbaar zonder coating of met een biocompatibele coating. Neem voor meer
informatie contact op met de klantenservice van Medtronic.
De wegwerp-inserts zijn gesteriliseerd met ethyleenoxide.
De transducer kan op een transducersteun worden geplaatst en aan een standaard worden bevestigd tijdens
een perfusieprocedure of tijdens opslag. Instructies voor het gebruik van de transducersteun vindt u in de
gebruikershandleiding van het desbetreffende model van de extracorporele Bio-Console-bloedpompconsole.
1.4. Inhoud van de verpakking
In de verpakking zit een gebruikershandleiding en een transducer voor volwassenen (Model TX50) of een
transducer voor kinderen (Model TX50P).
Gebruiksaanwijzing Nederlands 57

De wegwerp-insert wordt niet meegeleverd maar is een noodzakelijk onderdeel van het Bio-Probeflowbewakingssysteem.
1.5. Instrumentinspectie
Deze sectie bevat een lijst met inspectiepunten voor het Bio-Probe-flowbewakingssysteem voor technisch
personeel dat is opgeleid in en ervaring heeft met alle functies van het apparaat. Gebruikers dienen deze
handleiding volledig te lezen en te begrijpen voordat ze het Bio-Probe-flowbewakingssysteem gebruiken en
deze inspectie uitvoeren. De functies van dit apparaat dienen voor gebruik te worden gecontroleerd zoals
beschreven in deze handleiding. De instrumentinspectie, zoals beschreven in deze sectie, dient ten minste
eenmaal per 12 maanden te worden uitgevoerd.
Neem in geval van problemen te allen tijde contact op met de technische dienst van Medtronic.
De volgende controles dienen te worden uitgevoerd:
■
Visuele inspectie
■
Inscripties, informatie- en waarschuwingsindicaties moeten goed en volledig zijn bevestigd
■
Mechanische schade aan het product, toebehoren en alle andere kabels
1.6. Omgevingsvereisten
Temperatuur in bedrijf (TX50, TX50P) 18 °C tot 33 °C (64 °F tot 92 °F)
Luchtvochtigheidsgraad in bedrijf (TX50, TX50P) 30% tot 75% relatieve luchtvochtigheid niet-conden-
Opslagtemperatuur
■
TX50, TX50P
■
DP38, DP38P, DP38T, DP38PT, BBDP38,
BBDP38P
■
CB2980POLY, CB4627
Luchtvochtigheidsgraad tijdens opslag (TX50, TX50P) 15% tot 95% relatieve luchtvochtigheid niet-conden-
1.7. Compatibiliteit met de extracorporele Bio-Console™-bloedpompconsole
Voordat u het Bio-Probe-flowbewakingssysteem gebruikt, moet u controleren of de juiste transducer voor de
console wordt gebruikt. Raadpleeg de gebruikershandleiding van de desbetreffende console voor meer
informatie.
1.7.1. Model 550 en 560
Deze consolemodellen zijn bedoeld voor gebruik met de transducers Model TX50 en TX50P.
1.8. Compatibiliteit van transducers met wegwerp-inserts
Raadpleeg de gebruiksaanwijzing bij de Bio-Probe-wegwerp-insert voor meer informatie over de coatings.
Voor gebruik met transducer
Model TX50 (voor volwasse-
Ongecoate insert DP38 DP38P
Insert met bioactieve Cortiva™-opper-
vlaktelaag
nen)
CB2980POLY CB4627
serend
-34 °C tot 66 °C (-29 °F tot 150 °F)
-34 °C tot 50 °C (-29 °F tot 122 °F)
-34 °C tot 40 °C (-29 °F tot 104 °F)
serend
Tabel 1.
Voor gebruik met transducer
Model TX50P (voor kinderen)
58 Gebruiksaanwijzing Nederlands

Voor gebruik met transducer
Model TX50 (voor volwasse-
Voor gebruik met transducer
Model TX50P (voor kinderen)
nen)
Insert gecoat met Trillium™-bio-oppervlaktelaag
Insert gecoat met Balance™-bio-oppervlaktelaag
a
b
a
b
Met toestemming van BioInteractions, Limited, Verenigd Koninkrijk.
Met toestemming van BioInteractions, Limited, Verenigd Koninkrijk.
DP38T DP38PT
BBDP38 BBDP38P
1.9. Bijwerkingen
De volgende bijwerkingen kunnen bij het gebruik van het Bio-Probe-flowbewakingssysteem optreden:
bloedverlies, coagulopathie, overmatig activeren van bloedcomponenten/trombogenese, hemolytische anemie,
hypotensie, infectie, ischemie, neurologische disfunctie en orgaanfalen.
1.10. Waarschuwingen en voorzorgsmaatregelen
Lees vóór gebruik aandachtig alle waarschuwingen, voorzorgsmaatregelen en gebruiksinstructies. Als u niet
alle instructies leest en opvolgt of niet alle gegeven waarschuwingen in acht neemt, kan de patiënt
ernstig letsel oplopen of zelfs overlijden.
1.10.1. Waarschuwingen
■
Lees vóór gebruik van dit systeem de gebruikershandleiding bij het betreffende model van de extracorporele
Bio-Console-bloedpompconsole.
■
Tijdens de perfusie dient een vervangende transducer klaar te staan. Als de transducer tijdens een
procedure moet worden vervangen, volgt u de instructies in het hoofdstuk Noodsituaties.
1.10.2. Voorzorgsmaatregelen
■
De wegwerp-inserts zijn ontworpen voor eenmalig gebruik bij één patiënt. Niet hergebruiken, herverwerken
of hersteriliseren. Door het opnieuw gebruiken, herverwerken of hersteriliseren kan het product worden
besmet en/of de structuur ervan worden aangetast, hetgeen kan resulteren in letsel, ziekte of overlijden van
de patiënt.
■
Als u de insert moet bevochtigen terwijl u de tubing aansluit, gebruik dan uitsluitend steriel water of
zoutoplossing. Gebruik geen vloeistoffen op basis van alcohol op de wegwerp-insert.
■
Voordat u de verpakking van de insert opent, inspecteert u de verpakking zorgvuldig. Als de verpakking
beschadigd of geopend is of als de insert beschadigd lijkt te zijn, gebruik de insert dan niet. Retourneer de
insert naar Medtronic.
■
Inspecteer de transducer, kabel en kabelconnector op mechanische schade. Als er schade wordt gevonden,
gebruik dan een vervangende transducer en neem contact op met de technische dienst van Medtronic.
■
Gebruik de transducers alleen met de compatibele wegwerp-inserts (zie Tabel 1). Als de verkeerde
wegwerp-insert gebruikt wordt, kan de transducer beschadigd raken.
■
Ga zorgvuldig met de transducers om. Vermijd scherpe buigingen, knikken, verdraaiingen en andere druk
op de transducerkabel. Houd de transducer voorzichtig vast en draag deze voorzichtig; draag de transducer
niet aan de kabel. Gebruik geen scalpels, vaatklemmen, tubingklemmen of andere chirurgisch gereedschap
op de transducer of de transducerkabel.
■
Sluit voor optimale flownauwkeurigheid een gevuld Bio-Probe-flowbewakingssysteem op de console aan.
■
Controleer of de pinnen van de transducer recht en schoon zijn.
■
Zorg dat de pinnen van de wegwerp-insert contact maken met de transducerpinnen (onder het
transducerdeksel).
■
Werp de producten weg volgens lokaal ziekenhuis-, administratie- en ander overheidsbeleid.
Gebruiksaanwijzing Nederlands 59

2. Montage
1
2
Sluit een gemonteerd en gevuld Bio-Probe-flowbewakingssysteem op de console aan voordat u een patiënt op
cardiopulmonale bypass zet. Raadpleeg de gebruikershandleiding van de desbetreffende extracorporele BioConsole-bloedpompconsole voor instructies voor het inschakelen van de console en het aansluiten van het BioProbe-flowbewakingssysteem op de console.
Let op: Controleer voordat u het systeem monteert of er ook daadwerkelijk een wegwerp-insert voor
volwassenen met de transducer Model TX50 voor volwassenen wordt gebruikt. Als er met de transducer voor
volwassenen een wegwerp-insert voor kinderen wordt gebruikt, kan de transducer beschadigd raken wanneer
het deksel wordt gesloten. Zie Tabel 1.
1. Voor volwassenen
2. Voor kinderen
Afbeelding 1
1. Gebruik een steriele techniek om de wegwerp-insert uit de steriele verpakking te halen.
Opmerking: De afstand tussen de pinnen bij de wegwerp-insert voor volwassenen is anders dan die bij de
insert voor kinderen.
60 Gebruiksaanwijzing Nederlands

Afbeelding 2
2. Sluit de arteriële lijn op de ene kant van de wegwerp-insert aan.
Let op: Gebruik uitsluitend steriele zoutoplossing of steriel water om de tubing en de insert te bevochtigen.
Gebruik geen alcohol of vloeistoffen op basis van alcohol.
Afbeelding 3
3. Blijft steriel werken en sluit een tubing van ten minste 15 cm (6 inch) aan op de andere kant van de
wegwerp-insert (a) en de uitgang van de centrifugaalpomp (b).
Gebruiksaanwijzing Nederlands 61

a
b
Afbeelding 4
4. Duw het transducerdeksel open en plaats de wegwerp-insert in de juiste vloeistofstroomrichting zoals
aangeduid op het transducerdeksel.
Opmerking: Als de transducer in een verkeerde richting ten opzichte van de vloeistoftraject wordt geplaatst,
wordt er een negatieve flowwaarde op de console weergegeven.
Afbeelding 5
5. Duw de insert stevig in de transducer.
62 Gebruiksaanwijzing Nederlands

Afbeelding 6
6. Druk het transducerdeksel dicht tot de vergrendeling vastklikt.
Afbeelding 7
Het Bio-Probe-flowbewakingssysteem is nu gemonteerd. Raadpleeg de gebruikershandleiding van de
extracorporele Bio-Console-bloedpompconsole voor instructies voor het aansluiten van de transducer, het
vullen van het circuit (met een gebalanceerde elektrolytische of normale zoutoplossing) en het kalibreren van de
transducer.
3. Noodsituaties
Mocht de transducer niet meer werken, vervang deze dan door een vervangende tranducer:
1. Koppel de niet-werkende transducer los van de console en sluit de vervangende transducer aan op de
console.
2. Verwijder de wegwerp-insert met aangesloten tubing uit de niet-werkende transducer.
Gebruiksaanwijzing Nederlands 63

Afbeelding 8
3. Duw het transducerdeksel van de vervangende transducer open. Plaats de wegwerp-insert in de juiste
vloeistofstroomrichting zoals aangeduid op het transducerdeksel van de werkende transducer.
Opmerking: Als de transducer in een verkeerde richting ten opzichte van de vloeistoftraject wordt geplaatst,
wordt er een negatieve flowwaarde op de console weergegeven.
Afbeelding 9
4. Duw de insert stevig in de vervangende transducer.
64 Gebruiksaanwijzing Nederlands

Afbeelding 10
5. Druk het transducerdeksel dicht tot de vergrendeling vastklikt.
Afbeelding 11
Het Bio-Probe-flowbewakingssysteem is nu gemonteerd met de vervangende transducer. Raadpleeg de
gebruikershandleiding van de extracorporele Bio-Console-bloedpompconsole voor instructies voor het
kalibreren van de transducer.
Gebruiksaanwijzing Nederlands 65

4. Problemen oplossen
Situatie Oorzaak Actie
Knipperend digitaal
display
Negatieve flowmeterwaarde bij een
bevestigde positieve pomp-output
of -flow
Andere problemen Onbekend Neem contact op met de technische dienst van
De primevloeistof is geen gebalanceerde
elektrolytische of normale zoutoplossing.
Het contact tussen de pinnen van de
wegwerp-insert en de pinnen onder het
transducerdeksel is verstoord.
De pinnen onder het transducerdeksel
zijn niet goed uitgelijnd.
De transducerkabel is niet goed aangesloten.
Er is interferentie van een cauterisatieeenheid, lamp of andere elektrische
stroom in de omgeving.
De pinnen van de wegwerp-insert of de
pinnen in het transducerdeksel zijn vochtig.
De transducer zit verkeerd om in de
vloeistoftraject. (Let op de pijl op het
transducerdeksel.)
Het flowdisplay is niet correct op nul
gezet.
Gebruik een gebalanceerde elektrolytische of
normale zoutoplossing als primevloeistof.
Zorg ervoor dat het deksel van de transducer
(op de wegwerp-insert) goed is gesloten.
Zorg ervoor dat er goed contact is tussen de
pinnen van de transducer en de wegwerpinsert.
Controleer of de transducerpennen vrij zijn van
vuil en vloeistof.
Gebruik de juiste maat wegwerp-insert voor dit
transducermodelnummer.
Zorg ervoor dat de transducerkabel in de console is gestoken.
Controleer de transducerkabel op breuken.
Ga na wat de oorzaak is en verhelp deze zo
mogelijk.
Droog de pennen.
Open het transducerdeksel, til de wegwerpinsert eruit, draai de transducer (of de wegwerp-insert) 180°, plaats de wegwerp-insert
terug onder het transducerdeksel en kalibreer
de transducer opnieuw.
Raadpleeg de procedure voor het kalibreren
('op nul zetten') van de flowtransducer in de
desbetreffende gebruikershandleiding voor de
extracorporele Bio-Console-bloedpompconsole.
Medtronic.
5. Reiniging
De transducer werkt efficiënter en gaat langer mee als deze wordt gereinigd en bewaard zoals beschreven:
1. Open het transducerdeksel na een perfusieprocedure en verwijder de wegwerp-insert.
Let op: De wegwerp-insert mag nooit opnieuw gesteriliseerd of hergebruikt worden.
66 Gebruiksaanwijzing Nederlands

Afbeelding 12
2. Reinig de transducer, transducerpennen (onder het transducerdeksel) en de kabel door deze af te vegen
met een met water en milde zeep bevochtigde doek. Vermijd sterke en schurende reinigingsmiddelen. Veeg
de zeep eraf met een met zuiver water bevochtigde doek of spons.
Opmerking: Dompel geen enkel onderdeel van de transducer, kabel of kabelconnector onder.
Afbeelding 13
Opmerking: De transducer hoeft niet te worden gesteriliseerd.
3. Droog de transducer, transducerpennen en kabel met een doek af.
4. Controleer of de pinnen onder het transducerdeksel recht zijn.
Als een pin niet recht is, neemt u contact op met de technische dienst van Medtronic.
5. Bewaar de transducer op de transducersteun of koppel de transducer los en plaats deze in de
beschermende plastic hoes.
Gebruiksaanwijzing Nederlands 67

Instructies voor het gebruik van de transducersteun vindt u in de gebruikershandleiding van het desbetreffende
model van de extracorporele Bio-Console-bloedpompconsole.
6. Onderhoud
Als de transducer beschadigd is, neemt u contact op met de klantenservice van Medtronic. U moet goedkeuring
van Medtronic krijgen alvorens een transducer ter reparatie te retourneren.
Opmerking: Repareer de transducer niet, daardoor vervalt de garantie.
Medtronic beschikt over een professionele staf die technische adviezen kan verstrekken aan de gebruikers van
haar producten. Als u antwoord op een vraag wilt krijgen of producttraining wilt plannen, neem dan contact op
met Medtronic.
7. Beperkte garantie bij apparatuur
(VOOR REPAREERBARE EXTERNE APPARATUUR/BUITEN DE VS)
De volgende beperkte garantie 3 geldt alleen voor klanten buiten de Verenigde Staten:
A. Deze BEPERKTE GARANTIE geeft de koper van de flowtransducers Model TX50 en TX50P van Medtronic
voor het Bio-Probe-flowbewakingssysteem, hierna de “Transducer” genoemd, de verzekering dat, indien de
Transducer niet volgens de specificaties functioneert binnen een periode van 1 jaar vanaf de afleverdatum
van de Transducer aan de koper door een defect in het materiaal of door een fabricagefout, Medtronic naar
keuze: (a) het defecte onderdeel/de defecte onderdelen van de Transducer kan repareren of vervangen;
(b) een vergoeding kan toekennen gelijk aan de oorspronkelijke koopprijs (doch niet hoger dan de waarde
van de vervangende Transducer) voor de aankoop van een vervangende Transducer, of (c) kosteloos een
functioneel vergelijkbare Transducer kan verstrekken.
B. Om in aanmerking te komen voor deze reparatie, vervanging of vergoeding, moet aan de volgende
voorwaarden zijn voldaan:
(1) De Transducer moet binnen 60 dagen nadat het defect is opgemerkt, worden geretourneerd aan
Medtronic. (Medtronic kan naar haar keuze de Transducer ter plaatse repareren).
(2) De Transducer mag niet zijn gerepareerd of veranderd door iemand anders dan Medtronic, zodanig dat,
volgens het oordeel van Medtronic, de degelijkheid en betrouwbaarheid van de Transducer aangetast
worden.
(3) De Transducer mag niet zijn blootgesteld aan verkeerd gebruik, misbruik of onregelmatigheden.
C. Deze BEPERKTE GARANTIE is beperkt tot de uitdrukkelijk vermelde voorwaarden. In het bijzonder is
Medtronic niet verantwoordelijk voor enige incidentele of gevolgschade, veroorzaakt door om het even welk
gebruik, defect of falen van de Transducer, ongeacht of de vordering is gebaseerd op een garantie,
contract, onrechtmatige daad of anderszins.
D. De uitsluitingen en beperkingen die hierboven uiteengezet zijn, zijn niet bedoeld, en moeten niet
geïnterpreteerd worden als een inbreuk op dwingende bepalingen van de van toepassing zijnde wet. Indien
enig onderdeel of enige bepaling van deze BEPERKTE GARANTIE door een daartoe bevoegde rechtbank
als onrechtmatig, onuitvoerbaar of in strijd met de van toepassing zijnde wet beschouwd wordt, zal dit de
geldigheid van het overige deel van deze BEPERKTE GARANTIE niet aantasten en zullen alle rechten en
plichten worden uitgelegd en ten uitvoer worden gebracht alsof deze BEPERKTE GARANTIE het
desbetreffende ongeldig verklaarde gedeelte niet bevatte.
8. Beperkte garantie
(VOOR WEGWERP-PRODUCTEN/BUITEN DE VS)
De volgende beperkte garantie 4 geldt alleen voor klanten buiten de VS:
3
Deze Beperkte Garantie wordt gegeven door Medtronic, 710 Medtronic Parkway, Minneapolis, MN 55432. Klanten buiten de
Verenigde Staten kunnen contact opnemen met hun lokale Medtronic-vertegenwoordiger voor meer informatie over de
voorwaarden van de Beperkte Garantie.
68 Gebruiksaanwijzing Nederlands

A. Deze BEPERKTE GARANTIE geeft de koper van de Bio-Probe-wegwerp-inserts van Medtronic voor het
Bio-Probe-flowbewakingssysteem, hierna het "Product" genoemd, de verzekering dat, indien het Product
niet volgens de specificaties functioneert, Medtronic een vergoeding zal toekennen gelijk aan de
oorspronkelijke koopprijs (doch niet hoger dan de waarde van het vervangende product) voor de
vervangingsaankoop van enig Medtronic-product bestemd voor de desbetreffende patiënt. DE
WAARSCHUWINGEN IN DE PRODUCTDOCUMENTATIE VORMEN EEN INTEGRAAL ONDERDEEL
VAN DEZE BEPERKTE GARANTIE. NEEM CONTACT OP MET MEDTRONIC VOOR INFORMATIE OVER
DE WIJZE WAAROP AANSPRAKEN KRACHTENS DEZE BEPERKTE GARANTIE BEHANDELD MOETEN
WORDEN.
B. Om in aanmerking te komen voor de BEPERKTE GARANTIE, moet aan de volgende voorwaarden zijn
voldaan:
(1) Het Product moet gebruikt zijn vóór het verstrijken van de uiterste gebruiksdatum.
(2) Het Product moet binnen 60 dagen na gebruik worden geretourneerd aan Medtronic en zal eigendom
worden van Medtronic.
(3) Het Product mag niet voor een andere patiënt zijn gebruikt.
C. Deze BEPERKTE GARANTIE is beperkt tot de uitdrukkelijk vermelde voorwaarden. In het bijzonder:
(1) In geen geval zal een vervangingsvergoeding worden toegekend wanneer er tekenen zijn van
oneigenlijk hanteren, oneigenlijk gebruik of wijzigingen in het Product.
(2) Medtronic is niet verantwoordelijk voor welke incidentele of gevolgschade dan ook, voortkomend uit om
het even welk gebruik, defect of falen van het Product, ongeacht of de vordering is gebaseerd op een
garantie, contract, onrechtmatige daad of anderszins.
D. De uitsluitingen en beperkingen die hierboven uiteengezet zijn, zijn niet bedoeld, en moeten niet
geïnterpreteerd worden als een inbreuk op dwingende bepalingen van de van toepassing zijnde wet. Indien
enig onderdeel of enige bepaling van deze BEPERKTE GARANTIE door een daartoe bevoegde rechtbank
als onrechtmatig, onuitvoerbaar of in strijd met de van toepassing zijnde wet beschouwd wordt, zal dit de
geldigheid van het overige deel van deze BEPERKTE GARANTIE niet aantasten en zullen alle rechten en
plichten worden uitgelegd en ten uitvoer worden gebracht alsof deze BEPERKTE GARANTIE het
desbetreffende ongeldig verklaarde gedeelte niet bevatte.
4
Deze Beperkte Garantie wordt gegeven door Medtronic, 710 Medtronic Parkway, Minneapolis, MN 55432. Klanten buiten de
Verenigde Staten kunnen contact opnemen met hun lokale Medtronic-vertegenwoordiger voor meer informatie over de
voorwaarden van de Beperkte Garantie.
Gebruiksaanwijzing Nederlands 69

Medtronic, Inc.
*M973271A001*
710 Medtronic Parkway
Minneapolis, MN 55432
USA
www.medtronic.com
+1 763 514 4000
LifeLine Technical Support, 24 hour consultation
service:
1 877 526 7890
Medtronic Perfusion Systems
7611 Northland Drive
Minneapolis, MN 55428
USA
+1 763 391 9000
Customer service and product orders:
1 800 854 3570
www.perfusionsystems.com
Medtronic B.V.
Earl Bakkenstraat 10
6422 PJ Heerlen
The Netherlands
+31 45 566 8000
Australia
Medtronic Australasia Pty Ltd
5 Alma Road
Macquarie Park, NSW 2113
Australia
1800 668 670
Canada
Medtronic of Canada Ltd
99 Hereford Street
Brampton, Ontario L6Y 0R3
Canada
1 800 268 5346
© 2004-2017 Medtronic
M973271A001 Rev. 1A
 Loading...
Loading...