Medtronic 8626, 8627L, 8626L, 8627 User Manual
SYNCHROMED® EL
8626
Programmable pumps
8626L
8627
8627L
Implant manual
! USA
Rx only
1999

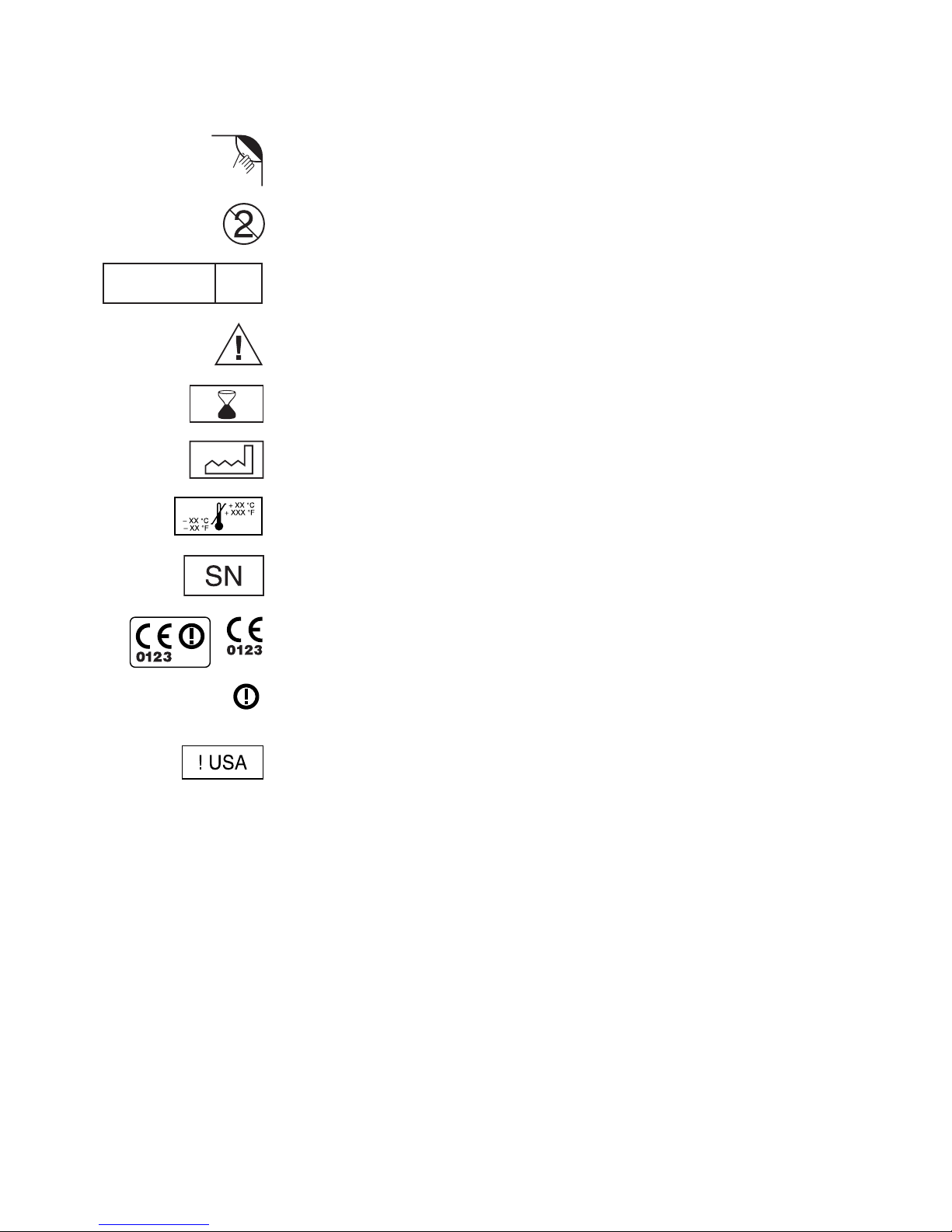
Explanation of symbols on product or package labeling
Refer to the appropriate product for symbols that apply.
Open here
Do not reuse
Sterilization: ethylene-oxide gas
STERILE
EO
Caution, consult accompanying documents
Use by
Manufacturing date
Storage temperature
Serial number
Conformité Européenne (European Conformity). This symbol means
that the device fully complies with AIMD Directive 90/385/EEC and
R&TTE Directive 1999/5/EC.
The use of this device might be subject to individual country licensing
regimes in Europe.
For USA audiences only
1

Medtronic® and SynchroMed® are registered trademarks of Medtronic, Inc.
2

Table of contents
Device description 5
Package contents 6
Patient identification card and registration 6
Device specifications 7
Device longevity 10
Flow rate accuracy 14
Declaration of conformity 14
Instruction for use 15
Preparing for pump implant 15
Sterile procedure 17
Emptying the pump 17
Preparing to fill the pump 18
Filling the pump 18
Replacing an implanted pump 19
Preparing the pump pocket 21
Implanting the pump 21
Programming the pump 22
Refilling the pump 23
Accessing the catheter access port 23
Appendix A — Refilling the pump 25
Contraindication 25
Preliminary procedures 25
Emptying the pump 26
Performing a reservoir rinse 27
Refer to the Indications, Drug Stability, and Emergency Procedures, reference
manual for indications and related information.
Refer to the appropriate information for prescribers booklet for contraindications,
warnings, precautions, adverse events summary, individualization of treatment,
patient selection, use in specific populations, and component disposal.
Refer to the appropriate drug labeling for indications, contraindications, warnings,
precautions, dosage and administration information, and screening procedures.
8626/8627 2005-11 English 3

Refilling the pump 28
Programming the pump 30
After the refill procedure 30
Appendix B — Accessing the catheter access port 31
Contraindications 31
Preliminary procedures 32
Preparing the access site 32
Intraspinal applications only 32
Vascular applications only 34
Flushing the catheter access port 35
Clearing a catheter occlusion 36
4 English 8626/8627 2005-11

Device description
The implantable Medtronic SynchroMed EL programmable pump is part of an infusion system
that stores and delivers a prescribed drug to a specific site. The implanted infusion system
consists of a SynchroMed EL pump and a catheter.
The catheter connects to the pump catheter port. The pump is anchored in the pump pocket
using the suture loops on the outside of pump models 8626L-10, 8626L-18, 8627L-10, and
8627L-18. Pump models 8626-10, 8626-18, 8627-10, and 8627-18 are anchored to the pump
pocket using a mesh pouch.
The drug is stored in the pump reservoir. Per a programmed prescription, the drug moves from
the pump reservoir, through the pump tubing, catheter port, and catheter, to the infusion site.
Pump models 8627-10, 8627-18, 8627L-10, and 8627L-18 have a catheter access port (CAP)
(Figure 1). The CAP allows injection of drug directly into the implanted catheter for drug
administration and diagnostic purposes. Drug injected into the CAP bypasses the pump
mechanism and goes directly through the catheter port into the implanted catheter to the
infusion site. The CAP allows entry of a 25-gauge noncoring needle to prevent accidental
injection during refill procedures (which use the 22-gauge noncoring needle supplied in the
refill kit).
Catheter
access port
Pump with catheter access port Pump without catheter access port
Catheter port
Reservoir fill
port
Figure 1. Pump exterior view.
8626/8627 2005-11 English 5

Package contents
■
Pump
■
Needle, 22-gauge (black sheath)
■
Needle, 25-gauge (orange sheath) (models 8627-10, 8627-18, 8627L-10, and 8627L-18)
■
Mesh pouch (models 8626-10, 8626-18, 8627-10, and 8627-18)
■
Product literature
■
Registration form
■
Patient identification card
■
c
Warranty card
Patient identification card and registration
A patient identification card is packaged with this device. Advise the patient to carry the
identification card at all times.
c
The patient identification card packaged with the device is temporary; a permanent card
is mailed to the patient when Medtronic receives the registration form.
c
The implant registration form registers the device warranties and creates a record of the
device in the Medtronic implant data system.
6 English 8626/8627 2005-11

Device specifications
Table 1. Shipping and operating values for the SynchroMed EL pumps
Shipping Operating
Fluid in reservoir 10 mL models:
minimum 8 mL sterile
water when
manufactured
18 mL models:
minimum 15 mL sterile
water when
manufactured
Shipping flow rate 0.007 to 0.012 mL/day
Infusion modes
Simple continuous Single bolus
a
a
Priming bolus
Purge bolus
Stopped pump
Periodic bolus
Simple continuous
Complex continuous
Bridge bolus and simple continuous
Priming bolus and simple continuous
Single bolus and simple continuous
Alarms
Low battery alarm
Low reservoir alarm
Pump memory error
Disabled
Enabled
Enabled
Enabled/disabled
Enabled/postponed
Enabled
Audible alarms Enabled Enabled/disabled
a
The volume of sterile water in the pump reservoir when the package is opened depends on the amount of
time that has elapsed since the pump was manufactured (the more time that has elapsed, the less sterile
water will be in the pump reservoir). The volume of sterile water that is removed from the pump reservoir
before implant will be at least the amount that the programmer calculates is in the pump during the initial
interrogation.
8626/8627 2005-11 English 7

Table 2. Device specifications for the SynchroMed EL 10 mL pumps
a
Models 8626-10 and
8626L-10
Models 8627-10 and
8627L-10
Pump
Thickness
Weight (empty)
Displacement volume
Diameter (without CAP or
21.6 mm
165 g
83.1 mL
70.4 mm
21.6 mm
185 g
105.0 mL
70.4 mm
silicone rubber surface pad)
Diameter (with CAP or silicone
85.2 mm
85.2 mm
rubber surface pad)
Pump reservoir
Volume
Residual volume
10.0 mL
1.2 mL
10.0 mL
1.2 mL
Pump tubing
Volume 0.23 or 0.32 mL 0.26 or 0.36 mL
Reservoir fill port
Septum puncture life 500 punctures 500 punctures
Catheter access port
Prime volume
Septum puncture life
Recommended maximum
injection rate
-----
-----
-----
0.4 mL
2000 punctures
15 mL/min saline/drug;
3 mL/min radiopaque
compounds
Flow rate
Maximum programmable
Minimum programmable
Stopped pump maximum
b
c
21.6 mL/day
0.048 mL/day
0.120 mL/day
21.6 mL/day
0.048 mL/day
0.120 mL/day
leakage
Bacterial retentive filter
Pore size 0.22 µm (micron) 0.22 µm (micron)
Power source
Battery
Longevity
a
All measurements are approximate.
b
Actual limits depend on pump calibration constant and selected infusion mode. The programmer may further
narrow these limits.
c
At rates less than 0.048 mL/day, the flow accuracy may exceed the ±15% specification.
Lithium thionyl-chloride
Rate dependent
(Figure 3)
Lithium thionyl-chloride
Rate dependent
(Figure 3)
8 English 8626/8627 2005-11

Table 3. Device specifications for the SynchroMed EL 18 mL pumps
a
Models 8626-18 and
8626L-18
Models 8627-18 and
8627L-18
Pump
Thickness
Weight (empty)
Displacement volume
Diameter (without CAP or
27.5 mm
185 g
107.0 mL
70.4 mm
27.5 mm
205 g
125.0 mL
70.4 mm
silicone rubber surface pad)
Diameter (with CAP or silicone
85.2 mm
85.2 mm
rubber surface pad)
Pump reservoir
Volume
Residual volume
18.0 mL
2.4 mL
18.0 mL
2.4 mL
Pump tubing
Volume 0.23 or 0.32 mL 0.26 or 0.36 mL
Reservoir fill port
Septum puncture life
500 punctures 500 punctures
Catheter access port
Prime volume
Septum puncture life
Recommended maximum
injection rate
-----
-----
-----
0.4 mL
2000 punctures
15 mL/min
saline/drug; 3 mL/min
radiopaque compounds
Flow rate
Maximum programmable
Minimum programmable
Stopped pump maximum
b
21.6 mL/day
0.048 mL/day
0.120 mL/day
21.6 mL/day
0.048 mL/day
0.120 mL/day
leakage
Bacterial retentive filter
Pore size 0.22 µm (micron) 0.22 µm (micron)
Power source
Battery
Longevity
a
All measurements are approximate.
b
Actual limits depend on pump calibration constant and selected infusion mode. The programmer may further
narrow these limits.
Lithium thionyl-chloride
Rate dependent
(Figure 2)
Lithium thionyl-chloride
Rate dependent
(Figure 2)
8626/8627 2005-11 English 9
Table 4. Material of components in the SynchroMed EL sterile package
Component Material Material contacts
Material contacts
human tissue
Pump
Exterior Titanium Yes No
Reservoir Titanium No Yes
Reservoir valve Titanium No Yes
Tubing Silicone rubber No Yes
Reservoir fill port
Silicone rubber Yes Yes
septum
Catheter access
port septum
a
Silicone rubber Yes Yes
Catheter port Titanium Yes Yes
Bacterial
retentive filter
Suture loops
b
Polyvinylidene
No Yes
fluoride
Titanium Yes No
Propellant Inert gas No No
drug
Needles Stainless steel Yes Yes
a
Models 8627-10, 8627-18, 8627L-10, and 8627L-18
b
Models 8626L-10, 8626L-18, 8627L-10, and 8627L-18
Device longevity
Pump service life is a function of flow rate and shelf time. Shelf time is the number of months
between the date of manufacture and the implant date. (The date of manufacture is located on
the package label.) Service life is the number of months from implant to pump stoppage due to
battery depletion. With a higher flow rate and longer shelf time, service life is reduced
(Figure 2).
To determine service life, first determine the shelf time and flow rate. Then refer to
Figures 3 to 6 to read service life for 15-, 24-, 36-, or 48-month shelf time.
10 English 8626/8627 2005-11
 Loading...
Loading...