
Reduced Profile
X Small (1.4cc) & XX Small (0.7cc)
INFUSE® Bone Graft Kits ONLY
IMPORTANT MEDICAL INFORMATION
In USA
Customer Service Division Telephone: 800-933-2635
Medtronic Sofamor Danek USA, Inc
. or
1800 Pyramid Place 901 396 3133
Memphis, Tennessee 38132 Telefax: 901 396 0356
USA
Supplied by
Medtronic Sofamor Danek USA, Inc.
2015-06-10
and XX Small (0.7cc) INFUSE® Bone Graft kit for the corresponding Medtronic Titanium Threaded
Interbody Fusion Device component size:
SINGLE CAGE USE
In the case of only one cage needing INFUSE® Bone Graft, due to the loss or contamination of a
sponge or sponges, single cages can be filled using the X Small (1.4cc) and/or XX Small (0.7cc)
kits as shown below:
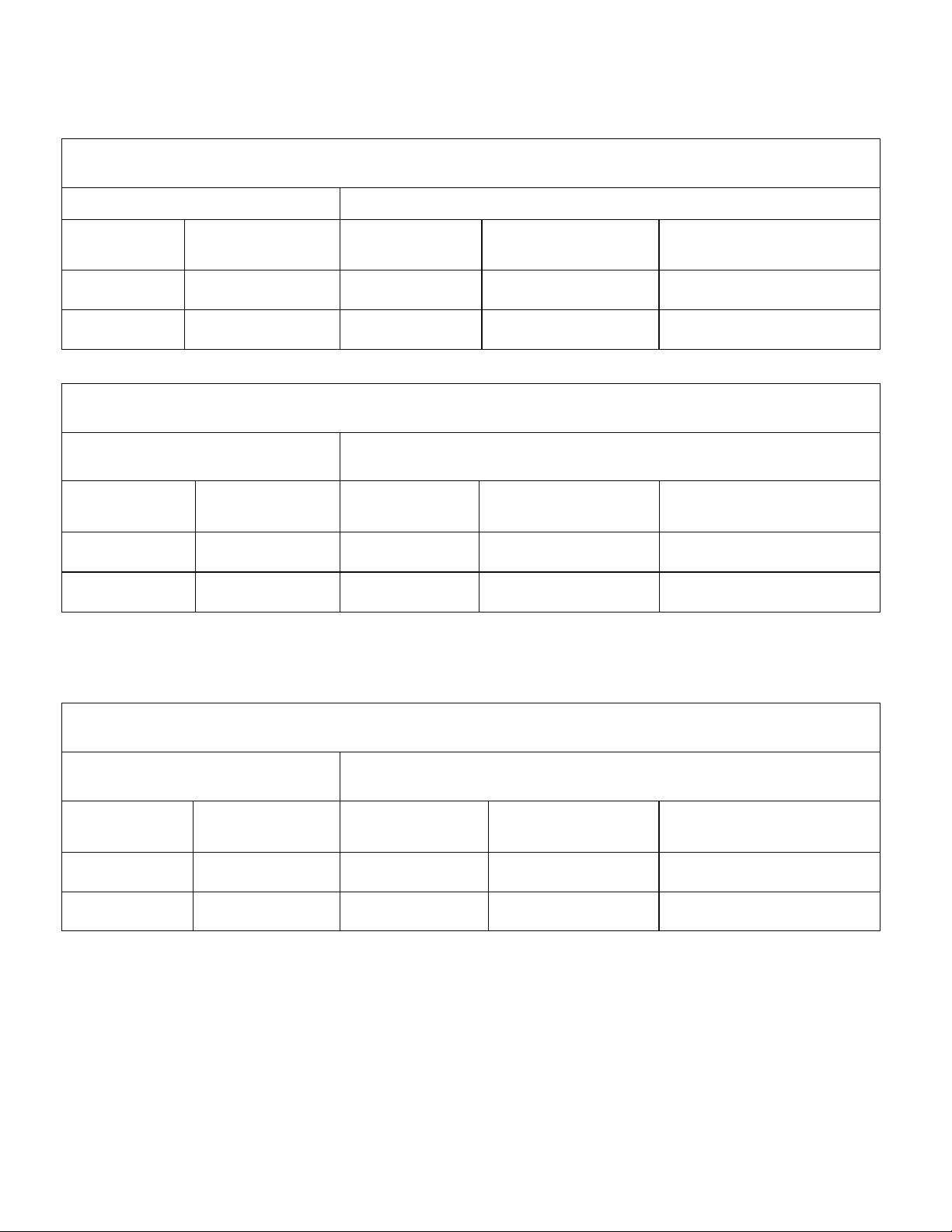
INTER FIX™ Threaded Fusion Device Recommended INFUSE® Bone Graft Kit(s)
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume
(cc)
890120 12x20 7510100 X Small 1.4
890125 12x25
7510100
INTER FIX™ and INTER FIX™ RP
Threaded Fusion Devices
Part # of
INTER FIX™
Devices
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
890120+9011221 12x20 7510100 X Small 1.4
890125+9011225 12x25
†
The INTER FIX™ Threaded Fusion Device is to be used with the corresponding size of the INTER FIX™ RP Threaded
Fusion Device.
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume
(cc)
9011221 12x20 7510100 X Small 1.4
9011225 12x25
7510100
Part #
Size
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
8941420 14x20 7510100 X Small 1.4
8941423 14x23 7510100 X Small
8941620 16x20
7510050 +
INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device Combinations
Single LT-CAGE® Device Fill
LT-CAGE® Lumbar
Tapered Fusion Device
Recommended INFUSE® Bone Graft Kit(s)
Part #
Size
(lead diameter, mm
x length, mm)
Part #
Kit size
Reconstituted
rhBMP-2/ACS
graft volume
(cc)
8941420
14x20
7510100
X Small
1.4
8941423
14x23
7510100
X Small
1.4
8941620
16x20
7510050 +
7510100
XX Small + X Small
2.1
8941623
16x23
7510050 +
7510100
XX Small + X Small
2.1
M705106B018E Rev. D
INFUSE
INFUSE
®
BONE GRAFT/INTER FIX™ RP THREADED FUSION DEVICE - REDUCED PROFILE
®
BONE GRAFT/LT-CAGE® LUMBAR TAPERED FUSION DEVICE
INFUSE
®
BONE GRAFT/INTER FIX™ THREADED FUSION DEVICE
X Small (1.4cc) & XX Small (0.7cc) INFUSE® Bone Graft Kits ONLY
GREFFON OSSEUX INFUSE®/DISPOSITIF CONIQUE DE FUSION LOMBAIRE LT-CAGE
GREFFON OSSEUX INFUSE®/DISPOSITIF DE FUSION FILETÉ INTER FIX™
®
GREFFON OSSEUX INFUSE®/DISPOSITIF DE FUSION FILETÉ À PROFIL RÉDUIT INTER FIX™ RP
Kits de greffon osseux INFUSE® XS (1,4 cc) et XXS (0,7 cc) UNIQUEMENT
IN USA
2015-06-10
Customer Service Division Telephone: 800-933-2635
Medtronic Sofamor Danek USA or
1800 Pyramid Place 901-396-3133
Memphis, Tennessee 38132 Telefax: 901-396-0356
USA
ENGLISH
Supplied by
Medtronic Sofamor Danek USA, Inc.
IMPORTANT MEDICAL INFORMATION
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician with
appropriate training.
The following contains important medical information on the use of INFUSE® Bone Graft with a variety
of Medtronic metallic interbody fusion cages. These interbody fusion devices include the LT-CAGE®
Lumbar Tapered Fusion Device, the INTER FIX™ Threaded Fusion Device, and the INTER FIX™
RP Threaded Fusion Device. Hereafter in this insert, these cages will be referred to collectively as
Medtronic Titanium Threaded Interbody Fusion Device.
DESCRIPTION
The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device consists of two
components containing three parts – a metallic spinal fusion cage, a recombinant human bone
morphogenetic protein, and a carrier/scaffold for the bone morphogenetic protein and resulting bone.
The INFUSE® Bone Graft component is inserted into the Medtronic Titanium Threaded Interbody
Fusion Device component to form the complete INFUSE® Bone Graft/Medtronic Titanium Threaded
Interbody Fusion Device. These components must be used as a system for the prescribed
indication described below. The bone morphogenetic protein solution component must
not be used without the carrier/scaffold component or with a carrier/scaffold component
different from the one described in this document. The INFUSE® Bone Graft component must
not be used without the Medtronic Titanium Threaded Interbody Fusion Device component.
Medtronic Titanium Threaded Interbody Fusion Device Component
LT-CAGE® Lumbar Tapered Fusion Device
The LT-CAGE® Device consists of a hollow, perforated, machined cylinder with opposing flat sides.
The cage has a tapered design with an angle of 8.8° and is available in diameters ranging from
14mm to 18mm at the narrow end of the taper to 17mm to 22 mm at the wide end of the taper and
in lengths ranging from 20mm to 26mm. There are two holes on each of the two flat sides. On each
of the two rounded aspects, there is a single rounded slot. The implants have a helical screw thread
on the outer surface. One end of the device is closed. The other end is open to be filled with the
INFUSE® Bone Graft component.
The LT-CAGE® implants are made from implant-grade titanium alloy (Ti-6Al-4V) described by such
standards as ASTM F136 or its ISO equivalent.

in lengths ranging from 20mm to 26mm. There are two holes on each of the two flat sides. On each
of the two rounded aspects, there is a single rounded slot. The implants have a helical screw thread
on the outer surface. One end of the device is closed. The other end is open to be filled with the
INFUSE® Bone Graft component.
The LT-CAGE® implants are made from implant-grade titanium alloy (Ti-6Al-4V) described by such
standards as ASTM F136 or its ISO equivalent.
CONTRAINDICATIONS
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device is contraindicated
for patients with a known hypersensitivity to recombinant human Bone Morphogenetic Protein-2,
bovine Type I collagen or to other components of the formulation.
!USA
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume
(cc)
7510100
Part # of
Devices
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume
(cc)
7510100
Part #
Size
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
8941420 14x20 7510100 X Small 1.4
8941423 14x23 7510100 X Small
8941620 16x20
7510050 +
8941623 16x23
7510100
INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device Combinations
Single LT-CAGE® Device Fill
LT-CAGE® Lumbar
Tapered Fusion Device
Recommended INFUSE® Bone Graft Kit(s)
Part #
Size
(lead diameter, mm
x length, mm)
Part #
Kit size
Reconstituted
rhBMP-2/ACS
graft volume
(cc)
8941420
14x20
7510100
X Small
1.4
8941423
14x23
7510100
X Small
1.4
8941620
16x20
7510050 +
7510100
XX Small + X Small
2.1
8941623
16x23
7510050 +
7510100
XX Small + X Small
2.1
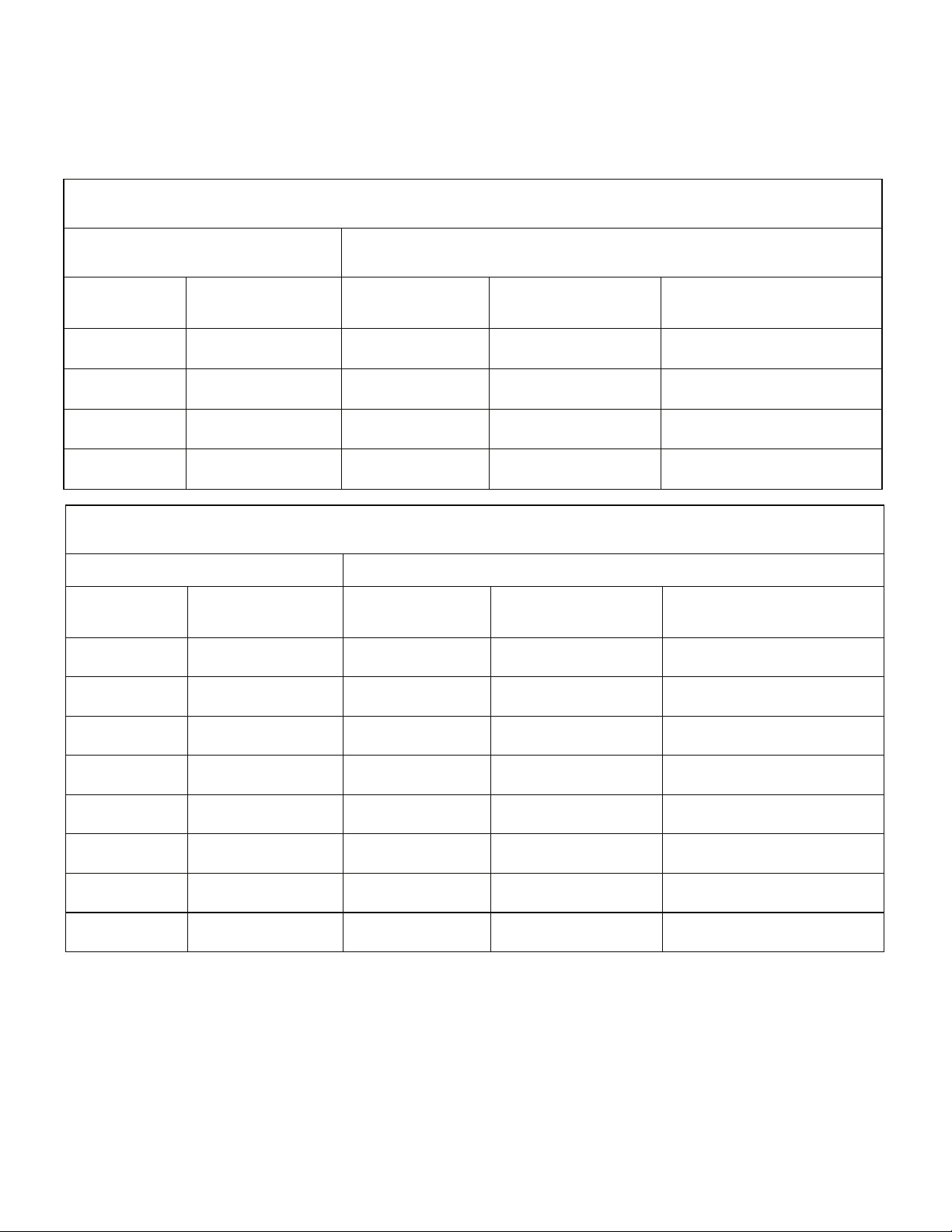
INTER FIX™ Threaded Fusion Device Recommended INFUSE® Bone Graft Kit(s)
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
890120 12x20 7510050 XX Small 0.7
890125 12x25 7510100 X Small 1.4
890140 14X20 7510100 X Small 1.4
890143 14X23 7510100 X Small 1.4
890146 14X26
7510050 +
890149 14X29
7510050 +
890160 16X20
7510050 +
890163 16X23
7510050 +
INTER FIX™ Threaded Fusion Device Recommended INFUSE® Bone Graft Kit(s)
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
9011221 12x20 7510050 XX Small 0.7
9011225 12x25 7510100 X Small 1.4
9011420 14X20 7510100 X Small 1.4
9011423 14X23 7510100 X Small 1.4
9011426 14X26
7510050 +
7510100
9011429 14X29
7510050 +
7510100
9011620 16X20
7510050 +
7510100
9011623 16X23
7510050 +
7510100
The LT-CAGE® Lumbar Tapered Fusion Device component is sold separately from the INFUSE®
Bone Graft component; however, these two components must be used together. The package
labeling for the LT-CAGE® Lumbar Tapered Fusion Device contains complete product information
for this component.
INTER FIX™ Threaded Fusion Device
The INTER FIX™ Device consists of a hollow, perforated, cylinder with parallel sides and an endcap.
The cage is available in diameters ranging from 12mm to 24mm and in lengths ranging from 20mm to
29mm. The endcaps of the INTER FIX™ cages are sized according to the diameter of the cylinders
and are applied to the open end of the cylinders after they are filled with INFUSE® Bone Graft.
The INTER FIX™ Threaded Fusion Device implants are made from implant-grade titanium alloy
(Ti-6Al-4V) described by such standards as ASTM F136 or its ISO equivalent.
The INTER FIX™ Threaded Fusion Device component is sold separately from the INFUSE® Bone
Graft component; however, these two components must be used together. The package labeling for
the INTER FIX™ Threaded Fusion Device contains complete product information for this component.
INTER FIX™ RP Threaded Fusion Device
The INTER FIX™ RP Device consists of a hollow, perforated, cylinder with a single, large, outerradiused groove along the entire longitudinal axis that extends into the inside diameter of the device.
Both ends of the INTER FIX™ RP implant are closed. The cage is available in diameters ranging
from 12mm to 24mm and in lengths ranging from 20mm to 29mm.
The INTER FIX™ RP Threaded Fusion Device implants are made from implant-grade titanium alloy
(Ti-6Al-4V) described by such standards as ASTM F136 or its ISO equivalent.
The INTER FIX™ RP Threaded Fusion Device component is sold separately from the INFUSE® Bone
Graft component; however, these two components must be used together. The package labeling for the
INTER FIX™ RP Threaded Fusion Device contains complete product information for this component.
NOTE: The INTER FIX™ Threaded Fusion Device and the INTER FIX™ RP Threaded Fusion
Device may be used together to treat a spinal level. LT-CAGE® Lumbar Tapered Fusion Device
implants are not to be used in conjunction with either the INTER FIX™ OR INTER FIX™ RP
implants to treat a spinal level.
INFUSE® Bone Graft Component
INFUSE® Bone Graft consists of recombinant human Bone Morphogenetic Protein-2 (rhBMP-2,
known as dibotermin alfa) placed on an absorbable collagen sponge (ACS). The INFUSE® Bone
Graft component induces new bone tissue at the site of implantation. Based on data from non-clinical
studies, the bone formation process develops from the outside of the implant towards the center until
the entire INFUSE® Bone Graft component is replaced by trabecular bone.
rhBMP-2 is the active agent in the INFUSE® Bone Graft component. rhBMP-2 is a disulfide-linked
dimeric protein molecule with two major subunit species of 114 and 131 amino acids. Each subunit
is glycosylated at one site with high-mannose-type glycans. rhBMP-2 is produced by a genetically
engineered Chinese hamster ovary cell line.
rhBMP-2 and excipients are lyophilized. Upon reconstitution, each milliliter of rhBMP-2 solution
contains: 1.5 mg of rhBMP-2; 5.0 mg sucrose, NF; 25 mg glycine, USP; 3.7 mg L-glutamic acid,
FCC; 0.1 mg sodium chloride, USP; 0.1 mg polysorbate 80, NF; and 1.0 mL of sterile water. The
reconstituted rhBMP-2 solution has a pH of 4.5, and is clear, colorless to slightly yellow, and essentially
free from plainly visible particulate matter.
The ACS is a soft, white, pliable, absorbent implantable matrix for rhBMP-2. ACS is made from
bovine Type I collagen obtained from the deep flexor (Achilles) tendon. The ACS acts as a carrier
for the rhBMP-2 and acts as a scaffold for new bone formation.

implants are not to be used in conjunction with either the INTER FIX™ OR INTER FIX™ RP
implants to treat a spinal level.
INFUSE® Bone Graft Component
INFUSE® Bone Graft consists of recombinant human Bone Morphogenetic Protein-2 (rhBMP-2,
known as dibotermin alfa) placed on an absorbable collagen sponge (ACS). The INFUSE® Bone
Graft component induces new bone tissue at the site of implantation. Based on data from non-clinical
studies, the bone formation process develops from the outside of the implant towards the center until
the entire INFUSE® Bone Graft component is replaced by trabecular bone.
rhBMP-2 is the active agent in the INFUSE® Bone Graft component. rhBMP-2 is a disulfide-linked
dimeric protein molecule with two major subunit species of 114 and 131 amino acids. Each subunit
is glycosylated at one site with high-mannose-type glycans. rhBMP-2 is produced by a genetically
engineered Chinese hamster ovary cell line.
rhBMP-2 and excipients are lyophilized. Upon reconstitution, each milliliter of rhBMP-2 solution
contains: 1.5 mg of rhBMP-2; 5.0 mg sucrose, NF; 25 mg glycine, USP; 3.7 mg L-glutamic acid,
FCC; 0.1 mg sodium chloride, USP; 0.1 mg polysorbate 80, NF; and 1.0 mL of sterile water. The
reconstituted rhBMP-2 solution has a pH of 4.5, and is clear, colorless to slightly yellow, and essentially
free from plainly visible particulate matter.
The ACS is a soft, white, pliable, absorbent implantable matrix for rhBMP-2. ACS is made from
bovine Type I collagen obtained from the deep flexor (Achilles) tendon. The ACS acts as a carrier
for the rhBMP-2 and acts as a scaffold for new bone formation.
CONTRAINDICATIONS
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device is contraindicated
for patients with a known hypersensitivity to recombinant human Bone Morphogenetic Protein-2,
bovine Type I collagen or to other components of the formulation.
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not
be used in the vicinity of a resected or extant tumor, in patients with any active malignancy or
patients undergoing treatment for a malignancy.
• INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not be
used in patients who are skeletally immature (<18 years of age or no radiographic evidence of
epiphyseal closure).
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not
be used in pregnant women. The potential effects of rhBMP-2 on the human fetus have not
been evaluated.
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not be
implanted in patients with an active infection at the operative site or with an allergy to titanium
or titanium alloy.
WARNINGS
• In an experimental rabbit study, rhBMP-2 has been shown to elicit antibodies that are capable
of crossing the placenta. Reduced ossification of the frontal and parietal bones of the skull was
noted infrequently (<3%) in fetuses of rabbit dams immunized to rhBMP-2; however, there was
no effect noted in limb bud development. There are no adequate and well-controlled studies in
human pregnant women. Women of child bearing potential should be warned by their surgeon
of potential risk to a fetus and informed of other possible orthopedic treatments.
• Women of childbearing potential should be advised that antibody formation to rhBMP-2 or its
influence on fetal development has not been completely assessed. In the clinical trial supporting
the safety and effectiveness of the INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion
Device, 2/277 (0.7%) patients treated with INFUSE® Bone Graft component and 1/127 (0.8%)
patients treated with autograft bone developed antibodies to rhBMP-2. The effect of maternal
antibodies to rhBMP-2, as might be present for several months following device implantation,
on the unborn fetus is unknown. Additionally, it is unknown whether fetal expression of BMP-2
could re-expose mothers who were previously antibody positive. Theoretically, re-exposure
may elicit a more powerful immune response to BMP-2 with possible adverse consequences
for the fetus. However, pregnancy did not lead to an increase in antibodies in the rabbit study.
Studies in genetically altered mice indicate that BMP-2 is critical to fetal development and that
a lack of BMP-2 activity may cause neonatal death or birth defects. It is not known if anti-BMP-2
antibodies may affect fetal development or the extent to which these antibodies may reduce
BMP-2 activity.
• INFUSE® Bone Graft should not be used immediately prior to or during pregnancy. Women of
childbearing potential should be advised not to become pregnant for one year following treatment
with the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device.
!USA
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume
(cc)
7510100
Part # of
Devices
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume
(cc)
7510100
Part #
Size
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
7510050 +
7510100
INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device Combinations
Single LT-CAGE® Device Fill
LT-CAGE® Lumbar
Tapered Fusion Device
Recommended INFUSE® Bone Graft Kit(s)
Part #
Size
(lead diameter, mm
x length, mm)
Part #
Kit size
Reconstituted
rhBMP-2/ACS
graft volume
(cc)
8941420
14x20
7510100
X Small
1.4
8941423
14x23
7510100
X Small
1.4
8941620
16x20
7510050 +
7510100
XX Small + X Small
2.1
8941623
16x23
7510050 +
7510100
XX Small + X Small
2.1
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
7510050 +
7510050 +
7510050 +
7510050 +
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
9011221 12x20 7510050 XX Small 0.7
9011225 12x25 7510100 X Small 1.4
9011420 14X20 7510100 X Small 1.4
9011423 14X23 7510100 X Small 1.4
9011426 14X26
7510050 +
7510100
9011429 14X29
7510050 +
7510100
9011620 16X20
7510050 +
7510100
9011623 16X23
7510050 +
7510100
Bone Graft. See the insert for the other INFUSE® Bone Graft kits (Small, Medium, Large, and Large
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device in nursing mothers has not been established. It is not known if BMP-2 is excreted
in human milk.
General
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not
be used in patients suspected of having a malignancy at the site of application.
• The safety and effectiveness of the use of the INFUSE® Bone Graft component with other spinal
implants, implanted at locations other than the lower lumbar spine, or used in surgical techniques
other than anterior open (LT-CAGE®, INTER FIX™, INTER FIX™ RP Devices) or anterior
laparoscopic (LT-CAGE® Device) approaches have not been established.
• The implantation of the INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device
using an anterior laparoscopic surgical approach is associated with a higher incidence of
retrograde ejaculation (10.5%, 6/57 male patients) when compared to implantation using an
anterior open surgical approach (6.4%, 5/78 male patients). Both of these rates are greater
The tables provided in the “Directions for Use” of this insert describe how to use the X Small (1.4cc)
& XX Small (0.7cc) kits to fill smaller single cages if the need arises during surgery. For larger size
cages, the following is recommended: If a Large kit was used originally to fill two cages, use a Small
plus an X Small (1.4cc) kit to fill a single cage; if a Medium kit was used originally, use a Small kit
to fill a single cage; if a Small kit was used originally, use an X Small (1.4cc) kit to fill a single cage;
and if an X Small (1.4cc) kit was used originally, use an XX Small (0.7cc) kit to fill a single cage.
Each kit contains all the components necessary to prepare the INFUSE® Bone Graft component:
the rhBMP-2, which must be reconstituted; sterile water; absorbable collagen sponge; syringe(s) with
needle(s); this package insert; and instructions for preparation. The number of each item may vary
depending on the size of the kit. If two kits are going to be used (e.g., X Small (1.4cc) & XX Small
(0.7cc)), open both kits simultaneously and mix each according to the directions.
The rhBMP-2 is provided as a lyophilized powder in vials delivering 1.05 mg of protein for the X
Small and XX Small kits. After appropriate reconstitution, the configuration results in the formulation
and concentration (1.5 mg/mL) of rhBMP-2. The solution is then applied to the provided absorbable
collagen sponge. The INFUSE® Bone Graft component is prepared at the time of surgery and
allowed a prescribed amount of time (no less than 15 minutes) before placement inside of the
Medtronic Titanium Threaded Interbody Fusion Device component(s). The Instructions for Preparation
contain complete details on preparation of the INFUSE® Bone Graft/Medtronic Titanium Threaded
Interbody Fusion Device.
Implied warranties of merchantability and fitness for a particular purpose or use are specifically
excluded. See the MDT Catalog or price list for further information about warranties and limitations
of liability.
INDICATIONS
The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device is indicated for
spinal fusion procedures in skeletally mature patients with degenerative disc disease (DDD) at one
level from L2-S1. DDD is defined as discogenic back pain with degeneration of the disc confirmed
by patient history and radiographic studies. These DDD patients may also have up to Grade I
spondylolisthesis or Grade 1 retrolisthesis at the involved level. Patients receiving the INFUSE® Bone
Graft/Medtronic Titanium Threaded Interbody Fusion Device should have had at least six months of
nonoperative treatment prior to treatment with the INFUSE® Bone Graft/Medtronic Titanium Threaded
Interbody Fusion Device. The INFUSE® Bone Graft with the LT-CAGE® Lumbar Tapered Fusion
Device is to be implanted via an anterior open or an anterior laparoscopic approach. The INFUSE®
Bone Graft with either the INTER FIX™ or the INTER FIX™ RP Threaded Fusion Device is to be
implanted via an anterior open approach.
DIRECTIONS FOR USE
The INFUSE® Bone Graft component is prepared at the time of surgery in the surgical suite by
reconstituting the lyophilized rhBMP-2 with sterile water (See Instructions for Preparation) and
then uniformly applying the reconstituted rhBMP-2 solution to the ACS. The INFUSE® Bone
Graft component is then inserted into the Medtronic Titanium Threaded Interbody Fusion Device
component. The complete device is then implanted through an anterior surgical approach. (See the
Surgical Technique manual.) If the INFUSE® Bone Graft component is not used within two hours
after reconstitution, it must be discarded.
The INFUSE® Bone Graft component must not be sterilized by the hospital. The Medtronic
Titanium Threaded Interbody Fusion Device component, if not supplied sterile, should be sterilized
before insertion of the INFUSE® Bone Graft component. Please refer to the specific Medtronic
Titanium Threaded Interbody Fusion Device package insert for information on packaging, cleaning/
decontamination, and sterilization of this component and its instruments.
This insert describes only the use of the X Small (1.4cc) and XX Small (0.7cc) kit sizes of INFUSE®
Bone Graft. See the insert for the other INFUSE® Bone Graft kits (Small, Medium, Large, and Large II)
DIRECTIONS FOR USE
The INFUSE® Bone Graft component is prepared at the time of surgery in the surgical suite by
reconstituting the lyophilized rhBMP-2 with sterile water (See Instructions for Preparation) and
then uniformly applying the reconstituted rhBMP-2 solution to the ACS. The INFUSE® Bone
Graft component is then inserted into the Medtronic Titanium Threaded Interbody Fusion Device
component. The complete device is then implanted through an anterior surgical approach. (See the
Surgical Technique manual.) If the INFUSE® Bone Graft component is not used within two hours
after reconstitution, it must be discarded.
The INFUSE® Bone Graft component must not be sterilized by the hospital. The Medtronic
Titanium Threaded Interbody Fusion Device component, if not supplied sterile, should be sterilized
before insertion of the INFUSE® Bone Graft component. Please refer to the specific Medtronic
Titanium Threaded Interbody Fusion Device package insert for information on packaging, cleaning/
decontamination, and sterilization of this component and its instruments.
This insert describes only the use of the X Small (1.4cc) and XX Small (0.7cc) kit sizes of INFUSE®
Bone Graft. See the insert for the other INFUSE® Bone Graft kits (Small, Medium, Large, and Large
II) for instructions for use on those size kits. The tables below list the appropriate X Small (1.4cc)
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device in nursing mothers has not been established. It is not known if BMP-2 is excreted
in human milk.
General
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not
be used in patients suspected of having a malignancy at the site of application.
• The safety and effectiveness of the use of the INFUSE® Bone Graft component with other spinal
implants, implanted at locations other than the lower lumbar spine, or used in surgical techniques
other than anterior open (LT-CAGE®, INTER FIX™, INTER FIX™ RP Devices) or anterior
laparoscopic (LT-CAGE® Device) approaches have not been established.
• The implantation of the INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device
using an anterior laparoscopic surgical approach is associated with a higher incidence of
retrograde ejaculation (10.5%, 6/57 male patients) when compared to implantation using an
anterior open surgical approach (6.4%, 5/78 male patients). Both of these rates are greater
than that for a control group implanted using an open anterior approach who did not receive
INFUSE® Bone Graft (1.5%, 1/68 male patients). In the randomized study of the anterior open
surgical approach, retrograde ejaculation occurred in the INFUSE® Bone Graft group in 17.6%
(3/17) of the male patients who underwent the surgery with a transperitoneal approach, as
Graft component is then inserted into the Medtronic Titanium Threaded Interbody Fusion Device
component. The complete device is then implanted through an anterior surgical approach. (See the
Surgical Technique manual.) If the INFUSE® Bone Graft component is not used within two hours
after reconstitution, it must be discarded.
The INFUSE® Bone Graft component must not be sterilized by the hospital. The Medtronic
Titanium Threaded Interbody Fusion Device component, if not supplied sterile, should be sterilized
before insertion of the INFUSE® Bone Graft component. Please refer to the specific Medtronic
Titanium Threaded Interbody Fusion Device package insert for information on packaging, cleaning/
decontamination, and sterilization of this component and its instruments.
This insert describes only the use of the X Small (1.4cc) and XX Small (0.7cc) kit sizes of INFUSE®
Bone Graft. See the insert for the other INFUSE® Bone Graft kits (Small, Medium, Large, and Large
II) for instructions for use on those size kits. The tables below list the appropriate X Small (1.4cc)
and XX Small (0.7cc) INFUSE® Bone Graft kit for the corresponding Medtronic Titanium Threaded
Interbody Fusion Device component size:
SINGLE CAGE USE
In the case of only one cage needing INFUSE® Bone Graft, due to the loss or contamination of a
sponge or sponges, single cages can be filled using the X Small (1.4cc) and/or XX Small (0.7cc)
kits as shown below:
General
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not
be used in patients suspected of having a malignancy at the site of application.
• The safety and effectiveness of the use of the INFUSE® Bone Graft component with other spinal
implants, implanted at locations other than the lower lumbar spine, or used in surgical techniques
other than anterior open (LT-CAGE®, INTER FIX™, INTER FIX™ RP Devices) or anterior
laparoscopic (LT-CAGE® Device) approaches have not been established.
• The implantation of the INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device
using an anterior laparoscopic surgical approach is associated with a higher incidence of
retrograde ejaculation (10.5%, 6/57 male patients) when compared to implantation using an
anterior open surgical approach (6.4%, 5/78 male patients). Both of these rates are greater
than that for a control group implanted using an open anterior approach who did not receive
INFUSE® Bone Graft (1.5%, 1/68 male patients). In the randomized study of the anterior open
surgical approach, retrograde ejaculation occurred in the INFUSE® Bone Graft group in 17.6%
(3/17) of the male patients who underwent the surgery with a transperitoneal approach, as
compared to 3.2% (2/ 61) of the males with a retroperitoneal approach. In the control group,
the retrograde ejaculation rate was 7.6% (1/13) in the males with a transperitoneal approach,
as compared to 0% (0/55) in the males with a retroperitoneal approach. With the two treatment
groups pooled, retrograde ejaculation occurred in 13.3% (4/30) of the males who underwent a
transperitoneal approach and in 1.8% (2/116) of the males who underwent a retroperitoneal
approach. This difference is statistically significant (p=0.017, Fisher exact test). Male patients
should be informed of this potential risk prior to considering the use of INFUSE® Bone Graft.
• The safety and effectiveness of the use of INFUSE® Bone Graft implanted in the cervical
spine has not been established. This product is only approved for use in the lumbar spine as
indicated above.
− When anterior cervical spinal fusions were performed using the INFUSE® Bone Graft
component, some cases of edema have been reported within the first postoperative week. In
some of these cases, this swelling has been severe enough to produce airway compromise,
sometimes requiring emergency surgery.
− In a clinical trial comparing single-level anterior cervical fusion using INFUSE® Bone Graft
to a control that did not use INFUSE® Bone Graft, 16.4% of patients treated with INFUSE®
Bone Graft reported dysphagia, compared to 7.3% of control patients. Most of the dysphagia
events occurred within the first four weeks after surgery, and most of these events were
classified as non-serious (e.g., non-life-threatening events not requiring hospitalization). While
dysphagia may occur following anterior cervical procedures, it may occur more frequently or
to a greater extent in the presence of INFUSE® Bone Graft.
− When anterior cervical fusions were performed using INFUSE® Bone Graft, the radiographic
appearance of anterior heterotopic ossification (HO) was noted in some patients, most commonly
observed anterior and superior to the treated level. In some of the cases of severe HO, adjacent-
level fusion and reduced motion were also noted. HO may occur more frequently or to a greater
extent with the use of INFUSE® Bone Graft.
Bone Formation
• Posterior bone formation outside of the disc space was observed in some patients when
degenerative disc disease was treated by a posterior lumbar interbody fusion procedure. Although
it was not clearly associated with key clinical outcome measures (e.g., leg pain) in most of the
cases, bone formation outside of the disc space is not desirable and may potentially lead to nerve
compression, requiring surgical intervention.
• Inappropriate use of the product, such as preparing it differently than prescribed, compressing
the rhBMP-2/ACS implant more than necessary, or overfilling the volume intended for new bone
formation, may change the concentration of the rhBMP-2, which may inhibit the ability of the
rhBMP-2/ACS to convert to bone and/or cause complications. Such use of the rhBMP-2/ACS
implant may result in radiographic evidence of resorption. These findings may be asymptomatic
or symptomatic. A sheep model developed to test the hypothesis that volume overfilling and/or
hyperconcentration of the rhBMP-2 solution results in radiographic evidence of bone resorption
has preliminarily been evaluated and appears to be supportive of the hypothesized mechanism.
• Placement of rhBMP-2/ACS can cause initial resorption of trabecular bone that may be transient.
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume
(cc)
Part # of
Devices
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume
(cc)
INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device Combinations
Single LT-CAGE® Device Fill
for instructions for use on those size kits. The tables below list the appropriate X Small (1.4cc
and XX Small (0.7cc) INFUSE® Bone Graft kit for the corresponding Medtronic Titanium Threaded
Interbody Fusion Device component size:
INTER FIX™ Threaded Fusion Device Recommended INFUSE® Bone Graft Kit(s)
INFUSE® Bone Graft/INTER FIX™ Threaded Fusion Device Combinations
Dual INTER FIX™ Devices
Size
(diameter, mm
rhBMP-2/ACS
890120 12x20 7510100 X Small 1.4
890125 12x25
INTER FIX™ and INTER FIX™ RP
INTER FIX™
890120+9011221 12x20 7510100 X Small 1.4
890125+9011225 12x25
†
The INTER FIX™ Threaded Fusion Device is to be used with the corresponding size of the INTER FIX™ RP Threaded
Fusion Device.
9011221 12x20 7510100 X Small 1.4
9011225 12x25
INFUSE® Bone Graft/INTER FIX™ and INTER FIX™ RP Threaded Fusion Device Combinations
INTER FIX™ RP Device with INTER FIX™ Device
Threaded Fusion Devices
Size
†
INTER FIX™ RP
Threaded Fusion Device
(diameter, mm
INFUSE® Bone Graft/INTER FIX™ RP Threaded Fusion Device Combinations
Size
(Diameter, mm
7510050 +
7510100
7510050 +
7510100
Dual INTER FIX™ RP Devices
7510050 +
7510100
XX Small + X Small 2.1
Recommended INFUSE® Bone Graft Kit(s)
XX Small + X Small 2.1
Recommended INFUSE® Bone Graft Kit(s)
XX Small + X Small 2.1
rhBMP-2/ACS
rhBMP-2/ACS
appearance of anterior heterotopic ossification (HO) was noted in some patients, most commonly
observed anterior and superior to the treated level. In some of the cases of severe HO, adjacent-
level fusion and reduced motion were also noted. HO may occur more frequently or to a greater
extent with the use of INFUSE® Bone Graft.
Bone Formation
• Posterior bone formation outside of the disc space was observed in some patients when
degenerative disc disease was treated by a posterior lumbar interbody fusion procedure. Although
it was not clearly associated with key clinical outcome measures (e.g., leg pain) in most of the
cases, bone formation outside of the disc space is not desirable and may potentially lead to nerve
compression, requiring surgical intervention.
• Inappropriate use of the product, such as preparing it differently than prescribed, compressing
the rhBMP-2/ACS implant more than necessary, or overfilling the volume intended for new bone
formation, may change the concentration of the rhBMP-2, which may inhibit the ability of the
rhBMP-2/ACS to convert to bone and/or cause complications. Such use of the rhBMP-2/ACS
implant may result in radiographic evidence of resorption. These findings may be asymptomatic
or symptomatic. A sheep model developed to test the hypothesis that volume overfilling and/or
hyperconcentration of the rhBMP-2 solution results in radiographic evidence of bone resorption
has preliminarily been evaluated and appears to be supportive of the hypothesized mechanism.
• Placement of rhBMP-2/ACS can cause initial resorption of trabecular bone that may be transient.
• Device migration has been reported with use of rhBMP-2/ACS in spinal fusion surgery. Device
migration has been reported in the presence and absence of bone resorption.
• Nerve compression associated with heterotopic bone formation has been reported in patients
undergoing spine surgery with rhBMP-2/ACS. Surgical intervention may be required to address
the symptoms.
Fluid Collection/Edema
• The formation of fluid collections (sometimes encapsulated) in some cases resulted in nerve
compression and pain, which may require clinical intervention (aspiration and/or surgical
removal) if symptoms persist. Many of these reports have occurred when rhBMP-2/ACS was
used in conjunction with unapproved approaches/devices or in a manner inconsistent with the
instructions for use.
• While there are currently anecdotal and literature evidence to suggest that volume overfilling and/
or hyperconcentration of the rhBMP-2 solution may lead to fluid formation and/or edema, animal
models for scientifically evaluating these events do not presently exist.
PRECAUTIONS
PHYSICIAN NOTE: Although the physician is the learned intermediary between the company and the
patient, the important medical information given in this document should be conveyed to the patient.
!USA
FOR US AUDIENCES ONLY
General
• The safety and effectiveness of repeat applications of the INFUSE® Bone Graft component
has not been established.
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should only
be used by surgeons who are experienced in spinal fusion procedures and have undergone
adequate training with this device, for anterior laparoscopic and/or anterior open procedures.
• Two Medtronic Titanium Threaded Interbody Fusion Device components should be implanted
side by side at the surgical level whenever possible.
• The Medtronic Titanium Threaded Interbody Fusion Device components and instruments must
be sterilized prior to use according to the sterilization instructions provided in the package insert
for that component, unless supplied sterile and clearly labeled as such.
• When using this device at spinal levels between L2 and L4, the potential impact of anatomical
structures (e.g., the aorta) on implant placement must be considered.
• The formation of exuberant or heterotopic bone growth at the upper lumbar levels (L2- L4) may
have a deleterious impact on certain neurovascular structures (e.g., the aorta and sympathetic
nerve chain).
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume
(cc)
7510100
Part # of
INTER FIX™
Devices
†
(diameter, mm
x length, mm)
Part #
Kit size
Reconstituted
rhBMP-2/ACS
graft volume (cc)
890120+9011221 12x20 7510100 X Small 1.4
890125+9011225 12x25
7510050 +
7510100
XX Small + X Small 2.1
†
The INTER FIX™ Threaded Fusion Device is to be used with the corresponding size of the INTER FIX™ RP Threaded
Fusion Device.
INFUSE® Bone Graft/INTER FIX™ RP Threaded Fusion Device Combinations
Dual INTER FIX™ RP Devices
INTER FIX™ RP
Threaded Fusion Device
Recommended INFUSE® Bone Graft Kit(s)
Part #
Size
(Diameter, mm
x length, mm)
Part #
Kit size
Reconstituted
rhBMP-2/ACS
graft volume
(cc)
9011221 12x20 7510100 X Small 1.4
9011225 12x25
7510050 +
7510100
XX Small + X Small 2.1
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device Combinations
Single LT-CAGE® Device Fill
LT-CAGE® Lumbar
Tapered Fusion Device
Recommended INFUSE® Bone Graft Kit(s)
Part #
Size
(lead diameter, mm
x length, mm)
Part #
Kit size
Reconstituted
rhBMP-2/ACS
graft volume
(cc)
8941420
14x20
7510100
X Small
1.4
8941423
14x23
7510100
X Small
1.4
8941620
16x20
7510050 +
7510100
XX Small + X Small
2.1
8941623
16x23
7510050 +
7510100
XX Small + X Small
2.1
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
DIRECTIONS FOR USE
The INFUSE® Bone Graft component is prepared at the time of surgery in the surgical suite by
reconstituting the lyophilized rhBMP-2 with sterile water (See Instructions for Preparation) and
then uniformly applying the reconstituted rhBMP-2 solution to the ACS. The INFUSE® Bone
Graft component is then inserted into the Medtronic Titanium Threaded Interbody Fusion Device
component. The complete device is then implanted through an anterior surgical approach. (See the
Surgical Technique manual.) If the INFUSE® Bone Graft component is not used within two hours
after reconstitution, it must be discarded.
The INFUSE® Bone Graft component must not be sterilized by the hospital. The Medtronic
Titanium Threaded Interbody Fusion Device component, if not supplied sterile, should be sterilized
before insertion of the INFUSE® Bone Graft component. Please refer to the specific Medtronic
Titanium Threaded Interbody Fusion Device package insert for information on packaging, cleaning/
decontamination, and sterilization of this component and its instruments.
This insert describes only the use of the X Small (1.4cc) and XX Small (0.7cc) kit sizes of INFUSE®
Bone Graft. See the insert for the other INFUSE® Bone Graft kits (Small, Medium, Large, and Large
II) for instructions for use on those size kits. The tables below list the appropriate X Small (1.4cc)
and XX Small (0.7cc) INFUSE® Bone Graft kit for the corresponding Medtronic Titanium Threaded
Interbody Fusion Device component size:
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device in nursing mothers has not been established. It is not known if BMP-2 is excreted
in human milk.
General
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not
be used in patients suspected of having a malignancy at the site of application.
• The safety and effectiveness of the use of the INFUSE® Bone Graft component with other spinal
implants, implanted at locations other than the lower lumbar spine, or used in surgical techniques
other than anterior open (LT-CAGE®, INTER FIX™, INTER FIX™ RP Devices) or anterior
laparoscopic (LT-CAGE® Device) approaches have not been established.
• The implantation of the INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device
using an anterior laparoscopic surgical approach is associated with a higher incidence of
retrograde ejaculation (10.5%, 6/57 male patients) when compared to implantation using an
anterior open surgical approach (6.4%, 5/78 male patients). Both of these rates are greater
than that for a control group implanted using an open anterior approach who did not receive
INFUSE® Bone Graft (1.5%, 1/68 male patients). In the randomized study of the anterior open
surgical approach, retrograde ejaculation occurred in the INFUSE® Bone Graft group in 17.6%
(3/17) of the male patients who underwent the surgery with a transperitoneal approach, as
compared to 3.2% (2/ 61) of the males with a retroperitoneal approach. In the control group,
the retrograde ejaculation rate was 7.6% (1/13) in the males with a transperitoneal approach,
as compared to 0% (0/55) in the males with a retroperitoneal approach. With the two treatment
groups pooled, retrograde ejaculation occurred in 13.3% (4/30) of the males who underwent a
SINGLE CAGE USE
In the case of only one cage needing INFUSE® Bone Graft, due to the loss or contamination of a
sponge or sponges, single cages can be filled using the X Small (1.4cc) and/or XX Small (0.7cc)
kits as shown below:
8941420 14x20 7510100 X Small 1.4
8941423 14x23 7510100 X Small
8941620 16x20
8941623 16x23
INTER FIX™ Threaded Fusion Device Recommended INFUSE® Bone Graft Kit(s)
890120 12x20 7510050 XX Small 0.7
890125 12x25 7510100 X Small 1.4
890140 14X20 7510100 X Small 1.4
890143 14X23 7510100 X Small 1.4
890146 14X26
890149 14X29
890160 16X20
890163 16X23
INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device Combinations
LT-CAGE® Lumbar
Tapered Fusion Device
Size
(lead diameter, mm
INFUSE® Bone Graft/INTER FIX™ Threaded Fusion Device Combinations
Size
(diameter, mm
Single LT-CAGE® Device Fill
7510050 +
7510100
7510050 +
7510100
Single INTER FIX™ Device Fill
7510050 +
7510100
7510050 +
7510100
7510050 +
7510100
7510050 +
7510100
Recommended INFUSE® Bone Graft Kit(s)
XX Small + X Small
XX Small + X Small
XX Small + X Small 2.1
XX Small + X Small 2.1
XX Small + X Small 2.1
XX Small + X Small 2.1
rhBMP-2/ACS
1.4
2.1
2.1
rhBMP-2/ACS

!USA
FOR US AUDIENCES ONLY
General
• The safety and effectiveness of repeat applications of the INFUSE® Bone Graft component
has not been established.
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should only
be used by surgeons who are experienced in spinal fusion procedures and have undergone
adequate training with this device, for anterior laparoscopic and/or anterior open procedures.
• Two Medtronic Titanium Threaded Interbody Fusion Device components should be implanted
side by side at the surgical level whenever possible.
• The Medtronic Titanium Threaded Interbody Fusion Device components and instruments must
be sterilized prior to use according to the sterilization instructions provided in the package insert
for that component, unless supplied sterile and clearly labeled as such.
• When using this device at spinal levels between L2 and L4, the potential impact of anatomical
structures (e.g., the aorta) on implant placement must be considered.
• The formation of exuberant or heterotopic bone growth at the upper lumbar levels (L2- L4) may
have a deleterious impact on certain neurovascular structures (e.g., the aorta and sympathetic
nerve chain).
• The safety and effectiveness of the device at spinal levels L2- L4 or in patients with up to Grade
1 retrolisthesis has not been established.
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device is intended
for single use only. Discard unused product and use a new device for subsequent applications.
• Prior to use, inspect the packaging, vials, and stoppers for visible damage. If damage is visible,
do not use the product. Retain the packaging and vials and contact a Medtronic representative.
• Do not use after the printed expiration date on the label.
Hepatic and Renal Impairment
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded
Interbody Fusion Device in patients with hepatic or renal impairment has not been established.
Pharmacokinetic studies of rhBMP-2 indicate that the renal and hepatic systems are involved
with its clearance.
Geriatrics
• Clinical studies of the INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device did not
include sufficient numbers of patients 65 years and older to determine whether they respond
differently from younger subjects.
Bone Formation
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device has not been demonstrated in patients with metabolic bone diseases.
• The potential for heterotopic or undesirable exuberant bone formation exists.
.
Antibody Formation/Allergic Reactions
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device has not been demonstrated in patients with autoimmune disease.
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device has not been demonstrated in patients with immunosuppressive disease or
suppressed immune systems resulting from radiation therapy, chemotherapy, steroid therapy, or
other treatments.
Immunogenicity
• As with all therapeutic proteins, there is a potential for immune responses to be generated
to the INFUSE® Bone Graft component. The immune response to the INFUSE® Bone Graft
components was evaluated in 349 investigational patients and 183 control patients receiving
lumbar interbody fusions.
– Anti-rhBMP-2 antibodies: 2/349 (0.6%) patients receiving the INFUSE® Bone Graft
component developed antibodies vs. 1/183 (0.5%) in the control group.
– Anti-bovine Type I collagen antibodies: 18.1% of patients receiving the INFUSE® Bone Graft
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume
(cc)
7510100
Part # of
Devices
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume
(cc)
7510100
Part #
Size
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
7510050 +
7510100
INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device Combinations
Single LT-CAGE® Device Fill
LT-CAGE® Lumbar
Tapered Fusion Device
Recommended INFUSE® Bone Graft Kit(s)
Part #
Size
(lead diameter, mm
x length, mm)
Part #
Kit size
Reconstituted
rhBMP-2/ACS
graft volume
(cc)
8941420
14x20
7510100
X Small
1.4
8941423
14x23
7510100
X Small
1.4
8941620
16x20
7510050 +
7510100
XX Small + X Small
2.1
8941623
16x23
7510050 +
7510100
XX Small + X Small
2.1
Part #
Size
(diameter, mm
x length, mm)
Part #
Kit size
Reconstituted
rhBMP-2/ACS
graft volume (cc)
890120 12x20 7510050 XX Small 0.7
890125 12x25 7510100 X Small 1.4
890140 14X20 7510100 X Small 1.4
890143 14X23 7510100 X Small 1.4
890146 14X26
7510050 +
7510100
XX Small + X Small 2.1
890149 14X29
7510050 +
7510100
XX Small + X Small 2.1
890160 16X20
7510050 +
7510100
XX Small + X Small 2.1
890163 16X23
7510050 +
7510100
XX Small + X Small 2.1
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
7510100
7510100
INTER FIX™ Threaded Fusion Device Recommended INFUSE® Bone Graft Kit(s)
9011221 12x20 7510050 XX Small 0.7
9011225 12x25 7510100 X Small 1.4
9011420 14X20 7510100 X Small 1.4
9011423 14X23 7510100 X Small 1.4
9011426 14X26
9011429 14X29
9011620 16X20
9011623 16X23
INFUSE® Bone Graft/INTER FIX™ RP Threaded Fusion Device Combinations
Single INTER FIX™ RP Device Fill
Size
(diameter, mm
7510050 +
7510050 +
7510050 +
7510050 +
7510100
7510100
XX Small + X Small 2.1
XX Small + X Small 2.1
XX Small + X Small 2.1
XX Small + X Small 2.1
rhBMP-2/ACS
CONTRAINDICATIONS
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device is contraindicated
for patients with a known hypersensitivity to recombinant human Bone Morphogenetic Protein-2,
bovine Type I collagen or to other components of the formulation.
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not
be used in the vicinity of a resected or extant tumor, in patients with any active malignancy or
patients undergoing treatment for a malignancy.
• INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not be
used in patients who are skeletally immature (<18 years of age or no radiographic evidence of
epiphyseal closure).
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not
be used in pregnant women. The potential effects of rhBMP-2 on the human fetus have not
been evaluated.
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not be
implanted in patients with an active infection at the operative site or with an allergy to titanium
or titanium alloy.
CONTRAINDICATIONS
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device is contraindicated
for patients with a known hypersensitivity to recombinant human Bone Morphogenetic Protein-2,
bovine Type I collagen or to other components of the formulation.
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not
be used in the vicinity of a resected or extant tumor, in patients with any active malignancy or
patients undergoing treatment for a malignancy.
• INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not be
used in patients who are skeletally immature (<18 years of age or no radiographic evidence of
epiphyseal closure).
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not
be used in pregnant women. The potential effects of rhBMP-2 on the human fetus have not
been evaluated.
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not be
implanted in patients with an active infection at the operative site or with an allergy to titanium
or titanium alloy.
!USA
• Do not use after the printed expiration date on the label.
Hepatic and Renal Impairment
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded
Interbody Fusion Device in patients with hepatic or renal impairment has not been established.
Pharmacokinetic studies of rhBMP-2 indicate that the renal and hepatic systems are involved
with its clearance.
Geriatrics
• Clinical studies of the INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device did not
include sufficient numbers of patients 65 years and older to determine whether they respond
differently from younger subjects.
Bone Formation
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device has not been demonstrated in patients with metabolic bone diseases.
• The potential for heterotopic or undesirable exuberant bone formation exists.
.
Antibody Formation/Allergic Reactions
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device has not been demonstrated in patients with autoimmune disease.
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device has not been demonstrated in patients with immunosuppressive disease or
suppressed immune systems resulting from radiation therapy, chemotherapy, steroid therapy, or
other treatments.
Immunogenicity
• As with all therapeutic proteins, there is a potential for immune responses to be generated
to the INFUSE® Bone Graft component. The immune response to the INFUSE® Bone Graft
components was evaluated in 349 investigational patients and 183 control patients receiving
lumbar interbody fusions.
– Anti-rhBMP-2 antibodies: 2/349 (0.6%) patients receiving the INFUSE® Bone Graft
component developed antibodies vs. 1/183 (0.5%) in the control group.
– Anti-bovine Type I collagen antibodies: 18.1% of patients receiving the INFUSE® Bone Graft
component developed antibodies to bovine Type I collagen vs. 14.2% of control patients. No
patients in either group developed anti-human Type I collagen antibodies.
– The presence of antibodies to rhBMP-2 was not associated with immune mediated adverse
events such as allergic reactions. The neutralizing capacity of antibodies to rhBMP-2 is not
known.
• The incidence of antibody detection is highly dependent on the sensitivity and specificity of the
assay. Additionally, the incidence of antibody detection may be influenced by several factors
including sample handling, concomitant medications and underlying disease. For these reasons,
comparison of the incidence of antibodies to the INFUSE® Bone Graft component with the
incidence of antibodies to other products may be misleading.
ADVERSE EVENTS
The INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device was implanted in 288
investigational patients and compared to 139 control patients who received the LT-CAGE® Lumbar
Tapered Fusion Device filled with iliac crest autograft. The investigational patients were implanted with
the device via either an open anterior surgical approach or a laparoscopic anterior surgical approach.
The control patients were implanted via the open anterior surgical approach only.
Adverse event rates presented are based on the number of patients having at least one occurrence
for a particular adverse event divided by the total number of patients in that treatment group. Because
no control subjects were evaluated at the 48- and 72-month timepoints, the reported events at these
timepoints are only from the investigational subjects.
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume
(cc)
7510100
Part # of
Devices
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume
(cc)
7510100
Part #
Size
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
7510050 +
7510100
INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device Combinations
Single LT-CAGE® Device Fill
LT-CAGE® Lumbar
Tapered Fusion Device
Recommended INFUSE® Bone Graft Kit(s)
Part #
Size
(lead diameter, mm
x length, mm)
Part #
Kit size
Reconstituted
rhBMP-2/ACS
graft volume
(cc)
8941420
14x20
7510100
X Small
1.4
8941423
14x23
7510100
X Small
1.4
8941620
16x20
7510050 +
7510100
XX Small + X Small
2.1
8941623
16x23
7510050 +
7510100
XX Small + X Small
2.1
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
7510050 +
7510050 +
7510050 +
7510050 +
Part #
x length, mm)
Part #
Kit size
Reconstituted
graft volume (cc)
9011221 12x20 7510050 XX Small 0.7
9011225 12x25 7510100 X Small 1.4
9011420 14X20 7510100 X Small 1.4
9011423 14X23 7510100 X Small 1.4
9011426 14X26
7510050 +
7510100
XX Small + X Small 2.1
9011429 14X29
7510050 +
7510100
XX Small + X Small 2.1
9011620 16X20
7510050 +
7510100
XX Small + X Small 2.1
9011623 16X23
7510050 +
7510100
XX Small + X Small 2.1
WARNINGS
• In an experimental rabbit study, rhBMP-2 has been shown to elicit antibodies that are capable
of crossing the placenta. Reduced ossification of the frontal and parietal bones of the skull was
noted infrequently (<3%) in fetuses of rabbit dams immunized to rhBMP-2; however, there was
no effect noted in limb bud development. There are no adequate and well-controlled studies in
human pregnant women. Women of child bearing potential should be warned by their surgeon
of potential risk to a fetus and informed of other possible orthopedic treatments.
• Women of childbearing potential should be advised that antibody formation to rhBMP-2 or its
influence on fetal development has not been completely assessed. In the clinical trial supporting
the safety and effectiveness of the INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion
Device, 2/277 (0.7%) patients treated with INFUSE® Bone Graft component and 1/127 (0.8%)
patients treated with autograft bone developed antibodies to rhBMP-2. The effect of maternal
antibodies to rhBMP-2, as might be present for several months following device implantation,
on the unborn fetus is unknown. Additionally, it is unknown whether fetal expression of BMP-2
General
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should not
could re-expose mothers who were previously antibody positive. Theoretically, re-exposure
may elicit a more powerful immune response to BMP-2 with possible adverse consequences
for the fetus. However, pregnancy did not lead to an increase in antibodies in the rabbit study.
Studies in genetically altered mice indicate that BMP-2 is critical to fetal development and that
a lack of BMP-2 activity may cause neonatal death or birth defects. It is not known if anti-BMP-2
antibodies may affect fetal development or the extent to which these antibodies may reduce
BMP-2 activity.
• INFUSE® Bone Graft should not be used immediately prior to or during pregnancy. Women of
childbearing potential should be advised not to become pregnant for one year following treatment
with the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device.
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device in nursing mothers has not been established. It is not known if BMP-2 is excreted
in human milk.
be used in patients suspected of having a malignancy at the site of application.
• The safety and effectiveness of the use of the INFUSE® Bone Graft component with other spinal
implants, implanted at locations other than the lower lumbar spine, or used in surgical techniques
other than anterior open (LT-CAGE®, INTER FIX™, INTER FIX™ RP Devices) or anterior
laparoscopic (LT-CAGE® Device) approaches have not been established.
• The implantation of the INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device
using an anterior laparoscopic surgical approach is associated with a higher incidence of
retrograde ejaculation (10.5%, 6/57 male patients) when compared to implantation using an
anterior open surgical approach (6.4%, 5/78 male patients). Both of these rates are greater
than that for a control group implanted using an open anterior approach who did not receive
INFUSE® Bone Graft (1.5%, 1/68 male patients). In the randomized study of the anterior open
surgical approach, retrograde ejaculation occurred in the INFUSE® Bone Graft group in 17.6%
(3/17) of the male patients who underwent the surgery with a transperitoneal approach, as
compared to 3.2% (2/ 61) of the males with a retroperitoneal approach. In the control group,
the retrograde ejaculation rate was 7.6% (1/13) in the males with a transperitoneal approach,
as compared to 0% (0/55) in the males with a retroperitoneal approach. With the two treatment
groups pooled, retrograde ejaculation occurred in 13.3% (4/30) of the males who underwent a
transperitoneal approach and in 1.8% (2/116) of the males who underwent a retroperitoneal
approach. This difference is statistically significant (p=0.017, Fisher exact test). Male patients
should be informed of this potential risk prior to considering the use of INFUSE® Bone Graft.

the retrograde ejaculation rate was 7.6% (1/13) in the males with a transperitoneal approach,
as compared to 0% (0/55) in the males with a retroperitoneal approach. With the two treatment
groups pooled, retrograde ejaculation occurred in 13.3% (4/30) of the males who underwent a
transperitoneal approach and in 1.8% (2/116) of the males who underwent a retroperitoneal
approach. This difference is statistically significant (p=0.017, Fisher exact test). Male patients
should be informed of this potential risk prior to considering the use of INFUSE® Bone Graft.
• The safety and effectiveness of the use of INFUSE® Bone Graft implanted in the cervical
spine has not been established. This product is only approved for use in the lumbar spine as
indicated above.
− When anterior cervical spinal fusions were performed using the INFUSE® Bone Graft
component, some cases of edema have been reported within the first postoperative week. In
some of these cases, this swelling has been severe enough to produce airway compromise,
sometimes requiring emergency surgery.
− In a clinical trial comparing single-level anterior cervical fusion using INFUSE® Bone Graft
to a control that did not use INFUSE® Bone Graft, 16.4% of patients treated with INFUSE®
Bone Graft reported dysphagia, compared to 7.3% of control patients. Most of the dysphagia
events occurred within the first four weeks after surgery, and most of these events were
classified as non-serious (e.g., non-life-threatening events not requiring hospitalization). While
dysphagia may occur following anterior cervical procedures, it may occur more frequently or
to a greater extent in the presence of INFUSE® Bone Graft.
− When anterior cervical fusions were performed using INFUSE® Bone Graft, the radiographic
appearance of anterior heterotopic ossification (HO) was noted in some patients, most commonly
observed anterior and superior to the treated level. In some of the cases of severe HO, adjacentlevel fusion and reduced motion were also noted. HO may occur more frequently or to a greater
extent with the use of INFUSE® Bone Graft.
Bone Formation
• Posterior bone formation outside of the disc space was observed in some patients when
degenerative disc disease was treated by a posterior lumbar interbody fusion procedure. Although
it was not clearly associated with key clinical outcome measures (e.g., leg pain) in most of the
cases, bone formation outside of the disc space is not desirable and may potentially lead to nerve
compression, requiring surgical intervention.
• Inappropriate use of the product, such as preparing it differently than prescribed, compressing
the rhBMP-2/ACS implant more than necessary, or overfilling the volume intended for new bone
formation, may change the concentration of the rhBMP-2, which may inhibit the ability of the
rhBMP-2/ACS to convert to bone and/or cause complications. Such use of the rhBMP-2/ACS
implant may result in radiographic evidence of resorption. These findings may be asymptomatic
or symptomatic. A sheep model developed to test the hypothesis that volume overfilling and/or
hyperconcentration of the rhBMP-2 solution results in radiographic evidence of bone resorption
has preliminarily been evaluated and appears to be supportive of the hypothesized mechanism.
• Placement of rhBMP-2/ACS can cause initial resorption of trabecular bone that may be transient.
• Device migration has been reported with use of rhBMP-2/ACS in spinal fusion surgery. Device
migration has been reported in the presence and absence of bone resorption.
• Nerve compression associated with heterotopic bone formation has been reported in patients
undergoing spine surgery with rhBMP-2/ACS. Surgical intervention may be required to address
the symptoms.
Fluid Collection/Edema
• The formation of fluid collections (sometimes encapsulated) in some cases resulted in nerve
compression and pain, which may require clinical intervention (aspiration and/or surgical
removal) if symptoms persist. Many of these reports have occurred when rhBMP-2/ACS was
used in conjunction with unapproved approaches/devices or in a manner inconsistent with the
instructions for use.
• While there are currently anecdotal and literature evidence to suggest that volume overfilling and/
or hyperconcentration of the rhBMP-2 solution may lead to fluid formation and/or edema, animal
models for scientifically evaluating these events do not presently exist.
PRECAUTIONS
PHYSICIAN NOTE: Although the physician is the learned intermediary between the company and the
patient, the important medical information given in this document should be conveyed to the patient.
!USA
FOR US AUDIENCES ONLY

patient, the important medical information given in this document should be conveyed to the patient.
!USA
FOR US AUDIENCES ONLY
General
• The safety and effectiveness of repeat applications of the INFUSE® Bone Graft component
has not been established.
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device should only
be used by surgeons who are experienced in spinal fusion procedures and have undergone
adequate training with this device, for anterior laparoscopic and/or anterior open procedures.
• Two Medtronic Titanium Threaded Interbody Fusion Device components should be implanted
side by side at the surgical level whenever possible.
• The Medtronic Titanium Threaded Interbody Fusion Device components and instruments must
be sterilized prior to use according to the sterilization instructions provided in the package insert
for that component, unless supplied sterile and clearly labeled as such.
• When using this device at spinal levels between L2 and L4, the potential impact of anatomical
structures (e.g., the aorta) on implant placement must be considered.
• The formation of exuberant or heterotopic bone growth at the upper lumbar levels (L2- L4) may
have a deleterious impact on certain neurovascular structures (e.g., the aorta and sympathetic
nerve chain).
• The safety and effectiveness of the device at spinal levels L2- L4 or in patients with up to Grade
1 retrolisthesis has not been established.
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device is intended
for single use only. Discard unused product and use a new device for subsequent applications.
• Prior to use, inspect the packaging, vials, and stoppers for visible damage. If damage is visible,
do not use the product. Retain the packaging and vials and contact a Medtronic representative.
• Do not use after the printed expiration date on the label.
Hepatic and Renal Impairment
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded
Interbody Fusion Device in patients with hepatic or renal impairment has not been established.
Pharmacokinetic studies of rhBMP-2 indicate that the renal and hepatic systems are involved
with its clearance.
Geriatrics
• Clinical studies of the INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device did not
include sufficient numbers of patients 65 years and older to determine whether they respond
differently from younger subjects.
Bone Formation
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device has not been demonstrated in patients with metabolic bone diseases.
• The potential for heterotopic or undesirable exuberant bone formation exists.
.
Antibody Formation/Allergic Reactions
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device has not been demonstrated in patients with autoimmune disease.
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device has not been demonstrated in patients with immunosuppressive disease or
suppressed immune systems resulting from radiation therapy, chemotherapy, steroid therapy, or
other treatments.
Immunogenicity
• As with all therapeutic proteins, there is a potential for immune responses to be generated
to the INFUSE® Bone Graft component. The immune response to the INFUSE® Bone Graft
components was evaluated in 349 investigational patients and 183 control patients receiving
lumbar interbody fusions.
– Anti-rhBMP-2 antibodies: 2/349 (0.6%) patients receiving the INFUSE® Bone Graft
component developed antibodies vs. 1/183 (0.5%) in the control group.
– Anti-bovine Type I collagen antibodies: 18.1% of patients receiving the INFUSE® Bone Graft
component developed antibodies to bovine Type I collagen vs. 14.2% of control patients. No
patients in either group developed anti-human Type I collagen antibodies.

!USA
• The INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device is intended
for single use only. Discard unused product and use a new device for subsequent applications.
• Prior to use, inspect the packaging, vials, and stoppers for visible damage. If damage is visible,
do not use the product. Retain the packaging and vials and contact a Medtronic representative.
• Do not use after the printed expiration date on the label.
Hepatic and Renal Impairment
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded
Interbody Fusion Device in patients with hepatic or renal impairment has not been established.
Pharmacokinetic studies of rhBMP-2 indicate that the renal and hepatic systems are involved
with its clearance.
Geriatrics
• Clinical studies of the INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device did not
include sufficient numbers of patients 65 years and older to determine whether they respond
differently from younger subjects.
Bone Formation
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device has not been demonstrated in patients with metabolic bone diseases.
• The potential for heterotopic or undesirable exuberant bone formation exists.
.
Antibody Formation/Allergic Reactions
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device has not been demonstrated in patients with autoimmune disease.
• The safety and effectiveness of the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device has not been demonstrated in patients with immunosuppressive disease or
suppressed immune systems resulting from radiation therapy, chemotherapy, steroid therapy, or
other treatments.
Immunogenicity
• As with all therapeutic proteins, there is a potential for immune responses to be generated
to the INFUSE® Bone Graft component. The immune response to the INFUSE® Bone Graft
components was evaluated in 349 investigational patients and 183 control patients receiving
lumbar interbody fusions.
– Anti-rhBMP-2 antibodies: 2/349 (0.6%) patients receiving the INFUSE® Bone Graft
component developed antibodies vs. 1/183 (0.5%) in the control group.
– Anti-bovine Type I collagen antibodies: 18.1% of patients receiving the INFUSE® Bone Graft
component developed antibodies to bovine Type I collagen vs. 14.2% of control patients. No
patients in either group developed anti-human Type I collagen antibodies.
– The presence of antibodies to rhBMP-2 was not associated with immune mediated adverse
Safety and Immune Response Evaluation
The assessment of safety consisted of an evaluation of the reported adverse events, as well as an
evaluation of antibodies to rhBMP-2, bovine Type I collagen, and human Type I collagen. The complete
list of complications, adverse events, and subsequent interventions is described in the Adverse
Events section above. The presence of antibodies was assessed at the pre-op and 3-month post-op
visits using ELISA. If there was a positive response to bovine Type I collagen, the serum was also
tested for antibodies to human Type I collagen. The screening ELISA cutpoint for positive antibody
responses was set to 5 times the standard deviation of sera from normal human donors. Subjects
were considered to have an elevated immune response if the preoperative test was negative (titer
< 50) and postoperative test was positive (titer ≥ 50) or if the preoperative test was positive and the
postoperative test was positive with a three-fold higher titer than the preoperative test.
There were 3 subjects who had positive antibody responses to rhBMP-2 – 1 subject in each of the
study groups. The rates of positive antibody response to rhBMP-2 were 0.7% in the open surgical
approach investigational group and 0.8% in the laparoscopic surgical approach investigational and
open surgical approach control groups. While there is a theoretical possibility that antibodies to
rhBMP-2 could neutralize endogenous BMP-2, thereby interfering with subsequent bone healing,
this was not observed during the course of the study.
Sixty-six subjects were considered to have an authentic elevated antibody response to bovine Type
I collagen – 18 open surgical approach investigational subjects, 32 laparoscopic surgical approach
investigational subjects, and 16 control subjects. No subjects had positive responses to human
Type I collagen.
An evaluation was performed on the impact of a positive antibody response on overall success and
fusion success. There was very little difference in overall and individual success when antibody
status was taken into consideration.
During the course of the study, 6 pregnancies were reported – one in the control group and five in
the investigational groups. Two of the four pregnancies that occurred in the laparoscopic approach
group resulted in first trimester miscarriages. The other three pregnancies in the investigational
groups resulted in live births with no reported complications. None of the pregnant subjects had
antibody responses to rhBMP-2 or Type I collagen (bovine or human) that were detectable to the
limits of the sensitivity of the assay.
Through the 24-month adverse event reporting window, three cases of cancer were diagnosed – two
in the investigational group and one in the control group. One investigational subject was found to
have pancreatic cancer while another was diagnosed with breast cancer. A control subject was also
found to have breast cancer. No additional information is available on these subjects (e.g., BMP-2
receptor expression).
Post-Approval Study
A total of 145 investigational subjects were evaluated out to 6 years (72 months) after the initial
surgery in a post-approval study. The patient population was obtained from participants in both the
open and laparoscopic arms of the IDE clinical trial. Control subjects from the IDE study were not
followed in the post-approval study. Based on criteria from the IDE study, information pertaining to
the safety and effectiveness of the product was obtained at four and six years post-implantation,
including adverse events, an independent radiographic assessment of fusion, and an assessment of
pain and function. The table below summarizes the clinical and radiographic data evaluated during
the post approval study, as well as the overall success rate at each timepoint.
Variable 4-Year Evaluation 6-Year Evaluation
Overall Success 60.6% (77/127) 60.4% (84/139)
Oswestry Pain Improvement 81.5% (106/130) 79.0% (109/138)
Fusion 98.3% (117/119) 98.5% (128/130)
Neurological Status
(Maintenance or Improvement) 73.3% (96/131) 81.2% (112/138)
HOW SUPPLIED
INFUSE® Bone Graft component is supplied in five kit sizes containing all the components necessary
to prepare this portion of the device (i.e., the collagen sponge(s), a vial with the lyophilized growth
factor, a vial with sterile water for reconstituting the growth factor, syringes and needles). This
insert describes only the use of two kit sizes, the X Small (1.4cc) and XX Small (0.7cc) of the
INFUSE® Bone Graft. The Medtronic Titanium Threaded Interbody Fusion Device component is
supplied in a variety of sizes, which must be properly selected based on a specific patient’s anatomy.
STORAGE CONDITIONS
Store the INFUSE® Bone Graft component at room temperature [15-30 degrees Centigrade (59
to 86° F)]. The Medtronic Titanium Threaded Interbody Fusion Device component should also be
ADVERSE EVENTS
(INFUSE® Bone Graft/LT-CAGE® Device data combined from all clinical trial experience with the device)
# of Patients Reporting &
Window)
Investigational #
Total Events
Control #
Total Events
Total #
Total Events
Total #
Total Events
Anatomical/Technical
Difficulty
11(3.8)
11
3(2.2) 3 0(0.0) 0 0(0.0)
0
70(24.3)
78
33(23.7)
37
23(17.2)
24
18(12.9)
21
2(0.7) 2 1(0.7) 1 4(3.0) 4 1(0.7)
1
17(5.9)
20
12(8.6)
14
7(5.2) 7 4(2.9)
4
0(0.0) 0 1(0.7) 1 2(1.5) 2 0(0.0)
0
0(0.0) 0 1(0.7) 1 0(0.0) 0 0(0.0)
0
56(19.4)
72
27(19.4)
32
14(10.4)
17
6(4.3)
8
0(0.0) 0 8(5.8) 8 0(0.0) 0 0(0.0)
0
36(12.5)
40
16(11.5)
17
4(3.0) 4 3(2.1)
3
5(1.7) 5 0(0.0) 0 0(0.0) 0 0(0.0)
0
39(13.5)
45
23(16.5)
24
12(9.0)
12
5(3.6)
5
6(2.1) 6 3(2.2) 3 0(0.0) 0 0(0.0)
0
10(3.5)
10
13(9.4)
13
0(0.0) 0 0(0.0)
0
62(21.5)
81
37(26.6)
43
22(16.4)
29
17(12.1)
18
31(10.8)
36
13(9.4)
16
15(11.2)
20
11(7.9)
12
5(1.7) 5 4(2.9) 4 1(0.7) 1 1(0.7)
1
Retrograde
Ejaculation
11(7.9)
1
12
1(1.4)2 1 0(0.0) 0 0(0.0)
0
30(10.4)
37
17(12.2)
18
9(6.7) 9 8(5.7)
8
7(2.4) 7 2(1.4) 2 0(0.0) 0 0(0.0)
0
68(23.6)
82
34(24.5)
39
21(15.7)
23
20(14.3)
22
41(14.2)
45
13(9.4)
14
2(1.5) 2 3(2.1)
3
14(4.9)
15
5(3.6) 5 1(0.7) 1 0(0.0)
0
1(0.3) 1 0(0.0) 0 0(0.0) 0 0(0.0)
0
*Non-union adverse events that have not resulted in second surgery.
**Non-union adverse events that have resulted in a second surgery.
Percent of 140 males.
Percent of 70 males.
for all treated subjects at all follow-up evaluation timepoints identified above. The primary clinical
events such as allergic reactions. The neutralizing capacity of antibodies to rhBMP-2 is not
known.
• The incidence of antibody detection is highly dependent on the sensitivity and specificity of the
assay. Additionally, the incidence of antibody detection may be influenced by several factors
including sample handling, concomitant medications and underlying disease. For these reasons,
comparison of the incidence of antibodies to the INFUSE® Bone Graft component with the
incidence of antibodies to other products may be misleading.
ADVERSE EVENTS
The INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device was implanted in 288
investigational patients and compared to 139 control patients who received the LT-CAGE® Lumbar
Tapered Fusion Device filled with iliac crest autograft. The investigational patients were implanted with
the device via either an open anterior surgical approach or a laparoscopic anterior surgical approach.
The control patients were implanted via the open anterior surgical approach only.
Adverse event rates presented are based on the number of patients having at least one occurrence
for a particular adverse event divided by the total number of patients in that treatment group. Because
no control subjects were evaluated at the 48- and 72-month timepoints, the reported events at these
timepoints are only from the investigational subjects.
Surgery
Complication Inves. Control Inves. Control Inves. Control Inves. Control Inves. Control Inves. Control Inves. Control
Back and/or Leg Pain 0 0 12 4 11 5 12 5 15 4 20 7 8 12
Cancer 0 0 0 0 0 0 0 1 0 0 1 0 1 0
Cardio/Vascular 1 0 6 5 5 2 1 3 2 1 4 2 1 1
Death 0 0 0 0 0 0 0 0 0 1 0 0 0 0
Dural Injury 0 0 0 0 0 0 0 1 0 0 0 0 0 0
Gastrointestinal 1 0 40 22 2 0 5 1 7 1 10 3 7 5
Graft Site Related 0 0 0 8 0 0 0 0 0 0 0 0 0 0
Implant
Displacement/
Loosening
Infection 0 0 19 9 8 4 5 1 5 1 3 0 0 2
Malpositioned Implant 5 0 0 0 0 0 0 0 0 0 0 0 0 0
Neurological 0 0 8 5 7 3 5 2 6 2 13 4 6 8
Non-Union* 0 0 0 0 0 0 0 0 1 2 4 0 1 1
Non-Union** 0 0 0 1 0 1 2 0 3 4 4 6 1 1
Other 5 6 18 11 9 2 3 4 11 4 15 8 20 8
Other Pain 0 0 3 1 2 1 4 2 6 1 10 8 11 3
Respiratory 0 0 3 2 1 0 0 0 1 0 0 1 0 1
Spinal Event 0 0 1 2 1 0 6 2 10 4 10 8 9 2
Subsidence 0 0 3 2 2 0 1 0 1 0 0 0 0 0
Trauma 0 0 4 5 5 3 11 7 14 5 28 11 20 8
Urogenital 0 0 24 5 3 0 2 3 6 3 3 1 7 2
Vascular Intra-Op 15 5 0 0 0 0 0 0 0 0 0 0 0 0
Vertebral Fracture 0 0 1 0 0 0 0 0 0 0 0 0 0 0
Any Adverse Event 228(79.2) 117(84.2) 84(62.7) 64(45.7)
1
2
11 3 0 0 0 0 0 0 0 0 0 0 0 0
0 0 1 1 3 0 1 0 0 0 0 0 0 0
0 0 5 1 4 0 1 0 0 0 2 0 0 0
Postoperative
(1 day -
<4 Weeks)
6 Weeks
(≥4 Weeks -
<9 Weeks)
3 Months
(≥9 Weeks -
<5 Months)
6 Months
(≥5 Months -
<9 Months)
12 Months
(≥9 Months -
<19 Months)
and bovine Type I collagen were assessed preoperatively and at 3 months post-operatively. Antibodies
to human Type I collagen were assessed if the antibody response to bovine Type I collagen was positive.
Primary and secondary clinical and radiographic effectiveness outcome parameters were evaluated
24 Months
(≥19 Months -
<30 Months)
Total Adverse Events
(Through 24-Month Reporting
(% of 288)
5(1.7) 5 1(0.7) 1 0(0.0)
(% of 139)
48 Months
(≥30 Months -
<60 Months)
(% of 134)
0
72 Months
(≥60 Months -
<84 Months)
(% of 140)
0(0.0)
0

ADVERSE EVENTS
(INFUSE® Bone Graft/LT-CAGE® Device data combined from all clinical trial experience with the device)
# of Patients Reporting &
Window)
Investigational #
Total Events
Control #
Total Events
Total #
Total Events
Total #
Total Events
Anatomical/Technical
Difficulty
11(3.8)
11
3(2.2)
3
0(0.0)
0
0(0.0)
0
70(24.3)
78
33(23.7)
37
23(17.2)
24
18(12.9)
21
2(0.7)
2
1(0.7)
1
4(3.0)
4
1(0.7)
1
17(5.9)
20
12(8.6)
14
7(5.2)
7
4(2.9)
4
0(0.0)
0
1(0.7)
1
2(1.5)
2
0(0.0)
0
0(0.0)
0
1(0.7)
1
0(0.0)
0
0(0.0)
0
56(19.4)
72
27(19.4)
32
14(10.4)
17
6(4.3)
8
0(0.0)
0
8(5.8)
8
0(0.0)
0
0(0.0)
0
Loosening
36(12.5)
40
16(11.5)
17
4(3.0)
4
3(2.1)
3
5(1.7)
5
0(0.0)
0
0(0.0)
0
0(0.0)
0
39(13.5)
45
23(16.5)
24
12(9.0)
12
5(3.6)
5
6(2.1)
6
3(2.2)
3
0(0.0)
0
0(0.0)
0
10(3.5)
10
13(9.4)
13
0(0.0)
0
0(0.0)
0
62(21.5)
81
37(26.6)
43
22(16.4)
29
17(12.1)
18
31(10.8)
36
13(9.4)
16
15(11.2)
20
11(7.9)
12
5(1.7)
5
4(2.9)
4
1(0.7)
1
1(0.7)
1
Retrograde
Ejaculation
11(7.9)
1
12
1(1.4)2
1
0(0.0)
0
0(0.0)
0
30(10.4)
37
17(12.2)
18
9(6.7)
9
8(5.7)
8
7(2.4)
7
2(1.4)
2
0(0.0)
0
0(0.0)
0
68(23.6)
82
34(24.5)
39
21(15.7)
23
20(14.3)
22
41(14.2)
45
13(9.4)
14
2(1.5)
2
3(2.1)
3
14(4.9)
15
5(3.6)
5
1(0.7)
1
0(0.0)
0
1(0.3)
1
0(0.0)
0
0(0.0)
0
0(0.0)
0
*Non-union adverse events that have not resulted in second surgery.
**Non-union adverse events that have resulted in a second surgery.
1
Percent of 140 males.
2
Percent of 70 males.
and bovine Type I collagen were assessed preoperatively and at 3 months post-operatively. Antibodies
to human Type I collagen were assessed if the antibody response to bovine Type I collagen was positive.
Primary and secondary clinical and radiographic effectiveness outcome parameters were evaluated
for all treated subjects at all follow-up evaluation timepoints identified above. The primary clinical
parameters assessed were of pain, function, and neurological status. The secondary clinical outcome
parameters assessed were general health status, back and leg pain, donor site pain (control subjects
only), patient satisfaction, and patient global perceived effect of the treatment. The primary radiographic
outcome parameter consisted of evaluations of fusion, while the secondary radiographic assessment
was disc height.
Fusion was evaluated at 6, 12, and 24 months post-op using plain radiographs (AP, lateral, and flexion/
extension films) and high resolution thin-slice CT scans (1mm slices with 1mm index on axial sagittal
and coronal reconstructions). Fusion was defined as the presence of bridging bone connecting the
inferior and superior vertebral bodies; a lack of motion on flexion/extension (≤ 3mm of translation and
< 5° of angulation); and no evidence of radiolucencies over more than 50% of either implant. Fusion
success was defined as the presence of all of these parameters plus the lack of a second surgical
intervention resulting from a non-union. All assessments were made from the plain films except for the
assessment of bridging bone, which was made using the CT scans only if bridging bone could not be
visualized on the plain film.
Pain and function were measured using the Oswestry Low Back Pain Disability Questionnaire. Success
was defined as a 15-point improvement in the Oswestry score from the pre-op baseline score.
Neurological status consisted of measurements of four parameters – motor, sensory, reflexes, and
straight leg raise (SLR). Neurological status success was defined as maintenance or improvement of
the pre-op baseline score for each parameter. Overall neurological status success required that each
individual parameter be a success for that subject to be counted as a success.
Patient Demographics and Accountability
A total of 143 open approach investigational and 136 control patients were enrolled in the randomized
arm of the study and received the device. A total of 134 subjects were enrolled in the non-randomized
arm of the study and received the device. For the majority of the demographic parameters, there were
no differences in pre-op demographics across the three populations.
Surgical Results and Hospitalization
Clinical and Radiographic Effectiveness Evaluation
Individual subject success was defined as success in each of the primary clinical and radiographic
outcome parameters. Success for these parameters included:
1. the presence of radiographic fusion;
2. an improvement of at least 15 points from the baseline Oswestry score;
3. maintenance or improvement in neurological status;
4. the presence of no serious adverse event classified as implant-associated or implant/surgical
procedure-associated; and
5. no additional surgical procedure classified as “Failure.”
Study success was expressed as the number of individual subjects categorized as a success divided
by the total number of subjects evaluated. The table below describes the success rates for the individual
primary outcome parameters and overall success. All success rates were based on the data from the
24-month follow-up evaluation, and posterior probabilities of success were calculated using Bayesian
statistical methods.
Surgical Results and Hospitalization
Surgical and Hospitalization Information
Investigational Open
Surgical Approach
Control Open
Surgical Approach
Investigational Laparoscopic
Surgical Approach
Mean Operative Time (hrs)
1.6* 2.0
1.9
Mean EBL (ml)
109.8
*
153.1
146.1
Hospitalization (days)
3.1
3.3
1.2
*
*
Statistically different from control
Surgical Results and Hospitalization
Surgical and Hospitalization Information
Investigational Open
Surgical Approach
Control Open
Surgical Approach
Investigational Laparoscopic
Surgical Approach
Mean Operative Time (hrs)
1.6* 2.0
1.9
Mean EBL (ml)
109.8
*
153.1
146.1
Hospitalization (days)
3.1
3.3
1.2
*
*
Statistically different from control
The reported rates of several adverse events were high but similar in both the investigational and
control groups. These events included back and leg pain, neurological events, gastrointestinal events,
spinal events, cardiovascular events, and infection.
Some of the reported adverse events required surgical interventions subsequent to the initial
surgery. The number of subjects requiring a second surgical intervention was 10.4% (30/288) in
the investigational groups and 13.7% (19/139) in the control group. The majority of supplemental
fixations were due to painful non-union.
Through the 24-month adverse event reporting window, urogenital events occurred with greater
frequency in the investigational groups (14.2%) compared to the control group (9.4%). Retrograde
ejaculation rates were greater in the investigational groups (11 subjects) compared to the control
group (1 subject) with the majority of events occurring in the early postoperative period.
The incidence of adverse events that were considered device related, including implant displacement/
loosening, implant malposition, and subsidence was all greater in the investigational groups compared
to the control group. The rates of these events were low, however, and may be partially attributed to
a learning curve associated with the laparoscopic surgical approach. The rate of non-union requiring
secondary surgery in the investigational groups was comparable to that of the control group. One
death was reported – a control group subject with cardiovascular disease.
POTENTIAL ADVERSE EVENTS
The following is a list of potential adverse events that may occur with spinal fusion surgery with
the INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device. Some of these
adverse events may have been previously reported in the adverse events table or have been reported
to the manufacturer:
• Allergic reaction.
• Anaphylactic reaction.
• Bone fracture.
• Bone resorption, which may be transient.
• Bowel or bladder problems.
• Cessation of any potential growth of the operated portion of the spine. Loss of spinal mobility
or function.
• Change in mental status.
• Damage to blood vessels and cardiovascular system compromise.
• Damage to internal organs and connective tissue.
• Death.
• Development of respiratory problems.
• Disassembly, bending, breakage, loosening, and/or migration of components.
• Dural tears.
• Elevated erythrocyte sedimentation rate.
• Encapsulated fluid collection.
• Erythematous tissue.
• Fetal development complications.
• Foreign body (allergic) reaction.
• Gastrointestinal complications.
• Hematoma
• Heterotopic and/or exuberant bone formation.
• Incisional complications.
• Infection.
• Inflammation.
• Insufflation complications.
• Itching.
• Localized edema (swelling).

ADVERSE EVENTS
(INFUSE® Bone Graft/LT-CAGE® Device data combined from all clinical trial experience with the device)
# of Patients Reporting &
Window)
Investigational #
Total Events
Control #
Total Events
Total #
Total Events
Total #
Total Events
Anatomical/Technical
Difficulty
11(3.8)
11
3(2.2)
3
0(0.0)
0
0(0.0)
0
70(24.3)
78
33(23.7)
37
23(17.2)
24
18(12.9)
21
2(0.7)
2
1(0.7)
1
4(3.0)
4
1(0.7)
1
17(5.9)
20
12(8.6)
14
7(5.2)
7
4(2.9)
4
0(0.0)
0
1(0.7)
1
2(1.5)
2
0(0.0)
0
0(0.0)
0
1(0.7)
1
0(0.0)
0
0(0.0)
0
56(19.4)
72
27(19.4)
32
14(10.4)
17
6(4.3)
8
0(0.0)
0
8(5.8)
8
0(0.0)
0
0(0.0)
0
Loosening
36(12.5)
40
16(11.5)
17
4(3.0)
4
3(2.1)
3
5(1.7)
5
0(0.0)
0
0(0.0)
0
0(0.0)
0
39(13.5)
45
23(16.5)
24
12(9.0)
12
5(3.6)
5
6(2.1)
6
3(2.2)
3
0(0.0)
0
0(0.0)
0
10(3.5)
10
13(9.4)
13
0(0.0)
0
0(0.0)
0
62(21.5)
81
37(26.6)
43
22(16.4)
29
17(12.1)
18
31(10.8)
36
13(9.4)
16
15(11.2)
20
11(7.9)
12
5(1.7)
5
4(2.9)
4
1(0.7)
1
1(0.7)
1
Retrograde
Ejaculation
11(7.9)
1
12
1(1.4)2
1
0(0.0)
0
0(0.0)
0
30(10.4)
37
17(12.2)
18
9(6.7)
9
8(5.7)
8
7(2.4)
7
2(1.4)
2
0(0.0)
0
0(0.0)
0
68(23.6)
82
34(24.5)
39
21(15.7)
23
20(14.3)
22
41(14.2)
45
13(9.4)
14
2(1.5)
2
3(2.1)
3
14(4.9)
15
5(3.6)
5
1(0.7)
1
0(0.0)
0
1(0.3)
1
0(0.0)
0
0(0.0)
0
0(0.0)
0
*Non-union adverse events that have not resulted in second surgery.
**Non-union adverse events that have resulted in a second surgery.
Percent of 140 males.
Percent of 70 males.
• Damage to internal organs and connective tissue.
• Death.
• Development of respiratory problems.
• Disassembly, bending, breakage, loosening, and/or migration of components.
• Dural tears.
• Elevated erythrocyte sedimentation rate.
• Encapsulated fluid collection.
• Erythematous tissue.
• Fetal development complications.
• Foreign body (allergic) reaction.
• Gastrointestinal complications.
• Hematoma
• Heterotopic and/or exuberant bone formation.
• Incisional complications.
• Infection.
• Inflammation.
• Insufflation complications.
• Itching.
• Localized edema (swelling).
• Neurological system compromise.
Clinical and Radiographic Effectiveness Evaluation
Individual subject success was defined as success in each of the primary clinical and radiographic
outcome parameters. Success for these parameters included:
1. the presence of radiographic fusion;
2. an improvement of at least 15 points from the baseline Oswestry score;
3. maintenance or improvement in neurological status;
4. the presence of no serious adverse event classified as implant-associated or implant/surgical
procedure-associated; and
5. no additional surgical procedure classified as “Failure.”
Study success was expressed as the number of individual subjects categorized as a success divided
by the total number of subjects evaluated. The table below describes the success rates for the individual
primary outcome parameters and overall success. All success rates were based on the data from the
24-month follow-up evaluation, and posterior probabilities of success were calculated using Bayesian
statistical methods.
The probability (also called the posterior probability) that the 24-month overall success rate for the
investigational groups was equivalent to the 24-month success rate for the control group was 99.4%
for the open surgical approach investigational group and almost 100% for the laparoscopic surgical
approach investigational group.
For a future patient receiving the INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device via
the open anterior surgical approach, the chance (the predictive probability) of overall success at 24
months would be 57.1% for the open surgical approach. Given the results of the trial, there is a 95%
probability that the chance of success ranges from 49.2% to 65.7%. For a future patient receiving the
INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device via the anterior laparoscopic surgical
approach, the chance of overall success at 24 months would be 68.0%. Given the results of the trial,
there is a 95% probability that the chance of success ranges from 59.3% to 76.5%. For a future patient
receiving the control treatment, the chance of overall success at 24 months would be 56.7%. Given the
results of the trial, there is a 95% probability that the chance of success ranges from 48.3% to 65.0%.
Surgical Results and Hospitalization
Surgical and Hospitalization Information
Investigational Open
Surgical Approach
Control Open
Surgical Approach
Investigational Laparoscopic
Surgical Approach
Mean Operative Time (hrs)
1.6* 2.0
1.9
Mean EBL (ml)
109.8
*
153.1
146.1
Hospitalization (days)
3.1
3.3
1.2
*
*
Statistically different from control
Surgical Results and Hospitalization
Surgical and Hospitalization Information
Investigational Open
Surgical Approach
Control Open
Surgical Approach
Investigational Laparoscopic
Surgical Approach
Mean Operative Time (hrs)
1.6* 2.0
1.9
Mean EBL (ml)
109.8
*
153.1
146.1
Hospitalization (days)
3.1
3.3
1.2
*
*
Statistically different from control
Posterior Probabilities of Success at 24 Months
Primary
Outcome
Variable
Investigational Open Surgical
Approach
Control Open Surgical
Approach
Investigational Laparoscopic
Surgical Approach
Posterior Mean
(95% HPD Credible Interval)
Posterior Mean
(95% HPD Credible Interval)
Posterior Mean
(95% HPD Credible Interval)
Fusion
92.8%
(88.5%, 96.9%)
88.1%
(82.6%, 99.3%)
93.0%
(87.9%, 97.5%)
Oswestry
71.0%
(63.4%, 78.7%)
70.9%
(63.1%, 79.1%)
83.0%
(75.6%, 90.5%)
Neurologic
81.0%
(74.5%, 87.9%)
81.7%
(74.9%, 88.7%)
89.0%
(83.1%, 94.8%)
Overall
Success
57.1%
(49.2%, 65.7%)
56.7%
(48.3%, 65.0%)
68.0%
(59.3%, 76.5%)
Type I collagen.
An evaluation was performed on the impact of a positive antibody response on overall success and
fusion success. There was very little difference in overall and individual success when antibody
status was taken into consideration.
During the course of the study, 6 pregnancies were reported – one in the control group and five in
the investigational groups. Two of the four pregnancies that occurred in the laparoscopic approach
group resulted in first trimester miscarriages. The other three pregnancies in the investigational
groups resulted in live births with no reported complications. None of the pregnant subjects had
antibody responses to rhBMP-2 or Type I collagen (bovine or human) that were detectable to the
limits of the sensitivity of the assay.
Through the 24-month adverse event reporting window, three cases of cancer were diagnosed – two
in the investigational group and one in the control group. One investigational subject was found to
have pancreatic cancer while another was diagnosed with breast cancer. A control subject was also
found to have breast cancer. No additional information is available on these subjects (e.g., BMP-2
receptor expression).
Post-Approval Study
A total of 145 investigational subjects were evaluated out to 6 years (72 months) after the initial
surgery in a post-approval study. The patient population was obtained from participants in both the
open and laparoscopic arms of the IDE clinical trial. Control subjects from the IDE study were not
followed in the post-approval study. Based on criteria from the IDE study, information pertaining to
the safety and effectiveness of the product was obtained at four and six years post-implantation,
including adverse events, an independent radiographic assessment of fusion, and an assessment of
pain and function. The table below summarizes the clinical and radiographic data evaluated during
the post approval study, as well as the overall success rate at each timepoint.
Overall Success 60.6% (77/127) 60.4% (84/139)
Oswestry Pain Improvement 81.5% (106/130) 79.0% (109/138)
Fusion 98.3% (117/119) 98.5% (128/130)
Neurological Status
(Maintenance or Improvement) 73.3% (96/131) 81.2% (112/138)
HOW SUPPLIED
INFUSE® Bone Graft component is supplied in five kit sizes containing all the components necessary
to prepare this portion of the device (i.e., the collagen sponge(s), a vial with the lyophilized growth
factor, a vial with sterile water for reconstituting the growth factor, syringes and needles). This
insert describes only the use of two kit sizes, the X Small (1.4cc) and XX Small (0.7cc) of the
INFUSE® Bone Graft. The Medtronic Titanium Threaded Interbody Fusion Device component is
supplied in a variety of sizes, which must be properly selected based on a specific patient’s anatomy.
STORAGE CONDITIONS
Store the INFUSE® Bone Graft component at room temperature [15-30 degrees Centigrade (59
to 86° F)]. The Medtronic Titanium Threaded Interbody Fusion Device component should also be
stored at room temperature.
DOSAGE AND ADMINISTRATION
INFUSE® Bone Graft component is prepared immediately prior to use from a kit containing all
necessary components. Once prepared, the INFUSE® Bone Graft component contains rhBMP-2
at a concentration of 1.5 mg/mL.
The size of the INFUSE® Bone Graft component kit and the volume of INFUSE® Bone Graft
component to be implanted are determined by the internal volume of the Medtronic Titanium Threaded
# of Patients Reporting &
Window)
Investigational #
Total Events
Control #
Total Events
Total #
Total Events
Total #
Total Events
11(3.8)
11
3(2.2)
3
0(0.0)
0
0(0.0)
0
70(24.3)
78
33(23.7)
37
23(17.2)
24
18(12.9)
21
2(0.7)
2
1(0.7)
1
4(3.0)
4
1(0.7)
1
17(5.9)
20
12(8.6)
14
7(5.2)
7
4(2.9)
4
0(0.0)
0
1(0.7)
1
2(1.5)
2
0(0.0)
0
0(0.0)
0
1(0.7)
1
0(0.0)
0
0(0.0)
0
56(19.4)
72
27(19.4)
32
14(10.4)
17
6(4.3)
8
0(0.0)
0
8(5.8)
8
0(0.0)
0
0(0.0)
0
5(1.7)
5
1(0.7)
1
0(0.0)
0
0(0.0)
0
36(12.5)
40
16(11.5)
17
4(3.0)
4
3(2.1)
3
5(1.7)
5
0(0.0)
0
0(0.0)
0
0(0.0)
0
39(13.5)
45
23(16.5)
24
12(9.0)
12
5(3.6)
5
6(2.1)
6
3(2.2)
3
0(0.0)
0
0(0.0)
0
10(3.5)
10
13(9.4)
13
0(0.0)
0
0(0.0)
0
62(21.5)
81
37(26.6)
43
22(16.4)
29
17(12.1)
18
31(10.8)
36
13(9.4)
16
15(11.2)
20
11(7.9)
12
5(1.7)
5
4(2.9)
4
1(0.7)
1
1(0.7)
1
11(7.9)
1
12
1(1.4)2
1
0(0.0)
0
0(0.0)
0
30(10.4)
37
17(12.2)
18
9(6.7)
9
8(5.7)
8
7(2.4)
7
2(1.4)
2
0(0.0)
0
0(0.0)
0
68(23.6)
82
34(24.5)
39
21(15.7)
23
20(14.3)
22
41(14.2)
45
13(9.4)
14
2(1.5)
2
3(2.1)
3
14(4.9)
15
5(3.6)
5
1(0.7)
1
0(0.0)
0
1(0.3)
1
0(0.0)
0
0(0.0)
0
0(0.0)
0
and bovine Type I collagen were assessed preoperatively and at 3 months post-operatively. Antibodies
to human Type I collagen were assessed if the antibody response to bovine Type I collagen was positive.
Primary and secondary clinical and radiographic effectiveness outcome parameters were evaluated
for all treated subjects at all follow-up evaluation timepoints identified above. The primary clinical
parameters assessed were of pain, function, and neurological status. The secondary clinical outcome
parameters assessed were general health status, back and leg pain, donor site pain (control subjects
only), patient satisfaction, and patient global perceived effect of the treatment. The primary radiographic
outcome parameter consisted of evaluations of fusion, while the secondary radiographic assessment
was disc height.
Fusion was evaluated at 6, 12, and 24 months post-op using plain radiographs (AP, lateral, and flexion/
extension films) and high resolution thin-slice CT scans (1mm slices with 1mm index on axial sagittal
and coronal reconstructions). Fusion was defined as the presence of bridging bone connecting the
• Non-union (or pseudarthrosis), delayed union, mal-union.
• Pain.
• Postoperative change in spinal curvature, loss of correction, height, and/or reduction.
• Retrograde ejaculation.
• Scar formation.
• Seroma.
• Tissue or nerve damage.
Note: Additional surgery may be necessary to correct some of these potential adverse events.
CLINICAL RESULTS
Clinical data to support the safety and effectiveness of the INFUSE® Bone Graft/LT-CAGE® Lumbar
Tapered Fusion Device were collected as part of a prospective, multi-center pivotal study that
consisted of randomized and non-randomized arms. The randomized arm contained two groups,
one investigational and one control. The control group was implanted with the LT-CAGE® Lumbar
Tapered Fusion Device filled with iliac crest autograft bone, while the investigational group was
implanted with the INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device. In both cases,
the surgical approach was an open anterior approach. The non-randomized arm contained only an
investigational group, where subjects were implanted with the INFUSE® Bone Graft/LT-CAGE®
Lumbar Tapered Fusion Device through a laparoscopic anterior approach. The control group from
the randomized arm was used as the control for the non-randomized arm.
Neither the investigators nor the subjects were blinded to the treatment. Subject blinding was not
possible due to the second surgical site resulting from the need to collect the iliac crest grafts.
The potential for investigator bias in the clinical outcome parameters was reduced by having the
subjects rate their outcome using objective self-assessments. The radiographic outcome parameters
were performed by independent radiologists who were blinded to treatment. These were the only
radiographic evaluations used for determining radiographic success.
The indication studied was degenerative disc disease (DDD) accompanied by back pain with or
without leg pain at a single level between L4 and S1 confirmed by history and radiographic studies.
Clinical and Radiographic Effectiveness Parameters
Patients were evaluated preoperatively (within 6 months of surgery), intraoperatively, and
postoperatively at 6 weeks, 3, 6, 12, and 24 months and biennially thereafter until the last subject
enrolled in the study had been seen for their 24-month evaluation. Complications and adverse events,
device-related or not, were evaluated over the course of the clinical trial. At each evaluation timepoint,
the primary and secondary clinical and radiographic outcome parameters were evaluated. Success
was determined from data collected during the initial 24 months of follow-up. Antibodies to rhBMP-2

at a concentration of 1.5 mg/mL.
The size of the INFUSE® Bone Graft component kit and the volume of INFUSE® Bone Graft
component to be implanted are determined by the internal volume of the Medtronic Titanium Threaded
Interbody Fusion Device components that are utilized. The patient’s anatomy will determine the
size of the Medtronic Titanium Threaded Interbody Fusion Device components to be used. Surgical
technique manuals for the INFUSE® Bone Graft with the specific Medtronic Titanium Threaded
Interbody Fusion Devices referenced in this package insert are available and will provide more
information on templating to determine the appropriate size Medtronic Titanium Threaded Interbody
Fusion Device component.
Only store the components in this INFUSE® Bone Graft kit in the manner described on the package,
only mix the components in the manner described in the directions, only add the reconstituted
rhBMP-2 to the ACS carrier provided in the manner described, and only use in the quantity and
indication specified in the package insert. Any other storage, mixture, or administration may cause
unanticipated adverse events.
PRODUCT COMPLAINTS
Any Health Care Professional (e.g., customer or user of this system of products) who has any
complaints or who has experienced any dissatisfaction in the product quality, identity, durability,
reliability, safety, effectiveness, and/or performance should notify the distributor or Medtronic. Further,
if any of the implanted INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device
component(s) ever “malfunctions,” (i.e., do not meet any of their performance specifications or
otherwise do not perform as intended), or are suspected of doing so, the distributor should be notified
immediately. If any Medtronic product ever “malfunctions” and may have caused or contributed to
the death or serious injury of a patient, the distributor should be notified immediately by telephone,
fax, or written correspondence. When filing a complaint, please provide the component(s) name
and number, lot number(s), your name and address, the nature of the complaint, and notification of
whether a written report from the distributor is requested.
DEVICE RETRIEVAL EFFORTS
Should it be necessary to remove an INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device, please call Medtronic prior to the scheduled surgery to receive instructions regarding
data collection, including histopathological, mechanical, and adverse event information.
Supplied by
Medtronic Sofamor Danek USA, Inc.
©2015 MEDTRONIC SOFAMOR DANEK USA, INC. All rights reserved.
INFUSE® Bone Graft in combination with LT-CAGE®, INTER FIX™ or INTER FIX™ RP devices
incorporate technology developed by Gary K. Michelson, M.D.
# of Patients Reporting &
Window)
Investigational #
Total Events
Control #
Total Events
Total #
Total Events
Total #
Total Events
11(3.8)
11
3(2.2)
3
0(0.0)
0
0(0.0)
0
70(24.3)
78
33(23.7)
37
23(17.2)
24
18(12.9)
21
2(0.7)
2
1(0.7)
1
4(3.0)
4
1(0.7)
1
17(5.9)
20
12(8.6)
14
7(5.2)
7
4(2.9)
4
0(0.0)
0
1(0.7)
1
2(1.5)
2
0(0.0)
0
0(0.0)
0
1(0.7)
1
0(0.0)
0
0(0.0)
0
56(19.4)
72
27(19.4)
32
14(10.4)
17
6(4.3)
8
0(0.0)
0
8(5.8)
8
0(0.0)
0
0(0.0)
0
36(12.5)
40
16(11.5)
17
4(3.0)
4
3(2.1)
3
5(1.7)
5
0(0.0)
0
0(0.0)
0
0(0.0)
0
39(13.5)
45
23(16.5)
24
12(9.0)
12
5(3.6)
5
6(2.1)
6
3(2.2)
3
0(0.0)
0
0(0.0)
0
10(3.5)
10
13(9.4)
13
0(0.0)
0
0(0.0)
0
62(21.5)
81
37(26.6)
43
22(16.4)
29
17(12.1)
18
31(10.8)
36
13(9.4)
16
15(11.2)
20
11(7.9)
12
5(1.7)
5
4(2.9)
4
1(0.7)
1
1(0.7)
1
11(7.9)
1
12
1(1.4)2
1
0(0.0)
0
0(0.0)
0
30(10.4)
37
17(12.2)
18
9(6.7)
9
8(5.7)
8
7(2.4)
7
2(1.4)
2
0(0.0)
0
0(0.0)
0
68(23.6)
82
34(24.5)
39
21(15.7)
23
20(14.3)
22
41(14.2)
45
13(9.4)
14
2(1.5)
2
3(2.1)
3
14(4.9)
15
5(3.6)
5
1(0.7)
1
0(0.0)
0
1(0.3)
1
0(0.0)
0
0(0.0)
0
0(0.0)
0
Fusion was evaluated at 6, 12, and 24 months post-op using plain radiographs (AP, lateral, and flexion/
extension films) and high resolution thin-slice CT scans (1mm slices with 1mm index on axial sagittal
and coronal reconstructions). Fusion was defined as the presence of bridging bone connecting the
inferior and superior vertebral bodies; a lack of motion on flexion/extension (≤ 3mm of translation and
< 5° of angulation); and no evidence of radiolucencies over more than 50% of either implant. Fusion
success was defined as the presence of all of these parameters plus the lack of a second surgical
intervention resulting from a non-union. All assessments were made from the plain films except for the
assessment of bridging bone, which was made using the CT scans only if bridging bone could not be
visualized on the plain film.
Pain and function were measured using the Oswestry Low Back Pain Disability Questionnaire. Success
was defined as a 15-point improvement in the Oswestry score from the pre-op baseline score.
Neurological status consisted of measurements of four parameters – motor, sensory, reflexes, and
straight leg raise (SLR). Neurological status success was defined as maintenance or improvement of
the pre-op baseline score for each parameter. Overall neurological status success required that each
individual parameter be a success for that subject to be counted as a success.
Patient Demographics and Accountability
A total of 143 open approach investigational and 136 control patients were enrolled in the randomized
arm of the study and received the device. A total of 134 subjects were enrolled in the non-randomized
arm of the study and received the device. For the majority of the demographic parameters, there were
no differences in pre-op demographics across the three populations.
Surgical Results and Hospitalization
Clinical and Radiographic Effectiveness Evaluation
Individual subject success was defined as success in each of the primary clinical and radiographic
outcome parameters. Success for these parameters included:
1. the presence of radiographic fusion;
2. an improvement of at least 15 points from the baseline Oswestry score;
3. maintenance or improvement in neurological status;
4. the presence of no serious adverse event classified as implant-associated or implant/surgical
procedure-associated; and
5. no additional surgical procedure classified as “Failure.”
Study success was expressed as the number of individual subjects categorized as a success divided
by the total number of subjects evaluated. The table below describes the success rates for the individual
primary outcome parameters and overall success. All success rates were based on the data from the
24-month follow-up evaluation, and posterior probabilities of success were calculated using Bayesian
statistical methods.
Surgical Results and Hospitalization
Surgical and Hospitalization Information
Investigational Open
Surgical Approach
Control Open
Surgical Approach
Investigational Laparoscopic
Surgical Approach
Mean Operative Time (hrs)
1.6* 2.0
1.9
Mean EBL (ml)
109.8
*
153.1
146.1
Hospitalization (days)
3.1
3.3
1.2
*
*
S
tatistically different from control
Surgical Results and Hospitalization
Surgical and Hospitalization Information
Investigational Open
Surgical Approach
Control Open
Surgical Approach
Investigational Laparoscopic
Surgical Approach
Mean Operative Time (hrs)
1.6* 2.0
1.9
Mean EBL (ml)
109.8
*
153.1
146.1
Hospitalization (days)
3.1
3.3
1.2
*
*
S
tatistically different from control
Posterior Probabilities of Success at 24 Months
Primary
Outcome
Variable
Investigational Open Surgical
Approach
Control Open Surgical
Approach
Investigational Laparoscopic
Surgical Approach
Posterior Mean
(95% HPD Credible Interval)
Posterior Mean
(95% HPD Credible Interval)
Posterior Mean
(95% HPD Credible Interval)
Fusion
92.8%
(88.5%, 96.9%)
88.1%
(82.6%, 99.3%)
93.0%
(87.9%, 97.5%)
Oswestry
71.0%
(63.4%, 78.7%)
70.9%
(63.1%, 79.1%)
83.0%
(75.6%, 90.5%)
Neurologic
81.0%
(74.5%, 87.9%)
81.7%
(74.9%, 88.7%)
89.0%
(83.1%, 94.8%)
Overall
Success
57.1%
(49.2%, 65.7%)
56.7%
(48.3%, 65.0%)
68.0%
(59.3%, 76.5%)

unanticipated adverse events.
PRODUCT COMPLAINTS
Any Health Care Professional (e.g., customer or user of this system of products) who has any
complaints or who has experienced any dissatisfaction in the product quality, identity, durability,
reliability, safety, effectiveness, and/or performance should notify the distributor or Medtronic. Further,
if any of the implanted INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device
component(s) ever “malfunctions,” (i.e., do not meet any of their performance specifications or
otherwise do not perform as intended), or are suspected of doing so, the distributor should be notified
immediately. If any Medtronic product ever “malfunctions” and may have caused or contributed to
the death or serious injury of a patient, the distributor should be notified immediately by telephone,
fax, or written correspondence. When filing a complaint, please provide the component(s) name
and number, lot number(s), your name and address, the nature of the complaint, and notification of
whether a written report from the distributor is requested.
DEVICE RETRIEVAL EFFORTS
Should it be necessary to remove an INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device, please call Medtronic prior to the scheduled surgery to receive instructions regarding
data collection, including histopathological, mechanical, and adverse event information.
Supplied by
Medtronic Sofamor Danek USA, Inc.
©2015 MEDTRONIC SOFAMOR DANEK USA, INC. All rights reserved.
INFUSE® Bone Graft in combination with LT-CAGE®, INTER FIX™ or INTER FIX™ RP devices
incorporate technology developed by Gary K. Michelson, M.D.
# of Patients Reporting &
Window)
Investigational #
Total Events
Control #
Total Events
Total #
Total Events
Total #
Total Events
11(3.8)
11
3(2.2)
3
0(0.0)
0
0(0.0)
0
70(24.3)
78
33(23.7)
37
23(17.2)
24
18(12.9)
21
2(0.7)
2
1(0.7)
1
4(3.0)
4
1(0.7)
1
17(5.9)
20
12(8.6)
14
7(5.2)
7
4(2.9)
4
0(0.0)
0
1(0.7)
1
2(1.5)
2
0(0.0)
0
0(0.0)
0
1(0.7)
1
0(0.0)
0
0(0.0)
0
56(19.4)
72
27(19.4)
32
14(10.4)
17
6(4.3)
8
0(0.0)
0
8(5.8)
8
0(0.0)
0
0(0.0)
0
36(12.5)
40
16(11.5)
17
4(3.0)
4
3(2.1)
3
5(1.7)
5
0(0.0)
0
0(0.0)
0
0(0.0)
0
39(13.5)
45
23(16.5)
24
12(9.0)
12
5(3.6)
5
6(2.1)
6
3(2.2)
3
0(0.0)
0
0(0.0)
0
10(3.5)
10
13(9.4)
13
0(0.0)
0
0(0.0)
0
62(21.5)
81
37(26.6)
43
22(16.4)
29
17(12.1)
18
31(10.8)
36
13(9.4)
16
15(11.2)
20
11(7.9)
12
5(1.7)
5
4(2.9)
4
1(0.7)
1
1(0.7)
1
11(7.9)
1
12
1(1.4)2
1
0(0.0)
0
0(0.0)
0
30(10.4)
37
17(12.2)
18
9(6.7)
9
8(5.7)
8
7(2.4)
7
2(1.4)
2
0(0.0)
0
0(0.0)
0
68(23.6)
82
34(24.5)
39
21(15.7)
23
20(14.3)
22
41(14.2)
45
13(9.4)
14
2(1.5)
2
3(2.1)
3
14(4.9)
15
5(3.6)
5
1(0.7)
1
0(0.0)
0
1(0.3)
1
0(0.0)
0
0(0.0)
0
0(0.0)
0
Neurological status consisted of measurements of four parameters – motor, sensory, reflexes, and
straight leg raise (SLR). Neurological status success was defined as maintenance or improvement of
the pre-op baseline score for each parameter. Overall neurological status success required that each
individual parameter be a success for that subject to be counted as a success.
Patient Demographics and Accountability
A total of 143 open approach investigational and 136 control patients were enrolled in the randomized
arm of the study and received the device. A total of 134 subjects were enrolled in the non-randomized
arm of the study and received the device. For the majority of the demographic parameters, there were
no differences in pre-op demographics across the three populations.
Surgical Results and Hospitalization
Clinical and Radiographic Effectiveness Evaluation
Individual subject success was defined as success in each of the primary clinical and radiographic
outcome parameters. Success for these parameters included:
1. the presence of radiographic fusion;
2. an improvement of at least 15 points from the baseline Oswestry score;
3. maintenance or improvement in neurological status;
4. the presence of no serious adverse event classified as implant-associated or implant/surgical
procedure-associated; and
5. no additional surgical procedure classified as “Failure.”
Study success was expressed as the number of individual subjects categorized as a success divided
by the total number of subjects evaluated. The table below describes the success rates for the individual
primary outcome parameters and overall success. All success rates were based on the data from the
24-month follow-up evaluation, and posterior probabilities of success were calculated using Bayesian
statistical methods.
The probability (also called the posterior probability) that the 24-month overall success rate for the
investigational groups was equivalent to the 24-month success rate for the control group was 99.4%
for the open surgical approach investigational group and almost 100% for the laparoscopic surgical
approach investigational group.
For a future patient receiving the INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device via
the open anterior surgical approach, the chance (the predictive probability) of overall success at 24
months would be 57.1% for the open surgical approach. Given the results of the trial, there is a 95%
probability that the chance of success ranges from 49.2% to 65.7%. For a future patient receiving the
INFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device via the anterior laparoscopic surgical
approach, the chance of overall success at 24 months would be 68.0%. Given the results of the trial,
there is a 95% probability that the chance of success ranges from 59.3% to 76.5%. For a future patient
receiving the control treatment, the chance of overall success at 24 months would be 56.7%. Given the
results of the trial, there is a 95% probability that the chance of success ranges from 48.3% to 65.0%.
Surgical Results and Hospitalization
Surgical and Hospitalization Information
Investigational Open
Surgical Approach
Control Open
Surgical Approach
Investigational Laparoscopic
Surgical Approach
Mean Operative Time (hrs)
1.6* 2.0
1.9
Mean EBL (ml)
109.8
*
153.1
146.1
Hospitalization (days)
3.1
3.3
1.2
*
*
S
tatistically different from control
Surgical Results and Hospitalization
Surgical and Hospitalization Information
Investigational Open
Surgical Approach
Control Open
Surgical Approach
Investigational Laparoscopic
Surgical Approach
Mean Operative Time (hrs)
1.6* 2.0
1.9
Mean EBL (ml)
109.8
*
153.1
146.1
Hospitalization (days)
3.1
3.3
1.2
*
*
S
tatistically different from control
Posterior Probabilities of Success at 24 Months
Primary
Outcome
Variable
Investigational Open Surgical
Approach
Control Open Surgical
Approach
Investigational Laparoscopic
Surgical Approach
Posterior Mean
(95% HPD Credible Interval)
Posterior Mean
(95% HPD Credible Interval)
Posterior Mean
(95% HPD Credible Interval)
Fusion
92.8%
(88.5%, 96.9%)
88.1%
(82.6%, 99.3%)
93.0%
(87.9%, 97.5%)
Oswestry
71.0%
(63.4%, 78.7%)
70.9%
(63.1%, 79.1%)
83.0%
(75.6%, 90.5%)
Neurologic
81.0%
(74.5%, 87.9%)
81.7%
(74.9%, 88.7%)
89.0%
(83.1%, 94.8%)
Overall
Success
57.1%
(49.2%, 65.7%)
56.7%
(48.3%, 65.0%)
68.0%
(59.3%, 76.5%)
Safety and Immune Response Evaluation
The assessment of safety consisted of an evaluation of the reported adverse events, as well as an
evaluation of antibodies to rhBMP-2, bovine Type I collagen, and human Type I collagen. The complete
list of complications, adverse events, and subsequent interventions is described in the Adverse
Events section above. The presence of antibodies was assessed at the pre-op and 3-month post-op
visits using ELISA. If there was a positive response to bovine Type I collagen, the serum was also
tested for antibodies to human Type I collagen. The screening ELISA cutpoint for positive antibody
responses was set to 5 times the standard deviation of sera from normal human donors. Subjects
were considered to have an elevated immune response if the preoperative test was negative (titer
< 50) and postoperative test was positive (titer ≥ 50) or if the preoperative test was positive and the
postoperative test was positive with a three-fold higher titer than the preoperative test.
There were 3 subjects who had positive antibody responses to rhBMP-2 – 1 subject in each of the
study groups. The rates of positive antibody response to rhBMP-2 were 0.7% in the open surgical
approach investigational group and 0.8% in the laparoscopic surgical approach investigational and
open surgical approach control groups. While there is a theoretical possibility that antibodies to
rhBMP-2 could neutralize endogenous BMP-2, thereby interfering with subsequent bone healing,
this was not observed during the course of the study.
Sixty-six subjects were considered to have an authentic elevated antibody response to bovine Type
I collagen – 18 open surgical approach investigational subjects, 32 laparoscopic surgical approach
investigational subjects, and 16 control subjects. No subjects had positive responses to human
Type I collagen.
An evaluation was performed on the impact of a positive antibody response on overall success and
fusion success. There was very little difference in overall and individual success when antibody
status was taken into consideration.
During the course of the study, 6 pregnancies were reported – one in the control group and five in
the investigational groups. Two of the four pregnancies that occurred in the laparoscopic approach
group resulted in first trimester miscarriages. The other three pregnancies in the investigational
groups resulted in live births with no reported complications. None of the pregnant subjects had
antibody responses to rhBMP-2 or Type I collagen (bovine or human) that were detectable to the
limits of the sensitivity of the assay.
Through the 24-month adverse event reporting window, three cases of cancer were diagnosed – two
in the investigational group and one in the control group. One investigational subject was found to
have pancreatic cancer while another was diagnosed with breast cancer. A control subject was also
found to have breast cancer. No additional information is available on these subjects (e.g., BMP-2
receptor expression).

Through the 24-month adverse event reporting window, three cases of cancer were diagnosed – two
in the investigational group and one in the control group. One investigational subject was found to
have pancreatic cancer while another was diagnosed with breast cancer. A control subject was also
found to have breast cancer. No additional information is available on these subjects (e.g., BMP-2
receptor expression).
Post-Approval Study
A total of 145 investigational subjects were evaluated out to 6 years (72 months) after the initial
surgery in a post-approval study. The patient population was obtained from participants in both the
open and laparoscopic arms of the IDE clinical trial. Control subjects from the IDE study were not
followed in the post-approval study. Based on criteria from the IDE study, information pertaining to
the safety and effectiveness of the product was obtained at four and six years post-implantation,
including adverse events, an independent radiographic assessment of fusion, and an assessment of
pain and function. The table below summarizes the clinical and radiographic data evaluated during
the post approval study, as well as the overall success rate at each timepoint.
Overall Success Rates – Post Approval Study
Variable 4-Year Evaluation 6-Year Evaluation
Overall Success 60.6% (77/127) 60.4% (84/139)
Oswestry Pain Improvement 81.5% (106/130) 79.0% (109/138)
Fusion 98.3% (117/119) 98.5% (128/130)
Neurological Status
(Maintenance or Improvement) 73.3% (96/131) 81.2% (112/138)
HOW SUPPLIED
INFUSE® Bone Graft component is supplied in five kit sizes containing all the components necessary
to prepare this portion of the device (i.e., the collagen sponge(s), a vial with the lyophilized growth
factor, a vial with sterile water for reconstituting the growth factor, syringes and needles). This
insert describes only the use of two kit sizes, the X Small (1.4cc) and XX Small (0.7cc) of the
INFUSE® Bone Graft. The Medtronic Titanium Threaded Interbody Fusion Device component is
supplied in a variety of sizes, which must be properly selected based on a specific patient’s anatomy.
STORAGE CONDITIONS
Store the INFUSE® Bone Graft component at room temperature [15-30 degrees Centigrade (59
to 86° F)]. The Medtronic Titanium Threaded Interbody Fusion Device component should also be
stored at room temperature.
DOSAGE AND ADMINISTRATION
INFUSE® Bone Graft component is prepared immediately prior to use from a kit containing all
necessary components. Once prepared, the INFUSE® Bone Graft component contains rhBMP-2
at a concentration of 1.5 mg/mL.
The size of the INFUSE® Bone Graft component kit and the volume of INFUSE® Bone Graft
component to be implanted are determined by the internal volume of the Medtronic Titanium Threaded
Interbody Fusion Device components that are utilized. The patient’s anatomy will determine the
size of the Medtronic Titanium Threaded Interbody Fusion Device components to be used. Surgical
technique manuals for the INFUSE® Bone Graft with the specific Medtronic Titanium Threaded
Interbody Fusion Devices referenced in this package insert are available and will provide more
information on templating to determine the appropriate size Medtronic Titanium Threaded Interbody
Fusion Device component.
Only store the components in this INFUSE® Bone Graft kit in the manner described on the package,
only mix the components in the manner described in the directions, only add the reconstituted
rhBMP-2 to the ACS carrier provided in the manner described, and only use in the quantity and
indication specified in the package insert. Any other storage, mixture, or administration may cause
unanticipated adverse events.

HOW SUPPLIED
INFUSE® Bone Graft component is supplied in five kit sizes containing all the components necessary
to prepare this portion of the device (i.e., the collagen sponge(s), a vial with the lyophilized growth
factor, a vial with sterile water for reconstituting the growth factor, syringes and needles). This
insert describes only the use of two kit sizes, the X Small (1.4cc) and XX Small (0.7cc) of the
INFUSE® Bone Graft. The Medtronic Titanium Threaded Interbody Fusion Device component is
supplied in a variety of sizes, which must be properly selected based on a specific patient’s anatomy.
STORAGE CONDITIONS
Store the INFUSE® Bone Graft component at room temperature [15-30 degrees Centigrade (59
to 86° F)]. The Medtronic Titanium Threaded Interbody Fusion Device component should also be
stored at room temperature.
DOSAGE AND ADMINISTRATION
INFUSE® Bone Graft component is prepared immediately prior to use from a kit containing all
necessary components. Once prepared, the INFUSE® Bone Graft component contains rhBMP-2
at a concentration of 1.5 mg/mL.
The size of the INFUSE® Bone Graft component kit and the volume of INFUSE® Bone Graft
component to be implanted are determined by the internal volume of the Medtronic Titanium Threaded
Interbody Fusion Device components that are utilized. The patient’s anatomy will determine the
size of the Medtronic Titanium Threaded Interbody Fusion Device components to be used. Surgical
technique manuals for the INFUSE® Bone Graft with the specific Medtronic Titanium Threaded
Interbody Fusion Devices referenced in this package insert are available and will provide more
information on templating to determine the appropriate size Medtronic Titanium Threaded Interbody
Fusion Device component.
Only store the components in this INFUSE® Bone Graft kit in the manner described on the package,
only mix the components in the manner described in the directions, only add the reconstituted
rhBMP-2 to the ACS carrier provided in the manner described, and only use in the quantity and
indication specified in the package insert. Any other storage, mixture, or administration may cause
unanticipated adverse events.
PRODUCT COMPLAINTS
Any Health Care Professional (e.g., customer or user of this system of products) who has any
complaints or who has experienced any dissatisfaction in the product quality, identity, durability,
reliability, safety, effectiveness, and/or performance should notify the distributor or Medtronic. Further,
if any of the implanted INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody Fusion Device
component(s) ever “malfunctions,” (i.e., do not meet any of their performance specifications or
otherwise do not perform as intended), or are suspected of doing so, the distributor should be notified
immediately. If any Medtronic product ever “malfunctions” and may have caused or contributed to
the death or serious injury of a patient, the distributor should be notified immediately by telephone,
fax, or written correspondence. When filing a complaint, please provide the component(s) name
and number, lot number(s), your name and address, the nature of the complaint, and notification of
whether a written report from the distributor is requested.
DEVICE RETRIEVAL EFFORTS
Should it be necessary to remove an INFUSE® Bone Graft/Medtronic Titanium Threaded Interbody
Fusion Device, please call Medtronic prior to the scheduled surgery to receive instructions regarding
data collection, including histopathological, mechanical, and adverse event information.
Supplied by
Medtronic Sofamor Danek USA, Inc.
©2015 MEDTRONIC SOFAMOR DANEK USA, INC. All rights reserved.
INFUSE® Bone Graft in combination with LT-CAGE®, INTER FIX™ or INTER FIX™ RP devices
incorporate technology developed by Gary K. Michelson, M.D.

FRANÇAIS
GREFFON OSSEUX INFUSE®/DISPOSITIF CONIQUE DE FUSION LOMBAIRE LT-CAGE®
GREFFON OSSEUX INFUSE®/DISPOSITIF DE FUSION FILETÉ INTER FIX™
GREFFON OSSEUX INFUSE®/DISPOSITIF DE FUSION FILETÉ À PROFIL RÉDUIT INTER FIX™ RP
KITS DE GREFFON OSSEUX INFUSE® XS (1,4CC) ET XXS (0,7CC) UNIQUEMENT
INFORMATIONS MÉDICALES IMPORTANTES
ATTENTION: La loi fédérale (aux États-Unis) n’autorise la vente de ces dispositifs que par un médecin ou sur ordre d’un médecin ayant une formation
adéquate.
Les pages suivantes contiennent des informations médicales importantes sur l’utilisation du greffon osseux INFUSE® avec différentes cages
métalliques Medtronic pour arthrodèse intersomatique. Ces dispositifs d’arthrodèse intersomatique incluent le dispositif conique de fusion lombaire
LT-CAGE®, le dispositif de fusion fileté INTER FIX™ et le dispositif de fusion fileté à profil réduit INTER FIX™ RP. Dans la notice ci-après, ces cages seront
collectivement désignées sous le terme de dispositif d’arthrodèse intersomatique fileté en titane de Medtronic.
DESCRIPTION
Le greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic est constitué de deux composants décomposés en
trois parties: une cage métallique pour fusion rachidienne, une protéine morphogénétique recombinante d’os humain et une structure porteuse/
échafaudage pour la protéine morphogénétique d’os humain et l’os en résultant. Le composant greffon osseux INFUSE® est inséré dans le composant
dispositif d’arthrodèse intersomatique fileté en titane de Medtronic pour former l’ensemble greffon osseux INFUSE®/dispositif d’arthrodèse
intersomatique fileté en titane de Medtronic. Ces composants doivent être utilisés en tant que système pour les indications prescrites ci-
dessous. Le composant de solution de protéine morphogénétique d’os humain ne doit pas être utilisé sans le porteur/échafaudage ou
avec un autre porteur/échafaudage que celui décrit dans ce document. Le composant greffon osseux INFUSE® ne doit pas être utilisé sans
le composant dispositif d’arthrodèse intersomatique fileté en titane de Medtronic.
Composant dispositif d’arthrodèse intersomatique fileté en titane de Medtronic
Dispositif conique de fusion lombaire LT-CAGE®
Le dispositif LT-CAGE® est constitué d’un cylindre usiné creux et perforé avec deux côtés plats qui se font face. La cage de forme conique, avec un
angle de 8,8°, est disponible en diamètres de 14mm à 18mm à l’extrémité étroite du cône et de 17mm à 22mm à l’extrémité large du cône, et en
longueurs de 20mm à 26mm. Deux perforations sont pratiquées dans chacun des deux côtés plats. Une seule ouverture arrondie est pratiquée
dans chacun des deux côtés arrondis. Les implants sont pourvus d’un filetage hélicoïdal sur leur surface extérieure. Une extrémité du dispositif est
fermée. L’autre extrémité est ouverte pour pouvoir être remplie par le composant greffon osseux INFUSE®.
Les implants LT-CAGE® sont fabriqués en alliage de titane de qualité chirurgicale (Ti-6Al-4V) tel que décrit dans la norme ASTMF136 ou son
équivalent ISO.
Le composant dispositif conique de fusion lombaire LT-CAGE® est vendu séparément du composant greffon osseux INFUSE®; toutefois, ces deux
composants doivent être utilisés ensemble. L’étiquette de l’emballage du dispositif conique de fusion lombaire LT-CAGE® contient toutes les
informations produit relatives à ce composant.
Dispositif de fusion fileté INTER FIX™
Le dispositif INTER FIX™ est constitué d’un cylindre creux et perforé avec deux côtés parallèles et un embout. La cage est disponible en diamètres de
12mm à 24mm et en longueurs de 20mm à 29mm. Les embouts des cages INTER FIX™ sont dimensionnés en fonction du diamètre des cylindres
et sont appliqués sur l’extrémité ouverte des cylindres après leur remplissage par le greffon osseux INFUSE®.
Les implants dispositif de fusion fileté INTER FIX™ sont fabriqués en alliage de titane de qualité chirurgicale (Ti-6Al-4V) tel que décrit par la norme
ASTMF136 ou son équivalent ISO.
Le composant dispositif de fusion fileté INTER FIX™ est vendu séparément du composant greffon osseux INFUSE®; toutefois, ces deux composants
doivent être utilisés ensemble. L’étiquette de l’emballage du dispositif de fusion fileté INTER FIX™ contient toutes les informations produit relatives
à ce composant.
Dispositif de fusion fileté à profil réduit INTER FIX™ RP
Le dispositif INTER FIX™ RP est constitué d’un cylindre creux et perforé doté d’une unique rainure extérieure, large et incurvée, pratiquée le long
de l’ensemble de l’axe longitudinal qui se prolonge dans le diamètre intérieur du dispositif. Les deux extrémités de l’implant INTER FIX™ RP sont
fermées. La cage est disponible en diamètres de 12mm à 24mm et en longueurs de 20mm à29mm.
Les implants dispositif de fusion fileté INTER FIX™ RP sont fabriqués en alliage de titane de qualité chirurgicale (Ti-6Al-4V) tel que décrit par la
norme ASTMF136 ou son équivalent ISO.
Le composant dispositif de fusion fileté INTER FIX™ RP est vendu séparément du composant greffon osseux INFUSE®; toutefois, ces deux composants
doivent être utilisés ensemble. L’étiquette de l’emballage du dispositif de fusion fileté INTERFIX™ RP contient toutes les informations produit
relatives à ce composant.
REMARQUE : le dispositif de fusion fileté INTER FIX™ et le dispositif de fusion fileté INTER FIX™ RP peuvent être utilisés ensemble pour un
traitement au niveau rachidien. Les implants dispositif conique de fusion lombaire LT-CAGE® ne peuvent pas être utilisés conjointement
avec les implants INTER FIX™ OU INTER FIX™ RP pour un traitement au niveau rachidien.
Composant greffon osseux INFUSE®
Le greffon osseux INFUSE® est constitué de la protéine morphogénétique recombinante d’os humain de type2 (rhBMP-2, connue sous le nom de
dibotermine alfa) placée sur une éponge de collagène résorbable (absorbable collagen sponge, ACS). Le composant greffon osseux INFUSE® produit
un nouveau tissu osseux dans la zone de l’implantation. Selon les données d’études non cliniques, le processus de formation osseuse se développe
de l’extérieur de l’implant vers le centre, jusqu’à ce que l’ensemble du composant greffon osseux INFUSE® soit remplacé par de l’os trabéculaire.

La rhBMP-2 est l’agent actif du composant greffon osseux INFUSE®. Le rhBMP-2 est une molécule protéique dimérique reliée par un pont bisulfure,
comprenant deux protomères majeurs de 114 et 131acides aminés. Chaque sous-unité est glycosylée à un endroit, avec des glycanes de type
haut-mannose. La rhBMP-2 est produite par une lignée de cellules ovariennes de hamster chinois génétiquement modifiée.
La rhBMP-2 et ses excipients sont lyophilisés. Lors de la reconstitution, chaque millilitre de solution rhBMP-2 contient: 1,5mg de rhBMP-2; 5,0mg
de sucrose, NF; 25mg de glycine, USP; 3,7mg d’acide L-glutamique, FCC; 0,1mg de chlorure de sodium, USP; 0,1mg de polysorbate 80, NF; et
1,0ml d’eau stérile. La solution reconstituée de rhBMP-2 a un pH de4,5, est limpide, va de incolore à jaune clair et ne comprend quasiment aucune
particule visible.
L’ACS est une matrice implantable pour rhBMP-2 douce, blanche, souple et absorbante. L’ACS est constituée de collagène bovin de typeI obtenu sur
le tendon fléchisseur profond (tendon d’Achille). L’ACS agit comme porteuse pour la rhBMP-2 et comme échafaudage pour la nouvelle formation
osseuse.
Les tableaux fournis dans le «Mode d’emploi» de cette notice décrivent comment utiliser les kits XS (1,4cc) et XXS (0,7cc) pour remplir des cages
uniques plus petites si cela s’avère nécessaire pendant la chirurgie. Pour des cages de taille supérieure, les recommandations sont les suivantes: Si
un kit L a été utilisé à l’origine pour remplir deux cages, utiliser un kit S plus un kit XS (1,4cc) pour remplir une cage unique; si un kit M a été utilisé
à l’origine, utiliser un kit S pour remplir une cage unique;si un kit S a été utilisé à l’origine, utiliser un kit XS (1,4cc) pour remplir une cage unique;
et si un kit XS (1,4cc) a été utilisé à l’origine, utiliser un kit XXS (0,7cc) pour remplir une cage unique.
Chaque kit contient tous les composants nécessaires pour préparer le composant greffon osseux INFUSE®: la rhBMP-2 qui doit être reconstituée, de
l’eau stérile, des éponges de collagène résorbables, une ou plusieurs seringues avec aiguille(s), la présente notice et les instructions de préparation.
Le nombre de chaque élément peut varier selon la taille du kit. S’il est prévu d’utiliser deux kits (p.ex. XS [1,4cc] et XXS [0,7cc]), ouvrir les deux kits
en même temps et mélanger chacun conformément aux instructions.
La rhBMP-2 est fournie sous forme de poudre lyophilisée conditionnée en flacons délivrant 1,05mg de protéine pour les kits XS et XXS. Après une
reconstitution appropriée, la configuration donne la formule et la concentration (1,5mg/ml) de rhBMP-2. La solution est ensuite appliquée sur
l’éponge de collagène résorbable fournie. Le composant greffon osseux INFUSE® est préparé au moment de la chirurgie et autorise un certain délai
(pas moins de 15minutes) avant la mise en place dans le(s) composant(s) dispositif d’arthrodèse intersomatique fileté en titane de Medtronic. Les
instructions de préparation contiennent les détails complets relatifs à la préparation du greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique
fileté en titane de Medtronic.
Les garanties implicites de qualité marchande, ainsi que l’aptitude à répondre à un objectif ou à un usage particulier, sont expressément exclues.
Se reporter au catalogue MDT ou aux tarifs pour obtenir davantage d’informations sur les garanties et les limites de responsabilité.
INDICATIONS
Le greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic est indiqué pour les procédures de fusion rachidienne
chez les patients ayant atteint leur maturité squelettique atteints de discopathie dégénérative (degenerative disc disease, DDD) à un niveau de L2S1. La discopathie dégénérative se définit comme une douleur dorsale d’origine discale avec dégénérescence discale confirmée par les antécédents
du patient et des examens radiographiques. Les patients atteints de DDD peuvent également être atteints de spondylolisthésis jusqu’au gradeI
ou de rétrolisthésis de grade1 au niveau concerné. Les patients recevant le greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté
en titane de Medtronic doivent avoir eu au moins six mois de traitement non chirurgical avant le traitement par greffon osseux INFUSE®/dispositif
d’arthrodèse intersomatique fileté en titane de Medtronic. Le greffon osseux INFUSE® avec dispositif conique de fusion lombaire LT-CAGE® doit
être implanté par une approche ouverte antérieure ou laparoscopique antérieure. Le greffon osseux INFUSE® avec dispositif fileté de fusion INTER
FIX™ ou INTER FIX™ RP doit être implanté par une approche ouverte antérieure.
MODE D’EMPLOI
Le composant greffon osseux INFUSE® est préparé au moment de la chirurgie, au bloc opératoire, en reconstituant la rhBMP-2 lyophilisée avec de
l’eau stérile (voir Instructions de préparation) puis en appliquant uniformément la solution de rhBMP-2 reconstituée sur l’ACS. Le composant greffon
osseux INFUSE® est ensuite inséré dans le composant dispositif d’arthrodèse intersomatique fileté en titane de Medtronic. L’ensemble du dispositif
est ensuite implanté par une approche chirurgicale antérieure (voir manuel de technique chirurgicale). Si le composant greffon osseux INFUSE®
n’est pas utilisé dans les deux heures suivant la reconstitution, il doit être mis au rebut.
Le composant greffon osseux INFUSE® ne doit pas être stérilisé par l’hôpital. S’il n’est pas fourni stérile, le composant dispositif d’arthrodèse
intersomatique fileté en titane de Medtronic doit être stérilisé avant l’insertion du composant greffon osseux INFUSE®. Se reporter à la notice
spécifique du dispositif d’arthrodèse intersomatique fileté en titane de Medtronic pour les informations relatives à l’emballage, le nettoyage/la
décontamination et la stérilisation de ce composant et ses instruments.
Cette notice décrit uniquement l’utilisation des kits de greffon osseux INFUSE® en tailles XS (1,4cc) et XXS (0,7cc). Se reporter à la notice des autres
tailles de kits de INFUSE® (S, M, L et LII) pour les instructions d’utilisation s’y rapportant. Les tableaux ci-dessous répertorient les correspondances
de taille entre les kits de greffon osseux INFUSE® XS (1,4cc) et XXS (0,7cc) et le composant dispositif d’arthrodèse intersomatique fileté en titane
de Medtronic:
Combinaisons greffon osseux INFUSE®/dispositif de fusion fileté INTER FIX™
Dispositif de fusion fileté INTER FIX™ Kit(s) de greffon osseux INFUSE® recommandé(s)
Nº de pièce
(diamètre principal en mm
Taille
x longueur en mm) Nº de pièce Taille de kit
Duo de dispositifs INTER FIX™
Volume de greffon
rhBMP-2/ACS
reconstitué (cc)
890120 12x20 7510100 XS 1,4
890125 12x25
7510050 +
7510100
XXS + XS 2,1

Combinaisons greffon osseux INFUSE®/dispositif de fusion fileté INTER FIX™ et INTER FIX™ RP
Dispositif INTER FIX™ RP avec dispositif INTER FIX™
Dispositifs de fusion filetés INTER FIX™ et
INTER FIX™ RP
Nº de pièce des
dispositifs INTER
FIX™
†
Taille
(diamètre en mm
x longueur en mm) Nº de pièce Taille de kit
Kit(s) de greffon osseux INFUSE® recommandé(s)
Volume de greffon
rhBMP-2/ACS
reconstitué (cc)
890120+9011221 12x20 7510100 XS 1,4
890125+9011225 12x25
7510050 +
7510100
XXS + XS 2,1
† La taille du dispositif de fusion fileté INTER FIX™ utilisée doit correspondre à celle du dispositif de fusion fileté INTER FIX™ RP.
Combinaisons du greffon osseux INFUSE®/dispositif de fusion fileté INTER FIX™ RP
Duo de dispositifs INTER FIX™ RP
Dispositif de fusion fileté
INTER FIX™ RP
Nº de pièce
x longueur en mm) Nº de pièce Taille de kit
Taille
(diamètre en mm
Kit(s) de greffon osseux INFUSE® recommandé(s)
Volume de greffon
rhBMP-2/ACS
reconstitué (cc)
9011221 12x20 7510100 XS 1,4
9011225 12x25
7510050 +
7510100
XXS + XS 2,1
UTILISATION D’UNE CAGE UNIQUE
Si une seule cage nécessite un greffon osseux INFUSE® en raison de la perte ou de la contamination d’une ou plusieurs éponges, des cages uniques
peuvent être remplies en utilisant des kits XS (1,4 cc) et/ou XXS (0,7 cc) comme indiqué
ci-dessous :
Combinaisons greffon osseux INFUSE®/dispositif conique de fusion lombaire LT-CAGE®
Dispositif conique de fusion
lombaire LT-CAGE®
Remplissage d’un dispositif LT-CAGE® unique
Kit(s) de greffon osseux INFUSE® recommandé(s)
Volume de greffon
rhBMP-2/ACS
reconstitué (cc)
Nº de pièce
(diamètre principal en mm
Taille
x longueur en mm) Nº de pièce Taille de kit
8941420 14x20 7510100 XS 1,4
8941423 14x23 7510100 XS 1,4
8941620 16x20
8941623 16x23
7510050 +
7510100
7510050 +
7510100
XXS + XS 2,1
XXS + XS 2,1
Combinaisons greffon osseux INFUSE®/dispositif de fusion fileté INTER FIX™
Remplissage d’un dispositif INTER FIX™ unique
Dispositif de fusion fileté
INTER FIX™
Nº de pièce
Taille
(diamètre en mm
x longueur en mm)
Nº de pièce Taille de kit
Kit(s) de greffon osseux INFUSE® recommandé(s)
Volume de greffon
rhBMP-2/ACS
reconstitué (cc)
890120 12x20 7510050 XXS 0,7
890125 12x25 7510100 XS 1,4
890140 14x20 7510100 XS 1,4
890143 14x23 7510100 XS 1,4

Combinaisons greffon osseux INFUSE®/dispositif de fusion fileté INTER FIX™
Dispositif de fusion fileté
INTER FIX™
890146 14x26
890149 14x29
890160 16x20
890163 16x23
Remplissage d’un dispositif INTER FIX™ unique
Kit(s) de greffon osseux INFUSE® recommandé(s)
7510050 +
7510100
7510050 +
7510100
7510050 +
7510100
7510050 +
7510100
XXS + XS 2,1
XXS + XS 2,1
XXS + XS 2,1
XXS + XS 2,1
Combinaisons du greffon osseux INFUSE®/dispositif de fusion fileté INTER FIX™ RP
Dispositif de fusion fileté
INTER FIX™ Kit(s) de greffon osseux INFUSE® recommandé(s)
Taille
Nº de pièce
9011221 12x20 7510050 XXS 0,7
9011225 12x25 7510100 XS 1,4
9011420 14x20 7510100 XS 1,4
9011423 14x23 7510100 XS 1,4
9011426 14x26
9011429 14x29
9011620 16x20
9011623 16x23
(diamètre en mm
x longueur en mm) Nº de pièce Taille de kit
Remplissage d’un dispositif INTER FIX™ RP unique
7510050 +
7510100
7510050 +
7510100
7510050 +
7510100
7510050 +
7510100
XXS + XS 2,1
XXS + XS 2,1
XXS + XS 2,1
XXS + XS 2,1
Volume de greffon
rhBMP-2/ACS
reconstitué (cc)
CONTREINDICATIONS
• Le greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic est contre-indiqué chez les patients présentant
une hypersensibilité connue à la protéine morphogénétique recombinante d’os humain de type2, au collagène bovin de typeI ou à d’autres
composants de la formule.
• Le greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic ne doit pas être utilisé à proximité d’une tumeur
réséquée ou existante, chez les patients avec une tumeur maligne active ou chez les patients actuellement traités pour une tumeur maligne.
• Le greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic ne doit pas être utilisé chez les patients n’ayant
pas atteint leur maturité squelettique (âge inférieur à 18ans ou absence de fermeture épiphysaire sur les radios).
• Le greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic ne doit pas être utilisé chez la femme enceinte.
Les effets potentiels de la rhBMP-2 sur le fœtus humain n’ont pas été évalués.
• Le greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic ne doit pas être implanté chez les patients
présentant une infection active au niveau de la zone opératoire ou une allergie au titane ou à un alliage de titane.
MISES EN GARDE
• Il a été démontré dans une étude expérimentale chez le lapin que la rhBMP-2 crée des anticorps capables de traverser le placenta. Il a été
évalué que l’ossification réduite des os frontaux et pariétaux n’était pas fréquente (<3%) chez les fœtus de lapins immunisés contre la rhBMP-2.
Cependant, aucun effet n’a été constaté sur le développement de bourgeon de membre. Il n’existe pas d’études adéquates et bien contrôlées
concernant les femmes enceintes. Les femmes en âge de procréer doivent être averties par leur chirurgien des risques potentiels pour le fœtus
et informées des autres traitements orthopédiques possibles.
• Les femmes en âge de procréer doivent être informées du fait que la formation d’anticorps à la rhBMP-2, ou son incidence sur le développement du
fœtus humain, n’ont pas été évaluées dans leur intégralité. Au cours de l’essai clinique validant la sécurité et l’efficacité du greffon osseux INFUSE®/
dispositif conique de fusion lombaire LT-CAGE®, 2 patients sur 277(0,7%) traités avec un composant greffon osseux INFUSE® et 1 patient sur 127
(0,8%) traités avec une autogreffe ont développé des anticorps anti-rhBMP-2. L’effet des anticorps maternels à la rhBMP-2 (pouvant être présent
plusieurs mois après l’implantation du dispositif) sur le fœtus est inconnu. De plus, on ignore si l’expression fœtale de BMP-2 pourrait réexposer
les mères auparavant positives aux anticorps. En théorie, la réexposition peut entraîner une réaction immunitaire à la BMP-2 plus importante
avec de possibles conséquences néfastes pour le fœtus. Cependant, dans l’étude sur les lapins, la grossesse n’a pas provoqué une augmentation
des anticorps. Les études sur les souris génétiquement modifiées ont indiqué que la BMP-2 est essentielle pour le développement du fœtus et
qu’un manque d’activité de la protéine BMP-2 peut entraîner une mort néonatale ou des malformations congénitales. On ignore si les anticorps
antiBMP-2 peuvent affecter le développement du fœtus ou dans quelle mesure ils peuvent réduire l’activité de la protéine BMP-2.

• Le greffon osseux INFUSE® ne doit pas être utilisé immédiatement avant ou pendant la grossesse. Les femmes en mesure de procréer doivent être
averties de ne pas tomber enceintes pendant l’année qui suit le traitement par greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique
fileté en titane de Medtronic.
• La sécurité et l’efficacité du greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic chez les mères qui
allaitent n’ont pas été établies. On ignore si la protéine BMP-2 est excrétée dans le lait humain.
Généralités
• Le dispositif d’arthrodèse intersomatique fileté en titane de Medtronic/greffon osseux INFUSE® ne doit pas être utilisé chez les patients susceptibles
de présenter une malignité au site d’implantation.
• La sécurité et l’efficacité du composant greffon osseux INFUSE® utilisé avec d’autres implants rachidiens implantés ailleurs que dans le rachis
lombaire inférieur ou utilisé dans le cadre de techniques chirurgicales autres que les approches ouvertes antérieures (dispositifs LT-CAGE®, INTER
FIX™ et INTER FIX™ RP) ou laparoscopiques antérieures (dispositif LT-CAGE®) n’ont pas été établies.
• L’implantation du greffon osseux INFUSE®/dispositif conique de fusion lombaire LT-CAGE® via une approche chirurgicale laparoscopique antérieure
est associée à une incidence supérieure d’éjaculation rétrograde (10,5%, 6patients sur57 de sexe masculin) en comparaison d’une implantation
via une approche chirurgicale ouverte antérieure (6,4%, 5patients sur78 de sexe masculin). Ces deux pourcentages sont supérieurs à celui du
groupe de contrôle avec implantation via une approche antérieure qui n’avait pas reçu le greffon osseux INFUSE® (1,5%, 1patient sur68 de sexe
masculin). Dans l’étude randomisée de l’approche chirurgicale ouverte antérieure, l’éjaculation rétrograde a été signalée dans le groupe greffon
osseux INFUSE® chez 17,6% (3/17) des patients de sexe masculin ayant subi une intervention par approche transpéritonéale, par rapport à 3,2%
(2/61) des patients de sexe masculin avec une approche rétropéritonéale. Dans le groupe de contrôle, le pourcentage d’éjaculation rétrograde était
de 7,6% (1/13) chez les hommes avec une approche transpéritonéale, comparé à 0% (0/55) chez les hommes avec une approche rétropéritonéale.
Dans les deux groupes de traitement regroupés, l’éjaculation rétrograde a été signalée chez 13,3% (4/30) des hommes ayant subi une approche
transpéritonéale et chez 1,8% (2/116) des hommes ayant subi une approche rétropéritonéale. Cette différence est statistiquement significative
(p=0,017, test exact de Fisher). Les patients de sexe masculin doivent être avertis de ce risque potentiel avant d’envisager l’utilisation du greffon
osseux INFUSE®.
• La sécurité et l’efficacité d’emploi du greffon osseux INFUSE® implanté dans la rachis cervical n’ont pu être établies. L’utilisation de ce produit n’est
approuvée que dans le rachis lombaire comme exposé ci-dessus.
– Quand les fusions rachidiennes cervicales antérieures ont été réalisées à l’aide d’un composant greffon osseux INFUSE®, des cas d’œdème ont
été rapportés durant la première semaine postopératoire. Dans certains de ces cas, le gonflement provoqué a été suffisamment grave pour
générer des troubles des voies respiratoires, nécessitant parfois une chirurgie en urgence.
– Au cours d’un essai clinique comparant la fusion cervicale antérieure sur un seul niveau avec greffon osseux INFUSE® par rapport à un contrôle
n’utilisant pas de greffon osseux INFUSE®, 16,4% des patients traités avec le greffon osseux INFUSE® ont manifesté une dysphagie, contre 7,3%
des patients du contrôle. L’essentiel des événements dysphagiques sont intervenus dans les quatre premières semaines après la chirurgie,
et la plupart des événements étaient classés comme non graves, p.ex. événements ne menaçant pas le pronostic vital et ne nécessitant pas
d’hospitalisation. Si la dysphagie peut survenir après une intervention cervicale antérieure, elle risque de se produire plus fréquemment ou
dans une plus large mesure en présence du greffon osseux INFUSE®.
– Lorsque les fusions cervicales antérieures étaient effectuées en utilisant le greffon osseux INFUSE®, l’aspect radiographique de la
paraostéoarthropathie (heterotopic ossification, HO) antérieure a été observé chez certains patients, le plus souvent en région antérieure et
supérieure au niveau traité. Dans certains cas de HO sévère, une fusion au niveau adjacent et une réduction du mouvement ont également
été observés. La HO est susceptible de se produire plus souvent ou dans une plus large mesure avec l’utilisation du greffon osseux INFUSE®.
Formation osseuse
• Quand la discopathie dégénérative était traitée par une procédure d’arthrodèse intersomatique lombaire postérieure, une formation osseuse
postérieure située à l’extérieur de l’espace intervertébral était observée chez certains patients. Bien qu’elle ne soit pas clairement associée à des
mesures de résultats cliniques principales (p.ex. douleurs dans les jambes), dans la plupart des cas, une formation osseuse à l’extérieur de l’espace
intervertébral n’est pas souhaitable, pouvant aboutir à une compression du nerf et nécessiter une intervention chirurgicale.
• Une utilisation inappropriée du produit, telle qu’une préparation ne respectant pas les indications, une compression de l’implant rhBMP-2/ACS plus
importante que nécessaire, ou un volume de remplissage excessif pour la nouvelle formation osseuse, peut modifier la concentration de rhBMP-2
et, par là même, inhiber la capacité du rhBMP-2/ACS à se transformer en os et/ou entraîner des complications. Une telle utilisation de l’implant
rhBMP-2/ACS peut entraîner des preuves radiographiques de résorption. Ces découvertes peuvent être asymptomatiques ou symptomatiques.
Un modèle de mouton développé pour tester l’hypothèse qu’un volume de remplissage excessif et/ou qu’une hyperconcentration de solution
rhBMP-2 montrent une preuve radiographique de résorption osseuse a été initialement évalué et semble confirmer l’hypothèse de ce mécanisme.
• La pose de rhBMP-2/ACS peut entraîner une résorption initiale de l’os trabéculaire pouvant être transitoire.
• La migration du dispositif a été signalée lors de l’utilisation de rhBMP-2/ACS dans le cadre d’une spondylodèse. La migration du dispositif a été
signalée en la présence et l’absence de résorption osseuse.
• Une compression du nerf associée à une formation osseuse hétérotopique a été rapportée chez des patients ayant subi une chirurgie rachidienne
avec le composant rhBMP-2/ACS. Une intervention chirurgicale peut être nécessaire pour remédier aux symptômes.
Accumulations de fluide et/ou œdème
• La formation d’accumulations de fluide (parfois encapsulées) a donné lieu dans certains cas à une compression du nerf et à des douleurs, qui
peuvent nécessiter une intervention clinique (aspiration et/ou retrait chirurgical) si les symptômes persistent. Bon nombre de ces cas ont été
rapportés lorsque le complexe rhBMP-2/ACS était utilisé avec des approches/dispositifs non approuvés ou d’une façon non conforme aux
instructions d’utilisation.
• Bien qu’il existe des preuves anecdotiques et documentaires suggérant que le volume de remplissage excessif et/ou l’hyperconcentration de
solution rhBMP-2 peuvent entraîner une formation de fluide et/ou un œdème, des modèles chez l’animal permettant une évaluation scientifique
de ces effets n’existent pas à l’heure actuelle.
PRÉCAUTIONS
REMARQUE À L’ATTENTION DU MÉDECIN : bien que le médecin soit un professionnel informé et qu’il représente l’intermédiaire entre l’entreprise
et le patient, les informations médicales importantes contenues dans ce document doivent être transmises au patient.
NE S’APPLIQUE QU’AUX ÉTATSUNIS

Généralités
• La sécurité et l’efficacité d’applications répétées du composant greffon osseux INFUSE® n’ont pas été établies.
• Le greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic ne doit être utilisé que par des chirurgiens
expérimentés en procédures de fusion rachidienne et ayant suivi une formation spécifique pour l’implantation de ce dispositif via une approche
laparoscopique antérieure et/ou ouverte antérieure.
• Deux composants dispositif d’arthrodèse intersomatique fileté en titane de Medtronic doivent si possible être implantés côte à côte au niveau
chirurgical.
• Les composants dispositif d’arthrodèse intersomatique fileté en titane de Medtronic et les instruments doivent être stérilisés avant leur utilisation
conformément aux consignes de stérilisation fournies dans la notice du composant en question, sauf s’il est fourni stérile et qu’il est clairement
étiqueté en tant que tel.
• Lorsque le dispositif est utilisé aux niveaux rachidiens entre L
anatomiques, p.ex. l’aorte, sur la mise en place de l’implant.
• Le développement d’une formation osseuse exubérante ou hétérotopique aux niveaux lombaires supérieurs (L
sur certaines structures neurovasculaires, p.ex. l’aorte et le système nerveux sympathique.
• La sécurité et l’efficacité du dispositif aux niveaux rachidiens L
établies.
et L4, il convient de prendre en considération l’éventuelle incidence de structures
2
- L4) peut avoir un effet délétère
2
- L4 ou chez les patients atteints de rétrolisthésis jusqu’au grade1 n’ont pas été
2
• Le greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic est prévu pour un usage unique exclusivement.
Mettre au rebut tout produit non utilisé et utiliser un nouveau dispositif pour les applications ultérieures.
• Avant chaque utilisation, vérifiez si l’emballage, les flacons et les opercules ne sont pas endommagés. Si tel est le cas, n’utilisez pas le produit.
Conservez l’emballage et les flacons et contactez un représentant Medtronic.
• N’utilisez pas le produit après la date d’expiration indiquée sur l’étiquette.
Insuffisance hépatique et rénale
• La sécurité et l’efficacité du greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic chez les patients avec
une atteinte hépatique ou rénale n’ont pas été établies. Les études pharmacocinétiques sur la rhBMP-2 indiquent que les systèmes rénaux et
hépatiques participent à sa clairance.
Gériatrie
• Le nombre de patients âgés au moins de 65ans inclus dans les études cliniques relatives au greffon osseux INFUSE®/dispositif conique de fusion
lombaire LT-CAGE® était insuffisant pour déterminer si leur réaction diffère de celle de sujets plus jeunes.
Formation osseuse
• La sécurité et l’efficacité du greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic n’ont pas été démontrées
chez les patients avec des maladies osseuses métaboliques.
• Il existe une possibilité de formation osseuse hétérotopique ou excessive indésirable.
Formation d’anticorps/réactions allergiques
• La sécurité et l’efficacité du greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic n’ont pas été démontrées
chez les patients avec une maladie auto-immune.
• La sécurité et l’efficacité du greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic n’ont pas été démontrées
chez les patients atteint d’une maladie immunosuppressive ou ayant une immunosuppression suite à une radiothérapie, une chimiothérapie,
un traitement stéroïdien ou à d’autres traitements.
Immunogénicité
• De même qu’avec toutes les protéines thérapeutiques, il est possible que des réponses immunitaires soient déclenchées contre le composant
greffon osseux INFUSE®. La réponse immunitaire contre les composants greffon osseux INFUSE® a été évaluée chez 349patients du groupe
expérimental et 183patients du groupe de contrôle subissant des arthrodèses intersomatiques lombaires.
– Anticorps anti-rhBMP-2 : 2patients sur349 (0,6%) recevant le composant greffon osseux INFUSE® ont développé des anticorps contre 1patient
sur183 (0,5%) dans le groupe de contrôle.
– Anticorps anticollagène bovin de type I : 18,1% des patients recevant le composant greffon osseux INFUSE® ont développé des anticorps
anticollagène bovin de typeI contre 14,2% dans le groupe de contrôle. Aucun patient des deux groupes n’a développé d’anticorps anticollagène
humain de typeI.
– La présence d’anticorps à la rhBMP-2 n’a pas été associée à des événements indésirables à médiation immunitaire, tels que des réactions
allergiques. On ignore la capacité de neutralisation des anticorps à la rhBMP-2.
• L’incidence de la détection des anticorps dépend étroitement de la sensibilité et de la spécificité du dosage. De plus, l’incidence de la détection
des anticorps peut être influencée par plusieurs facteurs, dont la manipulation des échantillons, les traitements concomitants et les maladies
sous-jacentes. Pour ces raisons, la comparaison entre l’incidence d’anticorps contre le composant greffon osseux INFUSE® et l’incidence d’anticorps
contre d’autres produits peut être trompeuse.
ÉVÉNEMENTS INDÉSIRABLES
Le greffon osseux INFUSE®/dispositif conique de fusion lombaire LT-CAGE® a été implanté chez 288patients du groupe expérimental et comparé à
139patients du groupe de contrôle qui ont reçu un dispositif conique de fusion lombaire LT-CAGE® rempli avec une autogreffe de la crête iliaque. Chez
les patients du groupe expérimental, le dispositif a été implanté soit via une approche chirurgicale ouverte antérieure, soit via une approche chirurgicale
laparoscopique antérieure. Chez les patients du groupe de contrôle, seule l’approche chirurgicale ouverte antérieure a été utilisée.
Les taux d’événements indésirables présentés sont calculés en divisant le nombre de patients ayant eu au moins une occurrence d’un événement
indésirable particulier par le nombre total de patients dans ce groupe de traitement. Comme aucun sujet du groupe de contrôle n’a été évalué aux
points temporels 48mois et 72mois, les événements rapportés à ces points temporels concernent uniquement les sujets du groupe expérimental.

ÉVÉNEMENTS INDÉSIRABLES
(Données pour le greffon osseux INFUSE®/dispositif LT-CAGE® regroupées à partir de toute l’expérience issue des essais cliniques avec ce dispositif)
Nb de patients ayant signalé
24 mois)
Expérimental Nb
Total d’événements
Contrôle Nb
d’événements
Nb total
d’événements
Nb total
otal
d’événements
Difficulté
anatomique/technique
11(3,8)
11
3(2,2) 3 0(0,0) 0 0(0,0)
0
Douleurs dorsales
et/ou dans les jambes
70(24,3)
78
33(23,7)
37
23(17,2)
24
18(12,9)
21
2(0,7) 2 1(0,7) 1 4(3,0) 4 1(0,7)
1
17(5,9)
20
12(8,6)
14
7(5,2) 7 4(2,9)
4
0(0,0) 0 1(0,7) 1 2(1,5) 2 0(0,0)
0
0(0,0) 0 1(0,7) 1 0(0,0) 0 0(0,0)
0
56(19,4)
72
27(19,4)
32
14(10,4)
17
6(4,3)
8
0(0,0) 0 8(5,8) 8 0(0,0) 0 0(0,0)
0
Déplacement/
desserrage de l’implant
5(1,7) 5 1(0,7) 1 0(0,0) 0 0(0,0)
0
36(12,5)
40
16(11,5)
17
4(3,0) 4 3(2,1)
3
5(1,7) 5 0(0,0) 0 0(0,0) 0 0(0,0)
0
39(13,5)
45
23(16,5)
24
12(9,0)
12
5(3,6)
5
Absence de
consolidation*
6(2,1) 6 3(2,2) 3 0(0,0) 0 0(0,0)
0
Absence de
consolidation**
10(3,5)
10
13(9,4)
13
0(0,0) 0 0(0,0)
0
62(21,5)
81
37(26,6)
43
22(16,4)
29
17(12,1)
18
31(10,8)
36
13(9,4)
16
15(11,2)
20
11(7,9)
12
5(1,7) 5 4(2,9) 4 1(0,7) 1 1(0,7)
1
12
1
0
0
30(10,4)
37
17(12,2)
18
9(6,7) 9 8(5,7)
8
7(2,4) 7 2(1,4) 2 0(0,0) 0 0(0,0)
0
68(23,6)
82
34(24,5)
39
21(15,7)
23
20(14,3)
22
41(14,2)
45
13(9,4)
14
2(1,5) 2 3(2,1)
3
Vasculaire
peropératoire
14(4,9)
15
5(3,6) 5 1(0,7) 1 0(0,0)
0
1(0,3) 1 0(0,0) 0 0(0,0) 0 0(0,0)
0
ÉVÉNEMENTS INDÉSIRABLES
(Données pour le greffon osseux INFUSE®/dispositif LT-CAGE® regroupées à partir de toute l’expérience issue des essais cliniques avec ce dispositif)
Chirurgie
En postopératoire
(1 jour -
< 4 semaines)
6 semaines
(≥ 4 semaines -
< 9 semaines)
3 mois
(≥ 9 semaines -
< 5 mois)
6 mois
(≥ 5 mois -
< 9 mois)
12 mois
(≥ 9 mois -
< 19 mois)
24 mois
(≥ 19 mois -
< 30 mois)
Nb de patients ayant signalé
des ÉI et nombre total d’ÉI
(Pour une période de suivi de
24 mois)
48 mois
(≥ 30 mois -
< 60 mois)
72 mois
(≥ 60 mois -
< 84 mois)
Complication
Expérim.
Contrôle
Expérim.
Contrôle
Expérim.
Contrôle
Expérim.
Contrôle
Expérim.
Contrôle
Expérim.
Contrôle
Expérim.
Contrôle
Expérimental Nb
Nb (% de 288)
Total d’événements
Contrôle Nb
(% de 139) Total
d’événements
Nb total
(% de 134) Total
d’événements
Nb total
(% de 140) Total
d’événements
Difficulté
anatomique/technique
11 3 0 0 0 0 0 0 0 0 0 0 0
0
11(3,8)
11
3(2,2) 3 0(0,0) 0 0(0,0)
0
Douleurs dorsales
et/ou dans les jambes
0 0 12 4 11 5 12 5 15 4 20 7 8
12
70(24,3)
78
33(23,7)
37
23(17,2)
24
18(12,9)
21
Cancer 0 0 0 0 0 0 0 1 0 0 1 0 1 0
2(0,7) 2 1(0,7) 1 4(3,0) 4 1(0,7)
1
Cardiovasculaire
1 0 6 5 5 2 1 3 2 1 4 2 1
1
17(5,9)
20
12(8,6)
14
7(5,2) 7 4(2,9)
4
Décès 0 0 0 0 0 0 0 0 0 1 0 0 0 0
0(0,0) 0 1(0,7) 1 2(1,5) 2 0(0,0)
0
Lésion de la dure-mère
0 0 0 0 0 0 0 1 0 0 0 0 0
0
0(0,0) 0 1(0,7) 1 0(0,0) 0 0(0,0)
0
Gastro-intestinal
1 0 40
22 2 0 5 1 7 1
10 3 7
5
56(19,4)
72
27(19,4)
32
14(10,4)
17
6(4,3)
8
Lié à la zone de greffe
0 0 0 8 0 0 0 0 0 0 0 0 0
0
0(0,0) 0 8(5,8) 8 0(0,0) 0 0(0,0)
0
Déplacement/
desserrage de l’implant
0 0 1 1 3 0 1 0 0 0 0 0 0
0
5(1,7) 5 1(0,7) 1 0(0,0) 0 0(0,0)
0
Infection 0 0
19 9 8 4 5 1 5 1 3 0 0
2
36(12,5)
40
16(11,5)
17
4(3,0) 4 3(2,1)
3
Implant mal positionné
5 0 0 0 0 0 0 0 0 0 0 0 0
0
5(1,7) 5 0(0,0) 0 0(0,0) 0 0(0,0)
0
Neurologique
0 0 8 5 7 3 5 2 6 2 13 4 6
8
39(13,5)
45
23(16,5)
24
12(9,0)
12
5(3,6)
5
Absence de
consolidation*
0 0 0 0 0 0 0 0 1 2 4 0 1
1
6(2,1) 6 3(2,2) 3 0(0,0) 0 0(0,0)
0
Absence de
consolidation**
0 0 0 1 0 1 2 0 3 4 4 6 1
1
10(3,5)
10
13(9,4)
13
0(0,0) 0 0(0,0)
0
Autre 5 6
18
11 9 2 3 4
11 4 15 8 20
8
62(21,5)
81
37(26,6)
43
22(16,4)
29
17(12,1)
18
Autre douleur
0 0 3 1 2 1 4 2 6 1 10 8 11
3
31(10,8)
36
13(9,4)
16
15(11,2)
20
11(7,9)
12
Respiratoire
0 0 3 2 1 0 0 0 1 0 0 1 0
1
5(1,7) 5 4(2,9) 4 1(0,7) 1 1(0,7)
1
Éjaculation rétrograde
0 0 5 1 4 0 1 0 0 0 2 0 0
0
11(7,9)
1
12
1(1,4)2
1
0(0,0) 0 0(0,0)
0
Événement rachidien
0 0 1 2 1 0 6 2 10 4 10 8 9
2
30(10,4)
37
17(12,2)
18
9(6,7) 9 8(5,7)
8
Affaissement
0 0 3 2 2 0 1 0 1 0 0 0 0
0
7(2,4) 7 2(1,4) 2 0(0,0) 0 0(0,0)
0
Traumatisme
0 0 4 5 5 3 11 7 14 5 28
11
20
8
68(23,6)
82
34(24,5)
39
21(15,7)
23
20(14,3)
22
Urogénital 0 0
24 5 3 0 2 3 6 3 3 1 7
2
41(14,2)
45
13(9,4)
14
2(1,5) 2 3(2,1)
3
Vasculaire
peropératoire
15 5 0 0 0 0 0 0 0 0 0 0 0
0
14(4,9)
15
5(3,6) 5 1(0,7) 1 0(0,0)
0
Fracture vertébrale
0 0 1 0 0 0 0 0 0 0 0 0 0
0
1(0,3) 1 0(0,0) 0 0(0,0) 0 0(0,0)
0
Tout événement
indésirable
228(79,2)
117(84,2)
84(62,7)
64(45,7)
*Événements indésirables d’une fracture non consolidée qui n’ont pas donné lieu à une deuxième chirurgie.
**Événements indésirables d’une fracture non consolidée qui ont donné lieu à une deuxième chirurgie.
Pourcentage parmi 140 hommes.
2
Pourcentage parmi 70 hommes.
Chirurgie
Complication Expérim. Contrôle Expérim. Contrôle Expérim. Contrôle Expérim. Contrôle Expérim. Contrôle Expérim. Contrôle Expérim. Contrôle
11 3 0 0 0 0 0 0 0 0 0 0 0 0
0 0 12 4 11 5 12 5 15 4 20 7 8 12
Cancer 0 0 0 0 0 0 0 1 0 0 1 0 1 0
Cardiovasculaire 1 0 6 5 5 2 1 3 2 1 4 2 1 1
Décès 0 0 0 0 0 0 0 0 0 1 0 0 0 0
Lésion de la dure-mère 0 0 0 0 0 0 0 1 0 0 0 0 0 0
Gastro-intestinal 1 0 40 22 2 0 5 1 7 1 10 3 7 5
Lié à la zone de greffe 0 0 0 8 0 0 0 0 0 0 0 0 0 0
0 0 1 1 3 0 1 0 0 0 0 0 0 0
Infection 0 0 19 9 8 4 5 1 5 1 3 0 0 2
Implant mal positionné 5 0 0 0 0 0 0 0 0 0 0 0 0 0
Neurologique 0 0 8 5 7 3 5 2 6 2 13 4 6 8
0 0 0 0 0 0 0 0 1 2 4 0 1 1
0 0 0 1 0 1 2 0 3 4 4 6 1 1
Autre 5 6 18 11 9 2 3 4 11 4 15 8 20 8
Autre douleur 0 0 3 1 2 1 4 2 6 1 10 8 11 3
Respiratoire 0 0 3 2 1 0 0 0 1 0 0 1 0 1
Éjaculation rétrograde 0 0 5 1 4 0 1 0 0 0 2 0 0 0
Événement rachidien 0 0 1 2 1 0 6 2 10 4 10 8 9 2
Affaissement 0 0 3 2 2 0 1 0 1 0 0 0 0 0
Traumatisme 0 0 4 5 5 3 11 7 14 5 28 11 20 8
Urogénital 0 0 24 5 3 0 2 3 6 3 3 1 7 2
15 5 0 0 0 0 0 0 0 0 0 0 0 0
Fracture vertébrale 0 0 1 0 0 0 0 0 0 0 0 0 0 0
Tout événement
indésirable
En postopératoire
(1 jour -
< 4 semaines)
6 semaines
(≥ 4 semaines -
< 9 semaines)
3 mois
(≥ 9 semaines -
< 5 mois)
228(79,2) 117(84,2) 84(62,7) 64(45,7)
6 mois
(≥ 5 mois -
< 9 mois)
12 mois
(≥ 9 mois -
< 19 mois)
24 mois
(≥ 19 mois -
< 30 mois)
des ÉI et nombre total d’ÉI
(Pour une période de suivi de
Nb (% de 288)
11(7,9)
1
(% de 139) Total
1(1,4)2
48 mois
(≥ 30 mois -
< 60 mois)
(% de 134) Total
0(0,0)
72 mois
(≥ 60 mois -
< 84 mois)
(% de 140) T
0(0,0)
1
Les pourcentages rapportés pour plusieurs événements indésirables étaient élevés, mais similaires dans le groupe expérimental et le groupe de
contrôle. Ces événements incluaient des douleurs dorsales et dans les jambes, des événements neurologiques, des événements gastro-intestinaux,
des événements rachidiens, des événements cardiovasculaires et des infections.
Certains des événements indésirables rapportés ont même requis des interventions chirurgicales ultérieurement à la chirurgie pratiquée initialement.
Le pourcentage de sujets ayant requis une seconde intervention chirurgicale était de 10,4% (30/288) dans les groupes expérimentaux et de 13,7%
(19/139) dans le groupe de contrôle. La majorité des fixations supplémentaires était due à une absence de consolidation douloureuse.
Lors de la période de 24mois pendant laquelle les événements indésirables ont été signalés, des événements indésirables au niveau du système
génito-urinaire ont été observés plus fréquemment dans les groupes expérimentaux (14,2%) que dans le groupe de contrôle (9,4%). Les pourcentages
d’éjaculation rétrograde étaient supérieurs dans les groupes expérimentaux (11sujets) comparativement au groupe de contrôle (1sujet), la majorité
des événements se produisant au début de la période postopératoire.
L’incidence d’événements indésirables considérés comme liés au dispositif, notamment les déplacements/desserrements, les mauvais positionnements
et les régressions de l’implant, était systématiquement supérieure dans les groupes expérimentaux comparativement au groupe de contrôle. Les
pourcentages de ces événements étaient toutefois faibles et peuvent partiellement être attribués à une phase d’apprentissage liée à l’approche
chirurgicale laparoscopique. Le pourcentage d’absence de consolidation nécessitant une deuxième chirurgie dans les groupes expérimentaux était
comparable à celui du groupe de contrôle. Un cas de décès a été rapporté, touchant un sujet du groupe de contrôle atteint d’une maladie cardiovasculaire.
Événements indésirables possibles
La liste ci-dessous énumère les événements indésirables éventuels associés à la fusion rachidienne avec greffon osseux INFUSE®/dispositif d’arthrodèse
intersomatique fileté en titane de Medtronic. Certains de ces événements indésirables peuvent avoir été rapportés précédemment dans le tableau
des événements indésirables ou avoir été rapportés au fabricant:
• Réaction allergique.
• Réaction anaphylactique.
• Fracture osseuse.

• Résorption osseuse, qui peut être transitoire.
• Troubles intestinaux ou urinaires.
• Arrêt de la croissance éventuelle de la partie opérée du rachis. Perte de la mobilité du rachis ou de sa fonction.
• Modification de l’état mental.
• Lésion des vaisseaux sanguins et affections de l’appareil cardiovasculaire.
• Lésion des organes internes et des tissus conjonctifs.
• Décès.
• Développement de problèmes respiratoires.
• Dislocation, torsion, rupture, desserrement et/ou migration des composants.
• Déchirure de la dure-mère.
• Vitesse de sédimentation des érythrocytes élevée.
• Accumulations de fluide encapsulées.
• Tissus érythémateux.
• Complications du développement fœtal.
• Réaction allergique au corps étranger.
• Complications gastro-intestinales.
• Hématomes.
• Formation osseuse hétérotopique et/ou exubérante.
• Complications cicatricielles.
• Infection.
• Inflammation.
• Complications liées à l’insufflation.
• Démangeaisons.
• Œdème localisé (gonflement).
• Affections du système neurologique.
• Absence de consolidation osseuse (ou pseudarthrose), consolidation retardée, cal vicieux.
• Douleur.
• Altérations postopératoires de la courbure du rachis, perte et/ou réduction de la correction ou de la hauteur.
• Éjaculation rétrograde.
• Formation de cicatrices.
• Séromes.
• Lésions tissulaires ou nerveuses.
Remarque: une intervention supplémentaire peut être nécessaire pour corriger certains de ces éventuels événements indésirables.
RÉSULTATS CLINIQUES
Les données cliniques appuyant la sécurité et l’efficacité du greffon osseux INFUSE®/dispositif conique de fusion lombaire LT-CAGE® ont été collectées
dans le cadre d’une étude pivot, prospective multicentrique avec bras randomisé et non randomisé. Le bras randomisé contenait deux groupes:
un groupe expérimental et un groupe de contrôle. Le groupe de contrôle a reçu le dispositif conique de fusion lombaire LT-CAGE® rempli d’une
autogreffe de crête iliaque, tandis que le groupe expérimental a reçu le greffon osseux INFUSE®/dispositif conique de fusion lombaire LT-CAGE®.
Dans les deux cas, l’approche chirurgicale était de type ouverte antérieure. Le bras non randomisé contenait uniquement un groupe expérimental,
dont les sujets ont reçu un greffon osseux INFUSE®/dispositif conique de fusion lombaire LT-CAGE® via une approche laparoscopique antérieure.
Le groupe de contrôle du bras randomisé a été utilisé comme groupe de contrôle pour le bras non randomisé.
Ni les examinateurs, ni les sujets n’étaient en aveugle. Il était impossible que les sujets soient en aveugle en raison de la seconde zone chirurgicale
résultant de la nécessité de collecter les greffons de crête iliaque. Le risque de partialité des examinateurs dans les paramètres de résultats cliniques
a été réduit en demandant aux sujets d’évaluer leur résultat à l’aide d’auto-évaluations objectives. Les paramètres de résultats radiographiques ont
été réalisés par des radiologues indépendants qui agissaient en aveugle. Seules ces évaluations radiographiques ont été utilisées pour déterminer
le résultat radiographique.
L’indication objet de l’étude était la discopathie dégénérative (DDD) à un seul niveau entre L4 et S1 accompagnée de douleurs dorsales avec ou
sans douleur dans les jambes et confirmée par les antécédents et les examens radiographiques.
Paramètres d’efficacité clinique et radiologique
Les patients ont été évalués en période préopératoire (dans les 6mois précédant l’intervention), en période peropératoire et en période postopératoire
à 6semaines et à 3, 6, 12, et 24mois, puis tous les deux ans jusqu’à ce que le dernier sujet inscrit à l’étude ait effectué l’évaluation à 24mois. Les
complications et événements indésirables, liés ou non au dispositif, ont été évalués tout au long de l’étude clinique. Lors de chaque point temporel
d’évaluation, les paramètres des résultats radiographiques et cliniques principaux et secondaires ont été évalués. Le succès a été déterminé à partir
des données recueillies pendant les 24premiers mois de la période de suivi. Les anticorps anti-rhBMP-2 et anticollagène bovin de typeI ont été
évalués avant l’opération et 3mois après l’opération. Les anticorps au collagène humain de typeI ont été évalués lorsque la réaction au collagène
bovin de typeI était positive.
Les paramètres de résultats d’efficacité cliniques et radiographiques principaux et secondaires ont été évalués pour tous les sujets traités à tous les
points temporels de l’évaluation de suivi indiqués ci-dessus. Les paramètres cliniques principaux évalués étaient la douleur, les fonctions et l’état
neurologique. Les paramètres cliniques secondaires évalués étaient l’état de santé général, les douleurs dorsales et dans les jambes, les douleurs
au niveau de la zone de prélèvement du greffon (sujets du groupe de contrôle uniquement), la satisfaction du patient et l’effet global du traitement
perçu par le patient. Le paramètre de résultat radiographique principal consistait en l’évaluation de la fusion, tandis que l’évaluation radiographique
secondaire concernait la hauteur du disque.
La fusion a été évaluée en période postopératoire à 6, 12 et 24mois à l’aide de radiographies standard (clichés antéro-postérieurs, latéraux et en
flexion/extension) et de tomodensitogrammes haute résolution à coupes fines (coupes de 1mm avec indice de 1mm en reconstructions axiales
sagittales et coronales). La fusion a été définie par la présence d’un pontage osseux raccordant les corps vertébraux inférieur et supérieur, une
perte d’amplitude en flexion/extension (£3mm de translation et <5° d’angulation) et l’absence de signes de radiotransparence sur plus de 50% de
chaque implant. Le succès de la fusion a été défini par la présence de tous ces paramètres, en plus de la non-nécessité d’une seconde intervention

chirurgicale résultant de l’absence de consolidation. Toutes les évaluations ont été effectuées à partir de clichés standard, à l’exception de l’évaluation
du pontage osseux, qui n’a été effectuée à l’aide des tomodensitogrammes que si le pontage n’était pas visible sur les clichés standard.
La douleur et les fonctions ont été mesurées à l’aide du questionnaire d’Oswestry sur la douleur et les incapacités rachidiennes. Le succès a été
défini par une amélioration de 15points du score d’Oswestry par rapport au score de référence calculé avant l’opération.
L’évaluation de l’état neurologique consistait à mesurer quatre paramètres: les paramètres moteurs, les paramètres sensitifs, les réflexes et le signe
de Lasègue (SLR). Le succès de l’état neurologique a été défini par le maintien ou l’amélioration du score de référence mesuré avant l’opération
pour chaque paramètre. Pour que le succès global de l’état neurologique soit comptabilisé en tant que tel, chaque paramètre individuel devait être
défini comme un succès pour le sujet en question.
Rapport démographique et nombre de patients
Au total, 143patients du groupe expérimental avec approche ouverte et 136patients du groupe de contrôle ont été intégrés au bras randomisé de
l’étude et ont reçu le dispositif. Au total, 134sujets ont été intégrés au bras non randomisé de l’étude et ont reçu le dispositif. Pour la majorité des
paramètres socio-démographiques tels que relevés avant l’opération, il n’y avait aucune différence entre les trois populations.
Résultats chirurgicaux et hospitalisation
Données chirurgicales et d’hospitalisation
Groupe expérimental
avec approche chirurgi-
cale ouverte
Durée opératoire moyenne (heures) 1,6
Perte de sang estimée (ml) 109,8
Hospitalisation (jours) 3,1 3,3 1,2
*
Statistiquement différent du contrôle
*
*
Groupe de contrôle avec
approche chirurgicale
ouverte
2,0 1,9
153,1 146,1
Groupe expérimental avec
approche chirurgicale laparos-
copique
*
Évaluation de l’efficacité clinique et radiographique
Le succès d’un sujet particulier était défini comme le succès de chacun des paramètres de résultats radiographiques et cliniques principaux. Le
succès de ces paramètres incluait:
1. la présence d’une fusion sur les images radiographiques;
2. une amélioration d’au moins 15points par rapport au score Oswestry de référence;
3. le maintien ou l’amélioration de l’état neurologique;
4. aucun événement indésirable grave classé comme associé à l’implant ou lié à l’implantation/l’intervention chirurgicale; et
5. aucune intervention chirurgicale supplémentaire considérée comme un «échec».
Le succès de l’étude était exprimé en divisant le nombre de sujets individuels classés en tant que succès par le nombre total de sujets évalués. Le
tableau ci-dessous représente les taux de succès pour les paramètres de résultats principaux individuels ainsi que le succès global. Tous les taux
de succès étaient basés sur les données provenant des résultats sur 24mois et les probabilités postérieures de succès étaient calculées au moyen
des procédés statistiques bayésiens.
Probabilités postérieures de succès à 24 mois
Variable de résultat principal
Fusion
Oswestry
Neurologique
Succès global
Groupe expérimental avec ap-
proche chirurgicale ouverte
Moyenne postérieure
(95 % de l’intervalle crédible de
la densité postérieure la plus
élevée [Highest Posterior Density
ou HPD])
92,8 %
(88,5 %, 96,9 %)
71,0 %
(63,4 %, 78,7 %)
81,0 %
(74,5 %, 87,9 %)
57,1 %
(49,2 %, 65,7 %)
Groupe de contrôle avec approche chirurgicale ouverte
Moyenne postérieure
(95 % de l’intervalle crédible
de l’HPD)
88,1 %
(82,6 %, 99,3 %)
70,9 %
(63,1 %, 79,1 %)
81,7 %
(74,9 %, 88,7 %)
56,7 %
(48,3 %, 65,0 %)
Groupe expérimental avec approche
chirurgicale laparoscopique
Moyenne postérieure
(95 % de l’intervalle crédible
de l’HPD)
93,0 %
(87,9 %, 97,5 %)
83,0 %
(75,6 %, 90,5 %)
89,0 %
(83,1 %, 94,8 %)
68,0 %
(59,3 %, 76,5 %)
La probabilité postérieure que le taux de succès global à 24mois pour les groupes expérimentaux soit équivalent au taux de succès à 24mois
pour le groupe de contrôle était de 99,4% pour le groupe expérimental avec approche chirurgicale ouverte et de presque 100% pour le groupe
expérimental avec approche chirurgicale laparoscopique.
Pour un futur patient recevant le greffon osseux INFUSE®/dispositif conique de fusion lombaire LT-CAGE® via l’approche chirurgicale ouverte
antérieure, la probabilité prédictive de succès global à 24mois serait de 57,1% pour l’approche chirurgicale ouverte. Étant donné les résultats
de l’essai, il existe 95% de chances que la probabilité de succès se situe entre 49,2% et 65,7%. Pour un futur patient recevant le greffon osseux
INFUSE®/dispositif conique de fusion lombaire LT-CAGE® via l’approche chirurgicale laparoscopique antérieure, la probabilité prédictive de succès
global à 24mois serait de 68,0%. Étantdonné les résultats de l’essai, il existe 95% de chances que la probabilité de succès se situe entre 59,3% et
76,5%. Pourun futur patient recevant le traitement de contrôle, la probabilité prédictive de succès global à 24mois serait de 56,7%. Étant donné
les résultats de l’essai, il existe 95% de chances que la probabilité de succès se situe entre 48,3% et 65,0%.

Évaluation de la sécurité et de la réaction immunitaire
L’évaluation de la sécurité portait sur les événements indésirables rapportés, ainsi que sur l’évaluation des anticorps anti-rhBMP-2, anticollagène bovin
de typeI et anticollagène humain de typeI. La liste exhaustive des complications, des événements indésirables et des interventions subséquentes est
fournie au paragraphe «Événements indésirables» ci-dessus. La présence d’anticorps a été évaluée par un test ELISA lors de la visite préopératoire
et de la visite postopératoire à 3mois. En cas de réaction positive au collagène bovin de typeI, les anticorps au collagène humain de typeI étaient
également recherchés dans le sérum. Le seuil du test ELISA pour la détection d’une réponse positive des anticorps a été fixé à 5fois l’écart-type de
sérum provenant de donneurs humains sains. Les sujets étaient considérés comme ayant une réaction immunitaire élevée si le test préopératoire
était négatif (titre <50) et si le test postopératoire était positif (titre > 50), ou si le test préopératoire était positif et le test postopératoire l’était aussi,
avec un titre trois fois supérieur au test préopératoire.
Trois sujets ont développé une réponse positive des anticorps anti-rhBMP-2, à raison de 1sujet dans chaque groupe de l’étude. Les taux de réponse
positive des anticorps anti-rhBMP-2 étaient de 0,7% dans le groupe expérimental avec approche chirurgicale ouverte et de 0,8% dans le groupe
expérimental avec approche chirurgicale laparoscopique et le groupe de contrôle avec approche chirurgicale ouverte. Bien qu’il soit théoriquement
possible que les anticorps anti-rhBMP-2 neutralisent la BMP-2 endogène, interférant ainsi avec le processus subséquent de guérison osseuse, aucun
phénomène de ce type n’a été observé tout au long de l’étude.
Soixante-six sujets ont été considérés comme présentant une vraie réponse élevée des anticorps anticollagène bovin de typeI, à raison de 18sujets
dans le groupe expérimental avec approche chirurgicale ouverte, 32sujets dans le groupe expérimental avec approche chirurgicale laparoscopique
et 16sujets dans le groupe de contrôle. Aucun sujet n’a développé de réponse positive au collagène humain de typeI.
Une évaluation a été réalisée au sujet de l’impact d’une réponse positive des anticorps sur le succès global et le succès de la fusion. Il y avait peu de
différence au niveau du succès global et individuel quand le statut des anticorps était pris en compte.
Au cours de l’étude, six grossesses ont été rapportées, à raison d’une dans le groupe de contrôle et cinq dans les groupes expérimentaux. Deux des
quatre grossesses qui se sont déclarées chez des sujets du groupe avec approche laparoscopique se sont terminées par des fausses couches durant
le premier trimestre. Les trois autres grossesses des groupes expérimentaux se sont terminées par des naissances vivantes sans aucune complication
rapportée. Aucune des patientes enceintes n’a développé de réponse des anticorps anti-rhBMP-2 ou anticollagène de typeI (bovin ou humain) qui
ait été détectable d’après les seuils de sensibilité de l’essai.
Lors de la période de 24mois pendant laquelle les événements indésirables ont été signalés, trois cas de cancer ont été diagnostiqués: deux dans
le groupe expérimental et un dans le groupe de contrôle. Un cancer du pancréas a été diagnostiqué pour un sujet du groupe expérimental, et un
cancer du sein a été identifié chez un autre sujet. Un cancer du sein a également été diagnostiqué pour un sujet du groupe de contrôle. Aucune
information supplémentaire n’est disponible sur ces sujets, notamment sur l’expression des récepteurs de la BMP-2.
Étude post-approbation
Un total de 145sujets du groupe expérimental a été évalué dans le cadre d’une étude post-approbation durant les 6années (72mois) qui ont suivi
l’intervention chirurgicale initiale. La population a été obtenue à partir des patients ayant participé à la fois aux bras avec approche ouverte et
laparoscopique de l’essai clinique pour l’exemption des dispositifs de recherche (Investigational Device Exemption ou IDE). Les sujets du groupe de
contrôle de l’étude IDE n’ont pas été suivis dans le cadre de l’étude post-approbation. En se basant sur les critères de l’étude IDE, les informations
relatives à la sécurité et l’efficacité du produit ont été recueillies à quatre et six ans après l’implantation. Elles comprenaient les événements indésirables,
une évaluation radiographique indépendante de la fusion et une évaluation de la douleur et des fonctions. Le tableau ci-dessous récapitule les
données cliniques et radiographiques évaluées durant l’étude post-approbation ainsi que le taux de succès global àchaque point temporel.
Taux de succès globaux – Étude post-approbation
Variable Évaluation sur 4 ans Évaluation sur 6 ans
Succès global 60,6 % (77/127) 60,4% (84/139)
Amélioration du score d’Oswestry de la douleur 81,5 % (106/130) 79,0 % (109/138)
Fusion 98,3 % (117/119) 98,5 % (128/130)
État neurologique
(maintien ou amélioration)
73,3 % (96/131) 81,2 % (112/138)
PRÉSENTATION
Le composant greffon osseux INFUSE® est disponible en différentes tailles de kit, qui contiennent chacune tous les composants nécessaires pour
préparer cette partie du dispositif, c’est-à-dire la ou les éponge(s) de collagène, un flacon avec le facteur de croissance lyophilisé, un flacon avec l’eau
stérile pour la reconstitution du facteur de croissance, des seringues et des aiguilles. Cette notice décrit uniquement l’utilisation des deux kits
de greffon osseux INFUSE® de taille XS (1,4 cc) et XXS (0,7 cc). Le composant dispositif d’arthrodèse intersomatique fileté en titane de Medtronic
est disponible dans différentes tailles; la taille qui convient doit être choisie en fonction de l’anatomie particulière du patient.
CONDITIONS DE STOCKAGE
Conserver le composant greffon osseux INFUSE® à température ambiante [de 15 à 30°C (de 59 à 86°F)]. Le composant dispositif d’arthrodèse
intersomatique fileté en titane de Medtronic doit également être conservé à température ambiante.
DOSAGE ET ADMINISTRATION
Le composant greffon osseux INFUSE® est préparé immédiatement avant utilisation à partir d’un kit contenant tous les composants nécessaires.
Une fois préparé, le composant greffon osseux INFUSE® contient la rhBMP-2 à une concentration de 1,5mg/ml.
La taille du kit du composant greffon osseux INFUSE® et le volume du composant greffon osseux INFUSE® à implanter sont déterminés par le volume
interne des composants dispositif d’arthrodèse intersomatique fileté en titane de Medtronic qui sont utilisés. L’anatomie du patient déterminera la
taille des composants dispositif d’arthrodèse intersomatique fileté en titane de Medtronic à utiliser. Les manuels de technique chirurgicale pour le
greffon osseux INFUSE® avec les dispositifs d’arthrodèse intersomatique filetés en titane de Medtronic spécifiques qui sont référencés dans cette
notice sont disponibles et fournissent davantage d’informations sur l’utilisation des gabarits pour déterminer la taille adaptée pour le composant
dispositif d’arthrodèse intersomatique fileté en titane de Medtronic.

Les composants de ce kit de greffon osseux INFUSE® doivent être uniquement conservés conformément aux indications figurant sur l’emballage;
les composants doivent être uniquement mélangés conformément à la méthode décrite dans les instructions; la rhBMP-2 reconstituée doit être
uniquement ajoutée à la structure porteuse ACS fournie conformément à la méthode décrite; le produit doit être uniquement utilisé conformément
aux quantités et indications précisées dans la notice. Tout autre mode de conservation, de mélange ou d’administration peut provoquer des
événements indésirables inattendus.
RÉCLAMATIONS CONCERNANT CE PRODUIT
Tout professionnel de santé (par exemple, un client ou un utilisateur de ce système de produits) ayant une réclamation ou un motif d’insatisfaction à
formuler sur la qualité du produit, son identité, sa durée de vie, sa fiabilité, sa sécurité d’emploi, son efficacité et/ou ses performances doit le notifier
au distributeur ou à Medtronic. Par ailleurs, dans l’éventualité où l’un des composants implantés du greffon osseux INFUSE®/dispositif d’arthrodèse
intersomatique fileté en titane de Medtronic «ne fonctionne pas correctement» (c’est-à-dire n’est pas conforme à une de ses spécifications de
performance ou ne fonctionne pas comme prévu), ou en cas de suspicion de mauvais fonctionnement, il convient de le signaler immédiatement
au distributeur. Dans l’éventualité où un produit Medtronic «ne fonctionne pas correctement» et est susceptible d’avoir entraîné le décès d’un
patient ou une lésion grave, ou y avoir contribué, le distributeur doit être immédiatement informé par téléphone, télécopie ou courrier postal.
Pour toute réclamation, veuillez indiquer le nom et le numéro du ou des composants, le ou les numéros de lot, vos nom et adresse, la nature de la
réclamation, et préciser si vous souhaitez un rapport écrit du distributeur.
EN CAS DE RETRAIT DU DISPOSITIF
Dans l’éventualité où le retrait du greffon osseux INFUSE®/dispositif d’arthrodèse intersomatique fileté en titane de Medtronic se révélait nécessaire,
appelez Medtronic avant la date prévue de l’intervention pour obtenir des instructions à propos de la collecte de données, y compris d’informations
histopathologiques, mécaniques et sur les événements indésirables.
©2015 MEDTRONIC SOFAMOR DANEK USA, Inc. Tous droits réservés.
Le greffon osseux INFUSE® combiné aux dispositifs LT-CAGE, INTER FIX™ ou INTER FIX™ RP intègre la technologie développée par Gary K. Michelson, M.D.
 Loading...
Loading...