
Simulus™
Ring/Band Accessories
Instructions for Use
Caution: Federal law (USA) restricts this
device to sale by or on the order of a physician.

The following list includes trademarks or registered trademarks of Medtronic in the United States and
possibly in other countries. All other trademarks are the property of their respective owners.
Medtronic, Simulus
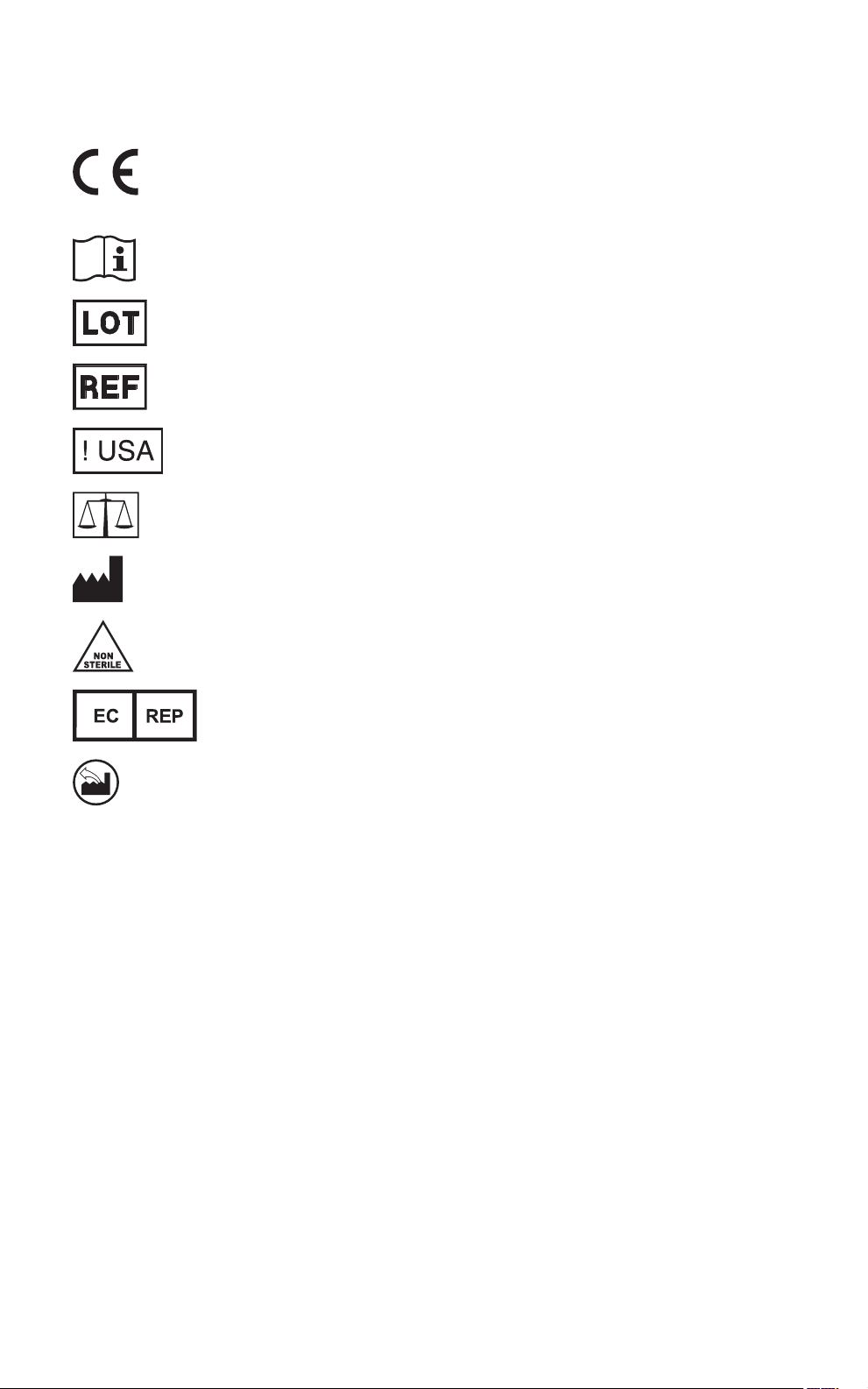
Explanation of symbols on package labeling
Refer to the device labeling to see which symbols apply to this product.
Conformité Européenne (European Conformity). This
symbol means that the device fully complies with applicable
European Union acts.
Consult instructions for use
Lot number
Catalog number
For US audiences only
Quantity
Manufacturer
Nonsterile
Authorized representative in the European Community
Manufactured in
3
1 Device description
The Simulus ring/band accessories consist of nonsterile reusable metal
handles, with Nitinol stems that straighten during the steam sterilization
cycle, and clear plastic sizers.
Use the following Simulus ring/band accessories for the Simulus flexible
annuloplasty ring, Model 700FF, and the Simulus flexible annuloplasty
band, Models 700FC and 725FC:
Accessory Model Product Description
750 Simulus flexible ring/band acces-
sory kit
• 9 rigid polysulfone sizers, sizes
23 mm through 39 mm
• 2 standard annuloplasty handles
• 1 sterilization tray
751 Simulus flexible ring/band polysul-
fone sizer set
• 9 rigid polysulfone sizers, sizes
23 mm through 39 mm
755 Simulus flexible ring/band robotic
accessory kit
• 9 polysulfone robotic sizers,
sizes 23 mm through 39 mm
• 1 sterilization tray
Use the following Simulus ring/band accessories for the Simulus semi-rigid
annuloplasty ring, Model 800SR, and the Simulus semi-rigid annuloplasty
band, Model 800SC:
Accessory Model
850 Simulus semi-rigid ring/band
Product Description
accessory kit
4

Accessory Model Product Description
• 9 rigid polysulfone sizers, sizes
24 mm through 40 mm
• 2 standard annuloplasty handles
• 1 sterilization tray
851 Simulus semi-rigid ring/band poly-
sulfone sizer set
• 9 rigid polysulfone sizers, sizes
24 mm through 40 mm
The handles have malleable Nitinol stems that straighten during the steam
sterilization cycle. The handles are available in the following lengths and
quantities:
Accessory Model Length Quantity
752 254 mm 2
2 Indications for use
The sizers are to be used to size a patient’s mitral or tricuspid valve annulus
in order to select the proper size Simulus ring or band.
Do not use other manufacturers’ annuloplasty sizers or sizers from other
annuloplasty products to size the Simulus ring or band. Other sizers may not
indicate the appropriate Simulus ring or band size.
3 Contraindications
The sizers are not intended for use with devices other than the Simulus ring
or band.
4 Warnings and precautions
4.1 Warnings
• The sizers and handles are provided NONSTERILE and must be
thoroughly cleaned and sterilized prior to use.
• Only surgeons who have received appropriate training in valve repair,
including ring implant and sizing techniques, should use this device.
• Correct annuloplasty ring or band sizing is an important element of a
successful valve repair. Undersizing the ring or band can result in:
– valve stenosis
– ring or band dehiscence
– ring or band fracture
5

• Oversizing the ring or band can result in:
– valve regurgitation
– ring or band fracture
• Do not force the sizer through the annulus as patient injury may occur.
• Carefully inspect each sizer and handle prior to use for cracks or flaws
that may result from sterilization, handling, or general use. Discard any
sizer or handle that shows signs of cracking or degradation.
4.2 Precautions
• Do not apply excessive force when engaging the handle to the sizer, as
this may damage the sizers.
• Do not use solvent-based cleaners (for example, acetone or toluene)
when cleaning the sizers or handles. Use water-soluble surfactant-type
soaps or mild detergents as cleaning agents.
5 Instructions for use
Refer to the Simulus ring or band Instructions for Use for information
regarding the sizing and implantation of the Simulus ring or band.
The handle has a spring quick-release catch that engages in a
corresponding square recess in each sizer. Two small protrusions on the
base of the handle correspond with recesses in the upper face of each sizer
to prevent the handle from being incorrectly snapped into place on the lower
face of the sizer. The metal shaft of the handle is made of bendable Nitinol
wire that straightens during the steam sterilization cycle. To engage the
sizer and the handle, gently insert the handle into the square recess of the
sizer. To disengage the sizer, gently squeeze the spring and then pull the
handle away from the sizer.
The flexible sizers have a small hole. Tying a length of 2/0 (or larger) suture
to the sizer using the side hole may help with sizer removal later. The flexible
silicone rubber sizers may be rolled up to fit the access port before
introduction to the right atrium. Once in place, the flexible sizer may be
gripped by a suitable instrument using the 2 off-center holes in the sizer.
6 How supplied
6.1 Packaging
The sizers and handles are supplied NONSTERILE. Thoroughly clean and
sterilize the sizers and handles prior to use and each reuse. For reuse,
completely disassemble the sizer from the handle prior to cleaning (for
example, detach the handle and sizer).
6

6.2 Storage
The sizers and handles are intended to be stored indefinitely and may be
reused with appropriate cleaning and sterilization.
7 Processing and Reprocessing
Medtronic has validated the following instructions for preparing this medical
device for reuse. It remains the responsibility of the processor to ensure that
the processing, as actually performed using equipment, materials, and
personnel in the processing facility, achieves the desired result. Processing
requires validation and routine monitoring. Likewise, any deviation by the
processor from the following instructions should be properly evaluated for
effectiveness and potential adverse consequences. Cleaning may be done
manually, or it may be automated according to these Instructions for Use or
an equivalent, validated method.
7.1 Cleaning
Warning: Thoroughly clean the device to eliminate soil prior to sterilization.
Warning: Do not use solvent-based cleaners (for example, acetone or
toluene).
• Do not allow contaminated devices to dry before implementing any
cleaning procedure.
• Reprocess devices within 2 hours after use. If transport to the
processing area will be delayed, place the device in a covered container
with an enzymatic detergent to prevent drying.
• If the device contains multiple components, completely disassemble
the components prior to cleaning per these Instructions for Use.
• Examine the device for cracking or degradation prior to use. Do not use
the device if any components show signs of cracking or degradation.
• Thermal disinfection is not required because the devices are terminally
sterilized.
7.1.1 Manual Cleaning
Medtronic has established the following manual cleaning procedure in
Table 1. These steps must be performed within a maximum of 2 hours after
use.
Note: If the device has movable parts, ensure that all surface areas have
been thoroughly cleaned.
7

Table 1. Manual Cleaning Instructions
Tempera-
Step Process
ture Cleaning Instructions
1 Rinsing >27°C
(>81°F)
Remove gross soil using running water
for 1 min. Use a soft-bristle brush (for
example, a nylon toothbrush) to clean
the device thoroughly.
2 Soaking >28°C
(>82°F)
Submerge the device completely for a
minimum of 5 min with 15.6 mL/1 L (or
2 ounces/1 gallon) of an enzymatic
detergent (for example, ENZOL™)
and water. Remove visible soil with a
soft-bristled brush.
3 Ultrasonic
Clean
>27°C
(>81°F)
Submerge the device completely in an
ultrasonic cleaner (for example,
Bransonic™) with 7.8 mL/1 L (or
1 ounce/1 gallon) of an enzymatic
detergent (for example, ENZOL) and
water. Sonicate for 10 min.
4 Rinsing >27°C
(>81°F)
Rinse the device with running water for
1 min.
5 Drying N/A Dry with a clean, lint-free wipe.
6 Inspection N/A Visually inspect each device for any
remaining soil or moisture. If soil
remains, repeat the process.
7.1.2 Automated Cleaning
Medtronic has established the following automated cleaning procedure.
The steps in Table 2 must be performed within a maximum of 2 hours after
use.
Note: If the device or component has movable parts, ensure that all surface
areas have been thoroughly cleaned.
Table 2. Pretreatment Instructions
Water tempera-
Step Process
ture Cleaning Instructions
1 Rinsing >25°C (>77°F) Remove gross soil using running
tap water.
2 Soaking >25°C (>77°F) Submerge the device com-
pletely for a minimum of 1 min
with 7.8 mL/1 L (or 1 ounce/1
gallon) of an enzymatic deter-
8

Table 2. Pretreatment Instructions (continued)
Water tempera-
Step Process
ture Cleaning Instructions
gent (for example, ENZOL) and
water. Remove visible soil with a
soft-bristled brush (for example,
a nylon toothbrush).
3 Rinsing >25°C (>77°F) Rinse the device for a minimum
of 2 min.
4 Inspec-
tion
N/A Visually inspect the device for
any remaining soil. If needed,
repeat the process.
Medtronic used the Prolystica™ family of cleaners according to the
manufacturer’s recommendations to validate the automated cleaning
process. It is the responsibility of the processor to ensure that the
processing is conducted in accordance with a validated method. Any
deviation by the processor from these recommendations should be
evaluated.
1. Place the device in an automated washer (for example, Steris
Reliance™ Genfore™ Washer/Disinfector).
Note: Avoid contact between devices while loading the washer.
2. Set the automated washer to run the parameters identified in Table 3
and allow the automated washer to complete the wash cycle.
Table 3. Automated Washer Cleaning Parameters
Treatment
Time
(min)
Tempera-
ture Cleaning Agent
Enzymatic
Wash
04:00 ≥60°C
(≥140°F)
Steris Prolystica™ Ultra Concentrate Enzymatic Cleaner diluted
as 1.0 mL/1 L (or 0.125 ounce/1
gallon).
Wash 02:00 ≥60°C
(≥140°F)
Steris Prolystica™ Ultra Concentrate Neutral Detergent diluted as
1.0 mL/1 L (or 0.125 ounce/1 gallon).
Rinse 02:00 ≥60°C
N/A
(≥140°F)
Dry 15:00 ≥82°C
N/A
(≥180°F)
Inspection N/A N/A Visually inspect each device for
any remaining soil or moisture. If
soil remains, repeat the process. If
9

Table 3. Automated Washer Cleaning Parameters (continued)
Treatment
Time
(min)
Tempera-
ture Cleaning Agent
needed, dry the device with filtered, compressed air or a lintfree wipe.
7.2 Steam Sterilization and Resterilization
• Use standard packaging material. Ensure that the pack is large enough
to contain the device without stressing the seals. When validating the
sterilization processes, Medtronic used the appropriate accessory tray
for each device.
• Medtronic validated steam cycles when the devices were wrapped by
CSR (Central Supply Room) wrap. However, the steam sterilization
process can be completed when the devices are either unwrapped or
wrapped in steam sterilization compatible materials.
• When sterilizing multiple devices in 1 autoclave cycle, do not exceed the
sterilizer’s maximum load.
• Examine the device for cracking or degradation prior to use. Do not use
the device if components show signs of cracking or degradation.
• Some non-US health care authorities recommend sterilization cycle
parameters that minimize the potential risk of transmitting
Creutzfeldt-Jakob Disease (CJD). This recommendation is especially
important for surgical instruments that could come into contact with the
central nervous system.
Table 4. Sterilization Cycle Parameters
Cycle type
Prevacuum
(Dynamic-
Air-Removal)
Prevacuum
(Dynamic-Air-
Removal)
Prevacuum
(Dynamic-Air-
Removal) for CJD
Temperature 132°C (270°F) 135°C (275°F) 134°C (273°F)
Exposure
4 min 3 min 18 min
Time
Dry Time
a
Medtronic recommends incinerating devices that have directly contacted patients
suspected or confirmed with Transmissible Spongiform Encephalopathy (TSE)/CJD
diagnosis. NHS Estates HTM 2010 Parts 4 & 6: Appendix 2, Items contaminated with
TSE Agents and WHO Infection Control Guidelines for Transmissible Spongiform
Encephalopathies refer to a TSE decontamination cycle using a steam autoclave at a
temperature of 134°C to 137°C (273°F to 279°F) for a single cycle of 18 min or multiple
cycles totaling 18 min (for example, six 3 min cycles).
b
The minimum dry times were validated using sterilizers with vacuum drying
capabilities. Drying cycles using ambient atmospheric pressure may require longer
dry times. Refer to the sterilizer manufacturer’s recommendations.
b
30 min 16 min 30 min
a
10

8 Disclaimer of Warranty
THE FOLLOWING DISCLAIMER OF WARRANTY APPLIES TO UNITED
STATES CUSTOMERS ONLY:
ALTHOUGH THE SIMULUS RING/BAND ACCESSORIES,
HEREAFTER REFERRED TO AS “PRODUCT”, HAVE BEEN
MANUFACTURED UNDER CAREFULLY CONTROLLED CONDITIONS,
MEDTRONIC HAS NO CONTROL OVER THE CONDITIONS UNDER
WHICH THIS PRODUCT IS USED. MEDTRONIC, THEREFORE,
DISCLAIMS ALL WARRANTIES, BOTH EXPRESS AND IMPLIED, WITH
RESPECT TO THE PRODUCT, INCLUDING, BUT NOT LIMITED TO,
ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR
A PARTICULAR PURPOSE. MEDTRONIC SHALL NOT BE LIABLE TO
ANY PERSON OR ENTITY FOR ANY MEDICAL EXPENSES OR ANY
DIRECT, INCIDENTAL, OR CONSEQUENTIAL DAMAGES CAUSED BY
ANY USE, DEFECT, FAILURE, OR MALFUNCTION OF THE PRODUCT,
WHETHER A CLAIM FOR SUCH DAMAGES IS BASED UPON
WARRANTY, CONTRACT, TORT, OR OTHERWISE. NO PERSON HAS
ANY AUTHORITY TO BIND MEDTRONIC TO ANY REPRESENTATION
OR WARRANTY WITH RESPECT TO THE PRODUCT.
The exclusions and limitations set out above are not intended to, and should
not be construed so as to, contravene mandatory provisions of applicable
law. If any part or term of this DISCLAIMER OF WARRANTY is held by any
court of competent jurisdiction to be illegal, unenforceable, or in conflict with
applicable law, the validity of the remaining portion of the DISCLAIMER OF
WARRANTY shall not be affected, and all rights and obligations shall be
construed and enforced as if this DISCLAIMER OF WARRANTY did not
contain the particular part or term held to be invalid.
9 Disclaimer of warranty
THE FOLLOWING DISCLAIMER OF WARRANTY APPLIES TO
CUSTOMERS OUTSIDE THE UNITED STATES:
ALTHOUGH THE SIMULUS RING/BAND ACCESSORIES,
HEREAFTER REFERRED TO AS “PRODUCT”, HAVE BEEN
CAREFULLY DESIGNED, MANUFACTURED, AND TESTED PRIOR TO
SALE, THE PRODUCT MAY FAIL TO PERFORM ITS INTENDED
FUNCTION SATISFACTORILY FOR A VARIETY OF REASONS. THE
WARNINGS CONTAINED IN THE PRODUCT LABELING PROVIDE
MORE DETAILED INFORMATION AND ARE CONSIDERED AN
INTEGRAL PART OF THIS DISCLAIMER OF WARRANTY.
MEDTRONIC, THEREFORE, DISCLAIMS ALL WARRANTIES, BOTH
EXPRESS AND IMPLIED, WITH RESPECT TO THE PRODUCT.
MEDTRONIC SHALL NOT BE LIABLE FOR ANY INCIDENTAL OR
CONSEQUENTIAL DAMAGES CAUSED BY ANY USE, DEFECT, OR
11

FAILURE OF THE PRODUCT, WHETHER THE CLAIM IS BASED ON
WARRANTY, CONTRACT, TORT, OR OTHERWISE.
The exclusions and limitations set out above are not intended to, and should
not be construed so as to, contravene mandatory provisions of applicable
law. If any part or term of this DISCLAIMER OF WARRANTY is held by any
court of competent jurisdiction to be illegal, unenforceable, or in conflict with
applicable law, the validity of the remaining portion of the DISCLAIMER OF
WARRANTY shall not be affected, and all rights and obligations shall be
construed and enforced as if this DISCLAIMER OF WARRANTY did not
contain the particular part or term held to be invalid.
12


Medtronic, Inc.
*M999028A001*
710 Medtronic Parkway
Minneapolis, MN 55432
USA
www.medtronic.com
+1 763 514 4000
LifeLine Technical Support, 24-hour
consultation service:
1 877 526 7890
Medtronic, Inc.
3800 Annapolis Lane
Minneapolis, MN 55447
USA
Customer service and product orders:
1 800 854 3570
Medtronic B.V.
Earl Bakkenstraat 10
6422 PJ Heerlen
The Netherlands
+31 45 566 8000
Canada
Medtronic of Canada Ltd
99 Hereford Street
Brampton, Ontario L6Y 0R3
Canada
1 800 268 5346
© 2020 Medtronic
M999028A001 A
2020-02-18
 Loading...
Loading...