Page 1

MAXIMO™ VR 7232Cx, 7232B,
7232E
Single Chamber Implantable Cardioverter Defibrillator (VVE-VVIR)
Reference Manual
Caution: Federal Law (USA) restricts this device to sale by or on
the order of a physician (or properly licensed practitioner).
Page 2

Page 3

MAXIMOTM VR 7232Cx,
7232B, 7232E
Reference Manual 0
A guide to the operation and programming of the Model
7232Cx, 7232B, and 7232E MaximoTM VR Single Chamber
Implantable Cardoverter Defibrillator
0
Page 4

The following are trademarks of Medtronic:
Active Can, Cardiac Compass, Checklist, Decision Channel, Flashback, GEM,
Leadless ECG, Marker Channel, Maximo, Patient Alert, Quick Look, QuickLink,
RapidRead, T-Shock, Wavelet
Page 5

Table of contents
Introduction 11
Abbreviations and acronyms 13
Part I Quick overview
1 Quick reference 17
Physical characteristics 18
Magnet application 22
Longevity projections 23
Replacement indicators 26
Typical charge times 27
High-voltage therapy energy 27
Stored data and diagnostics 29
New and enhanced features 31
2 The Maximo VR system 37
System overview 38
Indications and usage 41
Contraindications 41
Patient screening 41
Table of contents
5
3 Emergency therapy 43
Delivering emergency therapies 44
Part II Device implant and patient follow-up procedures
4 Implanting the ICD 53
Overview 54
Preparing for an implant 54
Replacing an ICD 57
Positioning the leads 58
Testing sensing and pacing thresholds 60
Connecting the leads to the ICD 61
Testing defibrillation operation and effectiveness 65
Positioning and securing the ICD 67
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 6

6
Table of contents
Completing the implant procedure 68
5 Conducting a patient follow-up session 69
Patient follow-up guidelines 70
Verifying the status of the implanted system 70
Verifying accurate detection and appropriate therapy 71
Verifying effective bradycardia pacing 73
Part III Configuring the ICD for the patient
6 Detecting tachyarrhythmias 77
Detection overview 78
Setting up sensing 81
Detecting VF episodes 85
Detecting VT episodes 90
Detecting FVT episodes 96
Detecting tachyarrhythmia episodes with Combined Count 101
Monitoring episodes for termination or redetection 104
Enhancing detection with Wavelet 107
Enhancing VT detection with the Onset criterion 116
Enhancing VT detection with the Stability criterion 121
Detecting prolonged tachyarrhythmias with
High Rate Timeout 124
Key terms 126
7 Treating tachyarrhythmia episodes 131
Treating VF with defibrillation 132
Treating VT and FVT with antitachycardia pacing 143
Treating VT and FVT with cardioversion 153
Optimizing therapy with Smart Mode and Progressive Episode
Therapies 161
Key terms 166
8 Treating bradycardia 169
Providing basic pacing therapy 170
Enhancing pacing for optimal cardiac output 174
Providing pacing after high-voltage therapies 185
Key terms 186
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 7

Table of contents
9 Optimizing charge time and device longevity 189
Optimizing charge time 190
Optimizing device longevity 193
Key terms 194
Part IV Evaluating and managing patient treatment
10 Using the programmer 197
Setting up and using the programmer 198
Display screen features 199
Viewing and programming device parameters 206
Starting and ending patient sessions 210
Viewing live waveform traces 212
Recording live waveform strips 219
Saving and retrieving device data 221
Printing reports 225
Key terms 230
11 Using system evaluation tools 231
A summary of system evaluation tools 232
Taking a quick look at device activity 233
Using the Patient Alert feature 235
Streamlining follow-ups with Checklist 243
Key terms 246
7
12 Setting up and viewing collected data 247
A summary of data collection 248
Setting up data collection 249
Collecting lead performance data 255
Viewing the episode and therapy efficacy counters 257
Viewing episode data 260
Viewing Flashback Memory 268
Viewing battery and lead status data 270
Viewing lead performance trends 272
Using Cardiac Compass to view long term clinical trends 274
Viewing and entering patient information 279
Automatic device status monitoring 283
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 8

8
Table of contents
Key terms 285
13 Testing the system 289
Testing overview 290
Evaluating the underlying rhythm 291
Measuring pacing thresholds 292
Testing the Wavelet criterion 295
Measuring lead impedance 299
Measuring EGM Amplitude 301
Testing the device capacitors 303
Key terms 305
14 Conducting electrophysiologic studies 307
EP Study overview 308
Inducing VF with T-Shock 310
Inducing VF with 50 Hz Burst 313
Inducing an arrhythmia with Manual Burst 316
Inducing an arrhythmia with PES 318
Delivering a manual therapy 320
Key terms 323
15 Solving system problems 325
Overview 326
Solving sensing problems 327
Solving tachyarrhythmia detection problems 328
Solving tachyarrhythmia therapy problems 330
Solving bradycardia pacing problems 331
Responding to device status indicators 332
Key terms 333
Appendices A Warnings and precautions 337
General warnings 338
Storage and handling 338
Resterilization 339
Device operation 340
Lead evaluation and lead connection 341
Follow-up testing 342
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 9

Explant and disposal 343
Medical therapy hazards 343
Home and occupational environments 345
BDevice parameters347
Emergency settings 348
Detection parameters 349
Therapy parameters 351
Bradycardia pacing parameters 353
System maintenance parameters 354
Data collection parameters 356
System test and EP study parameters 357
Fixed parameters 360
Patient information parameters 361
Programmer symbols 362
Parameter interlocks 364
Index 365
Table of contents
9
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 10

Page 11

Introduction
Using this manual
Before implanting the ICD, it is strongly recommended that you:
■
Refer to the product literature packaged with the ICD for
information about prescribing the ICD.
■
Thoroughly read this manual and the technical manuals for the
leads used with the device.
■
Discuss the procedure and the ICD system with the patient
and any other interested parties, and provide them with any
patient information packaged with the ICD.
Contacting technical support
Medtronic employs highly trained representatives and engineers
located throughout the world to serve you and, upon request, to
provide training to qualified hospital personnel in the use of
Medtronic products.
11
Introduction
In addition, Medtronic maintains a professional staff of consultants
to provide technical consultation to product users. For medical
consultation, Medtronic can often refer product users to outside
medical consultants with appropriate expertise.
For more information, contact your local Medtronic representative,
or call or write Medtronic at the appropriate address or telephone
number listed on the back cover.
Customer education
Medtronic invites physicians to attend an education seminar on
the complete ICD system. The course includes indications for use,
an overview of ICD system functions, implant procedures, and
patient management.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 12

12
Chapter
Introduction
References
Notice
The primary reference for background information is Zacouto FI,
Guize LJ. Fundamentals of Orthorhythmic Pacing. In: Luderitz B,
ed. Cardiac Pacing Diagnostic and Therapeutic Tools. New York:
Springer-Verlag; 1976: 212-218.
See these additional references for more background information:
■
Singer I, Ed. Implantable Cardioverter-Defibrillator. Armonk,
NY: Futura Publishing Co. 1994.
■
Singer I, Barold SS, Camm AJ, Eds. Nonpharmacological
Therapy of Arrhythmias for the 21st Century: The State of the
Art. Armonk, NY: Futura Publishing Co. 1998.
■
Estes M, Manolis AS, Wang P, Eds. Implantable
Cardioverter-Defibrillators. New York, NY: Marcel Dekker, Inc.
1994.
■
Kroll MW, Lehmann MH, Eds. Implantable
Cardioverter-Defibrillator Therapy: The Engineering-Clinical
Interface. Norwell, MA: Kluwer Academic Publishers 1996.
This software is provided as an informational tool for the end user.
The user is responsible for accurate input of patient information
into the software. Medtronic makes no representation as to the
accuracy or completeness of the data input into the software.
MEDTRONIC SHALL NOT BE LIABLE FOR ANY DIRECT,
INDIRECT, INCIDENTIAL OR CONSEQUENTIAL DAMAGES TO
ANY THIRD PARTY WHICH RESULTS FROM THE USE OF THE
INFORMATION PROVIDED IN THE SOFTWARE.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 13

Abbreviations and acronyms
ATP Antitachycardia Pacing
BOL Beginning of Life
bpm beats per minute
CNID Combined (VT and VF) Number of Intervals to Detect
CV Cardioversion
DF/Defib Defibrillation
ECG Electrocardiogram
EGM Electrogram
EOL End of Life
ERI Elective Replacement Indicator
FDI Fibrillation Detection Interval
FTI Fast Ventricular Tachycardia Detection Interval
13
Abbreviations and acronyms
FVT Fast Ventricular Tachycardia
ICD Implantable Cardioverter Defibrillator
J joules
-1
reciprocal minutes; for example, pacing pulses per minute
min
ms milliseconds
mV millivolts
NID Number of Intervals to Detect
NST Non-sustained Tachycardia
PES Programmed Electrical Stimulation
ppm paces or pulses per minute
PVC Premature Ventricular Contraction
RNID Number of Intervals to Redetect
R-R a ventricular interval
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 14

14
Chapter
Abbreviations and acronyms
ST/Sinus Tach Sinus Tachycardia
SVT Supraventricular Tachycardia
TDI Tachycardia Detection Interval
V volts
V- Ve n tr ic ul ar
VF Ventricular Fibrillation
VF NID VF Number of Intervals to Detect
VRS Ventricular Rate Stabilization
VT Ventricular Tachycardia
VT NID VT Number of Intervals to Detect
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 15

Quick overview
Part I
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 16

Page 17

Physical characteristics 18
Magnet application 22
Longevity projections 23
Replacement indicators 26
Typical charge times 27
High-voltage therapy energy 27
Stored data and diagnostics 29
New and enhanced features 31
Quick reference1
1
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 18

18
Chapter 1
Physical characteristics
Physical characteristics
Tab le 1- 1. ICD physical characteristicsa
Connector type Cx B or E
Volume 39 cc 45 cc
Mass 76 g 81 g
b
H x W x D
Surface area of
68 mm x 51 mm x
15 mm
2
67 cm
74 mm x 51 mm x
15 mm
2
64 cm
device can
Radiopaque IDc
PRN B-type connector PVF
E-type connector PVG
Materials in contact
with human tissue
Titanium / polyurethane /
d
silicone rubber
Battery Lithium silver vanadium
oxide
a
Measurements are nominal values based on CAD (computer aided design)
model measurements and are rounded to the nearest unit.
b
Grommets may protrude slightly beyond the can surface.
c
Engineering series number follows the radiopaque code.
d
These materials have been successfully tested for the ability to avoid biological
incompatibility. The device does not produce an injurious temperature in the
surrounding tissue.
Titanium / polyurethane /
silicone rubber
Lithium silver vanadium
oxide
Tab le 1- 2. Cx-type connector characteristics
General description Device
One IS-1 connector for pacing and
sensing, Two DF-1 connectors for
high-voltage therapy, Active Can
electrode (programmable).
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Port
Connector
Typ e
Software
Name
SVC DF-1 HVX
RV DF-1 HVB
Can — HVA, Can
V IS-1 bipolar
Page 19

Figure 1-1. Cx-type connector
2
Quick reference
Physical characteristics
1
19
5
3
4
1 Suture holes
2 SVC port (DF-1)
3RV port (DF-1)
4 programmable Active Can
5V port (IS-1)
Tabl e 1-3 . B-type connector characteristics
General description Device
Port
Three 6.5 mm unipolar
high-voltage ports and one
3.2 mm low profile bipolar
pace/sense (IS-1 compatible) port.
HVB 6.5 mm HVB
HVA
HVX
Connector
Typ e
a
6.5 mm HVX
a
6.5 mm HVX
Software
Name
Can — HVA, Can
P/S
bipolar
pace
3.2 mm
bipolar (IS-1
compatible)
sense
a
The HVA and HVX ports are electrically connected and are treated as the HVX
electrode. For more information see “B- and E-type connector pathways” on
page 136.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 20

20
Chapter 1
Physical characteristics
Figure 1-2. B-type connector
1
2
6
5
3
4
1 Suture holes
2 HVB port (6.5 mm)
3 HVA port (6.5 mm)
4 programmable Active Can
5 HVX port (6.5 mm)
6 P/S port (3.2 mm)
Tab le 1- 4. E-type connector characteristics
General description Device
Port
Connector
Typ e
Software
Name
P+/S 5.0 mm
Two 6.5 mm unipolar high-voltage
ports and two 5 mm unipolar
pace/sense ports.
HVA 6.5 mm HVX
HVB 6.5 mm HVB
a
Can — HVA, Can
P-/S 5.0 mm
a
The HVA port of the E-type connector can be used as the HVX electrode when
Active Can is programmed off. For more information, see “B- and E-type
connector pathways” on page 136.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 21

Figure 1-3. E-type connector
Quick reference
Physical characteristics
21
6
5
1 Suture holes
2 P+/S port (5.0 mm)
3 HVA port (6.5 mm)
4 programmable Active Can
5 HVB port (6.5 mm)
6 P-/S port (5.0 mm)
2
3
4
1
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 22

22
Chapter 1
Magnet application
Magnet application
Bringing a magnet close to the device triggers changes in device
operation as shown in Table 1-5. When the magnet is removed,
the device returns to its programmed operations.
Tab le 1- 5. Effects of magnet application on the device
Pacing mode as programmed
Pacing rate and interval as programmed
VF, VT, and FVT detection suspended
a
b
Patient Alert audible
tones (20 seconds
or less)
with programmable alert(s) enabled:
■
continuous tone (Test)
■
on/off intermittent tone (seek
c
follow-up)
■
high/low dual tone (urgent follow-up)
with programmable alerts disabled:
■
no tone
■
high/low dual tone (urgent follow-up)
a
Rate response adjustments are suspended while a Patient Alert tone sounds.
b
Detection resumes if telemetry is established and the application software is
running, or it resumes after the application software has started.
c
The Test tone does not sound if “VF Detection/Therapy Off” is the only alert
enabled.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 23

Longevity projections
Longevity estimates are based on accelerated battery discharge
data and device modeling with EGM pre-storage off, 60 ppm
-1
(min
) pacing rate, with
■
2.5 V pacing pulse amplitude, 0.4 ms pacing pulse width, and
35 J delivered therapy energy (see Table 1-6)
■
3 V pacing pulse amplitude, 0.4 ms pacing pulse width, and
35 J delivered therapy energy (see Table 1-7)
This model assumes default automatic capacitor formation
setting. As a guideline, each full energy charge decreases device
longevity by approximately 43 days.
Device longevity is affected by how certain features are
programmed, such as EGM pre-storage. For more information,
see “Optimizing device longevity” on page 193.
Considerations for using EGM pre-storage
When the EGM pre-storage feature is programmed off, the device
starts to store EGM following the third tachyarrhythmia event and
also provides up to 20 seconds of information before the onset of
the tachyarrhythmia, including:
■
VV intervals
■
Marker Channel
■
interval plot Flashback
Quick reference
Longevity projections
23
When the EGM pre-storage feature is programmed on, the device
also collects up to 20 seconds of EGM information before the
onset of the arrhythmia.
In a patient who uniformly repeats the same onset mechanisms,
the greatest clinical benefit of pre-onset EGM storage is achieved
after a few episodes are captured.To maximize the effectiveness
of the EGM pre-storage feature and optimize device longevity,
consider these programming options:
■
Turn pre-storage on to capture possible changes in the onset
mechanism following significant clinical adjustments, for
example, device implant, medication changes, and surgical
procedures.
■
Turn pre-storage off once you have successfully captured the
information of interest.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 24

24
Chapter 1
Longevity projections
Tab le 1- 6. Projected longevity in years with 2.5 V pacing amplitude and
0.4 ms pulse width
Percent
pacing
Maximum
energy charging
frequency
a
EGM
pre-storage
500 ohm
b
impedance
pacing
900 ohm
pacing
impedance
0% Semi-Annual Off 10.1 10.1
On 9.8 9.8
Quarterly Off 8.3 8.3
On 8.1 8.1
15% Semi-Annual Off 9.8 10.0
On 9.6 9.7
Quarterly Off 8.1 8.2
On 7.9 8.0
50% Semi-Annual Off 9.3 9.7
On 9.1 9.5
Quarterly Off 7.8 8.0
On 7.6 7.8
100% Semi-Annual Off 8.7 9.3
On 8.5 9.1
Quarterly Off 7.3 7.7
On 7.1 7.6
a
Maximum energy charging frequency may include full energy therapy shocks or
capacitor formations.
b
The data provided for programming EGM pre-storage on is based on a 6 month
period (two 3-month follow-up intervals) over the life of the device. Additional
use of EGM pre-storage reduces longevity by approximately 27% or 3 months
per year.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 25

Quick reference
Longevity projections
Tabl e 1-7 . Projected longevity in years with 3 V pacing amplitude and
0.4 ms pulse width
Percent
pacing
0% Semi-Annual Off 10.1 10.1
15% Semi-Annual Off 9.8 9.9
50% Semi-Annual Off 9.1 9.5
100% Semi-Annual Off 8.2 9.0
a
Maximum energy charging frequency may include full energy therapy shocks or
capacitor formations.
b
The data provided for programming EGM pre-storage on is based on a 6 month
period (two 3-month follow-up intervals) over the life of the device. Additional
use of EGM pre-storage reduces longevity by approximately 27% or 3 months
per year.
Maximum
energy charging
frequency
a
EGM
pre-storage
500 ohm
b
impedance
pacing
900 ohm
pacing
impedance
On 9.8 9.8
Quarterly Off 8.3 8.3
On 8.1 8.1
On 9.5 9.7
Quarterly Off 8.0 8.1
On 7.9 8.0
On 8.8 9.3
Quarterly Off 7.6 7.9
On 7.4 7.7
On 8.0 8.8
Quarterly Off 7.0 7.5
On 6.8 7.3
25
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 26

26
Chapter 1
Replacement indicators
Replacement indicators
Battery voltage and messages about replacement status appear
on the programmer display and on printed reports. Table 1-8 lists
the Elective Replacement Indicator (ERI) and the End of Life
(EOL) conditions.
Tab le 1- 8. Replacement indicators
Elective Replacement (ERI) ≤ 2.62 V
End of Life (EOL) 3 months after ERI
ERI date – The programmer displays the date when the battery
reached ERI on the Quick Look and Battery and Lead
Measurements screens.
Temporary voltage decrease – The battery voltage temporarily
decreases following a high-voltage charge. If a battery
measurement is taken immediately after a high-voltage charge,
the ERI or EOL indicator may be displayed. However, this is a
temporary status which will return to normal when the battery has
recovered from the charge.
EOL indication – If the programmer indicates that the device is at
EOL, replace the device immediately.
Post-ERI conditions – EOL device status is defined as three
months following an ERI indication assuming the following
post-ERI conditions: 100% VVI pacing at 60 ppm (min
0.4 ms; 500 Ω pacing load; and six 35 J charges. EOL may be
indicated before the end of three months if the device exceeds
these conditions.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
-1
), 3 V,
Page 27

Typical charge times
The most recent capacitor charge time appears on the
programmer display and on printed reports and can be evaluated
using the Charge/Dump test (see Table 1-9).
Tabl e 1-9 . Ty p ic ala full energy charge times
At Beginning of Life (BOL) 7.0 seconds
At Elective Replacement (ERI) 9.1 seconds
a
These charge times are typical when the capacitors are fully formed.
High-voltage therapy energy
The stored energy of the device is derived from the peak capacitor
voltage and is always greater than the energy delivered by the
device. Table 1-10 compares the programmed energy levels
delivered by the device to the energy levels stored in the
capacitors before delivery.
Quick reference
Typical charge times
27
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 28

28
Chapter 1
High-voltage therapy energy
Table 1-10. Comparing delivereda (programmed) and storedb energy levels
Energy (J) Charge
Delivered a/
Stored
b
Programmed
35 39 7.0 10 12 2.0
32 37 6.4 9 10.5 1.8
30 34 6.0 8 9.3 1.6
28 32 5.6 7 8.2 1.4
26 30 5.2 6 7.1 1.2
25 29 5.0 5 5.9 1.0
24 27 4.8 4 4.8 0.8
22 25 4.4 3 3.6 0.6
20 23 4.0 2 2.4 0.4
18 21 3.6 1.8 2.2 0.4
16 19 3.2 1.6 2.0 0.3
15 17 3.0 1.4 1.7 0.3
14 16 2.8 1.2 1.5 0.2
13 15 2.6 1.0 1.2 0.2
12 14 2.4 0.8 1.0 0.2
11 13 2.2 0.6 0.8 0.1
a
Energy delivered at connector block into a 75 ohm load.
b
Energy stored at end of charge on capacitor.
c
Typical charge time at Beginning of Life (BOL) with fully formed capacitors, rounded to the nearest tenth of
a second.
Time
c
(sec)
Delivered a/
Programmed
0.4 0.5 0.1
Energy (J) Charge
Timec (sec)
b
Stored
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 29

Stored data and diagnostics
Tabl e 1-1 1. Stored data and diagnostics
Episode data
Quick reference
Stored data and diagnostics
29
Tachy episodes 150 VF/VT/FVT episodes: intervals, text, EGM,
QRS Snapshot
EGM capacity for
tachy episodes
14 minutesb of dual-channel EGM, or
23.5 minutes
a
c
of single-channel EGM
SVT/NST episodes 50 SVT/NST episodes: intervals, text, EGM,
QRS Snapshota (the device does not usually
store detailed episode records for NST
episodes)
d
EGM capacity for
SVT/NST episodes
2 minutes
3.6 minutese of single-channel EGM
of dual-channel EGM, or
EGM sources Six options: ventricular / far-field
EGM options Store before onset; Store during charging
Flashback memory 2000 intervals (V-V): before latest VF, before
latest VT, and before interrogation
Counter data
Detection counters Lifetime total, since cleared, and since last
session
Episode counters Episodes:
■
VF, FVT, and VT
■
NST episodes and SVTs
Percentage pacing:
■
VS and VP percentages
Additional counters:
■
Single PVCs and PVC runs
■
Rate stabilization pulses and runs
Therapy efficacy
counters
Counts for each VF, FVT, VT Therapy:
■
Delivered
■
Successful
■
Unsuccessful
■
Intervention (manually aborted)
Total number of aborted shocks
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 30

30
Chapter 1
Stored data and diagnostics
Tab le 1- 11. Stored data and diagnostics (continued)
Other stored data
Patient Alert events Up to 10 log entries: text and date for the first
time an alert is triggered between interrogations
Battery and lead
measurements
Battery voltage, last capacitor formation, last
charge, lead impedance, EGM amplitude
measurements, last high-voltage therapy, and
sensing integrity counter
Lead performance
trends
14 days of daily measurements plus 80 weeks
of weekly minimum and maximum
measurements:
■
Lead impedance: ventricular pacing,
defibrillation pathway, and SVC lead
(if used)
■
Ventricular EGM amplitude (R-waves)
Cardiac Compass
trends
14 months of measurement trends:
■
VT and VF episodes per day
■
High-voltage therapies delivered per day
■
Ventricular rate during VT or VF
■
Episodes of non-sustained tachycardia
per day
■
Heart rate variability
■
Percent of time pacing is active
■
Patient activity
■
Average day and night ventricular
heart rate
a
When Wavelet is set to On or Monitor
b
13.5 minutes if Wavelet is set to On or Monitor
c
22 minutes if Wavelet is set to On or Monitor
d
1.6 minutes if Wavelet is set to On or Monitor.
e
2.75 minutes if Wavelet is set to On or Monitor.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 31

New and enhanced features
The following features are new or changed from the
7231 GEM III VR ICD.
Lead connection header options
The Maximo VR supports three types of lead connection headers.
The connection types are Cx, B, and E.
■
Cx-type connector is normally used with one multipolar
transvenous lead in the ventricle for sensing, pacing, and
delivering therapies.
■
B-type connector is normally used with one multipolar
transvenous lead in the ventricle for sensing and pacing and
with two or three high-voltage electrodes placed to deliver
cardioversion/defibrillation therapies.
■
E-type connector is normally used with two 5 mm unipolar
myocardial pacing and sensing lead connectors and two
6.5 mm high-voltage lead connectors.
Quick reference
New and enhanced features
31
Regardless of the connector type used, the Active Can feature
may be programmed so that the device Can serves as a second
high-voltage electrode and the HVX port
third high-voltage electrode if desired. For more information about
connection pathways, see “B- and E-type connector pathways” on
page 136.
Patient management
RapidRead telemetry – Communication between the device and
programmer is approximately 20 times faster than telemetry in
previous Medtronic ICD devices. The magnitude of improvement
depends on the amount and type of data that is interrogated.
RapidRead telemetry is more reliable and has an increased range
that makes placing the programming head easier.
Cardiac Compass trends report – This report displays up to
14 months of trend data related to tachyarrhythmia episodes,
heart rate, and patient activity. See “Using Cardiac Compass to
view long term clinical trends” on page 274.
1
1
can accommodate a
The HVA port of the B- or E-type connector serves as the HVX electrode.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 32

32
Chapter 1
New and enhanced features
Patient Alert – The alert duration when a magnet is applied to
the device is now 20 seconds. The device also provides several
new alerts:
■
SVC (HVX) lead impedance out of range
■
Active can off without SVC
■
VOO mode programmed
■
VF Detection programmed off, or fewer than four VF therapies
enabled for at least six hours
■
charge circuit timeout occurred
■
excessive charge time ERI
For more information, see “Using the Patient Alert feature” on
page 235.
EGM amplitude trends – The device automatically measures
R-wave EGM amplitudes every day. These daily measurements
are included in the data displayed on the Lead Performance
Trends screen. See “Collecting lead performance data” on
page 255.
1
EGM Amplitude test – You can use the EGM Amplitude test to
measure R-wave EGM amplitudes. The results are reported on
the EGM Amplitude test screen. See “Measuring EGM Amplitude”
on page 301.
Lead impedance measurements for SVC (HVX)
other lead impedance measurements, the device provides an
independent SVC (HVX) measurement to check the integrity of
the supplementary high-voltage electrode. See “Measuring lead
impedance” on page 299.
Leadless ECG signal – If a supplementary high-voltage
electrode is placed in the SVC
Leadless ECG signal through either the Can to SVC (HVX) or RV
(HVB) to SVC (HVX) EGM source. See “Setting up data collection”
on page 249.
1
SVC refers to the HVX electrode. For pathway information, see “B- and E-type
connector pathways” on page 136.
2
The HVA port of the B- or E-type connectors serves as the HVX electrode.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
2
– Along with
1
, the device provides the
Page 33

Quick reference
New and enhanced features
Expanded pre-onset EGM storage – The device can now store
up to 20 seconds of EGM before a tachycardia starts. See “Setting
up data collection” on page 249.
Smart Auto Cap Formation – When the Auto Cap Formation
Interval is set to Auto, the formation interval automatically adjusts
to optimize device longevity and charge times. See “Smart Auto
Cap” on page 192.
Ending a patient session – The device audits the programmed
parameter settings when you end a patient session and alerts you
if any of the settings are atypical. See “Starting and ending patient
sessions” on page 210.
Wavelet test – The Wavelet test allows you to manually collect
and assess the template used by the Wavelet criterion. See
“Testing the Wavelet criterion” on page 295.
Auto Collection for Wavelet – The Wavelet criterion includes the
option to have the device automatically collect and maintain the
template used to distinguish between SVT and ventricular
tachyarrhythmia. See “Details about Auto Collection” on page 114.
33
QRS Snapshot data – When the Wavelet criterion is
programmed to On or Monitor, the device stores QRS Snapshot
data with VT, VF, FVT and SVT episode records. This data
includes graphical representations of up to eight QRS complexes,
along with match scores and event classifications as “Match” or
“No Match.”
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 34

34
Chapter 1
New and enhanced features
Tachyarrhythmia detection
Wavelet Dynamic Discrimination criterion – The Wavelet
criterion is designed to prevent detection of rapidly conducted
SVTs as ventricular tachyarrhythmias. It compares the shape of
each QRS complex during a fast ventricular rate to a stored
template. If enough events occur that match this template, Wavelet
withholds detection. See “Enhancing detection with Wavelet” on
page 107.
VT Monitoring – VT detection can be set to Monitor, which allows
the device to detect and record VT episodes without delivering
therapy or influencing VF detection. See “VT monitoring” on
page 94.
Onset criterion – The Onset criterion is designed to prevent
detection of sinus tachycardia as VT by requiring that a rapid
increase in ventricular rate occurs before VT events can be
classified. See “Details about Onset” on page 117.
High Rate Timeout – High Rate Timeout can turn off detection
enhancements (Wavelet, Onset, or Stability) if a high rate episode
is longer than a programmed duration. See “Details about High
Rate Timeout” on page 125.
Tachyarrhythmia therapy
Episode confirmation during and after charging – The device
continually monitors the ventricular rhythm during and after
charging for cardioversion or defibrillation (when VF confirmation
is active) to ensure the arrhythmia is present before delivering the
high-voltage shock. See “Confirming VF after initial detection” on
page 138 and “Confirming VT or FVT after detection” on
page 158.
Programmable Active Can – If a supplementary electrode is
connected to the SVC (HVX) port, you can deselect the device
Can as a high-voltage electrode. For more information about
connection pathways, see “Delivery pathway electrodes” on
page 135.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 35

Output – The device has a maximum delivered energy of
35 joules.
Bradycardia pacing
Accelerometer-based rate response – The device uses an
accelerometer to provide rate responsive pacing.
Additional bradycardia pacing modes – The device provides
asynchronous pacing in the VOO pacing mode, and provides the
OVO mode to disable pacing. See Chapter 8, “Treating
bradycardia” on page 169.
EP studies
Defibrillation threshold testing support – The T-Shock and
50 Hz Burst induction screens allow you to monitor time between
inductions, program ventricular sensing and VF therapy settings,
adjust induction settings, select manual therapies, and retrieve
episode records after therapy. See “How to perform defibrillation
threshold testing” on page 66.
Quick reference
New and enhanced features
35
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 36

Page 37

System overview 38
Indications and usage 41
Contraindications 41
Patient screening 41
The Maximo VR system2
2
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 38

38
Chapter 2
System overview
System overview
The Model 7232 Maximo VR Single Chamber Implantable
Cardioverter Defibrillator (ICD) system is an implantable medical
device system that automatically detects and treats episodes of
ventricular fibrillation, ventricular tachycardia, fast ventricular
tachycardia, and bradyarrhythmia. The ICD system includes three
major components:
■
ICD
The ICD senses the electrical activity of the patient’s heart via
the sensing electrodes of the implanted ventricular leads. It
then analyze s the heart rhythm based on selectable sensing
and detection parameters. If the ICD detects a
tachyarrhythmia, it delivers defibrillation, cardioversion, or
antitachycardia pacing therapy to the patient’s heart. If the ICD
identifies a bradyarrhythmia, it delivers bradycardia pacing
therapy to the patient’s heart.
■
Leads
The ICD can be used with transvenous or epicardial
defibrillation leads. The lead system should consist of a
bipolar pacing/sensing lead (or paired unipolar
1
pacing/sensing leads) in the ventricle and one or two
high-voltage cardioversion/ defibrillation electrodes. You can
program the Active Can device case as a high-voltage
electrode. The pacing and sensing electrodes sense cardiac
activity and deliver pacing stimuli.
■
Programmer and software
The Medtronic programmer and Model 9979 application
software allow you to perform the following tasks:
■
configure the detection, therapy, and bradycardia features
for your patient
■
perform electrophysiological studies and system tests
■
monitor, display, or print patient cardiac activity information
■
view patient and device diagnostic data
1
With an appropriate unipolar to bipolar adapter kit.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 39

The Maximo VR system
System overview
The Maximo VR devices and Model 9979 application software
are compatible with the following programmer systems:
■
Medtronic CareLink Model 2090 programmer with a
Model 2067 or 2067L programming head
■
Medtronic Model 9790C programmer with a Model 9767 or
9767L programming head
For information about:
■
indications, contraindications, lead compatibility, warnings and
precautions, and patient selection, see the Maximo VR
7232Cx, 7232B, 7232E Implant Manual, which accompanies
each device.
■
basic programmer and software desktop functions that are not
included in Chapter 10, “Using the programmer” on page 197,
see the manual accompanying the programmer.
■
installing the programming head, see the manual
accompanying the programming head.
■
implanting leads, refer to the manuals accompanying
the leads.
39
Detecting and treating tachyarrhythmias
The ICD monitors the cardiac rhythm for short ventricular intervals
that may indicate the presence of VF, VT, or FVT.
■
Upon detection of VF, the ICD delivers a biphasic defibrillation
shock of up to 35 joules. If the VF episode persists, up to
five more individually programmed defibrillation shocks can
be delivered.
■
Upon detection of VT, the ICD delivers either a Ramp, Ramp+,
or Burst antitachycardia pacing therapy or a biphasic
cardioversion shock of up to 35 joules synchronized to a
ventricular depolarization. If the VT episode persists, up to five
more individually programmed VT therapies can be delivered.
You can also program the ICD to monitor the VT episode
without delivering therapy.
■
Upon detection of FVT, the ICD delivers either a Ramp,
Ramp+, or Burst antitachycardia pacing therapy, or a biphasic
cardioversion shock of up to 35 joules synchronized to a
ventricular depolarization. If the FVT episode persists, up
to five more individually programmed FVT therapies can
be delivered.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 40

40
Chapter 2
System overview
You can use the Wavelet, Stability and Onset criteria to help the
ICD distinguish between true ventricular arrhythmias and rapidly
conducted supraventricular tachycardia (SVT) and withhold
therapy for SVT.
Treating bradycardia
The ICD provides rate responsive ventricular pacing to treat
bradycardia. An internal accelerometer senses the patient’s
physical activity, allowing the ICD to increase and decrease the
pacing rate in response to changes in the level of activity.
Monitoring for real-time and stored data
The ICD and programmer provide real-time information on
detection and therapy parameters and status during a patient
session. The ICD also provides accumulated data on device
operation, including stored electrograms, detected and treated
tachyarrhythmia episodes, bradycardia interventions, and the
efficacy of therapy. The Cardiac Compass report provides up to
14 months of clinically significant data, including arrhythmia
episodes, therapies delivered, physical activity, heart rate, and
bradycardia pacing activities.
All of this information can be printed and retained in the patient’s
file or saved in electronic format on a floppy diskette.
Conducting electrophysiologic tests
You can use the system to conduct non-invasive
electrophysiologic studies including manual delivery of any of the
ICD therapies to manage an induced or spontaneous
tachyarrhythmia.
Alerting the patient to system events
You can use the programmable Patient Alert monitoring feature to
notify the patient with audible tones if certain conditions related to
the leads, battery, charge time and therapies occur. The patient
can then respond based on your prescribed instructions.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 41

Indications and usage
The implantable cardioverter defibrillator is intended to provide
ventricular antitachycardia pacing and ventricular defibrillation for
automated treatment of life threatening ventricular arrhythmias.
Contraindications
The Maximo VR system is contraindicated for
■
patients whose tachyarrhythmias may have transient or
reversible causes, such as: acute myocardial infarction,
digitalis intoxication, drowning, electric shock, electrolyte
imbalance, hypoxia, or sepsis.
■
patients with incessant VT or VF
■
patient who have a unipolar pacemaker
■
patients whose primary disorder is bradyarrhythmias or atrial
arrhythmias.
The Maximo VR system
Indications and usage
41
Patient screening
Prior to implant, patients should undergo a complete cardiac
evaluation, including electrophysiologic testing. Also,
electrophysiologic evaluation and testing of the safety and efficacy
of the proposed tachyarrhythmia therapies are recommended
during and after the implantation of the device.
Other optional screening procedures could include exercise stress
testing to determine the patient’s maximum sinus rate, and cardiac
catheterization to determine if there is a need for concomitant
surgery and/or medical therapy.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 42

Page 43

Emergency therapy3
Delivering emergency therapies 44
How to deliver emergency 35 joule defibrillation 46
How to deliver emergency cardioversion 47
How to deliver emergency fixed burst pacing 48
How to deliver emergency VVI pacing 50
3
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 44

44
Chapter 3
Delivering emergency therapies
Delivering emergency therapies
The device provides the following emergency therapies:
■
defibrillation
■
cardioversion
■
fixed burst pacing
■
emergency VVI pacing
The default emergency therapy is 35 joule defibrillation. When you
select [Emergency] and [DELIVER], the device charges and
delivers a biphasic 35 joule shock along the AX>B pathway
The programmer resets the emergency defibrillation energy to
35 joules each time you select [Emergency]. Emergency
cardioversion and fixed burst values remain as selected for the
duration of the session.
To return to other programming functions from an Emergency
screen, select [Exit Emergency].
1
.
Effect on system operation
The device suspends the automatic detection features when
emergency defibrillation, cardioversion, or fixed burst pacing
therapies are delivered. Detection is not suspended during
emergency VVI pacing. Removing the programming head or
pressing [Resume] turns detection on again.
Aborting an emergency therapy
As a safety precaution, the programmer also displays an [ABORT]
button which immediately terminates any emergency therapy
in progress.
1
If Active Can is turned off, the defibrillation is delivered between the HVX and
HVB electrodes.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 45

Emergency therapy
Delivering emergency therapies
Mechanical Emergency buttons on the Model 9790C programmer
If you press the red mechanical [Emergency] button on the
programmer display panel, the programmer displays the
Emergency screen. The mechanical yellow-on-blue [Deliver]
button activates the emergency therapy displayed on the
programmer screen. This button functions only when the
Emergency screen is displayed.
Mechanical Emergency VVI button on the CareLink Model 2090
programmer
If you press the red Emergency VVI button on the programmer
display panel, the device initiates Emergency VVI pacing and the
programmer displays the Emergency screen.
Temporary parameter values
45
Emergency tachyarrhythmia therapies use temporary values that
1
do not change the programmed parameters of the device.
These
values are not in effect until you select [DELIVER]. After the
tachyarrhythmia therapy is complete, the device reverts to its
programmed values.
1
Delivery of Emergency VVI Pacing changes the programmed bradycardia
pacing values to the emergency values (see page 49).
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 46

46
Chapter 3
Delivering emergency therapies
Delivering an emergency defibrillation therapy
The default emergency therapy is a full-energy defibrillation. When
you select [Emergency] and [DELIVER], the device charges and
delivers a biphasic full-energy shock. The programmer resets the
emergency defibrillation energy to its maximum value each time
you select [Emergency] or select the [Defibrillation] option from an
Emergency screen.
Parameters
* Medtronic nominal setting
Energy – Amount of energy delivered to the
heart by the therapy.
a
Pathway
through the heart.
a
If Active Can is Off, the HVA (Can) electrode is not used as part of the
high-energy delivery pathway.
– Direction the electrical current flows
How to deliver emergency 35 joule defibrillation
3
4
2
10, 11, …16
18, 20, 22, 24, 25,
26, 28, 30, 32, 35*J
AX>B (fixed)
1. Position the programming
head over the device.
2. Select [Emergency].
3. Accept the defibrillation
energy shown on the screen,
or select Energy and select a
new value from the window.
4. Select [DELIVER].
If delivery is not confirmed,
verify that the programming
head is properly positioned
and select [Retry] or [Cancel].
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 47

Delivering emergency therapies
Delivering an emergency cardioversion therapy
When you initiate an emergency cardioversion therapy, the device
charges its capacitors to the selected energy and attempts to
deliver therapy synchronized with a sensed tachyarrhythmia event.
If the cardioversion therapy cannot be synchronized, it is aborted.
See “Synchronizing cardioversion after charging” on page 159.
Parameters
* Medtronic nominal setting
Emergency therapy
47
Energy – Amount of energy delivered to the
heart by the therapy.
a
Pathway
through the heart.
a
If Active Can is Off, the HVA (Can) electrode is not used as part of the
high-energy delivery pathway.
– Direction the electrical current flows
How to deliver emergency cardioversion
3
4
5
2
0.4, 0.6, …1.8,
2, 3, …16
18, 20, 22, 24, 25,
26, 28, 30, 32, 35*J
AX>B (fixed)
1. Position the programming
head over the device.
2. Select [Emergency].
3. Select [Cardioversion].
4. Accept the cardioversion
energy shown on the screen,
or select Energy and select a
new value from the window.
5. Select [DELIVER].
If delivery is not confirmed,
verify that the programming
head is properly positioned
and select [Retry] or [Cancel].
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 48

48
Chapter 3
Delivering emergency therapies
Delivering emergency fixed burst pacing
Emergency fixed burst pacing delivers maximum output pacing
pulses to the ventricle at a selectable interval. The therapy
continues for as long as you keep the programmer stylus on the
[BURST Press and Hold] button.
Parameters
How to deliver emergency fixed burst pacin
* Medtronic nominal setting
Interval – Time interval between pacing
pulses delivered during the fixed burst
therapy.
V. Amplitude – Voltage of the ventricular
pacing pulses delivered during the fixed
burst therapy.
V. Pulse Width – Duration of the
ventricular pacing pulses delivered during
the fixed burst therapy.
How to deliver emergency fixed burst pacing
3
4
2
5
100, 110, …350*
360, 370, … 600 ms
8V(fixed)
1.6 ms (fixed)
1. Position the programming
head over the device.
2. Select [Emergency].
3. Select [Fixed Burst].
4. Accept the pacing interval
shown on the screen, or
select Interval for a new
interval value.
5. Select [BURST Press
and Hold].
If delivery is not confirmed,
the programmer displays
an error window. Verify that
the programming head is
properly positioned. Select
[OK] from the window and
reselect [BURST Press
and Hold].
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 49

Enabling emergency VVI pacing
Emergency VVI pacing programs the device to deliver high-output
ventricular pacing. You can initiate emergency VVI pacing from the
Emergency screen or by pressing the red mechanical button on
the programmer display panel. To disable emergency VVI pacing,
reprogram the bradycardia pacing parameters from the
Parameters screen.
Emergency therapy
Delivering emergency therapies
49
Parameters
How to deliver emergency fixed burst pacin
Pacing Mode – NBG Codea for the pacing mode
VVI
provided during emergency VVI pacing.
Lower Rate – Minimum pacing rate to maintain
70 ppm
adequate heart rate during periods of inactivity.
V. Amplitude – Voltage of the ventricular pacing
8V
pulses delivered during emergency VVI pacing.
V. P u l se W i d t h – Duration of the ventricular pacing
1.6 ms
pulses delivered during emergency VVI pacing.
V. Pace Blanking – Time interval during which
320 ms
sensing is disabled after a pacing pulse.
Hysteresis – Enables tracking of intrinsic heart
Off
rate below programmed Lower Rate to prevent
pacing during extended periods of inactivity, such
as when a patient is sleeping.
V. Rate Stabilization – Modifies the pacing rate to
Off
eliminate the long pause that typically follows a
premature ventricular contraction.
a
N–North American Society of Pacing and Electrophysiology (NASPE), B–British
Pacing and Electrophysiology Group (BPEG), G–Generic Pacemaker Code
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 50

50
Chapter 3
Delivering emergency therapies
How to deliver emergency VVI pacing
4
2
1. Position the programming
head over the device.
2. Select [Emergency].
3. Select [VVI Pacing].
4. Select [PROGRAM]. A
successful programming
sets the device to the
3
following maximum output
bradycardia pacing values.
■
Pacing Mode: VVI
■
Lower Rate: 70 ppm
-1
(70 min
■
V. Amplitude: 8 V
■
V. Width: 1.6 ms
■
V. Pace Blanking: 320 ms
■
Hysteresis: Off
■
Ventricu l a r R a te
)
Stabilization: Off
If programming is not
confirmed, verify that the
programming head is
properly positioned and
select [Retry] or [Cancel].
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 51

Part II
Device implant and patient follow-up procedures
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 52

Page 53

Implanting the ICD4
Overview 54
Preparing for an implant 54
Replacing an ICD 57
Positioning the leads 58
Testing sensing and pacing thresholds 60
Connecting the leads to the ICD 61
Testing defibrillation operation and effectiveness 65
Positioning and securing the ICD 67
Completing the implant procedure 68
4
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 54

54
Chapter 4
Overview
Overview
Preparing for an implant
The tasks for implanting an ICD include
1. Preparing for an implant
2. Replacing an ICD
3. Positioning the leads
4. Testing sensing and pacing thresholds
5. Connecting the leads to the ICD
6. Testing defibrillation operation and effectiveness
7. Positioning and securing the ICD
8. Completing the implant procedure
These tasks are described in the sections that follow.
Warning: Keep a back-up external defibrillator available
during the implant for transthoracic rescue when arrhythmias
are induced.
Equipment for an implant
The equipment that is needed for an implant is as follows:
■
Medtronic CareLink Model 2090 programmer with a
Model 2067 or 2067L programming head, or a Model 9790C
programmer with a Model 9767 or 9767L programming head.
■
Maximo Model 9978/9979 software application
■
Model 2290 or 8090 Analyzer lead analysis device or
equivalent pacing system analyzer
■
external defibrillator
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 55

Sterile supplies for an implant
The sterile supplies that are needed for an implant are as follows:
■
implantable device and lead system components
■
programming head sleeve or programming head
■
analyzer cables
■
lead introducers appropriate for the lead system
■
extra stylets of appropriate length and shape
Implanting the ICD
Preparing for an implant
55
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 56

56
Chapter 4
Preparing for an implant
How to prepare for implanting
Set up the programmer and start the application
Preprogram the device
Before opening the sterile package, prepare the device for implant as
follows:
1. Set up the programmer as described in the instructions provided with
the programmer.
2. Install the Maximo VR Model 9979 software on the programmer, if it is
not already installed.
3. Place the programming head over the device and start the application.
Select the device model or select [Auto identify].
Note: The programmer automatically interrogates the device when the
application starts.
1. Check the “use by” date printed on the package. Do not implant the
device after the “use by” date because the battery’s longevity could be
reduced.
2. Interrogate the device and print a full summary report.
Note: The If the programmer reports that an electrical reset occurred,
do not implant the device. Contact a Medtronic representative.
3. Confirm that the battery voltage displayed on the Quick Look screen is
at least 3.0 V at room temperature
a
(see page 233).
4. Set up data collection parameters and the device internal clock (see
page 251).
5. Perform a manual capacitor formation (see page 303).
■
Dump any charge on the capacitors.
■
Perform a test charge to full energy.
■
Retrieve the charge data.
■
Do not dump the stored charge. Allow it to dissipate, thus reforming
the capacitors.
■
If the reported charge time is clinically unacceptable, contact a
Medtronic representative.
6. Program the therapy and pacing parameters to values appropriate for
the patient (see page 172 ). Ensure that all tachyarrhythmia detection
is programmed Off (see page 80).
7. Interrogate the device again.
a
If the device has been exposed to lower temperatures or has delivered a recent
high-voltage charge, the battery voltage may be temporarily lower than 3.0 V.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 57

Replacing an ICD
If you are replacing a previously implanted ICD, turn off ICD
detection and therapies before explanting.
When implanting the ICD with a chronic lead system, perform the
following evaluations to ensure appropriate detection and therapy:
■
■
■
■
■
■
Notes:
■
■
Implanting the ICD
Replacing an ICD
Check the integrity of the chronic high-voltage leads with a test
shock, chest x-ray, and inspection.
Perform chronic pacing and sensing measurements.
Measure high-voltage lead impedances.
Test defibrillation efficacy.
Confirm adequate sensing during VF.
Ensure proper fit of the lead connectors in the ICD
connector block.
To meet the implant requirements, it may be necessary to
reposition or replace the chronic leads or to add a third
high-voltage electrode.
Any unused leads that remain implanted must be capped.
57
How to explant and replace an ICD
1. Program all tachyarrhythmia detection Off.
2. Dissect the leads and the device free from the pocket. Be careful
not to nick or breach the lead insulation.
3. Loosen each setscrew, and gently retract the lead from the
connector block.
4. Remove the device from the surgical pocket.
5. If the connector pin of any implanted lead shows signs of pitting or
corrosion, replace the implanted lead with a new lead. The
damaged lead should be replaced to ensure the integrity of the
device system.
6. Measure sensing, pacing, and defibrillation efficacy using the
replacement device.
7. Evaluate the defibrillation efficacy of the replacement system.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 58

58
Chapter 4
Positioning the leads
Positioning the leads
Using transvenous leads
Implant endocardial leads according to the supplied instructions,
unless suitable chronic leads are already in place. Do not use any
lead with this device without first verifying connector compatibility
(refer to the Maximo VR 7232Cx, 7232B, 7232E Implant Manual).
Transvenous or epicardial leads may be used.
Use standard transvenous implant techniques to position the
ventricular lead tip in the right ventricular apex.
Follow the general guidelines below for initial positioning of other
transvenous leads (the final positions are determined by
defibrillation efficacy tests):
■
SVC (HVX) lead: Place the lead tip high in the innominate
vein, approximately 5 cm proximal to the right atrium (RA) and
SVC junction.
■
SQ patch: Place the patch along the left mid-axillary, centered
over the fourth-to-fifth intercostal space.
■
CS lead: Advance the lead tip to just under the left atrial
appendage, if possible.
If using a subclavian approach, position the lead laterally to avoid
pinching the lead body between the clavicle and the first rib.
Warning: Pinching the lead can damage the lead conductor
or insulation, which may cause unwanted high-voltage
therapies or result in the loss of sensing or pacing therapy.
Using epicardial leads
A variety of surgical approaches can be used to implant epicardial
leads, including a limited left thoracotomy or median sternotomy.
A typical placement may use an anterior right ventricular patch as
the RV (HVB) and a posterolateral left ventricular patch as
SVC (HVX).
Follow the general guidelines below for positioning epicardial
leads:
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 59

Surgical incisions
Implanting the ICD
Positioning the leads
■
If unipolar epicardial pacing leads are used, position the
electrodes about 1 to 2 cm apart to reduce electromagnetic
interference, and route the leads together with several
loose twists.
■
Suture the smooth face of each patch lead against the
epicardium or pericardium in locations that produce optimal
defibrillation.
■
Place the patches so that they encompass the maximum
amount of cardiac mass and they have approximately equal
amounts of mass between them.
■
Ensure that the patches do not overlap and the electrode
portions do not touch.
■
Avoid placing extra-pericardial patches over the phrenic nerve.
A single-incision submuscular or subcutaneous approach is
recommended when the ICD is implanted in the pectoral region.
Make the implant pocket about 1.5 times the size of the ICD.
59
Submuscular implant – An incision extending over the
deltoid-pectoral groove typically provides access to the cephalic
and subclavian veins as well as the implant pocket. Place the ICD
sufficiently medial to the humeral head to avoid interference with
shoulder motion.
Subcutaneous implant – A transverse incision typically permits
isolation of the cephalic vein. Place the ICD far medially to keep
the leads away from the axilla. Make sure that the upper edge of
the ICD remains inferior to the incision.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 60

60
Chapter 4
Testing sensing and pacing thresholds
Testing sensing and pacing thresholds
Sensing and pacing tests include the following measurements:
■
EGM amplitude
■
slew rate
■
pacing threshold
■
pacing lead impedance
Medtronic recommends that you use a Model 2290 or 8090
Analyzer lead analysis device to perform sensing and pacing
measurements. If you use a Pacing System Analyzer (PSA),
perform ventricular measurements via the ventricular channel of
the PSA.
Refer to the technical manual for the Analyzer you use to find
details on performing sensing and pacing measurements.
Parameters
Measured sensing and pacing values must meet the following
specific requirements at implant.
Tab le 4- 1. Sensing and pacing values at implant
Measurement Acute Transvenous Leads Chronic Leads
R- wave amplitude ≥ 5 mV ≥ 3 mV
Ventricular slew rate: ≥ 0.75 V/s ≥ 0.5 V/s
Ventricular capture
threshold:
a
At 0.5 ms pulse width
a
Considerations
When measuring sensing and pacing values, measure between
the tip (cathode) and ring or coil (anode) of each bipolar
pacing/sensing lead.
For unipolar epicardial pacing leads, either electrode can be the
cathode; use the configuration that yields the lower pacing
threshold.
Note: Do not measure the intracardiac EGM telemetered from the
ICD to assess sensing.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
≤ 1.0 V ≤ 3.0 V
Page 61

Connecting the leads to the ICD
For more detailed information about lead/connector compatibility,
see the Maximo VR 7232Cx, 7232B, 7232E Implant Manual, or
contact Medtronic Technical Services at 1-800-723-4636.
Warning: Loose lead connections may result in inappropriate
sensing and failure to deliver necessary arrhythmia therapy.
Caution: Use only the torque wrench supplied with the device.
It is designed to prevent damage to the device from
overtightening a setscrew
Tabl e 4-2 . Cx-type connector characteristics
General description Device
One IS-1 connector for pacing and
sensing, Two DF-1 connectors for
high-voltage therapy, Active Can
electrode (programmable)
Implanting the ICD
Connecting the leads to the ICD
Port
SVC DF-1 HVX
RV DF-1 HVB
Can — HVA, Can
V IS-1 bipolar
Connector
Typ e
Software
Name
61
Figure 4-1. Cx-type connector
2
5
1 Suture holes
2 SVC port (DF-1)
3RV port (DF-1)
4 Programmable Active Can
5V port (IS-1)
3
4
Maximo VR 7232Cx, 7232B, 7232E Reference manual
1
Page 62

62
Chapter 4
Connecting the leads to the ICD
For easier lead insertion into the Cx-type connector, insert the
ventricular IS-1 leg before the other legs.
Tab le 4- 3. B-type connector characteristics
General description Device
Three 6.5 mm unipolar
high-voltage ports and one
3.2 mm low profile bipolar
pace/sense (IS-1 compatible) port.
a
The HVA and HVX ports are electrically connected and are treated as the HVX
electrode. For more information, see “B- and E-type connector pathways” on
page 136.
Figure 4-2. B-type connector
Port
Connector
Typ e
Software
Name
HVB 6.5 mm HVB
a
HVA
HVX
6.5 mm HVX
a
6.5 mm HVX
Can — HVA, Can
P/S
bipolar
pace
3.2 mm
bipolar (IS-1
compatible)
sense
1
2
6
5
1 Suture holes
2 HVB port (6.5 mm)
3 HVA port (6.5 mm)
4 Programmable Active Can
5 HVX port (6.5 mm)
6 P/S port (3.2 mm)
Maximo VR 7232Cx, 7232B, 7232E Reference manual
3
4
Page 63
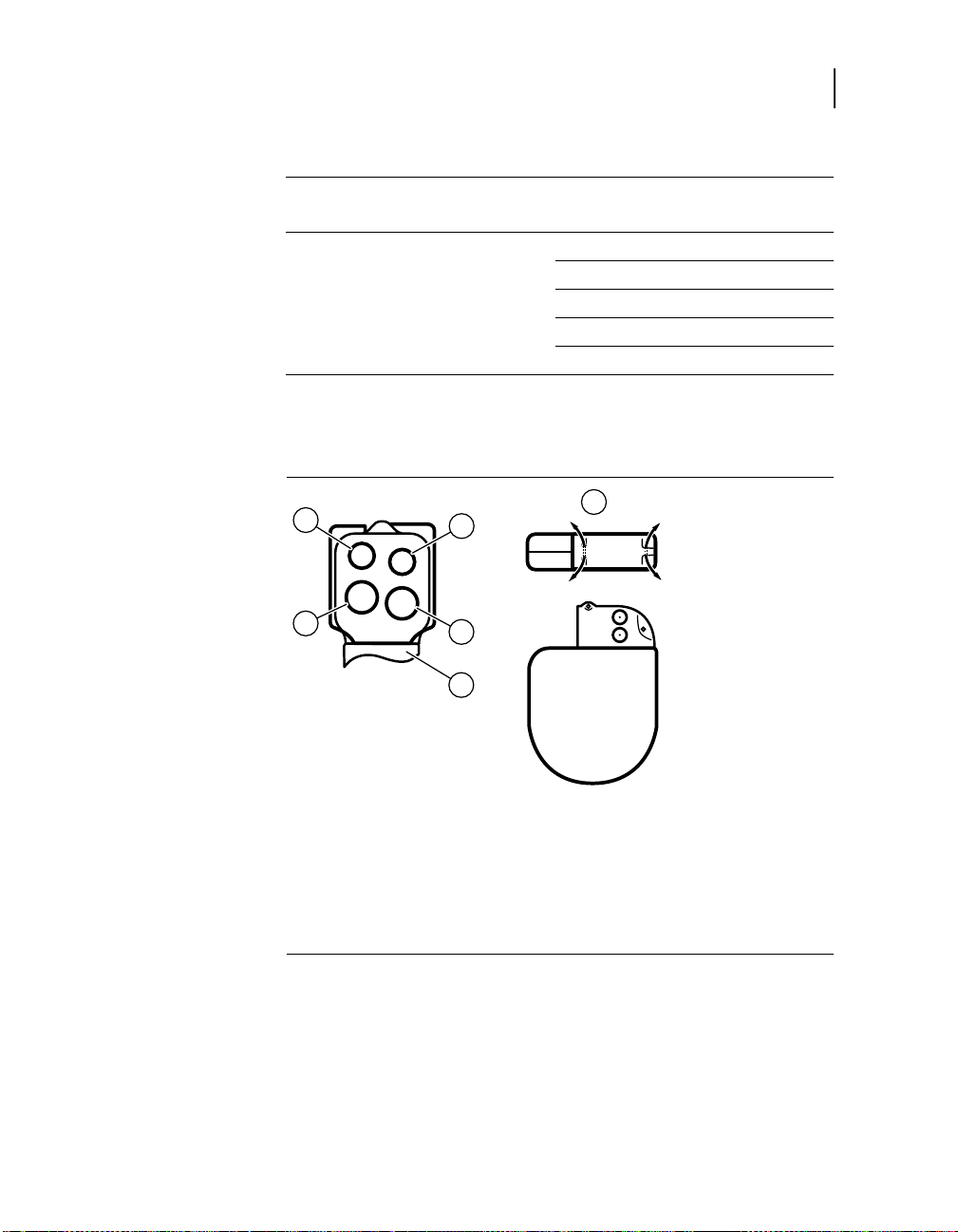
Implanting the ICD
Connecting the leads to the ICD
Tabl e 4-4 . E-type connector characteristics
General description Device
Port
P+/S 5.0 mm
Two 6.5 mm unipolar high-voltage
ports and two 5 mm unipolar
pace/sense ports.
HVA 6.5 mm HVX
HVB 6.5 mm HVB
Can — HVA, Can
P-/S 5.0 mm
a
The HVA port of the E-type connector can be used as the HVX electrode when
Active Can is programmed off. For more information, see “B- and E-type
connector pathways” on page 136.
Figure 4-3. E-type connector
Connector
Typ e
Software
Name
a
63
6
5
2
3
4
1 Suture holes
2 P+/S port (5.0 mm)
3 HVA port (6.5 mm)
4 Programmable Active Can
5 HVB port (6.5 mm)
6 P-/S port (5.0 mm)
1
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 64

64
Chapter 4
Connecting the leads to the ICD
How to connect the lead to the device
How to connect the lead to the device
1
a
b
30SetScrew.eps
2
30LeadTIp.eps
Model 7232Cx connector shown in example
1. Insert the torque wrench into the
appropriate setscrew.
a. If the port is obstructed, retract the
setscrew to clear it. Take care not to
disengage the setscrew from the
connector block.
b. Leave the torque wrench in the setscrew
until the lead is secure. This allows a
pathway for venting trapped air when the
lead is inserted.
2. Push the lead or plug into the connector
port until the lead pin is clearly visible in the
pin viewing area. No sealant is required, but
sterile water may be used as a lubricant.
3. Tighten the setscrew by turning clockwise
until the torque wrench clicks.
4. Tug gently on the lead to confirm a secure
fit. Do not pull on the lead until all setscrews
have been tightened.
5. Repeat these steps for each lead.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 65

Implanting the ICD
Testing defibrillation operation and effectiveness
Testing defibrillation operation and effectiveness
Demonstrate reliable defibrillation effectiveness with the implanted
lead system by using your preferred method to establish that a
10 J (minimum) safety margin exists.
Note: If the 10 J (minimum) safety margin cannot be ensured, see
“Solving tachyarrhythmia therapy problems” on page 330.
High-voltage implant values
Measured values must meet the following requirements at implant.
Tabl e 4-5 . High-voltage therapy values at implant
Measurement Acute or Chronic Leads
V. Defib impedance
SVC (HVX) impedance (if applicable)
Defibrillation threshold
Warning: Ensure that an external defibrillator is charged for a
rescue shock.
20 - 200 ohms
20 - 200 ohms
≤ 25 J
65
How to prepare for defibrillation threshold testing
1. Place the programming head over the ICD, start a patient session,
and interrogate the device, if you have not already done so.
2. Observe the Marker Channel telemetry annotations and the
programmer ECG display to verify that the ICD is sensing properly.
3. Conduct a manual Lead Impedance Test
lead connections. Perform this test with the ICD in the surgical
pocket and keep the pocket very moist. If the impedance is out of
range, perform one or more of the following tasks:
■
Recheck lead connections and electrode placement.
■
Repeat the measurement.
■
Inspect the bipolar EGM for abnormalities.
■
Measure the defibrillation impedance with a manual test shock.
4. Program the ICD to properly detect VF with an adequate safety
margin (1.2 mV sensitivity).
a
See “Measuring lead impedance” on page 299.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
a
to verify the defibrillation
Page 66

66
Chapter 4
Testing defibrillation operation and effectiveness
How to perform defibrillation threshold testing
3
2
4
6
5
7
1. Select Tests > EP Study.
2. Select either 50 Hz BURST or
T-shock induction.
3. Select [Resume at BURST] or
[Resume at DELIVER].
4. Select [Adjust Permanent...].
1
5. Program VF Enable On.
6. Program the automatic therapy
energy settings. Therapies 2-6
should be set to the maximum
energy.
8
7. Select [Program].
8. Select [Close].
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 67

13
11
12
Implanting the ICD
67
Positioning and securing the ICD
9. If performing a T-Shock
induction, select the [Enable]
checkbox.
10. Select [DELIVER], or [50 Hz
BURST Press and Hold].
If necessary, you can abort an
9
10
14
induction or therapy in
progress by pressing [ABORT].
11. Observe the live rhythm
monitor for proper post-shock
sensing.
12. Use the [Adjust Permanent...]
button to program the energy
level.
13. Wait until the on-screen timer
reaches 5 minutes, then repeat
steps 9 through 12 as desired.
14. Select Params > Detection and
program VF, FVT, and VT
detection Off before closing.
Positioning and securing the ICD
Cautions:
■
If no SVC electrode is implanted, the pin plug provided
with the device must be secured in the SVC port.
■
Program tachyarrhythmia detection Off before closing.
How to position and secure the device
1. Ensure that each lead pin or plug is fully inserted into
the connector block and that all setscrews are tight.
2. Coil any excess lead length beneath the device. Avoid
kinks in the lead conductors.
3. Implant the device within 5 cm of the skin. This
position optimizes the ambulatory monitoring
operations.
4. Suture the device securely within the pocket to
minimize post-implant rotation and migration of the
device. Use a surgical needle to penetrate the
suture holes.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 68

68
Chapter 4
Completing the implant procedure
Completing the implant procedure
After implanting the device, x-ray the patient to verify the device
and leads placement. To complete programming the device, select
parameters that are appropriate for the patient.
How to complete programming the device
1. After closing the pocket, program detection On. Program ventricular
tachyarrhythmia therapies On as desired.
2. Monitor the patient after the implant, and take x-rays as soon as
possible to document and assess the location of the leads.
3. Program patient information. See “How to view and enter new patient
information” on page 282.
4. Configure the Patient Alert feature. See “Using the Patient Alert
feature” on page 235.
5. Set up data collection parameters. See “Setting up data collection” on
page 249.
6. Interrogate the device after any spontaneous episodes to evaluate the
detection and therapy parameter settings.
7. If the patient has not experienced spontaneous episodes, you may
induce the clinical tachyarrhythmias using the non-invasive EP Study
features to further assess the performance of the system. See Chapter
14, “Conducting electrophysiologic studies” on page 307.
8. Recheck pacing and sensing values, and adjust if necessary.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 69

Conducting a patient follow-up
Patient follow-up guidelines 70
Verifying the status of the implanted system 70
Verifying accurate detection and appropriate therapy 71
Verifying effective bradycardia pacing 73
session
5
5
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 70

70
Chapter 5
Patient follow-up guidelines
Patient follow-up guidelines
Schedule regular patient follow-up sessions to monitor the
condition of the ICD and leads and to verify that the ICD is
configured appropriately for your patient.
During the first few months after receiving a new device, the
patient may require close monitoring. Schedule an office visit at
least every three months.
The Quick Look screen, which is displayed after you interrogate
the device, provides a good beginning for the follow-up review.
Using this screen you can
■
verify that the device is functioning correctly.
■
review the clinical performance and long term trends.
■
print appropriate reports1 to compare the results to the
patient’s history and to retain for future reference.
Note: The Checklist feature provides a standard list of tasks to
perform at a complete follow-up visit. You can also customize your
own checklists if you wish. See (“Streamlining follow-ups with
Checklist” on page 243) for more information.
Verifying the status of the implanted system
To verify that the ICD and leads are functioning correctly, review
the following information from the Quick Look screen and perform
follow-up tests as indicated:
■
Review the displayed battery voltage for comparison to the
Elective Replacement Indicator value (see page 26).
Remember that battery voltage may be low if high-voltage
charging has occurred within 24 hours.
■
Review the last full energy charge.
– For information about adjusting the capacitor formation
interval, see (“Optimizing charge time” on page 190).
– If the programmer displays an Excessive Charge Time ERI,
the ICD should be replaced immediately.
1
See (“Using Cardiac Compass to view long term clinical trends” on page 274) for
information on this new report.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 71

Conducting a patient follow-up session
Verifying accurate detection and appropriate therapy
■
Review the defibrillation and pacing lead impedance values for
inappropriate values or large changes since the last follow-up.
See (“Measuring lead impedance” on page 299).
■
Perform an EGM Amplitude test for comparison to previous
EGM Amplitude measurements. See (“How to perform an
EGM Amplitude test” on page 302).
■
To review longer term trends in sensing and impedance
measurements, select the [>>] button from the lead
impedance area of the Quick Look screen. The programmer
displays a detailed history of automatic sensing and
impedance measurements. See (“Taking a quick look at
device activity” on page 233).
Verifying accurate detection and appropriate therapy
To verify that the ICD is providing effective tachyarrhythmia
detection and therapy, review the following information from the
Quick Look screen and investigate as indicated:
■
Review Quick Look Observations that relate to patient history
and device operation. To display more detailed information
about any observation, select the observation and then select
the [>>] button.
■
Review any Patient Alerts listed in the Observations of the
Quick Look screen. For the most detailed information about
Patient Alerts, select Patient Alert from the Data icon and
select [Events].
■
Check stored episode records for appropriate sensing and
detection of arrhythmias. See (“Viewing episode data” on
page 260).
■
Check stored SVT episode records for appropriate
identification of SVTs.
71
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 72

72
Chapter 5
Verifying accurate detection and appropriate therapy
Considerations
Review the following information before verifying detection and
therapy.
Flashback memory – In addition to the episode text and stored
electrograms, use Flashback memory and interval plots to help
investigate the accuracy and specificity of ventricular detection.
Episode misidentification – If the episode records indicate that
false detections have occurred, the Sensing Integrity counter may
help in determining the prevalence of oversensing. For more
information, see (“Sensing integrity counter” on page 256).
If the ICD is oversensing, consider these programming options:
■
Increase the Pace Blanking value.
■
Increase the sensitivity threshold.
Caution: Do not re-program the ICD to decrease oversensing
without assuring that appropriate sensing is maintained, See
(“Setting up sensing” on page 81).
If the episode records reveal that a stable monomorphic VT has
been identified and treated as VF, consider these options to
improve the detection accuracy:
■
Review the Interval Plot for the episode, and adjust
VF Interval, if necessary. Use caution when reprogramming
the VF Interval, because changes to this value can adversely
affect VF detection.
■
Consider enabling FVT via VF detection. See (“Detecting FVT
episodes” on page 96).
If the SVT episode records include episodes of true VT, review the
SVT episode record to identify the SVT detection criterion that
withheld detection. Adjust the SVT detection criteria parameters
as necessary. See (“Enhancing detection with Wavelet” on
page 107), (“Enhancing VT detection with the Onset criterion” on
page 116), and (“Enhancing VT detection with the Stability
criterion” on page 121).
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 73

Conducting a patient follow-up session
Verifying effective bradycardia pacing
Verifying effective bradycardia pacing
To verify that the ICD is sensing and pacing appropriately, review
the following information from the Quick Look screen and
investigate as indicated:
■
Confirm that the patient is receiving adequate cardiac support
for daily living activities.
■
Review the pacing history for comparison to the patient
history. A sharp increase in the paced beats percentage may
indicate a need for investigation and analysis.
■
Review the Cardiac Compass report for comparison to patient
history (see page 274 ).
■
Conduct a pacing threshold test (see page 294) to verify that
the programmed pacing parameters provide a sufficient
safety margin.
Considerations
Review the following information before verifying bradycardia
pacing.
73
Ventricular Pacing – If the ventricle is predominantly paced and
the patient exhibits adequate ventricular response, consider
decreasing the Lower Rate.
% Pacing – Because the percentages for this counter are
rounded to the nearest unit, they may not add up to 100%.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 74

Page 75

Part III
Configuring the ICD for the patient
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 76

Page 77

Detecting tachyarrhythmias6
Detection overview 78
Setting up sensing 81
Detecting VF episodes 85
Detecting VT episodes 90
Detecting FVT episodes 96
Detecting tachyarrhythmia episodes with Combined Count 101
Monitoring episodes for termination or redetection 104
Enhancing detection with Wavelet 107
Enhancing VT detection with the Onset criterion 116
Enhancing VT detection with the Stability criterion 121
6
Detecting prolonged tachyarrhythmias with High Rate Timeout 124
Key terms 126
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 78

78
Chapter 6
Detection overview
Detection overview
The device detects ventricular tachyarrhythmias (VF, VT, and FVT)
by comparing the time intervals between sensed ventricular
events to a set of programmable detection intervals. If enough
intervals occur that are shorter than the programmed intervals, the
device detects a tachyarrhythmia, and responds automatically with
a programmed therapy. After delivering the therapy, the device
either redetects the arrhythmia and delivers the next programmed
therapy or detects episode termination.
To avoid detecting rapidly conducted SVTs (for example, sinus
tachycardia or atrial fibrillation) as ventricular tachyarrhythmias,
the device provides several detection enhancements, including
the Wavelet Dynamic Discrimination criterion, the Onset criterion,
and the Stability criterion.
Figure 6-1 shows how all of these detection features interact
during initial detection. During redetection, the device does not
apply the Wavelet and Onset detection criteria.
Note: Detection functions can be turned off by programming the
VF Enable, FVT Enable, and VT Enable parameters to Off. For an
example, see “How to program VF detection” on page 88.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 79

Detecting tachyarrhythmias
Figure 6-1. How detection features interact during initial detection
79
Detection overview
No
No
Ye s
No
No / suspended by
High Rate Timeout
Update counts and pattern information
Has High Rate Timeout suspended detection
Is the Onset criterion allowing VT event counting? (VT and
Does Stability reset the VT event count?
Ventricular Event
Is the interval in the
VF, FVT, or VT detection zone?
Ye s
enhancements?
No
FVT via VT detection only)
Ye s
(VT and FVT via VT detection only)
No
Has a tachyarrhythmia event count
reached an NID?
Ye s
Is Wavelet on?
Ye s
Ye s
Tachy
Episode
Detected
Ye s
No
Is the median ventricular interval less than the
SVT Limit?
No
Is Wavelet withholding detection?
Ye s
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 80

80
Chapter 6
Detection overview
Suspending tachyarrhythmia detection
When detection is suspended, the device temporarily stops
classifying and counting tachyarrhythmia intervals. Sensing and
bradycardia pacing remain active, and the programmed detection
settings are not modified.
Detection is suspended
■
when the device senses the presence of a strong magnet. The
programmer head contains a magnet which suspends
detection, but once telemetry between the device and
programmer is established, detection resumes.
■
while performing any of the manual system tests, including
Underlying Rhythm, Pacing Threshold, Lead Impedance,
EGM Amplitude, Wavelet, and Charge/Dump. Detection
automatically resumes once the test is complete.
■
while performing a T-Shock, 50 Hz Burst, Manual Burst, or
PES Induction. You can choose to have the device
automatically resume detection after delivering the induction.
■
when you deliver a Manual or Emergency therapy. You can
resume detection by selecting the [Resume] button or
removing the programming head from the device.
■
when you select the on-screen [Suspend] button. You can
resume detection by selecting the [Resume] button or by
removing the programming head from the device.
■
during the automatic daily lead impedance measurements.
Detection resumes when the measurements are complete.
■
while the device is delivering an automatic tachyarrhythmia
therapy (including capacitor charging for defibrillation and
cardioversion). However, the device does continue to confirm
the detected episode during charging. Detection resumes
when the therapy is complete.
Note: The device suspends VT detection (and Combined
Count detection; see page 101) for 17 events following a
defibrillation therapy delivered in response to a detected VF.
■
during charging for Automatic Capacitor Formation. Detection
resumes when charging is complete.
1
1
If the defibrillation therapy is delivered as a result of a High Rate Timeout
Therapy operation, VT detection is not suspended (see page 126).
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 81

Setting up sensing
The device provides bipolar sensing in the ventricle via the
sensing electrodes of the implanted ventricular lead. You can
adjust the sensitivity to intracardiac signals using the ventricular
sensitivity setting. This setting defines the minimum electrical
amplitude recognized by the device as a ventricular sensed event.
Proper sensing is essential for the safe and effective use of the
device. To provide appropriate sensing, the device uses an
auto-adjusting ventricular sensing threshold.
See “Details about sensing” on page 83.
Parameters
V. Sensitivity (mV) – Minimum amplitude of
electrical signal that registers as a sensed
ventricular event.
Considerations
Detecting tachyarrhythmias
Setting up sensing
* Medtronic nominal setting
0.15, 0.3*, 0.45, 0.6,
0.9, 1.2
81
Review the following information before programming sensing
parameters.
Sensitivity thresholds – The programmed ventricular sensitivity
threshold applies to all features related to sensing, including
detection and bradycardia pacing.
Bradycardia pacing and sensing – A combination of high
pacing pulse width or high amplitude with a low sensitivity
threshold may cause inappropriate sensing. Programming a lower
pulse width, lower amplitude, longer pace blanking, or a higher
sensitivity threshold may eliminate this inappropriate sensing.
Recommended ventricular sensitivity threshold – A ventricula r
sensitivity threshold of 0.3 mV is recommended to maximize the
probability of detecting VF and to limit the possibility of
oversensing.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 82

82
Chapter 6
Setting up sensing
High ventricular sensitivity threshold – Setting V. Sensitivity to
a value greater than 0.6 mV is not recommended except for
testing. Doing this may cause undersensing, which can cause any
of the following situations:
■
delayed or aborted cardioversion therapy
■
delayed defibrillation therapy (when VF confirmation is active)
■
asynchronous pacing
■
underdetection of tachyarrhythmias.
Low ventricular sensitivity threshold – If you set V. Sensitivity
to its most sensitive value of 0.15 mV, the device will be more
susceptible to EMI and oversensing.
Testing sensitivity after reprogramming – If you change the
ventricular sensitivity threshold, evaluate for proper sensing and
detection by inducing VF and allowing the device to automatically
detect and treat the arrhythmia.
Sensing during VF – Always verify that the device senses
properly during VF. If the device is not sensing or detecting
properly, program detection and therapies off, and evaluate the
system (making sure to monitor the patient for life-threatening
arrhythmias until you enable detection and therapies again). You
may need to reposition or replace the ventricular sensing lead to
achieve proper sensing.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 83

How to program sensitivity
Details about sensing
Auto-adjusting sensitivity thresholds
Detecting tachyarrhythmias
83
Setting up sensing
1. Select Params > Detection
2. Select the desired
V. Sensitivity parameter
value.
3. Select [PROGRAM].
1
2
3
The device automatically adjusts the sensitivity thresholds after
certain paced and sensed events to help reduce oversensing from
T-waves and pacing. Figure 6-2 shows how sensitivity thresholds
are adjusted after different types of events.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 84

84
Chapter 6
Setting up sensing
Figure 6-2. Auto-adjusting sensitivity threshold
Sensitivity threshold
1 2
Rectified and Filtered
V. E G M
Marker Channel
30Autoadjust.eps
V
S
V
P
1 After a ventricular sensed event, the ventricular sensitivity threshold
increases to 75% of the EGM peak (maximum: 8x the programmed
value, decay constant: 450 ms).
a
2 After the ventricular pace blanking period is finished, the ventricular
threshold increases to 4.5x the programmed value (maximum:
1.8 mV, decay constant: 450 ms).
a
The exponential decay continues through a subsequent ventricular pacing pulse
and its blanking period.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 85

Detecting tachyarrhythmias
Detecting VF episodes
Blanking periods
During a blanking period, the device does not sense electrical
signals. This helps prevent sensing of device pacing,
cardioversion and defibrillation pulses, post-pacing depolarization,
T-waves, and multiple sensing of the same event. The blanking
periods following paced events are longer than those following
sensed events to avoid sensing ventricular depolarizations.
Table 6-1 shows the duration of the fixed blanking periods. For
information on programmable pace blanking periods (see
page 186).
Tabl e 6-1 . Fixed blanking periods
Ventricular blanking after sensed ventricular event 120 ms
85
Ventricular blanking after delivered
cardioversion or defibrillation therapy
Refractory periods
During a refractory period, the device senses normally, but
classifies sensed events as refractory and limits its response to
these events. Refractory periods are used during synchronization
to help prevent the device from delivering cardioversion and
defibrillation therapies at inappropriate times. See “Synchronizing
defibrillation without confirming VF” on page 137 and
“Synchronizing cardioversion after charging” on page 159.
Note: Refractory periods do not affect tachyarrhythmia detection.
Detecting VF episodes
The device detects VF episodes by examining the cardiac rhythm
for short ventricular intervals. If a predetermined number of
intervals occurs that are short enough to be considered VF events,
the device detects VF and delivers the first programmed VF
therapy. After therapy, the device continues to evaluate the
ventricular rhythm to determine if the episode is ongoing.
See “Details about VF detection” on page 88.
520 ms
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 86

86
Chapter 6
Detecting VF episodes
Parameters
* Medtronic nominal setting
Considerations
VF Detection Enable – Turns VF
detection on or off.
VF Interval (ms) – V-V intervals shorter
than this value are counted as VF events.
VF Initial NID – Number of Intervals to
Detect: number of VF events the device
must count to detect a VF episode.
VF Redetect NID – Number of Intervals
to Redetect: number of VF events the
device must count to redetect a
continuing VF after a therapy.
On*, Off
240, 250, …320*, …400
12/16, 18/24*,
24/32,30/40, 45/60, 60/80,
75/100, 90/120,
105/140, 120/160
6/8, 9/12, 12/16*, 18/24,
21/28, 24/32, 27/36, 30/40
Review the following information before programming
VF detection parameters.
VF Interval minimum setting – To ensure proper VF detection,
you should not program the VF Interval less than 300 ms.
VF Interval maximum setting – Programming the VF Interval to
a value greater than 350 ms may cause inappropriate detection
of rapidly conducted atrial fibrillation as VF or FVT via VF. Intervals
shorter than the VF Interval are counted using the VF event
counter, which is more sensitive than the consecutive VT
event counter.
VF, FVT, and VT Intervals – To allow for normal variations in the
patient’s tachycardia interval, you should program the VF, FVT,
and VT intervals at least 40 ms apart.
Episode redetection – You can expedite redetection by
programming the VF and VT Redetect NIDs lower than the
Initial NIDs.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 87

Restrictions
Detecting tachyarrhythmias
Detecting VF episodes
Enabling VF detection – When VF Detection Enable is
programmed On for the first time, the device
■
enables Automatic Capacitor Formation
■
starts recording Cardiac Compass data
■
starts recording lead performance trends (starting at
3:00 AM, by the device clock)
■
clears all brady pacing counters
VF Detection and Wavelet – You can program the device to
exclude rapidly conducted SVTs from VF detection by enabling the
Wavelet Dynamic Discrimination criterion. Note that the SVT Limit
must be programmed shorter than the VF Interval in order for
Wavelet to affect VF detection. See “Enhancing detection with
Wavelet” on page 107.
Review the following information before programming
VF detection parameters.
87
Tachyarrhythmia detection and bradycardia pacing – To e n s u r e
reliable ventricular tachyarrhythmia detection, the programmer
regulates the values available for bradycardia pacing and
tachyarrhythmia detection. See (“Parameter interlocks” on
page 364).
VF detection backup – To ensure VF detection backup during
VT and FVT episodes, if VF Detection is off, both VT Detection
and FVT Detection must also be off.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 88

88
Chapter 6
Detecting VF episodes
How to program VF detection
2
Details about VF detection
To program VF detection:
1. Select Params > Detection
2. Select the desired values for
VF Enable, VF Initial NID,
VF Redetect NID and
VF Interval.
3. Select [PROGRAM].
1
3
The device detects VF by counting the number of VF events, which
are V-V intervals shorter than the programmed VF Interval. On
each event, the device counts the number of recent VF events.
The number of recent events examined is called the VF detection
window. The size of the VF detection window is the second
number in the programmed VF NID (for example, 24 events if the
VF Initial NID is 18/24).
The threshold for detecting VF is the first number in the
programmed VF NID (for example, 18 events if the VF Initial NID
is 18/24). This threshold is always 75% of the VF detection
window. That is, if 75% of the events in the VF detection window
are VF events, the device detects a VF episode (see Figure 6-3).
After the device detects VF, it delivers the first programmed VF
therapy. Following the therapy, if the number of VF events reaches
the programmed VF Redetect NID, the device redetects VF and
delivers the next programmed VF therapy.
Note: The device can also detect VF Episodes via the Combined
Count detection criterion (see page 101).
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 89

Figure 6-3. Device detects VF
ECG
Marker Channel
Detecting tachyarrhythmias
Detecting VF episodes
1 2 3
89
FSFSFSFSFSFSFSFSFSF
F
F
S
VSVSV
D
S
VF Event Count
V
S
V
S
FSFSF
V
S
1 2 3 4 5 6 6 7 8 9 10 11 12 13 14 15 16 17 18
FSFSF
V
S
S
S
S
VF Interval
200 ms
1 VF starts, and the device begins counting VF events (intervals less than the programmed
VF Interval).
2 A ventricular interval occurs outside the VF detection zone. The VF event count is not
incremented.
3 The VF event count reaches the programmed VF NID value of 18 events out of 24, and the
device detects VF.
30VFDetection.eps
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 90

90
Chapter 6
Detecting VT episodes
Detecting VT episodes
Parameters
The device detects VT episodes by examining the cardiac rhythm
for short ventricular intervals. If enough intervals occur that are
short enough to be considered VT events (but are not VF or FVT
events), the device detects VT and delivers the first programmed
VT therapy. After therapy, the device continues to evaluate the
ventricular rhythm to determine if the episode is ongoing.
You can program the device to detect and record VT episodes
without treating them with VT therapies by setting VT Detection
Enable to Monitor. If a patient’s VT episodes are well-tolerated,
this feature allows you to collect data about these episodes without
delivering therapy or affecting VF detection.
See “Details about VT detection” on page 92.
* Medtronic nominal setting
VT Detection Enable – Turns VT
detection on or off, or enables VT
monitoring.
VT Interval (Rate) (ms) – V-V intervals
shorter than this value are counted as
VT events.
VT Initial NID – Number of Intervals to
Detect: number of VT events the device
must count to detect a VT episode.
VT Redetect NID – Number of Intervals
to Redetect: number of VT events the
device must count to redetect a
continuing VT after a therapy.
On, Off*, or Monitor
280, 290, …400*, …600
12, 16*, …52,
76, 100
4, 8, 12*,. . ., 52
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 91

Considerations
Detecting tachyarrhythmias
Detecting VT episodes
Review the following information before programming
VT detection parameters.
VF, FVT, and VT Intervals – To allow for normal variations in the
patient’s tachycardia interval, you should program the VF, FVT,
and VT intervals at least 40 ms apart.
Episode redetection – You can expedite redetection by
programming the VF and VT Redetect NIDs lower than the
Initial NIDs.
VT detection and Combined Count detection – When
VT Detection is On, the device applies the Combined Count
detection criterion to help speed detection of rhythms that
fluctuate between detection zones. Combined Count detection is
disabled if VT Detection is set to Off or Monitor. See “Detecting
tachyarrhythmia episodes with Combined Count” on page 101.
VT detection and rapidly conducted SVTs – You can program
the device to exclude rapidly conducted SVTs from VT detection
by enabling the Wavelet, Onset, or Stability detection criteria. See
“Enhancing detection with Wavelet” on page 107, “Enhancing VT
detection with the Onset criterion” on page 116, and “Enhancing
VT detection with the Stability criterion” on page 121.
91
Restrictions
Review the following information before programming
VT detection parameters.
Tachyarrhythmia detection and bradycardia pacing – To e n s u r e
reliable ventricular tachyarrhythmia detection, the programmer
regulates the values available for bradycardia pacing and
tachyarrhythmia detection. See “Parameter interlocks” on
page 364.
VF detection backup – To ensure VF detection backup during
VT and FVT episodes, if VF Detection is off, both VT Detection
and FVT Detection must also be off.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 92

92
Chapter 6
Detecting VT episodes
How to program VT detection
2
Details about VT detection
To program VT detection:
1. Select Params > Detection
2. Select the desired values for
VT Enable, VT Initial NID,
VT Redetect NID and
VT Interval.
3. Select [PROGRAM].
1
3
The device detects VT by counting the number of consecutive VT
events. A VT event is a V-V interval shorter than the programmed
VT Interval but greater than or equal to the VF Interval. If the
number of consecutive VT events reaches the programmed VT
Initial NID, the device detects VT (see Figure 6-4).
The VT event count resets to zero whenever an interval occurs
that is greater than or equal to the programmed VT Interval. The
count remains at the current value if an interval is shorter than the
programmed VF Interval.
After the device detects VT, it delivers the first programmed VT
therapy. Following the therapy, if the VT event counter reaches the
VT Redetect NID, the device redetects VT and delivers the next
programmed therapy.
Note: The device can also detect VT Episodes via the Combined
Count detection criterion (see page 101).
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 93

Figure 6-4. Device detects VT
ECG
Marker Channel
132
Detecting tachyarrhythmias
Detecting VT episodes
93
VT Event Count
V
S
VSV
S
V
T
S
10123456
TSTSTSTSTST
S
S
VT Interval
ECG
Marker Channel
T
D
V
S
30VTDetection.eps
VT Event Count
TSTSTSTSTSTSTSTST
78910111213141516
S
VT Interval
200 ms
1 VT starts, and the device begins counting VT events (intervals less than the programmed
VT Interval, but greater than or equal to than the VF Interval).
2 A ventricular interval occurs outside VT detection zone. The VT event count resets to zero.
3 The VT event count reaches the programmed VT NID of 16 events, and the device
detects VT.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 94

94
Chapter 6
Detecting VT episodes
VT monitoring
You can program the device to record VT episodes without
delivering VT therapy by setting VT Detection to Monitor. When VT
monitoring is enabled, the device detects VT episodes but does
not deliver VT therapy (see Figure 6-5). Instead, it records VT
episodes, labeling them as “monitored,” and waits for episode
termination to occur.
When VT Detection is set to Monitor, several detection operations
work differently.
VT event counting – Before the device detects an episode, it
counts VT events normally. However, once the VT Initial NID is
reached, the device sets the VT event count to zero and suspends
VT event counting for the rest of the episode.
VF and FVT detection – VF and FVT detection operate as if VT
detection is off. Specifically, Combined Count detection is
disabled, and FVT via VT detection is not selectable. If a
monitored VT episode accelerates into the FVT or VF detection
zone, the device applies the VF Initial NID to detect the new
tachyarrhythmia. Once an episode is in progress, VT event
counting doesn’t resume until the episode ends.
Caution: Programming the VF Interval greater than 350 ms
may result in inappropriate detection of rapidly conducted
atrial fibrillation as VF or FVT via VF. Intervals shorter than the
VF Interval are counted using the VF event counter, which is
more sensitive than the consecutive VT event counter.
Wavelet, Onset, and Stability criteria – Before the device
detects a tachyarrhythmia episode, the Wavelet, Onset, and
Stability criteria, if turned on, are applied. If a monitored VT
episode accelerates into the FVT or VF detection zone, the device
continues to apply Wavelet as initial VF or FVT detection begins.
However, since Stability and Onset do not affect either VF
detection or FVT via VF detection, they are not applied.
Episode termination – The device compares ventricular
intervals to the VT Interval to identify when a VT monitored
episode has ended. However, if a VF episode or FVT via VF
episode occurs when VT monitoring is enabled, the device
compares ventricular intervals to the VF Interval to identify
episode termination.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 95

Figure 6-5. Device detects and monitors VT
ECG
Marker Channel
Detecting tachyarrhythmias
Detecting VT episodes
2 31
95
TSTSTSTDTSTST
S
13 14 15 16 0 0 0
S
30VTMonitor.eps
200 ms
VT Event Count
VT Interval
V
S
VSTSTSTST
1234
1 VT starts, and the device begins counting VT events (intervals less than the programmed
VT Interval but greater than or equal to the VF Interval).
2 The VT event count reaches the programmed VT NID of 16 events, and the device
detects VT.
3 After detecting the VT episode, the device resets the VT event count to zero and monitors
the episode until termination.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 96

96
Chapter 6
Detecting FVT episodes
Detecting FVT episodes
Parameters
The device detects episodes of Fast Ventricular Tachycardia (FVT)
by examining the cardiac rhythm for short ventricular intervals. If
enough intervals occur in the programmed FVT detection zone,
the device detects FVT and delivers the first programmed FVT
therapy. After therapy, the device continues to evaluate the
ventricular rhythm to determine if the episode is ongoing. To make
sure it delivers sufficiently aggressive therapies, the device can
merge the programmed detection zones during redetection to
increase sensitivity.
See “Details about FVT detection” on page 98.
* Medtronic nominal setting
Considerations
FVT Detection Enable – Enables FVT
detection via the VF or the VT
detection algorithm.
FVT Interval (Rate) (ms) – V-V
intervals between this value and the
programmed VF Interval are marked
as FVT events.
Off*, via VF, or via VT
200, 210, …600
Review the following information before programming
FVT detection parameters.
VF, FVT, and VT Intervals – To allow for normal variations in the
patient’s tachycardia interval, you should program the VF, FVT,
and VT intervals at least 40 ms apart.
Episode redetection – You can expedite redetection by
programming the VF and VT Redetect NIDs lower than the
Initial NIDs.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 97

Detecting tachyarrhythmias
Detecting FVT episodes
FVT Detection Enable – Your choice for an appropriate setting
for FVT Detection should depend on the patient’s VF and VT cycle
lengths. After determining a reliably sensitive VF Interval, consider
the following suggestions:
■
If the patient presents with a clinical VT interval in the VF
zone, select via VF to ensure reliable detection of VF. (VT
detection need not be enabled at all.)
■
If the patient presents with two clinical VTs, both outside
the VF zone, select via VT to allow for correct classification
of the faster VT and to offer a separate therapy regimen for
each VT.
■
If the patient presents with only one clinical VT which is
outside the VF zone, select VF and VT detection only, and
set FVT Enable to Off.
FVT detection and Wavelet – You can program the device to
exclude rapidly conducted SVTs from FVT detection by enabling
the Wavelet detection criterion. Note that the SVT Limit must be
programmed shorter than the VF Interval for Wavelet to affect FVT
via VF detection. See “Enhancing detection with Wavelet” on
page 107.
97
Restrictions
Review the following information before programming
FVT detection parameters.
Tachyarrhythmia detection and bradycardia pacing – To
ensure reliable ventricular tachyarrhythmia detection, the
programmer regulates the values available for bradycardia pacing
and tachyarrhythmia detection. See “Parameter interlocks” on
page 364.
VF detection backup – To ensure VF detection backup during
VT and FVT episodes, VT and FVT Detection cannot be on unless
VF Detection is also on.
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 98

98
Chapter 6
Detecting FVT episodes
How to program FVT detection
2
FVT detection – To ensure reliable ventricular tachyarrhythmia
detection, the programmer regulates the values available for the
FVT parameter as follows:
■
VT Detection must be set to On if FVT Detection is set to
via VT.
■
If FVT Detection is set to via VF, the FVT Interval must be
programmed to a value shorter than the VF Interval.
■
If FVT Detection is set to via VT, the FVT Interval must be
programmed to a value greater than the VF Interval and less
than or equal to the VT Interval.
To program FVT detection:
1. Select Params > Detection
2. Select the desired values for
FVT Enable and FVT Interval.
3. Select [PROGRAM].
3
Details about FVT detection
You can program the device to detect FVT episodes via the VF or
VT detection zone and NID.
When FVT Detection is set to via VF, a V-V interval within the FVT
detection zone is marked as an “FVT via VF” event. When the
VF NID is reached, the device reviews the last eight intervals:
■
If any of the last eight intervals are in the VF zone, it detects
the episode as VF.
■
If all of the last eight intervals are outside the VF zone, it
detects the episode as FVT (see Figure 6-6).
Maximo VR 7232Cx, 7232B, 7232E Reference manual
1
Page 99

When FVT Detection is set to via VT, a V-V interval within the FVT
detection zone is marked as an “FVT via VT” event. When the VT
NID is reached, the device reviews the last eight intervals:
■
If any of the last eight intervals are in the VF or FVT zones, it
detects the episode as FVT.
■
If all of the last eight intervals are outside the FVT and VF
zones, it detects the episode as VT.
Note: The device can also detect FVT episodes via the Combined
Count detection criterion (see page 101).
Figure 6-6. Device detects FVT via VF
Detecting tachyarrhythmias
Detecting FVT episodes
99
1 2
3
ECG
Marker Channel
VF Event Count
V
S
V
S
V
T
T
S
F
1 1 2345
TFTFTFT
S
TFT
F
TFTFTFTFTFV
F
13 14 15 16 17 18
VF and FVT Intervals
1 A fast ventricular tachycardia starts, and the first event falls into the FVT detection zone.
2 The second event of the FVT episode has an interval that falls into the VT zone. The VF
event count is not incremented.
3 The device detects FVT after the VF event count reaches the VF Initial NID.
S
30FVTDetect.eps
200 ms
Maximo VR 7232Cx, 7232B, 7232E Reference manual
Page 100

100
Chapter 6
Detecting FVT episodes
Figure 6-7. FVT zone merging
Zone merging after detection
To ensure the device delivers sufficiently aggressive therapies
during an extended or highly variable tachyarrhythmia episode,
the device merges detection zones during redetection in some
instances, as shown in Figure 6-7. The merged zone configuration
uses the event counting and therapies for the faster arrhythmia
and remains in effect until episode termination.
FVT set to “via VF” FVT set to “via VT”
Before
detection:
After VF
detection:
After FVT
detection:
VF
FVT
VT
VF and FVT zones merge, leaving
a larger VF zone.
VF
FVT
VT
VF
FVT
VT
VT and FVT zones merge, leaving a
larger FVT zone.
VF
FVT
VT
All zones remain unchanged. VT and FVT zones merge, leaving a
larger FVT zone.
VF
FVT
VT
Detection Intervals: VF Interval: 320 ms, FVT Interval: 280 ms / 360 ms, VT Interval: 400 ms
VF
FVT
VT
FVT1.eps
FVT2.eps
FVT3.eps
Maximo VR 7232Cx, 7232B, 7232E Reference manual
 Loading...
Loading...