Page 1

Disassembly Instructions for CD
M708348B492E Rev. A
HORIZON® Percutaneous Inserter,
P/N 6640002
2015-11-19
Refer to the section entitled “Reprocessing – General Considerations” in the Medtronic Reusable Instruments Instructions for
Use prior to reprocessing this instrument. This device should be disassembled for cleaning and reassembled prior to
sterilization.
Caution: federal law (USA) restricts these devices to sale by or on the order of a physician.
Figure 1: Disassembly Step 1
Page 2
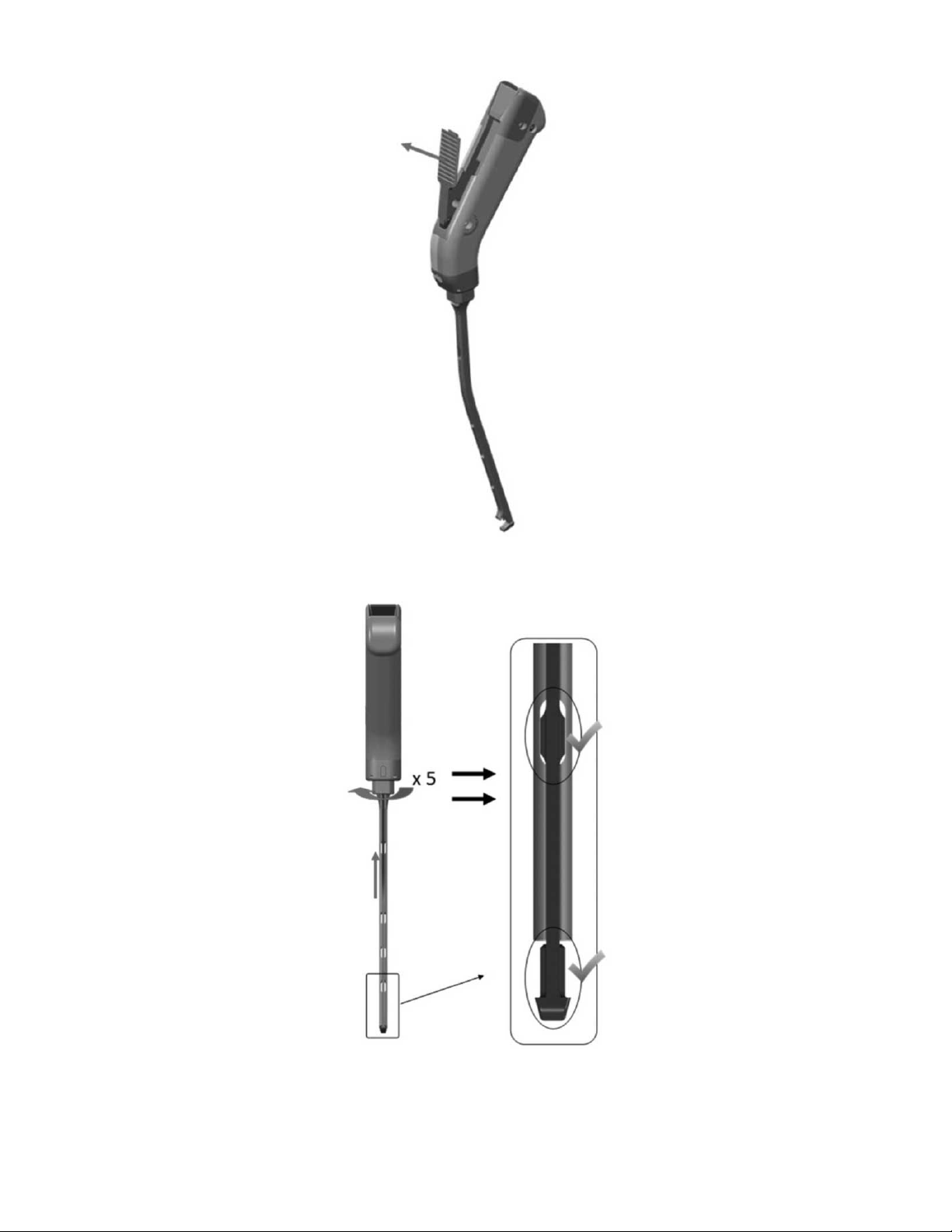
Figure 2: Disassembly Step 2
Figure 3: Disassembly Step 3
Page 3
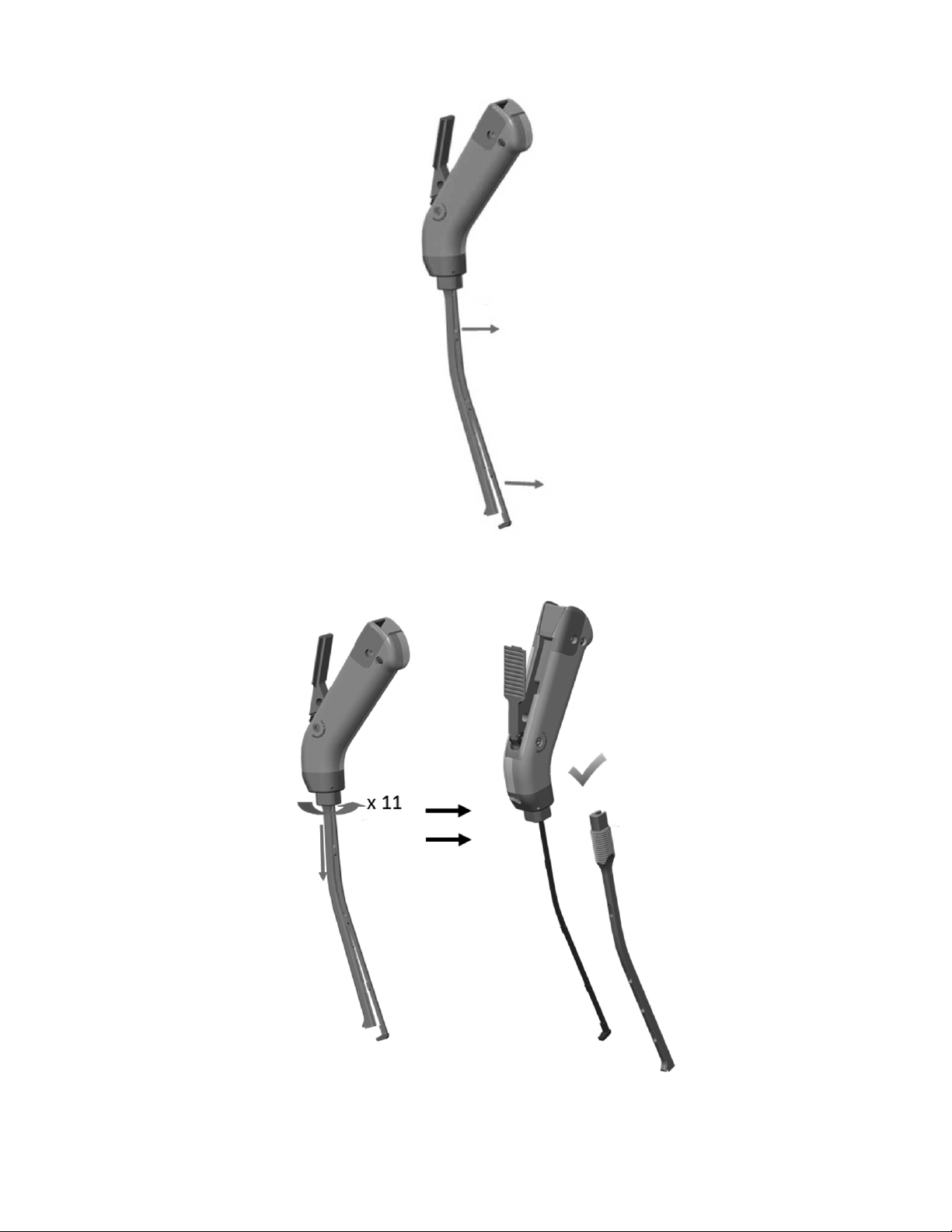
Figure 4: Disassembly Step 4
Figure 5: Disassembly Step 5
Page 4

Figure 6: Disassembly Step 6
Figure 7: Disassembly Step 7
Page 5

Figure 8: Fully Disassembled Step 8
Figure 9: Assembly Step 9
Page 6

Figure 10: Assembly Step 10
Figure 11: Assembly Step 11
Page 7

Figure 12: Assembly Step 12
Figure 13: Assembly Step 13
Page 8

Figure 14: Assembly Step 14
Figure 15: Fully Assembled Step 15
©2015 Medtronic Sofamor Danek USA, Inc. All rights reserved.
Page 9

AUSTRALIAN SPONSOR:
Medtronic Australasia Pty Ltd
97 Waterloo Rd
North Ryde, NSW 2113
Australia
EXPLANATION OF SYMBOLS
Authorized representative in the European Community
CAUTION: Federal law (USA) restricts these devices to
sale by or on the order of a physician.
Batch code
Manufacturer
Catalogue number
For US audiences only
The device complies with European Directive MDD
93/42/EEC
Medtronic Sofamor Danek USA, Inc.
1800 Pyramid Place
Memphis, TN 38132
Telephone: 800 933 2635 (USA)
901 396 3133 (Outside USA)
Fax: 901 396 0356
Medtronic B.V.
Earl Bakkenstraat 10
6422 PJ Heerlen
The Netherlands
Tel: + 31 45 566 80 00
Non-sterile
Consult instructions for use
 Loading...
Loading...