Page 1

ATTAIN CLARITY™ 6225
Venogram Balloon Catheter
Technical manual
Caution: Federal (USA) law restricts this product to sale
by or on the order of a physician.
Page 2

The following list includes trademarks or
registered trademarks of Medtronic in the
United States and possibly in other countries.
All other trademarks are the property of their
respective owners.
Attain Clarity, Medtronic
Page 3
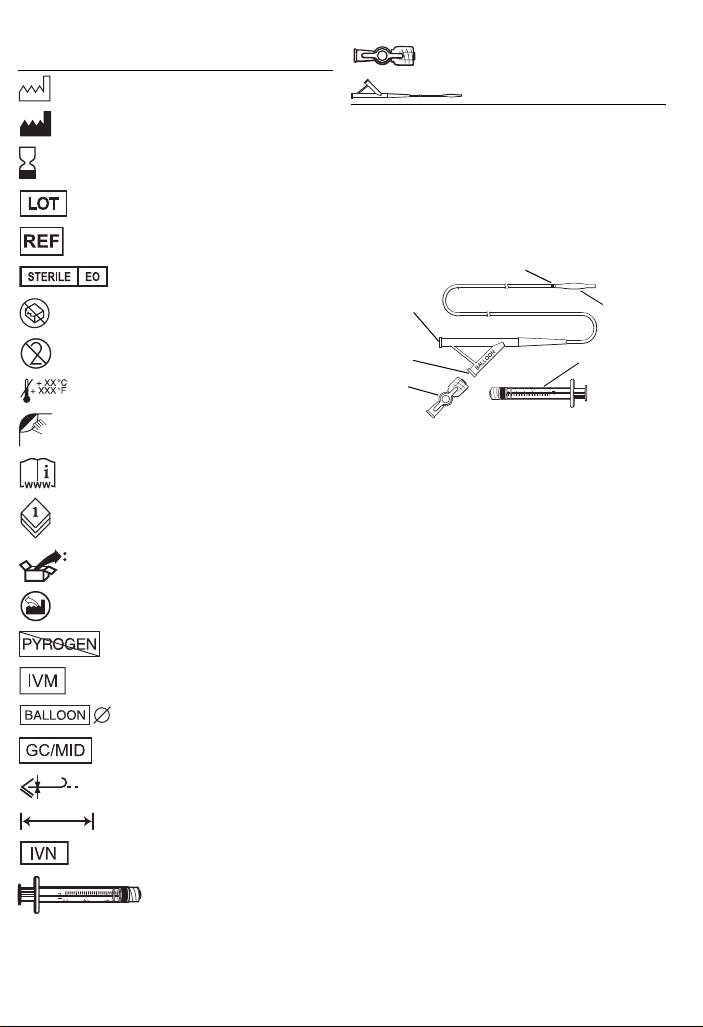
Explanation of symbols on package l abeling
Infusion port
Balloon
Inflation port
Marker band
Syringe
Stopcock
Refer to the package labels to see which symbols apply to this product.
Date of manufacture
Manufacturer
Use by
Lot number
Catalogue number
Sterilized using ethylene oxide
Do not use if package is damaged
Do not reuse
Maximum storage temperature
Stopcock
Venogram balloon catheter
1 Device description
The Medtronic Attain Clarity Model 6225 Venogram Balloon Catheter is
designed for use within the coronary sinus to infuse contrast solutions for
occlusive venogram imaging. The balloon catheter consists of polymeric
tubing with an infusion port and an inflation port in the main body of the
catheter (Figure 1). The inflation port features a luer lock at the proximal
end. Inflation is achieved through the use of a controlled stroke fixed
volume syringe attached to a stopcock. The stopcock is manually
attached to the balloon catheter. Both the stopcock and t he syringe are
supplied with the device. A silicone balloon is located near the distal end;
a marker band is located proximally to the balloon to help with visualizing
the location of the balloon under fluoroscopy. The infusion port has a luer
lock at the proximal end.
Peel here
Consult instructions for use at
www.medtronic.com/manuals.
Contents: one device
Package contents
Manufactured in
Nonpyrogenic
Inflation Volume Maximum
Balloon diameter at nominal
Guide Catheter/Minimum inner
diameter
Maximum guide wire diameter
Usable length
Inflation Volume Nominal
Syringe
Figure 1. Model 6225 Venogram Balloon Catheter
1.1 Contents of package
Each package contains the following:
■
One venogram balloon catheter
■
One 2.5 ml syringe
■
One stopcock
■
Product documentation
2 Indications for use
The venogram balloon catheter is indicated for use within the coronary
sinus; it is intended for infusing contrast solutions into the coronary
vasculature for occlusive venogram imaging.
3 Contraindications
The venogram balloon catheter is contraindicated for patients with a
known allergy to contrast medium.
4 Warnings and precautions
For single use only – This device is provided sterile and is for single
use, for one procedure only. This device is intended to contact bo dy
tissues. Do not reuse, reprocess, or resterilize. Reprocessing,
resterilization, or reuse may comprom ise the mechanic al or structural
integrity of this device and may compromise essential material and
design characteristics leading to device failure. Reprocessing,
resterilization, or reuse of this device may compromise the st erility of the
device and create a potential risk of patient or user infections due to the
use of a contaminated or unsterile device. Modification of packaging and
labeling may also lead to product mi x-up or use beyond shelf life,
possibly leading to patient injury or death.
Maximum inflation volume – Do not exceed the recommended
maximum balloon inflation volume (2.75 ml). Exceeding this volume will
not appreciably increase the diameter of the balloon and will increase the
probability of balloo n r upture. Refer to the “ Specifications” section f or
maximum inflatio n volume.
Inflation media – Do not use liquids as a balloon inflation media this
impacts catheter performance and the ability to deflate the balloon.
Injecting contrast solution – Do not inject contrast solution into the
infusion port if blood cannot be aspirated, t o avoid pulmonary
extravasation.
Recommended trai ning – Although diagnostic cardiac catheterization
procedures have proven to be safe, certain complications can occur. It is
recommended that the user of this product becomes familiar with the
evidence based guidelines for the safe u se of the venogram balloon
catheter.
Guide catheter compatibility – Use the Attain Clarity only with
compatible guide catheters. Refer to the section “Specifications” on
page 4 for additional information on compatible guide catheters. No test
data is available to demonstrate compatibility of Attain Clarity with nonMedtronic guide catheters. Consequences of using At tain Clarity with
3
Page 4

incompatible guide catheters may include the inability to deliver the
device or damage to the device during delivery. Contact your Medtronic
representative regarding compatibility quest ions.
Sterilization – The Model 6225 has been sterilized in ethylene oxide
prior to shipment. Do not clean or resterilize the venogram balloon
catheter. Destroy the venogram balloon catheter after use.
Inspecting the sterile package – Inspect the s terile pac ka ge before
opening.
■
Contact your local Medtronic representative if the seal or package is
damaged.
■
Do not store this product above 104 °F (40 °C).
■
Do not use the product after its expiration date.
Wiping or flushing the cathete r – Do not use alcohol to wipe or flush
the catheter because the balloon may be damaged.
5 Potential complications
Potential complications related to the use o f the venogram balloon
catheter include, but are not limited to, the following patient-related
conditions:
■
air embolism
■
allergic reaction to contrast media
■
bleeding at the insertion site
■
brachial plexus injury
■
cardiac tamponade
■
dissection
■
endocarditis
■
hematoma formation
■
hemothorax
■
infection
■
irregular heart beat
■
perforation
■
pneumothorax
■
subclavian artery puncture
■
thrombophlebitis
■
thrombosis
■
valve damage
■
vascular occlusion
■
vessel damage
Other potential complications related to the venogram balloon catheter
include, but are not limited to the following:
■
balloon rupture
■
catheter kinking
6 Directions for use
Some techniques vary according to physician preference and the
patient’s anatomy or physical condition. The following instructions
suggest one or two possible techniques; additional methods may exist.
Note: A guide wire may be used according to physician preference.
Note: Creating a venogram using the venogram bal loon catheter
requires the use of a contrast solution. A syringe containing contrast
solution should be prepared prior to using the venogram balloon
catheter. Do not use the syringe supplied with the balloon catheter for
this purpose. Refer to the documentation packaged with the contrast
medium used for the solution to determine the amount of solution
required for venogram imaging.
Note: Testing with out anticoagulation sh ows variable amounts of sleeve
thrombus formation on the device surface. Thrombogeni city evaluations
conducted using a heparinized model do no t indic ate thrombus
formation. If your patient is not appropriately anticoagulated, thrombus
formation may occur. Appropriate anticoagulation therapy should be
administered to reduce potential thrombosis.
6.1 Test ing the balloon before use
1. Using aseptic technique, remove the balloon catheter and
accessories from the sterile package.
2. Remove the stylet from the distal end of the catheter and discard it.
3. Attach the stopcock to the inflation port. The inflation port is labeled
with “BALLOON”.
Caution: Do not inflate the balloon using a syringe other than the
one provided with the venogram balloon catheter. Do not inflate the
balloon above the maximum inflation volume (2.75 ml).
4. Fill the syringe with 2.5 ml of air before attac hing it to the stopcock.
5. Open the stopcock on the inflation port by moving the lever so that
it is parallel with the inflation port.
6. Inject the air into the inflation port. The balloo n should expand; close
the stopcock. If the balloon does not inflate, verify that the stopcock
and syringe are on the correct port.
7. Inspect the balloon. Put the balloon in a bowl of sterile wat er to
check the integrity of the balloon. No bubbles should be observed.
8. Open the stopcock and deflate the balloon by pulling back the
syringe plunger to use the vacuum t o deflate the balloon.
6.2 Placing the venogram balloon catheter
The venogram balloon catheter should be introduced in to the body and
placed within the coronary sinus by inserting it through an appropriate
guide catheter that has an inner diameter of 5.7 Fr or greater.
Note: Push the venogram balloon catheter slowly whe n in serting it into
the guide catheter. The venogram balloon catheter may k ink if inserted
too rapidly.
Refer to the documentation packaged with the guide catheter for
additional instructions on accessing the coronary sinus.
6.3 Obtaining a venogram
1. Prior to use, flush the infusion port with saline.
2. After testing the venogram balloon catheter and passing the
venogram balloon catheter through a g uide catheter positioned in
the coronary sinus, use fluoroscopy to check the placement of the
balloon. The balloon should be in a proximal coronary sinus
position, and the balloon should be completely past the distal end of
the guide catheter. The radiopaque marker band should be outside
of the guide catheter.
3. Attach the syringe containing the contrast solution to the infusion
port. Aspirate blood through the infusion port to remove air from the
infusion port.
4. Prior to inflating the balloon catheter, inject a small amount of
contrast solution through the infusion port of the venogram balloon
to verify balloon catheter position and coronary sinus size.
5. Fill the syringe supplied with the venogram balloon catheter with
2.5 ml of air.
Caution: Do not inflate the balloon using a syringe other than the
one provided with the venogram balloon cat hete r.
6. Attach the air-filled syringe to the inflation port. Open the stopcock
and slowly inject the air to inflate the balloon. Only inflate the balloon
relative to the vessel size. Stop inflating the balloon when resistance
is felt or when occlusion is viewable on fluoroscopy. Close the
stopcock.
7. With the inflated balloon occluding the coronary sinus, slowly inject
the contrast solution through the infusion port and into the coronary
vasculature.
8. Record a fluoroscopic image of the contrast solution in the coronary
vasculature.
9. Occlude the coronary sinus as long as necessary. Open the
stopcock and deflate the balloon by pulling back the syringe plunger
to use the vacuum to deflate the balloon.
10. Remove the venogram balloon catheter from the guide catheter.
7 Specifications
Parameter Model 6225
Guide catheter minimum inner
diameter
1.9 mm (5.7 Fr, 0.075 in)
Usable length 90 cm (35.4 in)
Inflation volum e maximum 2.75 ml
Inflation volume nominal 2.5 ml
Maximum injection pressure 300 kPa (43.5 psi)
Maximum injectio n flow rate 0.6 mL/sec
Nominal balloon OD 13 mm (0.512 in)
Maximum guide wire diameter 0.018 in (0.46 mm)
Materials
Catheter body
Balloon
Marker band
8 Medtronic disclaimer of warranty
For complete warranty information, see the accom panyin g warranty
document.
Polyurethane
Silicone
Gold
4
Page 5

9 Service
Medtronic employs highly trained representatives and engineers located
throughout the world to serve you and, upon request, to provide training
to qualified hospital personnel in the use of Medtronic products.
Medtronic also maintains a professional staff to provide technical
consultation to product users. For more information, contact your local
Medtronic representative, or call or write Medtronic at the appropriate
address or telephone number listed on th e back cover.
5
Page 6

Page 7

Manufacturer
Medtronic, Inc.
710 Medtronic Parkway
Minneapolis, MN 55432
USA
www.medtronic.com
Tel. +1 763 514 4000
Fax +1 763 514 4879
Medtronic E.C. Authorized
Representative
Medtronic B.V.
Earl Bakkenstraat 10
6422 PJ Heerlen
The Netherlands
Tel. +31 45 566 8000
Fax +31 45 566 8668
Europe/Africa/Middle East
Headquarters
Medtronic International Trading Sàrl
Route du Molliau 31
Case Postale 84
CH-1131 Tolochenaz
Switzerland
Tel. +41 21 802 7000
Fax +41 21 802 7900
Technical manuals:
www.medtronic.com/manuals
© Medtronic, Inc. 2012
M732059B001 1A
2012-11-06
*M732059B001*
 Loading...
Loading...