Page 1

CARDIOBLATE® LP
Surgical Ablation Device
60841
Instructions for Use
Caution: Federal law (USA) restricts
this device to sale by or on the order of a
physician.
Page 2

Page 3
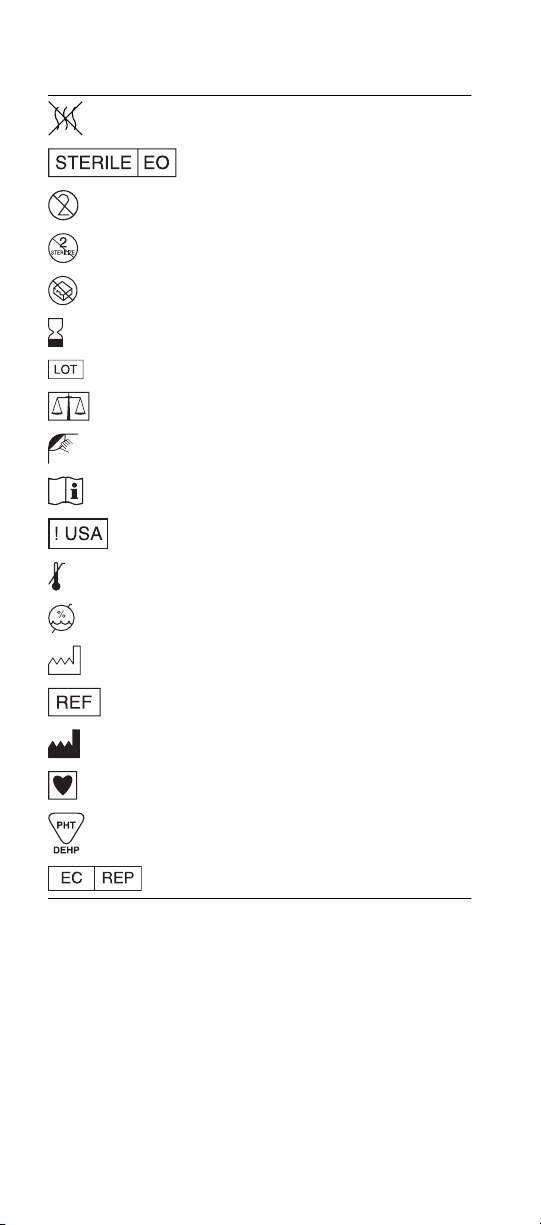
Explanation of symbols on package labeling
Refer to the device labeling to see which symbols apply to this product
Nonpyrogenic
Sterilized Using Ethylene Oxide
Do Not Reuse
Do Not Resterilize
Do not use if package is damaged
Use By
Lot Number
Quantity
Open Here
Consult Instructions for Use
For US Audiences Only
Upper Limit of Temperature
Humidity Limitation
Date of Manufacture
Catalog Number
Manufacturer
Type CF Applied Part
Contains di(2-ethylhexyl)phthalate (DEHP)
Authorized Representative in the European Community
1
Page 4

7
6
4
3
1
2
9
8
5
Figure 1
1.
Rigid, slim line jaws, 7 cm (2.76 in) in length
2. Seven cm (2.76 in) electrodes with embedded weeping polymer
3. 300° rotation of jaw assembly
4. Flexible neck
5. Trigger
6. Trigger lock with raised thumb slide
7. Molded handle
8. Saline fluid delivery tubing 304.8 cm (10 ft) in length with a female
luer for connection to standard IV tubing systems
9. Electrical cable 304.8 cm (10 ft) in length that terminates into a multipin connector
2
Page 5

CARDIOBLATE® LP
Surgical Ablation Device
Model
60841
Description
Cardioblate® LP Surgical Ablation Device is a hand-held, single-use,
The
bipolar, radiofrequency ablation device for use in cardiac surgery. It has
a saline irrigation system to deliver fluid at the contact point between the
tissue and electrode to cool the tissue during radiofrequency energy
delivery. The device is intended for intermittent operation.
Sterile, nonpyrogenic, disposable, single use only.
Indications for Use
The Cardioblate® LP Surgical Ablation Device is intended to ablate
cardiac tissue during cardiac surgery using radiofrequency energy.
Contraindications
The Cardioblate® LP Surgical Ablation Device should not be used for:
■
patients that have active endocarditis at time of surgery
■
ablation in a pool of blood (eg, through a purse string suture
on a beating heart) (Effects of this type of ablation are
unknown.)
Adverse Effects
Possible adverse effects related to the ablation of cardiac tissue in
combination with open heart surgery are:
■
tissue perforation
■
extension of extracorporeal bypass
■
perioperative heart rhythm disturbances (atrial and/or
ventricular)
■
postoperative embolic complications
■
pericardial effusion or tamponade
■
injury to the great vessels
■
valve leaflet damage
■
conduction disturbances (SA/AV node)
■
acute ischemic myocardial event
■
thrombus formation
■
nerve damage
■
unintentional burns
■
pericarditis
■
pleural effusion
■
esophageal perforation
■
death
■
coronary sinus perforation
■
coronary artery spasm
■
atrial lead dislodgement
■
hypotension
■
pulmonary vein stenosis
■
cerebrovascular accident
■
transient ischemic attack
■
blood loss
Warnings
Do not activate device if saline is not flowing freely. The cooling of
the outer tissue prevents the charring and tissue desiccation that
may occur when applying radiofrequency energy without irrigation.
Instructions for Use English 3
Page 6

The device may be damaged before or during the procedure by improper
handling or other intervening acts. If damaged, the device may fail to
function properly, and may result in the following medical complications
including, but not limited to:
■
damage to the physiological conduction system
■
peripheral tissue ablation due to breach of insulation
covering
Device shall be removed from the patient before defibrillation.
representation or warranty is made that failure or cessation of function
No
of the device will not result in an adverse event, or that medical
complications (including perforation of cardiac tissue) will not follow the
procedure, or that the use of the device will in all cases restore adequate
cardiac function.
Precautions
Caution: Federal law (USA) restricts this device to sale by or on the order
of a physician.
Proper surgical procedures and techniques are necessarily the
responsibility of the medical professional. Each surgeon must evaluate
the appropriateness of any procedure based on his or her own medical
training and experience, and the type of surgical procedure.
Patient and procedure selection is solely a medical responsibility and the
outcome is dependent on many variables including patient pathology,
surgical and perfusion procedures.
Use caution to avoid trauma to tissues not within the target area of
ablation.
Purge air from system to ensure saline fluid flows from the electrodes
during use.
Separation of electrodes from tissue contact during activation will result
in a high impedance shutdown placing generator power condition to OFF.
Placing electrodes in direct contact during radiofrequency energy
activation results in a low impedance shut down and places the generator
power condition to OFF. In both situations, three short audible alarm
beeps will be heard. Refer to the Cardioblate generator technical manual
for reset instructions.
Maximum storage temperature: 40°C (104°F)
Storage relative humidity: 5% to 85%, noncondensing
This device was designed for single patient use only. Do not reuse,
reprocess, or resterilize this product. Reuse, reprocessing, or
resterilization may compromise the structural integrity of the device
and/or create a risk of contamination of the device, which could result in
patient injury, illness, or death. Multiple uses can result in the occlusion
of the irrigation openings and affect the device’s performance. To prevent
this from occurring, the generator has the capability to detect previously
used ablation devices and will not allow their reuse.
It is the responsibility of the user to dispose of the devices in accordance
with local regulations and hospital procedures.
Due to the presence of phthalates in the product, the clinician must weigh
the medical benefits of product use against the drawbacks of phthalate
exposure for male children and pregnant or nursing women.
Connections to External Devices
1. Connection to the Cardioblate generator is as follows:
a. Position the multi-pin connector of the bipolar device so the
alignment indicator matches the generator port indicator.
b. Insert the multi-pin connector of the bipolar device into the device
port on the generator until it locks in place. Do not force the
connection.
Note: Connect to RF generators (only IEC / EN 60601-1 Type CF)
with the following specifications:
4 Instructions for Use English
Page 7

RF power output
(Transmurality
Mode)
RF power output fre-
quency
Maximum RF volt-
age, between electrodes
Note: If additional devices are to be used, refer to their specific
instructions for connections to external devices.
Sterile normal saline, commercially available pressure cuff, and IV
2.
tubing set are required for use with the bipolar device. These are not
included with the Cardioblate® LP Surgical Ablation Device.
Model 60880/60890 Model 68000
25 - 40 W
(automatically varying)
479 - 489 kHz 468 - 478 kHz
159 Vrms 180 Vrms ±10%
15 - 40 W
(automatically vary-
ing)
Instructions for Use
1. Inspect the package and device to ensure the expiration date has not
passed and damage has not occurred to the device during shipping
and handling. Do not use the device if the packaging or device is
damaged or if the expiration date has elapsed. Open the package
and transfer the device onto the sterile field utilizing aseptic
technique.
2. Uncoil both the saline delivery tubing and the electrical cable carefully
to avoid kinking and tangling.
3. Attach the saline delivery tubing female luer connector to an IV tubing
set and a 1000 mL bag of sterile normal saline. (0.9% normal saline
is recommended because it provides a low impedance pathway for
the radiofrequency energy to the tissue.)
4. If both a bipolar and monopolar device are being used, remove the
sterile caps from the “Y” connector (Model 10004D) provided and
attach the female luer of both devices. Then insert the IV line into the
“Y” connector.
Figure 2.
Insert a 1000 mL bag of 0.9% normal saline into commercially
5.
available pressure cuff and inflate to between 150 and 300 mm Hg.
6. Open the IV line and squeeze the trigger to purge the system of air
until fluid is seen coming from the electrodes on the bipolar device.
Release the trigger to stop IV flow.
Instructions for Use English 5
Page 8

1
1. 7 cm (2.76 in) active
electrodes
Figure 3.
7. a. Attach the bipolar electrical cable to the generator per
instructions outlined in “Connections to External Devices.”
b.
Use the flexible neck and 300° roll to assist in positioning the
electrodes on the targeted tissue. Once the electrodes are
correctly positioned, engage the lock and squeeze the trigger to
bring the electrodes in full contact with the tissue. The lock must
be engaged to ensure accurate transmurality readings.
c. Confirm the generator is set to the bipolar mode.
8. Prior to tissue ablation:
a. Squeeze the trigger and make certain that saline is flowing freely
out of the electrodes. Continuous flow of saline is necessary
during ablation to cool the outer tissue. Cooling of the outer tissue
prevents charring and tissue desiccation and allows
radiofrequency energy to be delivered deeper into the tissue to
more effectively create transmural lesions.
Note: While irrigation increases the effectiveness of
radiofrequency ablation, ablating in a pool of fluid can have a
detrimental effect. Radiofrequency energy can shunt between
the two electrodes and the transmurality algorithm may be less
effective. Suctioning of excessive fluid at the ablation site is
recommended to prevent this from occurring.
b. When using a bipolar device, the radiofrequency ablation
generator will default to the TRANSMURALITY control mode.
c. In the TRANSMURALITY mode, all generator parameters will be
automatically adjusted.
9. The RF energy is activated in the device using the RF energy button
or the foot switch provided with the Cardioblate generator. The
current flows between the electrodes.
Table 1. Anticipated Energy Delivery at Various Tissue
Thicknesses
Tissue Thickness (7 cm length) Energy Delivered (W)
10. The lesion becomes visible by a white coloration on the tissue. In
TRANSMURALITY
once a transmural lesion is created. This is the indication to the
surgeon to stop ablating by releasing the foot switch or pressing the
RF energy power button on the generator.
1 mm 439±40
3 mm 505±25
5 mm 557±28
control mode, the generator will emit a solid tone
6 Instructions for Use English
Page 9

0
2
0
3
04
0
5
06
07
08
0
9
0
01
0
2000
4000
6000
8000
10000
12000
14000
16000
18000
20000
0
1
5
1
02
5
2
03
53
04
54
05
1
3
2
3
1
1. Average Impedance (Ohms)
Time (msec)
2.
3. Power (Watts)
Figure 4. Typical Tissue
Impedance Values with
Corresponding Power Levels.
(Approximate total energy
delivered 570 W)
11. Ablating beyond recommended ablation times may result in tissue
perforation.
Note: To prevent the porous jaws from drying out and clogging,
maintain the jaws immersed in saline or covered with a wet sponge
between ablations.
12.
Upon completion of the surgical procedure, disconnect the device
from the generator and discard.
Clinical Data on Conduction Block
In vitro testing was conducted creating lesion sets on porcine atria. In vivo
testing was performed on porcine cardiac tissue for the Cardioblate BP2
Surgical Ablation Device1. Transmural lesions were demonstrated with a
95/95 confidence level. The testing resulted in the following lesion
dimensions:
n=3
Max Transmurality Depth (mm) 2.4 ± 2.3
Lesion Length (cm) 3.2 ± 0.6
Lesion Width (mm) 2.8 ± 0.5
These results are consistent with a small, retrospective study conducted
at 2 investigational centers of 69 patients during 2006 and 2007. Thirtyfive of those patients received cardiac tissue ablation using irrigated
radiofrequency (RF) energy. Of the 35 patients, 29 (82.9%) underwent
conduction block assessment confirming transmurality of cardiac tissue
lesions. The remaining 6 patients were not assessed.
1
Testing was performed on the Cardioblate® BP2 Surgical Ablation Device, which
has mechanical and electrode designs identical with the Cardioblate LP Surgical
Ablation Device.
Instructions for Use English 7
Page 10

The following disclaimer of warranty applies to United
States customers only:
Disclaimer of Warranty
ALTHOUGH THE CARDIOBLATE® LP SURGICAL ABLATION
DEVICE, HEREAFTER REFERRED TO AS “PRODUCT” HAS BEEN
MANUFACTURED UNDER CAREFULLY CONTROLLED
CONDITIONS, MEDTRONIC HAS NO CONTROL OVER THE
CONDITIONS UNDER WHICH THIS PRODUCT IS USED.
MEDTRONIC, THEREFORE DISCLAIMS ALL WARRANTIES, BOTH
EXPRESS AND IMPLIED, WITH RESPECT TO THE PRODUCT,
INCLUDING, BUT NOT LIMITED TO, ANY IMPLIED WARRANTY OF
MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE.
MEDTRONIC
FOR ANY MEDICAL EXPENSES OR ANY DIRECT, INCIDENTAL OR
CONSEQUENTIAL DAMAGES CAUSED BY ANY USE, DEFECT,
FAILURE OR MALFUNCTION OF THE PRODUCT, WHETHER A
CLAIM FOR SUCH DAMAGES IS BASED UPON WARRANTY,
CONTRACT, TORT OR OTHERWISE. NO PERSON HAS ANY
AUTHORITY TO BIND MEDTRONIC TO ANY REPRESENTATION OR
WARRANTY WITH RESPECT TO THE PRODUCT.
The exclusions and limitations set out above are not intended to, and
should not be construed so as to, contravene mandatory provisions of
applicable law. If any part or term of this Disclaimer of Warranty is held
to be illegal, unenforceable or in conflict with applicable law, by a court
of competent jurisdiction, the validity of the remaining portions of this
Disclaimer
shall be construed and enforced as if this Disclaimer of Warranty did not
contain the particular part or term held to be invalid.
SHALL NOT BE LIABLE TO ANY PERSON OR ENTITY
of Warranty shall not be affected, and all rights and obligations
8 Instructions for Use English
Page 11

Page 12

Europe
*M940538A001*
Europe/Africa/Middle East
Headquarters
Medtronic International Trading
Sàrl
Route du Molliau 31
Case Postale 84
CH - 1131 Tolochenaz
Switzerland
Internet: www.medtronic.co.uk
Tel. 41-21-802-7000
Fax 41-21-802-7900
Authorized Representative in
the European Community
Medtronic B.V.
Earl Bakkenstraat 10
6422 PJ Heerlen
The Netherlands
Tel. 31-45-566-8000
Fax 31-45-566-8668
Asia-Pacific
Japan
Medtronic Japan
Comodio Shiodome 5F
2-14-1 Higashi-Shimbashi,
Minato-ku
Tokyo 105-0021
Japan
Tel. 81-3-6430-2011
Fax 81-3-6430-7140
Australia
Medtronic Australasia Pty. Ltd.
97 Waterloo Road
North Ryde NSW 2113
Australia
Tel. 61-2-9857-9000
Fax 61-2-9878-5100
Asia
Medtronic International Ltd.
1602 16/F, Manulife Plaza
Suite
The Lee Gardens,
33 Hysan Avenue
Causeway Bay
Hong Kong
Tel. 852-2891-4068
Fax 852-2591-0313
Americas
Latin America
Medtronic Latin America
3750 NW 87th Avenue
Suite 700
Miami, FL 33178
USA
Tel. 305-500-9328
Fax 786-709-4244
Canada
Medtronic of Canada Ltd.
99 Hereford Street
Brampton, Ontario L6Y 0R3
Canada
Tel. 905-460-3800
Fax 905-826-6620
Toll-free: 1-800-268-5346
United States
Manufacturer:
Medtronic, Inc.
710 Medtronic Parkway
Minneapolis, MN 55432
USA
Internet: www.medtronic.com
Tel. 763-514-4000
Fax 763-391-9100
Toll-free: 1-800-328-2518
(24-hour consultation service)
© 2006, 2009, 2011
Medtronic
M940538A001 Rev. 1B
 Loading...
Loading...