Medtronic 5388 Technical Manual

5388
Dual Chamber Temporary Pacemaker
Technical Manual
c
Caution: Federal Law (USA) restricts this device to sale by
or on the order of a physician.
0123

MODEL 5388 0
Technical Manual 0
Dual Chamber Temporary Pacemaker
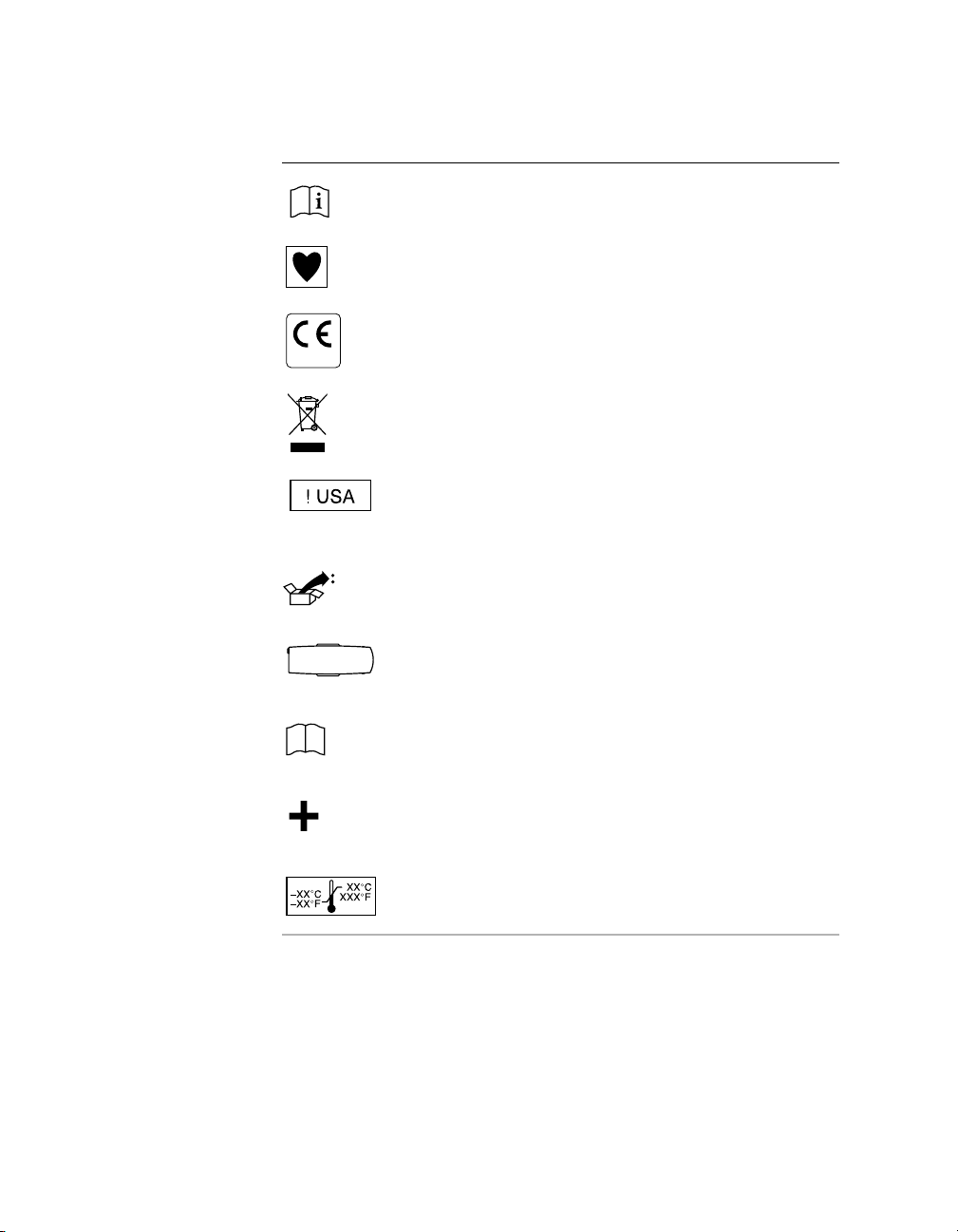
Symbols 0
Explanation of symbols
Consult instructions for use
Type CF applied part
Conformité Européenne (European Conformity)
This symbol means that the device fully complies with European
0123
Directive 93/42/EEC .
Do not dispose of this product in the unsorted municipal waste stream.
Dispose of this product according to local regulations.
http://recycling.medtronic.com for instructions on proper disposal of this
product.
For US audiences only
Package contents
Temporary pacemaker
Product documentation
Accessories
Storage temperature limitation
See
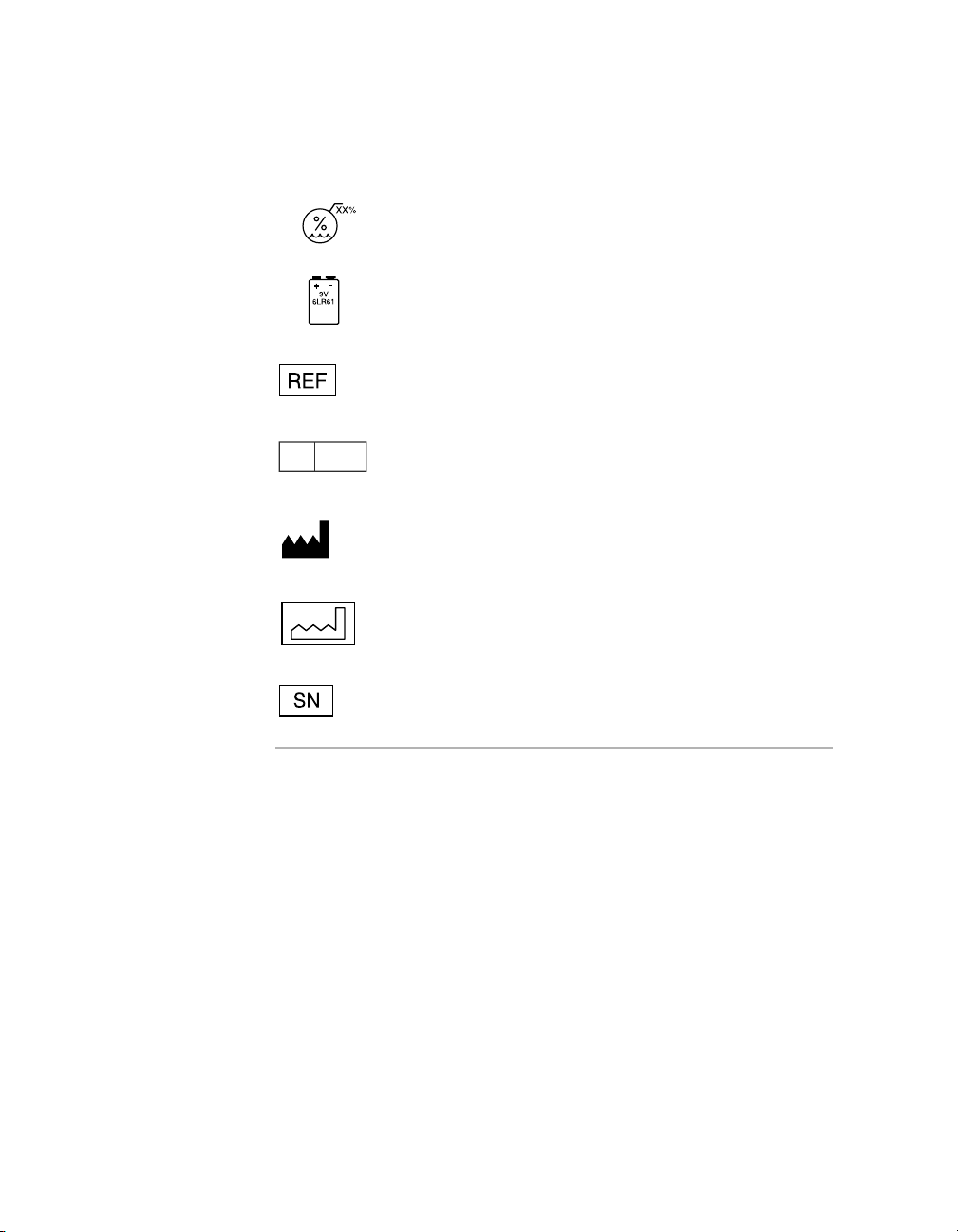
Explanation of symbols
Humitidy limitation
Battery
Reorder number
5
EC REP
Authorized representative in the European Community
Manufacturer
Date of manufacture
Serial number
5388 Technical manual

Contents
1 Overview 9
General Description 10
Intended Use 12
Contraindications 12
2 Warnings, precautions, and adverse effects 15
Warnings 16
Precautions 18
Environmental Precautions 22
Adverse Effects 23
3 Controls, indicators, and other features 25
Controls 26
Light-Emitting Diodes 32
Upper Screen 33
Lower Screen 37
Physical Features 46
Functional Features 49
Timing Violations 53
4 Preparation for use 57
Battery Installation 58
Disposable Pouch 59
Disposable Cover 59
Cables 60
Connector Setup 61
5 User guide 67
Overview 68
Indicators 69
Basic Operation 70
Connector Setup 77
Pacing Parameter Adjustments 79
Thresholds 83
Pacing Setup 88
5388 Technical manual

8
Contents
RAP (Rapid Atrial Pacing) 90
Battery Replacement 92
Ta bl e s 94
6 Device maintenance 97
Cleaning and Sterilization 98
Safety and Technical Checks 99
Service 100
7 Specifications 101
Device Specifications 102
Device Accessories 105
8 Warranty information 107
Special Notices 108
A Pacemaker diagnostic diagrams 109
About the Chapter 110
Definitions 110
Single Chamber Modes 113
Dual Chamber Modes 118
5388 Technical manual
Index 139
General Description 10
Intended Use 12
Contraindications 12
Overview1
1
10
Chapter 1
General Description
General Description
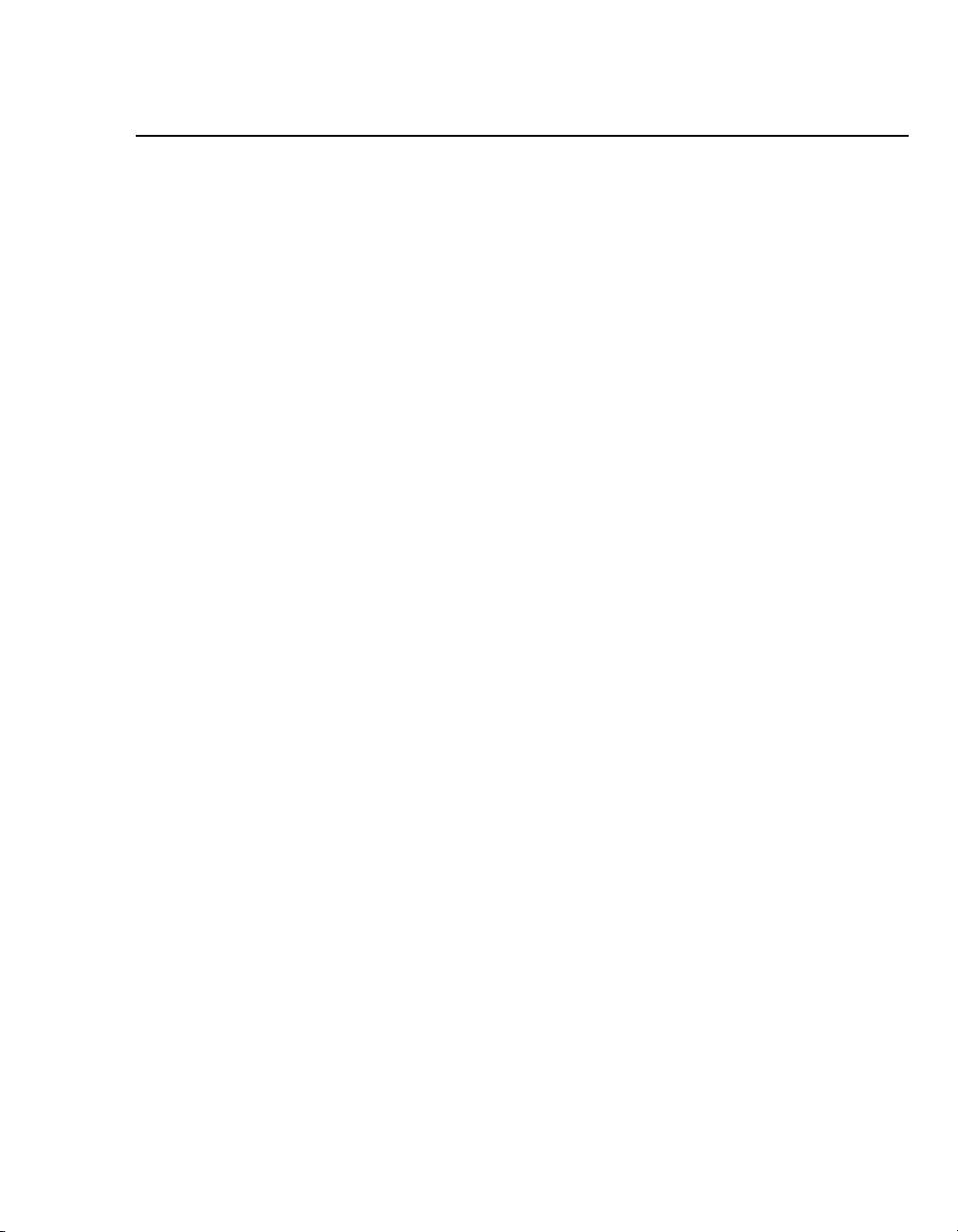
The Medtronic® Model 5388 is a battery-powered, temporary, dual
chamber pacemaker designed primarily for temporary
antibradycardia pacing therapy. The device provides eight
selectable modes of pacing therapy: DDD, DVI, DDI, DOO, VOO,
VVI, AOO, and AAI. High-rate, burst pacing therapy up to
800 min
available in the asynchronous mode.
-1
(reciprocal minutes), for atrial tachyarrhythmias, is
1
The device is typically connected to temporary transvenous,
epicardial or myocardial pacing leads, in a bipolar configuration,
using patient cables (Medtronic Models 5433A and 5433V) or
surgical cables (Medtronic Model 5832 or 5832S).
5388 Technical manual
l
p
p
n
n
s
c
rb
.
x
k
n
T
rtg
p
r V
S
s
N
n
m
N
b
F
R
B
rtg
x
s
P
Figure 1-1. The Medtronic Model 5388 Dual Chamber Temporary
Pacemaker and the Model 5433A or 5433V Patient Cable.
The device operates using a 9-volt alkaline or lithium battery,
installed in the battery drawer at the lower right side of the device.
Note: The Model 5388 is a constant current device. When it emits
a pulse, the current output is maintained at a constant value. This
value is set by the output control and does not vary.
1
For atrial use only.

Safety Features
Overview
General Description
The Medtronic Model 5388 is designed to be reliable, easy to
operate, and comfortable to hold. Safety features of the
Model 5388 include:
■
Cables with no exposed electrical connections;
■
Self-test function;
■
Low Battery indicator;
■
Lock feature to prevent accidental change of parameters;
■
Safe, two-step operation to turn the device off;
■
Runaway rate protection;
■
Protection from defibrillation shock;
■
Continuous operation during battery replacement (see
page 105);
■
Reversible battery polarity;
■
Electrostatic protection;
■
Minimized susceptibility to electromagnetic and magnetic
interference; and
■
Rubber seals to cover the connector pin receptacles.
11
Package Contents
Registration Card
See the enclosed card for a detailed list of package contents.
Check the package prior to use. Damaged packages should be
returned to Medtronic (see back cover for address).
Please complete the registration card and return it to Medtronic.
Consult the back cover of this manual for the address.
5388 Technical manual

12
Chapter 1
Intended Use
Intended Use
The Medtronic Model 5388 temporary pacemaker is intended to
be used in conjunction with a cardiac pacing lead system for
temporary single or dual chamber pacing in a clinical environment
by trained personnel. The Model 5388 can be used where
short-term demand (synchronous) or asynchronous pacing is
indicated for therapeutic, prophylactic or diagnostic purposes.
Specific indications for temporary cardiac pacing include, but are
not limited to, the following:
■
Complete heart block;
■
Sinus bradycardia;
■
Sick sinus syndrome;
■
Bradycardia with congestive heart failure;
■
Atrial and/or ventricular arrhythmias;
■
Cardiac arrest;
■
Support, management, and evaluation of a patient prior to
permanent pacemaker implantation;
■
Support during permanent pacemaker replacement;
■
Cardiac complications during invasive or surgical procedures;
■
Support following cardiac surgery;
■
Acute myocardial infarction complicated by heart block; and
■
High-rate burst pacing for treatment of atrial tachyarrhythmias.
The Model 5388 can be used to determine sensing thresholds of
temporary and permanently implanted lead systems. When
implanting a permanent pacemaker, however, Medtronic
recommends the use of a Medtronic Pacing System Analyzer.
Contraindications
There are no known contraindications to the use of temporary
pacing as a means to control the heart rate. The patient’s age and
medical condition, however, may dictate the type of temporary
pacemaker and lead system used by the physician.
5388 Technical manual

Atrial Sensing
Pacing modes which allow sensing in the atrium to trigger a
ventricular response are contraindicated in the presence of rapid
atrial arrhythmias such as atrial fibrillation or atrial flutter.
Atrial Pacing
Atrial pacing is ineffective in the presence of atrial fibrillation or
flutter.
Single chamber atrial pacing is contraindicated in the presence of
AV conduction disorders.
Asynchronous Pacing
Asynchronous pacing is contraindicated in the presence of
intrinsic cardiac rhythms.
Atrial High-Rate Burst Pacing Therapy
Atrial high-rate burst pacing therapy is intended for use in the
atrium only. High-rate burst pacing in the ventricle may result in
life-threatening arrhythmias.
Overview
Contraindications
13
5388 Technical manual

Warnings, precautions, and
Warnings 16
Precautions 18
Environmental Precautions 22
Adverse Effects 23
adverse effects
2
2

16
Chapter 2
Warnings
Warnings
Equipment Modification
Defibrillation/Cardioversion
Do not modify this equipment. Modifications could impact device
effectiveness and adversely affect patient safety.
Defibrillation discharges up to 360 watt-seconds have not affected
the Model 5388 in laboratory tests. However, for maximum safety,
it is recommended that paddles be placed at least 15 cm
(6 inches) away from the Model 5388 or the lead system.
Whenever possible, for the safety of the patient, disconnect the
pacemaker from the lead system before defibrillating or
cardioverting. A relatively low resistance pathway exists between
the positive (+) and negative (–) electrodes of the implanted lead
system. During defibrillation a large current could flow across this
pathway, causing myocardial damage.
Line-powered Equipment
An implanted lead or lead with extension cable constitutes a direct,
low-resistance current pathway to the myocardium. Due to the
danger of tachyarrhythmias resulting from alternating current
leakage, extreme caution must be taken to properly ground all
line-powered equipment used on or in the vicinity of the patient.
Electrosurgical Units (Cautery)
Electrosurgical units can cause tachyarrhythmias by inducing
current on the leads, and thus should never be used within 15 cm
(6 inches) of the pacemaker/lead system.
Electromagnetic Interference (EMI)
Pacemakers operating in the demand mode respond to
intracardiac potentials with magnitudes of a few millivolts. This
level of sensitivity makes the pacemaker inherently sensitive to
some external fields. In the presence of excessive levels of
interference, the Model 5388 may inhibit completely or revert to
asynchronous operation, pacing at the rate set by the RATE dial.
5388 Technical manual

Warnings, precautions, and adverse effects
It is recommended that the device be set to an asynchronous
mode at a rate higher than the patient’s intrinsic rate when
operated in the presence of strong electromagnetic interference
(EMI).
Sources of excessively strong EMI which may temporarily affect
the operation of the Model 5388 include:
■
Electrosurgical equipment;
■
Diathermy equipment;
■
Some medical telemetry equipment [when operated within
one meter (about three feet) of the pacemaker];
■
Communication transmitters such as cellular phones, “walkie
talkies”, and transmitters in emergency transport vehicles; and
■
Magnetic Resonance Imaging (MRI) equipment.
Atrial High-Rate Burst Pacing Therapy
Use of high rates in the atrium could result in high-rate conduction
to the ventricle. Defibrillation equipment should be on standby,
immediately available during atrial high-rate burst pacing therapy.
17
Warnings
There is no ventricular back-up pacing during delivery of atrial
high-rate burst pacing therapy.
Connecting the Lead System
The patient cable should be connected to the temporary
pacemaker before the lead(s) is connected to the patient cable(s).
To prevent pacing into the vulnerable period of the T-wave, turn the
temporary pacemaker ON and turn A and V OUTPUT down to the
minimum amplitude before connecting the temporary pacemaker
to the patient’s lead system. Determine sensing thresholds (see
“Sensing Threshold” on page 84) before turning A and V OUTPUT
up to threshold levels.
Handling Implanted Leads
When handling implanted leads (temporary or permanent), the
terminal pins or exposed metal must not be touched nor be
allowed to contact electrically conductive or wet surfaces.
5388 Technical manual

18
Chapter 2
Precautions
Precautions
Random Failures
The physician should be aware that operational failure of the
Model 5388 temporary pacemaker can occur as the result of
battery depletion, mishandling, or random component failure.
Possible operational failures of the Model 5388 can include:
■
No output or erratic output;
■
No sensing or erratic sensing;
■
False indicator light signals;
■
Inappropriate variance of rate, output pulse width, or output
amplitude;
■
Reversion to asynchronous pacing; and
■
Loss of control of rate, output, sensitivity or power.
If loss of control of rate, output, sensitivity or power occurs, and it
is not due to a low battery, disconnect the device from the patient
and return it to Medtronic for service.
Batteries
5388 Technical manual
Use of batteries with different physical dimensions from that of the
recommended batteries may result in erratic, or no, pacing output.
Replace the battery for each new patient, and when the low
battery indicator appears during device operation (see page 33).
Check the battery status at least twice daily. Replace alkaline
batteries at least once every week when the temporary pacemaker
is in continuous use or when the low battery indicator is displayed.
When replacing the battery, make sure the battery drawer is fully
closed and latches in place with an audible click.
Inspect the contacts on the battery for visible signs of
contamination prior to use. Use of batteries with contamination on
the contacts may result in erratic, or no output.
Failure to ensure that the battery drawer is fully latched may result
in a loss of power. Continued device operation is NOT an
indication that the battery drawer is properly latched.

Pacing Leads and Cables
Improper connection, displacement or fracture of leads or cables
may result in pacemaker system failure. Inspect leads and cables
for damage prior to each use.
Pacing System Adjustments
Monitor the patient’s ECG and blood pressure and keep
defibrillation equipment on standby, immediately available for
emergency use during evaluation of stimulation and sensing
thresholds, pacemaker and pacing lead connections and
adjustments, and atrial high-rate burst pacing therapy.
Bipolar Lead Systems
Bipolar lead systems are recommended because they are less
susceptible to electromagnetic interference. Separation between
the positive (+) electrode and negative (–) electrode of the same
lead system should not exceed 15 mm (0.6 inches). Also, the atrial
and ventricular lead systems should be positioned so that the
electrodes of one system are a minimum of 4 cm (1.5 inches) from
the electrodes of the other system and are at right angles to each
other.
Warnings, precautions, and adverse effects
Precautions
19
Unipolar Lead Systems
Unipolar lead systems are not recommended because they are
more susceptible to electromagnetic interference, which may
result in inappropriate pacing. Unipolar lead systems should not
be used in the dual-chambered pacing modes because the current
path of one lead system may interfere with the current path of the
other.
Atrial Sensing
The atrial sensing threshold should be evaluated to ensure
maximum electrogram amplitude and that an adequate atrial
sensing threshold is obtained prior to programming to a mode that
requires atrial sensing (DDD, DDI, or AAI).
5388 Technical manual

20
Chapter 2
Precautions
Sensitivity Settings
Place the wires on the right atrial free wall, oriented along the
direction of the myocardial fibers, approximately 1 cm apart. It is
important to achieve a sensing threshold of at least 1.0 mV. The
atrial sensitivity should be set to a minimum of one-half the
measured threshold. This ensures a minimum safety margin of 2x
the sensing threshold. Failure to follow this procedure can lead to
delivery of asynchronous pulses.
Since the sensitivity setting determines the smallest signal that
can be sensed by the pacemaker, set the sensitivity dial to
one-half the mV value of the patient’s sensitivity threshold (see
“Sensing Threshold” on page 84). This setting will provide a 2x
safety margin to ensure proper sensing.
A more sensitive setting may be chosen to provide a greater safety
margin. However, be aware that setting the sensitivity value too
low (too sensitive) could result in inappropriate sensing of far field
signals (e.g., sensing of R- or T-waves on the atrial channel or
P-waves on the ventricular channel), leading to inappropriate
inhibition of pacing pulses.
High Output and Maximum Sensitivity
Although the pacemaker contains a safety pacing feature that
prevents inappropriate inhibition of ventricular pacing due to
far-field sensing, the simultaneous use of high output and
maximum sensitivity (i.e., the lowest mV value) should be avoided.
Electrostatic Discharge (ESD)
The pacing lead(s) provides a low-impedance pathway to the
heart. Therefore, it is recommended that attending health care
professionals discharge any static electricity by touching a large
metal or conductive, grounded surface prior to touching the
patient, the cable, the leads or the pacemaker. Also, neutralize any
static electricity from the patient by touching the patient away from
(i.e., distal to) the leads.
5388 Technical manual

Retrograde Conduction
If retrograde P-waves are being sensed outside the
rate-dependent, automatic Post-Ventricular-Atrial-Refractory
Period (PVARP) setting, manually increase the PVARP until the
retrograde waves fall inside the PVARP. Failure to follow this
procedure may lead to a pacemaker mediated tachycardia (PMT).
Termination of Pacing
Abrupt termination of pacing stimuli may result in intervals of
asystole before an intrinsic rhythm is reestablished. Prior to
terminating pacing, set the pacemaker to a demand mode, then
gradually reduce the pacing rate below the patient’s intrinsic rate.
PAUSE Key
Use the PAU SE key with care, since the patient is without pacing
support (for a maximum of 10 seconds at a time) when PAUSE is
pressed and held.
A-V Interval
Warnings, precautions, and adverse effects
Precautions
21
EMERGENCY Key
Programming long A-V intervals may result in pacing the ventricle
during the vulnerable period of ventricular repolarization, thus
precipitating ventricular arrhythmias in unstable patients.
Use the EMERGENCY key only when high-output asynchronous
pacing (DOO) is needed. When the EMERGENCY key is pressed,
the emergency pacing mode is entered and remains in effect until
the emergency pacing mode is deactivated. Press the ON key to
deactivate emergency pacing mode. For more information, see
“EMERGENCY (ASYNC.) Key” on page 29.
5388 Technical manual

22
Chapter 2
Environmental Precautions
Environmental Precautions
The Model 5388 has been carefully designed and tested to ensure
reliability during normal use. However, electronic devices are
susceptible to many environmental stresses. Precautions should
be taken to avoid damage to the unit, including (but not limited to)
the precautions listed in this chapter.
■
Do not drop the unit or handle it in a way that might physically
damage the device. The device may appear to work
appropriately immediately after being dropped or mishandled,
but operational damage may have occurred.
■
Do not place the Model 5388 in any area where a patient may
interact with it. Tampering with programmed parameters may
have direct and serious patient health effects. The temporary
pacemaker should be placed in an area that minimizes
tampering with the device by unauthorized personnel
(patients, visitors, etc.). Medtronic recommends the use of a
protective cover, such as the Medtronic Model 5441 clear
plastic cover, to minimize tampering.
■
Avoid spilling fluid on the unit. The Model 5388 was carefully
designed to minimize leakage, but fluid incursion may still
occur. Medtronic recommends the use of a protective cover,
such as the Medtronic Model 5409 plastic pouch, to minimize
fluid incursion.
■
Avoid contaminating the safety cable receptacle and
connector pin receptacles with blood or other body fluids.
■
Always use safe electrostatic discharge (ESD) procedures;
this device could be adversely affected by ESD.
■
Do not open the device. The seam joining the unit is designed
to minimize fluid incursion and may not be effective if
improperly opened and resealed. Furthermore, removing the
label on the back of the unit may compromise the ESD barrier.
Opening this unit will void the warranty.
■
Do not sterilize the Model 5388 by gamma irradiation or steam
(autoclave). See “Cleaning and Sterilization” on page 98 for
more information.
■
Rapid temperature changes may affect proper operation.
Always allow the temperature of the device to stabilize in the
environment in which the device will be used before
attachment and operation (see page 104 for recommended
storage and operation temperatures).
5388 Technical manual

■
Prolonged storage or operation of the device in high humidity
may affect proper operation. Allow the device to completely
dry after exposure to humidity.
Other environmental factors may impact proper performance of
the unit in the hospital setting. Use of appropriate environmental
health and safety practices will help prevent environmental
damage to the unit.
Adverse Effects
Temporary Pacemakers
Potential adverse effects related to the use of temporary external
pacemakers such as the Model 5388 include, but are not
limited to:
■
Asystole following abrupt cessation of pacing;
■
Inhibition or reversion in the presence of strong
electromagnetic interference; and
■
Initiation of a tachyarrhythmia or acceleration of an existing
tachyarrhythmia.
Warnings, precautions, and adverse effects
Adverse Effects
23
Atrial High-Rate Burst Pacing
Atrial high-rate burst pacing may result in the onset of tachycardia,
acceleration of an existing tachycardia, or fibrillation. Application
of temporary atrial high-rate burst pacing should be performed in
a carefully monitored and controlled patient environment. Monitor
the patient’s ECG and blood pressure, and keep defibrillation
equipment on standby, immediately available for emergency use.
Dual Chamber Modes
In the DVI, DDI, and DDD modes, the ventricular sense amplifier
may sense the atrial pacing pulse. Reducing the atrial amplitude,
the ventricular sensitivity, and/or repositioning the electrodes may
be necessary to avoid this situation.
5388 Technical manual

24
Chapter 2
Adverse Effects
Safety Margins
Lead Systems
Determine an adequate safety margin for sensing and pacing in
both the ventricle and atrium (see Chapter 5). Failure to do so may
result in inappropriate pacing.
Potential adverse effects related to the use of pacing lead systems
used in conjunction with the Model 5388 temporary pacemaker
include, but are not limited to:
■
Inappropriate lead connections;
■
Inadvertent disconnection of the lead system;
■
Lead fracture or displacement causing intermittent or
complete loss of capture and/or sensing; and
■
Perforation and tamponade.
Other potential adverse effects related to the use of any implanted
lead system include, but are not limited to:
■
Myocardial irritability resulting in fibrillation;
■
Infarction;
■
Pericarditis;
■
Body rejection phenomena (local tissue reaction);
■
Muscle and nerve stimulation; and
■
Infection.
5388 Technical manual
Nerve or muscle stimulation can be caused by pacing lead contact
with the nerve or muscle tissue and/or by high-output settings. The
stimulation may be controlled by repositioning or replacing the
electrode, or by reducing the output pulse amplitude.
Controls, indicators, and other
Controls 26
Light-Emitting Diodes 32
Upper Screen 33
Lower Screen 37
Physical Features 46
Functional Features 49
Timing Violations 53
features
3
3

26
Chapter 3
Controls
Controls
Lock/Unlock Key
The dials and keys used to control the functions and parameter
settings of the Model 5388 are described below.
Note: All adjustments to the RATE, A (Atrial) OUTPUT, and
V (Ventricular) OUTPUT dials take effect within the next two
pacing cycles.
This key allows the user to “lock” and “unlock” the upper screen
parameter values, RATE, AOUTPUT, and VOUTPUT (see “Lock
Feature” on page 49).
When the upper screen is unlocked, press this key to lock the
upper screen parameters at their current settings. The backlight
will turn off and the lock indicator appears in the upper right-hand
corner of the upper screen. Pressing this key while a lower menu
is active causes the device to exit the menu.
Note: If this key is not pressed, the device automatically locks the
parameters on the upper screen 60 seconds after the last device
adjustment, with the exception of Menu 3. When in Menu 3 (Rapid
Atrial Pacing), the device waits five minutes before locking.
5388 Technical manual
When the upper screen is locked, press this key to unlock the
upper screen, allowing the upper screen parameters to be
adjusted again. The backlight turns on, unless the low battery
indicator is flashing (see “Low Battery Indicator” on page 33).
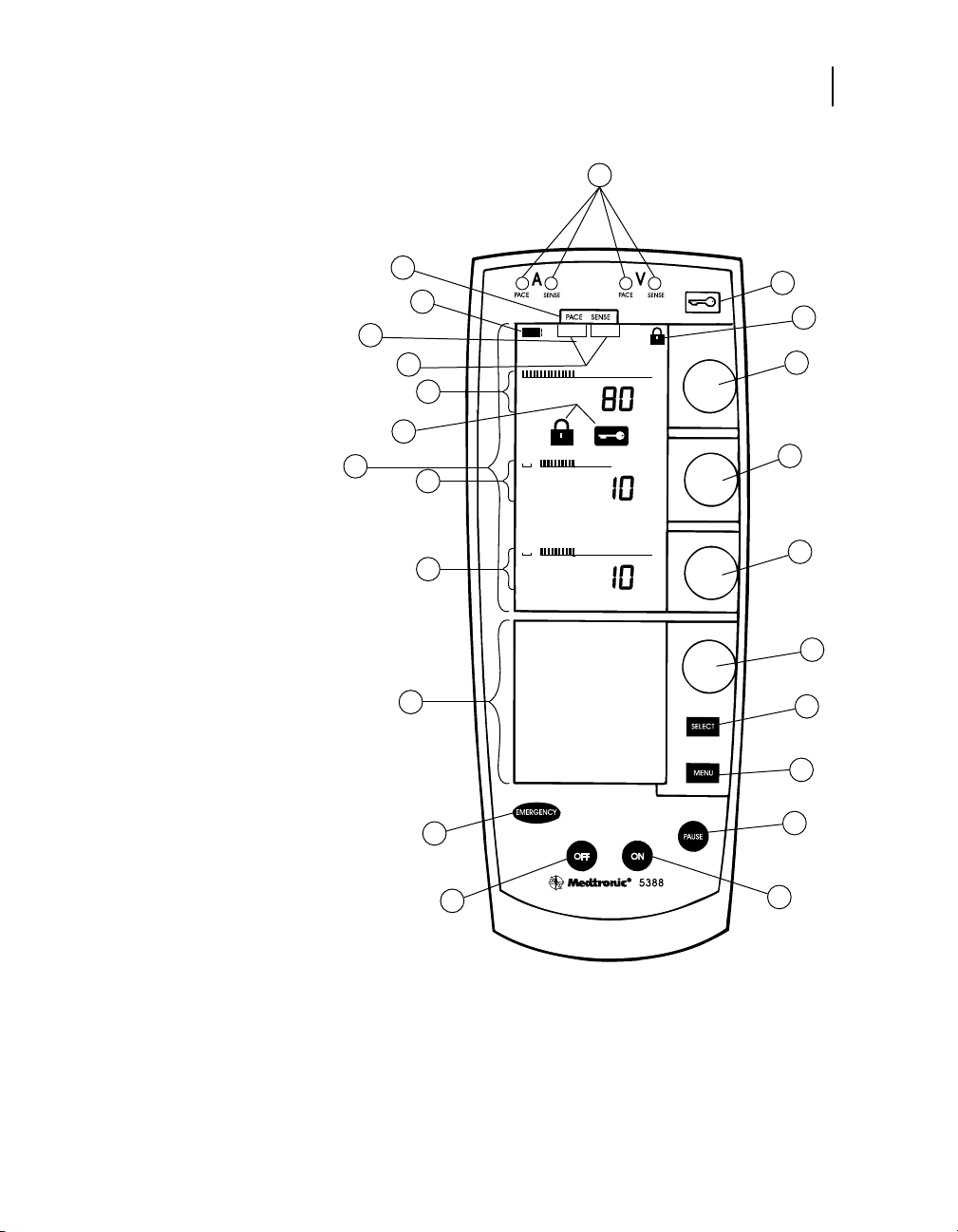
Controls, indicators, and other features
Controls
27
1. Pace/Sense LEDs
2. Lock/Unlock Key
3. Lock Indicators
4. Rate Dial
5. Atrial Output Dial
6. Ventricular Output Dial
7. Menu Parameter Dial
8. Parameter Selection Key
9. Menu Selection Key
10. Pause Key
11. Power On Key
12. Power Off Key
13. Emergency/Asynchronous
Pacing Key
14. Lower Screen
15. Ventricular Output
Graphics
16. Atrial Output Graphics
17. Upper Screen
18. Rate Graphics
19. Setup Indicators
20. DDI Indicator
21. Low Battery Indicator
22. Setup Labels
17
20
22
19
3
14
21
18
16
15
13
+
–
30
RATE
OFF
0.1
A OUTPUT
OFF
0.1
V OUTPUT
A
S
Y
N
1
2
A + V
DDI
A + V
3
4
20012080
ppm
20
10
10
mA
20
25
mA
5
6
7
8
9
.
C
10
Dual Chamber
12
Temporary Pacemaker
Figure 3-1. Controls and Indicators of the Model 5388.
5388 Technical manual
11
28
Chapter 3
Controls
ON Key
ON
Press the ON key once to power the device up. The device first
senses, then begins sensing and pacing in both chambers (DDD
mode).
The upper screen and the backlight illuminate and a self-test is
initiated (see “Self-test” on page 50). After the self-test is
successfully completed the following occurs:
■
If the battery has sufficient power, the device begins sensing
and pacing in both the atrium and ventricle at the following
nominal parameter values:
Tab le 3-1. Power-on values.
ON
110 min
300 ms
170 ms
-1
a
a
-1
Base RATE 80 min
AOUTPUT and VOUTPUT 10 mA
Atrial pulse width 1.0 ms
Ventricular pulse width 1.5 ms
A SENSITIVITY 0.5 mV
V SENSITIVITY 2.0 mV
A TRACKING
UPPER RATE
PVARP
A-V INTERVAL (paced)
a
Pulse width is not adjustable.
b
For a description of atrial tracking, see page 40.
c
These parameters are set to the automatic, rate-dependent values
(see “Device Specifications” on page 103).
b
c
c
c
5388 Technical manual
■
If the battery is nearing depletion, the low-battery indicator is
displayed.
■
If the battery is depleted, the LEDs may come on (see
“Light-Emitting Diodes” on page 32) while ON is pressed, but
the device will not operate.
When the upper screen is locked, press the ON key once to unlock
the upper screen (see “Lock/Unlock Key” on page 26). The
backlight comes on and the upper screen parameters may be
adjusted. The device continues to pace at the currently selected
values.
OFF Key
OFF
Controls, indicators, and other features
Controls
When the device is pacing asynchronously, to return the device to
demand (synchronous) pacing:
■
Press the ON key once if the asynchronous pacing message
(see page 30) is displayed in the lower screen.
■
Press the ON key twice if the asynchronous pacing message
is not displayed in the lower screen. (After the first press of the
ON key, the asynchronous pacing message appears).
The device begins pacing synchronously at the following values:
Tabl e 3-2. ON key synchronous values.
RATE current setting
A OUTPUT and V OUTPUT current settings
A SENSITIVITY
V SENSITIVITY
a
If the corresponding OUTPUT is not OFF.
0.5 mV (nominal)
2.0 mV (nominal)
a
a
To turn the device off, press the OFF key twice within 5 seconds.
(After the first press, a message appears in the lower screen
telling the user to press OFF a second time to turn the device off.)
■
The backlight turns off, the screens blank, and three LEDs
illuminate, then turn off, when OFF is pressed the second time.
29
Note: If the OFF key is pressed once when the upper screen
To SHUT DOWN,
Press again.
OFF
parameters are locked:
■
The backlight illuminates and the upper screen unlocks,
allowing the parameters to be adjusted.
■
The lower screen displays the message telling the user to
press OFF a second time (Ignoring this message allows the
device to continue pacing at the currently selected values).
EMERGENCY (ASYNC.) Key
A single press of this key selects high-output, dual-chamber
EMERGENCY
A
S
C
Y
N
.
asynchronous pacing (DOO) at any time, including when the
device is off. Avoid accidentally activating the Emergency key.
Note: Asynchronous pacing can also be reached by adjusting
A and V SENSITIVITY on Menu 1 (see “Menu 1” on page 38).
5388 Technical manual
30
Chapter 3
Controls
To initiate dual-chamber asynchronous pacing, press the
EMERGENCY/ASYNC. key once at any time (that is, while the
device is on, off, in a Menu or locked). The device will pace at the
following values:
Tab le 3-3. Emergency values.
RATE current setting, or
80 min-1if device was off
AOUTPUT
VOUTPUT
A SENSITIVITY
V SENSITIVITY
A TRACKING,
UPPER RATE, PVARP
20 mA
25 mA
ASYNC (i.e., no sensing)
ASYNC (i.e., no sensing)
not applicable
ASYNCHRONOUS
PACING
To Resume
Synchronous Pacing
Press ON
PAUSE Key
PAUSE
A-V INTERVAL
automatic rate-dependent, or
current manual setting
Note: If the device is locked when EMERGENCY/ASYNC. is
pressed, the upper screen parameters unlock, the backlight turns
on and the device immediately begins to pace at emergency
values.
The RATE, AOUTPUT, and VOUTPUT can be adjusted using the
three upper dials. The A-V INTERVAL can be manually adjusted
or allowed to adjust automatically with the RATE (see
“A-V Interval” on page 40).
The message to the left appears in the lower screen.
Note: The asynchronous pacing message disappears after one
minute. The message reappears anytime the ON key is pressed
(see “ON Key” on page 28) during asynchronous pacing.
To resume demand (synchronous) pacing, press the ON key (see
“ON Key” on page 28), or access Menu 1 and adjust
A SENSITIVITY and/or V SENSITIVITY (see page 39).
This key interrupts pacing and sensing to allow the user to view the
patient’s intrinsic rhythm.
Caution: Use the PAUSE key with care, since the patient is
without pacing support (for a maximum of 10 seconds at a
time) when PAUSE is pressed and held.
5388 Technical manual
 Loading...
Loading...