Medtronic 5348 Technical Manual

0123
5348
Single Chamber Temporary PaceMaker (AAI/VVI)
Technical Manual
Caution: Federal Law (USA) restricts this device to sale by
or on the order of a physician.
c


MEDTRONIC MODEL 5348 0
Technical Manual 0
Single Chamber Temporary Pacemaker
Explanation of symbols 0
Consult instructions for use
Type CF applied part
Conformité Européenne
This symbol means that the device fully complies with European Directive 93/42/EEC.
Do not dispose of this product in the unsorted municipal waste stream. Dispose of this product
according to local regulations.
See http://recycling.medtronic.com for instructions on proper disposal
of this product.
For US audiences only
Package contents
Temporary pacemaker
Product documentation
Accessories
Storage temperature limitation
0123
5348 Technical manual
5
Humitidy limitation
Battery
Reorder number
Authorized representative in the European Community
Manufacturer
Date of manufacture
Serial number
EC REP


5348 Technical manual
Contents
1 General description 11
Package Contents 12
Safety Features 12
Registration Card 12
Medtronic Warranty 13
2 Intended use 15
3 Contraindications 17
Atrial Pacing 17
Asynchronous Pacing 17
High-Rate Burst Therapy 17
4 Warnings 19
Equipment Modification 19
Line-powered Equipment 19
Electrosurgery 19
Electromagnetic Interference (EMI) 19
Defibrillation/Cardioversion 20
High-Rate Burst Therapy 20
Connecting the Lead System 20
Handling Indwelling Leads 21
Turning the Device On 21
5 Precautions 23
Random Failures 23
Pacing Leads and Cables 23
Pacing System Adjustments 23
Unipolar Lead Systems 24
Sensitivity Settings 24
Electrostatic Discharge (ESD) 24
Termination of Pacing 24
Battery 24
Unauthorized Changes of Pacemaker Settings 25
6 Environmental precautions 27
7 Potential adverse effects 29
Pacemakers 29

5348 Technical manual
Contents
8
High Rate Pacing 29
Lead Systems 29
8 Controls, indicators, and other features 31
Base Level Pacing Controls 31
RATE 31
OUTPUT 31
SENSITIVITY 31
ON and OFF 33
Rapid Atrial Pacing (RAP) Controls 33
ENABLE/DISABLE 33
HOLD TO DELIVER 34
OUTPUT 34
Indicators 34
PAC E 3 5
SENSE 35
LOW BATT. 35
Physical Features of the Model 5348 35
Control Covers 35
Battery 35
Connector Block 36
Attachment Ring and Bails 37
Functional Features of the Model 5348 37
Self-Testing 37
RAP Standby 38
Rate-Runaway Protection 38
Pulse Width 38
Synchronous (Demand) Modes (AAI/VVI) 38
Asynchronous Modes (AOO/VOO) 39
Blanking Periods 39
Refractory Periods 39
Reversion Response 39
Cables 40
Medtronic Models 5433A and 5433V Patient Cables 40
Medtronic Surgical Cable Models 5832 and 5832S 41
The Model 5409 Disposable Pouch 41
Description 41
Procedure for use 42
9 Preparation for use 43
Battery Installation 43

5348 Technical manual
Contents
9
Connecting the Model 5433A or 5433V Patient Cable to the
Model 5348 44
Connecting the Pacing Lead System to the Model 5433A or
5433V Patient Cable 45
Connecting the Pacing Lead System Directly to the Model 5348
Pacemaker 46
10 Instructions for use 49
Turning the Model 5348 On and Off 49
Power-on Self-test 49
Procedures for Basic Pacing 50
Determining the Pacing Mode 50
Adjusting the Pacing Parameters 51
Determining Sensing Potentials 51
Determining Stimulation Thresholds 52
Procedure for Rapid Atrial Pacing (RAP) 53
Verify Connections 53
Enable the RAP Standby State 53
Adjust the RAP Rate 53
Delivering a RAP Burst 53
Adjusting Parameters During RAP Delivery 54
Returning to Basic Pacing Operation (Disabling RAP Standby)
54
11 Service information 55
Cleaning and Disinfection 55
Model 5348 Temporary Pacemaker 55
Model 5433A and 5433V Patient Cables 55
Safety and Technical Checks 56
Visual Inspection: 56
Functional Inspection: 56
Practical Measurements: 56
Service 57
12 Specifications 59

1
General description1
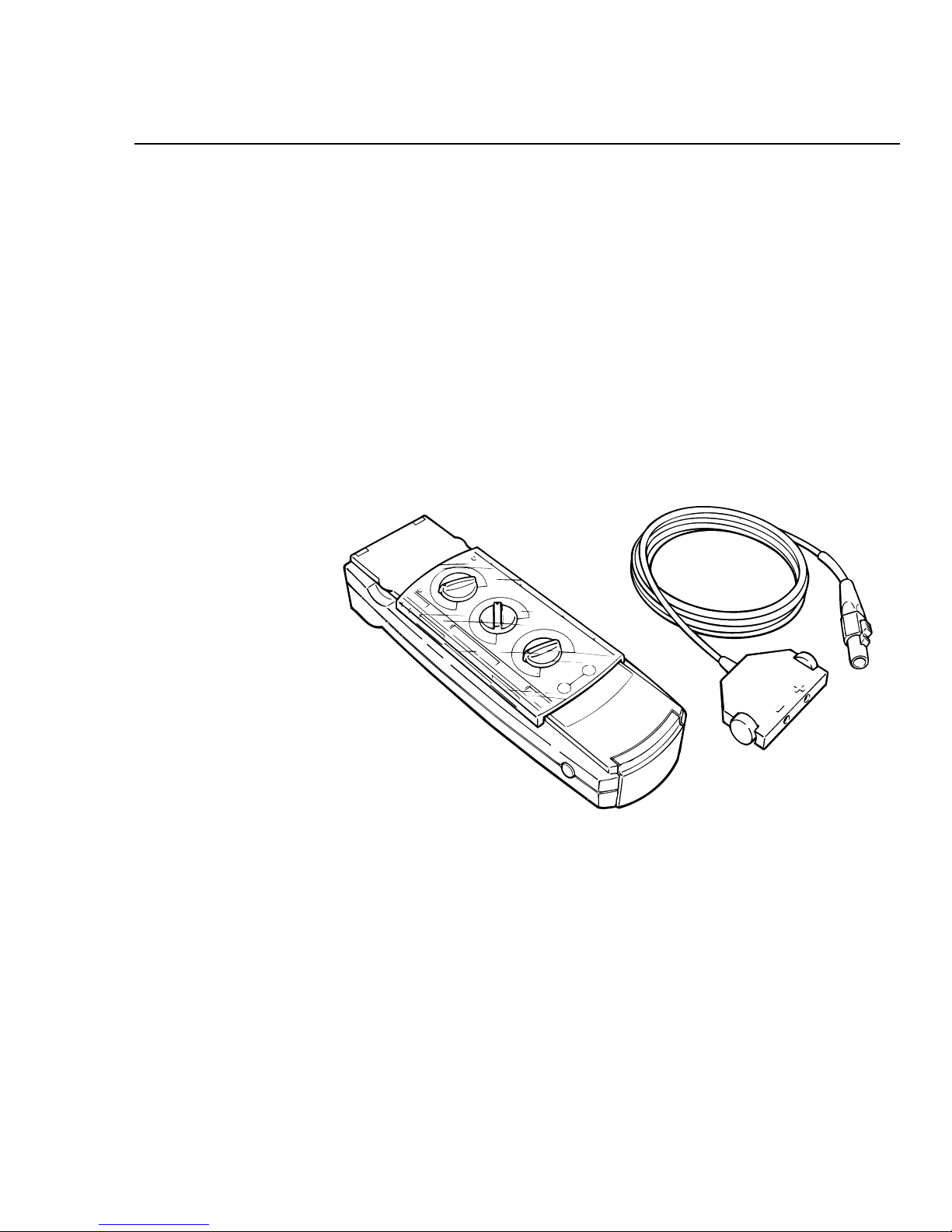
The Medtronic® Model 5348 is a temporary, battery-powered, single
chamber pacemaker designed primarily for temporary antibradycardia
pacing therapy in asynchronous or demand (synchronous) modes.
High-rate burst pacing therapy up to 800 min
-1
(reciprocal minutes)
(ppm [pulses per minute]), for tachyarrhythmias, is available in the
asynchronous mode
1
.
The device is typically connected to temporary transvenous, epicardial
or myocardial pacing leads, in a bipolar configuration, using a patient
cable (Medtronic Model 5433A or 5433V) or a surgical cable (Medtronic
Model 5832 or 5832S).
Figure 1-1. The Medtronic Model 5348 Single Chamber Temporary
Pacemaker and the Model 5433A or 5433V Patient Cable.
The device operates using a 9-volt alkaline or lithium battery, which is
installed in a battery drawer at the bottom end of the pacemaker.
Note: The Model 5348 is a constant current device; it emits a pulse
with a current output that is maintained at a constant value. This value
is set by the output control and does not vary with respect to the
myocardium/lead impedance (as long as the myocardium/lead
impedance stays between 200 Ω and 1000 Ω).
1
For atrial use only.

5348 Technical manual
Chapter 1
Package Contents
12
Package Contents
The Model 5348 is supplied with a 9-volt alkaline battery, technical
literature, one Model 5409 disposable pouch, one Model 5433A
atrial patient cable, one Model 5433V ventricular patient cable, a
package of heartwire seals, and a carrying case. Check the
package prior to use. Damaged packages should be returned to
Medtronic (see back cover for the address).
Safety Features
The Medtronic Model 5348 is designed to be reliable, simple to
operate, and comfortable to hold. Safety features of the
Model 5348 include:
■
Self-tests;
■
Low Battery indicator;
■
Continuous operation during battery replacement
(at 80 min
-1
[ppm], 10 mA) for a minimum of 15 seconds;
■
Reversible battery polarity;
■
Protective covers over the controls and a rubber seal covering
the heartwire receptacles;
■
Safe “power-off” operation (Two buttons must be pressed
simultaneously to turn the device off.);
■
Cautionary label at the Rapid Atrial Pacing (RAP) controls;
■
Detents (mechanical restrictions of dial movement) on the
RATE and SENSITIVITY dials to highlight extremes or
potentially hazardous settings;
■
Safety cables (recessed pins);
■
Runaway rate protection;
■
Protection from defibrillation shock up to 360 watt-seconds;
■
Electrostatic protection; and
■
Minimized susceptibility to electromagnetic and magnetic
interference.
Registration Card
Please complete the registration card and return it to Medtronic.
Consult the back cover of this manual for the address. U.S.
customers: use the address labels provided.

5348 Technical manual
General description
Medtronic Warranty
13
Medtronic Warranty
For complete device warranty and accessories disclaimer of
warranty, see the accompanying warranty documents.


2
Intended use2
The Medtronic Model 5348 pacemaker is intended to be used in
conjunction with a cardiac pacing lead system for temporary atrial or
ventricular pacing in a clinical environment. The Model 5348 can be
used where short-term demand (synchronous) or asynchronous pacing
is indicated for therapeutic, prophylactic or diagnostic purposes.
Specific indications for temporary cardiac pacing include, but are not
limited to, the following:
■
Complete heart block;
■
Sinus bradycardia;
■
Sick Sinus Syndrome;
■
Bradycardia with congestive heart failure;
■
Atrial and/or ventricular arrhythmias;
■
Cardiac arrest;
■
Temporary support, management, and evaluation of a patient prior
to permanent pacemaker implantation;
■
Support during permanent pacemaker replacement;
■
Cardiac complications during invasive or surgical procedures;
■
Temporary support of a patient following cardiac surgery;
■
Acute myocardial infarction complicated by heart block; and
■
High-rate burst pacing for the treatment of supraventricular
tachyarrhythmias.
The Model 5348 can be used to determine sensing potentials of
temporary and permanently implanted lead systems. When implanting
a permanent pacemaker, however, Medtronic recommends the use of
a Medtronic Pacing System Analyzer.


3
Contraindications3
There are no known contraindications to the use of temporary pacing
as a means to control the heart rate. The patient’s age and medical
condition, however, may dictate the type of temporary pacemaker and
lead system used by the physician.
Atrial Pacing
Single chamber atrial pacing is contraindicated in the presence of AV
conduction disorders.
Asynchronous Pacing
Asynchronous pacing is contraindicated in the presence of intrinsic
cardiac rhythms.
High-Rate Burst Therapy
High-rate burst therapy is intended for use in the atrium only. Use in the
ventricle could result in life-threatening arrhythmias.


4
Warnings4
Equipment Modification
Do not modify this equipment. Modifications could impact device
effectiveness and adversely affect patient safety.
Line-powered Equipment
An implanted lead or lead with extension cable constitutes a direct,
low-resistance current pathway to the myocardium. Due to the danger
of fibrillation resulting from alternating current leakage, extreme caution
must be taken to properly ground all line-powered equipment used on
or in the vicinity of the patient.
Electrosurgery
Electrosurgery can induce ventricular fibrillation, and thus should never
be used within 15 cm (6 inches) of an implanted lead system.
Electromagnetic Interference (EMI)
All pacemakers operating in the demand mode respond to intracardiac
potentials of a magnitude of a few millivolts. They are inherently
sensitive to some external fields. In the presence of excessive levels of
interference the Model 5348 may inhibit completely or revert to
asynchronous operation, pacing at the rate set by the RATE dial.
It is recommended that the device be set to an asynchronous mode
when operated in the presence of strong electromagnetic interference
(EMI).
Some sources of excessively strong EMI which may temporarily affect
the operation of the Model 5348 are:
■
Electrosurgical equipment;
■
Diathermy equipment;
 Loading...
Loading...