Page 1

Medtronic Open Pivot™
Heart Valve
500, 501, 505
Instructions for Use
Caution: Federal law (USA) restricts this device to sale by or on the
order of a physician.
Page 2

Trademarks may be registered and are the property of their respective owners.
Page 3
Explanation of symbols on package labeling
Refer to the device labeling to see which symbols apply to this product
Nonpyrogenic
Sterilized Using Steam
Size
Do Not Reuse
Do Not Resterilize
Use-By Date
Quantity
Open Here
Consult Instructions for Use
For US Audiences Only
Keep Dry
Date of Manufacture
Serial Number
Catalog Number
Manufacturer
Authorized Representative in the European Community
MR Conditional
Do Not Use if Package is Damaged
Manufactured In
1
Page 4
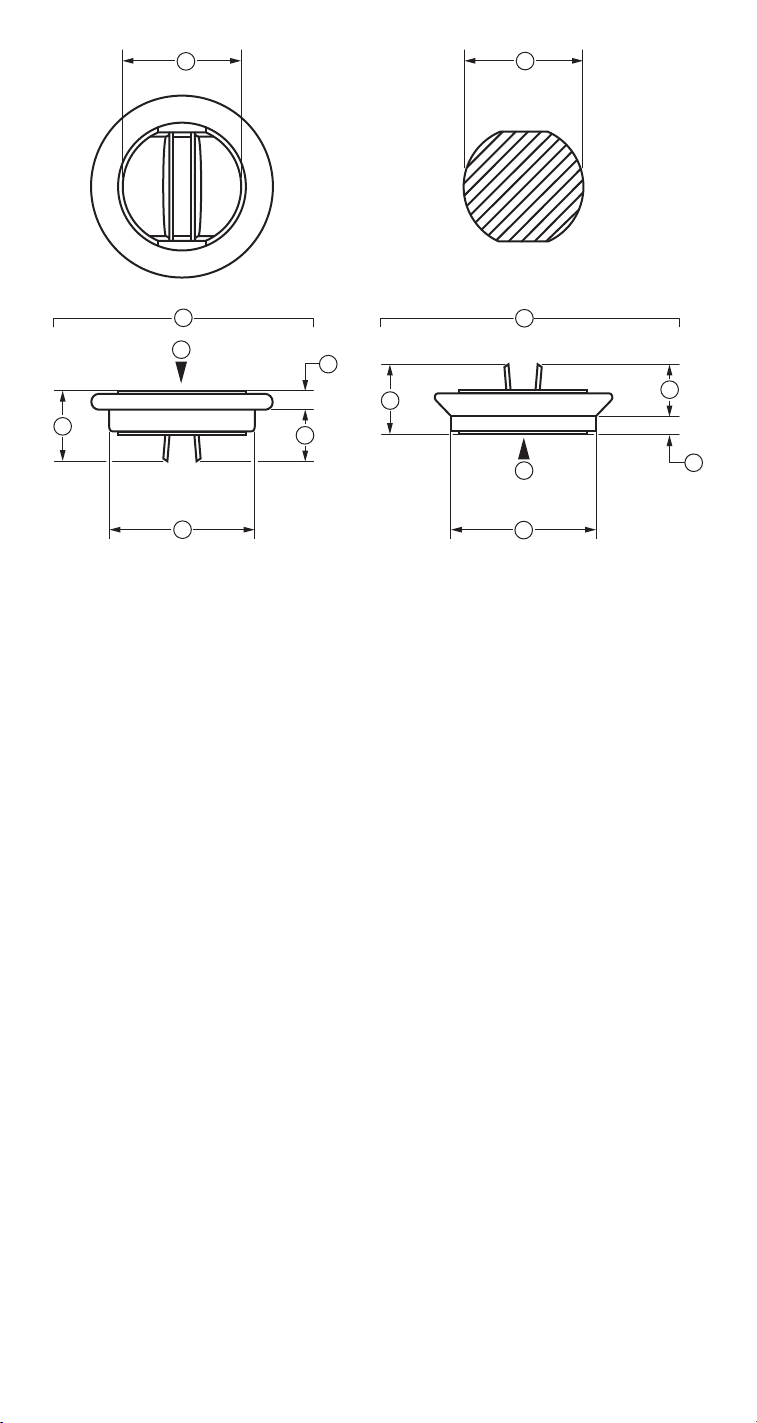
1
2
3
4
5
9
7
6
8
5
7
8
9
6
1. Inside diameter
2. Geometric orifice
3. Mitral valve
4. Aortic valve
5. Profile height
6. Inflow height
7. Outflow height
8. Tissue annulus
9. Blood flow
Figure 1. Standard heart valve
2
Page 5
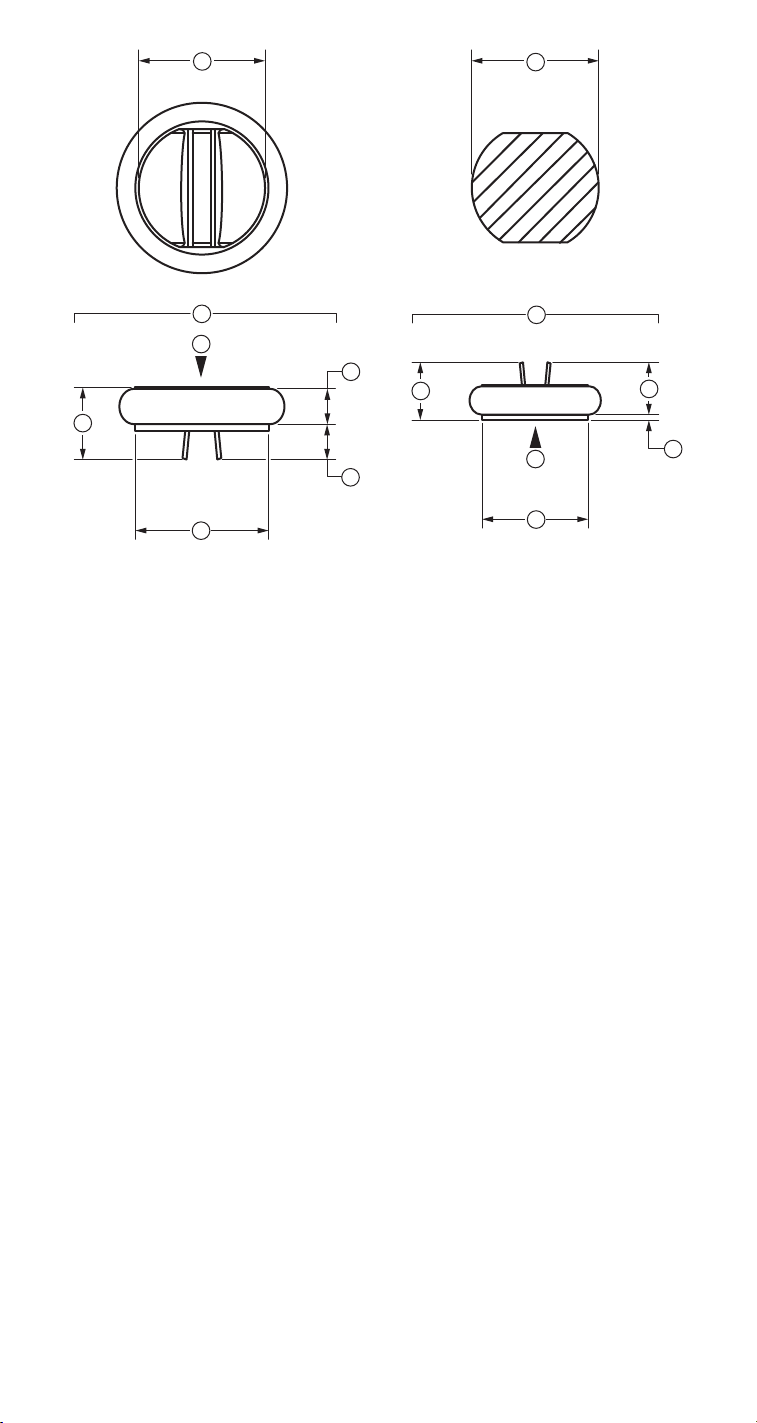
1
3
4
2
5
5
7
9
9
7
6
8
8
6
1. Inside diameter
2. Geometric orifice
3. Mitral valve
4. Aortic valve
5. Profile height
6. Inflow height
7. Outflow height
8. Tissue annulus
9. Blood flow
Figure 2. AP™ heart valve
3
Page 6

1
2
9
7
6
5
3
4
5
8
9
6
7
8
1. Inside diameter
2. Geometric orifice
3. Mitral valve
4. Aortic valve
5. Profile height
6. Inflow height
7. Outflow height
8. Tissue annulus
9. Blood flow
Figure 3. AP360™ heart valve
4
Page 7
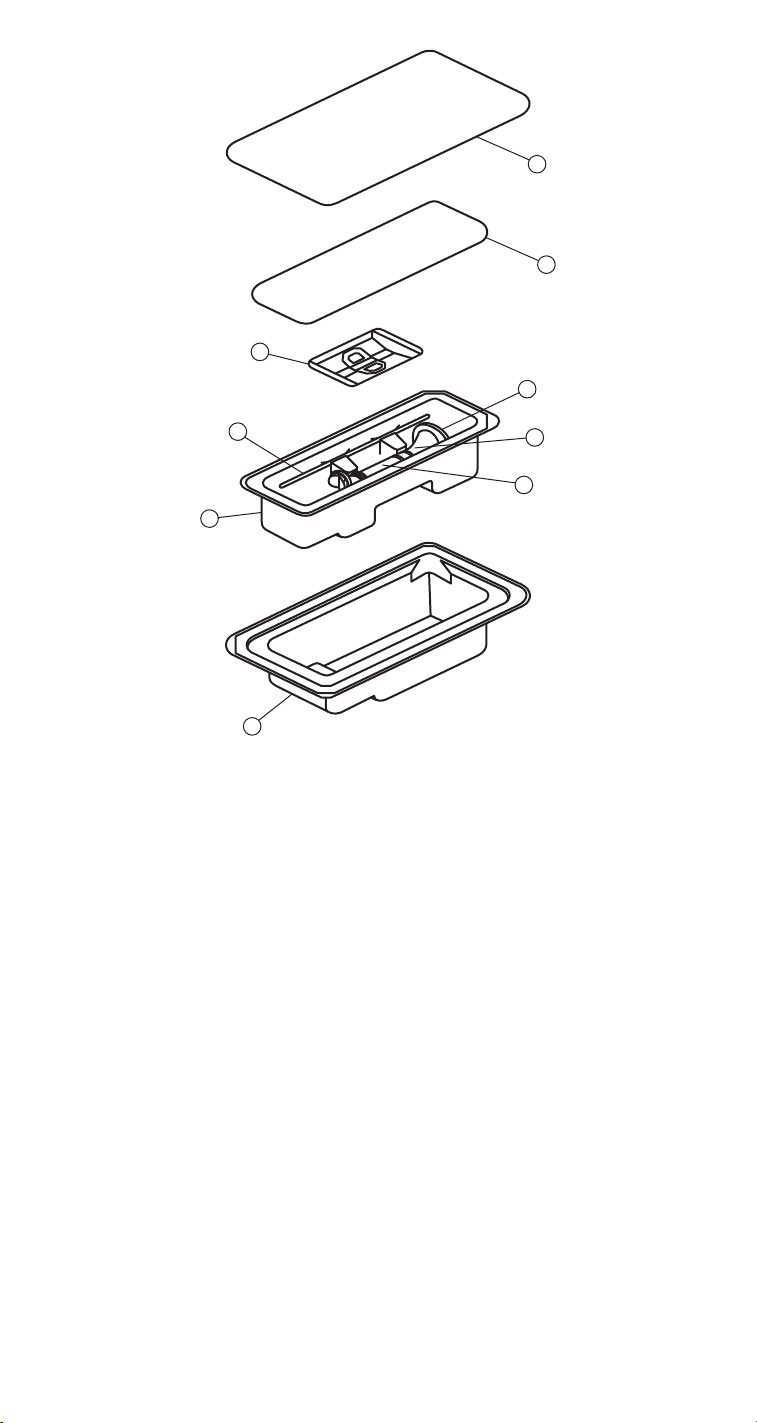
1
3
2
5
9
7
6
8
4
1. Outer lid
2. Inner lid
3. Retainer
4. Leaflet actuator
5. Heart valve
6. Valve holder
7. Handle rotator
8. Inner tray
9. Outer tray
Figure 4. Double barrier tray package
5
Page 8

1
Figure 5. Aortic valve holder and handle rotator assembly (leaflets inside holder)
1
1. Radial ridge
1. Radial ridge
Figure 6. Mitral valve holder and handle rotator assembly
6
Page 9

Figure 7. Standard heart valve sizer
7
Page 10

Figure 8. AP™ heart valve sizer
8
Page 11

1
1. Flexible support
Figure 9. Bendable handle
9
Page 12

Heart Valve
1. Device Description
The Medtronic Open Pivot™ heart valve (Models 500, 501, and 505) is a low profile bileaflet valve
prosthesis consisting of pyrolytic carbon orifice ring and leaflets (Figure 1, Figure 2, and Figure 3).
The prosthesis consists of an orifice housing 2 mirror image leaflets. The low profile of the prosthesis
results from the bileaflet design where the pivot areas are located entirely within the orifice ring, which
minimizes the overall height of the valve. Pivot guides located on the inner circumference of the orifice ring
control the range of leaflet motion. The pivot geometry consists of arc-shaped notches at either end of
each leaflet and spherical protrusions at 4 places on the orifice. Each leaflet rotates around 2 opposing
spheres. There are inflow and outflow stops adjacent to each sphere on the orifice. The inflow and outflow
stops limit the rotation of the leaflets. There are no recesses or cavities in the pivot area. The leaflets open
to an approximate angle of 85 degrees and close to a maximum angle of approximately 25 degrees.
The leaflets consist of pyrolytic carbon coated over a graphite substrate. The graphite substrate is
impregnated with 20% tungsten for radiopacity. The orifice consists entirely of pyrolytic carbon.
The valve sewing cuff is constructed of double velour polyester fabric mounted on the orifice using a
titanium or MP35N, a cobalt-chromium alloy, stiffening ring and secured with two titanium lock rings and a
lock wire. This method of sewing cuff attachment to the orifice allows for rotation of the sewing cuff in situ
during surgical placement. The sewing cuff of mitral sizes 25 mm through 33 mm contains a
polytetrafluoroethylene (PTFE) liner inside the double velour polyester fabric.
The Medtronic Open Pivot heart valve is available in the aortic and mitral configurations in several sewing
cuff styles: the standard, AP™, and AP360™. The AP™ series valves are supra-annular configurations of
the standard heart valve. Three cuff markers are located in the aortic cuff and 4 cuff markers are located in
the mitral cuff to assist in the uniform placement of sutures around the valve annulus.
The valves are available in the dimensions and sizes shown in Table 1 through Table 6.
Table 1. Medtronic Open Pivot Standard Heart Valve Specifications (Aortic)
Valve Size
(mm)
19 500FA19 9.3 14.8 19.2 1.55
21 500FA21 10.3 16.8 21.2 2.02
23 500FA23 11.3 18.8 23.2 2.56
25 500FA25 12.3 20.8 25.2 3.17
27 500FA27 13.3 22.8 27.2 3.84
29 500FA29 14.3 24.8 29.2 4.59
Size
Valve
(mm)
25 500DM25 12.3 20.8 25.2 3.17
27 500DM27 13.3 22.8 27.2 3.84
29 500DM29 14.3 24.8 29.2 4.59
31 500DM31 15.4 26.8 31.2 5.35
33 500DM33 15.4 26.8 33.2 5.35
35 500DM35 15.4 26.8 35.2 5.35
Size
Valve
(mm)
16 501DA16 9.3 14.8 16.2 1.55
18 501DA18 10.3 16.8 18.2 2.02
20 501DA20 11.3 18.8 20.2 2.56
22 501DA22 12.3 20.8 22.2 3.17
24 501DA24 13.3 22.8 24.2 3.84
26 501DA26 14.3 24.8 26.2 4.59
Size
Valve
(mm)
22 501DM22 12.3 20.8 22.2 3.17
24 501DM24 13.3 22.8 24.2 3.84
26 501DM26 14.3 24.8 26.2 4.59
28 501DM28 15.4 26.8 28.2 5.35
Model Number Overall Profile
Height (mm)
Table 2. Medtronic Open Pivot Standard Heart Valve Specifications (Mitral)
Model Number Overall Profile
Height (mm)
Table 3. Medtronic Open Pivot AP Heart Valve Specifications (Aortic)
Model Number Overall Profile
Height (mm)
Table 4. Medtronic Open Pivot AP Heart Valve Specifications (Mitral)
Model Number Overall Profile
Height (mm)
Inside Diameter (mm)
Inside Diameter (mm)
Inside Diameter (mm)
Inside Diameter (mm)
Tissue Annulus
Diameter (mm)
Tissue Annulus
Diameter (mm)
Tissue Annulus
Diameter (mm)
Tissue Annulus
Diameter (mm)
Orifice Area
(cm2)
Orifice Area
(cm2)
Orifice Area
(cm2)
Orifice Area
(cm2)
10 Instructions for Use English
Page 13

Table 5. Medtronic Open Pivot AP360 Heart Valve Specifications (Aortic)
Valve Size
(mm)
16 505DA16 9.3 14.8 16.2 1.55
18 505DA18 10.3 16.8 18.2 2.02
20 505DA20 11.3 18.8 20.2 2.56
22 505DA22 12.3 20.8 22.2 3.17
24 505DA24 13.3 22.8 24.2 3.84
26 505DA26 14.3 24.8 26.2 4.59
Valve Size
(mm)
22 505DM22 12.3 20.8 22.2 3.17
24 505DM24 13.3 22.8 24.2 3.84
26 505DM26 14.3 24.8 26.2 4.59
28 505DM28 15.4 26.8 28.2 5.35
Model Number Overall Profile
Height (mm)
Table 6. Medtronic Open Pivot AP360 Heart Valve Specifications (Mitral)
Model Number Overall Profile
Height (mm)
Inside Diameter (mm)
Inside Diameter (mm)
Tissue Annulus Diameter
(mm)
Tissue Annulus Diameter
(mm)
Orifice Area
(cm2)
Orifice Area
(cm2)
2. Indications for Use
The Medtronic Open Pivot heart valve is indicated for the replacement of diseased, damaged, or
malfunctioning native or prosthetic aortic or mitral valves.
3. Contraindications
The Medtronic Open Pivot heart valve is contraindicated in patients unable to tolerate anticoagulation
therapy.
4. Warnings and Precautions
4.1. Warnings
■
This device was designed for single patient use only. Do not reuse, reprocess, or resterilize this
product. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device
and/or create a risk of contamination of the device, which could result in patient injury, illness, or death.
■
Avoid damaging the prosthesis. Only handle the prosthesis with the accessories provided by
Medtronic. Touching the valve with gloved fingers or any surgical instrument may cause damage to the
valve surface that cannot be seen with the unaided eye. Such damage may serve as a nidus for
thrombus formation. The damage may also lead to leaflet escape or accelerated structural
deterioration of the valve.
■
Do not pass a catheter through the prosthesis as this may cause valvular insufficiency, leaflet
dislodgment, or catheter entrapment.
■
Do not apply force to the leaflets, attempt to change the position of the leaflets, or remove a leaflet.
■
Patients allergic to cobalt-chromium or nickel may suffer an allergic reaction, specifically to the AP360
device.
4.2. Precautions Prior to Use
■
Do not use the Medtronic Open Pivot heart valve if the prosthesis has been dropped, damaged, or
mishandled in any way. Should the valve be damaged during implantation or removal from the
package, do not use for implantation.
■
Do not use the Medtronic Open Pivot heart valve if the tamper evident seal is broken or the Use-By
Date has elapsed.
4.3. Precautions During Use
■
Use only the Medtronic Open Pivot heart valve sizer to select the proper valve size as other sizers
may result in improper valve selection.
■
When a valve is released from a holder and implanted without using the holder, implant it with
attention to the valve orientation.
■
When seating the valve, ensure that no suture material or anatomic structures interfere with leaflet
motion. Rotate the valve to avoid abnormal residual pathology that could interfere with leaflet motion.
■
Use only taper point needles for suturing the cuff as taper cut or other cutting needles may cut the cuff
fibers.
5. Adverse Events
A total of 965 Medtronic Open Pivot heart valves were implanted in 965 patients at 20 centers. The mean
follow-up was 1.4 years (range 0 to 5 years) with a total of 1323 patient-years. A total of 56 deaths
occurred during the study and 20 of these were characterized as valve related. The causes of valverelated deaths were endocarditis (2 patients), paravalvular leak (1 patient), thromboembolism (3 patients),
anticoagulant-related hemorrhage (10 patients), and unknown (4 patients).
5.1. Observed Adverse Events
Table 7 shows the observed adverse events for early events (occurring ≤30 days postimplant), the
linearized rates for late events (occurring >30 days postoperatively), and the actuarial adverse event rates
at 1 and 5 years postoperatively.
Instructions for Use English 11
Page 14

Table 7. Observed Adverse Events (Aortic Valve Replacement)
Early Events Late Events Actuarial Freedom by Kaplan-
All patients implanted: N=685, Cumulative Follow-up=866.4 patient years
Deaths (all causes) 2.04% (14) 2.77% ± 1.06% (24) .9735 [± .01] .9031 [± .05]
Death (valve-related/unexplained)
Anticoagulant-Related
Hemorrhage (All)
Anticoagulant-Related
Hemorrhage (Major)
Thromboembolism (All) 1.75% (12) 2.08% ± 0.93% (18) .9733 [± .01] .9283 [± .05]
Permanent Neurological Events
Transient Neurological
Events
Valve Thrombosis 0.00% (0) 0.00% ± 0.00% (0) 1.000 [± .00] 1.000 [± .00]
Perivalvular Leak (All) 0.15% (1) 0.46% ± 0.52% (4) .9966 [± .00] .9898 [± .01]
Perivalvular Leak
(Major)
Endocarditis 0.00% (0) 0.35% ± 0.46% (3) .9960 [± .01] .9908 [± .01]
Hemolysis 0.00% (0) 0.00% ± 0.00% (0) 1.000 [± .00] 1.000 [± .00]
Structural Dysfunction 0.00% (0) 0.00% ± 0.00% (0) 1.000 [± .00] 1.000 [± .00]
Nonstructural Dysfunction 0.00% (0) 0.00% ± 0.00% (0) 1.000 [± .00] 1.000 [± .00]
Reoperation 0.15% (1) 0.35% ± 0.46% (3) .9961 [± .01] .9914 [± .01]
Explant 0.00% (0) 0.23% ± 0.41% (2) .9978 [± .00] .9931 [± .01]
Notes:
1. Cumulative probability of freedom from event estimate at the end of the interval (Pc) is based on the
Kaplan-Meier method.
2. The 95% confidence interval bound for the cumulative freedom rate at the end of the
interval = 1.96 X SE, where SE is the standard error estimate of the cumulative probability freedom
from a heart valve related or an unexplained event estimate calculated using Greenwood’s formula.
3. The actuarial hazard rate estimates are calculated at the midpoint of each interval.
4. The 95% confidence interval bound for the hazard rate at the midpoint of each interval = 1.96 X SE,
where SE is the standard error estimate of the hazard rate estimate at the midpoint of the interval.
All patients implanted: N=280, Cumulative Follow-up=374.7 patient years
Deaths (all causes) 1.79% (5) 3.47% ± 1.88% (13) .9814 [± .02] .8099 [± .11]
Death (valve-related/unexplained)
Anticoagulant-Related
Hemorrhage (All)
Anticoagulant-Related
Hemorrhage (Major)
Thromboembolism (All) 3.21% (9) 4.00% ± 2.00% (15) .9534 [± .03] .8589 [± .09]
Permanent Neurological Events
Transient Neurological
Events
Valve Thrombosis 0.00% (0) 0.53% ± 0.95% (2) .9947 [± .01] .9866 [± .02]
Perivalvular Leak (All) 0.71% (2) 1.07% ± 1.19% (4) .9819 [± .02] .9819 [± .02]
Perivalvular Leak
(Major)
Endocarditis 0.36% (1) 0.53% ± 0.95% (2) .9957 [± .01] .9861 [± .02]
Hemolysis 0.00% (0) 0.53% ± 0.95% (2) .9952 [± .01] .9814 [± .03]
Structural Dysfunction 0.00% (0) 0.00% ± 0.00% (0) 1.000 [± .00] 1.000 [± .00]
Nonstructural Dysfunction 0.00% (0) 0.00% ± 0.00% (0) 1.000 [± .00] 1.000 [± .00]
Reoperation 0.71% (2) 1.07% ± 1.19% (4) .9874 [± .01] .9697 [± .03]
Explant 0.36% (1) 0.53% ± 0.95% (2) .9959 [± .01] .9783 [± .03]
% of pts (N) %/pt-yr (N) 1 Year [95% CI] 5 Years [95% CI]
0.58% (4) 1.15% ± 0.74% (10) .9817 [± .01] .9539 [± .03]
4.67% (32) 1.96% ± 0.91% (17) .9781 [± .01] .9340 [± .04]
3.21% (22) 1.27% ± 0.76% (11) .9878 [± .01] .9473 [± .04]
0.88% (6) 0.69% ± 0.60% (6) .9920 [± .01] .9706 [± .04]
0.88% (6) 1.39% ± 0.78% (12) .9812 [± .01] .9564 [± .03]
0.15% (1) 0.12% ± 0.33% (1) .9983 [± .00] .9983 [± .00]
Table 8. Observed Adverse Events (Mitral Valve Replacement)
Events Late Events Actuarial Freedom by Kaplan-
Early
% of pts (N) %/pt-yr (N) 1 Year [95% CI] 5 Years [95% CI]
0.71% (2) 1.07% ± 1.19% (4) .9831 [± .02] .9342 [± .07]
3.21% (9) 0.53% ± 0.95% (2) .9958 [± .01] .9673 [± .06]
3.21% (9) 0.53% ± 0.95% (2) .9958 [± .01] .9673 [± .06]
1.79% (5) 0.80% ± 1.08% (3) .9910 [± .01] .9807 [± .02]
1.43% (4) 3.20% ± 1.82% (12) .9621 [± .03] .8758 [± .09]
0.36% (1) 0.53% ± 0.95% (2) .9915 [± .01] .9915 [± .01]
Meier
Meier
12 Instructions for Use English
Page 15

Notes:
1. Cumulative probability of freedom from event estimate at the end of the interval (Pc) is based on the
Kaplan-Meier method.
2. The 95% confidence interval bound for the cumulative freedom rate at the end of the
interval = 1.96 X SE, where SE is the standard error estimate of the cumulative probability freedom
from a heart valve related or an unexplained event estimate calculated using Greenwood’s formula.
3. The actuarial hazard rate estimates are calculated at the midpoint of each interval.
4. The 95% confidence interval bound for the hazard rate at the midpoint of each interval = 1.96 X SE,
where SE is the standard error estimate of the hazard rate estimate at the midpoint of the interval.
5.2. Potential Adverse Events
Adverse events potentially associated with the use of prosthetic aortic heart valves include, but are not
limited to:
■
angina
■
cardiac arrhythmia
■
endocarditis
■
hemolysis
■
hemolytic anemia
■
hemorrhage, anticoagulation-related
■
myocardial infarction
■
leaflet entrapment (impingement)
■
nonstructural dysfunction
■
pannus
■
perivalvular leak
■
transvalvular regurgitation
■
structural dysfunction
■
thrombosis
■
stroke
■
thromboembolism
It is possible that these complications could lead to:
■
reoperation
■
explantation
■
permanent disability
■
heart failure
■
death
6. Instructions for Use
6.1. Physician Training
No special training is required to implant the Medtronic Open Pivot heart valve. The techniques for
implanting this valve are similar to those used for any bileaflet mechanical valve.
6.2. Handling and Preparation Instructions
Proper prosthesis size selection is an important part of the heart valve replacement. The size of the
Medtronic Open Pivot heart valve is determined using the Medtronic Open Pivot™ heart valve sizers.
Caution: The valve accessories (sizer and handle) should not be used unless cleaned and sterilized as
per the recommended instructions in the Medtronic Open Pivot™ heart valve accessories Instructions for
Use.
The valve, valve holder, handle rotator, and leaflet actuator are supplied sterile in the same package. The
contents of the package should be handled in an aseptic manner to prevent contamination.
1. Remove the package from the white shipping box along with the Patient Registration Form. Verify that
the valve size, type, and serial number as marked on the tray label, match the information on the white
shipping box label. If any differences are noted, do not use the valve for implantation.
2. Examine the lid to verify that the prosthesis container has not been damaged or previously opened. If
resterilization of accessories is necessary prior to implantation, follow the recommended cleaning and
sterilization guidelines.
3. Hold the outer tray and gently peel the lid back using sterile techniques until the lid has been
completely removed from the tray.
4. Record the serial number of the valve in the patient’s record using the stickers provided on the Patient
Registration Form.
5. Remove the inner tray from the outer tray by grasping the inner tray by its lip.
6. Holding the bottom of the inner tray, grasp the inner tray lid tab and peel back until the lid has been
completely removed.
7. Using sterile technique, carefully remove the retainer insert and discard.
8. Verify that the valve holder is secured to the handle rotator before removing the valve holder and
handle rotator assembly from the inner tray. There should be no visible screw threads at the
connection between the colored handle rotator and the valve holder when secured properly.
9. Remove the blue leaflet actuator from the tray and set aside for later use.
Instructions for Use English 13
Page 16

10. Grasp the handle rotator in the center and gently lift the valve, valve holder, and handle rotator out of
the inner tray. Do not remove the valve from the valve holder unless instructed by the implanting
surgeon.
6.3. Device Implantation
Proper prosthesis size selection is an important part of heart valve replacement. Care must be exercised
to avoid using too large or too small of a prosthesis. Size of the prosthesis is determined using the
Medtronic Open Pivot heart valve sizers (Figure 7 and Figure 8).
Inspection of the valve should be performed immediately before implantation.
Warning: Do not touch the valve (leaflets/orifice) except with the leaflet actuator supplied with the valve.
Touching the valve with gloved fingers or any surgical instrument may cause damage to the valve surface
that cannot be seen with the unaided eye. Such damage may serve as a nidus for thrombus formation.
The damage may also lead to leaflet escape or accelerated structural deterioration of the valve.
Caution: Do not use the valve if it has been dropped, damaged, or mishandled in any way. Should the
valve be damaged during implantation or removal from the package, do not use it for implantation.
Use only accessories provided by Medtronic to handle the valve. All accessories should be inspected prior
to use. Do not use any accessory if the surface appears damaged or cracked.
6.4. Suturing Techniques
Ensure proper orientation of the valve. The blood flow in either the aortic or mitral position is always into
the straight edge of the leaflets. The markers on the sewing cuff (3 on the aortic cuff and 4 on the mitral
cuff) are useful in proper placement of the sutures and orientation of the valve. The aortic markers are
120° apart and may be placed at the approximate location of the commissures of the excised valve. The
mitral cuff has 4 markers 90° apart, that can be placed at each commissure, in the center of the anterior
leaflet, and in the center of the posterior leaflet. If necessary, further orientation of the valve can be done
with the handle rotator once the valve is seated in the annulus.
Medical practice has evolved numerous acceptable suture methods. The technique used should be based
on the patient’s anatomy, procedure requirements, and surgeon preference. Pledgets may be used at the
discretion of the surgeon for support of the annulus if necessary. Care should be taken to assure that
pledgets or tissue do not interfere with leaflet motion. Everting stitches with pledgets may be useful in
avoiding situations where tissue might impede leaflet motion.
After sutures are placed in the valve cuff and the valve is seated in the tissue annulus, the valve is
released from the valve holder by sliding a scalpel down the holder slot to cut the green suture and
release the valve holder. The green suture and valve holder remain attached as the holder and handle
rotator are carefully withdrawn. The suture knots can be tied at this point.
Caution: Long suture ends must be avoided since these can interfere with leaflet motion.
6.4.1. Suturing Technique for Standard Cuffs
Surgeons performing mitral and aortic valve replacements frequently use interrupted everting mattress
sutures. Alternatives include interrupted figure of 8 sutures or interrupted simple mattress sutures.
6.4.2. Suturing Technique for AP Series Heart Valves
The Medtronic Open Pivot AP series heart valves have a supra-annular configuration. Different suture
techniques, such as horizontal mattress or simple stitch, may be necessary. Deep needle placement will
contact the strengthening band inside the sewing cuff, impeding the smooth placement of sutures. Sutures
should be placed in the outer 1/3 of the cuff.
6.5. Leaflet Motion Assessment and Valve Rotation
Leaflet motion is assessed using the blue leaflet actuator.
To test leaflet motion, use the blue leaflet actuator. If the leaflets do not move freely, the valve orifice may
be rotated either clockwise or counterclockwise to a more optimal position. Before implanting the device,
check freedom of rotation by holding the valve cuff and gently turning the valve holder and handle rotator.
Rotate the orifice only after the suture knots have been tied to secure the cuff to the tissue annulus. Use
only the appropriate rotators provided to rotate the valve. The handle rotator seats properly into the valve
orifice when the radial ridge on the handle side of the rotator head (Figure 5 and Figure 6) is aligned with
the straight edge of the leaflets. The rotator head is designed to contact the flat surfaces of the orifice near
the pivot areas. After rotation of the valve orifice, verify leaflet motion with the blue actuator.
Caution: The valve orifice and leaflets may be rotated in situ using only the appropriate handle rotator
provided. Do not use any other instruments for valve rotation.
Caution: If mitral valve apparatus preservation techniques are used, it is important to check leaflet motion
following valve implantation.
6.6. Sterilization Information
The Medtronic Open Pivot heart valve and package contents have been steam sterilized within the trays.
The valve is ready for use when received. Do not use the valve if the Use-By Date has passed or if, upon
removal from the outer box, the valve container is damaged or the sterility barrier is broken. Call a
Medtronic local representative and arrange to return the valve and receive a replacement.
The sizers and bendable handles are supplied nonsterile and must be cleaned and sterilized prior to use.
Refer to the accessories Instructions for Use for cleaning and sterilization instructions.
Warning: If, during surgery, the valve is removed from its container but not used, it must not be
repackaged or resterilized.
Caution: Extreme care must be exercised when handling the valve holder assembly to avoid structural
damage to the valve. Inspect each accessory before each use. Cracked or damaged accessories must not
be used.
14 Instructions for Use English
Page 17

7. Clinical Studies
Duration of Follow-up (Years)
Number of Patients
Total Patients Aortic Patients Mitral Patients
1000
800
600
400
200
0
0 1 2 3 4 5
This section contains the results of 3 separate sets of clinical data.
1. Prospective investigational studies, 1994 to 1999.
2. Additional clinical data on sizes 16/19 mm aortic valves and 24/27 mm mitral valves.
3. Additional clinical data on size 22/25 mm mitral valves.
7.1. Prospective Investigational Studies, 1994 to 1999
Patients requiring isolated aortic or mitral heart valve replacement were enrolled from 1994 to 1999 at
20 centers (17 domestic and 3 international) in a multicenter, international, prospective, nonrandomized
study. NYHA classification and blood data were obtained preoperatively, intraoperatively, and
postoperatively at 3 to 6 months, at 1 year, and annually thereafter. Hemodynamic data was obtained at
discharge and at 1 year. Patients were monitored throughout the postoperative period for possible adverse
events. The antiplatelet and anticoagulant agents used were reported. Target INRs were as follows: mitral
position, 2.5 to 3.5, and aortic position, 2.0 to 3.0 (when in normal sinus rhythm).
The cohort included 965 patients (580 men, 385 women), ages from 2 to 88 years (mean of 60.7). The
cumulative follow-up was 1323 patient-years with a mean follow-up of 1.4 years (SD = 1.2 years,
range = 0 to 5.0 years). Table 9 shows patient characteristics by age, gender, and etiology of valve
disease.
Table 9. Patient Characteristics
All patients implanted, N=965, 1323 patient-years
Description of Patients Aortic Valve n=685 (70.98%) Mitral Valve n=280 (29.02%)
Age at implant in years
0–9 5 (0.7%) 6 (2.1%)
10–19 4 (0.6%) 0 (0.0%)
20–29 11 (1.6%) 2 (0.7%)
30–39 33 (4.8%) 14 (5.0%)
40–49 72 (10.5%) 31 (11.1%)
50–59 142 (20.7%) 52 (18.6%)
60–69 238 (34.7%) 107 (38.2%)
70–79 155 (22.6%) 62 (22.1%)
80 & over 25 (3.6%) 6 (2.1%)
Gender
Male 460 (67.2%) 120 (42.9%)
Female 225 (32.8%) 160 (57.1%)
Etiology of valve disease
Stenosis 541 (79.0%) 118 (42.1%)
Insufficiency 330 (48.2%) 210 (75.0%)
Mixed 205 (29.9%) 63 (22.5%)
Other 12 (1.8%) 12 (4.3%)
Figure 10 shows the number of patients implanted versus duration of follow-up with a breakdown by valve
location (aortic and mitral). Table 10 shows the number of patients implanted for whom hemodynamic data
was collected. Table 11 shows the number of patient-years by implant size and
location. Table 12, Table 13, Table 14,Table 15, and Table 16 show effectiveness outcomes.
Figure 10. Number of patients by implant location over time
Instructions for Use English 15
Page 18

Year 0 1 2 3 4 5
All patients implanted, N=965
Total Patients 965 624 331 135 92 2
Aortic
685 436 231 93 58 2
Patients
Mitral
280 188 100 42 34 0
Patients
Table 10. Number of Patients Implanted and Maximum Number of Patients with Hemodynamic Data at
>6 Months Follow-up
By implant location and valve size, N/n, n=575, N=965
Implant Location Valve size (mm)
16/19 18/21 20/23 22/25 24/27 26/29 28/31/33 Total
Aortic
Mitral 0/0 3/1 5/4 3/2
2
100/61 202/108 206/113 111/70 39/32 4/4 685/405
23/17
23/18
2
71/39 175/106 280/170
Total 23/17 103/62 207/112 209/115 134/88 110/71 179/110 965/575
Notes:
1. n = number of patients with hemodynamic data; N = number of patients implanted
2. Includes data from 8 patients for the 16/19 mm aortic valve and 10 patients for the 24/27 mm mitral
valve not included in the analysis of the original patient cohort.
Table 11. Number of Patient-Years by Implant Location and Valve Size
By implant location and valve size, all patients implanted, N=965
Implant
Loca-
tion
16/19 18/21 20/23 22/25 24/27 26/29 28/31 33 Total
Valve size (mm)
Aortic 15.7 159.3 275.0 255.6 150.1 61.1 7.4 0.0 924.2
Mitral 0.0 2.2 4.8 2.4 17.6 99.8 97.7 174.0 398.5
Total 15.7 161.5 279.8 258.0 167.7 160.9 105.1 174.0 1322.7
Table 12. Effectiveness Outcomes, Functional New York Heart (NYHA) Classification
NYHA
Class
Pre-op 1 Year
(11–14 Months)
2 Year
(23–26 Months)
3 Year
(35–38 Months)
4 Year
(47–50 Months)
n/N % n/N % n/N % n/N % n/N %
Aortic Valve Replacement, N=685
I 4/685 0.6% 399/431 92.5% 205/231 88.7% 85/94 90.4% 53/58 91.4%
II 267/685 38.9% 25/431 5.8% 20/231 8.7% 7/94 7.4% 5/58 8.6%
III 340/685 49.6% 1/431 0.2% 1/231 0.4% 0/94 0% 0/58 0%
IV 68/685 9.9% 1/431 0.2% 1/231 0.4% 0/94 0% 0/58 0%
MISSING 6/685 0.9% 5/431 1.2% 4/231 1.7% 2/94 2.1% 0/58 0%
Mitral Valve Replacement, N=280
I 0/280 0% 158/182 86.8% 78/100 78.0% 33/42 78.5% 28/34 82.4%
II 74/280 26.4% 19/182 10.4% 19/100 19.0% 8/42 19.0% 5/34 14.7%
III 155/280 55.4% 3/182 1.6% 2/100 2.0% 1/42 2.4% 1/34 2.9%
IV 45/280 16.1% 0/182 0% 0/100 0% 0/42 0% 0/34 0%
MISSING 6/280 2.1% 2/182 1.1% 1/100 1.0% 0/42 0% 0/34 0%
Note: N = all values reported; n = number in subgroup
Table 13. Late Effectiveness Outcomes — Hemodynamics, Valvular Regurgitation (Aortic Valves)
Size
Valve
(mm)
No Regurgita-
tion
% patients, n/N
Trivial Regurgi-
tation
% patients, n/N
Mild Regurgi-
tation
% patients, n/N
Moderate
Regurgitation
% patients, n/N
Severe Regur-
gitation
% patients, n/N
Aortic Valves, N=685
16/19 mm 33.3%, 3/9 66.7%, 6/9 0%, 0/9 0%, 0/9 0%, 0/9
18/21 mm 39.6%, 21/53 58.5%, 31/53 1.9%, 1/53 0%, 0/53 0%, 0/53
20/23 mm 33.0%, 31/94 67.0%, 63/94 0%, 0/94 0%, 0/94 0%, 0/94
22/25 mm 28.3%, 28/99 71.7%, 71/99 0%, 0/99 0%, 0/99 0%, 0/99
24/27 mm 42.6%, 26/61 55.7%, 34/61 1.6%, 1/61 0%, 0/61 0%, 0/61
26/29 mm 27.3%, 6/22 72.7%, 16/22 0%, 0/22 0%, 0/22 0%, 0/22
28/31 mm 100%, 4/4 0%, 0/4 0%, 0/4 0%, 0/4 0%, 0/4
Aortic Totals 34.8%, 119/342 64.6%, 221/342 0.6%, 2/342 0%, 0/342 0%, 0/342
Notes:
1. Late is defined as 11–14 months postimplantation
2. N = all values reported; n = number in subgroup
16 Instructions for Use English
Page 19

Table 14. Late Effectiveness Outcomes — Hemodynamics, Valvular Regurgitation (Mitral Valves)
Valve Size
(mm)
No Regurgita-
tion
% patients, n/N
Trivial Regurgi-
tation
% patients, n/N
Mild Regurgi-
tation
% patients, n/N
Moderate
Regurgitation
% patients, n/N
Severe Regur-
gitation
% patients, n/N
Mitral Valves, N=280
16/19 mm 0%, 0/0 0%, 0/0 0%, 0/0 0%, 0/0 0%, 0/0
18/21 mm 0%, 0/0 0%, 0/0 0%, 0/0 0%, 0/0 0%, 0/0
20/23 mm 66.7%, 2/3 33.3%, 1/3 0%, 0/3 0%, 0/3 0%, 0/3
22/25 mm 50.0%, 1/2 50.0%, 1/2 0%, 0/2 0%, 0/2 0%, 0/2
24/27 mm 83.3%, 5/6 16.7%, 1/6 0%, 0/6 0%, 0/6 0%, 0/6
26/29 mm 54.5%, 18/33 42.4%, 14/33 3.0%, 1/33 0%, 0/33 0%, 0/33
28/31/33 mm 61.2%, 52/85 37.6%, 32/85 1.2%, 1/85 0%, 0/85 0%, 0/85
Mitral Totals 60.5%, 78/129 38.0%, 49/129 1.6%, 2/129 0%, 0/129 0%, 0/129
Notes:
1. Late is defined as 11–14 months postimplantation
2. N = all values reported; n = number in subgroup
Table 15. Effectiveness Outcomes — Hemodynamics, Mean Pressure Gradient and Effective Orifice Area
(Aortic Valve Replacement)
Endpoint
n/N, mean ± SD (min, max)
Early
Late
n/N, mean ± SD (min, max)
Aortic Valve Replacement, N=685
Mean Gradient (mm Hg)
16/19 mm 15/23, 25.8 ± 5.1 (10.5, 49.0) 9/23, 20.2 ± 2.8 (15.0, 26.6)
18/21 mm 87/100, 18.7 ± 1.7 (2.4, 42.0) 61/100, 18.0 ± 1.6 (7.0, 36.0)
20/23 mm 181/202, 14.3 ± 0.8 (2.6, 37.0) 107/202, 13.1 ± 0.8 (5.1, 30.1)
22/25 mm 187/206, 12.8 ± 0.8 (2.6, 29.5) 112/206, 11.1 ± 0.8 (3.2, 26.0)
24/27 mm 102/111, 10.0 ± 0.7 (1.8, 22.0) 70/111, 8.0 ± 0.8 (1.3, 16.7)
26/29 mm 38/39, 9.2 ± 1.1 (3.5, 18.0) 32/39, 7.8 ± 1.1 (2.0, 13.0)
28/31 mm 3/4, 3.0 ± 0.7 (2.6, 3.8) 4/4, 5.1 ± 3.3 (1.4, 9.3)
Effective Orifice Area (cm2)
16/19 mm 11/23, 1.1 ± 0.2 (0.7, 1.8) 8/23, 1.2 ± 0.3 (0.8, 1.9)
18/21 mm 81/100, 1.5 ± 0.1 (0.8, 3.7) 60/100, 1.5 ± 0.1 (0.7, 3.4)
20/23 mm 165/202, 1.7 ± 0.1 (0.8, 6.6) 102/202, 1.7 ± 0.1 (0.9, 3.7)
22/25 mm 173/206, 2.0 ± 0.1 (1.1, 4.0) 103/206, 2.1 ± 0.1 (1.0, 4.9)
24/27 mm 97/111, 2.4 ± 0.2 (1.1, 4.8) 65/111, 2.5 ± 0.2 (1.5, 4.9)
26/29 mm 34/39, 3.0 ± 0.3 (1.4, 4.7) 28/39, 3.1 ± 0.4 (1.4, 5.4)
28/31 mm 3/4, 2.8 ± 0.8 (2.0, 3.4) 3/4, 3.1 ± 1.6 (1.6, 4.5)
Notes:
1. Early postoperative evaluation conducted at 30 days postimplantation or hospital discharge.
2. Late postoperative evaluation = 11–14 months postimplantation
3. N = all values reported; n = number in subgroup
4. Echo confirmed valve function.
Table 16. Effectiveness Outcomes — Hemodynamics, Mean Pressure Gradient and Effective Orifice Area
(Mitral Valve Replacement)
Endpoint
Early
n/N, mean ± SD (min, max)
n/N, mean ± SD (min, max)
Late
Mitral Valve Replacement, N=280
Mean Gradient (mm Hg)
16/19 mm 0 0
18/21 mm 0/3 1/3,6.0 (6.0, 6.0)
20/23 mm 3/5, 5.3 ± 3.6 (3, 9) 4/5, 4.6 ± 0.9 (4.0, 6.0)
22/25 mm 1/3, 10.0 (10, 10) 2/3, 5.4 ± 4.7 (3.0, 7.8)
24/27 mm 15/23, 4.4 ± 0.9 (2.3, 7.9) 8/23, 4.5 ± 0.9 (2.4, 6.2)
26/29 mm 66/71, 3.7 ± 0.4 (1.5, 10.0) 39/71, 3.7 ± 0.7 (1.3, 9.9)
28/31/33 mm 154/175, 3.5 ± 0.3 (0.7, 9.0) 106/175, 3.1 ± 0.2 (0.3, 7.3)
Effective Orifice Area (cm2)
16/19 mm 0 0
18/21 mm 0/3
1/3
4
20/23 mm 3/5, 2.9 ± 0.6 (2.3, 3.3) 3/5, 1.6 ± 0.3 (1.3, 1.8)
22/25 mm 1/3, 1.6 (1.6, 1.6) 2/3, 1.8 ± 0.5 (1.5, 2.0)
24/27 mm 15/23, 3.3 ± 0.5 (1.8, 5.2) 8/23, 2.9 ± 0.9 (1.6, 5.7)
Instructions for Use English 17
Page 20

Endpoint Early
n/N, mean ± SD (min, max)
Mitral Valve Replacement, N=280
26/29 mm 63/71, 3.3 ± 0.2 (1.6, 5.0) 38/71, 2.8 ± 0.3 (1.0, 4.6)
28/31/33 mm 140/175, 3.0 ± 0.2 (1.3, 7.6) 95/175, 2.9 ± 0.2 (1.5, 7.4)
Notes:
1. Early postoperative evaluation conducted at 30 days postimplantation or hospital discharge.
2. Late postoperative evaluation = 11–14 months postimplantation
3. N = all values reported; n = number in subgroup
4. Echo confirmed valve function.
7.2. Additional Clinical Data on Sizes 16/19 mm Aortic Valve and 24/27 mm Mitral Valve
Medtronic obtained additional follow-up clinical data for patients receiving the 16/19 mm aortic and the
24/27 mm mitral valves. The following are the clinical results of the additional patient data. This
information is presented both independently and in combination with the clinical data obtained in the
original patient cohort.
The only adverse events reported are 1 minor and 1 major late anticoagulant-related bleeding events for
the 16/19 mm aortic valve. No adverse events or deaths were reported for the 24/27 mm mitral valve. No
deaths were reported for the 16/19 mm aortic valve.
Table 17. Observed Adverse Events — Original Patient Cohort and Additional Data on 16/19 mm Aortic
Aortic Valve Replacement, All patients implanted: N=685, Cumulative Follow-up=887.0 patient years
Anticoagulant-Related Hem-
orrhage (All)
Anticoagulant-Related Hem-
orrhage (Major)
Table 18. Effectiveness Outcomes — Functional New York Heart (NYHA) Classification Additional Data
NYHA
Class
I 0/10 0% 9/10 90% 2/3 66.7% 0/0 0% 0/0 0%
II 3/10 30% 1/10 10% 0/3 0% 0/0 0% 0/0 0%
III 6/10 60% 0/10 0% 1/3 33.3% 0/0 0% 0/0 0%
IV 1/10 10% 0/10 0% 0/3 0% 0/0 0% 0/0 0%
MISSING 0/10 0% 0/10 0% 0/3 0% 0/0 0% 0/0 0%
I 0/10 0% 7/10 70% 2/3 66.7% 0/0 0% 0/0 0%
II 2/10 20% 2/10 20% 0/3 0% 0/0 0% 0/0 0%
III 5/10 50% 0/10 0% 1/3 33.3% 0/0 0% 0/0 0%
IV 3/10 30% 0/10 0% 0/3 0% 0/0 0% 0/0 0%
MISSING 0/10 0% 1/10 10% 0/3 0% 0/0 0% 0/0 0%
Note: N = all values reported; n = number in subgroup
Table 19. Effectiveness Outcomes — Functional New York Heart (NYHA) Classification Original Patient
NYHA
Class
I 4/685 0.6% 408/441 92.5% 207/234 88.5% 85/94 90.4% 53/58 91.4%
II 267/685 38.9% 26/441 5.9% 20/234 8.5% 7/94 7.4% 5/58 8.6%
III 340/685 49.6% 1/441 0.2% 2/234 0.9% 0/94 0% 0/58 0%
IV 68/685 9.9% 1/441 0.2% 1/234 0.4% 0/94 0% 0/58 0%
MISSING 6/685 0.9% 5/441 1.1% 4/234 1.7% 2/94 2.1% 0/58 0%
I 0/280 0% 165/192 85.9% 80/103 77.7% 33/42 78.6% 28/34 82.4%
II 74/280 26.4% 21/192 10.9% 19/103 18.4% 8/42 19.0% 5/34 14.7%
III 155/280 55.4% 3/192 1.6% 3/103 2.9% 1/42 2.4% 1/34 2.9%
IV 45/280 16.1% 0/192 0% 0/103 0% 0/42 0% 0/34 0%
MISSING 6/280 2.1% 2/192 1.6% 1/103 1.0% 0/42 0% 0/34 0%
Pre-op 1 Year
n/N % n/N % n/N % n/N % n/N %
16/19 mm Aortic Valve Replacement, N=10
Cohort and Additional Data on 16/19 mm and 24/27 mm Mitral Valves
Pre-op 1 Year
n/N % n/N % n/N % n/N % n/N %
Mean Gradient (mm Hg)
and 24/27 Mitral Valves
Early Events
% of pts (N) %/pt-yrs (N) 1 Year [95% CI] 5 Years [95% CI]
4.60% (32) 2.01% ± 0.88% (19) .9785 [± .01] .9320 [± .02]
3.16% (22) 1.27% ± 0.72% (12) .9881 [± .01] .9450 [± .02]
on 16/19 mm and 24/27 mm Mitral Valves
(11–14 Months)
24/27 mm Mitral Valve Replacement, N=10
(11–14 Months)
Aortic Valve Replacement, N=685
Mitral Valve Replacement, N=280
Late Events Actuarial Freedom by Kaplan-Meier
2 Year
(23–26 Months)
2 Year
(23–26 Months)
n/N, mean ± SD (min, max)
(35–38 Months)
(35–38 Months)
3 Year
3 Year
Late
4 Year
(47–50 Months)
4 Year
(47–50 Months)
18 Instructions for Use English
Page 21

Note: N = all values reported; n = number in subgroup
Table 20. Late Effectiveness Outcomes — Hemodynamics, Valvular Regurgitation; Additional Data on
Valve Size
(mm)
16/19 mm 20%, 2/10 80%, 8/10 0%, 0/10 0%, 0/10 0%, 0/10
24/27 mm 44.4%, 4/9 55.5%, 5/9 0%, 0/9 0%, 0/9 0%, 0/9
Notes:
1. Late is defined as 11–14 months postimplantation
2. N = all values reported; n = number in subgroup
Table 21. Late Effectiveness Outcomes — Hemodynamics, Valvular Regurgitation; Original Patient Cohort
Valve Size
(mm)
16/19 mm 26.3%, 5/19 73.7%, 14/19 0%, 0/19 0%, 0/19 0%, 0/19
24/27 mm 60%, 9/15 40%, 6/15 0%, 0/15 0%, 0/15 0%, 0/15
Notes:
1. Late is defined as 11–14 months postimplantation
2. N = all values reported; n = number in subgroup
Table 22. Effectiveness Outcomes — Hemodynamics, Mean Pressure Gradient and Effective Orifice
Endpoint
Mean Gradient (mm Hg)
16/19 mm 9/10, 24.3 ± 11.2 (10.5, 49.0) 9/10, 26.8 ± 5.5 (18.8, 34.0)
Effective Orifice Area (cm2)
16/19 mm 7/10, 1.1 ± 0.3 (0.8, 1.5) 9/10, 1.1 ± 0.3 (0.8, 1.9)
Mean Gradient (mm Hg)
24/27 mm 8/10, 4.3 ± 1.8 (2.4, 7.9) 9/10, 3.6 ± 0.9 (2.0, 5.2)
Effective Orifice Area (cm2)
24/27 mm 9/10, 3.3 ± 0.8 (1.8, 4.7) 10/10, 3.4 ± 0.7 (2.5, 4.3)
Notes:
1. Early postoperative evaluation conducted at 30 days postimplantation or hospital discharge.
2. Late postoperative evaluation = 11 to 14 months postimplantation
3. N = all values reported; n = number in subgroup
Table 23. Effectiveness Outcomes — Hemodynamics, Mean Pressure Gradient and Effective Orifice
Area — Original Patient Cohort and Additional Data on 16/19 mm Aortic and 24/27 mm Mitral Valves
Endpoint
Mean Gradient (mm Hg)
16/19 mm 19/23, 25.2 ± 9.6 (10.5, 49.0) 17/23, 23.3 ± 6.2 (13.0, 34.0)
Effective Orifice Area (cm2)
16/19 mm 19/23, 1.1 ± 0.3 (0.7, 1.8) 17/23, 1.1 ± 0.4 (0.5, 1.9)
Mean Gradient (mm Hg)
24/27 mm 18/23, 4.4 ± 1.7 (2.3, 7.9) 17/23, 4.0 ± 1.2 (2.0,6.2)
Effective Orifice Area (cm2)
24/27 mm 9/23, 3.4 ± 0.8 (1.8, 5.2) 18/23, 3.2 ± 1.0 (1.6, 5.7)
Notes:
1. Early postoperative evaluation conducted at 30-days postimplantation or hospital discharge.
2. Late postoperative evaluation = 11–14 months postimplantation
No Regurgita-
% patients, n/N
and Additional Data on 16/19 mm Aortic and 24/27 mm Mitral Valves
No Regurgita-
% patients, n/N
Area — Additional Data on 16/19 mm Aortic and 24/27 mm Mitral Valves
16/19 mm Aortic and 24/27 mm Mitral Valves
tion
tion
n/N, mean ± SD (min, max) n/N, mean ± SD (min, max)
Trivial Regurgi-
tation
% patients, n/N
Aortic Valves, N=10
Mitral Valves, N=10
Trivial Regurgi-
tation
% patients, n/N
Aortic Valves, N=695
Mitral Valves, N=290
Early Late
n/N, mean ± SD (min, max) n/N, mean ± SD (min, max)
Aortic Valve Replacement, N=10
Mitral Valve Replacement, N=10
Early Late
Aortic Valve Replacement, N=23
Mitral Valve Replacement, N=23
Mild Regurgita-
tion
% patients, n/N
Mild Regurgita-
tion
% patients, n/N
Moderate
Regurgitation
% patients, n/N
Moderate
Regurgitation
% patients, n/N
Severe Regur-
% patients, n/N
Severe Regur-
% patients, n/N
gitation
gitation
Instructions for Use English 19
Page 22

3. N = all values reported; n = number in subgroup
7.3. Additional Clinical Data on Size 22/25 mm Mitral Valve
Additional data were obtained through September 2005 for patients implanted with the Medtronic Open
Pivot 22/25 mm mitral heart valve. A total of 22 patients with a 22/25 mm mitral valve from 7 centers were
added to the previously approved cohort.
The 22/25 mm mitral heart valve patient cohort includes 22 patients. Cumulative follow-up was 106 pt-yrs
with mean follow-up of 4.8 ± 3.3 yrs (0.003 – 9.8 yrs). Table 24 presents the patient demographic
data. Table 25 represents the change in NYHA classification. Table 26 presents adverse event
rates. Table 27, Table 28, and Table 29 present the hemodynamic results.
Results:
Table 24. Preoperative Patient Demographics: 22/25 mm Mitral (N=22): 106 Pt Yrs
Age at Implant (Years) 0-9 0 0.0%
Gender Female 18 81.8%
Etiology Stenosis 5 22.7%
Table 25. Effectiveness Outcomes: New York Heart Association (NYHA) Classification
mm Mitral Early Events
22/25
Death (all causes) 4.55% (1) 0.94% ± 0.10% (1) 0.9545 [±.09] 0.8909 [±.14]
Death (valve-rela-
ted/unexplained)
Anticoagulant-Rela-
ted Hemorrhage
(All)
Anticoagulant-Related Hemorrhage
(Major)
Thromboembolism
(All)
Permanent Neurological Events
Transient Neurological Events
Valve Thrombosis 0.00% (0) 0.95% ± 0.19% (1) 1.000 [±.00] 0.8889 [±.21]
Variable
mm Mitral Pre-Op 1 Year
22/25
CLASS I (N)
% of Total
CLASS II (N)
% of Total
CLASS III (N)
% of Total
CLASS IV (N)
% of Total
Undetermined (N)
% of Total
Missing (N)
% of Total
Table 26. Adverse Event Rates
% of pts (N)
0.00% (0) 0.00% ± 0.00% (0) 1.000 [±.00] 1.000 [±.00]
4.55% (1) 0.00% ± 0.00% (0) 0.9500 [±.10] 0.9500 [±.10]
4.55% (1) 0.00% ± 0.00% (0) 0.9500 [±.10] 0.9500 [±.10]
4.55% (1) 0.95% ± 0.66% (1) 0.9524 [±.10] 0.8964 [±.14]
0.00% (0) 0.00% ± 0.00% (0) 1.000 [±.00] 1.000 [±.00]
4.55% (1) 0.95% ± 0.58% (1) 0.9524 [±.10] 0.8964 [±.14]
Category n % (n/N)
10-19 0 0.0%
20-29 1 4.5%
30-39 7 31.8%
40-49 1 4.5%
50-59 2 9.1%
60-69 6 27.3%
70-79 4 18.2%
80 & over 0 0.0%
Not Reported 1 4.5%
Male 3 13.6%
Not Reported 1 4.5%
Insufficiency 10 45.5%
Mixed 6 27.3%
Other 1 4.5%
Not Reported 0 0.0%
0
0%
2
10%
13
65%
3
15%
1
5%
1
5%
Late Events
%/pt-yrs (N)
Actuarial Freedom by Kaplan-Meier
1 Year [95% CI] 5 Years [95% CI]
8
40%
6
30%
1
5%
0
0%
0
0%
5
25%
20 Instructions for Use English
Page 23

22/25 mm Mitral Early Events
Perivalvular Leak
(All)
Perivalvular Leak
(Major)
Endocarditis 0.00% (0) 0.00% ± 0.00% (0) 1.000 [±.00] 1.000 [±.00]
Hemolysis 0.00% (0) 0.00% ± 0.00% (0) 1.000 [±.00] 1.000 [±.00]
Structural Dysfunc-
tion
Nonstructural Dys-
function
Reoperation 0.00% (0) 0.00% ± 0.00% (0) 1.000 [±.01] 1.000 [±.01]
Explant 0.00% (0) 0.00% ± 0.00% (0) 1.000 [±.00] 1.000 [±.00]
Notes:
1. N=22, Cumulative F/U = 106 pt yrs
2. Cumulative probability of freedom from event estimate at the end of the interval (Pc) is based on the
Kaplan-Meier method.
3. The 95% confidence interval bound for the cumulative freedom rate at the end of the interval =
1.96 X SE, where SE is the standard error estimate of the cumulative probability.
Discharge Echo
N 3
Mean (SD) 6.2 (3.3)
Late Echo (>11 mos.) 22/25 mm Mitral
N 19
Mean (SD) 4.7 (1.7)
Mean Follow-up: Mitral: 4.8 ± 3.3 years; Cumulative Follow-up: 106 pt yrs
Discharge
Late Echo (>11 mos.) 22/25 mm Mitral
Mean Follow-up: Mitral: 4.8 ± 3.3 years; Cumulative Follow-up: 106 pt yrs
Echo 22/25 mm Mitral
N 2
Mean (SD) 2.4 (1.1)
N 15
Mean (SD) 2.3 (0.7)
% of pts (N)
0.00% (0) 0.00% ± 0.00% (0) 1.000 [±.00] 1.000 [±.00]
0.00% (0) 0.00% ± 0.00% (0) 1.000 [±.00] 1.000 [±.01]
0.00% (0) 0.00% ± 0.00% (0) 1.000 [±.00] 1.000 [±.00]
0.00% (0) 0.00% ± 0.00% (0) 1.000 [±.00] 1.000 [±.00]
Table 27. Hemodynamic Data, Mean Gradient
Table 28. Hemodynamic Data, Effective Orifice Area
Table 29. Hemodynamic Data, Valvular Regurgitation
mm Mitral Early Late
22/25
No Regurgitation 5%
Trivial Regurgitation 5%
Mild Regurgitation 0.0%
Moderate Regurgitation 0.0%
Severe Regurgitation 0.0%
Missing 90%
Late Events
%/pt-yrs (N)
22/25 mm Mitral
Actuarial Freedom by Kaplan-Meier
1 Year [95% CI] 5 Years [95% CI]
25.0%
1/20
1/20
0/20
0/20
0/20
18/20
5/20
25.0%
5/20
0.0%
0/20
0.0%
0/20
0.0%
0/20
50.0%
10/20
8. Postoperative Information
8.1. MRI Safety Information
Nonclinical testing and modeling has demonstrated that the Medtronic Open Pivot heart valve
(Models 500, 501, and 505) is MR conditional. A patient with this device can be safely scanned in an MR
system meeting the following conditions:
■
static magnetic field of 1.5 T and 3 T
■
maximum spatial gradient magnetic field of 2500 gauss/cm (25 T/m)
■
maximum MR system reported, whole body averaged specific absorption rate (SAR) of ≤ 2.0 W/kg
(Normal Operating Mode)
Based on nonclinical testing and modeling, under the scan conditions defined above, the Medtronic Open
Pivot heart valve is expected to produce a maximum in vivo temperature rise of less than 1.8°C after
15 minutes of continuous scanning.
Instructions for Use English 21
Page 24

Models 500 and 501:
In nonclinical testing, the image artifact caused by the device extends no greater than 7 mm from the
Medtronic Open Pivot heart valve for a spin echo pulse sequence and 15 mm for a gradient echo pulse
sequence when imaged with a 3.0 T MRI system.
Model 505:
In nonclinical testing, the image artifact caused by the device extends no greater than 13 mm from the
Medtronic Open Pivot heart valve for a spin echo pulse sequence and 30 mm for a gradient echo pulse
sequence when imaged with a 3.0 T MRI system.
9. Individualization of Treatment
Adequate anticoagulant and/or antiplatelet therapy should be administered. Selection of an anticoagulant
and/or antiplatelet regimen is based on the particular needs of the patient and the clinical situation.
9.1. Specific Patient Populations
The safety and effectiveness of the Medtronic Open Pivot heart valve has not been established for the
following specific populations because it has not been studied in these populations:
■
patients who are pregnant
■
nursing mothers
■
patients with chronic endocarditis
■
patients requiring pulmonic or tricuspid replacement
There was limited use of the valve in patients requiring double or multiple valve replacement.
10. Patient Counseling Information
■
Patients with prosthetic valves who undergo dental or potentially bacteremic procedures should
receive antibiotic prophylaxis.
■
All mechanical prosthetic heart valves produce sound as a function of their operation. Patients should
be advised of this prior to implantation.
■
Patients require anticoagulation and/or antiplatelet therapy.
■
Patients should be encouraged to carry the Implanted Device Identification Card, provided by
Medtronic, with them at all times.
11. How Supplied
11.1. Packaging
The outer carton label serves as a tamper-resistant package seal. Medtronic Open Pivot heart valves are
packaged and steam sterilized in transparent double barrier trays (Figure 4). Examine the trays carefully to
verify that the seals and trays are intact. Do not use the valve if any seal is damaged or missing. Each
package is supplied sterile with a valve premounted onto a valve holder, a handle rotator, and a leaflet
actuator. The valve, valve holder, handle rotator, and leaflet actuator are ready for use when received
unless the package sterility has been compromised or the Use-By Date has occurred.
All package labeling and handle rotator accessories are color-coded and marked:
■
Aortic – Green
■
Mitral – Red
The Medtronic Open Pivot heart valve sizers are supplied nonsterile, and must be cleaned and sterilized
prior to use. The valve sizers are available to the implanting surgeon as an aid in selecting the appropriate
prosthesis size. Each set of sizers is packaged with Instructions for Use specific to the sizer.
The actuator is available for testing valve leaflet motion.
All packaging material is recyclable. Manage disposal in accordance with local statutory regulations.
11.2. Handling and Storage (Packaged Valve)
Care must be taken to ensure proper handling of the valve package during receiving, handling, and
storage.
Upon receipt of product, visually inspect the outer package for possible damage.
If evidence of damage is found, do not use.
For maximum protection and product identification, it is recommended that the valve be stored in its
original packaging. The storage environment should be clean, cool, and dry. The sterility and
nonpyrogenicity of the valve is validated to remain unaffected until the Use-By Date printed on the product,
provided the seals and containers are not opened or damaged.
11.3. Returned Product Information
For detailed information on the Medtronic returned product policy, please contact your local representative.
11.4. Return of Explanted Prosthetic Valves
Medtronic is interested in obtaining recovered clinical specimens of the Medtronic Open Pivot heart valve.
Specific studies of the explant may be performed and a written report summarizing the findings will be
returned to the implanting physician. Please contact Medtronic to obtain a Returned Material Authorization
(RMA). The explanted valve should be completely submersed in a 2% to 5% formalin solution immediately
after excision unless otherwise directed by your Medtronic representative.
12. Accessories
Refer to the Medtronic Open Pivot heart valve accessories Instructions for Use for cleaning instructions.
22 Instructions for Use English
Page 25

12.1. Sizers
A complete set of Medtronic Open Pivot™ heart valve sizers must be cleaned and sterilized prior to
surgery and made available in the operating room. Refer to the Medtronic Open Pivot heart valve
accessories Instructions for Use for cleaning and sterilization guidelines.
Use only Medtronic Open Pivot heart valve sizers to measure the patient’s tissue annulus. The Medtronic
Open Pivot heart valve sizers have a metal shaft that can be shaped allowing the same sizer to be used
for mitral or aortic tissue annulus measurement.
The sizer with the diameter marked in millimeters has a clear plastic ring that allows for easy tissue
annulus measurement. The sizer should pass easily through the patient’s tissue annulus with minimal
resistance. Do not force the sizer through the tissue annulus. Valve oversizing or undersizing should be
avoided.
Caution: Oversizing may cause complications including tissue interference with leaflet motion or improper
seating resulting in perivalvular leaks. Oversizing in the aortic position may result in blockage of the
coronary ostia. Undersizing may result in increased perivalvular leaks.
12.2. Standard Valve Sizing–White Handled Sizer
The white-handled sizer must be used for sizing of the Medtronic Open Pivot heart valve standard models.
The same Medtronic Open Pivot™ heart valve sizer set may be used for aortic or mitral sizing for standard
valves. The nominal diameter of the sizer in millimeters is printed onto the handle. The sizer selected
should pass easily through the patient’s annulus without resistance but still provide a snug fit between the
sizer ring and the tissue annulus.
12.3. AP Series Valve Sizing–Blue Handled Sizer
The Medtronic Open Pivot AP series heart valve sizers are intended to simulate supra-annular placement
of aortic or mitral valves. The blue-handled sizer must be used for sizing of the Medtronic Open Pivot
AP series heart valves. The AP series blue-handled sizer has 2 ring-shaped sizers. One end of the sizer
consists of a cylindrical ring used for measuring the tissue annulus. This end should pass easily through
the patient’s annulus without resistance, but provide a snug fit. The other sizer end consists of a cylindrical
ring (of the same diameter as the opposing ring) with a rounded protruding edge that simulates the supraannular cuff of the AP series heart valves, and can be used to estimate the diameter and height of the
supra-annular cuff of the AP series heart valves.
The center of the sizer ring is open so that the native annulus beyond the sizer ring can be examined for
intruding tissue that might touch the valve leaflets. Any intruding tissue should be removed. If it is not
possible to remove the intruding tissue or rotate the valve and position the leaflets to avoid leaflet
interference, a smaller size valve should be selected, sized, and evaluated in the same manner as
described above.
12.4. Valve Holder and Handle Rotator
The valve is mounted on a disposable holder that is attached to a color-coded handle rotator (green is
aortic; red is mitral). The rotator is used to rotate the valve in situ. The blood flow in either the aortic or
mitral position is always into the straight edge of the leaflets.
12.5. Bendable Handle
The bendable handle can be attached to the holder instead of the handle rotator assembly during use. The
handle has a metal shaft that can be easily shaped to provide access to the aortic or mitral annulus.
13. Patient Information
13.1. Registration Information
Note: Patient registration does not apply in countries where patient privacy laws conflict with providing
patient information, including countries from the European Union. A patient registration form is included in
each device package. After implantation, please complete all requested information. The serial number
may be found on the tray and box labels. Return the original form to the Medtronic address indicated on
the form, and provide the temporary identification card to the patient prior to discharge.
An Implanted Device Identification Card is provided to the patient. The card contains the name and
telephone number of the patient’s physician, as well as information that medical personnel would require
in the event of an emergency.
13.2. Patient Information Booklet
Medtronic has made available a Patient Information Booklet that the physician may choose to provide to
the patient prior to discharge. Copies of this booklet are available on request from your local sales
representative.
14. Disclaimer of Warranty
THE FOLLOWING DISCLAIMER OF WARRANTY APPLIES TO UNITED STATES CUSTOMERS ONLY:
ALTHOUGH THE MEDTRONIC OPEN PIVOT HEART VALVE, HEREAFTER REFERRED TO AS
“PRODUCT,” HAS BEEN MANUFACTURED UNDER CAREFULLY CONTROLLED CONDITIONS,
MEDTRONIC HAS NO CONTROL OVER THE CONDITIONS UNDER WHICH THIS PRODUCT IS
USED. MEDTRONIC, THEREFORE, DISCLAIMS ALL WARRANTIES, BOTH EXPRESS AND IMPLIED,
WITH RESPECT TO THE PRODUCT, INCLUDING, BUT NOT LIMITED TO, ANY IMPLIED WARRANTY
OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. MEDTRONIC SHALL NOT BE
LIABLE TO ANY PERSON OR ENTITY FOR ANY MEDICAL EXPENSES OR ANY DIRECT,
INCIDENTAL, OR CONSEQUENTIAL DAMAGES CAUSED BY ANY USE, DEFECT, FAILURE, OR
MALFUNCTION OF THE PRODUCT, WHETHER A CLAIM FOR SUCH DAMAGES IS BASED UPON
Instructions for Use English 23
Page 26

WARRANTY, CONTRACT, TORT, OR OTHERWISE. NO PERSON HAS ANY AUTHORITY TO BIND
MEDTRONIC TO ANY REPRESENTATION OR WARRANTY WITH RESPECT TO THE PRODUCT.
The exclusions and limitations set out above are not intended to, and should not be construed so as to
contravene mandatory provisions of applicable law. If any part or term of this DISCLAIMER OF
WARRANTY is held to be illegal, unenforceable, or in conflict with applicable law by a court of competent
jurisdiction, the validity of the remaining portions of this DISCLAIMER OF WARRANTY shall not be
affected, and all rights and obligations shall be construed and enforced as if this DISCLAIMER OF
WARRANTY did not contain the particular part or term held to be invalid.
24 Instructions for Use English
Page 27

Page 28

Authorized Representative in the European
*M951905A002*
Community
Medtronic B.V.
Earl Bakkenstraat 10
6422 PJ Heerlen
The Netherlands
Tel. +31 45 566 8000
Australia
Medtronic Australasia Pty. Ltd.
5 Alma Road
Macquarie Park, NSW 2113
Australia
1800 668 670
Canada
Medtronic of Canada Ltd.
99 Hereford Street
Brampton, Ontario L6Y 0R3
Canada
Tel. +1 905 460 3800
Toll-free: +1 800 268 5346
United States
Manufacturer:
Medtronic, Inc.
710 Medtronic Parkway
Minneapolis, MN 55432
USA
Internet: www.medtronic.com
Tel. +1 763 526 7890
Toll-free: +1 877 526 7890
(24-hour consultation service)
Manufactured In
Medtronic, Inc.
3800 Annapolis Lane
Minneapolis, MN 55447
USA
© 2013, 2016 Medtronic, Inc.
M951905A002 Rev. 1A
 Loading...
Loading...