Medtronic 37741 Users Manual
Neurological
Medtronic Confidential
Title
Model 7439 Patient Programmer User Manual -
Global
This Document is the Property of Medtronic, Inc. and must be
accounted for. Information hereon is confidential. DO NOT
reproduce it, reveal it to unauthorized persons or send it
outside Medtronic without proper authorization.
Medtronic Confidential
Part No. Part No. Rev Comments
221244001 D Release for translation
DO NOT PRINT THIS FORM WHEN PRINTING THE LITERATURE PIECE.
D
O NOT COUNT THIS FORM IN THE LITERATURE PIECE PAGE COUNT.
REVISION HISTORY: SEE PDM WEBSITE FOR INFORMATION.
1. Materials:
Covers
• Grade #1, 80 lb. white gloss coated cover stock, minimum brightness level: 89
Text
• 60 lb. white smooth opaque text stock
Labels
• N/A
2. Colors: To confirm all colors, graphics and text, refer to electronic file
Writer
Theresa King-Hunter
Date
11/03/04
Front Cover
• 4-color cover and solid gloss varnish
Back Cover
• Black text and graphics; solid gloss varnish
Body
• 2-color text and graphics (PMS 301, black, percentages of PMS 301 or percentages
of black)
Labels
• N/A
3. Size: 4.6″ ± 0.2 (w) x 6.0″ ± 0.2 (h)
4. Type of Binding: Clear plastic coil, MWE lay-flat, or perfect bound
5. Other:
• Cover and text whiteness must match visually.
6. Literature Piece Page Count (Including covers, excluding this form): 136
7. Note to print supplier: Supplier may add up to three blank pages at the end of the
document before the back cover sheet as needed. If more are required, contact the
purchasing agent.
8. Vendor-supplied information may appear at an appropriate location on the literature
piece (eg, part number, bar code, etc.).
9. Graphics Content and Layout to be as shown and per Medtronic electronic file that is
supplied (stored) by Medtronic Neuro.
Page 1 Form MEDN-0043 version 7.0

7439_FC.fm 10/13/04 9:26 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
PATIENT PROGRAMMER 7439
Pain therapy user manual
Medtronic Confidential
NeuroPatntR00
c Rx only
221244001 Rev X
2004
Printing instructions:
7439_FC.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
Medtronic Confidential
NeuroPatntR00
The following are trademarks of Medtronic, Inc.: Medtronic®,
MyStim™, SoftStart™, Synergy Plus+™, Synergy Compact+™,
and Synergy
®
.
c FCC Information
The following is communications regulation information on
the Model 7439 Patient Programmer.
FCC ID: LF537741
This device complies with Part 15 Rules. Operation is subject
to the following two conditions: (1) this device may not cause
harmful interference and (2) this device must accept any
interference received, including interference that may cause
undesired operation.
IMPORTANT: Changes or modifications to this product not
authorized by Medtronic, Inc., could void the FCC
Certification and negate your authority to operate this
product.
221244001 Rev X
Printing instructions:
7439TOC.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
Contents
Label symbols 7
1Introduction9
A company dedicated to patients 10
How to use this manual 11
Patient guides 13
Patient identification card 14
2 Important therapy information 15
Purpose of the neurostimulation system
(indications) 16
Therapies that may not be used with the
neurostimulation system
(contraindications) 16
Risks and benefits 17
Risks of surgery 17
Possible adverse effects 18
Changes in therapy 18
Possible system complications 18
Warnings 19
Precautions 26
System and therapy 26
Medtronic Confidential
NeuroPatntR00
Contents
221244001 Rev X
7439 2004-08 English
Printing instructions:
3
7439TOC.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
Patient activities 29
Individualization of treatment 31
3 Introduction to stimulation 33
How stimulation works 34
Parts of your system 36
Understanding your therapy 38
Controlling your stimulation 40
What your clinician controls 40
What you control 40
Recovery and care 41
Recovering from surgery 41
Activities 41
When to call your clinician 43
Care schedule 44
Medtronic Confidential
NeuroPatntR00
4 Using your patient programmer 45
How the patient programmer works 46
Synchronizing and displaying the THERAPY
screen 48
Guidelines for adjusting your stimulation 53
Turning your neurostimulator ON or
Contents
4
221244001 Rev X
OFF 55
English 7439 2004-08
Printing instructions:
7439TOC.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
Adjusting stimulation settings 57
Using the NAVIGATOR key 58
Changing a group 59
Increasing or decreasing a parameter
(amplitude, pulse width, or rate) 62
Patient programmer batteries 66
Checking patient programmer
batteries 66
Replacing patient programmer
batteries 68
Summary of keys 69
Preferences: Changing the audio, contrast,
and number format 72
Using the carrying case and labeling the
patient programmer 75
Optional detachable antenna 76
Connecting the antenna 76
Using the antenna 78
Medtronic Confidential
NeuroPatntR00
5 Troubleshooting 79
Programmer screens 80
221244001 Rev X
Warning screens 80
Communication screen 82
7439 2004-08 English
Printing instructions:
Contents
5
7439TOC.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
Information screens 83
Possible problems and solutions 88
User assistance 95
6 Maintenance 97
Cleaning and care 98
Safety and technical checks 100
Battery and programmer disposal 101
Neurostimulator disposal 101
Declaration of conformity 102
Specifications 103
7 Appendix A: Electromagnetic
interference (EMI) 105
Contraindication 106
Warnings 108
Precautions 116
Notes 120
Medtronic Confidential
NeuroPatntR00
Glossary 123
Index 129
Contents
6
221244001 Rev X
English 7439 2004-08
Printing instructions:
7439_Sym.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
Label symbols
Explanation of symbols on products and
packaging. Refer to the appropriate product
to see symbols that apply.
CE
0123
System meets the applicable Canadian
[C22.2-601.1-M90 (R2001)] and US
(UL 60601-1:2003) electrical safety
standard requirements.
Caution, consult accompanying
w
documents
Medtronic Confidential
NeuroPatntR00
Conformité Européenne
(European Conformity). This
symbol means that the device
fully complies with AIMD
Directive 90/385/EEC (NB 0123)
and R&TTE Directive 1999/5/EC.
The use of this device might be
subject to individual country
licensing regimes in Europe.
n
221244001 Rev X
Serial number
7439 2004-08 English
Printing instructions:
Label symbols
7
7439_Sym.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
Storage temperature
Relative humidity
Atmospheric pressure
IEC 60601-1/EN60601-1, Type BF
y
Equipment
Non-ionizing electromagnetic radiation
L
Screen light
Antenna jack
Medtronic Confidential
NeuroPatntR00
c For USA audience only
The Medtronic Model 7439 Patient
Programmer is designed to program the
adjustable settings of the Medtronic Model
7479 Synergy Plus+ and Model 7479B
Synergy Compact+ neurostimulators.
Label symbols
8
221244001 Rev X
English 7439 2004-08
Printing instructions:
7439_Ch01.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
Medtronic Confidential
NeuroPatntR00
1 Introduction
221244001 Rev X
Printing instructions:
7439_Ch01.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
A company dedicated to patients
Medtronic was founded in
1949 by Earl Bakken, a
graduate student in electrical
engineering, and his brotherin-law, Palmer J. Hermundslie.
Today Medtronic is the world
leader in medical technology,
pioneering therapies that
restore health, extend life and
alleviate pain.
From its modest beginnings in a 55-square
meter (600-square-foot) Minneapolis garage,
we have transformed Medtronic into a
worldwide company that serves customers in
more than 120 countries. Each year, millions
of patients are treated with Medtronic
products and therapies. We invest almost
$500 million each year in research and
development, working closely with the world’s
leading physicians and scientists to enhance
our current products and therapies, and to
Introduction 1
Medtronic Confidential
NeuroPatntR00
10
221244001 Rev X
English 7439 2004-08
Printing instructions:
7439_Ch01.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
develop new ones. Although we are a large
company, individual patients and their needs
are still the driving force behind what we do
and how we do it.
Our goal is to improve the quality of your life.
This booklet, which provides information
about your neurostimulation system, is one
small way we try to help.
Welcome to the Medtronic family. We wish
you well.
How to use this manual
Use this manual after receiving an implanted
neurostimulator. Ask your clinician to explain
anything that is unclear.
Medtronic Confidential
NeuroPatntR00
• Chapter 1, “Introduction,” describes the
patient documents your clinician should
have provided to you.
• Chapter 2, “Important therapy
information,” describes when you should
and should not use a neurostimulation
221244001 Rev X
7439 2004-08 English
Printing instructions:
Introduction 1
11
7439_Ch01.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
system, and the risks, benefits, warnings,
precautions, and patient activities related
to your neurostimulation system.
• Chapter 3, “Introduction to stimulation,”
describes the therapy, neurostimulation
system components, and recovery and
care information.
• Chapter 4, “Using your patient
programmer,” describes the patient
programmer and how to perform specific
tasks.
• Chapter 5, “Troubleshooting,” describes
patient programmer warning and
information screens, how to solve
possible problems, and who to contact if
your device is lost or broken.
Medtronic Confidential
NeuroPatntR00
• Chapter 6, “Maintenance,” describes how
to care for your patient programmer and
system specifications.
• Appendix A provides more information
about electromagnetic interference.
• A glossary is included at the end of this
Introduction 1
12
221244001 Rev X
manual.
English 7439 2004-08
Printing instructions:
7439_Ch01.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
Patient guides
You should receive the following documents
after a neurostimulator is implanted.
• Medtronic Model 7439 Patient
Programmer Quick Reference Guide:
provides instructions for common patient
programmer tasks.
• The Patient Identification Card: provides
information about you, your
neurostimulator, and your doctor.
• Medtronic Model 7439 Patient
Programmer Pain Therapy User Manual.
Medtronic Confidential
NeuroPatntR00
221244001 Rev X
7439 2004-08 English
Printing instructions:
Introduction 1
13
7439_Ch01.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
Patient identification card
When you leave the hospital, your doctor will
give you a patient identification card. This
card supplies information about you, your
implanted device, and your doctor. Your
identification card may allow you to bypass
security devices. Carry this card with you at
all times. If you move, change doctors, or
lose your card, contact Medtronic for a
replacement card. Refer to the Medtronic
contacts at the end of this manual.
c A temporary identification card will be
provided at the hospital. After Medtronic
receives your implant registration from the
hospital, you will receive a permanent
identification card.
Medtronic Confidential
NeuroPatntR00
Introduction 1
14
221244001 Rev X
English 7439 2004-08
Printing instructions:
7439_Ch02.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
Medtronic Confidential
NeuroPatntR00
2 Important therapy
221244001 Rev X
information
Printing instructions:
7439_Ch02.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
Purpose of the neurostimulation system (indications)
Refer to the indications sheet
that is packaged with the
patient programmer for the
purpose of the
neurostimulation system and
related information.
Therapies that may
not be used with the
neurostimulation system
Medtronic Confidential
NeuroPatntR00
(contraindications)
Diathermy – Inform anyone treating you that
you CANNOT have any shortwave diathermy,
microwave diathermy or therapeutic
ultrasound diathermy (all now referred to as
diathermy) anywhere on your body because
you have an implanted neurostimulation
system. Energy from diathermy can be
Important therapy information 2
transferred through your implanted system,
16
221244001 Rev X
English 7439 2004-08
Printing instructions:
7439_Ch02.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
and can cause tissue damage, resulting in
severe injury or death. Refer to “Appendix A:
Electromagnetic interference (EMI)” on
page 105 for more information.
Risks and benefits
Stimulation has helped thousands of patients
manage their pain and improve their quality
of life. Your neurostimulation system may be
used with other pain treatments. Stimulation
will not cure your pain. It can, however,
reduce your pain to a tolerable level and
allow you to resume many of your daily
activities.
Medtronic Confidential
NeuroPatntR00
Risks of surgery
Implanting a neurostimulation system has
risks similar to spinal procedures, including
spinal fluid leak, headaches, swelling,
bruising, bleeding, infection, or paralysis.
If you are on anticoagulation therapy you
might be at greater risk for postoperative
221244001 Rev X
7439 2004-08 English
Printing instructions:
Important therapy information 2
17
7439_Ch02.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
complications such as hematomas that could
result in paralysis.
Possible adverse effects
Adverse effects of stimulation are usually
mild and go away when stimulation is turned
OFF. These adverse effects could include
radicular chest wall stimulation,
uncomfortable stimulation, a jolting or
shocking sensation, or persistent pain at the
neurostimulator site.
Changes in therapy
Over time there could be changes in the level
of your symptom control. In most cases your
doctor can correct these changes without
surgery.
Medtronic Confidential
NeuroPatntR00
Possible system complications
The lead, extension, or neurostimulator could
migrate within the body or erode through the
skin. There could be undesirable changes in
stimulation, possibly related to cellular
Important therapy information 2
changes around the electrode(s), changes in
18
221244001 Rev X
English 7439 2004-08
Printing instructions:
7439_Ch02.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
the position of the electrode(s), loose
electrical connections, or lead or extension
fractures. It is also possible that the
implanted materials could cause an allergic
or immune system response.
Your neurostimulation system might
unexpectedly cease to function due to battery
depletion or other causes. These events,
which can include electrical shorts or open
circuits, conductor (wire) fractures, and
insulation breaches, cannot be predicted.
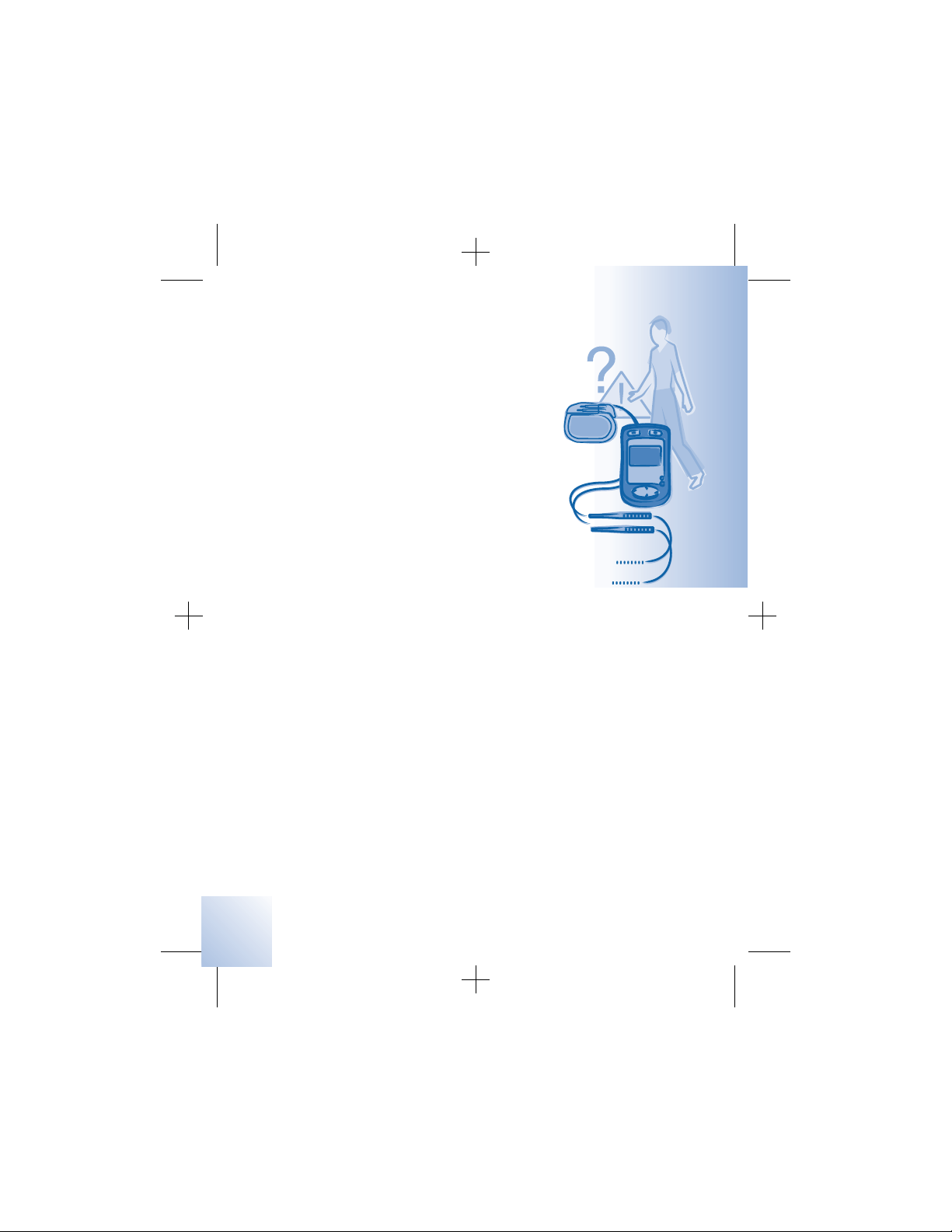
Warnings
Electromagnetic interference (EMI) –
Electromagnetic interference is a field of
energy generated by equipment found in the
home, work, medical or public environments
that is strong enough to interfere with
neurostimulator function. Neurostimulators
include features that provide protection from
EMI. Most electrical devices and magnets
encountered in a normal day are unlikely to
affect the operation of a neurostimulator.
Medtronic Confidential
NeuroPatntR00
Important therapy information 2
221244001 Rev X
7439 2004-08 English
Printing instructions:
19
7439_Ch02.fm 10/13/04 9:27 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
However, strong sources of EMI can result in
the following:
• Serious patient injury or death, resulting
from heating of the implanted
components of the neurostimulation
system and damage to surrounding
tissue.
• System damage, resulting in a loss of or
change in symptom control and requiring
additional surgery.
• Operational changes to the
neurostimulator that can cause it to turn
ON or OFF (particularly in a
neurostimulator enabled for magnet use)
or to reset to the power-on-reset (POR)
values, resulting in loss of stimulation,
return of underlying symptoms, and in the
case of POR, requiring your health care
provider to reprogram your
neurostimulator.
Medtronic Confidential
NeuroPatntR00
• Unexpected changes in stimulation,
causing a momentary increase in
stimulation or intermittent stimulation,
Important therapy information 2
20
221244001 Rev X
which some patients have described as a
English 7439 2004-08
Printing instructions:
7439_Ch02.fm 10/13/04 9:28 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
jolting or shocking sensation. Although
the unexpected change in stimulation
could feel uncomfortable, it does not
damage the device or injure a patient
directly. In rare cases, as a result of the
unexpected changes in stimulation,
patients have fallen down and been
injured.
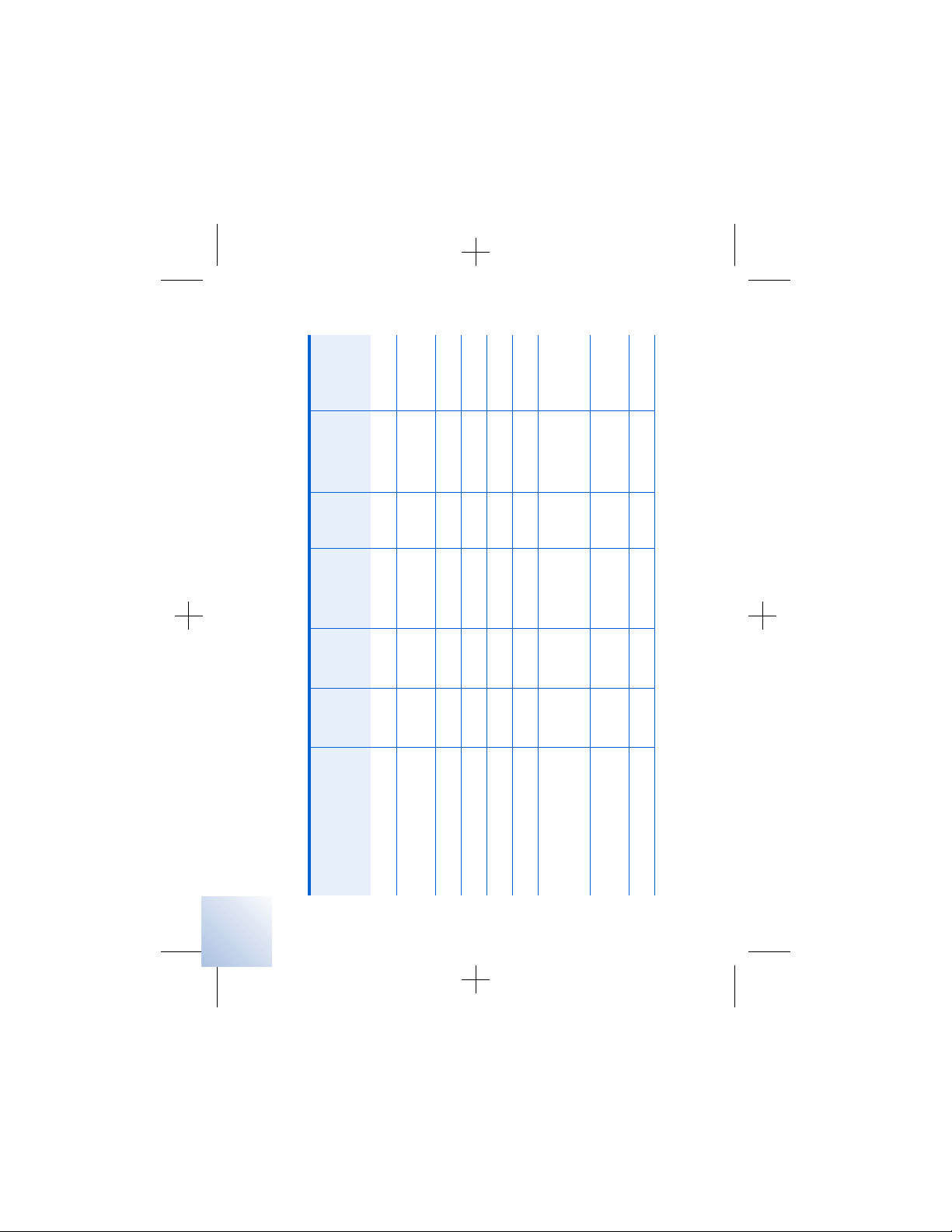
Refer to Table 2.1, on page 22, and
“Appendix A: Electromagnetic interference
(EMI)” on page 105 for information on the
sources of EMI, the effect of EMI on you and
your neurostimulation system, and
instructions on how to reduce the risk from
EMI.
Medtronic Confidential
NeuroPatntR00
221244001 Rev X
7439 2004-08 English
Printing instructions:
Important therapy information 2
21
7439_Ch02.fm 10/13/04 9:28 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
See
guidelines
stimulation
Intermittent
ON
turns
Device
OFF or
Medtronic Confidential
NeuroPatntR00
Table 2. 1 Potential effects of EMI from devices or procedures
Important therapy information 2
22
221244001 Rev X
English 7439 2004-08
in
increase
Momentary
Device
damage
patient
Serious
Device/procedure
✓✓ ✓page 117
stimulation
✓✓ ✓page 116
injury
Bone growth stimulators
✓✓ ✓ ✓page 108
Defibrillation/
cardioversion
✓ page 116
Dental drills and probes
✓✓ ✓page 106
✓✓ page 109
Diathermy, therapeutic
Electrocautery
Printing instructions:
✓✓ page 116
Electrolysis
Electromagnetic field
devices (eg, arc
welding, power stations)
✓ page 111
High-output ultrasonics
/lithotripsy
✓✓ page 120
Household items
7439_Ch02.fm 10/13/04 9:28 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
See
guidelines
stimulation
Intermittent
Medtronic Confidential
NeuroPatntR00
Device
Momentary
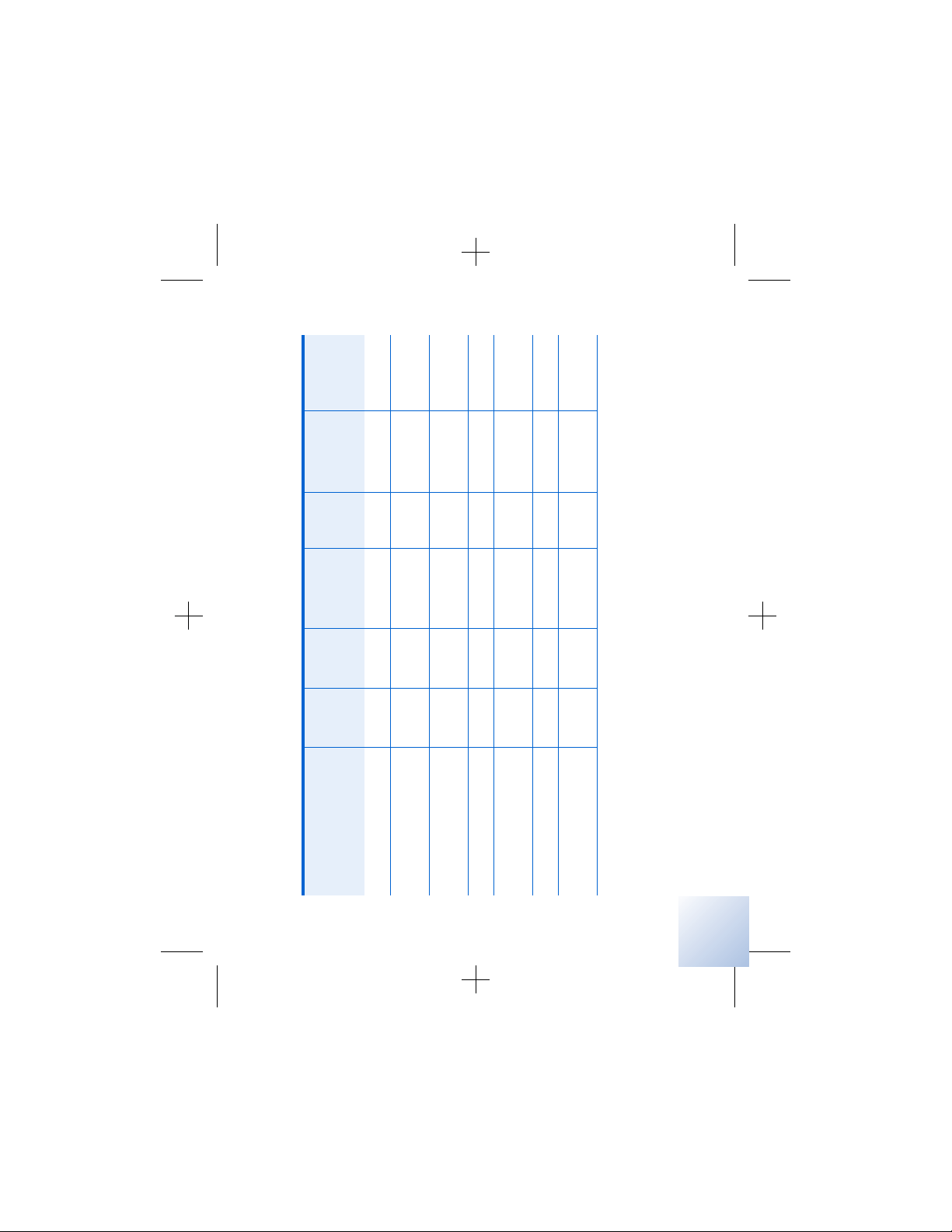
Table 2 . 1 Potential effects of EMI from devices or procedures (continued)
221244001 Rev X
ON
turns
OFF or
in
increase
stimulation
Device
damage
injury
patient
Serious
Device/procedure
✓ page 122
✓✓ ✓page 114
✓ page 118
Laser procedures
✓✓ ✓ ✓ ✓page 111
Magnetic resonance
✓✓✓✓page 119
✓ page 119
✓✓ ✓page 113
imaging (MRI)
Psychotherapeutic
procedures
Radiation therapy
Radiofrequency (RF)/
microwave ablation
Therapeutic magnets
Theft detectors/security
devices
7439 2004-08 English
Printing instructions:
Important therapy information 2
23
7439_Ch02.fm 10/13/04 9:28 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
See
guidelines
stimulation
Intermittent
ON
turns
Device
OFF or
Medtronic Confidential
NeuroPatntR00
Momentary
Important therapy information 2
24
221244001 Rev X
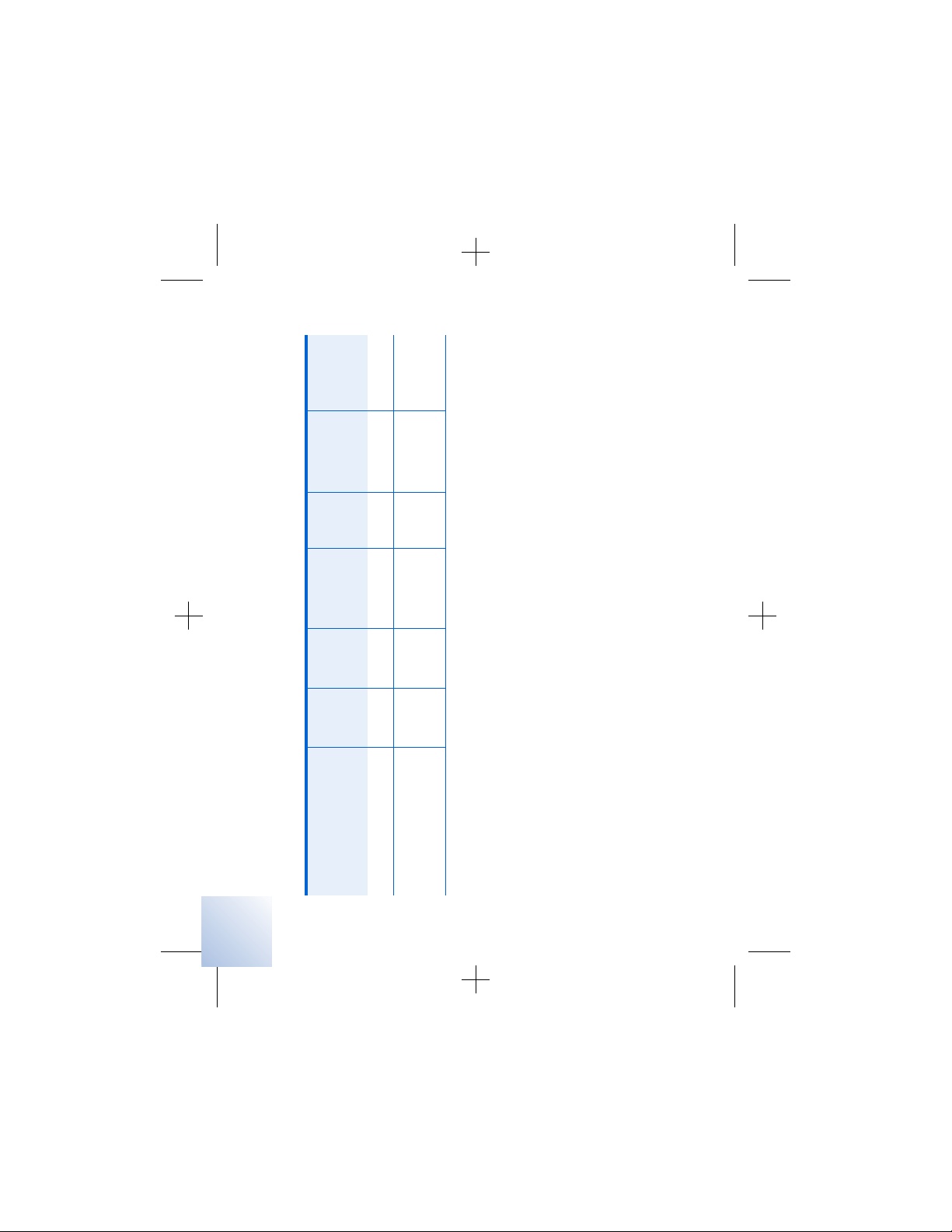
Table 2 . 1 Potential effects of EMI from devices or procedures (continued)
English 7439 2004-08
in
increase
stimulation
Device
damage
injury
patient
Serious
Device/procedure
✓✓ ✓page 106
Therapeutic ultrasound
✓✓ page 119
Transcutaneous
electrical nerve
stimulation (TENS)
Printing instructions:
7439_Ch02.fm 10/13/04 9:28 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
Case damage – If the neurostimulator case
is ruptured or pierced due to outside forces,
severe burns could result from exposure to
the battery chemicals.
Neurostimulator interaction with cardiac
implantable devices – When a
neurostimulator and an implanted cardiac
device (eg, pacemaker, defibrillator) are
required, the doctors involved with both
devices (neurologist, neurosurgeon,
cardiologist, cardiac surgeon) should
discuss the possible interaction between the
devices before surgery. To minimize or
prevent device damage or interactions, your
doctors should place the devices on the
opposite side of the body from one another.
Medtronic Confidential
NeuroPatntR00
• Defibrillation therapy from the implanted
defibrillator can damage the
neurostimulator.
• The electrical pulses from the
neurostimulation system could affect the
sensing operation of the cardiac device
and result in inappropriate responses
from the cardiac device. Your doctor
221244001 Rev X
7439 2004-08 English
Printing instructions:
Important therapy information 2
25
7439_Ch02.fm 10/13/04 9:28 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
should reprogram your neurostimulator to
a bipolar configuration and a minimum
rate of 60 Hz. The cardiac device should
be programmed to bipolar sensing.
Precautions
System and therapy
Clinician programmer interaction with a
cochlear implant – If you have a cochlear
implant, the external portion of the cochlear
system should be kept as far away as
possible from the clinician programmer or
the cochlear implant should be turned OFF
during programming to prevent unintended
audible clicks.
Medtronic Confidential
NeuroPatntR00
Clinician programmer interaction with
other active implanted devices – If you
have a neurostimulator and another active
implanted device, the radio-frequency signal
used to program either device can reset or
reprogram the other device, or the magnet in
a cardiac programmer can activate
magnetically controlled functions in the
Important therapy information 2
26
221244001 Rev X
English 7439 2004-08
Printing instructions:
7439_Ch02.fm 10/13/04 9:28 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
neurostimulator. To verify that inadvertent
programming did not occur, clinicians
familiar with each device should check the
programmed settings before you are sent
home from the hospital and after either
device is programmed (or as soon as
possible after these times).
Contact your doctor immediately if you notice
symptoms that could be related to either
device or to the medical condition treated by
that device.
Component compatibility – For proper
therapy, only components that are
compatible with the appropriate indication
(eg, spinal cord stimulation) should be used.
For a list of Medtronic-compatible
components, ask your doctor. No claims of
safety or efficacy are made about the
compatibility of non-Medtronic components
with Medtronic components.
Medtronic Confidential
NeuroPatntR00
Patient control devices – Do not place
patient control devices (eg, patient
programmer) over another device (eg,
221244001 Rev X
7439 2004-08 English
Printing instructions:
Important therapy information 2
27
7439_Ch02.fm 10/13/04 9:28 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
pacemaker, defibrillator, another
neurostimulator). The patient control device
could accidently change the operation of
another device.
Patient device handling – To avo i d
damaging the device, do not immerse it in
liquid; do not clean it with bleach, nail polish
remover, mineral oil, or similar substances;
and do not drop it or mishandle it in a way
that may damage it.
Patient device use – When operating an
external neurostimulator, patient
programmer, or charging system use special
care near flammable or explosive
atmospheres. An interaction between the
flammable or explosive atmospheres and the
battery in the device could occur. The
consequences of using a battery-powered
device near flammable or explosive
atmospheres are unknown.
Medtronic Confidential
NeuroPatntR00
Important therapy information 2
28
221244001 Rev X
English 7439 2004-08
Printing instructions:
7439_Ch02.fm 10/13/04 9:28 am
Size 4.625" x 6.0" (117 mm x 152 mm)
UC200xxxxxx EN
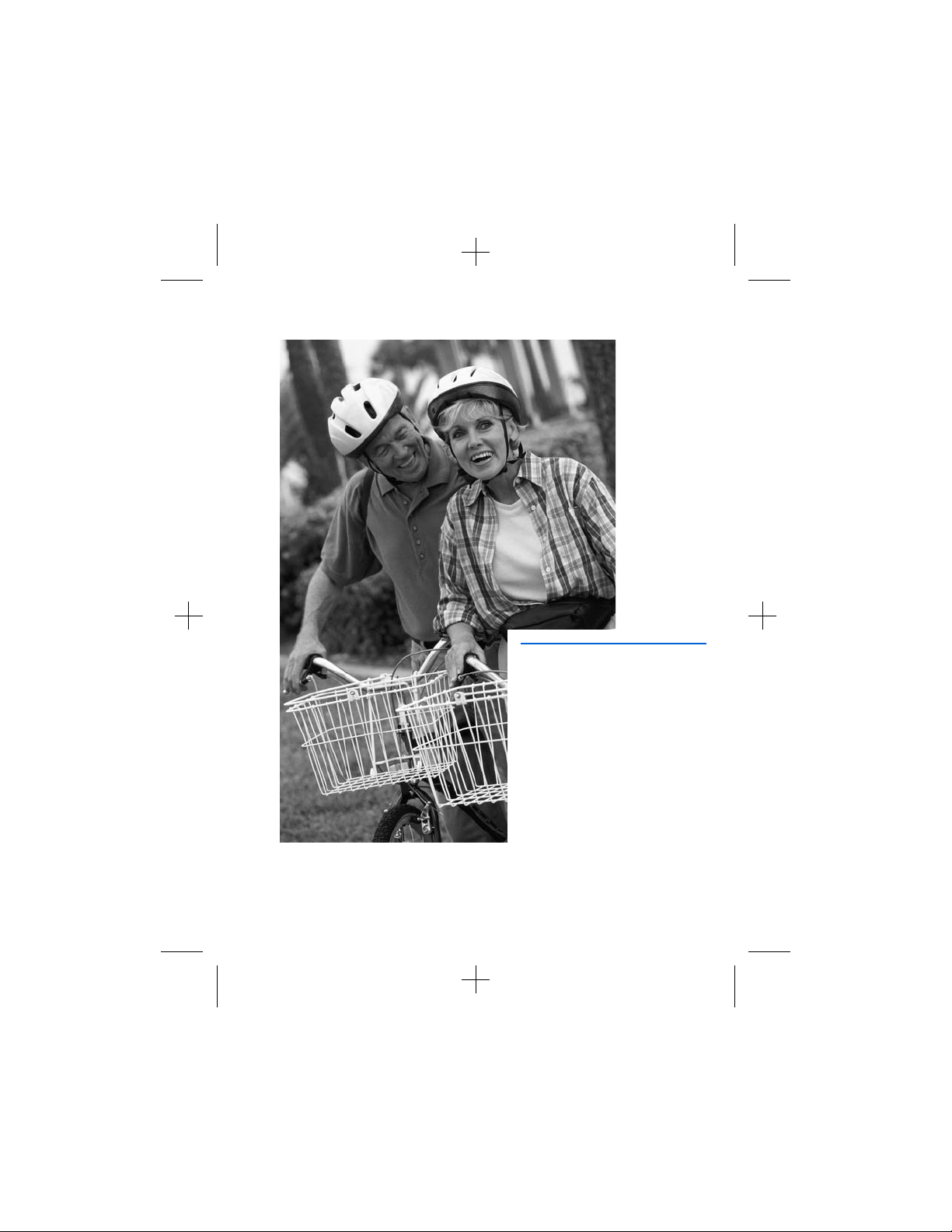
Patient activities
Activities requiring excessive twisting or
stretching – Avoid activities that put undue
stress on the implanted components of your
neurostimulation system. Activities that
include sudden, excessive, or repetitive
bending, twisting, bouncing, or stretching
can cause parts of your neurostimulation
system to fracture or migrate. This can result
in a loss of stimulation, intermittent
stimulation, stimulation at the fracture site,
and additional surgery. Spinal cord
stimulation patients in particular should
avoid excessive bending of the torso.
Component manipulation – Do not
manipulate or rub your neurostimulation
system through the skin, sometimes called
“Twiddler’s Syndrome.” Manipulation can
cause damage to your system, skin erosion,
or stimulation at the implant site.
Medtronic Confidential
NeuroPatntR00
221244001 Rev X
7439 2004-08 English
Printing instructions:
Important therapy information 2
29
 Loading...
Loading...