Page 1

ACHIEVE ADVANCE™
2ACH15, 2ACH20, 2ACH25
Mapping Catheter
Technical Manual
Caution: Federal law (USA) restricts
this device to sale by or on the order
of a physician.
Page 2
The following list includes trademarks or registered
trademarks of Medtronic in the United States and possibly in
other countries. All other trademarks are the property of their
respective owners.
Achieve Advance, Arctic Front, Medtronic
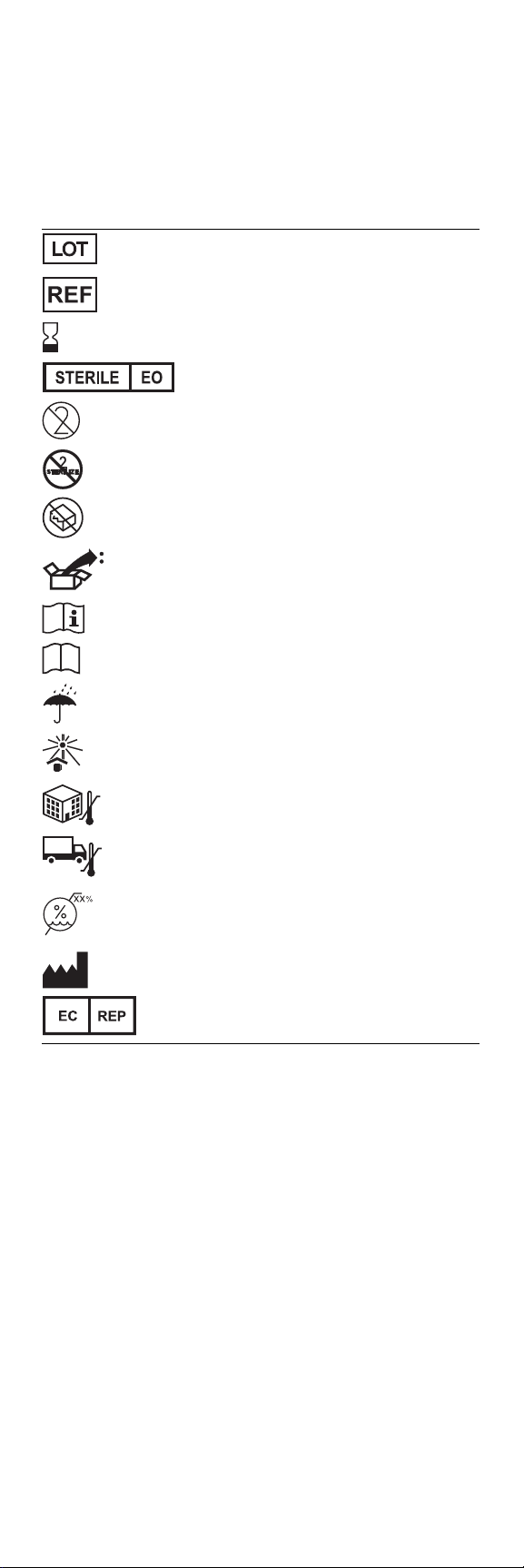
Explanation of symbols
Refer to the package labels to see which of the following symbols apply to this product.
Lot number
Reorder number
Use by
Sterilized using ethylene oxide
Do not reuse
Do not resterilize
t
Do not use if package is damaged
Do
Package contents
Consult instructions for use
Product documentation
Keep dry
Keep away from heat and sunlight
Storage temperature
Transit temperature
Humidity limitation
Manufacturer
Authorized representative in the European Community
Page 3

1 Description
1.1 mm (3.3 Fr)
165 cm
146 cm
15 mm 20 mm
6mm
4mm
2ACH15
2ACH20
25 mm
6mm
2ACH25
8 electrodes 8 electrodes
10 electrodes
This document is intended to be used with the Achieve Advance mapping catheter.
The mapping catheter is an intra-cardiac electrophysiology (EP) recording catheter and can
be used for cardiac stimulation during electrophysiology studies.
The mapping catheter is compatible for use with, and may be used to support and position,
all catheters in the Medtronic Arctic Front CryoAblation Catheter family. Refer to the
applicable Arctic Front Technical Manual for additional instructions for use.
Figure 1. Achieve Advance mapping catheter dimensions
The distal mapping section of the mapping catheter is a circular loop with eight or ten evenly
spaced electrodes to map electrical conduction between the left atrium and the pulmonary
veins. See Catheter specifications, section 7, for detail.
Figure 2. Achieve Advance mapping catheter loop
The mapping catheter should only be used with the Medtronic catheter connecting cable
(Model 2ACHC), which interfaces with standard EP recording equipment. For cable
instructions, see the 2ACHC catheter connecting cable instructions for use.
1.1 Contents of package
The mapping catheter is supplied sterile. The package contains the following items:
■
1 Achieve Advance mapping catheter
■
Product documentation
2 Indications for use
The Achieve Advance mapping catheter is indicated for multiple electrode
electrophysiological mapping of the cardiac structures of the heart, i.e. recording or
stimulation only. The Achieve Advance mapping catheter is designed to obtain electrograms
in the atrial regions of the heart.
3 Contraindications
The Achieve Advance mapping catheter is contraindicated as follows:
■
For use as an ablation device
■
For use with transseptal sheaths featuring side holes larger than 1.00 mm (0.04 in) in
diameter
■
Retrograde approach
Electrophysiology studies are contraindicated when the patient’s underlying cardiac disease
makes it likely that induced arrhythmias will be extremely difficult to terminate and carry a
high risk of death, as in the following conditions:
■
An active systemic infection
■
Left atrial thrombus
■
Pulmonary vein stents
■
Prosthetic heart valve (tissue or mechanical)
■
Myxoma
■
Interatrial baffle or patch
■
Conditions where the manipulation of the catheter within the heart would be unsafe
■
Acute myocardial infarction
4 Warnings and precautions
For single use only – This catheter is intended only to be used once for a single patient. Do
not reuse, reprocess, or resterilize these devices for purpose of reuse. Reuse, reprocessing,
or resterilization may compromise the structural integrity of the device or create a risk of
contamination of the device that could result in patient injury, illness, or death.
Do not resterilize – Do not resterilize the catheter for purpose of reuse. Resterilization may
compromise the structural integrity of the device or create a risk of contamination from the
device that could result in patient injury, illness, or death.
Qualified users – This equipment should be used only by or under the supervision of
physicians trained in cardiac catheterization procedures.
Device disposal – Dispose of catheter according to hospital biohazard requirements.
Expiration date – Check to verify the catheter is within the expiration date. Do not use if the
product date has expired.
Sterile package inspection – Inspect the sterile packaging prior to use. If the sterile
packaging of the device is damaged, do not use. Contact your local Medtronic
representative.
Improper connection – Do not connect the mapping catheter to a radiofrequency (RF)
generator or use it to deliver RF energy. Doing this may cause catheter malfunction or patient
harm.
Induced arrhythmias – Catheter procedures may mechanically induce arrhythmias.
Fluoroscopy required for catheter placement – Use of fluoroscopy during catheter
manipulation and placement is strongly advised. Manipulating the catheter without
fluoroscopy may result in damage to cardiac and vascular structures.
X-ray and fluoroscopic exposure – Minimize x-ray and fluoroscopic exposure. Due to the
intensity of the x-ray beam and the duration of the fluoroscopic imaging during catheter
procedures, patients and laboratory staff may be subjected to acute radiation injury and
increased risk for somatic and genetic effects. Take all appropriate measures to minimize
x-ray exposure to both patients and clinical staff. The long-term effects of protracted
fluoroscopy have not been established.
Catheter positioning around the chordae tendineae – Avoid positioning the catheter
around the chordae tendineae as this increases the likelihood of catheter entrapment within
the heart, which may necessitate surgical intervention or repair of injured tissues.
Other catheters, devices, or wires – Avoid catheter entanglement with other catheters,
devices, or wires. Such entanglement may necessitate surgical intervention.
Prosthetic heart valves – Do not pass the catheter through a prosthetic heart valve
(mechanical or tissue). The catheter may become trapped in the valve, damaging the valve
and causing valvular insufficiency or premature failure of the prosthetic valve.
Achieve Advance Technical Manual 3
Page 4

Embolism risk – Introducing any catheter into the circulatory system entails the risk of air
or gas embolism, which can occlude vessels and lead to tissue infarction with serious
consequences. Always advance and withdraw components slowly to minimize the vacuum
created and therefore minimize the risk of air embolism.
Catheter handling and care –
■
Use extreme care when manipulating the catheter. Lack of careful attention can result in
injury such as perforation or tamponade.
■
Do not use excessive force to advance or withdraw the catheter, especially if resistance
is encountered.
■
Do not use the catheter if it is kinked, damaged, or cannot be straightened.
■
Do not at any time preshape or bend the catheter shaft or distal (loop) segment. Bending
or kinking the catheter shaft may compromise the structural integrity of the device and
increase the risk of catheter failure. Prebending of the distal loop can damage the
catheter.
■
Always rotate the mapping catheter in a clockwise direction to prevent tissue damage.
Leakage current from connected devices – Use only isolated equipment (IEC 60601-1
Type CF equipment, or equivalent) with the mapping catheter or patient injury or death may
occur. Do not allow leakage current from any connected devices to the patient to exceed
10 µA under any circumstances.
Electrical isolation – Do not allow the patient to contact grounded equipment that might
produce electrical current leakage during ablation or DCCV (Direct Current CardioVersion).
Electrical current leakage may induce arrhythmias that may result in the patient’s death.
Cardioversion/defibrillation – Disconnect the catheter from the catheter connecting cable
prior to cardioversion/defibrillation. Failure to do so may result in damage to any attached
electrophysiological monitoring equipment. Do not touch the patient or the catheter during
cardioversion/defibrillation. Direct contact with the patient or the catheter during
cardioversion/defibrillation may result in shock.
System compatibility – Use the mapping catheter only with the Medtronic catheter
connecting cable Model 2ACHC, and a compatible mating device with a minimum internal
diameter of 1.12 mm (0.044 in) and standard 9 Fr hemostasis valve. The mapping catheter
is compatible for use with, and may be used to support and position, all catheters in the
Medtronic Arctic Front CryoAblation Catheter family. Refer to the applicable Arctic Front
Technical Manual for additional instructions for use. The mapping catheter is not
recommended for use with RF ablation catheters.
Catheter integrity – Do not use the catheter if it is kinked or damaged. If the catheter
becomes kinked or damaged while in the patient, remove it and use a new catheter.
Required use environment – Cardiac catheterization procedures should be performed
only in a fully equipped electrophysiology laboratory.
Fluid incursion – Do not immerse the electrical connectors in fluids or solvents. If these
components get wet, electrical performance may be compromised.
Anticoagulation therapy – Administer appropriate levels of peri-procedural
anticoagulation therapy for patients undergoing left-sided and transseptal cardiac
procedures. Administer anticoagulation therapy during and post-procedure according to the
institution's standards.
Rotation direct ion – Always rotate the mapping catheter in a clockwise direction to prevent
tissue damage.
Standards compliance – The mapping catheter must always be used with equipment that
complies with international safety standards.
Recommended storage conditions – Store the catheter in the box until ready for use.
Store the catheter in normal operating room temperatures (22 °C [72 °F]) and in a manner
that protects the integrity of the package and the sterile barrier. See “Catheter specifications”
on page 5 for more details. Keep in a dry location away from heat and all sources of light.
Exposure to light may cause damage to the catheter.
Organic solvents – Do not expose the catheter or cable to organic solvents such as
alcohol.
5 Adverse events
Potential adverse events associated with cardiac catheter procedures include, but are not
limited to the following conditions:
■
Access site vessel occlusion
■
Allergic reaction to x-ray contrast media
■
Arrhythmias, proarrhythmia
■
Arteriovenous fistula
■
Bleeding related to anticoagulation
■
Bradycardia
■
Cardiac perforation of the heart or other
organs during transseptal puncture or
other procedures
■
Cardiac tamponade
■
Cardiac thromboembolism
■
Cerebrovascular accident (CVA) or
transient ischemic attack (TIA)
■
Chest discomfort
■
Chronic cough
■
Death
■
Dislodgement of implantable cardioverter
defibrillator (ICD) or permanent pacing
leads
■
Fever
■
Heart failure
■
Hematoma
■
Hemoptysis
■
High creatinine phosphokinase or
troponin level
■
Hypotension
■
Infections
■
Mitral valve trauma
■
Myocardial infarction or ischemia
■
Obstruction, perforation, damage, or
spasm of the vascular system including
the coronary circulation system
■
Pericarditis or endocarditis
■
Pleural or pericardial effusion
■
Pneumonia
■
Pulmonary embolism
■
Pulmonary infiltrates
■
Pulmonary vein narrowing or stenosis
■
Pseudoaneurysm in groin
■
Radiation injury or damage and late
malignancy
■
Respiratory depression
■
Retroperitoneal bleed
■
Thrombotic or embolic events
■
Valvular insufficiency or damage
■
Vasovagal reaction
6 Instructions for use
The mapping catheter is compatible for use with, and may be used to support and position,
all catheters in the Medtronic Arctic Front CryoAblation Catheter family. Refer to the
applicable Arctic Front Technical Manual for additional instructions for use.
Caution: Carefully read all instructions prior to use. Observe all contraindications, warnings,
and precautions noted in these instructions. Failure to do so may result in patient harm or
impaired device function.
6.1 Preparing the mapping catheter
1. Prepare the mapping catheter under aseptic conditions.
Note: Carefully remove the catheter from the packaging and inspect the catheter before
use to verify it has not been compromised during shipping and handling.
2. Follow standard practice for vessel and transseptal puncture, guidewire insertion,
guiding sheath use, and aspiration procedures.
6.2 Inserting the mapping catheter
1. Slide the introducer over the distal loop.
2. Insert the mapping catheter through a compatible mating device with a minimum internal
diameter of 1.12 mm (0.044 in) until the catheter loop exits the distal tip of the compatible
device.
Caution: Avoid torquing the mapping catheter during delivery through the compatible
device.
Note: Use fluoroscopic guidance during the use of the catheter.
3. Connect the Medtronic catheter connecting cable to the mapping catheter. Ensure ECG
connections on the cable are connected to the EP recording system.
4 Achieve Advance Technical Manual
Page 5

Notes:
– Refer to the appropriate pacing and recording equipment operator manual for proper
set up and operation.
– When connecting the catheter connecting cable (Model 2ACHC) to the EP recording
system, pins 9 and 10 will not be used on the 8-electrode catheters (Models 2ACH15
and 2ACH20).
– Use of the Medtronic 8-pin cable (Model 990066) will result in a loss of functionality,
if used with the 25 mm Achieve Advance mapping catheter (2ACH25).
4. Advance the mapping catheter into the desired position.
Note: Mishandling of the mapping catheter could result in deformation of the distal loop.
Warn ing: Always rotate the connector in a clockwise direction to position the mapping
loop in the appropriate location to avoid tissue injury.
7 Catheter specifications
Maximum catheter shaft size 3.3 Fr
Compatible mating device
minimum internal diameter
Loop diameter 2ACH15: 15 mm (0.59 in)
Shaft length Overall: 165 cm (65.0 in)
Number of electrodes 2ACH15: 8
Electrode length 1.0 mm (0.039 in)
Electrode Spacing 2ACH15: 4 mm (0.16 in)
Environmental parameters
Operation 10 °C to 40 °C (50 °F to 104 °F)
Transit temperature -35 °C to 58 °C (-31 °F to 136 °F); up to 85% relative
Storage temperature 15 °C to 30 °C (59 °F to 86 °F)
1.1 mm (0.043 in)
3.4 Fr
1.12 mm (0.044 in)
2ACH20: 20 mm (0.79 in)
2ACH25: 25 mm (0.98 in)
Effective: 146 cm (57.5 in)
2ACH20: 8
2ACH25: 10
2ACH20: 6 mm (0.24 in)
2ACH25: 6 mm (0.24 in)
humidity (non-condensing)
8 Medtronic limited warranty
For catheter warranty information, see the accompanying limited warranty document.
9 Service
Medtronic employs highly trained representatives and engineers located throughout the
world to serve you and, upon request, to provide training to qualified hospital personnel in
the use of Medtronic products. Medtronic also maintains a professional staff to provide
technical consultation to product users. For more information, contact your local Medtronic
representative, or call or write Medtronic at the appropriate telephone number or address
listed on the back cover.
Achieve Advance Technical Manual 5
Page 6

Page 7

Page 8

Medtronic, Inc.
710 Medtronic Parkway
Minneapolis, MN 55432
USA
www.medtronic.com
+1 763 514 4000
Europe/Middle East/Africa
Medtronic International Trading Sàrl
Route du Molliau 31
Case Postale 84
CH-1131 Tolochenaz
Switzerland
+41 21 802 7000
Technical manuals
www.medtronic.com/manuals
© Medtronic, Inc. 2016
M426501B001 1B
2016-11-08
*M426501B001*
 Loading...
Loading...