Page 1

24967
Patient Connector
Technical Manual
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Page 2

The following list includes trademarks or registered trademarks of Medtronic in the United States and possibly in other countries. All other trademarks are the property
of their respective owners.
CareLink, Medtronic
Page 3

Contents
1 Introduction to the 24967 Patient Connector ............................................................... 4
1.1 Explanation of packaging and product symbols .......................................................... 4
1.2 Description ......................................................................................... 6
1.3 Intended use ....................................................................................... 6
1.4 Contraindications ................................................................................... 7
1.5 Warnings .......................................................................................... 7
1.6 Precautions ........................................................................................ 7
1.7 Regulatory compliance .............................................................................. 8
1.8 Patient Connector functions .......................................................................... 8
1.9 IT network, mobile device, and data information ......................................................... 8
2 Setup and configuration ................................................................................. 10
2.1 Contents of package ................................................................................ 10
2.2 System components ................................................................................ 10
2.3 Compatible accessories ............................................................................. 11
2.4 Setup ............................................................................................ 12
2.5 Charging the patient connector battery ................................................................ 13
2.6 Pairing the patient connector with the app ............................................................. 14
2.7 Troubleshooting potential interference ................................................................. 15
3 Conducting a patient session ............................................................................ 15
3.1 Positioning the patient connector ..................................................................... 15
3.2 Communicating with an implantable device ............................................................ 15
3.3 Troubleshooting .................................................................................... 16
4 Maintaining the patient connector ....................................................................... 17
4.1 Cleaning and disinfecting the 24967 patient connector .................................................. 17
4.2 Replacing the patient connector nose, cable, and weight ................................................. 21
4.3 Software updates .................................................................................. 23
4.4 Specifications ..................................................................................... 23
5 Electromagnetic compatibility declaration ................................................................ 24
3
Page 4
1 Introduction to the 24967 Patient Connector
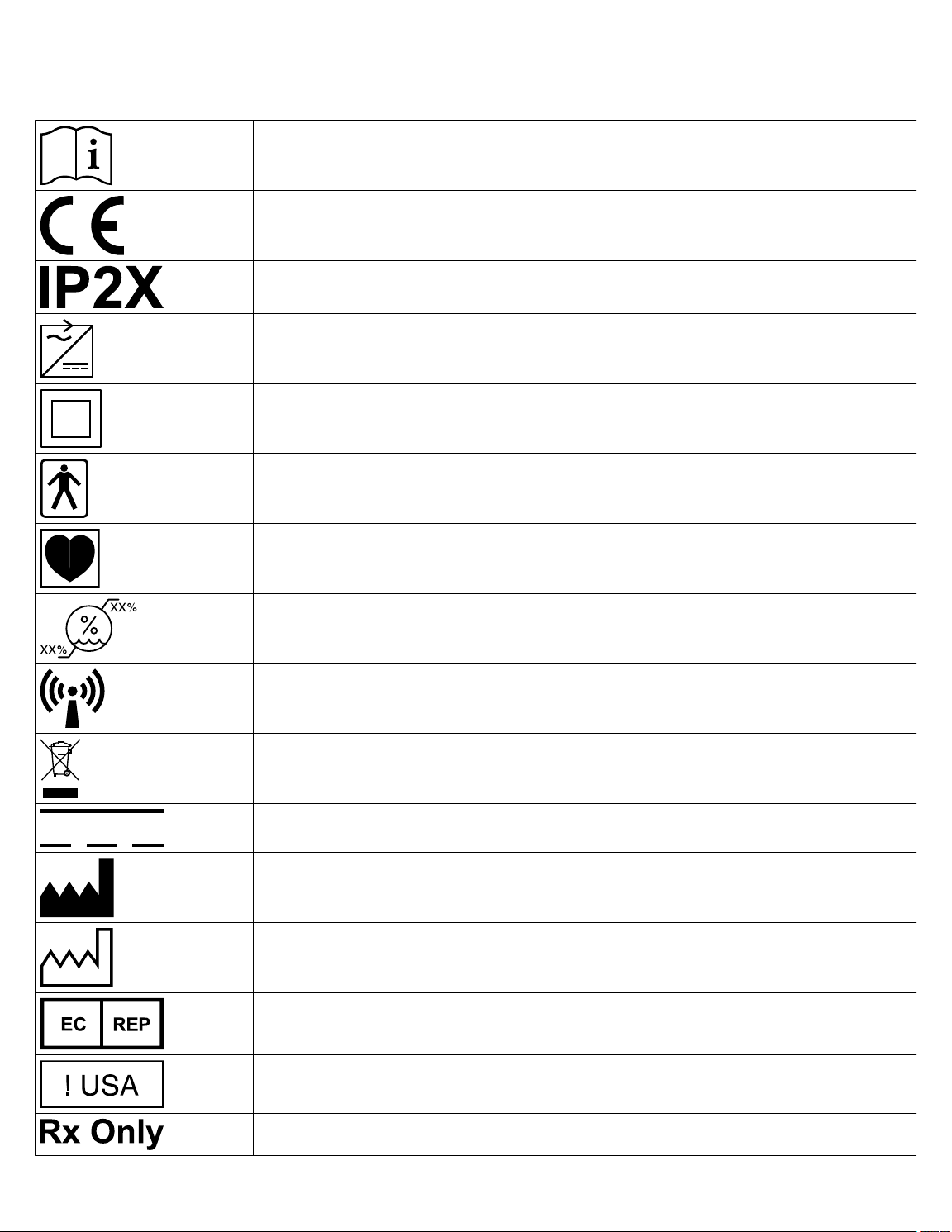
1.1 Explanation of packaging and product symbols
Refer to the package label and product to see which symbols apply to this product.
Consult instructions for use at www.medtronic.com/manuals.
Conformité Européenne (European Conformity). This symbol means that the device fully complies
with applicable European Union Acts.
Ingress protection
Use only with specified power supply
Class II ME equipment equipment
Type BF applied part
Type CF applied part
Humidity limitation
Non-ionizing electromagnetic radiation
Do not dispose of this product in the unsorted municipal waste stream. Dispose of this product
according to local regulations. See http://recycling.Medtronic.com for instructions on proper disposal of this product.
Direct current
Manufacturer
Date of manufacture
Authorized representative in the European community
For US audiences only
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician
4
Page 5
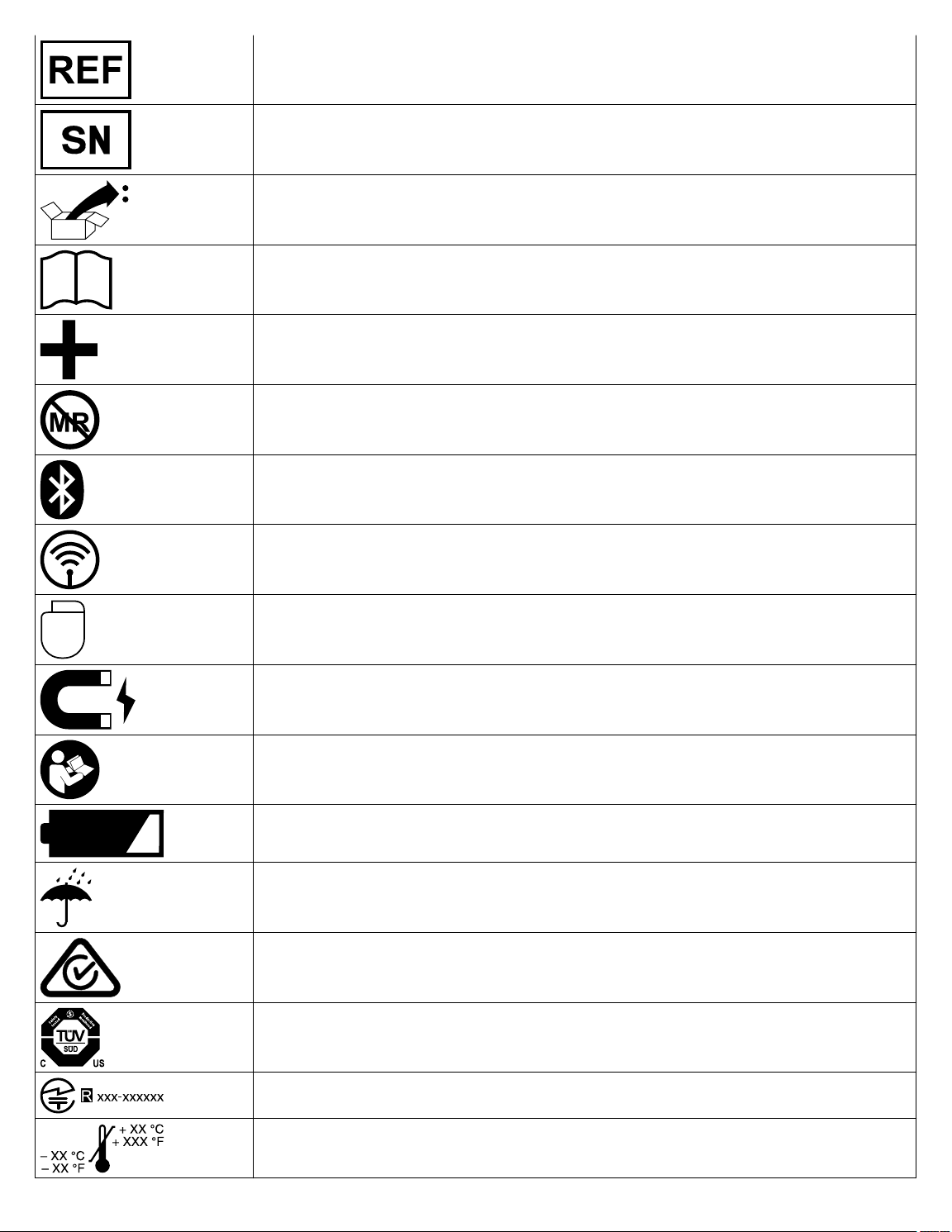
Re-order number
Serial number
Package contents
Product documentation
Accessories
Magnetic Resonance (MR) Unsafe
Bluetooth connection
Wireless communication enabled
Telemetry enabled
Caution: Strong magnet
Follow instructions for use (blue)
Low battery
Keep dry
ACMA (Australian Communications and Media Authority) and the New Zealand Ministry of Economic Development Radio Spectrum Management compliance mark for Australia and New Zealand
System meets the applicable Canadian and U.S. IEC safety standards
Technical Conformity (Ministry of Internal Affairs and Communications) mark for Japan
Operating temperature
5
Page 6

Transit temperature
Storage temperature
Security key
24970A Device manager
24967 Patient connector
249701 Power supply (for the 24970A base)
249705 Power cord (for the 24970A base)
249651 Power supply (for the 24967 patient connector)
249672 Tether Kit
249702 USB Cable
249671 Weight Kit
1.2 Description
The 24967 Patient Connector telemetry head (referred to from now on as the patient connector), pairs with Medtronic apps on your
mobile device to interrogate and/or program implantable Medtronic devices.
The patient connector includes these features:
• Low frequency inductive telemetry to communicate with implantable Medtronic devices.
• Bluetooth® wireless technology to communicate with implantable Medtronic devices and the apps running on the mobile device.
• Integration with the Medtronic 24970A Base for charging (not included).
1.3 Intended use
The patient connector is intended to be used with Medtronic apps to interrogate, analyze, and/or program implantable Medtronic
devices. The patient connector uses Bluetooth technology to transmit that data to a Medtronic app for further processing.
The patient connector is intended to be used by healthcare personnel only in a clinical or hospital environment
1
1
The Bluetooth® word mark and logos are registered trademarks owned by the Bluetooth SIG, Inc. and any such use of those marks by Medtronic
is under license.
6
Page 7

1.4 Contraindications
There are no known contraindications for the use of this device.
1.5 Warnings
These warnings apply in general to using the patient connector settings. For more information related to specific implantable device
models, see the reference guides for the implantable device and the software.
Battery exposure – Exposing the patient connector to cold temperatures may result in a loss of performance and shortened patient
connector service life.
Connection of external devices – Additional equipment connected to medical electrical equipment must comply with the respective
IEC or ISO standards. All configurations must comply with the requirements for medical electrical systems, see IEC 60601-1 and IEC
60601-1-1. Anyone connecting additional equipment to medical electrical equipment configures a medical system and is therefore
responsible that the system complies with the requirements for medical electrical systems. Local laws take priority over the above
mentioned requirements. If in doubt, consult your local Medtronic representative or the technical service department.
Damage due to impact – Do not use the patient connector if it has sustained impact damage. Internal components may be damaged
or exposed. Use of damaged equipment may impact user or patient safety.
Magnetic Resonance (MR) Unsafe – The patient connector is MR Unsafe. Do not bring the patient connector into Zone 4 (magnet
room), as defined by the American College of Radiology.
Modification of equipment – Do not modify this equipment. Modifications may reduce system effectiveness and impact user or
patient safety. Modifying the patient connector without the approval of Medtronic could void the user’s authority to operate the
equipment.
Radio-frequency (RF) interference – Portable and mobile RF communications equipment can interfere with the operation of the
patient connector. Although the patient connector has been approved, there is no guarantee that it will not receive interference or that
any particular transmission from the patient connector will be free from interference.
Unauthorized use – The patient connector can be used with any compatible mobile device onto which the app is installed.
Inappropriate programming could result if untrained persons obtain the patient connector and a patient with a Medtronic implantable
heart device allows them to use it with the patient’s device.
Use of unapproved power supply – Use only the Medtronic-supplied power supply with the patient connector. Use of an
unapproved power supply may damage equipment or impact user or patient safety.
1.6 Precautions
Attaching the tether kit – Do not overtighten the screw when attaching the tether kit.
Autoclaving – Do not autoclave the patient connector.
Damaged equipment – Periodically, inspect the patient connector, connection port, and cord for damage. If the case of the patient
connector is cracked, or if the power supply connector is damaged, contact your Medtronic representative. Replace the power supply
if there is damage to it. Dispose of the damaged power supply according to local regulations or return the part to Medtronic.
Do not immerse – Take care to prevent liquid from entering the patient connector. Do not immerse the patient connector or any
accessories in any liquid or clean them with aromatic or chlorinated hydrocarbons.
Maintenance and service – Do not modify or do any maintenance or service on the patient connector while you are using it. Doing
any of these tasks on the patient connector while it is in use can lower its effectiveness. Contact Medtronic at the number on the back
cover of this manual if your patient connector is not working properly.
Product and packaging labels and information – If labels or information appear to be missing from the product or packaging,
contact your local Medtronic representative at the address and telephone number located on the back cover of this document.
Security – Maintain adequate physical security of the patient connector to prevent unauthorized use that could lead to harm to
patients. Bluetooth communication in the patient connector is encrypted for security. Medtronic inductive telemetry uses short-range
communication to protect patient information. If the patient connector should fail, there is no risk of patient harm.
Sterility – The patient connector is not sterile and cannot be sterilized. For applications in which a sterile environment must be
maintained, place the patient connector inside of the Medtronic Model 6177 sterile sleeve.
Use of wireless devices – The patient connector incorporates radio-frequency (RF) communications components which may affect
other devices and equipment in the medical environment. The use of wireless devices in the medical environment must be evaluated
and authorized by the responsible organization. RF interference may affect device performance.
Electromagnetic Compliance (EMC) testing shows that the patient connector provides reasonable protection against harmful
interference and provides EMC immunity in a typical medical installation. The use of wireless devices in the medical environment must
be evaluated and authorized by the responsible organization. However, there is no guarantee that interference will not occur in a
particular installation.
7
Page 8

If the patient connector does cause harmful interference to other devices or is negatively impacted by other devices, correct the
interference by one or more of the following measures:
• Reorient or relocate the patient connector and other devices.
• Increase the separation between the patient connector and other devices by at least 2 m (approximately 6 feet). Other devices
include, but are not limited to, cellular phones, computer screens, wireless network devices, and ‘walkie-talkies’.
• Turn off any interfering equipment.
1.6.1 Environmental precautions
To ensure safe and effective operation, use the patient connector with care to avoid damage to the patient connector from
environmental factors that may impair its function. Care is exercised in design and manufacturing to minimize damage to the patient
connector under normal use. However, the patient connector is susceptible to many environmental stresses including, but not limited
to, the following examples.
• The patient connector is designed to be used indoors in a clinical or hospital environment.
• Do not drop or mishandle the patient connector or damage may occur. Damage can impair the functionality of the patient
connector. Even if the patient connector works immediately after being dropped, operational damage may have occurred that may
not be observed immediately.
• Do not spill fluid on the patient connector. Fluid incursion can occur and cause damage to the patient connector.
• The patient connector may be affected by electrostatic discharge (ESD). In an environment likely to cause ESD, such as a
carpeted floor, discharge any charge collected on your body before touching the patient connector.
• Do not open the case of the patient connector. The patient connector is constructed to minimize risk from environmental factors.
Opening the case of the patient connector can make it susceptible to environmental factors and can expose the patient or user
to hazardous voltage or current.
• Do not expose the patient connector to rapid temperature changes. Rapid temperature changes may affect proper operation of
the patient connector. If the patient connector is exposed to rapid temperature changes, allow the temperature to stabilize before
using it.
• Do not store or operate the patient connector for prolonged periods of time in high humidity. Prolonged storage or operation of the
patient connector in high humidity can affect proper operation.
If the patient connector is damaged, contact Medtronic at the telephone number on the back cover of this manual.
Other environmental factors can impair the performance of the patient connector. Always use good health management practices to
prevent environmental damage to the patient connector.
1.7 Regulatory compliance
1.7.1 US Federal Communications Commission (FCC)
This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This device may not cause
harmful interference, and (2) this device must accept any interference received, including interference that may cause undesired
operation.
1.8 Patient Connector functions
The patient connector communicates with an implantable device. The patient connector also communicates with the Medtronic app
running on a mobile device.
1.9 IT network, mobile device, and data information
1.9.1 Required IT network characteristics and configuration
The use of the patient connector requires your network to have Internet access for the following purposes:
• Download and install the Medtronic app from the app store.
• Download and install Medtronic app updates and software updates for the patient connector.
• Register, download, and install app configuration files from the Medtronic Application Services (MAS), if the Medtronic app allows
it.
• Send data to the CareLink Network, if the Medtronic app allows it.
Failure to provide IT network access results in the following effects:
• You cannot install or update the Medtronic app because the mobile device is unable to access the Internet.
• You cannot install periodic updates and security enhancements that maintain the security of the Medtronic app because the
mobile device is unable to access the Internet.
• You cannot send downloaded Medtronic implantable device data to the CareLink Network.
8
Page 9

To use the Medtronic app, you must also enable Bluetooth wireless technology on your mobile device. The patient connector
communicates with the Medtronic app installed on the mobile device through a Bluetooth connection.
Failure to provide Bluetooth wireless communication access prevents the patient connector from communicating with the Medtronic
app. If Bluetooth connectivity is disabled, the patient connector cannot interrogate, analyze or program Medtronic implantable
devices.
Follow your organization’s processes and policies to configure your network and your mobile device.
1.9.2 Supported mobile devices and technical specifications
For a list of supported mobile devices and their specifications, including network connectivity specifications, go to the following
website:
www.medtronic.com/24967
1.9.3 Intended information flows
Data is sent for processing through the system components in the following sequential order:
• Implantable device
• 24967 patient connector
• Medtronic app running on the mobile device
System logs are sent for processing through the system components in the following sequential order:
• 24967 patient connector
• Medtronic app running on the mobile device
• Internet
• Medtronic Application Services (MAS)
During installation and updates, the app sends clinic registration and device manager system credentials to Medtronic Application
Services via the Internet for authentication. In response, Medtronic Application Services returns configuration files to the app via the
Internet to install or update the app and component software as needed.
All information in transit is protected for security.
1.9.4 Precautions when connecting to your IT network
Connecting this system to an IT network that includes other equipment could result in unforeseen risks to patients, operators, or third
parties. Changes to your IT network, such as adding, disconnecting, and upgrading equipment or changing network configurations
could also introduce additional risks. Analyze, evaluate, report, and control any risks identified.
1.9.5 Data transmissions
The patient connector uses Bluetooth wireless technology to communication with the Medtronic app installed on the mobile device.
Data transmission rates over Bluetooth connections are highly dependent on the environment in which the patient connector and
mobile device are used. Transmission rates may degrade based on electrical interference from other radio emitters, distance between
the patient connector and the mobile device, and wireless or cellular settings on the mobile device. Maximum data throughput in an
ideal environment is 2.1 Mbit/s with a latency of 5 to 10 ms.
All data transmitted between the patient connector and the app is encrypted for data privacy. Data integrity is maintained through
standard communication protocols for error detection.
1.9.6 Security
Data in the Medtronic app is protected by application level encryption. The app does not provide data protection for data exported to
another destination. Data exported from the app should be handled in accordance with your clinic’s security policy for data handling
and storage.
To protect the system, Medtronic recommends you implement the following security measures:
• Use the Medtronic app and system components only on a managed, trusted network.
• Secure your network with anti-virus software or a firewall, in line with your IT department’s policies.
• To help protect patient health information, implement security measures such as a passcode and PIN to protect the mobile device.
• Ensure security certificates match before connecting to Medtronic Application Services for app updates.
If you suspect a cybersecurity event has occurred, stop using the app (if possible) and contact your IT Security or Biomedical
department for information on how to confirm and respond to the suspected incident.
9
Page 10

2 Setup and configuration
2.1 Contents of package
The package contains:
• Model 24967 Patient Connector
• MENB1020A0500XXX Power Supply (Medtronic re-order number: 249651)
• Model 249652 Tether Kit
• Literature
2.2 System components
Figure 1. Patient connector components
1 Weight (blue)
2 Patient connector
Patient connector – Provides the communication link between the app and the patient’s implantable device. The patient connector
contains a radio-frequency (RF) transmitter and receiver. It must be placed over the implantable device during a communication
session. The patient connector has a blue counter weight, and when it is unwrapped and placed over the patient’s shoulder can hold
the patient connector in place hands-free.
Mobile device (not included) – When running the Medtronic app and paired with the patient connector, provides the user interface
to communicate with the implantable device, depending on the app installed.
Power supply – Connects to an AC power outlet to charge the patient connector battery.
Tether kit – Semi-permanently connects the patient connector to the power cord. Contains a Torx wrench, screw, and cable retainer.
Warning: Do not modify this equipment. Modifications may reduce system effectiveness and impact user or patient safety. Modifying
the device without the approval of Medtronic could void the user’s authority to operate the equipment.
3 Tether kit
4 Power supply
10
Page 11

2.2.1 Indicator lights
Figure 2. Patient connector indicator lights
1 Telemetry status
2 Bluetooth connection status
Table 1. Patient connector icons and indicator lights Icon Indicator Color State Description
Telemetry status Amber Solid The patient connector is out of range of
Green Solid The patient connector is actively commu-
Bluetooth connection status
Battery status Amber Solid The patient connector battery is low.
Blue Flashing The patient connector is broadcasting its
Blue Solid The patient connector is connected to the
Green Flashing The patient connector battery is charg-
Green Solid The patient connector battery is fully
3 Battery status
the implanted device.
nicating with the implanted device. The
light becomes brighter when the signal
from the implanted device is stronger.
status or is being paired with the mobile
device.
mobile device.
ing.
charged.
2.3 Compatible accessories
The following compatible accessories are available for the patient connector:
• MENB1020A0500XXX power supply (Medtronic re-order number: 249651XX), 2 m (approximately 6.5 feet)
• Model 249672 tether kit
• Model 6177 sterile sleeve (not included)
• Model 24970A Base (not included)
• Model 249702 USB cable, approximately 3 m (10 feet) (not included)
Contact your local Medtronic representative to order them.
Warning: Use the Medtronic-supplied components only. Use of unapproved components may reduce device effectiveness or impact
user or patient safety.
11
Page 12

2.4 Setup
This section describes how to:
• Power on the patient connector
• Power off the patient connector
• Download the app
• Attach the optional tether kit
2.4.1 Powering on the patient connector
1. Locate the button on top of the patient connector. Figure 3. Locate the patient connector button
1 Patient Connector button
2. To power on the patient connector, press and release the button.
The Bluetooth connection status light flashes when the patient connector is turned on.
2.4.2 Powering off the patient connector
If the patient connector is not charging and has not been paired with the app, it powers off automatically after 5 minutes of inactivity.
If the patient connector is not charging and has been paired with the app, for example after a patient session, it powers off automatically
after 15 minutes of inactivity.
If the patient connector is charging, the Bluetooth radio turns off automatically after 5 minutes of inactivity.
2.4.3 Downloading the app
1. Make sure the mobile device is connected to the Internet.
2. Navigate to the app store and locate the app.
3. Tap the app download button to download the app onto the mobile device.
4. Install the app.
5. Tap the app icon to open it.
6. Follow the instructions in the app to complete the installation.
2.4.4 Attaching the optional tether kit
The tether kit provides a way to semi-permanently attach the patient connector to the power supply cord. This is an optional
configuration.
1. Locate the power supply connector cover on the bottom of the patient connector.
2. Use the Medtronic-supplied wrench to remove the screw.
12
Page 13

3. Remove the power supply cover from the patient connector.
4. Plug the power supply into the patient connector.
5. Slide the cable retainer over the power supply plug of the power cord.
6. Insert the screw into the bottom of the cable retainer and tighten the screw using the Medtronic-supplied wrench.
Caution: Do not over tighten the screw when attaching the tether kit.
Warning: Use the Medtronic-supplied components only. Use of unapproved components may reduce patient connector
effectiveness or impact user or patient safety.
2.5 Charging the patient connector battery
Caution: Charge the patient connector before use. If the patient connector is not adequately charged before beginning a procedure,
you may not be able to complete that procedure.
Charge the patient connector using the Medtronic-supplied power supply.
There are two additional ways to charge the patient connector using the 24970A base. For more information on charging, refer to the
instructions for use provided with the 24970A base. Contact your Medtronic representative for details.
Notes:
• The patient connector and power supply form a medical electrical system when connected.
• If you need to disconnect the patient connector from the AC power mains, the power supply is the power disconnect at the mains
outlet.
• Position the patient connector so that it can be easily disconnected or unplugged from the wall.
• The third conductor in the power supply plug, if present, is a functional earth connection.
• If the operational time between charges decreases or the patient connector internal rechargeable battery no longer retains a
charge, contact Medtronic for a replacement
2.5.1 Charging using the power supply
Connect the power supply to the patient connector and to an AC power outlet (AC mains) to charge the patient connector.
13
Page 14

1. Open the power supply connector cover located on the bottom edge of the patient connector.
2. Connect the power supply cord to the patient connector.
3. Plug the power supply into the AC power outlet (AC mains).
Warning: Use only the Medtronic-supplied power supply to power the patient connector. Use of an unapproved power supply may
damage equipment or impact user or patient safety.
2.6 Pairing the patient connector with the app
The patient connector uses a Bluetooth connection to communicate with the app running on the mobile device. You will need to pair
each patient connector to the mobile device.
1. Enable Bluetooth on the mobile device, if it is not enabled.
2. Open the app.
3. Follow the instructions in the app to continue.
4. Enter the patient connector model number, if you are prompted. The model number is located on the back of the patient
connector.
5. Turn on the patient connector by pressing the button when prompted by the app. The Bluetooth connection status light on the
patient connector flashes when it is being paired with the mobile device, and is solid when communication is established with the
mobile device.
6. When prompted by the app, select the patient connector you want to use from the list.
14
Page 15

7. Locate the security key code on the back of the patient connector. Figure 4. Patient Connector security key
1 Security key symbol
2 Security key code
8. Enter the security key code and follow the instructions in the app to continue.
9. Accept the Bluetooth pairing request on the mobile device.
10. Complete the configuration steps in the app.
2.7 Troubleshooting potential interference
Interference between the patient connector and other electronic equipment can result in reduced quality of service. Reduced quality
of service could result in slow data transmission speeds or poor communication strength.
For more information on app-specific error messages see the instructions for use provided with the Medtronic app.
To address possible interference between the patient connector and other electronic equipment, take one or more of the following
measures:
• Reorient or relocate the electronic equipment.
• Increase the separation between the patient connector and the electronic equipment.
• Connect the electronic equipment to an outlet on a different circuit, if the patient connector is plugged into the outlet for charging.
• Move the patient connector and mobile device closer together.
• Consult Medtronic for help.
3 Conducting a patient session
3.1 Positioning the patient connector
The patient connector can either be balanced over the patient’s shoulder using the blue weight for hands-free use or held over the
implanted device. The patient connector, blue weight, and cord come into physical contact with the patient during normal use of the
device. Use the Model 6177 sterile sleeve if sterility is a concern.
1. For hands-free use, unwrap the blue weight and cord from the patient connector and place the weight over the patient’s shoulder.
2. Position the patient connector over the implantable device.
Note: The bottom of the patient connector must be parallel to and typically within 5 cm of the implantable device.
3. Verify that the green telemetry status light on the patient connector is on. The telemetry status light will become brighter when
the signal from the device is stronger. This indicates better telemetry.
For best telemetry performance, do not use the patient connector close to sources of electrical noise or interference.
3.2 Communicating with an implantable device
To ensure good Bluetooth communication, make sure the mobile device is no further than 2 m (6.5 feet) from the patient connector
during the patient session.
Caution: Charge the patient connector before use. If the patient connector is not adequately charged before beginning a procedure,
you may not be able to complete that procedure.
Note: Place the Model 6177 sterile sleeve over the patient connector if the patient connector is being used in a sterile environment.
15
Page 16

1. Tap the app icon to open it.
2. If the patient connector isn’t already on, turn on the patient connector by pressing the button.
3. Place the patient connector over the implantable device. The telemetry status light on the patient connector is green when
communication is established with the implantable device. The telemetry status light is brighter when there is a stronger signal.
4. Follow the instructions in the app to start communicating with the implantable device.
5. Leave the patient connector over the implantable device until the communication is complete. Communication can take up to 5
minutes.
Caution: Only use the patient connector to communicate with the intended implanted device. Avoid placing the patient
connector over an unintended active implanted device. Placing the patient connector over an unintended active implanted
device could interfere with that device, potentially affecting its operation.
6. Follow the instructions in the app to complete the task.
3.3 Troubleshooting
An error or an informational message displays in the app if there is a problem with the patient connector. The message may have an
animated demo with directions on how to resolve the condition. Refer to the app online help for additional information about specific
error codes. For some conditions, contact Medtronic using the information found on the back cover of this manual.
3.3.1 Patient connector battery status
If the patient connector needs to be recharged, the following condition occurs:
Condition Action Result
The battery status light on the patient connector is amber.
Recharge the patient connector battery. When the patient connector is charging the
battery status light flashes green. When
the battery is fully charged the battery status light is solid green.
3.3.2 Patient connector position
Position the patient connector correctly over the implantable device to communicate with it. If you do not position the patient connector
properly over the implantable device, the following condition may occur:
Condition Action Result
The telemetry status light is amber. Reposition the patient connector over the
implantable device.
If the implantable device is implanted in the
patient, additional implanted devices may
interfere with the communication between
the patient connector and the patient’s
implanted device. If the patient has more
than one implanted device and you are
unable to communicate with the patient’s
implanted device, reposition the patient
connector closer to the patient’s implanted
device.
The telemetry status light is off. Confirm that the implantable device is a
Medtronic device. The patient connector
only works with Medtronic implantable
devices.
Reposition the patient connector over the
Medtronic implantable device.
3.3.3 Bluetooth connection lost
The patient connector pairs with a mobile device to send data. If the patient connector loses the Bluetooth connection with the mobile
device, the following condition occurs:
When the telemetry status light turns
green, the patient connector is communicating with the implantable device. The
telemetry status light is brighter when the
communication from the device is stronger.
When the telemetry status light turns
green, the patient connector is communicating with the implantable device. The
telemetry status light is brighter when the
communication from the device is stronger.
Condition Action Result
The Bluetooth connection status indicator
light flashes.
Check that Bluetooth is enabled on the
mobile device. Enable Bluetooth on the
mobile device if it is not enabled.
Move the mobile device closer to the
patient connector and confirm that the blue
light on the patient connector is on. Check
16
The Bluetooth connection status light is on.
When the blue light is on, Bluetooth has
established a connection between the
patient connector and the mobile device.
Page 17

Condition Action Result
that nothing physical is blocking the signal
between the patient connector and the
mobile device.
4 Maintaining the patient connector
4.1 Cleaning and disinfecting the 24967 patient connector
4.1.1 Cautions and notes for cleaning and disinfecting
Cautions:
• Clean and disinfect the patient connector as needed according to your organization’s policies. Use only the recommended
methods to clean and disinfect the patient connector. Depending on the level of contamination, such as exposure to blood or body
fluid, Medtronic recommends cleaning and disinfecting the patient connector promptly after use to minimize drying and cross
contamination.
• Use only recommended cleaning and disinfecting methods on the patient connector. Using other cleaners, solvents, or
disinfectants (such as bleach, ethers, acetone, or chlorinated solvents) may damage the patient connector plastic, circuitry, or
metal components.
• Do not immerse the patient connector in water or cleaning agents. Do not use automated machine washers. Severe damage to
the patient connector may occur.
• Do not sterilize the patient connector by ethylene oxide, gamma radiation or steam-sterilization (autoclave). Severe damage to the
device, housing, or labels may occur using these methods.
Notes:
• The patient connector is designed to withstand normal cleaning and disinfecting over its product life.
• The disinfecting procedure below has been tested and shown to achieve a log 4.8 or greater reduction in pathogens. If your
organization or application requires a higher level of disinfection, place the patient connector inside a sterile barrier (such as the
Medtronic Model 6177 sterile sleeve).
• During procedures in a sterile environment or when cross contamination is a concern, place the patient connector inside a sterile
barrier (such as the Medtronic Model 6177 sterile sleeve).
• The power cord, USB cable, and optional tether kit accessories cannot be disinfected effectively. If these accessories become
contaminated, discard the accessory and contact Medtronic for a replacement. When using the optional tether kit to secure the
USB cable or power cord, place the patient connector inside of the Medtronic Model 6177 sterile sleeve to limit patient contact and
reduce device exposure to contamination.
4.1.2 Preparing the patient connector
1. If the patient connector is connected plugged into a power source, unplug it. If you are using the optional tether kit to secure the
cord to the patient connector, only unplug the cord from the power source.
2. The patient connector powers off automatically in 5 min when it is not connected to a power source. Wait 5 min for the patient
connector to power off.
The LED lights turn off when the patient connector powers off.
17
Page 18

3. Unwrap the blue weight from around the patient connector and fully extend the cord.
4.1.3 Cleaning the patient connector
1. Clean the patient connector thoroughly using one of the following cleaning methods:
• A 70% isopropyl alcohol prep pad.
• A sterile gauze pad or sponge dampened with 70% isopropyl alcohol.
2. Wipe all external surfaces of the patient connector to remove all visible soil.
a. Wipe the blue weight and its cord.
b. Wipe all sides of the patent connector, including the cord retention channel and cable power supply connector cover or
optional tether kit.
c. Wipe the top of the patient connector.
d. Wipe the bottom of the patient connector.
18
Page 19

e. If the optional tether kit is installed to secure the power supply cord or USB cable, wipe the cord or cable for approximately
1 m (3 feet) extending from the patient connector.
3. Allow to air dry approximately 5 minutes or until dry. Leave the blue weight fully extended until it is dry.
4.1.4 Disinfecting the patient connector
1. Follow the steps in the previous section to thoroughly clean all external surfaces of the patient connector.
You do not need to allow the patient connector to dry if you are disinfecting it after cleaning.
2. If the optional tether kit is installed, remove the tether kit and unplug the USB cable or power cord. Reinstall the cable connector
port cover to seal the connector port contacts.
The USB cable, power cord, and optional tether kit accessories cannot be disinfected effectively. If these accessories become
contaminated, remove them from the patient connector and discard. Contact Medtronic for a replacement.
3. Disinfect the patient connector with one of the following materials:
• Several 70% isopropyl alcohol prep pads, or sterile gauze pads or paper wipes dampened with 70% isopropyl alcohol.
• Several 1.4% hydrogen peroxide wipes.
4. Fully wrap all external surfaces of the patient connector with the damp prep pads, sterile gauze pads, or wipes.
a. Wrap the blue weight and its cord lengthwise to completely cover both.
19
Page 20

b. Wrap all sides of the patient connector to completely cover it.
5. To maintain a wet or damp exposure time of 15 minutes and reduce evaporation, place all wrapped components of the patient
connector inside a plastic bag or container. Seal the bag or container to reduce evaporation.
6. After 15 minutes have elapsed, remove all wrapped components of the patient connector from the plastic bag or container.
7. Remove the damp prep pads, paper wipes, or sterile gauze from the exterior of the patient connector and all its components.
8. Safely discard the damp prep pads, paper wipes, or sterile gauze according the policies and procedures of your organization.
9. Allow to air dry approximately 5 minutes or until dry. Leave the blue weight fully extended from its cord during drying.
20
Page 21

4.1.5 Reassembling the patient connector
1. Rewrap the blue weight around the patient connector and secure it in the top of the retention channel.
2. Install a new replacement tether kit with a new USB cable or power supply, if needed. This is an optional configuration.
4.1.6 Additional resources
For additional information about cleaning and disinfecting the patient connector, contact Medtronic Instruments Technical Services:
• Phone: +1 800 638 1991
• Email: tshelp@Medtronic.com
For more information and resources on cleaning and disinfecting medical devices, visit the CDC and HICPAC websites.
4.2 Replacing the patient connector nose, cable, and weight
You can replace the patient connector nose, cable, and weight if they become damaged. Contact your Medtronic representative to
order the replacement parts.
1. Locate the screw on the nose of the patient connector.
2. Use the Medtronic-supplied wrench to loosen and remove the screw.
21
Page 22

3. Unwrap the blue weight from around the patient connector and fully extend the cord.
4. Remove the nose, the cable, and the weight.
5. Thread the cable with the weight through the hole in the nose.
6. Place the new nose on the patient connector and line up the screw hole.
22
Page 23

7. Wrap the blue weight around the patient connector and insert the screw into the screw hole. Use the Medtronic-supplied wrench
to tighten it.
4.3 Software updates
Software updates will be pushed automatically to the patient connector when you start a communication session in the app. Updates
can take up to 5 minutes. You must wait until the update is complete before you can continue with the session.
4.4 Specifications
Table 2. Patient connector specifications
Standards (The patient connector complies with the following:)
Radio frequency wireless specifications and applicable standards
EMC EN / IEC 60601-1-2
EN 300 328
EN 301 489
EN 302 195
EN 55011 Class B
EN 55024 Class B
Radio FCC CFR 47
Patient safety UL/CUL 60601-1, Type BF applied part
EN 60601-1, Class 2, continuous operation, Type BF
AC power requirement
Voltage 100–240 VAC nominal
Frequency 50/60 Hz nominal
Battery
Type Li-polymer, rechargeable
Capacity 1500 mAh
Charge duration Standby: 15 days
Operating: 2 hours (typical)
Voltage 3.7 V
Patient connector charger specifications
Power supply
Model MENB1020A0500XXX power supply (Medtronic re-order number 249651)
Voltage in 100-240 VAC 0.5A at 50-60 Hz
Voltage out 5 VDC 3 A
Electrical shock protection class Class II
Intended duty Continuous
USB cable
Model 249702 USB cable
Voltage 5 V 0.8 A
Power 4W
24970A charge cradle
Model 24970A
Voltage 5 V 3.0 A
Power 15 W
IEC 60529 Degrees of Protection Provided by Enclosures (IP Code)
Ingress This product complies with international electrical safety rating IP2X with regard to
ingress of dust, other foreign objects, and water as required by IEC 60601-1.
23
Page 24

Table 2. Patient connector specifications (continued)
Physical dimension and weight
Length
Width
Depth
Weight
Operating
Storage
Transport
Operating
Storage
Transport
Maximum 3000 m
Connectivity
Frequency range 150-200 kHz
Modulation frequency Frequency shift key, phase shift key, On/Off keying
Output power 30 dBµA/m @ 10 m
Frequency range 2.4-2.483 GHz
Modulation frequency Gaussian frequency shift key
Output power Less than 10 mW EIRP
16.7 cm (6.6 in)
7.3 cm (2.9 in)
3.0 cm (1.2 in)
0.25 kg (0.55 lbs)
Temperature limits
10°C to 35°C (50°F to 95°F)
15°C to 30°C (59°F to 86°F)
- 30°C to 55°C (-22°F to 131°F)
Humidity limits
8%-80%
15%-93% at 35°C (95°F)
15%-93% at 35°C (95°F)
Altitude
Low frequency inductive telemetry
Bluetooth wireless technology 2.1 and 4.0
4.4.1 Expected service life
The patient connector including the internal battery has an expected service life of 5 years. If you notice decreased operational time
between battery charges or the battery no longer holds its charge, contact Medtronic to get a replacement patient connector.
4.4.2 Disposal of the patient connector
Return the patient connector to Medtronic for proper disposal. Contact Medtronic at the address or telephone number on the back
cover for information on returning the patient connector.
5 Electromagnetic compatibility declaration
The following list of accessories were included in the system demonstrating compliance with the requirements of IEC 60601-1-2 2007
clauses 7 and 8:
Accessory Maximum length
MENB1020A0500XXX Power Supply
(Medtronic re-order number: 249651)
Use of accessories other than what is specifically listed may result in increased emissions or decreased immunity of the 24967 patient
connector.
The 24967 patient connector needs special precautions regarding electromagnetic compatibility (EMC) and needs to be installed and
used according to the EMC information provided in the accompanying documents.
The 24967 patient connector should not be used adjacent to or stacked with other equipment. If adjacent or stacked use is necessary,
the 24967 patient connector should be observed to verify normal operation in the configuration in which it will be used.
The 24967 patient connector contains RF transmission and receiving capabilities. Consequently, it is possible that other equipment
may interfere with the 24967 patient connector even if that other equipment complies with CISPR emission requirements. The
following is a technical summary of the RF communication properties:
Transmitting and receiving:
• Frequency of operation: 150 kHz to 200 kHz, 2400 MHz to 2483.5 MHz
• Modulation characteristics: 18K0M1D or 74K4F1D, FHSS, DTS
• Field strength: less than 30 dBµA/m @ 10 m, less than 10 mW EIRP
2 m (approximately 6.5 feet)
24
Page 25

Guidance and manufacturer’s declaration—electromagnetic emissions
The 24967 patient connector is intended for use in the electromagnetic environment specified below. The customer or the user of
the 24967 patient connector should assure that it is used in such an environment.
Emissions test Compliance Electromagnetic environment—guidance
RF emissions
CISPR 11
RF emissions
Group 1 The 24967 patient connector uses RF energy only for its internal func-
tion. Therefore, its RF emissions are very low and are not likely to cause
Class B
any interference in nearby electronic equipment.
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/flicker emissions
IEC 61000-3-3
Class A The 24967 patient connector is suitable for use in all establishments,
including domestic establishments and those directly connected to the
Complies
public, low-voltage power supply network that supplies buildings used
for domestic purposes.
Guidance and manufacturer’s declaration—electromagnetic immunity
The 24967 patient connector is intended for use in the electromagnetic environment specified below. The customer or the user of
the 24967 patient connector should assure that it is used in such an environment.
Immunity test IEC 60601 Test level Compliance level Electromagnetic environment—guid-
ance
Electrostatic discharge (ESD) ±6 kV contact ±8 kV contact Floors should be wood, concrete, or
IEC 61000-4-2 ±8 kV air ±15 kV air
ceramic tile. If floors are covered with synthetic material, the relative humidity should
be at least 30%.
Electrical fast transient/burst ±2 kV for power supply
lines
IEC 61000-4-4 ±1 kV for input/ output
lines
Surge ±1 kV differential
mode
±2 kV for power supply
lines
±1 kV for input/ output
lines
±1 kV differential
mode
Mains power quality should be that of a typical commercial or hospital environment.
IEC 61000-4-5 ±2 kV common mode ±2 kV common mode
Voltage dips, short interruptions, and
voltage variations on power supply
input lines
IEC 61000-4-11
Power frequency (50/60 Hz) magnetic field
IEC 61000-4-8
<5% U
T
(>95% dip in UT)
for 0.5 cycle
40% U
T
(60% dip in UT)
for 5 cycles
70% U
T
(30% dip in UT)
for 25 cycles
<5% U
T
(>95% dip in UT)
for 5 s
<5% U
T
(>95% dip in UT)
for 0.5 cycle
40% U
T
(60% dip in UT)
for 5 cycles
70% U
T
(30% dip in UT)
for 25 cycles
<5% U
T
(>95% dip in UT)
for 5 s
Mains power quality should be that of a typical commercial or hospital environment. If
the user of the 24967 Patient Connector
requires continued operation during power
mains interruptions, it is recommended that
the 24967 Patient Connector be powered
from an uninterruptible power supply or a
battery.
Note:UT is the AC mains voltage prior to
application of the test level.
3 A/m 30 A/m Power frequency magnetic fields should be
at levels characteristic of a typical location in
a typical commercial or hospital environment.
25
Page 26

Guidance and manufacturer’s declaration—electromagnetic immunity
The 24967 patient connector is intended for use in the electromagnetic environment specified below. The customer or the user of
the 24967 patient connectorshould assure that it is used in such an environment.
Immunity test IEC 60601 Test level Compliance level Electromagnetic environment—guid-
ance
Portable and mobile RF communications
equipment should be used no closer to any
part of the 24967 patient connector, including cables, than the recommended separation distance calculated from the equation
applicable to the frequency of the transmitter.
Recommended separation distance
Conducted RF 3 V
(volts root-
RMS
10 V d = 0.35√P
meansquare)
IEC 61000-4-6 150 kHz to 80 MHz
Radiated RF 3 V/m 10 V/m d = 0.35√P for 80 MHz to 800 MHz
IEC 61000-4-3 80 MHz to 2.5 GHz d = 0.70√P for 800 MHz to 2.5 GHz
where P is the maximum output power rating of the transmitter in watts (W) according
to the transmitter manufacturer and d is the
recommended separation distance in
meters (m).
Field strengths from fixed RF transmitters,
as determined by an electromagnetic site
survey,a should be less than the compliance
level in each frequency range.
b
Interference may occur in the vicinity of
equipment marked with the following symbol:
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects, and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM
and FM radio broadcast, and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to
fixed RF transmitters, consider an electromagnetic site survey. If the measured field strength in the location in which the 24967 patient connectoris
used exceeds the applicable RF compliance level above, observe the 24967 patient connectorto verify normal operation. If abnormal performance
is observed, additional measures may be necessary, such as re-orienting or relocating the 24967 patient connector.
b
Over the frequency range of 150 kHz to 80 MHz, field strengths should be less than 10 V/m.
Recommended separation distances between portable and mobile RF communications equipment and the 24970A base
The 24967 patient connector is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the 24967 patient connector can help prevent electromagnetic interference by maintaining a
minimum distance between portable and mobile RF communications equipment (transmitters) and the 24967 patient connector as
recommended below, according to the maximum output power of the communications equipment.
Rated maximum output power of
transmitter
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5 GHz
Separation distance according to frequency of transmitter
d = 0.35 √P d = 0.35 √P d = 0.70 √P
0.01 W 0.035 m 0.035 m 0.070 m
0.1 W 0.11 m 0.11 m 0.22 m
1 W 0.35 m 0.35 m 0.70 m
10 W 1.1 m 1.1 m 2.0 m
100 W 3.5 m 3.5 m 7.0 m
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be
estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
26
Page 27

Recommended separation distances between portable and mobile RF communications equipment and the 24970A base
Note 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects, and people.
27
Page 28

Page 29

Page 30

Medtronic, Inc.
*M966328A001*
710 Medtronic Parkway
Minneapolis, MN 55432
USA
www.medtronic.com
+1 763 514 4000
Medtronic USA, Inc.
Toll-free in the USA (24-hour technical consultation for physicians and
medical professionals)
Bradycardia: +1 800 505 4636
Tachycardia: +1 800 723 4636
Europe/Middle East/Africa
Medtronic International Trading Sàrl
Route du Molliau 31
Case Postale 84
CH-1131 Tolochenaz
Switzerland
+41 21 802 7000
Technical manuals
www.medtronic.com/manuals
© 2016 Medtronic
M966328A001 C
2016-08-16
 Loading...
Loading...