Page 1

MEDITOUCH 2 CONNECT
Blood glucose monitor
Memory slots
Test reminder
USB port
Art. 79046
0483
for self-testing
®
MediTouch 2
connect
Instruction manual
Please read carefully!
IVD
98/79 EG
Certified
medical device
3
Years warranty
Bluetooth 4.0
®
Page 2

Contents
Device and controls
1 Explanation of symbols
2 Safety information
2.1 Always observe the following points
2.2 Instructions for your health
2.3 Instructions on how to use the blood
glucose test strips
2.4 Instructions on how to use the control
solution
3 Useful information
3.1 Items supplied and packaging
3.2 Special features of the MEDISANA blood
glucose monitor MediTouch 2 connect
4 Getting started
4.1 Inserting the batteries
4.2 Setting the time and date
5 Operating
.............................................................
5.1 Using the control solution
5.2 Preparing the blood glucose test
5.3 Using the AST cap
5.4 Determining the blood glucose level
5.5 Discarding used lancets
5.6 Evaluating a test result
5.7 Typical symptoms of high or low blood
glucose
2
...............................................
.....................................
..............................................
...............
.............................
...........................................
...........................................................
..............................................
........................
®
..........
....................................................
.....................................
................................
...............................
....................
..........................................
..............
.................................
...................................
............................................................
6 Memory
3
6.1 Saving the test results
6
6.2 Accessing and deleting test results
8
7 Integration in VitaDock online
8
8 Data transfer to VitaDock+ app via
11
Bluetooth
8.1 Manual data transfer of measured results
13
to VitaDock+ app
9 Miscellaneous
14
9.1 Display and troubleshooting
15
9.2 Cleaning and maintenance
15
9.3 Reset the meter
9.4 Technical specifications
16
9.5 Accessories
17
9.6 Disposal
17
10 Warranty
19
10.1 Warranty and repair terms
20
10.2 Service address
20
..............................................................
.....................................
®
®
..........................................................
®
............................................
..................................................
...............................................
..................................
.....................................................
..........................................................
.........................................................
...........................................
24
27
29
32
33
35
.................
........................
®
..........................
.............................
............................
36
36
36
38
40
41
42
42
47
48
48
49
50
51
51
52
Page 3
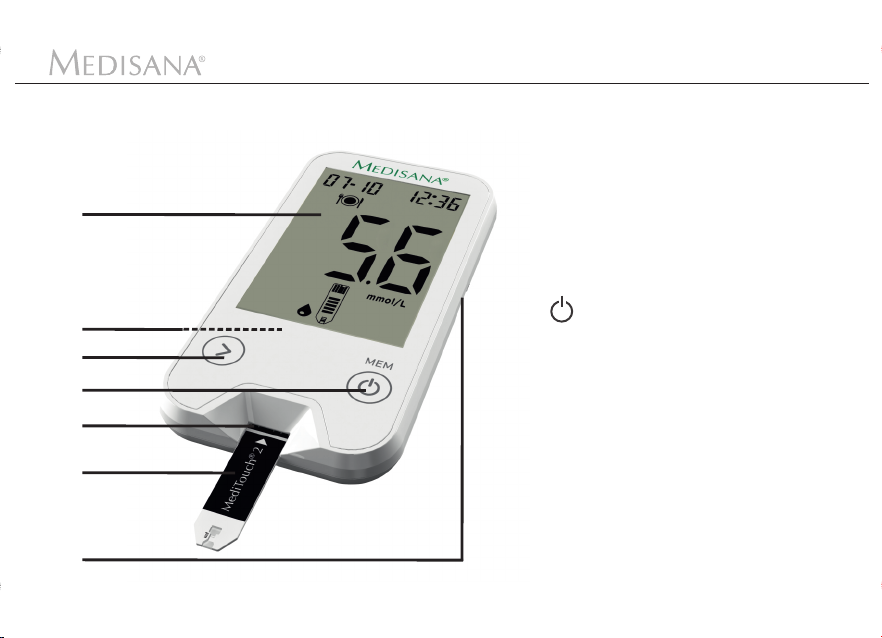
Device and controls
Monitor
1
2
3
4
5
6
7
Display
1
Battery compartment
2
(on the back)
- Button
3
>
for memory access, to enter
values and to look at test results
- Button
4
to confirm settings, delete results or to switch on the device
(press and hold for appr. 3
seconds).
Insertion slot for test strips
5
Test strip
6
Connection for USB cable
7
3
Page 4
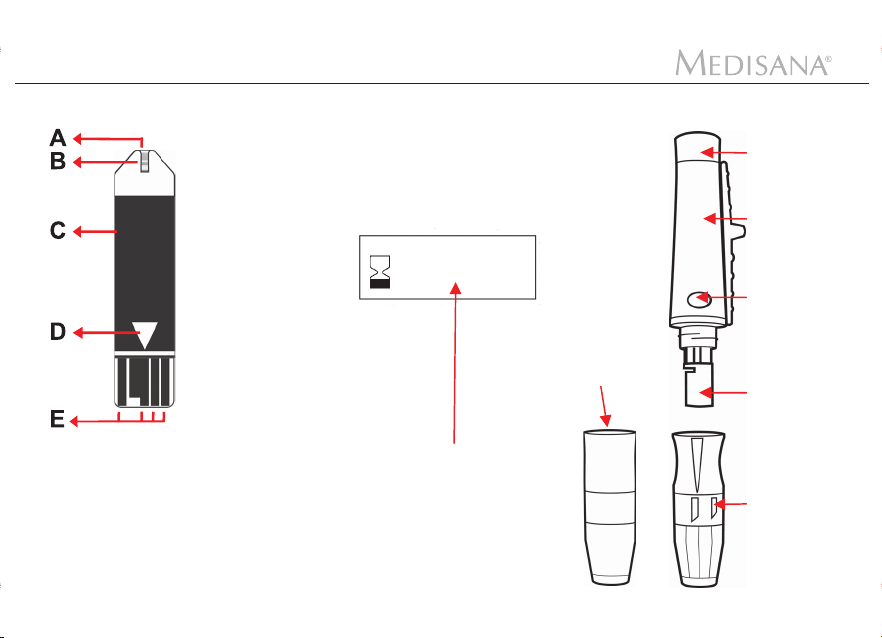
Device and controls
Test strips, control solution and lancing device
Sleeve
Hub
A. Blood sample area
(absorbent slot)
B. Reaction cell
C. Grip/holder
D. Insert in the direction
of the arrow
E. Contact electrode
4
YYYY-MM
Sample illustration
Expiration
date
AST cap
Trigger
button
Carrier
Cap end
(adjustable)
Page 5
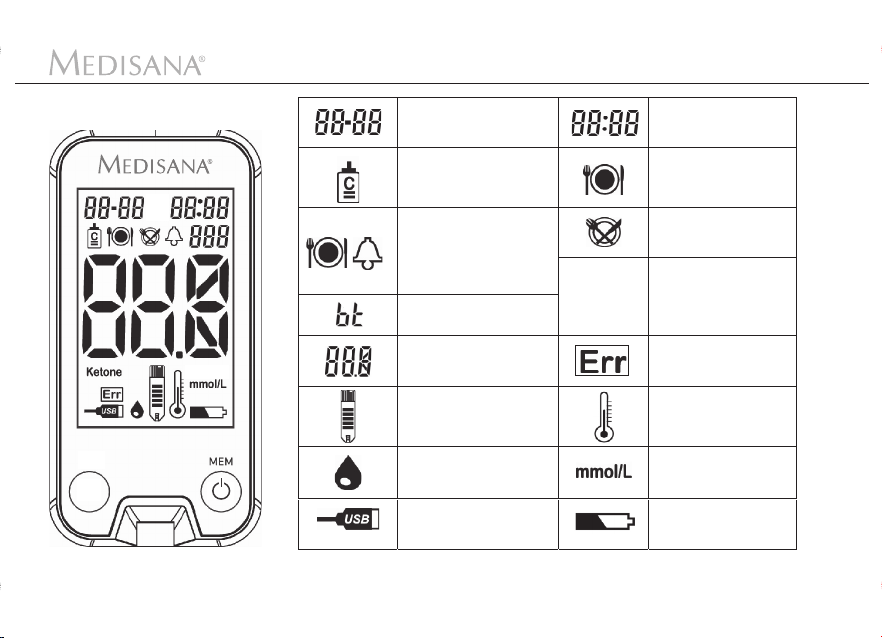
Device and controls
Display
>
Date (month:day)
Control solution
measurement mode
Alarm (before meals
with automatic alarm
after 2 hours to remind
you to perform a test
after meals)
Bluetooth -
Connection
Blood glucose
reading
Insert test
Apply blood or
control solution
Active USB
connection
®
strip
Ketone
Time
(hours:minutes)
Before
Meals (AC)
After
Meals (PC)
arning regarding
a possible diabe-
tical ketoacidosis.
Consult doctor!
System error
Ambient tempe-
ratur error
Unit of measure
Battery symbol
(low battery)
5
Page 6
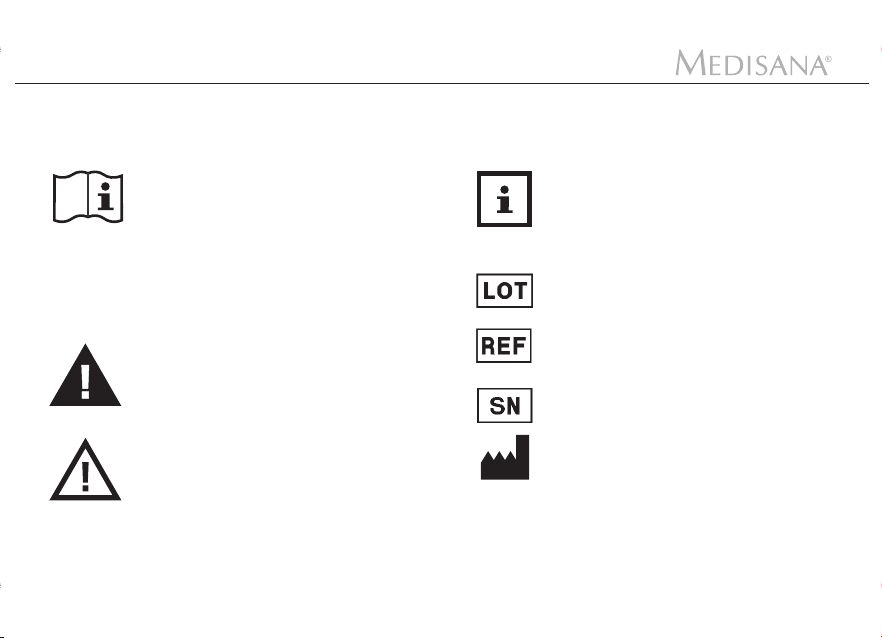
1 Explanation of symbols
The following signs and symbols on items, packaging and in the instruction manual bear important
information:
This instruction manual belongs to this
device. It contains important information
about starting up and operation. Read
the instruction manual thoroughly.
Non-observance of these instructions
can result in serious injury or damage
to the device.
WARNING
These warning notes must be observed
to prevent any injury to the user.
CAUTION
These notes must be observed to
prevent any damage to the device.
6
NOTE
These notes give you useful
additional information on the
installation or operation.
LOT number
Catalogue number
Serial number
Manufacturer
Page 7

1 Explanation of symbols
In vitro diagnostic medical device
(for external use only)
This blood glucose monitor corresponds to the requirements of the EU
guideline 98/79 for in vitro diagnostic devices.
Do not re-use
Use by
Storage temperature range
Contains sufficient for <n> tests
<n>
Control solution
Discard 6 months after opening
7
Page 8
2 Safety information
IMPORTANT INFORMATION!
RETAIN FOR FUTURE USE!
Read the instruction manual carefully before using this device, especially the
safety instructions, and keep the instruction manual for future use.
Should you give this device to another person, it is vital that you also pass on
these instructions for use.
2.1 Always observe the following points
Intended use
This system is intended for taking an adult’s blood glucose test from the finger tip, alternatively
•
from the ball of the hand or lower arm. Thereby it is a matter of a fast, electro-chemical
determination of the blood glucose level. The FAD-binding glucose-dehydrogenase converts
the glucose in human blood to gluconolactone. The device measures the current, which is
released by this reaction and which is in proportion to the blood glucose volume.
The system is intended for external use (in vitro) and can be used for self-testing by persons
•
with diabetes or in clinical settings by healthcare professionals as an aid to monitor the
effectiveness of diabetes control.
8
Page 9
2 Safety information
Contraindications
The system is not suitable for diagnosing diabetes for children younger than 12 years.
•
For use on older children ask your doctor.
It is not suitable for diagnosing diabetes or for testing the blood glucose levels of newborn
•
babies.
• This device measures in mmol/L.
• The unit is only to be used for the specific purpose described in this instruction manual.
• Any misuse will void the warranty.
• Only use the accessories which have been recommended by the manufacturer (test strips,
control solution) with this monitor.
• This device is not designed to be used by persons (including children) with limited physical,
sensory or mental abilities, or by persons with insufficient experience and/or knowledge, unless
under observation by a person responsible for their safety, or unless they have been instructed in
the use of the device.
• Children must be supervised to ensure that they do not play with the device.
• Do not operate the device in the vicinity of highfrequency transmitters, e.g. microwave and short
wave transmitters.
• Do not use the device if it is not working correctly, if it has been dropped or has fallen into water
or has been damaged.
• Protect the unit from moisture. Should moisture enter the unit, remove the batteries and stop
using it immediately. Contact your authorised service centre.
9
Page 10

2 Safety information
• If a fault occurs, do not try to repair the unit yourself. Attempts to do so will void the warranty.
Refer all servicing to authorized service personnel.
• Always keep the monitor clean and store it in a safe place. Protect the monitor from direct
sunlight to prolong its service life.
• Do not store the monitor and the test strips in a vehicle, bathroom or refrigerator.
• Extremely high humidity can affect the test results. A relative humidity of more than 90% can
cause incorrect readings.
• Store the monitor, the test strips and the lancing device out of reach of children and pets.
• Remove the battery if you do not intend to use the monitor for one month or longer.
• Consult your healthcare professional before making a blood glucose test with this device.
10
Page 11
2 Safety information
2.2 Instructions for your health
• This blood glucose monitor is intended for
actively testing a person’s blood glucose at
home. The reading from a blood glucose
home-test system does not replace a
professional test performed in a laboratory.
• The monitor is intended for external use only
(in vitro).
• Only use fresh, capillary whole blood from the
finger tips for the test.
• You may only adapt the procedure for using
products at home and self-monitoring, if you
have first received the appropriate training to
do so.
• Do not change any treatments as a result of
your blood glucose reading without first
consulting your doctor.
• The system is not suitable for testing critically
ill patients.
• Your monitor only requires one small drop of
blood to perform a test. You can get this from
a fingertip. Use different place for each test.
Repeated lancing in the same place can lead
to infection and numbness.
• Test results below 60 mg/dL (3.3 mmol/L) are
an indication of hypoglycemia, meaning the
blood glucose level is too low. If the reading is
above 240 mg/dL (13.3 mmol/L), symptoms of
a high blood glucose level (hyperglycemia)
can occur. Consult your doctor, if your readings
are regularly above or below these levels.
• If the test results display “HI” or “LO”, perform
the test once again. If you obtain similar rea dings again, consult your doctor immediately
and follow his instructions.
• If the proportion of red blood cells (haemotocrit
level) is very high (over 55 %) or very low
(under 30 %), this could distort the test results.
• Dehydration or a lack of water (such as from
sweating) can result in incorrect readings. If
you think you are suffering from dehydration or
a lack of water, consult your doctor immediate ly.
• If you have followed the instructions in this
manual and symptoms persist which are not
associated with your blood glucose level or
your blood pressure, consult your doctor.
11
Page 12
2 Safety information
• For additional advice on your health, read the
instruction manual for the test strips carefully.
WARNING
Risk of infection
Used test strips and lancets are considered to
•
be hazardous, biological non-biodegradable
waste. Carelessness when disposing of these
items can lead to the spread of infection.
If necessary, consult your local waste disposal
company, your doctor or pharmacist.
Dispose of your used test strips and used
•
lancets carefully. If you dispose of the used
parts with the household rubbish, make sure
you wrap them first to avoid harming or
infecting other people.
Medical staff or others who use this monitor
•
on more than one patient must be aware that
all products or objects which come into
contact with human blood must be dealt with
as if they are capable of spreading viral
diseases, even after cleaning.
Never use a lancet or the lancing device on
•
more than one person.
12
Use a new sterile lancet and a new test strip
•
for every test.
Lancets, test strips and alcohol pads are
•
disposable.
Avoid getting hand cream, oil or dirt in or on
•
the lancet, lancing device and test strip.
Page 13
2.3 Instructions for using the blood
glucose test strips
• Only use these with the MediTouch 2 connect
monitor.
• Store the test strips in their original container.
• To avoid contamination, only touch the test
strips with clean, dry hands. When removing
the strips from the container and inserting them
in the monitor, only touch them using the grip
(holder).
• Close the container again immediately once
you have removed the test strip. This keeps
the test strips dry and free of dust.
• Use the test strip within three minutes of
removing it from the container.
• The test strip is intended to be used only once.
Do not use it again.
• Write the opening date on the label of the
container when you open it for the first time.
Observe the expiry date. The test strips can be
used for approx. six months after opening
the container or until the expiry date, which ever comes first.
®
2 Safety information
• Do not use test strips which have already
exceeded the expiry date, as this can distort
the test result. The expiry date is printed on the
container.
• Store the test strips in a cool, dry place but not
in the refrigerator.
• Store the test strips between 2°C and 30°C
(35.6°F - 86°F). Do not freeze the test strips.
• Protect the strips from damp and direct sun light.
• Do not apply the blood sample or control
solution to the test strip before you have in serted it in the monitor.
• Only apply the blood sample or the control
solution provided on the test strip slot. Applying
any other substance will lead to an imprecise
or incorrect reading.
• The test strips can be used at altitudes of up to
3,048 m without having any impact on test
results.
• Do not bend, cut or adjust the test strips in any
way.
13
Page 14

2 Safety information
• Keep the container with the test strips away
from children. There is a risk of choking from
the lid. The lid also contains desiccative
substances which could be dangerous if they
are inhaled or swallowed. This could also lead
to skin and eye irritations.
2.4 Instructions on how to use the
control solution
• Only use MEDISANA MediTouch 2 connect
control solution.
• Only use with the MediTouch 2 connect
test strips.
• Write the opening date on the label of the
container. The control solution can be used for
approx. three months after opening the con tainer or until the expiry date, whichever comes
first.
• Do not use the control solution after the expiry
date.
• The ambient temperature for using the control
solution should lie between 10°C - 40°C
(50°F - 104°F).
14
®
®
• The temperatures for storing and transporting
the control solution should lie between min 2°C
and max 30°C (35.6°F – 86°F). Do not store
the solution in the refrigerator and do not
freeze it.
• Shake the bottle with the control solution well
before you open it. Wipe away the first drop
and use the second one to ensure a good
sample for a precise test result.
• To ensure the control solution does not get
contaminated, wipe away the remaining
solution on the tip of the container with a clean
cloth before closing it again.
• The control solution may stain your clothing.
Rinse the soiled clothing with water and
detergent.
• Do not put any excess control solution back in
the container.
• Close the container carefully after every use.
Page 15
3 Useful information
Thank you very much! G
Thank you for your confidence in us and congratulations! The blood glucose monitor Medi-
®
Touch 2 connect that you have purchased is
a top quality product from MEDISANA. i
In order to achieve the desired effect with your
MEDISANA MediTouch 2 connect blood
®
glucose monitor in the long term, we recommend
that you read the following information on its use
and maintenance carefully.
3.1 Items supplied and packaging
Please check first of all that the device is complete and is not damaged in any way. In case of
doubt, do not use and contact your supplier or
your service centre. G
The following parts are included:
• 1 MediTouch 2 connect blood glucose
monitor, (Art. 79046)
• 1 Medisana lancing device
• 10 MediTouch 2 blood glucose test strips,
(Art. 79038)
• 10 MediTouch lancets
• 1 MediTouch 2 connect control solution 4 ml,
(Art. 79039)
®
®
®
®
• 1 AST cap
• 2 CR2032 lithium batteries
• 1 Storage bag
• 1 Instruction manual & 1 Quick reference guide
• 1 USB-cable
• 1 Teststrips manual
• 1 Control solution manual
• 1 Diary for diabetics
The packaging can be reused or recycled.
Please dispose properly of any packaging material no longer required. If you notice any
transport damage during unpacking, please
contact your supplier without delay.
WARNING
Please ensure that the polythene packing is
kept away from the reach of children!
Risk of suffocation!
15
Page 16

3 Useful information
3.2 Special features of the MEDISANA
blood glucose monitor MediTouch 2
®
connect
Measuring your blood glucose level regularly can
be a great help when dealing with your diabetes.
This blood glucose monitor is designed so that
you can use it easily, regularly and anywhere you
choose. The lancing device can be set to the
sensitivity of your skin. The diabetic diary
provided helps you to recognise and record the
affect of your eating habits, sport activities or
medication on the test results. Always consult
your doctor about your test results and treatment.
This monitor is intended for actively testing a
person’s blood glucose at home. It is not suitable
for diagnosing diabetes or for testing the blood
glucose of newborn babies.
Your MediTouch 2 connect blood glucose
monitor from MEDISANA consists of five main
parts: the blood glucose monitor, the lancing
device, the lancets, the test strips and the control
solution.
16
®
These parts are specially designed to be used
together and for their quality to ensure precise
test results. Only use MediTouch 2 connect
®
approved test strips, lancets and control solution
for your blood glucose monitor. Precise test
results can only be ensured when the monitor is
used properly. Only use fresh, capillary whole
blood for the test, preferably from the finger tips.
The monitor measures the blood glucose level
very precisely. It has an automatic memory for
480 readings with the date and time.
Page 17
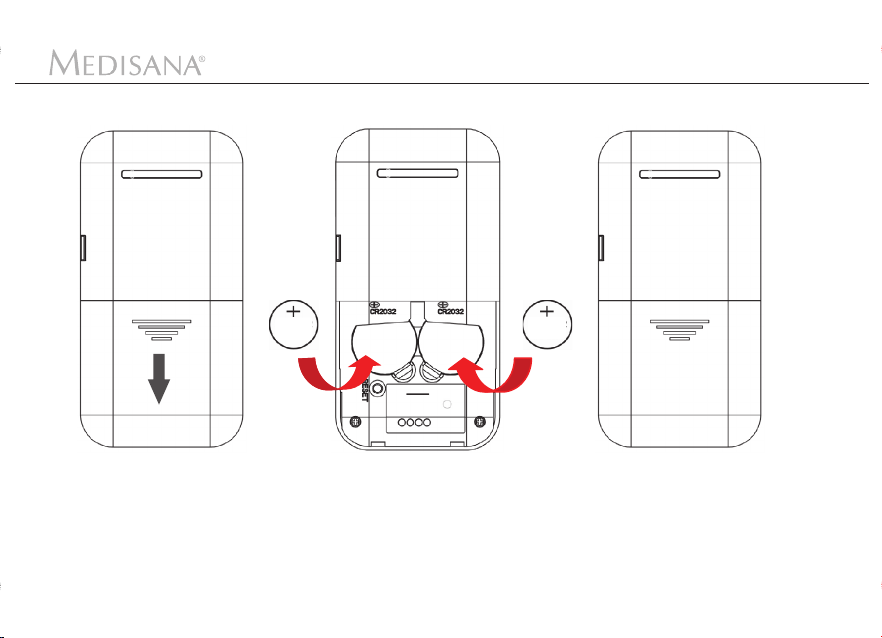
4.1 Inserting the batteries
4 Getting Started
1. Open the battery com-
partment on the back
side of the device by
sliding the lid in the
direction of the arrow.
2
CR2032
3V x 1
2. Insert two batteries as
shown. The device will
emit a short beep after
correct insertion.
CR2032
3V x 1
3. Close the lid of the
battery compartment.
It will "snap" into its place.
17
Page 18

4 Getting Started
WARNING
BATTERY SAFETY INFORMATION
• Do not disassemble batteries!
• Remove discharged batteries from the
device immediately!
• Increased risk of leakage, avoid contact
with skin, eyes and mucous membranes!
If battery acid comes in contact with any
of these parts, rinse the affected area with
copious amounts of fresh water and seek
medical attention immediately!
• If a battery has been swallowed seek
medical attention immediately!
• Only replace with batteries of the same
type, never use different types of batteries
together or used batteries with new ones!
• Insert the batteries correctly, observing
the polarity!
• Remove the batteries from the device if it
is not going to be used for an extended
period!
• Keep batteries out of children's reach!
18
• Do not attempt to recharge these
batteries!
There is a danger of explosion!
• Do not short circuit!
There is a danger of explosion!
• Do not throw into a fire!
There is a danger of explosion!
• Do not throw used batteries into the
household refuse; put them in a
hazardous waste container or take them
to a battery collection point, at the shop
where they were purchased!
Page 19

4 Getting Started
4.2 Setting the time and date
The correct setting of time and date is especially important, if you intend to use the memory
function of the device.
4. The icon
appears in
the display.
mmol/L
>
>
The device is
now ready for
the test with
control solution.
1. After insertion of the
batteries the monitor
switches on automatically.
2. The last two digits of the
year flash in the display.
Press to set the correct
value and press to
confirm.
>
3. Repeat step 2, to
enter the date and
time. The area
flashes for the
setting being made
at the time.
The unit of measure (mg/dL
or mmol/L) is installed in the
device. If you want to change
the unit of measure, contact
the customer service.
19
Page 20

5 Operating
5.1 Using the control solution
Why do I need to perform a test with control
solution?
When you perform a test with control solution,
you will find out whether your monitor and test
strips work properly and deliver exact readings.
You should perform a test in the following cases:
• You are using the monitor for the first time.
• You have a new container of test strips.
• You suspect the monitor or test strips might not
be working properly.
• The monitor has been dropped.
• You have repeated a test and the results are
still lower or higher than you expected.
• You are practising the test procedure.
20
Performing a test with control solution
WARNING
Before you perform a test with
control solution, read section 2 on
Safety instructions (p. 8 - 14),
carefully, especially items
2.3 Instructions on using the
blood glucose test strips and
2.4 Instructions on how to use the
control solution.
Page 21

You need the device, a test strip and the control solution.
5 Operating
mmol/L mmol/L
> > >
1. Insert a test strip into the
monitor in the direction
of the arrow.
The -symbol
appears automatically.
- The measurement results in the control solution measurement mode
will not be recorded in the memory for blood glucose readings.
- The measurement results in the control solution measurement mode
cannot be transferred via Bluetooth .
mmol/L
2. Press the - button, until
the symbol (control solution
measurement mode) appears.
The drop symbol flashes.
Press to confirm the setting.
>
®
3. Place the device on a flat and
even surface, e.g. like a table.
4. Remove the cap of the control
solution bottle and wipe the tip
of the bottle with a tissue.
21
Page 22

5 Operating
mmol/L
>
5. Press the container so that a
tiny droplet forms on the tip of
the container.
6. Place the drop onto the blood
sample area on the end of the
test strip.
7. Do not get any control solution
on the top of the test strip.
22
mmol/L
>
mmol/L
5.4 - 7.2 mmol/L
Sample illustration for a target range
on the test strips container
8. When a sufficient amount of
control solution has been ab sorbed by the reaction cell you
will hear a beep and in the display
" " will be shown.
9. The monitor starts a countdown
of approx. 5 seconds which is
shown in the display.
10. In the display, a test result
appears. Before you re move the test strip, check
whether the result lies
within the range indicated
on the test strips container.
11. After that, remove the test
strip and throw it away.
Page 23

5 Operating
Evaluating the control test result
The permissible range for the control solution
reading is indicated on the label of the test strip
container. Your test result must lie within the
range indicated. Make sure that you compare the
test result with the correct range. If the control
test result lies within the range indicated on the
test strip container, then the monitor and the
test strips are working accurately. If the control
test result does not lie within the range indicated
on the test strip container, then the following
options will be displayed to rectify the problem:
Cause Remedy
Has the test strip
been lying around
open for a long time?
Was the test strip
container properly
closed?
Repeat the test with a
test strip that has
been stored correctly.
The test strips are damp.
Replace the test strip.
Cause Remedy
Did the monitor work
properly?
Is the control
solution soiled or
has it exceeded the
expiry date?
Were the test strips
and control solution
stored in a cool, dry
place?
Did you follow the
steps of the test
procedure properly?
Repeat the test as described in section 5.1. If problems persist, get in touch
with the service centre.
Use new control solution
to check the performance
of the monitor.
Repeat the control test
using strips and solution
that have been stored
correctly.
Repeat the test as described in section 5.1.
If problems persist, get in
touch with the service
centre.
23
Page 24

5 Operating
5.2 Preparing the blood glucose test
Using the lancing device
The lancing device enables you to hygienically
and easily draw a drop of blood for the blood
glucose test and it is quick and painless. g
The lancing device can be set to the sensitivity
of your skin. You can adjust the tip to 6 different
lancing depths. Twist the cap end in the appropriate direction until the arrow is pointing to the
number for the lancing depth you want.
1) Consult the following for the
suitable lancing depth:
1 - 2 for soft or thin skin,
3 - 4 for normal skin and
5 - 6 for thick or callous skin.
2) Never use a lancet or a lancing
device that belongs to another
person.
Use a new sterile lancet for each
test.
24
WARNING
Lancets are intended to be used only once.
Used test strips and lancets are considered hazardous, biological non-biodegradable waste.
Dispose of them taking into account that
they are capable of spreading infection.
Dispose of the lancets so that there is no
risk of injury or infection to other people.
Inserting a lancet into the lancing device
Before using the lancing device you need to
insert a lancet.
WARNING
Before performing a blood glucose test and
before using the lancing device, make sure
you read section 2 on the safety instructions carefully, especially item 2.2 Instructions for your health and 2.3 Instructions
on how to use the blood glucose test
strips.
Page 25

5 Operating
1. Wash your hands with soap
and warm water. Rinse and
dry thoroughly. If needed,
you can also rub the area
of skin you have chosen for
the blood sample with a
special cleansing pad.
2. Open the lancing device by twisting
the protective cap in a clockwise
direction and then remove it. Insert
the lancet all the way (without turning
it) into the lancing device. Twist off
the protective cap on the lancet
carefully.
3. Replace the protective
cap on the lancing de vice and tighten by
turning it in an anti clockwise direction.
To use the AST cap,
check item 5.3 Using
the AST cap.
25
Page 26

5 Operating
Markings for lancing depths
4. Set the appropriate lancing
depth, as described on the
pages before.
1) Consult the following for the suitable lancing depth: 1 - 2 for soft or thin skin,
3 - 4 for normal skin and 5 - 6 for thick or callous skin.
2) Never use a lancet or a lancing device that belongs to another person. Sharing
lancing devices and lancets may transmit blood borne pathogens, such as
26
viral hepatitis.
5. Set the lancing device by
extending it until it clicks
into place. If it does not
click, it is probably already
in position from when you
inserted the lancet.
6. The lancing device is ready
to use. Do not lance your
finger before the monitor and
test strips are ready to use.
Page 27

5 Operating
5.3 Using the AST cap
It is generally recommended that a blood sample
for a blood glucose test performed at home is
taken from the fingertip. If you are not able to
take a blood sample from your fingertip, you
can also take one from another part of the body
(AST), such as the ball of the hand or lower arm
using the lancing device. g
In this case, exchange the protective cap on the
lancing device with the AST cap. After inserting
the lancet, put the transparent AST cap on the
lancing device instead of the protective cap and
tighten. Note that the AST cap is not intended to
be used for a blood sample from the fingertip.
We recommend, only to use the alternative site testing (AST), when:
• at least 2 hours passed after the
last meal
• at least 2 hours passed after the
last intake of insulin and / or
exercise
Discuss the readings from an AST
measurement with your doctor, if:
• The glucose results are often
fluctuating
• The results do not match the way
you feel
WARNING
In the case of low glucose (hypoglycemia),
the blood sample must be drawn from
the finger tip, as any changes in the blood
glucose level are quicker to detect from a
blood sample from the finger than from
any other part of the body.
The readings from a finger tip blood
sample and from another part of the body
can lead to the readings which radically
differ from one another. Therefore, always
consult your doctor before you perform a
blood glucose test using a blood sample
from another part of the body.
27
Page 28

5 Operating
The blood sample
can also be taken
from:
Ball of the hand
Lower
arm
This is how to do it:
1.
Select a part of the body that is soft,
not too densely covered with hair and
not near a bone or a vein.
2.
Massage the area gently to prepare
the skin and to improve the circulation.
3.
Hold the lancing device against the
lancing spot for a few seconds and
then press the trigger button.
4.
Wait until a blood drop forms under the
AST cap with a diameter of approx.
1.4 mm.
5.
Remove the lancing device from the
skin carefully and proceed in the same
way as for the normal protective cap
(see 5.4 Determining the blood
glucose level).
28
WARNING
Do NOT use the first drop of blood sample
when using the AST cap.
Page 29

5.4 Determining the blood glucose level
5 Operating
mmol/L
> >
mmol/L
1. Insert a test strip into the monitor in the
direction of the arrow. The symbol
appears automatically.
2. Press to set (before a meal),
(after a meal) or (before
a meal with alarm after 2 hours) and
press to confirm.
>
mmol/L
3. When the blood drop
symbol flashes in
the display, take a
drop of blood from your
finger tip. Massage the
area gently to stimulate
the blood circulation.
4. Place the lancing de vice on a finger tip
(preferably at the side)
and press the trigger
button.
Make sure, that the
blood droplet does not
smudge.
29
Page 30

5 Operating
mmol/L
>
mmol/L
5. Place the drop onto the blood sample area
on the end of the test strip. Pay attention,
that not any blood gets on the top of the
test strip.
To receive a correct test result, be sure to
apply enough blood into the test strip's blood
sample area - see the confirmation window
as per drawing above.
30
6. When a sufficient amount of blood has been
absorbed by the reaction cell you will hear a
beep and in the display " " is shown.
7. The device will count down from 5 seconds
(shown in display). After that, "OK" is shown
and then the result appears in the display.
Page 31

mmol/L
5 Operating
mmol/L
8. The „bt“-symbol flashes after a beep
and the device tries to establish a
Bluetooth -connection to the
VitaDock+ app.
®
®
9. When the test result has been up loaded, the „bt“-symbol stops flashing.
If it was not possible to transfer the test
result via Bluetooth , results may be
manually transferred later on to your
VitaDock+ app. You will find more
®
®
information on this topic in chapter
8.1 „Manual data transfer to VitaDock+
app via Bluetooth “.
®
®
31
Page 32

5 Operating
5.5 Discarding used lancets
1. Open the lancing device by
twisting the protective cap in
a clockwise direction and
then remove it.
32
2. Take out the used lancet
(without touching it directly)
and stick it into its protective
cover.
3. Move the sliding switch, loca ted on the other side of the
trigger button, which ejects
the lancet. Dispose of the
lancet in a container for bio hazard material carefully to
avoid harming other people.
4. After discarding, wash your
hands with soap and warm
water. Rinse and dry tho roughly.
Page 33

5 Operating
5.6 Evaluating a test result
WARNING
Never change the prescribed dose of
medicine or treatment on your own initiative on the basis of one test result from a
blood glucose test.
The MediTouch 2 blood glucose test strips
work based on an improved technology (GDHFAD) for a more exact and specific glucose
measurement. They are calibrated to easily compare with laboratory test results. G
The normal average blood glucose reading of an
adult without diabetes is between 3,9 and 7,2
mmol/L (70 - 130 mg/dL). The blood glucose
level of an adult without diabetes two hours after
a meal is less than 10 mmol/L (180 mg/dL). g
For those with diabetes: consult your doctor
about the range of blood glucose level valid for
you.
®
Unusual test results
If your test result is not what you expected, proceed as follows:
1. Perform a control test, see section 5.1 Using
the control solution
2. Repeat the blood glucose test, see section
5.4 Determining the blood glucose level
3. If your test result is still not what you think it
should be, consult your doctor immediately.
NOTES
• Extremely high humidity can affect the
test results. A relative humidity of more
than 90% can cause incorrect readings.
• If the proportion of red blood cells
(haemotocrit level) is very high (over 60%)
or very low (under 20%), this could distort
the test results.
33
Page 34

5 Operating
NOTES
• Studies have shown that electromagnetic
fields can affect the test results. Do not
perform a test near any devices which
emit strong electromagnetic rays, such
as microwaves, mobile phones etc.
Comparing your test results with a
laboratory result
The question of how you can compare the blood
glucose level of the monitor with tests performed
in the laboratory is frequently asked. Your blood
glucose level can change quickly, especially after
a meal, after taking medicine or strenuous activities. G
Your blood glucose is affected by various factors
and has different values at different times of the
day. If you would like to compare the test result
of your monitor with a laboratory result, you must
do the blood glucose test on an empty stomach.
Therefore, it is advisable to do this in the morning.
34
Take your monitor with you to the doctor’s and
test yourself five minutes before or after a trained
nurse has taken a blood sample from you. Take
into account that the technology in the laboratory
is different from your monitor and that blood glucose monitors for using at home generally produce slightly different results. To ensure the accuracy and precision of such important information,
read the instructions included with the blood
glucose test strips.
NOTE
Make sure that you always record your test
results with the date and time in your
diabetes diary and label with the appropriate symbol for:
before a meal , after a meal .
Page 35

5 Operating
5.7 Typical symptoms of high or low
blood glucose
In order to better understand your test results,
you can find some typical symptoms for high
and low blood glucose herewith. In each case,
you should contact your doctor about the therapy if you have noticed one of these symptoms.
Result is higher than 13,33 mmol/L
(240 mg/dL):
The test result is higher than reference normal
range (70 - 130 mg/dL resp. 3,89 - 7,22 mmol/L).
Possible symptoms may be:
Fatigue, increased appetite or thirst, frequent
urination, blurred vision, headache, general
aching, or vomiting.
What to do:
• Test your blood glucose level again
• If the result does not match how you feel,
follow the steps on page 33 "Unusual test
results"
• Contact your doctor
Result is lower than 3,33 mmol/L
(60 mg/dL):
The test result is lower than reference normal
range (70 - 130 mg/dL resp. 3,89 - 7,22 mmol/L).
Possible symptoms may be:
Sweating, trembling, blurred vision, rapid
heartbeat, tingling, or numbness around mouth
or fingertips.
What to do:
• Test your blood glucose level again
• If the result does not match how you feel,
follow the steps on page 33 "Unusual test
results"
• Contact your doctor
35
Page 36

6 Memory
6.1 Saving the test results
Your monitor can save up to 480 test results, including the time and date. You can access the
readings at any time. If the memory is full and
you want to add a new test result, the oldest test
result will be deleted automatically. g
Therefore, it is essential to enter the time and
date correctly in your monitor.
NOTES
• The content of the memory will not be
deleted if you change the batteries. Just
check that the time and date is still
correct. You may have to reset the time
and date after changing the batteries. To
do this read 4.2 Setting the time and date
• If the memory contains 480 test results
and you want to add a new test result,
the oldest test result saved will be
deleted.
36
6.2 Accessing and deleting test results
You can access test results anytime without
having to insert a test strip. Test results which
are under certain criteria are marked with the
appropriate symbol. When accessing saved test
results, you can select according to these
criteria by choosing the appropriate symbol:
before meals
after meals
before meals
with alarming
2 hours later
Page 37

1. Press and hold for 2 se conds to access the display
mode for single values in
memory. Press to display
the saved test results one
after the other in sequence
of 480 to 001 - in other words
the latest entry will be displayed
first and the oldest last.
>
6 Memory
2. To delete a test result, simulta-
neously press and hold and
for 3 seconds. “dEL” appears
in the display.
3. Presss to delete the result.
The display shows "OK".
4. Press to further display other
saved values. Press and hold
for 2 seconds to cancel this
mode. If no button is pressed,
the monitor switches off auto matically after approx. 1,5 minu tes.
>
>
37
Page 38

6 Memory / 7 Integration in VitaDock online
®
If the following display appears during memory
recall process, the device has no test results
in memory so far. Perform a blood glucose test
first, so that the device can save a test result.
mmol/L
38
MEDISANA MediTouch 2 connect
®
features an integration option for transferring
your measured values through the USB cable to
the VitaDock app resp. online. Therefore you
need the VitaDock software for your computer.
Download the software from
www.medisana.com/software as follows:
1. Go to www.medisana.com/software
2. Select "MediTouch 2" as your device.
®
3. Download and install the VitaDock software
on your computer
4. You will find a detailed instruction on the
website, how to install and use the software.
You may transfer data from your MediTouch 2
®
connect device to your computer in switchedon or -off state of the device.
Page 39

7 Integration in VitaDock online
> > >
®
1. Connect the monitor to your
personal computer with the
USB-cable. The symbol appears in the
display.
2. The transfer of your
measured values to the
VitaDock app resp. online
®
will be started automatically
if the software has been
installed properly. Please
check the page before for
how to properly install it.
3. After successful transfer,
"OK" appears in the display
and the device emits an
acoustic signal.
39
Page 40

8 Data transfer to VitaDock+ app via Bluetooth
After each measurement the device automatically tries to send the test results via Bluetooth to
® ®
®
your iOS- or Android mobile device. If this transfer is not successful, you may transfer multiple test
results later on manually - therefore check chapter 8.1 Manual data transfer of measured results to
VitaDock+ app.
®
After a measurement, the „bt“-symbol flashes after a beep and
the device tries to establish a Bluetooth -connection to the
VitaDock+ app.
®
®
When the test result has been uploaded, the „bt“-symbol stops
flashing.
mmol/L
mmol/L
HINTS
• For a successful synchronization of the device with the VitaDock+ app, a stable internet
connection is necessary.
• If the measured results have not been transferred to VitaDock+ app successfully, please
pay attention to chapter 8.1 Manual data transfer of measured results to VitaDock+ app.
®
®
®
40
Page 41

8.1 Manual data transfer of measured results to VitaDock+ app
The test results may also be transferred manually via Bluetooth to the VitaDock+ app. Therefore
make sure, Bluetooth is activated on your mobile device and follow these steps:
®
mmol/L
® ®
®
>
5. When „OK“ appears,
1. Switch on the MediTouch 2
®
connect device by pressing and
holding the - button for at least
2 seconds. The symbol „ “
flashes.
2. Afterwards, press twice , until the
„bt“-symbol for the Bluetooth -mode
>
®
appears.
3. As soon as „bt“ flashes,
the device searches for
the VitaDock+ app.
®
4. When „- - -“ appears,
the data transfer to
VitaDock+ app has
®
started.
the transfer has been
successfully completed.
NOTE
Never use Bluetooth and
®
USB connections simultaneously, as this would lead to
erroneous results.
41
Page 42

9 Miscellaneous
9.1 Display and troubleshooting
Display G
The monitor automatically checks its own system
when you switch it on and shows you any irregularities in the display. To ensure that the display
is working properly, switch on the monitor. Press
and hold down the -button for approx.
3 seconds so that you can see the whole display.
All the display elements must be clear to see with
the accompanying symbol (please compare with
the drawing on this page). If this is not the case,
get in touch with the service centre.
4
mmol/L
NOTES
• A description of the symbols shown can
be found at the beginning of this
instruction manual.
• The device may also be switched on by
inserting a test strip.
42
>
Error display G
On no account should you change your medication due to a measurement, which may be
incorrect. If in doubt, consult your service centre
or your doctor.
Page 43

9 Miscellaneous
Cause
Remedy
mmol/L
Damp / used
test strip
Replace with a
new test strip.
Low batteries
Replace with
new batteries.
The device will
not delete earlier
records when
batteries are
replaced.
Memory Error
First replace the
batteries. If error
005 appears again,
get in touch with
the service centre.
System error
First replace the
batteries. If error
001 appears again,
get in touch with
the service centre.
43
Page 44

9 Miscellaneous
mmol/L
Test result is higher than
35,0 mmol/L (630 mg/dL)
Repeat the test. If the result
does not change, consult your
doctor.
44
Test result is lower than
1,1 mmol/L (20 mg/dL)
Repeat the test. If the result
does not change, consult
your doctor.
mmol/L
mmol/L
mmol/L
Test result is higher than
13,8 mmol/L (250 mg/dL)
Repeat the test. If the result
does not change, consult your
doctor. The result may be an
indication to a diabetical ketoacidosis. The wording "Ketone"
appears in the display.
Page 45

9 Miscellaneous
"Ht" / "Lt" appears. Ambient temperature is too high or too low, not within
the required range of 10°C - 40°C
(50°F - 104°F). The user is warned about
a potentially incorrect test result if the
test proceeds.
Move your monitor to a place with a
temperature between 10°C and 40°C
(50°F - 104°F).
mmol/L
Blood sample or control
solution quantity is not
enough.
Repeat the test with a new
test strip and sufficient
sample quantity. If same
problem occurs again, get
in touch with the service.
centre.
45
Page 46

9 Miscellaneous
mmol/L
Bluetooth Transfer error during
®
upload of a single test result
If it was not possible to transfer the test
result after a measurement via Bluetooth ,
results may be manually transferred later
on. You will find more information on this
topic in chapter 8 „Data transfer to
VitaDock+ app via Bluetooth “.
® ®
46
Bluetooth Transfer error during upload of
®
multiple test results
®
Check the connection between your meter
and the mobile device first. Pay attention to
the information in chapter 8 „Data transfer to
VitaDock+ app via Bluetooth “. If still no
® ®
transfer is possible, contact the service centre.
Page 47

9 Miscellaneous
9.2 Cleaning and maintenance
Monitor G
Your MediTouch 2 connect blood glucose
monitor is a high-precision instrument. Handle
with care to avoid damaging the electronics and
prevent defects. You do not need any additional
cleaning to maintain your monitor, provided it has
not come into contact with blood or control solution. Keep the monitor free from dirt, dust, blood
and water stains. i
Observe the following instructions:
• Make sure that the monitor is switched off.
• Clean the surface of the monitor with a soft
cloth slightly dampened with 70-75 % ethanol.
• Never use abrasive detergents or strong
brushes.
• Never use cleaning agents on the monitor.
• Never immerse the monitor in water. Do not
allow water or other liquids to penetrate the
monitor. Dry the monitor with a lint-free cloth
after cleaning.
®
• Make sure that dirt, dust, blood, control so lution, water or alcohol do not penetrate the
test strip slot or get onto the buttons on the in side of the monitor.
• Do not expose the monitor to extreme tempe ratures.
• Store the monitor in the zip bag provided after
each use.
• Do not store the monitor and the test strips in
a vehicle, bathroom or refrigerator.
• Remove the battery if you do not intend to use
the monitor for one month or longer.
Lancing device G
Clean the lancing device with a slightly damp
cloth (with water and a mild detergent). Do not
immerse it in water or other liquids and never
allow water or other liquids to penetrate the inside
of the lancing device. To disinfect the protective
cap, soak it in 70 % - 75 % cleaning alcohol for
10 minutes once a week after cleaning. Leave the
cap to dry thoroughly after disinfecting.
47
Page 48

9 Miscellaneous
9.3 Reset the meter
Reset button
To reset the meter (Attention: All saved data
will be deleted!), open the cover of the battery
compartment on the back side of the unit.
Press and hold the reset button for approx.
3 seconds to reset the device.
48
2
9.4 Technical specifications
Name and model:
Measuring method:
Measuring range:
Measuring time:
Memory:
Operating conditions:
Storage / transport
conditions:
Blood sample volume:
Sample material:
Haemotocrit value (Htc):
Power supply:
Battery operation:
MEDISANA blood glucose monitor MediTouch 2
connect
electrochemical biosensor
technology
1.1 - 35.0 mmol/L
approx. 5 seconds
480 test results with time
and date
Temperature 10°C – 40°C
(50°F – 104°F), relative
humidity up to 90 %
Temperature 2°C – 30°C
(35.6°F - 86°F), relative
humidity up to 90 %
0,6 µL
Fresh blood from finger
tip, palm of the hand or
arm (capillary whole blood)
20 – 60 %
2 x 3V CR2032 lithium
batteries
over 2,000 tests
®
Page 49

9 Miscellaneous
Automatic switch-off:
Dimensions (display):
Dimensions (base unit):
Weight:
after approx. 1.5 minutes
approx. 37 x 56 mm
approx. 50 x 98 x 11 mm
approx. 47 g without
batteries
Article number:
EAN number:
79046
40 15588 79046 1
Compatible smartphones:
• iOS: iPhone 4S and newer, iPad 3 and newer.
• Android: Devices, which support Google Android-version 4.3 and Bluetooth 4.0
technology
• Compatible app: VitaDock+ App
®
®
Further information and the country-specific
service addresses can be found at:
www.medisana.com/meditouch2connect
0483
In accordance with our policy of continual
product improvement, we reserve the right
to make technical and visual changes
without notice.
The monitor is certified in accordance with the
requirements of EU guidelines 98/79 for in vitro
diagnostic devices and complies to the R&TTEdirective 1999/5/EC. The complete declaration of
conformity can be demanded from Medisana AG,
Jagenbergstr. 1, 41468 Neuss, Germany or can
be downloaded from the Medisana Homepage
(www.medisana.com).
Electromagnetic compatibility: g
The device complies with the EN 60601-1-2
standard for electromagnetic compatibility.
Enquire at MEDISANA for details on this
measurement data.
9.5 Accessories
Enquire at your local supplier or service centre.
• 100 MediTouch 2 lancets Art. nr. 79001
• 1 MediTouch 2 lancing device Art. nr. 79002
• 1 MediTouch 2 control solution
Art. nr. 79039
• 50 MediTouch 2 test strips Art.-Nr. 79038
®
®
®
®
49
Page 50

9 Miscellaneous
9.6 Disposal
This product must not be disposed of
together with domestic waste. All users
are obliged to hand in all electrical or
electronic devices, regardles s of
tances, at a municipal or commercial collection
point so that they can be disposed of in an environmentally acceptable manner. Please remove
the batteries before disposing of the device/unit.
Do not dispose of old batteries with your household waste, but at a battery collection station at a
recycling site or in a shop.
Teststrips and lancets G
Always dispose of the test strips and lancets in
a way that prevents injury and the spread of
infection to others. Consult your local authority
or your supplier for information about disposal.
50
whether or not they contain toxic subs-
Page 51

10 Warranty
10.1 Warranty and repair terms
Please contact your supplier or the service centre
in case of a claim under the warranty. If you have
to return the device, please enclose a copy of your
receipt and state what the defect is. G
The following warranty terms apply:
1. The warranty period for MEDISANA products
is three years from date of purchase. In case
of a warranty claim, the date of purchase has
to be proven by means of the sales receipt or
invoice.
2. Where defects in materials or workmanship
arise we will repair or replace free of charge
with in the warranty period.
3. Repairs under warranty do not extend the
warranty period either for the unit or for the
replacement parts.
4. The following is excluded under the warranty:
a. All damage which has arisen due to im-
proper treatment, e.g. non observance of
the user instructions.
b. All damage which is due to repairs or tam pering by the customer or unauthorised third
parties.
c. Damage which has arisen during transport
from the manufacturer to the consumer or
during transport to the service centre.
d. Accessory parts which are subject to nor mal wear and tear, such as battery, lancing
device and disposable items etc.
5. Liability for direct or indirect consequential
losses caused by the unit are excluded even if
the damage to the unit is accepted as a
warranty claim.
MEDISANA AG
Jagenbergstraße 19
41468 NEUSS
GERMANY
eMail: info@medisana.de
Internet: www.medisana.com
51
Page 52

10 Warranty
10.2 Service address
You can find out which is your responsible service centre
from your supplier
Further information and the country-specific
service addresses can be found at:
www.medisana.com/meditouch2connect
52
Page 53

Page 54

Page 55

Page 56

MEDISANA AG
Jagenbergstraße 19
41468 NEUSS
Germany
eMail: info@medisana.de
Internet: www.medisana.com
79046GB V1.2 04/2015
 Loading...
Loading...