
OPERATING INSTRUCTIONS
MEDAP
TAPPING UNIT
S VAC B 900 / D 200
GA 5752 5636 GB 08

Subject to technical modification!
4
Illustrations and technical specifications may vary slightly from those in these operating
instructions as a result of ongoing product development.
V08 2020-07
2
GA 5752 5636 GB 08

Table of contents
Table of contents
1 Introduction ..............................................................................................................................................6
1.1 Foreword .................................................................................................................................................... 6
1.2 How to use these operating instructions .................................................................................................... 6
1.2.1 Abbreviations ...............................................................................................................................6
1.2.2 Symbols .......................................................................................................................................6
1.2.2.1 Cross-references ....................................................................................................... 6
1.2.2.2 Actions and responses .............................................................................................. 6
1.2.3 Definitions ....................................................................................................................................7
1.2.3.1 Design of safety notes ............................................................................................... 7
1.2.3.2 Design of other notes ................................................................................................ 7
1.2.4 Symbols used ..............................................................................................................................7
1.2.5 Disposal .......................................................................................................................................8
1.2.5.1 Packaging .................................................................................................................. 8
1.2.5.2 ATMOS products ....................................................................................................... 9
1.3 Overview ....................................................................................................................................................9
1.3.1 S VAC B 900 / D 200.................................................................................................................... 9
1.3.2 Versions of the S VAC B 900 / D 200 ......................................................................................... 10
1.4 Basic requirements ...................................................................................................................................10
1.4.1 Use in accordance with the intended purpose ........................................................................... 10
1.4.2 Applicable standards .................................................................................................................. 11
1.4.3 Intended purpose ....................................................................................................................... 11
1.4.4 S VAC versions .......................................................................................................................... 12
1.4.5 Interface description ...................................................................................................................13
1.4.5.1 Vacuum connection tube ......................................................................................... 13
1.4.5.2 Hydrophobic bacterial and viral filter ....................................................................... 13
1.4.5.3 Septic fluid jar including septic fluid jar cap ............................................................. 14
1.4.5.4 Suction tube .............................................................................................................14
1.4.5.5 Fingertip ...................................................................................................................14
1.4.5.6 Utensil ......................................................................................................................14
1.4.5.7 Mechanical overflow protection ............................................................................... 15
2 Safety notes ............................................................................................................................................16
2.1 General safety notes ................................................................................................................................ 16
2.2 Product safety notes .................................................................................................................................16
3 Initial operation .......................................................................................................................................18
3.1 Product inspection .................................................................................................................................... 18
3.2 Connection to the terminal unit.................................................................................................................18
3.2.1 General ......................................................................................................................................18
3.2.2 Version A ....................................................................................................................................18
GA 5752 5636 GB 08
3

Table of contents
3.2.3 Version B ....................................................................................................................................19
3.2.4 Version C ...................................................................................................................................19
3.3 Mounting accessories ...............................................................................................................................20
3.3.1 General ......................................................................................................................................20
3.3.2 Mounting the mechanical overflow protection ............................................................................ 21
3.3.3 Connection of the mechanical overflow protection .................................................................... 21
3.3.4 Connection of the hydrophobic bacterial and viral filter ............................................................. 22
4 Operation ................................................................................................................................................23
4.1 Function test ............................................................................................................................................. 23
4.2 Use in conjunction with magnetic resonance imaging scanners .............................................................. 23
4.3 Setting the vacuum level .......................................................................................................................... 24
5 Taking the unit out of operation ............................................................................................................ 25
5.1 Completing the aspiration process ........................................................................................................... 25
5.2 Disassembly ............................................................................................................................................. 25
5.2.1 General ......................................................................................................................................25
5.2.2 Dismantling the mechanical overflow protection ........................................................................ 26
6 Cleaning and disinfection ......................................................................................................................27
6.1 General .....................................................................................................................................................27
6.2 Cleaning ................................................................................................................................................... 28
6.2.1 General ......................................................................................................................................28
6.2.2 Cleaning procedure ....................................................................................................................28
6.3 Disinfection ............................................................................................................................................... 28
6.3.1 General ......................................................................................................................................28
6.3.2 Suitable disinfectants ................................................................................................................. 29
6.3.3 Disinfection procedure ...............................................................................................................29
6.3.4 Disinfection procedures .............................................................................................................29
6.4 Product-specific safety notes ...................................................................................................................30
7 Maintenance ............................................................................................................................................31
7.1 General .....................................................................................................................................................31
7.2 Periodic tests ............................................................................................................................................ 31
7.3 Malfunctions and troubleshooting.............................................................................................................31
7.4 Repairs ..................................................................................................................................................... 32
7.5 Service hotline .......................................................................................................................................... 32
7.6 Type plate position ...................................................................................................................................32
7.7 Sending in the device ............................................................................................................................... 32
8 Technical specifications ........................................................................................................................ 34
8.1 General .....................................................................................................................................................34
8.2 Technical specifications ............................................................................................................................ 34
8.3 Ambient conditions ................................................................................................................................... 34
8.4 Dimensions and weight ............................................................................................................................ 34
4
GA 5752 5636 GB 08

Table of contents
9 Approved accessories ...........................................................................................................................35
9.1 Accessories .............................................................................................................................................. 35
9.2 Accessories for compact suction unit / basic vacuum equipment ............................................................ 35
9.3 Accessories for mobile suction unit / basic vacuum equipment ............................................................... 35
9.4 Consumables ...........................................................................................................................................36
GA 5752 5636 GB 08
5

Introduction
1
Foreword
1 Introduction
1.1 Foreword
Your facility has selected the leading-edge medical technology made by ATMOS. We sincerely
appreciate the trust you have placed in us.
1.2 How to use these operating instructions
These operating instructions are provided to familiarise you with the features of this ATMOS
product. They are subdivided into several chapters.
Please note:
• Please read these operating instructions carefully and completely before using the product for
the first time.
• Always proceed in accordance with the information contained herein.
• Store these operating instructions in a location near the product.
1.2.1 Abbreviations
EN European standard
EEC European Economic Community
VDE Verband der Elektrotechnik Elektronik Informationstechnik (Association for
Electrical, Electronic & Information Technology)
1.2.2 Symbols
1.2.2.1 Cross-references
References to other pages in these operating instructions are identified with a double arrow
symbol ‘’.
1.2.2.2 Actions and responses
The ‘’ symbol identifies an action taken by the user, while the ‘’ symbol identifies the reaction
that this will induce in the system.
Example:
Turn on the light switch.
Lamp lights up.
6
GA 5752 5636 GB 08

1.2.3 Definitions
4
1.2.3.1 Design of safety notes
Pictogram Descriptor Text
DANGER!
Indicates a direct and immediate risk to
persons which may be fatal or result in
most serious injury.
WARNING!
Indicates a potential risk to persons or
property which may result in health hazard
or grave property damage.
CAUTION!
Indicates a potential risk to property which
may result in property damage.
Introduction
How to use these operating instructions
The text for the safety note
describes the type of risk and
how to avert it.
1
Tab. 1: Design of safety notes
1.2.3.2 Design of other notes
Notes not referring to personal injury or property damage are used as follows:
Pictogram Descriptor Reference to
NOTE Supplementary assistance or further useful information.
ENVIRONMENT Information regarding proper disposal.
Tab. 2: Design of other notes
1.2.4 Symbols used
Symbols are attached to products, type plates and packaging.
Symbols Identification
Labelling for products which were developed and are marketed in
compliance with the Medical Devices Directive 93/42/EEC. Class Is, Im, IIa,
IIb and III products are also marked with the identifying number of the
Notified Body.
Labelling in compliance with the ISO 15223-1 standard.
Symbol for ‘Serial number’.
Labelling in compliance with the ISO 15223-1 standard.
Symbol for ‘Product number’.
GA 5752 5636 GB 08
7

1
Introduction
How to use these operating instructions
Symbols Identification
Labelling in compliance with the IEC 60601-1 standard.
Symbol for ‘Follow operating instructions’.
Labelling in compliance with the ISO 15223-1 standard.
Symbol for ‘Name and address of the manufacturer as well as date of
manufacture’.
Labelling in compliance with the IEC 62570 standard.
Symbol for ‘Conditionally MR safe’.
Packaging label.
Symbol for ‘Keep dry’.
Tab. 3: Symbols
1.2.5 Disposal
Packaging label.
Symbol for ‘Fragile! Handle with care’.
Packaging label.
Symbol for ‘Top’.
Labelling in compliance with the ISO 15223-1 standard.
Symbol for ‘Temperature limitations’.
Labelling in compliance with the ISO 15223-1 standard.
Symbol for ‘Relative humidity’.
Labelling in compliance with the ISO 15223-1 standard.
Symbol for ‘Atmospheric pressure’.
1.2.5.1 Packaging
The packaging is made of materials compatible with the environment. ATMOS will dispose of the
packaging materials upon request.
8
GA 5752 5636 GB 08
WARNING!
Infection hazard!
The product or some of its components may be contaminated after use.
Clean and disinfect the product before disposal.

1.2.5.2 ATMOS products
ATMOS will take back used products or those which are no longer in service. Please contact your
ATMOS representative for more detailed information.
1.3 Overview
1.3.1 S VAC B 900 / D 200
Introduction
Overview
1
1
2
4
7
3
8
9
5
6
Fig. 1: Overview of S VAC B 900 / D 200
1 S VAC B 900 5 S VAC D 200
2 Vacuum gauge 6 ON button
3 Control valve 7 Mechanical overflow protection
4 OFF button 8 Adapter for hydrophobic filter
9 Hydrophobic bacterial and viral filter
GA 5752 5636 GB 08
9

Introduction
1
Basic requirements
1.3.2 Versions of the S VAC B 900 / D 200
1
3
2
9
4
8
5
6
7
Fig. 2: Overview of versions S VAC B 900 / D 200
1 Version A 6 NIST screw connection
Tapping unit with integrated gas pin 7 Connection tube
2 Plug 8 Rail clamp
3 Terminal unit 9 Version C
4 Version B Tapping unit for screw connection, with NIST
Tapping unit with rail clamp and NIST
connection
5 NIST nipple
1.4 Basic requirements
1.4.1 Use in accordance with the intended purpose
Product
As per Annex IX to the Medical Devices Directive 93/42/EEC, this product belongs to class IIa.
In accordance with this directive, the product may only be used by persons who have been
instructed how to use this product by an authorised person.
This product is to be used exclusively for human medicine.
When employed in commercial or business use, this product must be entered in the inventory.
connection
10
GA 5752 5636 GB 08

Accessories
Accessories or combinations of accessories may be utilised only as and when indicated in these
operating instructions.
Other accessories, combinations of accessories and consumable items may be used only if they
have a valid certification, are intended expressly for the particular use and will not adversely
affect performance, the prescribed ambient conditions or safety requirements.
1.4.2 Applicable standards
The product satisfies the basic requirements set forth in Annex I to Council Directive 93/42/EEC
concerning medical devices (Medical Devices Directive) as well as the applicable national
(German) codes and the Medical Devices Act (MPG) in Germany. This is certified by compliance
with harmonised standards such as IEC 60601-1 and related standards and the respective
special sections.
1.4.3 Intended purpose
Name: S VAC B 900
S VAC D 200
Introduction
Basic requirements
1
Main function: Aspiration of secretion, blood, serous fluids, vomit and rinsing fluids
along with any contained particles (S VAC B 900).
Aspiration of secretion, blood and serous fluids (S VAC D 200).
Medical indications /
application:
Specification of the main
function:
User profile: Doctor, medically trained staff
Patient groups: Patients of all ages
Application organ: Natural and artificial body orifices
Application time: For continuous operation; in practice, short-term use on the patient
For all applications which require aspiration, e.g. general surgeries
(e.g. aspiration of wound cavities, abscesses), bronchial aspiration,
during endoscopy for aspiration of secretions and in neurosurgery
(S VAC B 900).
For precise regulation of vacuum for postoperative aspiration of
wound exudate, secretion, blood or serous fluids. Additional fields
of application are the aspiration of air as well as rinsing and wound
drainage (S VAC D 200).
Drainage and temporary collection of body fluids. For the supply of
vacuum, S VAC B 900 and S VAC D 200 are connected to a
terminal unit for vacuum of a central medical gas supply system
with a pressure of −100 kPa to −60 kPa. A septic fluid jar, which
has to be used, allows for temporary collection of drained body
fluids.
(< 30 days)
Application site: The application site is the clinical environment and doctor’s
practices which have a central vacuum source. The application of
the product may only be performed by medically trained and
instructed staff.
GA 5752 5636 GB 08
11

1
Introduction
Basic requirements
Contraindications: The S VAC B 900 and D 200 may not be used for the following
purposes:
• Outside the medical sector
• In MR areas > 4.7 Tesla
• In the home care sector
• Being operated directly by the patient
• For vacuum extraction
• For the aspiration of flammable or explosive liquids
• For the aspiration of smoke that is generated during HF and
laser surgery without the connection of an intermediate smoke
filter
• S VAC D 200 is not suitable for bronchial aspiration.
• S VAC B 900 is not suitable for drainages.
• S VAC B 900 and D 200 are not suitable for thoracic drainages.
Usage in combination with disposable thoracic drainage systems
with integrated vacuum regulation is excepted from this restriction.
• With central gas supply systems with supply pressures other
than −100 kPa to −60 kPa
The product is: Active
Sterility: Not a sterile product
Single-use product /
reprocessing:
1.4.4 S VAC versions
The connection of S VAC (regardless of which version S VAC B 900 or S VAC D 200) to the
vacuum terminal unit will depend on the product type.
Version A: Tapping unit with integrated gas pin
• S VAC is fitted directly to the terminal unit.
Version B: Tapping unit with rail clamp and NIST connection
• S VAC is designed for mounting to a 25–35 x 10 mm equipment rail in accordance with DIN
EN 19054 and is supplied via a NIST connection with vacuum from a terminal unit connected
using a connection tube with gas probe.
Version C: Tapping unit for screw connection, with NIST connection
• S VAC is designed for direct screw mounting onto mobile suction units (intensive care trolley /
surgical trolley) and compact suction units (solo carrier frame) and is supplied from a terminal
unit via a NIST connection with a connection tube and gas probe.
Products and accessories are only permitted with ISO colour coding. In Germany, Austria and
Switzerland, products with neutral colour coding are also permitted.
The device and parts of the accessories are reusable. For
information on reprocessing, cleaning and disinfection, please see
the operating instructions.
12
NOTE
The products are supplied with ISO coding. The scope of delivery for the versions
DIN, MEDAP, equipment rail and screw mount also includes a label for neutral
colour coding.
GA 5752 5636 GB 08

The S VAC B 900 is available in the following versions:
• S VAC B 900 Wall DIN (REF 5752 5619)
• S VAC B 900 Wall MEDAP (REF 5752 5620)
• S VAC B 900 Wall BOC (BS 5682) (REF 5752 5621)
• S VAC B 900 Wall Air Liquide (NF S 90-116) (REF 5752 5622)
• S VAC B 900 Wall AGA (SS 87 524 30) (REF 5752 5623)
• S VAC B 900 Equipment rail (REF 5752 5624)
• S VAC B 900 Screw connection (REF 5752 5625)
The S VAC D 200 is available in the following versions:
• S VAC D 200 Wall DIN (REF 5752 5626)
• S VAC D 200 Wall MEDAP (REF 5752 5627)
• S VAC D 200 Wall BOC (BS 5682) (REF 5752 5628)
• S VAC D 200 Wall Air Liquide (NF S 90-116) (REF 5752 5629)
• S VAC D 200 Wall AGA (SS 87 524 30) (REF 5752 5630)
• S VAC D 200 Equipment rail (REF 5752 5631)
• S VAC D 200 Screw connection (REF 5752 5636)
Introduction
Basic requirements
1
1.4.5 Interface description
All devices and accessories which are combined with the tapping unit must be listed in the
accessories list or meet the specifications of the interface description. Configuration of the overall
system as well as functional testing are subject to the overall responsibility of the medical staff.
Functionality and suitability of the connected accessory for each intended application must be
checked by the operator before every use. This includes the functionality of the connector
components, airtightness and suitability regarding material properties, working pressure and flow
rate.
1.4.5.1 Vacuum connection tube
The vacuum connection tube is used to connect the tapping unit and the septic fluid jar.
Technical specifications
• Shore hardness of 60
• Inner diameter 6 mm
• Length 50 cm (± 10 cm)
• Vacuum resistant down to −95 kPa (must not collapse)
Prerequisites
• The inner diameter of the vacuum connection tube should match the outer diameter of the
tube connector on the septic fluid jar cap of the pump.
The vacuum connection tube will be referred to only as ‘connection tube’ below.
1.4.5.2 Hydrophobic bacterial and viral filter
In its function as overflow protection, the hydrophobic bacterial and viral filter protects the product
against ingress of particles, fluid and foam. In its function as bacterial and viral filter, it protects
the product against the ingress of bacteria and viruses.
GA 5752 5636 GB 08
13

Introduction
1
Basic requirements
Prerequisites
• Pore size ≤ 1.0 μ.
• The tube connector must match the tube being used.
• The hydrophobic bacterial and viral filter must close tightly against water passage at an absolute pressure of up to 10 kPa.
• If required, observe the direction of flow (see note on the hydrophobic bacterial and viral filter).
1.4.5.3 Septic fluid jar including septic fluid jar cap
The septic fluid jar and septic fluid jar cap are used to collect the secretions extracted.
Prerequisites
• Low leakage.
• Always fasten the septic fluid jar securely.
• The outer diameter of the tube connector on the patient side should match the inner diameter
of the suction tube.
1.4.5.4 Suction tube
The suction tube is used to connect the tube connector on the septic fluid jar on the patient side
and the fingertip or the utensil.
Technical specifications
• Shore hardness of 60
• Inner diameter 6–8 mm
• Length 1.3–3.0 m
• Vacuum resistant down to −95 kPa (must not collapse)
Prerequisites
• The outer diameter of the tube connector on the patient side of the septic fluid jar cap must
match the inner diameter of the suction tube.
1.4.5.5 Fingertip
The fingertip serves to vent the suction tube in order to be able to quickly interrupt the aspiration
process.
Prerequisites
• It must be possible to sterilise the fingertip or it must be a sterilised disposable item.
• The outer diameter of the tube connector on the patient side should match the inner diameter
of the suction tube.
1.4.5.6 Utensil
The suction catheter, lance, etc., are referred to as utensils. The utensils are used to extract
septic fluids.
Prerequisites
• The inner diameter of the utensil's connector must match the outer diameter of the fingertip.
• The utensil must be sterilisable or a sterile single-use item.
• Biocompatibility.
• For endobronchial extraction, a utensil with side openings must be used.
14
GA 5752 5636 GB 08

1.4.5.7 Mechanical overflow protection
The mechanical overflow protection device protects the product against the ingress of particles
and fluid. The tube connector must match the vacuum connection tube.
Introduction
Basic requirements
1
GA 5752 5636 GB 08
15

Safety notes
2
General safety notes
2 Safety notes
2.1 General safety notes
DANGER!
Danger to life!
Danger due to unauthorised modifications.
The product may not be modified.
WARNING!
Risk of injury!
Hazard resulting from incorrect handling.
Be absolutely sure to observe the operating instructions for all the products used in
the configuration.
CAUTION!
ATMOS recommends always having another aspirator ready to hand. That way you
can perform aspiration even in the event of product failure.
2.2 Product safety notes
WARNING!
Risk of injury to mucous membranes!
Endobronchial aspiration in paediatrics and neonatology requires particularly
careful limitation of the vacuum. For regular endobronchial aspiration in paediatrics
and neonatology, the tapping unit S VAC D 200 from ATMOS should be used.
WARNING!
Risk of injury!
ATMOS products may be used only when fully functional.
Ensure that the ATMOS product is fully functional and in good working order prior
to use.
DANGER!
Infection hazard due to oversuction!
To avoid the ingress of fluid or foam into the product or the vacuum source, a
hydrophobic filter must be used. If secretion enters the inside of the unit, the
product must immediately be taken out of operation. Clean and disinfect the
product and have it repaired by a service technician authorised by ATMOS to do
so.
DANGER!
Infection hazard due to contamination!
To avoid the ingress of contaminants into the product or the vacuum source, a
bacterial and viral filter must be used. If bacteria or viruses enter the inside of the
unit, the product must immediately be taken out of operation. Clean and disinfect
the product and have it repaired by a service technician authorised by ATMOS to
do so.
16
GA 5752 5636 GB 08

Safety notes
Product safety notes
WARNING!
Impacts!
Impacts may cause damage to sensitive, precision mechanical components.
Do not expose the product to impacts.
WARNING!
Measuring accuracy / oversuction!
The product may only be operated in a vertical position.
WARNING!
Foaming!
Foam may be created when extracting secretion. Foam is detrimental to the
functioning of the mechanical overflow protection. This raises the risk that
secretions may penetrate the product and cause it to break down.
Use an ordinary foam inhibitor.
2
WARNING!
Non-permissible load!
If the permissible load is exceeded, leakages may occur at the connection between
the terminal unit and the gas probe.
In accordance with DIN EN ISO 9170-1, the overall weight of the product and
accessories may not exceed 2 kg.
WARNING!
Backflow of aspirated secretion!
In the event of oversuction, the aspirated secretion may flow back to the patient if
there is secretion still left in the suction tube.
Before replacing the septic fluid jar in the event of oversuction or switching off the
vacuum, always remove the tube from the patient first.
WARNING!
Risk of injury!
Immediately replace the hydrophobic bacterial and viral filter if it is discoloured,
contaminated or oversucked.
Furthermore, the filter must be changed if the vacuum displayed is above −0.3 bar /
−30 kPa when the vacuum controller is in the ‘max’ position and the suction tube is
open (S VAC D 200: −0.2 bar / −20 kPa).
WARNING!
Risk of injury!
The product may not be used for the following purposes or under the following
conditions:
• Never throw, hit or drop the product.
• The product is not suitable for vacuum extraction.
• The product may not be used without a hydrophobic filter.
• The product may not be used without a bacterial and viral filter.
• The product may not be used without a septic fluid jar.
• The product may not be used without a fingertip.
• The product is not autoclavable.
GA 5752 5636 GB 08
17

Initial operation
3
Product inspection
3 Initial operation
3.1 Product inspection
DANGER!
Product inspection!
Only product parts which are in perfect condition can ensure proper functioning of
the product.
The product parts will thus have to be carefully inspected before mounting.
WARNING!
Infection hazard!
Contaminated components may endanger the health of staff and patients.
Ensure the product is prepared as per hygiene standards before using it for the first
time.
NOTE
In order to ensure the functionality, carry out a function check prior to use.
3.2 Connection to the terminal unit
3.2.1 General
NOTE
Please refer to the manufacturer’s instructions for the particular terminal unit for
information on connecting the gas probe to the terminal unit.
3.2.2 Version A
2
1
Tapping unit with integrated gas pin
The tapping unit (1) is plugged directly into
the terminal unit (2).
18
Fig. 3: Version A
GA 5752 5636 GB 08
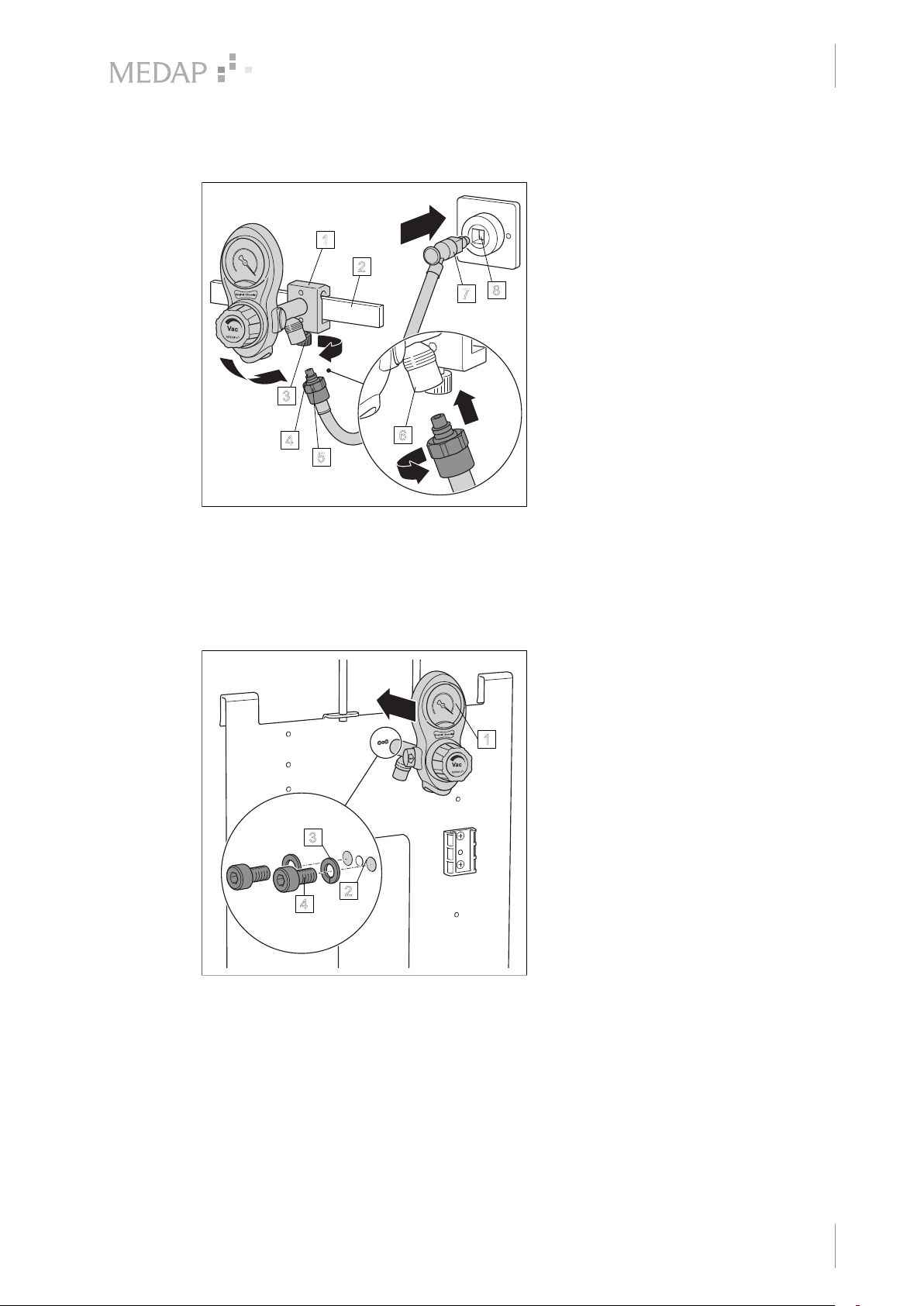
3.2.3 Version B
Fig. 4: Version B
Initial operation
Connection to the terminal unit
Tapping units with rail clamp and NIST
connection
1
2
8
7
3
4
5
6
With the upper edge of the guide groove at
the front, position the rail clamp (1) at a
slight angle on the equipment rail (2) and
then press it against the equipment rail and
allow it to click into place.
Tighten the handle screw (3) of the rail
clamp.
Make sure that the rail clamp is correctly
secured and that the tapping unit is in a
stable position on the equipment rail.
Insert the NIST nipple (4) of the connection
tube into the NIST connection (5) of the
tapping unit and tighten down the NIST
screw connection (6) by hand.
Plug the gas probe (7) of the connection
tube into the terminal unit (8).
3
3.2.4 Version C
The tapping unit for screw connection may be connected to an intensive care trolley, surgical
trolley or solo aspiration set carrier frame. The solo aspiration set carrier frame is used to show
the assembly.
Fig. 5: Version C
Tapping unit for screw connection, with
NIST connection
Press the tapping unit (1) up against the
carrier frame.
1
3
2
4
The drilled holes of the tapping unit are
positioned over the drilled holes (2) of
the carrier frame.
Put the lock washers (3) onto the hexagon
screws (4).
Use the hexagon screws to screw the
tapping unit into place.
GA 5752 5636 GB 08
19

3
Initial operation
Mounting accessories
5
Insert the NIST nipple (1) of the connection
tube into the NIST connection (2) of the
tapping unit and tighten down the NIST
2
1
4
3
screw connection (3) by hand.
Plug the gas probe (4) of the connection
tube into the terminal unit (5).
Fig. 6: Connection tube
3.3 Mounting accessories
3.3.1 General
WARNING!
Tensile forces!
The connected accessories must not exert any mechanical forces which could
adversely affect the secure fit of the product.
WARNING!
Tensile forces!
Hold the basic unit with one hand when installing or removing accessories in order
to compensate for the tensile forces which are created.
NOTE
Refer to the manufacturer's instructions for additional information on the use of the
septic fluid jar and the extraction utensil.
NOTE
The particle filter must be changed whenever the product is prepared for use.
20
GA 5752 5636 GB 08

3.3.2 Mounting the mechanical overflow protection
1
2
3
4
5
5
4
1
3
2
Initial operation
Mounting accessories
Mounting the mechanical overflow
protection
Insert the float (1) into the overflow
container (2).
Fit the sealing ring (3) onto the cap (4).
Insert the cap into the overflow container.
Fit the particle filter (5) onto the cap.
3
Fig. 7: Mounting the mechanical overflow protec-
tion
3.3.3 Connection of the mechanical overflow protection
Mounting the mechanical overflow
protection device
Plug the overflow protection device (1)
Turn the overflow protection device (1) to
Removing the mechanical overflow
2
1
Fig. 8: Mounting/removing the mechanical over-
flow protection device
protection device
Turn the overflow protection device (1) to
Pull the overflow protection device down.
directly onto the tapping unit (2) and press
it up as far as it will go.
the right.
The overflow protection device is locked.
the left.
The overflow protection device is
unlocked.
GA 5752 5636 GB 08
21

Initial operation
3
Mounting accessories
3.3.4 Connection of the hydrophobic bacterial and viral filter
Mounting the hydrophobic bacterial and
viral filter
Undo the grub screw (1) using an Allen key.
Insert the adapter (2) for the hydrophobic
filter directly into the tapping unit (3).
3
1
2
5
4
6
Rotate the adapter and, at the same time,
push up until the lug (4) engages in the slot
(5).
Tighten the grub screw using an Allen key.
Fit the hydrophobic bacterial and viral filter
(6) onto the adapter for the hydrophobic
filter and push up.
Fig. 9: Mounting the hydrophobic bacterial and vi-
ral filter
22
GA 5752 5636 GB 08

4 Operation
4.1 Function test
Prior to using the system, the operator should check that the product is fully functional and in
good condition.
Prior to each use, carry out the following functionality checks:
Version A: Tapping unit with integrated gas pin
• The tapping unit is correctly plugged into the terminal unit.
Version B: Tapping unit with rail clamp and NIST connection
• The tapping unit is locked firmly onto the equipment rail.
Operation
Function test
NOTE
Connecting several septic fluid jars in series can cause delayed suction effect and
reduced suction power.
4
Version C: Tapping unit for screw connection, with NIST connection
• The tapping unit is firmly screwed to the intensive care trolley, surgical trolley or aspiration set
carrier frame solo.
All versions:
• The product has been properly cleaned and neither residue nor contamination are present.
• The control valve can be easily turned and the ON/OFF button is functioning.
• The overflow protection device and the hydrophobic bacterial and viral filter are mounted, fully
functional and no residue is trapped in it.
• The tube connectors are firmly secured and tightly sealed and no mechanical forces are acting
on the tubes.
• The plastic and rubber product parts are in perfect condition and show no signs of ageing.
• A septic fluid jar is connected to the tapping unit.
4.2 Use in conjunction with magnetic resonance imaging scanners
WARNING!
Danger to life!
Please strictly observe the operating instructions of your magnetic resonance
imaging scanner.
WARNING!
Risk of injury!
Accessories of the product (e.g. trolley, carrier frame, connection tube, rail clamp)
may be affected by the magnetic field.
If the product is used in conjunction with accessories within the 0.5 mT line, all
connected accessories must be MR compatible. Observe the operating instructions
of all connected accessories or consult the manufacturer of the product.
NOTE
If the product is used in conjunction with accessories outside the 100 mT line, the
product does not create artefacts on the MR images.
GA 5752 5636 GB 08
23

Operation
4
Setting the vacuum level
4.3 Setting the vacuum level
DANGER!
Infection hazard!
In the event of oversuction, the hydrophobic bacterial and viral filter must no longer
be used.
Replace the hydrophobic bacterial and viral filter with a new one.
WARNING!
Vacuum setting!
Make the vacuum settings very carefully! The regulating mechanism is sensitive.
WARNING!
Air inlet!
The bore holes on the rear side of the unit must always be kept free so that airflow
is always ensured.
NOTE
Check the vacuum setting once again immediately before using the unit!
5
1
Fig. 10: Setting the vacuum level
2
4
Setting the vacuum for treatment
Press the OFF button (1) and connect the
tapping unit [ Page 18].
Turn the control valve (2) clockwise and
close as far as it will go.
Press the ON button (3).
Bend the connection tube (4) that leads to
the septic fluid jar.
3
Use the control valve to set the required
value for the treatment.
Increase the vacuum: Turn the control
valve anticlockwise.
Reduce the vacuum: Turn the control
valve clockwise.
Read the value on the vacuum gauge (5).
Should it not be possible to shut off or
increase the vacuum as required, refer to
the troubleshooting table to find the cause.
Release the connection tube (4) that leads
to the septic fluid jar.
Aspirate.
24
GA 5752 5636 GB 08

5 Taking the unit out of operation
5.1 Completing the aspiration process
NOTE
Please refer to the manufacturer’s instructions for the particular terminal unit for
information on detaching the gas probe from the terminal unit.
Remove the suction tube from the patient.
Press the OFF button and close the control valve.
Empty the septic fluid jar and clean or replace it.
Remove the connection tubes and the overflow protection device / the hydrophobic bacterial
and viral filter from the tapping unit and from the septic fluid jar and recondition or discard
them.
Clean the components [ Page 27]
Disconnect the gas probe from the terminal unit.
Taking the unit out of operation
Completing the aspiration process
5
Version B: Tapping unit with rail clamp and NIST connection
Remove the product from the equipment rail. For this purpose, undo the handle screw and lift
the product off the equipment rail.
5.2 Disassembly
5.2.1 General
WARNING!
Tensile forces!
Hold the basic unit with one hand when installing or removing accessories in order
to compensate for the tensile forces which are created.
WARNING!
Tensile forces!
The connected accessories must not exert any mechanical forces which could
adversely affect the secure fit of the product.
GA 5752 5636 GB 08
25

Taking the unit out of operation
5
Disassembly
5.2.2 Dismantling the mechanical overflow protection
1
2
5
4
3
Fig. 11: Dismantling the mechanical overflow pro-
tection
Remove the particle filter (1) from the cap
(2).
Remove the cap from the overflow
container (3).
Remove the sealing ring (4) from the cap
(2).
Pull the float (5) out of the overflow
container.
26
GA 5752 5636 GB 08

6 Cleaning and disinfection
6.1 General
The product must be wipe or spray disinfected after every use.
DANGER!
Risk due to incorrect use of detergents and disinfectants!
It is strictly advised to observe the manufacturer’s instructions regarding how to
use the detergents and disinfectants as well as to observe the valid hospital
hygiene rules.
WARNING!
Infection hazard!
Product may be contaminated.
Always wear gloves for cleaning and disinfection.
WARNING!
Infection hazard!
Particles of grime may become encapsulated and lead to the product not reaching
the desired germ reduction after disinfection.
Before disinfection, the product must be cleaned thoroughly of contamination and
encapsulated particles of grime.
Cleaning and disinfection
General
6
CAUTION!
Improper cleaning and disinfection can cause property damage!
Do not use the following products for cleaning and disinfection:
• Products containing alcohol (e.g. hand disinfectants)
• Halogenides (e.g. fluorides, chlorides, bromides, iodides)
• Dehalogenating compounds (e.g. fluorine, chlorine, bromine, iodine)
• Products that may scratch the surface (e.g. scouring agents, wire brushes, wire
wool)
• Standard commercial solvents (e.g. benzene, thinner)
• Water containing iron particles
• Cleaning sponges containing iron
• Products containing hydrochloric acid
Use a soft, lint-free cloth or a soft nylon brush to clean the product.
CAUTION!
Improper cleaning and disinfection can cause property damage!
Use only as much detergent and disinfectant as required.
CAUTION!
Improper cleaning and disinfection can cause property damage!
After each cleaning and disinfection process, carry out the functionality test.
GA 5752 5636 GB 08
27

Cleaning and disinfection
6
Cleaning
6.2 Cleaning
6.2.1 General
NOTE
Use only all-purpose cleaners which are slightly alkaline (soap solution) and
contain surfactants and phosphates as the active cleaning agents.
In the event of heavily contaminated surfaces, use concentrated all-purpose
detergent.
CAUTION!
Improper cleaning can cause property damage!
Residues of physiological saline solutions (e.g. sodium chloride) can attack the
surfaces of the product.
Remove residues of physiological saline solutions with a cloth dampened in clean
water. Then dry the product with a dry, lint-free cloth.
CAUTION!
Improper cleaning can cause property damage!
Do not spray cleaning agent directly into the joints or gaps and never use a highpressure cleaning unit!
6.2.2 Cleaning procedure
Use the correct dose of all-purpose detergent with water for the degree of surface
contamination and in accordance with the instructions of the detergent manufacturer.
Thoroughly wipe off the product with a soft cloth slightly dampened in an all-purpose detergent
solution.
Ensure that the product is free of contamination and encapsulated particles of grime.
Thoroughly wipe off the product with a soft cloth slightly dampened in clean water.
Ensure that the product is free of detergent residues.
Dry the product with a dry, absorbent and lint-free cloth.
This will help to reduce pathogen growth on the product's surface.
Wipe disinfect the product after every cleaning process.
6.3 Disinfection
6.3.1 General
28
NOTE
In the event that product surfaces are very dirty, carry out an additional cleaning
procedure before disinfecting.
DANGER!
Reduced performance!
Only clean the product by wipe disinfection.
Ensure that no disinfectants enter the unit. Check the functionality of the product
after each disinfection.
GA 5752 5636 GB 08

CAUTION!
Material damage due to excessive exposure times!
Exceeding the specified exposure time of the disinfectant may damage the
surfaces.
Observe the exposure time specified by the disinfectant manufacturer.
6.3.2 Suitable disinfectants
Only surface disinfectants based on the following combinations of active ingredients may be used
for disinfection:
• Aldehydes
• Quaternary compounds
• Guanidine derivatives
Ingredient group Active ingredients
Aldehydes 2-ethyl-1-hexanal, formaldehyde, glutardialdehyde, glyoxal,
o-phthaldialdehyde, succinaldehyde
Cleaning and disinfection
Disinfection
6
Quaternary compounds Alkyl-didecyl-polyoxethyl ammonium propionate, alkyl-dimethyl-
Guanidine derivatives Alkyl-biguanide, chlorhexidine-digluconate, cocospropylene-
Tab. 4: Active ingredients of disinfectants
6.3.3 Disinfection procedure
Wipe or spray disinfect the product in accordance with the instructions of the disinfectant
manufacturer.
Ensure that the product is free of disinfectant residue.
Perform visual and functional inspections.
alkylbenzyl ammonium chloride, alkyl-dimethyl-ethyl ammonium
chloride, alkyl-dimethyl-ethylbenzyl ammonium chloride,
benzalkonium propionate, benzalkonium chloride (alkyl-dimethylbenzyl ammonium chloride, coco-dimethyl-benzyl ammonium
chloride, lauryl-dimethylbenzyl ammonium chloride, myristyldimethyl-benzyl ammonium chloride), benzethonium chloride,
benzyl-dihydroxyethyl-coco-alkyl ammonium chloride, dialkyldimethyl ammonium chloride (didecyldimethyl ammonium chloride),
didecyl-methyl-oxyethyl ammonium propionate, mecetronium-ethyl
sulfate, methyl-benzethonium chloride, n-octyl-dimethyl-benzyl
ammonium chloride
diamine guanidinium diacetate, oliogomeric biguanide,
polyhexamethylene biguanide hydrochloride (oligo-diimino imiodocarbonyl imino-hexamethylene, polyhexanide)
6.3.4 Disinfection procedures
Different disinfection procedures may be used for the various components depending on the
properties of the materials. Before disinfection, remove contamination and residues from the
parts and dry well.
GA 5752 5636 GB 08
29

6
Cleaning and disinfection
Product-specific safety notes
Components Wipe, spray disinfection
S VAC X
Trolley X
Catheter holder
1 After exposure (as prescribed in the manufacturer's instructions), remove disinfectant residues
from the components using a moist cloth and dry them afterwards.
6.4 Product-specific safety notes
DANGER!
Health hazard!
The product may not be disassembled for cleaning or disinfection. During cleaning
and disinfection, pay attention that no cleaning agent, disinfectant or other
contamination is able to enter the product.
DANGER!
Risk to patient!
Oversuction of products results in them no longer being functional. There is
considerable risk to the patient if the tapping unit is not cleaned properly after being
exposed to oversuction, as safety equipment could be clogged.
After oversuction, products must be dismantled and cleaned thoroughly by
authorised service staff.
1
CAUTION!
Property damage due to sterilisation!
Do not sterilise the product.
CAUTION!
Property damage!
Using non-colour-fast surgical drapes can cause discolouration of surfaces.
Only use colour-fast surgical drapes.
NOTE
For the cleaning and disinfection of versions B and C, disconnect the connection
tube with the NIST screw connection from the tapping unit.
30
GA 5752 5636 GB 08

7 Maintenance
7.1 General
Maintenance, repairs and periodic tests may only be carried out by persons who have the
appropriate technical knowledge and are familiar with the product. To carry out these measures,
the person must have the necessary test devices and original spare parts.
ATMOS recommends: Work should be carried out by an authorised ATMOS service partner. This
ensures that repairs and testing are carried out professionally, original spare parts are used and
warranty claims remain unaffected.
DANGER!
Health hazard!
The product is used in the treatment of patients. The product or some of its
components may be contaminated.
Clean and disinfect the product before maintenance and repair.
Repair work may be performed by personnel authorised by ATMOS.
Maintenance
General
7
7.2 Periodic tests
At least every 5 years a test must be performed.
7.3 Malfunctions and troubleshooting
Defect Source of malfunction Corrective actions
• No or low vacuum
• No or reduced flow rate
• Regulation of flow rate not
possible
ON/OFF button is closed Open ON/OFF button
The connection tube is not
connected to tapping unit
Connection tube too long Shorten connection tube to a
Connection tubes collapse Use special connection tubes
Full septic fluid jar; overflow
protection system closed
Oversuction of hydrophobic
bacterial and viral filter
Seal damaged Replace seal
Suction system is leaking Check suction system
Connect connection tube
according to operating
instructions
maximum length of 50 cm
(vacuum proof up to –95 kPa)
Empty/replace septic fluid jar;
replace overflow protection
device
Replace hydrophobic bacterial
and viral filter
ON/OFF button is defective Contact Technical Service
Central supply system failure
Gas probe connection is
loose
Vacuum gauge is defective
Control valve is defective
GA 5752 5636 GB 08
31

7
Maintenance
Repairs
Defect Source of malfunction Corrective actions
Oversuction of product
despite protective system
Gas probe does not fit into
the terminal unit
Tab. 5: Malfunctions and troubleshooting
7.4 Repairs
The following may require repairs by the manufacturer or an authorised service partner:
• Liquid has penetrated the device.
• Performance has significantly decreased.
• Inexplicable notifications appear.
• Abnormal noises occur.
• Functional faults cannot be rectified according to the measures in chapter Malfunctions and
troubleshooting [ page 31].
If defects are detected, the product may not be used any longer.
Make a note of the defects and the REF number on the type plate and inform your ATMOS
representative.
Observe the information in chapter Sending in the device [ page 32].
Tapping unit mounted at an
angle
Overflow protection device
contaminated
No foam inhibitor used Use standard commercial foam
Terminal unit for the wrong
gas type
Operate tapping unit in a vertical
position only
Clean/replace the overflow
protection device
inhibitor
Check gas type
7.5 Service hotline
+49 7653 689-0
7.6 Type plate position
Fig. 12: Type plate position
7.7 Sending in the device
Remove and properly dispose of consumables.
Position of the type plate (1).
1
32
GA 5752 5636 GB 08

Maintenance
Sending in the device
Clean and disinfect the product and accessories according to the operating instructions.
Place used accessories with the product.
Fill in form QD 434 ‘Delivery complaint / return shipment’ and the respective decontamination
certificate.
This form is enclosed with each delivery and can be found at www.atmosmed.com.
The device must be well padded and packed in suitable packaging.
Place form QD 434 ‘Delivery complaint / return shipment’ and the respective decontamination
certificate in an envelope.
Affix the envelope to the outside of the package.
Send the product to ATMOS or to your dealer.
7
GA 5752 5636 GB 08
33

Technical specifications
8
General
8 Technical specifications
8.1 General
Classification as per Annex IX to Directive 93/42/EEC Class IIa
8.2 Technical specifications
Vacuum regulation range of S VAC B 900 0 to −100 kPa*
Vacuum regulation range of S VAC D 200 0 to −20 kPa*
Flow rate of S VAC B 900 (Freeflow)** min. 100 l/min
Flow rate of S VAC D 200 (Freeflow)** min. 90 l/min
Vacuum gauge Accuracy class 2.5
Use in MR environment MR conditional (tapping unit without
* 100 kPa = 1 bar = 1000 mbar = 750 mmHg
** in accordance with EN 10079-3. Depending on the design of the gas supply system, the actual
performance of the tapping unit may be reduced.
*** 1 T = 1000 mT= 10000 Gauss
trolley or carrier frame) up to 4.7 T***
within 100 mT
8.3 Ambient conditions
Temperature −15 °C to +50 °C (shipping)
Relative humidity less than 100% (shipping)
Atmospheric pressure 700 hPa to 1060 hPa (shipping)
8.4 Dimensions and weight
Dimensions S VAC B 900 and FINA S VAC D 200 (L x
W x H)
Weight S VAC B 900 310 to 510 g
Weight S VAC D 200 360 to 560 g
+10 °C to +40 °C (operation)
30% to 75% (operation)
700 hPa to 1060 hPa (operation)
165 x 80 x 125 to 165 mm
34
GA 5752 5636 GB 08

9 Approved accessories
The following accessories are not part of the scope of delivery and must be ordered separately:
9.1 Accessories
5752 5637 S VAC mobile suction unit basic equipment / vacuum
5752 5638 S VAC mobile suction unit complete unit / vacuum / 2 x 3 l
5752 5676 S VAC mobile suction unit complete unit / vacuum / 2 x 4 l / PSU
5752 5677 S VAC mobile suction unit complete unit / vacuum / 2 x 4 l / PC
5752 5639 S VAC compact suction unit basic equipment / vacuum
5752 5640 S VAC compact suction unit complete unit / vacuum
5752 5632 Mechanical overflow protection
5752 5634 Adapter for hydrophobic filter
5752 5256 Aspiration set for carrier frame solo
5752 5288 Equipment carrier for aspiration set carrier frame solo
5752 5314 Surgical trolley
5750 8021 Tube holder
see MEDAP
tube list
Tab. 6: Accessories
VAC connection tube with NIST screw connector
Approved accessories
Accessories
9
9.2 Accessories for compact suction unit / basic vacuum equipment
The suction units can be used in combination with the following application sets.
5752 5645 AS septic fluid aspiration / 2 x 1 l / reusable
Tab. 7: Accessories for compact suction unit / basic vacuum equipment
9.3 Accessories for mobile suction unit / basic vacuum equipment
The suction unit can be used as surgical aspirator by combining it with the following application
sets.
5752 2067 AS surgical aspiration / 2 x 5 l
5752 5664 AS surgical aspiration / 2 x 4 l / PSU
5652 5665 AS surgical aspiration / 2 x 4 l / PC
5752 2068 AS surgical aspiration / 2 x 3 l
5752 4940 AS surgical aspiration / 2 x 3 l / Serres
310.0401.0 Serres® disposable suction liner 3 l, with gelling agent (20 pieces)
310.0411.0 Serres® disposable suction liner 3 l, without gelling agent (24 pieces)
5752 2049 Vacuum shift
5752 4538 Bowl for trolley
®
Tab. 8: Accessories for mobile suction unit / basic vacuum equipment
GA 5752 5636 GB 08
35

Approved accessories
9
Consumables
9.4 Consumables
5752 5635 Hydrophobic bacterial and viral filter (disposable)
5752 5633 Particle filters (100 pieces)
006.0009.0 Suction tube, silicone, Ø 6 mm, 1 m
5750 5483 Vacuum connection tube, 8 x 14 mm, by the metre
000.0347.0 Fingertip
Tab. 9: Consumables
36
GA 5752 5636 GB 08

Notes

Notes

Notes

Manufacturer:
ATMOS
MedizinTechnik GmbH & Co. KG
Ludwig-Kegel-Str. 16
79853 Lenzkirch
GERMANY
Telephone: +49 7653 689-0
www.atmosmed.com
 Loading...
Loading...