
Avie™ A1c Reader
User’s Manual
TABLE OF CONTENTS
I. Installation and Information
Introduction
Installation
II. PRINCIPLES OF OPERATION
The Avie™ A1c Reader
The Avie™ A1c Test Cartridge
III. PERFORMANCE CHARACTERISTICS & PRODUCT SPECIFICATIONS
Performance Characteristics
Product Specifications
IV. OPERATING INSTRUCTIONS
Initial Setup
Performing a Test
Collecting a Fingerstick Sample
V. READER FUNCTIONS
VI. CALIBRATION AND QUALITY CONTROL
Calibration
Quality Control
VII. OPERATIONAL PRECAUTIONS AND LIMITATIONS
VIII. HAZARDS AND SYMBOLS
IX. MAINTENANCE AND CLEANING
Batteries
Troubleshooting
Warranty

I. INSTALLATION AND INFORMATION
1.1 Introduction
This manual will provide you with useful information that you will need to know in order
to perform Hemoglobin A1c (A1c) testing on the Avie™ A1c Reader.
Note: Please read the entire manual before using the Avie™ system
The Avie™ A1c System provides quantitative measurement of the percent of glycated
hemoglobin levels in fingerstick (fresh capillary) whole blood samples. The test is for
professional use and physician directed home use at the point of care, to monitor
glycemic control in diabetic patients. The Avie™ A1c Test System provides a simple,
reliable way to monitor glycemic control.
The Avie™ A1c System is for in vitro diagnostic use only.
Please see the Package Insert that accompanies the Avie™ A1c Cartridge for additional
information.
MEC Dynamics Technical Support and Customer Care contact information:
MEC Dynamics Corp
2225 Martin Ave. Suite I
Santa Clara, CA 95050
Phone: 408-844-9280
Toll Free: 888-376-1081
Fax: 408-844-9285
Hours: 6:00 am - 6:00 pm Pacific Standard Time, Monday - Friday
Web: www.mecdynamics.com
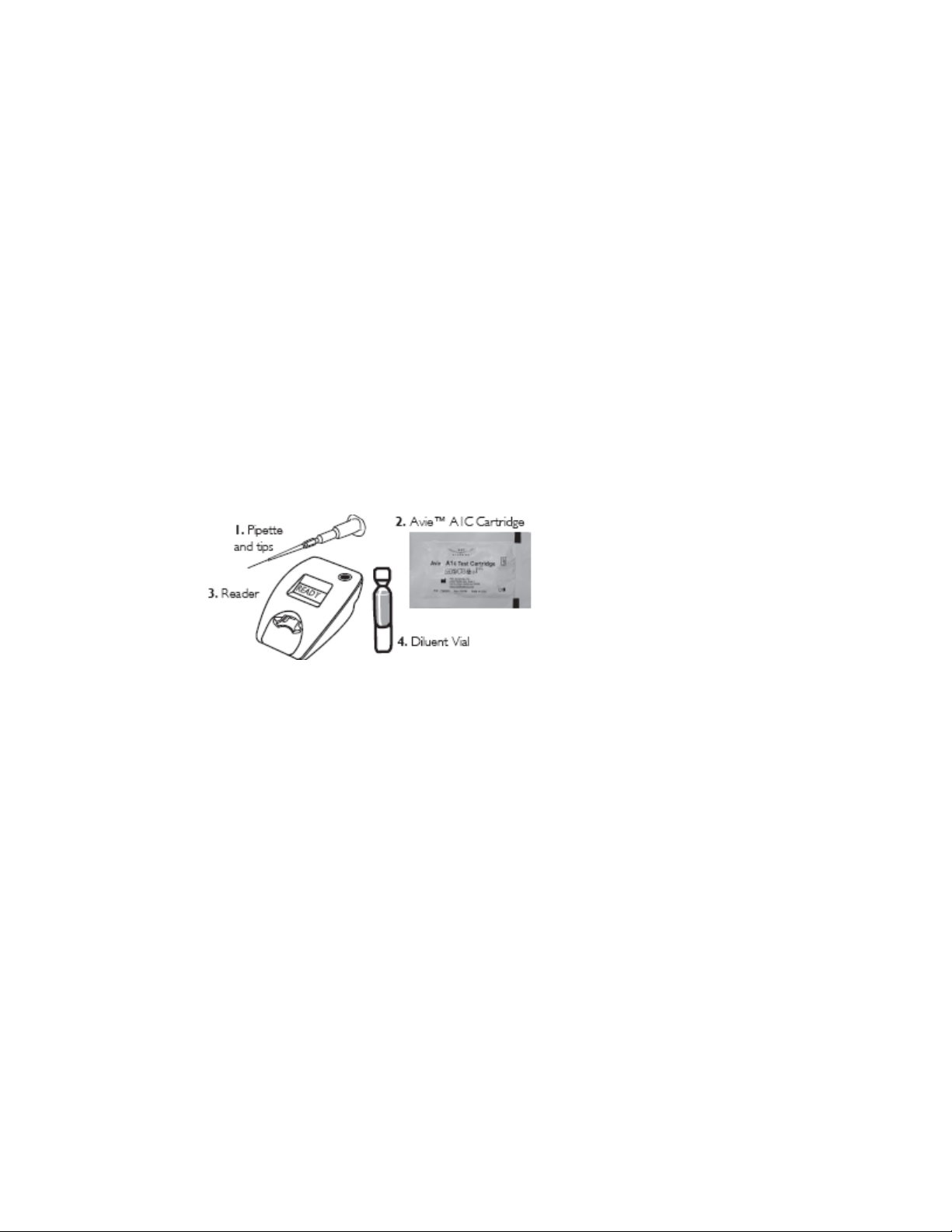
1.2 Installation
Your Avie™ A1c Test System includes:
• Avie™ A1c Reader (Battery power pack is optional)
• Universal Power adapter
• Avie™ A1c System Users Manual
• Quick Reference Guide
• Avie™ A1c Test Cartridge
• Pipette and Tips
• Diluent
• Warranty registration card
You will also need:
• Lancets
• Isopropyl alcohol or alcohol wipes
• Gauze or cotton balls
• Puncture resistant container (SHARPS container)
See the “Battery” section for more information and instructions on installing the
batteries if a battery power pack is to be used.
Special Requirements
To ensure that your Avie™ A1c Reader operates correctly, be sure the following
conditions are met:
• Room temperature should be between 65º F and 82º F (18º C and 28º C) for
testing.
• Relative humidity should be between 10% and 80%, without condensation, for
testing.
• The Avie™ A1c Reader should be transported in a secure container if there is a
need to move it.
• Avoid dropping the reader, or treating it roughly.
• Use the Reader only on a level non-vibrating surface.

II. PRINCIPLES OF OPERATION
The Avie A1C test system utilizes immunochemistry and general chemistry to quantify %A1C levels
(glycated hemoglobin) in whole blood. The system consists of a small electronic instrument (Reader),
a single-use diluent solution vial, and single-use reagent test cartridge.
To perform one test, the Reader is turned on. When the LCD display denotes “Ready” a test cartridge
is inserted into an unambiguous slot in the reader. A small amount of whole blood is added to the
diluent vial. This blood is immediately lysed and the hemoglobin is converted to met-hemoglobin.
At the instruction of the reader (LCD display), three drops of diluted blood are applied to the well on
the cartridge. The reactions then proceed automatically and no procedural steps are required. The test
is complete is less than 4 minutes.
The A1C test consists of two distinct quantitative areas of measurement. The first area consists of a
chamber where the met-hemoglobin is photometrical read at 420 nm. The optical density of the methemoglobin is proportional to the concentration of total hemoglobin. The second area consists of a
mixing chamber where the diluted blood is mixed with anti hemoglobin antibodies conjugated to blue
microparticles. After a predetermined time the microparticle mixture is automatically released onto a
reagent strip where the reacted and unreacted microparticle species are separated and read optically.
The concentration of these species is used to calculate the amount of A1c. The final displayed result is
expressed as;
%A1C = (A1C ÷ TOTAL HB) ×100
If attached to an ancillary printer, the test results will be automatically printed out at the end of the test.
1.3 Avie™ A1c Reader
Study the following illustrations to become familiar with the major components of your
Avie™ A1c Reader:

Avie™ A1c Test Cartridge
Power Button: Turns the Reader on
isplay: Screen shows test results and error
Test Cartridge Guide: Holds a test cartridge in
place during testing
Application Well

III. PERFORMANCE CHARACTERISTICS AND PRODUCT SPECIFICATIONS
1.4 Performance Characteristics
1.4.1 Expected Values
Approximate Mean Plasma Glucose*
GHB(%)
4 65
5 100
6 135
7 170 9.5 ADA Target#
8 205
9 240
10 275
11 310
12 345
* Mean blood glucose results are 10-15% lower. Most blood glucose Readers are calibrated to read as
mg/dL mmol/l
3.5
5.5
7.5
11.5
13.5
15.5
17.5
19.5
#
Diabetes Care 2004;27 (Suppl. 1):S91 - S93
plasma glucose.
Interpretation
Non-Diabetic
Range
Above Target

y
1.5 Product Specifications
Battery lifetime: Variable. Approximately 50 tests.
Mode of operation: Continuous
Operating Conditions:
Operating temperature: 65 to 82°F (18 to 28 °C)
Operating humidity: 10% to 80% (non-condensing)
Degree of protection against ingress of water: Ordinary equipment
Power supply: AC: Input 120V
4 AA Alkaline 1.5-volt batteries
Output: 6V @ 700 mA
Battery voltage: 6V @ 700 mA
Result range: A1c values 5% to 14% are reported.
Sample type: Fingerstick (fresh capillary) whole blood samples
Sample volume: 4 micro liters
Reader Storage and transportation
Temperature: 0° F to 131°F (-17° C to 55°C)
Humidity: l0% - 80% (non-condensing)
Atmospheric pressure: 500 hPa -1060 hPa
• Equipment complies with EM 61010-1
• Classification is with respect to electric shock, fire, and mechanical hazards only
in accordance with EN61010-1.
• Internally powered and Class II equipment.
• Type BF patient applied parts.
• System complies with EN 61326.
• EN61326-1 EMC Medical Products Warning: This equipment generates, uses,
and can radiate radio frequency energy and, if not installed and used in
accordance with the instructions, could cause interference to adjacent equipment.
There is no guarantee that interference will not occur in a particular installation if
the instructions are not followed. The user can determine if the interference is
caused by the equipment by turning the unit off. If interference is caused by the
equipment, you should try moving the equipment away from the other unit.
• This device complies with Part 15 of the FCC rules. Operation is subject to the
following two conditions : (1) This device may not cause harmful interference,
and (2) this device must accept any interference received, including interference
that may cause undesired operation.
1.6 Symbols and Explanations
In vitro Diagnostic: The Avie™ A1c system is for in vitro diagnostic use onl

Consult Instructions for Use prior to using the Avie™ A1c System
Serial Number: The Reader serial number is located on the bottom of the
Reader
Temperature Limitation: The Avie™ A1c system is intended to be used
between 65 to 82°F (18 to 28 °C) 10 to 80% non-condensing relative
humidity
Do Not Reuse: The Test Cartridges and Diluent vial are intended for one
time use only
Use By: Test Cartridge, Diluent, and Controls must be used by the
expiration date marked on their labels.
Batch Code: The lot number of the Test Cartridge may be found on the
cartridge vial and the Diluent on the vial and outer packaging.
Manufacturer: MEC Dynamics, Inc.
2225 Martin Ave. Suite I
Santa Clara, California 95050
1.7 Ordering Information
You may order the following products through your authorized MEC Dynamics Corp.
distributor or by contacting the Customer Service Department at MEC at 408 844 9280
Part Number Description
FIN0003 Avie™ A1c Physician Directed Home Use System Kit
FIN0004 Avie™ A1c Professional System Kit
FIN0005 Avie™ A1c Reader
FIN0006 Avie™ A1c Professional Test Cartridge (box of 100 with diluent)
FIN0007 Avie™ A1c Physician Directed Home use Test Cartridge (box of 5
with diluent)
FIN0009 Avie™ A1c Battery Power Pack
FIN0010 Avie™ A1c Printer and Cable
LBM0001 Avie™ A1c System User’s Manual
LPC0002 Avie™ A1c Use Quick Reference Guide
FIN011 Avie™ A1c System Control Level I
FIN012 Avie™ A1c System Control Level II

IV. OPERATING INSTRUCTIONS
1.8 Initial Set-up
Your Avie™ A1c Reader is ready to test when you receive it. Just plug it in and follow
the instructions below. If you prefer to run the Reader on batteries, see section IX.
Maintenance and Cleaning, for use of the battery pack.
Running an A1c Test
Check the expiration date on the cartridge vial, the diluent vial, and the control bottles. Do not use
items after their expiration date.
If the Test Cartridge Vial or Reader have been recently at high or low temperatures
(above 82°F or below 65°F), allow ALL parts to come to room temperature for at least 1
hour before running the test. Leave the cartridge in the sealed vial while doing this.
Avoid running the test in direct sunlight, on hot, cold, or vibrating surfaces, or near
sources of heat or cold. Do not reuse the cartridge or diluent vial.
1. Use the cartridge within 2 minutes after removing it from the vial.
2. Turn the Reader on by pressing the ON button. When “READY” is shown on the
display, the Reader is ready to start a test.
3. Touch the side of the cartridge vial to the middle side right side of the Reader to
transfer cartridge specific information. The reader will beep. Slide the test
cartridge into the Reader test cartridge guide in the direction of the arrow as far as
it will go.
4. The Reader will beep and show “APPLY DILUENT”.
5. The patient should wash their hands with soap and water.

6. Open the Diluent vial by holding on the bottom fin of the vial and twisting off the
vial cap. You may lay the vial down as it will not leak unless squeezed.
7. Firmly apply a clean unused pipette tip onto the end of the pipette giving it a
slight twist to make sure there is an airtight seal.
8. Obtain a sample by means of a fingerstick. If you are performing a control test
pipette a control sample directly from the control bottle (see step 9).
Performing a fingerstick
a. Clean the desired finger with alcohol or soap and warm water and then dry
hands thoroughly before lancing the finger.
b. Position the lancet on the puncture area (side of a fingertip, away from any
calluses or scars.
c. Use the lancet as directed to puncture the skin.
d. Apply gentle, continuous pressure across the entire finger to form a hanging
drop, do not milk the finger (see step 9 for collection of the sample).
e. Hold a cotton ball or gauze firmly over the puncture site until the bleeding
stops.
f. Place a band aid over the puncture on the finger if desired.
9. Collect the Sample:
a. Push the plunger on the pipettor all the way down and hold it down as far as it
will go.
b. Holding the pipettor at an upright angle, gently place the pipette tip in the
finger stick blood drop. If you are testing controls place the pipette into the
liquid sample in the control bottle.
c. Release the plunger slowly, drawing up the sample. Be careful not to have an
air gap or bubble form. The blood should be solid red with no spaces or
bubbles. Collect a new sample if this occurs.

10. Place the sample in the diluent vial:
a. Holding the vial upright by the fin, insert the pipette tip into the liquid.
b. Slowly depress the plunger to add all the blood to the liquid.
c. Keeping the pipettor depressed, remove the pipette tip out of the vial
before releasing the plunger. This will prevent blood sample from being
drawn back into the tip.
d. Remove the pipette tip and discard it in a biohazard waste container.
e. Invert the vial 5 times to mix the blood with the diluent. DO NOT
SHAKE. The tube will not spill in the inverted position unless squeezed.
11. Apply the sample. Squeeze 3 drops of the mixture (diluted sample) into the
application well located on the top of the cartridge.
12. The Reader will count down before the result is shown.
13. When the test is complete, the Reader will beep and the results will be shown on
the screen. Remove and discard cartridge in puncture proof container.
6.2 %
If the results are suspect and not in the expected range or if the controls are not
within the range given with the control sample repeat the test with a fresh sample.

14. If the meter has timed out (left idle for 5 minutes) and shutdown, press the power
button to briefly show the last test result.
15. Discard the lancet, pipette tip, and used cartridge using Universal Biohazard
Precautions.
V. READER FUNCTIONS
When the Reader is turned on, it performs a self-check (optics and software). If a
malfunction is detected, the Reader displays an error message on the LCD display. See
the troubleshooting section if this should occur.
VI. CALIBRATION AND QUALITY CONTROL
The MEC A1C System is Factory Calibrated. MEC Avie A1C System Control Level I
and Level II are available for purchase. MEC Dynamics recommends that external
controls be tested at the following times:
• Prior to home testing or at the start of each testing day.
• Upon receipt of each new shipment or use of a new lot of cartridges
• Whenever storage room conditions have been above 28°C (82°F).
• To become familiar with the process or to perform training or retraining of testing
personnel.
• Whenever Avie™ A1c results do not match other clinical findings or symptoms.
The controls are tested identically to a blood sample. See section IV Operating Instructions.
If the results are out of range when compared to the number supplied with the control bottle
repeat the test with a fresh sample. Check to ensure all materials are within dating (cartridge,
diluent, & control have not expired). If the result is still out of range contract the MEC
Technical Support at: 888-376-1081. Do not perform a test until the quality control sample
is within the given range.
VII. OPERATIONAL PRECAUTIONS AND LIMITATIONS
• The Avie™ A1c system is for in vitro diagnostic use only.
• Use fingerstick (fresh capillary) whole blood samples for testing.
• The fingerstick site must be completely dry. If any alcohol remains on the finger,
it may cause inaccurate results.
• The cartridges and diluent vials are for one use only. Do not reuse them.
• Do not move or touch the Reader while it is running a test.
VIII. HAZARDS AND SYMBOLS
Caution: The Reader generates, uses, and can radiate radio frequency
energy. If it is not installed and used in accordance with instructions, it may
cause interference to other devices in the vicinity. Call MEC Dynamics
Technical Support for help in determining whether your Reader is causing
interference and how to correct any interference.

Caution: Use only Avie™ A1c AC Adapter or damage to Reader may result.
Class II Equipment: The Reader is double insulated.
Biological Risk: Disposable items pose biological risks. Use Universal
Precautions
Type BF materials supplies
IX. MAINTENANCE AND CLEANING
Use Universal Biohazard Precautions. Clean the Reader only as needed. Do not turn the
meter on when cleaning.
External Cleaning
Use clean cloth lightly dampened with bleach solution. Use only 10% household bleach
solution, i.e., 10% Clorox® solution (1 part bleach + 9 parts water) or an alcohol wipe
(70% Isopropyl alcohol) to clean the Reader.
Internal Cleaning
Only the test cartridge guide needs cleaning.
CAUTION: DO NOT FLOOD READER WITH CLEANING SOLUTION!
1. Check for blood, lint or debris on the test cartridge guide. If present,
2. Remove by wiping with lint free cloth dampened with dilute (10%) bleach and
water solution or alcohol wipe (70% Isopropyl alcohol).
3. Dry the cartridge holder with a dry, lint free cloth.
Loading Batteries
Insert 4 AA batteries (only alkaline batteries) according to battery position diagrams
inside the battery pack, and then plug into power port.
Note: If Reader turns on at the push of the power button, the batteries are properly
installed.
Troubleshooting
The Avie™ A1c Reader displays error codes to indicate problem with the system. If you
are uncertain of an error or how to resolve a problem, contact MEC Technical Support at:
408 844 9280 or 888-376-1081
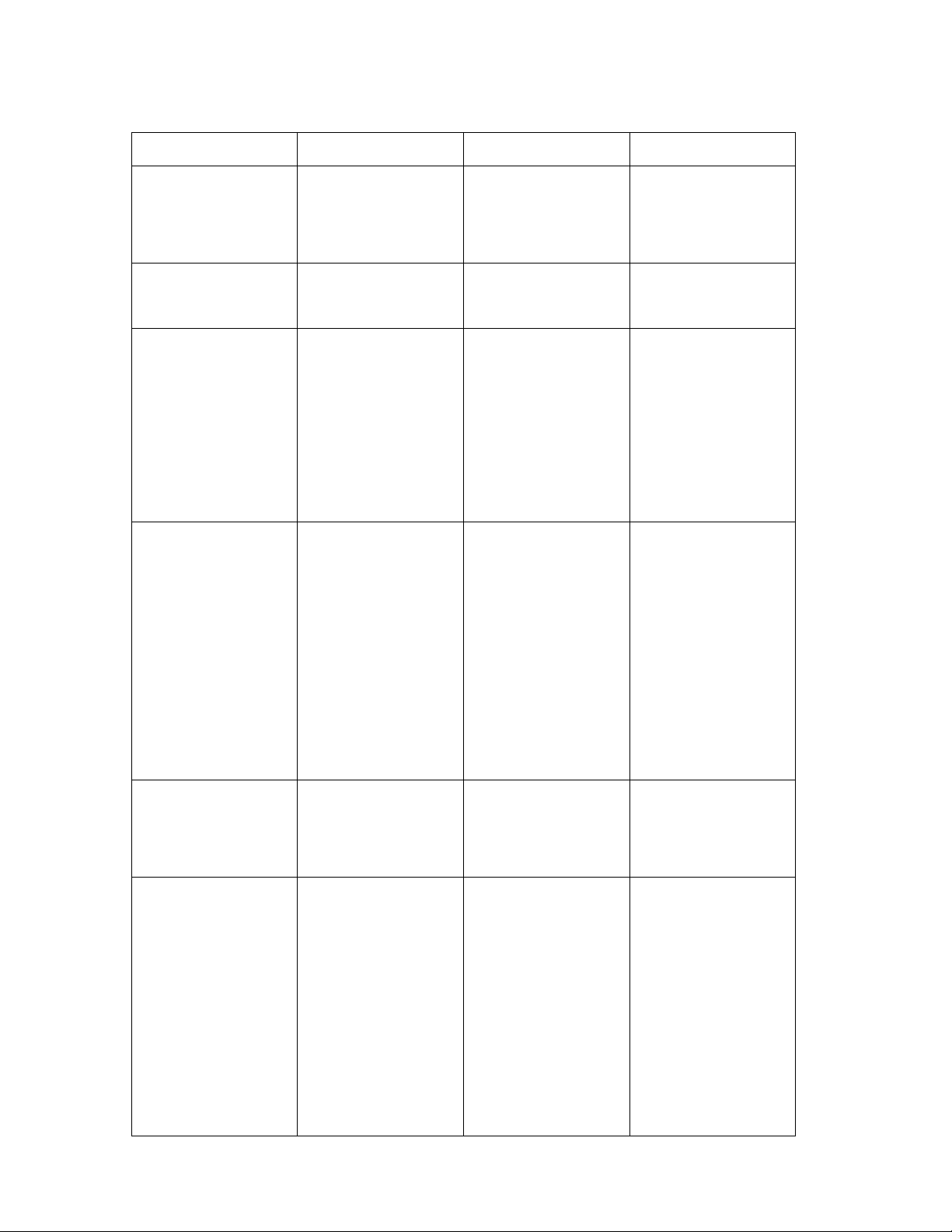
These messages are possible errors that may occur. If your problem still persists, please
MEC Dynamics Technical support at 408-844-9280 or 888-376-1081
Error Code ERROR CAUSE ACTION
LOWBAT
REMOVE STRIP
ERROR
REMOVE STRIP
No display
Low Batteries
Remove Cartridge
Remove Cartridge Cartridge not
Monitor has shut
down after sitting
idle for 5 minutes.
Battery power is
running low
Reader turned on
with test cartridge
already inserted
properly inserted or
used cartridge
Press power button.
Change batteries or
use AC adapter
Remove cartridge;
if new, restart
Reader and reinsert
cartridge. If
cartridge is used,
discard it.
Be sure cartridge is
fully inserted and is
properly aligned
ERROR 01
ERROR 02
inserted
Meter Error Meter issue
identified during
self check
Cartridge Error
All cartridge
malfunctions
during testing, or
cartridge is
removed before
completion of test
and has not been
previously used. If
error continues call
MEC Technical
Support
Call MEC
Technical Support
Repeat test with
new cartridge and
new sample. Be
sure cartridge is
fully inserted. If
error continues call
or delayed sample
application
MEC Technical
Support

Error Code
ERROR CAUSE ACTION
ERROR 03
ERROR LO
ERROR HI
ERROR LA
Meter Error Meter malfunctions
after self-check
Result out of Range Result below
5% AIC
Result out of Range Result more than
14% A1C
Result out of Range Result below
0.45 g/dL A1C
Repeat test with
new cartridge and
new sample. If
error continues call
MEC Technical
Support
Repeat test, if error
recurs, test by
another method
Repeat test, if error
recurs, test by
another method
Repeat test, if error
recurs, test by
ERROR HA
ERROR LH
ERROR HH
Warranty
30 Day Money Back Guarantee
Result out of Range Result above
Result out of Range Sample below
Result out of Range Sample above
2.8 g/dL A1C
9 g/dL Hb
20g/dL Hb
another method
Repeat test, if error
recurs, test by
another method
Repeat test, if error
recurs, test by
another method
Repeat test, if error
recurs, test by
another method
Rev. 11/08
 Loading...
Loading...